Abstract
Among conifer families, Podocarpaceae is the second largest, with amazing diversity and functional traits, and it is the dominant Southern Hemisphere conifer family. However, comprehensive studies on diversity, distribution, systematic and ecophysiological aspects of the Podocarpaceae are sparse. We aim to outline and evaluate the current and past diversity, distribution, systematics, ecophysiological adaptations, endemism, and conservation status of podocarps. We analyzed data on the diversity and distribution of living and extinct macrofossil taxa and combined it with genetic data to reconstruct an updated phylogeny and understand historical biogeography. Podocarpaceae today contains 20 genera and approximately 219 taxa (201 species, 2 subspecies, 14 varieties and 2 hybrids) placed in three clades, plus a paraphyletic group/grade of four distinct genera. Macrofossil records show the presence of more than 100 podocarp taxa globally, dominantly from the Eocene–Miocene. Australasia (New Caledonia, Tasmania, New Zealand, and Malesia) is the hotspot of living podocarps diversity. Podocarps also show remarkable adaptations from broad to scale leaves, fleshy seed cones, animal dispersal, shrubs to large trees, from lowland to alpine regions and rheophyte to a parasite (including the only parasitic gymnosperm—Parasitaxus) and a complex pattern of seed and leaf functional trait evolution.
Keywords: conservation, conifers, climate change, fossils, historical biogeography, IUCN red list, paleo-endemism, physiology, phylogenetics
1. Introduction
Conifers are economically and ecologically important, form extensive forests in both Hemispheres and are currently the most diverse gymnosperms. There are seven conifer families (Araucariaceae, Cupressaceae, Pinaceae, Podocarpaceae, Sciadopityaceae, Cephalotaxaceae and Taxaceae), including 72 genera and approximately 702 species [1]. They are estimated to have evolved in the late Devonian from progymnosperms, and then dominated the Mesozoic Era [2,3,4]. Leslie et al. [5] investigated the evolutionary dynamics of conifers on a hemispheric scale based on molecular studies of 489 species and concluded that extant conifers have diverged in the Neogene with older splits in the Southern Hemisphere. Pinaceae and Cupressaceae have their main distribution in the temperate and subtropical regions of the Northern Hemisphere, while the Southern Hemisphere conifers are dominated by the Araucariaceae, Podocarpaceae and the Callitroideae of the Cupressaceae. The podocarps are monoecious and dioecious evergreen trees, shrubs, and subshrubs with mostly spirally arranged leaves and fleshy cones [6]. They are morphologically highly diverse [7,8]. Although the ecological and environmental variation (mostly rainforest and wet montane) is restricted, the morphological variation in leaves and seed cones is very high [9,10]. The extant and extinct taxa present in Australasia and South America show the wider distribution of the family across Gondwana in the past. Phylogenetically, the Podocarpaceae are closest to the Araucariaceae, Sciadopityaceae and Taxaceae [11,12]. Podocarps are significant both ecologically and economically and are a vital component of global forests and biodiversity [4,13]. The Podocarpaceae are very important from an evolutionary and systematic point of view due to their remarkable eco-physiological adaptations as compared to other conifer families, and they can provide us with valuable information on evolution and response to climate change [4,14]. Podocarps provide an exceptional opportunity for the understanding of comparative diversification processes, the evolution of different functional traits and ecophysiological adaptations. However, comprehensive studies are lacking on the different taxonomic, phylogenetic, ecological, biogeographic, and evolutionary aspects of most Podocarpaceae due to fragmented data on living species, sparse fossil records, difficulty in sampling and ecological data collection and less attraction compared to other conifer families. Many species described from Papua New Guinea, Malaysia, Indonesia, and New Caledonia are based on single records, and these areas remain under-explored. To evaluate these different aspects and several other key features, updated checklists for living and extinct taxa, phylogenetic analysis and ecophysiological adaptation research is required.
In this study, we evaluate the diversity, distribution, taxonomy, phylogeny, ecophysiology, and ecology of podocarps with the following aims: 1. To tabulate updated podocarp checklists for both extant and extinct taxa. 2. To reconstruct a new dated phylogeny of Podocarpaceae using relevant macrofossil records. 3. To assess the historical overview of taxonomic classifications of podocarps. 4. To discuss the diversity and historical biogeography. 5. To consider ecophysiological adaptations and threats.
2. Material and Methods
2.1. Phylogenetic Studies
A new, dated phylogenetic tree was produced for the Podocarpaceae. For phylogenetic analysis, DNA sequences of Podocarpaceae and Araucariaceae were sourced from GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 25 January 2021). The sequences were cleaned and six markers, rbcL, matK, trnL-trnF, NEEDLY, PHYP and ITS species, were selected based on alignment confidence and availability of sequences. The final concatenated alignment consists of 190 taxa with 14 taxa as an out-group. The data were analyzed with BEAST version 2.6.3 at the CIPRES Science Gateway [15], set to run an uncorrelated lognormal relaxed clock and a GTR+I+G substitution model [16]. For calibration of the tree, we used the 20 oldest unequivocal macrofossil records (see supplementary file Table S1). Sixteen fossil constraints were assigned to the Podocarpaceae, two were assigned to the Araucariaceae and two were assigned to other conifers. The Fossil Birth–Death (FBD) model [17] was used as the tree prior, which imposes a time structure on the tree, while accounting for uncertainty in the placement of the fossil data by allowing all plausible placements for the fossil taxon on the extant tree [18].
2.2. Updated Checklist, Distribution, and IUCN Conservation Status of Podocarps
An updated checklist of all podocarp species was compiled based on the available literature [4], herbarium specimen observations and online databases, e.g., The Gymnosperm Database [19]; Global Biodiversity Information Facility-GBIF [20]; plants of the world online [21]; Australasian Virtual Herbarium-AVH [22]; Flora of China [23].
2.3. Distribution Data Analysis
The distribution of the species was analyzed in PC-ORD [24]. The Cluster Analysis (CA) and the Two-Way Cluster Analyses (TWCA) were used to identify significant and species-rich countries using Sorensen measures, based on presence/absence data.
2.4. Updated Checklist for Macrofossils of Extant Genera of Podocarpaceae
A checklist of fossil podocarps species belonging to extant genera was compiled using published literature and an online database Fossilworks [25].
3. Results
3.1. Phylogenetic Relationships
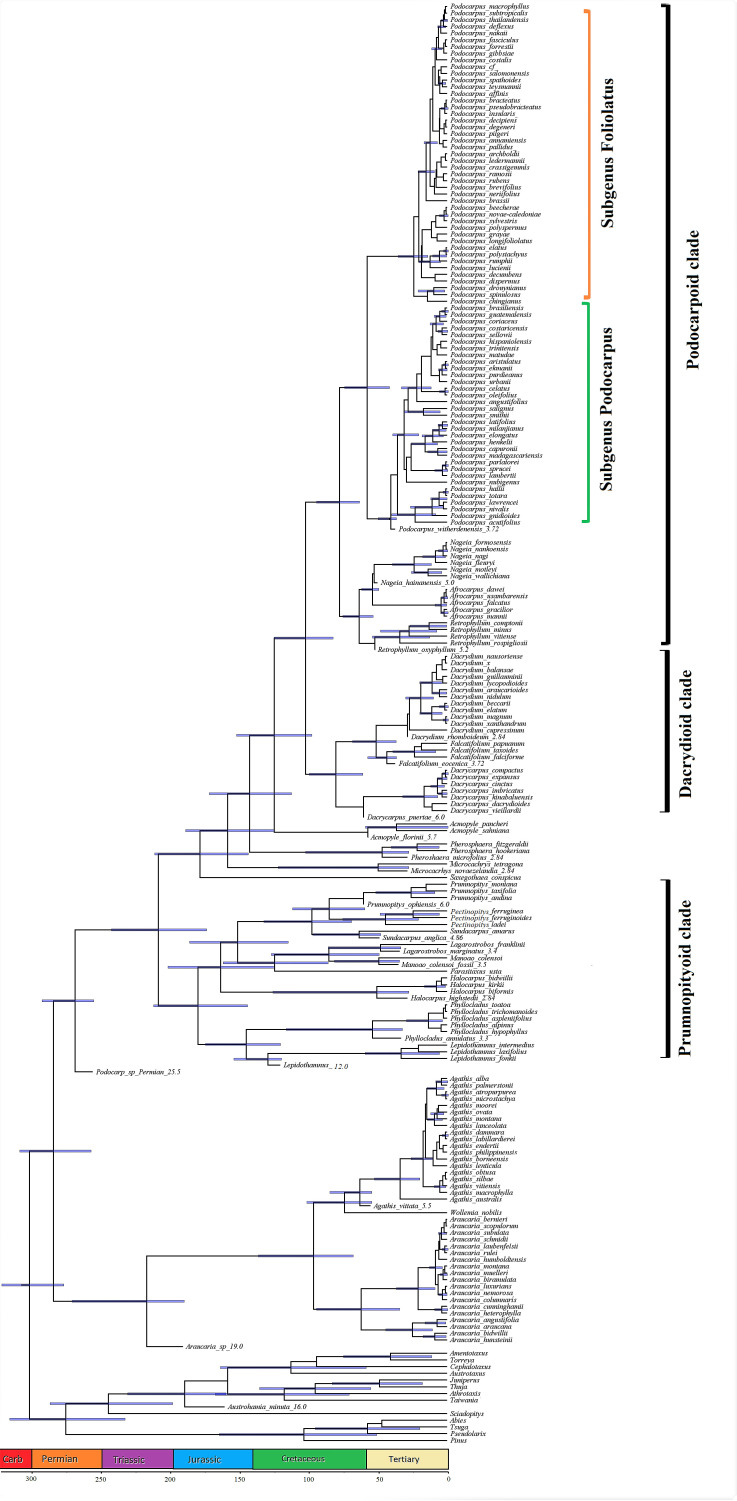
The fossil-calibrated phylogenetic tree under the FBD model indicates that the Podocarpaceae and Araucariaceae diverged around the early–mid Permian and the extant podocarp clades split during the mid–late Triassic. The extant podocarp genera have an estimated divergence time of the early Jurassic. The extant podocarp species predominantly show recent diversification from the Oligocene onwards. The phylogeny of the Podocarpaceae shows three major clades, I. Podocarpoid, II. Dacrydioid, III. Prumnopityoid, as well as a distinctive paraphyletic group/grade (Figure 1).
Figure 1.
The phylogenetic relationships and divergence time of the 20 extant podocarp genera within Podocarpaceae. Blue node bars indicate the 95% highest posterior density divergence time estimates for the corresponding node. Branch lengths are proportional to time (Ma, millions of years).
I. Podocarpoid clade—four genera, i.e., Afrocarpus, Nageia, Podocarpus and Retrophyllum. The suggested crown age for the Podocarpoid clade is approximately 75 Ma (54–85 Ma). The phylogeny also supports the split of Podocarpus into two subgenera i.e., Foliolatus and Podocarpus.
II. Dacrydioid clade—three genera, i.e., Dacrydium, Dacrycarpus and Falcatifolium. The suggested crown age for the Dacrydioid clade is approximately 75 Ma (54–95 Ma).
III. Prumnopityoid clade—nine genera, i.e., Lepidothamnus, Phyllocladus, Manoao, Lagarostrobos, Parasitaxus, Halocarpus, Sundacarpus, Pectinopitys and Prumnopitys. The newly dated phylogeny shows that the crown age for the Prumnopityoid clade is approximately 175 Ma (150–210 Ma).
IV. Paraphyletic group/grade—four genera, i.e., Acmopyle, Pherosphaera, Microcachrys and Saxegothaea.
3.2. Diversity at Genus Level and Distribution
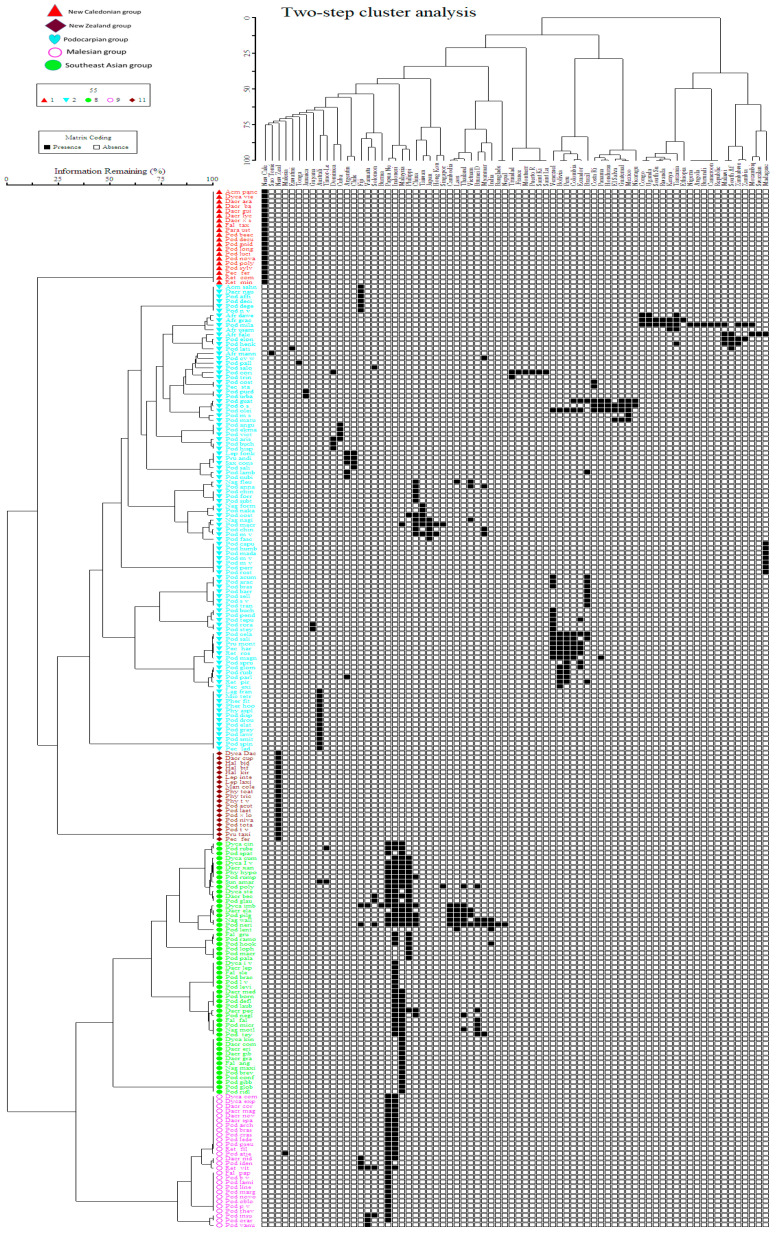
Currently, in the Podocarpaceae, 20 genera and approximately 219 taxa (201 species, 2 subspecies, 14 varieties and 2 hybrids) are recognized (Table 1). Podocarpus is the most speciose genus with approximately 120 species distributed in approximately 70 countries. Two-way cluster analysis of Podocarpaceae species distribution shows five major groups, I. New Caledonian group, II. New Zealand group, III. Malesian group, IV. Southeast Asian group and V. Podocarpian group, widely distributed across several countries (Figure 2). Some of the widely distributed species are Afrocarpus gracilior (7 countries) and A. falcatus (5 countries), Dacrycarpus imbricatus (11 countries), Dacrydium elatum (7 countries), Dacrydium pectinatum (5 countries), Nageia wallichiana (11 countries), Podocarpus coriaceus (7 countries), P. guatemalensis (9 countries), P. milanjianus (15 countries), P. neriifolius (16 countries), P. oleifolius (11 countries), P. pilgeri (9 countries), P. polystachyus (7 countries) and Sundacarpus amarus (6 countries). The current species diversity and distribution is listed in Table 1 and summarized here:
Table 1.
An updated checklist of living podocarp taxa.
| S# | Name | Synonyms | Common Name | Distribution in World | IUCN Status |
|---|---|---|---|---|---|
| 1 | Acmopyle pancheri (Brongn. and Gris) Pilg. | Acmopyle alba, Dacrydium pancheri, Nageia pancheri, Podocarpus pectinatus | New Caledonian acmopyle, Pancher’s acmopyle | New Caledonia | NT |
| 2 | Acmopyle sahniana Buchholz and N.E. Gray | Parasitaxus vodonaivalui | Fijian acmopyle | Fiji (Namosi and near Mt Tomanivi.) | CR |
| 3 | Afrocarpus dawei (Stapf) C.N.Page | Afrocarpus mannii subsp. dawei, Nageia mannii var. dawei, Podocarpus dawei, Podocarpus usambarensis var. dawei | - | Congo, Tanzania (Kagara and Mara provinces), Uganda | NT |
| 4 | Afrocarpus falcatus (Thunb.) C.N.Page | Afrocarpus falcatus subsp. gaussenii, Afrocarpus gaussenii, Decussocarpus falcatus, Nageia falcata, Nageia falcata var. gaussenii, Nageia meyeriana, Podocarpus falcatus, Podocarpus falcatus, Podocarpus gaussenii, Podocarpus gracillimus, Podocarpus meyerianus, Taxus falcata | Outeniqua yellowwood, Bastard Yellow wood | Malawi, Mozambique, South Africa (Eastern Cape Province, KwaZulu-Natal, Limpopo Province, Mpumalanga, Western Cape), Swaziland, Madagascar | LC |
| 5 | Afrocarpus gracilior (Pilg.) C.N.Page | Afrocarpus falcatus subsp. gracilior, Decussocarpus gracilior, Nageia falcata var. gracilior, Podocarpus gracilior | East African Yellow wood | Ethiopia, Kenya, Tanzania, Congo, Rwanda, South Sudan, Uganda | LC |
| 6 | Afrocarpus mannii (Hook.f.) C.N.Page | Decussocarpus mannii, Nageia mannii, Podocarpus mannii | - | Sao Tomé and Principe | VU |
| 7 | Afrocarpus usambarensis (Pilg.) C.N.Page | Afrocarpus mannii subsp. usambarensis, Nageia mannii var. usambarensis, Podocarpus usambarensis | African Yellowwood | Tanzania, Kenya (Kyulu Hills, Taita Taveta District) | EN |
| 8 | Dacrycarpus cinctus (Pilg.) de Laub. | Bracteocarpus cinctus, Bracteocarpus dacrydiifolius, Dacrycarpus dacrydiifolius, Podocarpus cinctus, Podocarpus dacrydiifolius | - | Indonesia (Maluku, Papua, Sulawesi), Malaysia (Sarawak), Papua New Guinea | LC |
| 9 | Dacrycarpus compactus (Wasscher) de Laub. | Bracteocarpus compactus, Podocarpus compactus | Binban Kadzinam, Kaibigl, Kaipik, Pau, Pawa, Uba, Umba, Umbwa | Indonesia and Papua New Guinea | LC |
| 10 | Dacrycarpus cumingii (Parl.) de Laub. | Bracteocarpus cumingii, Nageia cumingii, Podocarpus cumingii, Podocarpus imbricata var. cumingii, Podocarpus imbricatus var. cumingii | - | Indonesia (Sumatera), Malaysia (Sarawak), Philippines | LC |
| 11 | Dacrycarpus dacrydioides (A.Rich.) de Laub. | Dacrydium ferrugineum, Nageia dacrydioides, Nageia excesla, Podocarpus dacrydioides, Podocarpus thujoides | Kahikatea (Maori), White Pine | New Zealand | LC |
| 12 | Dacrycarpus expansus de Laub. | Bracteocarpus expansus | - | Indonesia (Papua), Papua New Guinea | LC |
| 13 | Dacrycarpus imbricatus (Blume) de Laub. | Bracteocarpus imbricatus, Bracteocarpus kawaii, Dacrycarpus imbricatus var. imbricatus, Dacrycarpus imbricatus var. patulus, Nageia cupressina, Podocarpus cupressinus, Podocarpus imbricatus, Podocarpus javanicus, Podocarpus kawaii, Thuja javanica | - | Cambodia, China (Guangxi, Hainan, Yunnan), Fiji, Indonesia (Jawa, Lesser Sunda Is., Papua, Sulawesi, Sumatera), Lao People’s Democratic Republic, Malaysia, Papua New Guinea (Bismarck Archipelago), Philippines, Thailand, Vanuatu, Vietnam | LC |
| 14 | Dacrycarpus imbricatus var. curvulus (Miq.) de Laub. | Podocarpus cupressina var. curvula, Podocarpus imbricatus var. curvula | Tjamarah | Indonesia (Sumatra and Java) | LC |
| 15 | Dacrycarpus imbricatus var. robustus de Laub. | Podocarpus papuanus, Podocarpus javanica, Podocarpus cupressina, Dacrycarpus papuana | Pierur, tupi, daru and umba | Papua New Guinea, Indonesia (Moluccas), Malaysia (Sarawak) and Philippines (Luzon, Mindanao) | LC |
| 16 | Dacrycarpus kinabaluensis (Wasscher) de Laub. | Bracteocarpus kinabaluensis, Podocarpus imbricatus var. kinabaluensis | - | Endemic to Mt. Kinabalu in Sabah (Borneo), Malaysia | LC |
| 17 | Dacrycarpus steupii (Wasscher) de Laub. | Bracteocarpus steupii, Podocarpus steupii | - | Indonesia (Kalimantan, Papua, Sulawesi), Papua New Guinea, Philippines | NT |
| 18 | Dacrycarpus vieillardii (Parl.) de Laub. | Dacrydium elatum var. compactum, Dacrydium elatum var. tenuifolium, Nageia tenuifolia, Nageia vieillardii, Podocarpus taxodioides var. tenuifolius, Podocarpus tenuifolius, Podocarpus vieillardii | - | New Caledonia | LC |
| 19 | Dacrydium araucarioides Brongn. and Gris | Athrotaxis araucarioides, Dacrydium arthrotaxoides, Metadacrydium araucarioides, Podocarpus araucarioides | - | New Caledonia | LC |
| 20 | Dacrydium balansae Brongn. and Gris | Metadacrydium balansae | - | New Caledonia | LC |
| 21 | Dacrydium beccarii Parl. | Nageia beccarii | - | Indonesia (Maluku, Papua, Sulawesi, Sumatera), Malaysia (Peninsular Malaysia, Sarawak), Papua New Guinea (Bismarck Archipelago), Philippines, Solomon Islands | LC |
| 22 | Dacrydium comosum Corner | Corneria comosa | - | Malaysia (Genting Highlands, Gunung Hulu Kali, Negeri Pahang) | EN |
| 23 | Dacrydium cornwallianum de Laub. | Corneria cornwalliana, Dacrydium nidulum var. araucarioides | - | Indonesia (Papua), Papua New Guinea | LC |
| 24 | Dacrydium cupressinum Sol. ex G.Forst. | Dacrydium cupressiforme, Thalamia cupressina | Rimu, Red pine | New Zealand | LC |
| 25 | Dacrydium elatum (Roxb.) Wall. ex Hook. | Corneria elata, Corneria pierrei, Dacrydium beccarii var. subelatum, Dacrydium junghuhnii, Dacrydium pierrei, Juniperus elata, Juniperus elatus, Juniperus philippsiana | Sempilor | Cambodia, Indonesia (Sumatera), Lao People’s Democratic Republic, Malaysia (Peninsular Malaysia, Sabah, Sarawak), Philippines, Thailand, Viet Nam | LC |
| 26 | Dacrydium ericoides de Laub. | Corneria ericoides | Sempilor, Bintulu | Malaysia (Borneo) | NA (IUCN) VU |
| 27 | Dacrydium gibbsiae Stapf | Corneria gibbsiae, Dacrydium beccarii var. kinabaluense | - | Malaysia (Mount Kinabalu in Sabah) | LC |
| 28 | Dacrydium gracile de Laub. | Corneria gracilis | - | Malaysia (Sabah, Sarawak) | NT |
| 29 | Dacrydium guillauminii J.Buchholz | Gaussenia guillauminii | - | New Caledonia (river Madeleine (Riviére des Lacs) and along the riverbanks of Lac en Huit) | CR |
| 30 | Dacrydium leptophyllum (Wasscher) de Laub. | Bracteocarpus leptophyllus, Corneria lepto. phylla, Dacrycarpus leptophyllus, Dacrydium leptophyllum, Podocarpus leptophylla, Podocarpus leptophyllus | - | Indonesia (Mt. Goliath, Papua) | VU |
| 31 | Dacrydium lycopodioides Brongn. and Gris | Gaussenia lycopodioides | Mou | New Caledonia (southern massif, from Mont Nekandi to Mont Dzumac and Mont Mou) | NT |
| 32 | Dacrydium magnum de Laub. | Corneria magna, Dacrydium beccarii var. rudens | - | Indonesia (Maluku), Papua New Guinea (Tagula Island, Normanby Island) | NT |
| 33 | Dacrydium medium de Laub. | Corneria media | Sangu, Gajo | Indonesia (Sumatera), Malaysia (Peninsular Malaysia) | VU |
| 34 | Dacrydium nausoriense de Laub. | Corneria nausoriensis | - | Fiji (Nausori Highlands, Sarava) | EN |
| 35 | Dacrydium nidulum de Laub. | Corneria nidula, Dacrydium nidulum var. vitiensis | - | Fiji, Indonesia (Lesser Sunda Is., Maluku, Papua, Sulawesi), Papua New Guinea | LC |
| 36 | Dacrydium novoguineense Gibbs | Corneria novoguineensis | - | Indonesia (Papua, Sulawesi), Papua New Guinea | LC |
| 37 | Dacrydium pectinatum de Laub. | Corneria pectinata, Dacrydium pectinatum var. robustum | - | Brunei Darussalam, China (Hainan), Indonesia (Kalimantan, Sumatera), Malaysia (Sabah, Sarawak), Philippines | EN |
| 38 | Dacrydium spathoides de Laub. | Corneria spathoides | - | Papua New Guinea (Irian Jaya), Indonesia | NT |
| 39 | Dacrydium × suprinii Nimsch | - | - | New Caledonia | NA |
| 40 | Dacrydium xanthandrum Pilg. | Corneria xanthandra | Kerapui | Indonesia (Kalimantan, Maluku, Papua, Sulawesi, Sumatera), Malaysia (Sabah, Sarawak), Papua New Guinea (Bismarck Archipelago, North Solomons), Philippines | LC |
| 41 | Falcatifolium angustum de Laub. | - | - | Malaysia (Sarawak) | EN |
| 42 | Falcatifolium falciforme (Parl.) de Laub. | Podocarpus falciformis, Nageia falciformis, Falcatifolium usan-apuensis, Falcatifolium falciforme var. usan-apuensis, Falcatifolium falciforme var. kinabaluensis, Falcatifolium falciforme var. kinabaluensis | - | Brunei Darussalam; Indonesia (Kalimantan); Malaysia (Peninsular Malaysia, Sabah, Sarawak) | NT |
| 43 | Falcatifolium gruezoi de Laub. | - | - | Indonesia (Maluku, Sulawesi), Philippines | NT |
| 44 | Falcatifolium papuanum de Laub. | Dacrydium papuanum | - | Papua New Guinea (Morobe) | LC |
| 45 | Falcatifolium sleumeri de Laub. and Silba | - | - | Indonesia (Papua) | NT |
| 46 | Falcatifolium taxoides (Brongn. and Gris) de Laub. | Dacrydium taxoides, Nageia taxoides, Pinus falciformis, Podocarpus taxodioides, Podocarpus taxodioides var. gracilis | - | New Caledonia | LC |
| 47 | Halocarpus bidwillii (Hook. f. ex Kirk) C.J.Quinn | Dacrydium bidwillii, Dacrydium bidwillii var. erectum, Dacrydium bidwillii var. relinatum | - | New Zealand (North Island, South Island, Stewart Island) | LC |
| 48 | Halocarpus biformis (Hook.) C.J.Quinn | Dacrydium biforme, Podocarpus biformis | Yellow pine, Pink pine, Bog Pine, Mountain Pine, Tarwood | New Zealand (North Island, South Island and Stewart Island) | LC |
| 49 | Halocarpus kirkii (F.Muell. ex Parl.) C.J.Quinn | Dacrydium kirkii | Monoao | New Zealand (Hokianga, Manukau Harbor and Coromandel Peninsula) | NT |
| 50 | Lagarostrobos franklinii (Hook.f.) Quinn | Dacrydium franklinii, Lepidothamnus franklinii | Huon Pine | Australia (Tasmania,) | LC |
| 51 | Lepidothamnus fonkii Phil. | Dacrydium fonckii, Dacrydium fonckii | Chilean Rimu | Argentina (Chubut, Santa Cruz); Chile (Aisén, Los Lagos, Magellanes) | LC |
| 52 | Lepidothamnus intermedius (Kirk) Quinn | Dacrydium intermedium | Yellow silver pine, Mountain pine | New Zealand (South Island, Stewart Island and North Island) | LC |
| 53 | Lepidothamnus laxifolius (Hook.f.) Quinn | Dacrydium laxifolium, Dacrydium laxifolium var. compactum, Dacrydium laxifolium var. debile | Mountain rimu, Pigmy pine, Pygmy pine | New Zealand (Tongariro National Park in the North Island southwards to Stewart Island) | LC |
| 54 | Manoao colensoi (Hook.) Molloy | Dacrydium colensoi, Dacrydium westlandicum, Lagarostrobos colensoi | Manoao, Silver pine, Westland pine, White silver pine | New Zealand (Lake Te Anau, Central Volcanic Plateau and Auckland) | LC |
| 55 | Microcachrys tetragona (Hook.) Hook.f. | Dacrydium tetragonu, Athrotaxis tetragona | Strawberry pine, Creeping Pine, | Australia (Tasmania) | LC |
| 56 | Nageia fleuryi (Hickel) de Laub. | Decussocarpus fleuryi, Podocarpus fleuryi | Kim giao | China (Guangdong, Guangxi, Yunnan), Laos, Vietnam | NT |
| 57 | Nageia formosensis (Dummer) C.N.Page | Decussocarpus nagi var. formosensis, Nageia nagi var. formosensis, Nageia nagi var. koshuensis, Nageia nankoensis, Podocarpus formosensis, Podocarpus formosensis var. koshuensis, Podocarpus koshunensis, Podocarpus nagi var. koshuensis | - | Taiwan | NA |
| 58 | Nageia maxima (de Laub.) de Laub. | Decussocarpus maximus, Podocarpus maxima, Podocarpus maximus | Landin paya | Malaysia (Sarawak) | EN |
| 59 | Nageia motleyi (Parl.) de Laub. | Agathis motleyi, Dammara motleyi, Decussocarpus motleyi, Nageia baccarii, Podocarpus beccarii, Podocarpus motleyi | Podo kebal musang, Kayu bawa, Setebal, Medang buloh | Brunei Darussalam, Indonesia (Kalimantan, Sumatera), Malaysia (Peninsular Malaysia, Sabah, Sarawak), Thailand | VU |
| 60 | Nageia nagi (Thunb.) Kuntze | Myrica nagi, Agathis veitchii, Dammara veitchii, Decussocarpus nagi, Nageia caesia, Nageia cuspidata, Nageia grandifolia, Nageia ovata, Podocarpus caesius, Podocarpus cuspidatus, Podocarpus grandifolius, Podocarpus nageia, Podocarpus nageia var. angustifolius, Podocarpus nageia var. rotundifolius, Podocarpus nagi, Podocarpus nagi var. angustifolius, Podocarpus nagi var. caesius, Podocarpus nagi var. ovatus, Podocarpus nagi var. rotundifolius, Podocarpus ovata, Podocarpus ovatus | Broad-leaved podocarp | Introduced China (Fujian, Guangdong, Guangxi, Hainan, Hunan, Jiangxi, Sichuan, Zhejiang), Japan (Honshu, Kyushu, Nansei-shoto, Shikoku), Taiwan (introduced), Vietnam (Lang Son) | NT |
| 61 | Nageia wallichiana (C.Presl) Kuntze | Decussocarpus wallichianus, Nageia blumei, Podocarpus blumei, Podocarpus wallichianus | Mala almaciga | Brunei Darussalam, Cambodia, China (Yunnan), India (Assam, Kerala, Nicobar Island), Indonesia (Jawa, Kalimantan, Maluku, Papua, Sulawesi, Sumatera), Laos, Malaysia (Peninsular Malaysia, Sabah, Sarawak), Myanmar, Papua New Guinea, Philippines, Thailand, Vietnam | LC |
| 62 | Parasitaxus usta (Vieill.) de Laub. | Dacrydium ustum, Nageia usta, Parasitaxus usta, Podocarpus ustus | Corail, Cèdre rabougri | New Caledonia [Grand Terre in the mountains of the far south (Dzumac, Montagne des Sources), central west (Paéoua and Tchingou) and far northeast (Colnett/Panie)] | VU |
| 63 | Pherosphaera fitzgeraldii (F.Muell.) Hook.f. | Dacrydium fitzgeraldii, Microstrobos fitzgeraldii | Dwarf mountain pine, Blue Mountain dwarf pine | Australia (New South Wales) | CR |
| 64 | Pherosphaera hookeriana W.Archer bis | Dacrydium hookerianum (W.Archer bis) Eichler, Microstrobos niphophilus J.Garden and L.A.S.Johnson, Pherosphaera niphophila (J.Garden and L.A.S.Johnson) Florin | Drooping pine, Mount Mawson Pine | Australia (Tasmania) | NT |
| 65 | Phyllocladus aspleniifolius (Labill.) Hook.f. | Brownetera aspleniifolia (Labill.) Tratt., Phyllocladus glaucus Carrière, Phyllocladus rhomboidalis Rich., Phyllocladus serratifolius Nois. ex Henkel and Hochst., Phyllocladus trichomanoides var. glaucus (Carrière) Parl., Podocarpus aspleniifolius Labill., Thalamia asplenifolia (Labill.) Spreng. | Celery top pine | Australia (Tasmania) | LC |
| 66 | Phyllocladus hypophyllus Hook.f. | Phyllocladus hypophyllus var. protractus Warb., Phyllocladus major Pilg., Phyllocladus protractus (Warb.) Pilg., Podocarpus hypophyllus (Hook.f.) Kuntze | Celery top pine | Philippines, Brunei, Malaysia (Celebes, Moluccas, Sulawesi), Papua New Guinea, Indonesia (Maluku) | LC |
| 67 | Phyllocladus toatoa Molloy | Phyllocladus glaucus Kirk | Toatoa (Maori), Blue celery pine | New Zealand (North Island) | LC |
| 68 | Phyllocladus trichomanoides D.Don | Phyllocladus cunninghamii, Podocarpus trichomanoides | Tanekaha (Maori), Celery pine | New Zealand (North Island and South Island) | LC |
| 69 | Phyllocladus trichomanoides var. alpinus (Hook.f.) Parl. | Phyllocladus alpinus, Phyllocladus aspleniifolius var. alpinus | Mountain toatoa | New Zealand | LC |
| 70 | Podocarpus acuminatus de Laub. | - | - | Brazil (Serra da Neblina in the state of Amazonas), Venezuela (Bolívar) | NT |
| 71 | Podocarpus acutifolius Kirk | Nageia acutifolia | Needle-leaved totara, Westland totara | New Zealand (South Island from Marlborough Sounds to S Westland) | LC |
| 72 | Podocarpus affinis Seem. | Nageia affinis | Kuasi | Fiji (Higher ridges on Viti Levu) | NT |
| 73 | Podocarpus angustifolius Griseb. | Podocarpus victorinianus, Podocarpus leonii, Podocarpus aristulatus | Sabina cimarrona | Cuba Cienfuegos (Sierra de Trinidad), Guantánamo (Sierra Maestra), Sancti Spíritus (Sierra de Sancti Spíritu), Holguín and Santiago de Cuba (Sierra de Nipe, Sierra del Cristal, and the Baracoa Ranges). | VU |
| 74 | Podocarpus annamiensis N.E.Gray | - | - | Myanmar, Vietnam, China | TH |
| 75 | Podocarpus aracensis de Laub. and Silba | - | - | Brazil [Amazonas (Serra Araca, Cerro Neblina] and Venezuela [Amazonas (Cerro Yaví)] | LC |
| 76 | Podocarpus archboldii N.E. Gray | Margbensonia archboldi, Podocarpus crassigemma | Baula, Jamekang, juba, Kaibigltuga, Morumba, Puling, Yamekange, Mu, Soa, Sarau | Indonesia (Papua), Papua New Guinea (Morobe District0 | VU |
| 77 | Podocarpus aristulatus Parl. | Nageia aristulata, Podocarpus angustifolius var. wrightii | Cuba, Dominican Republic, | TH | |
| 78 | Podocarpus atjehensis (Wasscher) de Laub. | Podocarpus neriifolius var. atjehensis, Margbensonia atjehensis, Margbensonia atjehense, Podocarpus neriifolius var. membranaceaus | Atjeh | Malesia [Sumatera (Aceh, Gajo Lands, Kemiri and Bandahara), New Guinea (Papua, Wissel Lakes). | NT |
| 79 | Podocarpus barretoi de Laub. and Silba | - | - | Brazil (Minas Gerais) | NT |
| 80 | Podocarpus beecherae de Laub. | - | - | New Caledonia | TH |
| 81 | Podocarpus borneensis de Laub. | Podocarpus polystachyus var. rigidus | Bisit, Bubung, Buloh | Indonesia (Kalimantan), Malaysia (Sabah, Sarawak) | LC |
| 82 | Podocarpus bracteatus Blume | Nageia bracteata, Podocarpus bracteatus var. brevipes, Podocarpus neriifolius var. bracteatus, Podocarpus neriifolius var. brevipes | Kayu unung unung, Toba Batak, Bima, Kimarak, Kipantjar, Ki putri | Indonesia (Jawa, Lesser Sunda Is., Sumatera) | LC |
| 83 | Podocarpus brasiliensis de Laub. | Podocarpus barretoi | - | Brazil (Bahia, Brasília Distrito Federal, Goiás, Mato Grosso, Minas Gerais, Roraima), Venezuela | LC |
| 84 | Podocarpus brassii Pilg. | - | Baugwa, Baula, Chuga, Kaibigltuga, Kaipil, Karbuku, Maja, Mbagua, Tsula | Indonesia (Papua), Papua New Guinea | LC |
| 85 | Podocarpus brassii var. humilis de Laub. | Podocarpus brassii subsp. humilis | - | Papua New Guinea | LC |
| 86 | Podocarpus brevifolius (Stapf) Foxw. | Podocarpus neriifolius | - | Malaysia (Sabah) | NT |
| 87 | Podocarpus buchholzii de Laub. | Podocarpus buchholzii var. neblinensis Silba, Podocarpus buchholzii subsp. neblinensis (Silba) Silba | - | Venezuela (Guyana Highlands) | LC |
| 88 | Podocarpus buchii Urb. | Podocarpus aristulatus var. buchii, Podocarpus angustifolius Griseb. ssp. buchii | Tachuela, Chicharrón, Palo de Cruz | Dominican Republic (Southeast Haiti) | EN |
| 89 | Podocarpus capuronii de Laub. | Podocarpus capuronii var. capuronii, Podocarpus woltzii | - | Madagascar (Itremo Massif, Manandona) | EN |
| 90 | Podocarpus celatus de Laub. | - | Cinqui-mase | Bolivia (Potosi), Brazil (Goiás, Mato Grosso), Colombia, Ecuador, Peru (Junin, Loreto, Montana, Puno), Venezuela (Amazonas, Bolivar, Tachira) | LC |
| 91 | Podocarpus chinensis Wall. ex J.Forbes | Podocarpus macrophyllus var. maki, Podocarpus japonicus, Podocarpus makoyi, Podocarpus appressus, Podocarpus macrophyllus subsp. maki, Podocarpus maki, Nageia appressa, Nageia japonica, Nageia chinensis, Nageia macrophylla var. maki, Myrica esquirolii | - | China (Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, Yunnan, and Zhejiang), Myanmar, Japan | LC |
| 92 | Podocarpus chinensis var. wardii de Laub. and Silba | Podocarpus chinensis subsp. wardii | - | Myanmar (N’mai Hka Valley) | LC |
| 93 | Podocarpus chingianus S.Y.Hu | Podocarpus macrophyllus var. chingiiI, Margbensonia chingiana | Zhu guan luo han song | China (Jiangsu, Zhejiang, Sichuan) | DD |
| 94 | Podocarpus confertus de Laub. | Podocarpus neriifolius var. penibukanensis | - | Malaysia (Sabah, Sarawak) | EN |
| 95 | Podocarpus coriaceus Rich. and A.Rich. | Nageia coriacea, Taxus lancifolia, Podocarpus coriaceus var. sulcatus | Yucca plum pine, Resinier moutaigue, Caoba del país | Dominican Republic, Guadeloupe, Martinique, Montserrat, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Trinidad, and Tobago | LC |
| 96 | Podocarpus costalis C.Presl | Nageia costalis, Podocarpus costalis var. taiwanensis | Lan yu luo han song, Arius | Philippines, Taiwan, China | EN |
| 97 | Podocarpus costaricensis de Laub. | - | Cipresillo | Costa Rica (San José) | CR (VU) |
| 98 | Podocarpus crassigemma de Laub. | Podocarpus crassigemmis | A-pul, Kaboga, Morumba, Baula, Iamuka, Jamekang, Juba, Kamga, Puling, Kabor, Kabiltugl, Kaibelparu, Kkaibig, Kaip, Nonofan, Ronohanini, Sula | Indonesia (Papua), Papua New Guinea (Bismarck Archipelago, Central highlands) | LC |
| 99 | Podocarpus decipiens N.E.Gray | - | - | Fiji (Viti Levu) | NA |
| 100 | Podocarpus decumbens N.E.Gray | - | - | New Caledonia (Grande Terre, Southern mountains) | CR |
| 101 | Podocarpus deflexus Ridl. | - | - | Indonesia (Sumatera), Malaysia (Peninsular Malaysia) | EN |
| 102 | Podocarpus degeneri (N.E.Gray) de Laub. | Margbensonia degeneri, Podocarpus neriifolius var. degeneri | - | Fiji | LC |
| 103 | Podocarpus dispermus C.T.White | Margbensonia disperma | Broad-leaved brown pine | Australia (Queensland) | LC |
| 104 | Podocarpus drouynianus F.Muell. | Nageia drouyniana, Margbensonia drouyniana, Podocarpus brownii | Emu Berry | Australia (Western Australia) | LC |
| 105 | Podocarpus ekmanii Urb. | - | Sabina Cimarrona | Cuba (Sierra del Cristal, Sierra de Moa and Sierra de Nipe) | LC |
| 106 | Podocarpus elatus R.Br. ex Endl | Margbensonia elata, Nageia elata | Illawarra plum, Brown pine, Plum pine, Turpentine pine, Yellow pine, Australian plum, White plum, Goongum, Native deal, Pencil cedar | Australia (New South Wales, Queensland) | LC |
| 107 | Podocarpus elongatus (Aiton) L’Hér. ex Pers. | Taxus elongatus, Taxus elongata, Taxus capensis, Nageia elongata, Podocarpus thunbergii var. angustifolia | Breede river yellowwood | Malawi, South Africa (Northern Cape Province, Western Cape), Zambia, Zimbabwe | LC |
| 108 | Podocarpus fasciculus de Laub. | Podocarpus macrophyllus var. liukiuensis, Podocarpus macrophyllus f. grandifolia | - | Japan (Nansei-shoto), Taiwan | VU |
| 109 | Podocarpus forrestii Craib and W.W.Sm. | Margbensonia forrestii, Podocarpus macrophyllus subsp. forrestii | - | China | VU |
| 110 | Podocarpus gibbsiae N.E.Gray | - | - | Malaysia (Endemic to Mt. Kinabalu in Sabah) | VU |
| 111 | Podocarpus glaucus Foxw. | - | Nipa | Indonesia (Maluku, Papua, Sulawesi), Papua New Guinea (Bismarck Archipelago), Philippines, Solomon Islands | LC |
| 112 | Podocarpus globulus de Laub. | - | Sapiro | Malaysia (Sabah, Sarawak) | EN |
| 113 | Podocarpus glomeratus D.Don | Nageia glomerata, Podocarpus rigidus, Podocarpus cardenasii | Pino de Monte, Intimpa, Huampo | Bolivia, Ecuador, Peru | NT |
| 114 | Podocarpus gnidioides Carrière | Nageia gnidioides, Podocarpus alpinus var. arborescens, Podocarpus alpinus var. caespitosus, Podocarpus caespitosus, Podocarpus gnidioides subsp. caespitosus | - | New Caledonia | NT |
| 115 | Podocarpus grayae de Laub. | - | Brown pine, Northern brown pine; Brown pine; Weeping brown pine | Australia (Northern Territory, Queensland) | LC |
| 116 | Podocarpus guatemalensis Standl. | Podocarpus allenii, Podocarpus guatemalensis var. allenii, Podocarpus guatemalensis subsp. allenii, Podocarpus guatemalensis subsp. pinetorum, Podocarpus guatemalensis var. pinetorum, Podocarpus pinetorum | Ocotillo de Llano, Alfajillo, Ciprecillo Amarillo, Ciprecillo Blanco, Cipresillo, Cypress de Montaña, Palo de Oro, Pinillo, Piño de Montaña | Belize, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico (Oaxaca, Veracruz), Nicaragua, Panama | LC |
| 117 | Podocarpus henkelii Stapf ex Dallim. and B.D.Jacks. | Podocarpus ensiculus, Podocarpus henkelii subsp. Ensiculus, Podocarpus thunbergii var. falcata | Henkel’s yellowwood, Falcate yellowwood, East griqualand yellowwood, Natal yellowwood, bastergeelhout, Nanjula | Malawi, South Africa (Eastern Cape Province, KwaZulu-Natal), Tanzania, Zimbabwe | CR |
| 118 | Podocarpus hispaniolensis de Laub. | - | - | Dominican Republic (Cordillera Central, San José de Ocoa, Cordillera Septentriona, Province Puerto Plata) | EN |
| 119 | Podocarpus hookeri de Laub. | Podocarpus neriifolius var. linearis, Podocarpus neriifolius var. staintonii | - | India (Sikkim), Indonesia (Sumatra, Java, Borneo), Philippines | LC |
| 120 | Podocarpus humbertii de Laub. | - | - | Madagascar (Mont Anjanaharibe, Mont Tsaratanana and Mont Marojezy) | EN |
| 121 | Podocarpus insularis de Laub. | - | Dala, tunum, ida-ayebo | Papua New Guinea (Bismarck Archipelago), Solomon Islands, Vanuatu | LC |
| 122 | Podocarpus idenburgensis N.E.Gray | - | - | Papua New Guinea, Fiji | NE |
| 123 | Podocarpus laetus Hooibr. ex Endl. | Podocarpus cunninghamii, Podocarpus hallii, Nageia hallii, Podocarpus totara var. hallii | Montane totara, Thin-bark totara, Hall’s totara | New Zealand (North Island, Tongariro National Park, South Island and Stewart Island) | LC |
| 124 | Podocarpus lambertii Klotzsch ex Endl. | Nageia lambertii, Podocarpus lambertii subsp. horsmanii, Podocarpus lambertii var. horsmanii, Podocarpus lambertii subsp. tigreensis, Podocarpus lambertii var. tigreensis | Pinheiro bravo | Argentina (Misiones), Brazil (Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina, São Paulo) | NT |
| 125 | Podocarpus laminaris de Laub. | Podocarpus rubens subsp. pabinamaensis, Podocarpus rubens var. pabinamaensis | - | Papua New Guinea | NA |
| 126 | Podocarpus latifolius (Thunb.) R.Br. ex Mirb. | Nageia latifolia, Nageia thunbergii, Podocarpus latifolius var. latior, Podocarpus latifolius subsp. latior, Podocarpus latior, Podocarpus nobilis, Podocarpus pinnata, Podocarpus thunbergii, Taxus latifolia | Broad-leaved yellowwood, Real yellowwood, True yellowwood, Upright yellowwood | Eswatini, South Africa (Eastern Cape Province, Free State, KwaZulu-Natal, Limpopo Province, Mpumalanga, Northern Cape Province, Western Cape) | LC |
| 127 | Podocarpus laubenfelsii Tiong | - | - | Indonesia (Kalimantan), Malaysia (Sabah, Sarawak) | EN |
| 128 | Podocarpus lawrencei Hook.f. | Podocarpus alpinus var. lawrencei, Podocarpus lawrencei subsp. errinundraensis | Mountain plum pine, Plum pine | Australia (Australian Capital Territory, New South Wales, Tasmania, Victoria) | LC |
| 129 | Podocarpus ledermannii Pilg. | Podocarpus idenburgensis | - | Indonesia (Papua), Papua New Guinea (Bismarck Archipelago) | LC |
| 130 | Podocarpus ledermannii Pilg. var. expansus de Laub. | - | - | Indonesia | LC |
| 131 | Podocarpus lenticularis de Laub. | - | - | Assam (India), Laos | NA |
| 132 | Podocarpus linearis de Laub. | - | - | Papua New Guinea | DD |
| 133 | Podocarpus levis de Laub. | - | Marisa, Sanru, Kayu tjina, Wasiwarare | Indonesia (Kalimantan, Maluku, Papua, Sulawesi) | LC |
| 134 | Podocarpus × loderi Cockayne | - | - | New Zealand | NA |
| 135 | Podocarpus longifoliolatus Pilg. | Podocarpus longifoliolatus | - | New Caledonia (Grande Terre) | EN |
| 136 | Podocarpus lophatus de Laub. | - | - | Philippines (Mt. Tapulao in Luzon and Mt. Halcon in Mindoro) | VU |
| 137 | Podocarpus lucienii de Laub. | - | - | New Caledonia (Massif du Colnett and the Massif du Tchingou) | LC |
| 138 | Podocarpus macrocarpus de Laub. | - | Malakawayan | Philippines (Luzon) | EN |
| 139 | Podocarpus macrophyllus (Thunb.) Sweet |
Margbensonia forrestii, Margbensonia macrophylla, Nageia macrophylla, Nageia macrophylla, Podocarpus forrestii, Podocarpus macrophyllus var. angustifolius, Podocarpus macrophyllus subsp. angustifolius, Podocarpus macrophyllus f. angustifolius, Podocarpus macrophyllus subsp. forrestii, Podocarpus macrophyllus var. macrophyllus, Podocarpus macrophyllus var. rubra, Podocarpus verticillatus, Taxus macrophylla, Taxus makoya |
Southern yew, Yew podocarp, Long-leaved podocarp, Buddhist pine, Kusamaki, Inumaki, luo han song | China (Anhui, Chongqing, Fujian, Guangxi, Guizhou, Hubei, Hunan, Jiangsu, Jiangxi, Sichuan, Yunnan, Zhejiang), Hong Kong, Japan (Honshu, Kyushu, Shikoku), Taiwan, Malaysia, Singapore | LC |
| 140 | Podocarpus macrophyllus var. piliramulus Zhi X. Chen and Zhen Q. Li | - | - | China (Anhui, Chongqing, Fujian, Guangdong, Guangxi, Guizhou, Hunan, Jiangxi, Yunnan, Zhejiang); Hong Kong; Japan (Honshu, Kyushu, Shikoku); Myanmar; Taiwan | NT |
| 141 | Podocarpus madagascariensis Baker | Nageia madagascariensis, Podocarpus madagascariensis var. madagascariensis | - | Madagascar | NT |
| 142 | Podocarpus madagascariensis var. procerus de Laub. | Podocarpus madagascariensis subsp. procerus | - | Madagascar (Tolanaro [Fort Dauphin] and Massif de Bekolosy). | EN |
| 143 | Podocarpus madagascariensis var. rotundus L.Laurent | Podocarpus madagascariensis subsp. rotundus (L. Laurent) Silba | - | Madagascar (Massif de Bekolosy and the Massif du Manongarivo) | DD |
| 144 | Podocarpus magnifolius J.Buchholz and N.E. Gray | - | Cinqui-masé | Bolivia (La Paz), Colombia, Panama, Peru (Pasco, Oxapampa), Venezuela (States of Bolívar, Amazonas, Aragua, Yaracuy) | LC |
| 145 | Podocarpus marginalis de Laub. | - | - | Papua New Guinea | DD |
| 146 | Podocarpus matudae Lundell | Podocarpus reichei, Podocarpus matudae var. reichei, Podocarpus matudae var. macrocarpus, Podocarpus matudae var. jaliscanus, Podocarpus matudae subsp. jaliscanus, Podocarpus matudae subsp. macrocarpus, Podocarpus matudae subsp. reichei | Sabino | Mexico (Chiapas, Jalisco, Michoacán, Nayarit, Oaxaca, Puebla, Querétaro, San Luis Potosí, Tamaulipas, Veracruz), El Salvador, Guatemala (Huehuetenango), Honduras | CR |
| 147 | Podocarpus matudae subsp. jaliscanus (de Laub. and Silba) Silba | Podocarpus matudae Lundell var. jaliscanus | - | Mexico (Jalisco) | VU |
| 148 | Podocarpus micropedunculatus de Laub. | - | Kayu china, kayu tjina | Brunei Darussalam, Indonesia, Malaysia (Sabah, Sarawak) | NT |
| 149 | Podocarpus milanjianus Rendle | Podocarpus ulugurensis | Lusamina | Angola, Burundi, Cameroon, Congo, Congo, Kenya, Malawi, Mozambique, Nigeria, Rwanda, Sudan, Tanzania, Uganda, Zambia, Zaire, Zimbabwe | LC |
| 150 | Podocarpus nakaii Hayata | Podocarpus macrophyllus var. nakaii | Nakai podocarp, Nakai yellowwood | Taiwan (Chianghua Co., Nantou Co., Taichung Co.) | EN |
| 151 | Podocarpus neriifolius D.Don | Nageia discolor, Nageia leptostachya, Nageia neriifolia, Nageia neglecta, Nageia decipiens, Nageia polyantha, Nageia annamiensis, Podocarpus discolor, Podocarpus leptostachya, Podocarpus annamiensis, Podocarpus epiphyticus, Podocarpus neglecta, Podocarpus junghuhniana, Podocarpus thailandensis, Podocarpus neriifolius var. polyanthus | Brown pine, Podo bukit, Ambai Ayam, Hatang, Hai nan luo han song, Thông tre, Thông lông gà | Brunei Darussalam, Cambodia, China (Guangxi, Yunnan), Fiji, India (Assam, West Bengal), Bangladesh, Indonesia (Bali, Jawa, Kalimantan, Lesser Sunda Is., Maluku, Papua, Sulawesi, Sumatera), Lao People’s Democratic Republic, Malaysia (Peninsular Malaysia, Sabah, Sarawak); Myanmar, Nepal, Papua New Guinea (Bismarck Archipelago), Philippines, Solomon Islands, Thailand, Vietnam | LC |
| 152 | Podocarpus neriifolius D.Don var. degeneri N.E.Gray | - | - | Fiji (Vanua Leva, Viti Levu) | LC |
| 153 | Podocarpus nivalis Hook. | Nageia nivalis, Podocarpus nivalis var. erectus, Podocarpus montanus | Alpine totara, Snow totara | New Zealand (North Island, South Island) | LC |
| 154 | Podocarpus novae-caledoniae Vieill. ex Brongn. and Gris | Nageia novae-caledoniae, Podocarpus beecherae, Podocarpus rivularis | - | New Caledonia (Grande Terre, Iledes Pins) | LC |
| 155 | Podocarpus neglectus Blume | Podocarpus discolor, Podocarpus leptostachyus, Podocarpus thailandensis, Nageia neglecta, Podocarpus discolor, Podocarpus junghuhnianus | - | Thailand, Indonesia, China (Hainan), Indonesia (Borneo, Java, Sumatra), Malaysia | NA |
| 156 | Podocarpus novoguineensis de Laub. | - | - | Papua New Guinea | NA |
| 157 | Podocarpus nubigenus Lindl. | Nageia nubigena, Saxegothaea gracilis | Huililahuani, Mañio, Mañío de Hojas Punzantes, Mañio Hembra, Mañio Macho, Mañiu de la Costa, Pino Amarillo | Argentina (Neuquén, Santa Cruz), Chile (Aisén, La Araucania, Los Lagos, Magellanes) | NT |
| 158 | Podocarpus oblongus de Laub. | Podocarpus rumphii subsp. Aruensis, Podocarpus rumphii var. aruensis | - | Papua New Guinea (Vogelkop) | DD |
| 159 | Podocarpus oleifolius D.Don | Nageia macrostachya, Nageia oleifolia, Podocarpus ballivianensis, Podocarpus ingensis, Podocarpus macrostachys, Podocarpus oleifolius subsp. equadorensis, Podocarpus oleifolius var. equadorensis, Podocarpus oleifolius var. macrostachys, Podocarpus oleifolius var. trujillensis, Podocarpus oleifolius subsp. trujillensis | Pino de pasto, Pinete | Bolivia, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico (Chiapas, Guerrero, Michoacán, Oaxaca, Puebla, Veracruz), Panama, Peru, Venezuela | LC |
| 160 | Podocarpus oleifolius subsp. costaricensis (J.Buchholz and N.E.Gray) Silba | - | - | Costa Rica, El Salvador, Guatemala, Honduras, Mexico Gulf, Mexico Southeast, Mexico Southwest, Nicaragua, Panamá | LC |
| 161 | Podocarpus orarius R.R.Mill and M.Whiting | Podocarpus spathoides subsp. solomonensis, Podocarpus spathoides var. solomonensis | - | Papua New Guinea (Solomon Island), Vanuatu | NT |
| 162 | Podocarpus palawanensis de Laub. and Silba | - | - | Philippines (Palawan) | CR |
| 163 | Podocarpus pallidus N.E.Gray | - | Uhiuhi | Tonga (island of Eua and islands of Vava’u) | VU |
| 164 | Podocarpus parlatorei Pilg. | Podocarpus angustifolius, Nageia angustifolia | Pino Blanco, Pino del Cerro | Argentina (Catamarca, Corrientes, Jujuy, Salta, Tucumán), Bolivia (Chuquisaca, Cochabamba, Potosí, Santa Cruz, Tarija, La Paz), Peru (Sierra de Chaglla) | NT |
| 165 | Podocarpus pendulifolius J.Buchholz and N.E.Gray | - | Pino Carbón, Pino Hayuco | Venezuela (Andes, Cordillera do Merida, Edo Lara, Merida, Tachira and Trujillo) | EN |
| 166 | Podocarpus perrieri Gaussen and Woltz | Podocarpus rostratus var. perrieri, Podocarpus rostratus subsp. perrieri | - | Madagascar (Andringitra Massif, Toamasina, Forêt d’Andasibé) | CR |
| 167 | Podocarpus pilgeri Foxw. | Podocarpus celebicus, Podocarpus pilgeri var. thailandensis, Podocarpus pilgeri subsp. wangii, Podocarpus schlechteri, Podocarpus wangii, Podocarpus tixieri | - | Cambodia (Kampuchea), China (Guangdong, Guangxi, Hainan, Yunnan), Indonesia (Maluku, Papua, Sulawesi), Laos, Malaysia (Sarawak), Papua New Guinea (Bismarck Archipelago); Philippines, Thailand, Vietnam | LC |
| 168 | Podocarpus polyspermus de Laub. | - | Mé Maoya podocarp | New Caledonia (Grande Terre) | EN |
| 169 | Podocarpus polystachyus R.Br. ex Endl. | Margbensonia polystachya, Nageia polystachya | Jati bukit, Kayu karamat, Podo laut, Mayu serai, Landin, Kandabang, kayu china, Saumah | Brunei Darussalam, Indonesia (Kalimantan, Maluku, Papua, Sumatera), Malaysia (Peninsular Malaysia, Sabah, Sarawak), Papua New Guinea, Philippines, Singapore, Thailand | VU |
| 170 | Podocarpus pseudobracteatus de Laub. | Podocarpus archboldii var. crassiramosus | Kaip, Kebu, Puling | Indonesia (Papua), Papua New Guinea | LC |
| 171 | Podocarpus pseudobracteatus de Laub. var. sicaris de Laub. | - | - | Papua New Guinea | LC |
| 172 | Podocarpus purdieanus Hook. | Nageia purdieana | Yacca, St. Ann Yacca | Jamaica (Claredon, St. Catherine, St. Ann, Trelawny, Sanders Hill, Mt. Diablo) | EN |
| 173 | Podocarpus ramosii R.R.Mill | Podocarpus rotundus | - | Indonesia (Kalimantan Timur), Philippines (Mt. Banahao in Luzon) | DD |
| 174 | Podocarpus ridleyi (Wasscher) N.E. Gray | Margbensonia ridleyi, Podocarpus neriifolius var. ridleyi | - | Malaysia (Peninsular Malaysia) | VU |
| 175 | Podocarpus roraimae Pilg. | Podocarpus buchholzii, Podocarpus buchholzii var. neblinensis, Podocarpus buchholzii subsp. neblinensis | Ai-yek | Guyana (Region of Cuyuni-Mazaruni on Mt. Roraima), Venezuela (Amazonas, Bolívar) | LC |
| 176 | Podocarpus rostratus J.Laurent | - | - | Madagascar (Antsiranana, Fianarantsoa, Mahajanga and Toamasina Provinces) | EN |
| 177 | Podocarpus rubens de Laub. | Podocarpus indonesiensis, Podocarpus rubens subsp. sumatranus, Podocarpus rubens var. sumatranu, Podocarpus rubens var. pabinamaensis, Podocarpus neriifolius var. timorensis | Bebi-è, Ungpop, Bin, Kaip, Nelil, Sukou | Indonesia (Maluku, Flores, Borneo, Sulawesi, Sumatera), Malaysia (Sabah), Papua New Guinea (Bismarck Archipelago, Papuasia, New Britain), Timor-Leste | LC |
| 178 | Podocarpus rumphii Blume | Cerbera nereifolia, Margbensonia koordersii, Margbensonia philippinensis, Margbensonia rumphii, Nageia rumphii, Podocarpus koordersii, Podocarpus philippinensis, Podocarpus rumphii subsp. arbainii, Podocarpus rumphii var. arbainii, Podocarpus sprengelii, Podocarpus rumphii var. aruensis | Nimsal | China (Hainan), Indonesia (Jawa, Lesser Sunda Island, Maluku, Papua, Sulawesi), Malaysia (Peninsular Malaysia, Sabah), Papua New Guinea (Bismarck Archipelago), Philippines (Luzon) | NT |
| 179 | Podocarpus rusbyi J.Buchholz and N.E.Gray | - | Pino Blanco, Pino del Monte | Bolivia (Cochabamba, La Paz, Santa Cruz), Peru (Cusco and near Machu Pichu) | VU |
| 180 | Podocarpus salicifolius Klotzsch and H.Karst. ex Endl. | Nageia salicifolia, Podocarpus pittieri | Pinabete | Venezuela, Brazil, Bolivia, Colombia, Peru | LC |
| 181 | Podocarpus salignus D. Don | Nageia chilina, Podocarpus chilinus, Podocarpus chilinus var. glaucus | Willow-leaf podocarp, Mañio, Mañío de Hojas Largas, Mañio de Hojas Punzantes, Mañio Hembra, Mañio Macho, Manique, Pino Amarillo | Chile (Biobío, La Araucania, Los Lagos, Maule) | VU |
| 182 | Podocarpus salomoniensis Wasscher | - | Dengali tolo | Solomon Islands (San Cristobal Island and San Jorge Island) | EN |
| 183 | Podocarpus sellowii Klotzsch ex Endl. | Nageia sellowii | - | Brazil (Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina, São Paulo) | EN |
| 184 | Podocarpus sellowii Klotzch ex Endl. var. angustifolius | - | - | Brazil (Rio de Janeiro) | CR |
| 185 | Podocarpus smithii de Laub. | - | Smith’s pine, Brown pine | Australia (Queensland) | LC |
| 186 | Podocarpus spathoides de Laub. | Podocarpus spathoides de Laub. var. solomonensis | - | Malaysia (Peninsular Malaysia, Sarawak, Maluku), Papua New Guinea (Solomon Islands) | DD |
| 187 | Podocarpus spinulosus (Sm.) R.Br. ex Mirb. | Margbensonia spinulosa, Nageia ensifolia, Nageia laeta, Nageia spinulosa, Podocarpus bidwillii, Podocarpus ensifolius, Podocarpus laetus, Podocarpus pungens, Taxus spinulosa | - | Australia (New South Wales, Queensland) | LC |
| 188 | Podocarpus sprucei Parl. | Nageia sprucei | Guabisay, Romerillo | Ecuador, Peru (Piura) | EN |
| 189 | Podocarpus steyermarkii J.Buchholz and N.E.Gray | - | - | Guyana (Pakaraima Mountains), Venezuela [Bolivar (Carrao-tepui, Uaipan-tepui, Cerro Jaua), Amazonas (Neblina Massif)] | LC |
| 190 | Podocarpus subtropicalis de Laub. | Podocarpus subtropicalis var. medogensis, Podocarpus subtropicalis subsp. medogensis | - | China (Sichuan, Yunnan) | DD |
| 191 | Podocarpus sylvestris J.Buchholz | Podocarpus colliculatus, Podocarpus novae-caledoniae var. colliculatus | - | New Caledonia (Grande Terre, Ile des Pins) | LC |
| 192 | Podocarpus tepuiensis J.Buchholz and N.E.Gray | - | - | Ecuador (Cordillera del Condo), Venezuela (Bolivar, Amazonas) | LC |
| 193 | Podocarpus teysmannii Miq. | Nageia teysmannii, Podocarpus epiphyticus, Podocarpus neriifolius var. polyanthus | Kalek rotan, Sikuju | Myanmar, Indonesia (Sumatera), Malaysia (Peninsular Malaysia, Sabah), Brunei Darussalam | NT |
| 194 | Podocarpus thevetiifolius Zipp. ex Blume | Margbensonia thevetiifolia, Nageia thevetiifolia, Podocarpus polystachyus subsp. thevetiifolius, Podocarpus polystachyus var. thevetiifolius | - | Papua New Guinea | NA |
| 195 | Podocarpus totara G.Benn. ex D.Don | Nageia totara, Podocarpus totara var. waihoensis, Podocarpus totara subsp. waihoensis | Totara | New Zealand (North Island and South Island) | LC |
| 196 | Podocarpus totara var. waihoensis Wardle | - | Totara, Westland totara | New Zealand (West Coast of the South Island) | NT |
| 197 | Podocarpus transiens (Pilg.) de Laub. | Podocarpus lambertii var. transiens, Podocarpus transiens var. harleyi, Podocarpus transiens subsp. harleyi | - | Brazil (Bahia, Goiás, Minas Gerais, Paraná, Santa Catarina) | EN |
| 198 | Podocarpus trinitensis J.Buchholz and N.E.Gray | - | - | Trinidad and Tobago (El Tucuche) | LC |
| 199 | Podocarpus urbanii Pilg. | - | Blue mountain yacca, Yacca | Jamaica (St. Andrew, Portland and St. Thomas within the Blue and John Crow Mountains) | CR |
| 200 | Podocarpus vanuatuensis de Laub. | - | - | Vanuatu | DD |
| 201 | Podocarpus victorinianus Carabia | Podocarpus leonii | - | Cuba | NE |
| 202 | Prumnopitys andina (Poepp. ex Endl.) de Laub. | Nageia andina, Nageia valdiviana, Podocarpus andinus, Podocarpus spicatus, Podocarpus valdivianus, Prumnopitys andina subsp. blijdensteinii, Prumnopitys elegans, Prumnopitys spicata, Stachycarpus andinus | Lleuque, Llleuqui, Uva de la Cordillera | Chile (Biobío, La Araucania, Maule), Argentina (Neuquen) | VU |
| 203 | Prumnopitys taxifolia (Sol. ex D.Don) de Laub. | Dacrydium mai, Dacrydium taxifolium, Nageia spicata, Podocarpus spicatus, Stachycarpus spicatus | Matai, Black pine | New Zealand (North Island and South Island) | VU |
| 204 | Prumnopitys montana (Humb. and Bonpl. ex Willd.) de Laub. | Botryopitys densifolia, Botryopitys meridensis, Botryopitys montana, Dacrydium distichum, Nageia montana, Podocarpus curvifolius, Podocarpus humboldtii, Podocarpus montanus var. densifolius, Podocarpus montanus var. diversifolius, Podocarpus montanus var. meridensis, Podocarpus taxifolius, Podocarpus taxifolius var. communis, Podocarpus taxifolius var. densifolius, Stachycarpus meridensis, Stachycarpus taxifolius, Taxus montana, Torreya montana | - | Bolivia (Cochabamba), Colombia (Belmira, San Andres, Arabuca, Villa de Leiva, Pensilvania, Cauca, Cesar, La Guajira, Magdalena, Quindío, Risarald, Tolima), Ecuado (Azuay, Cañar, Loja, Morona-Santiago, Zamora-Chinchipe), Peru (Cajamarca, Junín, Pasco, San Martín), Venezuela (Lara, Tachira, Zulia) | VU |
| 205 | Pectinopitys exigua (De Laub.) C.N.Page | - | Pino colorado, Jatun pino, Pino castilla, Pino negro | Bolivia (Cochabamba, Chuquisaca, Santa Cruz) | NT |
| 206 | Pectinopitys ferruginea (G.Benn. ex D.Don in Lamb.) C.N.Page | Nageia ferruginea, Podocarpus ferrugineus, Stachycarpus ferrugineus, Stachypitys ferruginea | Miro, Brown pine | New Zealand (North Island, South Island and Stewart Island) | LC |
| 207 | Pectinopitys ferruginoides (R.H.Compton) C.N.Page | Podocarpus distichus, Podocarpus distichus var. maialis, Podocarpus ferruginoides, Stachycarpus distichus, Stachycarpus ferruginoides, Stachypitys disticha, Stachypitys ferruginoides | - | New Caledonia | LC |
| 208 | Pectinopitys harmsiana (Pilg.) C.N.Page | Podocarpus harmsianus, Podocarpus utilior, Prumnopitys utilior | Uncumanu, Yellow miro | Bolivia (Abel Iturralde, Franz Tamayo, Sud Yungas), Colombia (Cauca, Quindío, Risaralda, Tolima, Sierra Nevada de Santa Marta), Ecuador (Loja), Peru (Ayacucho, Cajamarca, Cusco, Junín, Pasco, Piura, San Martín), Venezuela (Vargas, Miranda, Aragua, Yaracuy) | NT |
| 209 | Pectinopitys ladei (F.M.Bailey) C.N.Page | Stachypitys ladei, Podocarpus ladei, Stachycarpus ladei | Mount spurgeon black pine or Mount spurgeon brown pine | Australia (Queensland) | NT |
| 210 | Pectinopitys standleyi (J. Buchholz and N.E.Gray) C.N.Page | Podocarpus standleyi, Stachycarpus standleyi | Cipresillo, Ciprecillo, Ciprés lorito | Costa Rica (Alajuela, Cartago, Heredia, San José) | EN |
| 211 | Retrophyllum comptonii (J.Buchholz) C.N.Page | Decussocarpus comptonii, Nageia comptonii, Podocarpus comptonii | - | New Caledonia (Port Boise to Mt Ignambi) | LC |
| 212 | Retrophyllum filicifolium (N.E.Gray) R.R.Mill | Podocarpus filicifolius | Lehil, moegò | Indonesia (Maluku), Papua New Guinea (Bismarck Archipelago) | LC |
| 213 | Retrophyllum minus (Carrière) C.N.Page | Nageia minor, Podocarpus minor, Podocarpus palustris, Decussocarpus minor, Retrophyllum minor | Bois bouchon | New Caledonia (Grande Terre, Province Sud: Prony, Baie du Sud, Lac en Huit, Rivière des Lacs, Plaine des Lacs) | EN |
| 214 | Retrophyllum piresii (Silba) C.N.Page | Decussocarpus piresii, Nageia piresii | - | Brazil (Rondônia), Bolivia, Peru | DD |
| 215 | Retrophyllum rospigliosii (Pilg.) C.N.Page | Decussocarpus rospigliosii, Nageia rospigliosii, Podocarpus rospigliosii, Torreya bogotensis | Pino hayuelo, Diablo fuerte, Pino de monte, Pino real, Pino romero, Romerillo fino, Romerillo rojo, Saucecillo | Bolivia (Lap Paz), Colombia (Antioquia, Cundinamarca, Magdalena, Norte de Santander, Santander del Sur), Ecuador (Sucumbíos, Zamora-Chinchip), Peru (Junín, Pasco), Venezuela (Táchira, Mérida, Trujillo) | VU |
| 216 | Retrophyllum vitiense (Seem.) C.N.Page | Decussocarpus vitiensis, Nageia vitiensis, Podocarpus vitiensis | Ailumu, Dakua salusalu, Kau solo, Mungo | Fiji, Indonesia (Maluku, Papua), Papua New Guinea (Bismarck Archipelago), Solomon Islands (Santa Cruz Island), Vanuatu (Torba) | LC |
| 217 | Saxegothaea conspicua Lindl. | Squamataxus albertiana | Prince albert’s yew, Mañío, Mañío de hojas cortas, Mañío hembra, Mañío macho, Maniú | Argentina (Chubut, Neuquén, Rio Negro), Chile (Aisén, Biobío, La Araucania, Los Lagos, Maule) | NT |
| 218 | Sundacarpus amarus (Blume) C.N.Page | Nageia amara, Nageia eurhyncha, Podocarpus amara, Podocarpus dulcamara, Podocarpus eurhyncha, Podocarpus pedunculatus, Prumnopitys amara | Black pine, Choopoola | Australia (Queensland), Indonesia (Jawa, Maluku, Papua, Sulawesi, Sumatera), Malaysia (Sabah), Papua New Guinea (Bismarck Archipelago), Philippines, Timor-Leste | LC |
Figure 2.
Diagram of two-way cluster analysis. The vertical matrix axis consists of more than 200 podocarp species coded by scientific name and the horizontal axis is the distributed countries (74 countries). The matrix was constructed depending on the presence (black) and absence (white). The species were grouped into five main clusters, I. New Caledonian group, II. New Zealand group and III. Malesian group, IV. Southeast Asian group and V. Podocarpian group.
Acmopyle has two species in New Caledonia (A. pancheri) and Fiji (A. sahniana).
Afrocarpus has five species distributed across Africa.
Dacrycarpus has nine species and two varieties distributed in New Caledonia, New Zealand, some Pacific Islands and Southeast Asia (Figure 3A).
Dacrydium has 20 species and two hybrids distributed in New Caledonia, New Zealand, some Pacific Islands and Southeast Asia.
Falcatifolium has six species distributed in New Caledonia, Papua New Guinea, Indonesia, Malaysia, the Philippines and Brunei Darussalam.
Halocarpus has three species endemic to New Zealand.
Manoao has one species distributed in New Zealand.
Pherosphaera has two species endemic to Australia.
Lagarostrobos has one species endemic to Australia.
Microcachrys has one species endemic to Australia (Figure 3B).
Lepidothamnus has three species distributed in Argentina, Chile and New Zealand.
Nageia has six species distributed in Southeast Asia to India and Myanmar, Papua New Guinea, the Philippines and Japan.
Parasitaxus has one parasitic species endemic to New Caledonia.
Phyllocladus has four species and one variety distributed in Papua New Guinea, Indonesia, Malaysia, the Philippines, New Zealand and Australia.
Podocarpus is the largest genus, with 120 species, 4 subspecies, 9 varieties and one hybrid and has a wide distribution, occurring in all continents (approximately 70 countries) except Europe and Antarctica.
Prumnopitys has three species distributed in New Zealand and South America.
Pectinopitys has six species distributed in New Zealand, Australia, New Caledonia and South America.
Retrophyllum has six species distributed in New Caledonia, South America and the Pacific Islands.
Saxegothaea has one species distributed in South America.
Sundacarpus has one species distributed in Australia, Indonesia, Malaysia, Papua New Guinea (Timor-Leste) and the Philippines.
Figure 3.
(A) Dacrycarpus dacrydioides (White pine, Kahikatea) tree in the rainforest of Wellington Kaitoke Regional Park, New Zealand. (B) Microcachrys tetragona (Strawberry pine) is a creeping shrub in the alpine region of cradle mountain summit, Tasmania.
The Podocarpaceae has its highest diversity of genera in New Zealand (nine) with fewer in other regions New Caledonia and Malesia (eight), Australia (seven), South America (four) and Africa and Asia (two). Of the 20 genera, three are endemic to Australia and two are endemic to New Zealand. Countries with a rich diversity of podocarps include Indonesia (51 species), Papua New Guinea (43 species), Malaysia (39 species), the Philippines (23 species), New Caledonia (20 species), New Zealand (19 species), China (17 species), Venezuela (15 species), Australia (14 species), Brazil, Peru and Bolivia (12 species each), Fiji (11 species), Ecuador (9 species) and Madagascar, Thailand, Brunei Darussalam and Colombia (8 species each).
3.3. Podocarpaceae in Space and Time
This checklist consists of macrofossils assigned to extant podocarp genera and includes more than one hundred taxa from the Cretaceous to the Pleistocene (Table 2). The macrofossils are predominantly recorded from Eocene–Miocene deposits. Australian and New Zealand macrofossil records dominate. Most of the macrofossils are foliage but well-preserved reproductive parts (seed and pollen cones) are also recorded for Lepidothamnus, Lagarostrobos, Dacrycarpus, Phyllocladus, Podocarpus and Nageia. Extant podocarps dominate in the Southern Hemisphere and analysis of extinct taxa assigned to living podocarp genera supports their past importance in the Southern Hemisphere (Table 2). A number of extinct species assigned to Podocarpaceae genera have been reported from Australia, New Zealand and South America [26]. An analysis shows that:
Table 2.
An updated checklist of podocarps fossil sp.ecies of extant genera.
| S# | Fossil Taxa | Reported by | Part | Era/Period | Age Range Ma | Type Specimen | Distribution |
|---|---|---|---|---|---|---|---|
| 1 | Acmopyle antarctica | Florin, 1940 [27] | Leaf axis | Eocene | 99.7 to 55.8 | S132020-Swedish Museum of Natural History | Seymour Island (Antarctic Peninsula) |
| 2 | Acmopyle compactus | Pole, 1992 [28] | Leaf (Cuticle) | Middle–Late Eocene | 48.6 to 33.9 | S049-University of Tasmania | Hasties (Tasmania, Australia) |
| 3 | Acmopyle engelhardti | Florin, 1940 [27] | Leaf axis | Eocene–Miocene | 55.8 to 48.6 | PBDB 8673 | (9 records) Rio Negro, Argentina |
| 4 | Acmopyle florinii | Hill and Carpenter, 1991 [29] | Sterile axis (Cuticle) | Late Paleocene | 58.7 to 55.8 | LB-063-University of Tasmania | Lake Bungarby (New South Wales, Australia) |
| 5 | Acmopyle glabra | Hill and Carpenter, 1991 [29] | Sterile axis (Cuticle) | Oligocene–Eocene | 55.8 to 28.4 | RPE-006-University of Tasmania | (9 records) Cethana—Oligocene, Regatta Point—Eocene (Tasmania, Australia) |
| 6 | Acmopyle masonii | Pole, 1997 [30] | Sterile axis (Cuticle) | Miocene | - | SB 1149-University of Tasmania. | Manuherikia Group, Central Otago, New Zealand |
| 7 | Acmopyle setiger | Hill and Carpenter, 1991; Carpenter and Pole, 1995; Townrow, 1965 [29,31,32] | Leaf (Cuticle) | Early Eocene | 48.6 to 33.9 | B-001-University of Tasmania | Lake Lefroy, Buckland and Monitor Bores (Tasmania and Western Australia) |
| 8 | Acmopyle tasmanica | Hill and Carpenter, 1991 [29] | Leaf (Cuticle) | Eocene | 48.6 to 33.9 | LA-060-University of Tasmania | Loch Aber (Tasmania, Australia) |
| 9 | Dacrycarpus acutifolius | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene to Early Miocene | 28.4 to 15.97 | M-235- University of Tasmania | Monpeelyata (Tasmania, Australia) |
| 10 | Dacrycarpus arcuatus | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene to Early Miocene | 28.4 to 15.97 | LRR1-243-University of Tasmania | Little Rapid River (Tasmania, Australia) |
| 11 | Dacrycarpus carpenterii | Jordan, 1995 [34] | Leaf | Early Pleistocene | 2.588 to 0.126 | RPU 525-University of Tasmania | Regatta Point (Tasmania, Australia) |
| 12 | Dacrycarpus chilensis | Wilf, 2012 [35] | Leaf (Cuticle) | Eocene | MMG PB SAT | Chile | |
| 13 | Dacrycarpus crenulatus | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene to Miocene | 28.4 to 15.97 | P-221-University of Tasmania | Pioneer—tin mine (Tasmania, Australia) |
| 14 | Dacrycarpus cupressiformis | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Early Oligocene | 33.9 to 23.0 | LRR2-023-University of Tasmania | Little Rapid River 2 (Tasmania, Australia) |
| 15 | Dacrycarpus dacrydoides | Pole, 1992; 1997 [28,30] | Leaf (Cuticle) | Miocene | - | SB 1149-University of Tasmania. | Manuherikia Group, Central Otago, New Zealand |
| 16 | Dacrycarpus geminus | Pole, 1992 [28] | Leaf (Cuticle) | Eocene | 56 to 33.9 | S115-University of Tasmania | Hasties (Tasmania, Australia) |
| 17 | Dacrycarpus guipingensis | Wu et al., 2021 [36] | Seed cone+ Sterile axis | Miocene | - | GP109-Museum of Biology, Sun Yat-sen University | Guangxi, South China |
| 18 | Dacrycarpus involutus | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene to Miocene | 28.4 to 15.97 | M2023-University of Tasmania | Monpeelyata (Tasmania, Australia) |
| 19 | Dacrycarpus elandensis | Hill and Whang, 2000 [37] | Pollen cone, leaf | Miocene | - | ELD-005-University of Adelaide | Elands, New South Wales |
| 20 | Dacrycarpus falcatus | Carpenter, 1991 [38] | Sterile axis (Cuticle) | Oligocene | 35 | C-052, 203, 619-University of Tasmania | Cethana (Tasmania, Australia) |
| 21 | Dacrycarpus lanceolatus | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene to Miocene | 28.4 to 15.97 | M-1186-University of Tasmania | Monpeelyata (Tasmania, Australia) |
| 22 | Dacrycarpus latrobensis | Hill and Carpenter, 1991 [29] | Sterile axis (Cuticle) | Oligocene to Miocene | 28.4 to 23.03 | P 15714- Museum of Victoria, Melbourne | Southeastern Australia (Yallourn and Bacchus-3 locations) |
| 23 | Dacrycarpus linearis | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene | 28.4 to 23.03 | LRR2-051-University of Tasmania | Little Rapid River 2 (Tasmania, Australia) |
| 24 | Dacrycarpus linifolius | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Eocene to Oligocene | 55.8 to 28.4 | LRR1-851-University of Tasmania | Little Rapid River 1 (Tasmania, Australia) and Regatta Point (Tasmania, Australia) |
| 25 | Dacrycarpus microfolius | Jordan et al., 2011 [39] | Cuticle | Oligocene–Miocene | 28.4 to 15.97 | OU33024-Geology Museum (OU), University of Otago | F45/f0394, middle Gore Lignite Measure (Newvale Mine, New Zealand) |
| 26 | Dacrycarpus mucronatus | Wells and Hill, 1989; Carpenter, 1991; Lewis and Drinnan, 2013 [33,38,40] | Seed cone+ Sterile axis (Cuticle) | Eocene–Oligocene–Miocene | 48.6 to 15.97 | LRR2-044-University of Tasmania, (RPE-060-62, and RPE-4620 | Little Rapid River 2 (Tasmania); Regatta Point (Tasmania) Cethana (Tasmania); Lochaber (Naracoorte, South Australia) |
| 27 | Dacrycarpus patulus | Hill and Merrifield, 1993 [41] | Leaf | Eocene–Oligocene | 48.6 to 23.03 | WAM P.84.34-Western Australian Museum | West Dale (Western Australia, Australia) |
| 28 | Dacrycarpus praecupressinus | Greenwood, 1987; Mill and Hill, 2004 [42,43] | Sterile axis (Leaves) | Eocene | 37.2 to 33.9 | F 51245-Geological Survey of New South Wales | Vegetable Creek—Witherdens Tunnel (NSW, Australia) |
| 29 | Dacrycarpus puertae | Wilf, 2012 [35] | Seed cone+ Sterile axis | Eocene | 52–77.9 | MPEF-Pb 4983- | Patagonia, Argentina |
| 30 | Dacrycarpus sp. | Pole et al., 1993 [44] | Leaf (Cuticle) | Late Oligocene–Early Miocene | - | SB282 | Berwick Quarry, Victoria (Australia) |
| 31 | Dacrycarpus sp. | Carpenter and Pole, 1995 [32] | Leaf (Cuticle) | Middle Eocene | - | CD2999-University of Tasmania | Lefroy and Cowan Paleodrainages, Western Australia (Australia) |
| 32 | Dacrycarpus sp. | Carpenter, 1991 [38] | Foliage (Cuticle) | Oligocene | 35 | - | Cethana (Tasmania, Australia) |
| 33 | Dacrycarpus sp. | Carpenter et al., 1994 [45] | Foliage (Cuticle) | Oligocene–Early Miocene | - | R. J. Carpenter and R. S. Hill (unpublished data) | Lea River (Tasmania, Australia) |
| 34 | Dacrydium aciculare | Wells and Hill, 1989 [33] | Leaf (Cuticle) | Oligocene | 33.9 to 28.4 | LRR1-441-University of Tasmania | Little Rapid River 1 (Tasmania, Australia) |
| 35 | Dacrydium fimbriatus | Hill and Christophel, 2001 [46] | Sterile axis (Cuticle) | Middle Eocene | 48.6 to 37.2 | NC-004-University of Adelaide | Nelly Creek (South Australia, Australia) |
| 36 | Dacrydium microphyllum | Jordan et al., 2011 [39] | Cuticle | Oligocene–Miocene | 28.4 to 15.97 | OU33026-Geology Museum (OU), University of Otago | New Vale Mine, Waimumu (Coalfield, Southland, New Zealand) |
| 37 | Dacrydium mucronatus | Hill and Christophel, 2001 [46] | Fertile axis (Cuticle) | Eocene | 48.6 to 37.2 | NC-002- University of Adelaide | Nelly Creek (South Australia, Australia) |
| 38 | Dacrydium rhomboideum | Cookson and Pike, 1953; Blackburn, 1985 [47,48] | Foliage + Seeds | Oligocene–Miocene | 28.4 to 15.97 | P 209942 | Morwell and Yallourm, Victoria (Australia) |
| 39 | Dacrydium sinuosum | Wells and Hill, 1989 [33] | Leaf (Cuticle) | Oligocene–Miocene | 28.4 to 15.97 | P-631-University of Tasmania | Pioneer- tin mine (Tasmania, Australia) |
| 40 | Dacrydium tasmanicum | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene | 33.9 to 28.4 | LRR1-1031-University of Tasmania | Little Rapid River 1 (Tasmania, Australia) |
| 41 | Dacrydium waimumuensis | Jordan et al., 2011 [39] | Cuticle | Oligocene–Miocene | 28.4 to 15.97 | OU33025-Geology Museum (OU), University of Otago | F45/f0394, middle Gore Lignite Measure (Newvale Mine, New Zealand) |
| 42 | Dacrydium Sp1 | Carpenter, 1991 [38] | Foliage | Early Oligocene | 35 | C-202, 471-University of Tasmania | Cethana (Tasmania, Australia) |
| 43 | Dacrydium Sp2 | Carpenter, 1991 [38] | Foliage | Early Oligocene | 35 | C-517, 519-University of Tasmania | Cethana (Tasmania, Australia) |
| 44 | Dacrydium Sp1 | Carpenter and Pole, 1995 [32] | Foliage (disp.ersed cuticles) | Middle Eocene | - | CD2999 and DWT495-University of Tasmania | Lefroy and cowan paleodrainage, Western Australia |
| 45 | Dacrydium Sp2 | Carpenter and Pole, 1995 [32] | Foliage (disp.ersed cuticles) | Middle Eocene | - | CD2999-University of Tasmania | Lefroy and cowan paleodrainage, Western Australia |
| 46 | Dacrydium Sp | Blackburn, 1985 [48] | Foliage | Oligocene–Miocene | - | - | Morwell, Victoria (Australia) |
| 47 | Dacrydium Sp | Blackburn, 1985 [48] | Foliage | Oligocene–Miocene | - | - | Morwell, Victoria (Australia) |
| 48 | Falcatifolium eocenica | Hill and Scriven, 1999 [49] | Sterile axis (Cuticle) | Middle Eocene | 37.2 to 33.9 | 2351 and 2350-State Herbarium of South Australia. | ALCOA Anglesea Site II coal mine (Victoria, Australia) |
| 49 | Halocarpus highstedii | Jordan et al., 2011 [39] | Cuticle | Oligocene–Miocene | 28.4 to 15.97 | OU32899-Geology Museum (OU), University of Otago | F45/f0394, middle Gore Lignite Measure (Newvale Mine, New Zealand) |
| 50 | Lagarostrobos franklinii | Wells and Hill, 1989; Hill and Macphail, 1985; Carpenter et al., 1994; Jordan, 1995; Jordan et al., 2011 [33,34,39,45,50] | Seed cones and foliage | Late Pliocene–Early Pleistocene | 2.588 to 0.126 | RPU-190-University of Tasmania | Regatta Point (Tasmania, Australia) |
| 51 | Lagarostrobos marginatus | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene | 33.9 to 28.4 | LRR1-701-University of Tasmania | Little Rapid River 1 (Tasmania, Australia) |
| 52 | Lagarostrobos Sp | Peter, 1985 [51] | Sterile axis | Middle Cretaceous | 145-100.5 | - | Winton, Queensland |
| 53 | Lagarostrobos colensoi (correct name—Manoao colensoi) | Carpenter, 1991 [38] | Leaf (Cuticle) | Oligocene | 35 | - | Cethana (Tasmania, Australia) |
| 54 | Lepidothamnus intermedius | Pole, 1997 [30] | Sterile axis (Cuticle) | Miocene | - | S-632-University of Tasmania. | Manuherikia Group, Central Otago, New Zealand |
| 55 | Lepidothamnus diemenensis | Pole, 1992 [28] | Sterile axis (Cuticle) | Eocene | 48.6 to 33.9 | S014-University of Tasmania | Hasties (Tasmania, Australia) |
| 56 | Lepidothamnus | Peter, 1985 [51] | Seed cones and foliage | Middle Cretaceous | 145-100.5 | - | Winton, Queensland |
| 57 | Microcachrys tetragona | Jordan, 1995 [34] | Seed and sterile axes | Early Pleistocene | RPU2-University of Tasmania | Regatta Point (Tasmania, Australia) | |
| 58 | Microcachrys novaezelandiae | Carpenter et al., 2011 [52] | Leaf (Cuticle) | Oligocene–Miocene | 28.4 to 15.97 | OU32896-Geology Museum (OU), University of Otago | F45/f0394, middle Gore Lignite Measure (Newvale Mine, New Zealand) |
| 59 | Nageia hainanensis | Jin et al., 2010 [53] | Leaf (Cuticle) | Eocene | - | CC-1200 a, b-The Museum of Biology of Sun Yat-sen University, Guangzhou, China | Changchang Basin, Hainan Island, south China |
| 60 | Nageia maomingensis | Liu et al., 2015 [54] | Leaf (Cuticle) | Late Eocene | - | MMJ1-001-The Museum of Biology, Sun Yat-sen University, Guangzhou, China. | Maoming Basin, Jintang, Maoming, Guangdong Province, South China. |
| 61 | (Podocarpus) Nageia ryosekiensis | Kimura et al., 1988 [55] | Leafy branches, connected seed, detached leaves | Lower Cretaceous | - | Makino Botanical Garden, Kochi | Southwest Japan |
| 62 | (Podocarpus) Nageia sujfunensis | Krassilov, 1965 [56] | Leaf (Cuticle) | Early Cretaceous | - | 27/71-Far East Geological Institute, USSR Academy of Sciences | Far East Russia |
| 63 | Pherosp.haera sommervillae (Name correction Microstrobos sommervillae) | Townrow, 1965 [31] | Early Eocene | - | - | Buckland sediments in southeastern Tasmania | |
| 64 | Pherosp.haera microfolius (Name correction Microstrobos microfolius) | Wells and Hill, 1989 [33] | Sterile axis (Cuticle) | Oligocene–Miocene | 28.4 to 23.03 | M-1155-University of Tasmania | Mudstone lens cutting Monpeelyata canal (Tasmania, Australia) |
| 65 | Phyllocladus aberensis | Hill, 1989 [57] | Leaf | Oligocene (Middle–Late Eocene) | 28.4 to 23.03 | LRR1-951-University of Tasmania | Little Rapid River 2 (Tasmania, Australia) |
| 66 | Phyllocladus annulatus | Hill, 1989 [57] | Leaf (Cuticle) | Oligocene | 33.9 to 23.03 | P-742-University of Tasmania | Pioneer (Tasmania, Australia) |
| 67 | Phyllocladus aspleniifolius | Ettingshausen, 1887, 1888; Cookson and Pike, 1954; Hill and Macphail, 1985; Hill, 1989, 1988; Pole, 1992 [28,50,57,58,59,60] | Leaf (Cuticle) | Late Eocene (Quaternary, Eocene and Cretaceous) | 56-33.9 (84.9 to 0.012) | S 106-University of Tasmania | Deep leads NSW; Regatta point; Hasties, Tasmania (Australia) and Antarctica |
| 68 | Phyllocladus elongatus | Jordan et al., 2011 [39] | Leaf | Oligocene–Miocene | 28.4 to 15.97 | OU32901-Geology Museum (OU), University of Otago | F45/f0394, middle Gore Lignite Measure (Newvale Mine, New Zealand) |
| 69 | Phyllocladus lobatus | Hill, 1989 [57] | Cuticle | Oligocene | 28.4 to 23.03 | LRR1-1649- University of Tasmania | Little Rapid River 2 (Tasmania, Australia) |
| 70 | Phyllocladus morwellensis | Deane, 1925; Cookson and Pike, 1954; Hill, 1989 [57,60,61] | Leaf | Oligocene | 28.4 to 15.97 | P-15873-Museum of Victoria | Near Morwell, (Victoria, Australia) |
| 71 | Phyllocladus palmeri | Pole and Moore, 2011 [62] | Leaf (Cuticle) | Late Miocene | 6 to 6.5 | AU P340a and b-School of geography, geology and environmental science, University of Auckland | Near Matuora, (Coromandel Peninsula, New Zealand) |
| 72 | Phyllocladus Sp (P. lobatus) | Carpenter, 1991 [38] | Leaf (Cuticle) | Oligocene | - | - | Cethana, (Tasmania, Australia) |
| 73 | Phyllocladus Sp2 (P. hypophyllus) | Carpenter, 1991 [38] | Leaf (Cuticle) | Oligocene | - | - | Cethana, (Tasmania, Australia) |
| 74 | Phyllocladus Sp | Mc Loughlin and Hill, 1996; Mc Loughlin et al., 2001 [63,64] | Leaf (Cuticle) | Late Eocene | - | - | Kojonup, Western Australia |
| 75 | Phyllocladus Sp | Pole, 1992 [28] | Leaf (Cuticle) | Late Miocene | - | - | Near Cromwell, (South Island, New Zealand) |
| 76 | Phyllocladus Sp | Pole, 1992 [28] | Leaf (Cuticle) | Miocene | - | OU30068-Department of Geology, University of Otago. | Manuherikia Group, Central Otago, New Zealand |
| 77 | Phyllocladus Sp | Liz Kennedy, 2020 (unpublished) | Seed cones (Phyllocladus toatoa) | Miocene | 23 to 5.3 | - | Coromandel, North Island, New Zealand |
| 78 | Podocarpus andiniformis (Subgenus Foliatus) | Wilf et al., 2017/Berry, 1922 [65,66] | Leaf (Cuticle) | Late Triassic and Early Eocene | - | - | Patagonia, Argentina |
| 79 | Podocarpus alwyniae (Subgenus Podocarpus) | Pole, 1992 [28] | Sterile axis (Cuticle) | Miocene | - | OU29708, H411fD45-Department of Geology, University of Otago. | Manuherikia Group, Central Otago, New Zealand |
| 80 | Podocarpus oligocenicus | Awasthi et al., 1992 [67] | Leaf (Cuticle) | Oligocene | - | - | Mizoram, India; Manipur, India |
| 81 | Podocarpus araucoensis (as Decussocarpus araucoensis) | Berry, 1922; Mill and Hill, 2004 [43,66] | Foliage (Cuticle) | Eocene | 55.8 to 33.9 | - | Chile |
| 82 | Podocarpus brownei (probably Retrophyllum or Falcatifolium) | Greenwood, 1987 (Decussocarpus brownei); Mill and Hill, 2004 [42,43] | Foliage (Cuticle) | Eocene | 37.2 to 33.9 | 2345-State Herbarium of South Australia | ALCOA Anglesea Site II coal mine (Victoria, Australia) |
| 83 | Podocarpus fildesensis | Zhou and Li, 1994 [68] | Foliage | Cretaceous | 84.9 to 66.043 | - | Half Three Point assemblage (Antarctica) |
| 84 | Podocarpus inopinatus | Florin, 1940 [27] | Foliage | Paleogene, Eocene–Miocene | 55.8 to 33.9 | - | Chile |
| 85 | Podocarpus platyphyllum | Greenwood, 1987 [42] | Leaf (Cuticle) | Middle to Late Eocene | 37.2 to 33.9 | 1816-State Herbarium of South Australia | ALCOA Anglesea Site II coal mine (Victoria, Australia) |
| 86 | Podocarpus sinuatus | Pole, 1992 [28] | Leaf (Cuticle) | Eocene | 48.6 to 33.9 | S112-University of Tasmania | Hasties (Tasmania, Australia) |
| 87 | Podocarpus pliomacrophyllus (Subgenus Foliatus) | Chen et al., 2019; Wu et al., 2021 [69,70] | Leaf (Cuticle) | Lower Pliocene | - | MBU-16395- Institute of Palaeontology and Stratigraphy, Lanzhou University, Gansu Province, China. |
Mannong Village (western Yunnan, China); Tuantian Town, Yunnan Province, southwestern China |
| 88 | Podocarpus travisiae (Subgenus Podocarpus) | Pole, 1993 [71] | Leaf | Miocene | 23.03 to 15.97 | 0U30780- Department of Geology, University of Otago | Foulden Hills (New Zealand) |
| 89 | Podocarpus yunnanensis (Subgenus Foliatus) | Wu et al., 2021 [70] | Leaf (Cuticle) | Early Pliocene | - | MBU-19122301-Institute of Palaeontology and Stratigraphy, Lanzhou University | Tuantian Town, Yunnan Province, southwestern China |
| 90 | Podocarpus forrestii (Subgenus Foliatus) | Wu et al., 2021 [70] | Leaf (Cuticle) | Early Pliocene | - | MBU-20191221-Institute of Palaeontology and Stratigraphy, Lanzhou University | Tuantian Town, Yunnan Province, southwestern China |
| 91 | Podocarpus tasmanicus (Subgenus Podocarpus) | Townrow, 1965 [31] | Leaf (Cuticle) | Eocene | - | 81905-University of Tasmania | Bed of Tea Tree Rivulet, Buckland, Tasmania, Australia |
| 92 | Podocarpus strzeleckianus (Subgenus Podocarpus) | Townrow, 1965 [31] | Leaf (Cuticle) | Eocene | - | 81917-University of Tasmania | Bed of Tea Tree Rivulet, Buckland, Tasmania, Australia |
| 93 | Podocarpus witherdenensis (Subgenus Podocarpus) | Hill and Carpenter, 1991 [29] | Fertile axis+ seed cones (Cuticle) | Eocene | 37.2 to 33.9 | MMF 1201-Geological Survey of New South Wales, Sydney | Vegetable Creek—Witherdens Tunnel (NSW, Australia) |
| 94 | Podocarpus Sp | Carpenter et al., 1994 [45] | Foliage (Cuticle) | Oligocene–Early Miocene | - | R. J. Carpenter and R. S. Hill (unpublished data) | Lea River (Tasmania, Australia) |
| 95 | Podocarpus Sp1 | Carpenter, 1991 [38] | Foliage (Cuticle) | Oligocene | - | University of Tasmania | Cethana, (Tasmania, Australia) |
| 96 | Podocarpus Sp2 | Carpenter, 1991 [38] | Foliage (Cuticle) | Oligocene | - | C-251, 274, 275, 342, 492- University of Tasmania | Cethana, (Tasmania, Australia) |
| 97 | Podocarpus Sp | Carpenter et al., 1994 [45] | Foliage (Cuticle) | Oligocene–Early Miocene | - | R. S. Hill (unpublished data) | Little Rapid River (Tasmania, Australia) |
| 98 | Podocarpus Sp | Hill and Macphail, 1985 [50] | Foliage (Cuticle) | Late Pliocene–Early Pleistocene | - | University of Tasmania | Regatta Point (Tasmania, Australia) |
| 99 | Podocarpus Sp (Subgenus Podocarpus) | Jordan et al., 2011 [39] | Foliage (Cuticle) | Late Oligocene–Early Miocene | - | OU33027- Geology Museum, University of Otago | Newvale site, (South Island, New Zealand) |
| 100 | Podocarpus Sp | Pole, 1997 [72] | Foliage (Cuticle) | Miocene | - | H41/f74, S788-University of Tasmania. | Manuherikia Group, Central Otago, New Zealand |
| 101 | Podocarpus Sp | He and Wang, 2021 [73] | Leaf (Cuticle) | Miocene | 17 to 14 | - | Guangchang County, Jiangxi Province, southeastern China |
| 102 | Retrophyllum australe | Hill and Merrifield, 1993 [41] | Sterile axis | Eocene–Oligocene | 48.6 to 23.03 | WAM P.88.96-Western Australian Museum | West Dale (Western Australia, Australia) |
| 103 | Retrophyllum superstes | Wilf et al., 2017 [65] | Sterile axis (Leafy twig) | Cretaceous–Paleocene | 70.6 to 61.7 | MPEF-Pb 8910- Patagonia, Argentina | LefE (Chubut, Argentina) |
| 104 | Retrophyllum oxyphyllum (Retrophyllum sp.iralifolium) | Wilf, 2020 [74] | Sterile axis, cuticle, leaves, fertile axis | Eocene | 52 | MLP-4234 and MPEF–Pb 8915a Museo Paleontológico Egidio Feruglio | Trelew, Argentina |
| 105 | Retrophyllum vulcanense | Pole, 1992 [28] | Sterile axis (Cuticle) | Miocene | - | OU29857-Department of Geology, University of Otago. | Manuherikia Group, Central Otago, New Zealand |
| 106 | Prumnopitys tasmanica | Mill and Hill, 2004; Greenwood, 1987 [42,43] | Sterile axis (Cuticle) | Eocene | - | 81905-University of Tasmania | Alcoa Anglesea, Victoria, Australia |
| 107 | Prumnopitys montana | Pole, 1992 [28] | Cuticle | Eocene | 48.6 to 33.9 | S 110.-University of Tasmania | Hasties (Tasmania, Australia) |
| 108 | Prumnopitys opihiensis | Pole, 1997 [72] | Cuticle | Cretaceous/Eocene | 99.7 to 48.6 | OU30932- University of Otago | Taratu Formation (New Zealand) |
| 109 | Prumnopitys portensis | Pole, 1992 [28] | Leaf | Eocene | 48.6 to 33.9 | S056-University of Tasmania | Hasties (Tasmania, Australia) |
| 110 | Prumnopitys taxifolia (Leaf morphology is similar to that of Sundacarpus) | Pole, 1997 [30] | Leaf | Miocene | - | SB 1154--Department of Geology, University of Otago. | Manuherikia Group, Central Otago, New Zealand |
| 111 | Sundacarpus anglica | Page, 2019 [75] | Leaf (Cuticle) | Eocene | 48.6 to 33.9 | V.46883- Natural History Museum, BM | Bandulska (Bournemouth, England) |
| 112 | Sundacarpus tzagajanicus | Page, 2019 [75] | Leaf (Cuticle) | Uppermost Cretaceous (Earliest Paleocene—65.5–61.7 Ma) | 65.5–61.7 | 575-126-Far Eastern Scientific Centre, Vladivostok | Bureya River (Russia) |
Acmopyle has eight fossil species recorded (A. antarctica, A. compactus, A. engelhardti, A. florinii, A. glabra, A. masonii, A. setiger and A. tasmanica) from the Eocene–Oligocene of the Antarctic Peninsula, Australia, New Zealand and Argentina [27,28,29,30,31,32]. The fossil record shows a wider past distribution of Acmopyle compared to its current occurrence in New Caledonia and Fiji.
Dacrycarpus has approximately 25 fossil taxa reported (D. acutifolius, D. arcuatus, D. carpenterii, D. chilensis, D. crenulatus, D. cupressiformis, D. dacrydoides, D. geminus, D. guipingensis, D. involutus, D. elandensis, D. falcatus, D. lanceolatus, D. latrobensis, D. linearis, D. linifolius, D. microfolius, D. mucronatus, D. patulus, D. praecupressinus, D. puertae, Dacrycarpus sp. (four separate records)) from the Eocene–Early Pleistocene of Australia, New Zealand, China, Argentina [28,30,32,33,34,35,36,37,38,39,40,41,42,43,45,71,76]. Although Dacrycarpus currently has no living species in Australia or South America, the fossil record shows its extensive distribution in both those landmasses in the past.
Dacrydium has approximately 14 fossil taxa reported from Australia (D. aciculare, D. fimbriatus, D. mucronatus, D. sinuosum, D. rhomboideum, D. tasmanicum and six taxa described to genus level) and two species from New Zealand (D. microphyllum and D. waimumuensis) [32,33,38,39,47,48]. These suggest an Australasian origin in the Late Cretaceous [77].
Falcatifolium has only one fossil extinct species (F. eocenica) from the Middle Eocene of Victoria, Australia [49]. Currently, Australia has no living species of Falcatifolium.
Nageia has four extinct species (N. hainanensis, N. maomingensis, N. ryosekiensis, N. sujfunensis), from the Early Cretaceous–Eocene in China, Southwest Japan, and Far East Russia [53,54,55,56,78]. This demonstrates that Nageia had a wider past distribution and occurred in Japan and Russia.
Retrophyllum has four extinct species (R. australe, R. oxyphyllum, R. superstes, R. vulcanense) recorded from the Cretaceous–Miocene in Australia, Argentina, and New Zealand [28,41,65,74]. Currently, Australia and New Zealand have no living species of Retrophyllum.
Podocarpus has at least 16 extinct species reported and approximately seven taxa identified at the genus level. These are Podocarpus platyphyllum, P. sinuatus, P. strzeleckianus, P. tasmanicus and P. witherdenensis (Eocene) from Australia; P. travisiae and P. alwyniae (Miocene) from New Zealand; P. andiniformis, P. araucoensis and P. inopinatus (Eocene–Miocene) from South America; and P. pliomacrophyllus, P. yunnanensis and P. forrestii (lower Pliocene) from China [27,28,38,42,65,69,70,71,79].
Halocarpus has a single occurrence of one extinct species (Halocarpus highstedii) reported from the Oligocene–Miocene of New Zealand [39].
Manoao colensoi has a fossil record from the Oligocene of Cethana, Tasmania (Australia) (reported as Lagarostrobos colensoi) [38], showing that this current New Zealand endemic genus was once also distributed in Australia.
Lepidothamnus has three fossil records: L. intermedius from the Miocene in New Zealand [30], L. diemenensis from the Eocene of Hasties, Tasmania [28] and an undescribed extinct species from the middle Cretaceous of Winton, Queensland [51]. This indicates a wider distribution of Lepidothamnus in the Late Mesozoic across the Southern Gondwana regions [51].
Lagarostrobos has two fossil records, e.g., L. marginatus from the Early Oligocene and the extant L. franklinii from the Early Pleistocene in Tasmania, Australia [33,34,39,45,50]. Current and macrofossil records suggest a narrow distribution and endemism to Australia for this genus.
Phyllocladus has seven fossil species described, including records of the extant P. aspleniifolius (the fossil species are P. aberensis, P. annulatus, P. elongatus, P. lobatus, P. morwellensis, P. palmeri) and six at genus level from Late Eocene–Pliocene in Australia, New Zealand and Antarctica (Cretaceous) [38,39,47,50,57,58,59,61,62,63,64]. Protophyllocladus Berry [80], an extinct genus that resembles the foliage morphology of Phyllocladus, is recorded from the Jurassic and Cretaceous of the United States and southwestern Canada, Western Greenland, Serbia, Romania, Portugal, Kazakhstan, Japan, northeastern Russia, Germany [81]. Dörken et al. [82] considered that it is unlikely that Protophyllocladus is closely related to Phyllocladus. Wagstaff [83] suggested that extant species are the remnants of one of the recently diverged lineages of Phyllocladus, but there is no unequivocal fossil evidence to support this.
Prumnopitys has two extinct species (P. portensis and P. tasmanica) and a fossil record of one extant species (P. montana) from the Eocene in Australia and one extinct species (P. opihiensis) and a fossil record of one extant species (P. taxifolia) from the Cretaceous–Miocene in New Zealand [28,30,42,43,72].
Sundacarpus has two extinct species: S. anglica and S. tzagajanicus, from the Eocene–Oligocene in Australia and Cretaceous–Paleocene in Argentina, respectively [75].
Pherosphaera has two extinct species: P. sommervillae (Microstrobos sommervillae) from the Early Eocene and P. microfolius (Microstrobos microfolius) from the Oligocene–Miocene of Tasmania [31,33].
Microcachrys has one extinct species (Microcachrys novae-zelandiae) from the Oligo-Miocene of New Zealand and fossils of the extant Microcachrys tetragona from the Early Pleistocene of western Tasmania [34,45,52].
3.4. Chromosomal Number
The chromosomal number in the Podocarpaceae varies from x = 9 to x = 19 (Acmopyle x = 10, Afrocarpus x = 12, Dacrycarpus x = 10, Dacrydium x = 10, Falcatifolium x = 10, Halocarpus x = 9, 11, 12, Lagarostrobos x = 15, Lepidothamnus x = 14, 15, Manoao x = 10, Microcachrys x = 15, Nageia x = 18, Parasitaxus x = 18, Pectinopitys x = 19, Pherosphaera x = 13, Phyllocladus x = 9, Podocarpus x = 10, 11, 17, 18, 19, Prumnopitys x = 18, Retrophyllum x = 10, Saxegothaea x = 12 and Sundacarpus x = 12) [84].
4. Discussion
4.1. Phylogenetic History of the Podocarpaceae
Molecular studies suggest Araucariaceae as the sister family of Podocarpaceae, although these families are morpho-anatomically divergent [4,11,12,13], which was also supported by our results. Previous molecular and fossil records suggest that podocarps originated in the Triassic–Jurassic in Gondwana [12,85], or the Early Cretaceous [10], or Late Triassic [13], but recent podocarp fossils from Jordan push back the origin of the Podocarpaceae to the Permian (Figure 1) and show that they survived the “great dying” at the end of Permian [86,87]. Our results suggest that Lepidothamnus and Phyllocladus diverged in the Late Jurassic, when incorporating the oldest Lepidothamnus fossil record [51,88], which is earlier than the previous estimate of mid-Cretaceous–early Paleogene [10,12] and Early Jurassic [13]. Our studies recognized the presence of three major Prumnopityoid, Dacrydioid and Podocarpoid clades and a paraphyletic group similar to Chen et al. [13].
Several studies based on both morphological [7,89,90,91,92] and molecular [7,92,93,94,95,96] studies have been published evaluating the phylogenetic relationship among different genera of the Podocarpaceae. Based on morphology and 18S rDNA, Kelch [7] concluded that the Podocarpaceae are monophyletic except for Podocarpus (paraphyletic) and Dacrydium (polyphyletic). Conran et al. [93], based on molecular analysis (plastid rbcL), reported that Podocarpaceae are polyphyletic and supported the separation of Afrocarpus from Podocarpus and its placement as sister to Retrophyllum instead of Nageia, and also suggested that Podocarpus is monophyletic, a conclusion supported by Sinclair et al. [94]. Biffin et al. [85], based on their molecular studies of 94 Podocarpaceae species, reported that Podocarpus is closely related to Nageia, Afrocarpus and Retrophyllum. Knopf et al. [92] investigated the phylogeny of 145 species (including 77 species of Podocarpus) of Podocarpaceae based on morphological, anatomical and DNA sequences (rbcL, nrITS1 and NEEDLY). Their most significant findings were the support of subgenera in Podocarpus, the transfer of Sundacarpus amarus to Prumnopitys and the incorporation of the Phyllocladaceae into the Podocarpaceae as Phyllocladus. Lu et al. [11] reported two monophyletic sister groups: the Dacrydioid group (Dacrycarpus, Dacrydium and Falcatifolium) and the Podocarpoid group (Retrophyllum–Nageia subclade and the Afrocarpus–Podocarpus subclade). Little et al. [95] used DNA barcoding (matK, rbcL and nrITS2 DNA barcodes) for the identification of Podocarpaceae (18 genera and 145 species) and to construct a phylogenetic tree. Quiroga et al. [97], based on molecular and fossil data, reported that Podocarpus originated in late Cretaceous–early Paleogene (~63 Ma) and supported the two subgenera in Podocarpus. Leslie et al. [96], using more comprehensive sampling and markers, recognized 19 genera and supported the division of Podocarpus into two subgenera. Recently, Page [75] again split the genus Prumnopitys into three genera (Prumnopitys, Sundacarpus and the new genus Pectinopitys) based on morphological and molecular data. The current phylogeny supports the division of the 20 genera of podocarps into main three clades and a paraphyletic grade (Figure 1). Similarly, the current phylogeny also recognizes and supports the division of Podocarpus into two subgenera, i.e., Podocarpus and Foliolatus [12,13,92,97].
4.2. Historical Taxonomic Treatment
The most extensive taxonomic studies on the Podocarpaceae have been by de Laubenfels, Buchholz, Gray and Page, with many other contributions, which are summarized in Table 3.
Table 3.
A brief historical overview of major taxonomic classifications of Podocarpaceae (Type genus Podocarpus elongatus).
| Taxonomist | Taxonomic Treatment |
|---|---|
| Endlicher, 1847 [98] | He classified Podocarpaceae into three genera i. Podocarpus (with four sections i. Eupodocarpus, ii. Stachycarpus, iii. Nageia and iv. Dacrycarpus), 2. Dacrydium Sol. ex G. Forst, 3. Microcachrys Hook. f. |
| Pilger, 1926 [99] | He considered Podocarpaceae as subfamilies Podocarpoideae with Subgenus I. Protopodocarpus (with section i. Eupodocarpus, ii. Dacrycarpus), II. Stachycarpus with section B. i. Nageia ii. Saxegothaea iii. Microcachrys iv. Pherosphaera v. Acmopyle vi. Dacrydium, vii. section A, viii. Microcarpus and Phyllocladoideae with i. Phyllocladus |
| Buchholz and Gray, 1948 [100,101] | Classified Podocarpus into nine sections (P. sect. Eupodocarpus, P. sect. Nageia, P. sect. Afrocarpus, P. sect. Polypodiopsis, P. sect. Microcarpus, P. sect. Dacrycarpus, P. sect. Sundacarpus, P. sect. Stachycarpus) |
| Keng, 1973 [102] | Divided into two families, i.e., Podocarpaceae and Phyllocladaceae |
| Gaussen, 1974 [103] | Raised this group into suborder Podocarpineae and divided into three families, i.e., Podocarpaceae, Phyllocladaceae and Saxegothaeaceae. |
| de Laubenfels, 1985 [104] | Classified Podocarpus into two subgenera and 18 sections (subgenus Podocarpus: sect. Podocarpus, sect. Scytopodium, sect. capitulatis, sect. Australis, sect. Crassiforms, sect. Pratensis, sect. Lanceolatis, sect. Pumilis, sect. Nemoralis, subgenus Foliolatus: sect. Globulus, sect. Foliolatus, sect. Acuminatis, sect. Longifoliolatus, sect. Gracilis, sect. Macrostachyus, sect. Spinulosus, sect. Rumphius, sect. Polystachyus.) |
| Quinn, 1987 [105] | Placed back Phyllocladus in Podocarpaceae |
| Hart, 1987 [90] | Recognized 15 genera Lagarostrobos, Microstrobos (Pherosphaera), Microcachrys, Lepidothamnus, Halocarpus, Parasitaxus, Dacrycarpus, Falcatifolium, Dacrydium, Acmopyle, Nageia, Saxegothaea, Phyllocladus, Prumnopitys and Podocarpus |
| Page, 1988 [106] | Recognized eight genera in s.l. Podocarpus and five in Dacrydium |
| Page, 1990 [107] | Classified Podocarpaceae into Acmopyle, Falcatifolium, Dacrydium, Halocarpus, Lagarostrobos, Lepidothamnus, Microcachrys, Microstrobos (Pherosphaera), Phyllocladus and Podocarpus (P. subg. Podocarpus and P. subg. Foliolatus) Nageia (N. sect. Nageia, N. sect. Afrocarpus, N. sect. Polypodiopsis), Dacrycarpus, Parasitaxus, Prumnopitys, Sundacarpus, Saxegothaea |
| Dezhi, 1992 [108] | Placed Nageia into a new family Nageiaceae |
| Kelch, 1998 [7] | Produced the phylogeny of Podocarpaceae-based molecular markers (18S RNA) of 10 genera in the following sequences: Podocarpus, Dacrycarpus, Pherosphaera, Microcachrys, Afrocarpus, Saxegothaea, Dacrydium, Parasitaxus, Lagarostrobos and Phyllocladus. |
| Conran et al., 2000 [93] | Produced the phylogeny of Podocarpaceae-based molecular markers (rbcL) of 16 genera in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Acmopyle, Pherosphaera, Microcachrys, Lagarostrobos, Manoao, Prumnopitys, Halocarpus, Phyllocladus, Lepidothamnus and Saxegothaea. |
| Kelch, 2002 [14] | Produced the phylogeny of Podocarpaceae-based molecular markers (18S RNA) of 16 genera in the following sequences: Dacrydium, Falcatifolium, Dacrycarpus, Pherosphaera, Microcachrys, Saxegothaea, Acmopyle, Nageia, Afrocarpus, Podocarpus, Lagarostrobos, Halocarpus, Parasitaxus, Phyllocladus, Lepidothamnus and Prumnopitys. |
| Sinclair et al., 2002 [94] | Constructed the phylogeny of 18 genera-based molecular markers (trnL-trnF+ITS2) in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Acmopyle, Pherosphaera, Microcachrys, Saxegothaea, Lagarostrobos, Manoao, Parasitaxus, Halocarpus, Prumnopitys, Lepidothamnus and Phyllocladus. |
| Wagstaff, 2004 [83] | Constructed the phylogeny of 9 genera-based molecular markers (rbcL+matK) in the following sequences: Afrocarpus, Podocarpus, Dacrydium, Saxegothaea, Halocarpus, Lepidothamnus, Prumnopitys and Phyllocladus. |
| Biffin et al., 2012 [8] | Constructed the phylogeny of 18 genera based molecular markers (matK+ trnL-trnF+ITS2) in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Acmopyle, Pherosphaera, Microcachrys, Saxegothaea, Lagarostrobos, Manoao, Parasitaxus, Halocarpus, Prumnopitys, Lepidothamnus and Phyllocladus. |
| Knopf et al., 2012 [92] | Constructed the phylogeny of 18 genera-based molecular markers (ITS1+NEEDLY intron 2+ anatomy and morphology) in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Pherosphaera, Microcachrys, Halocarpus, Lepidothamnus, Lagarostrobos, Manoao, Phyllocladus, Prumnopitys and Saxegothaea. |
| Little et al., 2013 [95] | Used DNA barcoding (matK, rbcL and nrITS2 DNA barcodes) for the identification of Podocarpaceae (18 genera and 145 species) and to construct the phylogenetic tree |
| Lu et al., 2014 [11] | Constructed the phylogeny of 18 genera-based molecular markers (LEAFY+NEEDLY CDS+ introns) in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Acmopyle, Pherosphaera, Saxegothaea, Microcachrys, Lagarostrobos, Manoao, Parasitaxus, Phyllocladus, Lepidothamnus, Halocarpus and Prumnopitys. |
| Contreras et al., 2017 [109] | Constructed the phylogeny of 18 genera-based molecular markers in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrydium, Falcatifolium, Dacrycarpus, Acmopyle, Pherosphaera, Microcachrys, Saxegothaea, Halocarpus, Phyllocladus, Lepidothamnus, Prumnopitys, Lagarostrobos, Manoao and Parasitaxus. |
| Leslie et al., 2018 [12] | Recently constructed the phylogeny of 19 genera-based molecular markers (18S, rbcL and matK) in the following sequences: Podocarpus, Afrocarpus, Nageia, Retrophyllum, Falcatifolium, Dacrydium, Dacrycarpus, Acmopyle, Pherosphaera, Microcachrys, Saxegothaea, Prumnopitys, Sundacarpus, Manoao, Lagarostrobos, Parasitaxus, Halocarpus, Phyllocladus and Lepidothamnus. |
| Sudianto et al., 2019 [110] | Constructed the phylogeny tree of 12 genera based on Plastome in the following sequences: Afrocarpus, Nageia, Retrophyllum, Podocarpus, Dacrycarpus, Dacrydium, Pherosphaera, Saxegothaea, Phyllocladus, Lagarostrobos, Lepidothamnus and Prumnopitys. |
| Page, 2019 [75] | Recently divided the genus Prumnopitys into two genera, Prumnopitys (Subgenus Prumnopitys and Subgenus Botryopitys) and Pectinopitys. |
| Khan et al., 2023 [current classification] | Dacrycarpus, Halocarpus, Lepidothamnus, Manoao, Dacrydium, Lagarostrobos, Microcachrys, Pherosphaera, Parasitaxus, Acmopyle, Falcatifolium, Phyllocladus, Retrophyllum, Prumnopitys, Pectinopitys, Afrocarpus, Nageia, Podocarpus, Sundacarpus and Saxegothaea. |
Initially, podocarps were placed in two genera, Podocarpus and Dacrydium, mainly based on leaf morphology [98]. Several early taxonomists including Gordon [111] and Philippi [112] recognized variation in Podocarpus and Dacrydium and classified them into several sections, subgenera, and subgroups. From the 1960s onwards, Podocarpus was then divided into eight genera and Dacrydium into five. Based on leaf morphology and anatomy, Podocarpus was initially divided into eight sections (Afrocarpus, Dacrycarpus, Eupodocarpus, Microcarpus, Nageia, Polypodiopsis, Stachycarpus and Sundacarpus). After a more detailed examination, de Laubenfels [113] raised section Dacrycarpus to the genus Dacrycarpus. Quinn [114] suggested raising the eight sections of Podocarpus to generic level and de Laubenfels [115] raised the section Microcarpus to generic level as Parasitaxus. Later, de Laubenfels [104] revised the genus Podocarpus into 18 sections and described 94 species. Page [106] raised section Sundacarpus into the genus Sundacarpus, section Polypodiopsis to the genus Retrophyllum, section Nageia to the genus Nageia and section Afrocarpus to the genus Afrocarpus. Some taxonomists reject these changes of status [116,117,118]. Page [107] divided the Podocarpaceae into 17 genera (Phyllocladus was excluded and Sundacarpus was included). Biffin et al. [85] recognized three major clades, i.e., the Podocarpoid clade (Afrocarpus, Nageia, Podocarpus, Retrophyllum), the Dacrydioid clade (Dacrydium, Dacrycarpus, Falcatifolium) and the Prumnopityoid clade (Halocarpus, Lagarostrobos, Manoao, Parasitaxus, Prumnopitys).
The concept of the separate family Phyllocladaceae has been supported in several different studies [93,107,119], and while this is no longer regarded as valid, its status as the genus Phyllocladus has been well supported by other taxonomists and recent phylogenetic studies [12,13,83,92,94,95,120,121,122,123,124].
Our studies recognize 20 extant genera (Acmopyle, Afrocarpus, Dacrycarpus, Dacrydium, Falcatifolium, Halocarpus, Lagarostrobos, Lepidothamnus, Manoao, Microcachrys, Nageia, Parasitaxus, Pectinopitys, Pherosphaera, Phyllocladus, Podocarpus, Prumnopitys, Retrophyllum, Saxegothaea and Sundacarpus), two subgenera in both Podocarpus (Podocarpus and Foliolatus) and Prumnopitys (Prumnopitys and Botryopitys) in Podocarpaceae, similar to Page [75] and Yang et al. [1], Chen et al. [13] proposes the splitting of Prumnopitys into Prumnopitys and Pectinopitys but reported 19 genera for Podocarpaceae. Our checklist enlists 201 living species for Podocarpaceae than previously reported 181 species by Yang et al. [1], which will increase the total number of conifer species from 702 to 722.
4.3. Current Diversity and Distribution of Podocarpaceae
Podocarps occur mainly in the Southern Hemisphere, although some genera extend northward, i.e., subtropical China and Japan and to Mexico and the Caribbean [125]. The living species of Podocarpaceae are a small representation of a once highly diverse group [55,126]. Today, several genera have low species representation (e.g., monospecific in Manoao, Lagarostrobos, Microcachrys, Parasitaxus and Saxegothaea and two in Acmopyle and Pherosphaera), although the fossil record suggests a more extensive diversity for at least some of these genera in the past. The center of diversity for the Podocarpaceae is Australasia (New Caledonia, Tasmania, New Zealand and Malesia), South America (Andes mountains), Indo-China and the Philippines [125,127].
Podocarps favor mostly cool and wet climates but usually do not tolerate extreme cold like some Northern Hemisphere conifers [128]. However, some temperate Podocarpaceae species occur as shrubs and prostrate woody plants above the tree line in the alpine ecosystems of Tasmania, Victoria, and New Zealand (Figure 4).
Figure 4.
(A) Podocarpus lawrencei in the alpine region, Mount McKay Falls Creek, Australia. (B) Pherosphaera hookeriana and Microcachrys tetragona populations in the alpine region of Cradle Mountain summit, Tasmania.
The tropical podocarps are mostly confined to mountain forests and heathlands and nutrient-poor habitats in the lowlands, although some also grow in forest understories. Temperate podocarps are good competitors in nutrient-poor soils probably because the light is more easily available within the incomplete canopies, but they are outcompeted in nutrient-rich soil as the canopy and forest floor is occupied by angiosperms and the growth of new individuals is slow due to shading. Such conditions favor broad-leaved podocarps (Nageia and broad-leaved Podocarpus species are shade-tolerant) and exclude imbricate-leaved podocarps due to competition [128]. This is supported by Adie and Lawes [129], who concluded that African podocarps are not lowland rainforest survivors but are temperate forest relicts.
4.4. Historical Biogeography
The historical reconstruction of Podocarpaceae confirms that it is a Southern Hemisphere family that was initially centered in Gondwana [130]. Leslie et al. [12] suggest that Podocarpaceae diversified in the Cretaceous and earliest Cenozoic after its appearance in the Triassic of Gondwana. Klaus and Matzke [10], based on the reconstruction of ancestral ranges, suggested that podocarps originated in the Early Jurassic in what is today Central–South America, Australia, and New Zealand. The family subsequently dominated Australasia and Southern America and later (and through to the present) in Malesia [77]. However, the discovery of macrofossils of podocarps from the early Permian of Jordan [86,87] will require a re-assessment of the early history of the family [88].
Klaus and Matzke [10] used living taxa to reconstruct the ancestral ranges and suggested that the ancestral area for the Podocarpoid clade is the Australia–New Guinea–Malesian region; for the Dacrydioid clade it is New Caledonia; for the Prumnopityoid clade it is New Zealand and for the paraphyletic group/grade, South America to Australia. Macrofossil evidence and the historical biogeographic reconstruction by Klaus and Matzke [10] support an Australian origin of Podocarpus and multiple dispersals to South America, Asia, New Zealand, Malesia, and New Caledonia. Morley [77] concluded that Podocarpus dispersed into South Asia in the Late Eocene, either by dispersal from India or by multiple long-distance dispersal events from Australia. Similarly, he concluded that Podocarpus was possibly present in Africa during the mid-Cenozoic but its dispersal to West Africa occurred by island-hopping in the late Pliocene. According to Nieto-Blázquez et al. [131], Podocarpus species in the Caribbean are the result of colonization from the Andes during the Eocene to Oligocene (c. 45–31 Ma). Fossil records and living species distributions of Nageia support an Asian origin [10,54]. The living species of Afrocarpus strongly support an African origin for that genus. The living taxa and fossil record suggest a Gondwanan origin of Retrophyllum, with it evolving by the early Eocene [65,74]. Although the historical biogeographic reconstruction produced by Klaus and Matzke [10] suggests the origin of Dacrydium in New Caledonia, the macrofossil record strongly suggests an Australasian origin [32,39]. Morley [77] also concluded that Dacrydium originated in Australasia in the Late Cretaceous and dispersed to Southeast Asia in the Early Oligocene, probably by island-hopping (e.g., it dispersed to the Ninety East Ridge by the Paleocene and to India by the Early Eocene and later expanded to Japan during the Middle Miocene climatic optimum). According to Wu et al. [36,76], Dacrycarpus also had an Australasian origin during the Late Cretaceous. Dacrycarpus was present by the Eocene in Patagonia, supporting biogeographic connections during the warm Eocene from Patagonia to Australasia across Antarctica [35]. According to Morley [77], Dacrycarpus dispersed to New Guinea from Australia by the late Miocene and then during the mid-Pliocene, it island-hopped to Borneo, and during the Pleistocene, to Sumatra and the Malay Peninsula. However, the Dacrycarpus megafossil from the Miocene of South China shows its earlier arrival to Asia from the Southern Hemisphere and China during Late Miocene [36]. Paleoclimatic studies also support the existence of Dacrycarpus in high-precipitation areas and explain its possible extinction in Australia as that continent dried [36,85]. Dacrycarpus possibly became extinct around the Paleogene–Neogene transition from both South America and Antarctica and during the Neogene from Australia [36]. Klaus and Matzke’s [10] historical biogeographic reconstruction suggests that Falcatifolium originated in the Fiji–New Guinea region around the Late Eocene. However, the fossil record of Falcatifolium from the Middle Eocene of Australia suggests an Australian origin [49], Falcatifolium probably dispersed later to New Caledonia and Papua New Guinea [84].
Klaus and Matzke [10] also concluded that the Prumnopityoid clade originated in New Zealand around the mid-Cretaceous. However, a recent phylogeny of the podocarps suggests an Early to Mid-Jurassic origin for this clade (Figure 1). Leslie et al. [5] and Wang and Ran [84] reported that the phylogenetic divergence of Podocarpaceae shows that the three genera (Lepidothamnus, Podocarpus and Prumnopitys) were dispersed from Australia to South America through Antarctica. A Lepidothamnus macrofossil from the middle Cretaceous of Winton, Queensland [51,88] also supports its Australian origin. The living and macrofossil records of Phyllocladus indicates a Gondwanan origin and wider distribution. Phyllocladus dispersed to New Guinea by the late Miocene and then, during the mid-Pliocene, it island-hopped to Borneo [77]. The extant and extinct species (Halocarpus highstedii from the Oligocene–Miocene) are endemic to New Zealand [39]. Today, Manoao is a monotypic endemic genus to New Zealand but one fossil specimen from the Oligocene (35 Ma) of Cethana, Tasmania (Australia) is similar to that of Manoao colensoi (reported as Lagarostrobos colensoi), showing this genus was once present in Australia [38]. Parasitaxus is a monotypic endemic genus to New Caledonia with no macrofossil records. Lagarostrobos is also a monotypic endemic genus in Tasmania and the macrofossil records from Early Oligocene to Early Pleistocene are also restricted to Tasmania [33,34].
Prumnopitys has three living species distributed in New Zealand and South America. The macrofossil records (Cretaceous–Miocene) demonstrate a Gondwanan origin and wider distribution [43,75]. Although Sundacarpus is now a monotypic genus, the macrofossil records (S. anglica from England and S. tzagajanicus from Russia) from the Uppermost Cretaceous and Eocene show a wider past distribution [75]. Pectinopitys is widely distributed in New Zealand, Australia, New Caledonia, and South America, but with no macrofossil record.
Klaus and Matzke [10] conclude that Acmopyle originated in New Caledonia, but macrofossils from the Eocene–Oligocene suggest a Gondwanan origin [27,28,29,30,31,32]. Microcachrys is now endemic to Australia but is also present in the Oligocene–Miocene of New Zealand [52]. Saxegothaea is the oldest genus in the family and is part of an ancient lineage endemic to South America. Pherosphaera has two living species and two macrofossils from Australia [33].
4.5. Eco-Physiological Adaptations
Most podocarps have evolved flattened leaves and fleshy seed cones, which enable them to survive in low-light conditions beneath the tree canopy and disperse their seeds biotically [85,88,132,133]. Podocarps mature as trees or shrubs. Some of the most significant ecophysiological adaptations and strategies are discussed here.
4.5.1. Seed Cone Morpho-Anatomy
The Podocarpaceae have evolved distinct seed cone morphotypes and display marked variation in functional traits across the 20 genera [88,133,134]. Most podocarp genera produce fleshy seed cones utilizing the epimatium, aril, bracts, receptaculum or a combination of these [109]. Podocarpus is the largest genus in the Podocarpaceae and has a cone composed of one or two seeds covered mostly by a papery and sometimes a fleshy epimatium [10,109]. Several podocarp genera have cones with a brightly colored, fleshy receptaculum [10,88,134].
4.5.2. Leaf Morpho-Anatomy
The Podocarpaceae is prominent in many mixed conifer/broadleaf vegetation types in the Southern Hemisphere, and they exhibit great variation in leaf morphology across the 20 genera [135]. The diversity in leaf morphology of Podocarpaceae is remarkable, ranging from uni-veined needle and scale-like leaves to multi-veined broad leaves. Podocarpaceae foliage can be divided into two main types, imbricate (Dacrycarpus, Dacrydium, Halocarpus, Manoao, Lagarostrobos, Lepidothamnus, Microcachrys, Pherosphaera and Parasitaxus) and broad (flattened) leaved (Acmopyle, Nageia, Afrocarpus, Falcatifolium, Phyllocladus, Podocarpus, Retrophyllum, Pectinopitys, Sundacarpus, Prumnopitys and Saxegothaea). These genera have leaves either spirally arranged or in opposite pairs. Most Podocarpaceae species possess flattened or composite leaves (in 11 genera and more than 140 species) and this may be an adaptation to light requirements, as most of these species grow in the understory of forests with a low-light environment and are unable to reach the canopy level and high sunlight [9] unless a canopy gap occurs. Nageia is characterized by having leaves with multiple parallel veins [55]. All Phyllocladus species have evolved multi-veined phylloclades (Supplementary Figure S1), probably to compete with angiosperms for light [9,82,136]. Acmopyle, Dacrycarpus and Falcatifolium have bilaterally flattened leaves, lacking a true petiole. Leaf dimorphism is present in many genera of Podocarpaceae (Supplementary Figure S2). All other broad-leaved species have bifacially flattened broad leaves [135].
4.5.3. Pollen Morphology
All conifer species are wind-pollinated and those in the Podocarpaceae (except Saxegothaea) and Pinaceae have developed special wing-like structures called sacci [2]. The Podocarpaceae usually have saccate pollen with a tectate exine but usually with a smaller grain than the Pinaceae [137]. Pollen of all genera are bi-saccate except Microcachrys, Pherosphaera and Dacrycarpus, which are tri-saccate, and Saxegothaea which does not have sacci [91,138,139]. Because of this, Erdtman [138] suggested shifting Saxegothaea to the Araucariaceae, while Gaussen [103] and Woltz [140] suggested promoting it to the new family Saxegothaeaceae. The fossil pollen record of the Podocarpaceae is not considered here but is in need of revision, with much important data currently difficult to assess without expert comment on the validity of published interpretations.
4.6. Dispersal Biology
The Podocarpaceae are predominantly zoochorous as their main seed dispersal mechanism, although some genera have other dispersal strategies [141]. The zoochorous mode of dispersal is reported in Dacrycarpus, Halocarpus, Dacrydium, Microcachrys, Afrocarpus, Nageia¸ Podocarpus, Lepidothamnus, Phyllocladus, Parasitaxus, Manoao, Sundacarpus, Falcatifolium, Retrophyllum, Prumnopitys and Pectinopitys [88,134]. Klaus and Matzke [10] reported that 11 genera of Podocarpaceae show endozoochory, two (Prumnopitys and Afrocarpus) epizoochory and seven genera are not ornithochorous. Barochory is present in Pherosphaera and Saxegothaea. Hydrochory and zoochory are reported in Retrophyllum comptonii, R. minor and Lagarostrobos [109].
Leslie et al. [96] reported that cone morphology and seed size are co-evolved in a correlated pattern in animal-dispersed conifers and animal-dispersed species have a relatively larger seed size to attract animals. Similarly, climate change (higher temperatures or water stress in drier conditions) can affect the evolution of cone shape. Interpreting the cone morphology and animal dispersal in Podocarpaceae is difficult because animal-dispersed seeds (fleshy cones) evolved many times in the deep past (from the Cretaceous or even earlier, based on ancestral reconstruction) [88,96,134]. Podocarpus can be interpreted as zoochorous and mainly bird-dispersed due to their colorful fleshy receptacle and epimatium. Bird and bat dispersal have been reported from South African podocarps [142]. The Emu (Dromaius novaehollandiae) is a large bird with a wide distribution range in Australia and it is the main disperser of Podocarpus drouynianus in southwestern Australia, keeping the seeds for up to 50 h in the digestive tract and dispersing them several kilometers [143].
4.7. Ecology of Podocarpaceae
The major Southern Hemisphere conifer family Podocarpaceae is different in morphology, functional physiology, and ecology from the Northern Hemisphere’s major conifer family Pinaceae. Pinaceae are successful in Northern Hemisphere forests, where angiosperms are outcompeted during freezing temperatures, and also occur in low-rainfall areas. Podocarp species are more abundant and compete more successfully with broadleaf angiosperms in the tropical montane forest through multiple morphological and anatomical adaptations but in most cases avoid low-rainfall areas [144]. Ecologically, podocarps have a highly conserved association with the conifer families Araucariaceae and Cupressaceae and with the angiosperm families Nothofagaceae, Winteraceae and Cunoniaceae [9,136]. However, ecological data are lacking for most of the species in these families [4].
Podocarps are unable to bear extreme cold temperate but can tolerate moderate frosts [128] and some exist as alpine shrubs in relatively cold climates (e.g., alpine Tasmania) where permanent snow is uncommon (Figure 4). They possess broad to scale leaves, phylloclades and fleshy cones and they are adapted to a range of conditions from alpine to lowland, understory environments beneath a dense canopy, semi-aquatic (Retrophyllum minus), drought-and fire-prone conditions (Podocarpus drouynianus). The only parasitic gymnosperm (Parasitaxus usta) grows on the roots of another podocarp species (Falcatifolium taxoides). The occurrence of extant species of Podocarpaceae in angiosperm-dominated humid forests is of great interest to ecologists and paleontologists. The Podocarpaceae have preferred wet climates throughout their history [77] and nutrients are a stronger limiting factor for their distribution than the temperature [145], with Coomes and Bellingham [128] reporting that within temperate and tropical rainforests with few exceptions, podocarps are well adapted to nutrient-poor soils.
Coomes and Bellingham [128] evaluated the ecological similarities and differences of temperate and tropical podocarps. They concluded that angiosperm diversification and expansion during the Late Cretaceous was responsible for driving conifers from the lowland tropics and mesic temperate regions due to inferior reproductive competitiveness. However, Bond [146] and Midgley and Bond [147] challenged this view and hypothesized that the physiological traits of conifers (slow seedling establishment and later growth) put them at a disadvantage in competitive regeneration in changing climates (increasing cold and droughts) and habitats (nutrient-poor soil, poorly drained soil, and low light). Podocarps are predominantly slow-growing with low photosynthetic capacity per unit leaf mass and per unit leaf area compared with angiosperms with the same leaf are [128]. The studies that evaluated the growth of podocarps in different habitats lead to the conclusion that podocarp growth is slow compared to other conifers and to angiosperms (e.g., in lowland cool temperate forest, the growth rate is half that of angiosperms [148], and in subalpine shrublands, podocarps grow more slowly than several angiosperm species [149]. In the nutrient-rich soil of southern New Zealand, even tree ferns grow faster than podocarps [150,151].
Brodribb [144] argues that drought is one of the major agents that prevents podocarp success at high altitudes in the Southern Hemisphere. The Late Cenozoic was a major drying period in the temperate region and resulted in the contraction and extinction of Australian and other southern podocarps [152]. The cool and wet conditions (on the continental margins of Gondwana) necessary for the diversification of the Podocarpaceae favor the theory of the drought sensitivity of Podocarps [135,153]. High wood density (that lowers hydraulic efficiency) and leaf sclereids (that collapse under water tension, which results in a loss of hydraulic and photosynthetic function in the leaf) are also present in the broad-leaved tropical podocarps and may be the cause of poorer drought performance and weak competition in drier forests but favor cool, shady, and wet regions of the Southern Hemisphere for podocarp persistence [135,144,154]. In contrast, the Pinaceae have tough and waxy needle-like leaves, lower wood density, fewer sclereids and a high photosynthetic rate, making them resistant and adaptable to drought and freezing temperatures that are common in parts of the Northern Hemisphere [144,155]. This also provides a possible insight into why podocarps are today almost absent from the Northern Hemisphere, despite their potential for long-distance dispersal. A few podocarps are tolerant of drier regions, e.g., Afrocarpus falcatus (southern Africa), Podocarpus drouynianus (Western Australia) and Halocarpus bidwillii, Phyllocladus alpinus and Podocarpus laetus (dry lowland forests of New Zealand) [134]. Podocarp morphology is unusual compared to other conifers, since, despite possessing thick tracheid walls that are vulnerable to embolism at low tensions [154]. (Pittermann et al., 2006b), they also have high hydraulic resistance across pit membranes [156] and that makes the implosion of sclereids in podocarp leaves under tension a real possibility [157].
4.8. IUCN Conservation Status and Threats
The analysis of the available data on the IUCN conservation status of Podocarpaceae shows that 8 species (1 variety) are Critically Endangered (CR), 27 species (2 varieties) are Endangered (EN), 23 species (one subspecies) are Vulnerable (VU), 3 species are Threatened (TH), 33 species (2 varieties) are Near Threatened (NT), 89 species (8 varieties and one subspecies) are Least Concern (LC), 10 species are Data Deficient (DD) and 7 species (2 hybrids) are Not Evaluated (NE) for IUCN status (Figure 5). The Critically Endangered (CR) species are Acmopyle sahniana (Fiji), Pherosphaera fitzgeraldii (Australia), Dacrydium guillauminii (New Caledonia), Podocarpus urbanii (Jamaica), P. costaricensis (Costa Rica and Panama), P. decumbens (New Caledonia), P. palawanensis (the Philippines), P. perrieri (Madagascar) and P. sellowii var. angustifolius (Brazil). The IUCN conservation status for tropical podocarps states that 5 species are considered critically endangered, 18 species are endangered, and 16 species are vulnerable (Cernusak et al., 2011). The New Caledonian podocarp species are facing serious conservation threats due to their restricted populations (Enright and Jaffré, 2011); i.e., Retrophyllum minus (endangered), Podocarpus decumbens (critically endangered) P. longefolaliatus (endangered), Dacrydium guillauminii (critically endangered), Acmopyle pancheri (nearly threatened) and Parasitaxus usta (vulnerable).
Figure 5.
The proportion of current IUCN conservation status of Podocarpaceae species. The conservation status is evaluated according to IUCN Red List categories and criteria, version 3.1 (IUCN Council, Geneva, Switzerland).
Deforestation associated with mining, expansion of tropical agricultural activities and other anthropogenic activities poses a serious threat to tropical podocarps [158]. Deforestation and climate change are also posing a serious threat to montane endemic podocarps [159]. Similarly, more extreme dry seasons are also damaging for tropical podocarps because they are drought and fire intolerant [158]. Wildfire is posing a huge threat to Australian podocarps (Figure 6) and in some areas, the podocarp population has been driven to extinction by these fires [160]. The harvest of podocarp timber has been an important industry, but their slow growth makes it detrimental and unsustainable for the species involved [161]. Mill [162] reported habitat loss, climate change and deforestation as major threats causing the extinction of Podocarpus species. Failure of regeneration and aging of the current populations are two major threats for at least some podocarp species [128,163,164].
Figure 6.
A wildfire in 2020 burnt the Tahune rainforest, Tasmania. This photo is of a burnt Celery-top Pine (Phyllocladus aspleniifolius) tree.
4.9. Current Gaps and Future Perspectives
Some clear gaps still exist that need to be filled in order for us to gain a better understanding of the Podocarpaceae and include some of the following aspects:
Descriptions and taxonomic treatments of several species from less explored/remote areas such as Papua New Guinea, Malaysia, Indonesia, and New Caledonia are based only on collections of one or a few specimens. Additionally, some of these areas are not well explored and may contain undescribed species.
Field-and laboratory-based studies on pollination biology, the reproductive cycle and anatomical structures are not well developed for most podocarps and require further detailed evaluation.
Extensive research is required to understand why Podocarpaceae have such remarkable seed cone and leaf morphology.
Very few studies report the dispersal biology of podocarp seeds and comprehensive assessments are required to understand the dispersal biology and ecology of podocarps.
Despite the several high-quality publications on the leaf cuticle morphology of various genera, a good quality publication is necessary that describes the taxonomic and phylogenetic authenticity of these foliar cuticular diagnostic characters. Similarly, studies are required to assess the infraspecific variation in the leaf morphology for different populations.
Phylogenomic and population-based studies are available only for a few Podocarpus species (P. matudae, P. nubigenus, P. parlatorei, P. salignus, P. latifolius, P. guatemalensis and P. oleifolius), and with fairly limited geographic scope (the Americas). With the availability of modern NGS techniques and bioinformatic tools, more comprehensive studies are required to unveil their phylogeny, historical biogeography, speciation, and population history.
Only a few studies are available on the historical biogeography of Podocarpaceae and the discovery of new podocarp fossils from the Early Permian (Paleozoic) of Jordan [86,87] questions the Gondwanan origin of the Podocarpaceae. The inclusion of well-placed podocarp fossils will help in better understanding the reconstruction of historical biogeography.
Comparative studies of the three Southern Hemisphere conifer families (Araucariaceae, Cupressaceae and Podocarpaceae) to evaluate the impact of these families on the habitats they occupy and their relationships with the rest of the Southern Hemisphere biota.
Evolution of photosynthetic units in these three families in response to the closed forests that predated the rise to dominance of the angiosperms and angiosperm-dominated rainforests and then the major aridification of the Southern Hemisphere.
A better understanding of the response of podocarp foliage to drought stress and the adaptations that have evolved to deal with the constraint of most podocarps in having only a single vein per leaf is required to better understand the distribution and ecology of the family.
Use of species distribution modelling to predict the possible ecological niche and the effect of climate change on species range dynamic.
A better understanding of the evolutionary history and biology, ecology and life history are important in conservation efforts, given that so many species are threatened.
5. Conclusions
The current study provides a comprehensive overview on the systematics, diversity, hotspots, evolutionary adaptations, and conservation status of podocarps. Podocarps are morphologically more diverse compared to other conifer families and the updated phylogeny based on more extensive macrofossil records broadens our understanding of the evolutionary history of Podocarpaceae. Most podocarp genera currently exhibit low species richness and high endemism and often have disjunct distributions. Today, the Malesian region is the diversity hotspot for living podocarp taxa. However, the fossil record demonstrates wider distributions in the past. Podocarpus, Dacrydium and Dacrycarpus are the most dominant genera (approximately 75% of living podocarps) and have acquired particular morpho-anatomical adaptations that help them to survive in tropical forests. Podocarps demonstrate a remarkable seed cone and leaf diversity compared with other conifers. The genera with fleshy seed cones predominantly rely on bird dispersal. Podocarps are facing serious threats from deforestation, climate change, drought and wildfire, and the need for further targeted research is urgent. Among the conifers, podocarps are less well known and receive less attention than their counterparts that dominate the Northern Hemisphere, despite their remarkable morphological diversity and long evolutionary history.
Acknowledgments
We acknowledge Adelaide Microscopy, University of Adelaide, Australian National Botanic Gardens, Canberra, Mount Lofty Botanical Garden, SA and The Tasmanian Arboretum, Devonport, National Natural Science Foundation of China.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12051171/s1. Figure S1. Phyllocladus aspleniifolius (with phylloclades) found in rainforest Tasmania. Figure S2. Leaf dimorphism in Dacrycarpus dacrydioides. Table S1. Fossil taxa used for calibration of the phylogeny [165,166].
Author Contributions
Conceptualization, R.K. and R.S.H.; methodology, E.B. and R.K.; software, E.B. and R.K.; validation, R.K., E.B. and R.S.H.; formal analysis, R.K., E.B. and R.S.H.; writing—original draft preparation, R.K.; writing—review and editing, E.B., J.L. and R.S.H.; supervision, R.S.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data available in article supplementary material. Additional supporting information may be found in the online version of the article at the publisher’s website.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Key Research Program of Frontier Sciences, CAS (ZDBS-LY-7001), National Natural Science Foundation of China (41971071, 42211540718), CAS “Light of West China” Program, and Top-notch Young Talents Project of Yunnan Provincial “Ten Thousand Talents Program” (YNWR-QNBJ-2018-146).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yang Y., Ferguson D.K., Liu B., Mao K.S., Gao L.M., Zhang S.Z., Wan T., Rushforth K., Zhang Z.X. Recent advances on phylogenomics of gymnosperms and an updated classification. Plant Divers. 2022;44:340–350. doi: 10.1016/j.pld.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens J.N., Takaso T., Runions C.J. Pollination in conifers. Trends Plant Sci. 1998;3:479–485. doi: 10.1016/S1360-1385(98)01337-5. [DOI] [Google Scholar]
- 3.Conway S. Beyond pine cones: An introduction to gymnosperms. Arnoldia. 2013;70:2–14. [Google Scholar]
- 4.Farjon A. The Kew review: Conifers of the world. Kew Bull. 2018;73:1–16. doi: 10.1007/s12225-018-9738-5. [DOI] [Google Scholar]
- 5.Leslie A.B., Beaulieu J.M., Rai H.S., Crane P.R., Donoghue M.J., Mathews S. Hemisphere-scale differences in conifer evolutionary dynamics. Proc. Natl. Acad. Sci. USA. 2012;109:16217–16221. doi: 10.1073/pnas.1213621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ran J.-H., Gao H., Wang X.-Q. Fast evolution of the retroprocessed mitochondrial rps3 gene in Conifer II and further evidence for the phylogeny of gymnosperms. Mol. Phylogenetics Evol. 2010;54:136–149. doi: 10.1016/j.ympev.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Kelch D.G. Phylogeny of Podocarpaceae: Comparison of evidence from morphology and 18S rDNA. Am. J. Bot. 1998;85:986–996. doi: 10.2307/2446365. [DOI] [PubMed] [Google Scholar]
- 8.Biffin E., Brodribb T.J., Hill R.S., Thomas P., Lowe A.J. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proc. R. Soc. B Biol. Sci. 2012;279:341–348. doi: 10.1098/rspb.2011.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodribb T., Hill R.S. The Evolution of Plant Physiology. Elsevier; Amsterdam, The Netherlands: 2004. The rise and fall of the Podocarpaceae in Australia—A physiological explanation; pp. 381–399. [Google Scholar]
- 10.Klaus K.V., Matzke N.J. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Syst. Biol. 2020;69:61–75. doi: 10.1093/sysbio/syz034. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y., Ran J.-H., Guo D.-M., Yang Z.-Y., Wang X.-Q. Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS ONE. 2014;9:e107679. doi: 10.1371/journal.pone.0107679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie A.B., Beaulieu J., Holman G., Campbell C.S., Mei W., Raubeson L.R., Mathews S. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 2018;105:1531–1544. doi: 10.1002/ajb2.1143. [DOI] [PubMed] [Google Scholar]
- 13.Chen L., Jin W.T., Liu X.Q., Wang X.Q. New insights into the phylogeny and evolution of Podocarpaceae inferred from transcriptomic data. Mol. Phylogenetics Evol. 2022;166:107341. doi: 10.1016/j.ympev.2021.107341. [DOI] [PubMed] [Google Scholar]
- 14.Kelch D.G. Phylogenetic assessment of the monotypic genera Sundacarpus and Manoao (Coniferales: Podocarpaceae) utilising evidence from 18S rDNA sequences. Aust. Syst. Bot. 2002;15:29–35. doi: 10.1071/SB01002. [DOI] [Google Scholar]
- 15.Miller M., Pfeiffer W., Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees; Proceedings of the Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; pp. 11572–11578. [DOI] [Google Scholar]
- 16.Drummond A.J., Ho S.Y.W., Phillips M.J., Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavryushkina A., Welch D., Stadler T., Drummond A.J. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput. Biol. 2014;10:e1003919. doi: 10.1371/journal.pcbi.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath T.A., Huelsenbeck J.P., Stadler T. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl. Acad. Sci. USA. 2014;111:E2957–E2966. doi: 10.1073/pnas.1319091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earle C.J. The Gymnosperm Database. 2022. [(accessed on 20 March 2022)]. Available online: https://www.conifers.org/
- 20.GBIF GBIF: The Global Biodiversity Information Facility. 2022. [(accessed on 22 September 2022)]. Available online: https://www.gbif.org/
- 21.Plants of the World Online. 2021. [(accessed on 10 May 2021)]. Available online: https://powo.science.kew.org/
- 22.AVH Australasian Virtual Herbarium, Council of Heads of Australasian Herbaria. 2022. [(accessed on 14 July 2022)]. Available online: https://avh.chah.org.au/
- 23.Flora of China. 2021. [(accessed on 4 June 2021)]. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2.
- 24.McCune B., Mefford M. Multivariate Analysis on the PC-ORD System. MjM Software Design; Gleneden Beach, OR, USA: 1999. Version 4. [Google Scholar]
- 25.Alroy J. Fossilworks: Gateway to the Paleobiology Database. 2022. [(accessed on 24 April 2022)]. Available online: http://www.fossilworks.org/
- 26.Mill R.R. Towards a biogeography of the Podocarpaceae. Acta Hortic. 2003;615:137–147. [Google Scholar]
- 27.Florin R. Die heutige und fruhere Verbreitung der Koniferengattung Acmopyle Pilger. Sven. Bot. Föreningen. 1940;34:117–140. [Google Scholar]
- 28.Pole M. Eocene vegetation from Hasties, north-eastern Tasmania. Aust. Syst. Bot. 1992;5:431–475. doi: 10.1071/SB9920431. [DOI] [Google Scholar]
- 29.Hill R., Carpenter R. Evolution of Acmopyle and Dacrycarpus (Podocarpaceae) foliage as inferred from macrofossils in south-eastern Australia. Aust. Syst. Bot. 1991;4:449–479. doi: 10.1071/SB9910449. [DOI] [Google Scholar]
- 30.Pole M. Miocene conifers from the Manuherikia group, New Zealand. J. R. Soc. N. Z. 1997;27:355–370. doi: 10.1080/03014223.1997.9517543. [DOI] [Google Scholar]
- 31.Townrow J.A. Notes on Tasmanian Pines. I-Some Lower Tertiary Podocarps. Pap. Proc. R. Soc. Tasman. 1965;99:87–108. [Google Scholar]
- 32.Carpenter R., Pole M. Eocene plant fossils from the Lefroy and Cowan paleodrainages, Western Australia. Aust. Syst. Bot. 1995;8:1107–1154. doi: 10.1071/SB9951107. [DOI] [Google Scholar]
- 33.Wells P., Hill R. Fossil imbricate-leaved Podocarpaceae from Tertiary sediments in Tasmania. Aust. Syst. Bot. 1989;2:387–423. doi: 10.1071/SB9890387. [DOI] [Google Scholar]
- 34.Jordan G.J. Extinct conifers and conifer diversity in the Early Pleistocene of western Tasmania. Rev. Palaeobot. Palynol. 1995;84:375–387. doi: 10.1016/0034-6667(94)00116-2. [DOI] [Google Scholar]
- 35.Wilf P. Rainforest conifers of Eocene Patagonia: Attached cones and foliage of the extant Southeast Asian and Australasian genus Dacrycarpus (Podocarpaceae) Am. J. Bot. 2012;99:562–584. doi: 10.3732/ajb.1100367. [DOI] [PubMed] [Google Scholar]
- 36.Wu X.K., Zavialova N.E., Kodrul T.M., Liu X.Y., Gordenko N.V., Maslova N.P., Jin J.H. Northern Hemisphere megafossil of Dacrycarpus (Podocarpaceae) from the Miocene of South China and its evolutionary and paleoecological implications. J. Syst. Evol. 2021;59:352–374. doi: 10.1111/jse.12534. [DOI] [Google Scholar]
- 37.Hill R.S., Whang S.S. Dacrycarpus (Podocarpaceae) macrofossils from Miocene sediments at Elands, eastern Australia. Aust. Syst. Bot. 2000;13:395–408. doi: 10.1071/SB98007. [DOI] [Google Scholar]
- 38.Carpenter R.J. Ph.D. Thesis. University of Tasmania; Hobart, Australia: 1991. [(accessed on 15 April 2021)]. Palaeovegetation and Environment at Cethana, Tasmania. Available online: https://eprints.utas.edu.au/18972/ [Google Scholar]
- 39.Jordan G.J., Carpenter R.J., Bannister J.M., Lee D.E., Mildenhall D.C., Hill R.S. High conifer diversity in Oligo-Miocene New Zealand. Aust. Syst. Bot. 2011;24:121–136. doi: 10.1071/SB11004. [DOI] [Google Scholar]
- 40.Lewis E.K., Drinnan A.N. The Miocene conifer flora of Balcombe Bay, Victoria, Australia. Aust. Syst. Bot. 2013;26:145–155. doi: 10.1071/SB11031. [DOI] [Google Scholar]
- 41.Hill R.S., Merrifield H.E. An early tertiary macroflora from West Dale, southwestern Australia. Alcheringa. 1993;17:285–326. doi: 10.1080/03115519308619596. [DOI] [Google Scholar]
- 42.Greenwood D.R. Early Tertiary Podocarpaceae-megafossils from the Eocene Anglesea locality, Victoria, Australia. Aust. J. Bot. 1987;35:111–133. doi: 10.1071/BT9870111. [DOI] [Google Scholar]
- 43.Mill R.R., Hill R.S. Validations of the names of seven Podocarpaceae macrofossils. Taxon. 2004;53:1043–1046. doi: 10.2307/4135571. [DOI] [Google Scholar]
- 44.Pole M.S., Hill R.S., Green N., Macphail M.K. The Oligocene Berwick Quarry flora—Rainforest in a drying environment. Aust. Syst. Bot. 1993;6:399–427. doi: 10.1071/SB9930399. [DOI] [Google Scholar]
- 45.Carpenter R., Hill R., Jordan G. History of the Australian Vegetation: Cretaceous to Recent. Cambridge University Press; Cambridge, UK: 1994. Cenozoic vegetation in Tasmania: Macrof ossil evidence; pp. 276–298. [Google Scholar]
- 46.Hill R.S., Christophel D.C. Two new species of Dacrydium (Podocarpaceae) based on vegetative fossils from Middle Eocene sediments at Nelly Creek, South Australia. Aust. Syst. Bot. 2001;14:193–205. doi: 10.1071/SB00025. [DOI] [Google Scholar]
- 47.Cookson I.C., Pike K.M. A contribution to the Tertiary occurrence of the genus Dacrydium in the Australian region. Aust. J. Bot. 1953;1:474–484. doi: 10.1071/BT9530474. [DOI] [Google Scholar]
- 48.Blackburn D. Palaeobotany of the Yallourn and Morwell Coal Seams. State Electricity Commission Victoria; Victoria, Australia: 1985. p. 108. Palaeobotanical Project, Report. [Google Scholar]
- 49.Hill R.S., Scriven L.J. Falcatifolium (Podocarpaceae) macrofossils from Paleogene sediments in south-eastern Australia: A reassessment. Aust. Syst. Bot. 1999;11:711–720. doi: 10.1071/SB97014. [DOI] [Google Scholar]
- 50.Hill R., Macphail M. A Fossil Flora from Rafted Plio-Pleistocene Mudstones at Regatta Point, Tasmania. Aust. J. Bot. 1985;33:497–517. doi: 10.1071/BT9850497. [DOI] [Google Scholar]
- 51.Peters M.D. Ph.D. Thesis. University of Adelaide; Adelaide, Australia: 1985. A Taxonomic Analysis of a Middle Cretaceous Megafossil Plant Assemblage from Queensland, Australia. [Google Scholar]
- 52.Carpenter R.J., Jordan G.J., Mildenhall D.C., Lee D.E. Leaf fossils of the ancient Tasmanian relict Microcachrys (Podocarpaceae) from New Zealand. Am. J. Bot. 2011;98:1164–1172. doi: 10.3732/ajb.1000506. [DOI] [PubMed] [Google Scholar]
- 53.Jin J., Qiu J., Zhu Y., Kodrul T.M. First fossil record of the genus Nageia (Podocarpaceae) in south China and its phytogeographic implications. Plant Syst. Evol. 2010;285:159–163. doi: 10.1007/s00606-010-0267-4. [DOI] [Google Scholar]
- 54.Liu X.Y., Gao Q., Jin J.H. Late Eocene leaves of Nageia (section Dammaroideae) from Maoming Basin, South China and their implications on phytogeography. J. Syst. Evol. 2015;53:297–307. doi: 10.1111/jse.12133. [DOI] [Google Scholar]
- 55.Kimura T., Ohana T., Mimoto K. Discovery of a podocarpaceous plant from the Lower Cretaceous of Kochi Prefecture, in the Outer Zone of Southwest Japan. Proc. Jpn. Acad. Ser. B. 1988;64:213–216. doi: 10.2183/pjab.64.213. [DOI] [Google Scholar]
- 56.Krassilov V. New coniferales from Lower Cretaceous of Primorye. Bot. J. 1965;50:1450–1455. [Google Scholar]
- 57.Hill R.S. New species of Phyllocladus (Podocarpaceae) macrofossils from southeastern Australia. Alcheringa. 1989;13:193–208. doi: 10.1080/03115518908527820. [DOI] [Google Scholar]
- 58.Ettingshausen C. V Beitrage zur Kenntniss der Tertiarflora australiens. Denkschriften der Kaiserlichen Akademie der Wissenschaften. Math.-Nat. Cl. 1886;53:81–142. [Google Scholar]
- 59.Von Ettingshausen C. Contributions to the Tertiary flora of Australia. Mem. Geol. Surv. New South Wales Palaeontol. 1888;2:1–189. doi: 10.1017/S001675680016604X. [DOI] [Google Scholar]
- 60.Cookson I.C., Pike K.M. The fossil occurrence of Phyllocladus and two other podocarpaceous types in Australia. Aust. J. Bot. 1954;2:60–68. doi: 10.1071/BT9540060. [DOI] [Google Scholar]
- 61.Deane H. Fossil leaves from the open cut, state brown coal mine, Morwell. Rec. Geol. Surv. Vic. 1925;4:492–498. [Google Scholar]
- 62.Pole M., Moore P.R. A late Miocene leaf assemblage from Coromandel Peninsula, New Zealand, and its climatic implications. Alcheringa. 2011;35:103–121. doi: 10.1080/03115518.2010.481829. [DOI] [Google Scholar]
- 63.McLoughlin S., Hill R. Gondwanan Heritage: Past, Present and Future of the Western Australian Biota. Surrey Beatty & Sons; Chipping Norton, Australia: 1996. The succession of Western Australian Phanerozoic Terrestrial Floras; pp. 61–80. [Google Scholar]
- 64.McLoughlin S., McNamara K., George A.S. Ancient Floras of Western Australia. Western Australian Museum; Perth, Australia: 2001. [Google Scholar]
- 65.Wilf P., Donovan M.P., Cúneo N.R., Gandolfo M.A. The fossil flip-leaves (Retrophyllum, Podocarpaceae) of southern South America. Am. J. Bot. 2017;104:1344–1369. doi: 10.3732/ajb.1700158. [DOI] [PubMed] [Google Scholar]
- 66.Berry E.W. The flora of the Concepción-Arauco coal measures of Chile. Johns Hopkins Univ. Stud. Geol. 1922;4:73–143. [Google Scholar]
- 67.Awasthi N., Mehrotra R.C., Lakhanpal R. Occurrence of Podocarpus and Mesua in the Oligocene sediments of Makum Coalfiedl, Assam, India. Geophytology. 1992;22:193–198. [Google Scholar]
- 68.Zhou Z., Li H. Some Late Cretaceous plants from King George Island, Antarctica. Stratigraphy and palaeontology of Fildes Peninsula, King George Island, Antarctica. State Antarctic Committee. Monograph. 1994;3:85–96. [Google Scholar]
- 69.Chen H., Tang D.-L., Zhang Y., An P.-C., Yan X.-Y., Ding S.-T., Wu J.-Y. Fossil Podocarpus (Podocarpaceae) from the lower Pliocene of Tengchong, Yunnan Province, China and its biogeographic significance. Hist. Biol. 2019;33:1352–1361. doi: 10.1080/08912963.2019.1697254. [DOI] [Google Scholar]
- 70.Wu J.-Y., Chen H., Ruan S.-C., Yang M., Mo L.-B., Ji B.-Q., Zhang J.-L., Ding S.-T. Fossil leaves of Podocarpus subgenus Foliolatus (Podocarpaceae) from the Pliocene of southwestern China and biogeographic history of Podocarpus. Rev. Palaeobot. Palynol. 2021;287:104380. doi: 10.1016/j.revpalbo.2021.104380. [DOI] [Google Scholar]
- 71.Pole M. Miocene broad-leaved Podocarpus from Foulden Hills, New Zealand. Alcheringa. 1993;17:173–177. doi: 10.1080/03115519308619601. [DOI] [Google Scholar]
- 72.Pole M. Paleocene plant macrofossils from Kakahu, South Canterbury, New Zealand. J. R. Soc. N. Z. 1997;27:371–400. doi: 10.1080/03014223.1997.9517544. [DOI] [Google Scholar]
- 73.He W.L., Wang X.J. A Miocene flora from the Toupi Formation in Jiangxi Province, southeastern China. Palaeoworld. 2021;30:757–769. doi: 10.1016/j.palwor.2020.12.006. [DOI] [Google Scholar]
- 74.Wilf P. Eocene “Chusquea” fossil from Patagonia is a conifer, not a bamboo. PhytoKeys. 2020;139:77. doi: 10.3897/phytokeys.139.48717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Page C.N. New and maintained genera in the taxonomic alliance of Prumnopitys s. l. (Podocarpaceae), and circumscription of a new genus: Pectinopitys. N. Z. J. Bot. 2019;57:137–153. doi: 10.1080/0028825X.2019.1625933. [DOI] [Google Scholar]
- 76.Wu X., Liu X., Kodrul T., Quan C., Jin J. Dacrycarpus pattern shedding new light on the early floristic exchange between Asia and Australia. Natl. Sci. Rev. 2019;6:1086–1090. doi: 10.1093/nsr/nwz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morley R.J. Dispersal and paleoecology of tropical podocarps. In: Turner B.L., Cemusak L., editors. Ecology of the Podocarpaceae in Tropical Forests. Smithsonian Institution Scholarly Press; Washington, DC, USA: 2011. pp. 21–41. [DOI] [Google Scholar]
- 78.Krassilov V. Podocarpus from the Upper Cretaceous of eastern Asia and its bearing on the theory of conifer evolution. Palaeontology. 1974;17:365–370. [Google Scholar]
- 79.Dutra T.L., Batten D.J. Upper Cretaceous floras of King George Island, West Antarctica, and their palaeoenvironmental and phytogeographic implications. Cretac. Res. 2000;21:181–209. doi: 10.1006/cres.2000.0221. [DOI] [Google Scholar]
- 80.Berry E.W. The American species referred to Thinnfeldia. Bull. Torrey Bot. Club. 1903;30:438–445. doi: 10.2307/2478730. [DOI] [Google Scholar]
- 81.Nosova N., Golovneva L. The Mesozoic genus Protophyllocladus Berry (Pinopsida) Rev. Palaeobot. Palynol. 2014;210:77–88. doi: 10.1016/j.revpalbo.2014.07.003. [DOI] [Google Scholar]
- 82.Dörken V.M., Hill R.S., Jordan G.J., Parsons R.F. Evolutionary and ecological significance of photosynthetic organs in Phyllocladus (Podocarpaceae) Bot. J. Linn. Soc. 2021;196:343–363. doi: 10.1093/botlinnean/boaa106. [DOI] [Google Scholar]
- 83.Wagstaff S.J. Evolution and biogeography of the austral genus Phyllocladus (Podocarpaceae) J. Biogeogr. 2004;31:1569–1577. doi: 10.1111/j.1365-2699.2004.01066.x. [DOI] [Google Scholar]
- 84.Wang X.-Q., Ran J.-H. Evolution and biogeography of gymnosperms. Mol. Phylogenetics Evol. 2014;75:24–40. doi: 10.1016/j.ympev.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Biffin E., Conran J.G., Lowe A.J. Podocarp evolution: A molecular phylogenetic perspective. In: Turner B.L., Cernusak L.A., editors. Ecology of the Podocarpaceae in Tropical Forests. Smithsonian Contributions to Botany, Smithsonian Institution Scholarly Press; Washington, DC, USA: 2011. pp. 1–19. [Google Scholar]
- 86.Pennisi E. Fossils push back origin of key plant groups millions of years. Am. Assoc. Adv. Sci. 2018;362:1340. doi: 10.1126/science.362.6421.1340. [DOI] [PubMed] [Google Scholar]
- 87.Blomenkemper P., Kerp H., Hamad A.A., DiMichele W.A., Bomfleur B. A hidden cradle of plant evolution in Permian tropical lowlands. Science. 2018;362:1414–1416. doi: 10.1126/science.aau4061. [DOI] [PubMed] [Google Scholar]
- 88.Khan R., Hill R.S., Dörken V.M., Biffin E. Detailed seed cone morpho-anatomy of the Prumnopityoid clade: An insight into the origin and evolution of Podocarpaceae seed cones. Ann. Bot. 2022;130:637–655. doi: 10.1093/aob/mcac097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keng H. The phylloclade of Phyllocladus and its possible bearing on the branch systems of progymnosperms. Ann. Bot. 1974;38:757–764. doi: 10.1093/oxfordjournals.aob.a084864. [DOI] [Google Scholar]
- 90.Hart J.A. A cladistic analysis of conifers: Preliminary results. J. Arnold Arbor. 1987;68:269–307. doi: 10.5962/p.185944. [DOI] [Google Scholar]
- 91.Kelch D.G. The phylogeny of the Podocarpaceae based on morphological evidence. Syst. Bot. 1997;22:113–131. doi: 10.2307/2419680. [DOI] [Google Scholar]
- 92.Knopf P., Schulz C., Little D.P., Stützel T., Stevenson D.W. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics. 2012;28:271–299. doi: 10.1111/j.1096-0031.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- 93.Conran J.G., Wood G.M., Martin P.G., Dowd J.M., Quinn C.J., Gadek P.A., Price R.A. Generic relationships within and between the gymnosperm families Podocarpaceae and Phyllocladaceae based on an analysis of the chloroplast gene rbcL. Aust. J. Bot. 2000;48:715–724. doi: 10.1071/BT99062. [DOI] [Google Scholar]
- 94.Sinclair W., Mill R., Gardner M., Woltz P., Jaffré T., Preston J., Hollingsworth M., Ponge A., Möller M. Evolutionary relationships of the New Caledonian heterotrophic conifer, Parasitaxus usta (Podocarpaceae), inferred from chloroplast trn LF intron/spacer and nuclear rDNA ITS2 sequences. Plant Syst. Evol. 2002;233:79–104. doi: 10.1007/s00606-002-0199-8. [DOI] [Google Scholar]
- 95.Little D.P., Knopf P., Schulz C. DNA barcode identification of Podocarpaceae—The second largest conifer family. PLoS ONE. 2013;8:e81008. doi: 10.1371/journal.pone.0081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leslie A.B., Beaulieu J.M., Mathews S. Variation in seed size is structured by dispersal syndrome and cone morphology in conifers and other nonflowering seed plants. New Phytol. 2017;216:429–437. doi: 10.1111/nph.14456. [DOI] [PubMed] [Google Scholar]
- 97.Quiroga M.P., Mathiasen P., Iglesias A., Mill R.R., Premoli A.C. Molecular and fossil evidence disentangle the biogeographical history of Podocarpus, a key genus in plant geography. J. Biogeogr. 2016;43:372–383. doi: 10.1111/jbi.12630. [DOI] [Google Scholar]
- 98.Endlicher S.F.L. Synopsis Coniferarum. Scheittin et Zollikofer; Switzerland: 1847. [Google Scholar]
- 99.Pilger R. Podocarpaceae. In: Engler A., editor. Die natürlichen Pflanzenfamilien. 2nd ed. Wilhelm Engelmann; Leipzig, Germany: 1925. pp. 211–249. 13 Band, Gymnospermae. [Google Scholar]
- 100.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus: I. the sections of the genus and their subdivisons with special reference to leaf anatomy. J. Arnold Arbor. 1948;29:49–63. doi: 10.5962/p.185589. [DOI] [Google Scholar]
- 101.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus: Ii. The American species of Podocarpus: Section Stachycarpus. J. Arnold Arbor. 1948;29:64–76. doi: 10.5962/p.185590. [DOI] [Google Scholar]
- 102.Keng H. On the family Phyllocladaceae. Taiwania. 1973;18:142–145. [Google Scholar]
- 103.Gaussen H. Les gymnospermes actuelles et fossiles Podocarpaces. Fasc. 12. Trav. Lab. Toulouse. 1974;12:1–143. [Google Scholar]
- 104.De Laubenfels D.J. A taxonomic revision of the genus Podocarpus. Blumea. 1985;30:251–278. [Google Scholar]
- 105.Quinn C.J. The phyllocladaceae Keng—A critique. Taxon. 1987;36:559–565. doi: 10.2307/1221846. [DOI] [Google Scholar]
- 106.Page C. New and maintained genera in the conifer families Podocarpaceae and Pinaceae. Notes R. Bot. Gard. Edinb. 1988;45:377–395. [Google Scholar]
- 107.Page C. Gymnosperms: Coniferophytina (conifers and ginkgoids) Fam. Genera Vasc. Plants. 1990;1:279–361. [Google Scholar]
- 108.Dezhi F. Nageia into a new family Nageiaceae F. Nageiaceae—A new Gymnosperm family. Acta Phytotaxon. Sin. 1992;30:515–528. [Google Scholar]
- 109.Contreras D., Duijnstee I., Ranks S., Marshall C., Looy C. Evolution of dispersal strategies in conifers: Functional divergence and convergence in the morphology of diaspores. Perspect. Plant Ecol. Evol. Syst. 2017;4:93–117. doi: 10.1016/j.ppees.2016.11.002. [DOI] [Google Scholar]
- 110.Sudianto E., Wu C.S., Leonhard L., Martin W.F., Chaw S.M. Enlarged and highly repetitive plastome of Lagarostrobos and plastid phylogenomics of Podocarpaceae. Mol. Phylogenetics Evol. 2019;133:24–32. doi: 10.1016/j.ympev.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 111.Gordon G. The Pinetum: Being a Synopsis of All the Coniferous Plants at Present Known with Descriptions, History and Synonymes, and Comprising Nearly One Hundred New Kinds. Bohn; Vancouver, BC, Canada: 1858. [Google Scholar]
- 112.Philippi R. Zwei neue Gattungen der Taxineen aus Chile. Linnaea. 1861;30:730–735. [Google Scholar]
- 113.De Laubenfels D.J. A revision of the Malesian and Pacific rainforest conifers, I. Podocarpaceae, in part. J. Arnold Arbor. 1969;50:315–369. doi: 10.5962/bhl.part.24691. [DOI] [Google Scholar]
- 114.Quinn C. Generic boundaries in the Podocarpaceae. Proc. Linn. Soc. NSW. 1970;94:166–172. [Google Scholar]
- 115.De Laubenfels D. Gymnosperms. Flore de la Nouvelle Calédonie et Dépendances. Muséum National D’Histoire Naturelle; Paris, France: 1972. p. 4. [Google Scholar]
- 116.Leistner O., Smith G., Glen H. Podocarpaceae. Bothalia. 1995;25:233–236. doi: 10.4102/abc.v25i2.731. [DOI] [Google Scholar]
- 117.Glen H. Podocarpaceae. In: Leistner S., editor. Seed Plants of Southern Africa: Families and Genera. Volume 10. National Botanical Institute; Pretoria, South Africa: 2000. pp. 30–31. [Google Scholar]
- 118.Barker N.P., Muller E., Mill R. A yellowwood by any other name: Molecular systematics and the taxonomy of Podocarpus and the Podocarpaceae in southern Africa. S. Afr. J. Sci. 2004;100:629–632. [Google Scholar]
- 119.Bobrov A.V.C., Melikian A.P., Yembaturova E.Y. Seed morphology, anatomy and ultrastructure of Phyllocladus LC & A. Rich. ex Mirb. (Phyllocladaceae (Pilg.) Bessey) in connection with the generic system and phylogeny. Ann. Bot. 1999;83:601–618. [Google Scholar]
- 120.Keng H. The genus Phyllocladus (Phyllocladaceae) J. Arnold Arbor. 1978;59:249–273. doi: 10.5962/bhl.part.22773. [DOI] [Google Scholar]
- 121.Chaw S.M., Sung H.M., Long H., Zharkikh A., Lie W.-H. The phylogenetic positions of the conifer genera Amentotaxus, Phyllocladus, and Nageia inferred from 18S rRNA sequences. J. Mol. Evol. 1995;41:224–230. doi: 10.1007/BF00170676. [DOI] [PubMed] [Google Scholar]
- 122.Tomlinson P.B., Braggins J.E., Rattenbury J.A. Contrasted pollen capture mechanisms in Phyllocladaceae and certain Podocarpaceae (Coniferales) Am. J. Bot. 1997;84:214–223. doi: 10.2307/2446083. [DOI] [PubMed] [Google Scholar]
- 123.Quinn C., Price R., Gadek P. Familial concepts and relationships in the conifer based on rbcL and matK sequence comparisons. Kew Bull. 2002;57:513–531. doi: 10.2307/4110984. [DOI] [Google Scholar]
- 124.Rai H.S., Reeves P.A., Peakall R., Olmstead R.G., Graham S.W. Inference of higher-order conifer relationships from a multi-locus plastid data set. Botany. 2008;86:658–669. doi: 10.1139/B08-062. [DOI] [Google Scholar]
- 125.Enright N.J., Jaffré T. Ecology and distribution of the Malesian podocarps. In: Turner B.L., Cernusak L.A., editors. Ecology of the Podocarpaceae in Tropical Forests. Smithsonian Institution Scholarly Press; Washington, DC, USA: 2011. pp. 57–77. [Google Scholar]
- 126.Hill R.S. Ecology of the Southern Conifers. Melbourne University Press; Melbourne, Australia: 1995. Conifer origin, evolution and diversification in the Southern Hemisphere; pp. 10–29. [Google Scholar]
- 127.Enright N.J., Hill R.S. Ecology of the Southern Conifers. Melbourne University Press; Melbourne, Australia: 1995. p. 342. [Google Scholar]
- 128.Coomes D.A., Bellingham P.J. Temperate and Tropical Podocarps: How Ecologically Alike Are They? In: Turner B.L.C., Lucas A., editors. Ecology of the Podocarpaceae in Tropical Forests. Smithsonian Contributions to Botany; Washington, DC, USA: 2011. pp. 119–140. [Google Scholar]
- 129.Adie H., Lawes M.J. Podocarps in Africa: Temperate zone relicts or rainforest survivors? Smithson. Contrib. Bot. 2011;95:79–100. doi: 10.5479/si.0081024X.95.79. [DOI] [Google Scholar]
- 130.Hill R.S., Khan R. Southern (Austral) Ecosystems in Encyclopedia of Reference Module in Life Sciences. Elsevier; Amsterdam, The Netherlands: 2022. [Google Scholar]
- 131.Nieto-Blázquez M.E., Peña-Castillo L., Roncal J. Historical biogeography of Caribbean Podocarpus does not support the progression rule. J. Biogeogr. 2021;48:690–702. doi: 10.1111/jbi.14034. [DOI] [Google Scholar]
- 132.Khan R., Hill R.S. Reproductive and leaf morpho-anatomy of the Australian alpine podocarp and comparison with the Australis subclade. Bot. Lett. 2022;169:237–249. doi: 10.1080/23818107.2022.2042381. [DOI] [Google Scholar]
- 133.Leslie A.B. How many ways can you build a conifer cone? A commentary on ‘Origin and evolution of Podocarpaceae seed cones’. Ann. Bot. 2022;130:i–iii. doi: 10.1093/aob/mcac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khan R., Hill R.S. Morpho-anatomical affinities and evolutionary relationships of three paleoendemic podocarp genera based on seed cone traits. Ann. Bot. 2021;128:887–902. doi: 10.1093/aob/mcab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hill R.S., Brodribb T. Turner Review No. 2-Southern conifers in time and space. Aust. J. Bot. 1999;47:639–696. doi: 10.1071/BT98093. [DOI] [Google Scholar]
- 136.Khan R. Ph.D. Thesis. School of Biological Sciences, University of Adelaide; Adelaide, Australia: 2022. Towards the Systematics and Evolution of the Conifer Family Podocarpaceae; New Insights into the Key Aspects; pp. 210–365. [Google Scholar]
- 137.Tomlinson P. Functional morphology of saccate pollen in conifers with special reference to Podocarpaceae. Int. J. Plant Sci. 1994;155:699–715. doi: 10.1086/297209. [DOI] [Google Scholar]
- 138.Erdtman G. Pollen and Spore Morphology-Plant Taxonomy: Gymnospermae, Bryophyta (Text): (An Introduction to Palynology. III) Almquist & Wiksell; New York, NY, USA: 1965. [Google Scholar]
- 139.Pocknall D.T. Pollen morphology of Phyllocladus LC et A. Rich. N. Z. J. Bot. 1981;19:259–266. doi: 10.1080/0028825X.1981.10426378. [DOI] [Google Scholar]
- 140.Woltz P. Place des gymnospermes endémiques des Adnes méridionales dans la végétation du Chili. Lazaroa. 1985;8:293–314. [Google Scholar]
- 141.Nanami S., Kawaguchi H., Yamakura T. Dioecy-induced spatial patterns of two codominant tree species, Podocarpus nagi and Neolitsea aciculata. J. Ecol. 1999;87:678–687. doi: 10.1046/j.1365-2745.1999.00392.x. [DOI] [Google Scholar]
- 142.Geldenhuys C. Reproductive biology and population structures of Podocarpus falcatus and P. latifolius in southern Cape forests. Bot. J. Linn. Soc. 1993;112:59–74. doi: 10.1006/bojl.1993.1041. [DOI] [Google Scholar]
- 143.Davies S.J., Bamford M. Ratites and Tinamous. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- 144.Brodribb T.J. A functional analysis of podocarp ecology. Smithson. Contrib. Bot. 2011;95:165–173. doi: 10.5479/si.0081024X.95.165. [DOI] [Google Scholar]
- 145.Kitayama K., Aiba S.-i., Ushio M., Seino T., Fujiki Y. The ecology of podocarps in tropical montane forests of Borneo: Distribution, population dynamics, and soil nutrient acquisition. Smithson. Contrib. Bot. 2011;95:101–117. doi: 10.5479/si.0081024X.95.101. [DOI] [Google Scholar]
- 146.Bond W. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 1989;36:227–249. doi: 10.1111/j.1095-8312.1989.tb00492.x. [DOI] [Google Scholar]
- 147.Midgley J.J., Bond W.J. Ecological aspects of the rise of angiosperms: A challenge to the reproductive superiority hypotheses. Biol. J. Linn. Soc. 1991;44:81–92. doi: 10.1111/j.1095-8312.1991.tb00608.x. [DOI] [Google Scholar]
- 148.Bentley W. Ph.D. Thesis. University of Cambridge; Cambridge, UK: 2008. Influences of Soil Nutrients, Waterlogging, and Disturbance Factors on Forest Processes along a New Zealand Soil Chronosequence. [Google Scholar]
- 149.Wardle P. Growth habits of New Zealand subalpine shrubs and trees. N. Z. J. Bot. 1963;1:18–47. doi: 10.1080/0028825X.1963.10429319. [DOI] [Google Scholar]
- 150.Gaxiola A., Burrows L.E., Coomes D.A. Tree fern trunks facilitate seedling regeneration in a productive lowland temperate rain forest. Oecologia. 2008;155:325–335. doi: 10.1007/s00442-007-0915-8. [DOI] [PubMed] [Google Scholar]
- 151.Coomes D.A., Kunstler G., Canham C.D., Wright E. A greater range of shade-tolerance niches in nutrient-rich forests: An explanation for positive richness—Productivity relationships? J. Ecol. 2009;97:705–717. doi: 10.1111/j.1365-2745.2009.01507.x. [DOI] [Google Scholar]
- 152.Kershaw A., Martin H., Mason J.M. The Neogene: A period of transition. Hist. Aust. Veg. Cretac. Recent. 1994;1:299–327. [Google Scholar]
- 153.Hill R.S., Lewis T., Carpenter R.J., Whang S.S. Agathis (Araucariaceae) macrofossils from Cainozoic sediments in south-eastern Australia. Aust. Syst. Bot. 2008;21:162–177. doi: 10.1071/SB08006. [DOI] [Google Scholar]
- 154.Pittermann J., Sperry J.S., Hacke U.G., Wheeler J.K., Sikkema E.H. Inter-tracheid pitting and the hydraulic efficiency of conifer wood: The role of tracheid allometry and cavitation protection. Am. J. Bot. 2006;93:1265–1273. doi: 10.3732/ajb.93.9.1265. [DOI] [PubMed] [Google Scholar]
- 155.Lusk C.H., Kelly C.K. Interspecific variation in seed size and safe sites in a temperate rain forest. New Phytol. 2003;158:535–541. doi: 10.1046/j.1469-8137.2003.00760.x. [DOI] [PubMed] [Google Scholar]
- 156.Pittermann J., Sperry J.S., Wheeler J.K., Hacke U.G., Sikkema E.H. Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant Cell Environ. 2006;29:1618–1628. doi: 10.1111/j.1365-3040.2006.01539.x. [DOI] [PubMed] [Google Scholar]
- 157.Brodribb T.J., Pittermann J., Coomes D.A. Elegance versus speed: Examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 2012;173:673–694. doi: 10.1086/666005. [DOI] [Google Scholar]
- 158.Cernusak L.A., Adie H., Bellingham P.J., Biffin E., Brodribb T.J., Coomes D.A., Dalling J.W., Dickie I.A., Enright N.J., Kitayama K. Podocarpaceae in tropical forests: A synthesis. Smithson. Contrib. Bot. 2011;95:189–195. doi: 10.5479/si.0081024X.95.189. [DOI] [Google Scholar]
- 159.Jump A.S., Mátyás C., Peñuelas J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009;24:694–701. doi: 10.1016/j.tree.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 160.Gill A.M. How Fires Affect Biodiversity. Biodiversity Series, 8, Footscray, Melbourne. [(accessed on 22 July 2021)];1996 Available online: https://www.cpbr.gov.au/fire_ecology/fire-and-biodiversity.html.
- 161.Lawes M.J., Griffiths M.E., Boudreau S. Colonial logging and recent subsistence harvesting affect the composition and physiognomy of a podocarp dominated Afrotemperate forest. For. Ecol. Manag. 2007;247:48–60. doi: 10.1016/j.foreco.2007.04.012. [DOI] [Google Scholar]
- 162.Mill R. A monographic revision of the genus Podocarpus (Podocarpaceae): I. Historical review. Edinb. J. Bot. 2014;71:309. doi: 10.1017/S0960428614000146. [DOI] [Google Scholar]
- 163.Holloway J.T. An ecological classification of the forest types of the Westland podocarp region. N. Z. J. For. 1954;7:24–33. [Google Scholar]
- 164.Wardle P. The regeneration gap of New Zealand gymnosperms. N. Z. J. Bot. 1963;1:301–315. doi: 10.1080/0028825X.1963.10429001. [DOI] [Google Scholar]
- 165.Bodnar J., Escapa I.H. Towards a whole plant reconstruction for Austrohamia (Cupressaceae): New fossil wood from the Lower Jurassic of Argentina. Rev. Palaeobot. Palynol. 2016;234:186–197. [Google Scholar]
- 166.Axsmith B.J., Escapa I.H., Huber P. An araucarian conifer bract-scale complex from the lower Jurassic of Massachusetts: Implications for estimating phylogenetic and stratigraphic congruence in the Araucariaceae. Palaeontol. Electron. 2008;11:9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in article supplementary material. Additional supporting information may be found in the online version of the article at the publisher’s website.