Abstract
A potential relationship between dysregulation of immune/inflammatory pathways and cognitive impairment has been suggested in severe mental illnesses (SMI), such as schizophrenia (SZ) and bipolar (BD) spectrum disorders. However, multivariate relationships between peripheral inflammatory/immune-related markers and cognitive domains are unclear, and many studies do not account for inter-individual variance in both cognitive functioning and inflammatory/immune status. This study aimed to investigate covariance patterns between inflammatory/immune-related markers and cognitive domains and further elucidate heterogeneity in a large SMI and healthy control (HC) cohort (SZ = 343, BD = 289, HC = 770). We applied canonical correlation analysis (CCA) to identify modes of maximum covariation between a comprehensive selection of cognitive domains and inflammatory/immune markers. We found that poor verbal learning and psychomotor processing speed was associated with higher levels of interleukin-18 system cytokines and beta defensin 2, reflecting enhanced activation of innate immunity, a pattern augmented in SMI compared to HC. Applying hierarchical clustering on covariance patterns identified by the CCA revealed a high cognition—low immune dysregulation subgroup with predominantly HC (24% SZ, 45% BD, 74% HC) and a low cognition—high immune dysregulation subgroup predominantly consisting of SMI patients (76% SZ, 55% BD, 26% HC). These subgroups differed in IQ, years of education, age, CRP, BMI (all groups), level of functioning, symptoms and defined daily dose (DDD) of antipsychotics (SMI cohort). Our findings suggest a link between cognitive impairment and innate immune dysregulation in a subset of individuals with severe mental illness.
Subject terms: Schizophrenia, Molecular biology, Bipolar disorder
Introduction
Schizophrenia (SZ) and bipolar (BD) spectrum disorders are complex severe mental illnesses (SMI) with shared genetic risk factors and neurobiological mechanisms [1]. Cognitive impairments are prevalent and considered a core feature of SMI [2]. These deficits may precede the onset of mental illness [3–5], often persist throughout the illness course [6, 7], and predict poor clinical and functional outcomes [8–11]. Although cognitive impairments are less severe in BD than in SZ, there is substantial heterogeneity within diagnostic categories [12, 13]. It is unclear what underlies the variation in cognitive functioning in SMI, though it is likely due to the complex interplay between genetic susceptibility, biological mechanisms, and environmental factors [14].
One potential biological correlate to cognitive impairment is systemic immune abnormalities such as dysregulated inflammatory pathways. Chronic, low-grade inflammation and immune activation is a risk factor for cognitive impairment in the general population [15–18]. Furthermore, dysregulated inflammatory pathways have been associated with the pathophysiology of SMI [19, 20]. Evidence of immune involvement is supported by genome-wide association studies (GWAS) identifying immune-related genotypes [21–23], and observations of dysregulated levels of inflammatory/immune markers in SMI [20, 24–27]. Such observations have been linked to the more frequent occurrence of somatic comorbidities, particularly cardiovascular disease [28, 29]. Importantly, inflammatory and immune-related processes may influence the central nervous system (CNS) through alteration of the blood-brain barrier (BBB) and modulation of immunocompetent brain cells such as astrocytes and microglia [30–33]. Experimental studies indicate that abnormal glial cell activation may impair neuronal development and homeostasis [34–37]. Thus, it has been suggested that dysregulated immune and inflammatory processes may contribute to cognitive impairments in SMI [38, 15–17].
A recent meta-analysis however, only showed weak associations between immune activation and cognition in case-control SMI studies [18]. Notably, previous studies have focused on individual markers, neglecting the complex interaction between different inflammatory and immune-related signalling systems [39]. Furthermore, recent studies indicate substantial inter-individual differences and potential subgroups of cognitive and inflammatory/immune profiles, which case control studies fail to detect [13, 16, 40]. Such findings are promising as identifying subgroups could help determine who may benefit from personalized treatments.
Recent work has identified subgroups based on comprehensive assessment of inflammatory/immune markers [41–45]. These studies have consistently identified two inflammatory subtypes, with a higher frequency of SMI and healthy controls (HC) in the high and low subtype, respectively. Moreover, the high inflammation subtype in SMI has been associated with poorer response to antipsychotic treatment, greater cortical thickness, and cognitive impairment, but with no differences in symptom severity [16, 41, 43, 44, 46, 47]. While these studies have increased our knowledge of inflammatory subtypes in SMI, interpretation and clinical relevance is limited due to low sample sizes.
Identifying subgroups based on biological data alone could capture variability unrelated to core SMI features such as cognitive functioning. One solution is to investigate immune/inflammatory markers that share variance with cognition [48]. To our knowledge, no previous studies have identified subgroups based on both cognitive functioning and inflammatory/immune markers in SMI. This could help elucidate whether cognitive impairment and elevated levels of immune/inflammatory markers co-occur. This approach has shown merit in SMI studies based on other biological and behavioral data [49, 50], using canonical correlation analysis (CCA), which is a dimension reduction technique that can identify multivariate associations between two sets of variables. Output from CCA can subsequently be used to identify subgroups that have potential clinical relevance [49].
The present study aimed to further elucidate the association between cognitive functioning and inflammation/immune activation in SMI and investigate heterogeneity across diagnostic categories. Using a novel multivariate approach, CCA, we investigated patterns of covariance between cognitive domains and a broad range of inflammatory/immune markers in a large SMI and HC cohort. This approach has the potential of shedding light on immune- and inflammatory pathways relevant for cognitive functioning. We then applied hierarchical clustering on their patterns of covariance to investigate heterogeneity across diagnostic categories. Here we include nine core cognitive domains that are sensitive to the range of cognitive impairments in SMI [6]. We investigate a large array of both novel and previously established immune/inflammatory markers associated with SMI that may link peripheral and neuroinflammation. These include markers related to neuroinflammation, BBB integrity, cell adhesion molecules (CAMs) that facilitate migration of leukocytes across the BBB, defensins secreted by neutrophils that may modulate innate and adaptive immune responses within the brain and potentially cause collateral damage to the BBB, chemokines that may promote migration to, and across, the BBB, and markers reflecting both adaptive and innate immunity including markers of the interleukin (IL)-18 family as part of the inflammasome system. We address previous concerns related to CCA and clustering techniques [48, 51] by performing cross-validation and stability analyses to evaluate model performance.
Methods
Sample
The current study is part of the ongoing Thematically Organized Psychosis (TOP)-study at the Norwegian Center for Mental Disorders Research (NORMENT) aimed at investigating the underlying mechanisms of SMI. Amongst other themes, the study evaluates specific research questions on the role of inflammation and immune activation in SMI, and the current study is part of this aim. Participants meeting the Diagnostic Manual of Mental Disorders (DSM)-IV criteria for SZ or BD spectrum disorders are recruited from psychiatric units (out-patient and in-patient) in the larger Oslo area. The public health care system in Norway offers treatment to all individuals with mental health problems within a given catchment area, resulting in a relatively high degree of patient representativity in the TOP-study. HC from the same catchment area are randomly selected through statistical records and invited by letter to participate. Exclusion criteria for all participants are: (1) outside the age range 18–65, (2) previous moderate to severe head injury, (3) severe somatic or neurological disease interfering with brain functioning, (4) not fluent in a Scandinavian language, and (5) pronounced intellectual disability (IQ < 70). In addition, HC were screened for drug abuse the past 12 months, current or previous history of mental illness, and first-degree relatives with SMI. For the current study, participants with signs of acute infections were excluded (CRP > 20 mg/L). The final sample with available cognitive and inflammatory/immune marker data included a total of 1402 individuals with SZ (n = 343) and BD (n = 289) spectrum disorders, and HCs (n = 770). Data was collected between 2004 and 2018. The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethics Committee, and all participants provided written informed consent.
Clinical assessments
Diagnoses were set by trained clinical psychologists or medical doctors using the Structured Clinical Interview for DSM-IV axis 1 disorders (SCID-I) [52], and included schizophrenia (n = 175), schizoaffective (n = 43), schizophreniform (n = 31), psychosis not otherwise specified (NOS, n = 94), bipolar I (n = 173), bipolar II (n = 103) and bipolar NOS (n = 14) disorders. Current positive, negative, disorganized, excited, and depressive symptom levels were assessed with the Positive and Negative Syndrome Scale (PANSS) [53, 54], and manic symptoms were assessed with the Young Mania Rating Scale (YMRS) [55]. Level of functioning was assessed using the split version of the Global Assessment of Functioning scale (GAF) [56], including symptoms (GAF-S) and function (GAF-F). Age at onset (AAO) of illness was defined as the age of the first SCID-verified psychotic episode for schizophrenia spectrum disorders and manic/hypomanic episode for bipolar spectrum disorders. Duration of illness was estimated by subtracting the AAO from age at assessment. All participants underwent physical examination with blood sampling including measurements of height and weight for calculation of body mass index (BMI). Clinical interviews, physical examination and cognitive testing all occurred within 35 days. The defined daily dose (DDD) of psychopharmacological treatment (antipsychotics, antidepressants, antiepileptics and lithium) was estimated according to guidelines from the World Health Organization Collaborating Center for Drug Statistics Methodology (https://www.whocc.no/atc_ddd_index). Somatic medication use (including anti-inflammatory/immunomodulatory; yes/no) in the SMI group is provided in Supplementary Table 1.
Cognitive assessments
Cognitive assessment was administered by clinical psychologists (clinical groups) and trained research personnel (HC). We used two test batteries: Battery 1 (from 2004–2012) and Battery 2 (from 2012–2018). To ensure a comprehensive selection of cognitive domains and the highest possible N, corresponding tests from the two batteries were merged to cover nine domains in addition to intellectual functioning: Intellectual functioning was assessed using the Matrix Reasoning and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) [57]. Fine-motor speed was assessed with the Grooved Pegboard test [58], Psychomotor processing speed with the Digit-Symbol Coding task from the Wechsler Adult Intelligence Scale (WAIS-III) [59] or the Digit Symbol task from the MATRICS Consensus Cognitive Battery (MCCB) [60, 61]. Mental processing speed (without a motor component) was measured with the color naming and reading subtests from the Color-Word Interference test, Delis Kaplan Executive Functioning System (D-KEFS) [62]. Attention was measured using Digit Span forward from the WAIS-III. Verbal learning was measured using total recall from the California Verbal Learning Test (CVLT-II) [63], or the Hopkins Verbal Learning Test- Revised (HVLT-R) from the MCCB. Verbal memory was measured using long-delay free recall from the CVLT-II, or delayed recall from HVLT-R [64]. For Semantic fluency the Category fluency subtest from the Verbal Fluency tests in D-KEFS or MCCB were used. Working memory was measured using the total score from the Letter Number Sequencing tests from MCCB or WAIS-III. Finally, cognitive control was assessed using the subtests inhibition and inhibition/switching from the Color-Word Interference Test in D-KEFS. See Supplementary Table 2 for descriptives of tests in Battery 1 and Battery 2.
Peripheral inflammatory and immune markers
Blood was sampled from the antecubital vein in EDTA vials, stored at 4 °C overnight, before isolation of plasma that was stored at −80 °C. Average freezer storage time was 6 years (range 1–14), with shorter duration in HC (included as covariate). Markers associated with neuroinflammation included serpin family A member 3 (SA3), alpha-2-macroglobulin (A2M), B-cell activating factor (BAFF), and A proliferation-inducing ligand (APRIL). Neuronal-glial markers reflecting neuroimmune modulation and related to BBB integrity were S100 calcium binding protein B (S100b), furin, glial fibrillary acidic protein (GFAP), neuron specific enolase (NSE/ENO2). The CAMs included were mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1), junctional adhesion molecule-A (JAMA), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and P-selectin (PSEL). The IL-18 system markers analyzed were IL-18 and its binding protein (IL-18BP), as well as IL-18 receptor 1 (IL-18R1), and IL-18 accessory protein (IL-18RAP) reflecting systemic inflammasome activity. The defensins were human neutrophil peptides 1–3 (HNP1–3), beta defensin 1 (BD-1) and beta defensin 2 (BD-2). Lastly, the chemokines included growth-regulated oncogene alpha (GROα/CXCL1), stromal cell-derived factor 1 alpha (SDF1α/CXCL12), eotaxin (CCL11), and regulated upon activation normal T-cell expressed and secreted (RANTES/CCL5). Case-control studies on CAMs, NSE, BAFF, APRIL and IL-18 system components, as well as composite scores based on all markers with overlapping samples have been previously published [65–69].
Plasma levels of the above biomarkers were measured in duplicate by enzyme immunoassays (EIA) by applying commercially available antibodies (R&D Systems, Minneapolis, MN, USA) in a 384-format using a combination of SELMA (Jena, Germany) and a BioTek (Winooski, VT, USA) dispenser/washer. Absorption was read at 450 nm with wavelength correction set to 540 nm using an ELISA plate reader (Bio-Rad, Hercules, CA, USA). All EIA’s had intra- and inter-assay coefficients <10%. A validation of the stability of the markers regarding effects of diurnal and postprandial variation has been published previously [69] and in Supplementary Table 3, we show data from 4 samples exposed to 4 °C for 24 h before processing, indicating marginal effects of storage.
Statistical procedure
Data preprocessing
All preprocessing, statistical analyses and visualization of results were conducted in the R- environment (https://www.r-project.org/; v.4.0.3; R-packages reported in Supplementary methods 1). We used a complete-case approach for the cognitive tests which were z-score standardized and some were combined to create the relevant cognitive domain. The inflammatory/immune markers were standardized, outliers removed and replaced with NA using 1.5 x IQR below or above the 25th and 75th percentile, respectively. Missing data on the biomarkers were imputed using Multiple Imputation by Chained Equations (MICE), with predictive mean matching (m = 5). No variable had >15% missing data (Supplementary Table 4 for missing per variable; Supplementary Fig. 1 for MICE output). One-way analyses on plasma levels of the measured inflammatory/immune markers and cognitive domain test scores are found in Supplementary Tables 5, 6. Sample and clinical characteristics were compared across groups using permutation (n = 10,000) based t-tests and one-way analysis of variance (ANOVA) for continuous variables, and chi-squared tests for categorical variables.
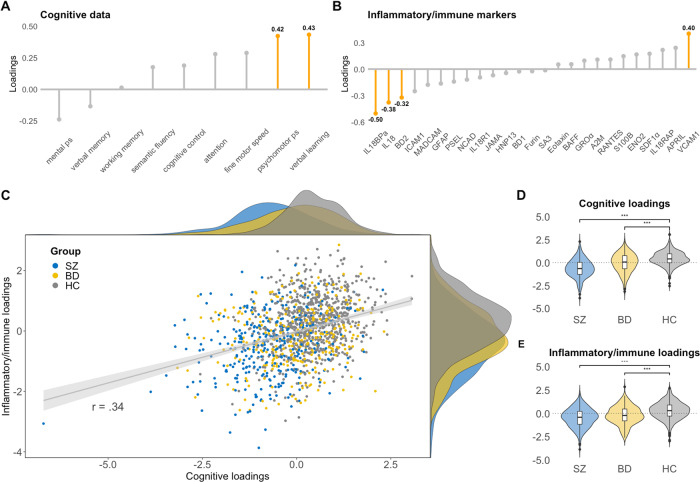
Fig. 1. Multivariate mode of covariation between cognitive and inflammatory/immune marker data in SZ, BD and HC.
A, B Shows the highest contributions (in orange) of each variable in the cognitive and inflammatory/immune marker datasets (numbers in bold represents the loading score for the variables with the highest contributions). C Scatterplot of the individual loading scores, including density plots (top = cognitive loading scores, right = inflammatory/immune marker loading scores). D, E Violin/box plots showing differences in the loading scores between SZ, BD and HC.
Canonical correlation analysis
We applied canonical correlation analysis (CCA) to identify patterns of covariation between cognitive functioning and inflammatory/immune markers [70]. The new linear combinations (i.e. canonical variates) of the variables generated by the CCA reflect modes of covariance (i.e. canonical variate pairs) between the variable sets. The significance of each mode was assessed by permutation testing (n = 10,000), repeating the CCA on the entire sample for each permutation by randomly shuffling the rows of the inflammatory marker data. The participant loading scores (i.e. mode weights) for the cognitive and inflammatory/immune canonical variates on significant modes were used for interpretation, plotting and in further analyses to investigate the presence of subgroups in the data. Further details on CCA and permutation testing are found in Supplementary Methods 2.
Out-of-sample cross-validation
To get a more unbiased estimate of the performance of the CCA model in an out-of-sample variable set, we performed a 10-fold cross-validation procedure with 100 repetitions. For each iteration, a new fold was allocated as the test set (20% of the participants), and the remaining 80% of the participants (training set) was submitted to CCA. We then calculated the average canonical correlation from the training set and applied it to the out-of-sample test set to assess generalizability.
Stability of canonical loadings
The stability of the canonical loadings (i.e. contribution of each variable on significant modes) was examined following the procedure reported by Dinga and colleagues [48], using their shared R-code at github (https://github.com/dinga92/niclin2019-biotypes). We resampled the data, using their delete-one jack-knife procedure, and replotted the distribution of the canonical loadings for each resample to assess the stability of the loadings.
Assessing the influence of covariates on significant modes
Associations between individual loading scores for canonical variates and diagnosis (HC, BD, SZ) were assessed using linear regression, adjusting for age, sex, DDD of psychopharmacological treatments (antipsychotics, antidepressants, antiepileptics and lithium), and BMI, and freezer storage time (where relevant). In addition, as we wanted to pinpoint specific inflammatory pathways as reflected by the wide array of inflammatory markers, we also adjusted for CRP, as a robust marker of non-specific subclinical inflammation.
Hierarchical clustering
We performed hierarchical clustering to investigate the presence of subgroups in the cognitive and inflammatory/immune canonical variates by generating a distance matrix using the Euclidean distance between the loading scores. To minimize the total within cluster variance, the agglomerative coefficient for several linkage methods (average-linkage, single-linkage, complete-linkage, and Ward’s linkage method) was evaluated. The optimal number of clusters was determined by inspecting the corresponding dendrogram, the elbow method and the average silhouette index. Pairwise-comparisons of clusters with inflammatory/immune marker levels, cognitive domains and demographic characteristics were performed using permutation-based t-tests (Bonferroni corrected).
Clustering significance and stability
The significance of the observed silhouette index was tested using a previously reported procedure [48]. Briefly, we first simulated a bivariate Gaussian distribution by taking random samples (n = 1000) of the covariance matrix for the canonical variates. Next, we applied hierarchical clustering to each random sample and the highest silhouette index was obtained. We then compared the number of times the silhouette index was smaller for the null distribution, compared to the observations on non-simulated data. Clustering stability was assessed using a bootstrapping resampling procedure. Replicates of the loading scores from the CCA were generated by randomly picking out observations and then replacing them (n bootstraps = 1000). Hierarchical clustering was performed on each bootstrapped resample. We then computed the Jaccard similarity index ranging from 0–100% and considered an index >0.7 as stable.
Results
Sample demographics
Sample demographics are provided in Table 1.
Table 1.
Sample demographics.
| SZ (N = 343) | BD (N = 289) | HC (N = 770) | p-value | Group comparisons | |
|---|---|---|---|---|---|
| Age | 30.2 (10.2) | 32.8 (11.5) | 32.9 (9.1) | p < 0.001 | SZ < HC, BD |
| Sex (m/f) | 188/155 | 114/175 | 423/347 | p < 0.001 | SZ, HC ~ BD |
| Education (years) | 12.3 (2.3) | 13.6 (2.2) | 14.5 (2.2) | p < 0.001 | SZ < BD < HC |
| WASI IQ (2-subtests) | 101 (13.6) | 109 (11.8) | 113 (10.4) | p < 0.001 | SZ < BD < HC |
| BMI (kg/m2) | 26.3 (5.5) | 25.9 (4.6) | 24.7 (3.7) | p < 0.001 | SZ, BD > HC |
| CRP | 3.3 (3.5) | 2.7 (3.0) | 2.2 (2.7) | p < 0.001 | SZ > HC, BD |
| PANSS Negative | 13.4 (5.8) | 8.4 (3.4) | p < 0.001 | SZ > BD | |
| PANSS Positive | 9.8 (4.2) | 5.8 (2.8) | p < 0.001 | SZ > BD | |
| PANSS Disorganized | 5.6 (2.6) | 4.3 (1.6) | p < 0.001 | SZ > BD | |
| PANSS Excited | 5.7 (2.1) | 5.2 (1.6) | p < 0.001 | SZ > BD | |
| PANSS Depressed | 8.3 (3.3) | 7.7 (2.8) | ns | - | |
| YMRS | 4.3 (4.8) | 3.4 (4.8) | ns | - | |
| GAF Symptom | 45.1 (13.1) | 57.9 (11.2) | p < 0.001 | BD > SZ | |
| GAF Function | 45.4 (13.0) | 55.8 (13.1) | p < 0.001 | BD > SZ | |
| Age at onset | 23.8 (8.6) | 25.2 (9.5) | ns | - | |
| Duration of illness (years) | 6.7 (8.0) | 7.4 (9.2) | ns | - | |
| Antipsychotics, DDD | 1.0 (0.9) | 0.4 (0.7) | p < 0.001 | SZ > BD | |
| Antidepressants, DDD | 0.4 (0.8) | 0.4 (0.8) | ns | - | |
| Antiepileptics, DDD | 0.1 (0.2) | 0.3 (0.5) | p < 0.001 | BD > SZ | |
| Lithium, DDD | 0.0 (0.1) | 0.2 (0.5) | p < 0.001 | BD > SZ | |
| Total, DDD | 1.5 (1.4) | 1.3 (1.2) | ns | - |
Presented are means and standard deviations and results from pairwise comparisons.
HC healthy controls, SZ schizophrenia, BD bipolar disorder, WASI Wechsler Abbreviated Scale of Intelligence, BMI body mass index, CRP C-reactive Protein, PANSS Positive and Negative Syndrome Scale, YMRS Young Mania Rating Scale, GAF Global Assessment of Functioning scale, DDD defined daily dosage.
Canonical correlation analysis (CCA)
CCA significance and out-of-sample cross-validation
The CCA revealed two significant modes of covariation between the cognitive and inflammatory/immune markers after permutation testing. The first mode had a canonical correlation of 0.34 (p < 0.001), and the second mode had a canonical correlation of .22 (p < 0.001). The null distribution of the canonical correlations from the permutation test is visualized in Supplementary Fig. 2. Cross-validation showed that the first mode performed better on unseen data (canonical correlation: meantraining = 0.34, meantest = 0.26), compared to the second mode where the canonical correlation was substantially lower (meantraining = 0.22, meantest = 0.09). Due to poor performance of the second mode in the out-of-sample variable set, suggestive of low generalizability, we only considered the first mode moving forward. The variables in each variable set with the largest contributions to the canonical correlation are depicted in Fig. 1A, B.
The significant mode of covariation captured verbal learning and psychomotor processing speed which was correlated with a combination of markers of innate immune activation including IL-18, IL-18BP, BD-2 and VCAM-1, with 11% of variance explained. The directionality of the loading scores and the positive correlation indicates that as loading scores decrease on both canonical variates, there is lower cognitive functioning and more severe immune dysregulation (except VCAM-1), including higher inflammasome activation (higher circulating levels of IL-18 system cytokines). Likewise, as loading scores increase, there is higher cognitive functioning and lower degree of immune activation. The scatter plot of the individual loading scores for each canonical variate (Fig. 1C), indicates inter-individual heterogeneity and potential subgroups. Small perturbations in the data by leaving one participant out of the CCA did not cause large variations in the canonical loadings, suggesting robust loadings even in the presence of outliers (Supplementary Fig. 3).
Influence of covariates
We next investigated the association between the loading scores for the cognitive canonical variate and diagnosis, while controlling for age, sex and DDD of psychopharmacological treatment (Fig. 1C, D). Both the BD group (loading score estimate = −0.35 ± 0.07, t = −4.9, p < 0.001) and SZ group (loading score estimate = −0.97 ± 0.07, t = −13.7, p < 0.001) had significantly lower loading scores on the cognitive canonical variate relative to the HC group. The same pattern was observed for the inflammatory/immune canonical variate for both BD (loading score estimate = −0.41 ± 0.07, t = −5.2, p < 0.001) and SZ (loading score estimate = −0.70 ± 0.08, t = −8.5, p < 0.001), also after controlling for CRP, BMI, and freezer storage time.
Hierarchical clustering
Clustering structure and significance
Out of the linkage methods evaluated, Ward’s came out with the highest agglomerative coefficient (0.99). A 2-cluster solution had the highest average silhouette index (0.37), which was also indicated by visual inspection of the corresponding dendrogram and the elbow method (Supplementary Fig. 4). Using a previously described simulation approach [48], the average silhouette index was statistically significant (p = 0.03), indicating a presence of clusters in the data. The stability analysis suggested a relatively robust cluster assignment, with an average Jaccard similarity Index ~0.73 (73% overlap) for cluster 1, and ~0.77 (77% overlap) for cluster 2.
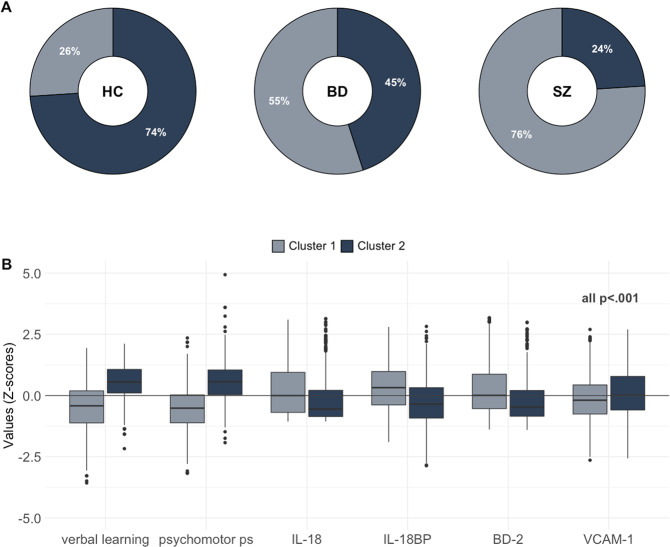
The first cluster identified a subgroup of participants (total n = 625; SZ = 264 [76%], BD = 160 [55%], HC = 201 [26%]) with negative loading scores on both the cognitive and inflammatory/immune marker canonical variates, whereas the second cluster (total n = 777; SZ = 79 [24%], BD = 129 [45%], HC = 569 [74%]) was characterized by positive loading scores (all p < 0.001). The first subgroup (cluster 1) was characterized by higher IL-18, IL-18BP and BD-2 levels, lower VCAM-1 levels, and lower cognitive scores on verbal learning and psychomotor processing speed relative to the subgroup in cluster 2 (all p < 0.001). See Fig. 2 for differences across clusters. Next, we investigated differences between the clusters across demographic and clinical data (SMI only). The subgroup in cluster 1 had lower IQ and years of education, and higher age, CRP, and BMI, relative to the subgroup in cluster 2 (all p < 0.001). Among the participants with SMI, the subgroup in cluster 1 had lower functioning (GAF-S, GAF-F), more positive, negative, and disorganized symptoms and used a higher DDD of antipsychotics compared to the subgroup in cluster 2 (all p < 0.001). See Table 2 for comparisons.
Fig. 2. Hierarchical clustering of CCA data in SZ, BD and HC.
A Percentage of SZ, BD and HC in cluster 1 and cluster 2. B Boxplots showing differences between the subgroup cluster 1 and cluster 2 on the cognitive domains and inflammatory/immune markers identified from the CCA.
Table 2.
Differences between subgroups on demographic and clinical data.
| Cluster 1 (N = 625) | Cluster 2 (N = 777) | p valuea | 95% CIb | Cluster comparisons | |
|---|---|---|---|---|---|
| Age | 33.2 (11.1) | 31.4 (8.9) | p < 0.001 | [0.8, 2.9] | Cluster 1 > Cluster 2 |
| Sex (m/f) | 370/255 | 355/422 | p < 0.001 | - | Cluster 1 ~ Cluster 2 |
| Education (years) | 12.9 (2.3) | 14.4 (2.3) | p < 0.001 | [−1.7, −1.2] | Cluster 1 < Cluster 2 |
| WASI IQ (2 subtests) | 103.7 (13.1) | 114.1 (9.9) | p < 0.001 | [−11.7, −9.1] | Cluster 1 < Cluster 2 |
| BMI (kg/m2) | 26.5 (5.0) | 24.4 (3.7) | p < 0.001 | [1.5, 2.5] | Cluster 1 > Cluster 2 |
| CRP | 3.1 (3.3) | 2.2 (2.7) | p < 0.001 | [0.6, 1.2] | Cluster 1 > Cluster 2 |
| SMI group | BD = 160 SZ = 264 | BD = 129 SZ = 79 | |||
| PANSS Negative | 11.9 (5.6) | 9.7 (4.6) | p < 0.001 | [1.3, 3.1] | Cluster 1 > Cluster 2 |
| PANSS Positive | 8.6 (4.4) | 6.8 (3.3) | p < 0.001 | [1.1, 2.4] | Cluster 1 > Cluster 2 |
| PANSS Disorganized | 5.3 (2.4) | 4.3 (1.7) | p < 0.001 | [0.6, 1.4] | Cluster 1 > Cluster 2 |
| PANSS Excited | 5.5 (2.0) | 5.3 (1.8) | ns | [−0.2, 0.5] | - |
| PANSS Depressed | 8.1 (3.1) | 7.9 (2.9) | ns | [−0.3, 0.7] | - |
| YMRS | 4.0 (4.9) | 3.6 (4.4) | ns | [−0.4, 1.2] | - |
| GAF Symptom | 48.8 (13.6) | 55.6 (13.3) | p < 0.001 | [−0.9, −4.5] | Cluster 1 < Cluster 2 |
| GAF Function | 47.73 (12.9) | 55.2 (14.9) | p < 0.001 | [−9.8, −5.1] | Cluster 1 < Cluster 2 |
| Age at onset | 24.9 (9.6) | 23.4 (7.8) | ns | [−0.0, 3.0] | - |
| Duration of illness (years) | 7.5 (9.2) | 6.0 (7.0) | ns | [−0.0, 2.9] | - |
| Antipsychotics, DDD | 0.8 (0.9) | 0.5 (0.8) | p < 0.001 | [0.1, 0.4] | Cluster 1 > Cluster 2 |
| Antidepressants, DDD | 0.4 (0.9) | 0.4 (0.7) | ns | [−0.1, 0.2] | - |
| Antiepileptics, DDD | 0.2 (0.4) | 0.2 (0.4) | ns | [−0.1, 0.1] | - |
| Lithium, DDD | 0.1 (0.3) | 0.1 (0.4) | ns | [−0.1, 0.0] | - |
| Total, DDD | 1.5 (1.3) | 1.2 (1.3) | ns | [0.1, 0.5] | - |
Presented are means, standard deviations and results from permutation t-tests (and chi-square for sex) comparing clusters on demographic and clinical data.
HC healthy controls, SZ schizophrenia, BD bipolar disorder, WASI Wechsler Abbreviated Scale of Intelligence, BMI body mass index, CRP C-reactive Protein, PANSS Positive and Negative Syndrome Scale, YMRS Young Mania Rating Scale, GAF Global Assessment of Functioning scale, DDD defined daily dosage.
aBonferroni corrected p values (sample characteristics 0.05/5; clinical characteristics 0.05/15).
b95 percent (Monte-Carlo) permutation percentile confidence interval of the mean difference.
Discussion
In a large SMI and HC cohort, we identified shared covariance between verbal learning and psychomotor processing speed and markers of innate immune activation, including IL-18, IL-18BP, BD-2, and VCAM-1. Furthermore, the covariance patterns indicated two transdiagnostic subgroups with distinct cognition—immune dysregulation with differing demographics and clinical severity. Our findings suggest innate immune activation and cognitive impairment co-occur in a subgroup predominantly consisting of SMI, highlighting the importance of considering inter-individual variance in future research.
The cognitive domains that shared covariance with markers of innate immune activation, verbal learning and psychomotor processing speed, are among the most affected cognitive domains in SZ and BD [71–76]. Impairments are evident in clinical high-risk individuals with subsequent conversion to SMI [77] and potentially qualify as endophenotypes in both SZ and BD [71, 78]. A recent meta-analysis demonstrated modest correlations between IL-6 and it’s downstream mediator CRP and impairment in both of these domains [18]. However, as CRP is a non-specific marker of systemic inflammation, enhanced levels could reflect a range of comorbid conditions seen in SMI such cardio-metabolic disease, increased fat mass and gut microbiome dysbiosis. We therefore controlled for CRP and BMI, and our findings suggest that more specific markers reflecting other pathogenic processes, such as activation of innate immune responses, may be relevant for cognitive functioning.
Based on our findings we can begin to speculate about potential inflammatory/immune-related mechanisms that may influence cognitive functioning. IL-18 system components regulate innate immune responses and are broadly expressed by neurons, astrocytes and microglia, and may influence permeability of the BBB and induce neuroinflammatory states [79]. We have recently demonstrated increased levels of these IL-18 system components in SMI, associated with increased gene expression of the inflammasome components NLRP3 and NLRC4 in circulating immune cells [67]. The inflammasome is a key innate immune system function that is associated with many human diseases [80]. A growing number of studies suggest that inflammasome activation can influence cognitive functioning, particularly in autoimmune and neurodegenerative diseases [80–83]. In addition, experimental studies have shown promise in mitigating cognitive impairment by inhibiting inflammasome activation, which could be a potential treatment target for several pathologies [84].
Similar to IL-18, the small antimicrobial peptide BD-2, mainly produced by neutrophils and epithelial cells as well as macrophage cells, plays an important role in regulating innate immune responses. While representing a protective component against bacterial, viral and fungal infections, defensins may cause collateral damage in host cells by disrupting cellular membranes and have been shown to diffuse across the BBB [85]. Dysregulated expression of BD-2 in microglia and astrocytes has been suggested to prolong dendritic cell activity, which could mediate release of pro-inflammatory cytokines ultimately promoting loss of neuronal function and impacting cognition [86]. Based on the increased BD-2 levels indicated by the CCA, we speculate that similar mechanisms could be relevant in SMI. BD-2 has pleiotropic effects, acting as a chemokine binding to CCR6 with effects on T cells and dendritic cells [87], linking innate (inflammation) and adaptive (lymphocyte activation) immune responses. Furthermore, BD-2 induces IL-18 release in keratinocytes [88] and conversely, IL-18 may trigger BD-2 release in innate cells such as macrophages [89].
While we recently reported similar levels of sVCAM-1 in SMI and HC [90], our finding that low sVCAM-1 was associated with cognitive impairment could indicate an alternative role for the soluble form of this protein. VCAM-1 may mediate adhesion of monocytes, lymphocytes, and neutrophils to the vascular endothelium including immune cell trafficking via the BBB [91]. While increased VCAM-1 expression is a key marker for endothelial activation during vascular/systemic inflammation [92], inflammatory challenge may enhance shedding of VCAM-1 from human brain endothelial cells [93]. Additionally, in vitro, sVCAM-1 may act as a competitive inhibitor of ligand binding, blocking leukocyte adhesion to activated human brain endothelial cells [94]. Chronically elevated levels of circulating IL-18 may also downregulate sVCAM-1 in both immune and non-immune cells [95]. Taken together, we speculate that chronic dysregulation of innate immune regulatory loops in SMI could enhance systemic IL-18 signaling, together with BD-2 and sVCAM1 expression, impacting BBB permeability and neuroinflammation, thereby influencing cognition.
We identified two groups reflecting heterogeneity in cognitive functioning and inflammatory/immune status in SMI and HC. While there was a larger proportion of SMI participants in the more compromised group (low cognition – high immune dysregulation subgroup), they were also represented in the less compromised subgroup. We additionally found a proportion of HC in the compromised group sharing several characteristics with SMI. This is in line with findings of inter-individual variance in cognitive functioning and inflammatory/immune status in SMI and in the general population [96–98].
Importantly, our approach suggests that SMI individuals with lower cognitive functioning and higher immune/inflammatory dysregulation may experience more symptoms, worse functioning and have higher DDD of antipsychotics. Symptom severity has not previously been linked with subtypes based on inflammatory markers alone [16, 41, 43, 44, 47]. Additionally, we found no differences between subgroups for AAO or DOI, which could suggest a common cognitive and inflammatory/immune activation pattern independent of illness stage. This supports findings of cognitive deficits and increased inflammation in both first-episode psychosis and chronic illness [41, 99]. Notably, differences across studies based on the selection bias of inflammatory/immune markers and cognitive outcomes could contribute to differing observations from subgroup-studies.
Some limitations should be acknowledged. While cognitive functioning and immune/inflammatory markers can be influenced by multiple factors, an open question is whether they simply co-occur or whether there is a causal relationship between them. Due to the cross-sectional design and measures of peripheral inflammatory/immune markers we are unable to draw conclusions regarding causality. Longitudinal studies, and evaluation of the inflammatory and immune markers in CNS (i.e. cerebrospinal fluid) are needed to clarify this. An untargeted approach with omics technologies or using other inflammatory markers that have been shown dysregulate in SMI could give different answers but was not feasible in our large population. Hence, our findings do not disclude the importance of other inflammatory markers or pathways. The storage duration of samples is a limitation as we cannot exclude that some protein degradation has occurred, which could vary from protein to protein. However, we have previously measured CRP during isolation of plasma and CRP determined years later during bulk analysis in the same sample, finding a high degree of correlation (r = 0.86) [100]. In addition, we included freezer storage time in our models. The blood sampling protocol, with isolation of plasma the next day, was not optimal. However, our validation experiments found no systematic effects on the measured proteins during storage at 4 °C for up to 24 h. Another limitation includes the use of two different cognitive test batteries. Four domains were measured using identical tests in the two test batteries while five domains were measured employing different but very similar tests using the same stimuli and administration procedures, but with slight variations in time given to complete task (Psychomotor processing speed) or number of stimuli (Verbal learning). The study has several strengths including a robust methodology, a transdiagnostic approach, a large sample, and a comprehensive selection of cognitive domains and a large inflammatory/immune screening assay. Cross-validation, stability analyses, and evaluation of the cluster solution, further strengthen our findings, although replication in independent datasets is needed.
In conclusion, we identified patterns of covariance between cognitive functioning and inflammatory/immune markers, linking poor verbal learning and psychomotor processing skills to increased innate immune activation markers, including IL-18 system cytokines and BD-2, with the strongest associations in SMI. Based on covariance patterns we identified two subgroups of cognitive functioning and inflammation associated with differing patterns of functioning and symptom levels that transcended diagnostic categories. Our findings suggest that the IL-18 system, and perhaps inflammasome activation, could be an interesting path for future investigation of cognitive impairment in SMI.
Supplementary information
Acknowledgements
This study was funded by the South-Eastern Norway Regional Health Authority (grant number 2020089) and Research Council of Norway (#223273).
Author contributions
LSS: Conceptualization, methodology, statistical analysis, interpretation of results, graphical representations and writing original draft. Thor U & Torill U: Conceptualization and project development, methodology, supervision, interpretation of results, review and editing of manuscript. BH, AS, PA, CM, MBEGO: Interpretation of results, review and editing of manuscript. DR & LAM: Statistical and graphical visualization counseling, review and editing of manuscript. SD, TVL, NES, IM, OAA: Project administration, interpretation of results and editing of manuscript.
Code availability
Main analysis code is available at https://osf.io/qpdse/.
Competing interests
OAA is a consultant to HealthLytix. Remaining authors have no competing interests to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01924-w.
References
- 1.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 2.McCleery A, Nuechterlein KH. Cognitive impairment in psychotic illness: prevalence, profile of impairment, developmental course, and treatment considerations. Dialogues Clin Neurosci. 2019;21:239–48. doi: 10.31887/DCNS.2019.21.3/amccleery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42:743–55. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 4.Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:182. doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacCabe JH, Lambe MP, Cnattingius S, Torrång A, Björk C, Sham PC, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–40. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- 6.Flaaten CB, Melle I, Bjella T, Engen MJ, Åsbø G, Wold KF, et al. Domain-specific cognitive course in schizophrenia: Group- and individual-level changes over 10 years. Schizophr Res Cogn. 2022;30:100263. doi: 10.1016/j.scog.2022.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samamé C, Cattaneo BL, Richaud MC, Strejilevich S, Aprahamian I. The long-term course of cognition in bipolar disorder: a systematic review and meta-analysis of patient-control differences in test-score changes. Psychol Med. 2022;52:217–28. doi: 10.1017/S0033291721004517. [DOI] [PubMed] [Google Scholar]
- 8.Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2:531–6. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gitlin MJ, Miklowitz DJ. The difficult lives of individuals with bipolar disorder: A review of functional outcomes and their implications for treatment. J Affect Disord. 2017;209:147–54. doi: 10.1016/j.jad.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, et al. Neurocognitive Predictors of Work Outcome in Recent-Onset Schizophrenia. Schizophr Bull. 2011;37:S33–S40.. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowman M, Holleran L, Lonergan E, O’Connor K, Birchwood M, Donohoe G. Cognitive Predictors of Social and Occupational Functioning in Early Psychosis: A Systematic Review and Meta-analysis of Cross-Sectional and Longitudinal Data. Schizophr Bull. 2021;47:1243–53. doi: 10.1093/schbul/sbab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med. 2017;47:1848–64. doi: 10.1017/S0033291717000307. [DOI] [PubMed] [Google Scholar]
- 13.Vaskinn A, Haatveit B, Melle I, Andreassen OA, Ueland T, Sundet K. Cognitive Heterogeneity across Schizophrenia and Bipolar Disorder: A Cluster Analysis of Intellectual Trajectories. J Int Neuropsychol Soc. 2020;26:860–72. doi: 10.1017/S1355617720000442. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi A, Kar SK, Shukla R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin Psychopharmacol Neurosci. 2018;16:7–17. doi: 10.9758/cpn.2018.16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamminga CA, Clementz BA, Pearlson G, Keshavan M, Gershon ES, Ivleva EI, et al. Biotyping in psychosis: using multiple computational approaches with one data set. Neuropsychopharmacology. 2021;46:143–55. doi: 10.1038/s41386-020-00849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop JR, Zhang L, Lizano P. Inflammation subtypes and translating inflammation-related genetic findings in schizophrenia and related psychoses: A perspective on pathways for treatment stratification and novel therapies. Harv Rev Psychiatry. 2022;30:59–70. doi: 10.1097/HRP.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15:317–28. doi: 10.1038/s41582-019-0174-4. [DOI] [PubMed] [Google Scholar]
- 18.Morrens M, Overloop C, Coppens V, Loots E, Van Den Noortgate M, Vandenameele S, et al. The relationship between immune and cognitive dysfunction in mood and psychotic disorder: a systematic review and a meta-analysis. Mol Psychiatry. 2022:1–10. [DOI] [PMC free article] [PubMed]
- 19.Horváth S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry. 2014;75:316–23. doi: 10.1016/j.biopsych.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mørch RH, Dieset I, Færden A, Hope S, Aas M, Nerhus M, et al. Inflammatory evidence for the psychosis continuum model. Psychoneuroendocrinology. 2016;67:189–97. doi: 10.1016/j.psyneuen.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29. doi: 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Dushlaine C, Rossin L, Lee PH, Duncan L, Parikshak NN, Newhouse S, et al. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9:1–13.. doi: 10.1038/s41398-019-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: A systematic review and meta-analysis. Schizophr Res. 2017;190:18–27. doi: 10.1016/j.schres.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med. 2019;49:1971–9. doi: 10.1017/S0033291719001685. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieset I, Andreassen OA, Haukvik UK. Somatic Comorbidity in Schizophrenia: Some Possible Biological Mechanisms Across the Life Span. Schizophr Bull. 2016;42:1316–9. doi: 10.1093/schbul/sbw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PloS One. 2011;6:e24597. doi: 10.1371/journal.pone.0024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Räuber S, Heming M, Repple J, Ruland T, Kuelby R, Schulte-Mecklenbeck A, et al. Cerebrospinal fluid flow cytometry distinguishes psychosis spectrum disorders from differential diagnoses. Mol Psychiatry. 2021;26:7661–70. doi: 10.1038/s41380-021-01244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer JH, Cervenka S, Kim M-J, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7:1064–74. doi: 10.1016/S2215-0366(20)30255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi: 10.1016/j.neulet.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 33.van Kesteren CFMG, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075. doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penadés R, García-Rizo C, Bioque M, González-Rodríguez A, Cabrera B, Mezquida G, et al. The search for new biomarkers for cognition in schizophrenia. Schizophr Res Cogn. 2015;2:172–8. doi: 10.1016/j.scog.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tchessalova D, Posillico CK, Tronson NC. Neuroimmune Activation Drives Multiple Brain States. Front Syst Neurosci. 2018;12:1–9. doi: 10.3389/fnsys.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Picker LJ, Morrens M, Chance SA, Boche D. Microglia and Brain Plasticity in Acute Psychosis and Schizophrenia Illness Course: A Meta-Review. Front Psychiatry. 2017;8:238. doi: 10.3389/fpsyt.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9:5790. doi: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagi K, Nosaka T, Dickinson D, Lindenmayer JP, Lee J, Friedman J, et al. Association Between Cardiovascular Risk Factors and Cognitive Impairment in People With Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021;78:510–8. doi: 10.1001/jamapsychiatry.2021.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller BJ, Goldsmith DR. Towards an Immunophenotype of Schizophrenia: Progress, Potential Mechanisms, and Future Directions. Neuropsychopharmacology. 2017;42:299–317. doi: 10.1038/npp.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller BJ, Goldsmith DR. Inflammatory biomarkers in schizophrenia: Implications for heterogeneity and neurobiology. Biomark. Neuropsychiatry. 2019;1:100006. [Google Scholar]
- 41.Lizano P, Lutz O, Xu Y, Rubin LH, Paskowitz L, Lee AM, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry. 2020. 10.1038/s41380-020-00914-0. [DOI] [PMC free article] [PubMed]
- 42.Zhang L, Lizano P, Guo B, Xu Y, Rubin LH, Hill SK, et al. Inflammation subtypes in psychosis and their relationships with genetic risk for psychiatric and cardiometabolic disorders. Brain Behav Immun - Health. 2022;22:100459. doi: 10.1016/j.bbih.2022.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21:1090–8. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang D, Xu Y, Lutz O, Bannai D, Zeng V, Bishop JR, et al. Inflammatory Subtypes in Antipsychotic-Naïve First-Episode Schizophrenia are Associated with Altered Brain Morphology and Topological Organization. Brain Behav Immun. 2022;100:297–308. doi: 10.1016/j.bbi.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boerrigter D, Weickert TW, Lenroot R, O’Donnell M, Galletly C, Liu D, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation. 2017;14:188. doi: 10.1186/s12974-017-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, et al. Cortisol and Inflammatory Biomarkers Predict Poor Treatment Response in First Episode Psychosis. Schizophr Bull. 2015;41:1162–70. doi: 10.1093/schbul/sbv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry. 2014;4:e365–e365. doi: 10.1038/tp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinga R, Schmaal L, Penninx BWJH, van Tol MJ, Veltman DJ, van Velzen L, et al. Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017) NeuroImage Clin. 2019;22:101796. doi: 10.1016/j.nicl.2019.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T, Tang X, Li H, Woodberry KA, Kline ER, Xu L, et al. Clinical subtypes that predict conversion to psychosis: A canonical correlation analysis study from the ShangHai At Risk for Psychosis program. Aust N. Z J Psychiatry. 2020;54:482–95. doi: 10.1177/0004867419872248. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Hayes DN, Nobel A, Marron JS. Statistical Significance of Clustering for High-Dimension, Low–Sample Size Data. J Am Stat Assoc. 2008;103:1281–93. [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-P, Version 2.0). New York State Psychiatric Institute, New York: Biometrics Research Department; 1995.
- 53.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 54.Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–50. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry J Ment Sci. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning–Split version. Compr Psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Wechsler D. Wechsler Abbreviated Scale of Intelligence-Second Edition. 2011. 2011. 10.1037/t15171-000.
- 58.Klove H. Clinical Neuropsychology. Med Clin North Am. 1963;47:1647–58. [PubMed] [Google Scholar]
- 59.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. 1997. 1997. 10.1037/t49755-000.
- 60.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 61.Mohn C, Sundet K, Rund BR. The Norwegian standardization of the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery. J Clin Exp Neuropsychol. 2012;34:667–77. doi: 10.1080/13803395.2012.667792. [DOI] [PubMed] [Google Scholar]
- 62.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. 2001. 2001. 10.1037/t15082-000.
- 63.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Second Edition. 1987. 1987. 10.1037/t15072-000.
- 64.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 65.Andreou D, Steen NE, Jørgensen KN, Smelror RE, Wedervang-Resell K, Nerland S, et al. Lower circulating neuron-specific enolase concentrations in adults and adolescents with severe mental illness. Psychol Med. 2021:1–10. 10.1017/S0033291721003056. [DOI] [PMC free article] [PubMed]
- 66.Engh JA, Ueland T, Agartz I, Andreou D, Aukrust P, Boye B, et al. Plasma Levels of the Cytokines B Cell-Activating Factor (BAFF) and A Proliferation-Inducing Ligand (APRIL) in Schizophrenia, Bipolar, and Major Depressive Disorder: A Cross Sectional, Multisite Study. Schizophr Bull. 2021;48:37–46. doi: 10.1093/schbul/sbab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabo A, O’Connell KS, Ueland T, Sheikh MA, Agartz I, Andreou D, et al. Increased circulating IL-18 levels in severe mental disorders indicate systemic inflammasome activation. Brain Behav Immun. 2022;99:299–306. doi: 10.1016/j.bbi.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 68.Hjell G, Szabo A, Mørch-Johnsen L, Holst R, Tesli N, Bell C, et al. Interleukin-18 signaling system links to agitation in severe mental disorders. Psychoneuroendocrinology. 2022;140:105721. doi: 10.1016/j.psyneuen.2022.105721. [DOI] [PubMed] [Google Scholar]
- 69.Elkjaer Greenwood Ormerod MB, Ueland T, Frogner Werner MC, Hjell G, Rødevand L, Sæther LS, et al. Composite immune marker scores associated with severe mental disorders and illness course. Brain Behav Immun - Health. 2022;24:100483. doi: 10.1016/j.bbih.2022.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hotelling H. Relations Between Two Sets of Variates. Biometrika. 1936;28:321–77. [Google Scholar]
- 71.Luperdi SC, Correa-Ghisays P, Vila-Francés J, Selva-Vera G, Salazar-Fraile J, Cardoner N, et al. Is processing speed a valid neurocognitive endophenotype in bipolar disorder? Evidence from a longitudinal, family study. J Psychiatr Res. 2021;141:241–7. doi: 10.1016/j.jpsychires.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Mathias SR, Knowles EEM, Barrett J, Leach O, Buccheri S, Beetham T, et al. The Processing-Speed Impairment in Psychosis Is More Than Just Accelerated Aging. Schizophr Bull. 2017;43:814–23. doi: 10.1093/schbul/sbw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vöhringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D, et al. Cognitive Impairment in Bipolar Disorder and Schizophrenia: A Systematic Review. Front Psychiatry. 2013;4:87. doi: 10.3389/fpsyt.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr Res. 2013;150:42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ojeda N, Peña J, Schretlen DJ, Sánchez P, Aretouli E, Elizagárate E, et al. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophr Res. 2012;135:72–78. doi: 10.1016/j.schres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 77.Carrión RE, Walder DJ, Auther AM, McLaughlin D, Zyla HO, Adelsheim S, et al. From the psychosis prodrome to the first-episode of psychosis: No evidence of a cognitive decline. J Psychiatr Res. 2018;96:231–8. doi: 10.1016/j.jpsychires.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, et al. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet Publ Int Soc Psychiatr Genet. 2007;144B:242–9. doi: 10.1002/ajmg.b.30446. [DOI] [PubMed] [Google Scholar]
- 79.Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:1–12.. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheon SY, Kim J, Kim SY, Kim EJ, Koo B-N. Inflammasome and Cognitive Symptoms in Human Diseases: Biological Evidence from Experimental Research. Int J Mol Sci. 2020;21:1103. doi: 10.3390/ijms21031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol Balt Md. 1950;2010:974–81. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang T, Zhang Y, Wu S, Chen Q, Wang L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front Pharmacol. 2022;13:1–21. doi: 10.3389/fphar.2022.845185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schluesener H, Meyermann R. Neutrophilic defensins penetrate the blood-brain barrier. J Neurosci Res. 1995;42:718–23. doi: 10.1002/jnr.490420515. [DOI] [PubMed] [Google Scholar]
- 86.Williams WM, Castellani RJ, Weinberg A, Perry G, Smith MA. Do β-Defensins and Other Antimicrobial Peptides Play a Role in Neuroimmune Function and Neurodegeneration? Sci World J. 2012;2012:905785. doi: 10.1100/2012/905785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 88.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol Balt Md 1950. 2005;175:1776–84. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 89.Yang R, Yang E, Shen L, Modlin RL, Shen H, Chen ZW. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J Immunol Balt Md. 1950;2018:2405–17. doi: 10.4049/jimmunol.1701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheikh MA, O’Connell KS, Lekva T, Szabo A, Akkouh IA, Osete JR, et al. Systemic cell-adhesion molecules (CAM) in severe mental illness-potential role of intracellular CAM-1 in linking peripheral and neuro-inflammation. Biol Psychiatry. 2022;93:187–96. doi: 10.1016/j.biopsych.2022.06.029. [DOI] [PubMed] [Google Scholar]
- 91.Dietrich J-B. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 92.Kong D-H, Kim YK, Kim MR, Jang JH, Lee S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int J Mol Sci. 2018;19:E1057. doi: 10.3390/ijms19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hummel V, Kallmann BA, Wagner S, Füller T, Bayas A, Tonn JC, et al. Production of MMPs in human cerebral endothelial cells and their role in shedding adhesion molecules. J Neuropathol Exp Neurol. 2001;60:320–7. doi: 10.1093/jnen/60.4.320. [DOI] [PubMed] [Google Scholar]
- 94.Kallmann BA, Hummel V, Lindenlaub T, Ruprecht K, Toyka KV, Rieckmann P. Cytokine-induced modulation of cellular adhesion to human cerebral endothelial cells is mediated by soluble vascular cell adhesion molecule-1. Brain. 2000;123:687–97. doi: 10.1093/brain/123.4.687. [DOI] [PubMed] [Google Scholar]
- 95.Morel JC, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001;276:37069–75. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- 96.Stenfors CUD, Jonsdottir IH, Magnusson Hanson LL, Theorell T. Associations between systemic pro-inflammatory markers, cognitive function and cognitive complaints in a population-based sample of working adults. J Psychosom Res. 2017;96:49–59. doi: 10.1016/j.jpsychores.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 97.Beydoun MA, Dore GA, Canas J-A, Liang H, Beydoun HA, Evans MK, et al. Systemic Inflammation Is Associated With Longitudinal Changes in Cognitive Performance Among Urban Adults. Front Aging Neurosci. 2018;10:1-12. [DOI] [PMC free article] [PubMed]
- 98.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wedervang-Resell K, Ueland T, Aukrust P, Friis S, Holven KB, H. Johannessen C, et al. Reduced levels of circulating adhesion molecules in adolescents with early-onset psychosis. Npj Schizophr. 2020;6:1–8. doi: 10.1038/s41537-020-00112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reponen EJ, Dieset I, Tesli M, Mørch RH, Aas M, Vedal TSJ, et al. Atherogenic lipid ratios related to myeloperoxidase and C-reactive protein levels in psychotic disorders. Front Psychiatry. 2020;11:672. doi: 10.3389/fpsyt.2020.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Main analysis code is available at https://osf.io/qpdse/.