Abstract
Objectives
In this study, we aimed to study the rate of autoantibodies against type I interferons (IFNs) in patients with COVID-19 and analyze its dependence on severity of infection and some other variables.
Methods
A systemic review with the search terms: “COVID-19” or “SARS-CoV-2” and “autoantibodies” or “autoantibody” and “IFN” or “interferon” for the period 20 December 2019 to 15 August 2022 was carried out using PubMed, Embase, Cochrane, and Web of Science. R 4.2.1 software was used for meta-analysis of the published results. Pooled risk ratios and 95% confidence intervals (CIs) were calculated.
Results
We identified eight studies involving 7729 patients, of whom 5097 (66%) had severe COVID-19 and 2632 (34%) had mild or moderate symptoms. The positive rate of anti-type-I-IFN-autoantibodies in the total dataset was 5% (95% CI, 3-8%), but reached 10% (95% CI, 7-14%) in those with severe infection. The most common subtypes were anti-IFN-α (89%) and anti-IFN-ω (77%). The overall prevalence in male patients was 5% (95% CI, 4-6%), and in female patients 2% (95% CI, 1-3%).
Conclusion
Severe COVID-19 is associated with high rates of autoantibodies against type-I-IFN and more so in male than female patients.
Keywords: COVID-19, Anti-type-I-IFN-autoantibodies, Meta-analysis
Introduction
COVID-19 induced by the SARS-CoV-2 has spread to numerous countries, with currently (February 2023) approximately 680 million confirmed cases (the real number could be much higher but cannot be determined) and 6.8 million deaths (which should be relatively close to the real number) (https://www.worldometers.info/coronavirus/). COVID-19 was declared a pandemic by the World Health Organization already in March 2020 and it is still a public health emergency of international concern [1]. This infection has also affected social life and the global economy significantly.
Patients infected with SARS-CoV-2 generally develop a range of symptoms, with the most common being fever, dry cough, and dyspnea [2]. Respiratory failure and organ failures occur in severe cases. Usually, patients older than 60 years, or those with severe pre-existing ailments, are at a comparably greater risk of developing life-threatening signs [3]. Like in other infections, laboratory tests in patients with COVID-19 indicate the presence of general inflammatory responses and specific ones reflected by immunologically active cells, specific antibodies, and the cytokine cascades that elicit these responses. Autoantibodies, that is, antibodies that react with self-antigens are not unusual in chronic infections but the initial event that leads to their initiation is unknown. Autoantibodies, including those with affinity to type I interferons (IFNs), have been found in SARS-CoV-2 infections [4].
The innate immune responses serve as a first line of antiviral defense and are essential for immunity to viruses; these responses have been shown to recognize the SARS-CoV-2 virus and activate signals governing host pattern recognition receptors, involving toll-like receptors and retinoic acid-inducible gene I-like receptors, which are of critical importance for eliciting the innate immune response that results in the production of type-I-IFN [5]. Typically, type-I-IFNs play an important role in the defense against viruses and are associated with a broad spectrum of antiviral infections [6]. The presence of type-I-IFN-neutralizing autoantibodies in patients with COVID-19 leads to impaired viral clearance, while patients lacking these autoantibodies can reduce their viral load over time suggesting that anti-type-I-IFN-autoantibodies weaken the ability to control viral replication [4,7,8]. These autoantibodies could therefore play a role in modulating the severity of COVID-19 infections. Bastard et al. [7] have reported the presence of anti-type-I-IFN-autoantibodies in approximately 10% of patients with life-threatening COVID-19. Another similar study found an approximately 9% positive rate of anti-type-I-IFN-autoantibodies in severe and critical patients with COVID-19 [9], while another research team found these autoantibodies in 5.2% of hospitalized patients with COVID-19 [4]. The existence of this kind of autoantibodies in vivo provides a new direction for the study of host immune responses and mechanisms after SARS-CoV-2 infection, reflecting the complexity of the immune response in COVID-19 infection.
We conducted a systemic literature review and collected a number of key papers dealing with autoantibodies against type-I-IFN associated with SARS-CoV-2 infection to be used for a meta-analysis with the aim of finding how common these autoantibodies and their subtypes are in patients with COVID-19. We also wished to investigate the association of anti-type-I-IFN-autoantibodies with disease severity and gender of the patients.
Materials and methods
Search and selection of published reports
We searched PubMed, Embase, Cochrane, and Web of Science to find articles reporting anti-IFN-autoantibodies and SARS-CoV-2 infection from 20 December 2019 to 15 August 2022 (PROSPERO registration number CRD42022354364). The following keywords were used: “COVID-19" or “SARS-CoV-2” and “autoantibodies” or “autoantibody” and “IFN” or “interferon”. The search strategy is available in supplementary materials. Two researchers independently screened each report. The inclusion criteria were: (i) subjects diagnosed with COVID-19 by the reverse transcription-polymerase chain reaction or serological testing for specific antibodies; (ii) subjects tested for autoantibodies against type-I-IFN, including subtypes; and (iii) articles published in English. The exclusion criteria were articles without data relevant to the study or where the full text was not available; case reports and preprints. Moreover, we also excluded studies in which autoantibodies were not assayed for neutralization; studies that dealt with specific populations, such as patients with only severe or only mild COVID-19; only male or only female patients; patients with COVID-19 with other diseases; and patients with COVID-19 receiving unconventional or special treatment. For studies with duplicate subjects, we included the one with the larger sample size if it was impossible to distinguish the duplicate patients from the others; if the duplicate patients could be identified, the non-replicated data were included for analysis.
Studies included in the analysis were subjected to a final test as advised by the Agency for Health Care Research and Quality (AHRQ), a United States governmental organization (https://www.ahrq.gov/) that assists health systems and clinicians in delivering high-quality healthcare. Its scale for cross-sectional and prevalence studies was used to evaluate the quality of the articles finally included in the systemic review [10]. Each item was awarded one score if it matched the AHRQ criteria, otherwise not. A quality score of 0-3 was regarded as low, 4-7 as moderate, and 8-11 as high (Table 1 ).
Table 1.
Characteristics of the patient cohorts included in the meta-analysis.
| Authorship | Year | Acronym of the consortia | Study quality | No. | Male | severe | Type of autoantibody found |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | IFN-α | IFN-ω | IFN-β | |||||||
| Frasca et al. | 2022 | IMH;SUR | moderate | 360 | 249 | 60 | 13 | 13 | 9 | 1 |
| Akbil et al. | 2022 | DFG;BIH,et al | moderate | 403 | 291 | 210 | 13 | 13 | 8 | NA |
| Eto et al. | 2022 | JSPS KAKENHI;MHLW;AMED;OCU-SRG,et al. | moderate | 622 | 439 | 405 | 26 | 20 | 21 | NA |
| Yee et al. | 2021 | EPICC | moderate | 127 | 86 | 49 | 4 | NA | NA | NA |
| Bastard et al. | 2021 | NIH;CTSA;FRM; REACTing,et al. | moderate | 5756 | NA | 4117 | 540 | 397 | 418 | NA |
| Abers et al. | 2021 | NIH;CTSA;NHGRI;ANR;FRM,et al. | moderate | 83 | NA | 44 | 3 | 0 | 3 | 0 |
| Goncalves et al. | 2021 | HCL | moderate | 94 | NA | 84 | 15 | 15 | 10 | 1 |
| van der Wijst et al. | 2021 | NIAID;DRC;NIH;UCSF;ANR,et al. | moderate | 284 | 196 | 128 | 10 | NA | NA | NA |
IFN, interferon; NA, not applicable.
Data extraction
The data were extracted by two researchers and assessed by a third when disputed. Data extracted from each article included the number of participants, age, gender, antibody detection method, and type of autoantibodies detected. If relevant data were not reported, we examined also supplementary data if given. Notably, not every study used the terms “severe” and “mild” to group patients. Therefore, we classified patients in critical condition or treated in the intensive care unit (ICU) in the hospital as “severe” and patients referred to as non-ICU, moderate, mild, or asymptomatic as “not severe”.
Statistical analysis
R-software version 4.2.1 (https://cran.r-project.org/) was used for the statistical data analysis. Quantitative meta-analysis of individual rates was first performed with the “metaprop” function in its Meta package to obtain the total combined positive rate of anti-Type-I-IFN-autoantibodies and 95% confidence interval (CI). A homogeneity test (Q test) was used to test for homogeneity and heterogeneity among the included studies, where the former was defined as P ≥ 0.10 and I 2 ≤50% indicating that the fixed effect model should be used. In case of the presence of heterogeneity, the random effect model should be used. This methodology was likewise applied for analyzing the effect of disease severity and gender, as well as type of the autoantibodies. The “metabin” function of R-4.2.1 was used to compare the positive rate of anti-Type-I-IFN-autoantibodies between severe and not severely ill patients and between male and female patients, with the results expressed as the risk ratio (RR). Assessment for publication bias was not done because less than 10 studies were included in each analysis. Sensitivity analysis was performed with the “metainf” function to assess the stability of results.
Results
Study selection results and characteristics
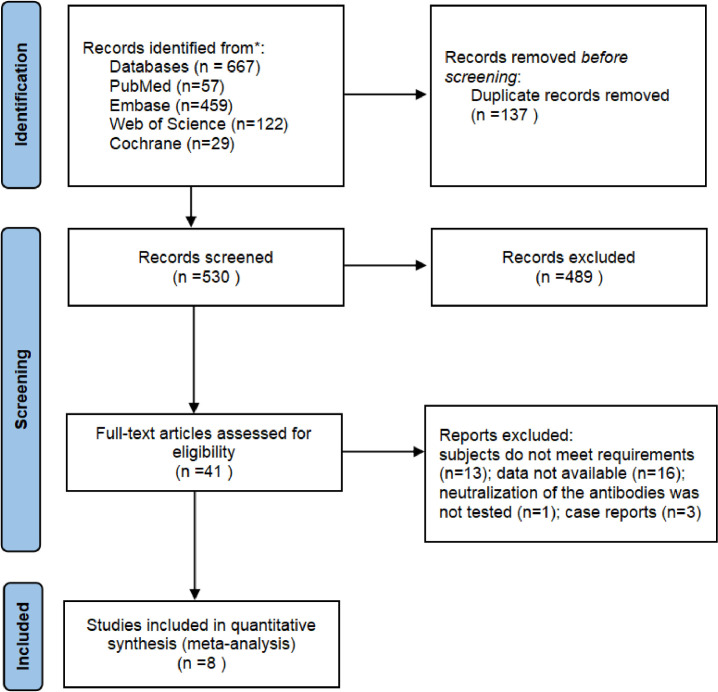
We initially identified 667 relevant studies. After screening according to inclusion/ exclusion criteria, a total of eight studies were included for meta-analysis [8,9,[11], [12], [13], [14], [15], [16]] (Figure 1 ). They had a wide geographical distribution and included a total number of 7729 patients with COVID-19, 5097 of whom (66%) were termed severely ill. Five of the eight studies used the enzyme-linked immunosorbent assay to measure anti-IFN autoantibody levels [[11], [12], [13],15,16]. One study also used the multiplex particle-based assay and the Gyros method [15], another used the radio-ligand-binding assay [9], while two did not describe which method was used [8,14]. Relevant information from these articles and the estimated study quality are shown in Table 1.
Figure 1.
Literature search and selection process.
Anti-type-I-interferon-autoantibodies in patients with COVID-19
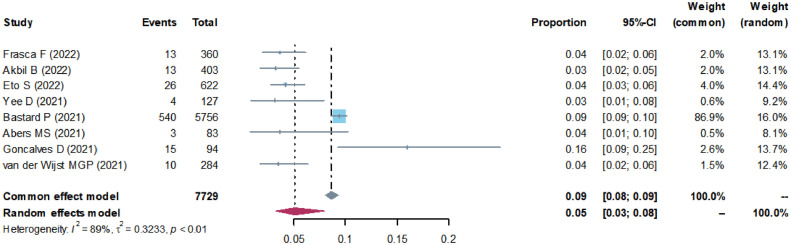
All 7729 patients included in this analysis were confirmed to be infected with SARS-CoV-2. We chose log-transformed data for the meta-analysis. As shown in Figure 2 , there was significant heterogeneity among the studies (I 2 = 89%, >50%, P < 0.01), therefore, the random effect model was chosen. Of the 7729 patients, the weighted, pooled positive rate of anti-type-I-IFN-autoantibodies was 5% (Figure 2). Sensitivity analysis showed that the main result did not change significantly after excluding any study (Supplementary Figure 1).
Figure 2.
Forest plot of the total positive rate of anti-type-I- interferon autoantibodies in patients with COVID-19.
CI, confidence interval.
Six of the studies were subjected to statistical analysis of subtypes of autoantibodies against type-I-IFN, i.e., IFN-α, IFN-ω, and IFN-β (Table 1). The combined positive rate of each type of autoantibody was calculated by meta-analysis, which yielded 89% positives for anti-IFN-α and 77% for anti-IFN-ω (Supplementary Figure 2).
Anti-type-I-interferon-autoantibodies in patients with severe and not severe COVID-19
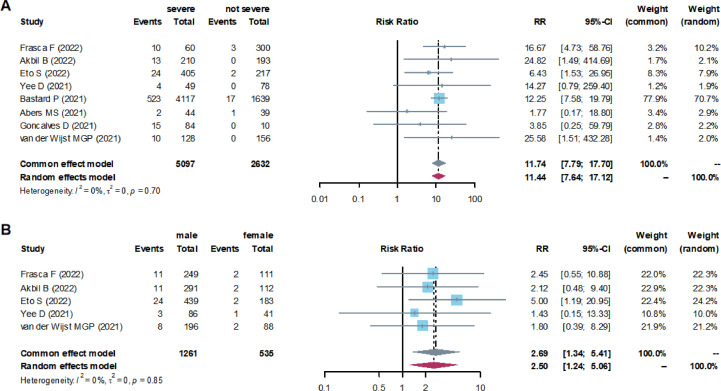
The meta-analysis showed that the total combined positive rate of anti-type-I-IFN-autoantibodies in the severely ill patients was 10% (95% CI, 7-14%), while in the patients diagnosed as not severely ill, only 1% (95% CI, 1-2%) was discovered (Table 2 ). At the same time, the level of anti-type-I-IFN-autoantibodies in patients with severe COVID-19 was significantly higher than that in the ones with less severe symptoms (RR = 11.74). The I 2 test showed no significant heterogeneity between these studies (I 2 = 0%) (Figure 3 a).
Table 2.
Positive rates of anti-type-I-IFN-autoantibodies in different groups of patients with COVID-19.
| Group | Positive rate (%, 95%CI) | I2 test |
|---|---|---|
| Disease severity | ||
| Severe | 10% (95% CI, 7-14%) | 76%, P <0.01 |
| Not severe | 1% (95% CI, 1-2%) | 0%, P = 0.79 |
| Sex | ||
| Male | 5% (95% CI, 4-6%) | 0%, P = 0.81 |
| Female | 2% (95% CI, 1-3%) | 0%, P = 0.95 |
CI, confidence interval; IFN, interferon.
Figure 3.
(a) Forest plots of the RR of anti-type-I-interferon-autoantibodies in patients with severe and not severe COVID-19 and (b) in male and female patients.
CI, confidence interval; RR, risk ratio.
Gender distribution of anti-type-I-interferon-autoantibodies
To further understand whether there is a correlation between anti-type-I IFN-autoantibodies and gender, we analyzed five of the eight studies that reported this kind of data. The results showed that the positive rate of these autoantibodies was 5% (95% CI, 4-6%) in male patients and 2% (95% CI, 1-3%) in female patients (Table 2). Thus, male patients had a considerably higher positive rate than female patients (RR = 2.69). The I 2 test showed no significant heterogeneity in these studies (Figure 3b).
Discussion
The mechanism of autoimmunity is complex and includes many factors, such as chronic infection, autoimmunity, hormone effects, genetic propensity, and environmental factors, that all are capable of triggering such responses. Some viral infections have additionally been linked to autoimmunity [17]. Studies in the past 2 years have found an association between SARS-CoV-2 infection and host autoimmunity, with both reactivity and variety of autoantibodies significantly increased [4]. Examples include not only anti-type-I-IFN autoantibodies of different subtypes but also general antinuclear antibodies and anti-neutrophil cytoplasmic antibodies [18]. The presence of these autoantibodies is associated with an increased antiviral humoral immune response and a stronger inflammatory immune response than normal [19], which counter-intuitively can impair resistance to viral infection by inhibiting immune receptor signaling and altering the composition of the peripheral immune cells [4].
We observed that the autoantibodies against type-I-IFN reported in these studies were mainly of the anti-IFN-α and anti-IFN-ω types, which are present in only 0.3% of the general population [15]. Under appropriate conditions, anti-cytokine autoantibodies could neutralize the biological function of the target cytokines by depleting them through formation of immune complexes [20], however, not all autoantibodies are able to neutralize their target cytokines in this way [21]. Because autoantibodies with neutralizing properties are more biologically relevant [20], we focused on those capable of neutralizing type-I-IFNs. In our analysis, these autoantibodies were more prevalent among severely ill patients; and it is thus of great significance to find them in SARS-CoV-2-infected patients with severe signs of disease.
Age and gender influence the outcome of COVID-19, and it has been reported that infected individuals over 65 years of age as well as men (in comparison to women) are more likely to develop severe pneumonia [22]. Our analysis showed that patients characterized by this symptom had a higher positive rate of anti-type-I-IFN-autoantibodies than less severely ill patients. Therefore, we speculate that a high level of this type of autoantibody can significantly reduce the level of type-I-IFN in patients with SARS-CoV-2 infection and that these autoantibodies may be an important factor contributing to severe pneumonia (and other symptoms). Because most of them can neutralize type-I-IFN in vivo [7,16], deficiency of this cytokine can be considered a marker of severe COVID-19 [23], which supports the idea that the presence of anti-type-I-IFN-autoantibodies constitutes a detrimental factor. Indeed, the clearance of the virus is delayed in patients with high levels of these autoantibodies because they block IFN-I signaling [8]. These autoantibodies also hinder the activation of myeloid and lymphoid cells in response to SARS-CoV-2 infection [4]. If anti-type-I-IFN-autoantibodies are rather the cause than the consequence of severe COVID-19, which has been suggested [4], it would be highly meaningful to screen for these autoantibodies in patients with COVID-19 early on. Administration of type-I-IFNs would be the treatment of choice for patients shown to produce high levels of such antibodies because it would presumably help prevent them from developing severe symptoms.
It is worth noting that some patients with pre-existing high titers of neutralizing anti-type-I-IFN-autoantibodies, mainly young women, showed only mild symptoms after infection with SARS-CoV-2 [24]. Although the presence of these neutralizing autoantibodies is a strong risk factor for exacerbating disease, not all patients with the antibodies develop severe or critical COVID-19 disease [13]. Most patients with critical COVID-19 pneumonia and neutralizing autoantibodies against type-I-IFN were men over 65 years [7]. Moreover, it has been reported that the prevalence of anti-type-I-IFN-autoantibodies increased with age [7,13]. Although we were unable to perform an age-stratified analysis because specific age data for the included patients were not available in all studies, age is an important factor that cannot be ignored because of its potential contribution to severe disease. There are gender differences in COVID-19, with male patients more commonly experiencing severe disease and higher mortality [15], a fact supported by our finding of a higher positive rate of anti-type-I-IFN-autoantibodies in men. Thus, it is concluded that anti-type-I-IFN-autoantibodies are more common in older and male patients, and these autoantibodies appear to be more likely to be a risk factor for severe COVID-19 in the elderly and men, but much less so in the young and women.
Although generally autoantibody production is typical of autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis [25], anti-type-I-IFN-autoantibodies in patients with COVID-19 are likely to have been present before infection, and such neutralizing antibodies have even been associated with an increased risk of death [26]. Moreover, these autoantibody responses in most patients with COVID-19 are highly dynamic, peaking in the acute phase and then gradually decreasing, which is in sharp contrast to the stable high level of anti-type-I-IFN IgG in patients with autoimmune-polyendocrine-syndrome type 1 [27]. Interestingly, in contrast, it has been suggested SARS-CoV-2 infection can lead to the emergence of new IgG anti-type-I-IFN-autoantibodies, which are positively correlated with the immune response to viral proteins [14]. Emerging autoimmune diseases have also been observed during or after SARS-CoV-2 infection [28].
There is obviously an interaction between SARS-CoV-2 infection and autoimmunity, where the latter seems to aggravate the infection; there is then a question if severe SARS-CoV-2 infection induces autoimmunity or if it is the result of pre-existing autoantibodies and molecular mimicry induced by other infections. Molecular mimicry may be an important mechanism of autoimmunity caused by COVID-19 [17]; In the analysis of the SARS-CoV-2 proteome, Fath et al. [29] found that SARS-CoV-2 and the human proteome share 23 peptides derived from the ORF1ab polyprotein, non-structural protein NS7a, surface glycoprotein, and the SARS-CoV-2 envelope protein. That these viral antigens have significant homology with human proteins can be an important reason why autoimmunity arises during COVID-19 infection. Although vaccination clearly prevents severe symptoms of the disease and helps reduce viral transmission, the autoimmune responses occurring in some individuals vaccinated against COVID-19 cannot be ignored [30].
A limitation is there may be a considerable recruitment bias as almost all the studies collected for this review were cross-sectional by design and the patients recruited were usually from a certain hospital. Therefore, it is very likely that most of the enrolled patients were hospitalized or severely ill patients, while patients with mild symptoms, or those who were not admitted to the hospital for examination, were rarely included in any studies. Although we found a significantly higher rate of anti-type-I-IFN-autoantibodies in severely ill patients, the heterogeneity that was found among the studies analyzed for this result was considerable. This may be because of the rules applied for the groups, for example, severely ill patients included “severe", “critical” or “ICU” patients, while the group of not severely ill patients included “non-ICU", “moderate", “mild” or “asymptomatic” patients. The difference between these two groups may not have been as rigorous as desired because different countries and regions may have different diagnostic criteria. In addition, because the research subjects in the eight studies came from various countries it could have affected immune functionality related to genetic differences making the autoimmune disease status of all patients before SARS-CoV-2 infection somewhat unclear. In addition, not all the included studies established a specific positive cut-off value for the autoantibodies in question. Therefore, we did not determine a uniform positive cut-off for the values reported but relied on the data as given in each article. It is also possible that other unreported factors contributed to the observed heterogeneity.
In conclusion, the results of our study suggest that anti-type-I-IFN-autoantibodies in patients with COVID-19 are associated with disease severity and it is also gender-related. Importantly, there is growing evidence that COVID-19 is associated with autoimmunity, but several questions remain. They include the specific mechanism of anti-type-I-IFN-autoantibody production in patients with COVID-19 and how these autoantibodies regulate the disease process. With reference to the IFN subtype, further studies are needed to find the reason why the autoantibodies against IFN-α and IFN-ω are more common while those against IFN-β are rare. Finally, it is also necessary to find out if there is a way to neutralize the development of autoimmunity after COVID-19 infection (and also after vaccination), so that COVID-19 can be effectively prevented and controlled.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
The work was supported by Key Techniques in Collaborative Prevention and Control of Major Infectious Diseases in the Belt and Road Initiative (Grant No. 2018ZX10101002-004). The funder was not involved in the design, data collection, analysis, or writing of the manuscript.
Ethical approval statement
Not applicable.
Acknowledgments
We thank Dr. Menbao Qian for his kind assistance and advice during the study.
Author contributions
Zhiqiang Qin initiated and conceptualized the project. Xi Wang and Qi Tang pursued the systematic review and collected the data. Xi Wang, Qi Tang, Hongmei Li, Honglin Jiang, and Zhiqiang Qin analyzed the data. Xi Wang wrote the original draft, and Jing Xu, Honglin Jiang, Robert Bergquist, and Zhiqiang Qin helped wrote the article and critically edited and revised the manuscript. All authors approved the final version.
Data sharing
Please contact author for data requests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.03.011.
Appendix. Supplementary materials
References
- 1.World Health Organization. Director General's opening remarks at the media briefing on COVID-19, https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-20-march-2020; 2020 [accessed 22 November 2022].
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 5.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy S, Subbian S. Critical Determinants of cytokine Storm and Type I interferon Response in COVID-19 pathogenesis. Clin Microbiol Rev. 2021;34 doi: 10.1128/CMR.00299-20. e00299-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abers MS, Rosen LB, Delmonte OM, et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol Cell Biol. 2021;99:917–921. doi: 10.1111/imcb.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Wijst MGP, Vazquez SE, Hartoularos GC, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med. 2021;13:eabh2624. doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Dong Y, Chen X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. 2015;61:78–89. doi: 10.1016/j.comppsych.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Frasca F, Scordio M, Santinelli L, et al. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur J Immunol. 2022;52:1120–1128. doi: 10.1002/eji.202249824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbil B, Meyer T, Stubbemann P, et al. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J Clin Immunol. 2022;42:1111–1129. doi: 10.1007/s10875-022-01252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eto S, Nukui Y, Tsumura M, et al. Neutralizing Type I interferon autoantibodies in Japanese patients with severe COVID-19. J Clin Immunol. 2022;42:1360–1370. doi: 10.1007/s10875-022-01308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee D, Tso M, Shaw E, et al. Type-I interferon autoantibodies are detected in those with critical COVID-19, including a young female patient. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab466.649. S325–6. [DOI] [Google Scholar]
- 15.Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6:eabl4340. doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncalves D, Mezidi M, Bastard P, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunology. 2021;10:e1327. doi: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moody R, Wilson K, Flanagan KL, Jaworowski A, Plebanski M. Adaptive immunity and the risk of autoreactivity in COVID-19. Int J Mol Sci. 2021;22:8965. doi: 10.3390/ijms22168965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchi MC, Tamiazzo S, Stobbione P, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci. 2021;14:898–907. doi: 10.1111/cts.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77:2415–2430. doi: 10.1111/all.15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku CL, Chi CY, von Bernuth H, et al. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet. 2020;139:783–794. doi: 10.1007/s00439-020-02180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kärner J, Pihlap M, Ranki A, et al. IL-6-specific autoantibodies among APECED and thymoma patients. Immun Inflamm Dis. 2016;4:235–243. doi: 10.1002/iid3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Bastard P, Bolze A, et al. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Med (N Y) 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meisel C, Akbil B, Meyer T, et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Invest. 2021;131 doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao ZX, Miller JS, Zheng SG. An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2020.102743. [DOI] [PubMed] [Google Scholar]
- 26.Chauvineau-Grenier A, Bastard P, Servajean A, et al. Autoantibodies neutralizing Type I interferons in 20% of COVID-19 deaths in a French hospital. J Clin Immunol. 2022;42:459–470. doi: 10.1007/s10875-021-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw ER, Rosen LB, Cheng A, et al. Temporal dynamics of anti-type 1 interferon autoantibodies in patients with coronavirus disease 2019. Clin Infect Dis. 2022;75:e1192–e1194. doi: 10.1093/cid/ciab1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10:3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karami Fath M, Jahangiri A, Ganji M, et al. SARS-CoV-2 proteome harbors peptides which are able to trigger autoimmunity responses: implications for infection, vaccination, and population coverage. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.705772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bril F, Al Diffalha S, Dean M, et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.