Abstract
Jabuticaba (Plinia cauliflora) and jambolan (Syzygium cumini) fruits are rich in phenolic compounds with antioxidant properties, mostly concentrated in the peel, pulp, and seeds. Among the techniques for identifying these constituents, paper spray mass spectrometry (PS-MS) stands out as a method of ambient ionization of samples for the direct analysis of raw materials. This study aimed to determine the chemical profiles of the peel, pulp, and seeds of jabuticaba and jambolan fruits, as well as to assess the efficiency of using different solvents (water and methanol) in obtaining metabolite fingerprints of different parts of the fruits. Overall, 63 compounds were tentatively identified in the aqueous and methanolic extracts of jabuticaba and jambolan, 28 being in the positive ionization mode and 35 in the negative ionization mode. Flavonoids (40%), followed by benzoic acid derivatives (13%), fatty acids (13%), carotenoids (6%), phenylpropanoids (6%), and tannins (5%) were the groups of substances found in greater numbers, producing different fingerprints according to the parts of the fruit and the different extracting solvents used. Therefore, compounds present in jabuticaba and jambolan reinforce the nutritional and bioactive potential attributed to these fruits, due to the potentially positive effects performed by these metabolites in human health and nutrition.
Keywords: jabuticaba, jambolan, peel, pulp, seed, chemical profile, PS-MS
1. Introduction
Jabuticabeira (Plinia cauliflora) and jambolan (Syzygium cumini) are plants that belong to the Myrtaceae family, whose fruits have a sweet and astringent flavor, and a purplish color due to the presence of anthocyanins [1,2]. Studies have reported the presence of several phenolic compounds in different parts of the fruit, such as flavonoids, tannins, gallic and ellagic acids, and quercetin, among others. These phytochemicals possess several bioactivities, responsible for many beneficial activities on human health and nutrition, such as antioxidant, antimicrobial, antimutagenic, and anti-inflammatory activities [3,4,5,6,7,8].
The fruits of Plinia cauliflora and Syzygium cumini have been processed and used to develop new products in different food industry sectors and in the pharmaceutical area, such as jams, desserts, wines, teas, and microcapsules [9,10]. The fruit processing chain generates a considerable number of residual co-products, mainly represented by their peel and seeds, usually discarded and underexplored [10].
Different techniques have been used to identify bioactive compounds in plant materials. High-performance liquid chromatography with an evaporative light-scattering detector and electrophoresis in polyacrylamide gel are examples of these methodologies, however, they require extensive laboratory preparation, time-consuming analyses, and high operational costs, limiting the potential of several studies [11].
Paper-spray mass spectrometry (PS-MS) has been shown to be efficient in overcoming these limitations, as it allows the rapid acquisition of fingerprints of different matrices at a considerably accessible analytical cost and without the generation of chemical residue [12,13,14,15]. Advantages of paper-spray mass spectrometry range from higher replicability to shorter data acquisition time to stronger signal stability [12]. In this method, the compounds present in the raw material are extracted by a solvent, with the drag of the extracted analytes recorded on paper and a spray ionization utilized due to the high voltage applied [16].
The factors that influence an extraction process are diverse and may be related to the conditions and time elapsed, the temperature used during the process, and the sample–solvent ratio, mainly associated with the polarity of the metabolites [17]. Therefore, the objective of this study was to determine the chemical profiles of jabuticaba and jambolan fruits by paper spray mass spectrometry (PS-MS) and to verify the influence of using different solvents (water and methanol) in obtaining fingerprints of their parts, such as the peel, pulp, and seeds.
2. Materials and Methods
2.1. Plant Material and Chemicals
Ripe jabuticaba and jambolan fruits were collected in Paraopeba—Minas Gerais, Brazil (latitude 19° 16′ 54″ S, longitude 44° 24′ 32″ W, altitude 741 m), between August and December 2020. The fruits were harvested manually in the morning from three different matrices. After harvest, they were packed in polyethylene bags, labeled, and kept in thermal boxes until transport to the Organic Chemical Laboratory of the University Federal de São João del Rei—Campus Sete Lagoas. They were then sanitized with sodium hypochlorite and fractionated into their constituent parts: peel, pulp, and seeds (the seeds were ground in a batch mill, IKA A11 basic). The samples were stored separately in a freezer at −18 °C until analysis.
Analytical grade methanol was acquired from Neon (São Paulo, Brazil), and the ultrapure water used was Milli-Q quality.
2.2. Physicochemical Characterization
For the physical analyses, 150 fruits (50 from each of the three matrices) of each species were evaluated. The fruit weight (g) was determined using a digital scale (Diamond, model 500). The longitudinal diameter (mm) and the transversal diameter (mm) were obtained with the aid of a digital caliper (Insize, digital caliper 0–300 mm/0–12″) with a sensitivity of 0.01 mm. To determine the soluble solids (SS) and pH of jabuticaba and jambolan pulps, a benchtop refractometer (AAKER, model: Q767BOV-OW) and pH meter (MS Tecnopon), respectively, were used [18].
2.3. Obtaining Extracts
The peel, pulp, and seeds of the fruits were used to prepare samples of 1.0 g each, which were added to 10 mL, in proportion 1:10 (m/v), of the different solvents analyzed: water and methanol, separately. The samples were stirred for 30 s in a vortex mixer and then kept at rest for 60 min in the dark at room temperature (±25 °C). They were centrifuged at 3500 rpm for 15 min in a bench centrifuge, and the supernatant subsequently transferred to Eppendorf tubes and kept at −18 °C in a cold chamber until further evaluations.
2.4. Mass Spectrometry with Paper Spray Ionization
The chemical profiles of the peel, pulp, and seeds of the fruits under study were analyzed using an LCQ mass spectrometer (Thermo Scientific, San Jose, CA, USA) equipped with a paper spray ionization source. For analysis, 2 μL of samples and 40 μL of methanol were applied on chromatographic paper and cut into a triangular shape (equilateral 1.5 cm) coupled to the equipment. The instrumental conditions of the PS-MS analysis were source voltage equal to −3.0 kV (negative mode) and +4.0 kV (positive mode), a capillary voltage of 40 V, a tube lens voltage of 120 V, transfer tube temperature of 275 °C, and mass range of 100 to 1000 for positive and negative ionization modes. A comparison was made between the mass/charge ratios (m/z) obtained in the study with those found in literature, through fragmentation by sequential mass spectrometry to identify the compounds under analysis. The collision energy used to fragment the compounds ranged from 15 to 40 V [11,18,19].
2.5. Statistical Analysis
The Xcalibur software version 2.1 (Thermo Fisher Scientific Inc., San Jose, CA, USA) processed the mass spectra obtained and tabulated in both ionization modes in Excel 2016 spreadsheets. The fingerprints of the samples in the positive and negative ionization modes were arranged in an X/Y matrix, with data centered on the mean, and the analysis of the principal components was performed in the MatLab software with the aid of the PLS Toolbox.
3. Results and Discussion
3.1. Physicochemical Characterization
Table 1 presents the mean values of the physicochemical characteristics analyzed in the fruits of jabuticaba and jambolan. The results showed that jabuticaba fruits presented an average pH equal to 3.12, quite similar to that of the jambolan sample (pH 3.47).
Table 1.
Physicochemical characteristics of jabuticaba and jambolan fruits: soluble solids (SS) (°Brix), average weight (g), transversal (mm) and longitudinal (mm) diameter.
| Properties | Jabuticaba * | Jambolan * |
|---|---|---|
| pH | 3.12 ± 0.03 | 3.47 ± 0.05 |
| SS | 18.93 ± 2.62 | 16.08 ± 2.17 |
| Weight | 5.63 ± 0.92 | 4.36 ± 1.04 |
| Transversal diameter (TD) | 20.51 ± 1.18 | 15.22 ± 1.29 |
| Longitudinal diameter (LD) | 20.59 ± 1.29 | 21.46 ± 0.83 |
* Means ± standard deviation.
According to Costa et al. [20], the soluble solids content is an extremely important parameter for estimating fruit quality. It is typically associated with the contents of sugars, organic acids, and other microconstituents of plant samples. This characteristic is also related to in natura consumption of these raw materials by the population, and their industrialization, due to the concentrations of nectars produced by the amount of pulp.
The ratio between the diameters was calculated (LD/TD), and the results (1.004 for jabuticaba and 1.409 for jambolan) indicated a spherical shape for jabuticaba and an ellipsoidal or oval shape for jambolan (LD/TD > 1) [18]. The mean fruit weights in this analysis corroborate the values found in other studies involving these materials, such as that of Zerbielli et al. [21] when evaluating jabuticaba and Steiner et al. [22] when studying jambolan fruit samples.
3.2. Chemical Profile
The analyses of the spectra of aqueous and methanolic extracts of jabuticaba and jambolan fruits in ionization modes allowed the identification of 63 compounds, comprising flavonoids (40%, n = 25), benzoic acid derivatives (13%, n = 8), fatty acids (13%, n = 8), carotenoids (6%, n = 4), phenylpropanoids (6%, n = 4), tannins (5%, n = 3), terpenes (5%, n = 3), sugars (5%, n = 3), organic acids (3%, n = 2), amino acids (3%, n = 2), and esters (1%, n = 1), 28 being compounds tentatively identified in the positive ionization mode and 35 in the negative ionization mode.
According to the information base, the compounds found were provisionally identified through fragmentation and comparison with data already reported in the scientific literature. The attempt to identify compounds using PS-MS in the positive ionization mode (+) distinguished eight chemical classes: flavonoids (15), carotenoids (4), sugars (2), amino acids (2), fatty acids (2), one phenylpropanoid (1), one ester (1), and one benzoic acid derivative (1), verified in the subsequent constituent fractions of peel, pulp, and seeds.
Table 2 shows that all 28 compounds identified in the positive ionization mode were present in jabuticaba, while 27 substances were found in jambolan: 5-pyranopelargonidin-3-O-glucoside was not detected in jambolan. In the positive mode, some flavonoids maintain an isomeric relationship and evidently could not be differentiated by their exact mass; examples are delphinidin and peonidin (m/z 303), and some carotenoids, such as 9-cis-β-carotene, all-trans-β-carotene and 13-cis-β-carotene (m/z 537), all-trans-zeaxanthin, all-trans-lutein and cis-lutein (m/z 569), and cis-neoxanthin and cis-violaxanthin (m/z 601).
Table 2.
Compounds provisionally identified in the peel (PL), pulp (PP) and seeds (SD) of jabuticaba and jambolan fruits by (+) PS/MS.
| Jabuticaba | Jambolan | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Water | Methanol | ||||||||||||
| m/z | Compounds | PL | PP | SD | PL | PP | SD | PL | PP | SD | PL | PP | SD | MS-MS | Ref. |
| Benzoic acid derivatives | |||||||||||||||
| 138 | p-Hydroxybenzoic acid | nd | nd | nd | X | nd | X | nd | nd | nd | X | X | nd | 159 | [23] |
| Ester | |||||||||||||||
| 333 | Galloyl-glucose ester | nd | nd | nd | nd | nd | X | nd | nd | nd | nd | X | X | 153 | [23] |
| Amino acid | |||||||||||||||
| 175 | L-Arginine | X | nd | X | X | nd | X | X | X | nd | nd | X | X | 70, 129 | [24] |
| 205 | Tryptophan | X | X | nd | X | X | nd | X | X | nd | X | X | nd | 188 | [25] |
| Phenylpropanoids | |||||||||||||||
| 181 | Caffeic acid | X | X | X | X | X | X | X | nd | nd | X | nd | nd | - | [23] |
| 203 | Glucose | X | X | X | X | X | X | X | X | X | X | X | X | - | [26] |
| 365 | Sucrose | X | X | X | X | X | X | X | X | X | X | X | X | 203 | [25] |
| Flavonoids | |||||||||||||||
| 270 | Apigenin | X | X | nd | X | X | nd | X | X | nd | X | X | nd | - | [27] |
| 287 | Cyanidin | X | X | X | X | X | X | X | X | nd | X | X | nd | - | [23] |
| 301 | Diosmetin | X | X | X | X | X | X | X | X | nd | X | X | nd | 286 | [25] |
| 303 | Delphinidin/ Peonidin |
X | nd | X | X | nd | X | X | X | nd | X | X | nd | - | [23] |
| 317 | Petunidin | X | X | X | X | X | X | X | X | X | X | X | X | - | [23] |
| 331 | Malvidin | X | X | X | X | X | X | X | X | X | X | X | X | - | [23] |
| 433 | Pelargonidin-3-O-glucoside | X | nd | X | X | nd | X | X | nd | nd | X | nd | nd | 271 | [28] |
| 501 | 5-Pyranopelargonidin-3-O-glucoside | X | X | X | X | X | X | nd | nd | nd | nd | nd | nd | - | [25] |
| 611 | Rutin | X | X | X | X | X | X | X | X | X | X | X | X | 303 | [27] |
| 625 | Peonidin-3,5-diglucoside | X | nd | X | X | nd | X | X | nd | nd | X | nd | nd | 301 | [29] |
| 627 | Delphinidin-3,5-O-diglucoside | X | nd | X | X | nd | X | X | X | nd | X | X | nd | 465 | [30] |
| 629 | Quercetin-3-7-diglycoside | X | X | X | X | X | X | X | X | nd | X | X | nd | 383 | [31] |
| 641 | Petunidin-3,5-diglucoside | X | X | X | X | X | X | X | X | X | X | X | X | 479, 317 | [32] |
| 644 | Dihydromyricetin diglucoside | X | nd | X | X | nd | X | X | nd | nd | X | nd | nd | - | [23] |
| 655 | Malvidin-3,5-diglucoside | X | X | X | X | X | X | X | X | X | X | X | X | 493 | [33] |
| Fatty acids | |||||||||||||||
| 285 | Octadecanoic acid | nd | nd | nd | nd | nd | X | nd | nd | nd | X | X | X | - | [34] |
| 293 | Lycanic acid | nd | nd | nd | X | nd | X | nd | nd | nd | X | X | X | 257 | [25] |
| Carotenoids | |||||||||||||||
| 537 | 9-cis-β-Carotene/ All-trans-β-carotene/ 13-cis-β-Carotene |
nd | nd | nd | X | X | X | nd | nd | nd | X | X | X | - | [32] |
| 553 | All-trans-β-cryptoxanthin | nd | nd | nd | X | X | X | nd | nd | nd | X | X | X | - | [32] |
| 569 | All-trans-zeaxanthin/all-trans-lutein/ cis-lutein |
nd | nd | nd | X | X | X | nd | nd | nd | nd | X | X | 551 | [25,32] |
| 601 |
cis-Neoxanthin/ cis-Violaxanthin |
nd | nd | nd | X | nd | X | nd | nd | nd | X | X | nd | - | [32] |
X: detected, nd: not detected.
Ripe fruits contain high levels of carbohydrates, such as glucose and sucrose, which can be identified by PS-MS. These nutrients were also found in several other fruits, such as cagaita [24], cambuí [18], ripe banana peel [19], grumixama [25], pequi [13] and cerrado pear [35], using identical methodologies.
In both fruits under study, the presence of flavonoids stands out, mainly derived from glycosidic conjugates, a form that confers better performance to plants, such as protection against UV radiation and microbial pathogens [36,37]. This group of secondary metabolites is responsible for several beneficial health properties, such as antioxidant, antimicrobial, antimutagenic, antihypertensive, antidiabetic, and anti-inflammatory activities [3,8,12].
Among the class of flavonoids, the anthocyanins represented by the ions at m/z 287, 303, 317, 331, 433, 625, 627, 641, and 655 stand out, totaling nine tentatively identified compounds. According to Minighin et al. [38], anthocyanins are natural pigments belonging to the large class phenolic compounds that can vary from bright red to violet/blue, being water-soluble compounds, which may explain why they are all found in the aqueous extracts evaluated.
Anthocyanins are located mainly in the peel and seeds of jabuticaba and jambolan fruits. However, they were also tentatively identified in the pulp of these materials, which have a colorless hue. This is due to the migration of these constituents, possibly as a result of fruit ripening or during sample preparation [30].
The results obtained in the present study reinforce the potential for using jabuticaba peel and other dark-colored fruits, such as jambolan, in the development of natural dyes, due to the presence of anthocyanins as the main class of flavonoids tentatively identified.
Tavares et al. [30] identified nine anthocyanins, mainly derived from delphinidin, petunidin, and malvidin, in the edible parts of jambolan. The same number was obtained in another study by Tavares et al. [8] in jambolan fruit, juice, and powder. The species of the Myrtaceae family are generally considered as good sources of anthocyanins, and the higher their content, the greater the intensity of the peel color [30].
Carotenoids were also significantly represented in jabuticaba and jambolan samples in the positive ionization mode. These substances play an important role in plant metabolism and have beneficial effects at the level on human health [39]. The carotenoids all-trans-β-cryptoxanthin, 13-cis-β-carotene, all-trans-β-carotene, and 9-cis-β-carotene, tentatively identified in jabuticaba and jambolan, were also present in Syzygium cumini in the study reported by Faria et al. [32].
The frozen jabuticaba peel and pulp (−18 °C) were characterized by Dessimoni-Pinto et al. [40]. According to the authors, it was possible to observe that the highest concentrations of nutrients were found in the peel of this fruit, with significant levels of natural pigments, such as certain flavonoids, fibers, and carbohydrates, represented mainly by sugars.
In terms of the nutritional potential of seeds, Fidelis et al. [3] and Khan et al. [5] reinforced the activities of these constituent fractions of jabuticaba and jambolan, respectively, by observing the influence of compounds identified in these materials, with promising antimutagenic and DNA-protective properties for S. cumini seeds, and antioxidant, antimicrobial, anti-hyperglycemic, anti-inflammatory, and antihypertensive activities for jabuticaba seeds.
The jabuticaba contains all 35 compounds tentatively identified in the negative ionization mode, whereas for jambolan, 33 compounds were found (gallic and abscisic acids were not detected; Table 3). In the negative mode, some flavonoids also show an isomeric relationship and evidently could not be differentiated by their exact mass; examples are provided by epicatechin and catechin (m/z 289), as well as some tannins, such as castalagin and vescalagin (m/z 933).
Table 3.
Compounds provisionally identified in the peel (PL), pulp (PP) and seeds (SD) of jabuticaba and jambolan fruits by (-) PS/MS.
| Jabuticaba | Jambolan | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Water | Methanol | ||||||||||||
| m/z | Compounds | PL | PP | SD | PL | PP | SD | PL | PP | SD | PL | PP | SD | MS-MS | Ref. |
| Organic acid | |||||||||||||||
| 133 | Malic acid | X | X | nd | X | X | nd | X | X | X | X | X | X | 89, 115 | [24] |
| 191 | Citric acid | X | X | X | X | X | X | X | X | X | X | X | X | 85, 111 | [24] |
| Benzoic acid derivatives | |||||||||||||||
| 137 | Salycilic acid | nd | nd | nd | nd | nd | X | nd | nd | nd | nd | X | X | - | [41] |
| 169 | Gallic acid | X | nd | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | - | [42] |
| 197 | Syringic acid | X | X | X | X | X | X | X | X | X | X | X | X | - | [41] |
| 299 | Hydroxybenzoic-O-hexoside acid | X | X | X | X | X | X | X | X | nd | X | X | nd | - | [41] |
| 301 | Ellagic acid | nd | nd | nd | X | nd | X | nd | nd | nd | X | X | nd | - | [43] |
| 307 | Protocatechuic acid | X | X | X | X | X | X | X | X | X | X | X | X | - | [44] |
| 329 | Vanillic acid hexoside | X | X | nd | X | X | nd | X | X | X | X | X | X | - | [45] |
| Sugar | |||||||||||||||
| 179 | Hexose | X | X | X | X | X | X | X | X | X | X | X | X | 89 | [24] |
| Fatty acids | |||||||||||||||
| 255 | Palmitic acid | nd | nd | nd | nd | X | X | nd | nd | nd | nd | X | X | - | [26] |
| 277 | α-Linolenic acid | nd | nd | nd | X | X | X | nd | nd | nd | X | X | X | - | [26] |
| 279 | Linoleic acid | nd | nd | nd | nd | X | X | nd | nd | nd | nd | X | X | - | [26] |
| 281 | Oleic acid | nd | nd | nd | nd | X | X | nd | nd | nd | nd | X | X | - | [26] |
| 283 | Stearic acid | nd | nd | nd | X | X | X | nd | nd | nd | X | X | X | - | [26] |
| 367 | Lignoceric acid | nd | nd | nd | X | X | nd | nd | nd | nd | X | X | X | - | [26] |
| Flavonoids | |||||||||||||||
| 285 | Kaempferol | X | nd | nd | X | nd | nd | X | X | nd | X | X | nd | 197, 213, 217, 241 | [25] |
| 289 | Epicatechin/ Catechin |
X | X | X | X | X | X | X | X | nd | X | X | nd | 245 | [46,47] |
| 301 | Quercetin | X | X | X | X | X | X | X | X | X | X | X | X | - | [46] |
| 303 | Taxifolin | X | X | nd | X | X | nd | X | X | nd | X | X | nd | 241, 285 | [25] |
| 305 | Gallocatechin | X | X | X | X | X | X | X | X | nd | X | X | nd | 137, 261 | [25] |
| 315 | Isorhamnetin | X | nd | X | X | nd | X | nd | nd | X | nd | nd | X | 300 | [41] |
| 317 | Myricetin | X | nd | nd | X | nd | nd | X | nd | nd | X | nd | nd | - | [46] |
| 431 | Vitexin | X | X | X | X | X | X | X | X | X | X | X | X | - | [24] |
| 447 | Cyanidin-3-O-glucoside | X | nd | X | X | nd | X | X | X | nd | X | X | nd | 285 | [48] |
| 463 | Isoquercetin | X | X | nd | X | X | nd | X | X | X | X | X | X | - | [41] |
| Phenylpropanoids | |||||||||||||||
| 311 | Caftaric acid | X | X | X | X | X | X | X | X | X | X | X | X | - | [24] |
| 325 | p-Coumaric acid hexoside | X | X | X | X | X | X | X | X | X | X | X | X | 145 | [25] |
| 339 | Cafeoil-D-glucose | X | X | X | X | X | X | X | X | X | X | X | X | - | [24] |
| Terpenes | |||||||||||||||
| 263 | Abscisic acid | nd | nd | nd | X | nd | nd | nd | nd | nd | nd | nd | nd | 309, 527 | [49] |
| 485 | Annurcoic acid | nd | nd | nd | X | nd | X | nd | nd | nd | nd | X | X | 441 | [26] |
| 501 | Guavenoic acid | nd | nd | nd | nd | X | X | nd | nd | nd | X | X | X | 467, 503 | [26] |
| Tannin | |||||||||||||||
| 783 | Pedunculagin | nd | nd | nd | X | nd | X | nd | nd | nd | X | X | X | 257, 301 | [46] |
| 933 | Castalagin/ Vescalagin |
nd | nd | nd | X | nd | X | nd | nd | nd | X | X | X | 301, 915 | [46] |
| 935 | Potentillin | nd | nd | nd | X | nd | X | nd | nd | nd | X | X | X | 301, 633 | [46] |
X: detected, nd: not detected.
Table 3 shows the compounds possibly identified in the negative mode (-), which can be grouped into eight chemical classes, namely flavonoids (10), benzoic acid derivatives (7), fatty acids (6), tannins (3), phenylpropanoids (3), terpenes (3), organic acids (2), and one sugar (1), with a differential distribution among the fruit fractions under study.
The flavonoid at m/z 447, cyanidin-3-O-glucoside, is the most abundant anthocyanin in dark-skinned fruits [50] and was detected in both jabuticaba and jambolan, as well as in the study by Lequiste et al. [51] in freeze-dried jabuticaba peel and the aqueous extract of jabuticaba peel, by Quatrin et al. [7] in powdered jabuticaba peel, by Fidelis et al. [3] in jabuticaba seeds, and by Tavares et al. [8] in jambolan (fruit, juice, and powder).
Another group of compounds tentatively identified in the present study was that of fatty acids, with six representative compounds comprising saturated (palmitic, stearic, and lignoceric acids), monounsaturated (oleic acid), and polyunsaturated (α-linolenic and linoleic acids) fatty acids. Of these, oleic and linoleic acids play an important role in human nutrition [35]. Using PS-MS, Mariano et al. [35] also identified the presence of oleic acid in cerrado pear (Eugenia klotzschiana Berg), another fruit of the Myrtaceae family.
The tentatively identified phenylpropanoids are also highlighted. This group of substances has demonstrated several pharmacological activities, such as anti-inflammatory, antitumor, antibacterial, and antifungal action [18]. In addition, it is reported in the literature that many chronic non-communicable diseases, such as cardiovascular diseases, type II diabetes, and various types of cancer, have their development reduced by the preventive action of phenylpropanoids [52]. When studying the chemical profile of cambuí pulp, a fruit of the Myrtaceae family, García et al. [18] observed that benzoic acid derivatives were also one of the most predominant classes.
In terms of comparing the solvents used, fatty acids, carotenoids, terpenes, and tannins were not detected in aqueous extracts. Fatty acids and terpenes have low affinity for water; carotenoids are fat-soluble molecules and are soluble in organic solvents such as petroleum ether, methanol, and acetone [53]. According to Três et al. [54], knowledge of the solubility of carotenoids in organic solvents is of fundamental importance for the understanding of recrystallization processes using solvents.
According to Bazykina et al. [55], alcohol is more effective in extracting tannins and the anthocyanins have a comparatively higher affinity for water [56]. However, some scientific studies report that it is possible to improve the solubility of anthocyanins and other phenolic compounds by using a mixture of solvents, using aqueous solutions and organic solvents (e.g., hydroethanolic mixtures) [57,58,59].
Quatrin et al. [7] also identified castalagin/vescalagin and pedunculagin in powdered jabuticaba peel. According to these authors, hydrolysable tannins play an important role in the antioxidant capacity of jabuticaba. Furthermore, as Tavares et al. [30] reported, these compounds can cause the astringent sensation of jambolan.
Preferential solubility in each solvent is a peculiar characteristic of certain phytochemical classes, such as flavonoids, fatty acids, sugars, and other phenolics, which explains, for example, the lack of a universal extraction procedure. Studies report that high polarity solvents are more effective in extracting phenolic compounds [60].
3.3. Principal Component Analysis (PCA)
In addition to the attempt to identify the chemical constituents of jabuticaba and jambolan, a PCA was performed among the samples. A data matrix was generated using the presence or absence of compounds found in the peel, pulp, and seeds of the fruits, when performing a fusion of the results obtained in the two ionization modes evaluated.
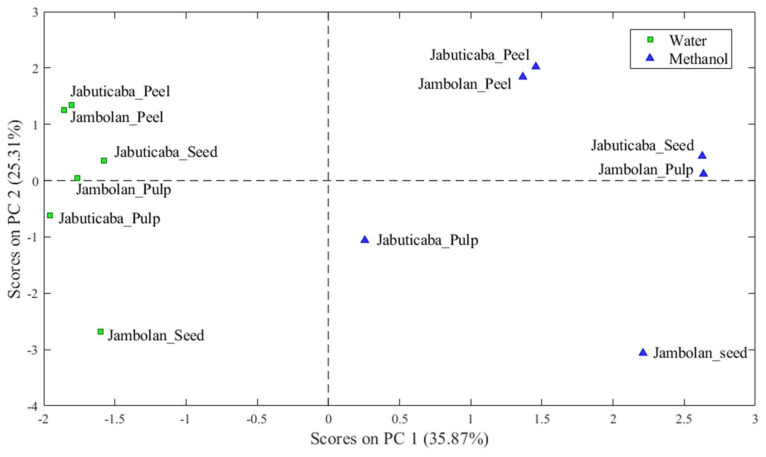
The models were built by selecting two main components of analysis (PC1 and PC2), which explained 61.18% (PC1 35.87% and PC2 25.31%) of the total variability of the data related to the effect of the use of different solvents and evaluation of the different parts of the fruits (Figure 1).
Figure 1.
PC1 and PC2 jabuticaba and jambolan samples.
Through the loads PC1 and PC2, it is possible to observe that, depending on the solvent used, the jabuticaba and jambolan samples were grouped according to their constituent fractions, which leads to the realization that there was a significant difference in terms of the use of water or methanol in extracting substances.
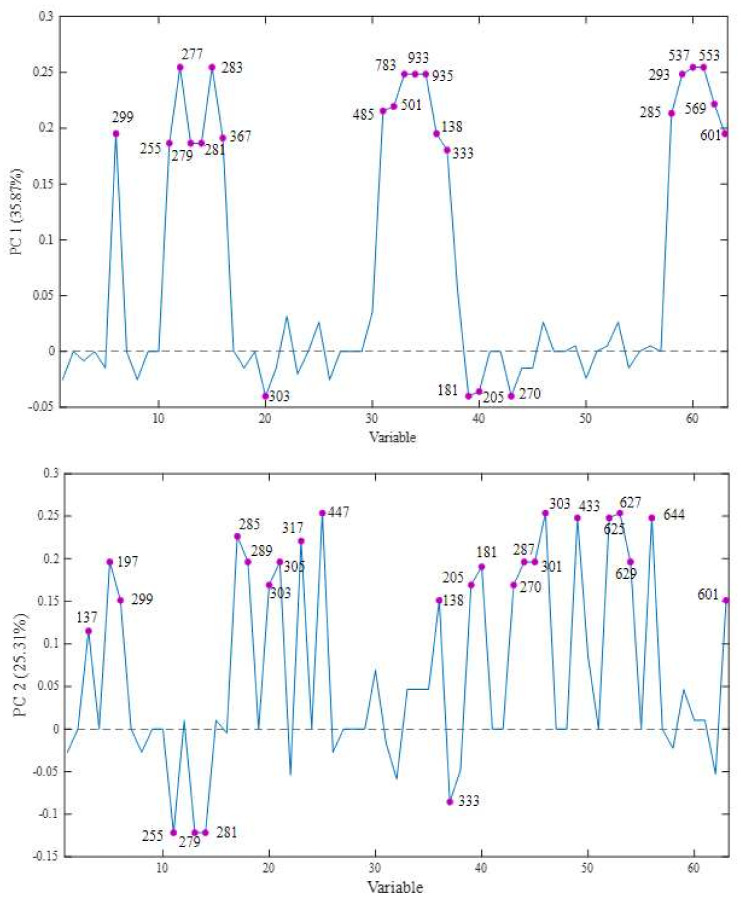
PC1 showed the discrimination of the fruits through its positive scores, where all the methanolic fractions were found, both from jabuticaba and jambolan. The compounds responsible for this separation were hydroxybenzoic acid-O-hexoside, the fatty acids palmitic, α-linolenic, linoleic, oleic, octadecanoic, licanic, stearic and lignoceric, the tannins pedunculagin, castalagin/vescalagin and potentilin, the terpenes annurcoic acid and guavenoic acid, p-hydroxybenzoic acid, galloyl-glucose ester, and the carotenoids 9-cis-β-carotene/all-trans-β-carotene/13-cis-β-carotene, all-trans-β-cryptoxanthin, all-trans-zeaxanthin/all-trans-lutein/cis-lutein and cis-neoxanthin/cis-violaxanthin (positive score), and taxifolin, caffeic acid, tryptophan and apigenin (negative score), as shown in Figure 2.
Figure 2.
Representation of the loads responsible for the discrimination of the scores of the samples in PC 1 and PC 2.
In turn, PC2 shows the distinction between the constituent fractions, mainly the peel of the two fruits (positive) and the seeds (negative) of jambolan. The following compounds responsible for this differentiation are: salicylic acid, syringic acid, hydroxybenzoic acid-O-hexoside, kaempferol, epicatechin/catechin, taxifolin, gallocatechin, myricetin, cyanidin-3-O-glycoside, p-hydroxybenzoic, tryptophan, caffeic acid, apigenin, cyanidin, diosmetin, delphinidin/peonidin, perlagonidin-3-O-glucoside, peonidin-diglucoside, delphinidin-3,5-O-diglucoside, quercetin-3-7-diglucoside, dihydromyricetin diglucoside and cis-neoxanthin/cis-violaxanthin (positive scores), and palmitic, linoleic, oleic and galloyl-glucose ester acids (negative scores).
4. Conclusions
The use of mass spectrometry in the ambient ionization mode by paper spray provided comprehensive information associated with the chemical composition of jabuticaba and jambolan fruits. Compounds present in these fruits, such as flavonoids, carotenoids, tannins, and organic acids reinforce the potential bioactive effect attributed to these materials, as well as the possibility of technological and sensory use due to their physicochemical properties.
In terms of distinction between solvents, it is concluded that both water and methanol were efficient for obtaining fingerprints of different parts of the fruits (pulp, peel, and seed). Although some metabolites have not been detected in aqueous extracts, there is the possibility of using water as a solvent to extract compounds for PS-MS analysis, as it is a non-toxic solvent with good affinity for anthocyanins, the main class of flavonoids tentatively identified in this study.
Acknowledgments
The authors thank the Universidade Federal de Minas Gerais (UFMG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (88887.503309/2020-00), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Pró-Reitoria de Pesquisa (PRPq) da Universidade Federal de Minas Gerais (UFMG), Universidade Federal de São João del-Rei (UFSJ) and Instituto Federal de Educação Ciência e Tecnologia de Minas Gerais (IFMG) for financial support.
Author Contributions
Conceptualization, V.T.d.V.C., J.O.F.M. and C.A.F.; methodology, V.T.d.V.C. and A.L.C.C.R.; validation, V.T.d.V.C. and H.d.O.P.M.; formal analysis, V.T.d.V.C., H.d.O.P.M. and A.L.C.C.R.; investigation, V.T.d.V.C., V.D.M.S. and M.R.S.; resources, R.A., A.C.C.F.F.d.P., R.M.d.S.B.F., J.O.F.M. and C.A.F.; data curation, V.T.d.V.C.; writing—original draft preparation, V.T.d.V.C., V.D.M.S. and A.L.C.C.R.; writing—review and editing, M.R.S., R.M.d.S.B.F., J.O.F.M. and C.A.F.; visualization, V.T.d.V.C. and V.D.M.S.; supervision, M.R.S., R.A., J.O.F.M. and C.A.F.; project administration, J.O.F.M. and C.A.F.; funding acquisition, R.A., A.C.C.F.F.d.P., R.M.d.S.B.F., J.O.F.M. and C.A.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sanches M.A.R., Camelo-Silva C., Tussolini L., Tussolini M., Zambiazi R.C., Pertuzatti P.B. Development, characterization and optimization of biopolymers films based on starch and flour from jabuticaba (Myrciaria cauliflora) peel. Food Chem. 2021;343:128430. doi: 10.1016/j.foodchem.2020.128430. [DOI] [PubMed] [Google Scholar]
- 2.Seraglio S.K.T., Schulz M., Nehring P., Della Betta F., Valese A.C., Daguer H., Gonzaga L.V., Fett R., Costa A.C.O. Nutritional and bioactive potential of Myrtaceae fruits during ripening. Food Chem. 2018;239:649–656. doi: 10.1016/j.foodchem.2017.06.118. [DOI] [PubMed] [Google Scholar]
- 3.Fidelis M., Do Carmo M.A.V., Azevedo L., Cruz T.M., Marques M.B., Myoda T., Sant’Ana A.S., Furtado M.M., Wen M., Zhang L., et al. Response surface optimization of phenolic compounds from jabuticaba (Myrciaria cauliflora [Mart.] O.Berg) seeds: Antioxidant, antimicrobial, antihyperglycemic, antihypertensive and cytotoxic assessments. Food Chem. Toxicol. 2020;142:111439. doi: 10.1016/j.fct.2020.111439. [DOI] [PubMed] [Google Scholar]
- 4.Galvão B.V.D., Araujo-Lima C.F., Dos Santos M.C.P., Seljan M.P., Carrão-Dantas E.K., Aiub C.A.F., Cameron L.C., Ferreira M.S.L., Gonçalves É.C.B.A., Felzenszwalb I. Plinia cauliflora (Mart.) Kausel (Jaboticaba) leaf extract: In vitro anti-Trypanosoma cruzi activity, toxicity assessment and phenolic-targeted UPLC-MSE metabolomic analysis. J. Ethnopharmacol. 2021;277:114217. doi: 10.1016/j.jep.2021.114217. [DOI] [PubMed] [Google Scholar]
- 5.Khan M.S., Qais F.A., Ahmad I., Hussain A., Alajmi M.F. Genotoxicity inhibition by Syzygium cumini (L.) seed fraction and rutin: Understanding the underlying mechanism of DNA protection. Toxicol. Res. 2018;7:165–171. doi: 10.1039/C7TX00269F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qamar M., Akhtar S., Ismail T., Wahid M., Ali S., Nazir Y., Murtaza S., Abbas M.W., Ziora Z.M. Syzygium cumini (L.) Skeels extracts; in vivo anti-nociceptive, anti-inflammatory, acute and subacute toxicity assessment. J. Ethnopharmacol. 2022;287:114919. doi: 10.1016/j.jep.2021.114919. [DOI] [PubMed] [Google Scholar]
- 7.Quatrin A., Pauletto R., Maurer L.H., Minuzzi N., Nichelle S.M., Carvalho J.F.C., Maróstica Junior M.R., Rodrigues E., Bochi V.C., Emanuelli T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019;78:59–74. doi: 10.1016/j.jfca.2019.01.018. [DOI] [Google Scholar]
- 8.Tavares I.M.C., Nogueira T.Y.K., Mauro M.A., Goméz-Alonso S., Gomes E., Da Silva R., Hermosín-Gutiérrez I., Lago-Vanzela E.S. Dehydration of jambolan [Syzygium cumini (L.)] juice during foam mat drying: Quantitative and qualitative changes of the phenolic compounds. Food Res. Int. 2017;102:32–42. doi: 10.1016/j.foodres.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 9.Albuquerque B.R., Pereira C., Calhelha R.C., Alves M.J., Abreu R.M.V., Barros L., Oliveira M.B.P.P., Ferreira I.C.F.R. Jabuticaba residues (Myrciaria jaboticaba (Vell.) Berg) are rich sources of valuable compounds with bioactive properties. Food Chem. 2020;309:125735. doi: 10.1016/j.foodchem.2019.125735. [DOI] [PubMed] [Google Scholar]
- 10.da Veiga Correia V.T., da Silva P.R., Ribeiro C.M.S., Ramos A.L.C.C., Mazzinghy A.C.d.C., Silva V.D.M., Júnior A.H.O., Nunes B.V., Vieira A.L.S., Ribeiro L.V., et al. An integrative review on the main flavonoids found in some species of the Myrtaceae family: Phytochemical characterization, health benefits and development of products. Plants. 2022;11:2796. doi: 10.3390/plants11202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campelo F.A., Henriques G.S., Simeone M.L.F., Queiroz V.A.V., Silva M.R., Augusti R., Melo J.O.F., Lacerda I.C.A., Araújo R.L.B. 2020 Study of thermoplastic extrusion and its impact on the chemical and nutritional characteristics in two sorghum genotypes SC 319 and BRS 332. J. Braz. Chem. Soc. 2020;31:788–802. doi: 10.21577/0103-5053.20190243. [DOI] [Google Scholar]
- 12.Nascimento C.D., De Paula A.C.C.F.F., Oliveira Junior A.H., Mendonça H.O.P., Reina L.C.B., Augusti R., Figueiredo-Ribeiro R.C.L., Melo J.O.F. Paper Spray Mass Spectrometry on the Analysis of Phenolic Compounds in Rhynchelytrum repens: A Tropical Grass with Hypoglycemic Activity. Plants. 2021;10:1617. doi: 10.3390/plants10081617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos B.O., Tanigaki M., Silva M.R., Ramos A.L.C.C., Labanca R.A., Augusti R., Melo J.O.F., Takahashi J.A., Araújo R.L.B. Development and Chemical Characterization of Pequi Pericarp Flour (Caryocar brasiliense Camb.) and Effect of in vitro Digestibility on the Bioaccessibility of Phenolic Compounds. J. Braz. Chem. Soc. 2022;33:1058–1068. doi: 10.21577/0103-5053.20220022. [DOI] [Google Scholar]
- 14.Silva V.D.M., Macedo M.C.C., Santos A.N., Silva M.R., Augusti R., Lacerda I.C.A., Melo J.O.F., Fante C.A. Bioactive activities and chemical profile characterization using paper spray mass spectrometry of extracts of Eriobotrya japonica Lindl. leaves. Rapid Commun. Mass Spectrom. 2020;34:e8883. doi: 10.1002/rcm.8883. [DOI] [PubMed] [Google Scholar]
- 15.Silva G.G., Pimenta L.P.S., Melo J.O.F., Mendonça H.O.P., Augusti R., Takahashi J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules. 2022;27:1892. doi: 10.3390/molecules27061892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y., Wang H., Liu J., Zhang Z., Mcluckey M.N., Ouyang Z. Analysis of Biological Samples Using Paper Spray Mass Spectrometry: An Investigation of Impacts by the Substrates, Solvents and Elution Methods. Chromatographia. 2013;76:1339–1346. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García Y.M., Ramos A.L.C.C., Oliveira Júnior A.H., De Paula A.C.C.F.F., De Melo A.C., Andrino M.A., Silva M.R., Augusti R., de Araújo R.L.B., de Lemos E.E.P., et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria floribunda (H. West ex Willd.) O. Berg Accessions. Molecules. 2021;26:7206. doi: 10.3390/molecules26237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva V.D.M., Arquelau P.B.F., Silva M.R., Augusti R., Melo J.O.F., Fante C.A. Use of paper spray-mass spectrometry to determine the chemical profile of ripe banana peel flour and evaluation of its physicochemical and antioxidant properties. Quím. Nova. 2020;43:579–585. doi: 10.21577/0100-4042.20170521. [DOI] [Google Scholar]
- 20.Costa D.S., Plácido G.R., Takeuchi K.P., Sousa T.L. Physical and biometric characterization of jabuticaba variety ‘Pingo De Mel’ oriunda of cerrado goiano. Res. Soc. Dev. 2020;9:e146953323. doi: 10.33448/rsd-v9i5.3323. [DOI] [Google Scholar]
- 21.Zerbielli L., Nienow A.A., Dalacorte L., Jacobs R., Daronch T. Diversidade físico-química dos frutos de jabuticabeiras em um sítio de ocorrência natural. Rev. Bras. Frutic. 2016;38:107–116. doi: 10.1590/0100-2945-267/14. [DOI] [Google Scholar]
- 22.Steiner F., Zuffo A.M., Zoz T. Physical characterization of fruits and seeds of jambolan [Syzygium cumini (L.) Skeels] (Myrtaceae) Acta Iguazu. 2017;6:79–90. doi: 10.48075/actaiguaz.v6i3.16551. [DOI] [Google Scholar]
- 23.Sharma R.J., Gupta R.C., Bansal A.K., Singh I.P. Metabolite Fingerprinting of Eugenia jambolana Fruit Pulp Extracts using NMR, HPLC-PDA-MS, GC-MS, MALDI-TOF-MS and ESI-MS/MS Spectrometry. Nat. Prod. Commun. 2015;10:969–976. doi: 10.1177/1934578X1501000644. [DOI] [PubMed] [Google Scholar]
- 24.Silva M.R., Freitas L.G., Souza A.G., Araújo R.L.B., Lacerda I.C.A., Pereira H.V., Augusti R., Melo J.O.F. Antioxidant Activity and Metabolomic Analysis of Cagaitas (Eugenia dysenterica) using Paper Spray Mass Spectrometry. J. Braz. Chem. Soc. 2019;30:1034–1044. doi: 10.21577/0103-5053.20190002. [DOI] [Google Scholar]
- 25.Ramos A.L.C.C., Mendes D.D., Silva M.R., Augusti R., Melo J.O.F., Araújo R.L.B., Lacerda I.C.A. Chemical profile of Eugenia brasiliensis (Grumixama) pulp by PS/MS paper spray and SPME-GC/MS solid-phase microextraction. Res. Soc. Dev. 2020;9:e318974008. doi: 10.33448/rsd-v9i7.4008. [DOI] [Google Scholar]
- 26.Ramos A.S., Souza R.O.S., Boleti A.P.A., Bruginski E.R.D., Lima E.S., Campos F.R., Machado M.B. Chemical characterization and antioxidant capacity of the araçá-pera (Psidium acutangulum): An exotic Amazon fruit. Food Res. Int. 2015;75:315–327. doi: 10.1016/j.foodres.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Siebert D.A., Bastos J., Spudeit D.A., Micke G.A., Alberton M.D. Determination of phenolic profile by HPLC-ESI-MS/MS and anti-inflammatory activity of crude hydroalcoholic extract and ethyl acetate fraction from leaves of Eugenia brasiliensis. Rev. Bras. Farmacogn. 2017;27:459–465. doi: 10.1016/j.bjp.2017.01.008. [DOI] [Google Scholar]
- 28.Oliveira P.S., Chaves V.C., Bona N.P., Soares M.S.P., Cardoso J.S., Vasconcellos F.A., Tavares R.G., Vizzotto M., da Silva L.M.C., Grecco F.B., et al. Eugenia uniflora fruit (red type) standardized extract: A potential pharmacological tool to diet-induced metabolic syndrome damage management. Biomed. Pharmacother. 2017;92:935–941. doi: 10.1016/j.biopha.2017.05.131. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Adams L.S., Chen S., Killian C., Ahmed A., Seeram N.P. Eugenia jambolana Lam. Berry Extract Inhibits Growth and Induces Apoptosis of Human Breast Cancer but Not Non-Tumorigenic Breast Cells. J. Agric. Food Chem. 2009;57:826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavares I.M.C., Lago-Vanzela E.S., Rebello L.P.G., Ramos A.M., Gómez-Alonso S., García-Romero E., da Silva R., Hermosín-Gutiérrez I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels) Food Res. Int. 2016;82:1–13. doi: 10.1016/j.foodres.2016.01.014. [DOI] [Google Scholar]
- 31.Furlan C.M., Santos D.Y.A.C., Motta L.B., Domingos M., Salatino A. Guava flavonoids and the effects of industrial air pollutants. Atmos. Pollut. Res. 2010;1:30–35. doi: 10.5094/APR.2010.005. [DOI] [Google Scholar]
- 32.Faria A.F., Marques M.C., Mercadante A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011;126:1571–1578. doi: 10.1016/j.foodchem.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira C.T., Ramos A.L.C.C., Mendonça H.O.P., Consenza G.P., Silva M.R., Fernandes C., Augusti R., Melo J.O.F., Ferreira A.V.M., Araújo R.L.B. Quantification of 6-gingerol, metabolomic analysis by paper spray mass spectrometry and determination of antioxidant activity of ginger rhizomes (Zingiber officinale) Res. Soc. Dev. 2020;9:e366984822. doi: 10.33448/rsd-v9i8.4822. [DOI] [Google Scholar]
- 34.Calloni C., Agnol R.D., Martínez L.S., Marcon F.S., Moura S., Salvador M. Jaboticaba (Plinia trunciflora (O. Berg) Kausel) fruit reduces oxidative stress in human fibroblasts cells (MRC-5) Food Res. Int. 2015;70:15–22. doi: 10.1016/j.foodres.2015.01.032. [DOI] [Google Scholar]
- 35.Mariano A.P.X., Ramos A.L.C.C., Augusti R., Araújo R.L.B., Melo J.O.F. Analysis of the chemical profile of cerrado pear fixed compounds by mass spectrometry with paper spray and volatile ionization by SPME-HS CG-MS. Res. Soc. Dev. 2020;9:e949998219. doi: 10.33448/rsd-v9i9.8219. [DOI] [Google Scholar]
- 36.Cavaliere C., Foglia P., Pastorini E., Samperi R., Lagana A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:3143–3158. doi: 10.1002/rcm.2185. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira Júnior A.H., Ramos A.L.C.C., Guedes M.N.S., Fagundes M.C.P., Augusti R., Melo J.O.F. Chemical profile and bioprospecting of cocoa beans analyzed by paper spray mass spectrometry. Res. Soc. Dev. 2020;9:e975986882. doi: 10.33448/rsd-v9i8.6882. [DOI] [Google Scholar]
- 38.Minighin E.C., Anastácio L.R., Melo J.O.F., Labanca R.A. Açai (Euterpe oleracea) and its contributions to achieve acceptable daily intake of essential fatty acids. Res. Soc. Dev. 2020;9:e760986116. doi: 10.33448/rsd-v9i8.6116. [DOI] [Google Scholar]
- 39.Rodrigues C.G., Silva V.D.M., Loyola A.C.F., Silva M.R., Augusti R., Melo J.O.F., Carlos L.A., Fante C.A. Characterization and identification of bioactive compounds in agro-food waste flours. Quím. Nova. 2022;45:403–409. doi: 10.21577/0100-4042.20170853. [DOI] [Google Scholar]
- 40.Dessimoni-Pinto N.A.V., Moreira W.A., Cardoso L.M., Pantoja L.A. Jaboticaba peel for jelly preparation: An alternative technology. Ciênc. Tecnol. Aliment. 2011;31:864–869. doi: 10.1590/S0101-20612011000400006. [DOI] [Google Scholar]
- 41.Tenfen A., Siebert D.A., Spudeit D., Cordova C.M.M., Micke G.A., Alberton M.D. Determination of phenolic profile by HPLC-ESI-MS/MS and antibacterial activity of Eugenia platysema against mollicutes strains. J. Appl. Pharm. Sci. 2017;7:7–11. doi: 10.7324/JAPS.2017.70502. [DOI] [Google Scholar]
- 42.Loyola A.C.F., Silva V.D.M., Silva M.R., Rodrigues C.R., Santos A.N., Melo J.O.F., Augusti R., Fante C.A. Use of Paper Spray Mass Spectrometry for Determining the Chemical Profile of Green Cavendish Banana (Musa AAA) Peel and Pulp Flours and Evaluation of Its Functional Potential. J. Braz. Chem. Soc. 2021;32:953–963. doi: 10.21577/0103-5053.20200243. [DOI] [Google Scholar]
- 43.Rodrigues S., Fernandes F.A.N., Brito E.S., Sousa A.D., Narain N. Ultrasound extraction of phenolics and anthocyanins from jabuticaba peel. Ind. Crops Prod. 2015;69:400–407. doi: 10.1016/j.indcrop.2015.02.059. [DOI] [Google Scholar]
- 44.Dastmalchi K., Flores G., Wu S.B., Ma C., Dabo A.J., Whalen K., Reynertson K.A., Foronjy R.F., Armiento J.M.D., Kennelly E.J. Edible Myrciaria vexator fruits: Bioactive phenolics for potential COPD therapy. Bioorg. Med. Chem. 2012;20:4549–4555. doi: 10.1016/j.bmc.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva N.A., Rodrigues E., Mercadante A.Z., Ross V.V. Phenolic Compounds and Carotenoids from Four Fruits Native from the Brazilian Atlantic Forest. J. Agric. Food Chem. 2014;62:5072–5084. doi: 10.1021/jf501211p. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira L.L., Bertoldi F.C., Lajolo F.M., Hassimotto N.M.A. Identification of Ellagitannins and Flavonoids from Eugenia brasilienses Lam. (Grumixama) by HPLC-ESI-MS/MS. J. Agric. Food Chem. 2015;63:5417–5427. doi: 10.1021/acs.jafc.5b01195. [DOI] [PubMed] [Google Scholar]
- 47.Alves A.M., Dias T., Hassimotto N.M.A., Naves M.M.V. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian Savannah native fruits. Food Sci. Technol. 2017;37:564–569. doi: 10.1590/1678-457x.26716. [DOI] [Google Scholar]
- 48.Celli G.B., Pereira-Netto A.B., Beta T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res. Int. 2011;44:2442–2451. doi: 10.1016/j.foodres.2010.12.036. [DOI] [Google Scholar]
- 49.Flores G., Wu S.B., Negrin A., Kennelly E.J. Chemical composition and antioxidant activity of seven cultivars of guava (Psidium guajava) fruits. Food Chem. 2015;170:327–335. doi: 10.1016/j.foodchem.2014.08.076. [DOI] [PubMed] [Google Scholar]
- 50.Lenquiste S.A., Lamas C.A., Marineli R.S., Moraes E.A., Borck P.C., Camargo R.L., Quitete V.H.A.C., Carneiro E.M., Maróstica Junior M.R. Jaboticaba peel powder and jaboticaba peel aqueous extract reduces obesity, insulin resistance and hepatic fat accumulation in rats. Food Res. Int. 2019;120:880–887. doi: 10.1016/j.foodres.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 51.Lenquiste S.A., Marineli R.S., Moraes E.A., Dionísio A.P., Brito E.S., Maróstica Junior M.R. Jaboticaba peel and jaboticaba peel aqueous extract shows in vitro and in vivo antioxidant properties in obesity model. Food Res. Int. 2015;77:162–170. doi: 10.1016/j.foodres.2015.07.023. [DOI] [Google Scholar]
- 52.Hýsková V., Ryšlavá H. Antioxidant Properties of Phenylpropanoids. Biochem. Anal. Biochem. 2019;8:e171. doi: 10.35248/2161-1009.19.8.e171. [DOI] [Google Scholar]
- 53.Silva M.L.C., Costa R.S., Santana A.S., Bello Koblitz M.G. Compostos fenólicos, carotenóides e atividade antioxidante em produtos vegetais. Semin. Cienc. Agrar. 2010;31:669–681. doi: 10.5433/1679-0359.2010v31n3p669. [DOI] [Google Scholar]
- 54.Três M.V., Francheschi E., Borges G.R., Dariva C., Corazza F.C., Oliveira J.V., Corazza M.L. Influência da temperatura na solubilidade de β-caroteno em solventes orgânicos à pressão ambiente. Ciênc. Tecnol. Aliment. 2007;27:737–743. doi: 10.1590/S0101-20612007000400011. [DOI] [Google Scholar]
- 55.Bazykina N.I., Nikolaevskii A.N., Fillipenko T.A., Kaloerova V.G. Optimization of conditions for the extraction of natural antioxidants from raw plant materials. Pharm. Chem. J. 2002;36:46–49. doi: 10.1023/A:1016024300843. [DOI] [Google Scholar]
- 56.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 57.Lao F., Giusti M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018;80:87–93. doi: 10.1016/j.jcs.2018.01.001. [DOI] [Google Scholar]
- 58.Setford P.C., Jeffery D.W., Grbin P.R., Muhlack R.A. Mathematical modelling of anthocyanin mass transfer to predict extraction in simulated red wine fermentation scenarios. Food Res. Int. 2019;121:705–713. doi: 10.1016/j.foodres.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 59.Tarone A.G., Silva E.K., Barros H.D.F.Q., Cazarin C.B.B., Marostica Junior M.R. High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: Effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds. Food Res. Int. 2021;140:110048. doi: 10.1016/j.foodres.2020.110048. [DOI] [PubMed] [Google Scholar]
- 60.Melo E.A., Maciel M.I.C., Lima V.L.A.G., Nascimento R.J. Capacidade antioxidante de frutas. Braz. J. Pharm. Sci. 2008;44:193–201. doi: 10.1590/S1516-93322008000200005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.