Abstract
Background
Surgical treatment of pituitary lesions causing hormonal overproduction or mass effect is standard procedure. There are few reports on the results and complications related to these surgeries from Northern Europe. Our aim was to evaluate the outcome and complications of a single tertiary surgical center over more than a decade.
Methods
This was a retrospective study on all patients that underwent pituitary surgery from 1st of January 2005 to 31st of December 2017. The analysis included type of lesion, surgical method, pre- and postoperative need for hormonal substitution, hormonal outcome, complications to surgery, survival, need for revision surgery, or stereotactic radiation. Appropriate statistical analyses were made to evaluate surgical results, complications, and survival.
Results
Five hundred seventy-eight patients were included in the study. Remission was achieved in 58% of patients with GH-producing and 94% of ACTH-releasing adenomas. Sixty-six percent had no preoperative hormonal substitution compared to 39% postoperatively. Rhinosinusitis (10%) was the most commonly reported postoperative complication followed by leakage of cerebrospinal fluid (8%) and meningitis (4%). Standardized mortality rate for the study population was higher (p = 0.18) when compared to the general population.
Conclusion
Our results regarding remission rates and complications are in comparison with previous studies. Surgery of pituitary lesion can be considered a safe and efficient surgery. We noted lower rates of CSF leakage in the later part of the study period and believe that this, in part, was an effect by the introduction of a multidisciplinary surgical skull base team and increased surgical experience.
Keywords: Transsphenoidal, Pituitary surgery, Surgical results, Complications, Morbidity, Mortality, Survival
Introduction
Pituitary adenomas (PA) are common, with a reported prevalence of 16.7% of intracranial tumors [9]. In addition to adenomas, other lesions originating from the pituitary may arise and provoke symptoms, most commonly cysts. PAs can be classified into hormone-releasing or hormonally inactive tumors (non-functioning pituitary adenomas, NFPA). Symptoms arise either due to affected hormonal production or compression of nearby structures, usually the optic chiasm [29]. PAs are also classified according to size, i.e., micro (< 10 mm), macro (> 10 mm), and giant adenomas (> 40 mm) [6, 16, 18]. With the exception for prolactinomas, surgery is the preferred treatment of PAs[29]. Pituitary surgery has undergone major changes over time, mainly due to technical development of surgical equipment. Nowadays, an endoscopic approach to the sellar region, with a dedicated skull base team, is commonly practiced[38]. The intricate anatomy surrounding the pituitary offers a surgical challenge and it is considered the main reason why non-radical resections are common [1]. A well-established complement to surgery, especially in Cushing disease, is stereotactic radio surgery (SRS)[5, 28, 32].
The literature contains numerous scientific publications on surgical results and techniques in endoscopic pituitary surgery[1, 4, 23, 29, 31]. However, few originate from northern Europe and none from Sweden, although these procedures have been performed here for many decades.
This study had multiple aims. Our first aim was to evaluate complication rates after primary transsphenoidal pituitary surgery in patients treated at Karolinska University Hospital 2005–2017. Secondarily, we wanted to evaluate survival rates in patients that have undergone transsphenoidal pituitary surgery of PA (both hormone-releasing PAs and NFPAs) compared to an age- and gender-matched general population. Thirdly, our aim was to evaluate remission rates (in hormone-releasing PAs), with or without complementary SRS and the need for postoperative hormonal substitution in patients with PAs.
Materials and methods
Study design
This was a retrospective observational study based on medical records from individual patients that underwent primary transsphenoidal pituitary surgery, with or without complementary SRS, due to assumed PA at the Karolinska University Hospital 1 Jan 2005 to 31 Dec 2017. The patients were identified by the surgical codes for transsphenoidal surgery of the pituitary gland and SRS.
Patients with craniopharyngeomas, germinomas, or chordomas were excluded as these are not considered true lesions of the pituitary gland. Patients who had either revision surgery at the Karolinska University Hospital or had postoperative follow-up at another hospital were also excluded from the study.
The medical records were reviewed for age, gender, date of surgery, date of death, hormonal release (pre- and postoperatively), hormonal substitution (pre- and postoperatively) type of surgery (± SRS), radiologic examinations, type of pituitary lesion (pathological-anatomical diagnosis), and postoperative complications.
Remission was defined as biochemical normalization of hormone hypersecretion (normal IGF, growth hormone (GH)) < 0.4 ng/mL after OGTT, and random GH < 1.0 ng/mL), normal Urinary Free Cortisol (UFC), and adequate inhibition after dexamethazone test. Detailed information regarding endocrinological follow-up is described in a later section.
Complications were defined as cerebrospinal fluid (CSF) leakage (verified using Beta transferrin protein test) requiring surgical treatment, postoperative sinonasal infection (signs of rhinosinusitis at follow-up endoscopic examination), bleeding which required intervention, diagnosed meningitis, sepsis, mortality or neurological deficits within 48 h from surgery, deep venous thrombosis (DVT), and pulmonary embolism (PE) during postoperative hospital stay.
All surgeries were performed in the Karolinska University Hospital, either at the Department of Neurosurgery or at the Department of Otorhinolaryngology, while the SRS was performed at the Department of Neurosurgery alone.
The date for the survival analysis was set to 18th of May 2018. Statistics for general population was collected from Statistics Sweden (www.scb.se).
The study was approved by the Central Ethical Review Board in Stockholm, Sweden (EBN 8,930,234).
Surgical technique
All patients were operated in general anesthesia and received prophylactic perioperative corticosteroids (Solu-cortef ©) and a single dose of i.v. cephalosporine antibiotics. Patients allergic to beta-lactam antibiotics received i.v. clindamycine. The surgical method used was either a minimally invasive endoscopic transsphenoidal approach to the sella or transsphenoidal microscope-assisted surgery. In both methods, intracranial removal of adenoma was performed with cold-steel curettes and microforceps. In case of perioperative CSF leakage, this was sealed using autologous free fat graft (harvested from the lateral side of the right thigh) followed by one or more layers with absorbable hemostat (Surgicel©, Ethicon) and fibrin sealant (Tisseel©, Baxter).
Follow-up
Postoperatively, all patients were followed up at the ENT department after 4–6 weeks for postoperative sinonasal endoscopic examination, evaluation of CSF leakage, and sinonasal status. Evaluation of the pituitary function was performed 4–8 weeks after the pituitary surgery. Prolactin, TSH (thyroid stimulating hormone), free T4 (thyroxine) and T3 (triiodothyronine), testosterone/estradiol, FSH (follicle stimulating hormone), LH (lutenizing hormone), and IGF-I (insulin-like growth factor) were analyzed. The HPA (hypothalamic–pituitary–adrenal) axis was evaluated with the short Synachten test. Function of the posterior pituitary lobe was assessed with measurement of urine osmolality. Remission of GH, ACTH, and Prolactin hypersecretion was assessed with OGTT (oral glucose tolerance test) and/or repeated measurements of GH in the morning and IGF-I, 24-h U-cortisol, repeated measurements during 24 h of S-cortisol, P-ACTH, saliva cortisol, dexamethazone test, and prolactin respectively. MRI was performed 3 months after surgery and discussed at multidisciplinary conference and patients were thereafter evaluated at the neurosurgery outpatient clinic.
Statistical methods
Descriptive statistics and variate analyses were calculated using statistical software (SPSS, version 24, IBM©). Crosstabulation for odds ratios and chi2 test for bivariate analysis for complications were used and the Kaplan–Meier graphs and standardized mortality rate for comparison of survival rate, compared to general population where data was obtained and matched from Statistics Sweden.
Results
In total, 578 patients that met inclusion criteria were identified. There were slightly fewer females 282 (49%) in the population. The mean age was 53±16 y. The general characteristics of the study population are presented in Table 1. In all patients, the indication for surgery was either a tumor mass effect on nearby structures, mainly optic chiasm, hormone-secreting adenoma, or a combination of the two. The majority of the pituitary adenomas were hormonally inactive 355 (61%), followed by GH-producing adenomas 104 (18%), and ACTH-producing adenomas 50 (9%).
Table 1.
General characteristics of the study population
| Study population, n | 578 |
|---|---|
| Mean age, mean (SD) | 53±16 |
| Gender, n (%) | |
| Female | 282 (49) |
| Male | 296 (51) |
| Lesion type, n (%) | |
| NFPA | 355 (61) |
| GH | 104 (18) |
| ACTH | 50 (9) |
| PRL | 20 (3) |
| TSH | 4 (1) |
| Cyst | 34 (6) |
| Other | 11 (2) |
| Lesion size, n (%) | |
| > 10 mm | 498 (86) |
| < 10 mm | 80 (14%) |
| Surgical technique, n (%) | |
| Microscope | 61 (11) |
| Endoscope | 517 (89) |
| Stereotactic radio surgery | 64 (11) |
Up until early 2010, microscope-assisted surgery was performed in 61 patients; thereafter, the remaining 517 was treated purely endoscopically.
Remission
The remission rates are presented in Table 2. In mono-hypersecretional PAs, remission was achieved with primary surgery in 49/104 (47%) of GH-producing PAs and 40/50 (80%) in ACTH-releasing PAs. Eleven patients with GH overproduction received complementary treatment due to either mass effect or persistent hypersecretion. Of these, 5 underwent a secondary surgery while 6 received SRS in order to achieve hormonal cure. In ACTH-releasing adenomas, 2 had complementary surgery and 5 received SRS until cured. Consequently, a total remission rate of 60 (58%) out of 104 patients and 47 (94%) out of 50 patients with GH- and ACTH-releasing adenomas respectively was achieved.
Table 2.
Remission rates of hormone-releasing adenomas
| Type | Primary surgery | Revision surgery | Surgery and stereotactic radio surgery | Total remission |
|---|---|---|---|---|
| GH | 49/104 (47%) | 5/104 (5%) | 6/104 (6%) | 60/104 (58%) |
| ACTH | 40/50 (80%) | 2/50 (4%) | 5/50 (10%) | 47/50 (94%) |
Hormonal substitution
Data on hormonal substitution is presented in detail in Tables 3 and 4. The majority of patients (380 (66%)) did not need hormonal substitution prior to surgery. However, the proportion of patients in need of substitution increased from 35% before treatment to 61% after surgery. The majority of patients (342 (59%)) had no alteration in the number of substituted hormones before and after treatment and 29 (5%) patients had a decreased need of hormone treatment after surgery.
Table 3.
Pre- and postoperative hormonal substitution
| Hormonal substitution | Preop, n (%) | Postop, n (%) |
|---|---|---|
| No axis | 380 (66) | 225 (39) |
| 1 axis | 111 (19) | 167 (29) |
| 2 axis | 56 (10) | 113 (20) |
| 3 axis | 28 (5) | 62 (10) |
| 4 axis | 3 (1) | 11 (2) |
Table 4.
Difference in hormonal substitution 6–8 weeks after transsphenoidal pituitary surgery
| n (%) | |
|---|---|
| No difference in the number of substituted axis | 342 (59) |
| One additional substituted axis | 144 (25) |
| Two additional substituted axes | 56 (10) |
| Three additional substituted axes | 26 (4) |
| Four additional substituted axes | 5 (1) |
| One less substituted axis | 25 (4) |
| Two less substituted axes | 4 (1) |
Complications
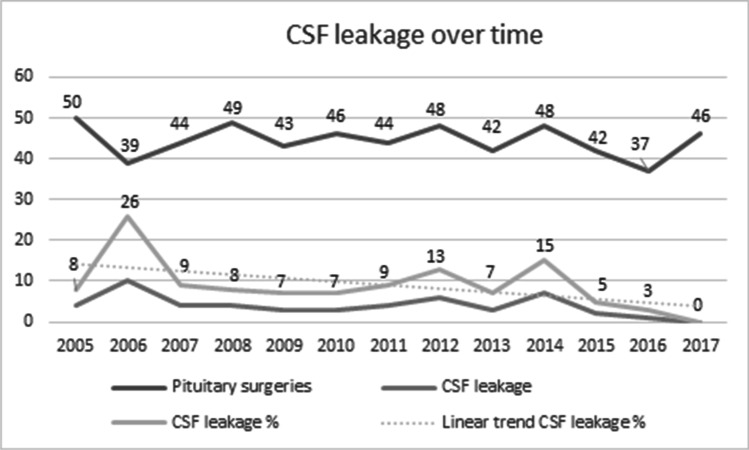
The rates of complications are presented in Table 5. The most frequent complication was rhinosinusitis in 63 patients (10%). The second most frequent complication was CSF leakage in 51 patients (8.4%). The rates of CSF leakage decreased over the later period of the study period (Fig. 1).
Table 5.
Surgical complications
| Surgical complications | n | % |
|---|---|---|
| Rhinosinusitis | 63 | 11 |
| CSF leakage | 49 | 8 |
| Meningitis | 26 | 4 |
| Sepsis | 12 | 2 |
| Neurological deficits | 5 | < 1 |
| Bleeding | 4 | < 1 |
| DVT/PE | 2 | < 1 |
| Surgical mortality < 48 h | 2 | < 1 |
Fig. 1.
CSF leakage over time
Meningitis was recorded in 26 patients (4%), whereas postoperative sepsis was recorded in 12 (2%) of the cases. Neurological deficits related to surgery, postoperative bleeding, DVT/PE, and surgical mortality < 48 h were observed in 5 (< 1%), 4 (< 1%), 2 (< 1%), and 2 (< 1%) patients, respectively (Table 5).
There were no statistically significant differences for any of the studied complications after surgery regarding gender (female vs male), size of adenomas (micro vs macro), hormonal release (PAs vs NFPAs), or surgical method.
Patient survival
The overall survival in the studied population is shown in Fig. 2. Comparison with the general population and study population was made through register data (SCB) and matched for age, gender, and place of residency. Standardized mortality rate for the study population over time was 1.2 (p = 0.18) as compared to the general population (Table 6) and 5- and 10-year survival was 94.2% and 88.2% respectively (Table 7).
Fig. 2.
Kaplan–Meier graph showing survival over time as compared to general population (n 578)
Table 6.
Standardized mortality rate (SMR) for the study population
| Label | Number of observations | Observed person years | Observed events | Expected events | SMR value | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| SMR | 578 | 3777.4 | 53 | 43.5 | 1.21827 | 0.913–1.594 | 0.18 |
Table 7.
1-, 5-, and 10-year survival rates
| Years | Survival rate | 95& CI low | 95% CI high |
|---|---|---|---|
| 1 | 97.7% | 96.1% | 98.7% |
| 5 | 94.2% | 91.7% | 95.9% |
| 10 | 88.2% | 83.9% | 91.4% |
Discussion
This is, to our knowledge, the first Swedish study on overall remission and complications after surgery of pituitary adenomas. We found that our surgical results regarding remission in hormone-releasing adenomas were comparable to international published results[8] and that they improved by the combination with SRS. Our overall complication rates were generally low although CSF leakage was more common than expected. However, there was decreasing tendency in the later study period. Furthermore, we found that surgery of pituitary adenomas may increase the need for postoperative hormonal substitution. Finally, we found that the survival rate for the study population was slightly lower than that of the general population but comparable to other reported material from northern Europe[11]
In an international compilation of surgical results using endoscopic transsphenoidal approach from 2015, Dallapiazza et al. presented remission rates of 68–95% in ACTH-releasing adenomas. Using consensus remission criteria published in 2010[19], the surgical success rate varied from 46 to 70% in GH-producing adenomas overall[8]. Comparing our findings, we conclude that our results fall somewhere within the boundaries of previously published data regarding remission. In our study, primary surgery of ACTH- and GH-releasing adenomas accomplished remission in 80% and 47%, respectively. After revision surgery, remission was accomplished in 84% and 52%, respectively, and when combined with SRS, the cure rate was improved to 94% in ACTH-releasing adenomas and 56% in GH-releasing adenomas. SRS also showed to be an effective method in treating PA extending towards surgically challenging areas such as the cavernous sinus. According to the general opinion, SRS seems to be a viable option in the treatment of pituitary adenomas[5, 27, 34]. The risk of secondary brain tumors after SRS has been discussed in other studies, and although low, should be taken into consideration [20, 28, 30]. Too few other hormonally secreting tumors had undergone surgery to be conclusively assessed in our material [25].
Rhinosinusitis was the most commonly observed complication at first postoperative follow-up in our study. A fact for consideration is that 4 weeks after surgery, most patients have not achieved mucosal recovery. Additionally, our definition of RS was based either on patient complaints or objectives findings of crusting in the sinonasal cavity. Admittedly, this wide definition will probably over-estimate the number of patients with postoperative true bacterial infection. However, a number of publications have focused on PROMs including disease-specific quality of life instruments, relating to rhinosinusitis [2, 7, 13–15, 33, 36]. It seems that a major patient complaint, after pituitary surgery, is due to sinonasal morbidity, something that needs to be taken into account, when planning patients for surgery[2, 7, 33, 37, 40].
The rates of postoperative CSF leakage in our study seem to be high from an international perspective. However, the frequency of CSF leak varied over the years and in more recent years, our rates were comparable to those published from other high-volume centers [12, 24]. Several studies have indicated an advantage of using a multidisciplinary team consisting of rhinologists and neurosurgeons when performing endoscopic approach to the sellar region[23]. This method of surgical collaboration, probably in combination with increased surgical experience, has had a positive effect on surgical outcome and complication rate[35, 41]. Initially in our study period, surgery of PAs was performed in either the ENT or neurosurgery department separately, but in recent years, a dedicated “high volume,” multidisciplinary surgical skull base team has been formed that does nearly all the endoscopic surgeries together. This change of surgical cooperative paradigm seems to have had a positive effect on the surgical results.
The majority of patients in our study were treated with transsphenoidal endoscopic technique, which today is the most widely used and commonly accepted approach [25, 39]. Several studies have compared the surgical success between this technique and the previously more commonly used microscopical transsphenoidal approach and shown similar results[3, 15, 21, 22, 31].
An interesting finding in our study is the fact that a fairly large group of patients increased their need for postoperative hormonal substitution after surgery. The majority of these patients were treated with postoperative hydrocortisone as additional hormonal substitution and we know from a previous study that hydrocortisone treatment is more commonly used in our institution compared to international standards[10]. This fact may very well influence the true need for postoperative hormonal substitution, and should perhaps be analyzed in detail separately.
Limitations
This was a retrospective investigation. However, the study population is large, and the time period long. Data regarding visual field was not achievable due to inconsistent/incomplete medical journals which was unfortunate, as this is a major indication for surgery on clinically inactive macroadenomas. In this study, we chose to classify the PAs based on clinically significant hormonal secretion. The major reason why, was that in our center, this classification was used to decide the treatment. Other reasons were the retrospective design and the long period (2005–2017) when data was collected. During this period, the WHO has proposed novel recommendations on the classification of PAs [17]. However, we acknowledge that a more detailed classification of PAs, such as the WHO or the classification proposed by Mindermann in 1997, could have strengthened the results [26]. Another limitation was the definition of rhinosinusitis where we, in our follow-up after pituitary surgery, did not use any standardized definition. Therefore, the quality of variables studied (including rhinosinusitis) is limited to what can be discerned from the medical records. This means that the relatively high rate of postoperative rhinosinusitis found in our study should be interpreted with caution as follow-up was made early after surgery, prior to complete mucosal recovery.
Conclusion
In summary, this is the first Swedish study of overall surgical success and complications after surgery of pituitary lesions. When compared to international studies, our results regarding hormonal remission rates are comparable. The most commonly noted complication was rhinosinusitis, followed by CSF leakage. In the latter, we saw although a high overall rate, but with a positive trend towards lower rates in the later part of the study period. We believe that this, at least in part, was an effect of the introduction of a multidisciplinary surgical skull base team and increased surgical experience.
Abbreviations
- PA
Pituitary adenoma
- NFPA
Non-functioning pituitary adenoma
- SRS
Sterotactic radiation surgery
- OGTT
Oral glucose tolerance test
- IGF
Insuline-like growth factor
- GH
Growth hormone
- ACTH
Adrenocorticotrophic hormone
- CSF
Cerebrospinal fluid
- UFC
Urinary free cortisol
- DVT
Deep venous trombose
- PE
Pulmonary embolism
- ENT
Ear, nose, and throat
- TSH
Thyroid-stimulating hormone
- T4
Thyroxine
- T3
Triiodothyronine
- FSH
Follicle-stimulating hormone
- LH
Lutenizing hormone
- HPA
Hypothalamic-pituitary-adrenal
- MRI
Magnetic resonance imaging
Funding
Open access funding provided by Karolinska Institute.
Data Availability
The data that support the findings in this study is available from corresponding author, upon reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Central Ethical Review Board in Stockholm, Sweden (EBN 8930234) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
No need for informed consent was necessary from individual participants included in the study.
Footnotes
This article is part of the Topical Collection on Pituitaries
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Almutairi RD, Muskens IS, Cote DJ, Dijkman MD, Kavouridis VK, Crocker E, Ghazawi K, Broekman MLD, Smith TR, Mekary RA, Zaidi HA. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir (Wien) 2018;160:1005–1021. doi: 10.1007/s00701-017-3438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzhrani G, Sivakumar W, Park MS, Taussky P, Couldwell WT. Delayed complications after transsphenoidal surgery for pituitary adenomas. World Neurosurg. 2018;109:233–241. doi: 10.1016/j.wneu.2017.09.192. [DOI] [PubMed] [Google Scholar]
- 3.Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of complications, trends, and costs in endoscopic vs microscopic pituitary surgery: analysis from a US health claims database. Neurosurgery. 2017;81:458–472. doi: 10.1093/neuros/nyx350. [DOI] [PubMed] [Google Scholar]
- 4.Buchfelder M, Schlaffer SM, Zhao Y. The optimal surgical techniques for pituitary tumors. Best Pract Res Clin Endocrinol Metab. 2019;33:101299 . doi: 10.1016/j.beem.2019.101299. [DOI] [PubMed] [Google Scholar]
- 5.Bunevicius A, Laws ER, Vance ML, Iuliano S, Sheehan J. Surgical and radiosurgical treatment strategies for Cushing’s disease. J Neurooncol. 2019;145:403–413. doi: 10.1007/s11060-019-03325-6. [DOI] [PubMed] [Google Scholar]
- 6.Chabot JD, Chakraborty S, Imbarrato G, Dehdashti AR. Evaluation of outcomes after endoscopic endonasal surgery for large and giant pituitary macroadenoma: a retrospective review of 39 consecutive patients. World Neurosurg. 2015;84:978–988. doi: 10.1016/j.wneu.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Caulley L, Uppaluri R, Dunn IF. Perioperative nasal and paranasal sinus considerations in transsphenoidal surgery for pituitary disease. Br J Neurosurg. 2020;34(3):246–252. doi: 10.1080/02688697.2020.1731424. [DOI] [PubMed] [Google Scholar]
- 8.Dallapiazza RF, Jane JA., Jr Outcomes of endoscopic transsphenoidal pituitary surgery. Endocrinol Metab Clin North Am. 2015;44:105–115. doi: 10.1016/j.ecl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 10.Fridman-Bengtsson O, Hoybye C, Porthen L, Stjarne P, Hulting AL, Sunnergren O. Evaluation of different hydrocortisone treatment strategies in transsphenoidal pituitary surgery. Acta Neurochir (Wien) 2019;161:1715–1721. doi: 10.1007/s00701-019-03885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halvorsen H, Ramm-Pettersen J, Josefsen R, Ronning P, Reinlie S, Meling T, Berg-Johnsen J, Bollerslev J, Helseth E. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir (Wien) 2014;156:441–449. doi: 10.1007/s00701-013-1959-7. [DOI] [PubMed] [Google Scholar]
- 12.Hannan CJ, Almhanedi H, Al-Mahfoudh R, Bhojak M, Looby S, Javadpour M. Predicting post-operative cerebrospinal fluid (CSF) leak following endoscopic transnasal pituitary and anterior skull base surgery: a multivariate analysis. Acta Neurochir (Wien) 2020;162:1309–1315. doi: 10.1007/s00701-020-04334-5. [DOI] [PubMed] [Google Scholar]
- 13.Hanson M, Patel PM, Betz C, Olson S, Panizza B, Wallwork B. Sinonasal outcomes following endoscopic anterior skull base surgery with nasoseptal flap reconstruction: a prospective study. J Laryngol Otol. 2015;129(Suppl 3):S41–46. doi: 10.1017/s002221511500047x. [DOI] [PubMed] [Google Scholar]
- 14.Hong SD, Nam DH, Kong DS, Kim HY, Chung SK, Dhong HJ. Endoscopic modified transseptal transsphenoidal approach for maximal preservation of sinonasal quality of life and olfaction. World Neurosurg. 2016;87:162–169. doi: 10.1016/j.wneu.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 15.Hong SD, Nam DH, Seol HJ, Choi NY, Kim HY, Chung SK, Dhong HJ. Endoscopic binostril versus transnasal transseptal microscopic pituitary surgery: sinonasal quality of life and olfactory function. Am J Rhinol Allergy. 2015;29:221–225. doi: 10.2500/ajra.2015.29.4165. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias P, Rodríguez Berrocal V, Díez JJ. Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine. 2018;61:407–421. doi: 10.1007/s12020-018-1645-x. [DOI] [PubMed] [Google Scholar]
- 17.Inoshita N, Nishioka H. The 2017 WHO classification of pituitary adenoma: overview and comments. Brain Tumor Pathol. 2018;35:51–56. doi: 10.1007/s10014-018-0314-3. [DOI] [PubMed] [Google Scholar]
- 18.Garibi J, Pomposo I, Villar G, Gaztambide S. Giant pituitary adenomas: clinical characteristics and surgical results. Br J Neurosurg. 2002;16:133–139. doi: 10.1080/02688690220131723. [DOI] [PubMed] [Google Scholar]
- 19.Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S, Acromegaly Consensus G. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141–3148. doi: 10.1210/jc.2009-2670. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez B, Vargas G, Espinosa-de-los-Monteros AL, Sosa E, Mercado M. Efficacy and safety of radiotherapy in acromegaly. Arch Med Res. 2011;42:48–52. doi: 10.1016/j.arcmed.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Kahilogullari G, Beton S, Al-Beyati ES, Kantarcioglu O, Bozkurt M, Kantarcioglu E, Comert A, Unlu MA, Meco C. Olfactory functions after transsphenoidal pituitary surgery: endoscopic versus microscopic approach. Laryngoscope. 2013;123:2112–2119. doi: 10.1002/lary.24037. [DOI] [PubMed] [Google Scholar]
- 22.Kassis S, De Battista JC, Raverot G, Jacob M, Simon E, Rabilloud M, Froehlich P, Trouillas J, Borson-Chazot F, Perrin G, Jouanneau E. Endoscopy versus microsurgery: results in a consecutive series of nonfunctioning pituitary adenomas. Neurochirurgie. 2009;55:607–615. doi: 10.1016/j.neuchi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi R, Toda M, Tomita T, Ogawa K, Yoshida K. Surgical outcome of endoscopic endonasal surgery for non-functional pituitary adenoma by a team of neurosurgeons and otolaryngologists adenoma by a team of neurosurgeons and otolaryngologists. Turk Neurosurg. 2017;27:1–7. doi: 10.5137/1019-5149.JTN.14354-15.0. [DOI] [PubMed] [Google Scholar]
- 24.Lee CY, Chen YC, Wang YP, Chen SJ. Difference in the incidence of cerebrospinal fluid leakage and residual tumors between functional and nonfunctional pituitary adenomas treated by endoscopic transsphenoidal pituitary adenomectomy. J Int Med Res. 2019;47:5660–5670. doi: 10.1177/0300060519875857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikhaylov NI, Kalinin PL, Savin IA. Complications after endoscopic endonasal transsphenoidal removal of pituitary adenomas. Zh Vopr Neirokhir Im N N Burdenko. 2018;82:105–113 . doi: 10.17116/neiro201882061105. [DOI] [PubMed] [Google Scholar]
- 26.Mindermann T. Classification of pituitary adenomas. Acta Neurochir (Wien) 1997;139:267–270. doi: 10.1007/BF01844766. [DOI] [PubMed] [Google Scholar]
- 27.Minniti G, Flickinger J, Tolu B, Paolini S. Management of nonfunctioning pituitary tumors: radiotherapy. Pituitary. 2018;21:154–161. doi: 10.1007/s11102-018-0868-4. [DOI] [PubMed] [Google Scholar]
- 28.Minniti G, Flickinger J. The risk/benefit ratio of radiotherapy in pituitary tumors. Best Pract Res Clin Endocrinol Metab. 2019;33:101269 . doi: 10.1016/j.beem.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317:516–524. doi: 10.1001/jama.2016.19699. [DOI] [PubMed] [Google Scholar]
- 30.Norberg L, Johansson R, Rasmuson T. Intracranial tumours after external fractionated radiotherapy for pituitary adenomas in northern Sweden. Acta Oncol. 2010;49:1276–1282. doi: 10.3109/02841861003782025. [DOI] [PubMed] [Google Scholar]
- 31.Perry A, Graffeo CS, Meyer J, Carlstrom LP, Oushy S, Driscoll CLW, Meyer FB. Beyond the learning curve: comparison of microscopic and endoscopic incidences of internal carotid injury in a series of highly experienced operators. World Neurosurg. 2019;131:e128–e135. doi: 10.1016/j.wneu.2019.07.074. [DOI] [PubMed] [Google Scholar]
- 32.Pomeraniec IJ, Kano H, Xu Z, Nguyen B, Siddiqui ZA, Silva D, Sharma M, Radwan H, Cohen JA, Dallapiazza RF, Iorio-Morin C, Wolf A, Jane JA, Grills IS, Mathieu D, Kondziolka D, Lee CC, Wu CC, Cifarelli CP, Chytka T, Barnett GH, Lunsford LD, Sheehan JP. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J Neurosurg. 2018;129:648–657. doi: 10.3171/2017.5.Jns163069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber A, Bertazzoni G, Ferrari M, Rampinelli V, Verri P, Mattavelli D, Fontanella M, Nicolai P, Doglietto F. Nasal morbidity and quality of life after endoscopic transsphenoidal surgery: a single-center prospective study. World Neurosurg. 2019;123:e557–e565. doi: 10.1016/j.wneu.2018.11.212. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL, Lee JY, Kano H, Park KJ, Niranjan A, Kondziolka D, Barnett GH, Rush S, Golfinos JG, Lunsford LD. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;119:446–456. doi: 10.3171/2013.3.Jns12766. [DOI] [PubMed] [Google Scholar]
- 35.Soliman MAR, Eaton S, Quint E, Alkhamees AF, Shahab S, O'Connor A, Haberfellner E, Im J, Elashaal AA, Ling F, Elbreki M, Dang T, Morassutti DJ, Shamisa A. Challenges, learning curve, and safety of ndoscopic endonasal surgery of sellar-suprasellar lesions in a community hospital. World Neurosurg. 2020;138:e940–e954. doi: 10.1016/j.wneu.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soyka MB, Serra C, Regli L, Meier E, Holzmann D. Long-term olfactory outcome after nasoseptal flap reconstructions in midline skull base surgery. Am J Rhinol Allergy. 2017;31:334–337. doi: 10.2500/ajra.2017.31.4463. [DOI] [PubMed] [Google Scholar]
- 37.Suberman TA, Zanation AM, Ewend MG, Senior BA, Ebert CS., Jr Sinonasal quality-of-life before and after endoscopic, endonasal, minimally invasive pituitary surgery. Int Forum Allergy Rhinol. 2011;1:161–166. doi: 10.1002/alr.20029. [DOI] [PubMed] [Google Scholar]
- 38.Wang EW, Gardner PA, Zanation AM. International consensus statement on endoscopic skull-base surgery: executive summary. Int Forum Allergy Rhinol. 2019;9:S127–S144. doi: 10.1002/alr.22327. [DOI] [PubMed] [Google Scholar]
- 39.Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND, Bettag M, Bleier BS, Cappabianca P, Carrau RL, Casiano RR, Cavallo LM, Ebert CS, Jr, El-Sayed IH, Evans JJ, Fernandez-Miranda JC, Folbe AJ, Froelich S, Gentili F, Harvey RJ, Hwang PH, Jane JA, Jr, Kelly DF, Kennedy D, Knosp E, Lal D, Lee JYK, Liu JK, Lund VJ, Palmer JN, Prevedello DM, Schlosser RJ, Sindwani R, Solares CA, Tabaee A, Teo C, Thirumala PD, Thorp BD, de Arnaldo Silva Vellutini E, Witterick I, Woodworth BA, Wormald PJ, Snyderman CH. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019;9:S145–s365. doi: 10.1002/alr.22326. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Chen Y, Li J, Wei L, Wang R. Olfactory function and quality of life following microscopic endonasal transsphenoidal pituitary surgery. Medicine (Baltimore) 2015;94:e465 . doi: 10.1097/md.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaidi HA, Awad AW, Bohl MA, Chapple K, Knecht L, Jahnke H, White WL, Little AS. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. J Neurosurg. 2016;124:596–604. doi: 10.3171/2015.4.jns15102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings in this study is available from corresponding author, upon reasonable request.