Abstract
Background
Some studies suggested that the patients included in the Z0011 trial may represent patients with ultrasound-negative axillary nodes and axillary invasion diagnosed by sentinel node (SN) biopsy. Nevertheless, the National Comprehensive Cancer Network (NCCN) guidelines recommend SN mapping if 1 or 2 suspicious lymph nodes are identified on axillary ultrasound (AU). The aim of this preliminary phase of the Multimodal Targeted Axillary Surgery (MUTAS) trial was to establish the accuracy of SN mapping in patients with axillary involvement undergoing upfront surgery.
Methods
Between September 2019 and March 2022, we recruited patients with biopsy-proven metastatic axillary nodes and upfront surgery from a single center. We performed SN mapping in these patients before the surgical intervention, which included axillary lymph node dissection. The biopsy-proven metastatic node, SNs and the remaining axillary nodes were excised separately. SN status was considered representative of the status of the remaining axillary nodes. We calculated the sensitivity, specificity, negative predictive value and positive predictive value of the SN, overall and in patients with palpable nodes, in those with non-palpable nodes and an AU leading to diagnosis of axillary involvement, in those with 1 or 2 suspicious nodes on AU, and in patients with a single suspicious node on AU. We evaluated clinical, imaging and pathology features as predictors of the status of the remaining axillary nodes, false-negatives, and false-positives.
Results
We included 25 patients in this phase. The false-negative rate of SN mapping was 28% overall, 21.42% for patients with palpable nodes, 36.36% for patients with non-palpable nodes and an AU diagnosis of axillary involvement, 28.75% for those with 1 or 2 suspicious nodes on AU, and 15.38% in patients with a single suspicious node on AU. The negative predictive value was highest in patients with a single suspicious node on AU (75%). The only significant predictive factor was that FN showed a higher Ki67 index score.
Conclusions
In this study, SN mapping was not reliable in patients with biopsy-proven metastatic axillary nodes and upfront surgery for any of the subgroups studied. Further research should elucidate the best staging pathways in these patients to avoid premature de-escalation.
Keywords: Breast cancer, sentinel nodes (SNs), biopsy-proven lymph nodes, false-negatives, non-palpable lymph nodes
Highlight box.
Key findings
• The sentinel node technique has low accuracy to predict the axillary tumor load in patients with one or two suspicious nodes on the ultrasound and proven infiltration of the axilla in breast cancer patients.
What is known and what is new?
• The NCCN guidelines recommend proceeding to the sentinel node mapping when axillary ultrasound shows one or two suspicious lymph nodes in breast cancer patients.
• In the present study, the sentinel node technique showed a false-negative rate of 28.75% in patients with one or two suspicious nodes on the ultrasound and proven infiltration of the axilla.
What is the implication and what should change now?
• Premature de-escalation of treatment should be avoided in patients with suspicious lymph nodes on axillary ultrasound before further research elucidates the best staging and therapeutic pathways in these patients.
Introduction
The publication of the ACOSOG Z0011 clinical trial (1) in 2011 represented a radically new approach to the surgical treatment of axillary lymph nodes in breast cancer. In the first 10 years of the 21st century, it was well established that, in patients with no evidence of axillary lymph node involvement on either physical examination or imaging tests, axillary lymph node dissection could be avoided if the sentinel node (SN) was negative, given that the probability of invasion of other axillary nodes was less than 10% (2,3). The Z0011 trial also demonstrated that lymph node dissection could be avoided in patients with 1 or 2 positive SNs and T1 or T2 invasive breast cancer, who underwent breast-conserving surgery and could receive adjuvant radiation therapy, as lymph node dissection provided no advantage in terms of overall survival or local recurrence in these patients (1,4).
One of the most controversial features of the Z0011 trial was that the trial did not include the results of preoperative axillary ultrasound (AU). The authors concluded that AU was not needed in clinically negative patients, and therefore that these patients could benefit from the de-escalation of axillary surgery proposed in the trial (1,4).
This assertion has been refuted by multiple authors, who have stressed the need for AU in patients with a negative physical examination, arguing that ultrasound identifies patients with a high axillary nodal burden, irrespective of the result of the physical examination. Numerous retrospective series were published (5-8) whose results were summarized in 2017 in a meta-analysis by Ahmed et al. (9): ultrasound-positive patients more frequently had 3 or more metastatic lymph nodes than ultrasound-negative patients whose axillary involvement was diagnosed with SN biopsy (56.8% vs. 21.3%). Of note, in the Z0011 trial, only 21% of the patients undergoing axillary lymph node dissection had 3 or more metastatic lymph nodes, a percentage identical that of ultrasound-negative patients of the above-mentioned meta-analysis. Consequently, the patients included in the Z0011 trial seem to mostly represent ultrasound-negative patients, but not clinically negative patients with suspicious nodes on AU.
The aim of the Multimodal Targeted Axillary Surgery trial (MUTAS trial; ClinicalTrials.gov Identifier: NCT04039893) was to investigate how to individualize axillary surgery, depending on the various parameters of physical examination, imaging tests and pathological findings. In this first part of the trial, we performed a pilot study to determine whether SN data could be useful to predict the status of remaining axillary nodes in patients with breast cancer and demonstrated axillary involvement during the staging performed prior to any treatment, who were initially recommended surgery. In addition, we studied whether the utility of this information changed, depending on the results of the physical examination and AU. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-480/rc).
Methods
Ethics committee
The trial was prospective and conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Ethics Committee of the IMIM [Spanish acronym for Instituto Municipal de Investigación Médica (Municipal Institute of Medical Research)] (No. 2018/8361/I). Informed consent was taken from all individual participants.
Patient recruitment
This preliminary phase of the MUTAS trial was performed exclusively in Hospital del Mar, Parc de Salut Mar, in Barcelona, Spain. All patients with a first diagnosis of breast cancer underwent routine AU in the Breast Diseases Unit of the hospital as part of disease staging. Axillary lymph nodes were considered suspicious and underwent biopsy if they showed features of type 4 (generalized lobulated hypoechoic cortex), type 5 (focal hypoechoic cortical lobulation) or type 6 (hypoechoic node with absent hilum) categories of the Bedi classification (10). For this pilot phase, when axillary involvement was suspected on ultrasound, the number of nodes presumed metastatic were quantified. Patients ≥18 years old with cytologically- or biopsy-confirmed axillary involvement and who were recommended to undergo upfront surgery including lymph node dissection by the Breast Diseases Committee were invited to participate in the trial. Exclusion criteria were a previous history of breast cancer in the same or the contralateral breast, patients <18 years old, inability to understand the trial, and non-proven metastatic axillary lymph nodes on either cytology or biopsy.
Among patients accepting to participate, the metastatic biopsied lymph node was marked with an ultrasound visibility marker if the node was non-palpable and had not been marked in the initial diagnostic biopsy.
Identification of the SN and axillary surgery
Trial participants underwent SN mapping according to the standard protocol in our Breast Disease Unit, where we perform the SN identification exclusively with radiotracer in patients treated with upfront surgery.
The day before surgery, the protocol included the intratumoral injection of 111 MBq of 99mTc-nanocolloid (GE Healthcare) to all the patients. The radiotracer volume used was 0.5 mL for palpable tumors and 0.3 mL for non-palpable tumors. The injection in non-palpable tumors was guided with ultrasound. After 30–120 minutes, imaging was obtained. The first SN was identified with the following criteria: the first node to appear, a node appearing in a specific location in the sequential images, a node with a visible lymphatic channel to the injection site or a combination of any of these criteria. The secondary SNs were identified if any lymph node appeared later in the sequential imaging performed.
Axillary surgery was carried out in several stages:
First, the biopsy-proven metastatic lymph node was identified, either by palpation or by ultrasound if it was non-palpable and was then excised. A gamma probe was used to confirm whether the abnormal node was also a hot (sentinel) node.
Second, the hot nodes identified were excised using the gamma probe. Counts were recorded per unit of time with the probe in the operating field before excision (in vivo) and after excision (ex vivo). The wound site was checked for remaining activity. For the study, these nodes were designated as SNs.
Third, Berg level I and II axillary lymph node dissection was performed. Level III was only excised if it contained palpable abnormal nodes. We studied these nodes separately and designated them as remaining axillary nodes.
Pathological analysis
Tumoral study was performed following the routine protocols in our center. The information contained in the standardized pathological report included the tumor size, the pathological subtype, pathological grade, presence or absence of necrosis, perineural invasion, lymphovascular invasion and the immunohistochemistry results, including ki67 index, estrogen receptor (ER) expression, progesterone receptor (PR) expression, c-erbB2 expression and gene amplification status whenever in situ hybridization was performed. We adopted the American Society of Clinical Oncology/College of American Pathologists guideline (11) criteria and considered hormone receptors to be positive if more than 1% of cells were positive on immunohistochemistry. We also adopted the criteria of the American Society of Clinical Oncology/College of American Pathologists clinical guideline (12) regarding c-erbB2: an intense staining on immunohistochemistry or gene amplification by fluorescent in situ hybridization defined the positive cases.
The biopsy-proven metastatic lymph node and the SNs were examined by serial sectioning. Nodes were cut into 2-mm slices and for each slice, six 4-µm sections were obtained, leaving a 20-µm separation between them. Three alternate sections were stained with routine hematoxylin-eosin (HE), and in the case of negative SNs, the remnant sections were studied immunohistochemically using cytokeratin. Information collected included the following: overall number of SLN removed, number of positive SLN removed, size of node metastases [macrometastases, micrometastases or isolated tumor cells (ITCs), as defined in the tumor, node, metastasis (TNM) classification], and the presence or absence of extracapsular invasion.
Regarding the pathological study of the remaining axillary nodes, 3-mm slices were obtained for each node and for each slice a single 4-µm section was stained with routine HE. The total number of nodes excised in this step and the number of invaded nodes were noted.
Data were anonymously collected with Microsoft Access as patient identity was blinded, and patients were identified by only a reference number.
Assessment of SNs as predictors of axillary status
To assess whether the status of SNs was predictive of the status of the remaining axillary nodes, we considered that the absence of involvement of any of the SNs should indicate the absence of involvement in the remaining axillary nodes. In contrast, a finding of metastatic SNs should indicate the presence of remaining metastatic axillary nodes. Therefore, true-negatives were cases with no involvement of either the SNs or the remaining axillary nodes, while true-positives were cases with at least one metastatic SN and at least one remaining metastatic axillary node. False-negatives were cases with negative SNs but at least one remaining metastatic axillary node, while false-positives were defined as cases with at least one metastatic SN but no involvement of any of the remaining axillary nodes.
We calculated the sensitivity, specificity, negative predictive value, and positive predictive value for the total number of patients, for those with palpable nodes, those with non-palpable nodes and an ultrasound diagnosis of axillary involvement, those with 1 or 2 suspicious nodes on AU and those with a single suspicious node on AU.
As the SN technique accuracy has already been stablished, we calculated the intended sample size assuming that in the routinary use of the technique the false-negative rate is 5%. In this new population of patients with biopsy-proven metastatic axillary lymph nodes, we expected a false-negative rate of 20%. To prove this difference in a unilateral test, with a 95% confidence level and 80% statistical power, 59 patients were deemed necessary.
Predictive factors of involvement of the remaining axillary nodes, false-negatives, and false-positives
We studied distinct clinical, AU, and pathological variables as predictors of involvement of the remaining axillary tissue as well as SN false-negatives and false-positives.
Clinical variables consisted of age, body mass index, menopausal status and whether the biopsy-proven metastatic lymph node identified was palpable. Imaging variables comprised the size of the invaded node identified, identification of more than one suspicious node on AU, magnetic resonance (if performed) or computed tomography (routinely performed in our unit in patients with lymph node involvement). Based on the pathological report, as predictive variables of the tumor, we assessed the percentages of ER expression, PR expression, the Ki67 percentage score, lobular carcinoma as a pathological type, histological grade III, the presence of lymphovascular invasion, the size of the largest focus of invasion and total tumor size; as predictive variables of the lymph nodes, we assessed the size of the abnormal node identified, the number of SNs excised, the number of remaining axillary nodes excised, and extranodal extension.
Statistical analysis
Statistical analyses were performed using PASW version 18 (IBM SPSS software, USA). Continuous data were compared using the Mann-Whitney U test as continuous parameters were non-normally distributed. Categorical data were compared using the Fisher exact test. All statistical analyses were two-sided and P values <0.05 were considered significant.
Results
Due to slow accrual, we decided to end this pilot phase of the study in March 2022. From the start of recruitment in September 2019 until the end of the pilot study in March 2022, we identified 61 patients with breast cancer and demonstrated axillary involvement. These patients were recommended surgery as the initial treatment and therefore met the inclusion criteria. Of the 32 patients who accepted to participate, we excluded 7 [in 4, the intervention was postponed due to a positive coronavirus disease (COVID) screening test when the preoperative lymphoscintigraphy had already been performed and they were excluded to avoid them undergoing a second procedure; in 3 patients, the surgeon could not locate the biopsy-proven metastatic lymph node either by palpation or ultrasound]. We report the results of the 25 included patients.
The mean age of the patients was 55.6 [standard deviation (SD): 11.85] years and the median tumor size in the pathology report was 22.55, range 3.1–130 mm. All participants were hormone-receptor-positive and c-erbB2 HER2 negative. Of the 25 patients, the biopsy-proven metastatic lymph node was not a SN in 4 (16%): of these 4 patients, no other SNs were identified in two, 2 SNs were identified in one and 3 SNs were identified in the remaining patient. Of the two patients with no identified SNs, only one showed infiltration of the remaining axillary nodes. Given that the hypothesis of our study was that the presence of infiltrated SNs (other than the cytology- or biopsy-proven infiltrated lymph node) predicted infiltration of the remaining axillary nodes, and no infiltrated SNs were identified in these two patients, we decided to include them in the group of patients with negative SNs: one was a false-negative and the other was a true-negative.
The clinical, imaging and pathology features of the patients are summarized in Table 1. The percentage of patients showing 3 or more metastatic lymph nodes was 56% overall (14/25), 71.43% (10/14) in patients with palpable metastatic nodes, 36.36% (4/11) in patients with non-palpable nodes and an ultrasound diagnosis of axillary involvement, 57.14% (12/21) in those with 1 or 2 suspicious nodes on AU, 46.15% (6/13) in those with a single suspicious node on AU, and 44.44% (4/9) in patients with non-palpable nodes and 1 or 2 suspicious nodes on AU.
Table 1. Clinical, imaging and pathology features of the patients included in this pilot phase of the MUTAS trial.
| Features | Measure or category | Value |
|---|---|---|
| Clinical features (n=25) | ||
| Age (years) | Mean (SD) | 55.6 (11.85) |
| Range | 37–81 | |
| Menopausal status | Premenopausal | 15 |
| Menopausal | 10 | |
| Palpable lymph nodes | Yes | 14 |
| No | 11 | |
| cT | cT1 | 13 |
| cT2 | 12 | |
| cN | cN1 | 25 |
| Imaging features | ||
| Breast cancer focality (n=25) | Unifocal | 24 |
| Multifocal | 1 | |
| Number of suspicious nodes on US (n=25) | 1 | 13 |
| 2 | 8 | |
| 3 | 4 | |
| Number of suspicious nodes on CT (n=25) | 0 | 10 |
| 1 | 9 | |
| 2 | 6 | |
| Number of suspicious nodes on MRI (n=20) | 0 | 2 |
| 1 | 9 | |
| 2 | 9 | |
| Axillary node positivity confirmation (n=25) | Cytology | 18 |
| Biopsy | 7 | |
| Surgical specimen features (n=25) | ||
| Tumor pathology size (mm) | Median (range) | 22.55 (3.1–130.0) |
| Pathology type | Invasive carcinoma NST | 18 |
| Lobular invasive carcinoma | 7 | |
| ER (%) | Mean (SD) | 92.24 (14.22) |
| Range | 30–99 | |
| PR (%) | Mean (SD) | 64.00 (38.11) |
| Range | 0–99 | |
| Ki67 index (%) | Mean (SD) | 20.80 (13.12) |
| Range | 5–60 | |
| C-erbB2/HER2 status | Negative | 25 |
| Histological grade | I | 4 |
| II | 16 | |
| III | 5 | |
| Tumor necrosis | No | 23 |
| Yes | 2 | |
| Tumor lymphatic invasion | No | 12 |
| Yes | 13 | |
| Number of excised ALN (overall)* | Median [range] | 15 [9–25] |
| Number of infiltrated ALN (overall)* | Median [range] | 3 [1–8] |
| pT | pT1 | 15 |
| pT2 | 7 | |
| pT3 | 2 | |
| pT4b | 1 | |
| pN | pN1 | 17 |
| pN2 | 8 | |
*, includes the marked infiltrated node, the SNs, and the remaining axillary lymph nodes. MUTAS, Multimodal Targeted Axillary Surgery; SD, standard deviation; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; ER, estrogen receptor; PR, progesterone receptor; ALN, axillary lymph node; SN, sentinel node.
Ability of SNs to predict the status of the remaining axillary nodes
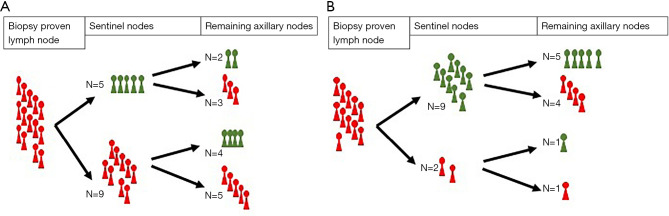
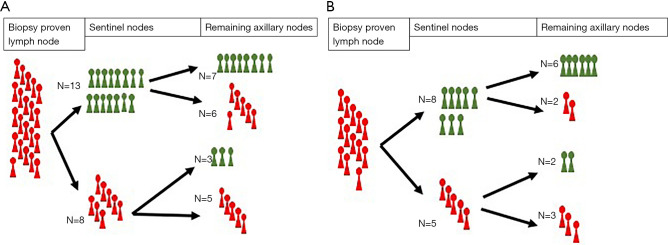
Figure 1A,1B together with Figure 2A,2B illustrate the ability of SNs to establish the status of the remaining axillary nodes. False-negatives represented 28% (7/25) of the patients overall, 21.42% in patients with palpable nodes, 36.36% in patients with non-palpable nodes and an ultrasound diagnosis of axillary involvement, 28.57% in patients with 1 or 2 suspicious nodes on AU, and 15.38% in patients with a single suspicious node on AU. The percentages of false-positives were 20% (5/25), 28.57% (4/14), 9.09% (1/11), 14.29% (3/21) and 23.08% (3/13), respectively. Table 2 shows the sensitivity, specificity, negative predictive value, and positive predictive value in each of these patient groups: the negative predictive value was highest in the group of patients with a single suspicious node on AU.
Figure 1.
Representation of the number of patients with and without metastatic axillary LNs in each level. (A) Patients with palpable LNs, N=14. (B) Patients with non-palpable LNs and an ultrasound diagnosis of axillary involvement, N=11. LN, lymph node.
Figure 2.
Representation of the number of patients with and without metastatic axillary LNs in each level. (A) Patients with 1 or 2 suspicious LNs on ultrasound, N=21. (B) Patients with a single suspicious LN on ultrasound, N=13. LN, lymph node.
Table 2. Overall sensitivity, specificity, PPV and NPV of SN biopsy and in the different studied groups.
| Measures | Overall | Palpable LN | Non-palpable LNs US diagnosis | 1 or 2 suspicious LN on US | Single suspicious LN on US |
|---|---|---|---|---|---|
| Sensitivity | 0.46 | 0.63 | 0.20 | 0.45 | 0.60 |
| Specificity | 0.58 | 0.33 | 0.83 | 0.70 | 0.75 |
| PPV | 0.45 | 0.56 | 0.50 | 0.63 | 0.60 |
| NPV | 0.50 | 0.40 | 0.56 | 0.54 | 0.75 |
PPV, positive predictive value; NPV, negative predictive value; SN, sentinel node; LN, lymph node; US, ultrasound.
Factors predictive of involvement of the remaining axillary nodes and of SN false-negatives and false-positives
Table 3 shows the results of analysis of the quantitative variables as possible predictors of involvement of the remaining axillary nodes and SN false-negatives and false-positives. In patients with involvement in the remaining axillary nodes, the size of the invading component and the total tumor size were larger, although this difference was not statistically significant. Among false-negatives, the Ki67 index score was significantly higher and the size of the focus of invasion was larger, although this latter difference was not statistically significant. There were no significant differences in the number of false-positives, although the mean size of the focus of invasion was lower in these cases.
Table 3. Analysis of the quantitative variables as possible predictors of involvement of the remaining axillary nodes and SN false-negatives and false-positives, Mann-Whitney U test.
| Features | Remaining axillary nodes status | SN false-negatives | SN false-positives | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None metastatic (n=12), mean (SD) | >1 metastatic LNs (n=13), mean (SD) | P | No FN (n=18), mean (SD) | FN (n=7), mean (SD) | P | No FP (n=20), mean (SD) | FP (n=5), mean (SD) | P | |||
| Age (years) | 51.66 (7.88) | 59.23 (13.93) | 0.13 | 58.85 (17.78) | 54.3 (8.9) | 0.74 | 56.45 (12.73) | 52.20 (7.39) | 0.53 | ||
| Body mass index (kg/m2) | 27.61 (6.46) | 24.52 (4.48) | 0.20 | 26.41 (5.71) | 24.95 (5.70) | 0.53 | 26.29 (5.62) | 24.85 (6.16) | 0.45 | ||
| Suspicious node US size (mm) | 14.08 (4.94) | 16.07 (7.02) | 0.61 | 14.94 (5.10) | 15.57 (8.58) | 0.83 | 14.75 (6.12) | 16.60 (6.30) | 0.45 | ||
| ER (%) | 94.08 (6.11) | 90.53 (19.08) | 0.76 | 95 (5.47) | 85.14 (25.3) | 0.70 | 91.45 (15.72) | 95.40 (4.92) | 0.81 | ||
| PR (%) | 74.58 (32.54) | 54.23 (41.45) | 0.34 | 63.27 (36.26) | 65.85 (45.58) | 0.65 | 60.15 (39.93) | 79.40 (27.75) | 0.30 | ||
| Ki67 index (%) | 16.66 (12.30) | 24.61 (13.14) | 0.06 | 17.22 (10.60) | 30 (15.27) | 0.02 | 21.25 (12.12) | 19.00 (18.16) | 0.33 | ||
| Invasive tumor size (mm) | 27.60 (29.06) | 41.27 (68.30) | 0.61 | 27.52 (26.19) | 53.20 (92.60) | 0.70 | 38.56 (58.36) | 19.32 (7.87) | 0.92 | ||
| Tumor size including DCIS (mm) | 30.50 (35.24) | 48.50 (67.87) | 0.25 | 29.65 (30.36) | 65.97 (89.76) | 0.13 | 43.99 (61.18) | 26.02 (15.52) | 0.89 | ||
| Biopsy proven LN size on the pathology report (mm) | 21.50 (5.97) | 22.15 (7.25) | 0.89 | 21.27 (6.25) | 23.28 (7.54) | 0.458 | 22.10 (7.25) | 20.80 (2.28) | 0.86 | ||
| Number of SNs excised | 1 (1.04) | 1.69 (1.49) | 0.27 | 1.55 (1.33) | 0.85 (1.21) | 0.27 | 1.25 (1.40) | 1.8 (0.83) | 0.30 | ||
| Final number of LNs excised | 14.41 (6.21) | 12.76 (4.85) | 0.61 | 13.66 (5.48) | 13.28 (5.93) | 0.79 | 13.85 (5.77) | 12.40 (4.50) | 0.76 | ||
SN, sentinel node; SD, standard deviation; LN, lymph node; FN, false negative; FP, false positive; US, ultrasound; ER, estrogen receptor; PR, progesterone receptor; DCIS, ductal carcinoma in situ.
Table 4 shows the results of the bivariate analysis of the qualitative variables. Although there were no significant differences, the percentages of involvement of the remaining axillary nodes and false-negatives were higher if, on ultrasound, magnetic resonance or computed tomography, there was more than 1 suspicious node or if there was extranodal extension in the biopsy-proven metastatic lymph node. Equally, there were no significant differences in the number of false-positives, but they were more frequent in patients with palpable nodes, tumor histological grade III and lymphovascular tumoral invasion.
Table 4. Bivariate analysis of categorical variables as possible predictors of involvement of the remaining axillary nodes and SN false-negatives and false-positives, Fisher test.
| Features | Remaining axillary nodes status | SN false-negatives | SN false-positives | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None metastatic, fraction (%) | >1 metastatic, fraction (%) | P | No FN, fraction (%) | FN, fraction (%) | P | No FP, fraction (%) | FP, fraction (%) | P | |||
| Postmenopausal patient | 3/12 (25.0) | 7/13 (53.8) | 0.22 | 7/18 (38.9) | 3/7 (42.9) | 1.00 | 9/20 (45.0) | 1/5 (20.0) | 0.61 | ||
| Palpable metastatic node | 6/12 (50.0) | 8/13 (61.5) | 0.56 | 11/18 (61.1) | 3/4 (42.9) | 0.66 | 10/20 (50.0) | 4/5 (80.0) | 0.34 | ||
| >1 suspicious axillary node on US | 4/12 (33.3) | 8/13 (61.5) | 0.15 | 7/18 (38.9) | 5/7 (71.4) | 0.20 | 9/20 (45.0) | 3/5 (60.0) | 0.64 | ||
| >1 suspicious node on MRI | 4/11 (36.4) | 4/9 (55.6) | 0.65 | 6/15 (40.0) | 3/5 (60.0) | 0.61 | 8/15 (53.3) | 1/5 (20.0) | 0.31 | ||
| >1 suspicious node on CT | 2/12 (16.6) | 4/13 (30.7) | 0.10 | 4/18 (22.2) | 2/7 (28.5) | 0.37 | 4/20 (20.0) | 2/5 (40.0) | 0.43 | ||
| Pathological type: lobular carcinoma | 6/12 (50.0) | 7/13 (53.8) | 0.68 | 8/18 (44.4) | 3/7 (42.8) | 0.13 | 5/20 (25.0) | 2/5 (40.0) | 0.59 | ||
| Histological grade II in the surgical specimen | 2/12 (16.7) | 3/13 (23.1) | 0.10 | 4/18 (22.2) | 1/7 (14.3) | 0.46 | 3/20 (15.0) | 2/5 (40.0) | 0.37 | ||
| Lymphovascular invasion in the surgical specimen | 6/12 (50.0) | 7/13 (53.8) | 0.84 | 9/18 (50.0) | 4/3 (57.1) | 1.00 | 9/20 (45.0) | 4/5 (80.0) | 0.32 | ||
| Extracapsular nodal extension | 4/12 (33.3) | 7/13 (53.8) | 0.30 | 6/18 (33.3) | 5/7 (71.4) | 0.18 | 9/20 (45.0) | 2/5 (40.0) | 1 | ||
SN, sentinel node; FN, false negative; FP, false positive; US, ultrasound; MRI, magnetic resonance imaging; CT, computed tomography.
Discussion
In this initial analysis of the results of the MUTAS trial, the SN technique provided no valid information in patients with a diagnosis of axillary involvement and who underwent surgery as the initial treatment, given the very high number of false-positives and false-negatives. The results were similar in patients with palpable metastatic nodes and those with non-palpable nodes and an ultrasound diagnosis of axillary involvement. Both the negative predictive value and the positive predictive value improved in patients with a single suspicious node on AU.
Although the authors of the Z0011 trial defended the use of physical examination as a single step to assess the axilla if the results were negative (1,4), many authors roundly refuted this argument, given that the accuracy of palpation is only 20% (13,14). In the era of precision medicine and given the ever-greater availability of imaging tests, it seems reasonable to perform the most comprehensive staging possible to select the optimal therapeutic option.
We acknowledge that a direct statistical comparison between our study and the Z0011 trial would be inappropriate due to the low number of patients recruited. Nevertheless, our figures show that all the subgroups studied had a considerably higher percentage of patients with 3 or more metastatic lymph nodes than that reported in the Z0011 study (1,4). Even in the most favorable subgroup, i.e., patients with non-palpable metastatic lymph nodes and a single suspicious node on AU, this percentage was 36.36%, which is substantially higher than the 21% reported in Z0011. This difference could be attributed to the fact that the median tumor size is larger in our study. However, as previously mentioned, patients included in the Z0011 trial seem to be more representative of patients with non-suspicious nodes on AU than of patients with 1 or 2 suspicious nodes. This statement is supported not only by the 2017 meta-analysis by Ahmed et al. (9) but also by subsequent studies (15-17).
In view of all the previously mentioned published studies, we believe the recommendation of the National Comprehensive Cancer Network (NCCN) guidelines to perform SN mapping if only 1 or 2 suspicious lymph nodes are identified on AU (18) to be premature. Currently, the accuracy of AU is unclear, as some authors report insufficient precision to detect limited axillary disease (19,20), while others consider its sensitivity to be similar to that of SN mapping (21). These contradictory findings, together with the results of our study showing a high false-negative rate in patients with 1 or 2 suspicious nodes on ultrasound, should prompt cautious recommendations before new ongoing trials elucidate when to rely on ultrasound quantification of axillary involvement.
In patients with a diagnosis of breast cancer and axillary lymph node involvement, it is as important to determine the optimal technique to quantify the tumor burden as it is to select the most effective treatment. In a recent retrospective review of the Surveillance, Epidemiology and End Results (SEER) program database by Gou et al., in patients with 3 affected SNs, survival was better with axillary lymph node dissection than with sentinel lymph node biopsy alone (22). Prospective studies should define the role of axillary lymph node dissection, radiotherapy, or both together, in the treatment of axillary node involvement, which could change depending on axillary tumor load (23). It is therefore crucial to identify the most precise technique to quantify tumor burden.
The small number of patients included in this initial phase of the MUTAS trial limits the value of the bivariate analysis of the factors predictive of involvement of the remaining axillary nodes and SN false-negatives and false-positives. Although we report some differences in the percentages in this bivariate analysis, most of them were non-significant. In addition, because of the low number of cases analyzed, we did not evaluate as predictors of involvement of the remaining axillary tissue other important features, such as tumor multifocality, because they were sparsely represented in this cohort. New studies with a larger number of patients should elucidate if any of these differences could be significant. Of note, in such a small number of patients, the Ki67 index score was significantly higher in patients with a false-negative SN result. This suggests that the SN technique is even less reliable in patients with axillary lymph node involvement and a more proliferative breast cancer, which is therefore more aggressive. Future trials should analyze all the techniques available to quantify axillary tumor load considering the histopathological, immunohistochemical and molecular characteristics of the tumors, given that it has been proven that these features influence the axillary tumor load (24,25) and the accuracy of diagnostic tests (26,27)
An obvious limitation of this initial phase of the MUTAS trial is the very small number of patients analyzed that did not reach the predefined estimated sample size. The reason for the low recruitment was the outbreak of the coronavirus disease 2019 (COVID-19) pandemic a few months after the start of the study, 2.5 years ago. We were unable to recruit more patients into this surgical trial—always difficult even at the best of times—due to huge organizational and logistic obstacles. Nevertheless, we believe the results deserve to be reported as they may guide the design of further trials on axillary surgery that may be performed in the near future. Regarding the MUTAS trial, and after the results of this pilot phase, we are planning a multicenter study with techniques other than SN identification to identify the infiltrated axillary lymph nodes.
Conclusions
In this pilot study, SN mapping was unreliable to predict axillary tumor load in patients with confirmed axillary involvement during staging who underwent upfront surgery. The number of false-negatives was high in patients with palpable nodes, in those with non-palpable nodes and an ultrasound diagnosis of axillary involvement, and in those with 1 or 2 suspicious nodes on AU, as well as in patients with a single suspicious node on AU. Further research should elucidate the best staging and therapeutic pathways in these patients.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors wish to extend their most heartfelt thanks to the patients who agreed to participate in the study at a time when they not only faced a global pandemic that left nobody unscathed but also their own locally advanced breast cancer. Their courage is an inspiration and serves as a lesson to all health professionals and especially to the research team of the MUTAS trial. We also wish to thank all the remaining patients and extend our deep understanding and respect: no clinical trial should take priority over their wellbeing and we thank them for their trust in choosing our Breast Diseases Unit to join us in the fight against their breast cancer. We thank Gail Craigie for her help in translating and editing this manuscript. The abstract of this article was presented as a poster in the 13th European Breast Cancer Conference in November 2022, and published in the European Journal of Cancer 2022;175:s16. Available online: https://www.ejcancer.com/article/S0959-8049(22)01386-7/fulltext.
Funding: This work was supported by the 6th Ana Balil Grant of the GEICAM [Spanish acronym for the Grupo Español de Investigación en Cáncer de Mama (Spanish Breast Cancer Group)].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of the IMIM [Spanish acronym for Instituto Municipal de Investigación Médica (Municipal Institute of Medical Research)] (No. 2018/8361/I) and informed consent was taken from all individual participants.
Footnotes
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-480/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-480/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-480/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-480/coif). The authors have no conflicts of interest to declare.
References
- 1.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol 2006;7:983-90. 10.1016/S1470-2045(06)70947-0 [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. 10.1016/S1470-2045(10)70207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barco I, Chabrera C, García-Fernández A, et al. Role of axillary ultrasound, magnetic resonance imaging, and ultrasound-guided fine-needle aspiration biopsy in the preoperative triage of breast cancer patients. Clin Transl Oncol 2017;19:704-10. 10.1007/s12094-016-1589-7 [DOI] [PubMed] [Google Scholar]
- 6.Boone BA, Huynh C, Spangler ML, et al. Axillary Lymph Node Burden in Invasive Breast Cancer: A Comparison of the Predictive Value of Ultrasound-Guided Needle Biopsy and Sentinel Lymph Node Biopsy. Clin Breast Cancer 2015;15:e243-8. 10.1016/j.clbc.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 7.Moorman AM, Bourez RL, Heijmans HJ, et al. Axillary ultrasonography in breast cancer patients helps in identifying patients preoperatively with limited disease of the axilla. Ann Surg Oncol 2014;21:2904-10. 10.1245/s10434-014-3674-x [DOI] [PubMed] [Google Scholar]
- 8.Verheuvel NC, van den Hoven I, Ooms HW, et al. The role of ultrasound-guided lymph node biopsy in axillary staging of invasive breast cancer in the post-ACOSOG Z0011 trial era. Ann Surg Oncol 2015;22:409-15. 10.1245/s10434-014-4071-1 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M, Jozsa F, Baker R, et al. Meta-analysis of tumour burden in pre-operative axillary ultrasound positive and negative breast cancer patients. Breast Cancer Res Treat 2017;166:329-36. 10.1007/s10549-017-4405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol 2008;191:646-52. 10.2214/AJR.07.2460 [DOI] [PubMed] [Google Scholar]
- 11.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med 2020;144:545-63. 10.5858/arpa.2019-0904-SA [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 2018;142:1364-82. 10.5858/arpa.2018-0902-SA [DOI] [PubMed] [Google Scholar]
- 13.Angarita S, Ye L, Rünger D, et al. Assessing the Burden of Nodal Disease for Breast Cancer Patients with Clinically Positive Nodes: Hope for More Limited Axillary Surgery. Ann Surg Oncol 2021;28:2609-18. 10.1245/s10434-020-09228-5 [DOI] [PubMed] [Google Scholar]
- 14.Majid S, Tengrup I, Manjer J. Clinical assessment of axillary lymph nodes and tumor size in breast cancer compared with histopathological examination: a population-based analysis of 2,537 women. World J Surg 2013;37:67-71. 10.1007/s00268-012-1788-5 [DOI] [PubMed] [Google Scholar]
- 15.Defer A, Tessier V, Haudebourg J, et al. Is preoperative axillary radio-cytology justified after ACOSOG Z001? Bull Cancer 2021;108:605-13. 10.1016/j.bulcan.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto N, Aruga T, Horiguchi S, et al. Ultrasound-guided fine-needle aspiration of axillary lymph nodes in breast cancer: Diagnostic accuracy and role in surgical management. Diagn Cytopathol 2019;47:788-92. 10.1002/dc.24203 [DOI] [PubMed] [Google Scholar]
- 17.Cipolla C, Valerio MR, Grassi N, et al. Axillary Nodal Burden in Breast Cancer Patients With Pre-operative Fine Needle Aspiration-proven Positive Lymph Nodes Compared to Those With Positive Sentinel Nodes. In Vivo 2020;34:729-34. 10.21873/invivo.11831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. Breast Cancer (version 4.2022). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419.
- 19.Riedel F, Schaefgen B, Sinn HP, et al. Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur J Radiol 2021;135:109468. 10.1016/j.ejrad.2020.109468 [DOI] [PubMed] [Google Scholar]
- 20.Le Boulc'h M, Gilhodes J, Steinmeyer Z, et al. Pretherapeutic Imaging for Axillary Staging in Breast Cancer: A Systematic Review and Meta-Analysis of Ultrasound, MRI and FDG PET. J Clin Med 2021;10:1543. 10.3390/jcm10071543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radosa JC, Solomayer EF, Deeken M, et al. Preoperative Sonographic Prediction of Limited Axillary Disease in Patients with Primary Breast Cancer Meeting the Z0011 Criteria: an Alternative to Sentinel Node Biopsy? Ann Surg Oncol 2022;29:4764-72. 10.1245/s10434-022-11829-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gou Z, Lu X, He M, et al. Trends in axillary surgery and clinical outcomes among breast cancer patients with sentinel node metastasis. Breast 2022;63:9-15. 10.1016/j.breast.2022.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Rashdan A, Deban M, Quan ML, et al. Locoregional Management of Breast Cancer: A Chronological Review. Curr Oncol 2022;29:4647-64. 10.3390/curroncol29070369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolau P, Gamero R, Rodríguez-Arana A, et al. Imaging and pathology features to predict axillary tumor load in breast cancer. J Obstet Gynaecol Res 2018;44:331-6. 10.1111/jog.13490 [DOI] [PubMed] [Google Scholar]
- 25.Rossing M, Pedersen CB, Tvedskov T, et al. Clinical implications of intrinsic molecular subtypes of breast cancer for sentinel node status. Sci Rep 2021;11:2259. 10.1038/s41598-021-81538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ensenyat-Mendez M, Rünger D, Orozco JIJ, et al. Epigenetic Signatures Predict Pathologic Nodal Stage in Breast Cancer Patients with Estrogen Receptor-Positive, Clinically Node-Positive Disease. Ann Surg Oncol 2022;29:4716-24. 10.1245/s10434-022-11684-0 [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Huang Y, Peng J, et al. Optimal Selection of Imaging Examination for Lymph Node Detection of Breast Cancer With Different Molecular Subtypes. Front Oncol 2022;12:762906. 10.3389/fonc.2022.762906 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as