Abstract
Carotenoids were synthesized in the plant cells involved in photosynthesis and photo-protection. In humans, carotenoids are essential as dietary antioxidants and vitamin A precursors. Brassica crops are the major sources of nutritionally important dietary carotenoids. Recent studies have unraveled the major genetic components in the carotenoid metabolic pathway in Brassica, including the identification of key factors that directly participate or regulate carotenoid biosynthesis. However, recent genetic advances and the complexity of the mechanism and regulation of Brassica carotenoid accumulation have not been reviewed. Herein, we reviewed the recent progress regarding Brassica carotenoids from the perspective of forward genetics, discussed biotechnological implications and provided new perspectives on how to transfer the knowledge of carotenoid research in Brassica to the crop breeding process.
Keywords: Brassica, carotenoid, QTL, genetics, biotechnological implications
1. Introduction
Carotenoids are red, orange, and yellow pigments that are widely distributed in nature, mainly comprising C40 isoprenes. Studies have shown that lutein and β-carotene are the main components of carotenoids, which also include zeaxanthin, cryptoxanthin, astaxanthin, and lycopene [1]. Carotenoids have important functions in plant photosynthesis and are auxiliary pigments in light absorption. Carotenoid molecules contain multiple conjugated double bonds that can absorb energy and protect plants from reactive oxygen species [2]. Carotenoids endow fruit and flowers with bright colors [3], enabling them to attract the attention of pollinating insects and animals. In addition to their roles in plants, carotenoids are vital for the health and nutrition of humans. People take carotenoids to supplement vitamin A, which is used to treat night blindness [4]. Lutein, as a main component of carotenoids, can protect human vision to a certain extent, prevent vision deterioration, and prevent cataracts, and other eye diseases [5]. Astaxanthin also helps the body fight inflammation and boosts immunity [6]. There have been significant efforts to increase the carotenoid content in agricultural crops to improve their nutritional value and health benefits. Manipulating carotenoid metabolism using biotechnology and genetic engineering has been successfully implemented in many crops, and golden rice is the most relevant example of improving β-carotene in food [7].
The metabolism of carotenoids in plants has been the subject of extensive research because of the significance of carotenoids to both plants and humans. The genus Brassica includes many vegetable crops, such as B. rapa (AA genome), B. nigra (BB genome), B. oleracea (CC genome), B. juncea (AABB genome), B. napus (AACC genome), and B. carinata (BBCC genome) [8]. These species contain diverse carotenoid metabolites and high levels of nutritionally significant components. Genus and species, as well as genotype and agricultural conditions, affect the carotenoid composition and content of these organisms. The present review focuses on current situation and recent advances in carotenoid metabolism of Brassica crops from the perspective of forward genetics. Moreover, we discuss the evolution of carotenoid composition and certain key carotenoid metabolism genes during the formation of the Brassica crops. Lastly, we review the agricultural application and the prospects of carotenoid metabolism and its genetic manipulation in Brassica.
2. Overview of the Metabolic Pathway of Carotenoids
2.1. Carotenoid Biosynthesis
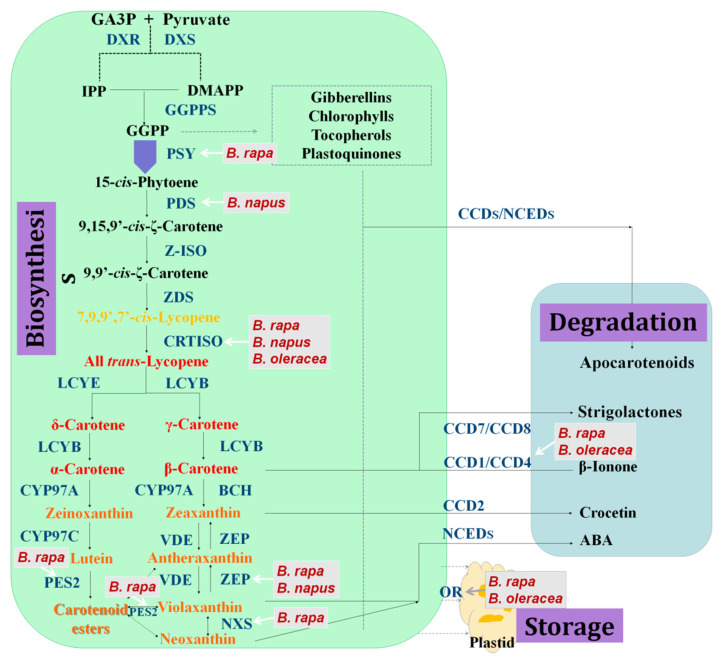
Carotenoid biosynthesis pathway components in higher plants have been gradually clarified through biochemical analysis, classical genetics, and molecular genetics. In plants, the methylerythritol phosphate (MEP) pathway is the major carotenoid production route (Figure 1). It can produce the isopentenyl diphosphate (IPP) and its allyl isomer dimethylallyl diphosphate (DMAPP) [9], which are used as substrates to synthesize the C20 geranylgeranyl diphosphate (GGPP) [10]. GGPP is the most direct precursor of plant carotenoids and participates in the synthesis of the earliest carotenoid in plants [11]. The first compound in the carotenoid biosynthesis pathway, 15-cis-phytoene, is synthesized by the condensation of two GGPP molecules, catalyzed by phytoene synthase (PSY) [12]. Phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS) catalyze a four-step dehydrogenation reaction that produces 9,15,9′-cis-ζ-carotene, which is then isomerized into yellow 9,9′-cis-ζ-carotene by ζ-isomerase (ZISO) [13]. Subsequently, carotenoid isomerase (CRTISO) converts yellow prolycopene into red all-trans lycopene [14]. If lycopene β-cyclase (LCYB) acts on both ends of the lycopene molecule, β-carotene is formed. If one of the ends of the lycopene molecule is affected by lycopene ε-cyclase (LCYE), δ-carotene is formed [15]. Then, δ-carotene can be catalyzed by LYCB into α-carotene. Cytochrome P450 carotene β-hydroxylase (CYP97A) and cytochrome P450 carotene ε-hydroxylase (CYP97C) then catalyze α-carotene to create lutein [16]. In addition, β-carotene hydroxylase (BCH) catalyzes β-carotene to produce β-cryptoxanthin, which is converted into zeaxanthin. Zeaxanthin can be further converted to antherxanthin and then to violaxanthin, both of which are catalyzed by zeaxanthin epoxidase (ZEP) [17]. Violaxanthin can also be reversed to form zeaxanthin under the catalysis of violaxanthin de-epoxidase (VDE) [18], via a process termed the xanthophyll cycle [19], which protects plants from light damage. Neoxanthin is then produced from violaxanthin under the catalysis of neoxanthin synthase (NXS) [20].
Figure 1.
Overview of carotenoid metabolism in Brassica.
2.2. Degradation of Carotenoids
Carotenoid cleavage dioxygenases (CCDs) lyse carotenoids to form products that provide leaves, flowers, and fruit with specific colors and flavors, and produces abscisic acid (ABA) and other plant hormones. A group of enzymes known as carotenoid cleavage oxygenases (CCOs) catalyze the particular enzymatic oxidative degradation of carotenoids. The varieties of apocarotenoid breakdown products are determined by the precise bonds at which CCOs cleave the carotenoids, with substrate specificity. The 9-cis-epoxycarotenoid dioxygenases (NCEDs) and carotenoid cleavage dioxygenases make up the plant CCO family (which are CCDs) [21]. CCDs are divided into a number of subfamilies, including CCD1, CCD2, CCD4, CCD7, and CCD8. Among them, CCD1 is mainly involved in the formation of plant aromatic substances, and can split β-carotene into β-ionone, an important aroma component [22]. CCD4 is mainly involved in the formation of pigment substances in flowers and fruit. The enzymes encoded by CCD7 and CCD8 are located in plastids and are mainly involved in the synthesis of strigolactone. NECDs are rate-limiting enzymes controlling the production of ABA from carotenoids, which mainly comprises cleaving violaxanthin or neoxanthin, thereby forming precursors of ABA [23]. In addition to the precise cleavage mediated by CCDs, nonspecific enzymes (lipoxygenases and peroxidases) and photochemical oxidation contribute to carotenoid degradation. Carotenoid nonspecific oxidation produces unspecific apocarotenoid factors via random cleavage.
Genes identified by forward genetic analysis which are involved in carotenoid metabolism in Brassica were labelled with grey boxes. Enzymes in the carotenoid metabolic pathway were in dark blue. GA3P, Glyceraldehyde 3-phosphate; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GGPP, geranylgeranyl diphosphate; DXS, 1-deoxy- D –xylulose 5-phosphate synthase; DXR, 1-deoxy- D -xylulose 5-phosphate reductoisomerase; GGPPS, GGPP synthase; PSY, phytoene synthase; PDS, phytoene desaturase; Z-ISO, ζ- carotene isomerase; ZDS, ζ-carotene desaturase; CrtISO, carotenoid isomerase; LCYE, lycopene ε-cyclase; LCYB, lycopene β-cyclase; BCH, β-carotene hydroxylase; CYP97A, cytochrome P450 carotene β-hydroxylase; CYP97C, cytochrome P450 carotene ε-hydroxylase; ZEP, zeaxanthin epoxidase; VDE, violaxanthin de-epoxidase; NXS, neoxanthin synthase; CCD, carotenoid cleavage dioxygenase; NCED, 9-cis-epoxycarotenoid dioxygenase; OR, ORANGE protein.
3. Genetic Study of Carotenoid Accumulation in Brassica Crops
Brassica carotenoid metabolism is important for the development of flower color, which is exploited for decorative and landscaping use. Brassica flowers are generally yellow, but can be dark yellow, orange, milky white, or white. Here, we used B. rapa (Chinese cabbage) as an example. The main carotenoids in the yellow petals of Chinese cabbage are violaxanthin and lutein [24], while there is more lutein and β-carotene in the orange petals. β-carotene and lutein contents are regarded as key elements related to the yellow pigmentation in the leaves of Chinese cabbage [25]. The lutein content in the internal leaves of yellow cultivars is greater than that in the internal leaves of orange cultivars. Similar observations were made for the inner yellow leaves compared with the white internal leaves. Collectively, these observations demonstrated that carotenoid profiles are distinctive in petals and leaves, and the carotenoid contents of Chinese cabbage with yellow or orange inner leaves are markedly higher than, and different from, those of common white leaf varieties.
CRTISO catalyzes the isomerization of poly-cis-carotenoids to all-trans-carotenoids. Together with PDS and ZDS, CRTISO is required to synthesize lycopene from phytoene. In B. rapa, the orange pigmentation of flowers and the inner leaves is under the control of the BrCRTISO gene. Using restriction fragment length polymorphism markers, the orange–yellow pigmentation gene (Oy) in Chinese cabbage was first mapped to linkage group 1 [26]. Linkage analysis finally located the candidate gene to the end of A09. Comparisons of the promoter regions of CRTISO promoter revealed insertions/deletions between the two parents, which identified CRTISO as the most likely candidate gene for Br-Oy. After using BC1 backcross population for high-quality mapping, three SNP markers delimited the Br-Oy locus was delimited to a 9.47 kb using three single nucleotide polymorphism (SNP) markers, which contained one functional gene, Bra031539 (BrCRTISO) [27]. The Br-Oy gene has many sequence variations, such as a 90-bp promoter deletion and a 501-bp 3′ end insertion. Meanwhile, a CRTISO mutation was found in an orange Chinese cabbage, which were used to develop molecular markers to differentiate orange from white genotypes [28]. Further study showed that these mutations in BrCRTISO reduced the flux of carotenoid synthesis, leading to the formation of orange leaves in Chinese cabbage. Genes involved in carotenoid metabolism were identified using white flowered varieties. According to genetic research, recessive loci Brwf1 and Brwf2 regulate Chinese cabbage’s white flowers. Insertion/deletion (InDel) and SNP tombstone analysis located Brwf1 in a 49.6-kb region of chromosome A01 comprising nine annotated genes. BrCRTISO (Bra031539) was mapped to a 59.3-kb gap on chromosome A09 comprising 12 known genes and Brwf2 [29]. To fine map the white flower gene BrWF3 in Chinese cabbage, an F2 population was created from the F1 plants of a cross between a white flowered line and a yellow flowered line. BrWF3 was precisely mapped to a 105.6 kb gap. Sequence variation analysis, functional elucidation, and expression profiling demonstrated that the BrWF3 gene was most likely Bra032957, an AtPES2 homolog. Carotenoid compound analysis and transmission electron microscopy revealed that BrWF3 might produce xanthophyll esters, primarily violaxanthin esters, which interfere with chloroplast formation and plastoglobule (PG) generation. The third exon of BrWF3 had an SNP deletion that prevented the protein from functioning properly and prevented PG assembly, accompanied by decreased expression of carotenoid metabolism-related genes [30]. In another study, a natural mutant of flowering Chinese cabbage (B. rapa ssp. chinensis var. parachinensis) with visually distinguishable pale-yellow petals was obtained in farmland. The pale-yellow petal was controlled by a single recessive gene BrPYP [31]. Further study showed that BrPYP was mapped to the same locus of BrWF3; however, different variations were identified. A functional 1148 bp deletion in the promoter region of BrPYP that reduces promoter activity and expression level was identified. In B. napus, mutation of BnaA09.CRTISO and BnaC08.CRTISO using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (CAS9) system caused the petals to turn milky white and the leaves to turn pale yellow. Thus, petal and leaf coloration in B. napus are regulated crucially and redundantly by BnaA09.CRTISO and BnaC08.CRTISO. The carotenoid concentrations in the petals and leaves of the BnaCRTISO double mutant were significantly decreased, according to subsequent observation. The levels of chalcone, a vital component of yellow color, were reduced dramatically in the mutant flower’s petals, yet its levels of lycopene, β-carotene, and α-carotene increased slightly [32]. These findings help explain how carotenoids are produced and how B. napus’s color variation is controlled. Chinese kale (B. oleracea var. alboglabra) contains abundant carotenoids, among which neoxanthin is one of the most important. When BoaCRTISO expression was downregulated in Chinese kale, virtually all carotenoid biosynthesis genes were downregulated, and the leaves turned yellow. The authors found that BoaCRTISO was a photoinduced gene, and a mixture of red, blue, and white light could increase the carotenoid content in plants [33]. Consequently, the CRISPR/Cas9 method was used to target and edit the Chinese kale BoaCRTISO gene. Decreases in the overall and individual carotenoid and chlorophyll levels were observed in the mutants. The color of the mutant changed from green to yellow, suggesting a reduced protective effect of carotenoids toward chlorophyll [34].
The hydroxylated β-rings of zeaxanthin were subjected to subsequent epoxidation by ZEAXANTHIN EPOXIDASE (ZEP) to produce antheraxanthin and later, violaxanthin. In addition to CRTISO, ZEPs were also identified to be widely involved in the coloration of flowers or leaves in different Brassica crops. In a study of the dark yellow petals of Chinese cabbage, bulked segregant RNA (BSR) sequencing combined with competitive allele-specific PCR (KASP) assays fine mapped Br-dyp1 to a 53.6 kb region on chromosome A09. Further expression analysis, functional annotation, and sequence variation assessment identified Bra037130 (BraA09.ZEP) as a potential candidate gene for Br-dyp1. Plants with dark yellow petals had a 679 bp insertion in BraA09.ZEP that created a premature stop codon, leading to ZEP loss-of-function. Loss of ZEP activity destroyed the carotenoid metabolism, and increased accumulation of total carotenoid, and finally changed the petals from yellow to dark yellow of Chinese cabbage. Large amounts of violaxanthin lead to yellow petals in B. napus, while the orange flowers were caused by the accumulation of lutein. In B. napus, the ratio of yellow- to orange-flowered plants was 15:1 in the F2 population and 3:1 in the BC1 population, indicating that two dominant nuclear genes controlled the yellow-flower phenotype [35]. Map-based cloning was used to isolate key genes to reveal the underlying molecular mechanism of the orange-flowered phenotype. Quantitative trait locus (QTL) cloning assigned the change in color from yellow to orange to the loss of BnaC09.ZEP and the deletion of a 1695 bp fragment of BnaA09.ZEP. Further CRISPR/Cas9 and genetic complementation analyses showed that the nullification of BnaA09.ZEP and BnaC09.ZEP contributed to markedly increased lutein levels and a large decrease in violaxanthin levels in petals rather than in leaves [36].
CCDs encompass a superfamily of mononuclear non-heme iron proteins that catalyze the oxygenolytic splitting of alkene bonds in carotenoids to develop apocarotenoid products. β-carotenoid cleavage dioxygenase 4 (CCD4) was reported to be the primary negative switch for seed carotenoids, particularly β-carotene, according to linkage mapping and genome-wide association studies of Arabidopsis carotenoids [37]. In B. napus, positional cloning identified a carotenoid cleavage dioxygenase 4 gene, BnaC3.CCD4, as being responsible for the composition of white or yellow flower color, with white-bloomed B. napus lines having higher expression of BnaC3.CCD4 in their petals. In yellow-flowered varieties, a CACTA-like transposable element 1 (TE1) is embedded in the coding region of BnaC3.CCD4, which disrupts BnaC3.CCD4 expression. Further investigation uncovered that this TE insertion occurred frequently in the BnaC3.CCD4 gene of yellow-flowered B. napus [38]. In B. oleracea, analyses demonstrated that this yellow-white petal characteristic was dependent a single locus on C03, and in 2019, Han mapped the gene to a 207-kb locus, suggesting that the candidate gene was BoCCD4. Further sequence analysis, expression pattern assessment, and functional complementation analyses in B. oleracea accessions showed that BoCCD4 functional failure resulted the yellow petal phenotype. Overexpression of BoCCD4 changed the color of the petals from yellow to white or pale yellow [39]. In cauliflower, the yellow-flower locus was fine-mapped, which identified BoCCD4 as the most likely candidate gene. Further investigation revealed the presence of a novel 10,608 bp CACTA-like transposon that inhibited the function of BoCCD4 [40]. The yellow-petal feature could be induced in a white-petal natural line by BoCCD4 functional complementation. BoCCD4 was observed to be particularly expressed in the petal tissue of white-petal plants, and a genetic study revealed that in carotenoid metabolism, CCD4 homologs might share evolutionarily conserved functions. BoAAO3 is a key enzyme that interacts with BoCCD4 to regulate petal carotenoid deterioration. Likewise, BoCCD4 co-regulates carotenoid metabolism accompanied by two key transcription components, Bo2g151880 (WRKY) and Bo3g024180 (SBP). Together, they regulate carotenoid biosynthesis in petals, which in turn adjusts whether petals are white or yellow [41].
The bioavailability of carotenoids from new and processed snacks heavily depends on their natural deposition form. In cauliflower (B. oleracea var botrytis), a spontaneous semidominant Orange (OR) mutant has a fascinating genetic mutation that results in the accumulation of β-carotene in commonly unpigmented tissues. Using positional cloning, the gene responsible for OR was determined, and functional complementation confirmed this identification in wild-type cauliflower. OR encodes a DnaJ Cys-rich domain-containing plastid-associated protein. The OR allele contains an inserted long terminal repeat retrotransposon, resulting in the OR gene mutation. Analyses of the gene, its output, and the effects of an OR transgene on cells indicated that OR’s function is associated with a biological process that promotes the differentiation of proplastids or other noncolored plastids into chromoplasts for carotene accumulation. Additionally, the study demonstrated that that regulating chromoplast formation is essential for the control of carotenoid production in plants [42]. Regarding leafy Brassica crops, cultivars with golden leafy heads are becoming increasingly recognized. The golden cultivars are abundant in β-carotene and lutein. In comparison with the white line, the β-carotene level was increased by 13.6−fold. Bulked-segregant study sequencing identified BraA09g007080.3C (encoding the ORANGE protein) as the candidate gene. A 4.67 kb long terminal repeat was observed to be inserted in exon three of BrGOLDEN, which resulted in the expression of three alternatively spliced transcripts. Spatiotemporal expression analysis showed that BrGOLDEN might affect the expression levels of carotenoid-related genes [43]. Further sequence analysis revealed that BrGOLDEN was probably transferred into B. rapa through distant hybridization between B. rapa and B. oleracea. In addition, in the first step of carotenoid biosynthesis, orange protein (OR) is known to interact with phytoene synthase (PSY) and is a major post-transcriptional regulator on PSY in Arabidopsis [44].
Additionally, turnip (B. rapa ssp. rapa) is a nutritious and fitness-promoting vegetable, with yellow and white flesh, among which yellow-fleshed turnips have a higher nutritional value. Combined transcriptomic and metabolomic investigations identified that PSY is the important gene that affects carotenoid creation in turnip. High expression of PSY results in yellow turnips rather than mutations in PSY. This suggested that carotenoids might be produced via a post-transcriptional regulatory mechanism.
In addition to CRTISO, ZEP, CCD, and OR, certain other genes were reported to be involved in carotenoid absorption. A mutant with yellowish-white flowers (ywf), which was developed from Zhongshuang 9 (ZS9) using ethyl methane sulfonate mutagenesis was analyzed. The ywf locus displayed a lower petal carotenoid content. A genetic study revealed that a single recessive gene regulated the yellowish-white trait. Bulked segregant analysis sequencing mapped the ywf locus to YWF, encoding phytoene desaturase 3 (PDS3). Moreover, ywf accommodated a C-to-T replacement in the coding region, which caused premature translation termination. RNA-seq and carotenoid constituent investigation showed that in ywf petals, the truncated BnaA08.PDS3 disrupted carotenoid biosynthesis [45]. In kale, the neoxanthin synthase gene (BoaNXS) functions to adjust leaf color, and in BoaNXS overexpressing plants, the color alternated from yellow-green to green, and the total carotenoids and individual carotenoid contents were considerably elevated [46].
4. Evolution of Carotenoid Biosynthesis and Some Key Carotenoid Genes in Brassica
The consumption of Brassica species confers unique health attributes, and they contain high carotenoid levels. The six genetically linked Brassica species share a common evolutionary history and are currently being bred using interspecific hybridization for species improvement [47]. In this background, to increase the nutritional value of Brassica, identifying the genetic connections among Brassica species related to carotenoid accumulation would promote their genetic improvement. The main carotenoids that accumulate in genetically-related Brassica species have been identified. B. rapa was reported to accumulate the highest concentrations of antheraxanthin, lutein, and zeaxanthin. The maximum concentrations of β-carotene and total chlorophyll were detected in B. juncea. B. nigra contained the highest levels of 5,6-epoxylutein and violaxanthin, while B. oleracea had the highest neoxanthin levels. Interestingly, the amphidiploids B. carinata and B. napus were found to contain significantly reduced levels of carotenoids compared with those in the diploid species and B. juncea [8].
Plant whole-genome triplication (WGT) resulted in multiple duplicates of carotenoid biosynthetic genes, most of which retain their syntenic relationships with their A. thaliana orthologs. Taking B. rapa as an example, B. rapa diverged from A. thaliana and its nucleus contains three subgenomes [48]. Gene loss events in the three subgenomes of B. rapa show bias. However, the proportions of carotenoid biosynthetic genes in the respective B. rapa subgenomes were not considerably different from those in the whole genome. Flower color is mostly determined by the presence or deficiency of carotenoid pigments. The production of carotenoids in chromoplasts of petal cells leads to the yellow color of petals. The prevailing flower color of Brassica spp. according to the triangle of U hypothesis is yellow. However, among the subspecies of the B. oleracea cytodeme, some white-flowered varieties are observed. Moreover, white flowers were reported in rapeseed lines created via interspecific hybridization between white-flowered B. oleracea and B. rapa. Herein, we used B. napus as an example to explain the evolution of carotenoids in Brassica. B. napus (2n = 4 = 38, AACC) is an allopolyploid crop plant formed by an interspecific crosses between B. rapa (2n = 2 = 20, AA) and B. oleracea (2n = 2 = 18, CC). Flower pigment divergence was evident in the selfed offspring from a single B. napus parent, varying from white to bright yellow. This variation was assumed to be genetically related because all offspring were identified under controlled environmental conditions. In B. napus and B. oleracea, the carotenoid cleavage dioxygenase 4 gene (CCD4) exhibits the white flower trait. Among B. carinata, B. rapa, and B. oleracea, five distinct alleles of C3.CCD4 were identified. The wild-type (WT) allele was designated as that contained by white-flowered lines of B. rapa and B. oleracea. The four variant alleles comprised two InDels (M2 and M3) and two with TE insertions (M1 and M4), and were contained in yellow-flowered Brassicas: B. oleracea and the allotetraploids B. napus and B. carinata. The color of the petals changed from white to yellow as a result of these variant alleles disrupting C3.CCD4’s function. Interestingly, all yellow-flowered B. napus and B. carinata plants and some yellow-flowered B. oleracea plants are homozygous for M1 or M4, suggesting that the CACTA-like TE insertion into C3.CCD4 occurred before B. napus and B. carinata allopolyploidization. The two InDels were only detected in B. oleracea BolC3.CCD4, but not in the CCD4 genes of B. napus or B. carinata. This suggested that mutations M2 and M3 occurred outside of the centers of origin of B. napus and B. carinata, making no contribution to their speciation. Using AtCCD4 as the outgroup, a phylogenetic tree of the five Brassica C3.CCD4 alleles was constructed, which suggested that the WT allele was the ancestral and functional type, and the others were loss-of-function types. This prompted the conclusion that the B. oleracea flower color diversified into yellow and white flowers prior to the emergence of the amphidiploids B. napus and B. carinata, resulting from CACTA-like TE insertions in the B. oleracea BolC3.CCD4 gene. Thus, we presumed that the development of blossom color in B. carinata, B. napus, and B. oleracea is governed by the evolution of CCD4 in the Brassica C genome [38].
In addition, the dynamic expression pattern of genes played a substantial role in the development of carotenoid biosynthesis during evolution. Brassica diploids were found to contain two ZEP homologs, while Brassica allotetraploids were found to have four. Genetic analyses allowed the assignment of Brassica ZEPs to subclades based on their localization to the A or C genome. ZEPs translated from genes located on chromosomeA07/C07 formed a subclade that was most closely related to Arabidopsis ZEP proteins, forming a sister cluster to the ZEPs located on Brassica chromosomes A09/C09. These results suggested that Brassica ZEPs diverged prior to the allopolyploidization event. Furthermore, BnaA09.ZEP and BnaC09.ZEP were reported to be mostly expressed in floral tissues, while homologous BnaA07.ZEP and BnaC07.ZEP were mainly expressed in leaves. These observations revealed that BnaZEPs were redundant and experienced tissue-specific diversification [36]. These discoveries, taken together with earlier data for CCD4, revealed the central role exerted by gene duplication and tissue-specific expression in the evolution of carotenoid accumulation in Brassica. Indeed, accumulating evidence demonstrated that the evolution of tissue-specific expression following gene duplication might be unique to plant carotenoid metabolism. In tomatoes, many carotenoid-metabolic genes, such as GGPPS, PSY, LCYB, and BCH, are present in multiple copies: one copy is preferentially expressed in green tissues, and the other in flowers or fruit.
5. Biotechnological Implications of Carotenoid Genes Identified by QTL-Mapping
Research has sought to develop crops with higher carotenoid contents because of their status as essential phytonutrients. Vegetable yield and quality are affected by cultivars, harvesting dates, climate, location, and conditions. However, conventional methods to improve quality, e.g., chemical spraying and hybridization, are often lengthy and ineffective. Plant disease resistance and yield have been improved using the efficient and convenient CRISPR/Cas9 system [49]. Such technology will drive innovative strategies for metabolic engineering to produce crops with higher nutritional value. Improved crop varieties with modified carotenoid pathways will have a wide application in horticulture.
The breeding of plants with different colors has been facilitated by the cloning and functional analysis of flower pigment regulatory gene. Indeed, certain transgenic ornamental plant varieties with modified flower color have been developed. Agro-transformation of B. napus has successfully altered its flower color. For example, B. napus with yellow flowers was transformed with PAP2 (encoding phytochrome-associated protein 2) controlled by a petal-specific promoter, which increased the petal anthocyanin content, generating red flowers [50]. Moreover, both BnaA09.ZEP and BnaC09.ZEP were mutated using CRISPR/Cas9, which changed the profile of carotenoids in the petals of the mutant plants, without disturbing their growth and development. The CRISPR/Cas9 system was also used to edit B. napus BnaA09.CRTISO and BnaC08.CRTISO, resulting in a mutant phenotype of yellowish leaves and creamy white petals in the double mutant plants. The carotenoid isomerase gene (BoaCRTISO) of Chinese kale was also targeted and edited, which decreased the chlorophyll content and the total and individual carotenoid levels. These studies showed that the manipulation of known genes that are genetically determined by QTL mapping represents an auspicious approach to alter the appearance of flowers and to breed Brassica crop varieties with different colored flowers with high ornamental worth.
6. Conclusions and Perspective
Consumption of carotenoids has been associated with various health benefits, including a reduced risk of age-related macular degeneration and cataract, some cancers and coronary heart disease [51]. There is also some evidence of a beneficial effect on cognitive function [52]. The content and composition of carotenoids have vital functions in the nutrition and quality of Brassica crops [53]. However, humans cannot synthesize carotenoids and must ingest them in food or via supplementation. In recent times, large numbers of genes and the mechanisms that regulate carotenoid biosynthesis and accumulation have been discovered (Figure 1 and Table 1), facilitating the smooth breeding of Brassica crop [54]. Here, we reviewed recent progress regarding carotenoids of Brassica from the perspective of forward genetics. We believe that this review provides biotechnological implications and some new perspectives on carotenoid research in Brassica, as well as other horticultural crops. However, the regulation of carotenoids in higher plants is complex and multi-faceted; thus, more effort should be made to elucidate the mechanisms of carotenoid function.
Table 1.
Enzymes and genes that regulate carotenoid accumulation in Brassica crops.
| Regulated Genes | Species | Major Changes | Genes (Gene Accession) | Color Change/Tissue | Reference |
|---|---|---|---|---|---|
| CRTISO | B. rapa | The mutant Br-oy protein cannot convert prolycopene to all- trans -lycopene. | BrOy (Bra031539) | white/yellow → orange inner leaf | [26,27] |
| The loss of BrCRTISO function leads to the accumulation of prolycopene. | Br-oy or BrCRTISO (Bra031539) | ||||
| Loss of BrWF3 function interferes with plastoglobules assembly and decreases expression levels of genes associated with carotenoid metabolism. | Brwf3 (Bra032957) | yellow → white/flower petal | [30] | ||
| The key factor for the pale-yellow color of petals was the decrease in esterified carotenoid content due to the loss of PYP function. | BrPYP (BraA02g037170.3C) | yellow → pale-yellow/flower petal | [31] | ||
| B. napus | The contents of carotenoids in petals and leaves of BnaCRTISO double mutant were reduced. In petals, the content of chalcone decreased, the content of some carotene (lycopene, α-carotene, γ-carotene) increased. |
BnaA09. CRTISO (BnaA09g49740D) BnaC08. CRTISO (BnaC08g44970D) |
yellow → milky white flower petals yellow → pale yellow leaves |
[32] | |
| B.oleracea | Carotenoid and chlorophyll levels were reduced in the mutant of BoaCRTISO. | BoaCRTISO (GenBank accession MN810158) | green → yellowing leaves | [33,34] | |
| ZEP | B. rapa | The loss of function of ZEP disrupts the metabolism of carotenoids and leads to the increase in total carotenoid accumulation. | Br-dyp1 (Bra037130) | yellow → dark yellow flower petal | [35] |
| B. napus | The abolishment of both genes led to a substantial increase in lutein content and a sharp decline in violaxanthin content in petals. |
BnaA09. ZEP (BnaA09g07610D) BnaC09. ZEP (BnaC07g16350D) |
yellow → orange flower petal | [36] | |
| CCD4 | B. napus | In yellow petals, a large amount of α-carotene, α-cryptoxanthin, β-cryptoxanthin, violaxanthin, 9-cis-violaxanthin, lutein, and cis-neoxanthinwere accumulated. | BnaC3.CCD4 (Bol029878) | white → yellow flower petal | [38] |
| B.oleracea | Not available. | BoCCD4 (Bol029878) | white/pale yellow → yellow flower petal | [39] | |
| These key genes may interact with BoCCD4 to jointly regulate carotenoid biosynthesis in petals. |
WRKY (Bo2g151880) SBP (Bo3g024180) |
[41] | |||
| OR | B.oleracea | The OR gene mutation confers the accumulation of high levels of β-carotene in various tissues normally devoid of carotenoids. | OR (GenBank accession DQ482460) | white → orange | [42] |
| B. rapa | The BrGOLDEN lines are rich in β-carotene and lutein. | BrGOLDEN (BraA09g007080.3C) | golden → light yellow inner leaf | [43] |
Acknowledgments
We gratefully acknowledge the support of our colleagues for critical review of the manuscript. We apologize to the colleagues whose work could not be discussed due to space limitations.
Author Contributions
F.S., L.C. and L.S. conceived the study and wrote the manuscript. T.S., F.Z., S.Y. and Y.D. discussed the focus, structure, and content of the review. Y.Y., D.Z., X.Z., W.W., P.L. and X.X. discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was funded by grants from the Innovation and Capacity-Building Project of BAAFS (KJCX20200204 and KYCX20210427), Beijing Joint Research Program for Germplasm Innovation and New Variety Breeding (G20220628003-01), and the Outstanding Scientists Training Program of Beijing Academy of Agriculture Forestry Sciences (JKZX201906).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.George B. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158699. doi: 10.1016/j.bbalip.2020.158699. [DOI] [PubMed] [Google Scholar]
- 2.Ho J., Kish E., Méndez-Hernández D.D., WongCarter K., Pillai S., Kodis G., Niklas J., Poluektov O.G., Gust D., Moore T.A., et al. Triplet-triplet energy transfer in artificial and natural photosynthetic antennas. Proc. Natl. Acad. Sci. USA. 2017;114:5513–5521. doi: 10.1073/pnas.1614857114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazzonelli C.I. Carotenoids in nature: Insights from plants and beyond. Funct. Plant Biol. 2011;38:833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 4.Palmer A.C., Healy K., Barffour M.A., Siamusantu W., Chileshe J., Schulze K.J., West K.P., Labrique A.B. Provitamin A Carotenoid-Biofortified Maize Consumption Increases Pupillary Responsiveness among Zambian Children in a Randomized Controlled Trial. J. Nutr. 2016;146:2551–2558. doi: 10.3945/jn.116.239202. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H.L., Rong B.Y., Rong L., Zhen X.H., Cheng C.H., Zhong H.Z., Le M. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients. 2014;6:452–465. doi: 10.3390/nu6010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M.X., Xiong F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules. 2020;25:5342. doi: 10.3390/molecules25225342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alós E., Rodrigo M.J., Zacarias L. Manipulation of Carotenoid Content in Plants to Improve Human Health. Sub-Cell. Biochem. 2016;79:311–343. doi: 10.1007/978-3-319-39126-7_12. [DOI] [PubMed] [Google Scholar]
- 8.Carl E.S., David E.K., Dean A.K., Scott M.J. Genetic variation in carotenoid concentrations among diploid and amphidiploid rapid-cycling Brassica species. HortScience. 2007;42:461–465. doi: 10.21273/HORTSCI.42.3.461. [DOI] [Google Scholar]
- 9.Cheng L., Charles A.S., Anthony J.S. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020;10:e00118. doi: 10.1016/j.mec.2019.e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramley P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 2002;53:2107–2113. doi: 10.1093/jxb/erf059. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Sola M.Á., Rodríguez-Concepción M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. Arab. Book. 2012;10:e0158. doi: 10.1199/tab.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barj M.V., Ezquerro M., Beretta S., Diretto G., FlorezSarasa I., Feixes E., Fiore A., Karlova R., Fernie A.R., Beekwilder J., et al. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol. 2021;231:255–272. doi: 10.1111/nph.17283. [DOI] [PubMed] [Google Scholar]
- 13.Hermanns A.S., Zhou X.S., Xu Q., Tadmor Y., Li L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020;6:343–360. doi: 10.1016/j.hpj.2020.10.002. [DOI] [Google Scholar]
- 14.Breitenbach J., Sandmann G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta. 2005;220:785–793. doi: 10.1007/s00425-004-1395-2. [DOI] [PubMed] [Google Scholar]
- 15.Francis X.C. Regulation of carotenoid synthesis and accumulation in plants. Pure Appl. Chem. 2013;74:1409–1417. doi: 10.1351/pac200274081409. [DOI] [Google Scholar]
- 16.Gupta P., Hirschberg J. The Genetic Components of a Natural Color Palette: A Comprehensive List of Carotenoid Pathway Mutations in Plants. Front. Plant Sci. 2022;12:806184. doi: 10.3389/fpls.2021.806184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luan Y.T., Fu X.M., Lu P.J., Grierson D., Xu C.J. Molecular Mechanisms Determining the Differential Accumulation of Carotenoids in Plant Species and Varieties. Crit. Rev. Plant Sci. 2020;39:125–139. doi: 10.1080/07352689.2020.1768350. [DOI] [Google Scholar]
- 18.Demmig A.B., Gilmore A.M., Adams W.W. Carotenoids 3: In vivo function of carotenoids in higher plants. FASEB J. 1996;10:403–412. doi: 10.1096/fasebj.10.4.8647339. [DOI] [PubMed] [Google Scholar]
- 19.Beatrycze N., Wojciech S., Kazimierz S. New transgenic line of Arabidopsis thaliana with partly disabled zeaxanthin epoxidase activity displays changed carotenoid composition, xanthophyll cycle activity and non-photochemical quenching kinetics. J. Plant Physiol. 2009;166:1045–1056. doi: 10.1016/j.jplph.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Wei Z., Arazi T., Hod N., Zohar M., Isaacson T., Doron-Faigenboim A., Reznik N., Yedidia I. Transcriptome Profiling of Ornithogalum dubium Leaves and Flowers to Identify Key Carotenoid Genes for CRISPR Gene Editing. Plants. 2020;9:540. doi: 10.3390/plants9040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Li J., Zhang J., Chen D., Zhang H., Liu C., Qin G. Genome-Wide Identification and Expression Analysis of the Carotenoid Cleavage Oxygenase Gene Family in Five Rosaceae Species. Plant Mol. Biol. Rep. 2021;39:739–751. doi: 10.1007/s11105-021-01284-9. [DOI] [Google Scholar]
- 22.Baldermann S., Kato M., Kurosawa M., Kurobayashi Y., Fujita A., Fleischmann P., Watanabe N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010;61:2967–2977. doi: 10.1093/jxb/erq123. [DOI] [PubMed] [Google Scholar]
- 23.Song H.X., Lu Q., Hou L.P., Li M.L. The genes crucial to carotenoid metabolism under elevated CO2 levels in carrot (Daucus carota L.) Sci. Rep. 2021;11:12073. doi: 10.1038/s41598-021-91522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N., Ma X.M., Li R., Xue Y.H., Sun Y.S., Nie S.S., Zhang L.G. Transcriptome-based analysis of carotenoid accumulation-related gene expression in petals of Chinese cabbage (Brassica rapa L.) 3 Biotech. 2019;9:274. doi: 10.1007/s13205-019-1813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung H.J., Manoharan R.K., Park J.I., Chung M.Y., Lee J., Lim Y.P., Hur Y., Nou I.S. Identification of Yellow Pigmentation Genes in Brassica rapa ssp. pekinensis Using Br300 Microarray. Int. J. Genom. 2014;2014:204969. doi: 10.1155/2014/204969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etsuo M., Chika Y., Michio O., Motohisa T. Linkage analysis of RFLP markers for clubroot resistance and pigmentation in Chinese cabbage (Brassica rapa ssp. pekinensis) Euphytica. 1998;104:79–86. doi: 10.1023/A:1018370418201. [DOI] [Google Scholar]
- 27.Su T.B., Yu S.C., Wang J., Zhang F.L., Yu Y.J., Zhang D.S., Zhao X.Y., Wang W.H. Loss of Function of the Carotenoid Isomerase Gene BrCRTISO Confers Orange Color to the Inner Leaves of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Plant Mol. Biol. Report. 2015;33:648–659. doi: 10.1007/s11105-014-0779-0. [DOI] [Google Scholar]
- 28.Seohee L., Sangchoon L., Donghae B., Dongyoung L., Jeeyoung P., Jonghoon L., Hyunoh L., Sanghyun S., Taejin Y. Association of molecular markers derived from the BrCRISTO1 gene with prolycopene-enriched orange-colored leaves in Brassica rapa. Theor. Appl. Genet. 2014;127:179–191. doi: 10.1007/s00122-013-2209-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N., Chen L., Ma S., Wang R.F., He Q., Tian M., Zhang L.G. Fine mapping and candidate gene analysis of the white flower gene Brwf in Chinese cabbage (Brassica rapa L.) Sci. Rep. 2020;10:6080. doi: 10.1038/s41598-020-63165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S.J., Tian X.X., Wang Z.Y., Wei X.C., Zhao Y.Y., Su H.N., Zhao X.B., Tian B.M., Yuan Y.X., Zhang X.W. Fine Mapping and Candidate Gene Identification of a White Flower Gene BrWF3 in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Front. Plant Sci. 2021;12:646222. doi: 10.3389/fpls.2021.646222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P., Lv S., Zhang D., Su T., Xin X., Wang W., Zhao X., Yu Y., Zhang Y., Yu S., et al. The Carotenoid Esterification Gene BrPYP Controls Pale-Yellow Petal Color in Flowering Chinese Cabbage (Brassica rapa L. subsp. parachinensis) Front. Plant Sci. 2022;13:844140. doi: 10.3389/fpls.2022.844140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H.L., Yu K.D., Amoo O., Yu Y.L., Guo M.X., Deng S.Y., Li M.T., Hu L.M., Wang J.Z., Fan C.C., et al. Site-Directed Mutagenesis of the Carotenoid Isomerase Gene BnaCRTISO Alters the Color of Petals and Leaves in Brassica napus L. Front. Plant Sci. 2022;13:801456. doi: 10.3389/fpls.2022.801456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M., Zhang F., Yuan Q., Lin P.X., Zheng H., Liang S., Jian Y., Miao H.Y., Li H.X., Wang Q.M., et al. Characterization of BoaCRTISO Reveals Its Role in Carotenoid Biosynthesis in Chinese Kale. Front. Plant Sci. 2021;12:662684. doi: 10.3389/fpls.2021.662684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bo S., Min J., Hao Z., Yue J., Wen L.H., Qiao Y., Ai H.Z., Qing C., Yun T.Z., Yuan X.L., et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020;7:94–102. doi: 10.1038/s41438-020-00379-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S.J., Liu H.L., Zhao Y.Y., Su H.N., Wei X.C., Wang Z.Y., Zhao X.B., Zhang X.W., Yuan Y.X. Map-Based Cloning and Characterization of Br-dyp1, a Gene Conferring Dark Yellow Petal Color Trait in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Front. Plant Sci. 2022;13:841328. doi: 10.3389/fpls.2022.841328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y.J., Ye S.H., Yuan G.G., Ma X.W., Heng S.P., Yi B., Ma C.Z., Shen J.X., Tu J.X., Fu T.D., et al. Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers. Plant J. 2020;104:932–949. doi: 10.1111/tpj.14970. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez J.S., Ha S.H., Magallanes L.M., Gilliland L.U., Zhou A., Lipka A.E., Nguyen Y.N., Angelovici R., Lin H.N., Cepela J., et al. Carotenoid cleavage dioxygenase4 is a negative regulator of β-carotene content in Arabidopsis seeds. Plant Cell. 2013;25:4812–4826. doi: 10.1105/tpc.113.119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Liu C., Wang Y.Q., Yao X., Wang F., Wu J.S., King G.J., Liu K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015;206:1513–1526. doi: 10.1111/nph.13335. [DOI] [PubMed] [Google Scholar]
- 39.Han F., Cui H., Zhang B., Liu X., Yang L., Zhuang M., Lv H., Li Z., Wang Y., Fang Z., et al. Map-based cloning and characterization of BoCCD4, a gene responsible for white/yellow petal color in B. oleracea. BMC Genom. 2019;20:242. doi: 10.1186/s12864-019-5596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan C., Huang Y., Liu Z., Guo F., Jiao Z., Yang W., Zhu F., Qiu Z. Rapid identification of yellow-flowered gene Bofc in cauliflower (Brassica oleracea var. botrytis) by bulked segregant analysis and whole-genome resequencing. Euphytica. 2020;216:1348–1351. doi: 10.1007/s10681-020-2560-9. [DOI] [Google Scholar]
- 41.Zhang B., Wang J., Chen L., Ren W.J., Han F.Q., Fang Z.Y., Yang L.M., Zhuang M., Lv H.H., Wang Y., et al. Transcriptome Analysis Reveals Key Genes and Pathways Associated with the Petal Color Formation in Cabbage (Brassica oleracea L. var. capitata) Int. J. Mol. Sci. 2022;23:6656. doi: 10.3390/ijms23126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S., Van E.J., Zhou X.J., Lopez A.B., O’Halloran D.M., Cosman K.M., Conlin B.J., Paolillo D.J., Garvin D.F., Vrebalov J., et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell. 2006;18:3594–3605. doi: 10.1105/tpc.106.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Zhang S.F., Dai Y., Wang S.X., Wang C.G., Li F., Zhang H., Chen G.H., Yuan L.Y., Hou J.F., et al. Mapping and Validation of BrGOLDEN: A Dominant Gene Regulating Carotenoid Accumulation in Brassica rapa. Int. J. Mol. Sci. 2022;23:12442. doi: 10.3390/ijms232012442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuto O., Miho T., Atsushi S., Norihiko M., Hiroshi S. Orange protein, phytoene synthase regulator, has protein disulfide reductase activity. Plant Signal. Behav. 2022;17:2072094. doi: 10.1080/15592324.2022.2072094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao C.J., Bin S.L., Xie M.L., Shi M.J., Dong Z.X., Yang L., Cheng X.H., Liu Y.Y., Bai Z.T., Xiang Y., et al. Mutation of the PHYTOENE DESATURASE 3 gene causes yellowish-white petals in Brassica napus. Crop J. 2021;9:1124–1134. doi: 10.1016/j.cj.2020.10.012. [DOI] [Google Scholar]
- 46.Jian Y., Zhang C.L., Wang Y.T., Li Z.Q., Chen J., Zhou W.T., Huang W.L., Jiang M., Zheng H., Li M.Y., et al. Characterization of the Role of the Neoxanthin Synthase Gene BoaNXS in Carotenoid Biosynthesis in Chinese Kale. Genes. 2021;12:1122. doi: 10.3390/genes12081122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elvis K., Daniela Q.M., Elizabeth I.K., Paula V.T., Annaliese S.M. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019;1:e190007. doi: 10.20900/cbgg20190007. [DOI] [Google Scholar]
- 48.Wang X.W., Wang H.Z., Wang J., Sun R.F., Wu J., Liu S.Y., Bai Y.Q., Mun J.H., Bancroft I., Cheng F., et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011;43:1035–1039. doi: 10.1038/ng.919. [DOI] [PubMed] [Google Scholar]
- 49.Raheeba T.N., Shaheen K.J., Farooq A.B., Tariq R.R., Asha N., Altaf A.W., Rehana A., Nahida A., Moneesa B., Ishfaq M.S., et al. Review on “Crispr-CAS9—A Genome editing tools for plant disease management”. Plant Cell Biotechnol. Mol. Biol. 2022;23:1–14. [Google Scholar]
- 50.Fu H., Chao H.B., Zhao X.J., Wang H.Y., Li H.X., Zhao W.G., Sun T., Li M.T., Huang J.Y. Anthocyanins identification and transcriptional regulation of anthocyanin biosynthesis in purple Brassica napus. Plant Mol. Biol. 2022;110:53–68. doi: 10.1007/s11103-022-01285-6. [DOI] [PubMed] [Google Scholar]
- 51.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017;174:1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012;96:1161S–1165S. doi: 10.3945/ajcn.112.034611. [DOI] [PubMed] [Google Scholar]
- 53.Cao W.X., Wang P., Yang L.M., Fang Z.Y., Zhang Y.Y., Zhuang M., Lv H.H., Wang Y., Ji J.L. Carotenoid Biosynthetic Genes in Cabbage: Genome-Wide Identification, Evolution, and Expression Analysis. Genes. 2021;12:2027. doi: 10.3390/genes12122027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pham A.T., Jae K.K., Jeongyeo L., Woo T.P., Do Y.K., Yeon B.K., Haeng H.K., Hye R.K. Analysis of carotenoid accumulation and expression of carotenoid biosynthesis genes in different organs of Chinese cabbage (Brassica rapa subsp. pekinensis) EXCLI J. 2012;11:508–516. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the study are available from the corresponding author upon request.