Abstract
Viral hepatitis is a leading cause of liver morbidity and mortality globally. The mechanisms underlying acute infection and clearance, versus the development of chronic infection, are poorly understood. In vitro models of viral hepatitis circumvent the high costs and ethical considerations of animal models, which also translate poorly to studying the human-specific hepatitis viruses. However, significant challenges are associated with modeling long-term infection in vitro. Differentiated hepatocytes are best able to sustain chronic viral hepatitis infection, but standard two-dimensional models are limited because they fail to mimic the architecture and cellular microenvironment of the liver, and cannot maintain a differentiated hepatocyte phenotype over extended periods. Alternatively, physiomimetic models facilitate important interactions between hepatocytes and their microenvironment by incorporating liver-specific environmental factors such as three-dimensional ECM interactions and co-culture with non-parenchymal cells. These physiologically relevant interactions help maintain a functional hepatocyte phenotype that is critical for sustaining viral hepatitis infection. In this review, we provide an overview of distinct, novel, and innovative in vitro liver models and discuss their functionality and relevance in modeling viral hepatitis. These platforms may provide novel insight into mechanisms that regulate viral clearance versus progression to chronic infections that can drive subsequent liver disease.
Keywords: hepatitis B virus, hepatitis C virus, SARS-CoV-2, liver-on-chip, disease model
Few viruses are able to establish long-term chronic infections in humans and most are cleared after only a short acute infection as is the case with SARS-CoV-2. The biological processes that facilitate these viruses to manifest only as acute infection and not frequently establish chronic infection and subsequent disease progression are not well characterized. 1 In the liver, hepatitis B, C, and D can all frequently establish chronic infection. 2 Chronic viral hepatitis infection is among the leading causes of hepatocellular carcinoma (HCC), which accounts for 1.3 million deaths per year and 90% of all primary liver cancer cases. 3 Chronic infections may manifest asymptomatically, but even asymptomatic infections can present significant risk for the development of liver disease in the future, and patients who were infected with chronic viral hepatitis still remain at an increased risk of developing HCC even after the virus has been cleared. 4 5 HCC is a burgeoning issue: it is the fastest rising cause of cancer-related deaths in the United States and the fourth most common cause of cancer-related death worldwide. 6 7 In 2012, a total of 170,000 new cancer cases were attributed to hepatitis C virus (HCV), and 420,000 were attributed to hepatitis B virus (HBV). 8
The mechanisms that facilitate the transition from chronic viral infection to HCC are poorly understood and require further investigation to devise therapies to combat the progression of disease post infection. Unfortunately, modeling chronic viral infection is extremely complex. Animal models have been indispensable in contributing to our understanding of viral hepatitis-induced liver disease progression and drug development. However, there is a dearth of intricate relevant physiological systems and these are limited by cost, ethical concerns related to the use of human tissue, and incongruent physiology. 9 Animal models are also challenged with accurately replicating a human's physiological immune response and sustaining chronic infection from human-specific viruses. 10 11 Even genetically humanized mice, which are transfected with human viral entry receptors genes to recapitulate human HCV infection, 12 are restricted in their capacity to generate physiologically relevant immune responses that are imperative to understanding mechanisms underlying disease progression. 13 14
In vitro liver models are necessary to complement the shortcomings of animal models, but are not without deficiencies. First, it is unclear exactly how long an infection must persist in vitro before cells exhibit phenotypic changes characteristic of a chronic infection. Establishing systems that can markedly increase the functional lifespan of in vitro culture is critical for determining this inflection point. Moreover, in vitro systems lack an immune system, and rely solely on the innate immunity of individual cells to study an immune response. By potentially incorporating multiple cell types, physiomimetic platforms offer the advantage of introducing a more dynamic interaction that includes cellular cross-talk and a more systemic approach to modeling an immune response. Conventional two-dimensional (2D) in vitro models that use monolayer culture are cost-effective and amenable to high-throughput experimentation, but their environment is not consistent with normal liver physiology. 15 As a result, primary hepatocytes typically suffer from lower viability and cannot sustain a functional phenotype for an extended period when plated in 2D in vitro. 16 This differentiated phenotype is necessary for modeling both acute and chronic viral hepatitis infection accurately and must be maintained throughout the duration of a study to replicate an accurate cellular response to infection. Physiology-mimicking/physiomimetic microsystems are one possible solution to the challenges of maintaining a differentiated and functional hepatocyte state, avoiding the attenuation of peak functionality that is observed out to roughly 2 weeks in vitro. 13 Though modeling “chronic” infection in vitro is supremely difficult and borderline impossible given the timescale by which chronic infection in vivo is defined (on the scale of years), physiomimetic microsystems may still indeed prolong hepatocyte functionality long enough to provide insight into the transition from acute to chronic infection that occurs. Such systems include micropatterned and three-dimensional (3D) substrates, spheroids, and microfluidic cultures known commonly as organs-on-chips. Many of these microsystems have been well-established as effective liver models for years, but far fewer have explored viral infection in the liver. 17 Here we investigate how physiomimetic liver microsystems are rapidly evolving to better maintain functional hepatocytes that will facilitate the execution of novel studies of viral infection in the liver.
We first describe the viral infections of interest along with the liver microenvironment and acinus architecture. We then explore the building blocks for recapitulating viral infection in the liver in vitro. Next, we investigate models specific to viral infections, and the ability of these models to faithfully recapitulate viral infections. Finally, we assess the future of in vitro models for the study of viral infection in the liver, and strategies for how microsystems previously designed for nonviral applications can be further optimized for pertinent studies.
Viral Infections in the Liver
The liver is susceptible to infection from a myriad of viruses, but here we highlight three which have been of particular interest due to their prevalence and contribution to morbidity and mortality: HBV, HCV, and severe acute respiratory syndrome 2 (SARS-CoV-2).
Hepatitis B Virus
HBV is a partially double-stranded DNA virus that can establish either acute or chronic infection. HBV infection remains a global health challenge because it lacks a finite cure, and chronic HBV infection has a clear link to HCC. 18 About 25% of patients with chronic HBV infection die prematurely from cirrhosis and liver cancer, the majority of which remain asymptomatic until the onset of more severe disease. The virus' surface antigen is extremely potent at generating functional neutralizing antibody responses and this has enabled the development of highly effective vaccines. 13 HBV enters the cell via a hepatocyte-specific receptor, Na + -taurocholate cotransporting polypeptide (NTCP). 19 It then translocates to the nucleus where its genome is modified to the covalently closed circular (cccDNA) form that exists stably as an extrachromosomal viral genome. cccDNA codes for the transcripts necessary for protein production and replication. This cccDNA is the primary therapeutic target for therapies that would enable a finite and durable cure for chronic HBV infection. 13 Neither an understanding of the mechanisms through which HBV drives progression to HCC nor an optimal treatment to achieve virus eradication have been realized; therefore, there is a persisting need for in-depth mechanistic studies on HBV infection in vitro. 18
Hepatitis C Virus
HCV is a single-stranded positive-sense RNA virus with six genotypes. Infection can occur acutely or chronically, but approximately 75 to 85% of people infected with HCV sustain chronic infection and many are asymptomatic for years. 20 HCV enters the cell via cluster of differentiation (CD81) and additional coreceptors and completes its life cycle in the cytoplasm. Viral RNA is translated to protein and continues to replicate in the endoplasmic reticulum. Virion morphogenesis is coupled to the very low-density lipoprotein (VLDL) pathway, forming lipoviral particles that are subsequently excreted. 13 HCV is curable through the use of potent direct-acting antiviral agents (DAAs) that target viral enzymes (protease and polymerase inhibitors), but there is currently no vaccine, and cirrhotic patients who have had chronic HCV infection are still at increased risk of developing HCC, even if the virus has been cleared. 21 22 23 24 25 Similar to HBV, even though there is a documented link between chronic HCV infection and HCC, the mechanisms which govern the transition from chronic infection to HCC are relatively unexplored because of difficulties modeling chronic viral infections in vitro. 26 27
SARS-CoV-2
SARS-CoV-2 has quickly emerged as one of the most transmissible and deadly viruses in modern history. Although the virus primarily targets alveolar cells, it has also been shown to infect hepatocytes, and liver injury has been reported in severe cases. SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor for entry, which is present on hepatocytes, and is also expressed on liver endothelial cells and biliary epithelial cells. 28 Patient studies measuring liver injury markers in cases of SARS-CoV-2 infection found higher levels of aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT), and total bilirubin in patients with more severe cases. 29 The virus has demonstrated higher rates of death in patients with preexisting liver disease, and the stage of liver disease is strongly associated with mortality. 30 Though the full impact of SARS-CoV-2 on the liver remains unclear, there is mounting evidence to suggest the virus has the capacity to directly cause hepatic damage. 31
The Hepatic Microenvironment
Hepatic Acinus
The hepatic lobule is the structural and functional unit of the liver ( Fig. 1 ). It is composed of plates of hepatocytes, and is vascularized by sinusoids that transport blood from the portal venules and hepatic arterioles (periportal sides) to the central vein (perivenous side), which carries blood back to the heart. 32 33 34 Chemical and functional gradients naturally form between the periportal and perivenous ends, creating zonation across the sinusoid. 35 The sinusoids are lined with liver sinusoidal endothelial cells (LSECs). Kupffer cells (KCs), the resident macrophages of the liver, neutrophils, and natural killer cells, are anchored to the surface of this endothelial lining. Hepatic stellate cells (HSCs), which support the deposition of collagen that can result in subsequent fibrosis, reside between the sinusoid and parenchymal tissue in the space of Disse. Bile ducts run between the hepatocyte plates, and flow opposite the blood toward the gall bladder via the common bile duct. A single sinusoid flanked by hepatocytes represents the smallest functional unit of the liver, the hepatic acinus. A lobule is composed of multiple sinusoids and acini, but a single acinus captures all of the liver's primary functions. Most physiomimetic liver systems that aim to recapitulate the microenvironmental architecture of the liver seek to reconstruct an individual acinus.
Fig. 1.
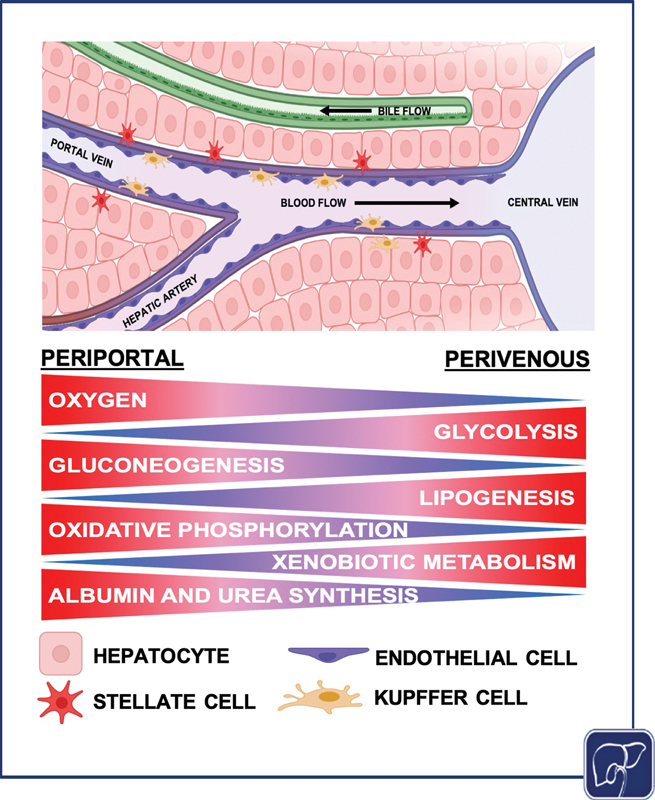
Diagram of the hepatic acinus, the smallest functional unit of the liver. Different hepatocyte functions occur at different points across the liver acinus. Gluconeogenesis, oxidative phosphorylation, and albumin and urea synthesis are higher in zone 1, while glycolysis, lipogenesis, and xenobiotic metabolism are elevated in zone 3. Oxygen concentration decreases across the sinusoid from the periportal region to the perivenous region (the image was generated using Biorender).
Parenchymal Cells
The hepatocyte is the parenchymal cell of the liver and serves critical roles in metabolism, detoxification, protein synthesis, and innate immunity. 36 Hepatocytes are the primary cell implicated in viral hepatitis infection and replication, and the predominate cell type of interest for studying infection in the liver. They make up approximately 60% of the total cells in the liver, and approximately 80% by mass. 37 Hepatocytes exhibit a highly polarized state, evidenced by their segregated membrane domains. The basal domain of the hepatocyte interfaces with blood flowing through the sinusoid, while the canicular domain makes up a lumen between adjacent hepatocytes and forms a network of bile canaliculi. 38 These canaliculi drain into the hepatic ducts, ultimately creating a flow system countercurrent to the flow of blood through the sinusoid. 39 The proximity of bile networks and vasculature renders them capable of rapid uptake and secretion, which is necessary for hepatocytes to carry out their metabolic functions.
Non-Parenchymal Cells
Non-parenchymal cells (NPCs) provide critical cell–cell interactions that support hepatocytes in performing their primary functions. Hepatocyte co-culture with NPCs has beneficial effects on hepatocyte viability and functionality in vitro. 40 NPCs also contribute significantly to the physiologic responses to viral infection and are implicated in the progression of disease. 41 42 43 Physical and chemical cues bolster synergistic cross-talk between different cell types, which helps mediate responses to viral infection. In the case of in vitro systems, this cross-talk may promote cell maturity and push cells toward a more physiologic phenotype. 40 44 All cell types implicated in viral infection are described in Table 1 .
Table 1. Overview of resident cell types in the liver and pertinence to viral hepatitis infection.
| Cell | Type | Proportion by number (%) | Diameter (µm) | Response to hepatitis infection |
|---|---|---|---|---|
| Hepatocytes | Epithelial | 65% | 20–30 | Directly infected, propagate virus, inflammatory protein secretion |
| Sinusoidal endothelial cells | Endothelial | 15% | 7–11 | Antiviral cytokine production |
| Kupffer cells | Macrophages | 12% | 10–13 | Inflammatory response, viral clearance |
| Stellate cells | Fibroblasts | 8% | 10–12 | Fibrogenic response |
Liver Sinusoidal Endothelial Cells
LSECs line the hepatic sinusoid and form fenestrations that perform important filtration functions between the blood and hepatocytes. They have high endocytic and metabolic capacity for various ligands (e.g., glycoproteins, lipoproteins, ECM components). They can act as APCs for both major histocompatibility complexes; secrete important chemokines and cytokines involved in inflammatory responses including interleukin (IL)-6, hepatocyte growth factor (HGF), and transforming growth factor (TGF-β) 45 ; and secrete interferons to inhibit HCV replication. 46 LSECs have demonstrated the capacity to inhibit hepatocarcinogenesis through cytokine secretion, and they support the health of hepatocytes via production of extracellular matrix (ECM) proteins. 40 In a damaged liver, LSECs acquire morphological abnormalities that inhibit their function, including supporting the maintenance of hepatic stellate cells in the quiescent state. These changes occur in the livers of patients with HBV and HCV infection. 46 47 48 49
Kupffer Cells
KCs are the liver resident macrophage, representing approximately 10 to 15% of the liver's total cells. 37 As macrophages, KCs play a critical role in the liver's immune response to viral infection. The role of KCs in response to HBV infection is unclear. After exposure to HBV, data have been published demonstrating both increased production of the inflammatory cytokines IL-6 and tumor necrosis factor (TNF), 50 but decreased production of the inflammatory cytokine IL-1β. 51 For HCV, KCs mount an inflammatory response upon binding, causing KCs to secrete IL-1B, IL-6, TNF-α, and the immune suppressing mediator IL-10. 43 KC-derived TNF-α can also incidentally increase hepatocyte permeability, indirectly promoting HCV infection. KCs play a critical role in the recruitment of immune cells that contribute to the clearance of HBV and HCV, but both viruses are particularly adept at evading the immune response. 52 KCs are also a significant contributor to liver damage postinfection, and are implicated in the progression of liver disease and HCC, likely modulated by the release of inflammatory and pro-fibrinogenic cytokines. 53
Hepatic Stellate Cells
HSCs are fibrotic lipid-storing cells that reside in the space of Disse between the sinusoid and parenchyma. In a healthy liver, HSCs are quiescent and represent 5 to 8% of cells in the liver. They store 80% of the body's vitamin A, are tasked with ECM turnover, and regulate contractility of the sinusoids. In a diseased liver, HSCs become active and transdifferentiate into ECM-secreting myofibroblasts. They can secrete ECM proteins, growth factors, cytokines, and metalloproteinases. HSCs are the primary cell type modulating fibrosis, and, as a result, can cause secondary damage to the liver through collagen deposition. 45 In models of HBV infection, HBV was found to promote the proliferation of HSCs through the platelet-derived growth factor (PDGF)-B/PDGF receptor-β signaling pathway. In addition, increased expression of collagen I, connective tissue growth factor, α-smooth muscle actin, matrix metalloproteinase-2, and TGF-β was observed. 54 55 In HCV, HSCs are activated by cytokines secreted from infected hepatocytes. 56 57 The HCV E2 protein has also demonstrated the ability of directly binding to HSCs inducing a fibrotic response. 41
Liver Zonation
Metabolism is central to the function of all hepatocytes, but hepatocyte metabolic functions vary depending on their position along the sinusoid (periportal vs. perivenous/centrilobular; Fig. 1 ). 58 This concept of metabolic zonation is established via chemical gradients, particularly oxygen, and manifests through variability in hepatocyte function. There is differential hepatocyte gene expression in specific locations along the sinusoid, including genes in the Wnt/β-catenin signaling pathway, demonstrating the regulatory effect of zonation on hepatocyte functionality. 59 The hepatic sinusoid is functionally segregated into three zones. In the oxygen-rich zone 1, near the portal vein and hepatic artery, hepatocytes are predominantly responsible for oxidative metabolism, fatty acid oxidation, gluconeogenesis, bile acid extraction, ammonia detoxification, and urea and glutathione conjugation. Conversely, hepatocytes in the oxygen-poor zone 3, near the central vein (centrilobular), are mainly responsible for glycolysis, liponeogenesis, and cytochrome P450 biotransformation. 58
Understanding the variable functionality of hepatocytes depending on zonation is not only crucial for evaluating drug toxicity but also for assessing responses to viral infection and the processes contributing to progression to HCC. For example, HCV preferentially infects perivenous hepatocytes and can perturb the metabolic function associated with zonation upon production of viral proteins. 60 61 62 Moreover, the same Wnt/β-catenin signaling pathway that plays a role in the establishment of liver zonation is also key in modulating the development and progression to HCC. It is clear that aberrations in zonation and subsequent effects on hepatocyte function are critical to processes implicated in liver disease progression. 18
In Vitro Human Models of Viral Hepatitis
Hepatocyte Sources
In vitro studies focused on viral hepatitis afford numerous options for modeling infection, but choice of cell source, particularly choice of hepatocyte source, holds the greatest impact in terms of experimental validity, efficacy, and ease-of-use. The different hepatocyte sources and their compatibility with distinct models to study viral infection are outlined in Table 2 .
Table 2. Overview of hepatocyte sources for study of viral infection in vitro.
| Functional conditions | Biological considerations | Primary human hepatocytes | Stem-cell–derived hepatocytes | HepaRG cell | HepG2 cell | HuH-7 cell |
|---|---|---|---|---|---|---|
| Culture conditions | Period of survival | ∼ 2 mo | < 1 mo | Indefinite | Indefinite | Indefinite |
| Propagation | No | Yes, contact-inhibited | Yes, contact-inhibited | Yes | Yes | |
| Matrix coating required | Collagen | Matrigel | None | Collagen | None | |
| Access conditions | Commercial availability | Low | Low | Moderate | High | High |
| Lot variability | High | Moderate | Moderate | Low | Low | |
| Infection Conditions | Innate immunity | Fully functional | Fully functional | Fully functional | Moderate | Low |
| Supports HBV infection | Yes | Yes | Yes, requires differentiation | No, requires NTCP | No, requires NTCP | |
| Supports HCV infection | Yes | Yes | Yes, requires differentiation | No, requires CD81 and miR-122 | Yes | |
| Supports SARS-CoV-2 infection | Yes | Yes | Likely | Yes | Yes |
Primary Human Hepatocytes
Advantages
Primary human hepatocytes (PHHs) are the gold standard for use in in vitro liver models. PHHs are at their in vitro functional peak when plated, and do not require any further differentiation. PHHs support HBV, HCV, and SARS-CoV-2 infection, though HCV replication is seldom observed due to the potent innate immune response. Regardless, these cells are the most useful tool for the general study of viral hepatitis. 13 63
Disadvantages
PHHs are sourced directly from a human patient or fetal liver which is costly and not readily available, and they do not divide once cultured in vitro. Without any advanced culture techniques, their functional peak typically lasts about 2 weeks in vitro, which is an insufficient time to establish a chronic viral infection model. PHHs vary from patient-to-patient isolates; so, robust studies typically require PHHs from multiple donors to achieve statistically reproducible results. 13 As an alternative, PHH pools from different donors can be utilized to generate data that may be more generalizable across donors.
Hepatoma Cell Lines
Compared with PHHs, hepatoma cell lines are a more accessible source of cells for modeling hepatic processes in vitro. The HuH7, HepG2, and HepaRG cells are among the hepatoma lines most commonly used to model viral hepatitis infection. Each of these cell lines vary in their functionality and state of differentiation, but all are immortalized, and can be continually passaged. 13
Huh7 Cells
Advantages
Huh7 cells can be used as a model for HBV, HCV, and SARS-CoV-2 infection. 13 64 This cell line is permissive to HCV infection and its derivatives; specifically, the Huh7.5 and Huh7.5.1 cell lines are often used to propagate the virus in vitro. 13
Disadvantages
Huh7 cells display minimal functional cell-intrinsic innate antiviral responses which render them permissive to viral infection, but they have an attenuated immune response, minimizing their utility in studying the progression of infection toward disease. 13
HepG2 Cells
Advantages
Genetically modified HepG2 cells are primarily used for the study of HBV, but also support SARS-CoV-2 infection. 13 65 Unlike Huh7 cells, they are polarized, and can sustain HBV infection if modified to express the NTCP viral entry receptor. HepG2 cells can mount a detectable innate antiviral response rendering them useful in the study of host defense pathways, specifically the type III interferon (IFN)-λ in response to HCV infection. 13
Disadvantages
Unmodified HepG2 cells do not support HBV or HCV virion infection without genetic manipulation/selection. They lack the NTCP receptor necessary for HBV infection. Even when expressing the NTCP receptor, HepG2 cells still remain less susceptible to HBV infection when compared with differentiated HepaRG cells. 66 They also lack micro-RNA (miRNA-122) necessary to support HCV life cycle, and demonstrate low expression of the CD81 receptor, necessary for HCV infection. HepG2 cells that are transfected and selected to stably express these proteins can serve as useful viral infection models. 13
HepaRG Cells
Advantages
The HepaRG cell line, that is immortalized but not transformed, is a bipotent progenitor that can differentiate to either the hepatocyte or the cholangiocyte lineage. When differentiated, HepaRG cells are similar but less effective versions of PHHs in terms of drug metabolism and their ability to support viral infection. 13 67
Disadvantages
As a bipotent progenitor cell, they may not have a well-established innate immune axis, which can compromise their utility for studying immune response to viral infection. 68 To support HBV and HCV infection, HepaRG cells must be differentiated from their hepatoblast (HB) state to more differentiated hepatocyte-like cells (HLCs), typically achieved through treatment with dimethyl sulfoxide (DMSO). 13 HCV has significant difficulty replicating in HepaRGs. At the time of this publication, SARS-CoV-2 infection in HepaRG cells has not been reported ( Table 2 ).
Stem-Cell–Derived Hepatocyte-Like Cells
Stem cells, whether sourced embryonically from blastocysts (hESCs) or through induction of pluripotency (iPSCs), are a promising source of HLCs that can be used to model viral infection. 13 69 iPSC-derived HLCs have an advantage over PHHs in that they can be grown in limitless quantities without donor variability. Stem cells must be differentiated to definitive endoderm (DE) and HB states before becoming HLCs. HLCs are not fully differentiated like PHHs because they continue to express AFP. 13 HLCs have demonstrated the capacity to support HBV, SARS-CoV-2, and HCV infection in 2D. 69 70 71 72
Sources of Viruses for In Vitro Study
HBV
HBV for in vitro research is sourced from either patient serum or via cell line production of recombinant virus. Patient serum has the advantage of being able to study distinct genotypes or variants, while recombinant viruses are favorable for investigating the roles of distinct proteins. 19 HepDE19 cells and HepAD38 cells are two examples of commonly used cell lines used to produce recombinant HBV. 73 74
HCV
HCV for in vitro research is available in several platforms and is sourced through patient serum (serum-derived HCV [HCVser]), as pseudoparticles (HCVpp), or through cell culture production (cell culture–derived HCV [HCVcc]). Pseudoparticles-derived HCV (HCVpp) is generated in a kidney cell line; so, these particles do not synthesize lipoproteins and are mainly used for studies on viral entry. 75 Recombinant cell culture–derived HCV (HCVcc), often replicated using the Huh7 cell line or one of its derivatives, synthesizes lipoproteins and is a more effective model to study the entire viral life cycle in vitro. The six HCV genotypes vary in both geographic distribution and in their antigenic and serologic properties. Understanding these differences is critical because responses to interferon and antiviral therapies are genotype dependent. 76 Similar to HBV, HCVser can be used to infect hepatocytes with different HCV genotypes and clones for all six HCV genotypes have been isolated, though the degree to which they are able to replicate in vitro varies. 77 The JFH1 recombinant strain (genotype 2a) replicates spontaneously in hepatoma cells and is commonly used to study infection in hepatoma cell lines. 78
SARS-CoV-2
The novel coronavirus, SARS-CoV-2, has very recently become an area of interest for research on viral infection in the liver. Due to its novelty, in vitro models of SARS-CoV-2 are in a nascent state, and study of the virus thus far has relied primarily on clinical data and clinical isolates. Unlike HBV and HCV, the liver is not the virus's primary target. Therefore, it may be more efficient to propagate the SARS-CoV-2 in non-liver cells. Vero E6 cells, kidney epithelial cells from a green African monkey, are the most commonly used to replicate the virus. 79 Wanner et al investigated the molecular consequences of liver tropism as a result of SARS-CoV-2 infection in autopsy samples, and used the homogenized tissue to infect Vero cells. 80 Huh7 and HepG2 cells have the capacity to be infected with SARS-CoV-2 and elicit an IFN response. 64 81 82 Hepatocytes have also proven capable of being infected by other coronaviruses including HCoV-229E and HCoV-OC43, which are less symptomatically severe but use the same mechanism of entry into cells. 83 84 There are seven coronaviruses in total including SARS-CoV-2, some of which are more accessible for in vitro experimentation and require less stringent safety protocols. 85 Other coronaviruses may serve useful in preliminary in vitro research on coronavirus infection in the liver.
Physiomimetic Models to Study Viral Infection
Physiomimetic platforms are in preliminary stages of modeling viral infections in the liver compared with their uses as platforms to identify and study drug toxicity. However, the principle of using a platform to optimally capture the liver microenvironment in vitro is consistent across applications, whether it is drug response or viral infection. The primary goals of traditional 2D in vitro infection models are to (1) investigate questions pertaining to viral infection and life cycle and (2) uncover cellular responses and related mechanisms. Although not the focus of this review, the development of standard 2D models to study viral hepatitis has been covered elsewhere. 86 87 The primary goals of physiomimetic or nontraditional in vitro models thus far are to (1) promote the functional longevity of hepatocytes and (2) establish more robust, long-term infections evocative of chronic infection in humans. In doing so, these models may help elucidate mechanisms underlying disease progression and expand our understanding of how viruses in the liver initiate and sustain chronic infections and drive subsequent inflammatory processes. Here we explore the progress on developing current physiomimetic viral infection models and their use to sustain long-term infection. These systems are outlined in Table 3 .
Table 3. Comparative overview of different physiomimetic culture platforms used to study viral hepatitis.
| Culture system | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Static systems | |||
| Sandwich culture | •High-throughput •Low maintenance •Accessible |
•Overly simplistic •Minimal benefits beyond traditional 2D culture |
80 |
| MPCCs | •Longevity •High-throughput •Low maintenance |
•Nonphysiological microenvironment •Incorporates nonhuman cells |
81 82 83 84 85 86 87 88 89 |
| Spheroids | •3D cell orientation •High-throughput •Low maintenance |
•Nonphysiological microenvironment •Necrotic cores •Variability between spheroids |
92 93 94 95 96 97 98 99 100 101 102 103 |
| Decellularized scaffolds | •Provide a physiological microenvironment •Versatile applications •3D cell orientation •Mimic physiological ECM interactions |
•Difficult to source •Extensive preparation and characterization required |
104 105 |
| Perfusion systems | |||
| Hollow-fiber bioreactors | •Provide a physiological microenvironment •3D cell orientation •Longevity |
•Large •Low-throughput •Low cell accessibility |
106 107 108 109 |
| Rotational bioreactors | •3D cell orientation •Longevity |
•Low-throughput •Low cell accessibility |
110 111 112 113 |
| Liver-on-chip platforms | •Provide physiological shear and nutrient exchange •Can recapitulate hepatic architecture •Small device |
•Nonphysiological cell orientation •Complex preparation |
114 115 116 117 118 119 |
Sandwich Culture
Sandwich culture is a layered culture approach, where cells are sandwiched between two matrix layers. Cells bind and form a monolayer on the matrix beneath them and are subsequently coated with a top layer of matrix, called an overlay. In a complex iteration of sandwich culture, Petropolis et al compared multiple different permutations of matrix and cell layering, and ultimately created two distinct Huh7-NTCP hepatocyte layers separated by collagen I matrix and topped with a layer of LSECs, to study HBV infection. 88 This model demonstrated HBV infection for 4 days. The authors did not observe significant differences in infection as compared with 2D, but were able to show nonsignificant differences in infection in the presence and absence of an endothelial barrier. They showed that they could incorporate another cell type that participates in cytokine signaling in response to infection, without hampering infection 88 ( Fig. 2A ). Though sandwich culture has fewer physiomimetic elements than the more complex platforms which are discussed later, a minimalist approach to building a model may be more cost-effective and amenable for high-throughput experimentation than more complex culture systems.
Fig. 2.
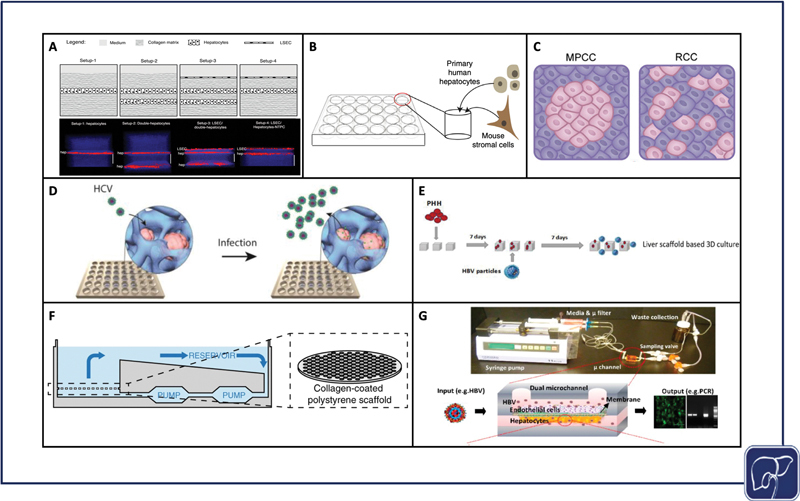
Models used to study viral hepatitis. Layered sandwich cultures that were infected with HBV (adapted from Petropolis et al under terms of CC BY) 80 ( A ), self-assembling 3D hepatocyte-stromal cell co-cultures that were infected with HBV (adapted from Winer et al under terms of CC BY) 90 ( B ), micropatterned co-cultures versus random co-cultures that were infected with HBV and HCV (adapted from Shlomai et al under terms of CC BY) 88 ( C ), spheroid cultures that were infected with HCV (reproduced with permission from Ananthanarayanan et al, ©2014 American Chemical Society) 95 ( D ), seeding and infection process of decellularized liver scaffolds that were infected with HBV (reproduced with permission from Zhang et al) 105 ( E ), CN Bio Physiomimix platform cross-section that was infected with HBV (adapted from Ortega-Prieto et al under terms of CC BY) 117 ( F ), PDMS liver chip that was infected with HBV (adapted from Kang et al under terms of CC BY) 118 ( G ). HBV, hepatitis B virus; HCV, hepatitis C virus; MPCC, micropatterned co-culture; RCC, random co-culture; PDMS, polydimethylsiloxane; PHH, primary human hepatocyte.
Micropatterned/Self-Assembling Co-culture
Micropatterned co-cultures (MPCCs) are deterministically patterned co-cultures, often achieved by selective matrix deposition or surface treatment. The original hepatocyte MPCC design from Khetani et al, now commercialized as HEPATOPAC, features groups of hepatocytes seeded on collagen, spatially arranged via stencil, and surrounded by 3T3-J2 mouse fibroblasts as feeder cells. 89 90 91 92 93 94 95 MPCCs help prolong a functional PHH phenotype, and therefore help promote long-term viral infection. Shlomai et al used MPCCs to culture PHHs and iPSC-derived HLCs with 3T3-J2s and demonstrated that HLCs support HBV in a differentiation-dependent manner. In this study, HBV infection permissiveness was also an evaluator of differentiative state 68 ( Fig. 2C ). For HCV, Ploss et al used the HEPATOPAC system to co-culture PHHs with 3T3-J2s, and they achieved productive HCV infection for up to 2 weeks. 96 Two weeks is a reasonable period of time for sustaining HCV infection in PHHs in vitro, but is also consistent with the timeframe in which PHHs begin to dedifferentiate. 13
Self-assembling co-cultures (SACCs), which were created using SACC plates from HμREL, combined PHHs and 3T3-J2 cells. The SACCs successfully supported HBV infection for 40 days, and HBV/HDV (hepatitis D virus) coinfection for 28 days 97 98 ( Fig. 2B ). Simple coculture systems may be highly effective for prolonging viral infection studies without sacrificing accessibility or throughput.
Spheroids and Organoids
3D spheroids (single cell type) and organoids (multiple cell types) are culture techniques that provide cells with points of contact in three dimensions to help better preserve hepatocyte function when compared with cell in 2D. 99 The same infection-permissive effects of SACCs and MPCCs are also observed in spheroid/organoid culture. Crignis et al infected healthy donor organoids (suspended in basement membrane matrix) with recombinant HBV and demonstrated robust production of HBV cccDNA up to 8 days postinfection. 100 Organoid cultures have also proven capable of increasing HBV infection in HLCs as a result of improving their differentiative state. Nie et al demonstrated productive HBV infection for up to 20 days using iPSC-derived organoids, and achieved results comparable to 2D PHH culture. 101 As a renewable source of cells with a functional interferon response, iPSC-derived HLC organoids offer the potential of reducing cell batch variability for modeling HBV infection in vitro.
In the context of HCV, spheroid cultures have been shown to be more permissive to viral infection when compared with traditional 2D culture. The establishment of hepatocyte polarity through spheroid formation may be linked to the upregulation and sustained expression of specific HCV entry receptors, and matrix-based spheroid cultures further assist in hepatocyte polarization 102 ( Fig. 2D ). For example, Tran et al used an optimal Ca-Na-alginate bead formulation to upregulate expression of HCV-specific receptors in the Huh7 cell line. 103 Molina-Jimenez et al, Cho et al, and Ananthanarayanan et al embedded Huh7 or Huh7.5 (Huh7 derivative) cells in Matrigel, polyethylene glycol diacrylate (PEGDA) hydrogel, and cellulosic hydrogel, respectively, which were subsequently infected with HCV. 102 104 105 Rajalakshmy et al infected 3D Huh7 cells cultured in Mebiol gel, a thermoreversible gelatin polymer. The authors observed results consistent with other 3D matrix encapsulation studies, and demonstrated increased expression of HNF4α and transthyretin, two key hepatocyte maturation markers. They demonstrated HCV infection for 10 days, but were able to support the growth of hepatocyte spheroids for 63 days. 106 Ananthanarayanan et al specifically incorporated PHHs into their model, but only compared HCV entry in the spheroids against standard 2D culture and they found a significant increase. 102 HCV infection in cell lines is clearly well established, but sustaining this infection in immunocompetent PHHs remains a challenge that may be facilitated through the use of immune modulators. 107
Although in their preliminary stages of development given the recent emergence of the virus, hepatic spheroids and organoids have shown to be highly permissible to SARS-CoV-2 infection as well. Yang et al created hepatic organoids from both iPSC-derived HLCs and isolated primary tissue, successfully infecting both cells types with this virus. 108 Similarly, Lui et al and Zhao et al infected cholangiocyte hepatic organoids derived from liver biopsies, which express the ACE2 receptor at high levels to support virus entry. 109 110
Decellularized Scaffolds
Decellularized scaffolds from both human and nonhuman livers provide a physiological matrix via enzymatic digestion of cellular material, but with preservation of liver-specific ECM proteins. This native ECM supports requisite signals for engraftment, survival, and function, upon hepatocyte reseeding. 111 Decellularized scaffolds can provide insight into the microenvironmental effects that may impact HBV infection. Zhang et al compared healthy and cirrhotic patient decellularized ECM (dECM) scaffolds with HBV-infected PHHs, and infected HepG2-NTCP cells. The authors observed increased viral replication in the diseased scaffold and used the healthy scaffold to establish a 3D infection model that demonstrated a significant increase in viral replication over the 2D condition. These data support the significance and contribution of microenvironment on viral infection in vitro 112 ( Fig. 2E ).
Bioreactors
Bioreactors are the trailblazing technologies that laid the foundation for the development of microfluidic chips. The purpose of bioreactors is to recapitulate an isolated system ex vivo. Hollow fiber (HF) bioreactors, the most common form, are physiomimetic perfusion devices that typically feature two sets of hydrophilic media capillaries and one set of hydrophobic oxygen capillaries. The media capillaries provide counter-current flow to increase nutrient exchange, while the oxygen capillaries promote gas exchange. Cells are cultured in 3D cell compartments between the HF capillary structures. 113 Aizaki et al maintained HCV infection in the FLC4 cell line, which readily translates HCV protein, 114 for over 100 days during culture in a radial flow, HF bioreactor. 115 116 Pihl et al established a similar HF bioreactor system and studied HCV infection with and without various antivirals in HuH7.5 cells. This study extended beyond promoting infection and replication in the bioreactor platform, and aimed to investigate mechanisms of viral resistance to antiviral agents. 117 Rotational bioreactors, or rotating wall vessels (RWVs), are platforms for spheroid generation and/or maintenance that continuously suspend cells and allow them to aggregate into spheroids. 118 119 120 Sainz et al used an RWV to create Huh7 spheroids for HCV infection and observed sustained levels of infection for 2 weeks in the system. 121 Of all the established HCV infection models, bioreactors hold the greatest potential for sustaining longer-term infections, but these platforms lack the capacity for high-throughput studies.
Liver-on-Chip Models
Liver-on-chip models are microfluidic devices that incorporate a variety of physiologically relevant hepatic microenvironmental factors like ECM proteins, improved spatial cell architecture, and media perfusion to induce healthy mechanical stimulation and nutrient exchange. These factors combined can promote prolonged cell viability and function to a degree that is otherwise unattainable in static culture. 122 123 Chip infection models offer dynamic conditions as a means of promoting viral infection. For example, systems that recirculate media may provide virions with additional opportunities for infection when compared with static media conditions. Ortega-Prieto et al infected PHHs using the CN Bio chip system that pneumatically circulates media, and compared the chip to spheroid cultures, SACCs, and traditional 2D culture. The authors observed the best infection results in their chip that maintained infection for 22 days. This model also incorporated other cell types, namely KCs, to study their response to HBV infection 124 125 ( Fig. 2F ). Kang et al used a perfused bilayer membrane chip design with bovine aortic endothelial cells and sustained HBV infection in PHHs for 14 days 126 ( Fig. 2G ). The longevity of these experiments is a strong indicator that chip microsystems are a tenable platform for informative chronic HBV infection studies in vitro.
As an HCV model, Natarajan et al infected primary liver organoids encapsulated in basement membrane matrix with HCV, and perfused the cells in co-culture with T cells on a commercially available chip, idenTx, from AIM Biotech. They used this co-culture platform to study the adaptive immune response in the context of HCV infection, and were able to recapitulate a more physiological interaction between infected hepatic organoids and T cells, by introducing T cells into the perfused media and allowing them to encounter the infected organoids independently. 127 This study highlights the utility of physiomimetic systems as more physiological models of the adaptive immune response.
Translating Nonviral Hepatitis Models to Viral Applications
At this time, viral-infection–focused physiomimetic liver models are limited; however, other disease models have utilized platforms highlighted in section “Physiomimetic Models to Study Viral Infection.” Previous use for studies on other distinct liver diseases provides valuable insight for a model's capacity to be successfully utilized to support studies on viral infection. Here, we discuss the foundational elements of current physiomimetic models that allow them to be used functionally to study viral infection. We also consider which design components best promote the establishment and maintenance of chronic infection in vitro.
Exploring Other Disease Models
While not specific to support viral infection studies, some liver disease models have significant design characteristics that overlap substantially with other viral infection models. For example, steatosis, fibrosis, and cirrhosis models simulate the same long-term effects that can be observed from chronic viral infection, despite using different drivers of liver disease for achieving these responses. Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are common liver diseases that are modeled in in vitro studies and they can cause inflammation in the liver which results in a fibrotic response similar to viral hepatitis. Even though NAFLD/NASH is triggered by entirely different mechanisms than viral hepatitis, both diseases activate many of the same inflammatory pathways that subsequently induce similar fibrotic responses. These similarities between the progression of these diseases to fibrotic states may influence the development of future viral infection models. NAFLD/NASH models specifically typically incorporate stellate cells, or some type of fibroblast substitute. Stellate cells are quiescent in a healthy liver, but induce a fibrotic response when activated via inflammatory pathways. Some in vitro studies modeling a fibrotic liver can be accomplished by comparing tissue from healthy and diseased donors, 128 but for most physiomimetic in vitro liver models, inflammation was induced. Induction of an inflammatory state was achieved through exposing cells to high concentrations of free fatty acids (FFAs), and other naturally occurring drivers of fatty liver disease, 129 130 131 132 133 134 135 136 and also by modulating matrix stiffness or drug treatment. 120 137 138 139 140 David Hughes's group studied the effects of FFAs in monoculture and co-culture, respectively, in two separate publications, using fat and lean hepatocyte cell culture media on the CN Bio chip. 131 132 The co-culture model included PHHs with primary KCs and HSCs and demonstrated stellate cell activation that contributed to the profibrotic response. 132 Ouchi et al and Pingitore et al offered similar FFA treatment conditions to their organoid cultures. 129 136
Changes in matrix stiffness have a reproducible effect on stellate cell activation since fibrosis is promoted under stiffer conditions. 141 142 Clark et al used the same CN Bio platform with hepatocyte and NPC co-culture, and manipulated matrix stiffness to modulate inflammatory response. 143 Drug treatment is also utilized as a means of inducing inflammatory responses and subsequent steatosis and these models have direct clinical applications. As an example of a platform to test treatments for viral infections, studying the tissue response to treatment is as critical as establishing infection, especially for therapeutics that target cellular factors. For general liver fibrosis models, methotrexate (MTX) is an attractive compound for experimental use because it is widely prescribed as a chemotherapeutic and immunosuppressant. 144 MTX has been used to induce fibrosis in vitro in a chip system, 139 organoid, 145 and in bioprinted models 137 138 alike, all of which demonstrated a profibrotic response. Bell et al demonstrated the capacity of their PHH/NPC organoid culture to model viral hepatitis via adenovirus infection, and then treated with trovafloxacin to induce hepatoxicity. 140 An alcoholic disease model was also developed from Deng et al, where the authors used a chip-based device to co-culture PHHs and NPCs, and examined the effects of treating the cells with varying concentrations of ethanol. 146 Two different groups developed bacterial infection models, specifically to study malaria. March et al used MPCCs to treat PHHs with antimalarial drugs, and Ng et al used MPCCs to study the effects of hypoxia on malaria infection in hepatocytes. 147 148 Each of the models described achieves a diseased-state liver that exhibits the capacity for use as viral infection platforms.
Multiorgan Platforms
The prospect of linking a liver platform with other organ systems to study specific viral infections is exiting and relatively unexplored and multiorgan platforms including multiorgan chips can be linked via perfusion of a common media. These more complex models would support studies offering a more systemic perspective on disease processes related to infections, diseases, and treatments. These multiorgan chips are an intermediate step in the pursuit of a full “body-on-chip,” which would effectively serve as a complete in vitro human model. Liver–gut chips have been the most commonly developed linked system that includes a liver platform. This is because most environmental contributors to steatosis, whether it be high-fat diets 149 or cytotoxic compounds, 150 151 152 can be mediated by the gut and its microbial community, upstream of the liver. Moreover, liver–skin, 153 liver–lung, 154 liver–heart, 155 and liver–kidney 156 chips have been developed to study drug toxicity on peripheral organs, downstream of the liver to account for hepatic drug metabolism. These two-organ chips can increase further in complexity by combining three or more organ systems. Maschmeyer et al established an aggregate model that includes four organ systems (gut, liver, lung, kidney) and used their platform to support viable culture of all four tissues for 28 days. 157 Ronaldson-Bouchard et al also developed a four-organ system (skin, bone, heart, liver) and maintained the culture for 4 weeks. 158 Other plate-based devices have not yet used liver cells specifically, but offer the potential for linking tissue from all major organs of the human body. 159 160 Miller and Shuler developed a system capable of culturing 14 different tissues, all connected on a pumpless device. 161 Multiorgan platforms have yet to be rigorously applied to the study of viral infections in the liver, but they provide an intriguing opportunity to study related systemic effects.
Design Considerations for Translating Platforms to Viral Applications
Established disease models naturally inform key design elements that would be needed to develop optimal virus infection models. Nonetheless, some disease models are better equipped than others for sustaining chronic infection in vitro for weeks to months. Here, we cite some considerations for expanding a platform's range of use to include amenability for supporting sustained chronic viral infection.
Size
The size of the platform is the primary consideration when fabricating/selecting a physiomimetic culture platform. Though less of a concern in static culture devices, considerations of working volume and media life cycle are inherent to the size of the system. Perfusion systems often recycle media, but the frequency at which recycled media has to be changed depends greatly on working volume. Furthermore, shear forces and nutrient exchange vary greatly depending on the ratio of size to flow rate. The dimensions of a device must be established before a flow rate that will replicate physiological shear and nutrient exchange can be determined. The size of the device also dictates the number of cells that can be seeded, which is critical when considering methods of comparison. Ideally, cell count in a physiomimetic platform can be standardized against traditional 2D culture formats, especially when quantifying replication of infection, that is, virions that have been released from infected cells into the spent media.
Materials
Selection of materials is crucial for optimizing a platform to its application, especially in the case of microfluidic devices that require precise fabrication of fine-tuned features. Though microfluidic devices in particular can be manufactured from numerous materials including silicon, ceramic, and even paper, 162 the three most widely used materials in microfluidic chips we have reviewed here are polydimethylsiloxane (PDMS), glass, and polymethyl methacrylate (PMMA) (acrylic). PDMS is gas-permeable and customizable at nanoscale resolution via casting with photolithographically patterned photoresist templates ( Fig. 3A, B ). However, PDMS is subject to nonspecific protein binding unless chemically modified, and lacks optical clarity making it difficult to image cells in situ without compromising sterility. Glass can be directly processed using photolithography and does not bind proteins nonspecifically, but glass is not gas-permeable, limiting its capacity for long-term culture if used as the chip's exclusive material. Photolithography can also be a cumbersome method of fabrication, and is less amenable to rapid prototyping in comparison to other methods. 162 163 164 Conversely, PMMA is a great candidate for rapid prototyping through laser machining, milling, injection-molding, embossing, casting, and reactive ion etching, and is not hindered with nonspecific protein binding to its surface. 165 PMMA is also optically clear and allows for easier live cell imaging. However, like glass, PMMA is also gas impermeable. Machining techniques reach only the microscale rather than the nanoscale, so this material is not ideal for creating small, high-resolution features that can be achieved via photolithography. Many chips combine materials or use PDMS with a glass substrate. 130 139 166 167 168 169 170 171 In this way, engineers can reduce the amount of PDMS in the chip, but still benefit from PDMS's capacity to form high-resolution features.
Fig. 3.
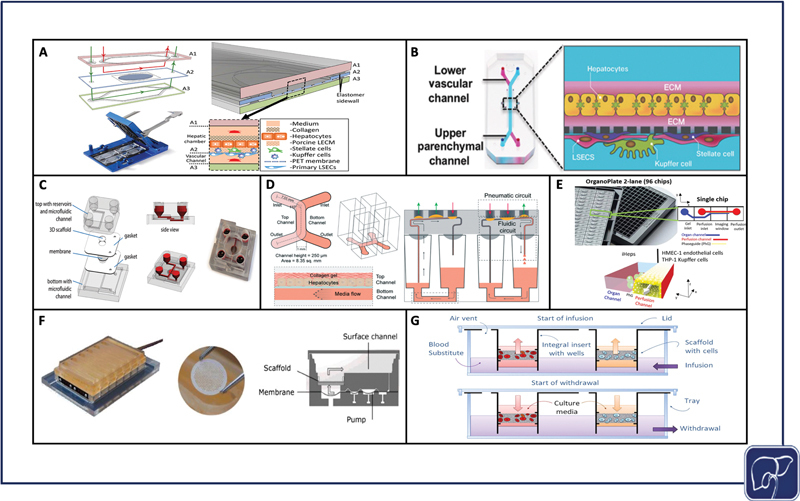
Significant physiomimetic perfusion platforms. PDMS zonation chip assembly (reproduced with permissions from Li et al) 122 ( A ), Emulate Liver-Chip assembly (reproduced with permissions from Jang et al) 169 ( B ), Hesperos liver chip assembly (reproduced with permissions from Esch et al) 164 ( C ), Draper PREDICT-96 chip assembly (reproduced with permissions from Tan et al) 165 ( D ), Mimetas OrganoPlate assembly (adapted from Bircsack et al under terms of CC BY) 166 ( E ), CN Bio Physiomimix assembly (reproduced with permissions from Long et al) 167 ( F ), Lena Biosciences PerfusionPal assembly (adapted from Shoemaker et al under terms of CC BY) 168 ( G ).
In addition to the materials used to assemble the platform, the cell matrix that is utilized has a significant impact on cell functionality and viability. 3D cell cultures in hydrogels and scaffolds have substantial benefits over monolayers ( Fig. 3C–G ), 172 173 174 175 176 but membrane monolayers provide opportunity for physiological cross-talk between co-cultures ( Fig. 3A, B ). 130 177 3D cultures separated by membranes combine some of the benefits of both 3D and membrane culture, but risk losing a degree of proximity when cells are encapsulated in separate gels, versus cultured opposite each other on the same membrane. For viral infection, it is imperative the material selection factors support the immediate access of virions to cell surface entry receptors. It is also critical that virions are able to pass readily through gels and scaffolds to reach the cell membrane for subsequent entry into the cytoplasm.
A final consideration for material choice is access to imaging, particularly for in situ imaging. Imaging intracellular viral proteins is an effective method for confirming the presence of productive infection. Transparent materials are crucial for any in situ imaging, and total thickness must be concordant with the focal length of the microscope being utilized. Any in situ fluorescence imaging also requires permeability to fluorescent dyes.
Accessibility and Throughput
Accessibility and throughput are critical for generating reproducible data that can be tested and corroborated through a multitude of different assays and multiple replicates. For viral infection, assaying cells for the production of viable virions and studying the virus life-cycle, at both transcriptional and protein levels, is common practice to support physiologically relevant viral studies. To run these analyses, the user must be able to access cells in the platform to isolate intracellular nucleic acids, protein, and any other cellular material that would provide insight on the establishment of a productive infection. Additional considerations include access to spent media to assay for viral replication and the production of inflammatory cytokines, and the device's amenability to imaging, both at end point and in situ, as described in the Materials section. HF bioreactors, for example, are useful tools for recreating a physiological microenvironment, but they are large systems that are not conducive to testing a large number of replicates or conditions. Plate-based formats that include open well access like the PREDICT-96 platform ( Fig. 3D ), 173 OrganoPlate ( Fig. 3E ), 174 CN Bio Physiomimix chip ( Fig. 3F ), 175 178 179 180 and PerfusionPal ( Fig. 3G ) 176 lend themselves most naturally to functional access to biologic materials and to support high throughput studies.
Media Dynamics and Composition
The benefit of perfusion in culture is well-documented, 181 but different platforms implement different methods for perfusing cells. Five commonly used methods include peristaltic pumping perfusion, 182 pneumatic pumping perfusion, 175 syringe pumping perfusion, 130 pumpless rocking perfusion, 174 and pumpless hydrostatic pressure-driven perfusion. 160 Peristaltic and pneumatic pumps create pulsatile flow while flow from syringe pumps is continuous. Peristaltic and pneumatic pumps are better for establishing unidirectional flow, especially at high flow rates. For devices that use perfusion to simulate blood flow and establish zonation, unidirectional flow is more physiologically relevant than bidirectional flow. Syringe pumps can also be used for unidirectional flow, but they are limited by volume, whereas peristaltic and pneumatic pumps can run indefinitely in one direction. Rocking perfusion is bidirectional, which is non-ideal for creating zonation, but it can still generate physiologic shear stress and nutrient exchange. Hydrostatic pressure-driven perfusion is passive and slow, so attaining physiological shear is more difficult, but these systems are able to promote nutrient delivery and flow unidirectionally. As stated previously, flow rate and size are completely intertwined, because a physiologically appropriate flow rate depends on the dimensions of the culture platform. Pump systems provide more accurate and tunable control over flow rate than pumpless systems, but pumpless systems are typically cheaper and easier to use.
Another consideration for sustaining cell co-cultures is media composition. Finding a culture medium that is agreeable to all cell types in the culture is a lengthy process, and can vary significantly when using different cell types and sources. For in vitro work, no universal media exists, so selecting a common media that can sustain all resident cells is of the utmost importance. This process becomes increasingly difficult with each cell type added, especially for multiorgan platforms that use different tissues in addition to different cell types within each tissue. No obvious solution to this issue exists, and approaches vary drastically depending on the setup of the platform and spatial distribution of cell types. There are several common approaches for solving the media dilemma with multiple cell types. The first, useful in cultures with a large variety of cell types, is to maintain a tissue-to-liquid ratio. In this way, the composition of the common media mirrors the ratio of each cell type. 157 A second option, for liver-based systems, is to cater specifically to hepatocytes and to utilize an optimal hepatocyte media as a common media for the entire system. 89 139 This approach is more frequently implemented when the system is designed to study one cell type specifically, and may be especially useful in the case of viral hepatitis infection models where only the hepatocyte is infected. The third method for media selection is specific to platforms that are spatially divided by cell type, but these systems allow for different cell types to receive their optimal media, to ensure the function of each cell type individually. 183 The challenge with this method is in preventing the mixture of the distinct medias into a homogenous solution, while still permitting cellular cross-talk between separate culture chambers. Without a nutrient-rich universal blood substitute, media composition will always be a necessary consideration for optimizing a co-culture platform. To improve infection specifically, media can be supplemented with a variety of small molecules and chemicals. Janus-kinase inhibitors, 107 polyethylene glycol (PEG), 184 and DMSO 184 185 may improve both the infectability and support maintenance of differentiation of more sophisticated in vitro liver platforms.
Clinical Translation of Physiomimetic Models
As physiomimetic models gain momentum as desirable alternatives to traditional in vitro models, it is important to consider the role they may play in facilitating the translation from in vitro study to clinical applications. Physiomimetic platforms may have the capacity to significantly expedite the transition from in vitro models to animal models. For example, Jang et al published cross-species drug toxicity data using the Emulate Liver Chip ( Fig. 3B ), and demonstrated species-dependent differences in toxicity response to a proprietary Janssen compound that was previously discontinued due to liver toxicity in rats. This high degree of toxicity was corroborated from results using rat hepatocytes on the in vitro platform, but when the same experiment was conducted on human hepatocytes, no such toxic effect was observed. Conversely, when testing a different proprietary compound that was discontinued due to hepatocellular necrosis in dogs, comparisons of dog and human hepatocyte data were aligned in their toxic response trends. 177 These findings are indicative of the potential for liver-on-chip platforms to evade species-dependent discrepancies during drug development, thereby expediting the process of translation from bench to clinic. While many of the advances in using liver-on-chip devices as a bridge to clinically relevant study have come in the context of drug discovery, 177 there is ripe potential to use these same platforms to conduct patient-specific studies to better understand case-by-case disease etiologies, make predictions on outcomes, and personalize treatments. The drug discovery pipeline typically progresses linearly, moving toward increasingly complex models; however, liver-on-chip platforms may soon be able to supplement and enhance this process, driving personalized medicine forward while supporting a bidirectional interplay between the clinic and bench ( Fig. 4 ).
Fig. 4.
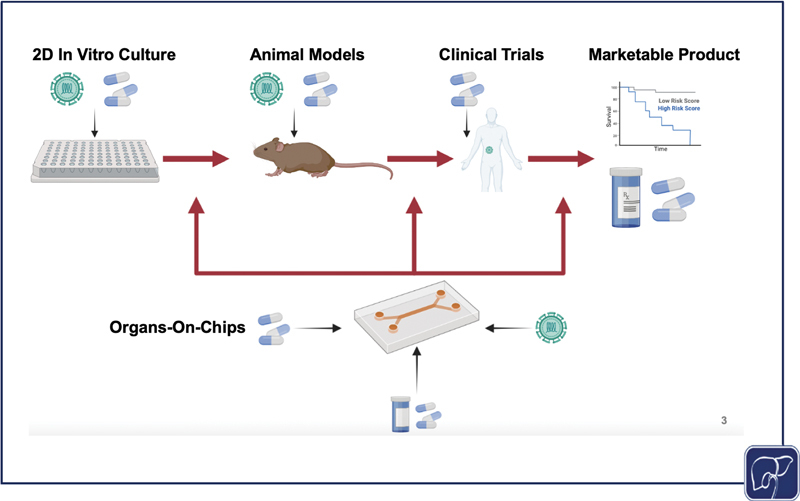
The standard process of disease study and drug development progresses linearly, starting with in vitro testing, moving next to animal models, then conducting clinical trials for drugs and using samples from diseased patients, finally resulting in compounds that are brought to market and clinical data from diseased patients that can be used to predict outcomes and further study the disease. However, a physiomimetic platform allows for a dynamic interplay between in vitro and animal models, and clinical data. If effective, in vitro models can be used to corroborate findings from animal models, predict outcomes from animal models, test and study clinical diseases and treatments in vivo, and predict treatment outcomes for patients as a personalized medicine approach. All of this information can enhance and expedite bench to clinic pipeline (the image was generated using Biorender).
Conclusions
Physiomimetic models are a promising tool for studying viral infection in the liver, but are currently in their nascent stages of development for this application. Initially, the focus for developing physiomimetic liver models has been directed primarily toward studying drug toxicity, which serves a paramount role in the pharmaceutical industry given that liver toxicity is a leading cause of failure in drug development. However, as these tools continue to develop and become more widely available, physiomimetic liver platforms hold the potential to greatly enhance how viral infection in the liver is studied in vitro, by promoting and sustaining a functional hepatocyte phenotype that is conducive to supporting long-term infection. Much of the foundation for using liver models to study viral infection has already been achieved through the development of drug toxicity and other disease models. It is now of utmost importance to find the best methods of translating these toxicity and disease models to viral infection applications specifically given the recent COVID-19 pandemic. The end goal for in vitro viral infection models would be that a physiomimetic system reaches an application convergence point, which can be used to study infection, subsequent disease, and pertinent therapeutics, all in a single study using an optimal platform.
Funding Statement
Funding This work was supported by NIH-NIGMS grant R35GM124915.
Conflict of Interest None declared.
Authors' Contribution
Paper concept, design, and drafting of the manuscript were performed by D.M., D.B., M.H., T.B., E.T., and A.A.
References
- 1.Rouse B T, Sehrawat S. Immunity and immunopathology to viruses: What decides the outcome? Nat Rev Immunol. 2010;10(07):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Do A, Reau N S. Chronic viral hepatitis: current management and future directions. Hepatol Commun. 2020;4(03):329–341. doi: 10.1002/hep4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringelhan M, McKeating J A, Protzer U.Viral hepatitis and liver cancerPhilos Trans R Soc Lond B Biol Sci 2017;372(1732):20160274 :20160274 [DOI] [PMC free article] [PubMed]
- 4.Kileng H, Bernfort L, Gutteberg T. Future complications of chronic hepatitis C in a low-risk area: projections from the hepatitis c study in Northern Norway. BMC Infect Dis. 2017;17(01):624. doi: 10.1186/s12879-017-2722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Ayer T, Adee M G, Wang X, Kanwal F, Chhatwal J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis C in the era of direct-acting antiviral agents. JAMA Netw Open. 2020;3(11):e2021173. doi: 10.1001/jamanetworkopen.2020.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawla P, Sunkara T, Muralidharan P, Raj J P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn) 2018;22(03):141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J D, Hainaut P, Gores G J, Amadou A, Plymoth A, Roberts L R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(09):e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 9.Low L A, Mummery C, Berridge B R, Austin C P, Tagle D A. Organs-on-chips: into the next decade. Nat Rev Drug Discov. 2021;20(05):345–361. doi: 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- 10.Mestas J, Hughes C CW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(05):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz S I, Zumbrun E E, Nalca A.Animal models of human viral diseasesAnim Models Study Hum Dis 2017 853–901
- 12.Dorner M, Horwitz J A, Robbins J B.A genetically humanized mouse model for hepatitis C virus infection Nature 2011474(7350):208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas E, Liang T J. Experimental models of hepatitis B and C - new insights and progress. Nat Rev Gastroenterol Hepatol. 2016;13(06):362–374. doi: 10.1038/nrgastro.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louz D, Bergmans H E, Loos B P, Hoeben R C. Animal models in virus research: their utility and limitations. Crit Rev Microbiol. 2013;39(04):325–361. doi: 10.3109/1040841X.2012.711740. [DOI] [PubMed] [Google Scholar]
- 15.Moradi E, Jalili-Firoozinezhad S, Solati-Hashjin M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 2020;116:67–83. doi: 10.1016/j.actbio.2020.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Rowe C, Gerrard D T, Jenkins R. Proteome-wide analyses of human hepatocytes during differentiation and dedifferentiation. Hepatology. 2013;58(02):799–809. doi: 10.1002/hep.26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers M J, Domansky K, Kaazempur-Mofrad M R. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78(03):257–269. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 18.Torresi J, Tran B M, Christiansen D, Earnest-Silveira L, Schwab R HM, Vincan E. HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models. BMC Cancer. 2019;19(01):707. doi: 10.1186/s12885-019-5916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allweiss L, Dandri M.Experimental in vitro and in vivo models for the study of human hepatitis B virus infection J Hepatol 201664(1, Suppl):S17–S31. [DOI] [PubMed] [Google Scholar]
- 20.Chen S L, Morgan T R. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(02):47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveria Andrade L J, D'Oliveira A, Melo R C, De Souza E C, Costa Silva C A, Paraná R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1(01):33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axley P, Ahmed Z, Ravi S, Singal A K. Hepatitis C virus and hepatocellular carcinoma: a narrative review. J Clin Transl Hepatol. 2018;6(01):79–84. doi: 10.14218/JCTH.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag H B.Hepatocellular carcinoma and hepatitis C in the United States Hepatology 200236(5, Suppl 1):S74–S83. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou G N, Beste L A, Green P K. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology. 2019;157(05):1264–1.278E7. doi: 10.1053/j.gastro.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahon P, Ganne-Carrié N. Management of patients with pre-therapeutic advanced liver fibrosis following HCV eradication. JHEP Rep. 2019;1(06):480–489. doi: 10.1016/j.jhepr.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi D, Mi G, Wang M, Webster T J. In vitro and ex vivo systems at the forefront of infection modeling and drug discovery. Biomaterials. 2019;198:228–249. doi: 10.1016/j.biomaterials.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dash S, Aydin Y, Widmer K E, Nayak L. Hepatocellular carcinoma mechanisms associated with chronic HCV infection and the impact of direct-acting antiviral treatment. J Hepatocell Carcinoma. 2020;7:45–76. doi: 10.2147/JHC.S221187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(05):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali N. Relationship between COVID-19 infection and liver injury: a review of recent data. Front Med (Lausanne) 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marjot T, Moon A M, Cook J A. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(03):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z H, Yang D L. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25(01):54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. Semin Immunopathol. 2009;31(03):399–409. doi: 10.1007/s00281-009-0155-6. [DOI] [PubMed] [Google Scholar]
- 33.Rocha F G. 5th ed. Philadelphia: W.B. Saunders; 2012. Liver blood flow: physiology, measurement, and clinical relevance [Internet] pp. 74–86. [Google Scholar]
- 34.Lautt W W. Hepatic circulation: physiology and pathophysiology . San Rafael, CA: Morgan & Claypool Life Sciences; 2009. Colloquium Series on Integrated Systems Physiology: From Molecule to Function to Disease; pp. 1–174. [PubMed] [Google Scholar]
- 35.Tonon F, Giobbe G G, Zambon A. In vitro metabolic zonation through oxygen gradient on a chip. Sci Rep. 2019;9(01):13557. doi: 10.1038/s41598-019-49412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Xu M-J, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13(03):301–315. doi: 10.1038/cmi.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng J, Wei W, Chen Z. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: a review. Micromachines (Basel) 2019;10(10):676. doi: 10.3390/mi10100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treyer A, Müsch A. Hepatocyte polarity. Compr Physiol. 2013;3(01):243–287. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCuskey R. 6th ed. Saint Louis: W.B. Saunders; 2012. Anatomy of the liver [Internet] [Google Scholar]
- 40.Bhatia S N, Balis U J, Yarmush M L, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Li J, Wang X, Sang M, Ho W. Hepatic stellate cells, liver innate immunity, and hepatitis C virus. J Gastroenterol Hepatol. 2013;28 01:112–115. doi: 10.1111/jgh.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12(46):7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyun J, McMahon R S, Lang A L. HIV and HCV augments inflammatory responses through increased TREM-1 expression and signaling in Kupffer and Myeloid cells. PLoS Pathog. 2019;15(07):e1007883. doi: 10.1371/journal.ppat.1007883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckwitt C H, Clark A M, Wheeler S. Liver ‘organ on a chip’. Exp Cell Res. 2018;363(01):15–25. doi: 10.1016/j.yexcr.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Z-Y, Weng S-Y, Yu Y. Signal molecule-mediated hepatic cell communication during liver regeneration. World J Gastroenterol. 2009;15(46):5776–5783. doi: 10.3748/wjg.15.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giugliano S, Kriss M, Golden-Mason L. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148(02):392–4.02E15. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni Y, Li J-M, Liu M-K. Pathological process of liver sinusoidal endothelial cells in liver diseases. World J Gastroenterol. 2017;23(43):7666–7677. doi: 10.3748/wjg.v23.i43.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baiocchini A, Del Nonno F, Taibi C. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci Rep. 2019;9(01):8760. doi: 10.1038/s41598-019-45114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poisson J, Lemoinne S, Boulanger C. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66(01):212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Boltjes A, van Montfoort N, Biesta P J. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211(08):1268–1278. doi: 10.1093/infdis/jiu599. [DOI] [PubMed] [Google Scholar]
- 51.Faure-Dupuy S, Delphin M, Aillot L. Hepatitis B virus-induced modulation of liver macrophage function promotes hepatocyte infection. J Hepatol. 2019;71(06):1086–1098. doi: 10.1016/j.jhep.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Yuan F, Zhang W, Mu D, Gong J. Kupffer cells in immune activation and tolerance toward HBV/HCV infection. Adv Clin Exp Med. 2017;26(04):739–745. doi: 10.17219/acem/62759. [DOI] [PubMed] [Google Scholar]
- 53.Boltjes A, Movita D, Boonstra A, Woltman A M. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61(03):660–671. doi: 10.1016/j.jhep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 54.Bai Q, An J, Wu X. HBV promotes the proliferation of hepatic stellate cells via the PDGF-B/PDGFR-β signaling pathway in vitro. Int J Mol Med. 2012;30(06):1443–1450. doi: 10.3892/ijmm.2012.1148. [DOI] [PubMed] [Google Scholar]
- 55.Martín-Vílchez S, Sanz-Cameno P, Rodríguez-Muñoz Y. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology. 2008;47(06):1872–1883. doi: 10.1002/hep.22265. [DOI] [PubMed] [Google Scholar]
- 56.Thomas E, Gonzalez V D, Li Q. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142(04):978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng J-C, Tseng C-P, Liao M-H. Activation of hepatic stellate cells by the ubiquitin C-terminal hydrolase 1 protein secreted from hepatitis C virus-infected hepatocytes. Sci Rep. 2017;7(01):4448. doi: 10.1038/s41598-017-04259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang Y BA, Eo J, Mert S, Yarmush M L, Usta O B. Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci Rep. 2018;8(01):8951. doi: 10.1038/s41598-018-27179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kietzmann T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 2017;11:622–630. doi: 10.1016/j.redox.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreau M, Rivière B, Vegna S. Hepatitis C viral proteins perturb metabolic liver zonation. J Hepatol. 2015;62(02):278–285. doi: 10.1016/j.jhep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Chang M-L. Metabolic alterations and hepatitis C: from bench to bedside. World J Gastroenterol. 2016;22(04):1461–1476. doi: 10.3748/wjg.v22.i4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wahlicht T, Vièyres G, Bruns S A. Controlled functional zonation of hepatocytes in vitro by engineering of Wnt signaling . ACS Synth Biol. 2020;9(07):1638–1649. doi: 10.1021/acssynbio.9b00435. [DOI] [PubMed] [Google Scholar]
- 63.Stebbing J, Sánchez Nievas G, Falcone M. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(01):eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Saccon E, Appelberg K S.Type-I interferon signatures in SARS-CoV-2 infected Huh7 cellsbioRxiv 2021 2021.02.04.429738 [DOI] [PMC free article] [PubMed]
- 65.Chen D-Y, Khan N, Close B J.SARS-CoV-2 desensitizes host cells to interferon through inhibition of the JAK-STAT pathwaybioRxiv 2020:2020.10.27.358259
- 66.Li J, Zong L, Sureau C, Barker L, Wands J R, Tong S. Unusual features of sodium taurocholate cotransporting polypeptide as a hepatitis B virus receptor. J Virol. 2016;90(18):8302–8313. doi: 10.1128/JVI.01153-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Geng X C, Wang J F, Miao Y F, Lu Y L, Li B. The HepaRG cell line, a superior in vitro model to L-02, HepG2 and hiHeps cell lines for assessing drug-induced liver injury. Cell Biol Toxicol. 2016;32(01):37–59. doi: 10.1007/s10565-016-9316-2. [DOI] [PubMed] [Google Scholar]
- 68.Shlomai A, Schwartz R E, Ramanan V. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci U S A. 2014;111(33):12193–12198. doi: 10.1073/pnas.1412631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Qu B, Zhang F. Stem cell-derived hepatocyte-like cells as model for viral hepatitis research. Stem Cells Int. 2019;2019:9.605252E6. doi: 10.1155/2019/9605252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simoneau C R, Ott M. Modeling multi-organ infection by SARS-CoV-2 using stem cell technology. Cell Stem Cell. 2020;27(06):859–868. doi: 10.1016/j.stem.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia Y, Carpentier A, Cheng X. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66(03):494–503. doi: 10.1016/j.jhep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz R E, Bram Y, Frankel A. Pluripotent stem cell-derived hepatocyte-like cells: a tool to study infectious disease. Curr Pathobiol Rep. 2016;4(03):147–156. doi: 10.1007/s40139-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai D, Mills C, Yu W. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56(08):4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seeger C, Sohn J A. Targeting hepatitis B virus with CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Catanese M T, Dorner M. Advances in experimental systems to study hepatitis C virus in vitro and in vivo. Virology. 2015;479-480:221–233. doi: 10.1016/j.virol.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 76.El-Shamy A, Hotta H. Impact of hepatitis C virus heterogeneity on interferon sensitivity: an overview. World J Gastroenterol. 2014;20(24):7555–7569. doi: 10.3748/wjg.v20.i24.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dustin L B, Bartolini B, Capobianchi M R, Pistello M. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22(10):826–832. doi: 10.1016/j.cmi.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y-P, Ramirez S, Gottwein J M. Robust full-length hepatitis C virus genotype 2a and 2b infectious cultures using mutations identified by a systematic approach applicable to patient strains. Proc Natl Acad Sci U S A. 2012;109(18):E1101–E1110. doi: 10.1073/pnas.1203829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takayama K. In vitro and animal models for SARS-CoV-2 research. Trends Pharmacol Sci. 2020;41(08):513–517. doi: 10.1016/j.tips.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wanner N, Andrieux G, Badia-I-Mompel P. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4(03):310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nie J, Li Q, Wu J. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9(01):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Zhu Y, Zhao X.ASGR1 is a candidate receptor for SARS-CoV-2 that promotes infection of liver cells [Internet]2022 [cited May 11, 2022];2022.01.15.476426. Accessed on Dec 29, 2022, at: https://www.biorxiv.org/content/10.1101/2022.01.15.476426v1.full.pdf
- 83.Mesel-Lemoine M, Millet J, Vidalain P-O. A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes. J Virol. 2012;86(14):7577–7587. doi: 10.1128/JVI.00269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao X, Guo F, Liu F. Interferon induction of IFITM proteins promotes infection by human coronavirus OC43. Proc Natl Acad Sci U S A. 2014;111(18):6756–6761. doi: 10.1073/pnas.1320856111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malik Y A. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42(01):3–11. [PubMed] [Google Scholar]
- 86.Xu R, Hu P, Li Y, Tian A, Li J, Zhu C. Advances in HBV infection and replication systems in vitro. Virol J. 2021;18(01):105. doi: 10.1186/s12985-021-01580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lohmann V. Hepatitis C virus cell culture models: an encomium on basic research paving the road to therapy development. Med Microbiol Immunol (Berl) 2019;208(01):3–24. doi: 10.1007/s00430-018-0566-x. [DOI] [PubMed] [Google Scholar]
- 88.Petropolis D B, Faust D M, Tolle M. Human liver infection in a dish: easy-to-build 3D liver models for studying microbial infection. PLoS One. 2016;11(02):e0148667. doi: 10.1371/journal.pone.0148667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khetani S R, Bhatia S N. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26(01):120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 90.Khetani S R, Kanchagar C, Ukairo O. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci. 2013;132(01):107–117. doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 91.Wang W W, Khetani S R, Krzyzewski S, Duignan D B, Obach R S. Assessment of a micropatterned hepatocyte coculture system to generate major human excretory and circulating drug metabolites. Drug Metab Dispos. 2010;38(10):1900–1905. doi: 10.1124/dmd.110.034876. [DOI] [PubMed] [Google Scholar]
- 92.Aleo M D, Ukairo O, Moore A, Irrechukwu O, Potter D M, Schneider R P. Liver safety evaluation of endothelin receptor antagonists using HepatoPac ® : a single model impact assessment on hepatocellular health, function and bile acid disposition . J Appl Toxicol. 2019;39(08):1192–1207. doi: 10.1002/jat.3805. [DOI] [PubMed] [Google Scholar]
- 93.Chan T S, Yu H, Moore A, Khetani S R, Tweedie D. Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac. Drug Metab Dispos. 2019;47(01):58–66. doi: 10.1124/dmd.113.053397fullarticlecorrection. [DOI] [PubMed] [Google Scholar]
- 94.Trask O J, Jr, Moore A, LeCluyse E L. A micropatterned hepatocyte coculture model for assessment of liver toxicity using high-content imaging analysis. Assay Drug Dev Technol. 2014;12(01):16–27. doi: 10.1089/adt.2013.525. [DOI] [PubMed] [Google Scholar]
- 95.Ware B R, Berger D R, Khetani S R. Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci. 2015;145(02):252–262. doi: 10.1093/toxsci/kfv048. [DOI] [PubMed] [Google Scholar]
- 96.Ploss A, Khetani S R, Jones C T. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci U S A. 2010;107(07):3141–3145. doi: 10.1073/pnas.0915130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winer B Y, Huang T S, Pludwinski E. Long-term hepatitis B infection in a scalable hepatic co-culture system. Nat Commun. 2017;8(01):125. doi: 10.1038/s41467-017-00200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winer B Y, Gaska J M, Lipkowitz G. Analysis of host responses to hepatitis B and delta viral infections in a micro-scalable hepatic co-culture system. Hepatology. 2020;71(01):14–30. doi: 10.1002/hep.30815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bachmann A, Moll M, Gottwald E. 3D cultivation techniques for primary human hepatocytes. Microarrays (Basel) 2015;4(01):64–83. doi: 10.3390/microarrays4010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crignis E D, Romal S, Carofiglio F. Human liver organoids; a patient-derived primary model for HBV infection and related hepatocellular carcinoma. bioRxiv. 2020:568147. doi: 10.7554/eLife.60747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nie Y-Z, Zheng Y-W, Miyakawa K. Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114–123. doi: 10.1016/j.ebiom.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ananthanarayanan A, Nugraha B, Triyatni M, Hart S, Sankuratri S, Yu H. Scalable spheroid model of human hepatocytes for hepatitis C infection and replication. Mol Pharm. 2014;11(07):2106–2114. doi: 10.1021/mp500063y. [DOI] [PubMed] [Google Scholar]
- 103.Tran N M, Dufresne M, Duverlie G.An appropriate selection of a 3D alginate culture model for hepatic Huh-7 cell line encapsulation intended for viral studies Tissue Eng Part A 201319(1-2):103–113. [DOI] [PubMed] [Google Scholar]
- 104.Molina-Jimenez F, Benedicto I, Dao Thi V L. Matrigel-embedded 3D culture of Huh-7 cells as a hepatocyte-like polarized system to study hepatitis C virus cycle. Virology. 2012;425(01):31–39. doi: 10.1016/j.virol.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Cho N-J, Elazar M, Xiong A. Viral infection of human progenitor and liver-derived cells encapsulated in three-dimensional PEG-based hydrogel. Biomed Mater. 2009;4(01):11001. doi: 10.1088/1748-6041/25/1/011001. [DOI] [PubMed] [Google Scholar]
- 106.Rajalakshmy A R, Malathi J, Madhavan H N, Samuel J KA. Mebiolgel, a thermoreversible polymer as a scaffold for three dimensional culture of Huh7 cell line with improved hepatocyte differentiation marker expression and HCV replication. Indian J Med Microbiol. 2015;33(04):554–559. doi: 10.4103/0255-0857.167330. [DOI] [PubMed] [Google Scholar]
- 107.Carpentier A, Sheldon J, Vondran F WR, Brown R J, Pietschmann T. Efficient acute and chronic infection of stem cell-derived hepatocytes by hepatitis C virus. Gut. 2020;69(09):1659–1666. doi: 10.1136/gutjnl-2019-319354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L, Han Y, Nilsson-Payant B E. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(01):125–1.36E9. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lui V C-H, Hui K P-Y, Babu R O.Human liver organoid derived intra-hepatic bile duct cells support SARS-CoV-2 infection and replication and its comparison with SARS-CoV [Internet]2021 [cited May 11, 2022]; 2021.02.10.21251458. Accessed on Dec 29, 2022, at: https://www.medrxiv.org/content/10.1101/2021.02.10.21251458v1.full.pdf [DOI] [PMC free article] [PubMed]
- 110.Zhao B, Ni C, Gao R. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rossi E A, Quintanilha L F, Nonaka C KV, Souza B SF.Advances in hepatic tissue bioengineering with decellularized liver bioscaffold[Internet]Stem Cells Int 201920192.693189E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Z, Xu H, Mazza G. Decellularized human liver scaffold-based three-dimensional culture system facilitate hepatitis B virus infection. J Biomed Mater Res A. 2019;107(08):1744–1753. doi: 10.1002/jbm.a.36690. [DOI] [PubMed] [Google Scholar]
- 113.Darnell M, Ulvestad M, Ellis E, Weidolf L, Andersson T B. In vitro evaluation of major in vivo drug metabolic pathways using primary human hepatocytes and HepaRG cells in suspension and a dynamic three-dimensional bioreactor system. J Pharmacol Exp Ther. 2012;343(01):134–144. doi: 10.1124/jpet.112.195834. [DOI] [PubMed] [Google Scholar]
- 114.Aoki Y, Aizaki H, Shimoike T. A human liver cell line exhibits efficient translation of HCV RNAs produced by a recombinant adenovirus expressing T7 RNA polymerase. Virology. 1998;250(01):140–150. doi: 10.1006/viro.1998.9361. [DOI] [PubMed] [Google Scholar]
- 115.Kawada M, Nagamori S, Aizaki H. Massive culture of human liver cancer cells in a newly developed radial flow bioreactor system: ultrafine structure of functionally enhanced hepatocarcinoma cell lines. In Vitro Cell Dev Biol Anim. 1998;34(02):109–115. doi: 10.1007/s11626-998-0092-z. [DOI] [PubMed] [Google Scholar]
- 116.Aizaki H, Nagamori S, Matsuda M. Production and release of infectious hepatitis C virus from human liver cell cultures in the three-dimensional radial-flow bioreactor. Virology. 2003;314(01):16–25. doi: 10.1016/s0042-6822(03)00383-0. [DOI] [PubMed] [Google Scholar]
- 117.Pihl A F, Offersgaard A F, Mathiesen C K. High density Huh7.5 cell hollow fiber bioreactor culture for high-yield production of hepatitis C virus and studies of antivirals. Sci Rep. 2018;8(01):17505. doi: 10.1038/s41598-018-35010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoffe B, Darlington G J, Soriano H E. Cultures of human liver cells in simulated microgravity environment. Adv Space Res. 1999;24(06):829–836. doi: 10.1016/s0273-1177(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 119.Chang T T, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng Part A. 2009;15(03):559–567. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leite S B, Wilk-Zasadna I, Zaldivar J M. Three-dimensional HepaRG model as an attractive tool for toxicity testing. Toxicol Sci. 2012;130(01):106–116. doi: 10.1093/toxsci/kfs232. [DOI] [PubMed] [Google Scholar]
- 121.Sainz B, Jr, TenCate V, Uprichard S L. Three-dimensional Huh7 cell culture system for the study of hepatitis C virus infection. Virol J. 2009;6:103. doi: 10.1186/1743-422X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalyanaraman B, Supp D M, Boyce S T. Medium flow rate regulates viability and barrier function of engineered skin substitutes in perfusion culture. Tissue Eng Part A. 2008;14(05):583–593. doi: 10.1089/tea.2007.0237. [DOI] [PubMed] [Google Scholar]
- 123.Huh D, Hamilton G A, Ingber D E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ortega-Prieto A M, Skelton J K, Cherry C, Briones-Orta M A, Hateley C A, Dorner M. “Liver-on-a-chip” cultures of primary hepatocytes and Kupffer cells for hepatitis B virus infection. J Vis Exp. 2019;(144):58333. doi: 10.3791/58333. [DOI] [PubMed] [Google Scholar]
- 125.Ortega-Prieto A M, Skelton J K, Wai S N. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat Commun. 2018;9(01):682. doi: 10.1038/s41467-018-02969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang Y, Rawat S, Duchemin N, Bouchard M, Noh M. Human liver sinusoid on a chip for hepatitis B virus replication study. Micromachines (Basel) 2017;8:27. [Google Scholar]
- 127.Natarajan V, Simoneau C R, Erickson A L. Modelling T-cell immunity against hepatitis C virus with liver organoids in a microfluidic coculture system. Open Biol. 2022;12(03):210320. doi: 10.1098/rsob.210320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Broutier L, Mastrogiovanni G, Verstegen M MA. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ouchi R, Togo S, Kimura M. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019;30(02):374–3.84E8. doi: 10.1016/j.cmet.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li X, George S M, Vernetti L, Gough A H, Taylor D LA. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip. 2018;18(17):2614–2631. doi: 10.1039/c8lc00418h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kostrzewski T, Cornforth T, Snow S A. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease . World J Gastroenterol. 2017;23(02):204–215. doi: 10.3748/wjg.v23.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kostrzewski T, Maraver P, Ouro-Gnao L. A microphysiological system for studying nonalcoholic steatohepatitis. Hepatol Commun. 2019;4(01):77–91. doi: 10.1002/hep4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gori M, Simonelli M C, Giannitelli S M, Businaro L, Trombetta M, Rainer A. Investigating nonalcoholic fatty liver disease in a liver-on-a-chip microfluidic device. PLoS One. 2016;11(07):e0159729. doi: 10.1371/journal.pone.0159729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feaver R E, Cole B K, Lawson M J. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight. 2016;1(20):e90954. doi: 10.1172/jci.insight.90954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mukherjee S, Zhelnin L, Sanfiz A. Development and validation of an in vitro 3D model of NASH with severe fibrotic phenotype. Am J Transl Res. 2019;11(03):1531–1540. [PMC free article] [PubMed] [Google Scholar]
- 136.Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D, Romeo S. Human multilineage 3D spheroids as a model of liver steatosis and fibrosis. Int J Mol Sci. 2019;20(07):1629. doi: 10.3390/ijms20071629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norona L M, Nguyen D G, Gerber D A, Presnell S C, LeCluyse E L. Editor's highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci. 2016;154(02):354–367. doi: 10.1093/toxsci/kfw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Norona L M, Nguyen D G, Gerber D A, Presnell S C, Mosedale M, Watkins P B. Bioprinted liver provides early insight into the role of Kupffer cells in TGF-β1 and methotrexate-induced fibrogenesis. PLoS One. 2019;14(01):e0208958. doi: 10.1371/journal.pone.0208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vernetti L A, Senutovitch N, Boltz R. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood) 2016;241(01):101–114. doi: 10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bell C C, Hendriks D FG, Moro S ML. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:25187. doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lachowski D, Cortes E, Rice A, Pinato D, Rombouts K, Del Rio Hernandez A. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. 2019;9(01):7299. doi: 10.1038/s41598-019-43759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wells R G.The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis J Clin Gastroenterol 200539(4, Suppl 2):S158–S161. [DOI] [PubMed] [Google Scholar]
- 143.Clark A M, Wheeler S E, Young C L. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip. 2016;17(01):156–168. doi: 10.1039/c6lc01171c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Methotrexate (Rheumatrex, Trexall) [Internet][cited March 10, 2021]. Accessed November 25, 2022 at:https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Treatments/Methotrexate-Rheumatrex-Trexall
- 145.Leite S B, Roosens T, El Taghdouini A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016;78:1–10. doi: 10.1016/j.biomaterials.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 146.Deng J, Chen Z, Zhang X. A liver-chip-based alcoholic liver disease model featuring multi-non-parenchymal cells. Biomed Microdevices. 2019;21(03):57. doi: 10.1007/s10544-019-0414-9. [DOI] [PubMed] [Google Scholar]
- 147.March S, Ng S, Velmurugan S. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax . Cell Host Microbe. 2013;14(01):104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ng S, March S, Galstian A. Hypoxia promotes liver-stage malaria infection in primary human hepatocytes in vitro. Dis Model Mech. 2014;7(02):215–224. doi: 10.1242/dmm.013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee S Y, Sung J H. Gut-liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol Bioeng. 2018;115(11):2817–2827. doi: 10.1002/bit.26793. [DOI] [PubMed] [Google Scholar]
- 150.Prot J M, Maciel L, Bricks T. First pass intestinal and liver metabolism of paracetamol in a microfluidic platform coupled with a mathematical modeling as a means of evaluating ADME processes in humans. Biotechnol Bioeng. 2014;111(10):2027–2040. doi: 10.1002/bit.25232. [DOI] [PubMed] [Google Scholar]
- 151.Esch M B, Ueno H, Applegate D R, Shuler M L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip. 2016;16(14):2719–2729. doi: 10.1039/c6lc00461j. [DOI] [PubMed] [Google Scholar]
- 152.Bricks T, Paullier P, Legendre A. Development of a new microfluidic platform integrating co-cultures of intestinal and liver cell lines. Toxicol In Vitro. 2014;28(05):885–895. doi: 10.1016/j.tiv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 153.Wagner I, Materne E-M, Brincker S. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. 2013;13(18):3538–3547. doi: 10.1039/c3lc50234a. [DOI] [PubMed] [Google Scholar]
- 154.Bovard D, Sandoz A, Luettich K. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip. 2018;18(24):3814–3829. doi: 10.1039/c8lc01029c. [DOI] [PubMed] [Google Scholar]
- 155.Vunjak-Novakovic G, Bhatia S, Chen C, Hirschi K. HeLiVa platform: integrated heart-liver-vascular systems for drug testing in human health and disease. Stem Cell Res Ther. 2013;4 01:S8. doi: 10.1186/scrt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Theobald J, Ghanem A, Wallisch P. Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater Sci Eng. 2018;4(01):78–89. doi: 10.1021/acsbiomaterials.7b00417. [DOI] [PubMed] [Google Scholar]
- 157.Maschmeyer I, Lorenz A K, Schimek K. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15(12):2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- 158.Ronaldson-Bouchard K, Teles D, Yeager K. A multi-organ chip with matured tissue niches linked by vascular flow. Nat Biomed Eng. 2022;6(04):351–371. doi: 10.1038/s41551-022-00882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim J-Y, Fluri D A, Kelm J M, Hierlemann A, Frey O. 96-well format-based microfluidic platform for parallel interconnection of multiple multicellular spheroids. J Lab Autom. 2015;20(03):274–282. doi: 10.1177/2211068214564056. [DOI] [PubMed] [Google Scholar]
- 160.Phan D TT, Wang X, Craver B M. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip. 2017;17(03):511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miller P G, Shuler M L. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng. 2016;113(10):2213–2227. doi: 10.1002/bit.25989. [DOI] [PubMed] [Google Scholar]
- 162.Ren K, Zhou J, Wu H. Materials for microfluidic chip fabrication. Acc Chem Res. 2013;46(11):2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 163.Qin D, Xia Y, Whitesides G M. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5(03):491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 164.Gökaltun A, Kang Y BA, Yarmush M L, Usta O B, Asatekin A. Simple surface modification of poly(dimethylsiloxane) via surface segregating smart polymers for biomicrofluidics. Sci Rep. 2019;9(01):7377. doi: 10.1038/s41598-019-43625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matellan C, Del Río Hernández A E. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci Rep. 2018;8(01):6971. doi: 10.1038/s41598-018-25202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee P J, Hung P J, Lee L P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng. 2007;97(05):1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 167.Deng J, Zhang X, Chen Z. A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. Biomicrofluidics. 2019;13(02):24101. doi: 10.1063/1.5070088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bavli D, Prill S, Ezra E. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2016;113(16):E2231–E2240. doi: 10.1073/pnas.1522556113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yajima Y, Lee C N, Yamada M, Utoh R, Seki M. Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers. J Biosci Bioeng. 2018;126(01):111–118. doi: 10.1016/j.jbiosc.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 170.Mi S, Yi X, Du Z, Xu Y, Sun W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication. 2018;10(02):25010. doi: 10.1088/1758-5090/aaa97e. [DOI] [PubMed] [Google Scholar]
- 171.Bhise N S, Manoharan V, Massa S. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(01):14101. doi: 10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 172.Esch M B, Prot J-M, Wang Y I. Multi-cellular 3D human primary liver cell culture elevates metabolic activity under fluidic flow. Lab Chip. 2015;15(10):2269–2277. doi: 10.1039/c5lc00237k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tan K, Keegan P, Rogers M. A high-throughput microfluidic microphysiological system (PREDICT-96) to recapitulate hepatocyte function in dynamic, re-circulating flow conditions. Lab Chip. 2019;19(09):1556–1566. doi: 10.1039/c8lc01262h. [DOI] [PubMed] [Google Scholar]
- 174.Bircsak K M, DeBiasio R, Miedel M. A 3D microfluidic liver model for high throughput compound toxicity screening in the OrganoPlate®. Toxicology. 2021;450:152667. doi: 10.1016/j.tox.2020.152667. [DOI] [PubMed] [Google Scholar]
- 175.Long T J, Cosgrove P A, Dunn R T., II Modeling therapeutic antibody-small molecule drug-drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab Dispos. 2016;44(12):1940–1948. doi: 10.1124/dmd.116.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shoemaker J T, Zhang W, Atlas S I, Bryan R A, Inman S W, Vukasinovic J. A 3D cell culture organ-on-a-chip platform with a breathable hemoglobin analogue augments and extends primary human hepatocyte functions in vitro. Front Mol Biosci. 2020;7:568777. doi: 10.3389/fmolb.2020.568777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jang K-J, Otieno M A, Ronxhi J. Reproducing human and cross-species drug toxicities using a liver-chip. Sci Transl Med. 2019;11(517):eaax5516. doi: 10.1126/scitranslmed.aax5516. [DOI] [PubMed] [Google Scholar]
- 178.Rowe C, Shaeri M, Large E. Perfused human hepatocyte microtissues identify reactive metabolite-forming and mitochondria-perturbing hepatotoxins. Toxicol In Vitro. 2018;46:29–38. doi: 10.1016/j.tiv.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 179.Sarkar U, Rivera-Burgos D, Large E M. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab Dispos. 2015;43(07):1091–1099. doi: 10.1124/dmd.115.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sarkar U, Ravindra K C, Large E. Integrated assessment of diclofenac biotransformation, pharmacokinetics, and omics-based toxicity in a three-dimensional human liver-immunocompetent coculture system. Drug Metab Dispos. 2017;45(07):855–866. doi: 10.1124/dmd.116.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee J-H, Ho K-L, Fan S-K. Liver microsystems in vitro for drug response. J Biomed Sci. 2019;26(01):88. doi: 10.1186/s12929-019-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Novik E, Maguire T J, Chao P, Cheng K C, Yarmush M L. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2010;79(07):1036–1044. doi: 10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jang M, Kleber A, Ruckelshausen T, Betzholz R, Manz A. Differentiation of the human liver progenitor cell line (HepaRG) on a microfluidic-based biochip. J Tissue Eng Regen Med. 2019;13(03):482–494. doi: 10.1002/term.2802. [DOI] [PubMed] [Google Scholar]
- 184.Michailidis E, Pabon J, Xiang K. A robust cell culture system supporting the complete life cycle of hepatitis B virus. Sci Rep. 2017;7(01):16616. doi: 10.1038/s41598-017-16882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dubois-Pot-Schneider H, Aninat C, Kattler K. Transcriptional and epigenetic consequences of DMSO treatment on HepaRG cells. Cells. 2022;11(15):2298. doi: 10.3390/cells11152298. [DOI] [PMC free article] [PubMed] [Google Scholar]


