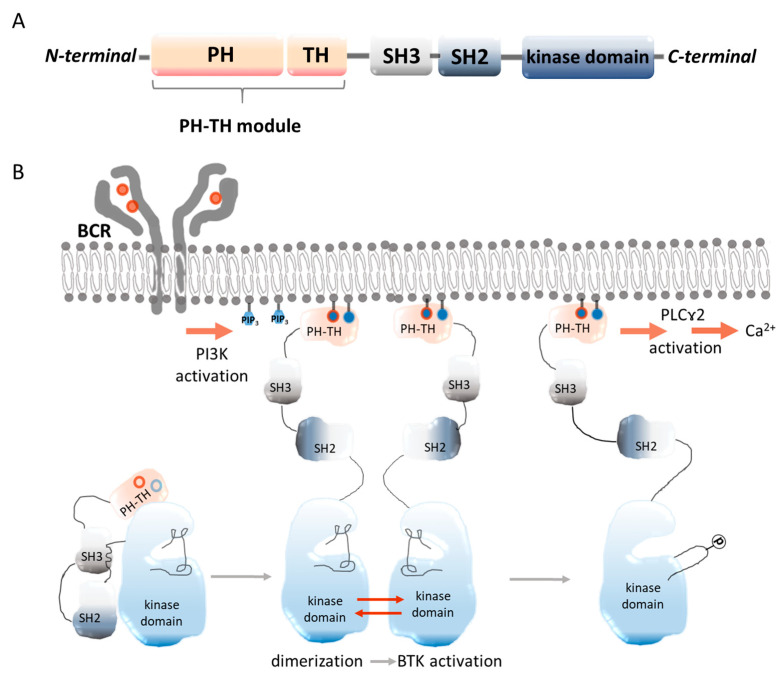
Figure 1.
Schematic representation of the BTK structure. (A) PH—the pleckstrin homology domain, which has the capacity to bind phospholipids, allowing BTK to be recruited from the cytosol to the plasma membrane. TH—the Tec homology domain, which is required for the stability of BTK. SH3, SH2—Src domains are important in protein–-protein interactions. SH2 is a phosphoamino acid binding domain that specifically recognizes phosphotyrosine residues. Kinase domain—the protein’s catalytic domain [7]; BTK activation in the B-cell receptor (BCR) pathway. When the SH3 domain of BTK binds to the SH2-kinase linker, it locks the kinase domain into an inactive conformation, resulting in a compact and autoinhibited Src-like module of BTK. Both the assembled conformation of the Src-like module of BTK and the inactive conformation of the kinase domain are stabilized by the PH-TH module. In the next step, the PH-TH module binds to two PIP3 lipids, which triggers the dimerization of the BTK PH-TH module on the membrane in a switch-like manner. This in turn activates BTK by trans-autophosphorylation [8,9] (B).