Abstract
Helicobacter pylori produces a number of proteins associated with the outer membrane, including adhesins and the vacuolating cytotoxin. These proteins are supposed to integrate into the outer membrane by β-barrel structures, characteristic of the family of autotransporter proteins. By using the SOMPES (shuttle vector-based outer membrane protein expression) system for outer membrane protein production, we were able to functionally express in H. pylori the cholera toxin B subunit genetically fused to the C-terminal VacA domain. We demonstrate that the fusion protein is translocated to the H. pylori outer membrane and that the CtxB domain is exposed on the H. pylori surface. Thus, we provide the first experimental evidence that the C-terminal β-domain of VacA can transport a foreign passenger protein to the H. pylori surface and hence acts as a functional autotransporter.
Virulence factors of gram-negative bacteria are generally secreted proteins which adapt the bacterium to a particular niche in the host organism or damage the host in a way that is favorable to the microorganism. In gram-negative bacteria, the outer membrane is a barrier which has to be overcome in order to reach the extracellular space or the bacterial surface. To date, secretion systems in gram-negative bacteria are classified from types I to V (11). Type I to type IV secretion systems rely on more or less complex secretion apparatuses which in most cases span the periplasmic space. The type V secretion systems, which may be considered a subgroup of type II secretion systems, since they use the common sec-dependent protein export pathway for reaching the periplasmic space (26), do not require accessory proteins and are thus also called autotransporter (AT) secretion systems (9, 12).
The AT family comprises some 30 proteins, most of which have been assigned to this group by structure predictions (13) or primary sequence features (17). Many of these proteins indeed represent bacterial virulence factors, such as adhesins, which remain associated with the outer membrane, or alternatively proteases or toxins, which are released into the extracellular milieu. The vacuolating cytotoxin VacA of Helicobacter pylori is an example of the latter category. It is a protein toxin that is thought to be involved in generating gastric epithelial damage (33), and there is indeed a contribution of VacA to ulcer development in a Mongolian gerbil model (22). In a mouse model, however, VacA was shown to be necessary for colonization (27). A multitude of effects have been reported for the VacA protein: it forms anion-selective channels (2, 32), it decreases the transepithelial resistance (24), it interferes with antigen presentation (19) and with intracellular vesicle trafficking (29), possibly by interacting with a vimentin-binding protein (3), and it induces apoptosis (7). Nevertheless, the in vivo function of VacA in the host-pathogen interaction is still unclear.
The VacA precursor protein has typical features of AT proteins, i.e., an N-terminal signal sequence, a region that becomes the mature cytotoxin containing only two cysteine residues, and a C-terminal extension which is able to form a set of 14 to 16 amphipathic β-strands that make up the translocating β-barrel structure in the outer membrane. This C-terminal part also contains a primary sequence “signature” characteristic for putative AT proteins (17). Despite the large number of putative AT proteins assigned to this group mainly by sequence features, only a small percentage of them have been experimentally shown to be able to translocate homologous or heterologous passenger proteins to the bacterial surface.
We recently described the SOMPES (shuttle vector-based outer membrane protein expression) system, which allows the expression of genes encoding proteins associated with the outer membrane, such as the vacA gene (6). Here we use the SOMPES system to show that the C-terminal domain of the VacA precursor protein acts as an AT capable of transporting a foreign passenger protein into or across the outer membrane of H. pylori cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori strains (Table 1) were grown on GC agar plates (Difco) supplemented with horse serum (8%), vancomycin (10 mg/liter), trimethoprim (5 mg/liter), and nystatin (1 mg/liter) (serum plates) and incubated for 36 to 60 h in a microaerophilic atmosphere (85% N2, 10% CO2, 5% O2) at 37°C. Escherichia coli DH5α (BRL) was grown on Luria-Bertani (LB) agar plates or in LB liquid medium (28) supplemented with ampicillin (100 mg/liter), chloramphenicol (30 mg/liter), or kanamycin (40 mg/liter), as appropriate. Strains β2150 and β2155 (4) were grown on the same media supplemented with diaminopimelic acid (0.2 mM).
TABLE 1.
H. pylori strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| P12(888-0) | Wild type | 30 |
| P14 | P12 vacA::TnMax5-73 | 30 |
| P128 | P12 ΔvacA | 6 |
| P132 | P128(pWS115) | This study |
| P162 | P12(pWS115) | This study |
DNA manipulations.
Standard cloning and DNA analysis procedures were performed according to Sambrook et al. (28). Plasmid DNA was purified from E. coli by the boiling procedure, and E. coli cells for electroporation were prepared according to the protocol recommended for the Gene Pulser (Bio-Rad). Plasmid DNA was isolated from H. pylori strains by using Wizard Minipreps (Promega) according to the protocol of the manufacturer. Amplification of DNA fragments by PCR was performed as described previously (8).
Natural transformation and bacterial conjugation.
Shuttle plasmids and suicide plasmids were introduced into H. pylori strains by conjugation or natural transformation as described previously (6). H. pylori transformants were selected on serum plates containing 6 mg of chloramphenicol or 8 mg of kanamycin/liter.
Plasmid constructions.
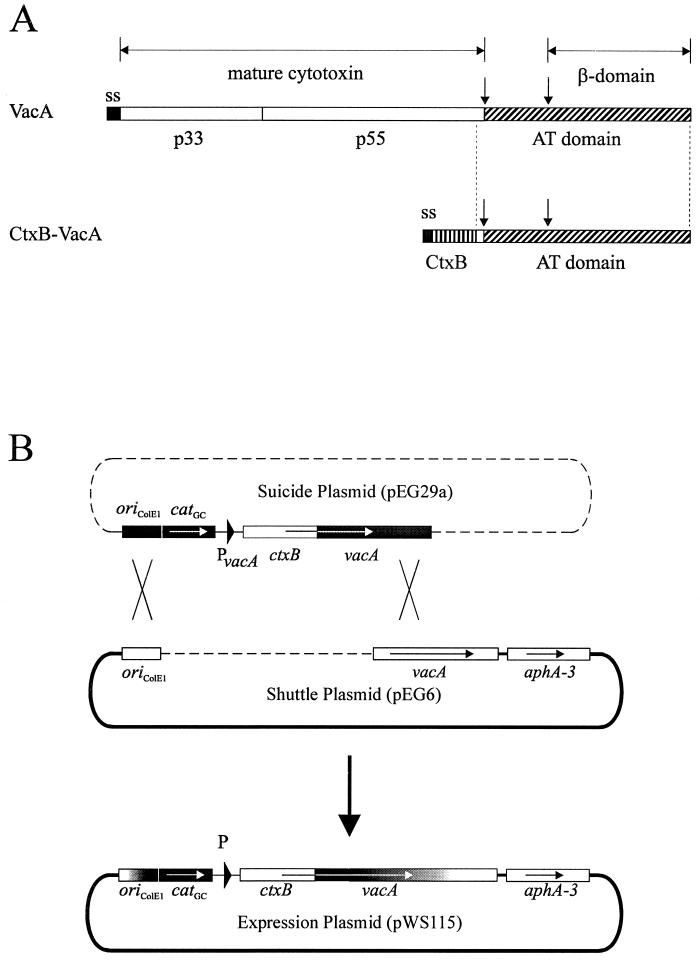
The shuttle plasmid pEG6 is an XbaI-XhoI deletion derivative (using the vacA internal XbaI site) of plasmid pDH64, the construction of which has been described elsewhere (6). For the construction of plasmid pEG29a, a ctxB fragment was PCR amplified by using primers WS65 and JM6 (Table 2) and plasmid pTK61 as a template (15). The PCR fragment was digested with BglII and KpnI and cloned into the corresponding sites on the minimal vector pMin1 (plasmid pWS69). An internal vacA fragment was amplified by using primers WS15 and WS43, digested with KpnI and SalI, and cloned into the KpnI and SalI sites of pWS69, yielding the ctxB-vacA fusion. The ctxB-vacA fusion was excised again with BglII and SalI and cloned, together with a vacA upstream fragment, amplified with primers WS13 and WS14, and digested with KpnI and BglII, into the KpnI and SalI sites of pDH59 (6). This resulted in plasmid pEG29a.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Gene | Positions | Sequence (5′-3′) |
|---|---|---|---|
| WS13 | vacA | 1–21 | GGGGTACCGCATGCTTTCTTCTACGAATG |
| WS14 | vacA | 599–580 | GAAGATCTATCGATATTTTCTTCCTTTCTTTTTG |
| WS15 | vacA | 3088–3107 | TCTTCTCGAGCTCCTAGACCTCCTGCGCCATCTACGCCTACTGAGAATGG |
| WS43 | vacA | 4141–4123 | CGCGTCGACACTTTTTGATTGCTGTTG |
| WS65 | ctxB | 156–177 | GAAGATCTTAGAATTAAGGATGAATTATGA |
| JM6 | ctxB | 515–495 | CATGGTACCAGGCGTTTTATTATTCCCTAC |
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (16) in a minislab apparatus. Proteins separated by SDS-PAGE were transferred to nitrocellulose membranes in a semidry blot apparatus at a current density of 0.8 mA/cm2. Unreacted sites of the nitrocellulose membrane were blocked with a 3% (wt/vol) solution of bovine serum albumin (BSA) in TBS (20 mM Tris-HCl, pH 7.5; 150 mM NaCl). The nitrocellulose membrane was then incubated with an appropriate dilution of antibody for 2 h and washed three times with TBS containing 0.5% (vol/vol) Tween 20. Subsequently, alkaline phosphatase conjugated to protein A was added in TBS containing 3% (wt/vol) BSA. After incubation for 1 h the nitrocellulose membrane was washed three times with TBS containing 0.5% (vol/vol) Tween 20 and developed by using BCIP (5-bromo-4-chloro-3-indolylphosphate) and nitroblue tetrazolium.
Production of antisera.
For the expression of a VacA fusion protein, the pEV40 expression vector system was used (25). An EcoRI/XhoI fragment was excised from the transposon-mutagenized plasmid pWS16-Tn76 (30) and cloned into pEV40b to obtain plasmid pWS31. This construct contains a 3′-vacA gene fragment from positions 3142 to 4474 (sequence accession no. Z26883). Plasmid pWS31 was transformed into E. coli 2136, carrying a temperature-sensitive λcIts repressor. A 500-ml culture of this clone was induced for expression at 42°C, and fusion protein inclusion bodies were harvested as described by Strebel et al. (31). The suspension with the enriched fusion protein was purified by Ni2+-nitrilotriacetic acid agarose affinity chromatography according to the protocol of the manufacturer (Qiagen). The purified protein was dialyzed against phosphate-buffered saline (PBS) and used to generate the antiserum AK204 in a rabbit. The anti-CtxB antiserum was purchased from Sigma. The production of the anti-AlpA antiserum has been described elsewhere (21).
Membrane preparations of H. pylori
Membrane preparations were performed essentially as described previously (23). H. pylori strains were grown on agar plates for 36 h. Bacteria were suspended in 10 mM HEPES-KOH (pH 7.4) and washed once. After three ultrasonications, each lasting 60 s, and separation from unlysed cells by centrifugation, the bacterial lysate was fractionated by ultracentrifugation at 100,000 × g for 1 h. The pellet was designated as a total membrane fraction, and the supernatant was designated as a cytosolic fraction. Total membranes were resuspended in 10 mM HEPES-KOH (pH 7.4) and layered on top of a continuous 50 to 70% (wt/vol) sucrose density gradient in the same buffer. After ultracentrifugation at 90,000 × g for 18 h, two visible bands (corresponding to sucrose densities of 62 to 66% [G1] or 54% [G2], respectively) were collected and centrifuged at 100,000 × g for 1 h to remove the sucrose. The pellets were resuspended in 10 mM HEPES-KOH (pH 7.4) and further analyzed by Western blot. The relative contents of cytoplasmic and outer membranes in the fractions G1 and G2 were estimated by determination of succinate dehydrogenase activities (14) for the cytoplasmic membrane and the amount of 2-keto-3-deoxyoctulonic acid (23) for the outer membrane.
Whole-cell protease treatment of H. pylori.
H. pylori cells were collected from serum plates, suspended in PBS, and adjusted to an optical density at 550 nm of 6.0. A 2-μl portion of trypsin or chymotrypsin stock solution was added to 200 μl of bacterial suspension to yield a final concentration of 50 μg of protease/ml. After incubation for 10 min at room temperature, digestion was stopped by washing the cells twice with 1 ml of PBS containing 10% fetal calf serum. Cells were collected by centrifugation and resuspended in sample buffer for SDS-PAGE.
RESULTS
Expression of a ctxB-vacA gene fusion in H. pylori.
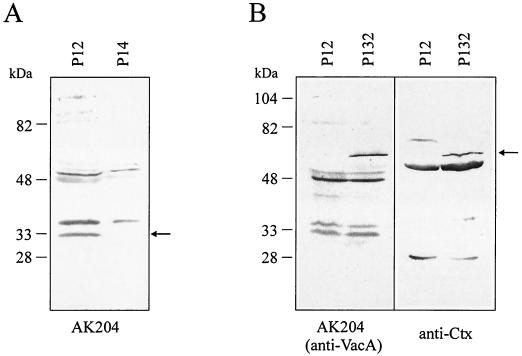
The secretion of VacA is generally postulated to be mediated by an AT mechanism, involving the C-terminal β-barrel domain in translocation across the outer membrane (1). This mechanism was deduced essentially from sequence and structural comparisons but has not been demonstrated experimentally so far. We therefore used the SOMPES technique to produce a fusion between a genetically engineered cholera toxin B subunit (CtxB) and the C-terminal part of the VacA precursor protein, which is thought to contain the AT function (AT domain). The fusion was constructed with a ctxB gene derived from plasmid pTK61, which contains its own Shine-Dalgarno sequence and two site-specific mutations resulting in exchange of both naturally occurring cysteine residues in CtxB (15). For the reconstruction of the ctxB-vacA gene fusion, a shuttle plasmid (pEG6) with a shortened 3′-vacA gene portion was constructed (Fig. 1B). This shuttle plasmid was introduced into H. pylori P128 (6) containing an unmarked chromosomal deletion of the vacA gene. The ctxB-vacA fusion was cloned into the suicide plasmid pEG29a. The reconstructed expression plasmid pWS115, obtained by recombination between pEG6 and pEG29a, contains the engineered ctxB gene fused to the AT domain of vacA (Fig. 1B). The ctxB-vacA fusion still contains a short sequence corresponding to the 27 C-terminal amino acids of the mature VacA protein (amino acids 798 to 824; accession no. Z26883). A whole-cell lysate of the resulting recombinant H. pylori P132 contained the expected 61-kDa fusion product, as shown in an immunoblot (Fig. 2B). This protein band was recognized by a polyclonal antiserum directed against cholera toxin (anti-Ctx), as well as by an antiserum reacting with the C-terminal VacA domain (AK204), confirming that the fusion protein contains both domains.
FIG. 1.
Domain structure of the VacA precursor protein and production of a CtxB-VacA fusion protein in H. pylori. (A) The VacA precursor consists of a signal sequence (ss), the cytotoxin domain, and an AT domain, the C-terminal part of which is predicted to fold into a β-barrel structure (β-domain). The putative A (p33) and B (p55) parts of the mature cytotoxin are indicated; molecular weights are as described elsewhere (20). The postulated AT domain including 27 C-terminal amino acids of the mature VacA protein was fused to CtxB. Processing sites between the mature 88-kDa VacA and the AT domain, as well as within the AT domain, are indicated by arrows. (B) Reconstruction principle of the SOMPES technique. A 3′ region of the vacA gene is cloned into the shuttle vector pHel3; the ctxB gene fused to an internal part (without the 3′ end) of the vacA gene is cloned on a suicide plasmid. After recombination, the ctxB-vacA fusion is produced on the shuttle plasmid.
FIG. 2.
(A) Detection of a putative β-domain fragment in the wild-type strain P12 by immunoblotting. A band of ca. 33 kDa, which is absent from a lysate of the vacA mutant P14, is recognized by the C-terminal VacA antiserum AK204. The missing 15-kDa fragment between the mature part of the cytotoxin and the β-domain could not be detected. (B) Evidence for the production of a CtxB-VacA fusion protein in H. pylori. Whole-cell lysates of H. pylori P12 and P132 were analyzed in an immunoblot with AK204 recognizing the VacA AT domain and with an anti-Ctx antiserum. The protein band of 61 kDa (arrow) is recognized by both antisera and corresponds to the expected fusion protein.
Localization of the CtxB-VacA fusion.
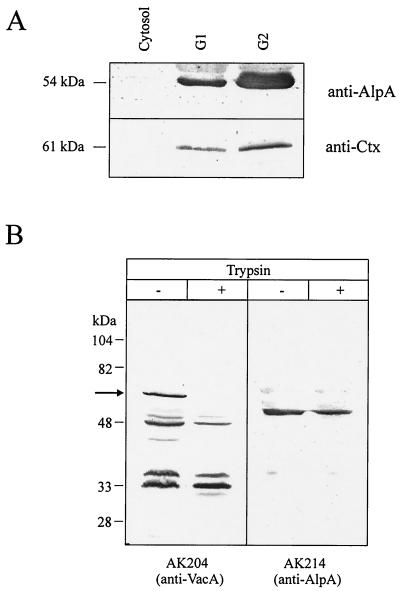
In order to localize the fusion protein within the bacteria, membrane preparations of strain P132 were made (see Materials and Methods). For unknown reasons, a complete separation of both membranes is hard to achieve for H. pylori (5). Membrane fractions G1 and G2 of H. pylori P132 prepared from a sucrose density gradient were estimated to have an outer membrane content of ca. 29 and 67%, respectively (calculated from succinate dehydrogenase activities of 8.4 versus 5.2 mU/mg of protein and 2-keto-3-deoxyoctulonic acid amounts of 4.1 versus 11.8 μmol/g of protein). These fractions were tested for their contents of the CtxB-VacA fusion and the outer membrane protein AlpA (21) (Fig. 3A). Both fractions contained AlpA and CtxB-VacA, the majority being found in the outer membrane-enriched fraction G2. No AlpA or CtxB-VacA protein was found in the cytosolic fraction, which also contains periplasmic proteins. The similar distribution of AlpA and CtxB-VacA indicates that the CtxB-VacA fusion is transported to the outer membrane of H. pylori.
FIG. 3.
(A) Localization of the CtxB-VacA fusion protein in the outer membrane fraction by immunoblotting. Fractions corresponding to the cytosol, the major part of the cytoplasmic membrane (G1), and the major part of the outer membrane (G2) were analyzed by Western blot with antisera AK214 (anti-AlpA; top) or anti-Ctx antiserum (bottom). The same amount of protein (25 μg) was loaded into all lanes. The CtxB-VacA fusion protein and AlpA are mainly found in the outer membrane fraction. (B) Trypsin digestion of whole cells of strain P132. Untreated control cells and trypsin-treated cells were analyzed by Western blot using AK204 (anti-VacA) or AK214 (anti-AlpA). The full-length CtxB-VacA fusion is completely processed (arrow), whereas AlpA is trypsin resistant.
Protease digestion experiments with untreated and with osmotically shocked bacteria.
The localization of the CtxB-VacA fusion in the outer membrane and the exposure of the CtxB domain to the cell surface was further confirmed by proteolytic digestion experiments. Treatment of whole cells of strain P132 with trypsin (Fig. 3B) or chymotrypsin (data not shown) resulted in complete processing of the full-length CtxB-VacA fusion. In contrast, AlpA (Fig. 3B) and other H. pylori proteins located in the cytoplasmic membrane (ComB3) or the cytoplasm (RecA) were not susceptible to protease treatment (data not shown). Since AlpA has an extensive N-terminal periplasmic domain (21), which should be degraded if trypsin enters the periplasm, this confirms the structural integrity of the outer membrane during trypsin treatment. After osmotic shock of the bacteria, AlpA (and also ComB3) was indeed partially degraded by trypsin treatment (data not shown). These observations indicate that the CtxB-VacA fusion protein is localized on the outer surface of H. pylori and hence confirm the role of the C-terminal VacA domain as a functional AT.
Detection and protease digestion of the pre-VacA β-domain.
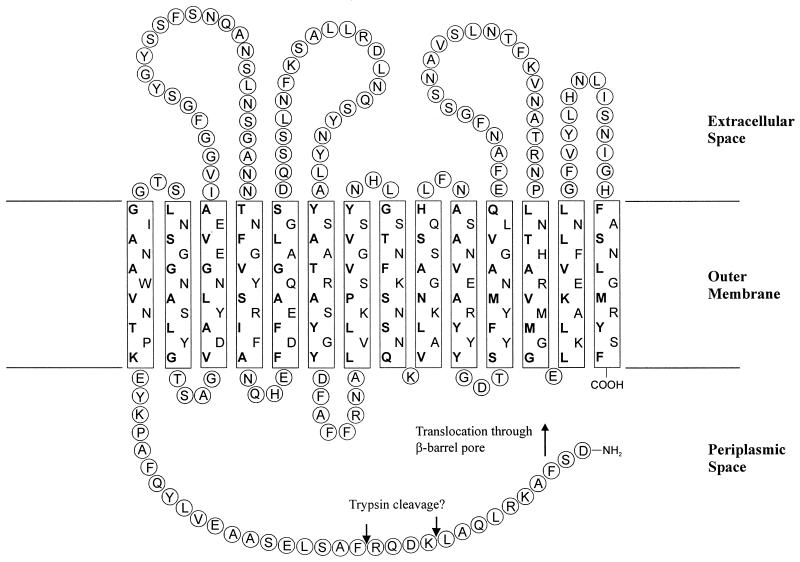
During secretion, the VacA precursor protein is proteolytically processed to produce the mature extracellular cytotoxin. Apart from the cleavage of the signal sequence by a LepB signal peptidase, this processing occurs at two sites, generating the mature cytotoxin, a 33-kDa C-terminal fragment (34) and a linker fragment of ca. 15 kDa. By Western blot analysis of total lysates of the wild-type strain P12 and its isogenic vacA mutant P14 with the antiserum AK204, we were able to detect a VacA fragment of ca. 33 kDa (Fig. 2A). This fragment most probably represents the β-domain of the precursor protein, the membrane-inserting part of which is predicted to have a size of ca. 29 kDa (Fig. 4).
FIG. 4.
One possible model for the insertion of the VacA β-domain in the H. pylori outer membrane. The localization of the membrane-spanning β-pleated sheets has been derived from hydrophobicity plots by using the Amphi program (35). Hydrophobic amino acids within the β-pleated sheets facing the lipid environment of the outer membrane are indicated in boldface. Amino acids 988 to 1291 of the VacA precursor from strain 185-44 are shown, corresponding to a domain of ca. 33 kDa. Note that the exact cleavage site is not known.
The 33-kDa β-domain fragment does not seem to have protease-sensitive extracellular loops since it can be degraded by externally added proteases but only to a protease-resistant core of ca. 31 kDa (Fig. 5). If the bacteria are osmotically shocked prior to protease treatment, the putative β-domain is not degraded to smaller fragments (data not shown). This suggests that 31 kDa of the β-domain is embedded in the outer membrane, with a small extension on its N terminus facing the bacterial surface through the β-barrel pore.
FIG. 5.
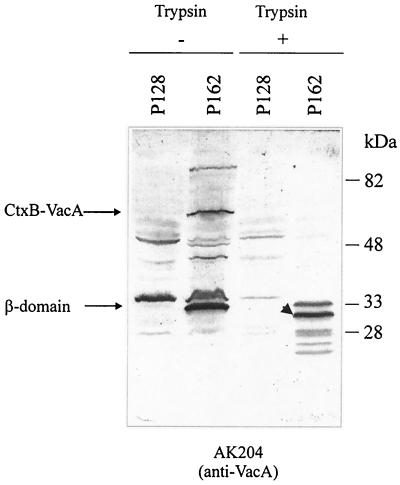
Coexpression of the ctxB-vacA fusion and the native vacA gene. In the bacterial lysate of strain P162, the CtxB-VacA fusion and the β-domain can be detected in an immunoblot by using antiserum AK204. For comparison, the P12 vacA strain P128 is shown. In spite of the presence of the native VacA precursor, the CtxB-VacA fusion is not completely processed. Trypsin digestion of whole bacteria results in degradation of the β-domain to a protease-resistant core of ca. 31 kDa (arrowhead). Possible trypsin cleavage sites are indicated in Fig. 4.
Processing of the CtxB-VacA fusion.
The CtxB-VacA fusion produced from plasmid pWS115 still contains both sites of the VacA precursor for proteolytic processing (Fig. 1A). The 33-kDa β-domain fragment can be detected in whole-cell lysates of strain P132 as well, but in contrast to the processing of the VacA precursor, processing of the CtxB-VacA fusion is incomplete (Fig. 2B). One reason for such an incomplete processing might be the lack of the proteolytic activity in strain P132, with some unspecific proteolysis accounting for the low efficiency. This would mean that the VacA precursor has an autoproteolytic activity which is absent in the CtxB-VacA fusion. In order to test this possibility, we prepared the ctxB-vacA shuttle plasmid pWS115 from strain P132 and used it to transform the wild-type strain P12, thus generating a strain, P162, that produces both the wild-type VacA precursor and the CtxB-VacA fusion. In this strain, a larger amount of the 33-kDa β-domain than of the 61-kDa CtxB-VacA fusion can be detected, but the complete CtxB-VacA fusion is still present (Fig. 5). This observation suggests that the CtxB-VacA fusion and the VacA precursor are not processed by autoproteolytic activity as in the case of the N. gonorrhoeae IgA protease.
DISCUSSION
The AT secretion pathway is now being recognized as a common mechanism for the secretion of proteins displaying a wide functional diversity, a pathway that is used throughout the gram-negative organisms (10). ATs are characterized by a rather conserved C-terminal domain, the β-domain, which is the region to be inserted in the outer membrane. However, an actual outer membrane localization, or the ability to translocate passenger domains across the outer membrane, has not been proven in many cases. Given the diversity in outer membrane composition among the gram-negative organisms putatively using this secretion mechanism, care should be taken when extrapolating features from one well-studied system to another. This diversity also became obvious when we tried to express H. pylori genes encoding outer membrane proteins in E. coli (6). In this study, we demonstrate that the β-domain of the VacA precursor protein of H. pylori, which has been termed a putative AT domain due to amphipathic β-strand predictions (30) and predictions of high surface probability (36), is indeed able to transport a heterologous passenger protein, i.e., the CtxB protein, to the bacterial surface.
Because of the problems with production of H. pylori outer membrane proteins in E. coli, we used the SOMPES method (6) for the expression of a heterologous fusion between CtxB as a passenger protein and the putative outer membrane transporter domain of VacA (Fig. 1). This construct could not be expressed in E. coli (data not shown). As demonstrated in Fig. 3, the CtxB-VacA fusion protein was translocated to the outer membrane of H. pylori. It was found enriched in the membrane fraction containing the majority of the outer membrane, indicating that it was inserted into the outer membrane, rather than into the cytoplasmic membrane, or into the periplasmic space. For the immunoglobulin A protease AT of Neisseria gonorrhoeae (15) and the AIDA-I AT from E. coli (18), the surface accessibility of the CtxB passenger protein could be demonstrated by immunofluorescence by using an anti-Ctx antiserum. Our attempts to localize the protein on the Helicobacter surface by immunofluorescence with an anti-Ctx antiserum failed due to the high background reaction of wild-type H. pylori with this antiserum. The absorption of the antiserum against a P12 lysate reduced the background signal, but the wild-type strain still reacted with the absorbed antiserum. Therefore, the surface accessibility of CtxB was shown by protease digestion experiments. Trypsin or chymotrypsin treatment of whole bacterial cells resulted in a complete removal of the full-length CtxB-VacA fusion construct. The integrity of the outer membrane in the CtxB-VacA expressing strain was demonstrated by the inability of trypsin to digest AlpA, which harbors an extended N-terminal periplasmic domain (21).
A further aspect is the processing of the VacA precursor protein in comparison to the CtxB-VacA fusion protein. The 87-kDa mature VacA is processed from the precursor either by autoproteolysis or by the activity of a membrane-bound protease. The AT domain is further processed into a 15-kDa fragment and a 33-kDa fragment (34) (Fig. 2A). We were unable to detect the 15-kDa linker fragment with our antiserum AK204. Even when we attempted to insert a sequence tag (Strep-tag II, WSHPQFEK; Institut für Bioanalytik, Göttingen, Germany) into the linker fragment, a detection or purification of the corresponding fragment was not successful (data not shown). This suggests that the linker fragment is rapidly degraded after proteolytic processing. In some preparations of strain P12, however, a protein of 103 kDa was detected in Western blots in addition to the mature 87-kDa VacA (data not shown). This band might represent an incomplete processing product consisting of the mature cytotoxin and the 15-kDa linker fragment.
The CtxB-VacA fusion protein was not completely processed in strain P132 (see Fig. 2B). Coproduction of the CtxB-VacA fusion protein and the wild-type VacA precursor did not significantly enhance the processing efficiency (Fig. 5). Since the CtxB-VacA fusion protein contains only 27 amino acids upstream of the recently determined processing site between mature cytotoxin and linker fragment (20), proteolytic processing of the CtxB-VacA fusion protein probably does not depend on the mature part of the cytotoxin. This might indicate that the processing site in the fusion protein is not well accessible for the putative membrane-bound protease. In that case, partial processing of the CtxB-VacA fusion protein might be an inefficient reaction, but not unspecific, since the correct β-domain fragment is produced. An alternative explanation would be that an oligomerization is necessary for processing, which may be inefficient in the case of the CtxB-VacA fusion. We cannot exclude, of course, that the VacA precursor has an autoproteolytic activity, but this seems unlikely as the only reason for partial processing of the CtxB-VacA fusion.
Taken together, our results show that the C-terminal VacA domain is embedded in the outer membrane of H. pylori. This domain is able to transport a foreign passenger protein to the cell surface and hence acts as a functional AT. Thus, the VacA secretion mechanism seems to be very similar to that used by other well-characterized type V secretion systems, although the ATs cannot be exchanged, possibly due to differences in their membrane insertion characteristics. A more detailed description of such characteristic features of different AT molecules will provide the basis for a better understanding of this growing family of secreted proteins.
ACKNOWLEDGMENTS
We thank B. P. Burns for critical reading of the manuscript. We are grateful for plasmid pTK61 from T. F. Meyer and E. coli strains β2150 and β2155 from C. Dehio.
This work was supported by the Deutsche Forschungsgemeinschaft (grant HA 2697/1–4 to R.H.).
REFERENCES
- 1.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 2.Czajkowsky D M, Iwamoto H, Cover T L, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bernard M, Moschioni M, Napolitani G, Rappuoli R, Montecucco C. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 2000;19:48–56. doi: 10.1093/emboj/19.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehio C, Meyer M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol. 1997;179:538–540. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exner M M, Doig P, Trust T J, Hancock R E W. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect Immun. 1995;63:1567–1572. doi: 10.1128/iai.63.4.1567-1572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer W, Schwan D, Gerland E, Erlenfeld G E, Odenbreit S, Haas R. A plasmid-based vector system for the cloning and expression of Helicobacter pylori genes encoding outer membrane proteins. Mol Gen Genet. 1999;262:501–507. doi: 10.1007/s004380051111. [DOI] [PubMed] [Google Scholar]
- 7.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard J C, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas R, Meyer T F, van Putten J P M. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol Microbiol. 1993;8:753–760. doi: 10.1111/j.1365-2958.1993.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 9.Henderson I R, Cappello R, Nataro J P. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 2000;8:529–532. doi: 10.1016/s0966-842x(00)01853-9. [DOI] [PubMed] [Google Scholar]
- 10.Henderson I R, Nataro J P. Virulence functions of autotransporter proteins. Infect Immun. 2001;69:1231–1243. doi: 10.1128/IAI.69.3.1231-1243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson I R, Nataro J P, Kaper J B, Meyer T F, Farrand S K, Burns D L, Finlay B B, St. Geme J W. Renaming protein secretion in the gram-negative bacteria. Trends Microbiol. 2000;8:352. [PubMed] [Google Scholar]
- 12.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 13.Jose J, Jähnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:378–380. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 14.King T E. Preparations of succinate-cytochrome c reductase and the cytochrome b-c1 particle, and reconstitution of succinate-cytochrome c reductase. Methods Enzymol. 1967;10:216–225. [Google Scholar]
- 15.Klauser T, Pohlner J, Meyer T F. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease b-domain: conformation-dependent outer membrane translocation. EMBO J. 1990;9:1991–1999. doi: 10.1002/j.1460-2075.1990.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Loveless B J, Saier M H J. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol Membr Biol. 1997;14:113–123. doi: 10.3109/09687689709048171. [DOI] [PubMed] [Google Scholar]
- 18.Maurer J, Jose J, Meyer T F. Autodisplay: one-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J Bacteriol. 1997;179:794–804. doi: 10.1128/jb.179.3.794-804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen V Q, Caprioli R M, Cover T L. Carboxy-terminal proteolytic processing of Helicobacter pylori vacuolating toxin. Infect Immun. 2001;69:543–546. doi: 10.1128/IAI.69.1.543-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 1999;31:1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- 22.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn M J, Gander J E, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J Biol Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 24.Pelicic V, Reyrat J M, Sartori L, Pagliaccia C, Rappuoli R, Telford J L, Montecucco C, Papini E. Helicobacter pylori VacA cytotoxin associated with the bacteria increases epithelial permeability independently of its vacuolating activity. Microbiology. 1999;145:2043–2050. doi: 10.1099/13500872-145-8-2043. [DOI] [PubMed] [Google Scholar]
- 25.Pohlner J, Krämer J, Meyer T F. A plasmid system for high-level expression and in-vitro processing of recombinant proteins. Gene. 1993;130:121–126. doi: 10.1016/0378-1119(93)90354-6. [DOI] [PubMed] [Google Scholar]
- 26.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama N R, Otto G, Tompkins L, Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun. 2001;69:730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 31.Strebel K, Beck E, Strohmaier K, Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986;57:983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford J L, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telford J L, Covacci A, Ghiara P, Montecucco C, Rappuoli R. Unravelling the pathogenic role of Helicobacter pylori in peptic ulcer: potential new therapies and vaccines. Trends Biotechnol. 1994;12:420–426. doi: 10.1016/0167-7799(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 34.Telford J L, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel H, Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986;190:191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang H J, Chang P C, Kuo C H, Tzeng C S, Wang W C. Characterization of the C-terminal domain of Helicobacter pylori vacuolating toxin and its relationship with extracellular toxin production. Biochem Biophys Res Commun. 1998;250:397–402. doi: 10.1006/bbrc.1998.9228. [DOI] [PubMed] [Google Scholar]