Abstract
One of the crucial challenges of our time is to effectively use metal and metal oxide nanoparticles (NPs) as an alternative way to combat drug-resistant infections. Metal and metal oxide NPs such as Ag, Ag2O, Cu, Cu2O, CuO, and ZnO have found their way against antimicrobial resistance. However, they also suffer from several limitations ranging from toxicity issues to resistance mechanisms by complex structures of bacterial communities, so-called biofilms. In this regard, scientists are urgently looking for convenient approaches to develop heterostructure synergistic nanocomposites which could overcome toxicity issues, enhance antimicrobial activity, improve thermal and mechanical stability, and increase shelf life. These nanocomposites provide a controlled release of bioactive substances into the surrounding medium, are cost effective, reproducible, and scalable for real life applications such as food additives, nanoantimicrobial coating in food technology, food preservation, optical limiters, the bio medical field, and wastewater treatment application. Naturally abundant and non-toxic Montmorillonite (MMT) is a novel support to accommodate NPs, due to its negative surface charge and control release of NPs and ions. At the time of this review, around 250 articles have been published focusing on the incorporation of Ag-, Cu-, and ZnO-based NPs into MMT support and thus furthering their introduction into polymer matrix composites dominantly used for antimicrobial application. Therefore, it is highly relevant to report a comprehensive review of Ag-, Cu-, and ZnO-modified MMT. This review provides a comprehensive overview of MMT-based nanoantimicrobials, particularly dealing with preparation methods, materials characterization, and mechanisms of action, antimicrobial activity on different bacterial strains, real life applications, and environmental and toxicity issues.
Keywords: antimicrobial, Ag, Cu, ZnO, montmorillonite, chitosan, food packaging, ion exchange
1. Introduction
Antimicrobial resistance (AMR) is one of the greatest threats to public health and the economy. Resistance of microorganisms towards antibiotics, antifungals, or disinfecting agents poses a great concern in scientific communities. The World Health Organization (WHO) outlines that antibiotic resistance is one of the top 10 public health threats [1]. Bacteria develop resistance and have the ability to survive harsh environments, forming biofilms. Biofilms are communities of microbes which can be formed on living surfaces such as skin, lungs, and bladder, or non-living surfaces such as wound dressings, medical implants, and devices (artificial hearts, catheters, and stents), and food packaging. [2]. Bacterial biofilms cause infections due to their intrinsic tolerance to antimicrobial therapies. Eradicating bacterial biofilms is a challenging task, owing to the formation of dense bacterial communities with a complex architecture, which provides protection from antibiotics and antibacterial agents. One of the strategies to inhibit biofilms is the development of novel nanoantimicrobials (NAMs) [3]. In fact, over a period of decades, research attention has been driven to synthesize novel antimicrobial agents by developing fast, environmentally friendly, easily scalable, cost effective, and facile approaches [4,5,6,7,8,9,10]. Several types of metal and metal oxide NPs such as Ag, Ag2O, Cu, CuO, ZnO, TiO2, Si, SiO2, Au, CaO, and MgO are considered as potent antimicrobial agents [11,12,13,14,15]. Among them, Ag-, Cu-, and ZnO NPs exhibit excellent antibacterial activity and have several advantages such as improved stability and safety, and longer active periods than that of organic nanomaterials (even at low concentrations with their multi-targeted mechanism of action) [15,16,17]. However, the direct use of these NPs may lead to instability, severe reactivity, and toxicity due to their high surface-to-volume ratio, and fast and uncontrolled release properties. Moreover, metal NPs can suffer from agglomeration which eventually leads to chemical and physical changes. As a result, antibacterial activity may be reduced [18]. Therefore, it is needed to overcome those shortcomings by developing sustainable, cheap, and environmentally friendly methods to prepare novel composite nanomaterials.
In general, the antimicrobial activity of metal nanoparticles is related to their size, shape, and ion release properties. Specifically, AgNPs, CuNPs, and ZnONPs can act as antimicrobial agents because they are able to go through the cell wall, if they are small enough (<10 nm) [19]. At the same time, all of them can work as bioactive ion reservoir. It is well known that AgNPs not only act as silver ion reservoirs but also provide a high enough concentration of silver antibacterial species in their surroundings. At the same time, all of them can work as bioactive ion reservoirs. AgNPs have the ability to adsorb onto the bacterial membrane, causing “pits” in the cell wall and consequent apoptosis. Therefore, antibacterial activity remains high for a long period of time [20,21]. On the other hand, the Cu(II) oxidation state of copper is highly effective towards microbial cells, owing to interaction with nucleic acids, enzyme active sites, and components of the cell membrane. As a consequence, the microbial cells end up dying [22]. It is also proven that ZnO produces hydroxyl radicals, which lead to bacterial cell damage [23].
The European Food Safety Authority (EFSA) highlights the use of antimicrobial NPs in food packaging. In particular, food supplements which contain AgNPs must not exceed 0.05 mg/L in water and 0.05 mg/Kg Ag migration in food [24]. Moreover, other factors such as size and shape of NPs, coating, life cycle, dosing, particle agglomeration, use of biosafe polymers and solid supports, control ion release, and synthetic route, play a vital role to address cytotoxicity issues and the antimicrobial activity of NPs for real life application by designing novel hybrid synergistic materials. Using NPs instead of ions is more effective because the latter deplete rapidly, even though their initial activity is higher. Therefore, the use of ions enables an immediate and high antimicrobial activity, which can be lost in a very small lapse of time. On the contrary, NPs act as a reservoir of ions with a possible control on the leaching rate and amount, ensuring focused and long-term activity of the nanomaterials [3,25]. The use of ions rather than the metal NPs as bioactive agents is sometimes preferred, because the release and associated potential accumulation of metal NPs in the organism could be dangerous, particularly if NP size is smaller than 10 nm. In this case, Cu ions are found to be less toxic than Ag ions [26]; however, the ecotoxicity of ions and NPs released from nanocomposites needs to be researched more, considering environmental health safety issues. Thus, choosing a carrier which can effectively lower the toxicity of Ag, Cu, or ZnO NPs and improve antimicrobial performance at a low concentration of antimicrobial NPs is vital. Apart from that, supports for deposition of nanomaterials are fundamental to control NP size, and attain improved stability with long-term activity [27,28,29,30].
Ag-, Ag2O-, Cu-, CuO-, and ZnO-based NPs with MMT play a vital role in shaping the current research scenario, such as fighting cross-infections and infectious diseases, providing safe food technology, and treating co-existing contaminates [31,32,33,34,35,36].
The reasons for considering Ag-, Cu-, and Zn-based particles as antimicrobial nanophases is to ensure their uniform dispersion in the supporting material and to control the release of their species. Synthesis of NPs on solid support such as MMT is highly recommended to achieve formation of practically applicable supported particles. This feature would be helpful to slow and control the release of bioactive metal ions from active films or coatings, for long-lasting antibacterial activity [37]. Moreover, incorporation of colloidal NPs into the MMT matrix could lead to the development of perspective additives for real life application. MMT is an ideal substrate for preparing 2D materials for its sheet-like morphology. MMT can be exfoliated into nanosheets used in a wide range of applications as two dimensional (2D) material. Metal NP–clay–polymer composite find potential applications in food packaging and biomedical fields, due to the formation of exfoliated nanocomposite heterostructures with a random dispersion of bioactive metal NPs on the polymer matrix, limiting the diffusion of metal ions, and slowing down their release, reducing water and oxygen permeability, and modifying antimicrobial activity [38,39,40]. In particular, the bacterial cell wall contains lipoprotein, which exhibits a net negative charge under physiological conditions because of the presence of functional groups such as phosphate, carboxyl, and hydroxyl. On account of that, metal ions are adsorbed on the cell wall by electrostatic attraction, causing shrinkage of the cytoplasm membrane or detachment of the cell wall, resulting in inactivation and inhibition in cell processes [17,41]. In this scenario, strong metal retention capacity of the solid support improves antimicrobial activity, which could be achieved by tailoring weak hydroxyl groups of polymer–metal interaction, and an optimum amount of metal-loaded polymer onto MMT substrate [42]. The adsorption of bacteria (Coliphages T1 and T7 of E. coli) on MMT clay was investigated for the first time by Schiffenbauer M. and Stotzky G. [43]. MMT clay as a natural material has always been worthy of consideration and has attracted intensive attention as a novel support, it absorbs a variety of materials on its surface because it has the advantage of a lamellar structure, a large surface area, and exhibits a negative surface charge (−60 ± 10 mV) [44,45,46], is biologically inert and it is effortless to remove it from the human organism [47]. MMT/metal NPs composites exhibit excellent adsorption, rheological, ion exchange, heat insulators and are heat-resistant, and swelling properties [48,49]. On top of that, nanosheets of MMT act as a stabilizing surface for the deposition of NPs [50], which prevent NP agglomeration. MMT has the capability of accommodating NPs in their lattice which open new perspectives in manufacturing medical devices, the textile industry, wastewater treatment, drug delivery, and food packaging application. MMT is not only cost effective and environmentally friendly, but also has ability to be pillared with metal ions, forming so-called Ag/MMT, Cu/MMT, or Zn/MMT nanocomposites, allowing for improved antibacterial activity [51,52,53,54,55]. However, in most of the cases, before the incorporation of MMT into materials, it is necessary to modify it by physical (grinding and calcination) or chemical exfoliation processes, in order to improve its surface area, changing particle size, and modifying surface charge, to improve its compatibility with other materials. To this aim, several exfoliation approaches have been investigated. The use of quaternary ammonium surfactant such as cetyltrimethylammonium bromide, which causes MMT clay to become partially organophilic; the surface potential of MMT shifts from negative to positive, facilitating the dispersion and occlusion of NPs in the MMT interparticle pore, and providing effective mutual interaction between NPs and microorganisms on the surface of MMT clay. This is also due to higher homogeneity and improved diffusion properties [56,57,58,59,60], and the introduction of functional groups on MMT surfaces [61]. It is imperative to perform washing and refining steps to make the MMT matrix pure and suitable for the accommodation of metal and metal oxides into/onto its layer/surface. Physical purification such as grinding for size fractionation separates some impurities while chemical dissolution–physical purification is highly recommended to separate most of the impurities from the MMT [62]. The capability of MMT for adsorbing target metals is a distinct advantage for efficient mechanisms which can be further adjusted based on the characteristics of NPs. MMT play significant roles in supporting metal and metal oxide NPs, acting as ligands and protecting agents for reactive and uncontrolled conditions, providing an active surface site which contributes directly to the reactivity of NPs for perspective application such as catalysts or antimicrobial agents [63,64].
Recently, MMT-based two-dimensional (2D) composite materials, such as MMT-layered double hydroxide and MMT–graphene have been reviewed focusing on their preparation strategies and applications in pollution adsorption, medical antibacterial, film thermal conduction, and flame retardant [65]. In 2016, Giraldo et al. critically reviewed an Ag incorporation technique into MMT phyllosilicates and further outlined that functionalization of the MMT can promote the Ag+ adsorption [66].
There are several reviews on Zeolite-supported silver [67,68,69,70,71] but no comprehensive review on NP-impregnated MMT dealing with Ag, Cu, and ZnO are available. In this review, we have focused our attention on preparation methods, potential antimicrobial activity, real life application, and environmental and toxicity issues of MMT-supported Ag, Cu, and ZnO nanoantimicrobials (NAMs). We aim to collect a library of available studies since the past two decades on the improvement of an antimicrobial effect of Ag-, Cu-, and ZnO-incorporated MMT-based composites and understanding the mechanisms involved in growth inhibition. Furthermore, their introduction into the polymer matrix dominantly for antimicrobial application is discussed using the data from >200 publications.
2. Nanoantimicrobials Based on Various Synthetic Routes
Several methods such as chemical, physical, biological, electrochemical, and hybrid methods have been applied for the preparation of Ag, Cu, and ZnO NAMs. Each method has its pros and cons. Details can be seen in the following reviews, which particularly deal with the synthesis, mechanism, characterization, and potential application of Ag, Cu, and ZnO NAMs [19,69,72,73,74,75]. In the following sections, the different studies dealing with MMT-supported Ag, Cu, and ZnO NAMs have been classified and characterized based on preparation methods, namely, ion exchange and chemical reduction, hybrid, irradiation, electrochemical, plasma resonance, and radio frequency (RF) approaches. Additionally, the study of adsorption, structural investigation, and molecular modelling of MMT-supported NAMs have been briefly discussed.
2.1. Ag/MMT Nanocomposite by Ion Exchange and Chemical Reduction
As MMT is negatively charged, it can adsorb metal cations, which could be easily incorporated by simple ion exchange methods within the interlayer space of MMT, and this can eventually result in improved antimicrobial behavior with a more controlled release of the active species. For example, in 2009, Ohashi et al. generated a silver chelate complex of 6-benzylaminopurine (Ag+(6-BAP)2) MMT by cation exchange reaction in aqueous medium. The planar Ag+(6-BAP)2 ions were arranged parallel to the silicate layers. In this approach, most of the interlayer cations are replaced by the silver chelate complexes. The interlayer silver chelate complex is decomposed between 250 °C and 400 °C, with increasing temperature, the Ag+ ions are reduced to form silver particles by carbothermal reduction. The slow release of silver chelate is attributed to the electrostatic adsorption state in the deep part of the nanolayers [76]. Following a similar approach, in 2015, Sohrabnezhad and Sadeghi prepared Ag2CO3-Mobile Crystalline Material (MCM-41) and Ag2CO3/MMT, and claimed that AgNPs are more stable in Ag2CO3/MMT than in Ag2CO3/(MCM-41). However, Ag2CO3/MCM-41 exhibited higher antibacterial activity towards E. coli [77], because of the binding of silver ions to functional groups of proteins and enzymes, which lead to cell process inhibition [78]. Citing a few related works, [79,80] describe the effect of microparticles in drug delivery and photocatalytic activity of Ag2CO3. In 2016, Sohrabnezhad et al. addressed silver halide (AgX, X = Cl, Br, I)/(MMT) nanocomposites as antibacterial agents using the same common ion exchange method. Furthermore, the study showed that AgCI/MMT is more active than AgBr/MMT and AgI/MMT against S. aureus, M. luteus, and E coli [81]. However, Naik et al. previously explored Ag/AgCl–mesoporous silica nanocomposites against E. coli [82]. In the following year, the study of silver histidine-loaded Na-MMT antibacterial agent showed higher cation exchange capacity than pristine Na-MMT [83]. Considering the incorporation of nano-clay into polymer composite to modify antimicrobial functionality, Ag-modified MMT (Ag/MMT) and Ag/MMT as powder coating in epoxy/polyester resin, was reported. The Ag/MMT exhibited antibacterial activity against E. coli over 24 h, and it was thermally stable even after annealing at 180 °C. While in contrast, powder coatings of Ag/MMT dispersed in epoxy/polyester resin showed no antimicrobial performance towards E. coli, due to poor wetting of the polymer coating, which limits the diffusion of Ag ion from the coating [84]. On the other hand, a study of chitosan (CS)-based Ag/MMT not only demonstrated it to be an efficient antimicrobial agent but also offered a better understanding of the synergy between chitosan and Ag/MMT, between pH 3.6 and 6. MMT clay containing Ag reduces the water uptake of the nano-bio-composites, because Ag+ ions corroborate chemical and physical cross-linking between the chitosan macromolecules. As a result, the proposed material showed improved stability in water, and could overcome some limitations to chitosan commercialization [85].
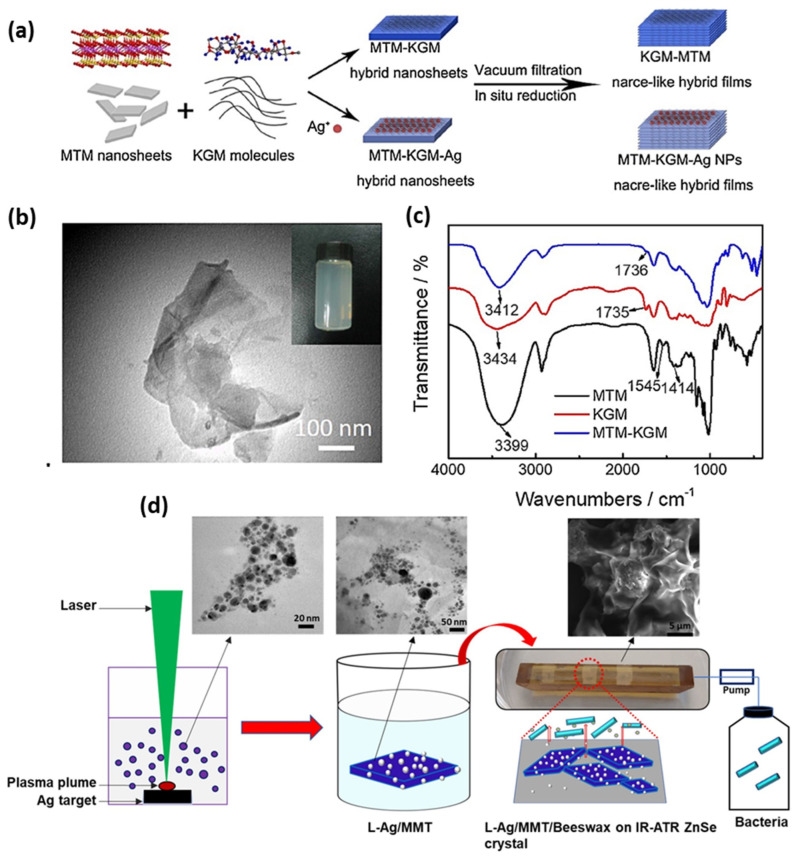
Chemical reduction is one of the most common and frequently applied strategies for the preparation of NAMs in water and organic solvents. Metal NPs can be embedded on organic or inorganic substrate, with improved antimicrobial efficacy with slow and control release of NPs and ions. Basically, NPs become crystallized over substrates such as MMT layers in situ or ex situ, in the presence of added reducing agents which facilitate ions to NP transformation. Several reduction media such as chemical salts, solvents, plant extracts, and biological species are being used to control NP diameter and size distribution. In most cases, antimicrobial efficacy depends on NP size and on the nature of reducing the medium which is responsible for NPs formation on supported layers. Therefore, controlling the nature and parameters of reductants also plays a significant role in the attainment of the biocidal effect in NP/substrate nanocomposites. Conventional chemical reduction methods need special attention to make stable colloids and controlled NP sizes and shapes. Several parameters such as solution temperature, concentrations of the reducing agent, precursor and surfactant concentration ratio and mixing speed, reaction time, and the use of additional supporting materials must be optimized. Preventing NP agglomeration, and controlling their size and shape may be challenging [86]. MMT could be considered as a protective agent to prevent the NP agglomeration and to stabilize metal particles in nanocomposite [50,87]. In light of this, Praus et al. intercalated MMT with silver cations by common reduction method: formaldehyde and sodium borohydride (NaBH4) were subsequently used as reductants. Silver NPs with sizes ranging from 3 nm to 13 nm, and 3 nm to 100 nm were observed after reduction with borohydride and formaldehyde, respectively [88]. It is well known that antibacterial activity of Ag/MMT is higher than pristine Ag. For instance, Malachova et al. showed that the minimum inhibitory concentration (MIC) values of Ag/MMT for E. coli (0.87 mg L−1) and Enterococcus faecium (E. faecium) (1.10 mg L−1) are lower than the MIC value of pristine Ag for E. coli (0.92 mg L−1) and E faecium (1.50 mg L−1). The reason behind this might be due to the controlled silver ions release from MMT [51]. In the year 2010, Miyoshi et al. reported on the preparation of AgNPs (approximately 2.5 ± 0.6 nm) on MMT, in n-hexanol. Average particle size ranged from 2.5 to 6.8 nm upon changing the Ag+ concentration, below 0.1 M, while it reached 400 nm for [Ag+] = 1 M, since AgNPs coagulated to form large particles on the clay at high concentrations of Ag+. Nevertheless, the clay prevented further coagulation of particles and surprisingly antibacterial activity of AgNP/MMT was observed against E. coli even after a long time [89]. It is known that in the High-Resolution Episcopic Microscopy (HREM) setup, extremely small particles are unstable in the presence of the electron beam [90]. However, Miyoshi et al.’s group claimed that the prepared AgNPs were extremely small but stable on the MMT clay, under HREM setup. In another study, Shameli et al. generated AgNPs (5–10 nm diameter) in the lamellar space of the MMT, where NaBH4 and AgNO3 were considered as the reducing agent and Ag precursor, respectively. After that, significant antibacterial activity was found against S. aureus, methicillin-resistant S. aureus (MRSA), E. coli O157:H7, and Klebsiella pneumoniae. Additionally, it has been observed that MMT acts as both reducing and protective agent, preventing AgNP aggregation. According to the literature, it has been hypothesized that, due to their small size, AgNPs with a diameter lower than 10 nm easily penetrate inside the bacterial cell. Otherwise, they are too big and are only able to attach onto negatively charged cell walls, thus disrupting bacterial integrity, and explore a larger surface area, resulting in improving antibacterial performance [91]. However, it is arguable that, in the case of solid support such as MMT, antibacterial activity depends on the release of Ag+ ions from the surface of AgNPs [92,93]. In 2015, the preparation of AgNPs in the presence of CO32− ions was reported, Ag2CO3/MMT and Ag/MMT nanocomposite were developed in ethylene glycol, which showed antibacterial activity against E. coli. According to the MIC value, it is apparent that Ag2CO3/MMT exhibited higher antibacterial performance than the Ag/MMT nanocomposite because Ag2CO3 NPs were found to be well dispersed in MMT, resulting in higher silver ion release [94]. Nevertheless, there is clear argument with others finding that antimicrobial performance depends on silver content [46,95], Later, Bonga et al. synthesized AgNPs on MMT by the chemical reduction of silver nitrate with sodium citrate and measured antibacterial performance towards S. aureus and E. coli. The effect of synthetic parameters, such as higher concentration of reductant, leading to a smaller AgNP size, was investigated [96]. It is worth describing that Zhu et al. fabricated Ag-introduced nacre-like konjac glucomannan–Montmorillonite (KGM–MMT) composite films with a pearl shell micro-layer (Figure 1a) by vacuum filtration and in situ reduction method. The very first-time nacre-like structure that contains KGM was reported, where assembly of a KGM–MMT hybrid was established. Transmission electron microscopy (TEM) images as shown in Figure 1b, confirm sheet-like morphologies of MMT, indicating that the composite could be dispersed in distilled water and then aligned to a nacre-like lamellar microstructure by vacuum-filtration-induced self-assembly. On the other hand, FTIR data reveal that the hydroxyl and carbonyl group of KGM and the silicate layer of MMT has strong interaction (Figure 1c) [97]. Following the chemical reduction method, the introduction of nanomaterials within the polymeric matrices is considered as an effective approach to further improve antimicrobial activity of nanocomposite. In this sense, Shameli et al. reported the synthesis of spherical AgNPs (around 6–9 nm in diameter) on modified MMT/CS by NaBH4 reduction, and investigated antibacterial activity towards S. aureus, E. coli, and P. aeruginosa by the disc diffusion method [98]. Similarly, Roy et al. proposed that hyperbranched epoxy resins modified Ag/MMT composite shows improved antibacterial activity, mechanical, and thermal properties [99]. The investigation contributed to the preparation of multifunctional epoxy/nanocomposites [100], cellulose–hyperbranched epoxy composites for coating applications [101], Ag/TiO2/bentonite nanocomposites for biological application [102], and homogeneous sponge microstructures of polycaprolactone Ag/MMT/PCL where a gradual increase in Ag release properties until 4 weeks of immersion in acidified water was observed [103,104]. The bioactivity mechanism of graphene oxide–silver nanocomposites [105,106], and of Ag/cyanobacteria composites was investigated too [107]. In 2017, Li et al. also applied specific synthetic routes to the investigation of the antibacterial activity of AgNPs/dry-fabricated biofilm (DFBF), Ag/MMT, and Ag/MMT/DFBF composites against S. aureus, E. coli, and P. aeruginosa. The laminated structure of MMT and the network of the DFBF lowered the release of both Ag+ ions and AgNPs [108]. Strikingly, biosynthesis of Ag/MMT nanocomposites was presented as a green chemical route, using water plant extracts Satureja hortensis as the reducing agent, tarragon leaf extract, Ocimum basilicum; Teucrium Polium plant extract as both reducing and capping agents, and ascorbic acid as the reducing agent. The reduction in AgNO3 was found to be possible due to the presence of phenolic agents and proteins in the tarragon. Nonetheless, the detailed reaction mechanism is unclear. However, controlled size and uniform distribution into MMT and lecithin (MMT/LEC) were demonstrated, with antimicrobial performance against S. aureus and E. coli. According to Dairi et al., cellulose acetate/triethyl citrate (CA/TEC) nano-bio-composite film was prepared by incorporating Ag/gelatin/organically modified montmorillonite (OMMT), and thymol, where Curcuma longa (C. longa) tuber extract was used for the biosynthesis of AgNPs. The nano-bio-composite film showed antioxidant and antimicrobial activities towards bacteria and fungi; particularly, E. coli was the most sensitive. OMMT was considered to control silver release from the films, which outlines a long-time antimicrobial effect and the presence of thymol was useful to inhibit the growth of E. coli [109,110,111,112,113].
Figure 1.
(a) Schematic preparation process of the composite films. (b) TEM images of MMT nanosheets, (c) FTIR spectra of MMT, KGM, and KGM–MMT composite films. Adapted from [97], with permission from Elsevier. Copyright 2018, Elsevier. (d) Schematic of preparation of Laser ablated Ag/MMT/Beeswax coating and IR-ATR monitoring of biofilms inhibition. Adapted from [150], distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
2.2. Cu/MMT Nanocomposite by Ion Exchange Method
Several studies have been devoted to the absorption of Cu2+ on montmorillonite by cation exchange. Cu2+ in the form of CuSO4, is used in large amount for antimicrobial applications; its excessive use affects environmental economic sustainability [114]. Incorporation of MMT clay could decrease the use of Cu2+ supplement, considering a large specific surface area of MMT efficiently adsorbs bacteria in water. In this regard, Cu/MMT nanocomposite is found to be active against E. coli and S. faecalis, Clostridium in broiler chicks, and jejunal contents. The improved antibacterial activity was attributed due to the attraction of Cu2+-exchanged MMT to bacteria cell wall by electrostatic forces [115,116,117]. Previously, the investigation of Cu/MMT nanocomposite showed strong antibacterial ability to inhibit Aeromonas hydrophila due to either adsorption of the bacteria from solution and immobilization on the surface of the Cu/MMT nanocomposite, or Cu2+ dissociation from MMT surface and exertion of their antibacterial effect on bacteria. The study suggests that the antibacterial activity of Cu/MMT nanocomposite was mainly due to the MMT surface, rather than to Cu2+ release [118]. Meanwhile, Tong et al. found 1024 and 2048 μg/mL MIC values for Cu/MMT against E. coli K88 and S. choleraesuis, respectively. In contrast, both bacteria still grew in the broth containing 32.768 μg/mL of MMT, which further indicated that MMT itself has no antimicrobial effect [119]. Later, the effects of Cu/MMT on intestinal microflora, digestibility, and digestive enzyme activities of Nile tilapia (Oreochromis niloticus) were studied [120]. In a different study, Bagchi et al. adapted an in situ reduction of copper–ammonia–complex exchanged MMT, explaining that CuNPs are both intercalated and adsorbed on MMT clay, being active against E. coli, S. aureus, P. aeruginosa, and E. faecalis with more than a 90% mortality rate after 12 h [121]. However, in case of Cu2+-exchanged MMT, detailed characterization including copper chemical state in the composite, copper interaction with the MMT center, and the solvation dynamics of hydroxide ions needs to be investigated for its industrial application as an antimicrobial agent. Cu/MMT composites could be used as precursors for the preparation of multicomponent hybrid synergistic NAMs.
2.3. Zn/MMT by Ion Exchange
Taking into account the synergy of multicomponent NAMs, Tan et al. developed Zn2+-Ce3+/MMT nanocomposite with improved antibacterial activity, which results from the increase in specific surface area and synergistic antibacterial effect. Due to the simultaneous presence of Zn2+ and Ce3+ in Zn-Ce/MMT nanocomposite, different targets on a cell membrane might be attacked and damaged at once. Ce3+ effectively promotes the production of more hydroxylic free radicals from Zn2+, which are harmful for microorganisms. The Zn-Ce/MMT nanocomposite displayed broad-spectrum antibacterial activity, possessing the MIC of 1500, 1000, 2000, and 3000 mg·L−1 against E coli, S aureus, Candida albicans, and Mucor, respectively [122]. In 2010, Shi et al. prepared Zn2+-exchanged MMT, and elucidated that Zn content is a very important factor: increasing Zn content improves the antibacterial performance of Zn/MMT nanocomposites towards E. coli and S. aureus [53].
2.4. Ag/MMT Nanocomposite by Hybrid Method
It is possible to modify clay surface area and cation exchange capacity by physical treatment. For instance, after calcination, bentonite adsorption capacity was found to be improved [123,124]. Hybrid methods can be considered efficient for the fabrication of NAMs with improved mechanical and thermal stability of nanocomposite, enhanced antibacterial performance, and with uniform NP distribution. Physical methods such as thermal treatment, calcination, grinding, dipping, rolling, melt combining, and stepwise assembly. could be combined with the ion exchange method. For example, Magana et al. modified physically treated (calcination and grinding) MMT with silver by the ion exchange method, to obtain Ag/MMT with a 13.3 mm mean diameter inhibition zone against E. coli, which was 45% larger than that of pristine MMT. After the ionic exchange, total Ag content was 3.36 and 8.37 wt% for the calcinated clay and ground sample, respectively. The MIC values of 2.5 and 1 mg/L were found for calcinated clay and ground sample, respectively. The lower MIC values obtained for the ground sample is due to the higher ionic silver content [95]. In another study, Ag+/MMT/suture was prepared to strengthen and improve the antibacterial activity, by the dipping and rolling approach. Ag+/MMT, prepared at several temperatures, showed different effects for either E. coli or S. aureus. [125]. Hybrid methods have also been considered for the fabrication of polymer-based nanocomposites. Thus, Incoronato et al. studied Ag+ ion-exchanged MMT, reduced by thermal treatment. The composite was embedded in agar, zein, and PCL polymer matrices, and antimicrobial activity was investigated against three strains of Pseudomonas spp. (foodborne bacteria) by in vitro tests. Ag/MMT NPs embedded into agar showed activity against bacteria, on account of the highest water uptake (WU) of agar hydrogel. The higher amount of absorbed water in the agar hydrogel improved macromolecular mobility for the polymeric network. As a result, small AgNPs and generated Ag+ were able to bind the bacterial cell wall to diffuse more rapidly toward the bacteria. However, in the case of Ag/MMT-incorporated zein and PCL polymer matrices, no substantial antimicrobial activity has been shown [126]. Similarly, Costa et al. applied ion exchange and thermal treatment to prepare Ag/MMT NPs. Following that, the activity of Ag/MMT on microbial and sensorial quality decay was investigated during the storage of packaged fruit salad (growth of mesophilic bacteria) and showed an effect of different concentrations of Ag/MMT NPs on the shelf life of fruit salad. Interestingly, a correlation was found between antimicrobial efficacy and Ag/MMT NPs concentration [127]. In the following year, the same group studied the influence of calcium–alginate coating loaded by Ag/MMT NPs on fresh-cut carrots [128]. Later on, Ibarra et al. proposed that 2-[2-(dimethylamine)]-ethoxy ethanol (DMAE) compatibilized nanocomposites exhibits uniform filler (Ag and MMT) dispersion and exfoliation [129]. Following a stepwise assembly and impregnation approach, in quaternized carboxymethyl chitosan of cetyltrimethylammonium bromide organic modified MMT silver (QCOM-Ag), composite quaternary ammonium groups were found to be more beneficial than carboxymethyl groups, to prepare silver-based nanomaterials [130]. Cetyltrimethylammonium bromide (CTAB) (Ag+/OMMT) filler placed into low density polyethylene could reinforce polymer NAM film and improve an effective E. coli bacterial reduction by as much as 70% [131]. Another study was dedicated to synthesizing quaternized chitosan (QCS)/(OMMT)/(AgNPs)(QOMA) nanocomposite by the one-step approach, and to investigate the antibacterial activity of QOMA coating on steel plates towards E. coli, S. aureus, and fungi. Moreover, physical and mechanical investigation revealed no negative effect of QOMA coating on steel plates; instead, hydroxyl groups in QOMA act as adhesive [39]. Later, migration of cetylpyridinium bromide (CPB) from the low density polyethylene/MMT/CPB composite to aqueous food simulant was studied, proposing MMT as potential surfactant carrier [132]. Golubeva et al. developed Ag/Lysozyme/MMT bio-nanocomposite, with Lysozyme (Lys) acting as an organic shell, by hybrid method. Furthermore, they proposed the potentiality of the prepared sample as antimicrobial [133]. In early 2020, Roy et al. performed in vitro and in vivo analyses of polymeric nanocomposites as antimicrobial agents for the first time. Here, polyethylene/AgMMT nanocomposite was fabricated by melt compounding route [134]. An antimicrobial test suggested biocidal activity of the polymeric nanocomposite against E. coli, S. aureus, and A. niger. In vitro cytocompatibility studies with human erythrocytes and histopathological studies using rat skin surgically stitched with nanocomposite films were carried out. Recently, Zhang et al. demonstrated the potentiality of copper-loaded black phosphorus nanocomposites [135].
Ag-embedded OMMT and Polyacrylonitrile (PAN)-modified Ag/OMMT nanocomposites were considered as effective antibacterial agents in a liquid phase [136,137]. Samples were found to have the potential to cultivate fungi, and the effect of PAN on graphene oxide was investigated [138], although the role and mechanism of PAN in the nanocomposites towards pathogens was not properly understood. Those studies stimulated the exploration of Ag-doped polyaniline–polyvinyl chloride nanocomposite films as either photocatalysts or antibacterial agents [139]. In Ag/MMT, Ag+ is bound to the inner surface [140], while the outer surface is bound to Al-OH or Si-OH by electrostatic bonding [141]. MMT-modified with soy lecithin and citronellol, OMMT modified with carboxymethyl chitosan (QC), and AgNPs, were applied as functionalized antimicrobial coatings [142,143]. Applying a solution casting method, Makwana et al. reported agar–carboxymethyl cellulose Ag/MMT/agar–CMC, where tea extract was used as the reducing agent, with Ag/MMT apparently improving the mechanical and antimicrobial properties of agar–CMC polymer films [144]. Abbasian et al. synthesized AgNPs (2–10 nm) using AgNO3 as a precursor, CS-grafting polymerizations of acrylic acid (g-PAA) as a stabilizer. It was prepared by reversible addition−fragmentation chain-transfer (RAFT) polymerization, and the introduction of MMT further improves the sample thermal stability. Ag/CS-g-PAA/MMTs exhibited higher activity towards E. coli and C. albicans than S. aureus. However, overall antimicrobial activity of Ag/CS-g-PAA was found to be higher than Ag/CS-g-PAA/MMTs. Nevertheless, the mechanism and reasons behind this apparently inconsistent performance were not fully addressed [145]. Golubeva et al. modified synthetic MMT by Ag/Lys using a hydrothermal method and explored its hemolytic activity on human erythrocytes for the first time. Further, they showed that the use of aluminum oxide on MMT eventually reduced the antibacterial activity, but improved adsorption capacities of the prepared samples [146]. It is important to highlight that hemolytic activity of silver nanomaterials towards human erythrocytes was first demonstrated by Bock and Muller [147]. Through a one-step, low-temperature solvothermal technique, Wu et al. proposed to use MMT as a support for Ag/TiO2 NPs; this composite was applied for visible-light bacteria photodegradation and showed good stability, recyclability, and improved antibacterial activities [148]. Meanwhile, Park et al. used an electrospinning method in order to fabricate poly-(vinyl alcohol) (PVA) hydrophilic polymer containing Ag/MMT in aqueous medium, as an efficient antibacterial agent [149]. Recently, a hybrid route (laser ablation synthesis in solution and wet chemical approach) was applied to the preparation of Ag/MMT-based antibiofilm coatings (Figure 1d). The advanced infrared attenuated total reflection (IR-ATR) spectroscopy technique allowed real-time in situ monitoring of histamine-producing Lentilactobacillus parabuchneri biofilms inhibition [150].
2.5. Cu/MMT by Hybrid Method
CuO/MMT nanohybrids open the potential to use CuO NPs as antimicrobial agents, while MMT support helps limiting toxicity issues [151]. CuO NPs immobilized into interlayers of MMT by thermal decomposition method show excellent antibacterial activity towards E. coli, as a result of Cu ion release while interacting with an aqueous phase. Pourabolghasem et al. demonstrated that the alkaline ion exchange method was a fast and simple route to synthesize Cu/MMT nanocomposites, which possess good antimicrobial activity against S. aureus and E. coli in broth media. A lower Cu2+ ion release from the nanocomposite was obtained thanks to the diffusion of Cu into the MMT, rather than the attachment of Cu NPs on MMT surface, which leads to longer antibacterial activity [152].
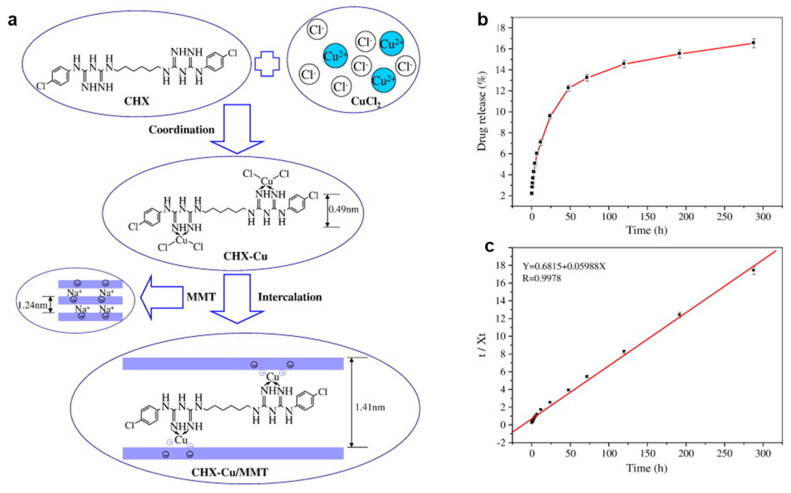
The development of polymeric nanocomposites with antibacterial properties opens up new perspectives in manufacturing medical devices, the textiles industry, wastewater treatment, and food packaging applications. Thus, many researchers have been focusing their attention on a variety of peculiar synthesis routes, including the incorporation of nanomaterials into polymer matrices. In light of this, Wu et al. reported the intercalation of chlorhexidine (CHX)–Cu(II) complexes into the interlayer of MMT (Figure 2a). To maintain the neutral charge of silica layers, a large amount of sodium chloride was introduced. After intercalation at the ratio of 5 for chlorhexidine (CHX)–Cu/MMT, the basal spacing reached 0.96 nm to 1.41 nm. Therefore, the interlayer height of CHX–Cu/MMT was found to be 0.45 nm. This value was nearly equaled to the height of the phenol ring of CHX itself (0.49 nm). Furthermore, it can be seen in Figure 2b that 16% of total CHX–Cu complexes were released over 288 h from CHX–Cu/MMT nanocomposite. Further, release kinetics data (Figure 2c) indicated that the pseudo-second-order mode is consistent with the release process. On the other hand, even a 0.25 mg/mL MIC value of the CHX–Cu/MMT nanocomposites completely inhibited the bacterial growth of both E. coli and S. aureus, owing to a synergistic effect of Cu and CHX [153]. Bruna et al. synthesized Cellulose acetate (CA)/copper montmorillonite (MMT/Cu2+) antimicrobial nanocomposites by the solution casting method. The nanocomposite exhibited improved antibacterial performance against E. coli, because of the diffusion of the antimicrobial agent in the CA [38]. Later, Jaiswal et al. analyzed the benefits of the Cu2+/MMT/CA nanocomposite application in food microbiology [154]. In 2018, Bruna et al. prepared low density polyethylene (LDPE)/Cu2+/MMT nanocomposite films by ion exchange and subsequently melt mixing in an extruder at 200 °C; the study showed a reduction of 94% of E. coli O157:H7 colonies, whilst 4 wt.% of MMT/Cu2+ was loaded to the polymer [155]. Later, the same authors developed poly (lactic acid) (PLA)/Cu2+MMT films, which revealed a reduction of 99% of E. coli ATCC 25922 and Listeria innocua ATCC 33090 at only 5% w/w Cu2+/MMT loading [156]. Previously, Nouri et al. claimed CuO/MMT interlayer sites cause increased electrostatic attraction between chitosan film and bacteria. Consequently, microbial cell wall can be damaged. The introduction of MMT/CuO (3% w/w) into chitosan films reduced water vapor permeability as the number of hydroxyl groups reduced by the increase in hydrogen bonding between chitosan and CuO/MMT [157], while improved mechanical properties and antimicrobial activity towards S. aureus and E. coli were observed. However, the antibacterial mechanism of the formed sample has not yet been identified [158]. Martucci and Ruseckaite incorporated Cu2+/MMT into a bovine gelatin matrix via a dissolution–intercalation method to fabricate functional nanocomposite films, and postulated that blending gelatin with 5% w/w of clay improved the tensile strength of the nanocomposite films up to 280%, while the elongation at break and the water vapor permeability decreased to about 42% and 30%, respectively. The nanocomposites showed a higher antibacterial effect on L. monocytogenes than on E. coli O157:H7, due to the different nature of the E. coli cell wall, which influenced their response to the active films [159]. Das et al. introduced Cu/OMMT into Mesua ferrea L.-seed oil-modified epoxy resin (BPSE), to obtain clay mineral polymer nanocomposites (CPN), which showed significant efficacy towards Gram-negative bacteria (K. pneumoniae). Thermostability, improved mechanical properties, and enhanced antibacterial performance were also shown. OMMT in CPN acts as reinforcing nanofiller, which improves the thermal and mechanical properties of CPN. H-bonding or other polar–polar interactions between hydroxyl groups of OMMT and epoxy/carboxyl/hydroxyl groups of the BPSE lead to the formation of stable and well-dispersed CPN. The presumable clay mineral binding to the bacterial surface helps CuNPs exert their action on bacteria. The authors also observed antihemolytic behavior of the CPN to mammalian red blood cells [160]. Later, Monsif et al. studied the effect of Natural Moroccan Clays on epoxy/nanocomposites and evaluated the antimicrobial performance of the composite [100]. Bartolozzi et al. demonstrated that binary and glass laminated reinforced epoxy resin nanocomposites showed outstanding antimicrobial activity due to the presence of MMT modified with Cu/MMT [161]. Previously, an antibiotic immobilized Cu2O/OMMT nanohybrid was prepared using carbon dot as the reducing agent in a hyperbranched epoxy matrix. The nanocomposite was investigated as a high performance antimicrobial material towards E. coli [162]. Recently, Sun et al. prepared QCS/MMT/5-FCCu as a drug nanoplatform, where 5-fluorocytosine (5-FC) antibiotic was coordinated with copper ions, and QCS coating on MMT was involved. Notably, more than 120 h of 5-FCCu drug release were found. Moreover, both in vitro and in vivo studies suggested the biocompatibility properties of the nanocomposite [163]. Carboxymethylcellulose (CMC) spray-coated Cu2+-intercalated MMT NAMs showed excellent in vitro antibacterial efficacy against Erwinia carotovora in controlling potato soft rot (Solanum tuberosum), exploring the release properties of Cu2+ release [164].
Figure 2.
(a) Schematic for CHX–Cu (II) complexes formation and intercalation into the interlayer of MMT. (b) The release profile of CHX–Cu complexes from the CHX–Cu/MMT nanocomposite in 0.9% NaCl solution at 37 °C; (c) linear regression curves of release data fitting with pseudo-second kinetic mode. Adapted from [153] with permission from Elsevier (Copyright 2013, Elsevier).
2.6. Zn/MMT by Hybrid Method
In 2017, Garshasbi et al. first used an alkaline ion exchange method to prepare Zn/MMT nanocomposites, and explored that, by increasing temperature and processing time, MMT pore sizes expanded, and Zn2+ ion loading increased, while no impact on the antibacterial activity was noted [165]. In contrast, Zou et al. applied a hydrothermal method to prepare Zn/MMT coatings which were effective antibacterial materials with good bone cell compatibility. Additionally, in vitro MTT tests and live–dead stains of osteoblast cells revealed the improved cytocompatibility of Na-MMT and Zn/MMT coatings [166]. Recently, Wang et al. investigated the biocompatibility of Mg alloy with MMT/bovine serum albumin (BSA) coating, and its in vivo and in vitro performance [167]. Hu et al. applied sol–gel intercalation to prepare MMT/ZnO composites with improved performance in respect to bare ZnO [168,169]. Since the introduction of nanomaterials into polymer composites emerged as a promising approach to develop novel antimicrobials, Mallakpour et al. developed poly(amide-imide) (PAI)/ZnO/OMMT nanocomposites by polycondensation and subsequent solution intercalation, and investigated the effect of ZnO and OMMT on the proposed nanocomposite [170]. In 2018, Zahedi et al. developed carboxymethyl cellulose (CMC)/MMT/ZnO nanocomposite using a solution casting method, and claimed that the introduction of MMT and ZnO NPs improved the functional characteristics of CMC film, which enhanced the resistance of the CMC/MMT film to water vapor transmission owing to ZnO NP loading [171]. In order to explore the importance of NP size and their distribution effect on antibacterial performance, Chitosan-grafted MMT/polyaniline (PANI)/ZnO nanocomposite was developed [172]. Later, Ramya et al. did optical studies based on MMT/Chitosan (CS)/phenylenediamine (pPDA) composite, and proposed the composite as optical limiter [173]. Vaezi et al. studied the dispersion of MMT and ZnO NPs with different percentages in cationic starch polymer matrix. Mechanical properties of starch polymer improved upon the incorporation of 3 wt% and 0.7 wt% contents of MMT and ZnO, respectively. Further, increasing the amount of ZnO enhanced the optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Those functional characteristics are highly appreciated for food packaging application [174]. Roy et at incorporated Zn/MMT into HDPE by melt compounding route in a co-rotating twin screw micro-extruder. The nanocomposite was effective against both bacteria and fungi. Further, the effect of the Zn valence state on the antimicrobial activity was reported [175]. Similarly, Na-MMT coated with ZnO nanorods was incorporated into biodegradable antibacterial PVA polymer matrix [176]. Recently, electro-spun polyacrylonitrile (PAN)/(ZnO/MMT) nanofibrous membrane was found to be effective against bacterial strains [177]. Synergistic cobalt-doped ZnO quantum dots (QDs)/cetyltributylphosphonium bromide (CTPB)–MMT NAMs were prepared via a facile chemical precipitation method. The study suggested that Co-ZnO QDs could be absorbed on the surface and into the layers of CTPB–MMT [178]. ZnO has an intrinsic tendency to be agglomerated during practical application, resulting in reduced antibacterial activity. To overcome this issue, ZnO-loaded tetraethyl orthosilicate (TEOS)-MMT composite could improve antibacterial activity of the nano ZnO due to uniform dispersion on MMT [179].
2.7. Ag/MMT Nanocomposite by Irradiation Method
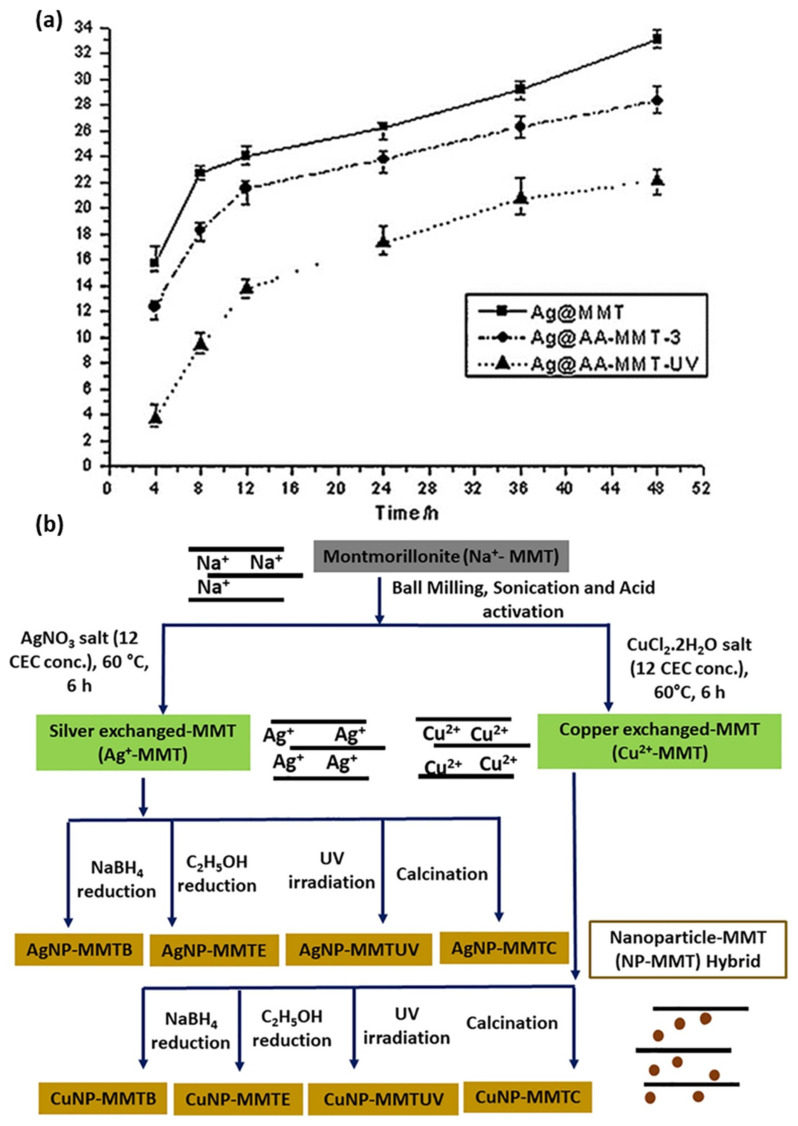
Modification of Ag/MMT by ion exchange method may take a long time to complete the reaction. Therefore, to accelerate the modification reaction of MMT, microwave irradiation has been considered as an alternative method, due to its various benefits such as rapid heating to the reaction temperature, enhanced reaction rates, and formation of novel phases [180]. Taking into account the biological toxicity and the environmental hazard of the residual reducing agent, reduction by different approaches (i.e., UV irradiation [181], and γ-ray irradiation [182], it has been proposed as an alternative way to eliminate the use of toxic reductant and purification steps. Moreover, controlled NP size, with uniform distribution and improved antibacterial activity, is achievable by microwave irradiation method [183,184]. Li et al. proposed that microwave irradiation accelerates the Ag/MMT ion exchange reaction rate, which eliminates the effect of the internal diffusion. As a result, Ag/MMT with a higher silver content and slow release property is obtained. The Ag/MMT shows an inhibition zone 38% larger than that of AgNO3, with the same concentration of AgNPs (wt%). In accordance with disk susceptibility test results, MIC values of the Ag/MMT and AgNO3 against S. aureus ATCC6535 were 1.34 and 1.48 mg/L, respectively; the lower MIC value of the Ag/MMT is due to the fact that the binding of the active agent to the bacteria depends on surface area. The large surface area and the negative surface charge of MMT contribute to the adsorption Ag/MMT on bacteria; consequently, improved antibacterial activity was achieved [185]. The following year, same authors prepared sulfur-containing amino acid (AA) (L-cystine)-modified MMT: Ag@AA-MMT-3, and Ag@AA-MMT-UV, by ion exchange and microwave irradiation method, respectively. Antibacterial activity of Ag@AA-MMT-UV was found to be higher than Ag@AA-MMT-3 and Ag@MMT towards S. aureus and E. coli, due to slow silver release properties (Figure 3a). Presumably, UV irradiation helps in reducing Ag(I) to Ag(0) [186]. The UV irradiation technique allows silver NP size to decrease while increasing irradiation time [37]. The effect of UV photoreduction in the presence of N-vinyl-2-pyrrolidone (PVP) on silver montmorillonite (Ag/MMT) reduces Ag+ into Ag(0), while the slow release property of silver (Ag+ and Ag0) is possible, particularly for those NPs entrapped in the interlayer of MMT, as NPs desorb more slowly due to the inhibition of MMT sheets. The antibacterial activity of Ag/MMT was attributed to a strong adsorption action of the MMT and electrostatic force between electropositive Ag+ and electronegative E. coli [187]. Recently, Roy et al. prepared Ag/MMT and Cu/MMT nanocomposites by chemical reduction, calcination, and UV irradiation methods to conduct a comparative study, as shown in Figure 3b. Toxicological properties of Ag/MMT and Cu/MMT hybrid on human RBC and fibroblast cell lines were here reported for the first time [31]. Sun et al. explored the treatment of mixed wound infections, and toxicity experiments based on a quaternary chitosan-coated MMT composite [163]. In 2010, Shameli et al. developed Ag/MMT nanocomposites, with 20–30 nm AgNPs, using γ-irradiation method, without heat treatment and chemical reduction: MMT protected the colloid against aggregation. With increasing γ-irradiation doses, the physical structure of MMT changed to increase holes in the surface of Ag/MMT; the interlayer structure of the MMT suspension gradually solvated eaq− electron concentration, which was increased in the solvent while Ag+ reduction process, indicating that MMT assisted the γ-reduction process [78]. A drawback could be underlined for the γ-radiation method: a γ source such as 60Co is necessary, which might not always be readily available.
Figure 3.
(a) Comparison of silver release properties of Ag@MMT, Ag@AA-MMT-3 and Ag@AA-MMT-UV. Adapted from [186] with permission from Elsevier (Copyright 2014). (b) Preparation protocol followed for synthesis of Ag/MMT and Cu/MMT nanohybrids. Adapted from [31], with permission from Elsevier (Copyright 2018).
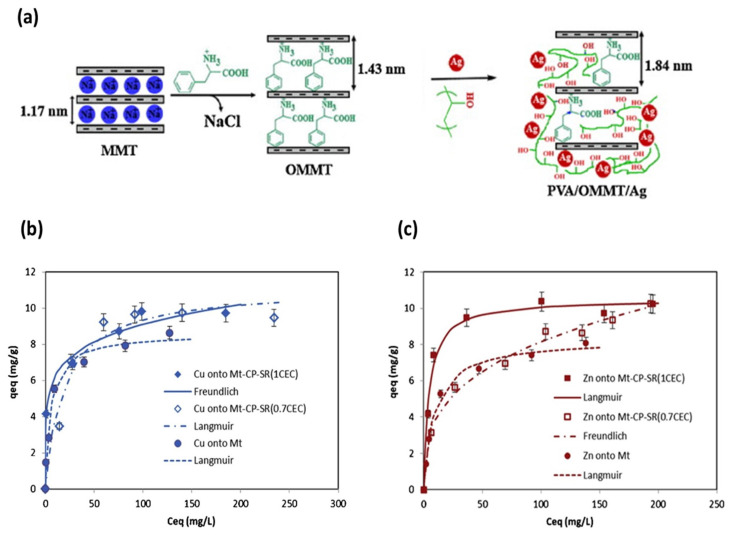
Shameli et al. applied a UV irradiation reduction method to prepare Ag/MMT/CS bio-nanocomposites (BNCs) without employing reducing agents or heat treatments. It was observed that increasing UV irradiation time from 3 to 96 h led to decreased AgNP size. It is apparent from the inhibition zone test that the Ag/MMT/CS bio-nanocomposites exhibited high antibacterial activity against S. aureus and E. coli. It is worth mentioning that MMT/CS assisted in the photoreduction process of silver. In addition, it prevented AgNP aggregation [188]. Following a similar approach, Liu et al. explored the synergistic effect of Ag-QCS/OMMT nanocomposite films. Exfoliated MMT adsorbs and fixes microorganisms, while QCS disrupts the cell membrane and allows the AgNPs to react with compounds in the cell wall. The sample showed activity towards Gram-negative bacteria [189]. Analogously, Gabriel et al. reported on the formation of small-sized and homogeneously distributed AgNPs on modified CS/MMT films, with outstanding antibacterial performance against E. coli and B. subtilis. The synergistic effect of several types of modified chitosan such as Ch30d (viscosity-average molar mass of 30,000 g mol−1; 98% deacetylation degree), Ch30-DEAE (diethylaminoethyl), Ch30-DEAE-Dod (dodecyl), and clay concentration on nanocomposite films has been discussed. On the one hand, DEAE groups in polymer chains increase chelation sites, which facilitate Ag+ reduction after the UV irradiation process. On the other hand, Ch30-DEAE-Dod is an amphiphilic compound which can act as a surfactant, controlling Ag NP size and distribution [190]. In another study, Mallakpour et al. prepared poly(vinyl alcohol)/organoclay/silver (PVA/OMMT/Ag) nanocomposite films with different silver compositions by ion exchange method under ultrasonic irradiation (Figure 4a); AgNPs were uniformly dispersed in the polymer matrix, which improved the thermal stability of the proposed sample [191]. Nonetheless, to the best of our knowledge, no significant effort has been devoted to the development of Cu/MMT nanocomposites by irradiation method.
Figure 4.
(a) Schematic illustrations for preparation of PVA/OMMT/Ag NC films. Adapted from [191], with permission from Elsevier (Copyright 2014). Adsorption of (b) Cu2+ and (c) Zn2+ onto the MMT-Na, MMT-CP-SR (0.7CEC), and MMT-CP-SR (1CEC). Adapted from [214], with permission from Elsevier (Copyright 2020, Elsevier).
2.8. Structural Investigation, Acid Treatment, and Adsorption Study Based on Ag/MMT Nanocomposite
Molecular modelling has been used to explore the structure of Ag/MMT [192]; structural study of Ag/MMT being fundamental to determine the exact localization of silver [193]; acid treatment eliminates the use of reducing agent [9,25]. The investigation of metal and metal oxide NPs adsorption on substrates could play a significant role in determining the antimicrobial performance and mechanism. For example, Zarei and Barghak et al. performed adsorption capacity tests and found that Na/MMT could remove a significant amount of AgNPs (more than 70 mg/g) in 10 min at pH 7. Further, it was proven that the Langmuir model is more accurate than the Freundlich one, describing the adsorption of AgNPs on MMT [194]. In this scenario, Syafiuddin et al. discussed the adsorption of AgNPs onto several types of adsorbents, such as core-shell mesoporous silica, glass beads, iron oxide magnetic particles, poly(ethylenimine) functionalized core-shell magnetic microspheres, and Fe3O4/polydopamine core-shell ones [195]. Interestingly, Solarte et al. studied hexadecyltrimethylammonium (CTA+) and Ag adsorption on MMT, with a synergistic effect towards S. aureus. However, the authors underlined that the synergistic effect is not visible for E. coli [196].
In 1995, Keller-Besrest et al. first investigated the structure of Ag/MMT composite by extended X-ray absorption fine structure (EXAFS) spectroscopy, and explored triangular silver cluster structures. The study apparently showed MMT containing Ag ions, AgNPs, and Ag2O with evidence of aggregation of the silver atoms in triangular clusters [193]. Previously, Busolo et al. described ionic Ag-exchanged MMT without the presence of AgNPs [197]. Later, Clegg et al. emphasized the production of AgNPs on bentonite clay surfaces [198]. With the aim of developing smaller AgNPs, Shabanzadeh et al. designed an artificial neural network (ANN), and outlined that AgNO3 concentration plays a significant role in determining NP size [199]. In another interesting study, Tokarsky et al. exploited molecular modelling (force field) for the investigation of the structure, stability, and adhesion energy of silver NPs on MMT clay. Further, they correlated the modelling data with results of spectroscopic analysis. It was found that AgNP adhesion on the substrate becomes weaker while increasing NP size and thickness. Van der Waals coulombic forces and bond length were taken into account into the model [192]. Following that, Valášková et al. used molecular modelling to investigate interlayer space arrangement of vermiculite during the interaction with α-Fe2O3 [200].
Tian et al. incorporated Ag/MMT into polycarbonate (PC); after that, the prepared samples were treated with 1,4-dimethylbenzene in order to obtain functional Ag/MMT/PC composite with super hydrophobicity. The combination of inorganic materials and polymers leads to excellent bio- and catalytic activity on the degradation reaction of methylene blue [201]. Roy et al. developed Ag/MMT by cation exchange method, and subsequent acid treatment, obtaining spherical AgNPs on MMT. Acid-activated Ag/MMT exhibited higher antibacterial efficacy than the untreated one, but lower than AgNPs, towards E. coli and S. aureus. Acid-treated Ag/MMT also showed a higher uptake of silver ions, which led to improved cation exchange capacity. It was hypothesized that the acid treatment leads to structural alterations such as an enrichment in crosslinked mesopores. Under these conditions, a higher negative charge was observed [25], which correlates with a higher surface area and pore volume [202,203]. In order to avoid NP agglomeration and to achieve the controlled slow release of bioactive ions, Ag/MMT was embedded in glass matrix [204]. It is worth noting that the study further led to the exploration of the potential application of inorganic metal nanomaterials in therapeutic and tissue engineering fields [205,206].
2.9. Adsorption Properties of Cu/MMT
Ma et al. showed the effect of temperature and ionic strength on the adsorption of methylene blue (MB) onto MMT- and Cu2+-exchanged MMT (CEM) [207]. It has been reported that Cu/MMT effectively reduces aflatoxin toxicity [208,209]. Moreover, Cu/MMT nanocomposite increases the adsorption of Aspergillus fungi aflatoxin B1 (AFB1), which is presumably due to the formation of chelation complexes of both Cu2+ and Ca2+ ions with the β-di-carbonyl system of AFB1 [209]. In 2009, Özdemir et al. reported the desorption of Cu2+ from the MMT surface in acidic solution at pH 2.0. The study suggested that the antibacterial activity of the cation mainly arose from adsorbed copper ions. It should not be left unmentioned that the low cation desorption in the aqueous medium caused a less negative impact on the environment [210]. Alternatively, Guo et al. explained the adsorption properties Cu/MMT composite towards E. coli K88. The rate of E. coli K88 adsorption on Cu/MMT composite was found to be higher in an acidic medium than in an alkaline one [211]. Based on this, Gu et al. developed algae removal nanocomposite with 5 to 10 nm sized Cu2O, by the reduction of MMT-absorbed Cu2+ using glucose and ethylene glycol as reductants [212]. Differently, Yan et al. synthesized MMT-reduced graphene oxide–Cu NPs (MMT-rGOx-CuNPs) and postulated that the nanocomposite adsorbed S. aureus more strongly than E. coli. Furthermore, based on bacteria morphological changes, they claimed that the outer membrane (OM) of E. coli defended cells from external attack compared to S. aureus. On the course of action, MMT-rGOx was selected as carrier; however, the role of rGOx in the nanocomposite and its effect on bacteria was not clear [213]. Recently, Özdemir et al. reported that the addition of N-lauroylsarcosinate (SR) to cetylpyridinium (CP) intercalated montmorillonite (MMT-CP) increased the adsorbed amount of Cu2+/Zn2+. The adsorbed amount of Cu2+ was found slightly higher than that of Zn2+, as shown in Figure 4b,c. The reason could be attributed to the ionic properties of Cu2+ and hydration degree, which ascertains the binding forces between the Cu2+/Zn2+ and MMT surface. The Langmuir isotherm model refers to homogeneous adsorption (all sites possess equal affinity for the adsorbate). The Freundlich model, instead, could be applied from a monolayer to an extended multilayer adsorption, with non-uniform distribution of affinities over the heterogeneous surface. Furthermore, the authors evaluated the effect of Cu2+/Zn2+ immobilization on OMMT, in which the structure was formed with cationic and anionic surfactants [214].
2.10. Adsorption Properties of Zn/MMT
Dakovic et al. determined the AFB1 adsorption isotherm by Zn/MMT at pH 3, with a capacity, which was higher than pristine MMT one by a factor of about 50%. This is probably due to the interactions between AFB1 and Zn ions in both the interlayer and at the surface [215]. Recently, Egirani et al. reported ZnO coating on Acros Organics (ACOR) MMT as an effective adsorbent for lead ion (Pb2+) removal. The study suggested that protonation was attenuated by the ZnO coating on ACOR MMT. The presence of the ZnO coating covered the acidic sites on the edges and planar surfaces of ACOR MMT. Three steps of mass transfer including film diffusion, adsorbent surface diffusion, and intraparticle diffusion were observed [216]. Previously, Ma et al. explored the adsorption properties of Cu2+–ZnO/cetylpyridinium (CP)-MMT in vitro against pathogenic E. coli. Further, they postulated that the bacterial adsorption on the (CP+)-MMT surface led to increased permeability, prolonged hydrophobicity and the reversal of surface charge from negative to positive, with consequent higher percentages of E. coli adsorption [217].
2.11. Ag/MMT, Cu/MMT, and ZnO/MMT Nanocomposite by Electrochemical, Plasma resonance, and Radio Frequency (RF) Methods
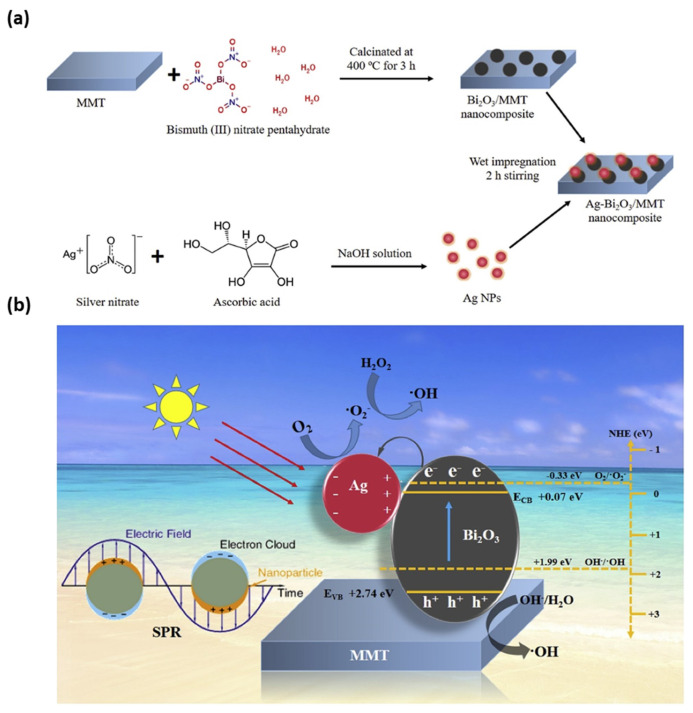
Taking environmental and health issues into account [218], the development of novel synthetic routes for the fabrication of multicomponent coatings with uniform thickness, strong adhesion to the substrate [219] with improved antimicrobial performance, is highly needed. In this regard, electrochemical synthetic routes are considered well advanced, inexpensive, and eco-friendly, and allow the obtaining of NPs with fine morphological and dimensional control and with high purity [72]. Huang et al. fabricated AgNPs by facile electrochemical method in the presence of Na-MMT; antibacterial activity of AgNP/MMT was investigated against S. aureus ATCC 6538P. In particular, AgNPs were prepared by a combination of two schemes, i.e., the electrochemical synthesis of stabilized metal clusters, and simultaneous reduction in AgNPs using methanol as the reducing agent. MMT served as both stabilizer and support during the electrochemical process. At reduction potentials of 5, 10, 15, and 20 V, the formed AgNPs in the aqueous solution had average sizes of 4, 6, 15, and 20 nm, respectively. In the prepared AgNP/MMT in aqueous solutions, AgNPs on the MMT surface had slightly higher average sizes of 5, 8, 17, and 25 nm [218]. In 2019, Iconaru et al. applied a radio frequency (RF) magnetron sputtering method to deposit MMT and Ag/MMT layers on Si substrates, studying their antifungal activity towards Candida albicans ATCC 101231. Both MMT and Ag/MMT layers showed good activity: after 72 h, the Candida albicans ATCC 101231 growth was inhibited significantly due to the presence of silver ions and their controlled release [220]. Tun et al. applied thermal and wet impregnation methods to fabricate functionalized plasmonic Ag-Bi2O3/MMT nanocomposites (Figure 5a), whereby surface plasmon resonance (SPR) of AgNPs appeared, and can be seen in Figure 5b [221].
Figure 5.
(a) Schematic diagram of the synthesis process of Ag–Bi2O3/MMT nanocomposite and (b) schematic representation of electron–hole separation of Ag–Bi2O3/MMT composite under visible light irradiation. Adapted from [221], with permission from Elsevier (Copyright 2020, Elsevier).
3. Comparison of Antimicrobial Performance and Mechanism among Ag/MMT, Cu/MMT, and Zn/MMT Nanocomposite
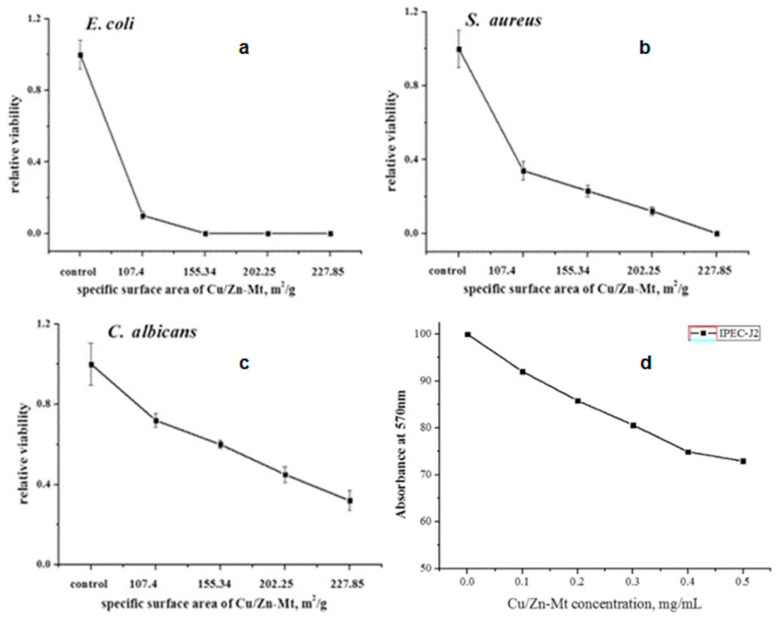
Özdemir et al. showed that Cu2+, Ag+-exchanged, and Ag0-covered MMT exhibited excellent antibacterial performance against P. aeruginosa and S. aureus. On the contrary, Zn2+/MMT was powerful only against S. aureus, which suggested the potential use of Zn/MMT instead of Ag/MMT for applications towards S. aureus [46]. In another interesting study, the antibacterial (E. coli) and antifungal (Pycnoporus cinnabarinus and Pleurotus ostreatus) activities of Ag/MMT, Cu/MMT, and Zn/MMT were evaluated. The study showed the strongest antibacterial performance for Ag/MMT and Ag+ ions. Cu/MMT was as effective as free Cu ions. Zn/MMT showed the weakest performance, similar to that of Zinc ions. Zn is an essential element, and can enhance bacterial growth at low concentrations. The antifungal effect of Zn/MMT and Cu/MMT was found to be higher than the inhibition by silver ions and Ag/MMT [222]. Previously, Kim et al. investigated the antibacterial effects of silver/hydroxyapatite (Ag-HA), Cu-HA, and Zn-HA composites. The antimicrobial ceramics (AC) based on HA were made by a wet chemical process. The authors noted an antimicrobial effect against E. coli in the case of Ag+/AC composite, whereas no antimicrobial effect was observed for Cu/AC and Zn/AC. The reason behind this inconsistency was not vivid [223]. The use of MMT-based adsorbents is considered as a potential technique to remove bacteria from contaminated water. In this regard, Al3+, Ag+, Cu2+, and Zn2+ played a significant role in antibacterial processes when the clay mineral was brought into contact with bacteria. The study suggested that the antibacterial effect of the metal cations is localized on the clay surface [224]. Recently, Roy et al. reported antimicrobial activity for metals containing HDPE-MMT composites, towards E. coli and S. aureus. In this regard, the efficiency was classified as follows: HDPE/Ag/MMT>HDPE/Cu-MMT>HDPE/Zn-MMT [225]. Alarmingly, the complex structure of bacteria continually adapts to the mechanism of action of well-known single metal and metal oxide NPs. Incorporation of dual active phases in the MMT matrix could be a promising way to find an effective alternative to combat colonies of drug resistant bacteria. Due to different modes of antimicrobial action of individual material in synergistic composites, they can attack peculiar bacteria at different stages of their life cycle. In this regard, researchers are focusing their attention on preparing multicomponent nanocomposites for antimicrobial application [226]. For example, Ma et al. impregnated Cu2+ and ZnO on MMT and further modified it with cetylpyridinium ions to investigate the antibacterial mechanism towards pathogenic E. coli and Salmonella typhimurium. The prepared Cu2+–ZnO/cetylpyridinium–montmorillonite (CZCM) nanocomposite attenuated the bacterial wall thanks to the interactions between proteins and enzymes of the cell wall. Moreover, the composite influenced the efflux of intracellular nutrients such as K+ and enzymes. The formed CZCM nanocomposite negatively affected the tricarboxylic acid pathway of the bacterial respiratory metabolism [227]. Following that, the same authors embedded Cu2+ and ZnO into zeolite in order to investigate its antibacterial performance against E. coli and S. aureus [228]. In 2017, Jiao et al. showed that Cu-Zn/MMT exhibited improved antibacterial and antifungal performance than bare Zn/MMT and Cu/MMT. The reason behind that could be due to the synergistic effect between Cu and Zn. Moreover, the antimicrobial activity was observed due to the enhancement of the specific surface area, as shown in Figure 6a–c [229]. In a recent study, carboxymethyl cellulose (CMC)-based films incorporating MMT modified with Ag and Cu ions were found to be less effective than Ag- or Cu-modified CMC-Cloisite 30B (additive for plastics) films against S. aureus and E. coli, and had a lower tensile strength, which is probably due to the better compatibility of C30B clay NPs in the biopolymer matrix than bare MMT. Particularly, Gram-positive bacteria were found to be more sensitive to these NPs than Gram-negative bacteria [230].
Figure 6.
(a–c) The antimicrobial activity of Cu/Zn-MMT-2 with different specific surface area, (d) the cytotoxicity of Cu/Zn-MMT. Adapted from [229], BMC part of Springer Nature, distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
4. Selected Cases of Study about Antimicrobial Applications of MMT-Based Materials
It is well known that Ag, Cu, and ZnO NPs show effective antibacterial activity [19,20,23]. The load of metal NPs on the surface of MMT and its incorporation within polymeric matrices (agar hydrogel, PVA, glassy matrix, calcium alginate, CA, CMC, konjac glucomannan/polycaprolactone, agar–CMC, (QCS)/(OMMT), CS/pPDA, polydimethylsiloxane (PDMS), KGM, PCL, poly (butylene adipate-co-terephthalate) (PBAT)) causes higher water uptake, improved thermal stability, and mechanical properties, along with changes in barrier and permeability properties. Most importantly, those nanocomposites exhibited improved antimicrobial activity towards a broad variety of pathogens, including E. coli, S. aureus, M. luteus, and I. orientails. Therefore, those nanocomposites are considered as potential active agents in food packaging, food bacteriology, agriculture, food technology, food preservation, biomedical field, wastewater treatment, clothing, marine biofouling on ship (Table 1) [38,39,40,97,126,128,144,149,171,173,204,231,232,233,234,235,236]. Pristine Ag/MMT is efficient in food technology since it is capable of reducing microbial viability [127]. Zhang et al. developed Ag/OMMT by a one-step solution–intercalation technique, and postulated that the composite is able to kill 100% of S. aureus, E. coli, and C. albicans, within 2 h, in solution; the approach is convenient to prepare surface-modified nanocomposites for adhesives, plastics, and paints manufacturing industries [136]. Functionalized plasmonic Ag-Bi2O3/MMT nanocomposites are potent in removing antibiotic pollutants and dyes from wastewater [221]. In another study, the slow release of silver chelators from (Ag+(6-BAP)2) MMT composite is attributed due to the electrostatic adsorption state in the deepest part of the nanolayers, making MMT clay a suitable host material for the delivery of bactericidal compounds [76]. Ag/MMT suspension can also be useful to modify bacterial cellulose (BC) membranes: MMT or Ag/MMT can diffuse through the BC matrix and adsorb on the surface of the polymeric networks. Ag/MMT/BC showed good properties towards wound healing, inhibiting biofilms of S. aureus and P. aeruginosa [237]. In 2016, Cu/MMT nanocomposite exhibited antibacterial performance towards S. aureus and E. coli, with excellent stability in water, which allowed for the consideration of the sample as a potential agent for wastewater treatment [152]. Later, Cu-Zn/MMT was found to be efficient in animal husbandry application because of its outstanding antimicrobial activity and relatively low toxicity [229]. The preparation of Cu-Zn/MMT hybrid nanocomposites by developing or adapting green, facile, scalable, and cost effective methods could open new perspectives for their real life application in the fight against antimicrobial resistance or biofilm. In a recent review, antimicrobial activity of nanomaterials towards foodborne pathogens in polymeric food packaging has been highlighted [238].
Table 1.
Summary of literature of nanocomposite prepared by several methods for potential application in different sectors.
| Type of Nanocomposite | Methods of Preparation | Application | Antimicrobial Result/Improved Properties | References |
|---|---|---|---|---|
| Ag/OMMT/QCS-QOMA | One-step approach. | Medical device and household. | Improved mechanical properties and confirmed activity towards E. coli, S. aureus, and fungi. | [39] |
| (Ag+(6-BAP)2)/MMT | Ion exchange method and carbothermal reduction. | Delivery of bacteriocidal compounds. | Slow release of silver chelate is confirmed. | [76] |
| (Ag-Nacre-like KGM)/MMT | Vaccum filtration and in situ reduction method. | Biomedical field. | Good mechanical properties and improved light transmission. | [97] |
| Ag/MMT/PCL | Reduction method. | Wastewater treatment. | Confirmed antibacterial activity towards S. aureus and E. coli | [103] |
| Ag/MMT/glassy matrix | Embedded. | Agriculture and food technology. | Confirmed activity towards E. coli, M. luteus, and I. orientails | [204] |
| Ag/MMT/PVA | Electrospinning method. | Food bacteriology. | Effective against S. aureus (ATCC6538) and E. coli (ATCC25922), improvement in thermal stability. | [149] |
| Ag/MMT/agar hydrogel | Ion exchange and thermal treatment method. | Food packaging. | Activite against selected spoilage microorganisms. | [126] |
| Ag/MMT | Ion exchange and thermal treatment method. | Fresh fruit salad. | Shelf life increase in fruit salad and reduced microbial viable count. | [127] |
| Ag/OMMT | One-step solution-intercalation technique. | Adhesives, plastics, and paints manufacturing industries. | Confirmed that in 2 h, 0.0125 mg/mL Ag/OMMT could kill 100% of S. aureus, E. coli, and C. albicans in solution. | [136] |
| Ag/MMT/Agar–CMC | Solution casting method. | Food preservation. | Improved mechanical and antimicrobial properties. | [144] |
| Ag/MMT | Aqueous extract of Acetabularia acetabulum. | Marine biofouling on ship. | Active against S. aureus and E coli | [236] |
| Ag-Bi2O3/MMT | Thermal and wet impregnation methods. | Refine antibiotic pollutants and dye from wastewater. | Showed outstanding visible light induced photocatalytic activities for tetracycline (TC) antibiotic and RhB degradation. | [221] |
| Ag/MMT/BC | Ion exchange, solution casting. | Scaffolds for wound dressing | Active against biofilms of S. aureus and P. aeruginosa. | [237] |
| Calcium–alginate coating loaded Ag/MMT | Ion exchange method. | Fresh-cut carrots | Improvement of sensory attributes, spoilage microorganisms reduced, Extension in shelf life of carrots. | [128] |
| Cu2+/MMT/CA | Solution casting. | Food packaging | The change in the oxygen barrier properties, confirmed antibacterial performance against E. coli. | [38] |
| Cu2+/MMTCarbon Paste Electrode (CPE) | Ion exchange and electrochemical method. | Detect propineb pesticide in river and sea water. | Confirmed sensitivity towards pesticide. | [239] |
| Cu/MMT | Alkaline ion exchange. | Wastewater treatment. | Confirmed antimicrobial activity against S. aureus and E. coli, with excellent stability in water. | [152] |
| Cu-Zn/MMT | Ion exchange reaction. | Animal husbandry. | Higher antimicrobial activity towards E. coli, S. aureus bacteria; and Candida albicans fungi, relatively low toxic. | [229] |
| Cu-Alpill/MMT | High power ultrasonic treatment and calcining. | Wastewater treatment. | [240] | |
| Cu2+/MMT/CMC | Ion exchange and spray coating. | Active against Erwinia carotovora in Potato (Solanum tuberosum L.) | [164] | |
| ZnO/MMT/CMC | Solution casting. | Food packaging. | Improved functional characteristics, and enhanced resistance to water vapour permeability, glass transition temperature increased, higher antibacterial activity against S. aureus than E. coli. | [171] |
| PCL/halloysite (HNT)/MMT | Heat treatment, precipitation | Beef meat packaging. | [241] |
5. Environmental and Toxicity Issue
Most environmental and toxicity studies focus on Ag, Cu, and ZnO colloids, and a very few studies report on Ag/MMT, Cu/MMT, and Zn/MMT nanocomposites. The nanotoxicology of Ag-, Cu-, and ZnO-containing nanomaterials has been the subject of several review papers [19,242,243,244,245,246,247,248,249]. In this present review, we have focused on supported nanophases, on substrates such as MMT, implementing, embedding, and impregnating Ag ions, AgNPs, Cu ions, CuNPs, and ZnONPs. Nanoparticles and microparticles supporting smaller nanophases exhibit lower toxicity, as to the bigger size of the overall size of the nano-/micro-structure. The cytotoxic effect of metal NPs on different mammalian cell lines has been widely investigated [250,251,252]. Nonetheless, the impregnation of NPs on MMT which is a non-toxic material and has a synergistic effect on its cytotoxic behavior, leads to the synthesis of novel cyto-compatible antimicrobial nanocomposites [31,204,220]. It is well known that NP toxicity depends on the particle properties such as size, shape, charge, surface energy, and chemical composition, and on the type of involved organisms. Antimicrobial NPs might cause adverse toxic effects in human organs and could damage DNA [253]. It is also proven that NPs smaller than 30 nm can easily penetrate skin, reaching the deepest layers of the stratum corneum and the outermost surface of the epidermis, and nasal/pharyngeal mucosa [71,254]. Due to uncontrolled release behavior, antimicrobial NPs could be toxic. When working with antimicrobials providing a controlled release, the metal ion concentration should be adjusted below 0.1 mg/L, which can be tolerated without health risks [51,76,255]. However, this concentration can be changed depending on the ways of interaction (skin penetration, respiratory tract, ingestion), type of metal, and prolonged or short-term exposure.
Toxicological properties of Ag/MMT and Cu/MMT hybrids on human RBC and fibroblast cell lines have been reported. Whereby, at a 10 μg/mL concentration, Ag/MMT exhibited higher hemolysis (55.2 ± 2.2%) than Ag/MMTUV (38.3 ± 1.5%), because of a smaller NP diameter and higher loading. In contrast, at 400 μg/mL concentration, Cu/MMTB and Cu/MMT-UV exhibited 30.1 ± 1.2% and 15.0 ± 0.6% hemolysis, respectively. In relation to the same conditions, the cytocompatibility of Ag/MMTUV and Cu-MMUV was found to be 96.6 ± 3.1% and 100 ± 12%, respectively. Hence, it appears that Cu/MMT hybrids are safer than Ag-based nanocomposites within human cell lines [31]. Ag+/MMT/sutures, prepared by a hybrid method, exhibited a hemolytic activity of 4.8% on blood cells and did not show significant cytotoxicity to human endothelial cell meridians, even after 7 days of continuous incubation [125]. Cytotoxicity studies on MMT and Ag/MMT showed that they did not reveal any considerable toxicity towards HeLa cells, in which viability decreased from 96% at 24 h, to 92% after 72 h of incubation [220]. In addition, Lal et al. investigated that MMT–insulin–PLGA composites were non-toxic towards human embryonic kidney HEK-293 [256]. Polycaprolactone)/MMT (PCL/MMT) composites do not damage plasma membrane. It was noted that the presence of MMT did not significantly affect the viability of fibroblasts [257]. Hemolytic activity of Ag/MMT/Lys towards human erythrocytes and the effect of aluminum oxide content on MMT was investigated. Increasing Al2O3 content in MMT caused the increase in negative surface zeta potential, resulting in the repulsion of Ag/Lyz NPs from the MMT surface. Consequently, silver content decreased at the MMT surface. This phenomena led to an increase in hemolytic activity (e.g., cytotoxicity) of the sample, which eventually reduced its antibacterial activity [146]. Previously, Ag/MMT nanomaterials were embedded in glassy matrix, which avoided NP agglomeration and mitigated toxicity in human cells, by slowing the release of nanomaterials [204]. HDPE-Zn/MMT nanocomposites exhibited 100% cytocompatibility in MTT assay, which suggests their inertness to dermal fibroblast cells, whereas HDPE/ZnO nanocomposites exhibited mild hemotoxic and cytotoxic behavior, since modified MMT/polymer matrix, intercalation, and exfoliation, affect the nanocomposite morphologies [175]. The in vitro and in vivo analyses of polymeric nanocomposite (Polyethylene/Ag/MMT) showed RBC hemolysis protection and cytocompatibility, and no viable cell count reduction to human erythrocytes and to dermal fibroblast cell lines [134]. It has been found that increasing the concentration of Cu-Zn-MMT causes moderate cytotoxicity to intestinal porcine epithelial cell line (IPEC-J2), as can be seen in Figure 6d [229]. In another study, the same authors showed that supplementing Cu-Zn-MMT in the diet of animals increased (p < 0.05) the Cu and Zn concentrations in serum, jejunum, and ileum mucosa. In the meantime, this enhanced the height of villus, while decreasing the depth of crypt [258]. Zhang et al. found the same trend using the zinc-bearing palygorskite diet supplement [259]. The study of toxicity and immunological activity of AgNPs in Swiss mice by using zerovalent Ag load on MMT showed no significant changes in mice behavior during 96 h of observation, which corresponded to no neurotoxic effects [260]. Cytotoxicity of the Nacre-like konjac glucomannan–Montmorillonite (KGM–MMM) nanocomposites was evaluated using RAW264.7 cells (monocyte/macrophage-like cells) [97]. Polymeric anthocyanin-containing OMMT has been found to be an efficient pH-sensitive antioxidant for nano-packaging systems. However, the toxicity of polymeric anthocyanin/OMMT nanocomposites actually limit their application in the food packaging system [261]. In contrast, due to beneficial interactions of AgNPs with living structures and their non-toxic effects on healthy human cells, they are considered a potential agent for various biomedical products [262]. The incorporation of non-toxic NPs into MMT open a new perspective.
6. Perspectives
Montmorillonite-based nanoantimicrobials open new perspectives in the manufacturing of medical devices, the textile industry, wastewater treatment, and food packaging. Either the in situ or ex situ preparation of metal and metal ions into and onto the MMT matrix, and the inclusion of bioactive polymers have been explored to control the size and shape of NPs, control the release of bioactive specimens, prevent NP agglomeration, develop synergistic and improved nanoantimicrobials, and address toxicity problems. Several approaches have been proposed: ion exchange, chemical reduction (involving surfactants, caping agents, chemical salts, solvents, plant extracts, biological species, γ-rays, microwave and UV irradiation), hydrothermal synthesis, solution casting, RAFT polymerization, electrospinning, dipping and rolling, thermal treatment, the dissolution–intercalation method, calcination and grinding, polycondensation, low-temperature solvothermal synthesis, melt compounding, electrochemical synthesis, plasma resonance, and radiofrequency.
Most of the published articles outline that nanocomposite antimicrobial performances depend on the slow release of active agents/ions, ion binding capabilities to bacterial functional groups, size, shape, the uniform distribution of NPs into MMT, increased electrostatic interaction between nanocomposite and bacteria, and a synergistic effect by using multicomponent materials. However, the role and mechanism of action, for each single component within the composites, are worth further investigation. Notably, the comparison of real performances among reported nanocomposites against bacteria is not accessible, due to the use of different growth conditions, bacterial concentrations, and the MMT/NP ratio. A small environmental change could cause unusual effects on bacteria, leading to ambiguous antimicrobial test results. In most of the papers, detailed spectroscopic study is missing: the latter is particularly important to understand the long time stability of nanocomposites.
In general, it is apparent that MMT-based NAMs are highly active towards Gram-positive and Gram-negative bacteria and can significantly inhibit and eradicate pathogens and harmful biofilms. Antimicrobial colloidal NPs have attracted enormous attention, and MMT-based materials sound mature for a wider use in the mentioned application fields. They could be dispersed in almost all kinds of organic and inorganic solvents, paving the way to the preparation of a novel class of hybrid materials to be used as additives in food and cosmetic industries, hard coatings, and biomedical devices.
7. Conclusions
This review provides a selection of the most convenient and easily scalable nanoantimicrobial fabrication routes, with a peculiar attention on the effect of synthetic parameters on the efficacy of these MMT-based composites against different bacterial strains, as a function of the final applications. After this literature overview, we can conclude that MMT is generally considered to be cost effective and easily available, and possesses a large surface area and highly negative surface charge, which effectively promotes electrostatic interaction between nanocomposites and bacteria. NP-modified MMT can be easily prepared on polymer matrix by either common polymer fabrication techniques or hybrid methods. NP-containing MMT can be effectively applied as coating on plastic, metal, food packaging, medical device, and textiles. Prolonged use of NPs/MMT/polymer nanocomposites with reduced toxicity is possible. Altering the surface charge of nanocomposites can promote electrostatic interactions with bacteria, improving the material antimicrobial activity. MMT could be considered a unique support which can accommodate multiple components and functionalities; hence, it could support the design of new materials and technological devices for industrial applications.
Author Contributions
Conceptualization, S.I.H., N.C. and M.C.S.; writing—original draft preparation, S.I.H.; writing—review and editing, S.I.H., E.A.K., M.I., R.A.P., M.C.S. and N.D.; supervision, N.C.; funding acquisition, N.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work is part of the “Break Biofilms” project, which has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska Curie Grant Agreement No. 813439.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jack A., Campbell C. The Threat of Antibiotic Resistance—In Charts. [(accessed on 23 January 2021)]. Available online: https://www.ft.com/content/d806dcf5-23f8-4714-ad04-ca11a66061e2.
- 2.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 3.Cioffi N., Torsi L., Ditaranto N., Tantillo G., Ghibelli L., Sabbatini L., Bleve-Zacheo T., D’Alessio M., Zambonin P.G., Traversa E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005;17:5255–5262. doi: 10.1021/cm0505244. [DOI] [Google Scholar]
- 4.Cioffi N., Torsi L., Ditaranto N., Sabbatini L., Zambonin P.G., Tantillo G., Ghibelli L., D’Alessio M., Bleve-Zacheo T., Traversa E. Antifungal Activity of Polymer-Based Copper Nanocomposite Coatings. Appl. Phys. Lett. 2004;85:2417–2419. doi: 10.1063/1.1794381. [DOI] [Google Scholar]
- 5.Ruparelia J.P., Chatterjee A.K., Duttagupta S.P., Mukherji S. Strain Specificity in Antimicrobial Activity of Silver and Copper Nanoparticles. Acta Biomater. 2008;4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Motshekga S.C., Ray S.S., Onyango M.S., Momba M.N.B. Microwave-Assisted Synthesis, Characterization and Antibacterial Activity of Ag/ZnO Nanoparticles Supported Bentonite Clay. J. Hazard. Mater. 2013;262:439–446. doi: 10.1016/j.jhazmat.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 7.Malarkodi C., Rajeshkumar S., Paulkumar K., Vanaja M., Gnanajobitha G., Annadurai G. Biosynthesis and Antimicrobial Activity of Semiconductor Nanoparticles against Oral Pathogens. Bioinorg. Chem. Appl. 2014;2014:e347167. doi: 10.1155/2014/347167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sportelli M.C., Nitti M.A., Valentini M., Picca R.A., Bonerba E., Sabbatini L., Tantillo G., Cioffi N., Valentini A. Ion Beam Sputtering Deposition and Characterization of ZnO-Fluoropolymer Nano-Antimicrobials. Sci. Adv. Mater. 2014;6:1019–1025. doi: 10.1166/sam.2014.1852. [DOI] [Google Scholar]
- 9.Roy A., Joshi M. Enhancing Antibacterial Properties of Polypropylene/Cu-Loaded Montmorillonite Nanocomposite Filaments through Sheath-Core Morphology: Enhancing Antibacterial Properties of PP/Cu-MMT Nanocomposite. Polym. Int. 2018;67:917–924. doi: 10.1002/pi.5580. [DOI] [Google Scholar]
- 10.Sportelli M.C., Picca R.A., Izzi M., Palazzo G., Gristina R., Innocenti M., Torsi L., Cioffi N. ZnO Nanostructures with Antibacterial Properties Prepared by a Green Electrochemical-Thermal Approach. Nanomaterials. 2020;10:473. doi: 10.3390/nano10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antony J.J., Sivalingam P., Siva D., Kamalakkannan S., Anbarasu K., Sukirtha R., Krishnan M., Achiraman S. Comparative Evaluation of Antibacterial Activity of Silver Nanoparticles Synthesized Using Rhizophora Apiculata and Glucose. Colloids Surf. B Biointerfaces. 2011;88:134–140. doi: 10.1016/j.colsurfb.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Castañón G.A., Niño-Martínez N., Martínez-Gutierrez F., Martínez-Mendoza J.R., Ruiz F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanoparticle Res. 2008;10:1343–1348. doi: 10.1007/s11051-008-9428-6. [DOI] [Google Scholar]
- 13.Shtykova L., Fant C., Handa P., Larsson A., Berntsson K., Blanck H., Simonsson R., Nydén M., Ingelsten Härelind H. Adsorption of Antifouling Booster Biocides on Metal Oxide Nanoparticles: Effect of Different Metal Oxides and Solvents. Prog. Org. Coat. 2009;64:20–26. doi: 10.1016/j.porgcoat.2008.07.005. [DOI] [Google Scholar]
- 14.Makhluf S., Dror R., Nitzan Y., Abramovich Y., Jelinek R., Gedanken A. Microwave-Assisted Synthesis of Nanocrystalline MgO and Its Use as a Bacteriocide. Adv. Funct. Mater. 2005;15:1708–1715. doi: 10.1002/adfm.200500029. [DOI] [Google Scholar]
- 15.Dutta R.K., Nenavathu B.P., Gangishetty M.K., Reddy A.V.R. Studies on Antibacterial Activity of ZnO Nanoparticles by ROS Induced Lipid Peroxidation. Colloids Surf. B Biointerfaces. 2012;94:143–150. doi: 10.1016/j.colsurfb.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.S., Kuk E., Yu K.N., Kim J.-H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.-Y., et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee A.K., Chakraborty R., Basu T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology. 2014;25:135101. doi: 10.1088/0957-4484/25/13/135101. [DOI] [PubMed] [Google Scholar]
- 18.Hamm S.C., Shankaran R., Korampally V., Bok S., Praharaj S., Baker G.A., Robertson J.D., Lee B.D., Sengupta S., Gangopadhyay K., et al. Sputter-Deposition of Silver Nanoparticles into Ionic Liquid as a Sacrificial Reservoir in Antimicrobial Organosilicate Nanocomposite Coatings. ACS Appl. Mater. Interfaces. 2012;4:178–184. doi: 10.1021/am2012273. [DOI] [PubMed] [Google Scholar]
- 19.Sportelli M.C., Picca R.A., Cioffi N. Recent Advances in the Synthesis and Characterization of Nano-Antimicrobials. TrAC Trends Anal. Chem. 2016;84:131–138. doi: 10.1016/j.trac.2016.05.002. [DOI] [Google Scholar]
- 20.Rojas-Andrade M., Cho A.T., Hu P., Lee S.J., Deming C.P., Sweeney S.W., Saltikov C., Chen S. Enhanced Antimicrobial Activity with Faceted Silver Nanostructures. J. Mater. Sci. 2015;50:2849–2858. doi: 10.1007/s10853-015-8847-x. [DOI] [Google Scholar]
- 21.Sheehy K., Casey A., Murphy A., Chambers G. Antimicrobial Properties of Nano-Silver: A Cautionary Approach to Ionic Interference. J. Colloid Interface Sci. 2015;443:56–64. doi: 10.1016/j.jcis.2014.11.074. [DOI] [PubMed] [Google Scholar]
- 22.Lejon D.P.H., Pascault N., Ranjard L. Differential Copper Impact on Density, Diversity and Resistance of Adapted Culturable Bacterial Populations According to Soil Organic Status. Eur. J. Soil Biol. 2010;46:168–174. doi: 10.1016/j.ejsobi.2009.12.002. [DOI] [Google Scholar]
- 23.Applerot G., Lipovsky A., Dror R., Perkas N., Nitzan Y., Lubart R., Gedanken A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009;19:842–852. doi: 10.1002/adfm.200801081. [DOI] [Google Scholar]
- 24.Carbone M., Donia D.T., Sabbatella G., Antiochia R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ.-Sci. 2016;28:273–279. doi: 10.1016/j.jksus.2016.05.004. [DOI] [Google Scholar]
- 25.Roy A., Butola B.S., Joshi M. Synthesis, Characterization and Antibacterial Properties of Novel Nano-Silver Loaded Acid Activated Montmorillonite. Appl. Clay Sci. 2017;146:278–285. doi: 10.1016/j.clay.2017.05.043. [DOI] [Google Scholar]
- 26.Tamayo L., Azócar M., Kogan M., Riveros A., Páez M. Copper-Polymer Nanocomposites: An Excellent and Cost-Effective Biocide for Use on Antibacterial Surfaces. Mater. Sci. Eng. C. 2016;69:1391–1409. doi: 10.1016/j.msec.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad M.B., Shameli K., Darroudi M., Yunus W.M.Z.W., Ibrahim N.A. Synthesis and Characterization of Silver/Clay Nanocomposites by Chemical Reduction Method. Am. J. Appl. Sci. 2009;6:1909–1914. doi: 10.3844/ajassp.2009.2030.2035. [DOI] [Google Scholar]
- 28.Wei J.-C., Yen Y.-T., Wang Y.-T., Hsu S., Lin J.-J. Enhancing Silver Nanoparticle and Antimicrobial Efficacy by the Exfoliated Clay Nanoplatelets. RSC Adv. 2013;3:7392–7397. doi: 10.1039/c3ra23476b. [DOI] [Google Scholar]
- 29.Rao F., Song S., Lopez-Valdivieso A. Synthesis and Characterization of Ag-PILC Through the Formation of Ag @Montmorillonite Nanocomposite. Nano. 2015;10:1550031. doi: 10.1142/S1793292015500319. [DOI] [Google Scholar]
- 30.Joshi M., Brahma S., Roy A., Wazed Ali S. Nano-Calcium Carbonate Reinforced Polypropylene and Propylene-Ethylene Copolymer Nanocomposites: Tensile vs. Impact Behavior. Fibers Polym. 2017;18:2161–2169. doi: 10.1007/s12221-017-7276-7. [DOI] [Google Scholar]
- 31.Roy A., Joshi M., Butola B.S., Malhotra S. Antimicrobial and Toxicological Behavior of Montmorillonite Immobilized Metal Nanoparticles. Mater. Sci. Eng. C. 2018;93:704–715. doi: 10.1016/j.msec.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Hong S.-I., Wang L.-F., Rhim J.-W. Preparation and Characterization of Nanoclays-Incorporated Polyethylene/Thermoplastic Starch Composite Films with Antimicrobial Activity. Food Packag. Shelf Life. 2022;31:100784. doi: 10.1016/j.fpsl.2021.100784. [DOI] [Google Scholar]
- 34.Wang W., Wen T., Bai H., Zhao Y., Ni J., Yang L., Xia L., Song S. Adsorption toward Cu(II) and Inhibitory Effect on Bacterial Growth Occurring on Molybdenum Disulfide-Montmorillonite Hydrogel Surface. Chemosphere. 2020;248:126025. doi: 10.1016/j.chemosphere.2020.126025. [DOI] [PubMed] [Google Scholar]
- 35.İlaslan K., Tornuk F. Characterization of Silver Ions-Doped Organomodified Nanoclays. Arab. J. Sci. Eng. 2023;48:327–340. doi: 10.1007/s13369-022-07046-3. [DOI] [Google Scholar]
- 36.Giannakas A.E., Salmas C.E., Moschovas D., Baikousi M., Kollia E., Tsigkou V., Karakassides A., Leontiou A., Kehayias G., Avgeropoulos A., et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials. 2022;12:1843. doi: 10.3390/nano12111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darroudi M., Ahmad M.B., Shameli K., Abdullah A.H., Ibrahim N.A. Synthesis and Characterization of UV-Irradiated Silver/Montmorillonite Nanocomposites. Solid State Sci. 2009;11:1621–1624. doi: 10.1016/j.solidstatesciences.2009.06.016. [DOI] [Google Scholar]
- 38.Bruna J.E., Galotto M.J., Guarda A., Rodríguez F. A Novel Polymer Based on MtCu2+/Cellulose Acetate with Antimicrobial Activity. Carbohydr. Polym. 2014;102:317–323. doi: 10.1016/j.carbpol.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 39.Chen K., Ye W., Cai S., Huang L., Zhong T., Chen L., Wang X. Green Antimicrobial Coating Based on Quaternised Chitosan/Organic Montmorillonite/Ag NPs Nanocomposites. J. Exp. Nanosci. 2016;11:1360–1371. doi: 10.1080/17458080.2016.1227095. [DOI] [Google Scholar]
- 40.Seray M., Hadj-Hamou A.S., uzunlu S., Benhacine F. Development of Active Packaging Films Based on Poly (Butylene Adipate-Co-Terephthalate) and Silver–Montmorillonite for Shelf Life Extension of Sea Bream. Polym. Bull. 2021;79:3573–3594. doi: 10.1007/s00289-021-03671-4. [DOI] [Google Scholar]
- 41.Bragg P.D., Rainnie D.J. The Effect of Silver Ions on the Respiratory Chain of Escherichia Coli. Can. J. Microbiol. 2011 doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 42.Noori F., Neree A.T., Megoura M., Mateescu M.A., Azzouz A. Insights into the Metal Retention Role in the Antibacterial Behavior of Montmorillonite and Cellulose Tissue-Supported Copper and Silver Nanoparticles. RSC Adv. 2021;11:24156–24171. doi: 10.1039/D1RA02854E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffenbauer M., Stotzky G. Adsorption of Coliphages T1 and T7 to Clay Minerals. Appl. Environ. Microbiol. 1982;43:590–596. doi: 10.1128/aem.43.3.590-596.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dermatas D., Dadachov M.S. Rietveld Quantification of Montmorillonites in Lead-Contaminated Soils. Appl. Clay Sci. 2003;23:245–255. doi: 10.1016/S0169-1317(03)00109-1. [DOI] [Google Scholar]
- 45.Carretero M.I. Clay Minerals and Their Beneficial Effects upon Human Health. A Review. Appl. Clay Sci. 2002;21:155–163. doi: 10.1016/S0169-1317(01)00085-0. [DOI] [Google Scholar]
- 46.Özdemir G., Limoncu M.H., Yapar S. The Antibacterial Effect of Heavy Metal and Cetylpridinium-Exchanged Montmorillonites. Appl. Clay Sci. 2010;48:319–323. doi: 10.1016/j.clay.2010.01.001. [DOI] [Google Scholar]
- 47.Bergaya F., Theng B.K.G., Lagaly G., editors. Handbook of Clay Science. Elsevier; Newnes, NSW, Australia: 2013. [Google Scholar]
- 48.Giovannini G., Garoli D., Rupper P., Neels A., Rossi R.M., Boesel L.F. Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection. Sensors. 2021;21:2655. doi: 10.3390/s21082655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi H., Xia L., Song S. Three-Dimensional Montmorillonite/Ag Nanowire Aerogel Supported Stearic Acid as Composite Phase Change Materials for Superior Solar-Thermal Energy Harvesting and Storage. Compos. Sci. Technol. 2022;217:109121. doi: 10.1016/j.compscitech.2021.109121. [DOI] [Google Scholar]
- 50.Valášková M., Simha Martynková G., Lešková J., Čapková P., Klemm V., Rafaja D. Silver Nanoparticles/Montmorillonite Composites Prepared Using Nitrating Reagent at Water and Glycerol. J. Nanosci. Nanotechnol. 2008;8:3050–3058. doi: 10.1166/jnn.2008.088. [DOI] [PubMed] [Google Scholar]
- 51.Malachová K., Praus P., Pavlíčková Z., Turicová M. Activity of Antibacterial Compounds Immobilised on Montmorillonite. Appl. Clay Sci. 2009;43:364–368. doi: 10.1016/j.clay.2008.11.003. [DOI] [Google Scholar]
- 52.Sohrabnezhad S., Mehdipour Moghaddam M.J., Salavatiyan T. Synthesis and Characterization of CuO–Montmorillonite Nanocomposite by Thermal Decomposition Method and Antibacterial Activity of Nanocomposite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;125:73–78. doi: 10.1016/j.saa.2014.01.080. [DOI] [PubMed] [Google Scholar]
- 53.Shi Q., Tan S., Yang Q., Jiao Z., Ouyang Y., Chen Y. Preparation and Characterization of Antibacterial Zn2+-Exchanged Montmorillonites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010;25:725–729. doi: 10.1007/s11595-010-0080-5. [DOI] [Google Scholar]
- 54.Kuorwel K.K., Cran M.J., Orbell J.D., Buddhadasa S., Bigger S.W. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015;14:411–430. doi: 10.1111/1541-4337.12139. [DOI] [Google Scholar]
- 55.Marin S., Mihail Vlasceanu G., Elena Tiplea R., Raluca Bucur I., Lemnaru M., Minodora Marin M., Mihai Grumezescu A. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr. Top. Med. Chem. 2015;15:1596–1604. doi: 10.2174/1568026615666150414142209. [DOI] [PubMed] [Google Scholar]
- 56.Zhan J., Chen H., Zhou H., Hao L., Xu H., Zhou X. Essential Oil-Loaded Chitosan/Zinc (II) Montmorillonite Synergistic Sustained-Release System as Antibacterial Material. J. Dispers. Sci. Technol. 2021;39:792–801. doi: 10.1080/01932691.2021.1947848. [DOI] [Google Scholar]
- 57.Yu L., He T., Yao J., Xu W., Peng S., Feng P., Shuai C. Cu Ions and Cetyltrimethylammonium Bromide Loaded into Montmorillonite: A Synergistic Antibacterial System for Bone Scaffolds. Mater. Chem. Front. 2022;6:103–116. doi: 10.1039/D1QM01278A. [DOI] [Google Scholar]
- 58.Abdel-Aziz M.S., Abou-El-Sherbini K.S., Hamzawy E.M.A., Amr M.H.A., El-Dafrawy S. Green Synthesis of Silver Nano-Particles by Macrococcus Bovicus and Its Immobilization onto Montmorillonite Clay for Antimicrobial Functionality. Appl. Biochem. Biotechnol. 2015;176:2225–2241. doi: 10.1007/s12010-015-1710-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J., Zhu L., Zhu R., Chen B. Microstructure of Organo-Bentonites in Water and the Effect of Steric Hindrance on the Uptake of Organic Compounds. Clays Clay Miner. 2008;56:144–154. doi: 10.1346/CCMN.2008.0560202. [DOI] [Google Scholar]
- 60.Reinert L., Batouche K., Lévêque J.-M., Muller F., Bény J.-M., Kebabi B., Duclaux L. Adsorption of Imidazolium and Pyridinium Ionic Liquids onto Montmorillonite: Characterisation and Thermodynamic Calculations. Chem. Eng. J. 2012;209:13–19. doi: 10.1016/j.cej.2012.07.128. [DOI] [Google Scholar]
- 61.Bertuoli P.T., Piazza D., Scienza L.C., Zattera A.J. Preparation and Characterization of Montmorillonite Modified with 3-Aminopropyltriethoxysilane. Appl. Clay Sci. 2014;87:46–51. doi: 10.1016/j.clay.2013.11.020. [DOI] [Google Scholar]
- 62.Veiskarami M., Sarvi M.N., Mokhtari A.R. Influence of the Purity of Montmorillonite on Its Surface Modification with an Alkyl-Ammonium Salt. Appl. Clay Sci. 2016;120:111–120. doi: 10.1016/j.clay.2015.10.026. [DOI] [Google Scholar]
- 63.Das T.K., Ganguly S., Ghosh S., Remanan S., Ghosh S.K., Das N.C. In-Situ Synthesis of Magnetic Nanoparticle Immobilized Heterogeneous Catalyst through Mussel Mimetic Approach for the Efficient Removal of Water Pollutants. Colloid Interface Sci. Commun. 2019;33:100218. doi: 10.1016/j.colcom.2019.100218. [DOI] [Google Scholar]
- 64.Feiona T.A., Sabeena G., Bagavathy M.S., Pushpalaksmi E., Samra J.J., Annadurai G. Synthesis and Characterization of ZnO-MMT Nanocomposite for Antibacterial Activity Studies. J. Appl. Sci. Environ. Manag. 2020;24:1079–1084. doi: 10.4314/jasem.v24i6.21. [DOI] [Google Scholar]
- 65.Wang R., Li H., Ge G., Dai N., Rao J., Ran H., Zhang Y. Montmorillonite-Based Two-Dimensional Nanocomposites: Preparation and Applications. Molecules. 2021;26:2521. doi: 10.3390/molecules26092521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giraldo L.F., Camilo P., Kyu T. Incorporation of Silver in Montmorillonite-Type Phyllosilicates as Potential Antibacterial Material. Curr. Opin. Chem. Eng. 2016;11:7–13. doi: 10.1016/j.coche.2015.11.003. [DOI] [Google Scholar]
- 67.Dutta P., Wang B. Zeolite-Supported Silver as Antimicrobial Agents. Coord. Chem. Rev. 2019;383:1–29. doi: 10.1016/j.ccr.2018.12.014. [DOI] [Google Scholar]
- 68.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 69.Khalandi B., Asadi N., Milani M., Davaran S., Abadi A.J.N., Abasi E., Akbarzadeh A. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of Their Actions on Bacteria. Drug Res. 2017;11:70–76. doi: 10.1055/s-0042-113383. [DOI] [PubMed] [Google Scholar]
- 70.Park H.J., Lim H.M. Antimicrobial Properties of Ag-Exchanged Natural and Synthetic Zeolites: A Short Review. Curr. Green Chem. 2015;2:354–361. doi: 10.2174/2213346102666150918190426. [DOI] [Google Scholar]
- 71.Marambio-Jones C., Hoek E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopart. Res. 2010;12:1531–1551. doi: 10.1007/s11051-010-9900-y. [DOI] [Google Scholar]
- 72.Longano D., Ditaranto N., Sabbatini L., Torsi L., Cioffi N. Synthesis and Antimicrobial Activity of Copper Nanomaterials. In: Nicola C., Mahendra R., editors. Nano-Antimicrobials: Progress and Prospects. Springer; Berlin/Heidelberg, Germany: 2012. pp. 85–117. [Google Scholar]
- 73.Simončič B., Klemenčič D. Preparation and Performance of Silver as an Antimicrobial Agent for Textiles: A Review. Text. Res. J. 2015;3:15–20. doi: 10.1177/0040517515586157. [DOI] [Google Scholar]
- 74.Giannossa L.C., Longano D., Ditaranto N., Nitti M.A., Paladini F., Pollini M., Rai M., Sannino A., Valentini A., Cioffi N. Metal Nanoantimicrobials for Textile Applications. Nanotechnol. Rev. 2013;2:307–331. doi: 10.1515/ntrev-2013-0004. [DOI] [Google Scholar]
- 75.Sportelli M.C., Picca R.A., Cioffi N. Novel Antimicrobial Agents and Strategies. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2014. Nano-Antimicrobials Based on Metals; pp. 181–218. [Google Scholar]
- 76.Ohashi F., Ueda S., Taguri T., Kawachi S., Abe H. Antimicrobial Activity and Thermostability of Silver 6-Benzylaminopurine Montmorillonite. Appl. Clay Sci. 2009;46:296–299. doi: 10.1016/j.clay.2009.08.025. [DOI] [Google Scholar]
- 77.Sohrabnezhad S., Sadeghi A. Matrix Effect of Montmorillonite and MCM-41 Matrices on the Antibacterial Activity of Ag2CO3 Nanoparticles. Appl. Clay Sci. 2015;105–106:217–224. doi: 10.1016/j.clay.2014.12.034. [DOI] [Google Scholar]
- 78.Shameli K., Ahmad M.B., Yunus W.M.Z.W., Ibrahim N.A., Gharayebi Y., Sedaghat S. Synthesis of Silver/Montmorillonite Nanocomposites Using γ-Irradiation. Int. J. Nanomed. 2010;5:1067–1077. doi: 10.2147/IJN.S15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ranjan S., Fontana F., Ullah H., Hirvonen J., Santos H.A. Microparticles to Enhance Delivery of Drugs and Growth Factors into Wound Sites. Ther. Deliv. 2016;7:711–732. doi: 10.4155/tde-2016-0039. [DOI] [PubMed] [Google Scholar]
- 80.Lakbita O., Rhouta B., Maury F., Senocq F., Amjoud M., Daoudi L. Influence of the Crystal Structure of Ag2CO3 on the Photocatalytic Activity under Visible Light of Ag2CO3-Palygorskite Nanocomposite Material. Appl. Surf. Sci. 2019;464:205–211. doi: 10.1016/j.apsusc.2018.09.053. [DOI] [Google Scholar]
- 81.Sohrabnezhad S., Rassa M., Mohammadi Dahanesari E. Spectroscopic Study of Silver Halides in Montmorillonite and Their Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2016;163:150–155. doi: 10.1016/j.jphotobiol.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 82.Naik B., Desai V., Kowshik M., Prasad V.S., Fernando G.F., Ghosh N.N. Synthesis of Ag/AgCl–Mesoporous Silica Nanocomposites Using a Simple Aqueous Solution-Based Chemical Method and a Study of Their Antibacterial Activity on E. coli. Particuology. 2011;9:243–247. doi: 10.1016/j.partic.2010.12.001. [DOI] [Google Scholar]
- 83.Duan S., Zhai Y.H., Qu Y.J. Synthesis and Characterization of Silver-Histidine Complex Doped Montmorillonite Antibacterial Agent. Adv. Mater. Res. 2012;510:757–761. doi: 10.4028/www.scientific.net/AMR.510.757. [DOI] [Google Scholar]
- 84.Armstrong G., Thornton R., Ryan M.P., Laffir F., Russell R.J., Bala T., Keely C., Babu R. Formulation of Epoxy–Polyester Powder Coatings Containing Silver-Modified Nanoclays and Evaluation of Their Antimicrobial Properties. Polym. Bull. 2012;68:1951–1963. doi: 10.1007/s00289-011-0695-5. [DOI] [Google Scholar]
- 85.Lavorgna M., Attianese I., Buonocore G.G., Conte A., Del Nobile M.A., Tescione F., Amendola E. MMT-Supported Ag Nanoparticles for Chitosan Nanocomposites: Structural Properties and Antibacterial Activity. Carbohydr. Polym. 2014;102:385–392. doi: 10.1016/j.carbpol.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 86.Bell W.C., Myrick M.L. Preparation and Characterization of Nanoscale Silver Colloids by Two Novel Synthetic Routes. J. Colloid Interface Sci. 2001;242:300–305. doi: 10.1006/jcis.2001.7833. [DOI] [Google Scholar]
- 87.Su H.-L., Chou C.-C., Hung D.-J., Lin S.-H., Pao I.-C., Lin J.-H., Huang F.-L., Dong R.-X., Lin J.-J. The Disruption of Bacterial Membrane Integrity through ROS Generation Induced by Nanohybrids of Silver and Clay. Biomaterials. 2009;30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 88.Praus P., Turicová M., Klementová M. Preparation of Silver-Montmorillonite Nanocomposites by Reduction with Formaldehyde and Borohydride. J. Braz. Chem. Soc. 2009;20:1351–1357. doi: 10.1590/S0103-50532009000700021. [DOI] [Google Scholar]
- 89.Miyoshi H., Ohno H., Sakai K., Okamura N., Kourai H. Characterization and Photochemical and Antibacterial Properties of Highly Stable Silver Nanoparticles Prepared on Montmorillonite Clay in N-Hexanol. J. Colloid Interface Sci. 2010;345:433–441. doi: 10.1016/j.jcis.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z.L. Transmission Electron Microscopy of Shape-Controlled Nanocrystals and Their Assemblies. J. Phys. Chem. B. 2000;104:1153–1175. doi: 10.1021/jp993593c. [DOI] [Google Scholar]
- 91.Shameli K., Mansor Bin Ahmad M., Mohsen Z., Yunis W.Z., Ibrahim N.A., Rustaiyan A. Synthesis of Silver Nanoparticles in Montmorillonite and Their Antibacterial Behavior. Int. J. Nanomed. 2011;6:581. doi: 10.2147/IJN.S17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 93.Lee D., Cohen R.E., Rubner M.F. Antibacterial Properties of Ag Nanoparticle Loaded Multilayers and Formation of Magnetically Directed Antibacterial Microparticles. Langmuir. 2005;21:9651–9659. doi: 10.1021/la0513306. [DOI] [PubMed] [Google Scholar]
- 94.Sohrabnezhad S., Pourahmad A., Mehdipour Moghaddam M.J., Sadeghi A. Study of Antibacterial Activity of Ag and Ag2CO3 Nanoparticles Stabilized over Montmorillonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;136:1728–1733. doi: 10.1016/j.saa.2014.10.074. [DOI] [PubMed] [Google Scholar]
- 95.Magaña S.M., Quintana P., Aguilar D.H., Toledo J.A., Ángeles-Chávez C., Cortés M.A., León L., Freile-Pelegrín Y., López T., Sánchez R.M.T. Antibacterial Activity of Montmorillonites Modified with Silver. J. Mol. Catal. A Chem. 2008;281:192–199. doi: 10.1016/j.molcata.2007.10.024. [DOI] [Google Scholar]
- 96.Bonga D.L.S., Pinto M.M.F.B., Tayad M.F.T. Synthesis and Characterization of Silver Nanoparticles Anchored on Montmorillonite via Chemical Reduction. Nano Hybrids Compos. 2016;11:30–37. doi: 10.4028/www.scientific.net/NHC.11.30. [DOI] [Google Scholar]
- 97.Zhu W., Li J., Lei J., Li Y., Chen T., Duan T., Yao W., Zhou J., Yu Y., Liu Y. Silver Nanoparticles Incorporated Konjac Glucomannan-Montmorillonite Nacre-like Composite Films for Antibacterial Applications. Carbohydr. Polym. 2018;197:253–259. doi: 10.1016/j.carbpol.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Shameli K., Mansor Bin Ahmad M., Yunis W.Z., Ibrahim N.A., Mohsen Z., Shabanzadeh P., Moghaddam M.G. Synthesis and Characterization of Silver/Montmorillonite/Chitosan Bionanocomposites by Chemical Reduction Method and Their Antibacterial Activity. Int. J. Nanomed. 2011;6:271. doi: 10.2147/IJN.S16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roy B., Bharali P., Konwar B.K., Karak N. Silver-Embedded Modified Hyperbranched Epoxy/Clay Nanocomposites as Antibacterial Materials. Bioresour. Technol. 2013;127:175–180. doi: 10.1016/j.biortech.2012.09.129. [DOI] [PubMed] [Google Scholar]
- 100.Monsif M., Zerouale A., Kandri N.I., Bertani R., Bartolozzi A., Bresolin B.M., Zorzi F., Tateo F., Zappalorto M., Quaresimin M., et al. Multifunctional Epoxy/Nanocomposites Based on Natural Moroccan Clays with High Antimicrobial Activity: Morphological, Thermal and Mechanical Properties. J. Nanomater. 2019;2019:e2810901. doi: 10.1155/2019/2810901. [DOI] [Google Scholar]
- 101.Gogoi B., Barua S., Sarmah J.K., Karak N. In Situ Synthesis of a Microbial Fouling Resistant, Nanofibrillar Cellulose-Hyperbranched Epoxy Composite for Advanced Coating Applications. Prog. Org. Coat. 2018;124:224–231. doi: 10.1016/j.porgcoat.2018.04.025. [DOI] [Google Scholar]
- 102.Krishnan B., Mahalingam S. Ag/TiO2/Bentonite Nanocomposite for Biological Applications: Synthesis, Characterization, Antibacterial and Cytotoxic Investigations. Adv. Powder Technol. 2017;28:2265–2280. doi: 10.1016/j.apt.2017.06.007. [DOI] [Google Scholar]
- 103.Benhacine F., Abdellaoui N., Arous O., Hadj-Hamou A.S. Behaviours of Poly(ε-Caprolactone)/Silver-Montmorillonite Nanocomposite in Membrane Ultrafiltration for Wastewater Treatment. Environ. Technol. 2018;40:2225–2231. doi: 10.1080/09593330.2018.1555283. [DOI] [PubMed] [Google Scholar]
- 104.Benhacine F., Hadj-Hamou A.S., Habi A. Development of Long-Term Antimicrobial Poly (ε-Caprolactone)/Silver Exchanged Montmorillonite Nanocomposite Films with Silver Ion Release Property for Active Packaging Use. Polym. Bull. 2016;73:1207–1227. doi: 10.1007/s00289-015-1543-9. [DOI] [Google Scholar]
- 105.Gurunathan S., Hyun Park J., Choi Y.J., Woong Han J., Kim J.H. Synthesis of Graphene Oxide-Silver Nanoparticle Nanocomposites: An Efficient Novel Antibacterial Agent. [(accessed on 18 April 2020)]. Available online: http://www.eurekaselect.com/144133/article.
- 106.Yun H., Ahmed M.S., Lee K., Jeon S., Lee C.W. Potential Enhancement of Antibacterial Activity of Graphene Oxide-Silver Nanocomposite by Introducing C2 Carbon Chain Linkage. Appl. Surf. Sci. 2016;360:915–920. doi: 10.1016/j.apsusc.2015.11.084. [DOI] [Google Scholar]
- 107.San Keskin N.O., Koçberber Kılıç N., Dönmez G., Tekinay T. Green Synthesis of Silver Nanoparticles Using Cyanobacteria and Evaluation of Their Photocatalytic and Antimicrobial Activity. J. Nano Res. 2016;40:120–127. doi: 10.4028/www.scientific.net/JNanoR.40.120. [DOI] [Google Scholar]
- 108.Li Y.-T., Lin S.-B., Chen L.-C., Chen H.-H. Antimicrobial Activity and Controlled Release of Nanosilvers in Bacterial Cellulose Composites Films Incorporated with Montmorillonites. Cellulose. 2017;24:4871–4883. doi: 10.1007/s10570-017-1487-3. [DOI] [Google Scholar]
- 109.Ghiassi S., Sedaghat S., Mokhtary M., Kefayati H. Plant-Mediated Bio-Synthesis of Silver–Montmorillonite Nanocomposite and Antibacterial Effects on Gram-Positive and -Negative Bacteria. J. Nanostructure Chem. 2018;8:353–357. doi: 10.1007/s40097-018-0280-7. [DOI] [Google Scholar]
- 110.Omidi S., Sedaghat S., Tahvildari K., Derakhshi P., Motiee F. Biosynthesis of Silver Nanocomposite with Tarragon Leaf Extract and Assessment of Antibacterial Activity. J. Nanostructure Chem. 2018;8:171–178. doi: 10.1007/s40097-018-0263-8. [DOI] [Google Scholar]
- 111.Moradi F., Sedaghat S., Arab-Salmanabadi S., Moradi O. Biosynthesis of Silver-Montmorillonite Nanocomposites Using Ocimum Basilicum and Teucrium Polium$\mathsemicolon$ a Comparative Study. Mater. Res. Express. 2019;6:125008. doi: 10.1088/2053-1591/ab5474. [DOI] [Google Scholar]
- 112.Ge M., Li J., Song S., Meng N., Zhou N. Development and Antibacterial Performance of Silver Nanoparticles-Lecithin Modified Montmorillonite Nanoparticle Hybrid. Appl. Clay Sci. 2019;183:105334. doi: 10.1016/j.clay.2019.105334. [DOI] [Google Scholar]
- 113.Dairi N., Ferfera-Harrar H., Ramos M., Garrigós M.C. Cellulose Acetate/AgNPs-Organoclay and/or Thymol Nano-Biocomposite Films with Combined Antimicrobial/Antioxidant Properties for Active Food Packaging Use. Int. J. Biol. Macromol. 2019;121:508–523. doi: 10.1016/j.ijbiomac.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 114.Scott A., Vadalasetty K.P., Chwalibog A., Sawosz E. Copper Nanoparticles as an Alternative Feed Additive in Poultry Diet: A Review. Nanotechnol. Rev. 2018;7:69–93. doi: 10.1515/ntrev-2017-0159. [DOI] [Google Scholar]
- 115.Zhou Y., Xia M., Ye Y., Hu C. Antimicrobial Ability of Cu-Montmorillonite. Appl. Clay Sci. 2004;27:215–218. doi: 10.1016/j.clay.2004.06.002. [DOI] [Google Scholar]
- 116.Xia M.S., Hu C.H., Xu Z.R. Effects of Copper-Bearing Montmorillonite on Growth Performance, Digestive Enzyme Activities, and Intestinal Microflora and Morphology of Male Broilers. Poult. Sci. 2004;83:1868–1875. doi: 10.1093/ps/83.11.1868. [DOI] [PubMed] [Google Scholar]
- 117.Xia M.S., Hu C.H., Xu Z.R., Ye Y., Zhou Y.H., Xiong L. Effects of Copper-Bearing Montmorillonite (Cu-MMT) on Escherichia Coli and Diarrhea on Weanling Pigs. Asian-Australas. J. Anim. Sci. 2004;17:1712–1716. doi: 10.5713/ajas.2004.1712. [DOI] [Google Scholar]
- 118.Hu C.H., Xu Z.R., Xia M.S. Antibacterial Effect of Cu2+-Exchanged Montmorillonite on Aeromonas Hydrophila and Discussion on Its Mechanism. Vet. Microbiol. 2005;109:83–88. doi: 10.1016/j.vetmic.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 119.Tong G., Yulong M., Peng G., Zirong X. Antibacterial Effects of the Cu(II)-Exchanged Montmorillonite on Escherichia Coli K88 and Salmonella Choleraesuis. Vet. Microbiol. 2005;105:113–122. doi: 10.1016/j.vetmic.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Hu C.H., Xu Y., Xia M.S., Xiong L., Xu Z.R. Effects of Cu 2+$ -Exchanged Montmorillonite on Intestinal Microflora, Digestibility and Digestive Enzyme Activities of Nile Tilapia. Aquac. Nutr. 2008;14:281–288. doi: 10.1111/j.1365-2095.2007.00531.x. [DOI] [Google Scholar]
- 121.Bagchi B., Kar S., Dey S.K., Bhandary S., Roy D., Mukhopadhyay T.K., Das S., Nandy P. In Situ Synthesis and Antibacterial Activity of Copper Nanoparticle Loaded Natural Montmorillonite Clay Based on Contact Inhibition and Ion Release. Colloids Surf. B Biointerfaces. 2013;108:358–365. doi: 10.1016/j.colsurfb.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 122.Tan S.-Z., Zhang K.-H., Zhang L.-L., Xie Y.-S., Liu Y.-L. Preparation and Characterization of the Antibacterial Zn2+ or/and Ce3+ Loaded Montmorillonites. Chin. J. Chem. 2008;26:865–869. doi: 10.1002/cjoc.200890160. [DOI] [Google Scholar]
- 123.Aguzzi C., Cerezo P., Viseras C., Caramella C. Use of Clays as Drug Delivery Systems: Possibilities and Limitations. Appl. Clay Sci. 2007;36:22–36. doi: 10.1016/j.clay.2006.06.015. [DOI] [Google Scholar]
- 124.Volzone C., Garrido L.B. Changes in Suspension Properties of Structural Modified Montmorillonites. Cerâmica. 2001;47:4–8. doi: 10.1590/S0366-69132001000100002. [DOI] [Google Scholar]
- 125.Cao G.-F., Sun Y., Chen J.-G., Song L.-P., Jiang J.-Q., Liu Z.-T., Liu Z.-W. Sutures Modified by Silver-Loaded Montmorillonite with Antibacterial Properties. Appl. Clay Sci. 2014;93–94:102–106. doi: 10.1016/j.clay.2014.03.007. [DOI] [Google Scholar]
- 126.Incoronato A.L., Buonocore G.G., Conte A., Lavorgna M., Del Nobile M.A. Active Systems Based on Silver-Montmorillonite Nanoparticles Embedded into Bio-Based Polymer Matrices for Packaging Applications. J. Food Prot. 2010;73:2256–2262. doi: 10.4315/0362-028X-73.12.2256. [DOI] [PubMed] [Google Scholar]
- 127.Costa C., Conte A., Buonocore G.G., Del Nobile M.A. Antimicrobial Silver-Montmorillonite Nanoparticles to Prolong the Shelf Life of Fresh Fruit Salad. Int. J. Food Microbiol. 2011;148:164–167. doi: 10.1016/j.ijfoodmicro.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 128.Costa C., Conte A., Buonocore G.G., Lavorgna M., Del Nobile M.A. Calcium-Alginate Coating Loaded with Silver-Montmorillonite Nanoparticles to Prolong the Shelf-Life of Fresh-Cut Carrots. Food Res. Int. 2012;48:164–169. doi: 10.1016/j.foodres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 129.Ibarra-Alonso M.C., Sánchez-Valdes S., Ramírez-Vargas E., Fernandez-Tavizón S., Romero-Garcia J., Ledezma-Perez A.S., de Valle L.F.R., Rodriguez-Fernandez O.S., Espinoza-Martinez A.B., Martinez-Colunga J.G., et al. Preparation and Characterization of Polyethylene/Clay/Silver Nanocomposites Using Functionalized Polyethylenes as an Adhesion Promoter. J. Adhes. Sci. Technol. 2015;29:1911–1923. doi: 10.1080/01694243.2015.1045247. [DOI] [Google Scholar]
- 130.Xia Z., Liu J., Zou J., Lin C. Assembly and Antibacterial Properties of Chitosan Derivatives Organic Montmorillonite Nanocomposites Doped with Silver. Mater. Res. Innov. 2015;19:S8-204–S8-211. doi: 10.1179/1432891715Z.0000000001657. [DOI] [Google Scholar]
- 131.Savas L.A., Hancer M. Montmorillonite Reinforced Polymer Nanocomposite Antibacterial Film. Appl. Clay Sci. 2015;108:40–44. doi: 10.1016/j.clay.2015.02.021. [DOI] [Google Scholar]
- 132.Muñoz-Shugulí C., Rodríguez F.J., Bruna J.E., Galotto M.J., Sarantópoulos C., Favaro Perez M.A., Padula M. Cetylpyridinium Bromide-Modified Montmorillonite as Filler in Low Density Polyethylene Nanocomposite Films. Appl. Clay Sci. 2019;168:203–210. doi: 10.1016/j.clay.2018.10.020. [DOI] [Google Scholar]
- 133.Golubeva O.Y., Shamova O.V., Yakovlev A.V., Zharkova M.S. Synthesis and Study of the Biologically Active Lysozyme–Silver Nanoparticles–Montmorillonite K10 Complexes. Glass Phys. Chem. 2016;42:87–94. doi: 10.1134/S1087659616010041. [DOI] [Google Scholar]
- 134.Roy A., Joshi M., Butola B.S., Ghosh S. Evaluation of Biological and Cytocompatible Properties in Nano Silver-Clay Based Polyethylene Nanocomposites. J. Hazard. Mater. 2020;384:121309. doi: 10.1016/j.jhazmat.2019.121309. [DOI] [PubMed] [Google Scholar]
- 135.Zhang D., Liu H.M., Shu X., Feng J., Yang P., Dong P., Xie X., Shi Q. Nanocopper-Loaded Black Phosphorus Nanocomposites for Efficient Synergistic Antibacterial Application. J. Hazard. Mater. 2020;393:122317. doi: 10.1016/j.jhazmat.2020.122317. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L., Chen J., Yu W., Zhao Q., Liu J. Antimicrobial Nanocomposites Prepared from Montmorillonite/Ag +$ /Quaternary Ammonium Nitrate. J. Nanomater. 2018;2018:1–7. doi: 10.1155/2018/6190251. [DOI] [Google Scholar]
- 137.Hwang J.-J., Ma T.-W. Preparation, Morphology, and Antibacterial Properties of Polyacrylonitrile/Montmorillonite/Silver Nanocomposites. Mater. Chem. Phys. 2012;136:613–623. doi: 10.1016/j.matchemphys.2012.07.034. [DOI] [Google Scholar]
- 138.Fryczkowska B., Biniaś D., Ślusarczyk C., Fabia J., Janicki J. Influence of Graphene Oxide on the Properties of Composite Polyacrylonitrile Membranes. DWT. 2017;81:67–79. doi: 10.5004/dwt.2017.21183. [DOI] [Google Scholar]
- 139.Ismayil K.M., Varghese A., Antony R. Silver-Doped Polyaniline–Polyvinyl Chloride Nanocomposite Films for Photocatalytic and Antibacterial Applications. J. Elastomers Plast. 2019;5:009524431881923. doi: 10.1177/0095244318819238. [DOI] [Google Scholar]
- 140.Fernández Solarte A.M., Villarroel-Rocha J., Morantes C.F., Montes M.L., Sapag K., Curutchet G., Torres Sánchez R.M. Insight into Surface and Structural Changes of Montmorillonite and Organomontmorillonites Loaded with Ag. Comptes Rendus Chim. 2019;22:142–153. doi: 10.1016/j.crci.2018.09.006. [DOI] [Google Scholar]
- 141.Lamarra J., Fernández M.A., Pascual Cosp J., De La Fournière S., Garbossa G., Torres Sanchez R.M. Water Decontamination by Silver and Copper Montmorillonite. Int. J. Environ. Health. 2014;7:15–30. doi: 10.1504/IJENVH.2014.060124. [DOI] [Google Scholar]
- 142.Ling Y., Luo Y., Luo J., Wang X., Sun R. Novel Antibacterial Paper Based on Quaternized Carboxymethyl Chitosan/Organic Montmorillonite/Ag NP Nanocomposites. Ind. Crop. Prod. 2013;51:470–479. doi: 10.1016/j.indcrop.2013.09.040. [DOI] [Google Scholar]
- 143.Fernández M.A., Barberia Roque L., Gámez Espinosa E., Deyá C., Bellotti N. Organo-Montmorillonite with Biogenic Compounds to Be Applied in Antifungal Coatings. Appl. Clay Sci. 2020;184:105369. doi: 10.1016/j.clay.2019.105369. [DOI] [Google Scholar]
- 144.Makwana D., Castaño J., Somani R.S., Bajaj H.C. Characterization of Agar-CMC/Ag-MMT Nanocomposite and Evaluation of Antibacterial and Mechanical Properties for Packaging Applications. Arab. J. Chem. 2020;13:3092–3099. doi: 10.1016/j.arabjc.2018.08.017. [DOI] [Google Scholar]
- 145.Abbasian M., Mahmoodzadeh F. Synthesis of Antibacterial Silver-Chitosan-Modified Bionanocomposites by RAFT Polymerization and Chemical Reduction Methods. J. Elastomers Plast. 2017;49:173–193. doi: 10.1177/0095244316644858. [DOI] [Google Scholar]
- 146.Golubeva O.Y., Brazovskaya E.Y., Shamova O.V. Biological Activity and Sorption Ability of Synthetic Montmorillonite Modified by Silver/Lysozyme Nanoparticles. Appl. Clay Sci. 2018;163:56–62. doi: 10.1016/j.clay.2018.07.015. [DOI] [Google Scholar]
- 147.Bock T.K., Müller B.W. A Novel Assay to Determine the Hemolytic Activity of Drugs Incorporated in Colloidal Carrier Systems. Pharm. Res. 1994;11:589–591. doi: 10.1023/A:1018987120738. [DOI] [PubMed] [Google Scholar]
- 148.Wu T.-S., Wang K.-X., Li G.-D., Sun S.-Y., Sun J., Chen J.-S. Montmorillonite-Supported Ag/TiO2$ Nanoparticles: An Efficient Visible-Light Bacteria Photodegradation Material. ACS Appl. Mater. Interfaces. 2010;2:544–550. doi: 10.1021/am900743d. [DOI] [PubMed] [Google Scholar]
- 149.Park J.H., Karim M.R., Kim I.K., Cheong I.W., Kim J.W., Bae D.G., Cho J.W., Yeum J.H. Electrospinning Fabrication and Characterization of Poly(Vinyl Alcohol)/Montmorillonite/Silver Hybrid Nanofibers for Antibacterial Applications. Colloid Polym. Sci. 2010;288:115–121. doi: 10.1007/s00396-009-2147-4. [DOI] [Google Scholar]
- 150.Hossain S.I., Bajrami D., Sportelli M.C., Picca R.A., Volpe A., Gaudiuso C., Ancona A., Gentile L., Palazzo G., Ditaranto N., et al. Preparation of Laser-Ablated Ag Nanoparticle–MMT Clay-Based Beeswax Antibiofilm Coating. Antibiotics. 2023;12:194. doi: 10.3390/antibiotics12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fernando L., Chen W.-T., Lai C.-W., Ye F.-Y., Lai P.-S., Lin J.-J., Yasuda K., Song T.-T., Song J.-M. Biocompatibility and Antimicrobial Activity of Copper(II) Oxide Hybridized with Nano Silicate Platelets. Surf. Coat. Technol. 2022;435:128253. doi: 10.1016/j.surfcoat.2022.128253. [DOI] [Google Scholar]
- 152.Pourabolghasem H., Ghorbanpour M., Shayegh R. Antibacterial Activity of Copper-Doped Montmorillonite Nanocomposites Prepared by Alkaline Ion Exchange Method. JPS. 2016;27:1–12. doi: 10.21315/jps2016.27.2.1. [DOI] [Google Scholar]
- 153.Wu Y., Zhou N., Li W., Gu H., Fan Y., Yuan J. Long-Term and Controlled Release of Chlorhexidine–Copper(II) from Organically Modified Montmorillonite (OMMT) Nanocomposites. Mater. Sci. Eng. C. 2013;33:752–757. doi: 10.1016/j.msec.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 154.Jaiswal L., Shankar S., Rhim J.-W. Chapter 3—Applications of Nanotechnology in Food Microbiology. In: Gurtler V., Ball A.S., Soni S., editors. Methods in Microbiology. Volume 46. Academic Press; Cambridge, MA, USA: 2019. pp. 43–60. Nanotechnology. [Google Scholar]
- 155.Bruna J.E., Peñaloza A., Guarda A., Rodríguez F., Galotto M.J. Development of MtCu2+/LDPE Nanocomposites with Antimicrobial Activity for Potential Use in Food Packaging. Appl. Clay Sci. 2012;58:79–87. doi: 10.1016/j.clay.2012.01.016. [DOI] [Google Scholar]
- 156.Bruna J.E., Quilodrán H., Guarda A., Rodríguez F., Galotto M.J., Figueroa P. Development of Antibacterial MtCu/PLA Nanocomposites by Casting Method for Potential Use in Food Packaging. J. Chil. Chem. Soc. 2015;60:3009–3014. doi: 10.4067/S0717-97072015000300007. [DOI] [Google Scholar]
- 157.Cárdenas G., Díaz V.J., Meléndrez M.F., Cruzat C.C., García Cancino A. Colloidal Cu Nanoparticles/Chitosan Composite Film Obtained by Microwave Heating for Food Package Applications. Polym. Bull. 2009;62:511–524. doi: 10.1007/s00289-008-0031-x. [DOI] [Google Scholar]
- 158.Nouri A., Yaraki M.T., Ghorbanpour M., Agarwal S., Gupta V.K. Enhanced Antibacterial Effect of Chitosan Film Using Montmorillonite/CuO Nanocomposite. Int. J. Biol. Macromol. 2018;109:1219–1231. doi: 10.1016/j.ijbiomac.2017.11.119. [DOI] [PubMed] [Google Scholar]
- 159.Martucci J.F., Ruseckaite R.A. Antibacterial Activity of Gelatin/Copper (II)-Exchanged Montmorillonite Films. Food Hydrocoll. 2017;64:70–77. doi: 10.1016/j.foodhyd.2016.10.030. [DOI] [Google Scholar]
- 160.Das G., Kalita R.D., Gogoi P., Buragohain A.K., Karak N. Antibacterial Activities of Copper Nanoparticle-Decorated Organically Modified Montmorillonite/Epoxy Nanocomposites. Appl. Clay Sci. 2014;90:18–26. doi: 10.1016/j.clay.2014.01.002. [DOI] [Google Scholar]
- 161.Bartolozzi A., Bertani R., Burigo E., Fabrizi A., Panozzo F., Quaresimin M., Simionato F., Sgarbossa P., Tamburini S., Zappalorto M., et al. Multifunctional Cu 2+$ -Montmorillonite/Epoxy Resin Nanocomposites with Antibacterial Activity. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.44733. [DOI] [Google Scholar]
- 162.De B., Gupta K., Mandal M., Karak N. Biocide Immobilized OMMT-Carbon Dot Reduced Cu2O Nanohybrid/Hyperbranched Epoxy Nanocomposites: Mechanical, Thermal, Antimicrobial and Optical Properties. Mater. Sci. Eng. C. 2015;56:74–83. doi: 10.1016/j.msec.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 163.Sun B., Xi Z., Wu F., Song S., Huang X., Chu X., Wang Z., Wang Y., Zhang Q., Meng N., et al. Quaternized Chitosan-Coated Montmorillonite Interior Antimicrobial Metal–Antibiotic in Situ Coordination Complexation for Mixed Infections of Wounds. Langmuir. 2019;35:15275–15286. doi: 10.1021/acs.langmuir.9b02821. [DOI] [PubMed] [Google Scholar]
- 164.Rienzie R., Sendanayake L., De Costa D., Hossain A., Brestic M., Skalicky M., Vachova P., Adassooriya N.M. Assessing the Carboxymethylcellulose Copper-Montmorillonite Nanocomposite for Controlling the Infection of Erwinia Carotovora in Potato (Solanum Tuberosum L.) Nanomaterials. 2021;11:802. doi: 10.3390/nano11030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garshasbi N., Ghorbanpour M., Nouri A., Lotfiman S., Garshasbi N., Ghorbanpour M., Nouri A., Lotfiman S. Preparation of Zinc Oxide-Nanoclay Hybrids by Alkaline Ion Exchange Method. Braz. J. Chem. Eng. 2017;34:1055–1063. doi: 10.1590/0104-6632.20170344s20150570. [DOI] [Google Scholar]
- 166.Zou Y.-H., Wang J., Cui L.-Y., Zeng R.-C., Wang Q.-Z., Han Q.-X., Qiu J., Chen X.-B., Chen D.-C., Guan S.-K., et al. Corrosion Resistance and Antibacterial Activity of Zinc-Loaded Montmorillonite Coatings on Biodegradable Magnesium Alloy AZ31. Acta Biomater. 2019;98:196–214. doi: 10.1016/j.actbio.2019.05.069. [DOI] [PubMed] [Google Scholar]
- 167.Wang J., Cui L., Ren Y., Zou Y., Ma J., Wang C., Zheng Z., Chen X., Zeng R., Zheng Y. In Vitro and in Vivo Biodegradation and Biocompatibility of an MMT/BSA Composite Coating upon Magnesium Alloy AZ31. J. Mater. Sci. Technol. 2020;47:52–67. doi: 10.1016/j.jmst.2020.02.006. [DOI] [Google Scholar]
- 168.Hu C.H., Gu L.Y., Luan Z.S., Song J., Zhu K. Effects of Montmorillonite–Zinc Oxide Hybrid on Performance, Diarrhea, Intestinal Permeability and Morphology of Weanling Pigs. Anim. Feed Sci. Technol. 2012;177:108–115. doi: 10.1016/j.anifeedsci.2012.07.028. [DOI] [Google Scholar]
- 169.Hu C.H., Qian Z.C., Song J., Luan Z.S., Zuo A.Y. Effects of Zinc Oxide-Montmorillonite Hybrid on Growth Performance, Intestinal Structure, and Function of Broiler Chicken. Poult. Sci. 2013;92:143–150. doi: 10.3382/ps.2012-02250. [DOI] [PubMed] [Google Scholar]
- 170.Mallakpour S., Aalizadeh R. Polymer Nanocomposites Containing 4,4′-Methylene Bis(3-Chloro-2,6-Diethylaniline) and N,N′-(Pyromellitoyl)-Bis-L-Phenylalanine Diacid Reinforced with Modified ZnO and Organo-Montmorillonite. Polym.-Plast. Technol. Eng. 2013;52:674–682. doi: 10.1080/03602559.2012.762665. [DOI] [Google Scholar]
- 171.Zahedi Y., Fathi-Achachlouei B., Yousefi A.R. Physical and Mechanical Properties of Hybrid Montmorillonite/Zinc Oxide Reinforced Carboxymethyl Cellulose Nanocomposites. Int. J. Biol. Macromol. 2018;108:863–873. doi: 10.1016/j.ijbiomac.2017.10.185. [DOI] [PubMed] [Google Scholar]
- 172.Samzadeh-Kermani A., Miri S. Synthesis, Characterization and Bactericidal Property of Chitosan-Graft-Polyaniline/Montmorillonite/ZnO Nanocomposite. Korean J. Chem. Eng. 2015;32:1137–1141. doi: 10.1007/s11814-014-0285-y. [DOI] [Google Scholar]
- 173.Ramya E., Rajashree C., Nayak P.L., Narayana Rao D. New Hybrid Organic Polymer Montmorillonite/Chitosan/Polyphenylenediamine Composites for Nonlinear Optical Studies. Appl. Clay Sci. 2017;150:323–332. doi: 10.1016/j.clay.2017.10.001. [DOI] [Google Scholar]
- 174.Vaezi K., Asadpour G., Sharifi H. Effect of ZnO Nanoparticles on the Mechanical, Barrier and Optical Properties of Thermoplastic Cationic Starch/Montmorillonite Biodegradable Films. Int. J. Biol. Macromol. 2019;124:519–529. doi: 10.1016/j.ijbiomac.2018.11.142. [DOI] [PubMed] [Google Scholar]
- 175.Roy A., Joshi M., Butola B.S. Preparation and Antimicrobial Assessment of Zinc-Montmorillonite Intercalates Based HDPE Nanocomposites: A Cost-Effective and Safe Bioactive Plastic. J. Clean. Prod. 2019;212:1518–1525. doi: 10.1016/j.jclepro.2018.11.235. [DOI] [Google Scholar]
- 176.Salmas C., Giannakas A., Katapodis P., Leontiou A., Moschovas D., Karydis-Messinis A. Development of ZnO/Na-Montmorillonite Hybrid Nanostructures Used for PVOH/ZnO/Na-Montmorillonite Active Packaging Films Preparation via a Melt-Extrusion Process. Nanomaterials. 2020;10:1079. doi: 10.3390/nano10061079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bansal P., Purwar R. Development of Efficient Antimicrobial Zinc Oxide Modified Montmorillonite Incorporated Polyacrylonitrile Nanofibers for Particulate Matter Filtration. Fibers Polym. 2021;22:2726–2737. doi: 10.1007/s12221-021-0914-0. [DOI] [Google Scholar]
- 178.Liu J., Wang Y., Fan X., Liu H., Li J., He X., Hui A., Wang A. The High-Efficiency Synergistic and Broad-Spectrum Antibacterial Effect of Cobalt Doped Zinc Oxide Quantum Dots (Co-ZnO QDs) Loaded Cetyltributylphosphonium Bromide (CTPB) Modified MMT (C-MMT) Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2021;613:126059. doi: 10.1016/j.colsurfa.2020.126059. [DOI] [Google Scholar]
- 179.Chen H., Deng H., Zhong X., Zhou H., Zhan J., Zhou X. Highly Dispersed Amorphous ZnO on a Petal-like Porous Silica-Clay Composite with Enhanced Antimicrobial Properties. Colloids Surf. B Biointerfaces. 2022;220:112978. doi: 10.1016/j.colsurfb.2022.112978. [DOI] [Google Scholar]
- 180.Baldassari S., Komarneni S., Mariani E., Villa C. Microwave versus Conventional Preparation of Organoclays from Natural and Synthetic Clays. Appl. Clay Sci. 2006;31:134–141. doi: 10.1016/j.clay.2005.09.005. [DOI] [Google Scholar]
- 181.Cheng D., Zhou X., Xia H., Chan H.S.O. Novel Method for the Preparation of Polymeric Hollow Nanospheres Containing Silver Cores with Different Sizes. Chem. Mater. 2005;17:3578–3581. doi: 10.1021/cm0503230. [DOI] [Google Scholar]
- 182.Chen P., Song L., Liu Y., Fang Y. Synthesis of Silver Nanoparticles by γ-Ray Irradiation in Acetic Water Solution Containing Chitosan. Radiat. Phys. Chem. 2007;76:1165–1168. doi: 10.1016/j.radphyschem.2006.11.012. [DOI] [Google Scholar]
- 183.Kesavan Pillai S., Sinha Ray S., Scriba M., Bandyopadhyay J., Roux-van der Merwe M.P., Badenhorst J. Microwave Assisted Green Synthesis and Characterization of Silver/Montmorillonite Heterostructures with Improved Antimicrobial Properties. Appl. Clay Sci. 2013;83–84:315–321. doi: 10.1016/j.clay.2013.08.014. [DOI] [Google Scholar]
- 184.Kheiralla Z.M.H., Rushdy A.A., Betiha M.A., Yakob N.A.N. High-Performance Antibacterial of Montmorillonite Decorated with Silver Nanoparticles Using Microwave-Assisted Method. J. Nanoparticle Res. 2014;16:2560. doi: 10.1007/s11051-014-2560-6. [DOI] [Google Scholar]
- 185.Li T., Zhang Y., Song Z., Wu H., Li S. Preparation and Characterization of Antibacterial Silver Loaded Montmorillonite under Microwave Irradiation. Sci. Eng. Compos. Mater. 2013;20:15–22. doi: 10.1515/secm-2012-0060. [DOI] [Google Scholar]
- 186.Li T., Lin O., Lu Z., He L., Wang X. Preparation and Characterization of Silver Loaded Montmorillonite Modified with Sulfur Amino Acid. Appl. Surf. Sci. 2014;305:386–395. doi: 10.1016/j.apsusc.2014.03.098. [DOI] [Google Scholar]
- 187.Xu G., Qiao X., Qiu X., Chen J. Preparation and Characterization of Nano-Silver Loaded Montmorillonite with Strong Antibacterial Activity and Slow Release Property. J. Mater. Sci. Technol. 2011;27:685–690. doi: 10.1016/S1005-0302(11)60126-6. [DOI] [Google Scholar]
- 188.Shameli K., Ahmad M.B., Yunus W.M.Z.W., Rustaiyan A., Ibrahim N.A., Zargar M., Abdollahi Y. Green Synthesis of Silver/Montmorillonite/Chitosan Bionanocomposites Using the UV Irradiation Method and Evaluation of Antibacterial Activity. Int. J. Nanomed. 2010;5:875–887. doi: 10.2147/IJN.S13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu B., Shen S., Luo J., Wang X., Sun R. One-Pot Green Synthesis and Antimicrobial Activity of Exfoliated Ag NP-Loaded Quaternized Chitosan/Clay Nanocomposites. RSC Adv. 2013;3:9714–9722. doi: 10.1039/c3ra41270a. [DOI] [Google Scholar]
- 190.Gabriel J.S., Gonzaga V.A.M., Poli A.L., Schmitt C.C. Photochemical Synthesis of Silver Nanoparticles on Chitosans/Montmorillonite Nanocomposite Films and Antibacterial Activity. Carbohydr. Polym. 2017;171:202–210. doi: 10.1016/j.carbpol.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 191.Mallakpour S., Barati A. A Straightforward Preparation and Characterization of Novel Poly(Vinyl Alcohol)/Organoclay/Silver Tricomponent Nanocomposite Films. Prog. Org. Coat. 2014;77:1629–1634. doi: 10.1016/j.porgcoat.2014.05.003. [DOI] [Google Scholar]
- 192.Tokarský J., Čapková P., Rafaja D., Klemm V., Valášková M., Kukutschová J., Tomášek V. Adhesion of Silver Nanoparticles on the Clay Substrates; Modeling and Experiment. Appl. Surf. Sci. 2010;256:2841–2848. doi: 10.1016/j.apsusc.2009.11.037. [DOI] [Google Scholar]
- 193.Keller-Besrest F., Bénazeth S., Souleau C. EXAFS Structural Investigation of a Silver-Added Montmorillonite Clay. Mater. Lett. 1995;24:17–21. doi: 10.1016/0167-577X(95)00086-0. [DOI] [Google Scholar]
- 194.Zarei A.R., Barghak F. Fast and Efficient Adsorption of Ag Nanoparticles by Sodium Montmorillonite Nanoclay from Aqueous Systems. J. Chem. Res. 2015;39:542–545. doi: 10.3184/174751915X14403380312326. [DOI] [Google Scholar]
- 195.Syafiuddin A., Salmiati S., Jonbi J., Fulazzaky M.A. Application of the Kinetic and Isotherm Models for Better Understanding of the Behaviors of Silver Nanoparticles Adsorption onto Different Adsorbents. J. Environ. Manag. 2018;218:59–70. doi: 10.1016/j.jenvman.2018.03.066. [DOI] [PubMed] [Google Scholar]
- 196.Fernández Solarte A.M., Blanco Massani M.R., Molina V.M., Benítez Guerrero M., Torres Sánchez R.M. Correlating Antimicrobial Activity and Structure in Montmorillonite Modified with Hexadecyltrimethylammonium and Silver. Sphinx Knowledge House; Maharashtra, India: 2018. [DOI] [Google Scholar]
- 197.Busolo M.A., Fernandez P., Ocio M.J., Lagaron J.M. Novel Silver-Based Nanoclay as an Antimicrobial in Polylactic Acid Food Packaging Coatings. Food Addit. Contam. Part A. 2010;27:1617–1626. doi: 10.1080/19440049.2010.506601. [DOI] [PubMed] [Google Scholar]
- 198.Clegg F., Breen C., Muranyi P., Schönweitz C. Antimicrobial, Starch Based Barrier Coatings Prepared Using Mixed Silver/Sodium Exchanged Bentonite. Appl. Clay Sci. 2019;179:105144. doi: 10.1016/j.clay.2019.105144. [DOI] [Google Scholar]
- 199.1Shabanzadeh P., Yusof R., Shameli K. Artificial Neural Network for Modeling the Size of Silver Nanoparticles’ Prepared in Montmorillonite/Starch Bionanocomposites. J. Ind. Eng. Chem. 2015;24:42–50. doi: 10.1016/j.jiec.2014.09.007. [DOI] [Google Scholar]
- 200.Valášková M., Tokarský J., Pavlovský J., Prostějovský T., Kočí K. α-Fe2O3 Nanoparticles/Vermiculite Clay Material: Structural, Optical and Photocatalytic Properties. Materials. 2019;12:1880. doi: 10.3390/ma12111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tian L., Yuanyuan Z., Yingying M., Ran H. Fabrication of Functional Silver Loaded Montmorillonite/Polycarbonate with Superhydrophobicity. Appl. Clay Sci. 2015;118:337–343. doi: 10.1016/j.clay.2015.10.016. [DOI] [Google Scholar]
- 202.Bhattacharyya K.G., Gupta S.S. Influence of Acid Activation on Adsorption of Ni(II) and Cu(II) on Kaolinite and Montmorillonite: Kinetic and Thermodynamic Study. Chem. Eng. J. 2008;136:1–13. doi: 10.1016/j.cej.2007.03.005. [DOI] [Google Scholar]
- 203.Komadel P. Acid Activated Clays: Materials in Continuous Demand. Appl. Clay Sci. 2016;131:84–99. doi: 10.1016/j.clay.2016.05.001. [DOI] [Google Scholar]
- 204.Esteban-Tejeda L., Malpartida F., Pecharromán C., Moya J.S. High Antibacterial and Antifungal Activity of Silver Monodispersed Nanoparticles Embedded in a Glassy Matrix. Adv. Eng. Mater. 2010;12:B292–B297. doi: 10.1002/adem.200980077. [DOI] [Google Scholar]
- 205.Diba M., Boccaccini A.R. 9-Silver-Containing Bioactive Glasses for Tissue Engineering Applications. In: Baltzer N., Copponnex T., editors. Precious Metals for Biomedical Applications. Woodhead Publishing; Sawston, UK: 2014. pp. 177–211. [Google Scholar]
- 206.Barui A.K., Nethi S.K., Haque S., Basuthakur P., Patra C.R. Recent Development of Metal Nanoparticles for Angiogenesis Study and Their Therapeutic Applications. ACS Appl. Bio Mater. 2019;2:5492–5511. doi: 10.1021/acsabm.9b00587. [DOI] [PubMed] [Google Scholar]
- 207.Ma Y.-L., Xu Z.-R., Guo T., You P. Adsorption of Methylene Blue on Cu(II)-Exchanged Montmorillonite. J. Colloid Interface Sci. 2004;280:283–288. doi: 10.1016/j.jcis.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 208.Tomašević-Čanović M., Daković A., Marković V., Stojšić D. The Effect of Exchangeable Cations in Clinoptilolite and Montmorillonite on the Adsorption of Aflatoxin B1. J. Serb. Chem. Soc. 2001;66:555–561. doi: 10.2298/JSC0108555T. [DOI] [Google Scholar]
- 209.Daković A., Matijašević S., Rottinghaus G.E., Ledoux D.R., Butkeraitis P., Sekulić Ž. Aflatoxin B1 Adsorption by Natural and Copper Modified Montmorillonite. Colloids Surf. B Biointerfaces. 2008;66:20–25. doi: 10.1016/j.colsurfb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 210.Özdemir G., Yapar S. Adsorption and Desorption Behavior of Copper Ions on Na-Montmorillonite: Effect of Rhamnolipids and PH. J. Hazard. Mater. 2009;166:1307–1313. doi: 10.1016/j.jhazmat.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 211.Guo T., Cao S., Su R., Li Z., Hu P., Xu Z. Adsorptive Property of Cu2+-Loaded Montmorillonite Clays for Escherichia Coli K88 in Vitro. J. Environ. Sci. 2011;23:1808–1815. doi: 10.1016/S1001-0742(10)60651-1. [DOI] [PubMed] [Google Scholar]
- 212.Gu N., Gao J., Li H., Wu Y., Ma Y., Wang K. Montmorillonite-Supported with Cu2O Nanoparticles for Damage and Removal of Microcystis Aeruginosa under Visible Light. Appl. Clay Sci. 2016;132–133:79–89. doi: 10.1016/j.clay.2016.05.017. [DOI] [Google Scholar]
- 213.Yan Y., Li C., Wu H., Du J., Feng J., Zhang J., Huang L., Tan S., Shi Q. Montmorillonite-Modified Reduced Graphene Oxide Stabilizes Copper Nanoparticles and Enhances Bacterial Adsorption and Antibacterial Activity. ACS Appl. Bio Mater. 2019;2:1842–1849. doi: 10.1021/acsabm.8b00695. [DOI] [PubMed] [Google Scholar]
- 214.Özdemir G., Yapar S. Preparation and Characterization of Copper and Zinc Adsorbed Cetylpyridinium and N-Lauroylsarcosinate Intercalated Montmorillonites and Their Antibacterial Activity. Colloids Surf. B Biointerfaces. 2020;188:110791. doi: 10.1016/j.colsurfb.2020.110791. [DOI] [PubMed] [Google Scholar]
- 215.Daković A., Kragović M., Rottinghaus G.E., Ledoux D.R., Butkeraitis P., Vojislavljević D.Z., Zarić S.D., Stamenić L. Preparation and Characterization of Zinc-Exchanged Montmorillonite and Its Effectiveness as Aflatoxin B1 Adsorbent. Mater. Chem. Phys. 2012;137:213–220. doi: 10.1016/j.matchemphys.2012.09.010. [DOI] [Google Scholar]
- 216.Egirani D.E., Poyi N.R., Wessey N. Synthesis of Zinc Oxide–Montmorillonite Composite and Its Effect on the Removal of Aqueous Lead Ions. Acta Geochim. 2019;38:120–130. doi: 10.1007/s11631-018-0285-4. [DOI] [Google Scholar]
- 217.Ma Y.-L., Yang B., Xie L. Adsorptive Property of Cu2+–ZnO/Cetylpyridinium–Montmorillonite Complexes for Pathogenic Bacterium in Vitro. Colloids Surf. B Biointerfaces. 2010;79:390–396. doi: 10.1016/j.colsurfb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 218.Huang R.-H., Chao W.-K., Yu R.-S., Huang R.-T., Hsueh K.-L., Shieu F.-S. Facile Synthesis of Silver Nanoparticles by Electrochemical Method in the Presence of Sodium Montmorillonite. J. Electrochem. Soc. 2012;159:E122–E126. doi: 10.1149/2.037206jes. [DOI] [Google Scholar]
- 219.Nikitenkov N. Modern Technologies for Creating the Thin-Film Systems and Coatings. BoD–Books on Demand; London, UK: 2017. [Google Scholar]
- 220.Iconaru S.L., Groza A., Stan G.E., Predoi D., Gaiaschi S., Trusca R., Chifiriuc C.M., Marutescu L., Tite T., Stanciu G.A., et al. Preparations of Silver/Montmorillonite Biocomposite Multilayers and Their Antifungal Activity. Coatings. 2019;9:817. doi: 10.3390/coatings9120817. [DOI] [Google Scholar]
- 221.Tun P.P., Wang J., Khaing T.T., Wu X., Zhang G. Fabrication of Functionalized Plasmonic Ag Loaded Bi2O3/Montmorillonite Nanocomposites for Efficient Photocatalytic Removal of Antibiotics and Organic Dyes. J. Alloys Compd. 2020;818:152836. doi: 10.1016/j.jallcom.2019.152836. [DOI] [Google Scholar]
- 222.Malachová K., Praus P., Rybková Z., Kozák O. Antibacterial and Antifungal Activities of Silver, Copper and Zinc Montmorillonites. Appl. Clay Sci. 2011;53:642–645. doi: 10.1016/j.clay.2011.05.016. [DOI] [Google Scholar]
- 223.Kim T.N., Feng Q.L., Kim J.O., Wu J., Wang H., Chen G.C., Cui F.Z. Antimicrobial Effects of Metal Ions (Ag+, Cu2+, Zn2+) in Hydroxyapatite. J. Mater. Sci. Mater. Med. 1998;9:129–134. doi: 10.1023/A:1008811501734. [DOI] [PubMed] [Google Scholar]
- 224.Unuabonah E.I., Ugwuja C.G., Omorogie M.O., Adewuyi A., Oladoja N.A. Clays for Efficient Disinfection of Bacteria in Water. Appl. Clay Sci. 2018;151:211–223. doi: 10.1016/j.clay.2017.10.005. [DOI] [Google Scholar]
- 225.Roy A., Joshi M., Butola B.S. Antimicrobial Performance of Polyethylene Nanocomposite Monofilaments Reinforced with Metal Nanoparticles Decorated Montmorillonite. Colloids Surf. B Biointerfaces. 2019;178:87–93. doi: 10.1016/j.colsurfb.2019.02.045. [DOI] [PubMed] [Google Scholar]
- 226.Cruces E., Arancibia-Miranda N., Manquián-Cerda K., Perreault F., Bolan N., Azócar M.I., Cubillos V., Montory J., Rubio M.A., Sarkar B. Copper/Silver Bimetallic Nanoparticles Supported on Aluminosilicate Geomaterials as Antibacterial Agents. ACS Appl. Nano Mater. 2022;5:1472–1483. doi: 10.1021/acsanm.1c04031. [DOI] [Google Scholar]
- 227.Ma Y.-L., Yang B., Guo T., Xie L. Antibacterial Mechanism of Cu2+–ZnO/Cetylpyridinium–Montmorillonite in Vitro. Appl. Clay Sci. 2010;50:348–353. doi: 10.1016/j.clay.2010.08.025. [DOI] [Google Scholar]
- 228.Ma X., Pei Y., Ma Y., Pu T., Lei Y. Antibacterial Activity of Cu2+-ZnO-Modified 13X Zeolite against E.Coli and S.Aureus. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2019;34:481–486. doi: 10.1007/s11595-018-2077-z. [DOI] [Google Scholar]
- 229.Jiao L., Lin F., Cao S., Wang C., Wu H., Shu M., Hu C. Preparation, Characterization, Antimicrobial and Cytotoxicity Studies of Copper/Zinc- Loaded Montmorillonite. J. Anim. Sci. Biotechnol. 2017;8:27. doi: 10.1186/s40104-017-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Peighambardoust S.J., Zahed-Karkaj S., Peighambardoust S.H., Ebrahimi Y., Peressini D. Characterization of Carboxymethyl Cellulose-Based Active Films Incorporating Non-Modified and Ag or Cu-Modified Cloisite 30B and Montmorillonite Nanoclays. Iran. Polym. J. 2020;29:1087–1097. doi: 10.1007/s13726-020-00863-z. [DOI] [Google Scholar]
- 231.Lin Z., Fu H., Zhang Y., Deng Y., Wei F., Li H., Xu C., Hua F., Lin B. Enhanced Antibacterial Effect and Biodegradation of Coating via Dual-in-Situ Growth Based on Carboxymethyl Cellulose. Carbohydr. Polym. 2023;302:120433. doi: 10.1016/j.carbpol.2022.120433. [DOI] [PubMed] [Google Scholar]
- 232.Lin W., Ni Y., Pang J. Size Effect-Inspired Fabrication of Konjac Glucomannan/Polycaprolactone Fiber Films for Antibacterial Food Packaging. Int. J. Biol. Macromol. 2020;149:853–860. doi: 10.1016/j.ijbiomac.2020.01.242. [DOI] [PubMed] [Google Scholar]
- 233.Subha V., Ranu A., Shankar A., Kirubanandan S., Satheeshkumar E., Suresh S., Pugazhendhi A., Ilangovan R. Functionalization of Spray Coated Cellulose Nanofiber Sheet with Montmorillonite (MMT) and Silver Nanoparticles (AgNPs) to Biomedical Nanocomposite as Wound Regeneration Scaffold. Prog. Org. Coat. 2022;166:106782. doi: 10.1016/j.porgcoat.2022.106782. [DOI] [Google Scholar]
- 234.Huang X., Ge M., Wang H., Liang H., Meng N., Zhou N. Functional Modification of Polydimethylsiloxane Nanocomposite with Silver Nanoparticles-Based Montmorillonite for Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2022;642:128666. doi: 10.1016/j.colsurfa.2022.128666. [DOI] [Google Scholar]
- 235.Sadeghianmaryan A., Zarbaf D., Montazer M., MahmoudiRad M. Green Synthesis of Organo-Montmorillonite/Silver Nanocomposites on Dyed Cotton with Vat Dyes to Achieve Biocompatible Antibacterial Properties on Fashionable Clothing. J. Nat. Fibers. 2022;19:10253–10268. doi: 10.1080/15440478.2021.1993494. [DOI] [Google Scholar]
- 236.Sahithya K., Karthika K. Green Synthesis of Silver Nanoparticles Using Marine Sea Weed Acetabularia Acetabulum and Their Activity as MMT-Ag Nanocomposites towards Antifouling Applications. Res. J. Pharm. Technol. 2022;15:5397–5404. doi: 10.52711/0974-360X.2022.00910. [DOI] [Google Scholar]
- 237.Horue M., Cacicedo M.L., Fernandez M.A., Rodenak-Kladniew B., Torres Sánchez R.M., Castro G.R. Antimicrobial Activities of Bacterial Cellulose–Silver Montmorillonite Nanocomposites for Wound Healing. Mater. Sci. Eng. C. 2020;116:111152. doi: 10.1016/j.msec.2020.111152. [DOI] [PubMed] [Google Scholar]
- 238.Ogunsona E.O., Muthuraj R., Ojogbo E., Valerio O., Mekonnen T.H. Engineered Nanomaterials for Antimicrobial Applications: A Review. Appl. Mater. Today. 2020;18:100473. doi: 10.1016/j.apmt.2019.100473. [DOI] [Google Scholar]
- 239.Abbaci A., Azzouz N., Bouznit Y. A New Copper Doped Montmorillonite Modified Carbon Paste Electrode for Propineb Detection. Appl. Clay Sci. 2014;90:130–134. doi: 10.1016/j.clay.2013.12.036. [DOI] [Google Scholar]
- 240.Tepmatee P., Siriphannon P. Nanoporous Copper Doped Aluminium Pillared Montmorillonite for Dye-Containing Wastewater Treatment. Water Environ. Res. 2016;88:2015–2022. doi: 10.2175/106143015X14362865227076. [DOI] [PubMed] [Google Scholar]
- 241.İlaslan K., Tornuk F., Durak M.Z. Development of Polycaprolactone Biodegradable Films Reinforced with Silver-Doped Organoclay and Effect on the Microbiological Quality of Ground Beef Meat. J. Food Process. Preserv. 2022;46:e16862. doi: 10.1111/jfpp.16862. [DOI] [Google Scholar]
- 242.Yang Z., Liu Z.W., Allaker R.P., Reip P., Oxford J., Ahmad Z., Ren G. A Review of Nanoparticle Functionality and Toxicity on the Central Nervous System. J. R. Soc. Interface. 2010;7:S411–S422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vandebriel R.J., Jong W.H.D. A Review of Mammalian Toxicity of ZnO Nanoparticles. [(accessed on 28 April 2020)]. Available online: https://www.dovepress.com/a-review-of-mammalian-toxicity-of-zno-nanoparticles-peer-reviewed-article-NSA.
- 244.Ivask A., Juganson K., Bondarenko O., Mortimer M., Aruoja V., Kasemets K., Blinova I., Heinlaan M., Slaveykova V., Kahru A. Mechanisms of Toxic Action of Ag, ZnO and CuO Nanoparticles to Selected Ecotoxicological Test Organisms and Mammalian Cells in Vitro: A Comparative Review. Nanotoxicology. 2014;8:57–71. doi: 10.3109/17435390.2013.855831. [DOI] [PubMed] [Google Scholar]
- 245.Minetto D., Volpi Ghirardini A., Libralato G. Saltwater Ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C60 Engineered Nanoparticles: An Overview. Environ. Int. 2016;92–93:189–201. doi: 10.1016/j.envint.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 246.Ema M., Okuda H., Gamo M., Honda K. A Review of Reproductive and Developmental Toxicity of Silver Nanoparticles in Laboratory Animals. Reprod. Toxicol. 2017;67:149–164. doi: 10.1016/j.reprotox.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 247.Sardoiwala M.N., Kaundal B., Choudhury S.R. Toxic Impact of Nanomaterials on Microbes, Plants and Animals. Env. Chem. Lett. 2018;16:147–160. doi: 10.1007/s10311-017-0672-9. [DOI] [Google Scholar]
- 248.Du J., Tang J., Xu S., Ge J., Dong Y., Li H., Jin M. ZnO Nanoparticles: Recent Advances in Ecotoxicity and Risk Assessment. Drug Chem. Toxicol. 2020;43:322–333. doi: 10.1080/01480545.2018.1508218. [DOI] [PubMed] [Google Scholar]
- 249.Bai C., Tang M. Toxicological Study of Metal and Metal Oxide Nanoparticles in Zebrafish. J. Appl. Toxicol. 2020;40:37–63. doi: 10.1002/jat.3910. [DOI] [PubMed] [Google Scholar]
- 250.AshaRani P.V., Low Kah Mun G., Hande M.P., Valiyaveettil S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 251.Hackenberg S., Scherzed A., Kessler M., Hummel S., Technau A., Froelich K., Ginzkey C., Koehler C., Hagen R., Kleinsasser N. Silver Nanoparticles: Evaluation of DNA Damage, Toxicity and Functional Impairment in Human Mesenchymal Stem Cells. Toxicol. Lett. 2011;201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 252.Gliga A.R., Skoglund S., Odnevall Wallinder I., Fadeel B., Karlsson H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014;11:11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rezić I. Determination of Engineered Nanoparticles on Textiles and in Textile Wastewaters. TrAC Trends Anal. Chem. 2011;30:1159–1167. doi: 10.1016/j.trac.2011.02.017. [DOI] [Google Scholar]
- 254.Larese F.F., D’Agostin F., Crosera M., Adami G., Renzi N., Bovenzi M., Maina G. Human Skin Penetration of Silver Nanoparticles through Intact and Damaged Skin. Toxicology. 2009;255:33–37. doi: 10.1016/j.tox.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 255.Foldbjerg R., Dang D.A., Autrup H. Cytotoxicity and Genotoxicity of Silver Nanoparticles in the Human Lung Cancer Cell Line, A549. Arch. Toxicol. 2011;85:743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 256.Lal S., Perwez A., Rizvi M.A., Datta M. Design and Development of a Biocompatible Montmorillonite PLGA Nanocomposites to Evaluate in Vitro Oral Delivery of Insulin. Appl. Clay Sci. 2017;147:69–79. doi: 10.1016/j.clay.2017.06.031. [DOI] [Google Scholar]
- 257.Corrales T., Larraza I., Catalina F., Portolés T., Ramírez-Santillán C., Matesanz M., Abrusci C. In Vitro Biocompatibility and Antimicrobial Activity of Poly(ε-Caprolactone)/Montmorillonite Nanocomposites. Biomacromolecules. 2012;13:4247–4256. doi: 10.1021/bm301537g. [DOI] [PubMed] [Google Scholar]
- 258.Jiao L.F., Zhang Q.H., Wu H., Wang C.C., Cao S.T., Feng J., Hu C.H. Influences of Copper/Zinc-Loaded Montmorillonite on Growth Performance, Mineral Retention, Intestinal Morphology, Mucosa Antioxidant Capacity, and Cytokine Contents in Weaned Piglets. Biol. Trace Elem. Res. 2018;185:356–363. doi: 10.1007/s12011-018-1259-4. [DOI] [PubMed] [Google Scholar]
- 259.Zhang R., Wen C., Chen Y., Liu W., Jiang Y., Zhou Y. Zinc-Bearing Palygorskite Improves the Intestinal Development, Antioxidant Capability, Cytokines Expressions, and Microflora in Blunt Snout Bream (Megalobrama amblycephala) Aquac. Rep. 2020;16:100269. doi: 10.1016/j.aqrep.2019.100269. [DOI] [Google Scholar]
- 260.Daniel S.C.G.K., Tharmaraj V., Sironmani T.A., Pitchumani K. Toxicity and Immunological Activity of Silver Nanoparticles. Appl. Clay Sci. 2010;48:547–551. doi: 10.1016/j.clay.2010.03.001. [DOI] [Google Scholar]
- 261.Gutiérrez T.J., León I.E., Ponce A.G., Alvarez V.A. Active and PH-Sensitive Nanopackaging Based on Polymeric Anthocyanin/Natural or Organo-Modified Montmorillonite Blends: Characterization and Assessment of Cytotoxicity. Polymers. 2022;14:4881. doi: 10.3390/polym14224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gherasim O., Puiu R.A., Bîrcă A.C., Burdușel A.-C., Grumezescu A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials. 2020;10:2318. doi: 10.3390/nano10112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.