Abstract
This research aimed to evaluate the effects of high-dose cholecalciferol (VD3) supplements (50,000 IU/week) on selected circulating cytokines associated with cytokine storms in adults with vitamin D deficiency. This clinical trial, based in Jordan, included 50 participants receiving vitamin D3 supplements (50,000 IU/week) for 8 weeks; the exact number was assigned to the control group. Interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-10 (IL-10), tumor necrotic factor-α (TNF-α), and leptin were measured in serum at baseline and 10 weeks (wash out: 2 weeks). Our results revealed that vitamin D3 supplementation significantly increased the serum levels of 25OHD, IL-6, IL-10, IL-1β, and leptin compared with baseline. In contrast, the serum level of TNF-α insignificantly increased in the group receiving vitamin D3 supplementation. Although the observations of this trial may refer to a potential negative effect of VD3 supplementation during cytokine storms, further trials are required to clarify the potential benefits of VD3 supplement during cytokine storms.
Keywords: vitamin D deficiency, vitamin D3, cytokine, storm, interleukin 6, interleukin 10, interleukin 1β, TNF-α
1. Introduction
The new coronavirus infection (COVID-19) is a global pandemic that has aggressively propagated worldwide [1]. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is responsible for the pandemic viral pneumonia known as COVID-19. A recent study observed elevated serum levels of specific cytokines, such as those seen during the COVID-19 cytokine storm (CS), which may be associated with severe complications resulting from the infection [2]. These cytokines include tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, IL-, 10, IL-17, interferon-gamma (IFN-γ), and many other cytokines [1]. Accordingly, hypercytokinemia has been recently suggested to be one of the main hallmarks of COVID-19 [1]. However, many COVID-19 symptoms can be treated based on the patient’s clinical condition. Hence, because there is no specific treatment for COVID-19 yet, supportive care, including dietary vitamins and minerals for infected persons, can be highly effective.
In this manner, vitamins D (VD) and C, in addition to zinc supplementations, are recommended by the Jordan Ministry of Health as a part of the treatment protocol for COVID-19 patients. VD increases innate cellular immunity and provides direct antibacterial activity against various microorganisms, including enveloped and nonenveloped viruses [3]. It has also been shown that VD may mitigate the CS induced by the innate immune system [4]. A previous study [5] concluded that VD modulates the immune response via its effects on dendritic cells (DCs) and T cells. This may enhance the clearance of the virus and decrease inflammatory reactions associated with symptoms. However, vitamin D deficiency (VDD) is still a global problem; over one billion people are either VD deficient or insufficient, and the incidence of VDD was reported to be higher in Mediterranean countries such as Jordan [6,7,8]. VDD is known to be directly associated with bone disorders, but nonskeletal outcomes grabbed the most attention [9]. It was linked with different disorders, including diabetes, cardiovascular disease (CVD), atherosclerosis, and cancer [10,11], and certain abnormal immune conditions, including infections [5]. Recent reviews indicated the ability of VD to reduce the risk of microbial infections through different potential mechanisms: physical barrier, natural cellular, and adaptive immunity [12,13].
Consequently, raising 25-hydroxyvitamin D (25OHD) levels by 1,25(OH)2D3 (VD3) supplementation is highly recommended to reduce the risk of infection and is advised as part of the treatment protocol for people who are sick with influenza [4]. However, the modulatory effects of VD on proinflammatory and anti-inflammatory, as well as cellular and humoral immune, responses are mixed and unclear. There is a study that showed that VD could suppress the production of T-helper (Th)1 proinflammatory cytokine [14] and augment Th2 cell development [15]. Furthermore, VD3 enhances T regulatory cell induction, thus inhibiting inflammatory processes [16].
A previous study [17] showed the safety of VD and a protection activity against acute respiratory tract infection. VDD has been correlated with acute respiratory distress syndrome (ARDS), and case fatality rates (CFRs) increase with age and comorbidity with chronic diseases, both of which are associated with lower 25OHD concentration [4]. Therefore, this randomized clinical trial (RCT) was designed to measure serum levels of IL-1β, IL-6, IL-10, and TNF-α as part of the immune response during CS before and 8 weeks after high-dose VD3 50,000 IU in adults with VDD.
2. Materials and Methods
2.1. Patient Characteristics
This RCT was approved by the Institutional Review Board of Applied Science Private University (ASU) (protocol number 2020-PHA-16) and undertaken between October 2020 and December 2020. The clinical trial was conducted following the Helsinki Declaration. Each individual who was enrolled provided informed consent for this clinical trial. With an average baseline age of 38.37 ± 9.77 years, volunteers included were Jordanian and from ASU staff and their families (ranging from 30 to 66). Eligible participants were included in the trial depending on a diagnosis of VDD confirmed by medical consultants at Ibn Al-Haytham clinical laboratories. Because prolonged VD3 administration is related to the formation of kidney stones, patients with kidney abnormalities were excluded from the study [18]. COVID-19 or chronic medical conditions, such as osteoporosis, cancer, endocrine disorders, and a history of allergic responses to VD3 supplements, were also among the exclusion criteria from this study.
2.2. Intervention
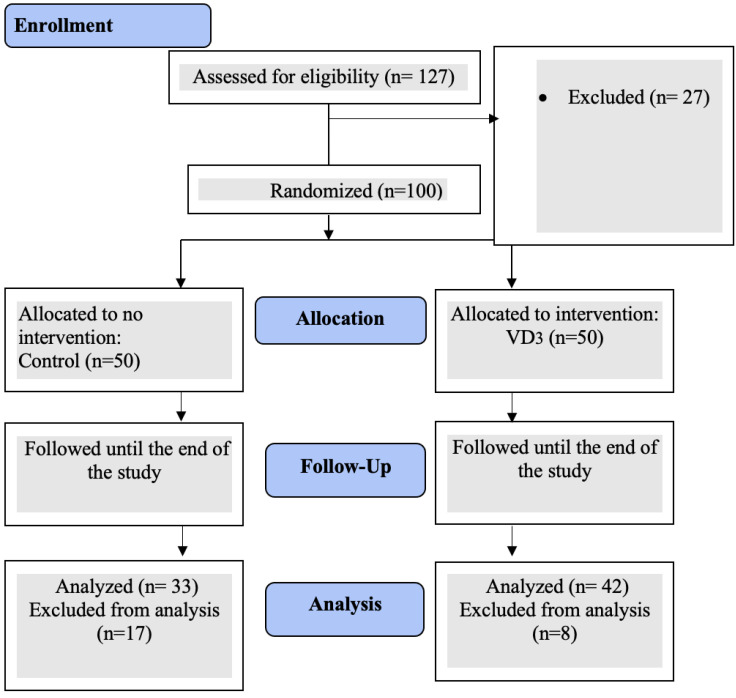
Before and after the VD3 supplement, baseline and follow-up values of anthropometric and clinical parameters were collected. At the conclusion of the 8-week interventional phase, the subjects underwent a 2-week washout phase before and following VD3 administration. VD3 is a fat-soluble vitamin with a long half-life; a washout period was achieved to avoid the potential effect of its cumulative dose. Then, all participants’ follow-up measurements were obtained. An independent statistician developed a computer-generated randomization process. According to the consortium chart (Figure 1), hundreds of eligible participants were divided into two groups: group 1 received once weekly 50,000 IU of VD3 in a Hi-Dee soft gelatin capsule (United Pharmaceuticals Company, Amman, Jordan). Participants in group 2 did not receive any supplementation and acted as the control group. In compliance with the Endocrine Society’s clinical guidelines for treating VDD in adults, therapeutic protocols for VD3 supplements were approved [19]. Similarly, administering VD3 to individuals throughout 12 months produced no toxicity [19]. All participants’ adherence to the therapy protocol was monitored by periodic text messages sent to their mobile phones.
Figure 1.
Consort flow diagram for the study.
2.3. Anthropometric Measurement
This RCT was conducted throughout the winter of 2020 at the ASU Pharmacy school laboratories to minimize seasonal fluctuations in vitamin D assays in the blood [20]. At the beginning and end of the experiment, anthropometric measurements, such as body mass index (BMI), body weight (BW), hip (H) circumference, waist/hip ratio (WHR), height (Ht), and waist (W) circumference, were recorded.
2.4. Clinical Parameter Assays
Serum assay of clinical parameters was collected into labeled Eppendorf tubes at Ibn Al-Haytham Hospital, Clinical Laboratories Department, Jordan.
The chemiluminescence immunoassay LIAISON 25OHD assay (DiaSorin, Saluggia, Italy) measured total serum 25OHD. The assay quantifies serum 25OHD and is cross-reactive with 25OHD2 and 25OHD3. Its lower limit was 4 ng/mL. An enzyme immunoassay kit measured serum leptin levels (leptin EIA-5302, DRG Diagnostics, Marburg, Germany). Test sensitivity was 0.1 ng/mL. An enzyme immunoassay kit tested serum PTH levels (PTH Intact EIA-3645, DRG Diagnostics, Marburg, Germany). The sensitivity was 1.57 pg/mL. The calcium-ARSENAZO kit (M11570i-15) and the phosphorus phosphomolybdate/Uv kit (M11508i-18, BioSystems, Barcelona, Spain) were used to measure the levels of calcium and phosphorus (PO4) in serum. Serum IL-6 concentration was measured using the Human ELISA KIT (ab178013, Abcam, Newark, NJ, USA). Using a Human ELISA Kit, serum IL-10 was measured (ab185986, Abcam). Human ELISA KIT assessed serum IL-1β (ab214025, Abcam). The Human ELISA KIT assay measured serum TNF-α (ab181421, Abcam).
2.5. Statistical Analysis
SPSS version 27 for Windows was used to execute the statistical analysis. A paired t-test was performed to determine any significant variations in each trial group before and after the delivery of the VD3 supplementation. Two independent sample t-tests were utilized to identify whether there were significant differences between all items of distinct groups (control, D3). Using correlation analysis, correlations were investigated between the serum levels of TNF, IL-1β, IL-6, IL-10, and 25-OHD, as well as between their ratios (TNF-α /IL-10, IL-1β /IL-10, and IL-6/IL-10). Simple linear regression was used to investigate the effect of VD3 supplementation on the items (TNF-α, IL-1β, IL6, IL10, ratio (TNF-α /IL-10), ratio (IL-1β/IL-10), ratio (IL-6/IL-10)), while multiple linear regression analysis was used to determine the predictors of the items (TNF-α, IL-1β, IL-6, IL-10, ratio (TNF-α /IL-10), ratio (IL-1β/IL-10), and ratio (IL-6/IL-10)) at the follow-up level for the (D) group. The Kolmogorov–Smirnov test was utilized to test the normality of distribution for laboratory measurements. The results displayed a normal distribution curve.
3. Results
3.1. Baseline Values of the Participants
A total of 75 out of 127 (59.1%) participants in the trial adhered to the protocol and finished the intervention period that lasted for 8 weeks. As indicated in Figure 1, the reasons why participants dropped out (n = 27) included noncompliance, not fulfilling inclusion criteria, and dropping out from the intervention group (n = 8) and the control group (n = 17). In this trial, 50.7% were female, and 49.2% were male. Morning sun exposure was practiced by 57.3% of participants. Other baseline percentages and frequencies of analyzed anthropometric and lifestyle variables are displayed in Table 1.
Table 1.
Frequencies and percentages of anthropometric and lifestyle variables at baseline (n = 75).
| Variable | Category | Frequency | % |
|---|---|---|---|
| Group | Control | 33 | 44.0 |
| VD3 | 42 | 56.0 | |
| Total | 75 | 100.0 | |
| Gender | Male | 37 | 49.3 |
| Female | 38 | 50.7 | |
| Total | 75 | 100.0 | |
| Morning sun exposure (20–30 min/day) |
Yes | 43 | 57.3 |
| No | 32 | 42.7 | |
| Total | 75 | 100.0 |
Abbreviations: VD3, VD3 supplementation group.
Participants’ average age was (38.37 9.77). When measured at baseline, all other serum markers had mean values that were within normal limits. Descriptive analysis for anthropometric parameters, including BMI (27.90 ± 4.76), waist and hip circumferences, and WHR, are shown in Table 2.
Table 2.
Statistical description of anthropometric parameters at baseline level (n = 75).
| Parameter | Mean (SD) |
|---|---|
| Age (year) | 38.37 (9.77) |
| Weight (kg) | 78.51 (15.79) |
| Height (cm) | 166.92 (7.42) |
| BMI (kg.m−2) | 27.90 (4.76) |
| Waist (cm) | 94.58 (14.12) |
| Hip (cm) | 106.13 (11.52) |
| WHR | 89.38 (11.39) |
Abbreviations: SD, standard deviation; BMI, body mass index; WHR, waist/hip ratio.
3.2. Baseline Clinical Characteristics
The baseline mean value for serum 25OHD was (17.29 ± 6.18) ng/mL (all participants were VD deficient). None of the participants presented in this trial with a baseline serum 25OHD level equal to or greater than 30 ng/mL. All participants’ baseline values of all serum parameters, including PTH, Ca, and PO4, were within normal ranges. A descriptive analysis of the clinical parameters is presented in Table 3. Table 4 shows baseline mean values for the serum levels of IL-1β, IL6, IL10, and TNF-α and their ratios.
Table 3.
Descriptive summary of clinical variables at baseline (n = 75).
| Parameter | Mean (SD) | Normal Range |
|---|---|---|
| 25OHD (ng/mL) | 17.29 (6.18) | 30–50 |
| PTH (pg/mL) | 37.38 (7.57) | 9–90 |
| Ca (mg/dl) | 9.30 (1.24) | 8.6–10.3 |
| PO4 (mg/dl) | 4.05 (0.18) | 2.5–4.5 |
| Leptin (ng/mL) | 7.86 (6.17) | NA |
Abbreviations: 25OHD, 25 hydroxy vitamin D; PO4, phosphorus; NA, not applicable; SD, standard deviation.
Table 4.
Levels of selected cytokines involved in the cytokine storm of COVID-19 at baseline in the entire study population with vitamin D deficiency (n = 75).
| Parameter | Mean (SD) | Range * |
|---|---|---|
| IL-1β | 3.24 (1.16) | 0.17–24 |
| TNF-α | 32.38 (5.95) | 0.93–26.8 |
| IL-6 | 5.08 (5.16) | 0.16–37.7 |
| IL-10 | 2.09 (0.56) | 0.01–19.8 |
| (TNF-α/IL10) | 1792.95 (1069.38) | |
| (IL-1β/IL10) | 173.84 (97.53) | |
| (IL6/IL10) | 243.21 (235.10) |
Abbreviations: IL-1β, interleukin-1 beta; IL-6, interleukin 6; IL-10, interleukin 10; TNF-α, tumor necrosis-alpha; SD, standard deviation. Note: * values are expressed as pg/mL—serum levels of cytokines for healthy people (age < 45 years) (23).
3.3. Connection between Selected Cytokine Variables and 25OHD Concentrations
In this trial, selected proinflammatory cytokines (IL-1β, TNF-α, and IL-6) and anti-inflammatory cytokine (IL-10) showed statistically significant intercorrelations (Table 5). At baseline, serum 25OHD levels showed a significant inverse correlation with serum IL-1β levels (R= −0.280, p = 0.015). Serum IL-1β levels also showed significant positive correlations with IL-6 (R = 0.236, p= 0.041) and IL-10 (R = 0.239, p = 0.039). Pearson correlation analysis showed no correlation between serum IL-6 and IL-10 levels. Other baseline intercorrelations between studied cytokines ratios are listed in Table 5.
Table 5.
Correlation of selected cytokine with baseline and follow-up 25OHD levels.
| Variable | Baseline | Follow-Up | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| TNF-α | −0.072 | 0.538 | −0.027 | 0.867 |
| IL1 | −0.280 | 0.015 | 0.215 | 0.171 |
| IL6 | −0.174 | 0.136 | 0.037 | 0.815 |
| IL10 | −0.206 | 0.076 | −0.059 | 0.712 |
Note: R, correlation coefficient.
The Pearson correlation analysis showed no intercorrelation between serum cytokine levels in the VD3 group, as shown in Table 5. Serum 25OHD levels showed a weak but insignificant positive correlation with serum IL-1β levels. However, this trial has shown a significant reverse correlation between proinflammatory cytokines and anti-inflammatory cytokines. Table 5 shows the correlation between each cytokine and 25OHD level.
3.4. Changes in the Serum Levels of 25OHD and PTH
Paired sample t-tests showed a significant difference in the follow-up mean 25OHD and PTH levels among participants of the D3 group (41.39 ± 12.19 vs. 16.41 ± 4.99 and 16.69 ± 8.72 vs. 37.88 ± 6.82, PA < 0.001, respectively). Independent sample t-tests determined significant differences in 25OHD and PTH levels between the control and D3 groups. There were significant differences in serum 25OHD and PTH between the control and D3 group at follow-up (17.31± 6.74 vs. 41.39 ± 12.19 and 33.85 ± 10.62 vs. 16.69 ± 8.72, PC < 0.001, respectively, PC < 0.001), as shown in Table 6.
Table 6.
Changes in the serum levels of 25OHD and PTH.
| Variable | Group | Control | D3 | p-Value |
|---|---|---|---|---|
| 25OHD | Baseline | 18.42 ± 7.36 | 16.41 ± 4.99 | PB = 0.163 |
| Follow-up | 17.31 ± 6.74 | 41.39 ± 12.19 | PC < 0.001 | |
| Change | −1.11 | 24.98 | ||
| PA | 0.062 | <0.001 | ||
| PTH | Baseline | 36.75 ± 8.49 | 37.88 ± 6.82 | PB = 0.524 |
| Follow-up | 33.85 ± 10.62 | 16.69 ± 8.72 | PC < 0.001 | |
| Change | −2.90 | −21.19 | ||
| PA | 0.052 | <0.001 |
Abbreviations: PA, p-value for paired sample t-test; PB, p-value for two independent sample t-tests at baseline; PC, p-value for two independent sample t-test at follow-up; CV%, the coefficient of variation; D3, vitamin D3 supplementation group; PTH, para thyroid hormone; 25OHD, 25hydroxy vitamin d.
3.5. Changes in the Serum Levels of Selected Cytokines Associated with Cytokine Storm at Baseline and 10-Week Follow-Up
At the end of this study, a paired t-test showed a significant difference in mean IL-1β, IL-6, and IL-10 levels. Mean IL-1β significantly increased with a change to 4.41 ng/mL (7.63 ± 2.36 vs. 3.22 ± 0.99, PA < 0.001) in the D3 group. The application of the statistically independent t-test showed a significant difference in mean IL-1β between D3 and the control group (3.59 ± 2.71 vs. 7.63 ± 2.36, PC < 0.001). There was a significant change between the mean IL-6 at baseline and follow-up among those in the D3 group (5.5 ± 6.51 vs. 26.99 ± 14.47, PC < 0.001). At the 10-week follow-up, mean IL-10 levels were significantly increased with a change to 2.45 ng/mL (2.01 ± 0.59 vs. 4.46 ± 4.67, PA = 0.001) in the D3 group. IL-10 levels were significantly higher in the D3 group compared with the control group, 4.46 ± 4.67 and 2.39 ± 1.39, respectively, with a p-value of PC = 0.016. Table 7 presents the baseline results and follow-up changes of the clinical variables studied in this trial.
Table 7.
Changes in the serum levels of selected cytokines associated with cytokine storm at baseline and 10-week follow-up.
| Variable | Group | Control | D3 | p-Value |
|---|---|---|---|---|
| IL-1β (pg/mL) | Baseline | 3.27 ± 1.37 | 3.22 ± 0.99 | PB = 0.852 |
| Follow-up | 3.59 ± 2.71 | 7.63 ± 2.36 | PC < 0.001 | |
| Change | 0.32 | 4.41 | ||
| PA | 0.574 | <0.001 | ||
| IL-6 (pg/mL) |
Baseline | 4.55 ± 2.62 | 5.5 ± 6.51 | PB = 0.431 |
| Follow-up | 4.85 ± 4.84 | 26.99 ± 14.47 | PC < 0.001 | |
| Change | 0.30 | 21.49 | ||
| PA | 0.750 | <0.001 | ||
| TNF-α (pg/mL) |
Baseline | 31.16 ± 5.22 | 33.34 ± 6.36 | PB = 0.116 |
| Follow-up | 33.65 ± 5.15 | 33.50 ± 6.18 | PC = 0.910 | |
| Change | 2.49 | 0.16 | ||
| PA | 0.100 | 0.899 | ||
| IL-10 (pg/mL) |
Baseline | 2.20 ± 0.52 | 2.01 ± 0.59 | PB = 0.159 |
| Follow-up | 2.39 ± 1.39 | 4.46 ± 4.67 | PC = 0.016 | |
| Change | 0.20 | 2.45 | ||
| PA | 0.433 | 0.001 |
Abbreviations: PA, p-value for paired sample t-test; PB, p-value for two independent sample t-test at baseline; PC, p-value for two independent sample t-test at follow-up of trial.
3.6. Stepwise Regression Analysis
The multivariate stepwise regression analysis revealed significant mediating factors (IDVs) on the circulatory levels of selected cytokines associated with CS at the 10-week follow-up supplementation of VD3 50,000 IU once a week. TNF levels were only mediated by age factor (R = 0.413, R2 = 0.170, p = 0.007). Changes in IL-1 level values observed in the VD3 interventional group were significantly mediated by body weight (R = 0.311, R2 = 0.097, p = 0.045).
Regarding the TNF/IL-10 ratio, WHR only was selected by the stepwise regression model among all IVDs to be involved in the positive relationship between elevated 25OHD levels and the TNF/IL-10 ratio (R = 0.348, R2 = 0.121, p = 0.024), as observed in Table 8.
Table 8.
Significant correlations of cytokine and ratio levels with trial variables at follow-up.
| Dependent Variable | Univariate Effect Estimate | Coefficient | ||||
|---|---|---|---|---|---|---|
| B | F | R | R2 | p-Value | ||
| TNF-α | Age | 0.257 | 8.220 | 0.413 | 0.170 | 0.007 |
| IL-1β | Weight | 0.053 | 4.294 | 0.311 | 0.097 | 0.045 |
| TNF-α /IL10 | WHR | 0.348 | 5.200 | 0.348 | 0.121 | 0.024 |
Abbreviations: WHR, waist/hip ratio.
4. Discussion
At the end of the trial, high doses of VD3 supplementation (50,000 IU/week) significantly increased serum IL-6, IL-1β, and IL-10 levels. These findings may refer to potential adverse effects during CS. High doses of VD3 significantly raised IL-6 levels, an important marker since an increase in its concentration is associated with an increase in the levels of CS. These findings confirmed that high or/and extensive doses of VD3 may potentially affect proinflammatory immune responses [21]. It has been demonstrated that 25OHD levels are lower in patients with many inflammatory diseases [22,23]. Further, inconclusive findings on the effects of VD3 supplementation for inflammatory conditions, including cytokines changes, were noted.
Results of the current trial were consistent with a prior RCT [24], showing that daily supplementation with 2000 IU of VD3 from baseline to 1 year had an 8% increase in IL-6 concentration in the intervention group compared with the placebo. Elevated IL-6 was also detected in children with multiple sclerosis who received VD3 [25]. Remarkably, the IL-10 findings of this trial were also consistent with other research reporting an elevation in IL-10 with no changes in IFN-γ levels in VD3-supplemented individuals [26]. Another study [27] also reported that after 6 months of VD3 supplementation, the levels of IL-6 were significantly elevated compared with baseline.
Previous clinical trials have typically been conducted under a treatment protocol close to or similar to our protocol, but they have been scarce. After extensive review, some clinical research studies were conducted under a protocol similar to this trial: four trials. In a trial conducted for 12 weeks on early chronic kidney disease [28], there were no changes in IL-6 levels. Conversely, dialysis patients, also studied for 12 weeks [29], showed a significant decrease in IL-6.
Patients with chronic renal impairment, such as hemodialysis patients, have elevated plasma IL-6 levels due to chronic inflammation and fluid overload. Reduced IL-6 clearance is noted with compromised kidney function, contributing to its retention. Therapeutic hemodialysis triggers inflammatory responses and increases IL-6 production [30,31].
The same dose of VD3 (50,000 IU per week) for 12 weeks lowered IL-6 levels in another RCT that aimed to examine the effect of VD3 and omega-3 fatty acid cosupplementation as an adjuvant chemotherapy [32].
It is important to note here that the observations of Al-Haidari and Khalighi were from trials conducted on patients under the influence of the treatment protocol for chronic diseases. In the Khalighi trial, all IBS patients received antispasmodic medication (Mebeverine, 135 mg twice daily) besides VD3 supplementation. Previous research has shown that the level of IL-6 in patients with diarrhea-predominant IBS was much greater than in healthy controls [33]. Therefore, the independent effect of VD3 supplementation on IL-6 has not been accurately evaluated.
Some studies have linked changes in the serum levels of IFN-γ and IL-10 observed after VD3 supplementation to the severity of VDD [34], suggesting that VD3 supplementation exerts the most influence on human immunity in the context of severe VDD. This is in contrast to many observational studies that support a potential inhibitory effect of VD3 supplementation on proinflammatory cytokines such as IL-6, IL-1β or/and TNF levels [35]. The effects of VD3 supplementation on human immunology have now been evaluated by large-scale RCTs that reported an absence of any effect of VD3 supplementation on IL-6 [36,37]. Notably, the majority of data are from Western countries. Ours is the first study to examine the effects of VD3 supplementation on the levels of CS-associated cytokines in the bloodstream of Jordanians with VDD. Similarly, VDD has been associated with elevated IL-6 levels [38], and VD3 downregulated IL-6 in some studies [39,40]. Contradictory results regarding IL-6 may be attributable to assessing the effects of VD3 supplementation on these cytokines in specific populations, multiple confounders, and discrepancies between research. These confounders include the duration and amount of VD3 supplementation, genetic background of patients, underlying clinical problems, impact of clinical therapy, and degree of VDD and insufficiency at baseline.
RCTs conducted on diabetic hemodialysis (HD) patients [41] or postmenopausal women without VDD [42] have revealed inconsistent results. Remarkably, results were quite different when smaller doses of VD3 over longer durations were used.
Considering the impact of a given dose in the different protocols, past clinical trials utilized different doses and durations of VD3 supplementation. Studies have shown that the effects of VD3 on reducing systemic inflammation may be greater in people who are overweight and have chronic inflammation and with more prolonged use [43,44]. Circulating IL-6 increases with age, BMI, and percentage of body fat mass. These factors such as being overweight with a slight elevation in serum leptin (approximately 8 ng/mL) and a mean age of around 40 years were detected in this trial. Nevertheless, stepwise regression did not show significant effects for these factors to be potential mediators in the association between 25OHD and IL-6 levels.
On the other hand, age and body weight factors are separately involved in the association between 25OHD and other proinflammatory cytokines (IL-1β and TNF). Elevated IL-6 and other cytokines observed in this trial contradict our previous hypothesis and are challenging to explain biologically. Considering whether the study sample is healthy people or patients, the effect of VD3 on those cytokines seems to be influenced by several factors, including baseline 25OHD levels and the dose, duration, and treatment protocol of VD3 [4,45]. The National Academy of Medicine of the U.S. deems a 600–800 IU VD daily intake adequate for most of the population. However, the U.S. Endocrine Society suggests daily 1500–2000 IU [46]. A total of 400 IU VD3 per day was suggested to treat individuals aged between 18 and 28 years [47]. This dose is approximately one-tenth the dose used in this trial, which is the most common treatment protocol in Jordan for patients with VDD. In the same context, 4000 IU VD3/day is the dose at which the risk of toxicity increases [48]. Therefore, a U-shaped association between serum 25OHD level and CVD risk has been proposed [49,50].
Further, the presence or absence of typical risk factors did not obscure the U-shape association [50]. Therefore, a U-shape association with extreme fluctuations in serum 25OHD levels may influence cytokine levels via its effects on the expression of their receptors. In this manner, we can explain the unexpected findings shown in this trial and previously [24]. Converse to previous studies and RCTs that reported the presence of a hypercytokiemia-reducing effect in VD3 therapy, Costenbader showed that an extensive VD3 dosage (2000 IU/day over 1 year) elevated 8% of IL-6 levels. According to these findings, high or/and extensive doses of these supplements, which are widespread in the community and a part of COVID-19 protocol treatment, require reconsideration, particularly during CS. Hence, it is improbable that these data can answer crucial issues about the possible effects of these supplements on the inflammatory pathway, even though they are frequently consumed by the general population [24]. Instead, it may induce a hypersensitive reaction accompanied by acute harmful consequences in people at risk of acute respiratory distress syndrome (ARDS), as observed in COVID-19 patients [51].
Although it has been established that VD3 supplementation reduces the incidence of influenza A [52], large amounts of IL-6 and IL-1β have been observed during CS [53]. There is new evidence that VDD is connected with higher levels of IL-6 in HIV patients [54]. There is currently no explanation for the variance in CS severity across COVID-19 patients. Accordingly, the results of this trial may point to a potential role for the sudden onset of 25OHD levels caused by high doses of VD3 supplements for this severity.
Another piece of evidence that came from a recent study showed that VD3 and IL-6 blockade (Tocilizumab) synergistically regulate rheumatoid arthritis by suppressing IL-17 [55]. Intriguingly, in the absence of serum VD3, the expression of IL-17A exhibited a positive feedback impact on the expression of IL-6. In contrast, under adequate conditions, IL-10 expression negatively impacted IL-17A and IL-6 expression; it raised the level of IL-10 mRNA expression in all groups. However, these effects were more pronounced in people with multiple sclerosis (MS). Eight weeks of treatment with 50,000 IU VD3 led to the downregulation of IL-6 and overexpression of IL-10 in 80% of MS patients [44]. Before this evidence, VD3 acted in synergy with Toll-like receptor (TLR) agonists and peptidoglycan (PGN) in inducing IL-6 and IL-10, whereas VD3 completely inhibited lipopolysaccharide (LPS) [55,56]. IL-6 and TGF-b may both have a role in developing Th17 cells that may play a vital function in antimicrobial immunity at mucosal barriers [57]. In response to the TLR activation of dendritic cells (DCs), it is well known that IL-6 blocks the inhibitory action of CD4, CD25, and regulatory T cells. It may interact with innate and adaptive immune responses [58].
Nevertheless, IL-6 decreases DC maturation and chemokine-receptor 7 expressions and may sometimes operate as an anti-inflammatory modulator [59]. Instead of exerting a general inhibitory impact on DCs, it has been suggested that VD3 promotes a delicate immunomodulation that inhibits adaptive immune responses while increasing innate immunological processes [60,61]. It is difficult to draw firm conclusions from this study due to its small sample size. Confirming these results, validating the reported cytokines as biomarkers of VD-mediated immune responses, and establishing the linkages between crucial immunological pathways and clinical outcomes all call for a larger clinical trial.
Contraindicating results might contribute to the varying doses and durations of VD3 supplements used in past trials. Therefore, based on the U-shaped curve, we hypothesize that high or extensive doses of VD3 may worsen serum cytokines associated with CS.
5. Conclusions
High doses of VD3 significantly raised IL-6 levels, which is an essential marker since elevated levels are linked to an increase in the severity of cytokine storms. Although the observations of this trial may refer to a potential negative effect of high-dose VD3 supplementation during a cytokine storm, careful implications are recommended, as this study did not investigate all cytokines involved in the cytokine storm. Accordingly, further trials are required to clarify the potential benefits of VD3 supplementation during a cytokine storm.
Acknowledgments
The authors appreciate the ASU, Amman, Jordan, for providing the required grant for this study.
Author Contributions
D.A.B.: Conceptualization, methodology, writing original article, data curation, investigation and review/editing the article. A.A. (Anas Abed): investigation, and review/editing. B.A.M.: methodology, writing and review/editing the article. A.A. (Ahmad Aljaberi): formal analysis and review/editing the article. A.S.: formal analysis and review/editing the article. M.H.: validation and review/editing the article. A.R.A.: resources, validation, and review/editing the article. M.A.: resources and writing and review/editing the article. S.A.-S.: resources and writing and review/editing the article. M.A.-S.: Conceptualization, methodology, supervision, writing original article, project administration, data curation, investigation and review/editing the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by ASU, Amman, Jordan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., François B., Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangalmurti N., Hunter C.A. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herr C., Shaykhiev R., Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin. Biol. Ther. 2007;7:1449–1461. doi: 10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- 4.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 2020;3:e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Samak M.S., AbuRuz M.E., Masa’Deh R., Khuzai R., Jarrah S. Correlation of selected stress associated factors with vitamin D deficiency in Jordanian men and women. Int. J. Gen. Med. 2019;12:225–233. doi: 10.2147/IJGM.S198175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shaer A.H., Abu-Samak M.S., Hasoun L.Z., Mohammad B.A., Basheti I.A. Assessing the effect of omega-3 fatty acid combined with vitamin D3 versus vitamin D3 alone on estradiol levels: A randomized, placebo-controlled trial in females with vitamin D deficiency. Clin. Pharmacol. Adv. Appl. 2019;11:25–37. doi: 10.2147/CPAA.S182927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barham A., Mohammad B., Hasoun L., Awwad S., Mosleh I., Aljaberi A., Abu-Samak M. The combination of omega-3 fatty acids with high doses of vitamin D3 elevate A1c levels: A randomized Clinical Trial in people with vitamin D deficiency. Int. J. Clin. Pract. 2021;75:e14779. doi: 10.1111/ijcp.14779. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M. An update on vitamin D and human immunity. Clin. Endocrinol. 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 10.Shirazi L., Almquist M., Borgquist S., Malm J., Manjer J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case-control study. Breast. 2016;28:184–190. doi: 10.1016/j.breast.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Kunadian V., Ford G.A., Bawamia B., Qiu W., Manson J.E. Vitamin D deficiency and coronary artery disease: A review of the evidence. Am. Heart J. 2014;167:283–291. doi: 10.1016/j.ahj.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Kast J.I., McFarlane A.J., Głobińska A., Sokolowska M., Wawrzyniak P., Sanak M., Schwarze J., Akdis C.A., Wanke K. Respiratory syncytial virus infection influences tight junction integrity. Clin. Exp. Immunol. 2017;190:351–359. doi: 10.1111/cei.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi G.A., Fanous H., Colin A.A. Viral strategies predisposing to respiratory bacterial superinfections. Pediatr. Pulmonol. 2020;55:1061–1073. doi: 10.1002/ppul.24699. [DOI] [PubMed] [Google Scholar]
- 14.White J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012;13:21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F., O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 16.Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., Walker L.S., Lammas D.A., Raza K., Sansom D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ (Clin. Res. Ed.) 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson R.D., LaCroix A.Z., Gass M., Wallace R.B., Robbins J., Lewis C.E., Bassford T., Beresford S.A., Black H.R., Blanchette P., et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 19.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Wacker M., Holick M.F. Sunlight and Vitamin D: A global perspective for health. Derm.-Endocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colotta F., Jansson B., Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017;85:78–97. doi: 10.1016/j.jaut.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Hirani V. Vitamin D status and pain: Analysis from the Health Survey for England among English adults aged 65 years and over. Br. J. Nutr. 2012;107:1080–1084. doi: 10.1017/S0007114511003965. [DOI] [PubMed] [Google Scholar]
- 23.D’Aurizio F., Villalta D., Metus P., Doretto P., Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015;14:363–369. doi: 10.1016/j.autrev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Costenbader K.H., MacFarlane L.A., Lee I.M., Buring J.E., Mora S., Bubes V., Kotler G., Camargo C.A., Jr., Manson J.E., Cook N.R. Effects of One Year of Vitamin D and Marine Omega-3 Fatty Acid Supplementation on Biomarkers of Systemic Inflammation in Older US Adults. Clin. Chem. 2019;65:1508–1521. doi: 10.1373/clinchem.2019.306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naghavi Gargari B., Behmanesh M., Shirvani Farsani Z., Pahlevan Kakhki M., Azimi A.R. Vitamin D supplementation up-regulates IL-6 and IL-17A gene expression in multiple sclerosis patients. Int. Immunopharmacol. 2015;28:414–419. doi: 10.1016/j.intimp.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Martínez E., López-Vancell M.R., Fernández de Córdova-Aguirre J.C., Rojas-Serrano J., Chavarría A., Velasco-Medina A., Velázquez-Sámano G. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNγ levels and cathelicidin expression. Cytokine. 2018;108:239–246. doi: 10.1016/j.cyto.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Yegorov S., Bromage S., Boldbaatar N., Ganmaa D. Effects of Vitamin D Supplementation and Seasonality on Circulating Cytokines in Adolescents: Analysis of Data From a Feasibility Trial in Mongolia. Front. Nutr. 2019;6:166. doi: 10.3389/fnut.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez J.A., Zughaier S.M., Law J., Hao L., Wasse H., Ziegler T.R., Tangpricha V. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur. J. Clin. Nutr. 2013;67:264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meireles M.S., Kamimura M.A., Dalboni M.A., Giffoni de Carvalho J.T., Aoike D.T., Cuppari L. Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: A randomized controlled trial. Clin. Nutr. 2016;35:1251–1258. doi: 10.1016/j.clnu.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Caglar K., Peng Y., Pupim L.B., Flakoll P.J., Levenhagen D., Hakim R.M., Ikizler T.A. Inflammatory signals associated with hemodialysis. Kidney Int. 2002;62:1408–1416. doi: 10.1111/j.1523-1755.2002.kid556.x. [DOI] [PubMed] [Google Scholar]
- 31.Pecoits-Filho R., Heimbürger O., Bárány P., Suliman M., Fehrman-Ekholm I., Lindholm B., Stenvinkel P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003;41:1212–1218. doi: 10.1016/S0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 32.Haidari F., Abiri B., Iravani M., Ahmadi-Angali K., Vafa M. Randomized Study of the Effect of Vitamin D and Omega-3 Fatty Acids Cosupplementation as Adjuvant Chemotherapy on Inflammation and Nutritional Status in Colorectal Cancer Patients. J. Diet. Suppl. 2020;17:384–400. doi: 10.1080/19390211.2019.1600096. [DOI] [PubMed] [Google Scholar]
- 33.Bashashati M., Moradi M., Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132–138. doi: 10.1016/j.cyto.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Barker T., Rogers V.E., Levy M., Templeton J., Goldfine H., Schneider E.D., Dixon B.M., Henriksen V.T., Weaver L.K. Supplemental vitamin D increases serum cytokines in those with initially low 25-hydroxyvitamin D: A randomized, double blind, placebo-controlled study. Cytokine. 2015;71:132–138. doi: 10.1016/j.cyto.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Khoo A.L., Chai L.Y., Koenen H.J., Sweep F.C., Joosten I., Netea M.G., van der Ven A.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011;164:72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamka M., Woźniewicz M., Walkowiak J., Bogdański P., Jeszka J., Stelmach-Mardas M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Eur. J. Nutr. 2016;55:2163–2176. doi: 10.1007/s00394-015-1089-5. [DOI] [PubMed] [Google Scholar]
- 37.Yusupov E., Li-Ng M., Pollack S., Yeh J.K., Mikhail M., Aloia J.F. Vitamin d and serum cytokines in a randomized clinical trial. Int. J. Endocrinol. 2010;2010:305054. doi: 10.1155/2010/305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubesch A., Quenstedt L., Saleh M., Rüschenbaum S., Schwarzkopf K., Martinez Y., Welsch C., Zeuzem S., Welzel T.M., Lange C.M. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS ONE. 2018;13:e0207162. doi: 10.1371/journal.pone.0207162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miroliaee A.E., Salamzadeh J., Shokouhi S., Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J. Crit. Care. 2018;44:300–305. doi: 10.1016/j.jcrc.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Goncalves-Mendes N., Talvas J., Dualé C., Guttmann A., Corbin V., Marceau G., Sapin V., Brachet P., Evrard B., Laurichesse H., et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddad Kashani H., Seyed Hosseini E., Nikzad H., Soleimani A., Soleimani M., Tamadon M.R., Keneshlou F., Asemi Z. The Effects of Vitamin D Supplementation on Signaling Pathway of Inflammation and Oxidative Stress in Diabetic Hemodialysis: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharm. 2018;9:50. doi: 10.3389/fphar.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bueloni-Dias F.N., Orsatti C.L., Cangussu L.M., Poloni P.F., Spadoto-Dias D., Nahas-Neto J., Nahas E.A.P. Isolated vitamin D supplementation improves the immune-inflammatory biomarkers in younger postmenopausal women: A randomized, double-blind, placebo-controlled trial. Menopause. 2018;25:897–903. doi: 10.1097/GME.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 43.Duggan C., de Dieu Tapsoba J., Mason C., Imayama I., Korde L., Wang C.Y., McTiernan A. Effect of Vitamin D3 Supplementation in Combination with Weight Loss on Inflammatory Biomarkers in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev. Res. 2015;8:628–635. doi: 10.1158/1940-6207.CAPR-14-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashemi R., Morshedi M., Asghari Jafarabadi M., Altafi D., Saeed Hosseini-Asl S., Rafie-Arefhosseini S. Anti-inflammatory effects of dietary vitamin D(3) in patients with multiple sclerosis. Neurol. Genet. 2018;4:e278. doi: 10.1212/NXG.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharifi A., Vahedi H., Nedjat S., Rafiei H., Hosseinzadeh-Attar M.J. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: A randomized placebo-controlled trial. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2019;127:681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- 46.Pramyothin P., Holick M.F. Vitamin D supplementation: Guidelines and evidence for subclinical deficiency. Curr. Opin. Gastroenterol. 2012;28:139–150. doi: 10.1097/MOG.0b013e32835004dc. [DOI] [PubMed] [Google Scholar]
- 47.Laaksi I., Ruohola J.P., Mattila V., Auvinen A., Ylikomi T., Pihlajamäki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish men. J. Infect. Dis. 2010;202:809–814. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 48.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium . The National Academies Collection: Reports funded by National Institutes of Health. In: Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B., editors. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US), National Academy of Sciences; Washington, DC, USA: 2011. [DOI] [PubMed] [Google Scholar]
- 49.Sondarwa K., Buttar R.S., Hensley V., Melamed M.L. Extraskeletal Effects of Vitamin D. Springer; Cham, Switzerland: 2018. Vitamin D and Cardiovascular Disease; pp. 151–164. [Google Scholar]
- 50.Dror Y., Giveon S.M., Hoshen M., Feldhamer I., Balicer R.D., Feldman B.S. Vitamin D levels for preventing acute coronary syndrome and mortality: Evidence of a nonlinear association. J. Clin. Endocrinol. Metab. 2013;98:2160–2167. doi: 10.1210/jc.2013-1185. [DOI] [PubMed] [Google Scholar]
- 51.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 53.Manion M., Hullsiek K.H., Wilson E.M.P., Rhame F., Kojic E., Gibson D., Hammer J., Patel P., Brooks J.T., Baker J.V., et al. Vitamin D deficiency is associated with IL-6 levels and monocyte activation in HIV-infected persons. PLoS ONE. 2017;12:e0175517. doi: 10.1371/journal.pone.0175517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H., Baek S., Hong S.M., Lee J., Jung S.M., Lee J., Cho M., Kwok S.K., Park S.H. 1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis. J. Korean Med. Sci. 2020;35:e40. doi: 10.3346/jkms.2020.35.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brosbøl-Ravnborg A., Bundgaard B., Höllsberg P. Synergy between vitamin D(3) and Toll-like receptor agonists regulates human dendritic cell response during maturation. Clin. Dev. Immunol. 2013;2013:807971. doi: 10.1155/2013/807971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones G.W., McLoughlin R.M., Hammond V.J., Parker C.R., Williams J.D., Malhotra R., Scheller J., Williams A.S., Rose-John S., Topley N., et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. [DOI] [PubMed] [Google Scholar]
- 58.Jones S.A. Directing transition from innate to acquired immunity: Defining a role for IL-6. J. Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 59.Park S.J., Nakagawa T., Kitamura H., Atsumi T., Kamon H., Sawa S., Kamimura D., Ueda N., Iwakura Y., Ishihara K., et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 60.Széles L., Keresztes G., Töröcsik D., Balajthy Z., Krenács L., Póliska S., Steinmeyer A., Zuegel U., Pruenster M., Rot A., et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 61.Liu P.T., Stenger S., Tang D.H., Modlin R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.