Abstract
Background:
The emergence of a plethora of new tobacco products marketed as being less harmful than smoking, such as electronic cigarettes and heated tobacco products, and an increased popularity of recreational marijuana, has raised concerns about the potential cardiovascular risk associated with their use.
Objective:
The present study aimed to investigate whether the use of novel tobacco products or marijuana can cause the development of proarrhythmic substrate and eventually lead to arrhythmias.
Methods and results:
Rats exposed to smoke from tobacco, marijuana, or cannabinoid-depleted marijuana, or to aerosol from electronic cigarettes or heated tobacco products, once per day for 8 weeks exhibited progressively increased systolic blood pressure, decreased cardiac systolic function with chamber dilation, and reduced overall heart rate variability, relative to the clean air negative control group. Atrial fibrillation and ventricular tachycardia testing by ex vivo optical mapping revealed a significantly higher susceptibility to each, with a shortened effective refractory period and prolonged calcium transient duration. Histological analysis indicated that in all exposure conditions except for air, exposure to smoke or aerosol from tobacco or marijuana products caused severe fibrosis with decreased microvessel density and higher level of sympathetic nerve innervation.
Conclusions:
These pathophysiological results indicate that tobacco and marijuana products can induce arrhythmogenic substrates involved in cardiac electrical, structural, and neural remodeling, facilitating the development of arrhythmias.
Keywords: Tobacco, Marijuana, Vaping, Atrial fibrillation, Ventricular tachycardia, Heart rate variability, Arrhythmogenic substrate
INTRODUCTION
The effect of conventional tobacco cigarette smoking on the pathophysiology of coronary artery disease is relatively well defined; however, the effects on cardiac arrhythmia and proarrhythmic mechanism are less well understood. The situation has become more complex recently with the advent of modern tobacco products like electronic cigarettes (e-cigs) and heated tobacco products (HTPs), and the increased popularity of legalized marijuana,1–4 all of which are popularly assumed to be safer than tobacco cigarettes. Although non-conventional tobacco products and marijuana may represent an emerging threat to cardiovascular health,5–8 current knowledge regarding the mechanism by which smoking/vaping leads to cardiac arrhythmias remains limited.
An enhanced substrate for both atrial and ventricular arrhythmias has been shown to result from any combination of cardiac neural, electrical, and structural remodeling. Neural control of the heart, which involves both sympathetic and parasympathetic nerves, plays a vital role in the initiation and perpetuation of arrhythmia diseases due to its regulation of automaticity and triggered activity.9 Electrical remodeling refers to alterations in ion channels and connexins that promote the development of arrhythmias by affecting action potential duration (APD) and reentrant activity.10 Structural remodeling is an advanced process that progressively affects myocytes and the myocardial interstitium, resulting in myocyte hypertrophy, interstitial fibrosis, and cardiac chamber enlargement, and eventually promotes reentry.11,12
Previous studies have suggested that conventional tobacco smoking can lead to the imbalance of cardiac autonomic control by causing sympathetic over-innervation and parasympathetic withdrawal.13 A recent study revealed that e-cigs can also promote the imbalance of cardiac automatic control and further the inducibility of ventricular tachycardia (VT).14 Nicotine-mediated fibrosis and the activation of nicotinic acetylcholine receptor are considered to be the common proarrhythmogenic substrate caused by all nicotine-containing products.15,16 However, the increased risk of arrhythmias caused by non-nicotine products such as marijuana suggests unexpected effects that cannot be fully explained by nicotine. The impacts of chronic smoking and vaping of this wide range of tobacco and marijuana products on the formation of arrhythmogenic substrate, including cardiac neural, electrical, and structural remodeling, have not been fully determined. The overall goal of this study was to test the hypothesis that smoking/vaping these tobacco products or marijuana may increase the susceptibility to inducible tachycardia including atrial fibrillation (AF) and VT comparably to smoking conventional tobacco cigarettes, and to investigate potentially related cardiac electro-pathophysiologic modifications.
METHODS
Animals.
Sprague-Dawley rats, 8–10 weeks old, of both genders, were used for this study. Group sizes varied from 5–16 depending on the experiment; a situation resulting from complications of the COVID-19 lab shutdowns although sufficient power was still achieved for an α of 0.05, two-tailed testing, and power of 0.8 (see Supplement for details). Animal procedures were approved and monitored by the Institutional Animal Care and Use Committee of the University of California, San Francisco. The research reported in this paper adhered to the ARRIVE guidelines for reporting animal research and the National Academies of Sciences, Engineering, and Medicine Guide for the Care and Use of Laboratory Animals.
Smoke/aerosol generation and animal exposure.
To mimic human active smoking/vaping, conscious rats in restrainers (Braintree Scientific) were exposed to pulsatile smoke/aerosol commencing after at least 3 days of acclimation to the restrainers, by which time the rats tolerated being held, as determined by breathing smoothly and not struggling. Each rat was exposed 5 days/week for 2 months, one session/day, with each session consisting of 10 cycles spread over 5 min, to approximate the consumption of a single cigarette or a single vaping session.17 Two out of 18 initial animals in the tobacco cigarette group died on days 1 and 14 of exposure and were replaced; no other mortalities occurred.
Rats were exposed to one of the following products: Marlboro Red tobacco cigarettes (CIG), HTPs (IQOS), e-cigs (JUUL, Virginia Tobacco flavor, 5% nicotine), marijuana (MJ, ~10% delta-9-tetrahydrocannabinol (THC)), or “Placebo marijuana” (pb-MJ, cannabinoid-depleted marijuana, <.01% THC). Marijuana was provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program. All required federal, state, and institutional approvals for acquisition and possession of marijuana and exposure of rodents were obtained.18 Air exposure was used as control. Group size was 8–16.
Pathophysiological assessments.
Systolic blood pressure (SBP), echocardiography, ECG telemetry, arrhythmia inducibility testing, and optical mapping were assayed during or after exposure as described previously (see Supplemental Materials).19–24 We measured conscious SBP by tail cuff on the first exposure day and at the end of the 2nd, 4th, 6th, and 8th week to determine progressively chronic effects. On each measurement day, SBP was measured twice, both before and after that day’s single exposure, to determine that day’s acute effect. Eight weeks post exposure, ex vivo heart optical mapping was performed as described previously to test the susceptibility to arrhythmias originating from left and right atria and ventricles and to evaluate their electrophysiological characteristics.19 The APD at 80% repolarization (APD80) and calcium transient duration at 80% repolarization (CATD80) were measured after a series of 20×S1 pacing trains at the pacing cycle lengths (PCL) of 150, 130, 120, 110, 100, 90, 80, and 70 ms. The effective refractory period (ERP) and the susceptibility to both AF and VT were tested via programmed stimulations including extra-stimuli and overdrive pacing. AF was diagnosed as fast and irregular beating lasting more than 2 seconds, whereas VT was determined as at least 6 non-driven consecutive ventricular premature beats.23,24
For histological analysis, hearts were weighed, fixed, and embedded in O.C.T compound for the following histological analyses.21 Heart coronal or transverse cryosections were stained with Sirius red/fast green to assess fibrosis or fluorescently stained for the assessment of the intrinsic cardiac nervous system (ICNS) and cardiac microvessels. ICNS as the autonomic nerve system inside the heart is vital for cardiac function and maintenance of normal heart rhythm.25 To visualize ICNS, we performed immunofluorescence staining for sympathetic and parasympathetic nerves. In order to quantify microvessels including capillaries and small precapillary arterioles with a cross area of 10–314 μm2,26 slides were incubated with biotinylated Griffonia simplicifolia I lectin (GS-I, Sigma-Aldrich) and then labeled with Alexa Fluor 488 Streptavidin (Invitrogen) as we previously described.27 Microvessel density (count/mm2 tissue) and area percentage were calculated. Ten views were taken randomly from subepicardium, midmural, and subendocardium for each section to calculate mean optical area that represented the average intensity level using Fiji ImageJ.28
Most procedures were analyzed by a blinded investigator. Some functional measurements were partially blinded due to contstraints imposed by the pandemic lab shutdowns (see Limitations section at the end).
Statistics.
Data are shown as mean ± SD. P<.05 was required for significance. See Supplement for details of statistical analysis.
RESULTS
Exposure to smoke or aerosol of tobacco and marijuana products increased SBP.
As shown in Fig. 1A, exposure to all non-air conditions altered SBP acutely (pre- vs. post-exposure). Tobacco cigarette smoke, JUUL aerosol, and IQOS aerosol acted similarly in substantially increasing SBP (>10 mmHg) at the first exposure, with relatively moderate acute increases in SBP on subsequent days. However, in contrast to tobacco products, marijuana (10% THC) decreased SBP during each acute exposure. The pb-MJ did not lower SBP, and actually increased it similarly to the tobacco products. Charting changes in the pre-exposure values reveals that chronic exposure to all products progressively elevated baseline SBP to >130 mmHg after 2 weeks of exposure, to >140 mmHg after 4 weeks of exposure, and steadily increases continued through the 8th week (Fig. 1B). The serum concentrations of norepinephrine, but not of angiotensin, were significantly different in rats exposed to tobacco or marijuana products compared to air at 8 weeks post-exposure (P<.001, Fig. 1C).
Fig. 1. Systolic blood pressure (SBP) was acutely raised or lowered depending on exposure condition, and chronically raised by all conditions.
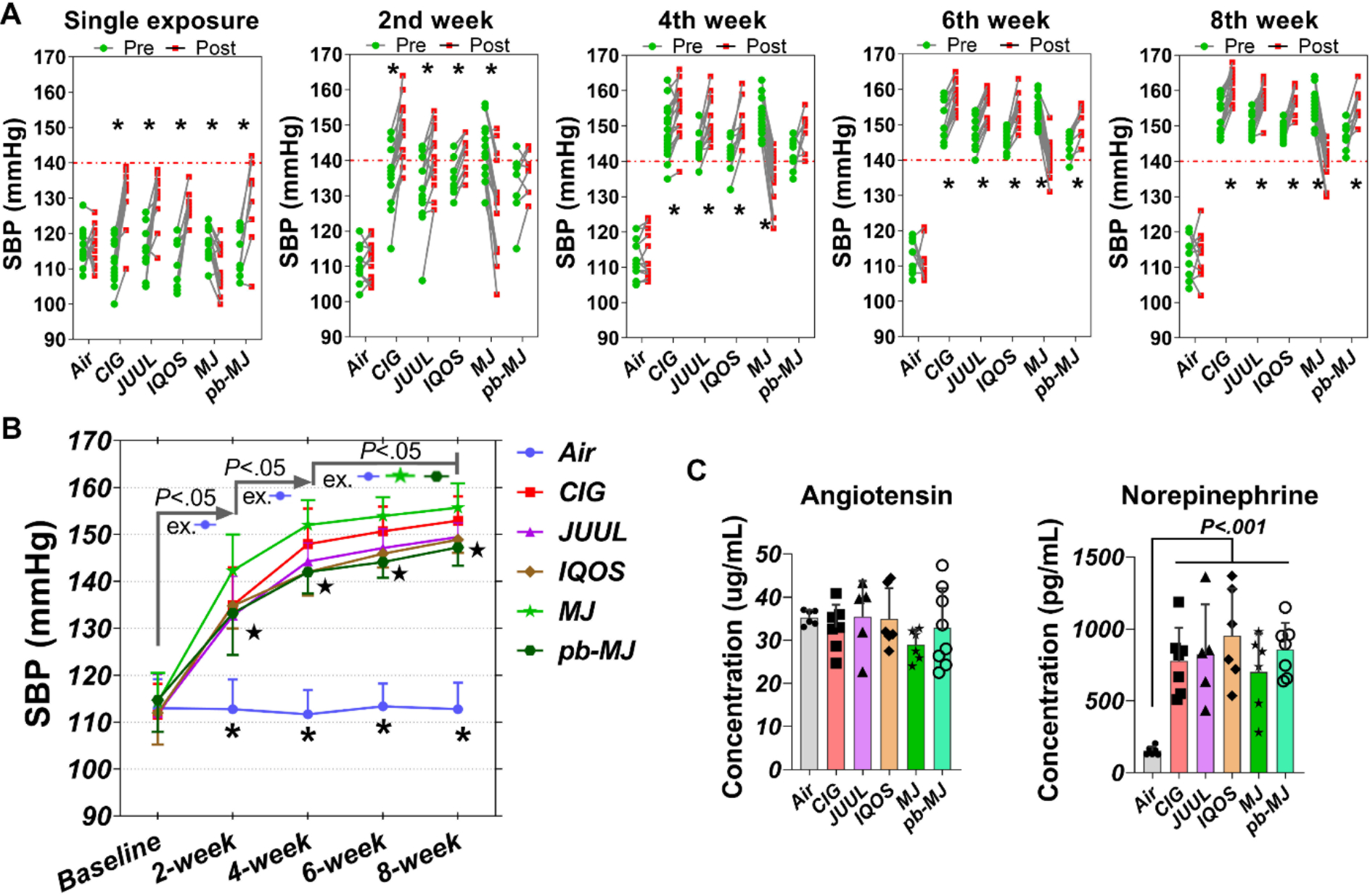
A. Acute effects on SBP of exposure to different tobacco or marijuana products or air. Tobacco products including conventional combustible tobacco cigarettes (CIG), e-cigs (JUUL), and HTPs (IQOS), and cannabinoid-depleted placebo marijuana (pb-MJ) acutely increased SBP, while marijuana (MJ) containing cannabinoids acutely reduced SBP. Two-way ANOVA was employed for comparisons. *P<.05. B. SBP development during chronic exposure to different smoke or aerosol. N = 10, 16, 14, 10, 16, and 8 respectively in Air, CIG, JUUL, IQOS, MJ, and pb-MJ. Data were shown as mean ± SD and analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test. *P<.05 Air compared to others; ★P<.01, JUUL, IQOS, pb-MJ compared to Marijuana group. C. Serum levels of total angiotensin and norepinephrine. N = 5–7, one-way ANOVA. In the analysis for angiotensin, P=0.4110, Air compared to MJ.
Left ventricular systolic function was reduced and cardiac chamber enlarged after chronic exposure.
To determine whether exposure to tobacco and marijuana smoke/aerosol affected cardiac function, we performed echocardiography. As shown in Fig. 2, exposure for 8 weeks progressively reduced both ejection fraction (EF) and fractional area change (FAC) in all non-air groups compared to their baseline levels. Left ventricular end-systolic volume (LVESV) and end-diastolic volume (LVEDV) were gradually increased in non-air groups; by 8 weeks post-exposure, LVESV and LVEDV were enlarged significantly in all non-air groups compared to baseline (P<.05), along with an increase in LV mass compared to baseline and to Air group (P<.001). Moreover, exposure to all conditions except air resulted in an enlargement of left atrial diameter by the 4th week of exposure and the enlargement continued to increase through the 8th week. These findings suggest that exposure to tobacco and marijuana products led to reduced LV function and enlarged cardiac chambers, indicating LV dysfunction and remodeling associated with smoking/vaping.
Fig. 2. Left ventricular (LV) systolic function was decreased and the LV volume and mass as well as the diameter of the left atrium were enlarged during exposure to tobacco or marijuana products.
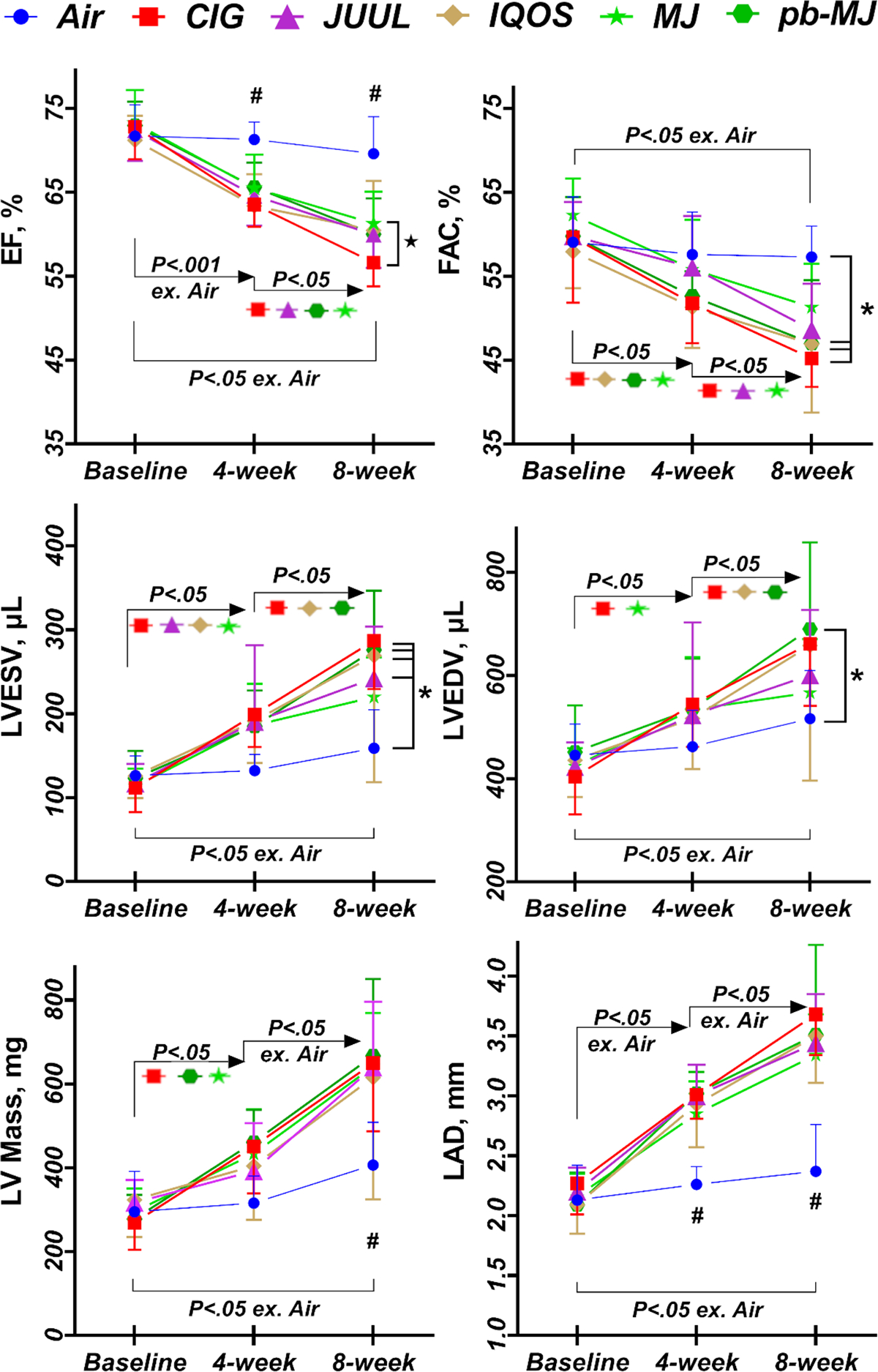
EF, ejection fraction; FAC, Fractional area change; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; LV, left ventricle; LAD, left atrial diameter. Data are shown as mean ± SD. Two-way ANOVA followed by Tukey’s multiple comparisons test was used for statistical analysis. *P<.05, compared to Air; #P<.05, Air compared to the other groups; ★P<.05 MJ compared to CIG. N = 8, 12, 10, 10, 16, and 8 respectively in Air, CIG, JUUL, IQOS, MJ, and pb-MJ. “ex.” is short for “except.”
Chronic use of tobacco, e-cigs, HTPs or marijuana reduced overall heart rate variability (HRV).
All of the normal-to-normal RR intervals (“NN interval”) of all conditions superimposed in Supplementary Fig.1 showed the same RR intervals distribution pattern in the form of a Poincaré plot, which plots each pair of RR intervals between 2 consecutive beats and thus quantifies the distribution pattern of heart rate mapping. Data in Fig. 3A indicated only exposure to JUUL led to a shortened average NN interval. Surprisingly, chronic exposure to smoke of CIG, IQOS, MJ, and pb-MJ did not shorten the NN interval and actually prolonged it. Using a time domain method to directly evaluate the degree of dispersion of NN interval revealed that exposure to tobacco and marijuana products compared to Air caused a significant reduction of the overall HRV indicators (Fig. 3B–E), including the average of 2 mins standard deviation of NN intervals (SDNN), the root mean square of successive differences between normal heartbeats (RMSSD), the number of pairs of successive NN intervals that differ by more than 9 ms (NN9), and the proportion of NN9 divided by the total number of NN intervals (pNN9), which suggests a depressed parasympathetic function. Frequency domain method for HRV analysis, an indirect method to reflect sympathetic and parasympathetic function, showed that all of these products decreased the overall HRV, especially the sympathetic modulations (Fig. 3F–J). Compared to Air, most of conditions had a lower total power (TP, P<.05 except JUUL), very-low frequency band (VLF, P<.05), and lower levels of low frequency band (LF, P<.05 except IQOS), ratio of low/high frequency (LF/HF, P<.05 except IQOS). Both LF and HF were depressed by IQOS exposure but apparently HF was influenced more and that caused an increased LF/HF value. As the VLF and LF are mainly driven by sympathetic activity, RMSSD, NN9, pNN9, and HF are driven by the parasympathetic, SDNN reflects the overall HRV, these results suggested that all of these products decreased the overall HRV, especially the sympathetic modulations.
Fig. 3. Heart rate variability (HRV).
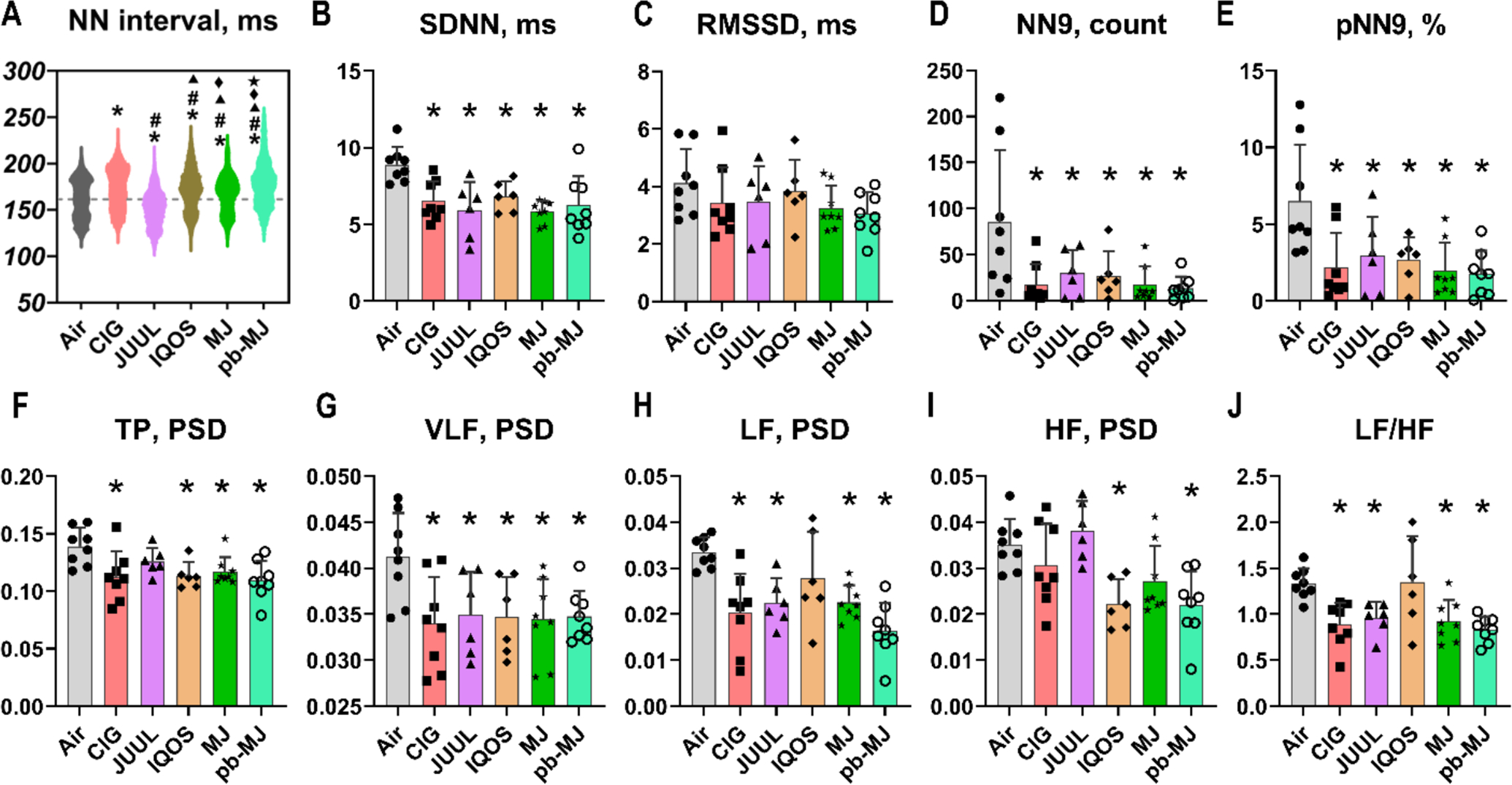
A-E. HRV analyzed by time domain method. NN interval, the normal-to-normal RR interval; SDNN, the standard deviation of the NN intervals; RMSSD, the root mean square of successive differences between normal heartbeats; NN9, the number of pairs of successive NN intervals that differ by more than 9 ms; pNN9, the proportion of NN9 divided by the total number of NN intervals. F-J. HRV analyzed by frequency domain method. TP, total power; VLF, very low frequency; LF, low frequency; HF, high frequency; LF/HF, ratio of LF to HF. PSD, power spectral density. Data are shown as mean ± SD and analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. N = 8, 8, 6, 6, 8, and 8 respectively in Air, CIG, JUUL, IQOS, MJ, and pb-MJ group. * P<.05 compared to Air respectively; #P<.05 compared to CIG; ▲P<.05 compared to JUUL; ◆P<.05 compared to IQOS; ★P<.05 compared to MJ. Some p-values that are greater than .05 but may be of interest are noted here. In panel C, P=.17, Air compared to MJ; in panel F, P=.43, Air compared to JUUL; in panel H, P=.36, Air compared to IQOS; in panel I, P=.13, Air compared to MJ.
Increased susceptibility, shortened ERP and APD80, and prolonged CaTD80.
Tachyarrhythmias were tested using programmed stimulation and burst pacing ex vivo. One heart from the JUUL group failed to connect to the Langendorff system due to technical difficulties. As shown in Fig. 4A&B and Table 1, all tobacco products caused a significant increase in overall AF inducibility compared to Air. An overall 37.5% of AF inducible rate was observed in rats exposed to MJ and 50% was evident for pb-MJ, whereas AF remained uninducible in rats exposed to Air. VT inducible rates were 0, 62.5%, 71.43%, 37.5%, 75%, and 37.5% in Air, CIG, JUUL, IQOS, MJ, and pb-MJ respectively, reaching significance (P<.05) for CIG, JUUL, and MJ. Over 50% of tachycardia events were induced by the overdrive pacing. Non-air groups developed shorter ERPs in the LA, RA, and LV (Fig. 4C–E). Compared to Air control, non-air conditions resulted in a shorter APD80 and longer CaTD80 at the different PCLs (Fig. 5). As a result, a bigger difference of CaTD80 – APD80 was found in those non-air groups.
Fig. 4. Chronic smoking or vaping increased the susceptibility to atrial fibrillation (AF) and ventricular tachycardias (VT) with shortened effective refractory period (ERP).
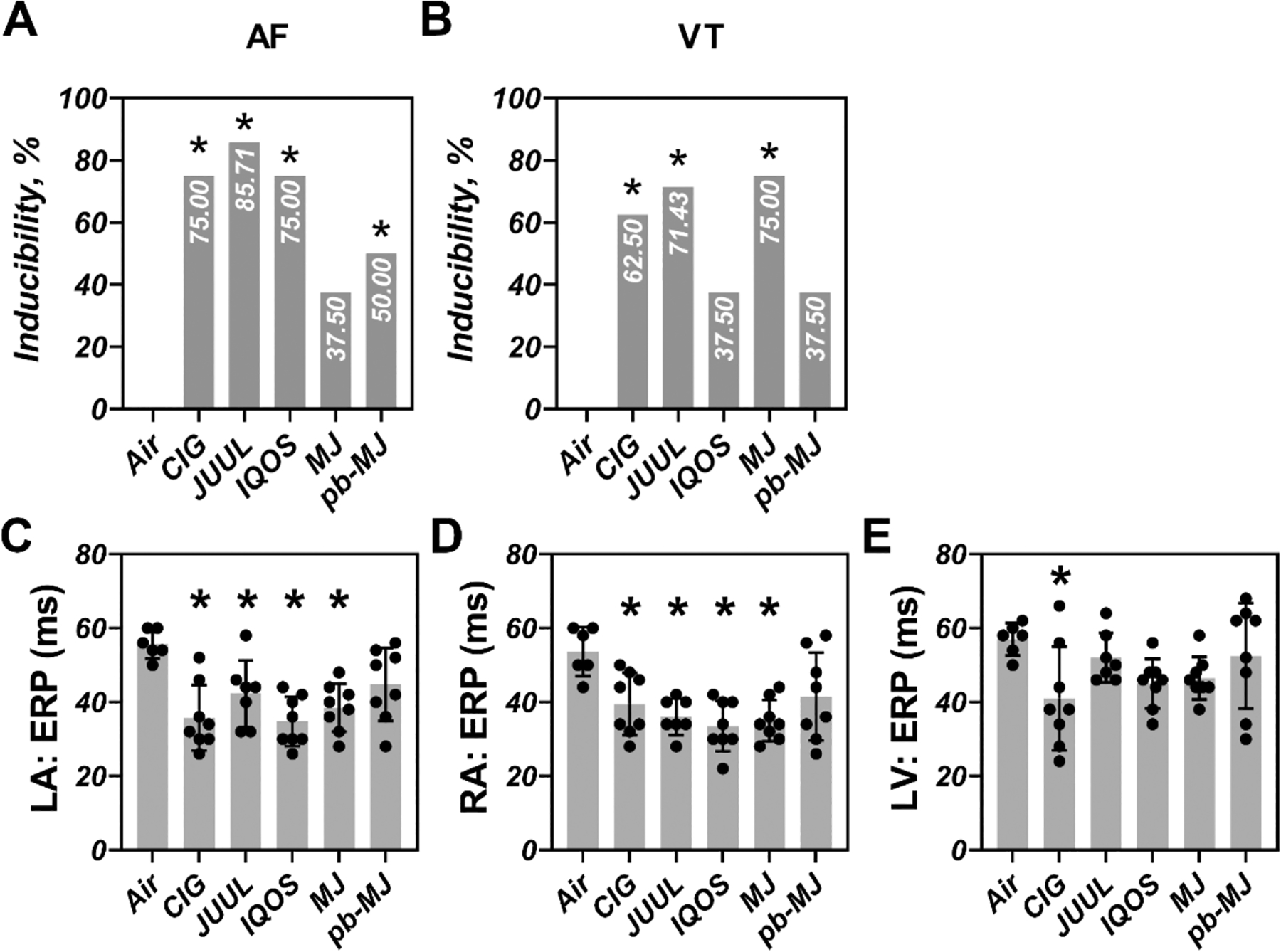
A-B. inducibilities of AF and VT. C-E. ERP. Pearson χ2 with a Bonferroni post hoc test or one-way ANOVA followed by Tukey’s multiple comparisons test was used to do statistical analysis. *P<.05, compared to Air; N = 6, 8, 7, 8, 8, and 8 in Air, CIG, JUUL, IQOS, MJ, and pb-MJ respectively. Of note, in panel A, P=.091, Air compared to MJ; in panel B, P=.091, Air compared to IQOS and pb-MJ; in panel C, P=.13, Air compared to pb-MJ; in panel D, P=.07, Air compared to pb-MJ; in panel E, P=.20, CIG compared to pb-MJ.
Table 1.
Inducibility of atrial fibrillation (AF) or ventricular tachycardia (VT).
| Inducibility (%) | Air (n=6) |
CIG (n=8) |
JUUL (n=7) |
IQOS (n=8) |
MJ (n=8) |
pb-MJ (n=8) |
|---|---|---|---|---|---|---|
| AF (in total) | 0 | 75 | 85.71 | 75 | 37.5 | 50 |
| LAF: | 0 | 62.5 | 28.57 | 50 | 12.5 | 12.5 |
| by extra-stimuli | 28.57 | 0 | 25 | 50 | 0 | |
| by overdrive | 71.43 | 100 | 75 | 50 | 100 | |
| RAF: | 0 | 50 | 85.71 | 50 | 37.5 | 50 |
| by extra-stimuli | 42.86 | 14.29 | 40 | 25 | 0 | |
| by overdrive | 57.14 | 85.71 | 60 | 75 | 100 | |
| VT | 0 | 62.5 | 71.43 | 37.5 | 75 | 37.5 |
| by extra-stimuli | 44.44 | 44.44 | 40 | 50 | 40 | |
| by overdrive | 55.56 | 55.56 | 60 | 50 | 60 |
AF, atrial fibrillation; VT, ventricular tachycardia. LAF, LA pacing-induced AF; RAF, RA pacing-induced AF.
Fig. 5. Relatively shortened APD80, prolonged CaTD80 and increased difference between CaTD80 and APD80 were observed in non-air groups.
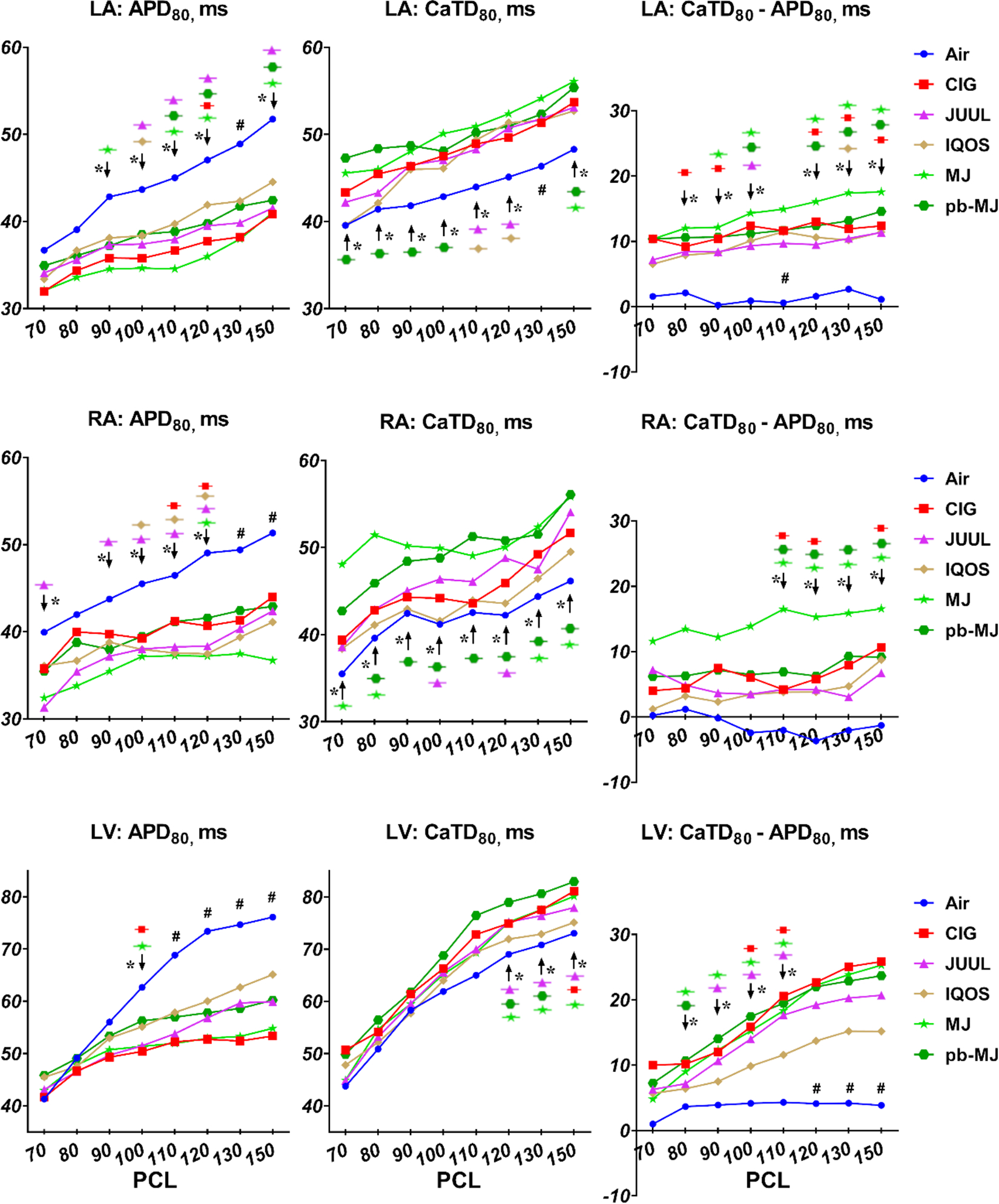
APD80, action potential duration at 80% repolarization; CaTD80, calcium transient duration at 80% repolarization. One way ANOVA followed by Tukey’s multiple comparisons test was used to do statistical analysis. *P<0.05, specific group(s) compared to Air; #P<0.05, all groups compared to Air. N = 6, 8, 7, 8, 8, and 8 in Air, CIG, JUUL, IQOS, MJ, and pb-MJ respectively.
Higher deposition of interstitial fibrosis and less microvessel density.
To determine if different smoking/vaping products may induce cardiac fibrosis, heart sections were stained for interstitial fibrosis in red and cardiomyocytes in green. As shown in Fig. 6, interstitial fibrosis in LA, RA, and LV was significantly increased in all non-air groups relative to the Air group (P<.001). There was no significant difference among non-air groups. Measurement of fibrotic biomarkers including galectin-3 (Gal-3), matrix metalloproteinase-9 (MMP-9), and its endogenous tissue inhibitor-1 (TIMP1) showed only MMP-9 was reduced comparably in all non-air groups, identifying MMP-9 potentially as the main mediator. Transverse sections stained with GS-I were analyzed to assess changes in microvessels. As shown in Fig. 7, both density and area percentage of microvessels were significantly decreased in non-air groups, suggesting adverse effects on the microcirculation.
Fig. 6. Fibrosis and fibrotic biomarkers.
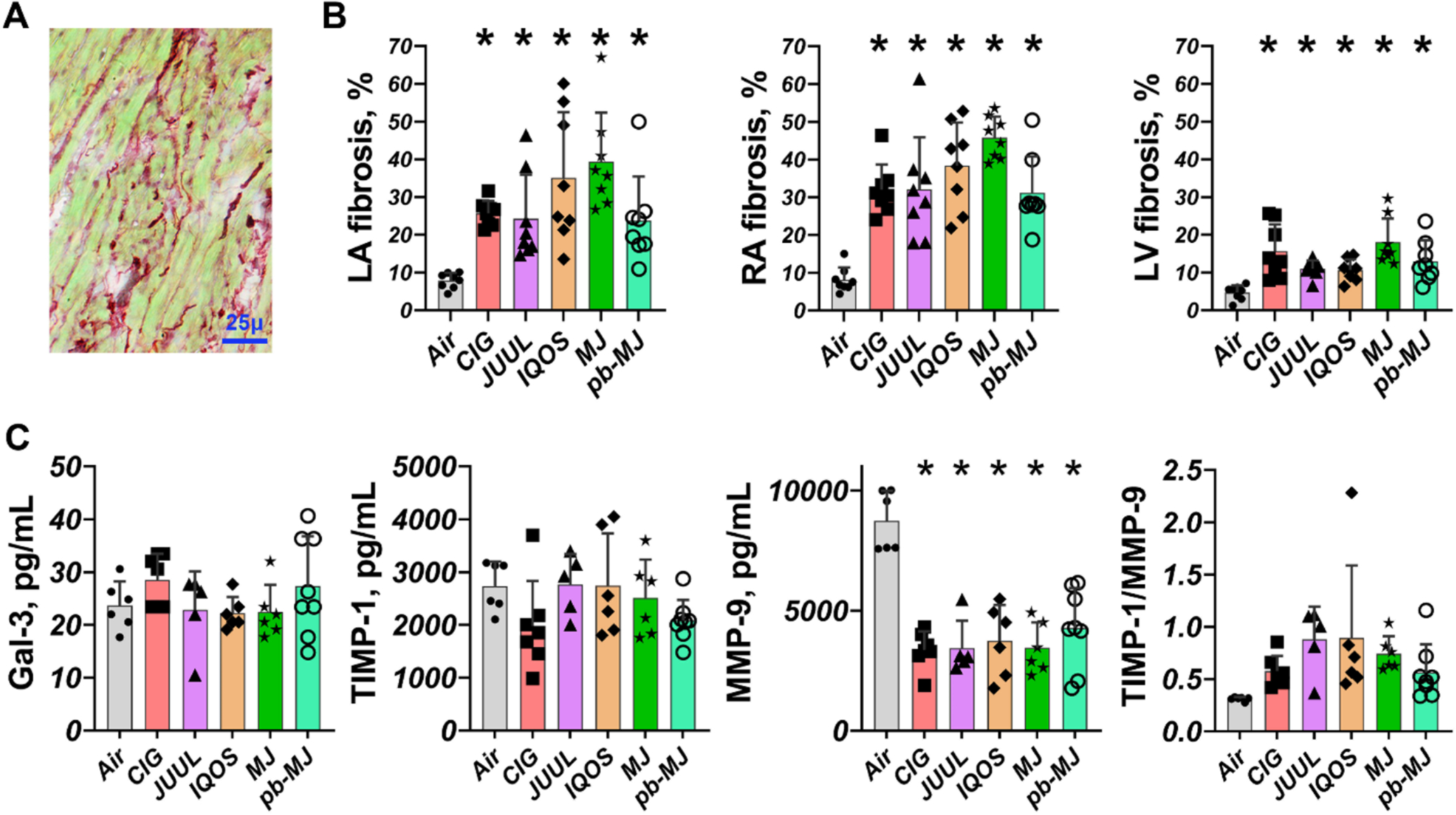
A. Sirius red staining is shown for example. B. Increased cardiac fibrosis in non-air groups. C. ELISA assays showed that there were no significant changes in Gal-3 and TIMP1; However, MMP-9 was significantly decreased in non-air groups and the ratio of TIMP1 to MMP-9 was elevated in non-air conditions. Data were expressed as mean ± SD and analyzed by one way ANOVA followed by Tukey’s multiple comparisons test. *P<.05, compared to Air. N = 8 each group for tissue fibrosis staining; and N = 6, 7, 5, 6, 6, and 8 in Air, CIG, JUUL, IQOS, MJ, and pb-MJ respectively for ELISA. Of note, in panel C, for Gal-3, P=.99, Air compared to CIG and pb-MJ. For TIMP-1, P=.30 and .71, Air compared to CIG and PB-MJ respectively. For TIMP1/MMP9, Air compared to CIG, JUUL, IQOS, MJ and PB-MJ P=.76, .11, .07, .34, and .76, respectively.
Fig. 7. Smoking or vaping reduced the density of microvessels in the left ventricle.
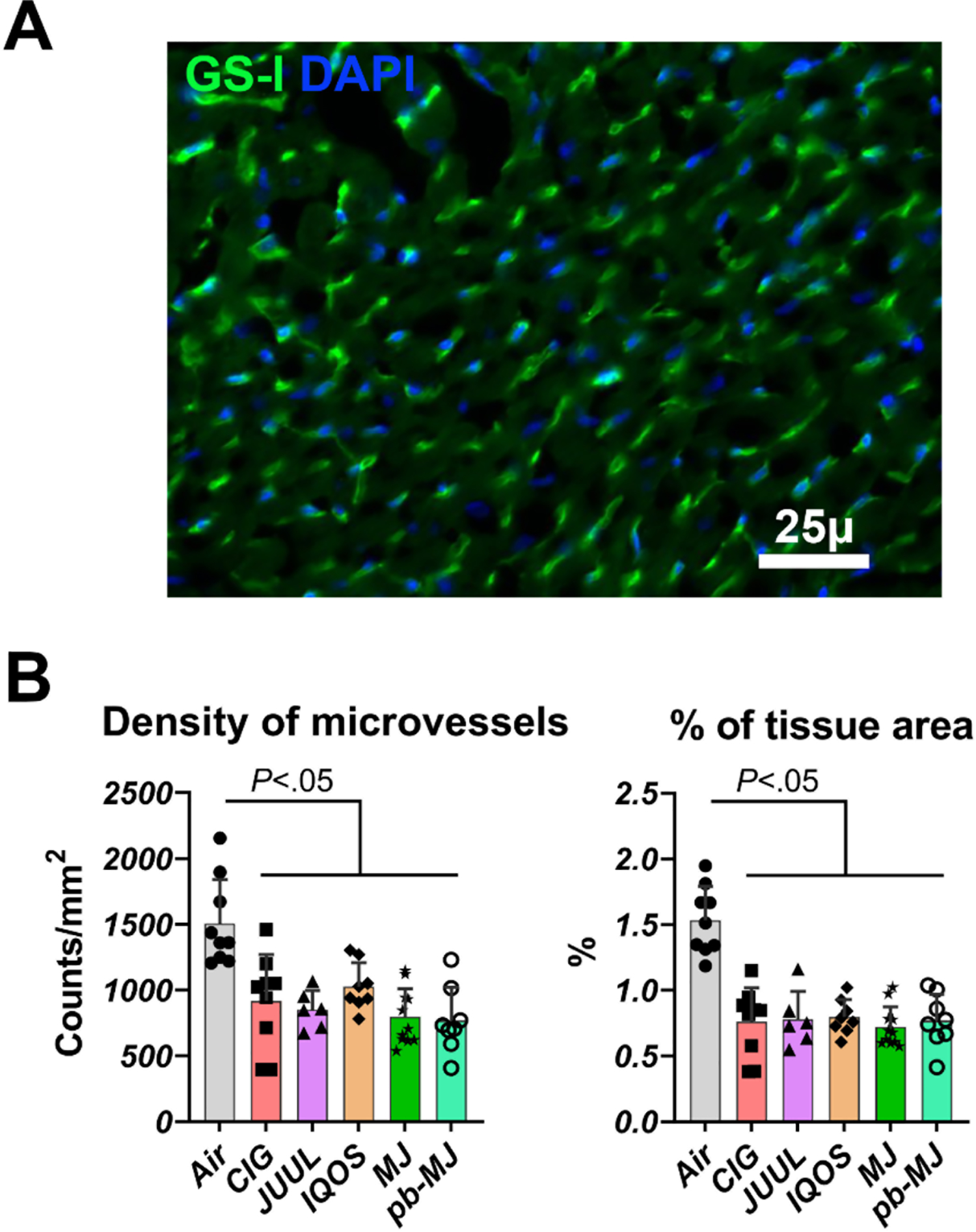
A. Capillaries are stained green in this example of staining; nuclei are blue. GS-I, Griffonia simplicifolia I, marker of microvessels. B. Density and proportion of microvessels in the tissue. Results were expressed as mean ± SD and analyzed by one way ANOVA followed by Tukey’s multiple comparisons test. N = 9, 9, 6, 8, 11, and 8 in Air, CIG, JUUL, IQOS, MJ, and pb-MJ respectively.
Intrinsic cardiac neural remodeling.
The densities of tyrosine hydroxylase (TH, as sympathetic marker)-positive nerves within LA, RA, LV, and RV were higher in rats exposed to tobacco or marijuana products than to Air (Fig. 8). Conversely, densities of choline acetyltransferase (ChAT, as parasympathetic marker)-positive nerves were relatively lower in non-air groups. These data suggest a potential cardiac neural remodeling of sympathetic hyperinnervation and parasympathetic withdrawal.
Fig. 8. Smoking or vaping promoted neural remodeling of the heart.
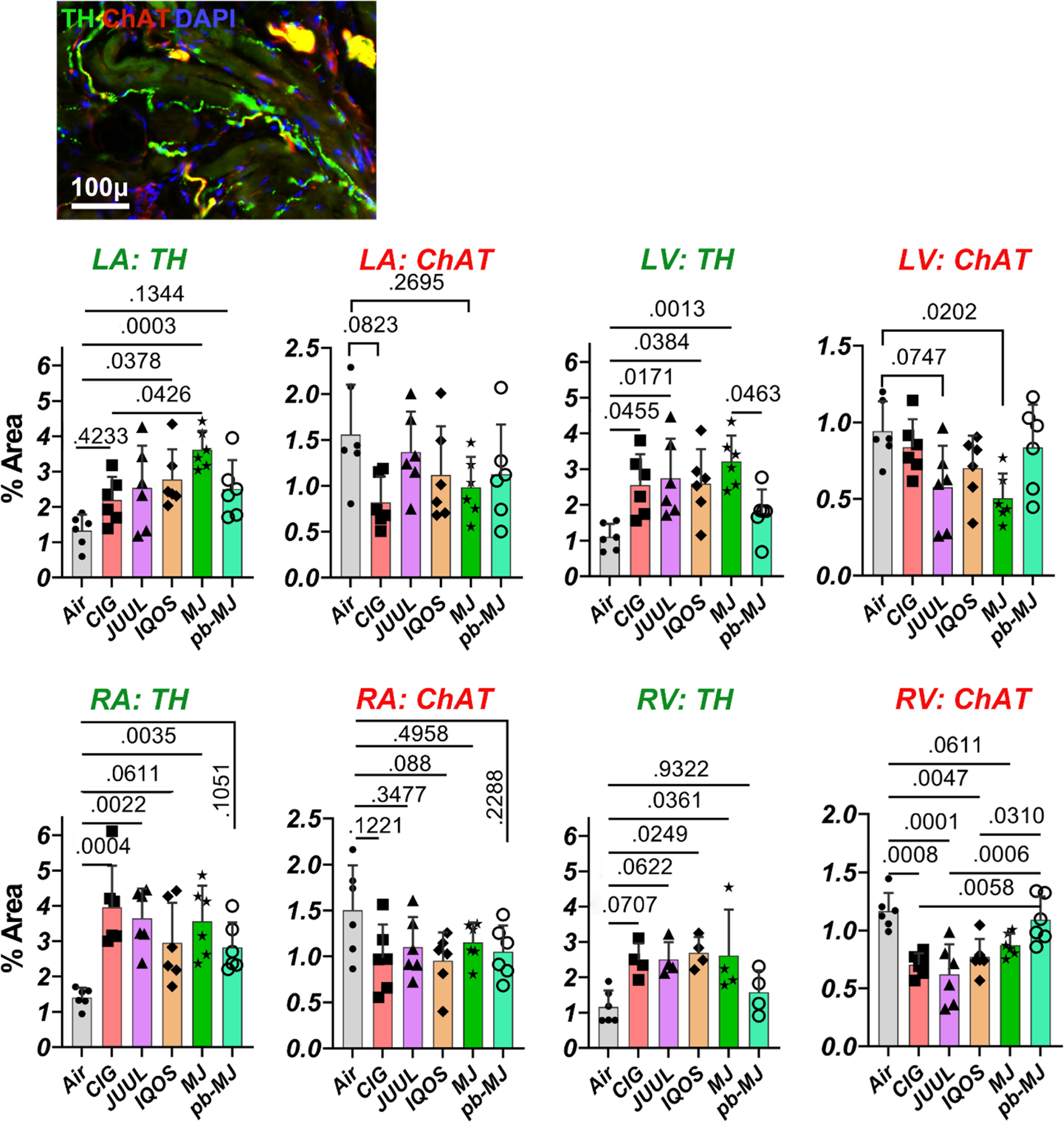
Fluorescent triple labeling of tyrosine hydroxylase (TH, as marker of sympathetic nerve component), choline acetyltransferase (ChAT, visualized the parasympathetic nerve component), and DAPI showed more sympathetic and less parasympathetic nerves in rats exposed to tobacco or marijuana versus to air. Data were expressed as mean ± SD and analyzed by one way ANOVA followed by Tukey’s multiple comparisons test; p values are shown in the graphs. N = 6 per group.
DISCUSSION
This study shows that 8 weeks of daily exposure to smoke or aerosol from tobacco cigarettes, e-cigs, HTPs, or marijuana can cause comparable pathophysiological changes, consequently leading to hypertension, cardiac dysfunction, and arrhythmias. Cardiac electrical, structural, and neural remodeling are all involved in inducible atrial fibrillation and ventricular tachycardia caused by smoking or vaping. It is notable that these adverse effects resulted from a single smoking/vaping session per day, with each session reflecting a relatively modest exposure mimicking 10 “puffs” over 5 minutes; i.e., we did not use an extreme exposure model. We have used the same conditions to study acute effects of a single session of cigarette smoking, IQOS use, and multiple types of e-cigarette vaping sessions.17,29,30 In the course of these studies, we have validated the relevance of our exposure conditions to human real-world use by (1) using nose cone pulsatile exposure to enable immediate switching between brief pulses of undiluted smoke/aerosol and interim periods of clean air, (2) showing that circulating plasma nicotine and cotinine levels after a single session of exposure to Marlboro Red cigarette smoke were comparable to circulating levels in humans after smoking one cigarette, and (3) confirming an approximate dose response relationship between number of exposure cycles in one session and resulting plasma nicotine levels.17
Smoking/vaping tobacco and marijuana products elevated SBP.
Exposure to tobacco and marijuana products progressively increased pre-exposure SBP. Within each individual measurement day, tobacco products and cannabinoid-depleted marijuana acutely increased SBP. In contrast, regular marijuana acutely reduced SBP but not all the way to baseline day 0 values, indicating that cannabinoids have a blood pressure lowering effect that may be not sufficient to counteract the prohypertensive effect of marijuana smoke. The increased norepinephrine levels in all non-air groups, with no change in angiotensin levels, suggests that the sympathetic neural drive may be more important in smoking-caused hypertension, rather than the renin-angiotensin system. Thus, beta-blockers may be better than angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the treatment of smoking-related hypertension.
Smoking or vaping led to alterations in autonomic nerve activity and cardiac neural remodeling.
Autonomic nerve function is an important determinant of arrhythmogenesis.25 It is intriguing that chronic exposure to e-cigs, HTPs, and marijuana all caused reduced overall HRV, of which both sympathetic and parasympathetic nerve function were downregulated as indicated by the results from the time domain method and change of LF band from frequency domain method. Previous studies have also suggested that chronic and acute tobacco smoking are associated with reduced overall HRV.13 A recent study suggested that exposure to vanillin-flavored e-cig aerosol for 10 weeks also influences autonomic nerve activity by increasing the predominance of sympathetic nerve function in mice.14 The reduced HRV is independently associated with the arrhythmogenesis of both AF and VT and is involved in the development of hypertension.31–33 Moreover, in the present study, we observed cardiac sympathetic hyperinnervation and parasympathetic withdrawal in rats exposed to tobacco and marijuana products. Cardiac neural remodeling plays an important role in arrhythmogenesis by inducing triggered activity and changing the automaticity of cardiomyocytes.34 The over-innervation of sympathetic nerves is associated with the arrhythmogenesis of both AF and VT.25 Both ECNS and ICNS are equally important in maintaining a normal cardiac physiology. Without ECNS control, pathological change of ICNS itself could be proarrhythmogenic.35 Our observed cardiac sympathetic hyperinnervation and parasympathetic withdrawal may be the cause of the subsequent cardiac electrical remodeling. Although we did not directly link the neural remodeling to electrical remodeling, previous studies have demonstrated that sympathetic hyperinnervation in the ICNS can promote Ca2+-initiated triggered activity.36 One reasonable explanation for the shortened APD80 and prolonged CaTD80 is that sympathetic nerves activate intracellular Ca2+ transients, while parasympathetic nerves activate IKAch, leading to triggered activity due to the late phase-3 early afterdepolarizations.25,36
Fibrosis and microvessel change.
The development of fibrosis and the decrease in capillary density in post-smoking/vaping hearts may be the consequence of the elevated SBP. In non-ischemic conditions, cardiac fibrosis leads to hypertension, cardiac hypertrophy, or heart failure with preserved ejection fraction, of which the latter is often accompanied by microvascular rarefaction.25 This provides an important indication that smoking or vaping may cause multiple aspects of cardiovascular disease (CVD) beyond arrhythmias, because the latter could be a symptom of other severe CVD; and the pathological findings including changes in fibrosis, microvessels, and nerves may cause more problems than we observed. Moreover, the increased ratio of TIMP1/MMP9 or the decreased MMP9 was known to accelerate fibrosis and microvessel remodeling.33 Accordingly, the increase of MMP9 may be able to inhibit fibrosis and restore vascular network.33 The present study therefore identified the important role of MMP9 in smoking-related cardiac fibrotic and microvessel remodeling.
Potential clinical application and limitations.
Smoking/vaping-related CVD affects countless people but is not named as a disease. Given the increasing use of marijuana and novel tobacco products including e-cigs and HTPs, and common perceptions that these products are relatively free of health risks, our results indicate that all of these products may still carry substantial risk of development of cardiac disease. Our findings may provide clues to treat smoking/vaping-related CVD by preventing hypertension, targeting HRV, intracellular calcium handling, and fibrosis. An improvement of calcium regulation may benefit heart function and reduce susceptibility to arrhythmia. In addition, since the neural remodeling of the heart, which here mainly refers to increased sympathetic innervation, is highly associated with CVD development, nerve ablation or stimulation may be a potential therapeutic measure applied to such pathophysiological changes. The improvement of HRV by multiple interventions (e.g., aerobic training,39 beta blockers,40 and calcium channel blockers41) may be accompanied by the improvements of sympatho-vagal rebalance and calcium handling. A limitation is that while most procedures underwent completely blinded analysis, only one investigator was able to access the lab during the COVID-19 shutdown and thus the exposures and the cardiac function and SBP data collection were performed by the same person. Those data were subsequently coded, randomized, and the investigator analyzed the data at least 2 weeks later, now blinded to the identity of each animal. Another limitation is that our study used entirely young, healthy rats; age or comorbidities presumably result in a more complex physiological effect. Whether smoking-related cardiac nerve activity correlates with frequency of arrhythmia episodes needs to be further investigated. This study was not designed to assess the effects of life-long product use, so we do not know whether the adverse effects that we observed were at their saturation point, or would have continued to worsen with continued chronic exposure.
CONCLUSIONS
A single daily smoking/vaping session with tobacco cigarettes, e-cigs, HTPs, or marijuana cigarettes results in an increased susceptibility to AF and VT with reduced HRV, cardiac sympathetic hyperinnervation, interstitial fibrosis, and electrophysiological changes in otherwise healthy rats. These pathophysiological changes may be secondary to the increase in blood pressure caused by chronic exposure.
Data Availability Statement
Raw data are accessible at https://doi.org/10.7272/Q66W98B3
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by grant U54 HL147127 from the NIH and FDA Center for Tobacco Products, and California TRDRP grant T29IP0490 to M.S., AHA postdoctoral fellowship 20POST35120455 to H.Q., and generous support from the Elfenworks Foundation (in memory of Deb O’Keefe) and the Roy E Thomas Medical Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Dai H, Catley D, Richter KP, Goggin K, Ellerbeck EF. Electronic cigarettes and future marijuana use: A longitudinal study. Pediatrics 2018; 141:2014–2015. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RD, Pacek LR, Copeland J, et al. Trends in daily cannabis use among cigarette smokers: United States, 2002–2014. Am J Public Health 2018; 108:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C-Y, Paek Y-J, Seo HG, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep 2020; 10:5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tattan-Birch H, Brown J, Shahab L, Jackson SE. Trends in use of e-cigarette device types and heated tobacco products from 2016 to 2020 in England. Sci Rep 2021; 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schweitzer RJ, Wills TA, Behner JD. E-cigarette Use and Indicators of Cardiovascular Disease Risk. Curr Epidemiol Reports 2017; 4:248–257. [Google Scholar]
- 6.Shah S, Patel S, Paulraj S, Chaudhuri D. Association of Marijuana Use and Cardiovascular Disease: A Behavioral Risk Factor Surveillance System Data Analysis of 133,706 US Adults. Am J Med 2021; 134:614–620.e1. [DOI] [PubMed] [Google Scholar]
- 7.Fried ND, Gardner JD. Heat-not-burn tobacco products: an emerging threat to cardiovascular health. Am J Physiol Circ Physiol 2020; 319:H1234–H1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatnagar A, Whitsel LP, Blaha MJ, et al. New and Emerging Tobacco Products and the Nicotine Endgame: The Role of Robust Regulation and Comprehensive Tobacco Control and Prevention: A Presidential Advisory From the American Heart Association. Circulation 2019; 139:E937–E958. [DOI] [PubMed] [Google Scholar]
- 9.Ai J, Epstein PN, Gozal D, Yang B, Wurster R, Cheng ZJ. Morphology and topography of nucleus ambiguus projections to cardiac ganglia in rats and mice. Neuroscience 2007; 149:845–860. [DOI] [PubMed] [Google Scholar]
- 10.Bosch R: Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res 1999; 44:121–131. [DOI] [PubMed] [Google Scholar]
- 11.Boixel C, Fontaine V, Rücker-Martin C, et al. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol 2003; 42:336–344. [DOI] [PubMed] [Google Scholar]
- 12.Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol 2017; 3:425–435. [DOI] [PubMed] [Google Scholar]
- 13.Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol 2013; 163:109–115. [DOI] [PubMed] [Google Scholar]
- 14.Abouassali O, Chang M, Chidipi B, et al. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am J Physiol Heart Circ Physiol 2021; 320:H133–H143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan H, Zhang Y, Lu Y, et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res 2009; 83:465–472. [DOI] [PubMed] [Google Scholar]
- 16.Shao XM, López-Valdés HE, Liang J, Feldman JL. Inhaled nicotine equivalent to cigarette smoking disrupts systemic and uterine hemodynamics and induces cardiac arrhythmia in pregnant rats. Sci Rep 2017; 7:16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabavizadeh P, Liu J, Havel CM, et al. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control 2018; 27:s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Derakhshandeh R, Liu J, et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J Am Heart Assoc 2016; 5:e003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding C, Gepstein L, Nguyen DT, Wilson E, Hulley G, Beaser A, Lee RJ, Olgin J. High-Resolution Optical Mapping of Ventricular Tachycardia in Rats with Chronic Myocardial Infarction. Pacing Clin Electrophysiol 2010; 33:687–695. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Sievers RE, Varga M, et al. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. J Appl Physiol 2013; 114:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Takagawa J, Lam VC, et al. Donor Myocardial Infarction Impairs the Therapeutic Potential of Bone Marrow Cells by an Interleukin-1–Mediated Inflammatory Response. Sci Transl Med 2011; 3:100ra90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Takagawa J, Sievers RE, et al. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol - Heart Circ Physiol 2007; 292:1187–1192. [DOI] [PubMed] [Google Scholar]
- 23.Qiu H, Ma J, Wu H, Ding C. DL-3-n-butylphthalide improves ventricular function, and prevents ventricular remodeling and arrhythmias in post-MI rats. Naunyn Schmiedebergs Arch Pharmacol 2018; 391:627–637. [DOI] [PubMed] [Google Scholar]
- 24.Qiu H, Ji C, Liu W, et al. Chronic Kidney Disease Increases Atrial Fibrillation Inducibility: Involvement of Inflammation, Atrial Fibrosis, and Connexins. Front Physiol 2018; 9:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S: Role of the Autonomic Nervous System in Atrial Fibrillation. Circ Res 2014; 114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM: Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeghiazarians Y, Zhang Y, Prasad M, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther 2009; 17:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao P, Han DD, Tan K, et al. Comparable Impairment of Vascular Endothelial Function by a Wide Range of Electronic Nicotine Delivery Devices. Nicotine Tob Res 2022;24(7):1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao P, Liu J, Springer ML. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob Regul Sci 2020; 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zandstra T, Kiès P, Maan A, et al. Association between reduced heart rate variability components and supraventricular tachyarrhythmias in patients with a systemic right ventricle. Auton Neurosci Basic Clin 2020; 227:102696. [DOI] [PubMed] [Google Scholar]
- 32.van den Berg MP, Haaksma J, Brouwer J, Tieleman RG, Mulder G, Crijns HJ. Heart Rate Variability in Patients With Atrial Fibrillation Is Related to Vagal Tone. Circulation 1997; 96:1209–1216. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, Blood Pressure, and Heart Rate Variability: The Atherosclerosis Risk in Communities (ARIC) Study. Hypertension 2003; 42:1106–1111. [DOI] [PubMed] [Google Scholar]
- 34.Pinnamaneni K, Sievers RE, Sharma R, et al. Brief Exposure to Secondhand Smoke Reversibly Impairs Endothelial Vasodilatory Function. Nicotine Tob Res 2014; 16:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapa S, Wang L, Francis Stuart SD, et al. Adrenergic supersensitivity and impaired neural control of cardiac electrophysiology following regional cardiac sympathetic nerve loss. Sci Rep 2020; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 2003; 107:2355–2360. [DOI] [PubMed] [Google Scholar]
- 37.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013; 28:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens 2010; 24:175–182. [DOI] [PubMed] [Google Scholar]
- 40.Sun YL, Hu SJ, Wang LH, Hu Y, Zhou JY. Effect of β-Blockers on Cardiac Function and Calcium Handling Protein in Postinfarction Heart Failure Rats. Chest 2005; 128:1812–1821. [DOI] [PubMed] [Google Scholar]
- 41.Tajiri K, Guichard JB, Qi X, et al. An N-/L-type calcium channel blocker, cilnidipine, suppresses autonomic, electrical, and structural remodelling associated with atrial fibrillation. Cardiovasc Res 2019; 115:1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are accessible at https://doi.org/10.7272/Q66W98B3


