Abstract
The Leishmania pifanoi amastigote antigen P-8 has been previously shown to induce protective immunity in a murine model of cutaneous leishmaniasis (L. Soong, S. M. Duboise, P. Kima, and D. McMahon-Pratt, Infect. Immun. 63:3559–3566, 1995). As this antigen is of interest for further vaccine studies, the biochemical characterization of P-8 was undertaken. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western-blot analysis, and gel filtration chromatography revealed that P-8 antigen consisted of two proteoglycolipid complexes. The P-8 epitope is associated with the L. pifanoi amastigote-specific glycolipid components found in the two complexes. The P-8 complex 1 (P-8c1) consists of a 56-kDa serine metalloproteinase, apolipoprotein E (derived from fetal bovine serum), and amastigote-specific glycolipids. The P-8 complex 2 (P-8c2) consists of a 31-kDa cysteine proteinase associated with amastigote glycolipids. Biochemical analyses suggest that the P-8 antigenic glycolipids may be distinct from previously described Leishmania glycolipids (glycosylinositolphospholipids and sphingoglycolipids). Protective immunity studies revealed that P-8c1 (serine metalloproteinase-glycolipid complex) confers comparable protection against infection as immunopurified P-8. The isolated P-8c2 (cysteine proteinase-glycolipid complex) does not provide significant protection, nor does stimulation with P-8c2 result in significant T-cell activation in P-8- or P-8c2-vaccinated mice. Consequently, the P-8c1 complex appears to be the immunodominant component of P-8.
Protozoan parasites of the genus Leishmania are associated with a broad spectrum of diseases, ranging from simple cutaneous to visceral leishmaniasis. Leishmania spp. are dimorphic obligate intracellular parasites. Flagellated promastigotes replicate and differentiate in the gut of the phlebotomine sandfly vector; transmission to humans or other vertebrate hosts occurs when the sandfly takes a blood meal. In the dermis, recently inoculated promastigotes are internalized by phagocytic cells and undergo transformation into amastigotes. Consequently, survival of the parasite within a mammalian host is dependent on successful entry into a macrophage and transformation into the nonmotile amastigote form. Amastigotes maintain the infection within the vertebrate hosts, replicating in the parasitophorous vacuole and eventually leading to the destruction of host cells and invasion of new macrophages.
The observations that protective immunity against Leishmania infection can be acquired in susceptible mice (23, 25, 34, 48–50) as well as in humans (2, 19, 37) have indicated the possibility for the development of a vaccine against leishmaniasis. In mice, studies of protection against cutaneous leishmaniasis have been observed after immunization with promastigote-derived molecules such as lipophosphoglycan, gp63, defined gp63 epitopes, and gp46 (also known as M-2) (8, 20, 24, 32, 45, 49, 50, 58, 59). In the search for immunogens that invoke potent host-protective responses, our group has been focused on molecules that are preferentially expressed in the amastigote stage, the primary form responsible for diseases in mammalian hosts; these studies have employed axenically cultured amastigotes of Leishmania (5, 11, 13, 14, 39). Since the curative response in naive infected animals requires a relatively long time to develop, it is likely that the antigens responsible for induction of self-healing in cutaneous leishmaniasis are synthesized by amastigotes (44).
The efficacy of immunization of BALB/c mice with purified L. pifanoi amastigote proteins has been evaluated previously; three amastigote antigens (P-2 [also known as A-2], P-4, and P-8) were found to provide significant (but varying degrees of) protection against infection (52). Among these, immunization with the amastigote external-membrane-associated antigen P-8 induced significant and reproducible protection in BALB/c mice against infection with L. pifanoi and L. amazonensis (52). L. amazonensis and L. pifanoi are members of the L. mexicana complex and are the causative agents of both cutaneous (limited) and diffuse cutaneous leishmaniasis (7, 18) in Central and South America. As an important factor for a potential vaccine candidate, the protection provided by vaccination with P-8 did not appear to be genetically restricted, as protection was induced in mice with different H-2 haplotypes (BALB/c, CBA, C57BL/6). As the P-8 antigen stimulates a predominately curative Th1-like lymphocyte response in cutaneous leishmaniasis patients infected with L. braziliensis (9), the potential of this immunogen as human vaccine candidate appears feasible.
In this study we present the biochemical characterization of the P-8 antigen, as an initial step for defining the nature of immunodominant epitopes of this vaccine candidate.
MATERIALS AND METHODS
Cell culture.
L. pifanoi MHOM/VE/60/Ltrod amastigotes were cultured at 31°C in simplified F29 medium containing 20% heat-inactivated fetal bovine serum (FBS) (39). Promastigote forms were derived from amastigotes by culture at 24°C in Schneider's Drosophila medium (GIBCO) supplemented with 20% heat-inactivated FBS and gentamicin (10 μg/ml).
Antibodies.
The preparation and characterization of the amastigote-specific monoclonal antibody (MAb) P-8 have been reported previously (38). A polyclonal serum was obtained by immunizing mice with the immunopurified antigen P-8, as described below. The sheep polyclonal antibody Sp180 raised against L. chagasi promastigote gp63 was a gift from M. Wilson (University of Iowa). Other antibodies and conjugates used were goat anti-human apolipoprotein E (ApoE) polyclonal antibody (Chemicon, Temecula, Calif.), anti-sheep immunoglobulin G (IgG) alkaline phosphatase conjugate (Sigma), alkaline phosphatase-conjugated affiniPure F(ab)2 fragment, rabbit anti-goat IgG, and goat anti-mouse IgG (Jackson Research Laboratory, West Grove, Pa.).
Purification of the P-8 antigen.
The P-8 antigen was purified as described previously (52), with the following modifications. Surface membranes of washed L. pifanoi amastigotes were isolated using nitrogen cavitation and differential centrifugation. Membrane proteins were solubilized with 1% decanoyl-N-methylglucamide (Mega-10; Sigma) at room temperature for 2 h, at a protein/detergent ratio of 1:1. In order to prevent aggregation, the mixture was centrifuged at 40,000 × g for 45 min at 4°C, and the supernatant was recovered and incubated with 14.2 mM 2-mercaptoethanol (Sigma) at room temperature for 30 min and subsequently alkylated by addition of iodoacetamide (final concentration, 30 mM). The reduced and alkylated solubilized membranes were fractionated by Sephadex G-25 (Pharmacia, Piscataway, N.J.) gel exclusion chromatography to remove the excess of reagents. The sample was then diluted with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) and subjected to P-8 immunoaffinity chromatography. After extensive washing with PBS containing 0.01% Mega-10, P-8 antigen was eluted with elution buffer (50 mM diethylamine, pH 11.5; 150 mM NaCl; 0.01% Mega-10) and 3-ml fractions were collected and immediately neutralized to pH 7 to 8 (using 1 M Tris-HCl, pH 5.3). The fractions eluted from the affinity column were assessed for protein by measuring the absorbance at 280 and 320 nm; protein fractions were pooled, concentrated, and then stored at −20°C.
SDS-PAGE and immunoblot analyses.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with a minigel system (Bio-Rad) and 12% acrylamide gels (27). Samples were pretreated as indicated (reduced, nonreduced, heat-denatured, or nondenatured). Proteins were visualized by Coomassie brillant blue staining. For immunoblots, proteins were transferred onto nitrocellulose membrane (Biotrace NT; Gelman Sciences), as described (56). Following transfer of proteins to nitrocellulose and staining with Ponceau S (Sigma), membranes were blocked for 1 h at room temperature in PBS containing 5% nonfat dried milk (Carnation). The filters were then incubated overnight at 4°C with the first antibody diluted, as indicated, in PBS containing 1% nonfat milk, followed by incubation with the appropriate alkaline phosphatase conjugate (1/5,000 dilution in PBS) for 2 h at room temperature. The reaction was detected with the substrate 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)–nitroblue tetrazolium (Kirkegaard & Perry Laboratories).
Detection of proteinase activity.
For the detection of proteinase activity in polyacrylamide gels, SDS-PAGE was performed as described above with the exceptions that the separating gel contained 0.5% gelatin (Bio-Rad) and samples were not heat denatured. Molecular mass markers were consistently employed to determine the relative migration of the proteinase activities within the gelatin-containing gels. Electrophoresis was conducted at 4°C. After electrophoresis, gels were washed for 30 min in acetate buffer, pH 5.3 (50 mM sodium acetate, 200 mM NaCl, 1 mM EDTA), containing 0.1% Triton X-100 and then washed twice (15 min) with the same buffer without detergent. For inhibition studies, samples were incubated for 30 min at 4°C with the inhibitor before electrophoresis, and then all the washing steps were done in the presence of inhibitor. Buffer-equilibrated gels were incubated overnight at 37°C in a humid chamber. The gels were then stained with Coomassie brillant blue; protease activity was detected as a clear band on a blue-stained gelatin background.
Extraction and analysis of glycolipids.
Glycolipids were extracted from either immunopurified P-8 or from total amastigote cells by the glycolipid extraction procedure of Svennerholm and Fredman (55). Briefly, starting material was homogenized on ice in 3 volumes of water and then 8 volumes of methanol was added. Following rehomogenization, 4 volumes of chloroform was added and the extract was rehomogenized again at room temperature. After overnight agitation, enough water was added to bring the solvent ratio to 4:8:5.6 (chloroform-methanol-water), thereby inducing phase separation. The resulting lower and upper phases were back extracted with upper and lower phases previously prepared from pure solvent. All upper phases and lower phases were combined separately and dried under a stream of nitrogen.
For subsequent analysis by high performance thin layer chromatography (HPTLC), the dried lower phase was resuspended in chloroform-methanol (1:2), and the dried upper phase was resuspended in chloroform-methanol-water (4:8:3). After application to glass-backed silica gel 60 HPTLC plates (Merck), glycolipid components were resolved by developing in solvent A (chloroform–methanol–0.25% aqueous KCl, 60:35:8) or in solvent B (chloroform–methanol–1 N NH4OH, 10:10:3).
Carbohydrate-containing bands were visualized by the orcinol spray reagent (Sigma). Resolved components on HPTLC plates were probed by immuno-overlay by the method of Magnani et al. (28). Briefly, after developing in solvent A or B, HPTLC plates were dried and then immersed in 0.001% poly(isobutyl methacrylate) (Aldrich) in hexane. Once freed of solvent by evaporation, the plates were blocked for 20 min in 1% bovine serum albumin in PBS. Anti-P-8 antibody was then placed on the plate at a 1:2 dilution in 0.1% bovine serum albumin in PBS. Bound anti-P-8 antibody was detected by incubation with peroxidase-conjugated anti-mouse IgG and followed by reaction with diaminobenzidine-H2O2.
Carbohydrate analysis.
Portions of each glycolipid were treated with hydrofluoric acid (HF) under conditions that selectively remove phosphate esters (15). Specifically, between 1 and 10 nmol of purified glycolipid was dried in a plastic screw-top Eppendorf tube and then chilled on ice. To each tube, 50 μl of cold (−20°C) HF was added and the sample was briefly (2 to 3 s) sonicated in a bath sonicator. Following incubation in a refrigerated bath at 0°C, the samples were frozen on dry ice and the acid was removed by lyophilization in a vacuum centrifuge. Hydrolysis was empirically determined to be complete by 72 h (51). Aliquots of 0.5 to 5 nmol of purified, HF-treated glycolipids were dried in glass crimp-top microvials (Agilent Technologies), resuspended in 100 μl of 2 M hydrochloric acid, and sealed. After 4 h at 100°C, the hydrolysates were quickly cooled on ice, decapped, and evaporated to dryness in a vacuum centrifuge. After resuspension in water, aliquots were injected onto a Dionex PA1 anion-exchange column on a Dionex DX500 high-performance liquid chromatography (HPLC) unit, equipped with PEAKNET software for resolution and detection of neutral and amino sugars by high-pH anion-exchange chromatography coupled to pulsed amperometric detection (Dionex AD20 detector) as described previously (21).
P-8 fractionation.
Immunopurified P-8 was fractionated by gel filtration on a Sepharose CL-6B column. Total antigen (500 μg) was applied to the column, and the different components were eluted with PBS–0.01% Mega-10. Individual fractions were collected and analyzed for proteinase activity using gelatin gels (as indicated above) and by Western blotting with MAb P-8. The column was calibrated with molecular mass standards (Bio-Rad), which included thyroglobulin (610 kDa), aldolase (158 kDa), ovalbumin (44 kDa), and myoglobin (17 kDa).
Immunization of mice and evaluation of infection.
Female BALB/c mice (five per group; Jackson Laboratories, Bar Harbor, Maine) were vaccinated with either 4 μg of whole immunopurified P-8 antigen or 2 μg of P-8c1 (56-kDa serine-metalloproteinase-glycolipid complex) or 2 μg of P-8c2 (31-kDa cysteine proteinase-glycolipid complex), together with 100 μg of the adjuvant Corynebacterium parvum. Three injections were given at biweekly intervals. Mice receiving PBS (used to dilute the purified proteins) or 100 μg of adjuvant alone served as controls. Before challenge, serum from the mice was collected. Four weeks after the last immunization, mice were challenged in the right hind foot with 105 stationary-phase L. pifanoi promastigotes. The course of infection was monitored by measuring the increase in footpad thickness, compared with the uninfected footpad, with a dial gauge caliper, and the result was expressed as a thickness ratio of the infected foot to the noninfected foot.
Proliferation assays.
P8 reactive cells used in this study were obtained from mice immunized with whole P8 in Freund's adjuvant in the hind footpad 9 days prior or mice infected with L. pifanoi for up to 3 months. Lymph nodes were removed aseptically, and a single-cell suspension was obtained. CD4+ T cells were enriched by negative selection to more than 90% by panning using MAbs to CD8, anti-major histocompatibility complex class II (212.A1), and CD16 (also known as CD32). CD4+ T lymphocytes (105/well) were then cultured in 96-well microtiter plates (Falcon) for 72 h in the presence of fixed 5 × 104 RAW 264.7 cells/well previously cultured with specific antigens as described previously (26). Cultures were then pulsed with [3H]thymidine (0.5 μCi/well) and incubated for 16 additional hours before harvesting. Stimulation indices were calculated by dividing the total radioactivity incorporated in the presence of antigen by the radioactivity incorporated in medium alone.
Amino acid sequencing.
For amino acid sequencing of the p34 component, immunopurified P-8 was separated using preparative SDS–12% PAGE. The 34-kDa band or component was excised from the gel and was then digested with trypsin. The resulting peptides were isolated using HPLC and sequenced by the W. M. Keck Foundation, Biotechnology Resource Laboratory (Yale University, New Haven, Conn.) according to methods previously described (53).
PI-PLC digestion of glycolipids.
Digestion with phosphatidylinositol (PI)-specific phospholipase C (PLC) (0.1 U/ml) was performed in 20 mM Tris-acetate buffer, pH 7.5, containing 0.1% Triton X-100 at 37°C for 60 min, as previously described (29).
RESULTS
Molecular complexity of immunopurified P-8 antigen.
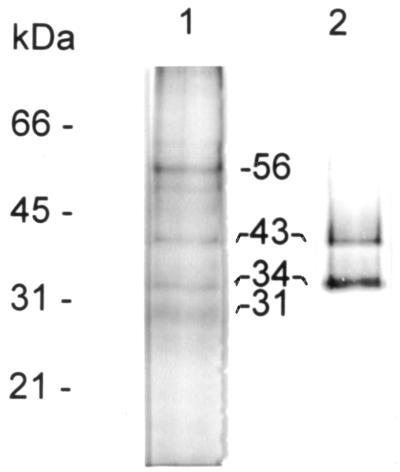
When immunopurified P-8 is analyzed by SDS-PAGE, consistently the preparations primarily contain four major components (Fig. 1, lane 1), with estimated molecular masses of 56, 43, 34, and 31 kDa. Although minor bands were observed, these differed in individual preparations and may represent, in part, degradation products. Interestingly, the immunoblot analysis of the same sample revealed that only the 34- and 43-kDa bands are directly recognized by the MAb used for the purification (Fig. 1, lane 2). When immunopurified P-8 was analyzed by silver stain, these two bands appeared to stain with a blue color rather than brown (data not shown), indicative of lipid content. Further studies (detailed below) confirmed this fact.
FIG. 1.
Analysis of immunopurified P-8. L. pifanoi axenic amastigote membrane-associated antigen P-8 was purified as indicated in Materials and Methods and then resolved by SDS–12% PAGE under reducing conditions, followed by either Coomassie blue staining (lane 1) or immunoblot analysis with MAb P-8 (lane 2).
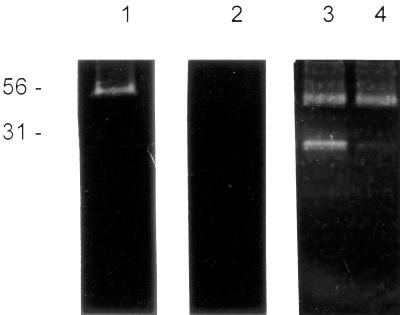
The purified P-8 antigen was initially analyzed on gelatin-copolymerized SDS-PAGE, under reducing and nonreducing conditions. As shown in Fig. 2 (lane 3), when 2-mercaptoethanol is added to the sample buffer, P-8 appears to contain two components capable of gelatin digestion. These proteinases have relative molecular masses of 56 and 31 kDa, respectively. Under nonreducing conditions, only the 56-kDa band can be detected (Fig. 2, lane 1).
FIG. 2.
Immunopurified P-8 gelatinase activity. Aliquots of immunopurified P-8 were incubated with different inhibitors, without preactivation (1,2) or after preactivation with 14 mM 2-mercaptoethanol (3, 4). For the serine proteinase, the inhibitor tested was 1 mM phenylmethylsulfonyl fluoride (2), and for the cysteine proteinase the inhibitor was 10 mM iodoacetamide (4). The enzymatic activities were developed as indicated in Materials and Methods. Migration of molecular mass markers (in kilodaltons) is indicated on the left.
To determine the identity of protein components associated with the 34- and 43-kDa bands present in the P-8 complex, the 34-kDa band was excised from a preparative SDS-polyacrylamide gel, digested with trypsin and the amino acid sequence was obtained from two tryptic peptides isolated by HPLC. As shown in Table 1, both peptides have 100% homology with the sequence of either mouse or rat ApoE. Peptide 56 had only a 50% homology with the corresponding bovine sequence; however, this area of sequence is known to reside in the hypervariable region of ApoE and thus might not be generically representative of all cattle. It should be noted that the Mr values for the 34- and 43-kDa antigenic components are consistent with those reported for the migration of ApoE (22). Given the conditions employed for the isolation of the P-8 antigen (see Materials and Methods), it is unlikely that the ApoE found represents nonspecifically bound host protein. In addition, gel densitometric analyses indicate that the ratio of the 56-kDa to 43- plus 34-kDa bands is consistently 1 to 2.3 (±0.2) which would not be expected to occur in the case of adventitiously bound molecules. Further, the 34- and 43-kDa components are observed in Western blot analyses of tissue-derived amastigotes (38) as well as cultured organisms. These results suggest that serum (host)-derived ApoE is a constituent of the P-8 antigen; however, further work is required to verify this point and the cellular mechanisms involved in the incorporation of ApoE into the P-8 antigen complex.
TABLE 1.
Comparison of amino acid sequences of two peptides from the 34-kDa proteoglycolipid component of P-8 and ApoE
| Protein | Sequence
|
|
|---|---|---|
| Peptide 94 | Peptide 56 | |
| P-8 (34 kDa) | FWDYLR | TANLGAGAAQPL |
| Mouse or rat ApoEa | FWDYLR | TANLGAGAAQPL |
| Bovine ApoEb | FWDYLR | AATLSTLAGQPL |
Peptides 94 and 56 span amino acids 18 to 23 and 177 to 188, respectively.
Peptides 94 and 56 span amino acids 50 to 55 and 209 to 220, respectively.
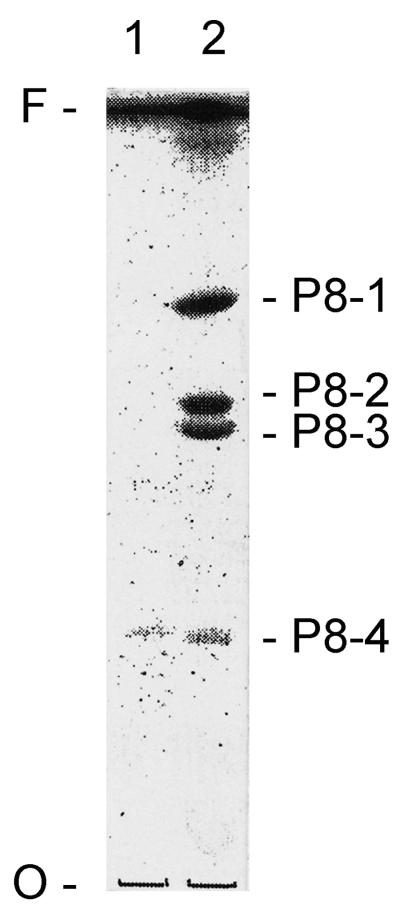
In order to investigate the potential glycolipid nature of the components present in the 34- and 43-kDa bands, total glycolipids were isolated from purified P-8, by extraction with chloroform-methanol-water (4:8:3). HPTLC analysis with the solvent A of the upper (aqueous)- and lower (organic and chloroform)-phase fractions revealed the presence of four glycolipid species in immunopurified P-8 recognized by the MAb (Fig. 3). The glycolipids P8-1, -2, and -3 partitioned only onto the lower phase and are strongly recognized by MAb P-8; glycolipid P8-4 was detected in both the aqueous and organic phases and had an apparently weaker reaction with MAb P-8. These results clearly indicate that the P-8 epitope (recognized by the P-8 MAb) is associated with glycolipid moieties and confirms the proteoglycolipid nature of the components migrating at Mr 34,000 and 43,000. It should be noted that Western blot analyses clearly indicate that the P-8 MAb does not recognize any component present in FBS (migrating as ApoE, glycolipid or other Mr constituent). Further, FBS extracted for glycolipids, concentrated and analyzed by HPTLC (as indicated above) does not possess components recognized by the P-8 MAb (data not shown). Consequently, the antigenic P-8 glycolipids appear to be of parasite derivation.
FIG. 3.
HPTLC analysis of the glycolipid components in immunopurified P-8. Glycolipids from immunopurified P-8 were isolated as described in Materials and Methods, and the fractionated upper (1) and lower (2) phases were analyzed by HPTLC using solvent A and immunostained with MAb P-8. O and F mark the origin and front of the TLC, respectively.
Immunopurified P-8 contains two independent protein-glycolipid complexes.
Based upon the results (above) concerning the biochemical composition and complexity of the P-8 antigen, two possibilities were hypothesized concerning the P-8 complex. One possibility is that the P-8 antigen is a large macromolecular complex consisting of four glycolipids, two proteinases, and ApoE; this tight complex can be dissociated by strong detergents (i.e., SDS) but not under high-pH or reducing conditions (used in the isolation of the antigen). The other possibility is that, independently, each protease forms a distinct or stable complex with the glycolipid components recognized by the P-8 MAb.
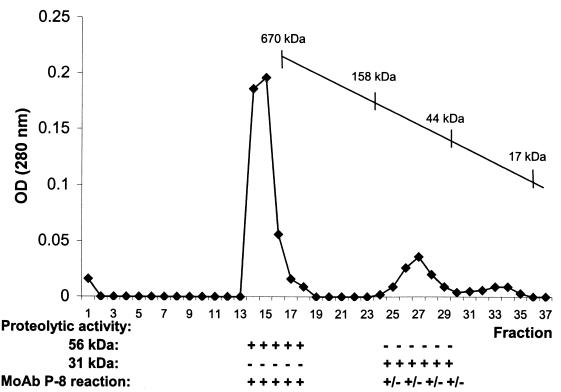
In order to determine the relation between the proteinases and the glycolipids present in P-8, the immunopurified complex was fractionated on a Sepharose CL-6B gel filtration column. Individual fractions were analyzed for the presence of the 56-kDa proteinase and the 31-kDa proteinase and also for reaction with MAb P-8 by Western blotting. As shown in Fig. 4, the two proteinase activities are recovered in different fractions; the 34- and 43-kDa components containing the P-8 glycolipids, migrated with the 56-kDa proteinase, forming a complex with a total molecular mass of >610 kDa.
FIG. 4.
Profile of P-8 fractionation by gel filtration chromatography. Immunopurified P-8 was applied to a Sepharose CL-6B column and eluted with PBS–0.01% Mega-10, and the various fractions were analyzed by SDS-PAGE copolymerized with gelatin and by Western blotting employing the MAb P-8, in order to detect the 56-kDa serine-metalloproteinase, the 31-kDa cysteine proteinase, and the 43- and 34-kDa proteoglycolipids, respectively.
The fractions containing either the 31-kDa or the 56-kDa component were pooled and the glycolipids were extracted and analyzed by HPTLC for reaction with MAb P-8. The 56-kDa proteinase complex contains all the glycolipid species detected in whole P-8 (P8-1 through P8-4), while the 31-kDa proteinase only contains the P8-3 component (data not shown). These results indicate that purified P-8 is comprised of two independent complexes: (i) a 56-kDa proteinase glycolipid complex (P-8c1) and (ii) a 31-kDa proteinase associated with the Leishmania amastigote glycolipid P8-3 (P-8c2).
Vaccine studies employing P-8c1 and P-8c2 proteoglycolipid complexes.
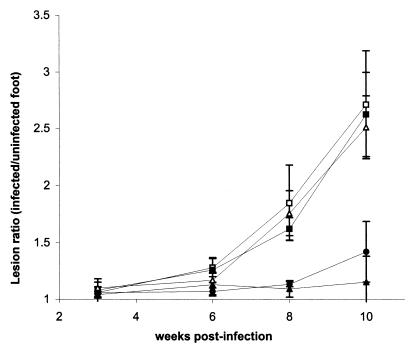
The interest in the P-8 antigen comes from its potential as a vaccine candidate against cutaneous leishmaniasis (52). In order to determine the relative contribution of the two P-8 complexes (P-8c1 and P-8c2) to the protection induced by immunization with whole P-8, BALB/c mice (five per group) were vaccinated with either the P-8c1 or P-8c2 complex (2 μg per immunization per mouse) together with C. parvum as the adjuvant. Control groups of mice either were immunized with whole immunopurified unfractionated P-8 (4 μg per immunization per mouse; positive control), or received C. parvum (adjuvant control) or PBS alone. All mice were then challenged with 105 stationary-phase L. pifanoi promastigotes, and the lesion size was measured at different times postinfection. As indicated in Fig. 5, the 56-kDa proteinase-glycolipid complex (P-8c1) confers comparable protection to unfractionated P-8; the 31-kDa cysteine proteinase-glycolipid complex does not appear to contribute significantly to protection against infection under these experimental conditions.
FIG. 5.
Comparison of the immunoprotective properties of the two complexes present in P-8. Immunopurified P-8 was fractionated by gel filtration chromatography, the two P-8 complexes (P-8c1, serine-metalloproteinase-glycolipid complex [▴]; P8-c2, cysteine proteinase-glycolipid complex (▵) separated and used to immunize mice. As controls, other mice were either nonimmunized (control, □), immunized with the adjuvant (C. parvum) alone (▪) or with whole P-8 and C. parvum (positive control, ●). After infection with L. pifanoi stationary-phase promastigotes, the development of cutaneous lesions was monitored as indicated in Materials and Methods. Error bars, standard deviations.
Antigenic properties of P-8 components.
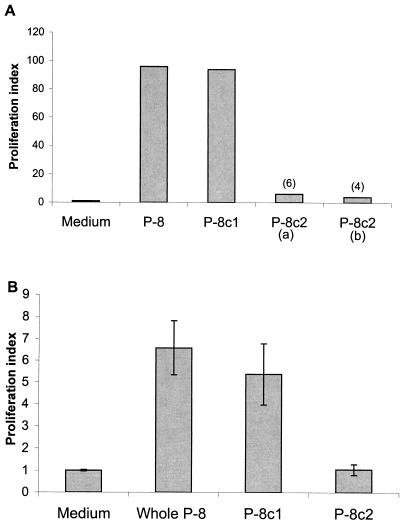
In order to determine if the antigenicity of the individual complexes might contribute to the overall protection observed, the immunologic properties of the 2 P-8 complexes were examined. The T-cell proliferative responses in nonvaccinated BALB/c mice infected with L. amazonensis (Fig. 6A) as well as uninfected BALB/c mice vaccinated with whole P-8 antigen (Fig. 6B) were determined. These results indicate that the P-8c1 complex is preferentially recognized by T cells derived from infected or vaccinated mice. Although there is a defined T-cell response by mice vaccinated with whole P-8 antigen to the P-8c2 cysteine proteinase complex, the stimulation indices (SI) (SI, 4 to 6) found are significantly less than those found for the P-8c1 serine proteinase complex (SI, 94 to 96). These results are consistent with antibody data found for P-8, P-8c1, and/or P-8c2 immunized mice. While a strong antibody response was found for the components of the P-8c1 complex, a negligible antibody response was found for the P-8c2 complex (data not shown). These data suggest that under the conditions employed for the vaccine studies, P-8c1 is the dominant antigenic complex and consequently appears to be primarily responsible for the protection observed.
FIG. 6.
Antigenic properties of P-8 components. CD4+-P8 reactive T cells were obtained from mice infected with L. pifanoi (3 months) (A) or mice immunized 9 days previously with whole P8 in incomplete Freund's adjuvant (B), as described in Materials and Methods. Stimulation indices were calculated by dividing the total radioactivity incorporated in the presence of antigen (whole immunopurified P-8, 5 μg; fractionated P-8c1, 5 μg; fractionated P-8c2, 10 μg [a] or 5 μg [b]), by the radioactivity incorporated in medium alone. Some values are indicated in parentheses. Error bars, standard deviations.
Characterization of the glycolipid and proteinase components present in the immunologically protective P-8c1 complex.
In order to compare the glycolipids present in P-8c1 with previously described L. mexicana GIPLs, total glycolipids from L. pifanoi amastigotes were extracted as described previously (30, 31), partitioned in upper and lower phases, and analyzed by HPTLC using solvent B and orcinol staining (Fig. 7). The glycolipids of L. pifanoi amastigotes recovered in the upper phase comigrate with previously characterized L. mexicana promastigote GIPLs (30), as determined by comparing the relative migration in this solvent.
FIG. 7.
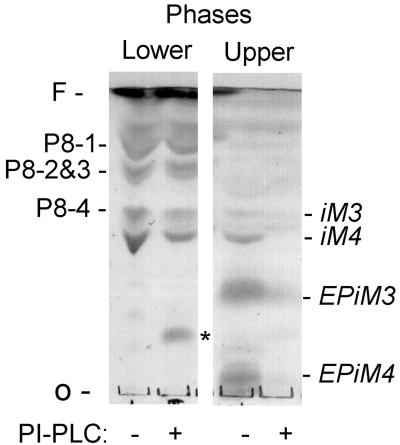
Characterization of the P-8 glycolipids. Total glycolipids of L. pifanoi axenic amastigote were extracted and partitioned into upper and lower phases. Glycolipids were subjected to PI-PLC digestion and compared to untreated samples by analysis on HPTLC using solvent B, and then detection with orcinol. The relative migration of specific GIPL components is indicated in the right margin. There is a clear loss of GIPL components upon PI-PLC treatment but little or no change in the P-8 associated glycolipids (P8-1 to P8-4). O and F mark the origin and front of the TLC, respectively. Asterisk marks migration of impurities from the enzyme preparation.
The susceptibility of the P-8 glycolipids to cleavage by PI-PLC was also determined. As shown in Fig. 7, the amastigote GIPLs recovered in the upper (aqueous) phase (iM3, iM4, EPiM3, and EPiM4) are sensitive to PI-PLC. These results are consistent with those previously described (31). The P8 species (P8-1, P8-2, P8-3, and P8-4), in contrast, are resistant to PLC digestion under these conditions. Therefore, these results suggest that the P-8 epitope is associated with glycolipid components different from previously described Leishmania GIPLs. In addition, preliminary results indicate that the purified bands corresponding to P8-1 to P8-4 consistently revealed the presence of mannose in all species. The carbohydrate composition of the various P-8 glycolipids was determined to be as follows: P8-1, 1GalN:1Gal:2Man; P8-2, 1Gal:2Man; P8-3, 1Gal:1Man; P8-4, 1GalN:1Gal:1Man. The presence of mannose indicates that the P-8 glycolipids are distinct from previously described amastigote glycosphingolipids (GSL) (54). Further analyses are in progress to determine the complete structure of these novel glycolipid components.
The 56-kDa proteinase present in P-8c1 can be detected on gelatin-copolymerized SDS-PAGE under nonreducing conditions, and this activity can be inhibited by the presence of the serine protease specific inhibitor phenylmethylsulfonyl fluoride (Fig. 2, lane 2) and also the metalloproteinase inhibitor 1,10 phenanthroline (data not shown). These results classify the 56-kDa band of P-8c1 as a serine-metalloproteinase.
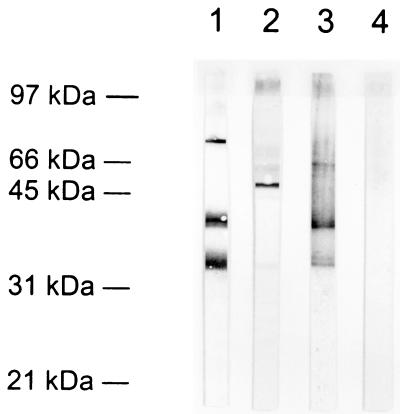
A major surface metalloproteinase of Leishmania promastigote (gp63) has been described previously and extensively studied. We have analyzed whether the 56-kDa serine-metalloproteinase present in P-8 might be the L. pifanoi axenic amastigote homologue of gp63. As shown in Fig. 8, the polyclonal antibody sp180 raised against gp63 recognized, by Western blotting, a major 63-kDa band in total amastigote protein (lane 2) but failed to detect any component present in immunopurified P-8 (lane 4). Moreover, the 63-kDa band detected by sp180 in L. pifanoi amastigotes was localized in the cytoplasmic fraction of the parasite (data not shown), as described by other authors (16, 47), rather than the membrane fraction used for P-8 antigen isolation. Another difference is that gp63 has been reported to be resistant to serine proteinase inhibitors (47). These biochemical and immunologic results suggest that the p56 proteinase is not a gp63 homologue.
FIG. 8.
Comparison of P-8 56-kDa serine-metalloproteinase and gp63. Total protein from L. pifanoi axenic amastigotes (lanes 1 and 2) or immunopurified P-8 (lanes 3 and 4) were resolved by SDS–12% PAGE, transferred to nitrocellulose, and immunostained with a polyclonal antibody raised against gp63 (lanes 2 and 4) or with a polyclonal serum against whole P-8 (lanes 1 and 3).
Consequently, the P-8c1 complex appears to represent a distinct membrane-associated antigen of Leishmania amastigotes. The role(s) of the glycolipid and protein components in the induction of protection against infection is of further interest.
DISCUSSION
The importance of the membrane-associated amastigote antigen P-8 comes from previous results, demonstrating that vaccination of mice with P-8 induces significant and reproducible protection against cutaneous leishmaniasis (52). In addition, Coutinho et al. (9) have found that, in patients suffering from American cutaneous leishmaniasis, cells activated in response to the antigen P-8 have predominantly a Th1-like cytokine profile, which is considered characteristic of a beneficial or curative T cell response.
In the present work we have analyzed the biochemical nature of P-8, as an initial step for defining immunodominant epitopes of this vaccine candidate. We have found that P-8 does not correspond to a simple antigen but instead consisted of two macromolecular complexes (P-8c1 and P-8c2), each containing several tightly bound components. P-8c1 contains a serine-metalloproteinase, host serum ApoE, and four amastigote-specific glycolipids. P-8c2 contains a cysteine proteinase and an amastigote glycolipid, P8-3. Even though the MAb used to purify this antigen recognizes the glycolipids present, the other components of immunopurified P-8 copurify due to an apparently tight association with the glycolipids. The complexes maintained their association throughout the purification procedure, which involves detergent solubilization (Mega-10) and alkaline pH treatment; however, a strong detergent such as SDS was capable of dissociating most complex components.
As indicated above, the initial interest in the characterization of the P-8 antigen was due to its immunologic and protective properties, as observed in human patients and vaccinated mice, respectively. As P-8 antigen can provide complete protection against cutaneous murine leishmaniasis, it was of interest to determine if one or both of the distinct P-8 complexes were critical to this protection. When mice were immunized with each of the two isolated P-8 complexes (P-8c1 or P-8c2), only mice vaccinated with the P-8c1 complex (containing the serine-metalloproteinase and the P8-1, -2, and -4 glycolipids) were protected against infection; no significant protection was induced by vaccination with the P-8c2 complex. The level of protection induced with vaccination with P-8c1 was comparable to that found for whole P-8. These results are in agreement with the differences in the humoral and cellular immune responses observed for the P-8c1 and P-8c2 complexes in infected or vaccinated mice.
The 31-kDa protein present in P-8c2 complex has been characterized as a cysteine protease due to its activation by thiols and inhibition by iodoacetamide. Abundant developmentally regulated cysteine proteinases are characteristic of the intracellular amastigote form of L. pifanoi and other species of the L. mexicana complex (12, 17, 40, 41). These enzymes appear to be essential to parasite growth (8, 17, 40) and have been proposed as potential chemotherapeutic targets. The cysteine proteases of L. mexicana complex described to date have been classified as cathepsin L-like or cathepsin B-like (4, 36, 42, 43). Without further molecular characterization, we cannot identify the P-8 31-kDa cysteine protease with a previously described enzyme. The interesting characteristic of this enzyme is that it is primarily localized in amastigote surface membrane; further, in axenically cultured amastigotes, the enzyme appears to be expressed as an inactive form, as it requires the presence of a thiol reagent for activation. These results suggest an enzymatic role only upon entry of the Leishmania amastigote into the parasitophorous milieu of the macrophage, which is predominantly reducing (1).
The 56-kDa protein present in P-8c1 is a serine-metalloproteinase. Serine proteases are members of one of the most biologically important and widely distributed families of enzymes found throughout nature. Members of this ubiquitous class of proteases are involved in a broad range of biological processes and have also been implicated in pathogenesis of a number of infectious diseases; parasite serine proteases may facilitate invasion of host tissue, metabolism of host proteins and evasion of the host immune response (46). In the case of Leishmania parasites, a serine oligopeptidase from L. amazonensis promastigotes has been described (10). This protease is localized in the cytoplasm and has an apparent molecular mass of 101 kDa, differing from the 56-kDa serine protease found in P-8c1. The Leishmania gp63 metalloproteinase is associated with both the promastigote and amastigote stages. However, immunologic and enzymatic data clearly indicate that the 56-kDa proteinase is distinct from the gp63 proteinase, which is primarily located within the megasomal compartment of the amastigote and resistant to serine protease inhibitors (16, 33, 47).
Glycosylphosphatidylinositol (GPI) glycolipids are major cell surface constituents in Leishmania parasites, and distinct classes are present as membrane anchors for several surface glycoproteins, the abundant lipophosphoglycan in promastigotes, and as a family of low-molecular-weight GPI glycolipids (GIPLs). The surface expression of both the lipophosphoglycan and GPI-anchored proteins is massively down-regulated in the intracellular amastigote stage of several species (3, 33, 57). The GIPLs are major surface constituents on both the promastigote and amastigote stages (29, 31, 57), while neutral GSL have been described as specific to the L. amazonensis amastigote (54). These GSL are ceramides that contain galactose and glucose but not mannose and, hence, differ from the P-8 glycolipids. The results presented here indicate that P-8 glycolipids also differ in their biochemical properties from previously described L. mexicana amastigote GIPLs. However given the general resistance of the P-8 glycolipids to digestion with PI-PLC, it is possible that they are related to recently described inositol-acylated GPI (glycolipids C and lyso-C′) in Trypanosoma brucei (35). The structural characterization of these glycolipids is currently in progress and should clarify these points.
It will be of interest to further define the immunologic components (glycolipid[s] and/or protein) of P-8c1 that are responsible for the protection observed. In addition, given the surface localization of this antigen, it will be of interest to further investigate the potential biological role of the P-8 complexes in Leishmania amastigote attachment, internalization, and survival within the host macrophage. This work is currently in progress.
ACKNOWLEDGMENTS
We thank Lynn Soong for helpful discussions and suggestions.
This work was supported by a grant from the National Institutes of Health to D.M.-P. (AI27811).
REFERENCES
- 1.Antoine J C, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 2.Antunes C M, Mayrink W, Magalhaes P A, Costa C A, Melo M N, Dias M, Michalick M S, Williams P, Lima A O, Vieira J B, et al. Controlled field trials of a vaccine against New World cutaneous leishmaniasis. Int J Epidemiol. 1986;15:572–580. doi: 10.1093/ije/15.4.572. [DOI] [PubMed] [Google Scholar]
- 3.Bahr V, Stierhof Y D, Ilg T, Demar M, Quinten M, Overath P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993;58:107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- 4.Bart G, Coombs G H, Mottram J C. Isolation of lmcpc, a gene encoding a Leishmania mexicana cathepsin-B-like cysteine proteinase. Mol Biochem Parasitol. 1995;73:271–274. doi: 10.1016/0166-6851(95)00113-f. [DOI] [PubMed] [Google Scholar]
- 5.Bates P A, Tetley L. Leishmania mexicana: induction of metacyclogenesis by cultivation of promastigotes at acidic pH. Exp Parasitol. 1993;76:412–423. doi: 10.1006/expr.1993.1050. [DOI] [PubMed] [Google Scholar]
- 6.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;56:3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convit J, Kerdel-Vegas F. Disseminated cutaneous leishmaniasis. Arch Dermatol. 1965;91:439–445. doi: 10.1001/archderm.1965.01600110025007. [DOI] [PubMed] [Google Scholar]
- 8.Coombs G H, Baxter J. Inhibition of Leishmania amastigote growth by antipain and leupeptin. Ann Trop Med Parasitol. 1984;78:21–24. doi: 10.1080/00034983.1984.11811768. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho S G, Da-Cruz A M, Bertho A L, Santiago M A, De-Luca P. Immunologic patterns associated with cure in human American cutaneous leishmaniasis. Braz J Med Biol Res. 1998;31:139–142. doi: 10.1590/s0100-879x1998000100019. [DOI] [PubMed] [Google Scholar]
- 10.de Andrade A S, Santoro M M, de Melo M N, Mares-Guia M. Leishmania (Leishmania) amazonensis: purification and enzymatic characterization of a soluble serine oligopeptidase from promastigotes. Exp Parasitol. 1998;89:153–160. doi: 10.1006/expr.1997.4269. [DOI] [PubMed] [Google Scholar]
- 11.Doyle P S, Engel J C, Pimenta P F, da Silva P P, Dwyer D M. Leishmania donovani: long-term culture of axenic amastigotes at 37 degrees C. Exp Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 12.Duboise S M, Vannier-Santos M A, Costa-Pinto D, Rivas L, Pan A A, Traub-Cseko Y, De Souza W, McMahon-Pratt D. The biosynthesis, processing, and immunolocalization of Leishmania pifanoi amastigote cysteine proteinases. Mol Biochem Parasitol. 1994;68:119–132. doi: 10.1016/0166-6851(94)00157-x. [DOI] [PubMed] [Google Scholar]
- 13.Eperon S, McMahon-Pratt D. Extracellular amastigote-like forms of Leishmania panamensis and L. braziliensis. II. Stage- and species-specific monoclonal antibodies. J Protozool. 1989;36:510–518. doi: 10.1111/j.1550-7408.1989.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 14.Eperon S, McMahon-Pratt D. Extracellular cultivation and morphological characterization of amastigote-like forms of Leishmania panamensis and L. braziliensis. J Protozool. 1989;36:502–510. doi: 10.1111/j.1550-7408.1989.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer W, Ishizuka I, Landgraf H R, Herrmann H. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from Streptococci. Biochim Biophys Acta. 1973;296:527–545. doi: 10.1016/0005-2760(73)90113-6. [DOI] [PubMed] [Google Scholar]
- 16.Frommel T O, Button L L, Fujikura Y, McMaster W R. The major surface glycoprotein (GP63) is present in both life stages of Leishmania. Mol Biochem Parasitol. 1990;38:25–32. doi: 10.1016/0166-6851(90)90201-v. [DOI] [PubMed] [Google Scholar]
- 17.Galvao-Quintao L, Alfieri S C, Ryter A, Rabinovitch M. Intracellular differentiation of Leishmania amazonensis promastigotes to amastigotes: presence of megasomes, cysteine proteinase activity and susceptibility to leucine-methyl ester. Parasitology. 1990;101:7–13. doi: 10.1017/s0031182000079683. [DOI] [PubMed] [Google Scholar]
- 18.Grimaldi G, Jr, Tesh R B. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230–250. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunders A E, Naggan L, Michaeli D. Follow-up study of a vaccination programme against cutaneous leishmaniasis. I. Vaccination with a 5 year-old human strain of L. tropica from the Negev. Trans R Soc Trop Med Hyg. 1972;66:235–238. doi: 10.1016/0035-9203(72)90152-6. [DOI] [PubMed] [Google Scholar]
- 20.Handman E, Mitchell G F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci USA. 1985;82:5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy M R, Townsend R R, Lee Y C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988;170:54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi K, Tajima S, Menju M, Yamamoto A. Structure and expression of mouse apolipoprotein E gene. J Biochem (Tokyo) 1989;106:98–103. doi: 10.1093/oxfordjournals.jbchem.a122828. [DOI] [PubMed] [Google Scholar]
- 23.Howard J G, Nicklin S, Hale C, Liew F Y. Prophylactic immunization against experimental leishmaniasis: I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982;129:2206–2212. [PubMed] [Google Scholar]
- 24.Jardim A, Alexander J, Teh H S, Ou D, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahl L P, Scott C A, Lelchuk R, Gregoriadis G, Liew F Y. Vaccination against murine cutaneous leishmaniasis by using Leishmania major antigen/liposomes. Optimization and assessment of the requirement for intravenous immunization. J Immunol. 1989;142:4441–4449. [PubMed] [Google Scholar]
- 26.Kima P E, Soong L, Chicharro C, Ruddle N H, McMahon-Pratt D. Leishmania-infected macrophages sequester endogenously synthesized parasite antigens from presentation to CD4+ T cells. Eur J Immunol. 1996;26:3163–3169. doi: 10.1002/eji.1830261249. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Magnani J, Smith D F, Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980;109:399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- 29.McConville M J, Blackwell J M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 30.McConville M J, Collidge T A, Ferguson M A, Schneider P. The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J Biol Chem. 1993;268:15595–15604. [PubMed] [Google Scholar]
- 31.McConville M J, Ferguson M A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMahon-Pratt D, Rodriguez D, Rodriguez J R, Zhang Y, Manson K, Bergman C, Rivas L, Rodriguez J F, Lohman K L, Ruddle N H, et al. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun. 1993;61:3351–3359. doi: 10.1128/iai.61.8.3351-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Acosta E, Karess R E, Schwartz H, Russell D G. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989;37:263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell G F, Handman E, Spithill T W. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. Int J Parasitol. 1985;15:677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- 35.Morita Y S, Acosta-Serrano A, Buxbaum L U, Englund P T. Glycosyl phosphatidylinositol myristoylation in African trypanosomes. New intermediates in the pathway for fatty acid remodeling. J Biol Chem. 2000;275:14147–14154. doi: 10.1074/jbc.275.19.14147. [DOI] [PubMed] [Google Scholar]
- 36.Mottram J C, Frame M J, Brooks D R, Tetley L, Hutchison J E, Souza A E, Coombs G H. The multiple cpb cysteine proteinase genes of Leishmania mexicana encode isoenzymes that differ in their stage regulation and substrate preferences. J Biol Chem. 1997;272:14285–14293. doi: 10.1074/jbc.272.22.14285. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento E, Mayrink W, da Costa C A, Michalick M S, Melo M N, Barros G C, Dias M, Antunes C M, Lima M S, Taboada D C, et al. Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect Immun. 1990;58:2198–2203. doi: 10.1128/iai.58.7.2198-2203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan A A, McMahon-Pratt D. Monoclonal antibodies specific for the amastigote stage of Leishmania pifanoi. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1988;140:2406–2414. [PubMed] [Google Scholar]
- 39.Pan A A, McMahon-Pratt D, Honigberg B M. Leishmania mexicana pifanoi: antigenic characterization of promastigote and amastigote stages by solid phase radioimmunoassay. J Parasitol. 1984;70:834–835. [PubMed] [Google Scholar]
- 40.Pral E M, Bijovsky A T, Balanco J M, Alfieri S C. Leishmania mexicana: proteinase activities and megasomes in axenically cultivated amastigote-like forms. Exp Parasitol. 1993;77:62–73. doi: 10.1006/expr.1993.1061. [DOI] [PubMed] [Google Scholar]
- 41.Pupkis M F, Coombs G H. Purification and characterization of proteolytic enzymes of Leishmania mexicana mexicana amastigotes and promastigotes. J Gen Microbiol. 1984;130:2375–2383. doi: 10.1099/00221287-130-9-2375. [DOI] [PubMed] [Google Scholar]
- 42.Robertson C D, Coombs G H. Cathepsin B-like cysteine proteases of Leishmania mexicana. Mol Biochem Parasitol. 1993;62:271–279. doi: 10.1016/0166-6851(93)90116-f. [DOI] [PubMed] [Google Scholar]
- 43.Robertson C D, Coombs G H. Multiple high activity cysteine proteases of Leishmania mexicana are encoded by the Imcpb gene array. Microbiology. 1994;140:417–424. doi: 10.1099/13500872-140-2-417. [DOI] [PubMed] [Google Scholar]
- 44.Russell D G. Immunity to leishmaniasis: what properties delineate a protective antigen? Ann Inst Pasteur Immunol. 1987;138:774–781. doi: 10.1016/s0769-2625(87)80037-5. [DOI] [PubMed] [Google Scholar]
- 45.Russell D G, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988;140:1274–1279. . (Erratum, 140:2858.) [PubMed] [Google Scholar]
- 46.Sakanari J A, Staunton C E, Eakin A E, Craik C S, McKerrow J H. Serine proteases from nematode and protozoan parasites: isolation of sequence homologs using generic molecular probes. Proc Natl Acad Sci USA. 1989;86:4863–4867. doi: 10.1073/pnas.86.13.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider P, Rosat J P, Bouvier J, Louis J, Bordier C. Leishmania major: differential regulation of the surface metalloprotease in amastigote and promastigote stages. Exp Parasitol. 1992;75:196–206. doi: 10.1016/0014-4894(92)90179-e. [DOI] [PubMed] [Google Scholar]
- 48.Scott P, Pearce E, Heath S, Sher A. Identification of T-cell-reactive antigens that protect BALB/c mice against Leishmania major. Ann Inst Pasteur Immunol. 1987;138:771–774. doi: 10.1016/s0769-2625(87)80036-3. [DOI] [PubMed] [Google Scholar]
- 49.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227. [PubMed] [Google Scholar]
- 50.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J Immunol. 1987;139:3118–3125. [PubMed] [Google Scholar]
- 51.Seppo A, Moreland M, Schweingruber H, Tiemeyer M. Zwitterionic and acidic glycosphingolipids of the Drosophila melanogaster embryo. Eur J Biochem. 2000;267:3549–3558. doi: 10.1046/j.1432-1327.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- 52.Soong L, Duboise S M, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–3566. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone K L, Williams K R. Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudarra P, editor. A practical guide to protein and peptide purification for microsequencing. 2nd ed. New York, N.Y: Academic Press; 1993. [Google Scholar]
- 54.Straus A H, Levery S B, Jasiulionis M G, Salyan M E, Steele S J, Travassos L R, Hakomori S, Takahashi H K. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993;268:13723–13730. [PubMed] [Google Scholar]
- 55.Svennerholm L, Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980;617:97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- 56.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter G, Fuchs M, McConville M J, Stierhof Y D, Overath P. Surface antigens of Leishmania mexicana amastigotes: characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107:2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- 58.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]
- 59.Yang D M, Rogers M V, Liew F Y. Identification and characterization of host-protective T-cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology. 1991;72:3–9. [PMC free article] [PubMed] [Google Scholar]