Abstract
The COVID-19 mRNA vaccine was developed by the scalable manufacture of lipid nanoparticles (LNPs) that encapsulate mRNA within the lipid. There are many potential applications for this large nucleic acid delivery technology, including the delivery of plasmid DNA for gene therapy. However, gene therapy for the brain requires LNP delivery across the blood–brain barrier (BBB). It is proposed that LNPs could be reformulated for brain delivery by conjugation of receptor-specific monoclonal antibodies (MAbs) to the LNP surface. The MAb acts as a molecular Trojan horse to trigger receptor-mediated transcytosis (RMT) of the LNP across the BBB and subsequent localization to the nucleus for transcription of the therapeutic gene. Trojan horse LNPs could enable new approaches to gene therapy of the brain.
Keywords: blood–brain barrier, Trojan horse, plasmid DNA, lipid nanoparticle, monoclonal antibody
Beyond current LNP technology and gene therapy of the brain
The mRNA coronavirus 2019 (COVID-19) vaccines were produced with a LNP technology platform that enables the delivery of large mRNA nucleic acids to tissues in vivo following systemic administration via an intramuscular injection [1,2]. Mass production of the COVID-19 vaccines was enabled by the development of a scalable manufacturing process for the encapsulation of large nucleic acids within the interior of the LNP. In addition to mRNA, plasmid DNA for gene therapy may be encapsulated within the lipids forming the LNP [3,4]. Following the lead of the COVID-19 vaccine, it is now possible to envisage the scalable manufacture of plasmid DNA gene nanomedicines using the LNP platform technology. The brain is a promising target for gene therapy, as plasmid DNA/LNP nanomedicines have been proposed for the treatment of multiple central nervous system (CNS) disorders, including gene silencing/editing [5,6], childhood genetic disorders [7], and adult illness such as Alzheimer’s disease [8,9], Parkinson’s disease (PD) [10], amyotrophic lateral sclerosis [11], brain cancer [12,13], and stroke [14]. However, a limiting problem in the development of DNA/LNP nanomedicines for the brain is that LNPs do not cross the BBB [15]. BBB passage of plasmid DNA carried within LNPs is possible following the attachment of a receptor-specific MAb to the surface of the LNP. The MAb binds a receptor, such as the insulin receptor (INSR) or transferrin receptor (TfR), on the BBB and acts as a molecular Trojan horse to enable RMT of the LNP across the BBB and into the extracellular space (ECS) of the brain [16]. Trojan horse LNPs have enabled the expression of plasmid DNA in the brain in vivo in mice, rats, and monkeys [17., 18., 19.]. The purpose of this opinion article is to propose that the combination of the BBB Trojan horse and LNP technologies can extend the therapeutic reach of LNPs beyond vaccines to plasmid-DNA-based gene nanomedicines for multiple brain disorders. This article first reviews the development of LNPs that gave rise to the current scalable manufacture of COVID-19 vaccines, then discusses the BBB Trojan horse technology applied to LNPs. It is possible to encapsulate within LNPs plasmid DNA as large as 22 kb, which includes an 18-kb gene expression cassette [20]. Therefore, the future development of Trojan horse LNPs may enable placement of the therapeutic gene under the influence of tissue-specific gene promoters.
Nucleic acid delivery with LNPs
Cationic lipoplexes
LNP is a generic term that includes both cationic lipoplexes and pegylated liposomes [21]. The COVID-19 vaccines encapsulate mRNA in the interior of the pegylated-liposome type of LNP. However, nucleic-acid-based vaccines were originally developed as a cationic-lipoplex type of LNP for either plasmid DNA [22] or mRNA [23]. Lipoplexes were formed by mixing the anionic nucleic acid with a 1:1 mixture of a cationic lipid, N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTMA), and a helper lipid, dioleoyl phosphatidylethanolamine (DOPE). DOTMA is a quaternary ammonium compound that is positively charged at both neutral and acidic pH. The DOTMA/DOPE mixture, known as LipofectinTM, is still used today for transfection of cultured cells. The DOTMA/DOPE LNPs were originally designated cationic liposomes. However, DNA lipoplexes do not form classical liposomal structures. Liposomes comprise a lipid bilayer forming a spherical shell housing an aqueous interior [24]. By contrast, the cationic lipid/anionic DNA lipoplexes form aggregated cylindrical micelles of the cationic lipid around the anionic DNA [25,26]. The DNA lipoplexes aggregate in saline [27] or serum [28] to form micron-sized structures, which trigger uptake via phagocytosis in cultured cells [29]. Following intravenous (IV) administration of the DNA lipoplexes, the aggregates are trapped in the lung microcirculation [28], which is the first vascular bed encountered following IV injection. Gene expression in the lung is 2–3 log orders greater than in other organs such as the liver following the IV administration of DNA lipoplexes [30,31]. Saline-induced aggregation of DNA lipoplexes enables the transfection of phagocytosing cells in culture. However, this aggregation restricts the utility of DNA or RNA lipoplexes for gene expression in vivo following systemic administration such as with an intramuscular injection. For these reasons, the cationic-lipoplex type of LNP was not used for the development of the COVID-19 vaccine.
Pegylated liposomes
The COVID-19 vaccines comprise mRNA encapsulated within pegylated liposomes. Pegylated liposomes are LNPs where the surface of the liposome is coated with a corona of polyethyleneglycol (PEG) (i.e., pegylation). The pegylation is important because the PEG corona prevents the adsorption of serum proteins on the liposome surface, which triggers rapid uptake in vivo of the LNP by the macrophage–phagocyte system [21,32]. The encapsulation of plasmid DNA in the interior of pegylated liposomes, which contain a small amount (e.g., 3–15%) of a quaternary cationic lipid such as dimethyldioctadecylammonium bromide (DDAB) or dioleyldimethylammonium chloride (DOTAC), was described using a thin-film/hydration/extrusion method [3] or a dialysis detergent method [4]. Plasmid DNAs encapsulated within pegylated liposomes do not aggregate and do not selectively target the lung in vivo [33]. However, the thin-film/hydration/extrusion and dialysis detergent methods are not scalable and do not allow commercialization of the LNP technology for nucleic acid delivery [21,34]. A pivotal innovation in the development of LNPs for nucleic acid delivery emerged with the description of a scalable manufacturing process to encapsulate plasmid DNA within LNPs using an ethanol-dilution method [35]. The lipids were diluted to 20 mM with 90% ethanol in parallel with suspension of the plasmid DNA in citrate buffer/pH 5 at a DNA concentration of ~1 mg/ml. The lipids/ethanol and DNA/citrate were rapidly mixed to a final ethanol concentration of 45%. Unencapsulated plasmid DNA was removed by filtration through an anion-exchange filter and the ethanol was removed by dialysis or filtration [35]. The lipids included 15% 1,2-dioleyloxy-3-dimethylaminopropane (DODMA), which is an ionizable tertiary amine lipid, 55% cholesterol, 20% 1,2-distearoyl-sn-glycero-3-phosphocholine, and 10% PEG lipid with a C18 acyl side chain [35]. The ethanol-dilution method for encapsulation of nucleic acids within LNPs parallels other work describing the production of small-vesicle liposomes by ethanol dilution [36,37] and the ethanol-induced condensation of single plasmid DNA molecules [38., 39., 40.]. Encapsulation of plasmid DNA within liposomes is enabled by the simultaneous condensation of the DNA and the formation of the small liposome vesicles that is induced during the rapid ethanol-dilution process. DNA condensation is necessary for encapsulation of DNA within the LNP as the gyration radius, 80–125 nm, of a single plasmid DNA is large compared with the radius, 50–75 nm, of the LNP [39].
COVID-19 mRNA vaccines
The COVID-19 mRNA vaccines were mass produced with the ethanol-dilution method [41,42]. The amount of ionizable lipid was increased to 46–50% of either ALC-0315, for the BNT162b2 vaccine, or SM-102, for the mRNA-1273 vaccine [42,43]. These ionizable lipids are positively charged at the pH 4–5 used to encapsulate the RNA within the LNP but are uncharged at the neutral pH of the cytosol, and this is believed to facilitate endosomal release of the mRNA within the cell [21,44]. The PEG lipids of either vaccine contain C14 acyl side chains [42]. The shorter-chain PEG lipids dissociate from the surface of the liposome in the bloodstream faster than the PEG lipids with C16 or C18 acyl chains [45]. Faster dissociation of the PEG lipid from the liposome surface is believed to allow faster coating of the liposome surface with plasma low-density lipoproteins (LDLs) such as ApoE [46]. The ApoE on the surface of the liposome is believed to trigger endocytosis via the LDL receptor (LDLR), primarily in the liver [44]. The high content of ionizable lipid, 46–50% of the total lipid, allows >70% mRNA encapsulation [41]. However, a part of the mRNA may not be encapsulated in the interior of the pegylated liposome, but may be bound at the exterior surface of the LNP by the cationic lipid. The external mRNA may play a proinflammatory role in vivo. The DNA component of DNA lipoplexes is proinflammatory in animals [47., 48., 49.], and evidence for an inflammatory reaction to the mRNA vaccines has been described [50., 51., 52.]. The external RNA on the surface of the LNP could be removed in the manufacturing process by RNase treatment followed by RNase removal. Although external RNA can be resistant to RNase when the liposome is formulated with a quaternary ammonium cationic lipid such as DDAB or DOTAP [53], the external RNA is degraded by RNase when the RNA is encapsulated within an LNP that is manufactured with an ionizable lipid [54].
The delivery of LNPs to the brain across the BBB, encapsulated with either RNA or DNA, is not enabled by the coating of a LDLR ligand, such as ApoE, on the liposome surface. This is because the LDLR is not expressed on the luminal side of the BBB in vivo [55]. Plasmid DNA encapsulated within LNPs can be formulated for BBB passage, but this requires the conjugation of a receptor-specific MAb, or Trojan horse, to the liposome surface to enable receptor-mediated transport across the BBB. An understanding of the biology of BBB transport, as well as the biology of receptor-mediated transport at the BBB, allows selection of the optimal receptor-specific antibodies for incorporation on the surface of the LNP. The selection of an effective MAb Trojan horse enables brain uptake and expression of a plasmid DNA encapsulated within the LNP.
BBB biology
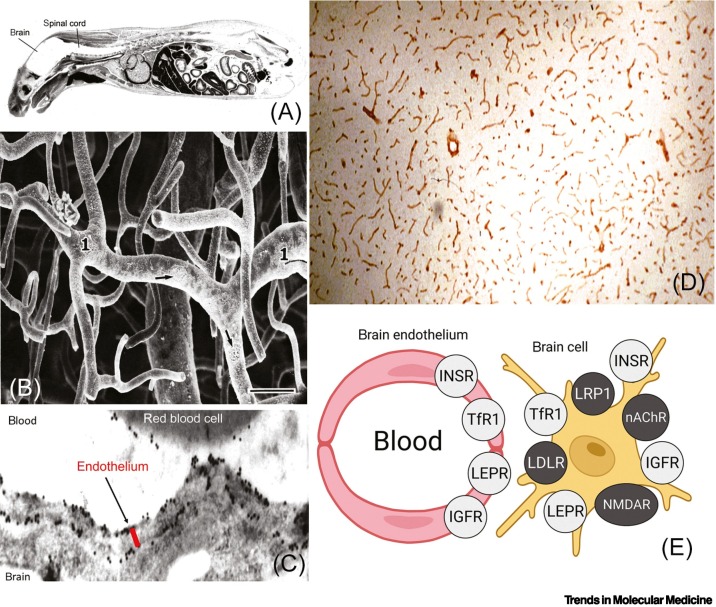
The BBB restricts brain uptake from blood of ~98% of small molecules and >99% of large molecules [55] The restricted brain uptake of histamine, a small molecule with a molecular weight (MW) of 111 Da, is shown in Figure 1A, which is a whole-body autoradiogram of a mouse following the IV administration of radiolabeled histamine. The small molecule enters all of the organs of the body except the brain and spinal cord [55]. The anatomical basis of the BBB is the presence of high-resistance tight junctions joining the endothelial cells of the brain capillaries [56]. The microvasculature of the human brain is revealed by vascular casts [57] and is shown in Figure 1B. The capillaries are about 40 μm apart [57], which is space for about two brain cells; therefore, every neuron in the brain is perfused by a brain capillary. Once the LNP crosses the BBB, the diffusion distance to the nearest brain cell is <20 μm. Diffusion of pegylated nanoparticles with a diameter of 100–150 nm through functional pores as large as 200 nm in the brain ECS was shown in vivo with fluorescence microscopy [58].
Figure 1.
Biology of blood–brain barrier (BBB) transport.
(A) Whole-body autoradiogram of mouse euthanized after intravenous (IV) injection of radiolabeled histamine. The BBB blocks the entry of this small molecule into the brain and spinal cord (shown in white), whereas histamine is taken up by all other organs. Reproduced from [55], CC BY 4.0 license. (B) The microvasculature of the brain is visualized by this plastic cast of the human cerebellar cortex, which shows an anastomosing network of capillaries in the brain. Bar, 40 μm; arrows point to endothelial nuclei. Position of parallel arterioles is indicated by '1'. Reproduced, with permission, from [57]; copyright © 1983 Elsevier. (C) Localization of the GLUT1 glucose transporter at the luminal and abluminal membranes of the endothelium of human brain is shown by the position of the gold particles (black dots) detected by this electron microscopy immunogold study. Post-embedding immune labeling of the GLUT1 transporter is detected with a primary antibody against the transporter and a 10-nm gold conjugate of a secondary antibody. The distance between the luminal and abluminal membranes, represented by the red bar, is only 0.3 μm [55]. The GLUT1 transporter is also localized to the plasma membrane of red cells in the capillary lumen of the human brain. Reproduced, with permission, from [59]; copyright © 1994 Sage Publications. (D) Immunohistochemistry of rat brain with a primary antibody against the leptin receptor (LEPR) shows high enrichment of the receptors in the microvasculature of the brain, which appear as red cylinders within the brain tissue. Reproduced, with permission, from [60]; copyright © 1998 John Wiley & Sons. (E) Summary of literature findings on the immunohistochemistry of the brain using primary antibodies against specific receptors [55]. Expression of the insulin receptor (INSR), transferrin receptor 1 (TfR), the LEPR, and the insulin-like growth factor 1 receptor (IGF1R) is detected at both the brain capillary endothelium, which forms the BBB, and the brain cell plasma membrane. By contrast, immunochemistry of the brain shows that other receptors, which have been targeted for brain drug delivery, including the low-density lipoprotein receptor (LDLR), LDLR-related protein 1 (LRP1), the nicotinic acetylcholine receptor (nAChR), and the N-methyl D-aspartate receptor (NMDAR), are expressed on brain cells on the abluminal side of the BBB. Reproduced from [55], CC BY 4.0 license.
LNPs may cross a vascular endothelial barrier by either a paracellular pathway via pores between endothelial cells or a transcellular pathway following endocytosis into the endothelium from the blood. The presence of tight junctions in the endothelium of brain capillaries eliminates any paracellular pathway for LNP transport across the brain microvascular barrier or BBB. Therefore, only a transcellular, or transcytosis, pathway is available for delivery to the brain [55]. The three pathways of BBB transport are carrier-mediated transport (CMT), absorptive mediated transport (AMT), and RMT [55]. The GLUT1 glucose transporter is an exemplary CMT system at the BBB. The expression of GLUT1 on both the luminal and the abluminal membrane of the capillary endothelium in the human brain is shown in Figure 1C, which is an electron microscopy immuno-gold study [59]. RMT systems include the INSR, the TfR, the leptin receptor (LEPR), and the insulin-like growth factor 1 receptor (IGF1R) [55]. The expression at the BBB of a RMT system, the LEPR, is shown with immunohistochemistry of the rat brain [60]. The LEPR is broadly expressed at the brain microvasculature, and the continuous immunostaining of the brain vessels is indicative of an endothelial origin of the LEPR (Figure 1D). Immunohistochemistry of the brain with receptor-specific antibodies shows that the INSR, TfR, LEPR, and IGFR are expressed at both the BBB and the brain cell membrane [55], as depicted in Figure 1E. Certain receptors are expressed on brain cells at the abluminal surface of the brain capillary without expression at the luminal membrane of the brain capillary endothelium (Figure 1E). These abluminal receptors include the LDLR, LDL-related protein 1 (LRP1), the nicotinic acetylcholine receptor (nAChR), and the N-methyl-D-aspartic acid receptor (NMDAR). BBB transport is enabled by the targeting of a receptor that is expressed on the luminal membrane of the brain capillary endothelium [55].
Receptor-mediated delivery of LNPs to the brain
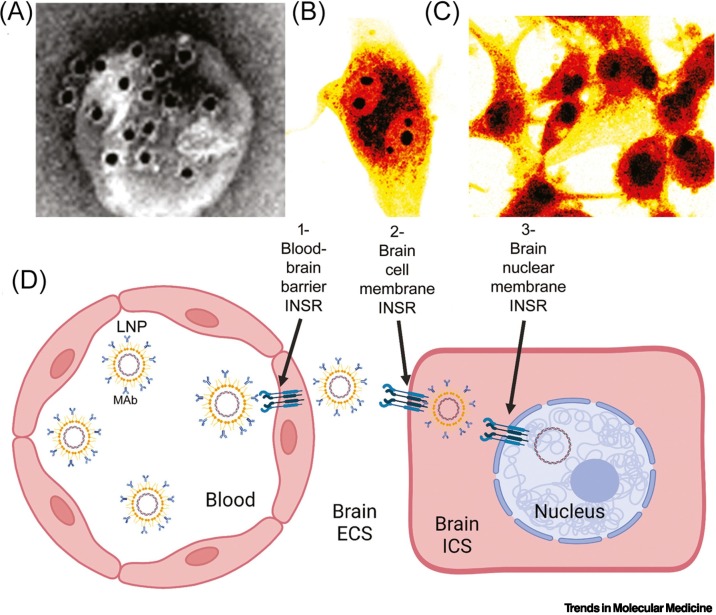
Conjugation of a Trojan horse to the surface of the LNP enables RMT through the BBB [15]. The optimal Trojan horse for a LNP is a MAb that binds exofacial epitopes on certain brain endothelial receptors, such as the INSR or TfR [16]. The MAb may be conjugated to the LNP via a thio-ether bond between the thiolated MAb and a maleimide moiety at the external tip of a fraction of the PEG strands on the surface of the LNP. The MAb-conjugated pegylated LNP is designated a Trojan horse LNP or a Trojan horse liposome (THL). An electron micrograph of a THL is shown in Figure 2A. A MAb targeting the rat TfR, designated TfRMAb, was conjugated to the surface of a 100-nm pegylated liposome encapsulating a plasmid DNA. The THL was mixed with a secondary antibody conjugated with 10-nm gold [16]. These gold particles are about the same size as the MAb on the LNP surface, and the electron micrograph shows that the MAb extended from the surface of the LNP via the PEG chain (Figure 2A). Optimization of the Trojan horse LNP for plasmid DNA delivery requires adequate conjugation of the MAb to the surface of the LNP, adequate encapsulation of the plasmid DNA within the interior of the LNP, the removal of unconjugated MAb, and the removal of plasmid DNA not encapsulated within the LNP [16].
Figure 2.
Trojan horse delivery of lipid nanoparticle (LNP)-encapsulated plasmid DNA across membrane barriers in brain.
(A) Electron micrograph of a Trojan horse LNP. The pegylated liposome encapsulating plasmid DNA is conjugated with a receptor-specific monoclonal antibody (MAb) at the tips of the polyethyleneglycol (PEG) strands on the surface of the LNP. The PEG-extended MAb is visualized with a 10-nm gold conjugate of a secondary antibody that binds the Trojan horse MAb on the LNP. Reproduced, with permission, from [16]; copyright © 2003 Elsevier. (B,C) Confocal microscopy of human U87 glioma cells at 3 h (B) and 24 h (C) of incubation with a fluoresceinated plasmid DNA encapsulated within a human insulin receptor (INSR) (HIR) MAb targeted LNP. At 3 h of incubation, the plasmid DNA is primarily located in the cytoplasm, with some distribution into the nucleolar compartment (B). At 24 h of incubation (C), the plasmid DNA is primarily localized to the nuclear compartment (C). Reproduced, with permission, from [61]; copyright © 2002 John Wiley & Sons. (D) Trojan horse LNPs encapsulated with plasmid DNA must traverse three barriers separating blood from the nuclear compartment of brain cells: (1) the blood–brain barrier (BBB), which comprises the luminal and abluminal membranes of the brain capillary endothelium; (2) the brain cell plasma membrane; and (3) the brain cell nuclear membrane. A MAb against the HIR, which is conjugated to the tips of the PEG strands at the surface of the LNP, binds the INSR expressed at the BBB to trigger receptor-mediated transcytosis of the LNP through the brain capillary endothelium. Following entry of the LNP into the brain extracellular space (ECS), HIRMAb then binds the INSR on the brain cell plasma membrane to trigger receptor-mediated endocytosis into the intracellular space (ICS) of brain cells. The INSR normally translocates to the nuclear membrane [63., 64., 65.], which enables delivery of the plasmid DNA encapsulated in LNPs to the nuclear compartment. Figure produced with Biorender.com.
Plasmid DNA delivery to human cells in culture or to Old World primates in vivo was enabled by the conjugation to the surface of the LNP of a MAb against the human INSR (HIR), designated HIRMAb. LNPs comprised 93% 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), a phospholipid, 3% cationic lipid (DDAB), and 4% C18 phospholipid conjugated with 2000 Da PEG [61]. POPC is a mixed saturated/unsaturated phospholipid that is abundant in mammalian cell membranes [62]. PEG lipids with long C18 acyl chains are used to produce THLs, as the shedding of the PEG lipid in vivo would result in loss of the targeting MAb from the surface of the LNP (see Outstanding questions). The intracellular delivery of the HIRMAb targeted LNPs was examined in human glioma cells. Fluoresceinated plasmid DNA was encapsulated within Trojan horse LNPs targeted with HIRMAb and applied to human U87 glioma cells in culture [61]. The intracellular distribution of the Trojan horse LNP at 3 and 24 h of incubation was examined by confocal microscopy. The THLs were primarily localized to the cytosolic compartment with some nucleolar distribution at 3 h (Figure 2B) and primarily to the nuclear compartment at 24 h (Figure 2C). The confocal microscopy study in Figure 2C demonstrates the delivery of the plasmid DNA to the nuclear compartment following uptake of the Trojan horse LNPs by the cell. The mechanism by which the plasmid DNA dissociates from the LNP is unknown. One factor underlying the nuclear delivery of plasmid DNA by HIRMAb-THLs is that the INSR normally translocates within the cell to the nuclear compartment [63., 64., 65.]. Recent evidence suggests that the INSR is internalized in the nucleus followed by binding to specific gene promoters [65]. Selective translocation of the INSR to the nucleus may underlie the greater expression of transgenes in the brain following THL delivery with HIRMAb compared with TfRMAb [19].
Outstanding questions.
What are the modifications in manufacture required to produce Trojan horse LNPs encapsulated with plasmid DNA, compared with the manufacture of mRNA LNP vaccines? Should quaternary ammonium or ionizable lipids be used? Ionizable lipid promotes early release of the mRNA into the cytosol. However, the plasmid DNA must bypass the cytosol to enter the nuclear compartment of the cell. Should PEG lipids with short C14 or long C18 acyl chains be used? The shorter C14 lipids, which promote early release in blood of the PEG strands from the LNP surface, may not be preferred when the Trojan horse is conjugated to the PEG.
How many grams of plasmid DNA encapsulated in Trojan horse LNPs must be manufactured on an annual basis to treat an orphan disease, which has a prevalence of about 1:100 000 compared with brain cancer, or PD, which has a higher disease prevalence?
What are the optimal gene promoters that should be used for plasmid-DNA-based gene therapy of the brain? Since plasmid DNA up to 22 kb has been encapsulated in Trojan horse LNPs, expression of the therapeutic gene can be regulated by large-sized tissue-specific gene promoters incorporated in the plasmid DNA.
Alt-text: Outstanding questions
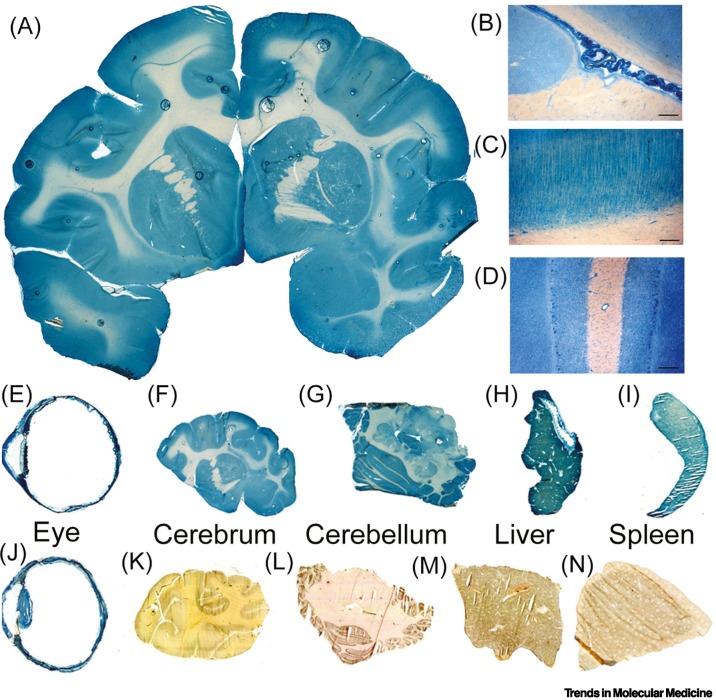
Expression of a transgene in the brain in vivo requires the THL to mediate the delivery of the plasmid DNA across three membranes in series: the BBB, the brain cell plasma membrane, and the brain cell nuclear membrane (Figure 2D). This was demonstrated in the adult rhesus monkey following IV administration of 12 μg/kg plasmid DNA encapsulated within HIRMAb targeted THLs [19]. The plasmid DNA encoded bacterial β-galactosidase (lacZ) under the influence of the widely expressed SV40 promoter. At 48 h after injection of the SV40-lacZ plasmid DNA encapsulated within THLs, the brain and peripheral organs were removed and examined with X-gal histochemistry. Global expression of the lacZ transgene throughout the primate brain was observed (Figure 3A). Light microscopy shows lacZ expression in the choroid plexus (Figure 3B), columns of the occipital cortex (Figure 3C), and the cerebellum (Figure 3D) of the brain. Similar global expression of the lacZ gene in the brain was observed in adult mice [17] and rats [18] following IV administration of TfRMAb-targeted THLs. However, when the THL was conjugated with the isotype control IgG, and not TfRMAb, no lacZ expression in mouse or rat brain was observed [17,18]. These findings show that the transgene expression in brain following THL administration is solely a function of the receptor specificity of the IgG Trojan horse conjugated to the THL.
Figure 3.
Delivery of a transgene to primate brain and peripheral organs and the effect of tissue-specific promoters on gene expression.
A plasmid DNA encoding the lacZ gene under the influence of either the widely expressed SV40 promoter (A–I) or the eye-specific promoter derived from the 5′ flanking sequence (FS) of the opsin gene (J–N) was encapsulated in human insulin receptor (HIR) monoclonal antibody (MAb) targeted lipid nanoparticles (LNPs) and injected intravenously in the adult rhesus monkey at a plasmid DNA dose of 12 μg/kg. Organs were removed at 48 h and lacZ gene expression was detected by X-gal histochemistry. (A) Coronal section of primate brain removed 48 h after LNP administration shows global expression of the SV40-lacZ transgene throughout the primate brain. (B,C,D) Light microscopy of the specific regions of the primate brain shows SV40-lacZ gene expression in the choroid plexus (B), the columns of the occipital cortex (C), and the cerebellum (D). The SV40-lacZ transgene is also expressed in the monkey eye (E), cerebrum (F), cerebellum (G), liver (H), and spleen (I). Conversely, the opsin-lacZ transgene is expressed only in the primate eye (J) and there is no expression of the opsin-lacZ in the monkey cerebrum (K), cerebellum (L), liver (M), or spleen (N). (A–I) reproduced from [19], CC BY 4.0 license. (J–N) reproduced from [66], CC BY NC ND 3.0 license.
Tissue-specific promoters and chromosomally derived genes
The IV administration of a lacZ expression plasmid, under the influence of the widely expressed SV40 promoter, encapsulated within HIRMAb-targeted THLs produced transgene expression in the primate cerebrum (Figure 3F) and cerebellum (Figures 3G) as well as in organs other than the brain such as the eye (Figure 3E), liver (Figure 3H), and spleen (Figure 3I). If the SV40 promoter was replaced with an eye-specific promoter taken from the 5′ flanking sequence (FS) of the opsin gene [66], lacZ expression in the primate eye was preserved (Figure 3J). However, no opsin-lacZ transgene expression was observed in the cerebrum (Figure 3K), cerebellum (Figure 3L), liver (Figure 3M), or spleen (Figure 3N) in the rhesus monkey [66]. These studies show that the combination of tissue-specific promoters and THL plasmid DNA delivery technology can restrict the expression of the transgene to the target organ. In addition to cDNAs under the influence of specific promoters, entire genes can be delivered to the brain with THLs. The 18-kb rat tyrosine hydroxylase (TH) gene, which comprises an 8.4-kb 5′-FS, a 7.3-kb coding region of 13 exons and 12 introns, and a 1.9-kb 3′-FS, was encoded in a 22-kb expression plasmid that was encapsulated within TfRMAb-targeted THLs [20]. Following IV administration in the rat, this chromosomal form of the TH transgene was expressed in the brain in vivo [20]. The ability to encapsulate plasmid DNA as large as 22 kb within Trojan horse LNPs will enable the future genetic engineering of expression plasmids encoding therapeutic genes under the influence of large tissue-specific gene promoters.
Therapeutic genes
Therapeutic genes encapsulated within Trojan horse LNPs have been delivered to rats with experimental PD, to mice with experimental brain cancer, and to transgenic mice with Niemann–Pick type C1 (NPC1) disease, and these investigations are summarized in Table 1 . PD is caused by degeneration of dopaminergic neurons in the nigral–striatal tract of the brain, and the rate-limiting enzyme in dopamine synthesis is TH. Expression of the TH gene in the brain is largely confined to the dopaminergic neurons of the nigral–striatal tract [67]. The goal of therapy in PD is to restore nigral–striatal TH enzyme activity, and this was accomplished with two types of therapeutic genes. In the first approach, a plasmid DNA was engineered where the expression cassette comprised the rat TH cDNA under the influence of a gene-specific promoter taken from 2 kb of the 5′-FS of the human glial fibrillary acidic protein (GFAP) gene [68]. Rats with experimental PD were treated with IV injections of THLs carrying the GFAP-TH plasmid DNA, and this treatment restored TH enzyme activity in the brain and reversed the abnormal motor activity associated with TH deficiency in the brain [68]. However, replacement of the TH gene in PD does not treat the underlying neural degeneration in this condition. Glial cell-derived neurotrophic factor (GDNF) exerts neurotrophic effects on the nigral–striatal region of the brain. Therefore, for GDNF gene therapy of PD, a 13-kb plasmid DNA was engineered that encoded human preproGDNF under the influence of the 8.4-kb rat TH promoter [69]. Weekly IV treatment of rats with PD resulted in prolonged normalization of both striatal TH enzyme activity and drug-induced motor behavior [69]. The therapeutic effects of THLs in brain cancer were examined in severe combined immunodeficiency disease (scid) mice implanted with intracranial U87 human glioma, which overexpresses the oncogenic human epidermal growth factor receptor (EGFR) [70]. The brain-tumor-bearing mice were treated with weekly IV injections of a plasmid DNA encapsulated within THLs; this plasmid DNA encoded an antisense RNA corresponding to nucleotide (nt) 2317–3006 of the human EGFR mRNA under the influence of the SV40 promoter. The repeat IV administration of THLs encapsulating plasmid DNA encoding EGFR antisense RNA resulted in a 100% increase in the survival time of the tumor-bearing mice, whereas the administration of THLs encapsulating a plasmid DNA encoding luciferase mRNA had no effect on mouse survival [70]. RNAi treatment of brain cancer was enabled by the engineering of a plasmid DNA that encoded a short hairpin RNA (shRNA) under the influence of the U6 promoter [71]. The shRNA targeted nt 2529–2557 of the human EGFR mRNA. Weekly IV administration of THLs encapsulating the plasmid DNA encoding this shRNA resulted in an 88% increase in the survival time of scid mice with intracranial U87 human gliomas [71]. For the treatment of NPC1 transgenic mice, a plasmid DNA was engineered where a 5.6-kb expression cassette comprised the 1.5-kb 5′-FS of the gene encoding human platelet-derived growth factor B (PDGFB), a neural specific promoter, the 3.9-kb human NPC1 open reading frame, and 0.2 kb of 3′ untranslated region [7]. NPC1 mice were treated chronically with weekly IV injections of THLs carrying the PDGFB-NPC1 plasmid DNA [7].
Table 1.
Therapy of preclinical models of neural disease with Trojan horse LNPs
Translation to humans of plasmid DNA delivery to the brain with Trojan horse LNPs requires both a favorable safety profile following chronic administration and downstream processing of the plasmid DNA encapsulated within the LNPs to enable long-term storage at non-freezing temperatures. The absence of toxicity of weekly IV injections of Trojan horse LNPs was demonstrated following chronic treatment of rats with weekly IV administration of THLs expressing the TH gene and targeted with TfRMAb. Chronic THL treatment showed no evidence of toxicity based on clinical chemistry, body weight, or organ histology, and immunohistochemistry of brain showed no evidence of inflammation [72]. The long-term storage and distribution under freezing temperatures of the COVID-19 mRNA vaccines is challenging [73,74]. Such problems may be avoided with freeze-dried formulations of plasmid DNA/LNP therapeutics. Following optimization of the lyoprotectant, plasmid DNA encapsulated within Trojan horse LNPs retains structural integrity and gene expression after hydration of lyophilized formulations [75].
Concluding remarks
The current clinical development of brain gene therapy utilizes viral vectors such as recombinant adenoassociated virus (rAAV), which have been FDA approved as single-use therapeutics for brain diseases such as spinal muscular atrophy [76]. However, the capsid proteins of rAAV are immunogenic [77]. In addition, the size of the expression cassette encoding the therapeutic gene is restricted to <2.3 kb and <4.7 kb for self-complementary and single-stranded rAAV, respectively [78]. Therefore, it is important to develop nonviral formulations of gene therapeutics, and this is possible with Trojan horse LNPs. To advance the Trojan horse LNP technology for brain gene therapy, what is needed is a clinical trial. The trial should target a serious inherited brain disorder for which there is no existing treatment, such as NPC1 disease. The preclinical drug development required for the submission of an Investigational New Drug (IND) application for the clinical trial can answer important questions related to manufacture and drug safety. An IND application provides data on the scalability and reproducibility of the manufacturing process, as well as preclinical data in animals on the dosage of the drug that causes no observable adverse effects. The Trojan horse LNP drug developer can use the COVID-19 vaccines as a model of a scalable manufacturing process for the encapsulation of large nucleic acids within LNPs. A clinical trial can address the fundamental questions of safety and efficacy in humans of nonviral gene therapy of the brain with Trojan horse LNPs and plasmid DNA gene therapeutics. The principal challenge in translating Trojan horse LNPs for gene therapy of the brain will be the manufacture of these formulations to insure both high conjugation of the antibody Trojan horse to the surface of the LNP and high encapsulation of the plasmid DNA in the interior of the LNP. The primary limitation of the technology is the lack of permanent expression of the transgene in the brain and the requirement for repeat administration of the therapeutic at intervals dictated by the stability of the plasmid DNA in brain cells.
Clinician’s corner.
The development of Trojan horse LNPs that encapsulate a plasmid DNA encoding a therapeutic gene for a CNS disorder would be a first-in-class therapy. The first clinical application should target an inherited brain disorder for which there is no treatment. As an example, NPC1 disease is a lysosomal storage disorder caused by mutations in an intracellular membrane cholesterol transporter, where enzyme replacement therapy is not possible. Acquired brain diseases that are candidates for gene therapy include neurodegenerative conditions such as PD, which can be treated with genes encoding neurotrophic factors, and brain cancer, which can be treated with antisense genes that knock down oncogenic proteins.
Whereas brain gene therapy with recombinant AAV is a single-dose treatment, gene therapy with plasmid DNA encapsulated within Trojan horse LNPs will involve chronic treatment of patients, as the plasmid DNA is degraded in the cell. Treatment regimens need to be developed (e.g., weekly, bimonthly, or monthly IV infusions). The frequency of administration is a function of the persistence of the plasmid DNA, and the transcribed mRNA, in the cell.
Alt-text: Clinician’s corner
Declaration of interests
The author has no interests to declare.
References
- 1.Corbett K.S., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 3.Monnard P.A., et al. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta. 1997;1329:39–50. doi: 10.1016/s0005-2736(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler J.J., et al. Stabilized plasmid–lipid particles: construction and characterization. Gene Ther. 1999;6:271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 5.Conniot J., et al. Revisiting gene delivery to the brain: silencing and editing. Biomater. Sci. 2021;9:1065–1087. doi: 10.1039/d0bm01278e. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y., et al. Blood–brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abc7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D., et al. Plasmid DNA gene therapy of the Niemann-Pick C1 mouse with transferrin receptor-targeted Trojan horse liposomes. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-70290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora S., et al. A review of brain-targeted nonviral gene-based therapies for the treatment of Alzheimer’s disease. Mol. Pharm. 2021;18:4237–4255. doi: 10.1021/acs.molpharmaceut.1c00611. [DOI] [PubMed] [Google Scholar]
- 9.Luo M., et al. Delivering the promise of gene therapy with nanomedicines in treating central nervous system diseases. Adv. Sci. (Weinh.) 2022;9 doi: 10.1002/advs.202201740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagaran K., Singh M. Lipid nanoparticles: promising treatment approach for Parkinson’s disease. Int. J. Mol. Sci. 2022;23:9361. doi: 10.3390/ijms23169361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ediriweera G.R., et al. Non-viral vector-mediated gene therapy for ALS: challenges and future perspectives. Mol. Pharm. 2021;18:2142–2160. doi: 10.1021/acs.molpharmaceut.1c00297. [DOI] [PubMed] [Google Scholar]
- 12.Luiz M.T., et al. Gene therapy based on lipid nanoparticles as non-viral vectors for glioma treatment. Curr. Gene Ther. 2021;21:452–463. doi: 10.2174/1566523220999201230205126. [DOI] [PubMed] [Google Scholar]
- 13.Tasset A., et al. Overcoming barriers in non-viral gene delivery for neurological applications. Nanoscale. 2022;14:3698–3719. doi: 10.1039/d1nr06939j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annu, et al. Nanoparticle mediated gene therapy: a trailblazer armament to fight CNS disorders. Curr. Med. Chem. 2022;30:304–315. doi: 10.2174/0929867329666220105122318. [DOI] [PubMed] [Google Scholar]
- 15.Huwyler J., et al. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardridge W.M. Gene targeting in vivo with pegylated immunoliposomes. Methods Enzymol. 2003;373:507–528. doi: 10.1016/S0076-6879(03)73032-8. [DOI] [PubMed] [Google Scholar]
- 17.Shi N., et al. Brain-specific expression of an exogenous gene after i.v. administration. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12754–12759. doi: 10.1073/pnas.221450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi N., et al. Receptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomes. Pharm. Res. 2001;18:1091–1095. doi: 10.1023/a:1010910523202. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., et al. Global non-viral gene transfer to the primate brain following intravenous administration. Mol. Ther. 2003;7:11–18. doi: 10.1016/s1525-0016(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 20.Xia C.F., et al. Comparison of cDNA and genomic forms of tyrosine hydroxylase gene therapy of the brain with Trojan horse liposomes. J. Gene Med. 2007;9:605–612. doi: 10.1002/jgm.1046. [DOI] [PubMed] [Google Scholar]
- 21.Hou X., et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felgner P.L., et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malone R.W., et al. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. U. S. A. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chonn A., Cullis P.R. Recent advances in liposomal drug-delivery systems. Curr. Opin. Biotechnol. 1995;6:698–708. doi: 10.1016/0958-1669(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 25.Radler J.O., et al. Structure of DNA–cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 26.Koltover I., et al. An inverted hexagonal phase of cationic liposome–DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 27.Finsinger D., et al. Protective copolymers for nonviral gene vectors: synthesis, vector characterization and application in gene delivery. Gene Ther. 2000;7:1183–1192. doi: 10.1038/sj.gt.3301227. [DOI] [PubMed] [Google Scholar]
- 28.Simberg D., et al. The role of organ vascularization and lipoplex–serum initial contact in intravenous murine lipofection. J. Biol. Chem. 2003;278:39858–39865. doi: 10.1074/jbc.M302232200. [DOI] [PubMed] [Google Scholar]
- 29.Matsui H., et al. Loss of binding and entry of liposome–DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J. Biol. Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- 30.Hong K., et al. Stabilization of cationic liposome–plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400:233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 31.Barron L.G., et al. Cationic lipids are essential for gene delivery mediated by intravenous administration of lipoplexes. Gene Ther. 1999;6:1179–1183. doi: 10.1038/sj.gt.3300929. [DOI] [PubMed] [Google Scholar]
- 32.Allen T.M. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol. Sci. 1994;15:215–220. doi: 10.1016/0165-6147(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 33.Monck M.A., et al. Stabilized plasmid–lipid particles: pharmacokinetics and plasmid delivery to distal tumors following intravenous injection. J. Drug Target. 2000;7:439–452. doi: 10.3109/10611860009102218. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni J.A., et al. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28:146–157. doi: 10.1089/nat.2018.0721. [DOI] [PubMed] [Google Scholar]
- 35.Jeffs L.B., et al. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm. Res. 2005;22:362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 36.Batzri S., Korn E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta. 1973;298:1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 37.Stano P., et al. Novel camptothecin analogue (gimatecan)-containing liposomes prepared by the ethanol injection method. J. Liposome Res. 2004;14:87–109. doi: 10.1081/lpr-120039794. [DOI] [PubMed] [Google Scholar]
- 38.Roy K.B., et al. Ethanol-induced condensation of calf thymus DNA studied by laser light scattering. J. Phys. Chem. B. 1999;103:5117–5121. [Google Scholar]
- 39.Bailey A.L., Sullivan S.M. Efficient encapsulation of DNA plasmids in small neutral liposomes induced by ethanol and calcium. Biochim. Biophys. Acta. 2000;1468:239–252. doi: 10.1016/s0005-2736(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., et al. Ethanol induces condensation of single DNA molecules. Soft Matter. 2011;7:4425–4434. [Google Scholar]
- 41.Hassett K.J., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carreno J.M., et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine. 2022;40:6114–6124. doi: 10.1016/j.vaccine.2022.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenmaker L., et al. mRNA–lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni J.A., et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 45.Mui B.L., et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids. 2013;2 doi: 10.1038/mtna.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akinc A., et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan Y., et al. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Mol. Ther. 2001;3:673–682. doi: 10.1006/mthe.2001.0311. [DOI] [PubMed] [Google Scholar]
- 48.Yew N.S., et al. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum. Gene Ther. 1999;10:223–234. doi: 10.1089/10430349950019011. [DOI] [PubMed] [Google Scholar]
- 49.Ito Y., et al. Evaluation of proinflammatory cytokine production and liver injury induced by plasmid DNA/cationic liposome complexes with various mixing ratios in mice. Eur. J. Pharm. Biopharm. 2009;71:303–309. doi: 10.1016/j.ejpb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Hernandez A.F., et al. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol. Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tahtinen S., et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022;23:532–542. doi: 10.1038/s41590-022-01160-y. [DOI] [PubMed] [Google Scholar]
- 52.Moghimi S.M., Simberg D. Pro-inflammatory concerns with lipid nanoparticles. Mol. Ther. 2022;30:2109–2110. doi: 10.1016/j.ymthe.2022.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brito L.A., et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014;22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blakney A.K., et al. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26:363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pardridge W.M. A historical review of brain drug delivery. Pharmaceutics. 2022;14:1283. doi: 10.3390/pharmaceutics14061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brightman M.W., Reese T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duvernoy H., et al. The vascularization of the human cerebellar cortex. Brain Res. Bull. 1983;11:419–480. doi: 10.1016/0361-9230(83)90116-8. [DOI] [PubMed] [Google Scholar]
- 58.Nance E.A., et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornford E.M., et al. The human brain GLUT1 glucose transporter: ultrastructural localization to the blood–brain barrier endothelia. J. Cereb. Blood Flow Metab. 1994;14:106. doi: 10.1038/jcbfm.1994.15. [DOI] [PubMed] [Google Scholar]
- 60.Boado R.J., et al. Up-regulation of blood–brain barrier short-form leptin receptor gene products in rats fed a high fat diet. J. Neurochem. 1998;71:1761–1764. doi: 10.1046/j.1471-4159.1998.71041761.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y., et al. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J. Gene Med. 2002;4:183–194. doi: 10.1002/jgm.255. [DOI] [PubMed] [Google Scholar]
- 62.Yepuri N.R., et al. Synthesis of perdeuterated 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine ([D82]POPC) and characterisation of its lipid bilayer membrane structure by neutron reflectometry. Chempluschem. 2016;81:315–321. doi: 10.1002/cplu.201500452. [DOI] [PubMed] [Google Scholar]
- 63.Podlecki D.A., et al. Nuclear translocation of the insulin receptor. A possible mediator of insulin's long term effects. J. Biol. Chem. 1987;262:3362–3368. [PubMed] [Google Scholar]
- 64.Amaya M.J., et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59:274–283. doi: 10.1002/hep.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batista T.M., et al. The insulin receptor goes nuclear. Cell Res. 2019;29:509–511. doi: 10.1038/s41422-019-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., et al. Organ-specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol. Vis. 2003;9:465–472. [PubMed] [Google Scholar]
- 67.Simunovic F., et al. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain. 2009;132:1795–1809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., et al. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental Parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Pardridge W.M. Near complete rescue of experimental Parkinson’s disease with intravenous, non-viral GDNF gene therapy. Pharm. Res. 2009;26:1059–1063. doi: 10.1007/s11095-008-9815-9. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., et al. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol. Ther. 2002;6:67–72. doi: 10.1006/mthe.2002.0633. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., et al. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin. Cancer Res. 2004;10:3667–3677. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y.F., et al. Absence of toxicity of chronic weekly intravenous gene therapy with pegylated immunoliposomes. Pharm. Res. 2003;20:1779–1785. doi: 10.1023/b:pham.0000003375.13655.f9. [DOI] [PubMed] [Google Scholar]
- 73.Uddin M.N., Roni M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines (Basel) 2021;9:1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J., et al. Dataset of ultralow temperature refrigeration for COVID 19 vaccine distribution solution. Sci. Data. 2022;9:67. doi: 10.1038/s41597-022-01167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H., et al. Lyoprotectant optimization for the freeze-drying of receptor-targeted Trojan horse liposomes for plasmid DNA delivery. Mol. Pharm. 2020;17:2165–2174. doi: 10.1021/acs.molpharmaceut.0c00310. [DOI] [PubMed] [Google Scholar]
- 76.Mendell J.R., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 77.Ertl H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hudry E., et al. Efficient gene transfer to the central nervous system by single-stranded Anc80L65. Mol. Ther. Methods Clin. Dev. 2018;10:197–209. doi: 10.1016/j.omtm.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]