Abstract
The rooting-zone water-storage capacity—the amount of water accessible to plants—controls the sensitivity of land–atmosphere exchange of water and carbon during dry periods. How the rooting-zone water-storage capacity varies spatially is largely unknown and not directly observable. Here we estimate rooting-zone water-storage capacity globally from the relationship between remotely sensed vegetation activity, measured by combining evapotranspiration, sun-induced fluorescence and radiation estimates, and the cumulative water deficit calculated from daily time series of precipitation and evapotranspiration. Our findings indicate plant-available water stores that exceed the storage capacity of 2-m-deep soils across 37% of Earth’s vegetated surface. We find that biome-level variations of rooting-zone water-storage capacities correlate with observed rooting-zone depth distributions and reflect the influence of hydroclimate, as measured by the magnitude of annual cumulative water-deficit extremes. Smaller-scale variations are linked to topography and land use. Our findings document large spatial variations in the effective root-zone water-storage capacity and illustrate a tight link among the climatology of water deficits, rooting depth of vegetation and its sensitivity to water stress.
Subject terms: Ecosystem ecology, Hydrology, Climate-change ecology
Global estimations of the water-storage capacity in the rooting zone from satellite data reveal plant access to deep water across a third of Earth’s vegetated surface.
Main
To sustain activity during dry periods and resist impacts of droughts, plants rely on water stored below the surface. The larger the rooting-zone water-storage capacity (S0), the longer plants can withstand soil moisture limitation1. S0 is therefore a key factor determining drought impacts, land–atmosphere exchanges and run-off regimes, particularly in climates with a seasonal asynchrony in radiation and precipitation (P)2–4. In models, S0 is commonly conceived as a function of the soil texture and the plants’ rooting depth (zr), limited to the depth of the soil3,5. Recent research has revealed a substantial component of S0 and contributions to evapotranspiration (ET) by water stored beneath the soil, in weathered and fractured bedrock and groundwater6–11. Plant access to such deep moisture plays an important role in controlling near-surface climate12–14, run-off regimes4, global patterns of vegetation cover15 and mitigating impacts of droughts16.
However, S0 is impossible to observe directly across large scales, and its spatial variations are poorly understood17. Global compilations of local plant zr measurements18,19 yield information related to S0 but have resolved this observational challenge only partly because of their limited size and large documented variations in zr across multiple scales7,18,18–21. Empirical approaches for estimating the global zr distribution have made use of relationships between in situ observations and climatic factors22. Modelling approaches for predicting zr have conceived their spatial variations as the result of optimal adaptation to the prevailing hydroclimate23–25 or as being adapted to just buffer water demand to sustain ET during dry periods2,26. Such mass-balance approaches make use of the maximum cumulative water deficit (CWD) during dry periods as an indication of the effective S0. An additional hypothesis posits that it would not be beneficial for plants to root even deeper and thus size their S0 even larger26. However, a link among the magnitude of CWD extremes, the sensitivity of vegetation activity to an increasing CWD and local zr observations remains to be shown, and the prevalence of plant access to water stored at depth (here taken as >2 m) across the globe remains to be quantified.
Despite its crucial role in controlling water and carbon fluxes and the scarcity of observations, virtually all models simulating water and carbon exchange between the land surface and the atmosphere rely on a specification of S0 either directly as the depth of a ‘water bucket’ or indirectly through prescribed zr and soil texture across the profile. Typically, water stored at depth and along the entire critical zone (including weathered bedrock) is not fully represented in models8,9, and the evident plasticity of zr and variations of S0 within plant types and along climatic and topographic gradients are often ignored. Implications of this simplification may be substantial for the simulation of land–atmosphere coupling and drought impacts8,12,13.
In this Article, we present a method for diagnosing S0 from the relationship between vegetation activity and CWD. By fusing multiple time series of Earth observation data streams with global coverage, we estimate the global distribution of S0 at a resolution of 0.05° (~5 km). Using a mass-balance approach2,26 and field observations of zr from a globally distributed dataset, we then show that the sensitivity of vegetation to water stress across the globe is strongly related to the magnitude of CWD extremes and reflects the rooting depth of plants.
Estimating S0 from Earth observations
We started by estimating S0 as the CWD at which vegetation ‘activity’ ceases. Our approach accounts for the constraint of the rooting-zone water availability on ET and photosynthesis and relates S0 to the sensitivity of vegetation activity to water stress. The parallel information of ET, P and the modelled snow mass balance enables a quantification of CWD over time. Vegetation activity was estimated from two alternative observations: from the evaporative fraction (EF, defined as ET divided by net radiation) and from sun-induced fluorescence (SIF, normalized by incident short-wave radiation (Methods)).
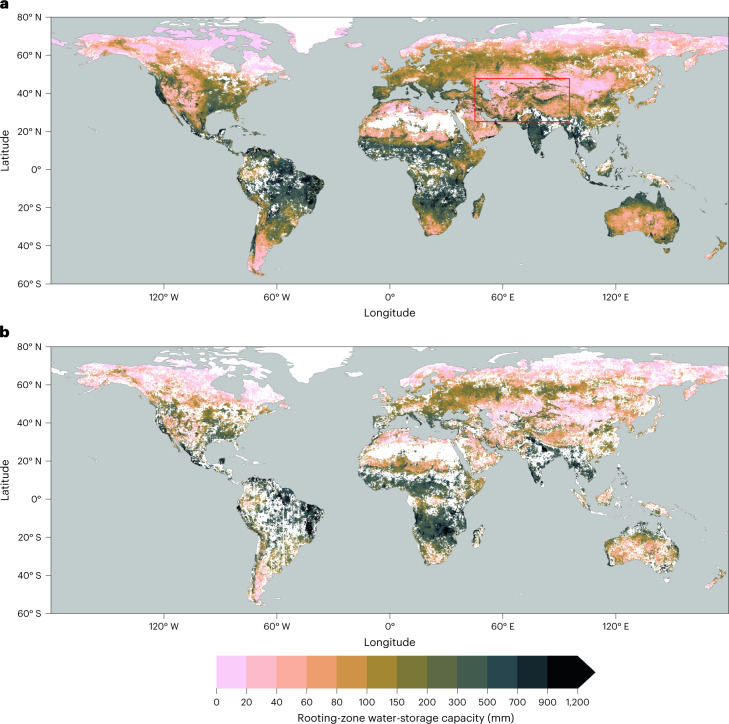
Figure 1 reveals large global variations in S0. Estimates based on EF and SIF correlate closely and agree in magnitude (R2 = 0.78; Supplementary Fig. 1). The lowest sensitivity of vegetation activity to an increasing CWD, and thus the largest apparent S0, is found in regions with a strong seasonality in radiation and water availability and substantial vegetation cover—particularly in monsoonal climates. By contrast, the lowest S0 values appear not only in regions where seasonal water deficits are limited due to short inter-storm duration (for example, western Amazon and Congo basin) and/or low levels of potential ET (for example, high latitudes), but also in deserts and arid grasslands. This probably reflects the limited water storage accumulating during rain events from which vegetation can draw during dry periods. In these regions, a rapid decline of ET and SIF with an increasing CWD is related to vegetation cover dynamics, governed by greening after rain pulses and browning during dry periods27.
Fig. 1. Rooting-zone water-storage capacity from vegetation activity.
a,b, Rooting-zone water-storage capacity estimated from SdEF (a) and SdSIF (b) to the CWD. The red box in a shows the outline of the magnified map provided in Fig. 2. Data shown are aggregated to 0.1° resolution. Blank cells (white) mark areas where all underlying cells at the original 0.05° resolution did not exhibit a significant and single, linearly declining relationship with increasing CWD.
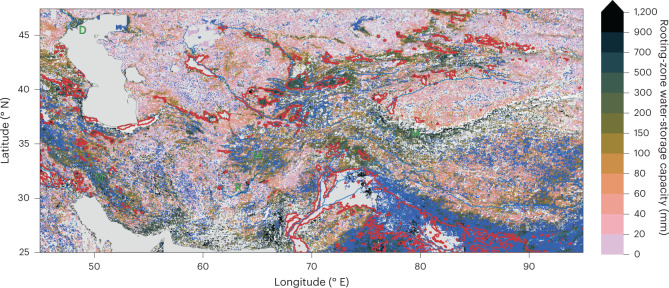
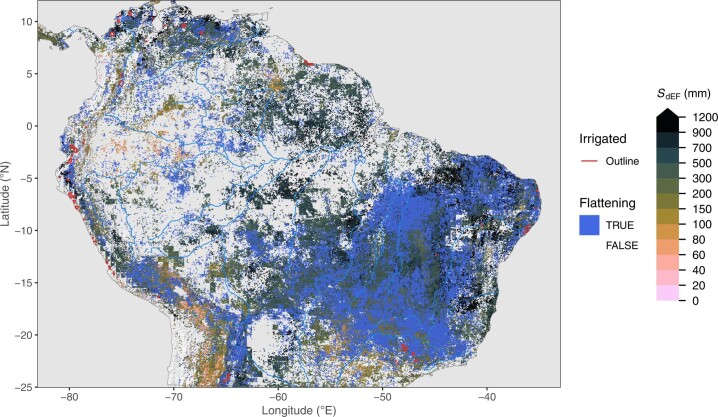
Clear patterns emerge also at smaller scales (Fig. 2 and Extended Data Figs. 1–3). The sensitivity of SIF (SdSIF) and sensitivity of the EF (SdEF) consistently (Supplementary Fig. 1) reveal how the sensitivity of photosynthesis and transpiration to drought stress varies across different topographical settings, indicating generally larger S0 in mountain regions (’M’ in Fig. 2) and along rivers (’R’) and deltas (’D’). We note, however, that ET estimates from the product used here (ALEXI28,29) may be biased high over mountainous terrain where low incident net radiation and surface temperatures are caused not by high EFs but rather by topography effects and local shading. The maps of SdSIF and SdEF also bear strong imprints of human land use. Major irrigated cropland areas are congruent with some of the highest apparent S0 values. In these areas, our analysis yields particularly high CWD values and a low sensitivity of SIF and EF to CWD, without using information about the location and magnitude of irrigation. Other major irrigated areas appear as blank cells in Fig. 2 because the algorithm used to calculate CWD (Methods) fails due to a long-term imbalance between P and ET and a ‘runaway CWD’. This indicates sustained overuse of water resources, caused by lateral water redistribution at scales beyond ~5 km via streamflow diversion or groundwater flow and extraction (or bias in P and ET estimates).
Fig. 2. Rooting-zone water-storage capacity in Central Asia, estimated from SdEF.
Blue areas (flattening) show grid cells where a significant reduction in the slope in EF versus CWD was identified beyond a certain threshold. SdEF values are not calculated for grid cells classified as flattening. Red lines outline major irrigated areas, where the irrigated land area fraction is above 30%41. Information about irrigated areas was used only for mapping here, but is not used for other parts of the analysis. Blank grid cells (white) indicate areas with a sustained imbalance of ET being greater than P. Green letters indicate locations of mountains (M), rivers (R) and delta (D), referred to in the main text. Additional regional maps are provided by Extended Data Figs. 1–3.
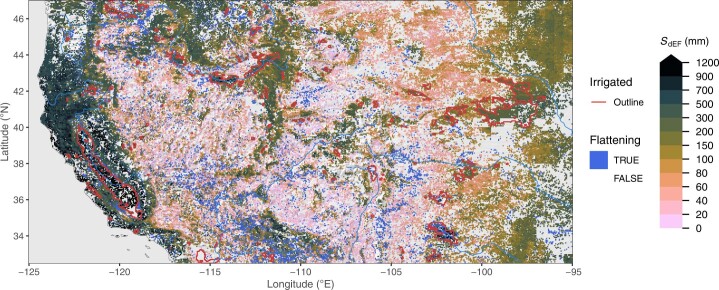
Extended Data Fig. 1. Rooting zone water storage capacity in the Western USA.
Estimated from the evaporative fraction (SdEF). Blue areas (‘flattening’) show grid cells where a significant reduction in the slope in EF vs. CWD was identified beyond a certain threshold. Red lines show outlines of major irrigated areas, that is, where the irrigated land area fraction is above 30%39. Blank grid cells indicate areas with a sustained imbalance of ET being greater than P.
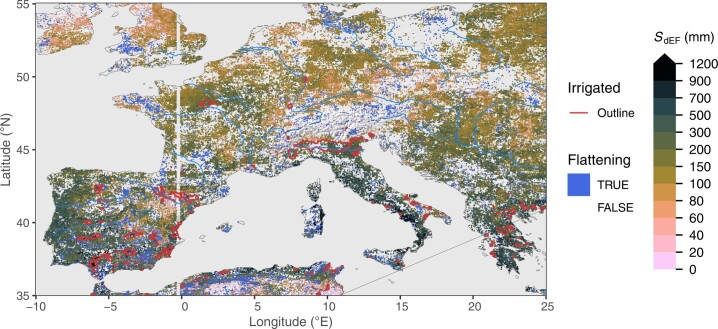
Extended Data Fig. 3. Rooting zone water storage capacity in Europe.
Estimated from the evaporative fraction (SdEF). Blue areas (‘flattening’) show grid cells where a significant reduction in the slope in EF vs. CWD was identified beyond a certain threshold. Red lines show outlines of major irrigated areas, that is, where the irrigated land area fraction is above 30%39. Blank grid cells indicate areas with a sustained imbalance of ET being greater than P.
Regressing vegetation activity against CWD also identifies locations where a decoupling of the two variables appears, that is, where the sensitivity of EF or SIF significantly decreases beyond a certain CWD threshold (‘flattening’ in Fig. 2; Methods). Such areas are particularly common in the vicinity of mountain regions, in areas with irrigated croplands and in savannahs (Supplementary Fig. 2). Related mechanisms may be at play. A flattening of the EF (SIF) versus CWD relationship is probably due to different portions of the vegetation having access to distinct water resources and respective storage capacities. In areas with large topographic gradients, this may be due to within-grid-cell heterogeneity in plant access to the saturated zone. Although relevant for land–atmosphere coupling12, land surface models typically do not account for such effects. This has potential implications for simulations of ET during prolonged dry periods in these regions. In savannahs, a shift in ET contributions from grasses and trees and a related shift in transpiration occurs as grasses, which are often more shallow rooted than trees30, senesce. In irrigated cropland areas, the flattening probably reflects land-use heterogeneity within ~5 km grid cells and the persistent water access on irrigated fields while EF and SIF are reduced more rapidly in surrounding vegetation.
What controls spatial variations in S0 and zr and the sensitivity of vegetation activity to water stress? Following ref. 2, we hypothesized that annual CWD maxima reflect the total amount of plant-accessible water. That is, zr and S0 are sized to just maintain transpiration and photosynthesis under extreme water deficits, commonly experienced over the course of a plant’s lifetime (recurring with a return period of T yr). Hence, a correlation between the magnitude of CWD extremes and the sensitivity of vegetation activity to an increasing CWD should emerge. For estimating CWD extremes, we started by using T = 80 yr and assessed other choices as described in Supplementary Text 1 (also see Extended Data Fig. 4).
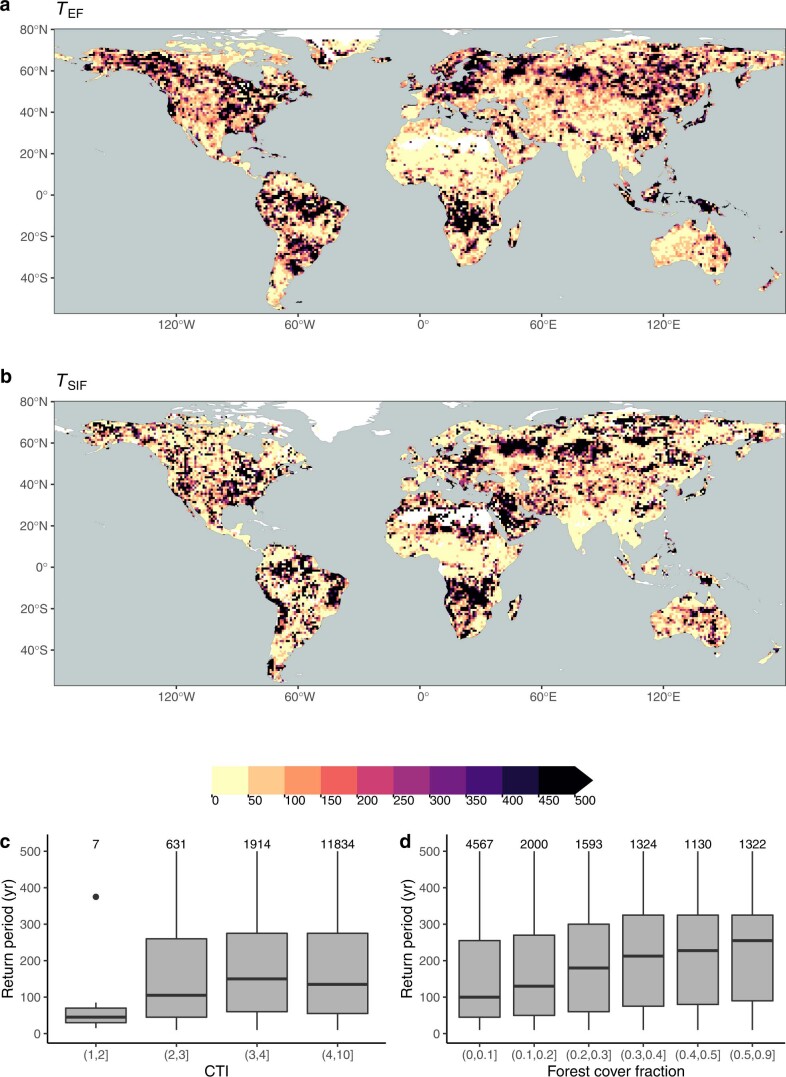
Extended Data Fig. 4. Return periods diagnosed from the evaporative fraction and sun-induced fluorescence.
Return periods T (yr), diagnosed from EF (a) and SIF (b). To diagnose T, a range of alternative values of T are screened and the corresponding range of values SCWDXT are compared to SdEF SdSIF) within 1∘ grid cells (resolution of maps shown here). The best matching T was retained for each gridcell, yielding a global distribution of TEF (TSIF). The bottom panel shows the distribution of diagnosed return periods T (mean of TEF and TSIF) within bins of the Compound Topography Index60 (c) and forest cover fractions (MOD44B62) (d). Boxes represent the interquartile ranges of binned values (Q25, Q75), and whiskers cover Q25 − 1.5(Q75 − Q25) to Q75 + 1.5(Q75 − Q25). Numbers of data points per bin are given above boxes.
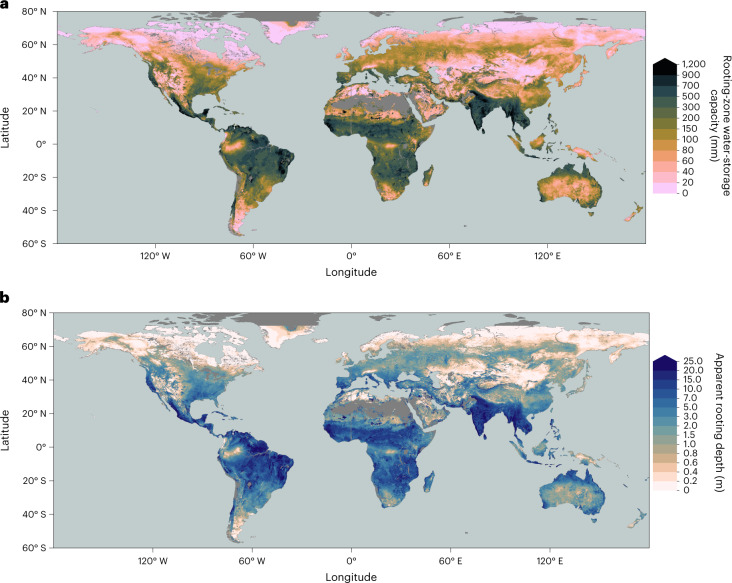
Figure 3a shows the global distribution of SCWDX80 and reveals patterns across multiple scales—in close agreement with SdSIF and SdEF (R2 = 0.76 and R2 = 0.83, respectively; Supplementary Fig. 3). This indicates that the sensitivity of vegetation activity to an increasing CWD (measured by SdSIF and SdEF) is strongly controlled by hydroclimate (as measured by SCWDX80). The agreement between S0 estimates based on water mass-balance approaches2,26 and vegetation activity suggests that plants tend to size their roots no deeper, and S0 no larger, than what is suggested by observed CWD extremes. Magnitudes of SCWDX80 inferred for 55% (37%) of Earth’s vegetated regions indicate plant access to water stored beyond 1 (2) m soil, assuming texture-dependent WHC31–33 (Extended Data Figs. 5 and 6).
Fig. 3. Rooting-zone water-storage capacity and apparent rooting depth from the water mass balance.
a,b, Spatial variations of the rooting-zone water-storage capacity, estimated by SCWDX80 (a) and the apparent rooting depth zCWDX80 (b). Values are remapped to 0.1° resolution. Blank grid cells (grey) are either permanent inland water bodies and ocean or locations with long-term accumulation of water deficits. Values are removed in grid cells where more than 99% is non-vegetation surface according to MODIS Landcover42.
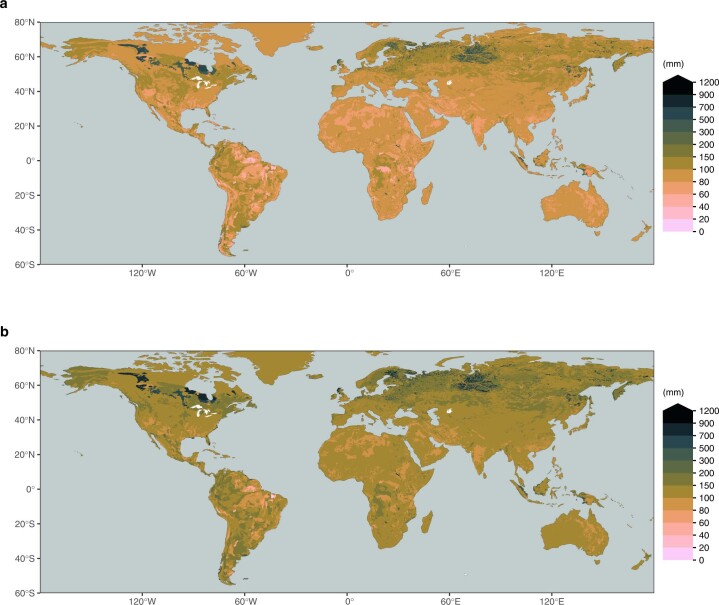
Extended Data Fig. 5. Integrated soil water holding capacity in the soil.
Integrated soil water holding capacity across the top 1 m (a) and the top 2 m (b). Values are calculated based on soil texture information from a gridded version30 of the Harmonized World Soil Database29 and pedo-transfer functions based on ref. 31. HWSD provides information for a top layer (0-30 cm depth) and a bottom layer (30-100 cm depth). For the top 2 m shown in (b), we assumed values from the bottom layer for 100-200 cm depth.
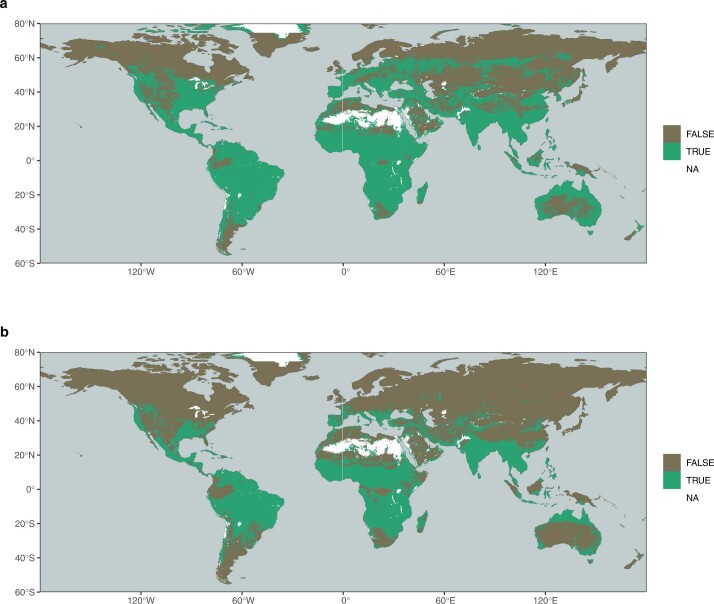
Extended Data Fig. 6. Locations of plant access to deep water storage.
Plant access to deep water storage. Green colors indicate regions where SCWDX80 suggests a rooting zone water storage capacity larger than the integrated water holding capacity across the top 1 m (a) and 2 m (b).
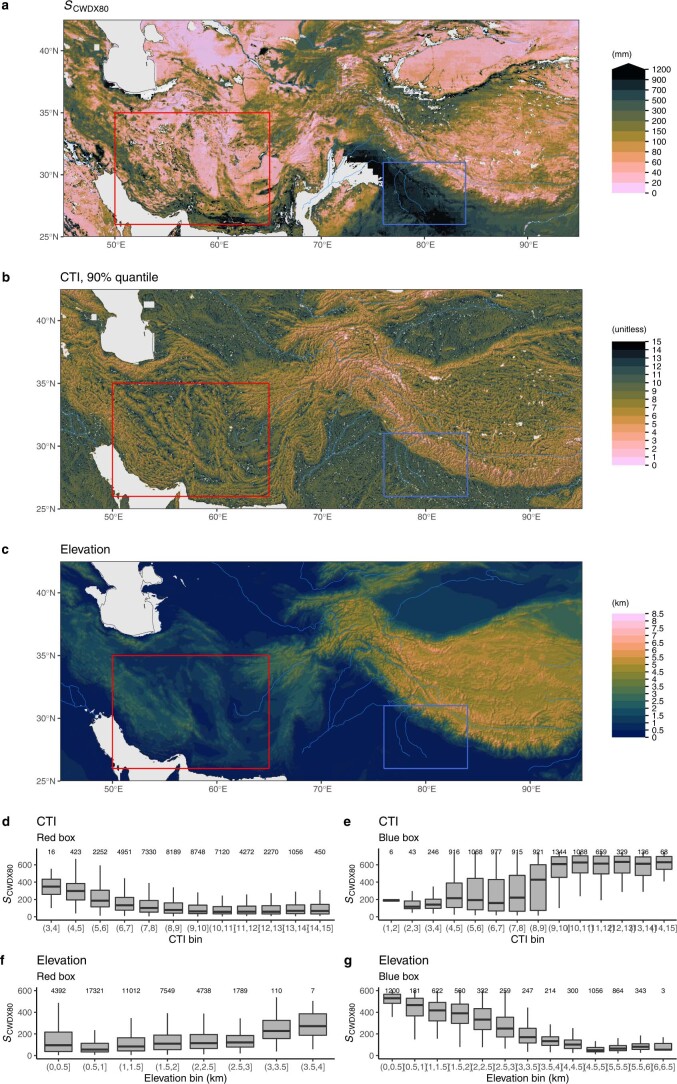
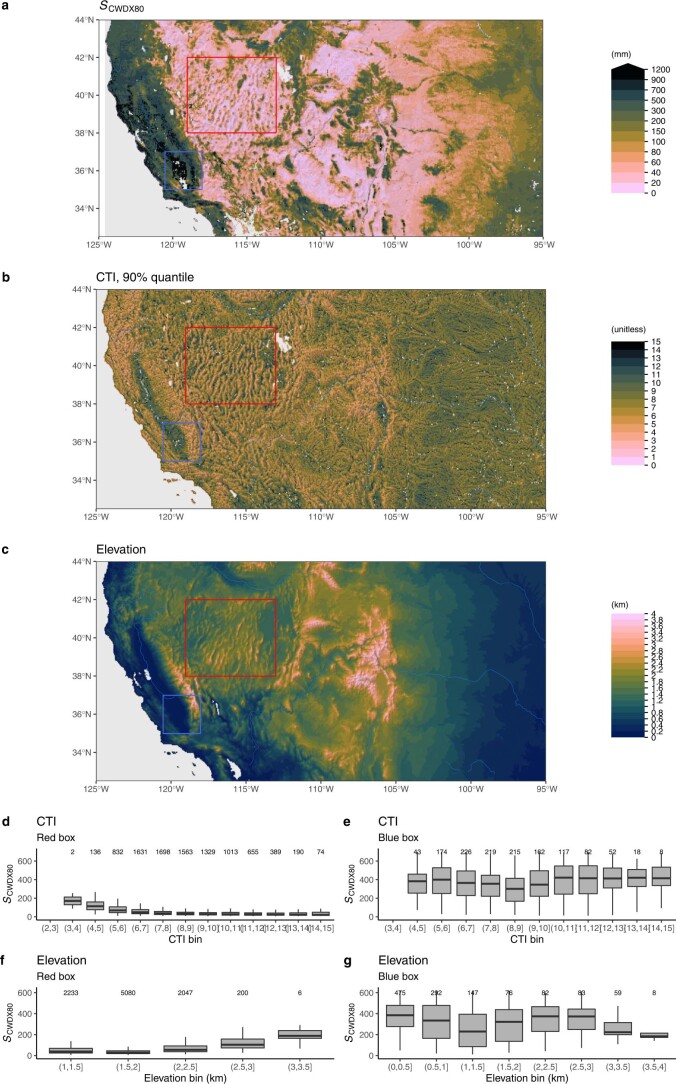
Fine granularity and large spatial heterogeneity of SCWDX80 at regional scales reveal the importance of land use and the local topographical setting for determining plant-available water-storage capacities (Extended Data Figs. 7 and 8). Complex patterns emerge. Mountainous areas feature higher SCWDX80 than their surrounding lowlands. In other regions, lowlands feature some of the highest recorded SCWDX80. In these regions, irrigated agriculture is widespread (Fig. 2 and Extended Data Fig. 1). Variations are likely to extend to even smaller scales along the hillslope topography7 and within individual forest stands34. These scales lie beyond the resolution of the satellite remote-sensing data used here to calculate CWD.
Extended Data Fig. 7. Rooting zone water storage capacity along topographic gradients in central Asia.
(a) Rooting zone water storage capacity in central Asia, estimated by the magnitude of cumulative water deficit extreme events with a return period of 80 years SCWDX80). (b) Compound Topography Index60, shown as 90% quantiles of underlying pixels, given at 15 arcsec, within matching 0.05∘ gridcells. (c) Elevation from ETOPO143. Red and blue rectangles indicate the domains for which SCWDX80 distributions along a CTI and an elevation gradient are shown in (d), (e), (f) and (g). The Compound Topography Index (CTI) is a measure for subsurface flow convergence and the water table depth based on the topographical setting61. Boxes represent the interquartile ranges of binned values (Q25, Q75), and whiskers cover Q25 − 1.5(Q75 − Q25) to Q75 + 1.5(Q75 − Q25). Numbers of data points per bin are given above boxes.
Extended Data Fig. 8. Rooting zone water storage capacity along topographic gradients in the western United States.
(a) Rooting zone water storage capacity in the western United States, estimated by the magnitude of cumulative water deficit extreme events with a return period of 80 years SCWDX80). (b) Compound Topography Index60, shown as 90% quantiles of underlying pixels, given at 15 arcsec, within matching 0.05∘ gridcells. (c) Elevation from ETOPO143. Red and blue rectangles indicate the domains for which SCWDX80 distributions along a CTI and an elevation gradient are shown in (d), (e), (f), and (g). The Compound Topography Index (CTI) is a measure for subsurface flow convergence and the water table depth based on the topographical setting61. Boxes represent the interquartile ranges of binned values (Q25, Q75), and whiskers cover Q25 − 1.5(Q75 − Q25) to Q75 + 1.5(Q75 − Q25). Numbers of data points per bin are given above boxes.
Evaluation with rooting-depth observations
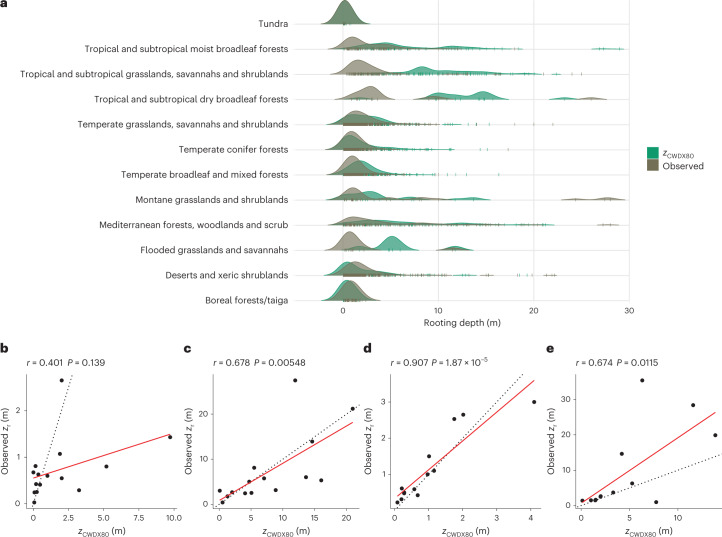
The S0 provides an estimate of the effective total plant-available water, independent of assumptions about physical constraint (limited soil depth, shallow bedrock or groundwater) and independent of uncertain soil texture and water-holding capacity (WHC). Due to the absence of direct observational constraints on S0, we converted S0 to a corresponding apparent zr, enabling an evaluation of S0 estimates against fully independent observations. We focused on comparing biome-level distributions of inferred apparent rooting depth (zCWDX80) with a dataset30 containing 5,524 individual field observations of plant rooting depth from 1,705 globally distributed sites (Supplementary Fig. 4). We thus tested the link between hydroclimate and below-ground vegetation structure across large climatic gradients.
Predicted and observed biome-level maximum rooting depth (90% quantiles) are correlated (Pearson’s r = 0.68; Fig. 4c) while the lower (10%) quantiles appear to be overestimated by zCWDX80 (Fig. 4b). Using a subset of the data where information about the water-table depth (WTD) is provided (489 entries from 359 sites), we limited values of zCWDX80 to the value of the observed local WTD (53% of all observations). This yields a strongly improved correlation of observed and estimated biome-level 10% rooting-depth quantiles (Pearson’s r = 0.91; Fig. 4d) compared with estimates that are not capped at the observed WTD (Fig. 4b). This suggests that inferred zr overestimates values where roots access the groundwater and indicates that groundwater access is relevant across more than half of the globally distributed sites in our dataset. While acting as a constraint on the rooting depth7, plant access to groundwater or a perched water table implies sustained transpiration during dry periods, correspondingly large CWDs and, by implication of the model design, large SCWDX80 and (apparent) zCWDX80.
Fig. 4. Modelled and observed rooting depth by biome.
a, Kernel density estimates of observed and predicted (zCWDX80) rooting depth by biome, based on data aggregated by sites, shown by vertical coloured tick marks. b–e, The 10% (b,d) and 90% (c,e) quantiles of observed versus predicted (zCWDX80) rooting depth by biome of all data (b,c) and of a subset of the data where the WTD was measured along with rooting depth (d,e). Classification of sites into biomes was done on the basis of ref. 43. Dotted lines in b–e represent the 1/1 line. r is the Pearson’s correlation coefficient, and P is the test statistic based on Pearson’s product moment correlation coefficient.
Influence of biotic and abiotic factors
Using first-principles modelling and integrating multiple data streams, we diagnosed a hydrologically effective ecosystem-level S0 from the sensitivity of vegetation activity to CWD. We found that large-scale variations in S0 are driven by the hydroclimate and that global patterns of seasonal water deficits are reflected in the rooting depth of plants. More fine-grained variations in S0 within regions and biomes are linked to land use and irrigation of agricultural land (Fig. 2), to topography (Extended Data Figs. 7 and 8) and to the WTD, as indicated by the comparison with plant-level rooting-depth observations. The method applied here makes use of the sensitivity of remotely sensed ET to an increasing CWD and thus provides estimates of S0 even if below-ground water stores are never fully depleted during the observational period. Additional analyses, where S0 was diagnosed from a simple water-balance model with prescribed S0, confirmed the reliability of the method across a broad range of hydroclimates (Supplementary Text 2 and Supplementary Fig. 5).
The S0 reflects a combination of biotic and abiotic factors. Biotic factors that determine the total plant-available water are, for example, the rooting depth of the vegetation and plant hydraulic properties. Abiotic factors include the hydroclimate and physical constraints to the rooting depth, related to the texture and depth of the soil and the weathered bedrock7. Similarly, human management activities such as irrigation and tile drainage can impact ET, and thus S0, in agricultural systems. Physical constraints to roots are largely unknown across large scales. Our estimation of S0 makes no assumptions about such constraints. Instead, the magnitude of the water-storage capacity is inferred from mass-balance considerations. The CWD we derive from the balances of ET and P imply that the corresponding amount of water is supplied by local storage or supplied from lateral subsurface water convergence—likely a smaller contributor at the ~5 km spatial resolution of the data analysed here35.
Diagnosed values of S0 implicitly include water intercepted by leaf and branch surfaces, internal plant water storage and moisture stored in the topsoil and supplied to soil evaporation. These components are generally smaller in magnitude compared with moisture storage supplied to transpiration36, and their contribution to ET declines rapidly as CWD increases. Hence, spatial variations in S0 reflect primarily variations mediated by moisture stored across the root zone.
Particularly in regions with pronounced dry seasons, our estimates of S0 greatly exceed typical values of the total soil WHC when considering the top 1 or 2 m of the soil column and texture information from global databases31 (Extended Data Fig. 5). The discrepancy in magnitude and spatial patterns of total 1 (2) m soil WHC and S0 diagnosed here hints at a critical role of plant access to deep water and the need to extend the focus beyond moisture in the top 1–2 m of soil for understanding and simulating land–atmosphere exchange10,11. Indications of widespread plant access to deep water stores are consistent with observations of bedrock-penetrating roots7,37 and with evidence for dry-season moisture withdrawal from the weathered bedrock9,11. We note that using the global map of SCWDX80 (zCWDX80) for directly parameterizing S0 (zr) in models may be misleading in areas with particularly small maximum CWDs and consequently small SCWDX80. Scaling relationships of above- and below-ground plant architecture30 and additional effects of how zr determines access to below-ground resources and function (for example, nutrients and mechanical stability) should be considered.
Underlying the estimates of SCWDX80 is the assumption that plant rooting strategies are reflected by CWD extremes with a return period T = 80 yr; SdSIF and SdEF provide an independent constraint to test this assumption. Extended Data Fig. 4 suggests that T is not a global constant. A tendency towards higher T emerges with an increasing grid-cell average forest-cover fraction.
Our analysis identified mountain regions as being characterized by particularly high S0, despite shallow soil and regolith depths38. This could be due to hillslope-scale variations in groundwater depth, enabling sustained transpiration during prolonged rain-free periods. Lateral subsurface flow at scales beyond the resolution of the data used here (~5 km) may additionally supply water for ET and thus contribute to large inferred S0 in valley bottoms of large drainage basins. Local convergence (divergence) acts to supply (remove) subsurface moisture and sustain (reduce) ET, leading to larger (smaller) CWD values. Without relying on a priori assumptions regarding S0 or functional dependencies of water stress effect on ET, thermal infrared- (TIR-) based remote-sensing data (as used here) offer an opportunity to detect such effects8. Our analysis yielded strong contrasts in diagnosed S0 along topographic gradients (Extended Data Figs. 7 and 8). However, further research should assess the accuracy of spatial variations in annual mean ET and potential effects of terrain, where land surface temperature signals on shaded slopes may be misinterpreted by the ALEXI algorithm as signatures of higher ET.
Our global S0 estimates are a ‘snapshot’ in time. Regional- to continental-scale variations in average tree ages may be associated with changes in rooting depth and S0. Furthermore, environmental change may trigger changes in vegetation composition and structure39, with consequences for S0. Similarly, deforestation implies changes in rooting depth18, S0 and the surface energy balance14. Such temporal changes are not considered here due to the limited length of available time series of satellite observations (16 yr). It remains to be seen whether plasticity in zr is sufficiently rapid to keep pace with a changing climate with strong and widespread increases in rainfall variability40 and to what degree rising CO2 alters plant water use and their carbon economy and thereby the costs and benefits of deep roots.
Taken together, constraints available from local zr observations and from global remote sensing of vegetation activity reveal consistent patterns across multiple spatial scales and suggest widespread plant access to deep water storage, including the weathered bedrock and groundwater, or to other ancillary sources of water, such as irrigation. Our study revealed a tight link of the climatology of water deficits and vegetation sensitivity to drought stress. We demonstrated how land–atmosphere interactions and the critical zone water-storage capacity are linked with the rooting depth of vegetation and how below-ground vegetation structure is influenced by the hydroclimate and topography across the globe.
Methods
Estimating ET
Unbiased estimates of ET during rain-free periods are essential for determining CWD and estimating S0 and implied zr. We tested different remote-sensing-based ET products and found that the ALEXI-TIR product, which is based on TIR remote sensing28,29, exhibits no systematic bias during progressing droughts (Supplementary Text 3 and Supplementary Fig. 6), in contrast to other ET estimates assessed here. The stability in ET estimates from ALEXI-TIR during drought is enabled by its effective use of information about the surface energy partitioning, allowing inference of ET rates without reliance on a priori specified and inherently uncertain surface conductances44 or shapes of empirical water stress functions45, and without assumptions of rooting depth or effective S0. ALEXI-TIR is thus well suited for estimating actual ET behaviour during drought without introducing circularity in inferring S0.
CWD estimation
The CWD is determined here from the cumulative difference of actual ET and the liquid-water infiltration to the soil (Pin). ET is based on thermal infrared remote sensing, provided by the global ALEXI data product at daily and 0.05° resolution, covering years 2003–2018. Values in energy units of the latent heat flux are converted to mass units accounting for the temperature and air-pressure dependence of the latent heat of vaporization following ref. 46. The Pin is based on daily reanalysis data of P in the form of rain and snow from WATCH-WFDEI47. A simple snow accumulation and melt model48 is applied to account for the effect of snowpack as a temporary water storage that supplies Pin during spring and early summer. Snow melt is assumed to occur above 1 °C and with a rate of 1 mm d−1 °C−1. The CWD is derived by applying a running sum of (ET – Pin), initiating on the first day when (ET – Pin) is positive (net water loss from the soil) and terminating the summation after rain has reduced the running sum to zero (Supplementary Fig. 7). This yields a continuous CWD time series of daily values. In general, P > ET for annual totals. This implies that the CWD summation is initiated at zero each year. In very rare cases, the CWD accumulates over more than one year, and data were discarded if the accumulation extended over five years (‘runaway CWD’). All P and snow melt (Pin) are assumed to contribute to reducing the CWD. This implicitly assumes that no run-off occurs while the CWD is above zero. The period between the start and end of accumulation is referred to as a CWD event. Within each event, co-varying data, used for analysis, are removed after rain has reduced the CWD to below 90% of its maximum value within the same event. This concerns the analysis of SIF and EF (see the following) and avoids effects of relieved water stress by re-wetting topsoil layers before the CWD is fully compensated. The algorithm to determine daily CWD values and events is implemented by the R package cwd49.
Diagnosing S0 from vegetation activity
By employing first principles for the constraint of the rooting-zone water availability on vegetation activity1, we developed a method to derive how the sensitivity of these fluxes to water stress relates to S0 and how this sensitivity can be used to reveal effects of access to extensive deep water stores. Two methodologically independent sources of information on vegetation activity were used: EF (defined as ET divided by net radiation) and SIF (normalized by incident short-wave radiation). SIF is a proxy for ecosystem photosynthesis50 and is taken here from a spatially downscaled data product51 based on GOME-2 data52,53. Since net radiation and short-wave radiation are first-order controls on ET and SIF, respectively, and to avoid effects by seasonally varying radiation inputs, we used EF instead of ET and considered the ecosystem-level fluorescence yield, quantified as SIF divided by short-wave radiation (henceforth referred to as ‘SIF’) for all analyses. The resulting estimates for S0 are referred to as SdEF and SdSIF, respectively.
The principles for relating vegetation activity to the rooting-zone water availability were considered as follows. As the ecosystem-level CWD increases, both gross primary production (ecosystem-level photosynthesis) and ET are limited by the availability of water to plants. In the following, we refer to gross primary production and ET as a generic ‘vegetation activity’ variable X(t). This principle can be formulated, in its simplest form, as a model of X(t) being a linear function of the remaining water stored along the rooting zone S(t), expressed as a fraction of the total rooting-zone water-storage capacity S0:
| 1 |
Following equation (1), S0 can be interpreted as the total rooting-zone water-storage capacity, or the depth of a water bucket that supplies moisture for ET. Following ref. 1 and with X(t) representing ET, the temporal dynamics during rain-free periods (where run-off can be neglected) are described by the differential equation
| 2 |
and solved by an exponential function with a characteristic decay timescale λ:
| 3 |
λ is related to S0 as S0 = λX0, where X0 is the initial ET at S(t0) = S0. In other words, the apparent observed exponential ET decay timescale λ, together with X0, reflects the total rooting-zone water-storage capacity S0.
Fitting exponentials from observational data is subject to assumptions regarding stomatal responses to declines in S(t) and is relatively sensitive to data scatter. Hence, resulting estimates of S0 may not be robust. With CWD(t) = S0 − S(t) and equation (1), the relationship of X(t) and CWD(t) can be expressed as a linear function
| 4 |
and observational data for X(t) can be used to fit a linear regression model. Its intercept a and slope b can then be used as an alternative, and potentially more robust, estimate for S0:
| 5 |
This has the further advantage that estimates for S0 can be derived using any observable quantity of vegetation activity X(t) (not just ET as in ref. 1) under the assumption that activity attains zero at the point when the CWD reaches the total rooting-zone water-storage capacity; that is, X(t*) = 0 for CWD(t*) = S0.
Here we use a spatially downscaled product of SIF51, normalized by incident short-wave radiation (WATCH-WFDEI data47), and the EF, defined as the ratio of ET (ALEXI-ET data29) over net radiation (GLASS data54), as two alternative, normalized proxies for water-constrained vegetation activity, termed . Normalization by net radiation and incident short-wave radiation, respectively, removes effects by seasonally varying energy available for vegetation activity. is thus assumed to be stationary over time, and the relationship of and CWD(t) is interpreted here as a reflection of effects by below-ground water availability and used to derive SdSIF and SdEF. All data used for are provided at 0.05° and daily resolution.
SdSIF and SdEF were then derived on the basis of the relationship of EF and normalized SIF versus CWD, guided by equation (5). The relationship was analysed for each pixel with pooled data belonging to the single largest CWD event of each year and using the 90% quantile of EF and normalized SIF within 50 evenly spaced bins along the CWD axis. Binning and considering percentiles were chosen to reduce effects of vegetation activity reduction due to factors other than water stress (CWD). We then tested, for each pixel, whether the data support the model of a single linear decline of SIF (EF) with increasing CWD (equation (5)) or, alternatively, a segmented regression model with one or two change points, using the R package segmented55. The model with the lowest Bayesian information criterion was chosen, and SdSIF and SdEF were quantified only for pixels where no significant change point was detected and where the regression of EF (SIF) versus CWD had a significantly negative slope. Flattening EF (SIF) versus CWD relationships were identified where a significant change point was detected and where the slope of the second regression segment was significantly less negative (P = 0.05 of t test) compared with the slope of the first segment. Examples, visualizing the diagnosing of S0 from EF, are given in Supplementary Fig. 8. We performed additional tests of the method’s reliability in estimating S0 by deriving SdEF from simulations of the ecosystem water balance and ET, where S0 was prescribed, using the SPLASH (Simple Process-Led Algorithms for Simulating Habitats) model46. This demonstrates that the method applied for SdSIF and SdEF yields accurate estimates of S0 across all climatic conditions and independent of the size of S0 (Supplementary Text 2 and Supplementary Fig. 5).
Diagnosing S0 from CWDs
Following ref. 2, the S0 is estimated on the basis of CWD extremes occurring with a return period of T years. Magnitudes of extremes with a given return period T (SCWDXT) are estimated by fitting an extreme value distribution (Gumbel) to the annual maximum CWD values for each pixel separately, using the extRemes R package56. Values SCWDXT. are translated into an effective depth zCWDXT using estimates of the plant-available soil WHC, on the basis of soil-texture data from a gridded version of the Harmonized World Soil Database31,32 and pedo-transfer functions derived by ref. 33. Associations of SCWDXT and topography were analysed considering the Compound Topography Index57 and elevation from ETOPO158. The Compound Topography Index is a measure for subsurface flow convergence and the WTB based on the topographical setting59.
Estimating return periods
Diagnosed values of SdSIF and SdEF provide a constraint on the return period T. To yield stable estimates of T and avoid effects of the strong nonlinearity of the function to derive T from the fitted extreme value distributions and magnitudes estimated by SdSIF and SdEF, we pooled estimates SdSIF (SdEF) and SCWDXT values within 1° pixels (≤400 values). A range of discrete values T was screened (10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 200, 250, 300, 350, 400, 450, 500 yr), and the best estimate T was chosen on the basis of comparison with SdSIF (TSIF) and to SdEF (TEF), that is, where the absolute value of the median of the logarithm of the bias was minimal. Relationships of best matching T with topography (measured by the Compound Topography Index57) and with the forest-cover fraction (MODIS MOD44B60) were analysed.
Rooting-depth estimation and observations
We converted root-zone water-storage capacity estimates, SCWDX80, to a corresponding apparent rooting depth (zCWDX80) using a global soil-texture map31,32. The conversion of SCWDX80 into a corresponding depth zCWDX80 accounts for topsoil and subsoil texture and WHC along the rooting profile (Methods and Fig. 3b) and, in view of lacking information with global coverage about the WHC of the weathered bedrock, assuming uniform subsoil texture extending below 30 cm depth. The comparison of biome-level quantities (instead of a direct point-by-point comparison) avoids the inevitable scale mismatch between in situ plant-level observations and global remote-sensing data.
The observational rooting-depth dataset (N = 5,524) was compiled by ref. 30 by combining and complementing published datasets from refs. 22,7. The data include observations of the maximum rooting depth of plants taken from 361 published studies plus additional environmental and climate data. The zr was taken as the plant’s maximum rooting depth. Data were aggregated by sites (N = 1,705) on the basis of longitude and latitude information. Sites were classified into biomes using maps of terrestrial ecoregions43. Quantiles (10%, 90%) were determined for each biome. For a subset of the data (359 sites) where parallel measurements of the WTD were available, we conducted the same analysis but took the minimum of WTD and zr.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41561-023-01125-2.
Supplementary information
Supplementary Figs. 1–8 and Texts 1–3.
Acknowledgements
B.D.S. was funded by the Swiss National Science Foundation grant no. PCEFP2_181115. This work is a contribution to the LEMONTREE (Land Ecosystem Models Based On New Theory, Observations and Experiments) project, funded through the generosity of Eric and Wendy Schmidt by recommendation of the Schmidt Futures programme (B.D.S.). We acknowledge ICDC, CEN, University of Hamburg for data support. S.J.T.-D. was funded by the NSF GRFP and LTER and the NASEM Ford Foundation Fellowship Program. A.G.K. was supported by NSF DEB-1942133.
Extended data
Extended Data Fig. 2. Rooting zone water storage capacity in the Amazon region.
Estimated from the evaporative fraction (SdEF). Blue areas (‘flattening’) show grid cells where a significant reduction in the slope in EF vs. CWD was identified beyond a certain threshold. Red lines show outlines of major irrigated areas, that is, where the irrigated land area fraction is above 30%39. Blank grid cells indicate areas with a sustained imbalance of ET being greater than P.
Author contributions
B.D.S. developed the methods, conducted the analysis and wrote the paper. S.J.T.-D. compiled the rooting-depth dataset and guided its analysis. A.G.K. helped design the analysis and paper. M.C.A. developed the algorithm for thermal infrared remote sensing of evapotranspiration. C.H. generated the evapotranspiration dataset. R.B.J. initiated the study and guided the analysis. All authors contributed to writing the paper.
Peer review
Peer review information
Nature Geoscience thanks Daniella Rempe, David Dralle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Tamara Goldin, in collaboration with the Nature Geoscience team.
Funding
Open access funding provided by University of Bern.
Data availability
Global datasets of SCWDX80 and zCWDX80 are available on Zenodo61. The rooting-depth data are published separately30.
Code availability
The CWD calculation from ET and Pin time series is implemented by the R package cwd49. All code for this analysis is published on Zenodo62.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41561-023-01125-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41561-023-01125-2.
References
- 1.Teuling, A. J., Seneviratne, S. I., Williams, C. & Troch, P. A. Observed timescales of evapotranspiration response to soil moisture. Geophys. Res. Lett. 33, L23403 (2006).
- 2.Gao H, et al. Climate controls how ecosystems size the root zone storage capacity at catchment scale. Geophys. Res. Lett. 2014;41:7916–7923. doi: 10.1002/2014GL061668. [DOI] [Google Scholar]
- 3.Milly PCD. Climate, soil water storage, and the average annual water balance. Water Resour. Res. 1994;30:2143–2156. doi: 10.1029/94WR00586. [DOI] [Google Scholar]
- 4.Hahm WJ, et al. Low subsurface water storage capacity relative to annual rainfall decouples Mediterranean plant productivity and water use from rainfall variability. Geophys. Res. Lett. 2019;46:6544–6553. doi: 10.1029/2019GL083294. [DOI] [Google Scholar]
- 5.Seneviratne SI, et al. Investigating soil moisture–climate interactions in a changing climate: a review. Earth Sci. Rev. 2010;99:125–161. doi: 10.1016/j.earscirev.2010.02.004. [DOI] [Google Scholar]
- 6.Thompson, S. E. et al. Comparative hydrology across AmeriFlux sites: the variable roles of climate, vegetation, and groundwater. Water Resour. Res. 47, W00J07 (2011).
- 7.Fan Y, Miguez-Macho G, Jobbágy EG, Jackson RB, Otero-Casal C. Hydrologic regulation of plant rooting depth. Proc. Natl Acad. Sci. USA. 2017;114:10572–10577. doi: 10.1073/pnas.1712381114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hain CR, Crow WT, Anderson MC, Tugrul Yilmaz M. Diagnosing neglected soil moisture source–sink processes via a thermal infrared-based two-source energy balance model. J. Hydrometeorol. 2015;16:1070–1086. doi: 10.1175/JHM-D-14-0017.1. [DOI] [Google Scholar]
- 9.Rempe DM, Dietrich WE. Direct observations of rock moisture, a hidden component of the hydrologic cycle. Proc. Natl Acad. Sci. USA. 2018;115:2664–2669. doi: 10.1073/pnas.1800141115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson TE, Jesse Hahm W, Crutchfield-Peters K. Digging deeper: what the critical zone perspective adds to the study of plant ecophysiology. N. Phytol. 2020;226:666–671. doi: 10.1111/nph.16410. [DOI] [PubMed] [Google Scholar]
- 11.McCormick EL, et al. Widespread woody plant use of water stored in bedrock. Nature. 2021;597:225–229. doi: 10.1038/s41586-021-03761-3. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell RM, Condon LE. Connections between groundwater flow and transpiration partitioning. Science. 2016;353:377–380. doi: 10.1126/science.aaf7891. [DOI] [PubMed] [Google Scholar]
- 13.Schlemmer L, Schär C, Lüthi D, Strebel L. A groundwater and runoff formulation for weather and climate models. J. Adv. Model. Earth Syst. 2018;10:1809–1832. doi: 10.1029/2017MS001260. [DOI] [Google Scholar]
- 14.Teuling AJ, et al. Contrasting response of European forest and grassland energy exchange to heatwaves. Nat. Geosci. 2010;3:722–727. doi: 10.1038/ngeo950. [DOI] [Google Scholar]
- 15.Koirala S, et al. Global distribution of groundwater–vegetation spatial covariation. Geophys. Res. Lett. 2017;44:4134–4142. doi: 10.1002/2017GL072885. [DOI] [Google Scholar]
- 16.Esteban EJL, Castilho CV, Melgaço KL, Costa FRC. The other side of droughts: wet extremes and topography as buffers of negative drought effects in an Amazonian forest. N. Phytol. 2021;229:1995–2006. doi: 10.1111/nph.17005. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Konings AG, Kennedy D, Gentine P. Global coordination in plant physiological and rooting strategies in response to water stress. Glob. Biogeochem. Cycles. 2021;35:e2020GB006758. doi: 10.1029/2020GB006758. [DOI] [Google Scholar]
- 18.Schenk HJ, Jackson RB. The global biogeography of roots. Ecol. Monogr. 2002;72:311–328. doi: 10.1890/0012-9615(2002)072[0311:TGBOR]2.0.CO;2. [DOI] [Google Scholar]
- 19.Canadell J, et al. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- 20.Weaver JE, Darland RW. Soil–root relationships of certain native grasses in various soil types. Ecol. Monogr. 1949;19:303–338. doi: 10.2307/1943273. [DOI] [Google Scholar]
- 21.Chitra-Tarak R, et al. Hydraulically-vulnerable trees survive on deep-water access during droughts in a tropical forest. N. Phytol. 2021;231:1798–1813. doi: 10.1111/nph.17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenk HJ, Jackson RB. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma. 2005;126:129–140. doi: 10.1016/j.geoderma.2004.11.018. [DOI] [Google Scholar]
- 23.Franklin O, et al. Organizing principles for vegetation dynamics. Nat. Plants. 2020;6:444–453. doi: 10.1038/s41477-020-0655-x. [DOI] [PubMed] [Google Scholar]
- 24.Kleidon A, Heimann M. A method of determining rooting depth from a terrestrial biosphere model and its impacts on the global water and carbon cycle. Glob. Change Biol. 1998;4:275–286. doi: 10.1046/j.1365-2486.1998.00152.x. [DOI] [Google Scholar]
- 25.Schymanski, S. J., Sivapalan, M., Roderick, M. L., Hutley, L. B. & Beringer, J. An optimality-based model of the dynamic feedbacks between natural vegetation and the water balance. Water Resour. Res. 45, W01412 (2009).
- 26.Wang-Erlandsson L, et al. Global root zone storage capacity from satellite-based evaporation. Hydrol. Earth Syst. Sci. 2016;20:1459–1481. doi: 10.5194/hess-20-1459-2016. [DOI] [Google Scholar]
- 27.Knapp AK, Smith MD. Variation among biomes in temporal dynamics of aboveground primary production. Science. 2001;291:481–484. doi: 10.1126/science.291.5503.481. [DOI] [PubMed] [Google Scholar]
- 28.Anderson M. A two-source time-integrated model for estimating surface fluxes using thermal infrared remote sensing. Remote Sens. Environ. 1997;60:195–216. doi: 10.1016/S0034-4257(96)00215-5. [DOI] [Google Scholar]
- 29.Hain CR, Anderson MC. Estimating morning change in land surface temperature from MODIS day/night observations: applications for surface energy balance modeling. Geophys. Res. Lett. 2017;44:9723–9733. doi: 10.1002/2017GL074952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumber-Dávila, S. J., Schenk, H. J., Du, E. & Jackson, R. B. Plant sizes and shapes above- and belowground and their interactions with climate. New Phytol. https://nph.onlinelibrary.wiley.com/doi/abs/10.1111/nph.18031 (2022). [DOI] [PMC free article] [PubMed]
- 31.Harmonized World Soil Database Version 1.0 (FAO, 2008).
- 32.Wieder, W. Regridded Harmonized World Soil Database Version 1.2 (ORNL DAAC, 2014); 10.3334/ORNLDAAC/1247
- 33.Balland V, Pollacco JAP, Arp PA. Modeling soil hydraulic properties for a wide range of soil conditions. Ecol. Model. 2008;219:300–316. doi: 10.1016/j.ecolmodel.2008.07.009. [DOI] [Google Scholar]
- 34.Agee E, et al. Root lateral interactions drive water uptake patterns under water limitation. Adv. Water Resour. 2021;151:103896. doi: 10.1016/j.advwatres.2021.103896. [DOI] [Google Scholar]
- 35.Krakauer NY, Li H, Fan Y. Groundwater flow across spatial scales: importance for climate modeling. Environ. Res. Lett. 2014;9:034003. doi: 10.1088/1748-9326/9/3/034003. [DOI] [Google Scholar]
- 36.Stoy PC, et al. Reviews and syntheses: turning the challenges of partitioning ecosystem evaporation and transpiration into opportunities. Biogeosciences. 2019;16:3747–3775. doi: 10.5194/bg-16-3747-2019. [DOI] [Google Scholar]
- 37.Jackson RB, Moore LA, Hoffmann WA, Pockman WT, Linder CR. Ecosystem rooting depth determined with caves and DNA. Proc. Natl Acad. Sci. USA. 1999;96:11387–11392. doi: 10.1073/pnas.96.20.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier JD, et al. A gridded global data set of soil, intact regolith, and sedimentary deposit thicknesses for regional and global land surface modeling. J. Adv. Model. Earth Syst. 2016;8:41–65. doi: 10.1002/2015MS000526. [DOI] [Google Scholar]
- 39.Parmesan C, Hanley ME. Plants and climate change: complexities and surprises. Ann. Bot. 2015;116:849–864. doi: 10.1093/aob/mcv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendergrass, A. G., Knutti, R., Lehner, F., Deser, C. & Sanderson, B. M. Precipitation variability increases in a warmer climate. Sci. Rep. 7, 17966 (2017). [DOI] [PMC free article] [PubMed]
- 41.Siebert S, et al. Development and validation of the global map of irrigation areas. Hydrol. Earth Syst. Sci. 2005;9:535–547. doi: 10.5194/hess-9-535-2005. [DOI] [Google Scholar]
- 42.Friedl MA, et al. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens. Environ. 2010;114:168–182. doi: 10.1016/j.rse.2009.08.016. [DOI] [Google Scholar]
- 43.Olson DM, et al. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience. 2001;51:933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2. [DOI] [Google Scholar]
- 44.Mu Q, Heinsch FA, Zhao M, Running SW. Development of a global evapotranspiration algorithm based on MODIS and global meteorology data. Remote Sens. Environ. 2007;111:519–536. doi: 10.1016/j.rse.2007.04.015. [DOI] [Google Scholar]
- 45.Fisher JB, et al. ECOSTRESS: NASA’s next generation mission to measure evapotranspiration from the international space station. Water Resour. Res. 2020;56:e2019WR026058. doi: 10.1029/2019WR026058. [DOI] [Google Scholar]
- 46.Davis TW, et al. Simple process-led algorithms for simulating habitats (SPLASH v.1.0): robust indices of radiation, evapotranspiration and plant-available moisture. Geosci. Model Dev. 2017;10:689–708. doi: 10.5194/gmd-10-689-2017. [DOI] [Google Scholar]
- 47.Weedon GP, et al. The WFDEI meteorological forcing data set: WATCH forcing data methodology applied to ERA-Interim reanalysis data. Water Resour. Res. 2014;50:7505–7514. doi: 10.1002/2014WR015638. [DOI] [Google Scholar]
- 48.Orth R, Koster RD, Seneviratne SI. Inferring soil moisture memory from streamflow observations using a simple water balance model. J. Hydrometeorol. 2013;14:1773–1790. doi: 10.1175/JHM-D-12-099.1. [DOI] [Google Scholar]
- 49.Stocker, B. cwd v.1.0: R package for cumulative water deficit calculation. Zenodo10.5281/zenodo.5359053 (2021).
- 50.Zhang Y, et al. Model-based analysis of the relationship between sun-induced chlorophyll fluorescence and gross primary production for remote sensing applications. Remote Sens. Environ. 2016;187:145–155. doi: 10.1016/j.rse.2016.10.016. [DOI] [Google Scholar]
- 51.Duveiller G, et al. A spatially downscaled sun-induced fluorescence global product for enhanced monitoring of vegetation productivity. Earth Syst. Sci. Data. 2020;12:1101–1116. doi: 10.5194/essd-12-1101-2020. [DOI] [Google Scholar]
- 52.Joiner J, et al. Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: methodology, simulations, and application to GOME-2. Atmos. Meas. Tech. 2013;6:2803–2823. doi: 10.5194/amt-6-2803-2013. [DOI] [Google Scholar]
- 53.Köhler P, Guanter L, Joiner J. A linear method for the retrieval of sun-induced chlorophyll fluorescence from GOME-2 and SCIAMACHY data. Atmos. Meas. Tech. 2015;8:2589–2608. doi: 10.5194/amt-8-2589-2015. [DOI] [Google Scholar]
- 54.Jiang B, et al. Validation of the surface daytime net radiation product from version 4.0 GLASS product suite. IEEE Geosci. Remote Sens. Lett. 2019;16:509–513. doi: 10.1109/LGRS.2018.2877625. [DOI] [Google Scholar]
- 55.Muggeo VMR. Estimating regression models with unknown break-points. Stat. Med. 2003;22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 56.Gilleland, E. & Katz, R. W. extRemes 2.0: an extreme value analysis package in R. J. Stat. Softw. 72, 1–39 (2016).
- 57.Marthews TR, Dadson SJ, Lehner B, Abele S, Gedney N. High-resolution global topographic index values for use in large-scale hydrological modelling. Hydrol. Earth Syst. Sci. 2015;19:91–104. doi: 10.5194/hess-19-91-2015. [DOI] [Google Scholar]
- 58.Etopo1, Global 1 Arc-Minute Ocean Depth and Land Elevation from the US National Geophysical Data Center (NGDC) (National Geophysical Data Center, NESDIS, NOAA and US Department of Commerce, 2011); 10.5065/D69Z92Z5
- 59.Beven KJ, Kirkby MJ. A physically based, variable contributing area model of basin hydrology. Hydrol. Sci. J. 1979;24:43–69. doi: 10.1080/02626667909491834. [DOI] [Google Scholar]
- 60.Hansen MC, Townshend JRG, DeFries RS, Carroll M. Estimation of tree cover using MODIS data at global, continental and regional/local scales. Int. J. Remote Sens. 2005;26:4359–4380. doi: 10.1080/01431160500113435. [DOI] [Google Scholar]
- 61.Stocker, B. D. Global rooting zone water storage capacity and rooting depth estimates. Zenodo10.5281/zenodo.5515246 (2021).
- 62.Stocker, B. stineb/mct: v3.0: re-submission to Nature Geoscience. Zenodo10.5281/zenodo.6239187 (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–8 and Texts 1–3.
Data Availability Statement
Global datasets of SCWDX80 and zCWDX80 are available on Zenodo61. The rooting-depth data are published separately30.
The CWD calculation from ET and Pin time series is implemented by the R package cwd49. All code for this analysis is published on Zenodo62.