Abstract
Coffee is one of the most widely consumed beverages, which has several effects on the human body. In particular, current evidence suggests that coffee consumption is associated with a reduced risk of inflammation, various types of cancers, and certain neurodegenerative diseases. Among the various constituents of coffee, phenolic phytochemicals, more specifically chlorogenic acids, are the most abundant, and there have been many attempts to utilize coffee chlorogenic acid for cancer prevention and therapy. Due to its beneficial biological effect on the human body, coffee is regarded as a functional food. In this review article, we summarize the recent advances and knowledge on the association of phytochemicals contained in coffee as nutraceuticals, with a particular focus on phenolic compounds, their intake, and nutritional biomarkers, with the reduction of disease risk, including inflammation, cancer, and neurological diseases.
Keywords: polyphenol, chlorogenic acid, inflammation, cancer progression, neurodegenerative diseases, cell membrane
1. Introduction
Coffee is one of the most widely consumed beverages globally [1]. Early records suggest that coffee was first discovered and consumed in Ethiopia, North Africa, in the 9th century AD [2]. Coffee plants belong to the genus Coffea, among which Coffea arabica and Coffea canephora var. Robusta, also known as Arabica and Robusta coffee, respectively, are the most well-known species [3]. Coffee beans are roasted, ground, and infused in hot water to produce a cup of coffee. In addition to its pleasantly bitter flavor, coffee has several effects on the human body and mind [4].
Coffee is popularly consumed as a caffeine-containing beverage (CCO) and has potential health benefits and risks based on the food-based dietary guidelines of the Food and Agriculture Organization (FAO) of the United Nations [5]. Epidemiological studies have demonstrated that coffee consumption reduces the risk of neurological diseases, including Alzheimer’s disease and Parkinson’s disease [6,7,8]. For example, coffee consumption ameliorated cognitive impairment induced by Alzheimer’s disease [7]. Another report showed a negative association between moderate consumption of coffee and the risk of age-related cognitive disorders and Parkinson’s disease [8]. Coffee consumption has been also linked to potential health benefits as a consequence of their chemopreventive and anti-inflammatory effects. It has been suggested that the reduction of inflammation under administration of coffee is attributed to the antioxidative features of certain ingredients of coffee [9]. Furthermore, current evidence suggests that coffee consumption is associated with a reduced risk of liver, kidney, and to a lesser extent, premenopausal breast and colorectal cancers, while it is unrelated to prostate, pancreas, and ovary cancers [10]. Although there are several plausible biologic mechanisms whereby coffee consumption might influence the risk of breast cancer, epidemiologic evidence is limited [11]. Meanwhile, Nkondjock et al. assessed the association between coffee consumption and breast cancer risk among high-risk women carrying breast cancer susceptibility genes (BRCA) mutations, and the results suggested that coffee is not only unlikely to be harmful but also high levels of coffee consumption may be linked to reduced breast cancer risk [12]. Many studies have demonstrated the relationship between coffee consumption and cancer risk [13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Therefore, in the present review, we summarize the recent advances and knowledge on the associations of phytochemicals present in coffee as nutraceuticals, particularly focusing on phenolic compounds in coffee, their intake, and nutritional biomarkers with reduction of disease risk including inflammation, cancer, and neurological diseases.
2. Chemical Ingredients of Coffee
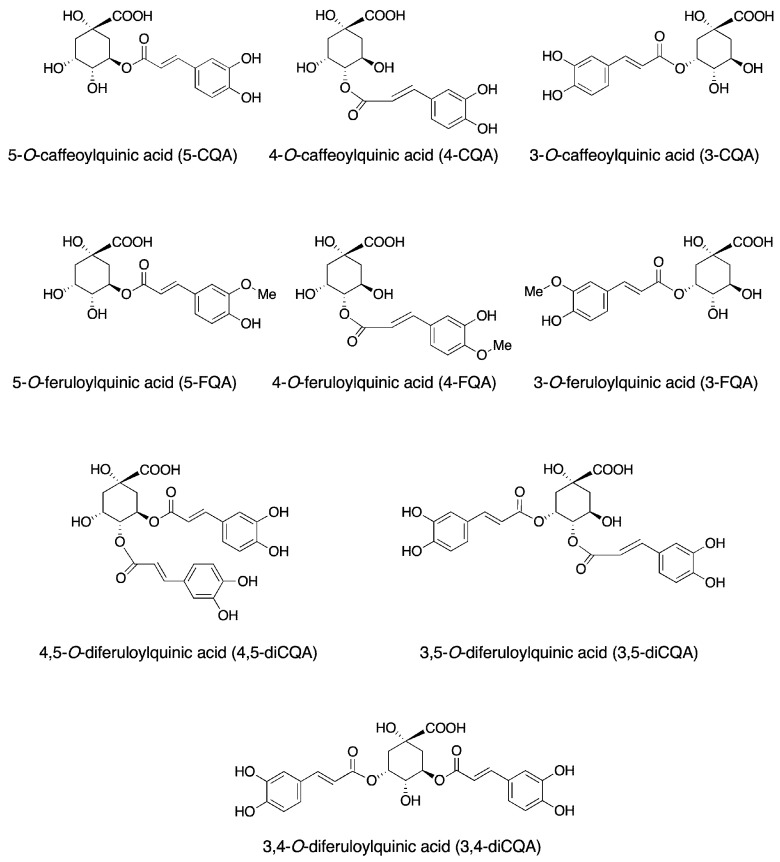
Coffee is a major dietary source of purine alkaloid caffeine (1,3,7-trimethylxanthine; 1,3,7-trimethyl-1H-purine-2,6-(3H,7H)-dione), which exerts various effects via the A1 and A2 adenosine receptor subtypes, effectively stimulating the sympathetic nervous system. However, caffeine intake also has negative effects although the amount of caffeine in a cup of coffee is influenced by the method of coffee preparation such as boiled coffee, filtered coffee, and espresso, and the half-life of caffeine in the human body is approximately 4–6 h [9]. Besides caffeine, coffee is a rich source of various phytochemicals naturally present as secondary plant metabolites. Coffee is abundant in several phenolic compounds, among which are chlorogenic acid and caffeic acid, lactones, diterpenes including cafestol and kahweol, and the niacin (vitamin B3) precursor trigonelline [27,28]. Particularly, green coffee contains various kinds of phenolic compounds accounting for 6–10% of its dry weight [5]. Chlorogenic acid, a type of acyl-quinic acid, i.e., a family of 1L-(−)-quinic acid esters combined with C6−C3 trans-hydroxycinnamic acid, is the major phenolic compound in coffee [9], which comprises three groups of chemical compounds, namely caffeoylquinic acids, feruloylquinic acids, and dicaffeoylquinic acids (Figure 1). The main components of the coffee polyphenols are caffeoylquinic acids. Coffee caffeoylquinic acids comprise three chemical compound isomers, and the most common form of chlorogenic acid is 5-O-caffeoylquinic acid, which is often called chlorogenic acid [29,30]; chlorogenic acid (5-O-caffeoylquinic acid; 5-CQA), neo-chlorogenic acid (3-O-caffeoylquinic acid; 3-CQA), and crypto-chlorogenic acid (4-O-caffeoylquinic acid; 4-CQA) (Figure 1). The chlorogenic acids found in green coffee beans contain 3-, 4-, and 5-caffeoylquinic acids and 3,4-, 3,5- and 4,5-caffeoylquinic acids, collectively referred to as total caffeoylquinic acids, and the composition depends on the type of coffee. They also contain 3-, 4-, and 5-feruloyl quinic acids and traces of at least one caffeoyl-feruloyl quinic acid, also known as total feruloyl quinic acids [31,32]. The chemical structures are shown in Figure 1. Among these chlorogenic acids, 5-CQA is the most abundant in coffee beans, accounting for approximately 50% of the total chlorogenic acids [33,34,35]. There are many attempts to utilize coffee chlorogenic acid for cancer prevention and therapy [36].
Figure 1.
The chemical structure of chlorogenic acids.
3. The Metabolism of Chlorogenic Acids
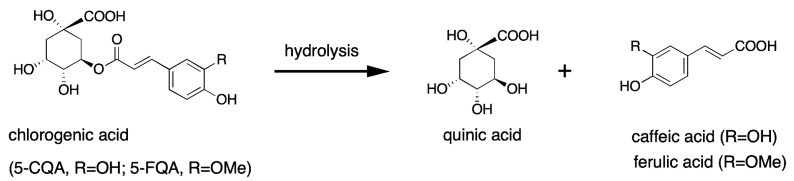
The biosynthesis of chlorogenic acids in humans is mainly mediated by three key enzymes, namely phenylalanine ammonia-lyase, shikimic acid/quinic acid hydroxyl cinnamyl transferase, and quinic acid cinnamate hydroxyltransferase [37]. The first enzyme, phenylalanine ammonia-lyase, acts as a rate-limiting enzyme of the chlorogenic acid biosynthetic pathway and catalyzes the dissociation reaction of an ammonia molecule from an l-phenylalanine to produce a trans-cinnamic acid. The second enzyme, quinic acid hydroxyl cinnamyl transferase, catalyzes the formation of p-coumaryl-quinic acid/shikimic acid, while quinic acid cinnamate hydroxyltransferase catalyzes the transesterification of cafeyl-CoA and quinic acid to generate chlorogenic acid. Dietary chlorogenic acids are hydrolyzed into quinic and caffeic or ferulic acid, and then further metabolized in the small intestine and colon before entering the bloodstream [37] (Figure 2). Caffeic acid, e.g., 3, 4-dihydroxycinnamic acid, is converted by the enzyme catechol-O-methyltransferase to another phenolic acid, ferulic acid. Both compounds may form an ester bond with quinic acid, and generate any of the many isomers included in the family of chlorogenic acids. Nonetheless, the most frequent isomer is the 5-O-caffeoylquinic acid that, because of that, is commonly called chlorogenic acid.
Figure 2.
The metabolism of chlorogenic acids.
4. Anti-Inflammatory Activity of Chlorogenic Acids
Reactive oxygen species (ROS) cause oxidative stress that contributes to the pathogenesis of various diseases, including inflammation, cancer, and neurodegenerative diseases [38,39]. ROS, including radical and non-radical derivatives, such as superoxide anions, hydroxyl radicals, and hydrogen peroxide, mainly derived from oxidative metabolism during inflammatory reactions [38], are generated by redox reactions during cellular metabolism. However, excess production of ROS can cause oxidative damage to essential molecules such as proteins, lipids, and DNAs [39]. Chlorogenic acids exert their antioxidant effect via their polyhydroxyl structure that directly scavenges free radicals and regulates the activity of the endogenous oxidase system and its associated proteins [40,41]. This natural antioxidant property depends on the chlorogenic acid’s unique molecular structure, which contains several active hydroxyl groups and one carboxyl group. Of these, the phenolic hydroxyl structure readily reacts with free radicals and forms hydrogen free radicals, which eliminate hydroxyl radicals and superoxide anions and exhibit a strong antioxidant effect [37,42]. Thus, under some circumstances, coffee might contribute to the endogenous systems which prevent oxidative damage to cell components, DNA, proteins, and lipids, which contribute to the pathogenesis of inflammation, cancer and neurodegenerative diseases [43]. Consequently, the consumption of instant coffee, which contains increased levels of chlorogenic acids, enables protection against oxidative damage in healthy adults [44].
Chlorogenic acids can eliminate superoxide anions and hydroxyl radicals through their antioxidant activities, rendering coffee an effective dietary antioxidant source due to its high chlorogenic acid content [45]. Chlorogenic acids directly act on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway and control the expression of both pro- and anti-inflammatory factors [46,47]. Studies have shown that chlorogenic acids can inhibit interleukin-8 (IL-8) production in human intestinal Caco-2 cells, induced by combined stimulation with tumor necrosis factor-alpha (TNF-α) and H2O2 [48]. IL-8 is a cytokine similar to platelet factor 4, with a chemoattractive activity. IL-8 is produced by phagocytes and mesenchymal cells exposed to inflammatory stimuli and activates neutrophils inducing chemotaxis. These results suggest that dietary chlorogenic acids might prevent intestinal inflammation [48]. Vascular endothelial cells exhibit upregulation of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which enable recruitment of immune cells, including monocytes, to the inflammation site. ICAM-1 and VCAM-1 are the members of the immunoglobulin superfamily of transmembrane adhesion molecules with an amino-terminus extracellular domain, a single transmembrane domain, and a carboxy-terminus cytoplasmic domain. VCAM-1 binds to its ligand, very late antigen-4 (VLA-4). Furthermore, chlorogenic acid attenuates the enhanced expression of ICAM-1 and VCAM-1 induced by interleukin-1β, highlighting its anti-inflammatory activities [49]. The recruitment of neutrophils to the site of inflammation is a typical process during inflammation, and the interactions of the circulating neutrophils with the vascular endothelial cells is the initial step of neutrophil recruitment, which is mediated by certain adhesion molecules expressed on the surface of neutrophils. In this context, an adhesion molecule CD62L, also called L-selectin, plays a pivotal role in cell–cell interactions. CD62L is a member of the carbohydrate-binding selectin family of cell adhesion molecules and forms a type-I transmembrane protein comprising an N-terminal lectin domain, an epidermal growth factor (EGF)- like domain, two short consensus repeats, a transmembrane region, and a short cytoplasmic domain. CD62L is expressed in leukocytes and located at the cell surface, which plays a pivotal role in multistep cell-cell adhesion interactions. A previous study reported that chlorogenic acid could attenuate the lipopolysaccharide-induced CD62L proteolytic processing of neutrophils and decrease the adhesion and chemotaxis of neutrophils to vascular endothelial cells [50]. Platelets are also key mediators of inflammation and platelet–endothelial cell interactions at the site of lesion trigger inflammatory responses. Upon platelet activation, the CD62P adhesion molecule, also called P-selectin, translocates from the Weibel–Palade bodies inside the cell body into the plasma membrane, where fibrinogen causes platelet aggregation. In addition, chlorogenic acid inhibits the expression of CD62P in human platelets and impair platelet–leukocyte interactions [51].
5. Anti-Cancer Activity of Chlorogenic Acids
Numerous epidemiological studies have indicated that coffee consumption might lower the risk of certain types of cancer. For instance, coffee consumption has been found to strongly and consistently reduce the risk of endometrial and hepatocellular cancer [10], and a modest or borderline negative association with breast and colorectal cancer has been reported. Contrastingly, no association was found with pancreatic, ovarian, prostate, or gastric cancer [10]. The epidemiologic evidence of coffee on each type of cancer is summarized in the literature [52].
One of the eight distinct hallmarks of cancer involves the acquired capability for sustaining proliferative signaling [53,54]. The first step of cancer progression toward poorly differentiated carcinomas is dedifferentiation which is not initially associated with increased proliferation. A study showed that chlorogenic acids could inhibit the proliferation of A549 human lung cancer cells in vitro by inhibiting activator protein-1, NF-κB, and mitogen-activated protein kinases (MAP kinases) [55]. NF-κB plays critical roles in inflammation, cell proliferation, differentiation, and survival. MAP kinases have three main families, extracellular-signal-regulated kinases (ERKs), jun amino-terminal kinases (JNKs), and p38/stress-activated protein kinases (SAPKs). These respond primarily to growth factors and mitogens to induce cell growth and differentiation. This suggests that the consumption of chlorogenic acids through coffee might prevent cancer [56,57].
In addition, matrix metalloproteinases (MMPs) are essential enzymes employed by tumor cells during metastasis that degrade proteins and regulate various cell behaviors [58,59]. These proteolytic enzymes are prevalent in cancer biology due to their capacity to promote cancer-cell growth, differentiation, apoptosis, migration, and invasion while they also regulate tumor angiogenesis and immune surveillance [58]. The MMPs belong to a family of zinc-dependent endopeptidases with more than 20 different members, and play pivotal roles in the degradation of the extracellular matrix which is composed of collagens, fibronectins, and laminins, which help maintain homeostasis. Based on their sub-cellular distribution and specificity for components of the extracellular matrix, the MMPs are divided into collagenases, gelatinases, stromelysins, matrilysins, and membrane-type matrix metalloproteases: MMPs that belong to collagenases are MMP-1, MMP-8, MMP-13, and MMP-18, which degrade triple-helical fibrillar collagen; MMPs that belong to gelatinases are MMP-2 and MMP-9; stromelysins include MMP-3 and MMP-10; MMP-11, and MMP-7 and MMP-26 are matrilysins. MMPs are inhibited by endogenous protein regulators, namely, the tissue inhibitors of MMPs (TIMPs). Most MMPs have consistently increased gene expression across cancers, and MMP1, MMP9, MMP10, MMP11, and MMP13 are almost universally upregulated across a wide variety types of cancers [59]. Of all MMPs, MMP-9 is the most essential for cancer-cell invasion and tumor metastasis [60]. Chlorogenic acids have been shown to inhibit MMP-9 activity in cultured hepatoma cells, indicating a possible cancer chemoprevention mechanism [61]. Furthermore, MMP-2, also called gelatinase A, also plays a significant role in ECM degradation because it can degrade collagen type IV during cancer progression, allowing cancer cells to migrate from the primary tumor to form metastasis [62]. However, chlorogenic acids have been found to inhibit cell migration and MMP-2 secretion of human glioma cells, highlighting their anti-cancer effects [63].
The signaling pathway comprising phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR)/phosphatase and tensin homologue deleted on chromosome ten (PTEN) plays a pivotal role in cancer progression, including cell proliferation and migratory activities [64]. Particularly, PI3K has been shown to induce the expression of the multidrug resistance-associated protein, suggesting that high PI3K activity facilitates drug resistance [64]. Therefore, the PI3K/AKT/mTOR/PTEN axis is an attractive target for targeted molecular therapy including cancer [65,66,67,68,69,70,71]. Moreover, germline mutations in the breast cancer susceptibility gene 1 (BRCA1) considerably increase the risk of breast and ovarian cancers and, thus, modulation of PTEN/BRCA1 proteins may prove therapeutically beneficial for breast, ovarian, and prostate cancer treatment [72]. Chlorogenic acids have a biological activity to modulate signal transduction through the PI3K/AKT/PTEN pathway, thereby suppressing cancer progression. Chlorogenic acid potentiated the apoptotic effect of certain anti-cancer agents via activation of apoptosis-related molecules, namely Bcl-2-associated X protein (Bax) and Caspase 3/7, and inhibition of anti-apoptotic molecules, namely B-cell/CLL lymphoma-2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xL), by modulating the PI3K/Akt signaling pathway [73]. Moreover, chlorogenic acid could selectively suppress the proliferation of human kidney cells by modulating the PI3K/Akt/mTORC signaling pathway [74].
Wnt signaling is another signaling pathway that plays an important role in cancer-cell signaling. The Wnt signal is transferred into the cytosol and thereby further transduced to the cell nucleus via the β-catenin–T-cell factor and lymphoid enhancer factor (TCF/LEF) complex to enhance the expression of targets, including Myc and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5). The Wnt pathway is involved in cell polarity formation and cell migration, and chlorogenic acid has been found to modulate the Wnt pathway in cancer cells, including colon cancer cells [75]. More specifically, chlorogenic acid has been confirmed to decrease the viability and migratory properties of colorectal cancer cells [76]. As summarized in Table 1, many other reports show that chlorogenic acid exhibits anti-cancer effects by inhibiting cell viability and migratory abilities [76,77], invasion with Akt [78], and ERK [79] inhibition. Chlorogenic acid affects apoptosis by acting on p53, p21, JNK, and nuclear factor erythroid 2-related factor 2 (Nrf2) molecules and also by regulating microRNA expression [80,81,82,83,84]. Furthermore, the combination of multiple phytochemicals is recently emerging as a promising cancer treatment therapy [85]. Chlorogenic acid in combination with cinnamaldehyde and arctigenin exhibited synergistic effects by increasing the number of pathways and systems that can be targeted at once [86].
Table 1.
The biological effects of CFGAs and the underlying molecular mechanisms.
| Effect | Mechanism | Cell | Reference |
|---|---|---|---|
| Anti-inflammatory effects | |||
| Adhesion molecule | ICAM-1, VCAM-1 | vascular endothelial cells | [48] |
| Chemotaxis | CD62L | neutrophil | [49] |
| Leukocyte rolling | CD62P | platelet | [50] |
| Anti-cancer effects | |||
| Proliferation | hepatoma | [56] | |
| Invasion | hepatoma | [57] | |
| MMP activity | MMP-9 | hepatoma | [61] |
| MMP activity | MMP-2 | glioma | [63] |
| Proliferation | PI3K/Akt/mTORC | hepatocellular carcinoma | [73] |
| Apoptosis | PI3K/Akt/mTORC | kidney cancer | [74] |
| Signaling | Wnt/β-catenin | colon cancer | [75] |
| Viability, migration | colorectal cancer | [76] | |
| Migration | DDR1 | ovarian cancer | [77] |
| Invasion | Akt | squamous cell carcinoma | [78] |
| Invasion | ERK, MMP-2/9 | hepatic cancer | [79] |
| Apoptosis | p53 | breast cancer | [80] |
| Apoptosis | p21 | breast cancer | [81] |
| Apoptosis | JNK | lung cancer | [82] |
| Apoptosis | Nrf2 | hepatocellular carcinoma | [83] |
| Carcinogenesis | mi-21a-5p | colon cancer | [84] |
| Neuroprotective effects | |||
| Glutamine release | c-Src | microglia | [87] |
| Glutamine release | neuron | [88] | |
| Cell viability | neuron | [89,90] | |
| Neurodegeneration | amyloid-β | neuron | [91] |
| Brain aging suppression | CREB | microglia | [92] |
6. Chlorogenic Acid and Neurological Diseases
Chlorogenic acid increases the levels of cyclic adenosine monophosphate (cAMP)-responsive element binding protein (CREB) in the hippocampus and suppresses inflammation in the old brain, facilitating a preventive effect against brain aging [92]. Alzheimer’s disease is the most common progressive neurodegenerative disorder associated with aging. The pathology of this disease is characterized by an earlier accumulation of extracellular amyloid-beta plaques and intracellular neurofibrillary tangles in the hippocampus, eventually leading to severe neurodegeneration, and cognitive and synaptic impairment over time [7]. Additionally, hyperphosphorylation of tau, neuronal inflammation, oxidative stress, and cellular apoptosis can contribute to the pathogenesis of Alzheimer’s disease [7]. Studies have shown that oxidative stress represents a major risk factor associated with the pathology of dementia [70]. More specifically, substantial evidence has confirmed that oxidative stress is associated with neuronal apoptosis and brain dysfunction in Alzheimer’s disease [70]. Due to the absence of actual treatments, brain dysfunction in Alzheimer’s disease is a prevalent public health anxiety. Therefore, a number of preventive factors have been proposed by epidemiological research, including modifiable lifestyle factors, such as healthy dietary habits. In fact, it has been revealed that dietary choices can play a certain role in neuroprotection against the Alzheimer’s disease [93]. However, the relationship between nutrient consumption and neuroprotection is fairly complex. In addition, the convolution of the human diet makes it difficult to examine its distinct effects. Although many lifestyle factors affect brain function, food-related involvements might be a promising strategy in preventing brain dysfunction [93]. Consumption of Arabian coffee containing moderate caffeine seems to ameliorate Alzheimer’s disease-induced cognitive impairment by decreasing amyloid-beta levels [7]. It is also believed that antioxidant nutraceuticals such as coffee phenolic compounds may have beneficial effects in the prevention of Alzheimer’s disease [94].
Parkinson’s disease is a brain disorder that is characterized by neuropsychiatric symptoms such as depression and anxiety preceding the onset of motor symptoms [95]. The major features of this disease include loss of dopaminergic neurons in the substantia nigra and Lewy body depositions [95]. It has been suggested that mitochondrial dysfunction, oxidative stress, and oxidative damage underlie the pathogenesis of Parkinson’s disease [95]. The activity of substantia nigra dopaminergic neurons is critical for striatal synaptic plasticity and associative learning, and degeneration of dopaminergic neurons leads to a disinhibition of the subthalamic nucleus, which in turn increases excitatory projections to the substantia nigra [95]. Environmental exposures to toxic mediators such as ROS may lead to the development of neurodegenerative disorders with similar clinical findings to Parkinson’s disease [95]. Consequently, it is critical to develop strategies to ensure that healthy neurons remain alive following ROS attack without using intricate medications [95]. Epidemiological studies have demonstrated that coffee consumption reduces the risk of Parkinson’s disease, in both case–control and cohort studies, yielding a 33% reduction in risk [96].
7. Membrane-Modulating Activity of Chlorogenic Acids
Recently, phenolic compounds have been found to exert modulatory effects in cells through selective action on multiple cell-signaling pathways involved in pathogenesis of degenerative diseases, indicating that the health effects go beyond simple antioxidant activity [43]. The cell membrane of human cells is a mixture of proteins and lipids and forms the boundary between the intracellular compartment and cellular space. With regards to the biological action of chlorogenic acid, current literature demonstrates that chlorogenic acid can alter the biological characteristics of basophil granulocytes by affecting the fluidity of the cell membrane and triggering pseudoallergic reactions [97]. In that study, the authors proposed a mechanism where chlorogenic acid may lead to the aggregation of membrane rafts on the cell membrane surface by altering the fluidity of the cell membrane, thus triggering Syk-related signal transduction and inducing a truncated type I such as an allergic reaction [97]. Another study showed that chlorogenic acid becomes localized mainly in the outer part of the cell membrane, does not induce hemolysis or change the osmotic resistance of erythrocytes, and induces the formation of echinocytes [98]. The values of generalized polarization and fluorescence anisotropy indicate that chlorogenic acid alters the hydrophilic region of the membrane, practically without changing fluidity in the hydrophobic region. The assay of electric parameters showed that chlorogenic acid reduces both the capacity and resistivity of black lipid membranes (BLMs). The overall result is that chlorogenic acid takes position mainly in the hydrophilic region of the membrane, modifying its properties. Such localization allows acids to reduce the concentration of free radicals in the immediate vicinity of the cell and hinder their diffusion into the membrane interior [98]. In addition, caffeic acid displays superficial interactions with cell membrane lipids that can be highly relevant to their biological action [99]. Frias and colleagues reported that chlorogenic acid is a strong phenolic antioxidant with antibacterial properties composed of a caffeoyl ester of quinic acid; however, details on its membrane action and the exact manner with which the composition and membrane state may affect this action, are yet to be fully explored [99]. In their recent study, the interaction of chlorogenic acid with lipid monolayers and bilayers composed by 1,2-di-istoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine (14:0 diether PC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and 1,2-di-O-hexadecyl-sn-glycero-3-phosphocholine (16:0 diether PC) were investigated at different surface pressures using Fourier transform infrared spectroscopy (FT-IR) measurements [99]. The authors found that the kinetics of interaction was more rapid in DMPC than in the absence of carbonyl groups.
The newly emerging field of membrane lipid therapy involves the pharmacological regulation of membrane lipid composition and structure for the treatment of diseases [100]. Membrane lipid therapy proposes the use of new molecules specifically designed to modify membrane lipid structures and microdomains as pharmaceutical disease-modifying agents by reversing the malfunction or altering the expression of disease-specific protein or lipid signal cascades [100]. As summarized in a semantic review article, the influence of lipids on protein function is reflected in the possibility to use these molecular species as targets for therapies against many diseases and disorders, including inflammation, cancer, and neurodegenerative disorders.
8. Conclusions and Future Perspective
As described above, coffee exhibits a variety of positive effects on the immune system by regulating inflammation exhibiting anti-cancer effects and inhibiting the progression of several pathologies of neurodegenerative diseases. In particular, phenolic compounds rich in coffee are considered the main substances that exhibit these effects. Particularly, daily dietary consumption of chlorogenic acids through drinking a cup of coffee has already demonstrated its great potential. Consequently, now that we have harnessed the fragmental evidence of coffee’s beneficial effects in the present review, it is extremely important to establish the deeper knowledge that accounts for the molecular mechanism underlying the beneficial effects of coffee. As a source of anti-inflammatory, anti-cancer and anti-neurodegenerative agents, a cup of coffee holds great promise as a kind of nutraceutical in the pursuit of a healthy human life. Functional foods and their bioactive ingredients are at the interface between nutrition and pharma, and will open the door to seeking new therapeutic intervention for the prevention of diseases [101,102].
Abbreviations
| caffeine-containing beverage (CCO) |
| Food and Agriculture Organization (FAO) |
| breast cancer susceptibility genes (BRCA) |
| 5-O-caffeoylquinic acid (5-CQA) |
| 3-O-caffeoylquinic acid (3-CQA) |
| 4-O-caffeoylquinic acid (4-CQA) |
| reactive oxygen species (ROS) |
| interleukin-8 (IL-8) |
| nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) |
| tumor necrosis factor-alpha (TNF-α) |
| intercellular adhesion molecule 1 (ICAM-1) |
| vascular cell adhesion molecule 1 (VCAM-1) |
| very late antigen-4 (VLA-4) |
| epidermal growth factor (EGF) |
| mitogen-activated protein kinase (MAP kinase) |
| extracellular-signal-regulated kinase (ERK) |
| jun amino-terminal kinases (JNK) |
| stress-activated protein kinases (SAPK) |
| matrix metalloproteinase (MMP) |
| tissue inhibitors of MMP (TIMP) |
| phosphatidylinositol 3-kinase (PI3K) |
| mammalian target of rapamycin (mTOR) |
| phosphatase and tensin homologue deleted on chromosome ten (PTEN) |
| breast cancer susceptibility gene 1 (BRCA1) |
| Bcl-2-associated X protein (Bax) |
| B-cell/CLL lymphoma-2 (Bcl-2) |
| B-cell lymphoma-extra-large (Bcl-xL) |
| T-cell factor and lymphoid enhancer factor (TCF/LEF) |
| leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) |
| nuclear factor erythroid 2-related factor 2 (Nrf2) |
| cyclic adenosine monophosphate (cAMP) |
| cyclic adenosine monophosphate responsive element binding protein (CREB) |
| glycosylphosphatidylinositol (GPI) |
| 1,2-di-istoyl-sn-glycero-3-phosphocholine (DMPC) |
| 1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine (14:0 diether PC) |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) |
| 1,2-di-O-hexadecyl-sn-glycero-3-phosphocholine (16:0 diether PC) |
| Fourier transform infrared spectroscopy (FT-IR) |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There are no data outside that reported in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by the All Japan Coffee Association to T.M.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Food and Agriculture Organization of the United Nations (FAO) Statistical Pocket Book, Coffee. FAO; Rome, Italy: 2015. [Google Scholar]
- 2.Wolf A., Bray G.A., Popkin B.M. A short history of beverages and how our body treats them. Obes. Rev. 2008;9:151–164. doi: 10.1111/j.1467-789X.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.United States Department of Agriculture Foreign Agricultural Service Coffee: World Markets and Trade. [(accessed on 31 January 2023)];2022 Available online: https://www.fas.usda.gov/data/coffee-world-markets-and-trade.
- 4.Gross M. What coffee does to body and mind. Curr. Biol. 2021;31:R311–R313. doi: 10.1016/j.cub.2021.03.080. [DOI] [Google Scholar]
- 5.Ludwig I.A., Mena P., Calani L., Cid C., Del Rio D., Lean M.E., Crozier A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014;5:1718–1726. doi: 10.1039/C4FO00290C. [DOI] [PubMed] [Google Scholar]
- 6.Mullee A., Romaguera D., Pearson-Stuttard J., Viallon V., Stepien M., Freisling H., Fagherazzi G., Mancini F.R., Boutron-Ruault M.C., Kühn T., et al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern. Med. 2019;179:1479–1490. doi: 10.1001/jamainternmed.2019.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zidan N.S., Omran A.M.E., Rezk S.M., Hebatallah H.A., Mohamed I.S. Anti-Alzheimer’s disease potential of Arabian coffee versus Date palm seed extract in male rats. J. Food Biochem. 2022;46:e14017. doi: 10.1111/jfbc.14017. [DOI] [PubMed] [Google Scholar]
- 8.Camandola S., Plick N., Mattson M.P. Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem. Res. 2019;44:214–227. doi: 10.1007/s11064-018-2492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpinska J., Świsłocka R., Lewandowski W. A mystery of a cup of coffee; an insight look by chemist. BioFactors. 2017;43:621–632. doi: 10.1002/biof.1371. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis M.C., Cornelis M.C. Caffeine in the diet: Country-level consumption guidelines. Nutrients. 2018;10:1772. doi: 10.3390/nu10111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nkondjock A. Coffee consumption and the risk of cancer: An overview. Cancer Lett. 2009;277:121–125. doi: 10.1016/j.canlet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Nkondjock A., Ghadirian P., Kotsopoulos J., Lubinski J., Lynch H., Kim-Sing C., Horsman D., Rosen B., Isaacs C., Weber B., et al. Coffee consumption and breast cancer risk among BRCA1 and BRCA2 mutation carriers. Int. J. Cancer. 2006;118:103–107. doi: 10.1002/ijc.21296. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.Y., Yoo D.M., Min C., Choi H.G. Association between coffee consumption/physical exercise and gastric, hepatic, colon, breast, uterine cervix, lung, thyroid, prostate, and bladder cancer. Nutrients. 2021;13:3927. doi: 10.3390/nu13113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawada T. Coffee consumption and risk of cancers: Kidney as an example for the assessment. Clin. Nutr. 2022;41:3122. doi: 10.1016/j.clnu.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y., Wu T., Luo G., Chen L. Exploring the casual association between coffee intake and bladder cancer risk using Mendelian Randomization. Front. Genet. 2022;13:992599. doi: 10.3389/fgene.2022.992599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferhatosmanoğlu A., Selcuk L.B., Arıca D.A., Ersöz Ş., Yaylı S. Frequency of skin cancer and evaluation of risk factors: A hospital-based study from Turkey. J. Cosmet. Dermatol. 2022;21:6920–6927. doi: 10.1111/jocd.15355. [DOI] [PubMed] [Google Scholar]
- 17.Li B.H., Yan S.Y., Li X.H., Huang Q., Luo L.S., Wang Y.Y., Huang J., Jin Y.H., Wang Y.B. Coffee and caffeine consumption and risk of renal cell carcinoma: A Mendelian randomization study. Front. Nutr. 2022;9:898279. doi: 10.3389/fnut.2022.898279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Y., Huang J., Wong M.C.S. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2022;62:749–756. doi: 10.1007/s00394-022-03002-x. [DOI] [PubMed] [Google Scholar]
- 19.Carter P., Yuan S., Kar S., Vithayathil M., Mason A.M., Burgess S., Larsson S.C. Coffee consumption and cancer risk: A Mendelian randomisation study. Clin. Nutr. 2022;21:2113–2123. doi: 10.1016/j.clnu.2022.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldato D., Havas J., Crane T.E., Presti D., Lapidari P., Rassy N., Pistilli B., Martin E., Del Mastro L., Martin A.L., et al. Coffee and tea consumption, patient-reported, and clinical outcomes in a longitudinal study of patients with breast cancer. Cancer. 2022;128:3552–3563. doi: 10.1002/cncr.34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crous-Bou M., Du M., Gunter M.J., Setiawan V.W., Schouten L.J., Shu X.O., Wentzensen N., Bertrand K.A., Cook L.S., Friedenreich C.M., et al. Coffee consumption and risk of endometrial cancer: A pooled analysis of individual participant data in the Epidemiology of Endometrial Cancer Consortium (E2C2) Am. J. Clin. Nutr. 2022;116:1219–1228. doi: 10.1093/ajcn/nqac229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salaroli L.B., Ferreira J.R.S., Prado C.B.D., De Podestá O.P.G., Carvalho A.L., Mercante A.M.D.C., Toporcov T.N. Cumulative coffee consumption as a protective factor for head and neck cancer in Brazil. Nutr. Cancer. 2023;75:228–235. doi: 10.1080/01635581.2022.2106377. [DOI] [PubMed] [Google Scholar]
- 23.Azzeh F.S., Hasanain D.M., Qadhi A.H., Ghafouri K.J., Azhar W.F., Ghaith M.M., Aldairi A.F., Almasmoum H.A., Assaggaf H.M., Alhussain M.H., et al. Consumption of food components of the mediterranean diet decreases the risk of breast cancer in the Makkah Region, Saudi Arabia: A case-control study. Front. Nutr. 2022;9:863029. doi: 10.3389/fnut.2022.863029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barré T., Fontaine H., Ramier C., Di Beo V., Pol S., Carrieri P., Marcellin F., Cagnot C., Dorival C., Zucman-Rossi J., et al. Elevated coffee consumption is associated with a lower risk of elevated liver fibrosis biomarkers in patients treated for chronic hepatitis B (ANRS CO22 Hepather cohort) Clin. Nutr. 2022;41:610–619. doi: 10.1016/j.clnu.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Imatoh T., Sawada N., Yamaji T., Iwasaki M., Inoue M., Tsugane S., JPHC Study Group Association between coffee consumption and risk of prostate cancer in Japanese men: A population-based cohort study in Japan. Cancer Epidemiol. Biomark. Prev. 2022;31:471–478. doi: 10.1158/1055-9965.EPI-21-0484. [DOI] [PubMed] [Google Scholar]
- 26.Rhee J., Lim R.K., Purdue M.P. Coffee consumption and risk of renal cancer: A meta-analysis of cohort evidence. Cancer Causes Control. 2022;33:101–108. doi: 10.1007/s10552-021-01506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Şemen S., Mercan S., Yayla M., Açıkkol M. Elemental composition of green coffee and its contribution to dietary intake. Food Chem. 2017;215:92–100. doi: 10.1016/j.foodchem.2016.07.176. [DOI] [PubMed] [Google Scholar]
- 28.Clifford M.N., Kerimi A., Williamson G. Bioavailability and metabolism of chlorogenic qcids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020;19:1299–1352. doi: 10.1111/1541-4337.12518. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Su C., Chen X., Wang Q., Jiao W., Luo H., Tang J., Wang W., Li S., Guo S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020;68:6464–6484. doi: 10.1021/acs.jafc.0c01554. [DOI] [PubMed] [Google Scholar]
- 30.Clifford M.N., Wight J. The measurement of feruloylquinic acids and, caffeoylquinic acids in coffee beans. Development of the technique and its, preliminary application to green coffee beans. J. Sci. Food Agric. 1976;27:73–84. doi: 10.1002/jsfa.2740270112. [DOI] [PubMed] [Google Scholar]
- 31.Jeon J.S., Kim H.T., Jeong I.H., Hong S.R., Oh M.S., Yoon M.H., Shim J.H., Jeong J.H., Abd El-Aty A.M. Contents of chlorogenic acids and caffeine in various coffee-related products. J. Adv. Res. 2019;17:85–94. doi: 10.1016/j.jare.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanlier N., Atik A., Atik I. Consumption of green coffee and the risk of chronic diseases. Crit. Rev. Food Sci. Nutr. 2019;59:2573–2585. doi: 10.1080/10408398.2018.1461061. [DOI] [PubMed] [Google Scholar]
- 33.Awwad S., Issa R., Alnsour L., Albals D., Al-Momani I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules. 2021;26:7502. doi: 10.3390/molecules26247502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alnsour L., Issa R., Awwad S., Albals D., Al-Momani I. Quantification of total phenols and antioxidants in coffee samples of different origins and evaluation of the effect of degree of roasting on their levels. Molecules. 2022;27:1591. doi: 10.3390/molecules27051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Pan X., Jiang L., Chu Y., Gao S., Jiang X., Zhang Y., Chen Y. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front Nutr. 2022;9:943911. doi: 10.3389/fnut.2022.943911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A., Atanasov A.G., Li Y., Kumar N., Bishayee A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022;186:106505. doi: 10.1016/j.phrs.2022.106505. [DOI] [PubMed] [Google Scholar]
- 37.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., FangFang X., Modarresi-Ghazani F., et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda Y., Nagase N., Tsuji A., Taniguchi K., Kitagishi Y., Matsuda S. Comprehension of the relationship between autophagy and reactive oxygen species for superior cancer therapy with histone deacetylase inhibitors. Oxygen. 2021;1:22–31. doi: 10.3390/oxygen1010004. [DOI] [Google Scholar]
- 39.Yoshikawa S., Taniguchi K., Sawamura H., Ikeda Y., Tsuji A., Matsuda S. Roles of reactive oxygen species and autophagy in the pathogenesis of of cisplatin-induced acute kidney injury. Oxygen. 2022;2:317–326. doi: 10.3390/oxygen2030022. [DOI] [Google Scholar]
- 40.Cheng D., Zhang X., Tang J., Kong Y., Wang X., Wang S. Chlorogenic acid protects against aluminum toxicity via MAPK/Akt signaling pathway in murine RAW264.7 macrophages. J. Inorg. Biochem. 2019;190:113–120. doi: 10.1016/j.jinorgbio.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Effect of chlorogenic acid on the physicochemical and functional properties of Coregonus peled myofibrillar protein through hydroxyl radical oxidation. Molecules. 2019;24:3205. doi: 10.3390/molecules24173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao M., Xiang L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020;87:71–88. doi: 10.1016/bs.apha.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig I.A., Clifford M.N., Lean M.E., Ashihara H., Crozier A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014;5:1695–1717. doi: 10.1039/C4FO00042K. [DOI] [PubMed] [Google Scholar]
- 44.Hoelzl C., Knasmüller S., Wagner K.H., Elbling L., Huber W., Kager N., Ferk F., Ehrlich V., Nersesyan A., Neubauer O., et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010;54:1722–1733. doi: 10.1002/mnfr.201000048. [DOI] [PubMed] [Google Scholar]
- 45.Agudelo-Ochoa G.M., Pulgarín-Zapata I.C., Velásquez-Rodriguez C.M., Duque-Ramírez M., Naranjo-Cano M., Quintero-Ortiz M.M., Lara-Guzmán O.J., Munoz-Durango K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016;146:524–531. doi: 10.3945/jn.115.224774. [DOI] [PubMed] [Google Scholar]
- 46.Melamed I., Kark J.D., Spirer Z. Coffee and the immune system. Int. J. Immunopharmacol. 1990;12:129–134. doi: 10.1016/0192-0561(90)90076-Y. [DOI] [PubMed] [Google Scholar]
- 47.Chen D., Pan D., Tang S., Tan Z., Zhang Y., Fu Y., Lü G., Huang Q. Administration of chlorogenic acid alleviates spinal cord injury via TLR4/NF-kappaB and p38 signaling pathway antiinflammatory activity. Mol. Med. Rep. 2018;17:1340–1346. doi: 10.3892/mmr.2017.7987. [DOI] [PubMed] [Google Scholar]
- 48.Shin H.S., Satsu H., Bae M.J., Zhao Z., Ogiwara H., Totsuka M., Shimizu M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015;168:167–175. doi: 10.1016/j.foodchem.2014.06.100. [DOI] [PubMed] [Google Scholar]
- 49.Chang W.C., Chen C.H., Lee M.F., Chang T., Yu Y.M. Chlorogenic acid attenuates adhesion molecules upregulation in IL-1beta-treated endothelial cells. Eur. J. Nutr. 2010;49:267–275. doi: 10.1007/s00394-009-0083-1. [DOI] [PubMed] [Google Scholar]
- 50.Hebeda C.B., Bolonheis S.M., Nakasato A., Belinati K., Souza P.D., Gouvea D.R., Lopes N.P., Farsky S.H. Effects of chlorogenic acid on neutrophil locomotion functions in response to inflammatory stimulus. J. Ethnopharmacol. 2011;135:261–269. doi: 10.1016/j.jep.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Fuentes E., Caballero J., Alarcón M., Rojas A., Palomo I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE. 2014;9:e90699. doi: 10.1371/journal.pone.0090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arab L. Epidemiologic evidence on coffee and cancer. Nutr. Cancer. 2010;62:271–283. doi: 10.1080/01635580903407122. [DOI] [PubMed] [Google Scholar]
- 53.Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 54.Pavlova N.N., Zhu J., Thompson C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian Y., Castranova V., Ding M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005;280:27888–27895. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- 56.Yagasaki K., Miura Y., Okauchi R., Furuse T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology. 2000;33:229–235. doi: 10.1023/A:1008141918852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlica S., Gebhardt R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic. Res. 2005;39:1377–1390. doi: 10.1080/09670260500197660. [DOI] [PubMed] [Google Scholar]
- 58.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 59.Gobin E., Bagwell K., Wagner J., Mysona D., Sandirasegarane S., Smith N., Bai S., Sharma A., Schleifer R., She J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019;19:581. doi: 10.1186/s12885-019-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors. 2018;18:3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin U.H., Lee J.Y., Kang S.K., Kim J.K., Park W.H., Kim J.G., Moon S.K., Kim C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus A latus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 62.Cabral-Pacheco G.A., Garza-Veloz I., Castruita-De la Rosa C. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020;21:9739. doi: 10.3390/ijms21249739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belkaid A., Currie J.C., Desgagnés J., Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuda S., Nakanishi A., Wada Y., Kitagishi Y. Roles of PI3K/AKT/PTEN pathway as a target for pharmaceutical therapy. Open Med. Chem. J. 2013;7:23–29. doi: 10.2174/1874104501307010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakanishi A., Kitagishi Y., Ogura Y., Matsuda S. The tumor suppressor PTEN interacts with p53 in hereditary cancer. Int. J. Oncol. 2014;44:1813–1819. doi: 10.3892/ijo.2014.2377. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda S., Ikeda Y., Murakami M., Nakagawa Y., Tsuji A., Kitagishi Y. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases. 2019;7:22. doi: 10.3390/diseases7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ikeda Y., Murakami M., Nakagawa Y., Tsuji A., Kitagishi Y., Matsuda S. Diet induces hepatocyte protection in fatty liver disease via modulation of PTEN signaling. Biomed. Rep. 2020;12:295–302. doi: 10.3892/br.2020.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikeda Y., Nagase N., Tsuji A., Kitagishi Y., Matsuda S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World J. Biol. Chem. 2021;12:104–113. doi: 10.4331/wjbc.v12.i6.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuda S., Murakami M., Ikeda Y., Nakagawa Y., Tsuji A., Kitagishi Y. Role of tumor suppressor molecules in genomic perturbations and damaged DNA repair involved in the pathogenesis of cancer and neurodegeneration. Biomed. Rep. 2020;13:10. doi: 10.3892/br.2020.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami M., Ikeda Y., Nakagawa Y., Tsuji A., Kitagishi Y., Matsuda S. Special bioactive compounds and functional foods may exhibit neuroprotective effects in patients with dementia. Biomed. Rep. 2020;13:1. doi: 10.3892/br.2020.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kitagishi Y., Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging. Int. J. Mol. Med. 2013;31:511–515. doi: 10.3892/ijmm.2013.1235. [DOI] [PubMed] [Google Scholar]
- 72.Minami A., Nakanishi A., Ogura Y., Kitagishi Y., Matsuda S. Connection between tumor suppressor BRCA1 and PTEN in damaged DNA repair. Front. Oncol. 2014;4:318. doi: 10.3389/fonc.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Refolo M.G., Lippolis C., Carella N., Cavallini A., Messa C., D’Alessandro R. Chlorogenic Acid Improves the Regorafenib Effects in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2018;19:1518. doi: 10.3390/ijms19051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019;71:1100–1109. doi: 10.1111/jphp.13095. [DOI] [PubMed] [Google Scholar]
- 75.Villota H., Santa-González G.A., Uribe D., Henao I.C., Arroyave-Ospina J.C., Barrera-Causil C.J., Pedroza-Díaz J. Modulatory effect of chlorogenic acid and coffee extracts on Wnt/β-catenin pathway in colorectal cancer cells. Nutrients. 2022;14:4880. doi: 10.3390/nu14224880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villota H., Moreno-Ceballos M., Santa-González G.A., Uribe D., Castañeda I.C., Preciado L.M., Pedroza-Díaz J. Biological impact of phenolic compounds from coffee on colorectal cancer. Pharmaceuticals. 2021;14:761. doi: 10.3390/ph14080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W., Ping Z., Xuemei G., Hongjuan M., Yi H., Xiaoli L., Zhongxiang Z. Chlorogenic acid regulates the proliferation and migration of high-grade serous ovarian cancer cells through modulating the miR199a5p/DDR1 axis. Acta Biochim. Pol. 2022;69:855–864. doi: 10.18388/abp.2020_6381. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y.K., Ngoc N.T.M., Chang H.W., Su Y.F., Chen C.H., Goan Y.G., Chen J.Y., Tung C.W., Hour T.C. Chlorogenic acid inhibition of esophageal squamous cell carcinoma metastasis via EGFR/p-Akt/Snail signaling pathways. Anticancer Res. 2022;42:3389–3402. doi: 10.21873/anticanres.15826. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., Feng Y., Li Y., Hu Y., Zhang Q., Huang Y., Shi K., Ran C., Hou J., Zhou G., et al. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020;11:867. doi: 10.3389/fphar.2020.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Changizi Z., Moslehi A., Rohani A.H., Eidi A. Chlorogenic acid inhibits growth of 4T1 breast cancer cells through involvement in Bax/Bcl2 pathway. J. Cancer Res. Ther. 2020;16:1435–1442. doi: 10.4103/jcrt.JCRT_245_19. [DOI] [PubMed] [Google Scholar]
- 81.Huang S., Wang L.L., Xue N.N., Li C., Guo H.H., Ren T.K., Zhan Y., Li W.B., Zhang J., Chen X.G., et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics. 2019;9:6745–6763. doi: 10.7150/thno.34674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamagata K., Izawa Y., Onodera D., Tagami M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell Biochem. 2018;441:9–19. doi: 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
- 83.Yin X., He X., Wu L., Yan D., Yan S. Chlorogenic acid, the main antioxidant in coffee, reduces radiation-induced apoptosis and DNA damage via NF-E2- related factor 2 (Nrf2) activation in hepatocellular carcinoma. Oxid. Med. Cell Longev. 2022;2022:4566949. doi: 10.1155/2022/4566949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartolomeu A.R., Romualdo G.R., Lisón C.G., Besharat Z.M., Corrales J.A.M., Chaves M.Á.G., Barbisan L.F. Caffeine and chlorogenic acid combination attenuate early-stage chemically induced colon carcinogenesis in mice: Involvement of oncomiR miR-21a-5p. Int. J. Mol. Sci. 2022;23:6292. doi: 10.3390/ijms23116292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y., Abe S.K., Inoue M., Yamaji T., Iwasaki M., Nomura S., Hashizume M., Tsugane S., Sawada N. JPHC Study Group. Green tea and coffee consumption and risk of kidney cancer in Japanese adults. Sci. Rep. 2022;12:20274. doi: 10.1038/s41598-022-24090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuster C., Wolpert N., Moustaid-Moussa N., Gollahon L.S. Combinatorial effects of the natural products Arctigenin, chlorogenic acid, and cinnamaldehyde commit oxidation assassination on breast cancer cells. Antioxidants. 2022;11:591. doi: 10.3390/antiox11030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Socodato R., Portugal C.C., Canedo T., Domith I., Oliveira N.A., Paes-de-Carvalho R., Relvas J.B., Cossenza M. c-Src deactivation by the polyphenol 3-O-caffeoylquinic acid abrogates reactive oxygen species-mediated glutamate release from microglia and neuronal excitotoxicity. Free Radic. Biol. Med. 2015;79:45–55. doi: 10.1016/j.freeradbiomed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 88.Mikami Y., Yamazawa T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015;139:69–74. doi: 10.1016/j.lfs.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Taram F., Winter A.N., Linseman D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neuron. Brain Res. 2016;1648:69–80. doi: 10.1016/j.brainres.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 90.Xue N., Zhou Q., Ji M., Jin J., Lai F., Chen J., Zhang M., Jia J., Yang H., Zhang J., et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017;7:39011. doi: 10.1038/srep39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuyama K., Kakio S., Nakazawa Y., Kobata K., Funakoshi-Tago M., Suzuki T., Tamura H. Roasted Coffee Reduces β-Amyloid Production by Increasing Proteasomal β-Secretase Degradation in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2018;62:e1800238. doi: 10.1002/mnfr.201800238. [DOI] [PubMed] [Google Scholar]
- 92.Unno K., Taguchi K., Hase T., Meguro S., Nakamura Y. Coffee polyphenol, chlorogenic acid, suppresses brain aging and its effects are enhanced by milk fat globule membrane components. Int. J. Mol. Sci. 2022;23:5832. doi: 10.3390/ijms23105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuda S., Nakagawa Y., Tsuji A., Kitagishi Y., Nakanishi A., Murai T. Implications of PI3K/AKT/PTEN signaling on superoxide dismutases expression and in the pathogenesis of Alzheimer’s disease. Diseases. 2018;6:28. doi: 10.3390/diseases6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Londzin P., Zamora M., Kąkol B., Taborek A., Folwarczna J. Potential of caffeine in Alzheimer’s disease-a review of experimental studies. Nutrients. 2021;13:537. doi: 10.3390/nu13020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakano N., Matsuda S., Ichimura M., Minami A., Ogino M., Murai T., Kitagishi Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson’s disease. Int. J. Mol. Med. 2017;39:253–260. doi: 10.3892/ijmm.2016.2833. [DOI] [PubMed] [Google Scholar]
- 96.Noyce A.J., Bestwick J.P., Silveira-Moriyama L., Hawkes C.H., Giovannoni G., Lees A.J., Schrag A. Meta-analysis of early non- motor features and risk factors for Parkinson disease. Ann. Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Q., Zhao Y., Zheng X., Chen Q., Zhang X. Chlorogenic acid alters the biological characteristics of basophil granulocytes by affecting the fluidity of the cell membrane and triggering pseudoallergic reactions. Int. J. Mol. Med. 2013;32:1273–1280. doi: 10.3892/ijmm.2013.1505. [DOI] [PubMed] [Google Scholar]
- 98.Bonarska-Kujawa D., Cyboran-Mikołajczyk S., Kleszczyńska H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015;32:46–54. doi: 10.3109/09687688.2015.1031833. [DOI] [PubMed] [Google Scholar]
- 99.Cejas J.P., Rosa A.S., Nazareno M.A., Disalvo E.A., Frias M.A. Interaction of chlorogenic acid with model lipid membranes and its influence on antiradical activity. Biochim. Biophys. Acta Biomembr. 2021;1863:183484. doi: 10.1016/j.bbamem.2020.183484. [DOI] [PubMed] [Google Scholar]
- 100.Escribá P.V., Busquets X., Inokuchi J., Balogh G., Török Z., Horváth I., Harwood J.L., Vígh L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015;59:38–53. doi: 10.1016/j.plipres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Domínguez Díaz L., Fernández-Ruiz V., Cámara M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020;60:1738–1746. doi: 10.1080/10408398.2019.1592107. [DOI] [PubMed] [Google Scholar]
- 102.Vega E.N., García-Herrera P., Ciudad-Mulero M., Dias M.I., Matallana-González M.C., Cámara M., Tardío J., Molina M., Pinela J.C.S.P., Pires T., et al. Wild sweet cherry, strawberry and bilberry as underestimated sources of natural colorants and bioactive compounds with functional properties. Food Chem. 2023;414:135669. doi: 10.1016/j.foodchem.2023.135669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data outside that reported in this article.