Abstract
To explore the roles of chemokines in type 1 and type 2 responses in vivo, we examined mRNA expression for a panel of up to 17 chemokines in experimental mouse models using Schistosoma mansoni. These studies revealed that Mig (monokine induced by gamma interferon), cytokine-responsive gene 2/10-kDa interferon-inducible protein, RANTES, lymphotactin, macrophage inflammatory protein 1β (MIP-1β), JE/monocyte chemoattractant protein 1, and MIP-2 are associated with type 1 egg-induced responses and that thymus-derived chemotactic agent 3 (TCA3), eotaxin, MIP-1α, and MIP-1γ are associated with type 2 egg-induced responses. After cercarial infection, both type 1-associated and type 2-associated chemokines were elevated in the livers of infected mice presensitized with eggs and recombinant interleukin-12 (rIL-12), a regimen that diminishes pathology. Neutralization of IL-12 or gamma interferon during egg deposition reversed the effects of prior treatment with rIL-12, leading to a return to larger granulomas; persistently elevated expression of TCA3, eotaxin, and MIP-1α; and a marked reduction in the expression of type 1-associated chemokines despite the maintenance of a dominant type 1 cytokine response in the draining lymph nodes. Our findings suggest that there are patterns of coordinate chemokine expression characteristic of type 1 and type 2 responses in vivo; that the cells recruited by a given pattern of chemokines may differ, depending on the composition of peripheral populations; and that patterns of tissue expression of chemokines may determine the character of an inflammatory response independently of the dominant pattern of differentiation of antigen-specific T cells. Our data reveal new relationships between chemokines and polarized immune responses and suggest that end organ inflammation might be altered by chemokine blockade without necessitating reversal of the phenotype of the majority of differentiated T cells.
Schistosomiasis is a parasitic disease affecting approximately 200 million people worldwide, constituting a significant problem in public health (4). Morbidity from infection with Schistosoma mansoni is primarily the result of an inflammatory reaction to eggs deposited in the liver, leading to fibrosis and the sequelae of portal hypertension (7). Mouse models have been used extensively to study the pathology of schistosomal infection and the immunopathology of the granulomatous response (48, 51), with the ultimate goal of being able to manipulate the response to minimize inflammation and fibrosis for clinical benefit (53). Pulmonary embolization of schistosomal eggs has provided an experimental system of synchronous development of granulomas. In naive animals, the granulomas increase in size over 2 weeks and then begin to resolve (51). For mice with prior sensitization, the pattern of inflammation is similar but accelerated, with peak granuloma size reached within the first week (34, 51).
Besides contributing to an understanding of schistosomiasis, these studies with mice have revealed general principles underlying the control of granuloma formation and inflammatory injury. To date, we have focused on the roles of pleiotropic cytokines such as interleukin-12 (IL-12), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-4, IL-5, IL-13, and IL-10 in determining the magnitude and character of the inflammatory response. We have investigated these roles by measuring cytokine expression in lymphoid and peripheral tissues, by blocking or eliminating cytokines by using antibodies or knockout mice, respectively, and by administering cytokine along with sensitizing antigen as part of strategies for immunization (8, 9, 14, 23, 55, 57). General conclusions from these studies have been that Th2 cytokines are primarily responsible for the granulomatous response to S. mansoni eggs; that in this context, Th1 cytokines function as endogenous down-regulators; and that unopposed activity at either polar extreme can be detrimental (23, 54).
Within the last 15 years, a family of more than 40 chemotactic factors has been described, now named chemokines, that signal through seven transmembrane domain receptors and that are critical for leukocyte trafficking, both as part of homeostasis and in inflammation and infection (32). Chemokines that are important in inflammatory reactions can be induced in many types of cells by an array of exogenous or endogenous factors, with pleiotropic cytokines being the primary endogenous regulators (2). Studies have been done to investigate the role of the chemokine system in the granulomatous inflammation associated with eggs from S. mansoni. These studies have analyzed the expression and activities of a limited number of chemokines and their receptors and have shown, for example, that the chemokines macrophage inflammatory protein 1α (MIP-1α) (29) and JE/monocyte chemoattractant protein 1 (MCP-1) (13) and chemokine receptor 1 (CCR1) (18) contribute to the size of the egg-induced granuloma, although the effects of individual elements on lesion size or cellular composition have not been found to be dramatic. Additional studies have used soluble egg antigen (SEA)-coated beads to model granulomatous inflammation, revealing the expression of multiple chemokines, some of which have been shown to influence granuloma size (12, 40).
In the present study, we addressed two general aspects of the relationship between the chemokine system and schistosomal inflammation. Firstly, we sought to examine the patterns of expression for as many as 17 chemokines in the lung and liver in response to egg embolization in the former and egg deposition following natural infection in the latter in order to create a more complete picture of the role of the chemokine system in these responses. We hypothesized that specific chemokines would be associated with the recruitment of leukocyte subsets into granulomas. Secondly, we sought to take advantage of protocols that we have developed to produce polarized type 1 or type 2 responses to schistosomal eggs in order to examine more generally the relationship between patterns of cytokine and chemokine expression. Experiments using these protocols had revealed previously that in mice lacking IFN-γ, the primary granulomas around eggs embolized to the lungs were increased in size compared with those in wild-type mice (58); that sensitization with eggs plus recombinant IL-12 (rIL-12) led to a type 1-deviated response to subsequent challenge either by pulmonary embolization of eggs (57) or by cercarial infection and egg deposition in the liver (55), resulting in smaller granulomas and diminished fibrosis; and that type 1 cytokines are required not only at the time of sensitization but also during the “effector” phase at the time of egg deposition in order to maintain the type 1 cytokine profile in tissue along with the associated attenuation of egg-induced pathology (22). We hypothesized that there would be patterns of chemokine expression characteristic of type 1 versus type 2 responses.
To facilitate the interpretation of our data, Table 1 lists the chemokines that we analyzed, along with their systematic names, inducers, and receptors and the cells that are their principal targets. In addition to validating our hypotheses described above, our results suggest both that the functions of chemokines in recruiting cells to tissue sites are influenced by (and need to be understood within) the context of the systemic immune response and that chemokines may themselves influence the character of tissue inflammation through selective recruitment from the pool of available cells. Importantly, these data suggest that end organ inflammation might be affected by inhibiting one component of the overall response—namely, chemokines and/or their receptors—and thereby changing the composition of recruited populations without reversing the dominant pattern of effector T-cell differentiation.
TABLE 1.
Chemokines analyzed in this studya
| Chemokine | Inducer(s) | Receptor(s) | Principal target(s) |
|---|---|---|---|
| CXC | |||
| KC (CXCL1-3)b | LPS, IL-1, TNF-α, PDGF | CXCR2 | N |
| MIP-2 (CXCL1-3) | LPS, IL-1, TNF-α | CXCR2 | N |
| LIX (CXCL5) | LPS | CXCR2 | N |
| Mig (CXCL9) | IFN-γ | CXCR3 | T, NK, B |
| CRG-2/IP-10 (CXCL10) | IFN-α, -β, -γ, LPS, IL-1, TNF-α | CXCR3 | T, NK, B |
| SDF-1 (CXCL12) | Constitutive | CXCR4 | T, B, D, M, Thy |
| CC | |||
| TCA3/I-309 (CCL1) | LPS, IL-1 | CCR8 | T, M, Thy |
| JE/MCP-1 (CCL2) | LPS, IL-1, TNF-α, IL-4, thrombin, IFN-γ, PDGF, GM-CSF, M-CSF | CCR2 | M, T, NK, Ba, B |
| MIP-1α (CCL3) | LPS, IL-1, TNF-α, IFN-γ, TCR, FCɛR | CCR1, CCR5 | T, M, N, D, E, Ba, NK, B, Thy |
| MIP-1β (CCL4) | LPS, IL-1, TNF-α, TCR, BCR | CCR5 | T, M, D, Thy |
| RANTES (CCL5) | LPS, IL-1, TNF-α, IFN-γ | CCR1, CCR3, CCR5 | T, M, E, Ba, D, NK, N, B, Thy |
| MCP-3/FIC (CCL7) | LPS, IL-1, TNF-α, IFN-γ, PDGF | CCR1, CCR2, CCR3 | M, T, E, Ba, D, NK, Thy |
| MIP-1γ (CCL9) | Constitutive | CCR1 | N, T |
| Eotaxin (CCL11) | IL-1, TNF-α, IL-4, IL-13, IFN-γ | CCR3 | E, Ba, T |
| MCP-5 (CCL12) | LPS, IFN-γ | CCR2 | M, T, B |
| C, lymphotactin (XCL1) | IL-4, TGF-β, TCR | XCR1 | T, NK |
Abbreviations: N, neutrophil; B, B lymphocyte; Ba, basophil; D, dendritic cell; E, eosinophil; M, monocyte; NK, natural killer cell; T, T lymphocyte; Thy, thymocyte; BCR, B-cell receptor; TCR, T-cell receptor; FCɛR, immunoglobulin E Fc receptor; PDGF, platelet-derived growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Systematic names are in parentheses beside common names. There are occasional ambiguities in applying the systematic names for human chemokines to their presumed mouse orthologues.
MATERIALS AND METHODS
Mice and parasites.
Approximately 6-week-old female C57BL/6 mice were obtained from the Division of Cancer Treatment, National Cancer Institute (Frederick, Md.). IFN-γ−/− mice were obtained from Genentech Inc. (San Francisco, Calif.) and maintained in the C57BL/6 background. Experiments were done at the National Institutes of Health under approved animal study protocols. S. mansoni eggs were isolated from the livers of infected mice and enriched for mature eggs (Biomedical Research Institute, Rockville, Md.). For infection studies, cercariae of a Puerto Rican strain of S. mansoni were obtained from infected Biomphalaria glabrata snails (Biomedical Research Institute).
Immunizations and infections.
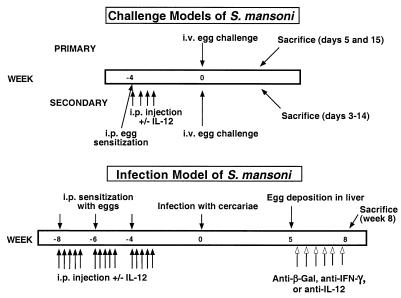
In primary egg challenge experiments, mice received 5,000 S. mansoni eggs intravenously (i.v.) and lungs were harvested on days 5 and 15 (Fig. 1). In sensitization and challenge experiments, mice were sensitized with 5,000 eggs intraperitoneally (i.p.) with or without rIL-12 (0.25 μg/dose given i.p. daily for 4 days). rIL-12 was obtained from the Genetics Institute, Cambridge, Mass., and was a kind gift from Joe Sypek. Four weeks later, mice received 5,000 eggs i.v. without exogenous cytokines. Mice were sacrificed at days 0, 3, 6, 10, and 14 after i.v. challenge with eggs. In the infection studies, mice were sensitized with 5,000 eggs i.p. three times, with 2 weeks between sensitizations, and treated with or without rIL-12 (0.25 μg/dose) on days 0, 1, 2, 3, and 5 after each egg exposure. Four weeks after receiving the last dose of rIL-12, mice were infected by percutaneous challenge of tail skin in water containing 20 to 25 cercariae for 40 min. Five weeks after infection (coinciding with egg deposition), mice received neutralizing antibodies against IFN-γ (XMG1.6), IL-12 (C17.8.20), or β-galactosidase (β-Gal) (GL113) at 1 mg/dose i.p. twice weekly until they were sacrificed 8 weeks after infection. XMG1.6 was kindly provided by DNAX, Palo Alto, Calif., and C17.8.20 was kindly provided by Georgio Trinchieri, Wistar Institute, Philadelphia, Pa.
FIG. 1.
Schematic of models of S. mansoni used in this study. In the primary-challenge model, C57BL/6 mice were sacrificed at days 5 and 15 after i.v. injection of 5,000 eggs. In the secondary-challenge model, mice were sensitized i.p. with 5,000 eggs. Mice cosensitized with rIL-12 received four daily injections i.p.(0.25 μg/dose) during egg sensitization. Four weeks later, mice received 5,000 eggs i.v. without exogenous cytokines. Mice were sacrificed at days 0, 3, 6, 10, and 14 after i.v. challenge. In the infection studies, mice were sensitized with 5,000 eggs i.p. three times, with 2 weeks between sensitizations, and treated with or without rIL-12 (0.25 μg/dose) on days 0, 1, 2, 3, and 5 after each egg exposure. Four weeks after administration of the last dose of rIL-12, mice were infected with cercariae by percutaneous challenge of the tail skin. Five weeks after infection (coinciding with egg deposition), mice received neutralizing antibodies against IFN-γ, IL-12, or, as a control, β-Gal at 1 mg/dose i.p. twice weekly until they were sacrificed 8 weeks after infection.
Preparation of mRNA from tissues.
Depending on the experimental model, the right lung or two 25-mg portions of the liver were placed in either 1 ml of Trizol (Life Technologies, Gaithersburg, Md.) or RNA STAT-60 (Tel-Test, Inc., Friendswood, Tex.) and immediately placed on dry ice. Tissues were disrupted by using a tissue homogenizer (Omni International, Waterbury, Conn.), and RNA was isolated in accordance with the manufacturers' protocols.
RT-PCR detection of chemokine mRNAs.
Reverse transcription (RT) of 1 μg of total RNA was performed by using the Superscript Preamplification System (Life Technologies), and the final cDNA preparation was diluted eightfold for use in a PCR. The oligonucleotide primers for the chemokines were selected such that the amplicon would span an intron (Table 2). The PCR conditions were strictly defined for each chemokine pair such that the relationship between the input RNA and the amplicon was linear. Threefold serial dilutions were made from the cDNA from each sample to verify the linearity of the assay. Twenty microliters of PCR product was analyzed by using a 1.5% agarose gel run in Tris-acetate-EDTA. Gels were washed in water, and the DNA was denatured with 1.5 M NaCl–0.4 N NaOH for 20 min. DNA was transferred onto Zeta-probe GT nylon membranes (Bio-Rad, Hercules, Calif.) in denaturing solution. After overnight transfer, blots were washed twice with 0.5 M Tris (pH 7.0) and then twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before baking under vacuum for 2 h at 80°C. Antisense oligonucleotide probes (5 pmol/reaction mixture) were end labeled by using 30 μCi of [γ-32P]ATP (NEN Life Sciences, Boston, Mass.) and T4 polynucleotide kinase (New England Biolabs, Beverly, Mass.), and probes were separated from unincorporated isotope by using NUCTRAP push columns (Stratagene, La Jolla, Calif.) in accordance with the suppliers' protocols. Blots were incubated for greater than 1 h at 50°C in prehybrization solution containing 10× Denhardt's solution, 6× SSC, 1% sodium dodecyl sulfate (SDS), 20 μg of yeast tRNA/ml, and 50 μg of salmon sperm DNA/ml before being placed in 6× SSC–1% SDS containing 5 × 106 to 10 × 106 cpm of probe/ml. Hybridization was done overnight at 50°C, and blots were washed three times (5 min each) with 6× SSC–1%SDS at 55°C, followed by a final 3-min wash in 1× SSC–1% SDS. Bands were quantified by using a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.) after overnight exposure. All chemokine expression was normalized to hypoxanthine phosphoribosyltransferase (HPRT) and expressed as the fold increase over levels in unsensitized, unchallenged mice.
TABLE 2.
Oligonucleotide primers and probes used for PCR-Southern blot analysis of murine chemokinesa
| Target, cycle no. | 5′ and 3′ oligonucleotides | Antisense probe | Product size (bp)c | |
|---|---|---|---|---|
| CRG-2/IP-10 (CXCL10), 24 | TGAGCAGAGATGTCTGAATC | ATTAGGACTAGCCATCCACTGGGTAAAGGG | 399 | |
| TCGCACCTCCACATAGCTTACAG | ||||
| Eotaxin (CCL11), 24 | TAGGTAAGCAGTAACTTCCATCTGTCTC | GATGCACCCTGAAAGCCATAGTCTTCAAGAC | 380 | |
| TGACTAAATCAAGCAGTTCTTAGGCTCTG | ||||
| JE/MCP-1 (CCL2), 26 | CACTCACCTGCTGCTACTCATTCAC | TCACTGTCACACTGGTCACTCCTACAGAAG | 505 | |
| GGATTCACAGAGAGGGAAAAATGG | ||||
| KC (CXCL1-3), 28 | TTGACCCTGAAGCTCCCTTGGTTC | CTCTCTGCACTTCTTTTCGCACAACACCCT | 521 | |
| CGTGCGTGTTGACCATACAATATG | ||||
| LIX (CXCL5), 30 | GGGATCTTGTCCACAATGAG | ACGGAGCTGCGTTGTGTTTGCTTAACCGTA | 547 | |
| AGGGACAATGGTTTCCCTT | ||||
| Lymphotactin (XCL1), 24 | TGCAATGGGTTTGGGAACTG | AACTTACAAACCCAGCGGCTGCCAGTTCAA | 439 | |
| CAAGACCTCAGCCATGAGAC | ||||
| MCP-3 (CCL7), 24 | GCCAGCTCTCTCACTCTCTTT | AGCTACAGAAGGATCACCAGTAGTCGGTGT | 430 | |
| CAACACATTTCATCAACAG | ||||
| MCP-5 (CCL12), 25 | GTTCCTGACTCCTCTAGCTTTC | GTTAAGCAGAAGATTCACGTGCGGAAGCTG | 397 | |
| ACGTAAGAGTTTTTGGAACTC | ||||
| Mig (CXCL9), 29 | GATCAAACCTGCCTAGATCC | CTCTTATGTAGTCTTCCTTGAACGACGACG | 399 | |
| GGCTGTGTAGAACACAGAGT | ||||
| MIP-2 (CXCL1-3), 29 | CCTGGTTCAGAAAATCATCC | AGACAGCGAGGCACATCAGGTACGATCCAG | 468 | |
| TCCCCAGTCTCTTTCACTGT | ||||
| MIP-1α (CCL3), 25 | CGGAAGATTCCACGCCAATTC | AGGAGATGGAGCTATGCAGGTGGCAGGAAT | 448 | |
| GGTTGAGGAACGTGTCCTGAAG | ||||
| MIP-1β (CCL4), 23 | CCCACTTCCTGCTGTTTCTCTTAC | AATCTGAACGTGAGGAGCAAGGACGCTTCT | 444 | |
| AGCAGAGAAACAGCAATGGTGG | ||||
| MIP-1γ (CCL9), 28 | GCCCACTAAGAAGATGAAGCCT | TGCTGCCTGTCCTATAACTCACGGATTCAG | 416 | |
| CCTTCTCTAAAGCAAATGTTA | ||||
| RANTES (CCL5), 23 | CCACGTCAAGGAGTATTTCTACACC | GCTAGGACTAGAGCAAGCAATGACAGGGAA | 326 | |
| CTGGTTTCTTGGGTTTGCTGTG | ||||
| SDF-1α (CXCL12), 24 | CTCCAAACTGTGCCCTTCAG | TGGCAAACCTTAGCATGACCCCAGTCAGTG | 348 | |
| AAAGCTCCATTGTGCACGGG | ||||
| SDF-1β (CXCL12), 26 | CTCCAAACTGTGCCCTTCAG | CAGCAAAACTGTGCAAAGCAAGTCCCTTGC | 368 | |
| GCCTGTCACCAATGACGTTG | ||||
| HPRT,b 22 | GTTGGATACAGGCCAGACTTTGTTG | GTTGTTGGATATGCCCTTGAC | 162 | |
| GATTCAACTTGCGCTCATCTTAGGC |
RT-PCR and Southern hybridization conditions are described in Materials and Methods.
Housekeeping gene used for normalization.
bp, base pairs.
RNase protection assay of chemokine and chemokine receptor mRNAs.
Five micrograms of total mRNA was used per hybridization reaction using the RiboQuant Multi-Probe RNase protection assay system (Pharmingen, San Diego, Calif.). Signals were quantified after overnight exposure by using a PhosphorImager as described above. Chemokine and chemokine receptor expression was normalized to the signals for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and then expressed as fold increases over the levels in unsensitized, unchallenged, or uninfected control mice.
Statistical analysis.
Values for mRNA expression were expressed as the mean ± the standard error of the mean. Differences between groups were evaluated by a two-tailed Student t test. P < 0.05 was considered significant.
RESULTS
Embolization of S. mansoni eggs to the lungs of wild-type and IFN-γ−/− mice reveals type 1 and type 2 response-associated chemokines.
We evaluated chemokine expression in mice following pulmonary embolization with eggs of S. mansoni or infection with cercariae in accordance with the protocols diagrammed in Fig. 1. The simplest of these was the primary-challenge model, in which lung tissue was harvested 5 or 15 days after injection of eggs and samples were analyzed for levels of chemokine and cytokine mRNAs, as well as for the size and composition of egg-induced granulomas. The evolution of pulmonary granulomas after embolization of eggs of S. mansoni in naive mice has been well characterized, showing maximal size and cellular infiltration between 1 and 4 weeks after injection, with the response decreasing by week 4 (31, 51). The infiltrating cells consist initially of lymphocytes, primarily CD4+ T cells and NK cells, and macrophages, which then diminish in favor of eosinophils (31, 39). The pattern of cytokine production by lymphocytes in the lymph nodes of egg-injected mice shows induction of IFN-γ within 1 day and subsequent loss of IFN-γ production by day 7, associated with large increases in the expression of IL-4 and IL-5 (47). The pattern in the lung is similar, although it is distinguished by the presence of a more mixed response at later times with lower but detectable expression of IFN-γ at up to 2 weeks, when IL-4, IL-5, and IL-13 are highly expressed (56, 57).
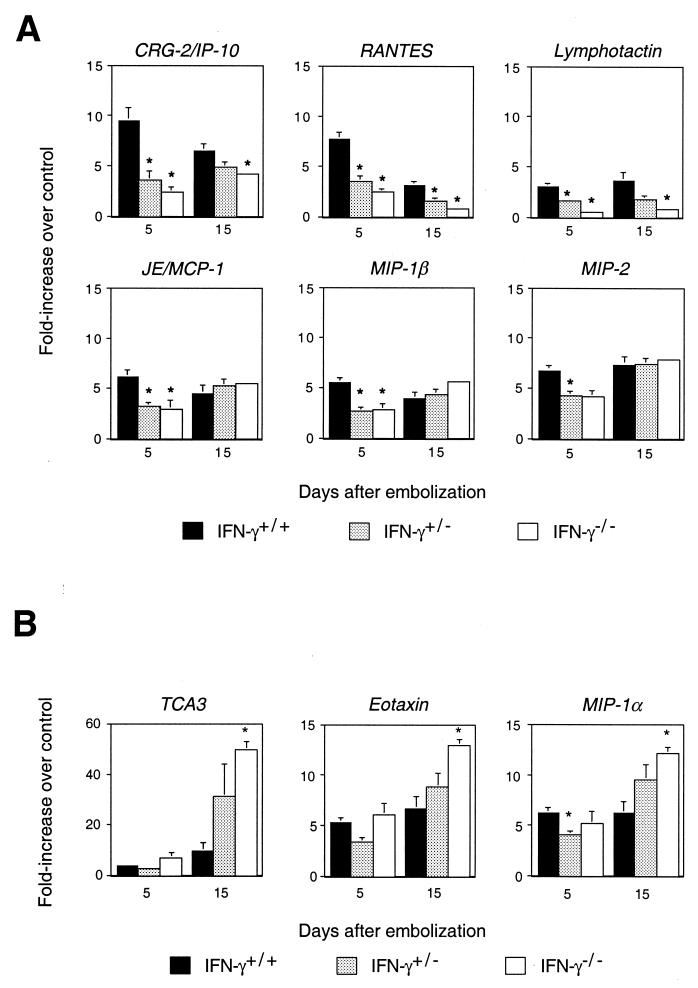
In order to characterize patterns of chemokine expression after primary challenge of mice that differed in the Th1 component of the inflammatory response, we challenged wild-type mice, as well as mice with targeted deletion of the gene for IFN-γ. We have demonstrated previously that blocking of IFN-γ leads to increased granuloma size, increased granuloma eosinophils, and increased expression of Th2 cytokines IL-5 and IL-13 at 2 weeks after embolization (57). As shown in Fig. 2A, the early induction of six chemokine genes, those for cytokine-responsive gene 2/10-kDa IFN-inducible protein (CRG-2/IP-10), RANTES, lymphotactin, JE/MCP-1, MIP-1β, and MIP-2, was diminished significantly in the IFN-γ−/− mice and in some cases in the IFN-γ+/− mice, while the later induction of three genes, those for thymus-derived chemotactic agent 3 (TCA3), eotaxin, and MIP-1α, was enhanced in the IFN-γ−/− mice compared with the wild-type mice. For the wild-type mice, consistent with the mixed pattern of expression of type 1 and type 2 cytokines at 5 and 15 days in naive mice, the differences in fold induction between the type 1- and type 2-associated chemokines at 5 compared with 15 days were not dramatic. However, the chemokines whose levels were most affected by IFN-γ—CRG-2/IP-10, RANTES, and TCA3—showed a pattern consistent with the known pattern of cytokine expression, with IFN-γ induced to the highest levels early and type 2 cytokines higher at 2 weeks.
FIG. 2.
Dual effects of IFN-γ on chemokine gene expression in the lungs of C57BL/6 mice after a primary challenge with S. mansoni. Chemokine gene expression was measured by RNase protection assays using RNA from homogenized lung tissue. Chemokine gene expression in the challenged animals was normalized to GAPDH and then divided by the GAPDH-normalized expression for the specific chemokine in the lungs of unmanipulated control mice. Values are the means of samples from four or five mice. (A) Chemokine genes showing diminished expression in IFN-γ+/− (hatched bars) and IFN-γ−/− (open bars) mice compared with that in IFN-γ+/+ (solid bars) mice. (B) Chemokine genes showing enhanced expression in IFN-γ+/− and IFN-γ−/− mice compared with that in IFN-γ+/+ mice. ∗, P < 0.05 by two-tailed Student t test for IFN-γ+/− or IFN-γ−/− mice compared with IFN-γ+/+ mice. Some error bars are too small to be seen.
Polarization of the response to embolized eggs of S. mansoni by pretreatment with rIL-12 is associated with dramatic changes in chemokine gene expression in the lung.
The experiment described above analyzed the effect of diminishing the type 1 component of the granulomatous response to eggs in naive mice. As a next step in associating patterns of chemokine expression with type 1- versus type 2-skewed responses to S. mansoni, we used a protocol in which, in contrast, we created highly and oppositely polarized responses in mice during sensitization to eggs. Early work on the murine models of schistosomiasis had shown that previously sensitized mice have exaggerated responses to embolized eggs, with large granulomas that peak in size at 1 week (34, 51). In these granulomas, in a pattern similar to but speeded up compared with that of naive mice, lymphocytes and macrophages infiltrated to the highest levels in the first few days, with their numbers falling thereafter along with an increase in the numbers of eosinophils (31, 34). We created highly polarized responses by injecting mice with eggs with or without rIL-12 i.p. 1 month before a challenge with eggs embolized to the lungs (Fig. 1). Whereas sensitization with eggs alone creates a type 2-polarized response upon challenge, we have shown that sensitization with eggs plus rIL-12 dramatically suppresses secondary granuloma formation and the production of Th2 cytokines (57).
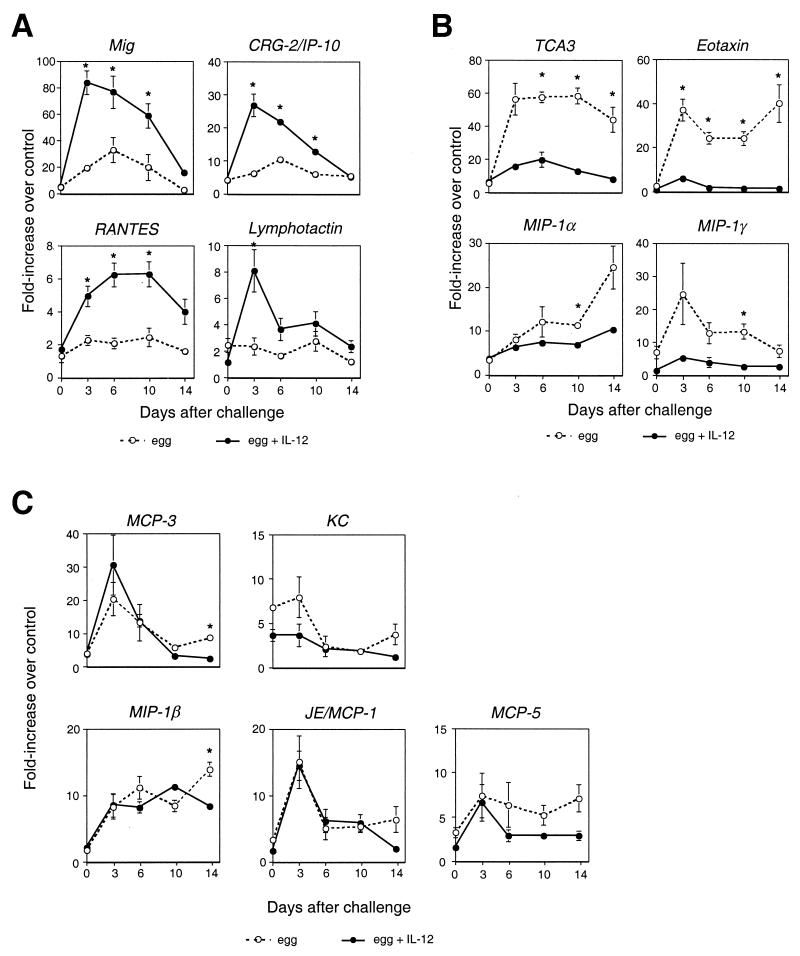
In analyzing this model, we expanded the number of chemokine mRNAs that we evaluated by using a semiquantitative RT-PCR. As shown in Fig. 3, a challenge after sensitization without rIL-12 led to induction of multiple chemokines, most dramatically, TCA3, eotaxin, MCP-3, MIP-1γ, and Mig (monokine induced by IFN-γ). In rIL-12-sensitized mice, as shown in Fig. 3A, expression of the IFN-γ-inducible chemokines Mig and CRG-2/IP-10 was dramatically enhanced. RANTES and lymphotactin were also significantly induced in rIL-12-treated mice. In contrast, as shown in Fig. 3B, rIL-12 treatment during sensitization reduced the induction of TCA3 and eotaxin from approximately 60-fold to 15-fold and from 35-fold to 6-fold, respectively, and also significantly diminished the induction of MIP-1γ and MIP-1α. As shown in Fig. 3C, the expression of MCP-3, KC, MIP-1β, JE/MCP-1, and MCP-5, while significantly induced after egg embolization, was not dramatically altered by pretreatment with rIL-12. MIP-2, stromal cell-derived factor 1α (SDF-1α), SDF-1β, and lipopolysaccharide (LPS)-inducible CXC chemokine (LIX) were not induced significantly in the lung by a challenge with eggs (data not shown). The general pattern of chemokine induction with a fall in expression levels during week 2 is consistent with the decrease in granuloma size and numbers of infiltrating cells during that time, as noted above. The resolution of the granulomas is due to a number of factors, including loss of egg viability and arrest of egg maturation (19).
FIG. 3.
Administration of rIL-12 during sensitization alters the pattern of chemokine gene expression after a secondary challenge with eggs of S. mansoni. With the exception of the gene for TCA3, chemokine gene expression was assayed by RT-PCR, as was expression of the control gene, that for HPRT. TCA3 gene expression was determined by RNase protection, as described in the legend to Fig. 2, because of difficulty in measuring TCA3 mRNA expression by RT-PCR. For the determinations by RT-PCR, chemokine gene expression was normalized to HPRT gene mRNA expression and then divided by the HPRT-normalized chemokine gene expression in the lungs of unmanipulated control mice. Values are the means of samples from three mice. (A) Chemokine genes showing enhanced expression in mice sensitized with eggs plus rIL-12 (filled circles, solid lines) compared with that in mice sensitized with eggs alone (open circles, dotted lines). (B) Chemokine genes showing diminished expression in mice cosensitized with eggs plus rIL-12 compared with that in mice sensitized with eggs alone. (C) Chemokine genes showing enhanced expression after egg challenge unaffected by prior treatment with rIL-12. Differences in chemokine gene expression were considered meaningful if there was a greater-than-twofold difference between groups at two or more time points, at least one of which was statistically significant. ∗, P < 0.05 by two-tailed Student t test for comparisons between rIL-12-treated and untreated mice at a given time point. Some error bars are too small to be seen.
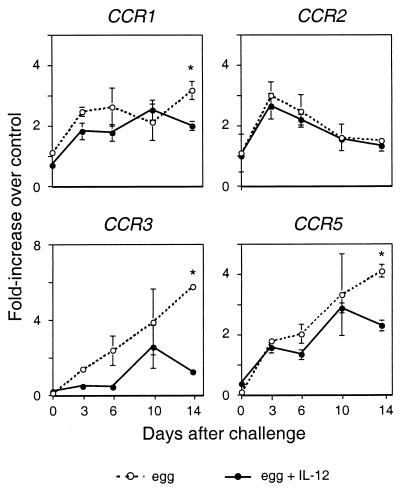
To complement the analysis of chemokines, we evaluated the expression of mRNAs for chemokine receptors by an RNase protection assay. As shown in Fig. 4, mRNAs for chemokine receptors expressed on monocytes/macrophages, CCR1, CCR2, and CCR5, all increased after egg embolization, although with different patterns. CCR1 mRNA levels did not change after an initial rise, CCR2 mRNA levels peaked on day 3 and then declined, and CCR5 levels continued to rise with time. Pretreatment with rIL-12 did not appreciably affect the levels of any of these mRNAs. In contrast, the mRNA of CCR3 demonstrated a clear difference between the two groups, increasing steadily after a challenge under type 2 conditions but without a significant or sustained increase in the mice treated with rIL-12.
FIG. 4.
Evolution of chemokine receptor gene expression in lungs of C57BL/6 mice after a secondary challenge with eggs of S. mansoni. Chemokine receptor gene expression was measured by RNase protection assays using RNA from lung homogenates as described in the legend to Fig. 2. Values are the means of samples from three mice. ∗, P < 0.05 by two-tailed Student t test for comparisons between rIL-12-treated (filled circles, solid lines) and untreated (open circles, dotted lines) mice at a given time point. Some error bars are too small to be seen.
Pretreatment with rIL-12 enhances expression of type 1-associated chemokines in the liver after egg deposition, but in contrast with the pulmonary embolization model, expression of type 2-associated chemokines remains elevated.
Beyond the models using egg embolization, we were interested in analyzing the patterns of chemokine expression after egg deposition in the liver in more complex and clinically relevant models of S. mansoni infection. Again, we compared chemokine gene expression in mice in which we had manipulated the polarization of the response by prior treatment with eggs plus rIL-12 (Fig. 1). This protocol provides a mouse model for vaccination targeted at reduction of hepatic pathology and is able to reduce granulomatous inflammation and collagen deposition (55). In addition, we analyzed how the expression of chemokine genes in rIL-12-treated mice was affected by blocking of IFN-γ or IL-12 during the response to egg deposition in the liver. We have shown previously that treatment of these mice with antibodies to IFN-γ or IL-12 leads to an increase in hepatic IL-4 and IL-5 back to levels seen in mice not pretreated with rIL-12 and that cytokine blockade partially or completely reverses the effects of rIL-12 pretreatment on granuloma size, granuloma eosinophil content, and hepatic fibrosis (22). In spite of these effects in the liver, blocking of IFN-γ or IL-12 during the effector phase of the response did not significantly restore the type 2 response in lymph node cells from the infected mice, which were characterized by stimulation in vitro with SEA or soluble worm antigen (22). We chose to analyze the livers at 8 weeks after infection because this is when the hepatic granulomas are fully developed, Th2 responses are well established, and cytokine mRNAs are maximally expressed (9, 55).
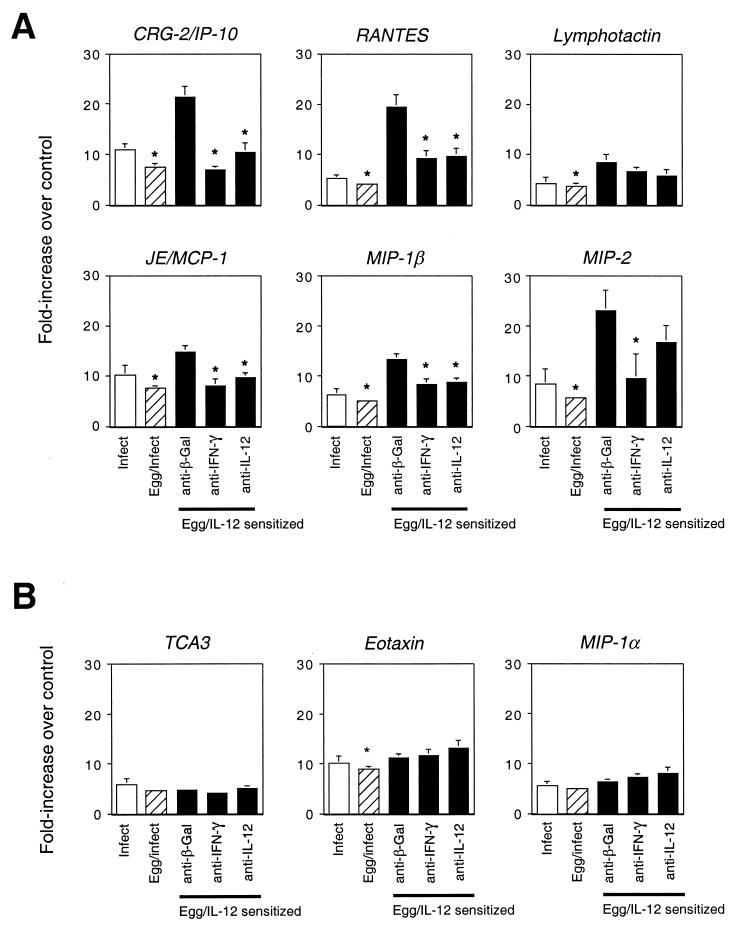
Figure 5 shows the results of RNase protection assays done on RNAs from livers harvested from these infected mice. Similar to the findings on the primary response to egg embolization to the lung in wild-type versus IFN-γ−/− mice (Fig. 2), as well as in the pulmonary response to eggs after pretreatment with rIL-12 (Fig. 3), CRG-2/IP-10, RANTES, and lymphotactin showed a type 1 bias, since they were increased in the livers of mice pretreated with rIL-12 compared to those sensitized with eggs alone. Consistent with the findings in the primary-response model (Fig. 2), JE/MCP-1 and MIP-1β also showed a modest type 1 bias in the liver, in that their expression was enhanced in the rIL-12-treated mice. Unlike the findings in the lung models, egg deposition in the liver led to a significant increase in MIP-2 gene expression, which was enhanced in mice pretreated with rIL-12. In contrast to the generally similar results obtained with the models for the type 1-biased chemokines, the chemokines whose gene expression showed a clear type 2 bias in the pulmonary responses failed to be inhibited after egg deposition in the livers of infected mice pretreated with rIL-12. Nonetheless, as in the lung models, TCA3, eotaxin, and MIP-1α behaved similarly to each other and were distinguished from the type 1-biased chemokines by not showing any noteworthy rIL-12-induced enhancement.
FIG. 5.
Prior treatment with rIL-12 and anti-cytokine antibodies at the time of egg deposition alters the pattern of expression of chemokine genes in livers of S. mansoni-infected C57BL/6 mice. Mice were infected without prior sensitization (Infect, open bars) or after sensitization with eggs alone (Eggs/Infect, striped bars) or with eggs plus rIL-12 (Egg/IL-12 sensitized, solid bars) (Fig. 1). Control (anti-β-Gal) or anti-cytokine antibodies were administered beginning at 5 weeks following infection, and livers were harvested at week 8 for determination of chemokine mRNA levels by RNase protection assays as described in the legend to Fig. 2. Values are the means of samples from five mice. (A) Expression of genes for type 1-associated chemokines. (B) Expression of genes for type 2-associated chemokines. ∗, P < 0.05 by two-tailed Student t test for comparisons with levels in the egg-sensitized, rIL-12-treated mice injected with control (anti-β-Gal) antibody. Some error bars are too small to be seen.
Not surprisingly for the type 1-biased chemokines, and similar to what we have reported for liver pathology, as well as liver cytokines (22), treatment of the mice with antibodies against IFN-γ or IL-12 during the time of egg deposition largely reversed the effects of prior treatment with rIL-12. Just as pretreatment with rIL-12 did not alter the expression of mRNAs for TCA3, eotaxin, and MIP-1α, the antibodies to IFN-γ or IL-12 had no effect on the expression of these chemokines in the infected livers.
DISCUSSION
Our study represents the most comprehensive analysis yet done on chemokine expression in mouse models of schistosomiasis. For some of the genes we evaluated, such as Mig, CRG-2/IP-10, MIP-1β, and KC, our data are the first description of expression in these models. For other genes, such as lymphotactin, MIP-1γ, and TCA3, our data are also among the first descriptions of their expression as part of an immune response in vivo. Overall, our expression data, together with the well-established information on cellular recruitment into schistosomal granulomas (31, 34, 39), support the association of specific chemokines with recruitment of defined leukocyte subsets. More importantly, the breadth of our data allows us to place the expression of multiple chemokine-encoding genes in a broader biological context in the production of the schistosomal granulomatous response and the Th1-Th2 paradigm generally, using immunologically complex challenges that mimic natural infection. Exposure of mice to schistosomal eggs elicits a mixed Th1-Th2 response with the predominance of IFN-γ early and IL-4, IL-5, and IL-13 later (21, 35, 47, 56). Perhaps not surprisingly, a mixed and moderated Th1-Th2 response forms the basis of a chronic and relatively benign parasitism (23). By using IFN-γ knockout mice and administering rIL-12, we created polarized responses to reveal and clarify relationships between patterns of cytokine and chemokine production. Overall, our data support the hypothesis that there are patterns of chemokine expression characteristic of type 1 versus type 2 responses. We have summarized these results in Table 3, and we will discuss the roles of the chemokines in schistosomal inflammation within this context. Nonetheless, it is evident that in the unmanipulated reactions to the parasite, the pattern of chemokine production is more balanced, faithfully reflecting the mixed and adaptive effects of opposing cytokines.
TABLE 3.
Association of chemokines with type 1 and type 2 responses in murine models using S. mansonia
| Chemokine | Primary embolization model | Secondary embolization model | Infection model |
|---|---|---|---|
| CXC | |||
| KC (CXCL 1-3) | NDb | Type 1/type 2 | ND |
| MIP-2 (CXCL 1-3) | Type 1 | NIc | Type 1 |
| LIX (CXCL5) | ND | NI | ND |
| Mig (CXCL9) | ND | Type 1 | ND |
| CRG-2/IP-10 (CXCL10) | Type 1 | Type 1 | Type 1 |
| SDF-1 (CXCL12) | ND | NI | ND |
| CC | |||
| TCA3/I-309 (CCL1) | Type 2 | Type 2 | Type 1/type 2 |
| JE/MCP-1 (CCL2) | Type 1 | Type 1/type 2 | Type 1 |
| MIP-1α (CCL3) | Type 2 | Type 2 | Type 1/type 2 |
| MIP-1β (CCL4) | Type 1 | Type 1/type 2 | Type 1 |
| RANTES (CCL5) | Type 1 | Type 1 | Type 1 |
| MCP (CCL7) | ND | Type 1/type 2 | ND |
| MIP-1γ (CCL9) | ND | Type 2 | ND |
| Eotaxin (CCL11) | Type 2 | Type 2 | Type 1/type 2 |
| MCP-5 (CCL12) | ND | Type 1/type 2 | ND |
| C, lymphotactin (XCL1) | Type 1 | Type 1 | Type 1 |
Responses are type 1 or type 2 as labeled. Type 1/type 2, induced but not polarized.
ND, not determined.
NI, not induced.
In our sensitization-pulmonary embolization model, MCP-3, MCP-5, and KC showed significant induction whether or not the mice had been pretreated with rIL-12. MCP-3 showed the most dramatic induction, to a peak of up to 30-fold over the control level at 3 days after challenge. MCP-3 is a highly promiscuous chemokine (Table 1) that has been associated with both type 1 (6) and type 2 (45) responses. MCP-5 and KC were induced to levels between 5- and 10-fold over the control level following egg embolization. The major cell targeted in common by MCP-3 and MCP-5 is the monocyte. And KC, although considered primarily a neutrophil chemotactic factor, might also recruit monocytes since, at least in humans, CXC chemokine receptor 2 (CXCR2) is well expressed on these cells (15). In the sensitization-pulmonary embolization protocol, MCP-3, together with JE/MCP-1 and, to a lesser extent, KC and MCP-5, peak within the first few days. Together, the data suggest that these chemokines may be important for the recruitment of monocytes/macrophages that occurs early after embolization in sensitized mice (34) and that would be expected to occur as part of both type 1 and type 2 responses.
Enhanced expression of CRG-2/IP-10, Mig, RANTES, and lymphotactin was consistently associated with manipulations in our models that favored type 1 responses and that reduced S. mansoni-induced granulomatous inflammation. As shown in Fig. 3A, inductions of Mig and CRG-2/IP-10 under type 1 conditions were more than 80- and 20-fold greater than the control levels, respectively. CRG-2/IP-10 and Mig, chemokines that share the receptor CXCR3, are the best-documented type 1-associated chemokines, being recognized since their discovery as IFN-γ-activated genes (16, 17, 30, 46). CRG-2/IP-10 and/or Mig have been shown to be expressed in multiple tissues in a variety of experimental infections (1) dominated by type 1 responses. In line with the induction of CRG-2/IP-10 and Mig by IFN-γ, their receptor, CXCR3, has been shown to be preferentially expressed on Th1 CD4+ (5, 41), as well as on CD8+, T cells and NK cells (27, 37). Both Mig and CRG-2/IP-10 tended to peak early (Fig. 2 and 3), which is consistent with the time courses that have been demonstrated for recruitment of T cells and NK cells into egg-induced granulomas (34, 39). The fall in expression of Mig and CRG-2/IP-10 over the 2-week time course shown in Fig. 2 and 3 is similar to what is seen for a number of other chemokines, such as MCP-3, and is, as noted in Results, also consistent with the general pattern of expansion and reduction in granuloma size during this time (34, 51). For RANTES, published data have also associated induction with type 1 responses (3, 24, 26, 42). For lymphotactin, there is little information on its expression in models of disease. Consistent with our findings, however, lymphotactin has been reported to be expressed selectively by activated Th1 CD4+ versus Th2 CD4+ T cells (6, 59).
In two of the models we evaluated, the primary response to egg embolization and the infection model, we found a modest association between MIP-1β expression and a type 1 response, consistent with a number of reports in the literature (25, 36, 42, 49) and with the preferential association of CCR5 with Th1 cells (37; P. Loetscher, M. Uguccioni, L. Bordoli, M. Baggiolini, B. Moser, C. Chizzolini, and J. M. Dayer, Letter, Nature 391:344–345, 1998). In the sensitization-pulmonary embolization model, levels of MIP-1β mRNA rose early and stayed elevated through the 2 weeks that we analyzed, similar to the pattern for another CCR5 ligand, MIP-1α. The pattern suggests that CCR5 and its ligands may be involved in maintaining lymphocytes in the granulomas throughout this time (34). MIP-2, a CXC chemokine that signals through CXCR2, was highly induced in the liver after egg deposition but only moderately expressed in the lung after egg embolization. In both tissues, there was an association with the type 1 response. Our data on MIP-2 in the liver are consistent with the increased numbers of neutrophils found in S. mansoni-induced hepatic granulomas of rIL-12-treated mice (21a; K.F.H. and T.A.W., unpublished data).
A noteworthy conclusion from the expression patterns of the type 1-associated chemokines is that expression of these “proinflammatory” proteins was enhanced under circumstances in which the net effect was to diminish the inflammatory response to eggs. These type 1 response-associated chemokines target primarily Th 1 cells and CD8+ T cells and are potent chemotactic factors for NK cells. Recruitment of all of these cell types through mutually reinforcing pathways presumably contributes to the production of IFN-γ and to the reduction of S. mansoni-induced inflammation. A potential role for NK cells, in particular, in the suppression of S. mansoni-induced inflammation, has been demonstrated in egg embolizations in mice following NK cell depletion (57).
In contrast to that of the chemokines described above, we found that expression of TCA3, eotaxin, and MIP-1α was associated with a type 2 response and an exacerbated granulomatous reaction. The induction of TCA3 in the type 2 environment was dramatic, up to 60-fold over control levels. Our results are consistent with published data that show a preferential expression of TCA3 in Th2 versus Th1 cells (59). The rIL-12-mediated inhibition of TCA3 was associated with diminished pathology, and we presume that TCA3 may be involved in recruitment of Th2 cells into the granulomatous reaction, since mouse CCR8 and human CCR8 were shown to be expressed preferentially on Th2 versus Th1 CD4+ T cells (60).
The preferential expression of eotaxin during the type 2-dominated responses associated with the prominent accumulation of eosinophils was not surprising. A number of experiments have indicated a role for eotaxin in the accumulation of eosinophils in allergic reactions in the lung and skin (20, 44). Just as for TCA3 expression, our experiments are the first to demonstrate that sensitization together with rIL-12 can almost completely suppress eotaxin expression after a subsequent pulmonary challenge with an agent that otherwise elicits a potent type 2 response. The time courses for expression of TCA3 and eotaxin in the sensitization-pulmonary embolization model were similar. Both showed sustained high levels, which is consistent with a role in the recruitment of eosinophils, whose numbers continue to increase during the first 2 weeks (31). An indirect role for TCA3 in eosinophil recruitment has been suggested by very recent data that CCR8 knockout mice showed decreased levels of IL-5 and IL-13 and diminished numbers of eosinophils in lungs challenged with SEA-coated beads (10).
The expression of MIP-1α showed a pattern similar to that of TCA3 and eotaxin in the three models tested, but the levels of induction and the differences between the type 1 and type 2 responses were less dramatic for MIP-1α, particularly in the pulmonary sensitization-challenge model. The relationship of MIP-1α to the type1-type2 paradigm is not clear-cut from the published data, and the role of MIP-1α in vivo is likely to be more model dependent than type 1 versus type 2 dependent. The last chemokine that was highly induced with a type 2 bias was MIP-1γ, which we evaluated in the pulmonary sensitization-challenge model. As far as we are aware, our data are the first to show induction of MIP-1γ in an in vivo model of inflammation. The time course of MIP-1γ expression shown in Fig. 3 and its receptor specificity suggest a role in the recruitment of lymphocytes and monocytes.
Despite the type 2-response bias shown for TCA3, eotaxin, and MIP-1α in the pulmonary embolization models, neither pretreatment with rIL-12 nor cytokine blockade affected their expression in the livers of infected mice. The differences between the findings in the liver model and those in the lung model could be the result of a number of factors. Our past work has demonstrated that it is much more difficult to redirect the immune response in hepatic infection, where three sensitizations with rIL-12 are required (55), compared with the pulmonary embolization model, where a single sensitization with rIL-12 suffices (57) (Fig. 1). This could be due to the chronic nature of the hepatic infection compared with the pulmonary embolization model and differences in the quality of the eggs deposited by the worms versus those that are injected. There could also be tissue-specific differences in the regulation of chemokine gene expression.
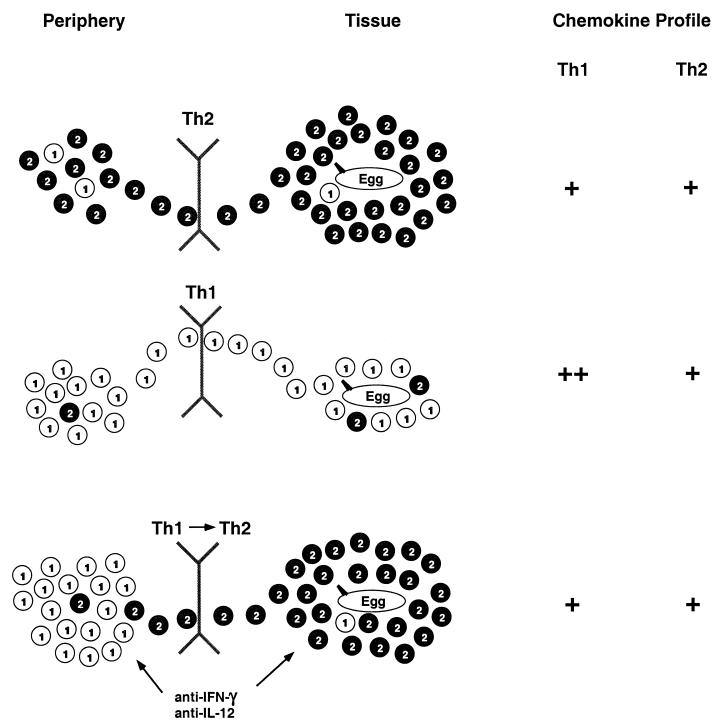
Our liver data, as interpreted in Fig. 6, suggest that the consequences of expression of a particular chemokine will differ depending on the context as part of an interactive relationship with cytokines, other chemokines, and the overall immune response, just as the pattern of chemokine expression will itself contribute to the outcome of the response. In the sensitized and infected but otherwise unmanipulated mice, chemokines that target Th2 cells and eosinophils act on a peripheral pool of T cells dominated by effector-memory Th2 cells and a pool of eosinophils expanded and mobilized through the actions of IL-5 (43). Together, these elements create large, eosinophil-rich granulomas expressing IL-4, IL-5, and IL-13 (57). Although type 1-associated chemokines are induced in these mice, the peripheral pool is poor in Th1 cells (55). In the mice treated with rIL-12 during sensitization, the type 1-associated chemokines are up-regulated. In the face of a peripheral pool that is now dominated by Th1 cells, the result is efficient recruitment of Th1 cells into the granulomas (55). The persistent expression of type 2-associated chemokines in the livers of these mice is inadequate, given the composition of the peripheral T-cell population, to recruit sufficient numbers of Th2 cells into the lesions to produce local type 2 cytokines and granulomas of the usual size and character (22). Blocking of IFN-γ or IL-12 in the rIL-12-treated mice during the time of egg deposition leads to down-regulation of the type 1-associated chemokines and restoration of the chemokine pattern to that seen in mice not treated with rIL-12. With this chemokine pattern and conditions that allowed the emergence of a small but increased number of Th2 cells in the peripheral pool, the type 2-associated chemokines are able to recruit selectively the minority Th2 population to produce Th2-dominated granulomas, leading to the restoration of pathology (22). These patterns suggest that by blocking the actions of one set of chemokines, such as those associated with the type 1 response, and allowing other chemokines to select out a subset of peripheral cells, it may be possible to alter the character of the tissue response without shifting the overall pattern of differentiation of effector-memory T cells.
FIG. 6.
Inflammatory response to eggs in the livers of S. mansoni-infected mice depends on both the peripheral T-cell pool and the tissue pattern of chemokine expression. Th1 and Th2 cells are represented by the numbered circles. In infected mice that have not been pretreated with rIL-12 (top panel), Th2 cells predominate within the population of reactive effector-memory CD4+ T cells, and type 2 response-associated chemokines, which target these cells, are induced in the liver, together leading to the efficient recruitment of Th2 cells and characteristic hepatic granulomas and pathology. In mice sensitized along with rIL-12 (middle panel), Th1 cells predominate within the population of reactive effector-memory CD4+ T cells, and type 1 response-associated chemokines, which target these cells, are highly induced in the liver, together leading to the efficient recruitment of Th1 cells and decreased Th2 cells with diminished granulomas and pathology. In mice sensitized along with rIL-12 and treated with anti-cytokine antibodies during the time that eggs are deposited (bottom panel), although Th1 cells still predominate, some Th2 cells emerge within the population of reactive effector-memory CD4+ T cells and the cytokine blockade results in a reversal of the chemokine pattern in the liver with decreased type 1 response-associated chemokines. Together, these changes lead to the return of Th2 cells to the liver with granuloma size, composition, and pathology being restored.
As noted in the introduction, studies published to date on chemokine expression using experimental models with S. mansoni have focused on a few chemokines in responses to embolized eggs. A number of studies have also evaluated responses to SEA-coated beads embolized to the lungs, which may partially mimic the responses to eggs. These studies have shown that eotaxin increases (40) and RANTES diminishes (12) lesion size. While this report was under review, Qiu et al. published an extensive analysis of chemokine gene expression in the lungs of sensitized mice injected with beads coated with purified protein derivative or SEA in order to characterize patterns of chemokine expression in type 1 versus type 2 responses (38). In many respects, the type 1-type 2 associations they observed were similar to ours, with Mig, CRG-2/IP-10, lymphotactin, and MIP-1β showing a type 1 association and eotaxin and TCA3 showing a type 2 association. Nonetheless, there are a number of differences between the studies, such as their finding of type 1 associations for MIP-1α and LIX and their failing to find any induction of MIP-1γ. These differences are likely due, at least in part, to the significant differences between the models used in the two studies. Instead of using antigen-coated beads, we challenged mice with viable schistosomal eggs; instead of using different antigens, we analyzed type 1 versus type 2 responses in the lung using an identical challenge; and in addition to the pulmonary embolization experiments, we analyzed a model of natural cercarial infection and egg deposition in the liver.
Besides our analyses of chemokine expression, we determined levels of chemokine receptor mRNAs in the lungs of sensitized mice after egg embolization. Given the cellular composition of the granulomas, the early increases in CCR1, CCR2, and CCR5 probably reflected the initial recruitment of monocytes, which express these receptors, from the peripheral blood to the granulomas. The subsequent down-regulation of CCR2 and the up-regulation of CCR5 were likely due to the differentiation of the monocytes to macrophages, since this switch in receptor expression has been shown to occur during maturation of human monocytes to macrophages in vitro (33), and an analogous difference in receptor expression has been found on peripheral blood monocytes that are CD14++ (immature, CCR2high, and CCR5low) versus CD14+ CD16+ (macrophage like, CCR2low, and CCR5high), although both populations express equivalent levels of CCR1 (52). To our knowledge, these are the first data to suggest that this switch in monocyte/macrophage receptor expression occurs in vivo during the evolution of an inflammatory reaction. The levels of mRNA for CCR3 rose dramatically in the mice not pretreated with rIL-12 but showed a much smaller rise in the rIL-12-treated mice, likely reflecting the significant difference that we have documented in the numbers of eosinophils recruited to the lungs in the two treatment groups (57).
A major impetus for studying the chemokine system is the possibility of using a chemokine-receptor blockade to treat inflammatory diseases. A complicating factor, however, is the expression of multiple chemokines, some with overlapping activities, as part of a given response. Of the 17 chemokine mRNAs that we evaluated, 11 showed significant induction in the pulmonary embolization models. This may be why blocking of individual chemokines (11–13, 28, 29, 40) or chemokine receptors (10, 18, 50) has had only modest effects on the size and/or composition of the granulomatous lesions. Together, our data suggest that an effective approach to manipulating the chemokine system in vivo requires both global analyses and a systematic approach, based on an understanding of the overall context of the response and likely on inhibition of chemokine activities not only singly but also in combination. Additional experiments will exploit the expression data presented above and use models such as those we have developed with S. mansoni in order to test the effects of such interventions.
ACKNOWLEDGMENTS
We thank Fred Lewis and Chris Rowe at the Biomedical Research Institute for providing parasite material and Joe Sypek for providing the rIL-12 used in this study.
This work was supported by funds from the Division of Intramural Research, NIAID, NIH.
REFERENCES
- 1.Amichay D, Gazzinelli R T, Karupiah G, Moench T R, Sher A, Farber J M. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–4520. [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Benyoucef S, Hober D, De Groote D, Bocket L, De La Tribonniere X, Mouton Y, Wattre P. RANTES production in HIV-1 antigen-stimulated whole blood culture: relationship with type 1 immune response and plasma viral load in individuals infected with HIV-1. Scand J Immunol. 1998;48:212–216. doi: 10.1046/j.1365-3083.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist N R, Colley D G. Schistosomiasis vaccines: research to development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi R, Bianchi G, Bordignon P P, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley L M, Asensio V C, Schioetz L K, Harbertson J, Krahl T, Patstone G, Woolf N, Campbell I L, Sarvetnick N. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J Immunol. 1999;162:2511–2520. [PubMed] [Google Scholar]
- 7.Cheever A W, Jankovic D, Yap G S, Kullberg M C, Sher A, Wynn T A. Role of cytokines in the formation and downregulation of hepatic circumoval granulomas and hepatic fibrosis in Schistosoma mansoni-infected mice. Mem Inst Oswaldo Cruz. 1998;93:25–32. doi: 10.1590/s0074-02761998000700004. [DOI] [PubMed] [Google Scholar]
- 8.Cheever A W, Poindexter R W, Wynn T A. Egg laying is delayed but worm fecundity is normal in SCID mice infected with Schistosoma japonicum and S. mansoni with or without recombinant tumor necrosis factor alpha treatment. Infect Immun. 1999;67:2201–2208. doi: 10.1128/iai.67.5.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheever A W, Williams M E, Wynn T A, Finkelman F D, Seder R A, Cox T M, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 10.Chensue S W, Lukacs N W, Yang T Y, Shang X, Frait K A, Kunkel S L, Kung T, Wiekowski M T, Hedrick J A, Cook D N, Zingoni A, Narula S K, Zlotnik A, Barrat F J, O'Garra A, Napolitano M, Lira S A. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chensue S W, Warmington K, Ruth J H, Lukacs N, Kunkel S L. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. . (Erratum, 162:3106, 1999.) [PubMed] [Google Scholar]
- 12.Chensue S W, Warmington K S, Allenspach E J, Lu B, Gerard C, Kunkel S L, Lukacs N W. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165–173. [PubMed] [Google Scholar]
- 13.Chensue S W, Warmington K S, Ruth J H, Sanghi P S, Lincoln P, Kunkel S L. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 14.Chiaramonte M G, Donaldson D D, Cheever A W, Wynn T A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Investig. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuntharapai A, Lee J, Hebert C A, Kim K J. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol. 1994;153:5682–5688. [PubMed] [Google Scholar]
- 16.Farber J M. A collection of mRNA species that are inducible in the RAW 264.7 mouse macrophage cell line by gamma interferon and other agents. Mol Cell Biol. 1992;12:1535–1545. doi: 10.1128/mcb.12.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farber J M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87:5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J L, Wynn T A, Chang Y, Lee E J, Broxmeyer H E, Cooper S, Tiffany H L, Westphal H, Kwon-Chung J, Murphy P M. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia E G, Mitchell G F, Tapales F P, Tiu W U. Reduced embryonation of Schistosoma japonicum eggs as a contributory mechanism in modulation of granuloma in chronically sensitized mice. Southeast Asian J Trop Med Public Health. 1983;14:272–273. [PubMed] [Google Scholar]
- 20.Gonzalo J A, Lloyd C M, Kremer L, Finger E, Martinez A C, Siegelman M H, Cybulsky M, Gutierrez-Ramos J C. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Investig. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzych J M, Pearce E, Cheever A, Caulada Z A, Caspar P, Hieny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 21a.Hesse M, Cheever A W, Jankovic D, Wynn T A. NOS-2 mediates the protective anti-inflammatory and antifibrotic effets of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann K F, Caspar P, Cheever A W, Wynn T A. IFN-gamma, IL-12, and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J Immunol. 1998;161:4201–4210. [PubMed] [Google Scholar]
- 23.Hoffmann K F, Cheever A W, Wynn T A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 24.John M, Hirst S J, Jose P J, Robichaud A, Berkman N, Witt C, Twort C H, Barnes P J, Chung K F. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: regulation by T helper 2 cytokines and corticosteroids. J Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- 25.Kawakami K, Shibuya K, Qureshi M H, Zhang T, Koguchi Y, Tohyama M, Xie Q, Naoe S, Saito A. Chemokine responses and accumulation of inflammatory cells in the lungs of mice infected with highly virulent Cryptococcus neoformans: effects of interleukin-12. FEMS Immunol Med Microbiol. 1999;25:391–402. doi: 10.1111/j.1574-695X.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim J J, Nottingham L K, Sin J I, Tsai A, Morrison L, Oh J, Dang K, Hu Y, Kazahaya K, Bennett M, Dentchev T, Wilson D M, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D B. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Investig. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loetscher M, Gerber B, Loetscher P, Jones S A, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu B, Rutledge B J, Gu L, Fiorillo J, Lukacs N W, Kunkel S L, North R, Gerard C, Rollins B J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukacs N W, Kunkel S L, Strieter R M, Warmington K, Chensue S W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med. 1993;177:1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luster A D, Unkeless J C, Ravetch J V. γΙnterferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 31.Moore D L, Grove D I, Warren K S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977;121:41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- 32.Murphy P M, Baggiolini M, Charo I F, Hebert C A, Horuk R, Matsushima K, Miller L H, Oppenheim J J, Power C A. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 33.Naif H M, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham A L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olds G R, Mahmoud A A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981;60:251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- 35.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearlman E, Lass J H, Bardenstein D S, Diaconu E, Hazlett F E, Jr, Albright J, Higgins A W, Kazura J W. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J Immunol. 1997;158:827–833. [PubMed] [Google Scholar]
- 37.Qin S, Rottman J B, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch A E, Moser B, Mackay C R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu B, Frait K A, Reich F, Komuniecki E, Chensue S W. Chemokine expression dynamics in mycobacterial (type 1) and schistosomal (type 2) antigen-elicited pulmonary granuloma formation. Am J Pathol. 2001;158:1503–1515. doi: 10.1016/S0002-9440(10)64101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remick D G, Chensue S W, Hiserodt J C, Higashi G I, Kunkel S L. Flow-cytometric evaluation of lymphocyte subpopulations in synchronously developing Schistosoma mansoni egg and Sephadex bead pulmonary granulomas. Am J Pathol. 1988;131:298–307. [PMC free article] [PubMed] [Google Scholar]
- 40.Ruth J H, Lukacs N W, Warmington K S, Polak T J, Burdick M, Kunkel S L, Strieter R M, Chensue S W. Expression and participation of eotaxin during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granuloma formation. J Immunol. 1998;161:4276–4282. [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrum S, Probst P, Fleischer B, Zipfel P F. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 43.Sher A, Coffman R L, Hieny S, Scott P, Cheever A W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira M M, Wells T N, Lukacs N W, Proudfoot A E, Kunkel S L, Williams T J, Hellewell P G. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J Clin Investig. 1997;100:1657–1666. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toney L M, Cattoretti G, Graf J A, Merghoub T, Pandolfi P P, Dalla-Favera R, Ye B H, Dent A L. BCL-6 regulates chemokine gene transcription in macrophages. Nat Immunol. 2000;1:214–220. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 46.Vanguri P, Farber J. Identification of CRG-2: an interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990;265:15049–15057. [PubMed] [Google Scholar]
- 47.Vella A T, Pearce E J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 48.von Lichtenberg F. Host response to eggs of S. mansoni. I. Granuloma formation in the unsensitized laboratory mouse. Am J Pathol. 1962;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Guan E, Roderiquez G, Norcross M A. Inhibition of CCR5 expression by IL-12 through induction of beta-chemokines in human T lymphocytes. J Immunol. 1999;163:5763–5769. [PubMed] [Google Scholar]
- 50.Warmington K S, Boring L, Ruth J H, Sonstein J, Hogaboam C M, Curtis J L, Kunkel S L, Charo I R, Chensue S W. Effect of C-C chemokine receptor 2 (CCR2) knockout on type 2 (schistosomal antigen-elicited) pulmonary granuloma formation: analysis of cellular recruitment and cytokine responses. Am J Pathol. 1999;154:1407–1416. doi: 10.1016/S0002-9440(10)65394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren K S, Domingo E O, Cowan R B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967;51:735–756. [PMC free article] [PubMed] [Google Scholar]
- 52.Weber C, Belge K U, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber K S, Ziegler-Heitbrock H W. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 53.Wynn T A. Immune deviation as a strategy for schistosomiasis vaccines designed to prevent infection and egg-induced immunopathology. Microbes Infect. 1999;1:525–534. doi: 10.1016/s1286-4579(99)80092-6. [DOI] [PubMed] [Google Scholar]
- 54.Wynn T A, Cheever A W. Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol. 1995;7:505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 55.Wynn T A, Cheever A W, Jankovic D, Poindexter R W, Caspar P, Lewis F A, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 56.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 57.Wynn T A, Eltoum I, Oswald I P, Cheever A W, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn T A, Jankovic D, Hieny S, Zioncheck K, Jardieu P, Cheever A W, Sher A. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. J Immunol. 1995;154:3999–4009. [PubMed] [Google Scholar]
- 59.Zhang S, Lukacs N W, Lawless V A, Kunkel S L, Kaplan M H. Cutting edge: differential expression of chemokines in Th1 and Th2 cells is dependent on Stat6 but not Stat4. J Immunol. 2000;165:10–14. doi: 10.4049/jimmunol.165.1.10. [DOI] [PubMed] [Google Scholar]
- 60.Zingoni A, Soto H, Hedrick J A, Stoppacciaro A, Storlazzi C T, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]