Abstract
Complement-mediated opsonization of bacteria by C3 binding is an important component of the host innate immune system. Little information is available concerning the interaction between complement proteins and capsule type 5 and 8 Staphylococcus aureus strains, even though these isolates are responsible for ∼70% of human staphylococcal infections. To investigate the importance of an intact complement pathway in an experimental staphylococcal infection, control and C3-depleted mice were challenged intravenously with 107 CFU of a serotype 5 S. aureus isolate. Whereas only 8% of the control mice succumbed to the infection, 64% of the complemented-depleted animals died. In vitro parameters of C3 binding to two heavily encapsulated (CP++) strains, three encapsulated (CP+) strains, and an isogenic capsule-negative (CP−) mutant were examined. The alternative pathway contributed 90% of C3 binding in 20% serum at 30 min, whereas it accounted for only 13% of C3 binding in 2% serum. Stationary-phase organisms bound only 10% as much C3 as mid-log-phase organisms; this was only in part due to capsule. When the S. aureus strains were cultivated on solid medium, the CP++ isolates bound 50% less C3 than CP+ strains; a CP+ strain bound 42% less C3 than the CP− mutant. Both C3b and iC3b fragments of C3 bound to S. aureus cells, and about one-third of the bound C3 was shed from the staphylococcal surface as iC3b, regardless of the CP phenotype of the strain. Thus, the phase of growth and presence of capsule are critical to opsonization.
Staphylococcus aureus is a major human pathogen, accounting for many community-acquired bacterial infections and the largest percentage of bacterial infections acquired in the hospital (5). This organism shows ever-increasing resistance to current antibiotic therapies (6). As antibiotics lose efficacy against this major bacterial pathogen, understanding the interactions between S. aureus and host defense mechanisms may ultimately prove critical in improving our ability to treat staphylococcal infections.
Two capsular serotypes (5 and 8) predominate among clinical isolates of S. aureus from humans (13, 18, 24). Isogenic, capsule-negative (CP−) mutants of encapsulated (CP+) strains are now available to more accurately evaluate the role of the capsule in the pathogenesis of staphylococcal infections (2, 3). Capsule expression has been reported to decrease phagocytic killing in vitro and to increase lethality in a mouse bacteremia model (25). In addition, CP+ strains have been shown to be more virulent than CP− mutants in animal models of arthritis, renal abscess formation, and subcutaneous abscess formation (19, 23).
For effective phagocytosis of most bacterial pathogens, opsonization of the bacterium is of major importance. Complement and antibody are the principal serum opsonins (reviewed in reference 10), and preliminary investigations have suggested that complement plays an important role in the control of S. aureus infections (15, 20, 26, 27). To date the role of complement in the control of CP+ S. aureus infections has not been addressed, and the complement-mediated opsonization of CP+ strains has not been studied in a systematic molecular fashion.
The complement system consists of an array of serum and cell surface proteins that are important components of the innate immune system. Complement peptides bind to organisms and are recognized by specific complement receptors on phagocytes that facilitate the opsonic process. The main activation pathways of the complement system are the classical pathway, which in general is antibody activated; the alternative pathway, which in general does not require antibody-mediated antigen recognition for activation; and the mannan-binding lectin (MBL) activation pathway, in which MBL binds to surface polysaccharides and then activates the complement cascade (8, 21). These pathways generate C3 convertases that cleave C3 to C3b, which may then bind to the cell surface. C3b and its immediate degradation fragment, iC3b, are the principal complement opsonins (9, 17).
In this study, we confirm the importance of complement in host defense against CP+ S. aureus bacteremia and investigate the binding of C3 fragments to CP+ strains. We evaluate C3 deposition kinetics, the pathways of complement activation, the contribution of antibody to C3 binding, the effect of the capsule on C3 binding, and the types of C3 fragments bound and released from CP+ S. aureus.
MATERIALS AND METHODS
Bacterial strains.
CP+ S. aureus includes serotype 5 strains Reynolds (13) and Lowenstein (ATCC 49521) and serotype 8 strain Wright (ATCC 49525). Heavily encapsulated (CP++) strains include S. aureus strains M (ATCC 49951; serotype 1) and Smith diffuse (ATCC 13709; serotype 2). CP− mutant JL022 (which carries a 727-bp deletion in the cap50 gene) was constructed by allelic replacement mutagenesis of strain Reynolds (23). S. aureus isolates were cultivated in Columbia medium supplemented with 2% NaCl to enhance capsule production. Fresh overnight cultures of staphylococci were inoculated into Columbia–2% NaCl broth and grown at 37°C with shaking to the mid-logarithmic phase or to stationary phase. Organisms grown on Columbia–2% NaCl agar plates were incubated for 16 h at 37°C.
Complement buffers.
Complement activation experiments were performed in buffers containing isotonic Veronal-buffered saline (VBS). All complement pathways are active in GVBS++ buffer (VBS with 0.1% gelatin, 0.15 mM CaCl2, and 1.0 mM MgCl2). All complement activation pathways are inhibited in EDTA-GVBS−− buffer (VBS with 0.1% gelatin, 0.01 M EDTA). The alternative complement pathway alone is active in Mg-EGTA-GVBS (VBS with 5 mM MgCl2 and 8 mM EGTA, pH 7.5).
Murine lethality.
Groups of 13 to 14 6-week-old female C57BL/6 mice (Charles River Labs, Wilmington, Mass.) were injected intraperitoneally on day −1 with cobra venom factor (CVF) (90 U/kg of body weight) or normal saline. All mice were injected intravenously (i.v.) with 107 CFU of S. aureus Reynolds on day 0 and monitored for 7 days. One day after bacterial challenge, the mice were reinjected with CVF or normal saline as on day −1. Mice were monitored in accordance with our Institutional Animal Care and Use Committee guidelines and sacrificed under anesthesia if in a moribund state.
C3 levels in the plasma of mice were tested by functional titration. Mouse plasma was collected using Microtainer brand tubes with EDTA (Becton Dickinson, Franklin Lakes, N.J.). Antibody-sensitized sheep erythrocytes (EA) were incubated in GVBS++ buffer with C3-deficient human serum and multiple dilutions of mouse plasma. Red cell lysis was measured by photospectroscopy at 412 nm to generate titration curves of C3 activity.
Complement and immunoglobulin sources.
Human serum was obtained from healthy volunteers and tested for normal total hemolytic complement (CH50) and normal alternative hemolytic complement levels (AH50). CH50 was calculated by measuring the hemolysis of EA in increasing concentrations of serum in GVBS++ buffer, and AH50 was calculated by measuring the hemolysis of rabbit erythrocytes in increasing concentrations of serum in Mg-EGTA-GVBS buffer. Blood samples for serum were collected in Vacutainer SST tubes and centrifuged to remove cellular blood components. Blood samples for plasma were collected in Vacutainer K3EDTA tubes to a final EDTA concentration of 4 mM. Plasma samples diluted in GVBS++ showed normal complement activity. Samples were stored at −80°C for a maximum of 3 months. Hypogammaglobulinemic serum was obtained from a patient after suitable informed consent and contained immunoglobulin A (IgA) (<7 mg/dl), IgG (<33 mg/dl), and IgM (9 mg/dl). IgG was prepared from Gamimmune N for i.v. injection (IVIg; Cutter Miles, Elkhart, Ind.). The IVIg was dialyzed overnight against VBS, filtered, and ultracentrifuged at 105 × g for 15 min prior to use.
Preparation of 125I–anti-C3 antibody and 125I-C3.
C3 binding was measured by two methods, the first using radiolabeled anti-C3 antibody to detect bound C3 fragments and the second using radiolabeled human C3 to quantitate bound C3 per bacterium. Goat anti-human C3 IgG antibody that recognizes all C3 fragments was prepared in our laboratory. The IgG was purified using caprylic acid and ammonium sulfate precipitation. The anti-C3 antibody was then iodinated using Iodobeads (Pierce Chemical Co., Rockford, Ill.) and 125I (Amersham Co., Arlington Heights, Ill.) to a specific activity of 3.3 × 105 cpm/μg. 125I-anti-C3 antibody was added to purified anti-C3 antibody in trace quantities (1:50 dilution).
Commercially available human C3 (Advanced Research Technologies Inc., San Diego, Calif.) was trace radiolabeled as described above to a specific activity of 106 cpm/μg. 125I-C3 was added to plasma at a ratio of 1:50 labeled to unlabeled molecules of C3. The S. aureus binding kinetics of 125I-C3 were the same as those of native C3, as detected by 125I-anti-C3 antibody (data not shown).
C3 binding kinetics.
S. aureus strains were grown as described above, washed with GVBS++, and suspended to a standardized concentration using photospectroscopy at 600 nm. Colony counting was performed using serial dilutions and plating to determine the total CFU in each assay. Bacteria at a concentration of ∼5 × 107 CFU/ml were incubated with normal human serum (NHS) in appropriate buffers at 37°C for specified intervals. At the end of the incubation period, the bacteria were washed in EDTA-GVBS−− to stop further complement activation before incubation in a saturating concentration of anti-C3 antibody (0.025 mg/ml) with trace quantities of 125I-anti-C3 antibody for 60 min at 25°C. After washing, counts per minute were estimated with a gamma spectrometer (Packard Cobra II, Meriden, Conn.). Specific binding was calculated by subtracting cpm/106 CFU in EDTA-GVBS−− from total cpm/106 CFU in GVBS++ or Mg-EGTA-GVBS buffer.
Determining molecules of bound C3 per bacterium.
S. aureus strains were grown as described above, and bacterial concentrations were standardized with photospectroscopy at 600 nm and CFU/ml determinations. S. aureus (∼5 × 107 CFU/ml) was added to human plasma containing trace labeled 125I-C3 diluted in standard complement buffers. The mixture was incubated at 37°C for specified intervals. After the bacteria had been washed, gamma emissions were recorded in counts per minute. From these data an absolute number of C3 fragments deposited per CFU was calculated using the following formula: [(cpm/cpm per molecule of 125I-C3) × (50 molecules of unlabeled C3/molecule of 125I-C3)]/CFU in pellet. Specific binding was calculated by subtracting C3 per CFU in EDTA-GVBS−− from total C3 per CFU in GVBS++.
Quantitation of capsule production by CP+ strains.
Staphylococci were grown as described above and suspended in 1 ml of phosphate-buffered saline. Determinations of CFU per milliliter were performed before the bacterial suspensions were autoclaved at 121°C for 45 min. The supernatants were filtered (0.45-mm pore size; Millipore Corp., Bedford, Mass.) and stored at −20°C. Rocket immunoelectrophoresis was performed in 1% agarose gels prepared in 0.05 M barbital buffer, pH 8.6, containing 0.8 to 1% CP5-specific rabbit serum (14). Purified CP5 standards and the capsule extracts were electrophoresed overnight at 20 V/cm at 4°C. The gel was washed in several changes of saline, dried, and stained with Coomassie blue. A standard curve of purified CP5 was used to estimate the amount of capsule in each sample and normalized to 1010 CFU.
C3 fragments deposited on CP+ S. aureus.
C3 fragments deposited on S. aureus were compared with those deposited on EA, a well-characterized standard. CP+ S. aureus strain Reynolds was grown in broth to the mid-logarithmic phase of growth. Sheep erythrocytes were sensitized with an optimal amount of rabbit anti-Forssman antigen antibody (EA). The bacteria were incubated with 20% NHS in GVBS++, and EA were incubated in 20% C8-deficient serum (to prevent lysis) for 30 min at 37°C. The C8 deficient serum was obtained from a human patient with congenital C8 deficiency, with appropriate informed consent. The samples were washed and incubated in 25 mM methylamine for 60 min at 37°C to release C3 fragments bound by ester bonds. Samples were centrifuged to separate the cell pellets from the supernatants. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on the supernatants and pellets dissolved in SDS buffer. Western blots were incubated with anti-C3 antibody, and the film was exposed by enhanced chemiluminescence.
C3 fragment shedding from S. aureus
125I-C3 fragments were deposited onto organisms as described above. Gamma emissions were counted for the washed, complement-coated organisms before they were reincubated in EDTA-GVBS−− for 60 min at 37°C. The supernatants were collected, gamma emissions were measured, and the percentage of C3 fragments shed from each sample was calculated. SDS-PAGE analysis was performed on the supernatants followed by autoradiography.
Quantitation of iC3 fragments deposited on S. aureus.
The results of previous studies have shown that iC3b, but not C3b, is exquisitely sensitive to cleavage by low concentrations of trypsin. Dose-response experiments with trypsin confirmed that a 2-μg/ml concentration was appropriate for cleaving iC3b from S. aureus. 125I-C3 fragments were deposited on S. aureus as described above. The staphylococci were washed, and gamma emissions were determined prior to incubation with either trypsin in Hanks buffered salt solution (HBSS) or HBSS alone for 10 min at 30°C (11). Supernatants were recovered, and gamma emissions were again measured to calculate the percentage of 125I-C3 released.
Optical densitometry.
Autoradiographs and Western blots were scanned using an Astra 2400S scanner (UMAX Technologies, Inc., Fremont, Calif.) with a transparency adapter to obtain a transilluminated image. The scanned image was rendered into a TIFF format using Adobe Photoshop (Adobe Systems Inc., San Jose, Calif.). From the TIFF formatted image of the film, calibrated optical densitometry measurements were made for area under the curve using NIH Image software (version 1.62; National Institutes of Health, Bethesda, Md.).
Statistical analysis.
Fisher's exact test (VassarStats [http://faculty.vassar.edu /∼lowry/VassarStats.html]) was used to compare lethality in the presence and absence of CVF. The unpaired two-tailed Student t test was used to compare relative quantities of C3 deposited per CFU (Microsoft Excel 98; Microsoft Co., Redmond, Wash.). Error bars indicate standard error of the sample mean (SEM) or standard deviation, as indicated, and were calculated using VassarStats.
RESULTS
Effects of complement depletion on S. aureus bacteremia in mice.
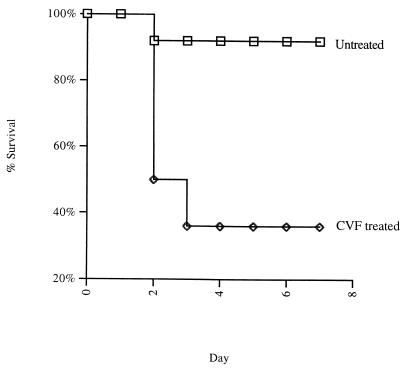
In order to test the importance of circulating complement levels in survival of CP+ S. aureus bacteremia, mice were injected with CVF to deplete them of complement 1 day prior to the experiment. After treatment with CVF, there was no detectable C3 in the mouse serum by functional titration (data not shown). As shown in Fig. 1, complement depleted mice injected i.v. with 107 CFU S. aureus Reynolds suffered 64% lethality compared to 8% lethality for untreated mice (P = 0.004).
FIG. 1.
Kaplan-Meier plot illustrating increased lethality of CP+ S. aureus Reynolds in mice after C3 depletion by injection with CVF compared with normal mice. Two experiments were performed, with a total of 14 CVF-treated mice and 13 mice not receiving CVF. All mice received 107 CFU of S. aureus Reynolds (CP5). The lethality for CVF-treated mice was 64%, compared with a lethality of 8% for mice not receiving CVF (P = 0.004).
C3 deposition relative to serum concentration.
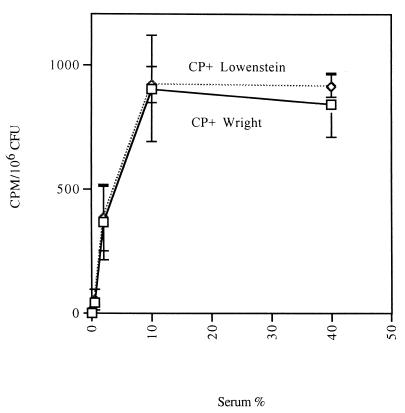
C3 deposition experiments were performed with two CP+ S. aureus strains (Lowenstein and Wright) grown to the mid-logarithmic phase of growth. The assays were performed in Veronal buffer in which all complement pathways are active. C3 deposition on both staphylococcal isolates increased to a maximum in 10% serum (Fig. 2), with no further increases seen in 40 or 95% serum (data not shown). CP++ S. aureus strains harvested in the logarithmic phase of growth also achieved maximal C3 deposition in serum concentrations of 10 to 20% (data not shown).
FIG. 2.
C3 deposition onto mid-logarithmic-phase CP+ strains Lowenstein (serotype 5) and Wright (serotype 8) as a function of increasing serum concentration. Deposited C3 molecules were detected using radiolabeled goat anti-human C3 antibody. Maximal C3 deposition on the organism was achieved in ≥10% serum. Each data point represents the mean of four independent experiments with single determinations. Error bars represent SEM.
Kinetics of C3 deposition by the classical and the alternative pathway.
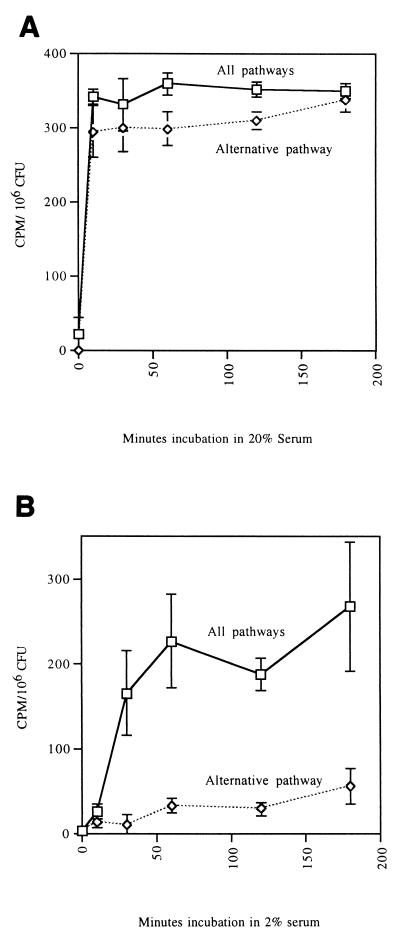
Many organisms bind complement in concentrated serum via activation of the alternative pathway. In tissue sites complement levels are likely to be limiting. Thus, we tested C3 deposition on logarithmic-phase S. aureus Lowenstein (CP+) in either 20% serum or in 2% serum. In 20% serum C3 deposition by the alternative pathway was rapid, reaching near maximal levels by 30 min (Fig. 3A). Moreover, the alternative pathway accounted for ∼90% of the total C3 binding. In 2% serum there was rapid deposition of C3 on S. aureus when all complement pathways were active (Fig. 3B), and maximal C3 deposition was achieved by 60 min. The alternative pathway contributed <25% of the total C3 deposited at 180 min. The kinetics of C3 binding to CP++ organisms was similar (data not shown).
FIG. 3.
C3 deposition on mid-log-phase CP+ strain Lowenstein over time in 20% serum (A) and 2% serum (B) for all complement pathways (GVBS++ buffer) and the alternative pathway only (Mg-EGTA-GVBS buffer). Deposited C3 molecules were detected using radiolabeled goat anti-human C3 antibody. In 20% serum the alternative pathway accounted for >90% of total C3 deposition by 30 min. In 2% serum the alternative pathway deposited C3 on the organisms relatively slowly compared to C3 deposition by all pathways. Each data point represents the mean of four independent experiments with single determinations. Error bars represent SEM.
C3 deposition in hypogammaglobulinemic serum and by the MBL pathway.
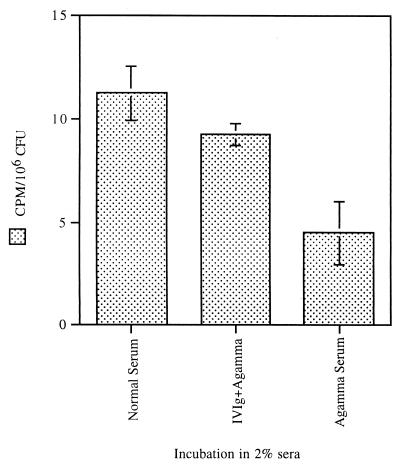
C3 deposition experiments were performed on CP+ strain Reynolds grown to the mid-logarithmic phase of growth. Levels of C3 deposited on strain Reynolds in 2% hypogammaglobulinemic serum were only ∼30% of those achieved in 2% NHS (Fig. 4). C3 deposited in hypogammaglobulinemic serum may reflect activation of both the alternative pathway and the classical pathway by trace amounts of antibody in the serum. Repletion of the IgG by adding IVIg to hypogammaglobulinemic serum restored C3 binding to 74% of the value for normal serum.
FIG. 4.
C3 deposition on mid-logarithmic-phase CP+ strain Reynolds for hypogammaglobulinemic serum and hypogammaglobulinemic serum + IVIg. Trace 125I-C3 was added to serum to detect C3 binding. One set of bacterial samples was coated with antibody by preincubation in IVIg (1 mg/ml) prior to incubation in hypogammaglobulinemic serum. In 2% hypogammaglobulinemic serum about two-thirds less C3 was deposited than in normal serum, but much of the C3 deposition was restored by precoating the bacteria with antibody contained in IVIg. The data are from a representative experiment. Error bars indicate standard deviations.
To investigate the role of MBL in this system, hypogammaglobulinemic serum was adsorbed at 0°C with mannan bound to agarose beads. Adequate removal of MBL was confirmed by Western blot analysis. The kinetics of C3 binding to strain Reynolds in mannan-adsorbed hypogammaglobulinemic serum was similar to that of nonabsorbed serum, suggesting that the MBL pathway contributed little to C3 binding.
C3 deposition relative to staphylococcal growth conditions.
C3 deposition experiments were performed on S. aureus strains cultivated under conditions likely to reflect the physiological milieu in different types of infection. CP+ strains Lowenstein and Reynolds and the isogenic CP− mutant JL022 were cultivated in well-aerated broth cultures to either the mid-logarithmic or the stationary phase of growth. In addition, the strains were cultivated on solid medium. As shown in Table 1, capsule was not detectable on either CP+ strain if the cells were harvested in the logarithmic phase of growth. Compared to logarithmic-phase cultures, strains Lowenstein and Reynolds produced >100-fold more CP when the bacteria were harvested from either stationary-phase broth cultures or from agar plates.
TABLE 1.
Capsule polysaccharide quantitation by rocket immunoelectrophoresis
| Strain | Mean polysaccharide ± SEM (μg/1010 CFU) in:
|
||
|---|---|---|---|
| Mid-log phase | Stationary phase | Agar grown cells | |
| Lowenstein | <0.17 | 32.9 ± 2.9 | 25.3 ± 4.0 |
| Reynolds | <0.03 | 11.9 ± 4.6 | 12.2 ± 2.0 |
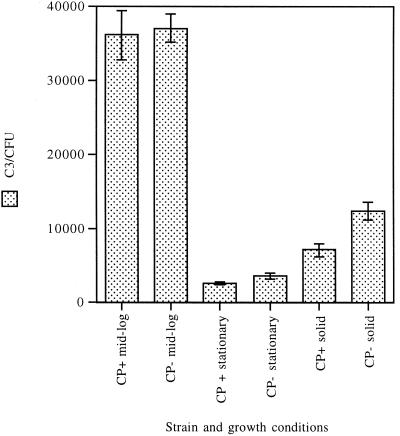
As shown in Fig. 5, strain Reynolds and the CP− mutant bound similar amounts of C3 when the strains were harvested from the mid-logarithmic-phase broth cultures. However, if the broth cultures were harvested during stationary phase, the amount of C3 bound to the bacterial cells was <10% of that deposited on logarithmic-phase cultures. Clearly, CP production alone was not responsible for this striking reduction, since C3 binding to strain Reynolds in stationary phase was only 26% less than that of mutant JL022 in stationary phase (P = 0.05). Likewise, agar-grown S. aureus cells did not bind C3 as well as logarithmic-phase cells harvested from broth cultures (Fig. 5). The results of complement deposition studies performed with agar-grown organisms indicated that capsule reduced C3 binding to S. aureus by 42% (P = 0.004).
FIG. 5.
C3 fragments deposited on CP+ strain Reynolds and CP− mutant JL022 grown in liquid media to mid-logarithmic phase or stationary phase or grown on solid medium. Trace 125I-C3 was added to 10% plasma to detect C3 binding. At least six separate determinations were made for each value. Error bars represent SEM.
C3 deposition on CP+ and CP++ S. aureus cultivated under different conditions.
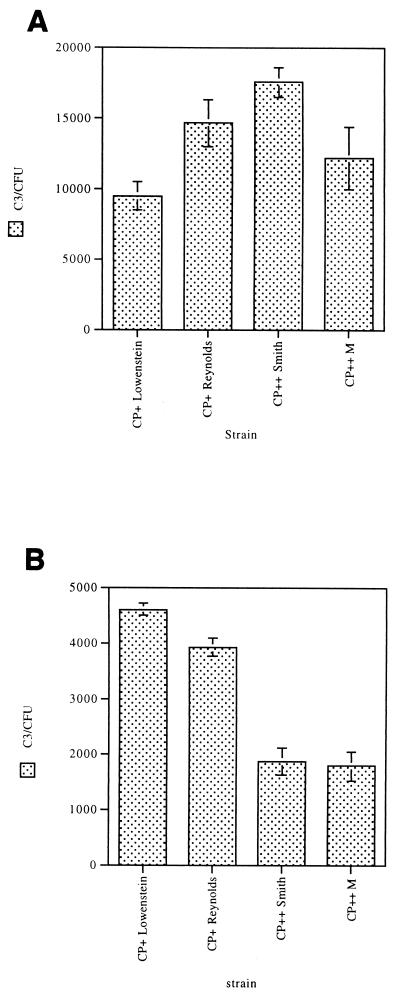
CP+ strains Lowenstein and Reynolds harvested in the mid-logarithmic phase of growth bound similar quantities of C3 fragments on their surface as CP++ strains M and Smith diffuse cultivated under the same conditions (Fig. 6A). In contrast, when the staphylococcal strains were cultivated on solid medium, average C3 binding to CP++ strains was ∼50% less than that of CP+ strains cultivated on solid medium (P < 0.001) (Fig. 6B). These results are consistent with the observation that fewer C3 molecules bind to S. aureus under growth conditions (such as solid medium) that result in more capsule expression than under growth conditions (such as logarithmic-phase broth cultures) that result in less capsule expression.
FIG. 6.
C3 deposition onto CP+ strain Lowenstein, CP+ strain Reynolds, CP++ strain Smith, and CP++ strain M grown to the mid-logarithmic-phase of growth (A) and grown on solid-media (B). Trace 125I-C3 was added to 10% serum to detect C3 binding. When grown to mid-log-phase, average C3 binding to CP+ and CP++ organisms is not significantly different (P >0.05), except between strains Smith and Lowenstein. When grown on solid media, average C3 binding to CP++ organisms is significantly less than C3 binding to CP+ organisms (P <0.001). At least three separate determinations were made for each value. Error bars represent SEM.
Forms of C3 deposited on CP+ S. aureus.
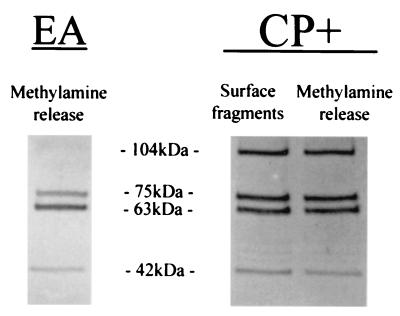
In this experiment, strain Reynolds was grown in broth to the logarithmic phase of growth. The cells were incubated with 20% NHS, and a portion of the cells were treated with methylamine to release C3 fragments bound by ester bonds. SDS-PAGE and Western blotting with polyclonal anti-C3 antibody revealed a relatively equal mixture of C3b and iC3b fragments deposited on the surface of the CP+ strain Reynolds (Fig. 7). Bound C3 fragments released by methylamine, which cleaves ester bonds, showed proportions of C3b and iC3b similar to those detectable on the CP+ surface. Nearly equal amounts of bound radiolabeled C3 fragments were released by methylamine as remained on the S. aureus surface, as determined by optical densitometry, suggesting that much of the C3 is amide bound (data not shown). This experiment was repeated for CP+ strain Lowenstein, CP− mutant JL022, and CP++ strain M with similar results (data not shown).
FIG. 7.
Deposited C3 fragments on EA or CP+ strain Reynolds that were unaffected or released by treatment with 25 mM methylamine. The samples were analyzed by SDS-PAGE and Western blotting with anti-C3 antibodies. The 104-kDa band represents the C3b α′ chain, the 75-kDa band represents the C3 β chain, the 63-kDa band represents iC3b α′1, and the 42-kDa band represents iC3b α′2. Whereas the C3 fragments bound to EA are almost all from iC3b, both C3b and iC3b forms are found bound to strain Reynolds.
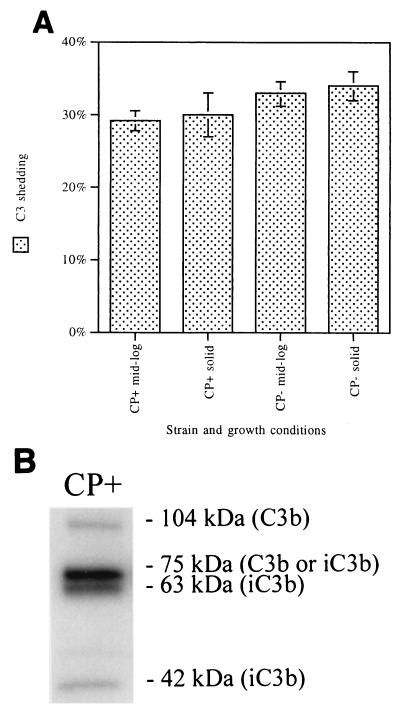
C3 shed from the S. aureus surface.
CP+ strain Reynolds and CP− mutant JL022 were cultivated either in liquid medium to the mid-logarithmic phase of growth or on solid medium. 125I-C3-coated organisms were incubated for 60 min at 37°C to allow release of C3 fragments from the bacterial cells. As shown in Fig. 8A, approximately one-third of deposited C3 fragments were shed from the S. aureus surface. Neither bacterial growth conditions nor capsule expression affected the release of C3 fragments. This experiment was repeated, except that after C3 binding and washing, the organisms were reincubated in 10% human plasma EDTA-GVBS−−. Reincubation in EDTA-plasma does not permit continued complement activation and C3 binding, but proteins in the plasma, like factors H and I, which facilitate complement degradation and release, might have acted to release remaining iC3b. The results were identical to those depicted in Fig. 8A (data not shown).
FIG. 8.
Total C3 shed (A) and C3 fragments shed (B) from opsonized CP+ strain Reynolds or CP− mutant JL022 cultivated in liquid medium to mid-log phase or on solid medium. 125I-C3-coated organisms were incubated in neutral buffer for 60 min at 30°C. Total C3 shed averaged ∼32% regardless of the CP phenotype of the S. aureus strain or the growth conditions. At least three separate determinations of each value were made. Error bars represent SEM. SDS-PAGE and autoradiography were used to detect C3 fragments shed from opsonized strain Reynolds. The 104-kDa band represents the C3b α′ chain, the 75-kDa band represents the C3 β chain, the 63-kDa band represents iC3b α′1, and the 42-kDa band represents iC3b α′2. The majority of the shed C3 fragments are from iC3b.
The dominant C3 α-chain fragment shed from CP+ strain Reynolds was a 63-kDa fragment from iC3b, termed α′1 (Fig. 8B). Optical densitometry measurements showed that the iC3b 63-kDa band represents 73% of all visible α-chain fragments released from the bacterial cells. The same C3 fragment pattern and similar densitometry measurements were found for C3 fragments shed from CP− mutant JL022 (data not shown).
Quantitation of iC3b fragments deposited on CP+ and CP− S. aureus
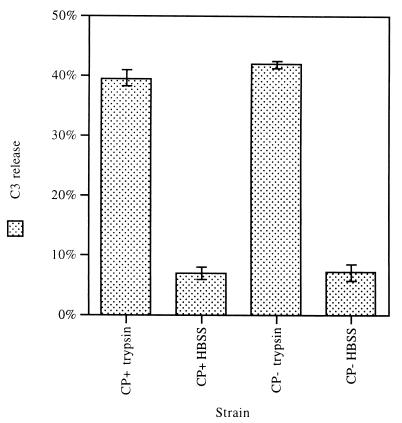
Studies of fragmentation of iC3b in other systems have shown that low concentrations of trypsin release bound iC3b preferentially over bound C3b (11). To determine how much of the bound C3 on S. aureus was in the iC3b form, CP+ strain Reynolds and CP− mutant JL022 were harvested from agar plates. 125I-C3-coated organisms were incubated in trypsin for 10 min at 30°C, and the amount of radioactivity released was measured. About 40% of the deposited 125I-C3 fragments on both CP+ and CP− S. aureus were released by trypsin, suggesting that this portion of the C3 fragments on the surface of S. aureus is the iC3b form (Fig. 9). The release of iC3b fragments by trypsin was confirmed by autoradiography, which showed that all of the α-chain product was present as a 23-kDa fragment, formed by trypsin conversion of iC3b to C3c (data not shown).
FIG. 9.
Deposited iC3b fragments released by incubating 125I-C3-coated CP+ strain Reynolds or CP− mutant JL022 (cultivated on solid medium) with trypsin (2 μg/ml) or control buffer (HBSS) for 10 min at 30°C. About 40% of deposited C3 fragments were in the iC3b form, as detected by their susceptibility to treatment with trypsin. Error bars represent SEM.
DISCUSSION
Complement is a critical element of the innate immune system, and in vitro studies support the importance of the complement system in the opsonophagocytic killing of both CP++, CP+, and CP− S. aureus strains (13, 28, 16). Verbrugh et al. (26) showed that the CP++ strain M incubated with NHS had C3 localized on the cell wall, beneath the capsular layer. The thick capsular layer was antiphagocytic because it interfered with the recognition of cell wall-bound C3b and iC3b molecules by the phagocytic cell receptors.
Little attention has been given to the details of the molecular interactions between S. aureus and complement proteins. Studies on the interaction between complement components and the clinically relevant serotype 5 and 8 strains of S. aureus have not been reported. One study examined the effects of complement depletion on staphylococcus-induced lethality in mice (7). Easmon and Glynn (7) compared the virulence of two CP++ strains, a CP+ strain (type 8), and two CP− strains in normal or complement-depleted mice that were challenged intraperitoneally. Their results indicated the C3 depletion increased mouse lethality following challenge with all of the strains except for one CP− strain. The results of our experiments expand the findings of Easmon and Glynn by challenging mice by the i.v. route with a clinically relevant S. aureus CP+ type 5 isolate. Only 8% of normal animals died from infection with CP+ strain Reynolds, whereas 64% of the complement-depleted animals succumbed to the infection.
Although the classical and alternative pathways both contribute to C3 deposition on CP+ S. aureus strains, we showed that the relative contribution of each pathway was dependent on complement protein concentrations. At a high serum concentration (20%) that may reflect that in blood, the alternative pathway was very active and accounted for 90% of the total C3 binding to S. aureus cells. At a lower serum concentration (2%) that might be present in tissues, the classical complement pathway predominated. Under the latter conditions with NHS, the classical pathway activated C3 binding to S. aureus, even in the absence of added specific antibody. IgG greatly enhanced C3 binding to a CP+ staphylococcal strain in hypogammaglobulinemic serum, confirming the importance of antibody in the classical pathway.
The results of C3 deposition experiments comparing the CP+ strain with its isogenic CP− mutant were dependent on the culture conditions under which the staphylococci were grown. C3 binding to S. aureus was evaluated under different bacterial growth conditions to reflect different disease processes. Mid-logarithmic-phase staphylococcal cultures may emulate organisms growing in the bloodstream, whereas organisms growing on solid medium may more closely mimic organisms growing on a surface, like a damaged heart valve or an intravascular catheter. For broth-grown S. aureus harvested in the mid-logarithmic phase of growth, the amounts of C3 bound to the CP+ and CP− isogenic pairs of organisms were identical. This is consistent with the observation that organisms in the logarithmic phase of growth do not produce detectable levels of CP5 (22; this study). Like other agr-regulated staphylococcal virulence factors, S. aureus CP is maximally expressed in post-exponential-phase cultures (22). When the CP+ strain Reynolds was cultivated under conditions of maximal capsule production (stationary-phase broth cultures or on agar plates), C3 deposition was reduced by ∼90%. Thus, an organism that may be adequately opsonized in bloodstream log-phase growth may not be adequately opsonized on a heart valve or catheter when in stationary phase. However, C3 deposition on the CP− mutant JL022 was also diminished markedly when the strain was grown to stationary phase or on solid medium. Thus, there are bacterial factors in addition to capsule that decrease C3 binding to stationary-phase staphylococci. The nature of these growth phase-dependent factors is unknown at the present time. In a comparison of bacterial strains grown on solid media, CP++ strains bound 50% fewer C3 molecules than did CP+ strains, and CP+ strains bound 42% fewer C3 molecules than CP− strains. These results suggest that C3 deposition also diminishes as capsule thickness increases.
The C3 binding data for S. aureus cultivated on solid medium are consistent with results obtained with capsule type 7 Streptococcus pneumoniae, in which increased capsule production correlated with decreased C3 binding (4). Previously reported studies, in which S. aureus was cultivated on solid media, revealed that the CP+ strain Reynolds showed increased lethality for mice in vivo compared to a CP− mutant strain (25). Similarly, strain Reynolds sustained a higher level of bacteremia in infected mice and was cleared from the bloodstream less readily than a CP− mutant strain (25). CP+ S. aureus strains have been shown to be susceptible to phagocytic killing only in the presence of specific capsular antibodies and complement, whereas CP− S. aureus strains were opsonized for phagocytosis by nonimmune serum with complement activity (16, 19, 25).
Studies by Gordon et al. (12) with S. aureus strain ATCC 25923 found significant amounts of C3b and iC3b bound to the surface as well as moderate amounts of C3d. We found that C3 fragments bound to the surface of CP+ S. aureus strains are a mixture of C3b and iC3b but did not find any C3d. There are receptors for these two forms of C3 on leukocytes, and both promote opsonophagocytosis (1, 4). A large proportion of the bound C3 fragments was shed from the surface of both CP+ and CP− organisms, predominantly in the iC3b form.
The ease of cleaving iC3b on the organisms with minute concentrations of trypsin suggests that iC3b on the surface of S. aureus is as exquisitely sensitive to cleavage as it is on the surface of particles such as sheep red blood cells. We speculate that in an abscess containing proteolytic enzymes released from dying neutrophils, the iC3b may be cleaved and shed from the organism surface to an even greater extent, further decreasing the susceptibility of S. aureus to phagocytosis.
In summary, we show in this report that complement is important in host immune defense against acute staphylococcal infection provoked by a clinically relevant CP+ S. aureus isolate. Both C3b and iC3b are deposited in approximately equal amounts on the surface of CP+ and CP− staphylococci. CP+ strains partially inhibit complement-mediated opsonization by diminished C3 binding and subsequent shedding of the C3 fragments from the bacterial surface. With the use of isogenic CP+ and CP− mutants, we show that decreased C3 binding is capsule dependent, whereas C3 shedding is not. Stationary-phase organisms had <10% binding of C3 and may be poorly opsonized and thus poorly phagocytosed in NHS. Further studies will examine whether these mechanisms are an important means of host defense evasion for S. aureus.
ACKNOWLEDGMENT
This work was in part supported by Public Health Service grant AI29040 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahern J M, Rosengard A M. Complement receptors. In: Volanakis J E, Frank M M, editors. The human complement system in health and disease. New York, N.Y: Marcel Dekker; 1998. pp. 167–202. [Google Scholar]
- 2.Albus A, Arbeit R D, Lee J C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule expression. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992;165:749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- 4.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National Nosocomial Infection Surveillance System report: data summary from October 1986–April 1996. U.S. Atlanta, Ga: Department of Health and Human Services; 1996. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—Illinois, 1999. Morb Mortal Wkly Rep. 2000;48:1165–1167. [PubMed] [Google Scholar]
- 7.Easmon C S, Glynn A A. Comparison of subcutaneous and intraperitoneal staphylococcal infections in normal and complement-deficient mice. Infect Immun. 1976;13:399–406. doi: 10.1128/iai.13.2.399-406.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz R A. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 9.Frank M M. The complement system. In: Frank M M, editor. Samter's immunologic diseases. Boston, Mass: Little, Brown and Co.; 1995. pp. 331–353. [Google Scholar]
- 10.Frank M M, Fries L F. Bailliere's clinical immunology and allergy. Vol. 2. Philadelphia, Pa: Bailliere Tindall; 1998. The role of complement in defense against bacterial disease; pp. 335–361. [Google Scholar]
- 11.Gaither T A, Hammer C H, Frank M M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of β1H and C3b inactivator (C3bINA) J Immunol. 1979;123:1195–1204. [PubMed] [Google Scholar]
- 12.Gordon D L, Rice J, Finlay-Jones J J, McDonald P J, Hostetter M K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988;157:697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- 13.Karakawa W W, Vann W F. Capsular polysaccharides of Staphylococcus aureus. In: Robbins J B, Hill J C, Sadoff J C, editors. Seminars in infectious diseases. IV. Bacterial vaccines. New York, N.Y: Thieme-Stratton Inc.; 1982. pp. 285–293. [Google Scholar]
- 14.Lee J C, Takeda S, Livolsi P J, Paoletti L C. Effect of in vitro and in vivo growth conditions in expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leijh P C, van den Barselaar M T, Daha M R, van Furth R. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun. 1981;33:714–724. doi: 10.1128/iai.33.3.714-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan B P. Physiology and pathophysiology of complement: progress and trends. Crit Rev Clin Lab Sci. 1995;32:265–298. doi: 10.3109/10408369509084686. [DOI] [PubMed] [Google Scholar]
- 18.Na'was T, Hawwari A, Hendrix E, Hebden J, Edelman R, Martin M, Campbell W, Naso R, Fattom A I. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J Clin Microbiol. 1998;36:414–420. doi: 10.1128/jcm.36.2.414-420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson I M, Lee J C, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson P K, Wilkinson B J, Kim Y, Schmeling D, Quie P G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978;19:943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petry F, Loos M. Bacteria and complement. In: Volanakis J E, Frank M M, editors. The human complement system in health and disease. New York, N.Y: Marcel Dekker; 1998. pp. 375–392. [Google Scholar]
- 22.Pohlmann-Dietze P M, Ulrich M, Kiser K B, Doring G, Lee J C, Fournier J M, Botzenhart K, Wolz C. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect Immun. 2000;68:4865–4871. doi: 10.1128/iai.68.9.4865-4871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portoles M, Kiser K B, Bhasin N, Chan K H N, Lee L C. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect Immun. 2001;69:917–923. doi: 10.1128/IAI.69.2.917-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sompolinsky D, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakker M, Park J S, Carey V, Lee J C. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–5189. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbrugh H A, Peterson P K, Nguyen B-Y T, Sisson S P, Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982;129:1681–1687. [PubMed] [Google Scholar]
- 27.Verhoef J, Peterson P K, Kim Y, Sabatu L D, Quie P G. Opsonic requirements for staphylococcal phagocytosis: heterogeneity among strains. Immunology. 1977;33:191–197. [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S, Arbeit R D, Lee J C. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1992;60:1358–1362. doi: 10.1128/iai.60.4.1358-1362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]