Abstract
This study aimed to increase the therapeutic potential of medicinal plants through inoculation with endophytic fungi. As endophytes influence medicinal plants’ biological properties, twenty fungal strains were isolated from the medicinal plant Ocimum tenuiflorum. Among all fungal isolates, the R2 strain showed the highest antagonistic activity towards plant pathogenic fungi Rosellinia necatrix and Fusarium oxysporum. The partial ITS region of the R2 strain was deposited in the GenBank nucleotide sequence databases under accession number ON652311 as Fusarium fujikuroi isolate R2 OS. To ascertain the impact of an endophytic fungus on the biological functions of medicinal plants, Stevia rebaudiana seeds were inoculated with Fusarium fujikuroi (ON652311). In the DPPH assay, the IC50 value of the inoculated Stevia plant extracts (methanol, chloroform, and positive control) was 72.082 µg/mL, 85.78 µg/mL, and 18.86 µg/mL, respectively. In the FRAP assay, the IC50 value of the inoculated Stevia extracts (methanol, chloroform extract, and positive control) was 97.064 µM Fe2+ equivalents, 117.662 µM Fe2+ equivalents, and 53.384 µM Fe2+ equivalents, respectively. In the extracts of the plant inoculated with endophytic fungus, rutin and syringic acid (polyphenols) concentrations were 20.8793 mg/L and 5.4389 mg/L, respectively, which were higher than in the control plant extracts. This approach can be further utilized for other medicinal plants to increase their phytochemical content and hence medicinal potential in a sustainable way.
Keywords: antagonistic activity, antioxidant activity, DNA sequencing, endophytic fungi inoculation, UHPLC
1. Introduction
Endophytic fungi spend all or part of their life cycle dominating in inter- and/or intracellular systems, mainly in leaves, stems, and roots, without causing disease signs in their hosts [1]. Endophytic fungi obtain shelter and nutrients from the host plant and protection from the harsh natural environment. In return, endophytic fungi enhance the host plant’s tolerance to abiotic or biotic stresses. The coexisting interaction has produced many beneficial aspects such as the production of phytohormones, siderophores, hydrogen cyanide, phosphate-solubilizing agents, and hydrolytic enzymes, etc. [2]. In return, endophytic fungi benefit the host plant, especially in the case of phytochemical production, where they are found to mimic the production of secondary metabolites of the host, e.g., Fusarium redolans, which was observed to produce taxol, an anticancer drug, and was isolated from Taxus plant as an endophytic fungi [3]. The metabolites produced by endophytic fungi may influence the intake or redistribution of resources and accumulation of bioactive metabolites and stimulate plant growth [4,5], which can significantly increase the host plant’s vigor. However, the knowledge of the precise interactions between the endophytic fungi and their host plants is still quite limited [5]. By modifying the conditions under which medicinal plants thrive, we can comprehend and take advantage of such interactions to produce better medications [6,7]. Maize plants inoculated with endophytic fungi (Piriformospora indica) have boosted the activity of antioxidant enzymes and raised resistance to parasitic root fungus [8]. It was discovered that F. fujikuroi produces several additional beneficial secondary metabolites, i.e., methylfusarubin, gibberelline, and bikaverin, etc., indicating that it has the potential to be used in the synthesis of other compounds. Consequently, the secondary metabolites produced by Fusarium strains can be thought of as possible biocontrol agents against mosquitoes or nematodes that carry various diseases impacting people and plants [9].
Two medicinal plants were selected for the present study, Ocimum and Stevia rebaudiana. Among the plants known for their medicinal value, the Ocimum plant of the Lamiaceae family has important therapeutic potential, which has been scientifically proven [10]. Ocimum sanctum, also known as Ocimum tenuiflorum and commonly known as Basil or Tulsi, is often used in the treatment of various diseases. It offers a variety of benefits, including adaptogenic, analgesic, anti-microbial, anti-fertility, and antispasmodic characteristics [11]. The key benefits of Ocimum in different therapies are its safety or harmless nature, as claimed by Ayurveda, in addition to being effective, less costly, and widely available [12,13]. Thus, the Ocimum plant was selected for the isolation of the beneficial endophytic fungi. Stevia rebaudiana (Asteraceae) is a medicinal plant used to produce steviol glycosides, a type of natural sweetener that has significant economic value in food goods [14]. Diabetic patients are thought to benefit most from Stevia-derived chemicals as an alternate sweetener. According to statistics, stevioside-like sweet products in some countries can replace up to 30% of the sugar that is normally consumed [15]. Stevia compounds are widely used in industry as energizers and in beverages, as well as in medicine in vasodilators, cardiotonics, anesthetics, anti-inflammatories, and uric acid reducers. Diterpene glycosides are a type of natural sweetener derived from the Stevia plant [16]. This study is an attempt to enhance the medicinal potential of this plant through endophytic fungi inoculation. Natural compounds originating from fungi are crucial in the search for novel medicines [17]. Therefore, the objective of the current study was to investigate the fungal strains that live as endophytes in the medicinal plant Ocimum tenuiflorum. Based on the antagonistic activity, the best fungal isolate was selected from a mythoreligious and important Ayurveda plant, Ocimum, and inoculated in S. rebaudiana to assess the antioxidant potential and the phytochemicals of two groups (plants with and without endophytic fungi inoculation). Thus, the S. rebaudiana plant, which has its own potential role for sugar patients, may improve its current significance to some extent by inoculation with potential endophytic fungi.
2. Results
2.1. Isolation and Morphological Characteristics of Endophytic Fungi
From the leaves of O. tenuiflorum, 20 fungal strains Trichoderma viride (R1), Fusarium fujikuroi (R2), Trichoderma sp. (R3), Alternaria alternata (R4), Alternaria sp. (R5), Alternaria sp. (R6), Trichoderma harzianum (R7), Fusarium solani (R8), Phoma setosa (R9), Penicillium citrinum (R10), Epicoccum nigrum (R11), Penicillium chrysogenum (R12), Absidia sp. (R13), Aspergillus versicolor (R14), Pythium sp. (R15), Pythium sp. (R16), Absidia ramosa (R17), Mortierella sp. (R18), Rhizopus oryzae (R19), and Rhizopus nigricans (R20) were isolated and identified. The R2 strain was selected based on antagonistic activity and identified as F. fujikuroi through sequence analyses of the fungal 18S ITS region. The isolated F. fujikuroi sequences were very like those in the NCBI’s GenBank database. The Fusarium isolate’s partial ITS regions of the rRNA sequences reported in this study have been deposited in the NCBI gene nucleotide sequence databases (http://www.ncbi.nlm.nih.gov; dated: 2 June 2022) with accession number ON652311 (Fusarium fujikuroi isolate R2 OS). The fungal isolates were classified as members of ascomycota (R1-R14), zygomycota (R15-R17), or oomycota (R18-R20).
2.2. Antagonistic Impact of Endophytic Fungi against Plant Pathogenic Fungi
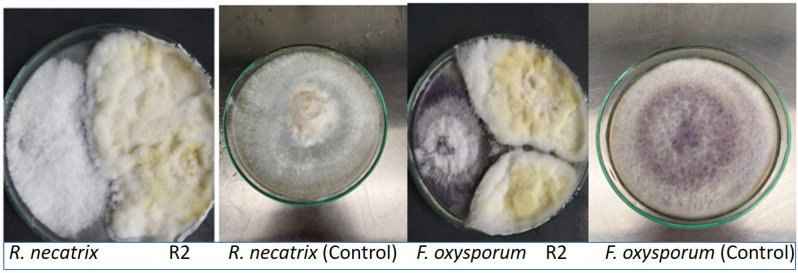
The antagonistic activity testing was performed on all the isolated endophytic fungi against two plant pathogens, Rosellinia necatrix and Fusarium oxysporum. As shown in Figure 1, antagonistic studies on endophytic fungal isolates revealed a significant reduction in pathogen development in terms of radial diameter. Out of the tested twenty fungal isolates, the R2 strain of endophytic fungi showed the highest level of antagonism (Table 1), followed by isolates R2, R11, R12, R1, and R15, with the remaining isolates showing very little activity against both pathogenic fungi.
Figure 1.
Antagonism shown by R2 strain of endophytic fungi isolated from O. tenuiflorum against the pathogenic fungi Rosellina necatrix and Fusarium oxysporum.
Table 1.
Antagonistic activity of fungal isolates from O. tenuiflorum against pathogenic fungi (F. oxysporum and R. necatrix).
| Pathogenic Fungi | Isolated Endophytic Fungi | Percentage of Growth Inhibition |
|---|---|---|
| Fusarium oxysporum | R1 | 38.09 ± 2.72 |
| R2 | 45.28 ± 2.72 | |
| R15 | 32.13 ± 3.09 | |
| Rosellinia necatrix | R11 | 26.98 ± 2.75 |
| R2 | 42.85 ± 2.75 | |
| R12 | 28.57 ± 2.38 |
Fungal strains were observed to be potentially antagonistic to plant pathogenic fungi in this screening study, with growth inhibition ranging from 26.98% to 42.85% in the case of R. necatrix and 32.13% to 45.82% in F. oxysporum. Table 1 shows that the highest antagonism of the R2 strain against both pathogenic fungi was 42.82% and 45.82% against R. necatrix and F. oxysporum, respectively.
2.3. Aligned Sequence Data of Sample—Fusarium Fujikuroi (551 bp)
The DNA sequence of the antagonistic fungi F. fujikuroi was determined, with accession number ON652311 (isolate R2 OS). The following is the 18S-ITS sequence of the isolated fungi:
TTCCGTAGGGTGAACCTGCGGAGGGATCATTACCGAGTTTACAACTCCCAAACCCCTGTGAACATACCAATTGTTGCCTCGGCGGATCAGCCCGCTCCCGGTAAAACGGGACGGCCCGCCAGAGGACCCCTAAACTCTGTTTCTATATGTAACTTCTGAGTAAAACCATAAATAAATCAAAACTTTCAACAACGGATCTCTTGGTTCTGGCATCGATGAAGAACGCAGCAAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACATTGCGCCCGCCAGTATTCTGGCGGGCATGCCTGTTCGAGCGTCATTTCAACCCTCAAGCCCAGCTTGGTGTTGGGACTCGCGAGTCAAATCGCGTTCCCCAAATTGATTGGCGGTCACGTCGAGCTTCCATAGCGTAGTAGTAAAACCCTCGTTACTGGTAATCGTCGCGGCCACGCCGTTAAACCCCAACTTCTGAATGATGACCTCGGATCAGGTAGGAATACCCGCTGAACTTAAGCATATCAATAAGCGGGAGGGAA
ITS1-5.8S rRNA-ITS2 Region Sequences Analyzed Phylogenetically
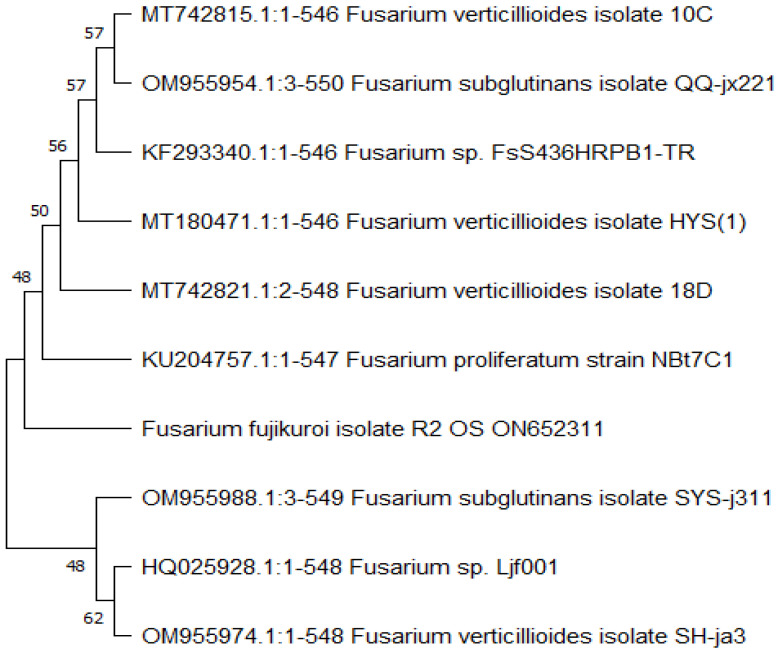
The ITS1-5.8S rRNA-ITS2 region of Fusarium isolate R2 OS proved to be extremely similar to F. fujikuroi in terms of data sequencing, with a total score of 1147 and 99.64% similarity. Multiple sequences from the GenBank database representing Fusarium species were used to construct a phylogenetic tree to evaluate the position of each strain in a phylogenetic analysis. The phylogenetic tree as given in Figure 2 revealed that Fusarium isolate (R2 OS) and F. fujikuroi endophytic fungi belonged to the same clade. As a result, Fusarium isolate R2 OS was named as F. fujikuroi.
Figure 2.
The phylogenetic relationship between isolate Fusarium fujikuroi and other related species.
2.4. Antioxidant Assay
The antioxidant potential of the plant extract after inoculation with endophytic fungi was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (Ferric reducing antioxidant power) assays.
2.4.1. DPPH Assay
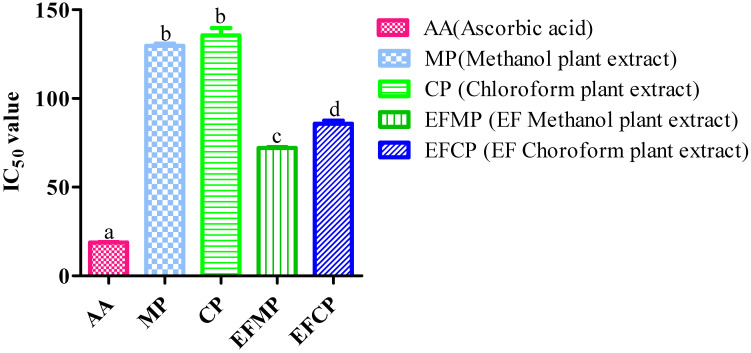
Both Stevia extracts (methanol and chloroform) reduced the oxidation activity of DPPH radicals because of the radical scavenging ability. The DPPH assay results revealed that when the extract concentration increased (20–100 µg/mL), the percentage inhibition of free radicals also increased. The greatest percentage inhibition was found to be 61.58 ± 0.51% in the methanol extract, while the chloroform extract demonstrated the least amount of inhibition at a concentration of 100 µg/mL (55.20 ± 0.81%). In the case of the control plants (non-inoculated), the DPPH radical scavenging activities of the extracts (methanol and chloroform) ranged from 19.46% to 41.94% and 17.16 % to 40.45%, respectively. The IC50 values of the control plant extracts (methanol and chloroform) were 129.676 µg/mL and 135.603 µg/mL, respectively, and in the case of the inoculated plant extracts (methanol, chloroform, and positive control), the IC50 values were 72.082 µg/mL, 85.78 µg/mL, and 18.86 µg/mL, respectively (Figure 3).
Figure 3.
Antioxidant activity (DPPH assay) of different extracts of S. rebaudiana. The values are calculated as mean ± standard deviation of the three replications (n = 3), and different letters are used for significant results (p < 0.05).
2.4.2. FRAP Assay
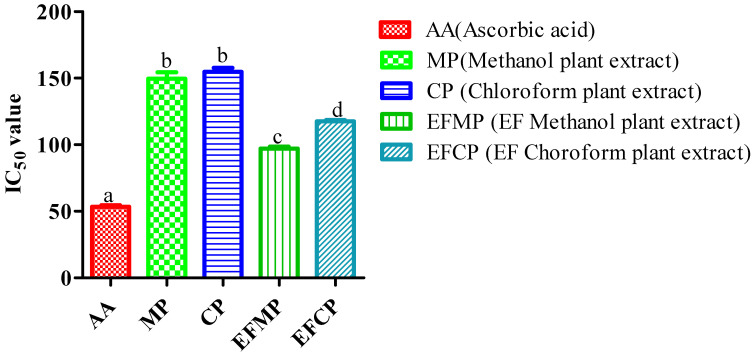
The plant extracts were tested for their oxidation-reduction ability by converting Fe3+ to Fe2+. Depending on the concentration and reduction power of the molecules present in the plant extracts, the test solution’s color changed from yellow to various shades of green and blue. Similar to the DPPH assay, plant extracts of methanol and chloroform were shown to have positive results for reducing power assay. Different concentrations of plant extracts (20–100 µg/mL) were used for the assessment of percent inhibition in the FRAP assay. At 100 µg/mL, the methanol extract (50.18 ± 0.40 M/mL FeSO4 equivalents) showed higher percentage inhibition than the chloroform extract (45.15 ± 0.56 M/mL FeSO4 equivalents). The antioxidant activity of the S. rebaudiana control plant extracts (methanol and chloroform) ranged from 19.14 to 38.09 M/mL and 15.05 to 35.47 M/mL, respectively. The IC50 value of the plant extracts (methanol and chloroform) in the control was 149.639 M Fe2+ equivalents and 154.788 M Fe2+ equivalents, respectively. The antioxidant potential of the Stevia extracts (with inoculated plants), viz., methanol and chloroform, was found to be 97.064 M Fe2+ equivalents and 117.662 M Fe2+ equivalents, respectively (Figure 4).
Figure 4.
Antioxidant activity (FRAP assay) of different extracts of S. rebaudiana. The results are presented as mean ± standard deviation where the tests were performed in triplicate, and different letters are used for significant results (p < 0.05).
2.5. UHPLC (Ultra-High-Performance Liquid Chromatography)
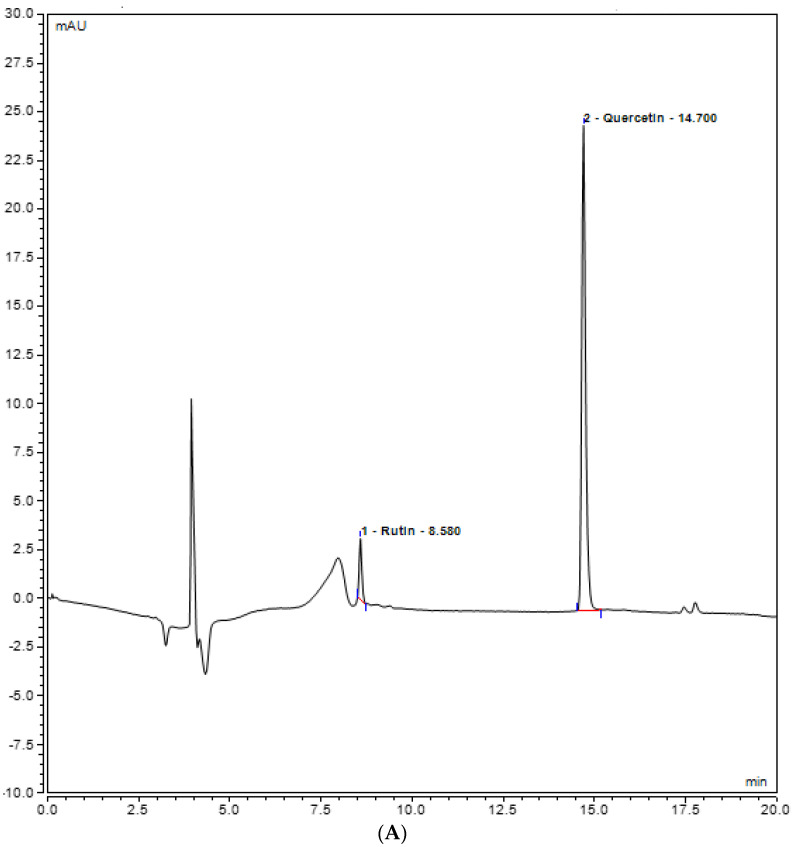
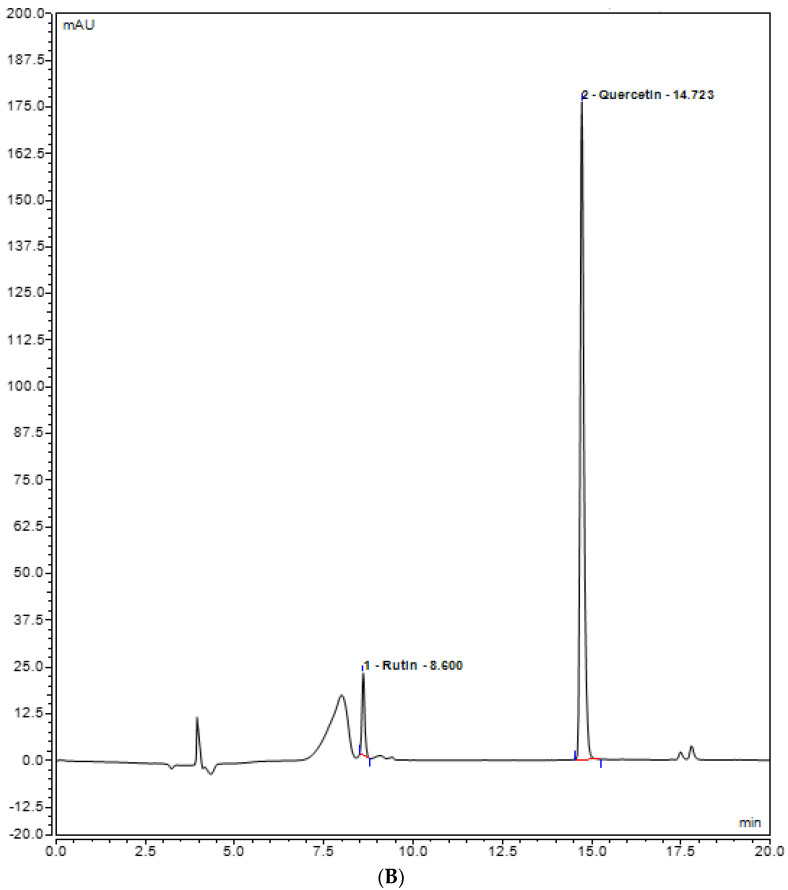
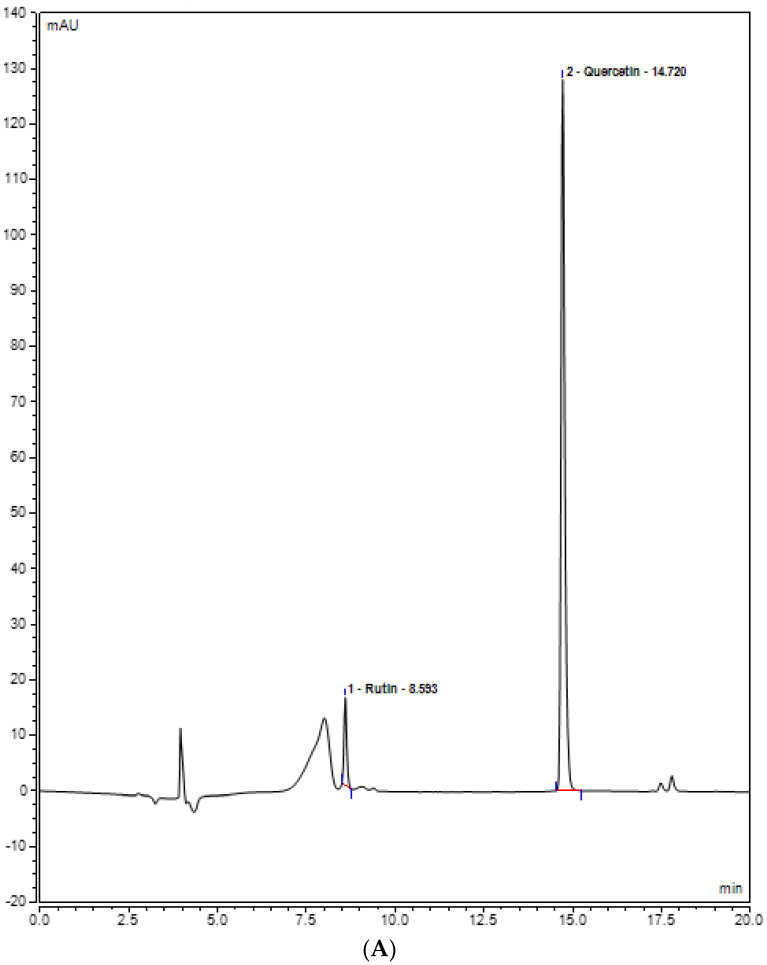
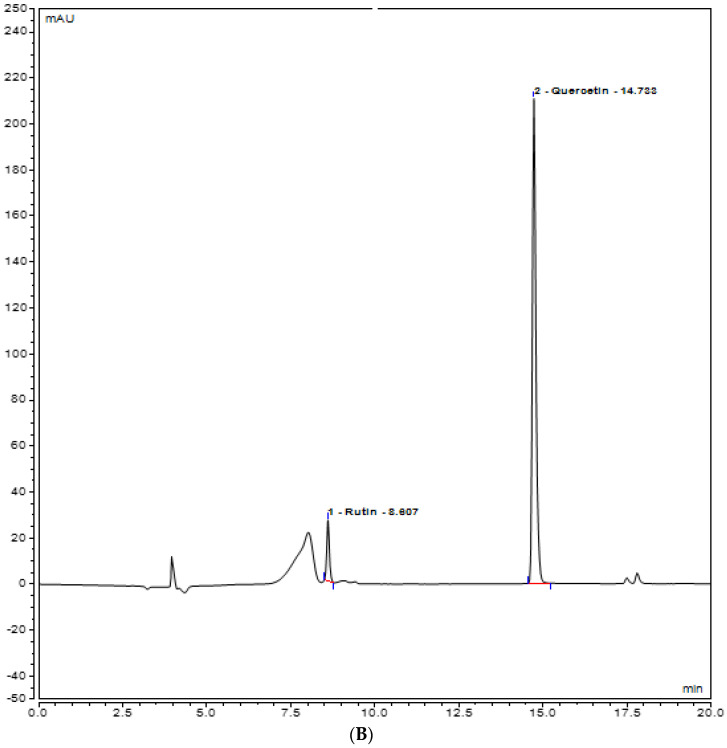
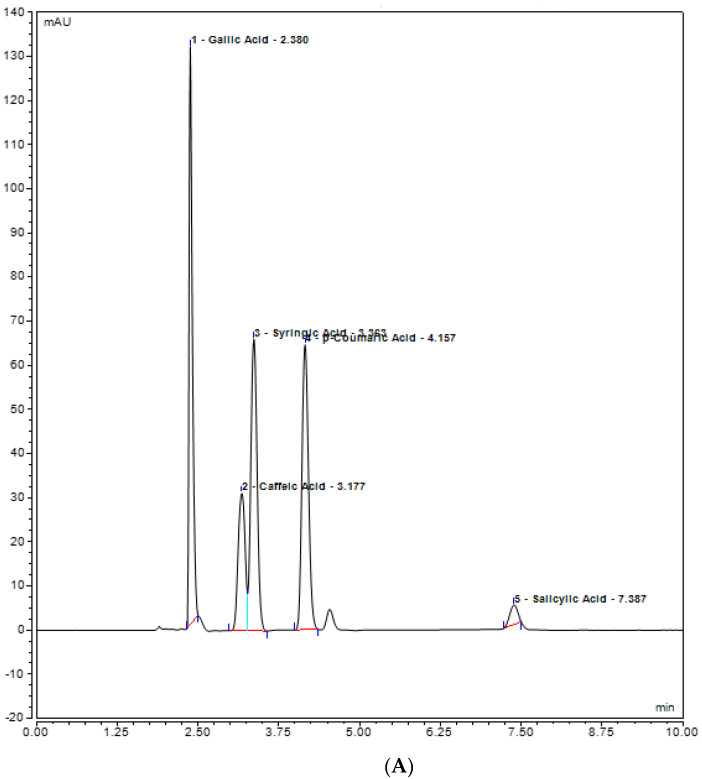
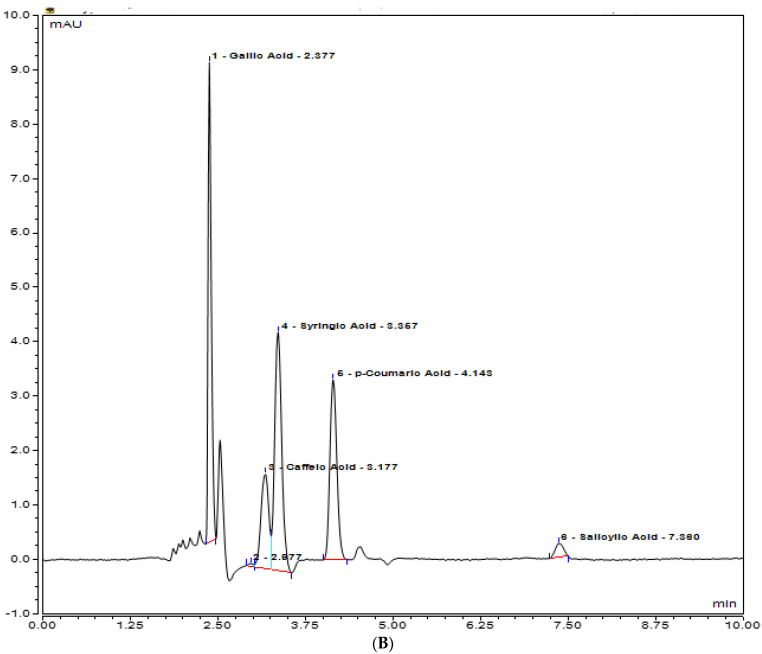
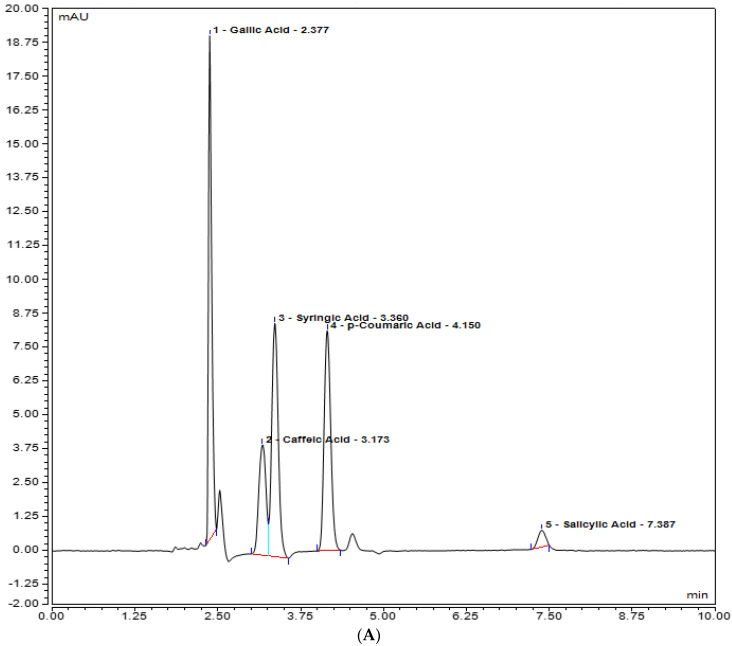
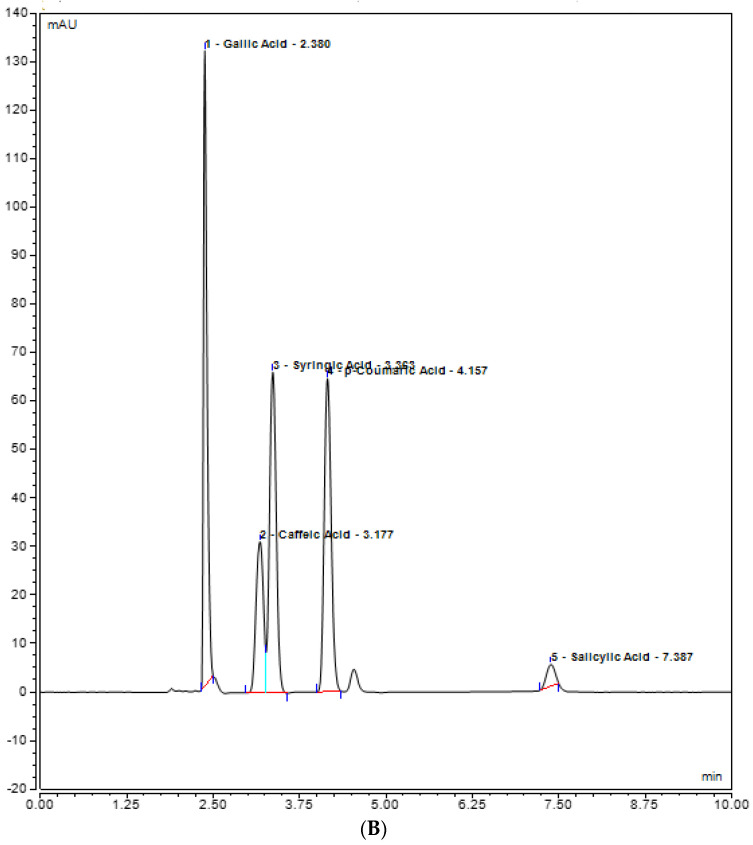
Rutin, quercetin, gallic acid, caffeic acid, and syringic acid (polyphenols) present in the plant extracts (methanol and chloroform) might be responsible for different bioactivities and were assessed for their presence in the plant extracts by UHPLC analysis. Various polyphenols were seen under the curve with different retention times. In the methanolic extract, rutin was observed with the highest concentration, i.e., 20.8793 mg/L, at 8.593 retention time (RT). At 350 nm, the absorbance of both quercetin and rutin was also measured, and it was observed that rutin was highest in Stevia methanolic plant extract at 8.593 RT when compared with the control plant extracts, i.e., methanol and chloroform (Figure 5). In contrast, UHPLC analysis of chloroform extracts of S. rebaudiana revealed the presence of the least amount of polyphenols when compared to methanolic extract of S. rebaudiana, as shown and summarized in Table 2 and Figure 6. Gallic acid, caffeic acid, syringic acid, p-coumaric acid, and salycylic acid standards were compared to the Stevia plant extracts to observe polyphenols such as gallic acid, caffeic acid, syringic acid, p-coumaric acid, and salycylic acid. It was observed that syringic acid in methanolic extract had a concentration of 5.4389 mg/L at 3.270 RT (Figure 7 and Table 2). Figure 8 shows the UHPLC results of polyphenols observed with chloroform plant extracts of S. rebaudiana.
Figure 5.
UHPLC chromatogram of polyphenols (Rutin and Quercetin) (A) methanol plant extract of inoculated S. rebaudiana with endophytic fungi, and (B) methanol plant extract of non−inoculated S. rebaudiana plant.
Table 2.
UHPLC analysis of polyphenols from different extracts of S. rebaudiana.
| Peak Name | Methanolic Extract of Inoculated Plant | Methanolic Plant Extract (Control) | Chloroform Extract of Inoculated Plant | Chloroform Plant Extract (Control) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retention time Min |
Relative Area % |
Amount (mg/L) |
Retention Time Min |
Relative Area % |
Amount (mg/L) |
Retention Time Min |
Relative Area % |
Amount (mg/L) |
Retention Time Min |
Relative Area % |
Amount (mg/L) |
|
| Gallic acid | 2.41 | 1.78 | 0.04 | 2.44 | 1.52 | 0.01 | 2.42 | 5.23 | 0.06 | 2.28 | 0.11 | 0.08 |
| Caffeic acid | 3.08 | 2.86 | 0.55 | 3.07 | 5.22 | 0.46 | 3.06 | 7.38 | 0.18 | 3.06 | 6.41 | 0.10 |
| Syringic acid | 3.27 | 44.01 | 5.44 | 3.25 | 54.22 | 3.06 | 3.33 | 2.24 | 1.59 | 3.31 | 0.48 | 0.34 |
| Rutin | 8.593 | 93.08 | 20.8793 | 8.567 | 100 | 3.2362 | 8.707 | 100.00 | 5.1032 | 8.713 | 100.00 | 0.0818 |
| Quercetin | 14.700 | 2.42 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| p-Coumaric acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Salicylic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Total | 100.00 | 100.00 | 1.855 | 100.00 | 100.00 | |||||||
N.D.—Not Determined.
Figure 6.
UHPLC chromatogram of polyphenols (Rutin and Quercetin) (A) chloroform plant extract of inoculated S. rebaudiana with endophytic fungi, and (B) chloroform plant extract of non−inoculated S. rebaudiana plant.
Figure 7.
UHPLC chromatogram of polyphenols (Gallic acid, Caffeic acid, Syringic acid, p-coumaric acid, Salicylic acid) (A) methanol plant extract of inoculated S. rebaudiana with endophytic fungi, and (B) methanol plant extract of non−inoculated S. rebaudiana plant.
Figure 8.
UHPLC chromatogram of polyphenols (Gallic acid, Caffeic acid, Syringic acid, p-coumaric acid, Salicylic acid) (A) chloroform plant extract of inoculated S. rebaudiana with endophytic fungi, and (B) chloroform plant extract of non−inoculated S. rebaudiana plant.
3. Materials and Methodology
3.1. Sample Collection
In separate sterile polythene bags, fresh, healthy, and mature plants of O. tenuiflorum were collected from the campus of Shoolini University, Solan district, Himachal Pradesh, India. The sampling site in Solan district lies in the latitudinal range of 30°44′53″ to 31°22′1″ N and longitudinal range of 76°36′10″ to 76°15′14″ E, with wide altitudinal ranges. Endophytic fungi were isolated from the fresh plants and used for the research. The Stevia and Ocimum plant samples were used for preparing the herbarium which was authenticated by BSI (Botanical Survey of India) in Nauni, Solan, Himachal Pradesh (Accession Nos. 00043 and 00044).
3.2. Endophytic Fungi Isolation and Cultivation
Cui et al.’s [18] method was adopted for isolating endophytic fungi from healthy O. tenuiflorum plant leaves. The sample was placed in a sterile bag, and within 24 h of collection the fungal endophytes were isolated. The plant samples were washed twice with autoclaved distilled water and three times with regular distilled water. Following a sterile distilled water rinse, the surface of each treatment sample was cleaned and disinfected using 70% ethanol for 60 s, 1% sodium hypochlorite (NaOCl) again for 60 s, and 70% ethanol for 30 s. To get rid of extra moisture, the treated samples were placed on sterilized blotting paper. Surface-sterilized samples were prepared with endophytic fungi cut into 6 to 6 mm long pieces and mounted on 90 mm Petri dishes with water agar medium (2% agar by volume). To stop bacterial growth, streptomycin sulphate (200 µg/mL) was added to the media. Until mycelium from the inoculated plant samples appeared, the Petri dishes were cultured for 3–12 days at 28 °C. Inoculated samples produced hyphal growth which was collected and put on potato dextrose agar media before being cultured at 28 °C to obtain a pure culture. Each isolate was grown on PDA agar and stored at 4 °C for later use. Morphological characteristics such as mycelium color, colony morphology, and reverse media color were used to identify endophytic fungi.
3.3. Fungal Isolates for Antagonistic Activity
The antagonistic effect of fungal endophytes on plant pathogenic fungi was investigated using the dual-culture technique [19]. On sterile PDA plates, the pathogen and antagonists were grown separately for 5 days. On sterile PDA plates, the antagonists and the pathogen were grown separately for five days. A 5 mm endophyte culture was positioned in one direction, 1 cm away from the PDA-media-containing Petri plate’s edge, and a test pathogen of the same size was obtained by placing it in another direction. Plant pathogenic fungi R. necatrix (accession no. ON652311) and F. oxysporum (accession no. SR266-9) were obtained from the School of Applied Sciences and Biotechnology in Shoolini University, Solan. Using the pathogen alone without the antagonist (endophytes) as control, the experiment was carried out in triplicate. The pathogen mycelia totally covered the control plates after 12 h in the dark and 12 h in the light at 25 ± 2 °C. To ensure equal growth opportunities, antagonist and pathogen paired cultures were placed at equal distances from the periphery. Following incubation, the radial growth of the control and treatment plates was measured, the radial growth of colonies was measured after 5 days, and the formula used to determine the percentage was:
| I = C − T × 100\C |
where I = mycelial growth inhibitor;
T = radial mycelial development of the antagonistic fungus toward the pathogen (T);
C = radial mycelial development of that on a control plate.
3.4. DNA Extraction and PCR Amplification
3.4.1. DNA Extractions
The isolated endophytic fungi from O. tenuiflorum were further subjected to molecular characterization. The CTAB method was used to extract the genomic DNA of the fungi. We added 100 mg of the sample and 1 mL of the extraction buffer in a crusher and pestle to homogenize it. To extract DNA, the homogenate was put in a 2 mL microfuge tube. The tubes were filled with equal amounts of phenol, chloroform, and isoamyl alcohol (25:24:1) and gently shaken to properly mix up the reagents. At room temperature, the tubes were centrifuged at 14,000 rpm for 15 min. The upper aqueous phase thus obtained was subjected to centrifugation at 14,000 rpm for 10 min and transferred to a fresh new tube. The DNA was precipitated from the solution by adding 0.1 volume of 3 M sodium acetate pH 7.0 and 0.7 volumes of isopropanol. Following a 15 min incubation period at ambient temperature, the tubes were centrifuged at 4 °C for 15 min at 14,000 rpm. The DNA pellet was thoroughly cleaned twice with 70% ethanol, quickly rinsed with 100% ethanol, and then left to dry naturally. To remove RNA, the DNA was treated with 5 μL of DNAse-free RNAse A (10 mg/mL) after dispersing in TE (Tris-Cl 10 mM, pH 8.0, EDTA 1 mM) [20]. A certain amount (50 μL) of total reaction volume, 10 pM of each primer, and a total of 127 ng of extracted DNA were used for amplification. High-fidelity PCR polymerase was used to amplify the 700 bp ITS fragment. The effectiveness of PCR amplification depends on using the proper primary pair and the appropriate annealing temperature for fungus. The main primer pairs employed in this investigation were ITS1 (forward), which served as a fungus-specific primer, and ITS4, which served as a universal primer. The annealing temperatures for primers were 57 °C and 53 °C, and the other details of the primers are mentioned in Table 3.
Table 3.
Primers used for amplification of sequences.
| S. No. | Oligo Name | Sequence (5`à 3`) | Tm (°C) | GC Content |
|---|---|---|---|---|
| 1. | ITS Forward | TCCGTAGGTGAACCTGCGG | 57 | 63.15% |
| 2. | ITS Reverse | TCCTCCGCTTATTGATATGC | 53 | 45% |
3.4.2. Quantity and Quality Determination
The amount of extracted gDNA was calculated using a Thermo Scientific Nano Drop 1000 spectrophotometer with measuring absorbance at 260 nm. The quality of the extracted gDNA and its eligibility for eventual use in Random Amplified Polymorphic DNA (RAPD) was used to assess the genomic relationship. The polycistronic gene and multi-copy genetic amplification were determined by putting the isolated gDNA through 0.8% agarose gel electrophoresis. We used 100 ng of genomic DNA, 2.5 mL of 10 mM dNTPs mix, 2 μL of random decamer oligo template OPA-1 (5′-CAGGCCCTTC-3′), and 1 µL (5 U/µL) of dNTPs in the PCR reactions for the RAPD evaluation in a 25 μL reaction volume (Sigma-Aldrich). An Eppendorf master cycler was used for amplification (Eppendorf, Hamburg). After three minutes of initial denaturation at 94 °C, the process went through 30 cycles of one minute of denaturation, one minute of annealing at 50 °C, two minutes of extension at 72 °C, and then extensions at 72 °C for ten minutes [21].
3.4.3. Sequence Alignment and Phylogenetic Analysis
The MEGA 11 software’s ClustlW algorithm was used to align the resulting DNA sequences. The BLASTn tool was used to perform a homology search on the NCBI GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi; dated: 2 June 2022), and the fungal isolates were identified using the percent homology scores. The phylogenetic tree was produced with MEGA version 11 using the neighbor-joining algorithm, the position estimation technique, and bootstrapping analyses for 1000 replicates. Partial ITS regions of the rRNA sequences of the Fusarium isolate reported in this study were deposited in the GenBank nucleotide sequence databases (http://www.ncbi.nlm.nih.gov; dated: 2 June 2022) with accession number ON652311 (Fusarium fujikuroi R2 OS).
3.5. Preparation of Fungal Inoculum
Minor modifications were made to the methods in [22] for the preparation of the fungal inoculum. Two or three pieces of the culture were inoculated into a 250 mL Erlenmeyer flask containing 100 mL potato dextrose broth, and the flask was then allowed to develop a pure culture for 15 days at 25 °C and 120 rpm. To detach the mycelia and filtrate from the broth culture, sterile cheesecloth was used as a filter.
3.5.1. Inoculation Method
Stevia seeds were surface-sterilized with 2% NaOCl (sodium hypochlorite) solution for 1 min before being washed with sterile water to remove traces of NaOCl. For the sterile soil assay, 15 × 10 cm plastic pots were used, and a potting mixture weighing 1.5 kg was placed inside each pot. A 10% (w/v) carboxyl methyl cellulose, or CMC, adhesive was used to wrap the Stevia seeds with an endophytic fungus cell suspension that had an antagonistic effect on pathogenic microbes. As sterilized air was being blown over them, the coated seeds were dried by air for a couple of hours. The coated seeds were then planted in 1.5 cm pots, with an average of four germinations occurring in each pot. The following treatments were studied: (i) control: no endophytic fungi inoculation (Figure 9A); (ii) endophytic fungi inoculation in S. rebaudiana plants under greenhouse conditions (Figure 9B). The pots were placed in a random pattern, and after two months of inoculation the plant leaves were collected for further examination [23].
Figure 9.
(A) Stevia rebaudiana plants without the inoculation of endophytic fungi, i.e., control group. (B) Plants after inoculation with endophytic fungi (F. fujikuroi).
3.5.2. Plant Extracts Preparation
With a few minor modifications, the Behera et al. [24] method was used to prepare the plant extracts. For this purpose, the effective plant part, i.e., leaves, were shade-dried for 10–15 days, grounded to powder form, and, using a conical flask, placed in a rotatory orbital shaker at 40 °C for 48 h. A total of 10 g of powder was extracted by adding 100 mL of polar solvent (methanol) and 100 mL of non-polar solvent (chloroform). The supernatant was then filtered using Whatman filter paper, dried in a water bath at 40 °C, and placed in the refrigerator for further use.
3.6. Antioxidant Activity
3.6.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
The Katalinic [25] method was used to assess the extract’s scavenging potential of DPPH. The activity was measured by preparing plant extract in methanol at different concentrations (5 µg/mL, 10 µg/mL, 20 µg/mL, and 40 µg/mL). Ascorbic acid was used as standard at a concentration comparable to that of the sample. A total of 100 µL of the extract was combined with 900 µL of DPPH methanolic solution (0.004%). The combination was kept at room temperature for 30 min. Following a 30 min incubation, the purple color disappearance was assessed with a UV/visible spectrophotometer.
We calculated the radical scavenging activity of DPPH using the following formula:
3.6.2. FRAP Assay
The FRAP activity was determined using the Benzie and Strain [26] methodology. To make the FRAP reagent, in a 10:1:1 ratio, 10.0 mM TPTZ (tripyridyltriazine) solution, 20.0 mM FeCl3·6H2O solution, and 300 mM sodium acetate buffer (pH 3.6) were combined. In order to analyze samples with different concentrations (20 µg/mL, 40 µg/mL, 60 µg/mL, and 80 µg/mL), three milliliters of FRAP reagent were applied. The reaction mixtures were maintained at 37 °C for 30 min. The absorbance was calculated at 593 nm. A fresh working solution of FeSO4 was utilized for calibration. Depending on how well the sample could begin decreasing ferrous ions, the antioxidant capacity was calculated and demonstrated as FeSO4 equivalents per gram of sample.
3.7. UHPLC
A Thermo-fisher scientific Dionex Ultimate 3000 series equipment (Thermo-fisher Scientific, USA) chromatographic system was used for all HPLC analyses (at Food Testing lab, Shoolini University, Solan, Himachal Pradesh), consisting of a vacuum degasser, quaternary pump, autosampler with thermostat, column compartment thermostat, and photodiode array detector (DAD). The system was coupled with Zorbax Eclipse C18 (4.6 mm × 250 mm, 5 µm) column (Agilent Technologies, Santa Clara, CA, USA). The mobile phase involved two components for the analysis of polyphenol (rutin and quercetin) content, combining HPLC water with pH 2.5 (solvent A) and HPLC-grade acetonitrile (solvent B) at a flow rate of 0.07 mL/min, column oven temp. 25 °C, and UV 350 nm in gradient mode. The compounds were detected using certified reference materials (CRMs) (Sigma Aldrich). The 20 uL sample was injected into the injector with the help of an autosampler. With the help of chromeleon 7 software in UHPLC, the compounds were identified [27]. For the analysis of polyphenols (gallic acid, caffeic acid, and syringic acid) content, the mobile phase was 1% acetic acid (A): acetonitrile (B) (70:30) in isocratic mode at flow rate 1 mL/min, column oven temp. 30 °C, and UV 280 nm. Polyphenols were detected using CRMs (Sigma Aldrich) with the help of chromeleon 7 software in UHPLC.
3.8. Statistical Analysis
The findings of the analyzed data were assessed by analysis of variance (one-way ANOVA) and are presented as mean ± standard deviation, with values calculated in triplicate. To identify significant differences, the Bonferroni multiple comparison test was applied. Graph Pad Prism software was utilized for statistical analysis.
4. Discussion
Similar rates of inhibition were observed against phytopathogenic fungi with the EF, and the R2 strain of the endophytic fungi showed the highest level of antagonism among the twenty fungal isolates examined. Morphological characteristics such as mycelium color, colony morphology, and reverse media color were used to identify the endophytic fungi [28,29,30]. Endophytes produce several chemicals that successfully suppress the growth of pathogenic fungi. Specific metabolic interactions can cause the production of secondary metabolites, and these metabolites may correspond to the fungal species’ ecological niche and taxon [31].
Some elicitors, such as glycoprotein, polysaccharides, and lipopolysaccharides, activate plant defense mechanisms and increase the secretion of secondary metabolites, effectively preventing pathogen attack [32]. The chemicals cannot be produced by many endophytic strains on their own. In fact, induced metabolism assists in metabolizing the byproduct of the other processes and boosts the production of metabolites [33]. Significant, physiologically active secondary metabolites called polyphenols are involved in the regulation of plant growth, development, and stress resistance. It has been demonstrated that a variety of biological or abiotic stimuli significantly enhance the number of phenolic compounds in plants [34]. The amount of polyphenols and alkaloids present in the roots and stems, as well as the activity of polyphenol oxidases in the stems and leaves and the activity of the acid phosphatase enzyme in the leaves, are all significantly affected by the inoculation of endophytic fungus [35]. These phenolic chemicals are also necessary for lowering oxidative stress as they are involved in the detoxification of reactive oxygen species (ROS) [36].
An endophytic fungi Piriformospora indica affected the gene expression of the plant Glycine max, thereby enhancing iron transport, lignin biosynthesis, hormone signaling, nutrient acquisition, and the biosynthesis of phenylpropanoids, flavonols, siderophores, and flavonoids. A total of 238 genes were involved in encoding the heat shock protein, and several other abiotic-stress-related defence responses [37]. There are several different bioactive secondary metabolites and phytohormones found in endophytic fungi [38]. Endophytic fungi may also be involved in biosynthesizing important secondary metabolites that are currently utilized commercially, including antibiotics, anticarcinogenics, cytotoxics, insecticides, and allelopathic compounds [39]. Additionally, endophytic fungi produce lignins, phenols and phenolic acids, flavonoids, saponins, alkaloids, terpenoids, polyketides, phenylpropanoids, and saponins when they are present in plants. This results in increased plant growth and development and pathogen resistance [38].
Secondary metabolism considerably changes in the symbionts after endophyte interaction with plants. According to Ludwig-Müller et al. [40], these modifications may result from (a) the endophyte impact on altering the host’s metabolism, (b) the host inducing endophyte metabolism, (c) the host and endophyte sharing some parts of a particular pathway, (d) the host metabolizing endophyte products, and (e) the endophyte’s capacity to degrade the host’s secondary metabolites. Endogenous fungal components as elicitors have several advantages. (i) Fungi can constantly interact with host cells, release metabolites, and expand along with the host’s growth. This can continuously activate the host’s defensive mechanism. (ii) Without showing obvious signs of infection, fungi can form a long-lasting symbiotic connection with the host [41]. The relationship between grass and Epichloe is a prime example of plant–endophyte mutualism [42]. The fungus secretes enzymes that weaken the cell walls of the epidermis to allow fungal proliferation into the cortical region. Endophytes in Echinacea plants produced indole-3-acetic acid, which altered the physiology and function of the roots [43]. Rhizopus oryzae, an endophytic fungus, was inoculated with Helianthus annuus and Glycine max and was shown to have significantly lower amounts of abscisic acid (ABA) and higher levels of proline, phenolics, flavonoids, ascorbic acid oxidase, and catalase [44]. The S. rebaudiana plant showed higher antioxidant potential after inoculation with F. fujikuroi, as compared with the control plant (S. rebaudiana) in the current study. Similarly, studies by Bagheri et al. [45]; Guler et al. [46]; and Hamayun et al. [47] observed that Piriformospora indica inoculated in Oryza sativa, Trichoderma atroviride inoculated in Zea mays under drought stress, and Gliocladium cibotii inoculated in Glycine max and Helianthus annuus under heat stress increased the antioxidant potential of the host plants. In our investigation, the methanol extract showed the highest percentage inhibition (61.58 ± 0.51%), whereas the chloroform extract showed the lowest percentage inhibition at 100 µg/mL (55.20 ± 0.81%) concentration, and the antioxidant potential was found to be at higher levels when compared with the control condition. Phenolic and flavonoid compounds are mainly responsible for the antioxidant property [48]. One of the beneficial flavonoid compounds found in high concentrations in plants is rutin [49]. Peres et al. [50] and Cardona et al. [51] found in their studies that rutin participated in antioxidant activity. In the UHPLC analysis, rutin in Stevia plants was 20.8793 mg/L which was higher thanthe control plants (not inoculated with any fungi). The Fusarium oxysporum species complex contains several strains which are commonly found in the soil. Bacopa monniera was co-cultivated with Piriformospora indica, and after that the plant was found to have increased biomass and antioxidant activity [52]. Recent research has demonstrated that endophytic fungi produce exopolysaccharides necessary for plant–endophyte interactions, and these biopolymers are distinguished by morphological structure and have potential antioxidant activity [53,54].
Phytochemicals are largely linked to the phenylpropanoid/polyphenol metabolism in plants [55]. Since many plant antioxidants are phenol derivatives, the inoculation’s minimal effect on total polyphenols suggests that the microbe–host symbiosis has a more focused physiological effect than a general increase in total polyphenol biosynthesis [55]. Phenolic buildup is facilitated by endogenous fungal components as elicitors. Additional signaling channels can also be involved, such as ROS signaling pathways, ion fluxes, and Ca2+ and Jasmonic acid pathways, which all influence phenolic accumulation [56,57]. According to Cappellari et al. [58], growth-promoting rhizobacteria treatments boosted Mentha piperita’s PAL activity, which led to the buildup of phenols. Thus, the conclusion can be made that endophytic fungi assist the plant in the formation and synthesis of secondary compounds, which enhances its antioxidant potential in mutualistic plant–fungus interactions.
Due to their wide range of advantageous effects on human health, phenolic compounds have recently received a lot of attention. The best-described property of practically all phenolic compound groups is their potential to eliminate free radicals and inhibit other oxidation reactions [59]. Gallic acid and quercetin were not detected in S. rebaudiana at the concentration of mg/100 g DW [60]. UHPLC analysis of S. rebaudiana ethanolic extracts revealed the presence of the least amount of quercetin, with 1.9 mg/L at 3.680 RT, and rutin, with 1.3 mg/L at 6.004 RT [61]. Caffeic acid and p-coumaric acid (polyphenols) were not found in the UHPLC study of S. rebaudiana by Oliveira et al. [60]. In our investigation, methanolic extract from S. rebaudiana plant inoculated with endophytic fungi had 0.46 mg/L of caffeic acid, as observed by UHPLC analysis.
5. Conclusions and Recommendations
Fungal isolates from the medicinal plant O. tenuiflorum have shown antagonistic activity towards plant pathogenic fungi, viz., R. necatrix (SR266-9) and F. oxysporum (HG964402.1). The isolate was inoculated on the S. rebaudiana plant, and the extracts of the same plant showed higher antioxidant potential as compared with the control plant (S. rebaudiana). In the UHPLC analysis, rutin and syringic acid (polyphenols) concentrations in Stevia plants inoculated with endophytic fungi were found to be comparatively higher than the control plants. Endophytic fungi’s impact on plant yield and quality should be identified and prioritized in all economic and medicinal plants for future use. This also serves as a sustainable way to increase the therapeutic potential of medicinal and aromatic plants, as fungal inoculation increases the phytochemical compounds in the host plants. Future research should focus on separating bioactive compounds from endophytic fungi to purify those molecules for use in the development of potential drugs. Identifying responsible biosynthetic genes for the numerous secondary metabolites from endophytic fungi opens the opportunity to explore the genetic potential of producer strains to discover novel secondary metabolites and enhance secondary metabolite production by metabolic engineering, resulting in novel and more affordable medicines and food additives.
Acknowledgments
We would like to express our special thanks to the Food Testing lab of Shoolini Life Sciences Pvt. Ltd., Solan, India, Himachal Pradesh-173229, for their support in analysis. The authors are also thankful to Anand Sagar from the Department of Biosciences, Himachal Pradesh University, Shimla for morphological identification of the endophytic fungi.
Author Contributions
R.V. and D.K.—conceptualization and interpretation of results; R.D.—performed the experiment; K.S. and A.A.—statistical analysis and writing the manuscript; R.Y., S.M. and A.K.—analysis of results and reviewing the manuscript; A.T.—edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors would like to thank the Deanship of Scientific Research, Jazan University. This research is part of the Research Group’s funding program (Vector-Borne Diseases Research Group, Grant no. RG-2-1) of Jazan University.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dutta D., Puzari K.C., Gogoi R., Dutta P. Endophytes: Exploitation as a tool in plant protection. Braz. Arch. Biol. Technol. 2014;57:621–629. doi: 10.1590/S1516-8913201402043. [DOI] [Google Scholar]
- 2.Tyagi J., Chaudhary P., Mishra A., Khatwani M., Dey S., Varma A. Role of endophytes in abiotic stress tolerance: With special emphasis on Serendipita indica. Int. J. Environ. Rese. 2022;16:62. doi: 10.1007/s41742-022-00439-0. [DOI] [Google Scholar]
- 3.Garyali S., Kumar A., Reddy M.S. Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J. Microbiol. Biotechnol. 2013;23:1372–1380. doi: 10.4014/jmb.1305.05070. [DOI] [PubMed] [Google Scholar]
- 4.Ye H.T., Luo S.Q., Yang Z.N., Wang Y.S., Ding Q., Wang K.F., Yang S.X., Wang Y. Endophytic fungi stimulate the concentration of medicinal secondary metabolites in Houttuynia cordata thunb. Plant Signal. Behavi. 2021;16:1929731. doi: 10.1080/15592324.2021.1929731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia M., Chen L., Xin H.L., Zheng C.J., Rahman K., Han T., Qin L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front Microbiol. 2016;9:906. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai D., Mao Z., Zhou Z., Zhao S., Xue M., Dai J., Zhou L., Li D. New chlamydosporol derivatives from the endophytic fungus Pleosporales sp. Sigrf05 and their cytotoxic and antimicrobial activities. Sci. Rep. 2020;10:8193. doi: 10.1038/s41598-020-65148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao Z., Xue M., Gu G., Wang W., Li D., Lai D., Zhou L. Lophiostomin A–D: New 3, 4-dihydroisocoumarin derivatives from the endophytic fungus Lophiostoma sp. Sigrf10. RSC Adv. 2020;10:6985–6991. doi: 10.1039/D0RA00538J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar M., Yadav V., Tuteja N., Johri A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology. 2009;155:780–790. doi: 10.1099/mic.0.019869-0. [DOI] [PubMed] [Google Scholar]
- 9.Janevska S., Tudzynski B. Secondary metabolism in Fusarium fujikuroi: Strategies to unravel the function of biosynthetic pathways. Appl. Microbiol. Biotechnol. 2018;102:615–630. doi: 10.1007/s00253-017-8679-5. [DOI] [PubMed] [Google Scholar]
- 10.Prakash P.A., Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J. Physiol. Pharmacol. 2005;49:125. [PubMed] [Google Scholar]
- 11.Pattanayak P., Behera P., Das D., Panda S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010;4:95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharjya D., Adhikari S., Biswas A., Bhuimali A., Ghosh P., Saha S. Ocimum phytochemicals and their potential impact on human health. Phytochemicals in Human Health. 2019;23:1–26. [Google Scholar]
- 13.Satyendra M. Phytochemical efficiency of Ocimum sanctum (Tulsi) in health enhancement and disease prevention: A review. IJARESM. 2020;9:1346–1351. [Google Scholar]
- 14.Benelli G., Pavela R., Drenaggi E., Desneux N., Maggi F. Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Ind. Crop. Prod. 2020;155:112844. doi: 10.1016/j.indcrop.2020.112844. [DOI] [Google Scholar]
- 15. [(accessed on 10 April 2016)]. Available online: http://eagri.tnau.ac.in/eagri50/HORT282/pdf/lec34.pdF.
- 16.Crammer B., Ikan R. Sweet glycosides from the Stevia plant. Chem. Ber. 1986;22:915–917. [Google Scholar]
- 17.Huang Y., Zhao J., Zhou L., Wang M., Wang J., Li X., Chen Q. Antimicrobial compounds from the endophytic fungus Fusarium sp. Ppf4 isolated from the medicinal plant Paris polyphylla var. yunnanensis. Nat. Prod. Commun. 2009;4:1455–1458. doi: 10.1177/1934578X0900401102. [DOI] [PubMed] [Google Scholar]
- 18.Cui W.G., Zheng H.L., Zhang F.B., Swingle B., Zhu H.T., Gao M. First report of Rhizopus oryzae causing potato soft rot in the hebei province of China. Plant Dis. 2019;103:773. doi: 10.1094/PDIS-09-18-1612-PDN. [DOI] [Google Scholar]
- 19.Vinayarani G., Prakash H.S. Fungal endophytes of turmeric (Curcuma longa L.) and their biocontrol potential against pathogens Pythium aphanidermatum and Rhizoctonia solani. World J. Microbiol. Biotechnol. 2018;34:49. doi: 10.1007/s11274-018-2431-x. [DOI] [PubMed] [Google Scholar]
- 20.Guo L.D., Hyde K.D., Liew E.C. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000;147:617–630. doi: 10.1046/j.1469-8137.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- 21.Aamir S., Sutar S., Singh S.K., Baghela A. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol. Quar. 2015;5:74–81. doi: 10.5943/ppq/5/2/6. [DOI] [Google Scholar]
- 22.Bhardwaj A., Sharma D., Jadon N., Agrawal P.K. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch. Clin. Microbiol. 2015;6:1–9. [Google Scholar]
- 23.Singh S.P., Gaur R. Evaluation of antagonistic and plant growth promoting activities of chitinolytic endophytic actinomycetes associated with medicinal plants against Sclerotium rolfsii in chickpea. J. Appl. Microbiol. 2016;121:506–518. doi: 10.1111/jam.13176. [DOI] [PubMed] [Google Scholar]
- 24.Behera B., Sinha P., Gouda S., Rath S.K., Barik D.P., Jena P.K., Panda P.C., Naik S.K. In Vitro propagation by axillary shoot proliferation, assessment of antioxidant activity, and genetic fidelity of micropropagated Paederia foetida L. J. Appl. Biol. Biotechnol. 2018;6:4–9. [Google Scholar]
- 25.Katalinic V., Milos M., Kulisic T., Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. doi: 10.1016/j.foodchem.2004.12.004. [DOI] [Google Scholar]
- 26.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Kaur N., Arora D.S., Kalia N., Kaur M. Antibiofilm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Mol. Biol. Rep. 2020;47:2901–2911. doi: 10.1007/s11033-020-05394-7. [DOI] [PubMed] [Google Scholar]
- 28.Hussein H.G., E.l-Sayed E.S., Younis N.A., Hamdy A.E., Easa S.M. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Express. 2022;12:68. doi: 10.1186/s13568-022-01408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Sayed E.S., Hazaa M.A., Shebl M.M., Amer M., Mahmoud S.R., Khattab A.A. Bioprospecting endophytic fungi for bioactive metabolites and use of irradiation to improve their bioactivities. AMB Express. 2022;12:46. doi: 10.1186/s13568-022-01386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashem A.H., Shehabeldine A.M., Abdelaziz A.M., Amin B.H., Sharaf M.H. Antifungal activity of endophytic Aspergillus terreus extract against some fungi causing mucormycosis: Ultrastructural study. Appl. Biochem. Biotechnol. 2022;194:3468–3482. doi: 10.1007/s12010-022-03876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caicedo N.H., Davalos A.F., Puente P.A., Rodríguez A.Y., Caicedo P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otobagracilipes. Microbiol. Open. 2019;8:903–910. doi: 10.1002/mbo3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadiji A.E., Babalola O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020;8:467. doi: 10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eid A.M., Salim S.S., Hassan S.E., Ismail M.A., Fouda A. Microbiome in Plant Health and Disease. Springer; Singapore: 2019. Role of endophytes in plant health and abiotic stress management; pp. 119–144. [Google Scholar]
- 34.Hoque M.N., Tahjib-Ul-Arif M., Hannan A., Sultana N., Akhter S., Hasanuzzaman M., Akter F., Hossain M.S., Sayed M.A., Hasan M.T., et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021;22:11445. doi: 10.3390/ijms222111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D., Wang K., Long F., Zhang W., Yao X., Chen S. Effects of endophytic fungi on the secondary metabolites of Hordeum bogdanii under alkaline stress. AMB Express. 2022;12:73. doi: 10.1186/s13568-022-01414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasanuzzaman M., Bhuyan M.B., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camehl I., Sherameti I., Venus Y., Bethke G., Varma A., Lee J. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 2010;185:1062–1073. doi: 10.1111/j.1469-8137.2009.03149.x. [DOI] [PubMed] [Google Scholar]
- 38.Alam B., Li J., Ge Q., Khan M.A., Gōng J., Mehmood S., Yuan Y., Gong W. Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Front. Plant Sci. 2021;12:3060. doi: 10.3389/fpls.2021.791033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj R., Huang Y., Gebrechristos S., Mikolajczyk B., Brown H., Prasad R., Varma A., Bushley K.E. Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 2018;8:10227. doi: 10.1038/s41598-018-26809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig-Müller J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015;37:1325–1334. doi: 10.1007/s10529-015-1814-4. [DOI] [PubMed] [Google Scholar]
- 41.Cui J.L., Wang Y.N., Jiao J., Gong Y., Wang J.H., Wang M.L. Fungal endophyte-induced salidroside and tyrosol biosynthesis combined with signal cross-talk and the mechanism of enzyme gene expression in Rhodiola crenulata. Sci. Rep. 2017;7:12540. doi: 10.1038/s41598-017-12895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott B., Green K., Berry D. The fine balance between mutualism and antagonism in the Epichloë festucae-grass symbiotic interaction. Curr. Opin. Plant Biol. 2018;44:32–38. doi: 10.1016/j.pbi.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Maggin V., De Leo M., Mengoni A. Plant-endophytes interaction influences the secondary metabolism in Echinacea purpurea (L.) Moench: An in vitro model. Sci. Rep. 2017;7:16924. doi: 10.1038/s41598-017-17110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail A.H., Mehmood A.S., Qadir M.U., Husna A.I., Hamayun M.U., Khan N.A. Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.) Pak. J. Bot. 2020;52:1857–1865. doi: 10.30848/PJB2020-5(10). [DOI] [Google Scholar]
- 45.Bagheri A.A., Saadatmand S., Niknam V., Nejadsatari T., Babaeizad V. Effect of endophytic fungus, Piriformospora indica, on growth and activity of antioxidant enzymes of rice (Oryza sativa L.) under salinity stress. Int. J. Adv. Biol. Biomed. Res. 2013;1:1337–1350. [Google Scholar]
- 46.Guler N.S., Pehlivan N., Karaoglu S.A., Guzel S., Bozdeveci A. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta. Physiologiae. Plantarum. 2016;38:132. doi: 10.1007/s11738-016-2153-3. [DOI] [Google Scholar]
- 47.Hamayun M., Hussain A., Iqbal A., Khan S.A., Khan M.A., Lee I.J. An endophytic fungus Gliocladium cibotii regulates metabolic and antioxidant system of Glycine max and Helianthus annuus under heat stress. Pol. J. Environ. Stud. 2021;30:1631–1640. [Google Scholar]
- 48.Jamwal M., Puri S., Prakash S., Sharma S., Pundir A., Singh D. Yearly variation in biochemical composition, morphological aspects, and antimicrobial activities of Justicia adhatoda L. growing wildly in Western Himalayas. J. Appl. Biol. Biotechnol. 2022;11:61–65. doi: 10.7324/JABB.2023.110108. [DOI] [Google Scholar]
- 49.Atanassova M., Bagdassarian V. Rutin content in plant products. J. Chem. Technol. Metall. 2009;44:201–203. [Google Scholar]
- 50.Peres D.A., De Oliveira C.A., Da Costa M.S., Tokunaga V.K., Mota J.P., Rosado C., Consiglieri V.O., Kaneko T.M., Velasco M.V., Baby A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Skin Res. Technol. 2016;22:325–333. doi: 10.1111/srt.12265. [DOI] [PubMed] [Google Scholar]
- 51.Cardona M.I., Toro R.M., Costa G.M., Ospina L.F., Castellanos L., Ramos F.A., Aragón D.M. Influence of extraction process on antioxidant activity and rutin content in Physalis peruviana calyces extract. J. Appl. Pharm. Sci. 2017;7:164–168. [Google Scholar]
- 52.Prasad K. Potential impact of seed coating with beneficial microorganisms to meticulousness sustainable organic agriculture for quality nutritive food production for modern lifestyle, improve global soil and environmental health towards green technology. Aditum J. Clin. Biomed. Res. 2021;2:1–9. [Google Scholar]
- 53.Chen M., Yang L., Li Q., Shen Y., Shao A., Lin S., Huang L. Volatile metabolites analysis and molecular identification of endophytic fungi bn12 from Cinnamomum camphora chvar. borneol. China Chin. Med. J. 2011;36:3217–3221. [PubMed] [Google Scholar]
- 54.Guo S., Mao W., Li Y., Gu Q., Chen Y., Zhao C., Li N., Wang C., Guo T., Liu X. Preparation, structural characterization and antioxidant activity of an extracellular polysaccharide produced by the fungus Oidiodendron truncatum GW. Process Biochem. 2013;48:539–544. doi: 10.1016/j.procbio.2013.01.014. [DOI] [Google Scholar]
- 55.Dong N.Q., Lin H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Int. Plant Biol. 2020;63:180–209. doi: 10.1111/jipb.13054. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Wu W. Angiotensin-converting enzyme inhibiting ability of ethanol extracts, steviol glycosides and protein hydrolysates from Stevia leaves. Food Fun. 2019;10:7967–7972. doi: 10.1039/C9FO02127B. [DOI] [PubMed] [Google Scholar]
- 57.Chen J., Li L., Tian P., Xiang W., Lu X., Huang R., Li L. Fungal endophytes from medicinal plant Bletilla striata (Thunb.) Reichb. F. promote the host plant growth and phenolic accumulation. South African J. Bot. 2021;143:25–32. doi: 10.1016/j.sajb.2021.07.041. [DOI] [Google Scholar]
- 58.Cappellari L.R., Chiappero J., Santoro M.V., Giordano W., Banchio E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting Rhizobacteria. Sci. Hortic. 2017;220:193–198. doi: 10.1016/j.scienta.2017.04.002. [DOI] [Google Scholar]
- 59.Kołodziej B., Kowalski R., Kędzia B. Antibacterial and antimutagenic activity of extracts aboveground parts of three Solidago species: Solidago Virgaurea L., Solidago Canadensis L. and Solidago Gigantea Ait. J. Med. Plants Res. 2011;5:6770–6779. doi: 10.5897/JMPR11.1098. [DOI] [Google Scholar]
- 60.Oliveira A.S., Ribeiro-Santos R., Ramos F., Castilho M.C., Sanches-Silva A. UHPLC-DAD multi-method for determination of phenolics in aromatic plants. Food Anal. 2018;11:440–450. doi: 10.1007/s12161-017-1015-y. [DOI] [Google Scholar]
- 61.Pacifico S., Piccolella S., Nocera P., Tranquillo E., Dal Poggetto F., Catauro M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharmed. Biomed. Anal. 2019;163:45–57. doi: 10.1016/j.jpba.2018.09.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.