Abstract
Background
This study aimed to evaluate the homologous recombination repair pathway deficiency (HRD) in ovarian high-grade serous carcinoma (HGSC).
Methods
In the ovarian cancer data from The Cancer Genome Atlas, we identified genes differentially expressed between tumours with and without HRD genomic scars and named these genes “HRDness signature”. We performed SNP array, RNA sequencing, and methylation array analyses on 274 HGSC tumours for which targeted sequencing of 51 genes and clinical data were available to generate JGOG3025-TR2 dataset. The HRDness signature was tested on external datasets, including the JGOG3025-TR2 cohort, by computational scoring and machine-learning prediction.
Results
High scores and positive predictions of the HRDness signature were significantly associated with BRCA alterations, genomic scar scores, and better survival. On the other hand, among cases with high scores and/or positive predictions, those with BRCA1 methylation showed poorer survival. In the JGOG3025-TR2 cohort, HRD status was significantly associated with the use of olaparib after relapse and progression-free survival after its initiation.
Conclusions
The HRDness gene expression signature is associated with a good prognosis, while BRCA1 methylation is associated with a poor prognosis. The newly generated JGOG3025-TR2 dataset will be useful in future HGSC studies.
Subject terms: Ovarian cancer, Ovarian cancer
Introduction
Ovarian cancer has the worst prognosis of all gynaecological cancers [1]. High-grade serous carcinoma (HGSC), the most common histologic type of ovarian cancer, is often clinically diagnosed at an advanced stage [2] and requires a combination strategy of surgery and drug therapy. HGSC is associated with germline or somatic BRCA1/2 mutations in about 20% of cases [3]. BRCA1 and BRCA2 are key molecules in the DNA homologous recombination repair (HRR) pathway, and their loss-of-function mutations result in HRR pathway deficiency (HRD). Chromosomal instability due to HRD contributes to carcinogenesis, and besides BRCA1/2 mutations, more than half of HGSCs exhibit HRD due to BRCA1 methylation, mutations in other HRR pathway genes, and unknown mechanisms [3].
Poly(ADP-ribose) polymerase (PARP) is involved in the repair of DNA single-strand breaks, and inhibition of PARP function by PARP inhibitors results in double-strand breaks. In addition, PARP inhibitors cause PARP trapping in DNA [4]. Since the HRR pathway plays a major role in the repair of double-strand breaks and PARP trapping, HRD tumour cells are sensitive to PARP inhibitors [5]. HRD tumours are also sensitive to platinum agents because they induce DNA double-strand breaks [6]. Therefore, it is important to examine the HRD status of the tumour to determine whether it is sensitive to platinum or PARP inhibitors. In addition, a recent meta-analysis reported that BRCA mutations correlate with improved survival, but BRCA1 methylation does not [7], suggesting that identifying not only whether a patient has HRD but also the cause of HRD may be important in individualising cancer treatment.
Chromosomal structural abnormalities detected by single-nucleotide polymorphism (SNP) array, namely, telomeric allelic imbalance (TAI) [8], large-scale state transition (LST) [9], and genomic loss of heterozygosity(LOH) [10], were reported to be indicators of tumour HRD status based on the data from ovarian HGSC and breast cancer. Subsequently, the HRD score, the sum of those scores, was reported as a “genomic scar” [11], and the MyChoice diagnostic system, which simultaneously detects tumour BRCA mutations and measures the HRD score, was developed. In the PAOLA-1 study, olaparib used in combination with bevacizumab in the maintenance setting was effective in HRD-positive patients determined by MyChoice, but not in HRD-negative patients [12]. On the other hand, the PRIMA study showed efficacy of niraparib also in non-HRD tumours determined by the same test [13], suggesting that the method currently used to identify HRD tumours may not be sufficiently accurate. Another indicator proposed as a genomic scar is the mutational signature 3, a pattern of genetic variation characteristic of HRD tumours [14], but its usefulness in clinical settings has yet to be fully evaluated.
Other than BRCA mutations and the above genomic scars, gene expression profile may be used as an indicator of HRD. In 2010, a “BRCAness signature,” gene expression profile consisting of 60 genes characteristic of tumours with BRCA1/2 mutations, was identified from training data of 61 cases and was reported to correlate with prognosis in 70 validation cases [15]. Although there have been several reports of similar methods since then, a more comprehensive gene expression profile that distinguishes HRD from non-HRD tumours has never been reported. This is because, besides The Cancer Genome Atlas (TCGA) data, there were no large multi-omics datasets, including SNP arrays or gene expression profiles, available for validation.
In this study, we report the establishment of a large multi-omics dataset of HGSC (JGOG3025-TR2 dataset) that allows for comprehensive HRD analysis and the development of a new gene expression signature to assess tumour HRD status. This is the largest multi-omics dataset of HGSC after TCGA report. The advantages of this study compared to TCGA include the relatively recent treatment period (since 2017) and the prospective collection of clinical information. This study will greatly contribute to the individualisation of clinical practice in HGSC.
Materials and methods
JGOG3025-TR2 cohort
The JGOG3025 study was conducted by the Japanese Gynecologic Oncology Group (JGOG) on patients with ovarian cancer (NCT03159572) [16]. In the study, clinical information was prospectively collected for epithelial ovarian cancer cases whose treatment started between March 2017 and March 2019 at 55 JGOG-affiliated centres, and a total of 701 cases were enrolled through central pathology review. In Japan, olaparib maintenance therapy for platinum-sensitive recurrence was approved by insurance in January 2018, and maintenance therapy with olaparib as first-line treatment for advanced ovarian cancer with BRCA mutations was approved by insurance in June 2019. Therefore, considering the study period, patients enrolled in the study could have received olaparib (but not other PARP inhibitors) under the health insurance as maintenance therapy only after relapse treatment, not after initial treatment.
In the JGOG3025-TR2 study, a total of 296 cases of FIGO Stage IIB-IV high-grade serous (HGSC) or high-grade endometrioid ovarian cancer were selected, and the present study used data from 274 cases with HGSC among them. Clinical data were obtained as part of the JGOG3025 study [16], with some modifications to the annotations for OS and surgeries performed (Supplementary Methods).
Multi-omics analysis of the JGOG3025-TR2 data
In the JGOG3025 study, targeted sequencing of a total of 51 genes (Supplementary Table S1), including DNA repair-related genes and genes frequently mutated in ovarian cancer, was performed using frozen tumour tissue samples [16]. In the JGOG3025-TR2 study, frozen tumour samples were sent to Takara Bio.inc. (Kusatsu, Japan), followed by DNA and RNA extraction for OncoScan CNV assay, DNA methylation array and RNA sequencing (RNA-seq) analysis (Supplementary Methods).
Gene mutations were annotated as previously reported [16] after excluding those with variant allele frequency >95%. For copy number and chromosomal instability analysis, from the OSCHP files, BAF and Log2 ratio were obtained for each SNP marker using Chromosome Analysis Suite (v4.2). ASCAT [17] (v3) was used to determine the segmented genome-wide allele-specific copy number profiles with the default setting. As we previously reported [18], we annotated the locus-specific LOH for each variant using the estimated copy number of the minor allele at the segment located in the locus of the variant; when the minor-allele copy number was equal to zero, it was determined to be “LOH,” when the number was one or more, determined to be “non-LOH.” A homozygous deletion for each gene in each sample was annotated when the region of the gene contained any segmented region with a total copy number equal to zero. Using GISTIC2 [19], gene amplification and deletion were annotated by the estimated focal copy number per gene, where a value greater than 0.4 was considered amplification and a value less than −0.4 deletion. scarHRD [20] was used to determine the scores for telomeric allelic imbalance (TAI) [8], large-scale state transition (LST) [9], and loss of heterozygosity (LOH) [10]. HRD scores were determined as the sum of these three scores. The HRD score calculated by scarHRD was strongly correlated with that by the method of Marquard et al. [21] (Supplementary Fig. S1).
For RNA-seq analysis, the raw fastq files were trimmed using Trim-Galore [22], and then mapped to the human reference genome (GRCh38) with the gene annotation of Ensemble GRCh38.v99 using STAR (v2.7.8a) [23]. The resulting read counts were normalised as transcripts per million (TPM) values and then, annotated with the gene symbol. The expression levels of transcripts duplicated by gene name were merged by geometric mean. We used ‘consensusOV’ R package [24] to classify each tumour into four molecular subtypes, namely, immune-reactive (IMR), mesenchymal (MES), differentiated (DIF), and proliferative (PRO), based on gene expression profiles.
BRCA1 and RAD51C promoter CpG island probes were selected when the mean beta was <0.25 and the Spearman correlation between mRNA expression and beta value was rS < −0.1, P value <0.01. Samples were annotated as silenced by methylation when at least 75% of the selected probes met the criteria of beta value >0.3 and mRNA expression <30th percentile.
Analysis of The Cancer Genome Atlas ovarian cancer (TCGA-OV) data
Somatic mutation profiles were obtained from the MC3 project [25] as a MAF format. The cosine similarity to signature 3 (Sig3 score) per sample was calculated using SigMA [26]. For somatic variants, we filtered out variants with variant allele frequency <5%, population minor-allele frequency >1%, no predicted LOF annotation in missense or splice site mutations, no COSMIC annotations, and “benign” or “likely benign” annotations in InterVar or ClinVar. For germline variants, annotations analysed in a previous paper [27] were used. From the raw CEL files of the SNP array analysis, Penn-CNV [28] and ASCAT [17] were used to calculate the segmented allele-specific copy numbers. The LOH/non-LOH annotation for each variant and the HRD score for each sample were calculated in the same method as above. BRCA1 methylation silencing was annotated using the Affymetrix Human Genome U133A microarray for gene expression and the Illumina HumanMethylation27 BeadChip array for DNA methylation, using the same criteria as above.
Differentially expressed gene (DEG) analysis between genomic scar-high vs. low (GS-high vs. GS-low) samples in the TCGA-OV cohort defined by two genomic scar score cutoffs that optimally determined BRCA1/2 mutations with LOH (Supplementary Fig. S2A) was performed for RNA-seq data using limma-voom [29, 30], where genes with Benjamini–Hochberg adjusted P value (FDR) < 0.05, average expression level (AveExpr) >2, absolute log2 fold change >0.25 were kept as significantly differentially expressed genes (DEGs). In addition, the same comparative analysis for the Affymetrix microarray data was performed using SAM [31], where genes of the probes with FDR < 0.05, absolute log2 fold change >0.05 were retained as DEGs. Genes with up- or downregulated expression in GS-high samples that overlapped between the above two platforms were defined as the HRDness signature (Supplementary Fig. S2B). The combined enrichment score of the 173 up- and 76 downregulated genes (Supplementary Table S2) was calculated in external datasets using ssGSEA [32], and used as the HRDness signature score. In addition, we developed a machine-learning method to perform binary classification based on the HRDness signature gene expression. Using the gene expression levels (fragments per kilobase of exon per million mapped reads in the RNA-seq and signal intensity in the microarray analysis) of the HRDness signature in the GS-high and GS-low samples as features, four prediction models were constructed based on four different algorithms: k-nearest neighbour, support vector machine, random forest, and linear regression. The four classification results were ensembled, and when all results matched, the matched classification was assigned to the sample; otherwise, the ‘undetermined’ classification was assigned to the sample. When applied the method to external data, the data were adjusted and calibrated to the TCGA platform using SVA::ComBat [33].
Validation datasets for correlation between HRDness signature and genomic scar
We obtained the whole-genome ovarian cancer dataset from Australia (OV-AU data) [34]. Only data from the primary tumour samples at the initial treatment were included in our analysis. Somatic mutation profiles were obtained from the supplementary file in the original paper (Supplementary Table S9.1 High Confidence Coding Somatic Variants). Using these profiles as input, the SigMA score was calculated using SigMA [26]. Annotations for BRCA1/2 germline and somatic mutations were obtained from the same paper (Supplementary Table 4.1. Germline loss-of-function mutations in homologous recombination pathway genes, Supplementary Table 4.2. Somatic loss-of-function mutations in homologous recombination pathway genes). The raw data from the SNP array analysis was obtained from Gene Expression Omnibus (GEO) (GSE61568), and the segmented allele-specific copy numbers were calculated using ASCAT [17], and the locus-specific LOH/non-LOH for BRCA1/2 was annotated in the same method described above. HRD scores were calculated using scarHRD [20], and BRCA1 methylation was annotated in the same way above.
We also used the Clinical Proteomic Tumour Analysis Consortium ovarian cancer (CPTAC-OV) data [35]. The somatic mutation profiles obtained from the GDC portal [36] in MAF format were used as input to calculate the SigMA score using SigMA [26]. Processed gene expression FPKM values were obtained from the same portal. BRCA1/2 somatic mutations were annotated using the same criteria for TCGA-OV data (see above). For germline BRCA1/2 mutations, we obtained normal BAM files and performed variant calling, annotation, and hard-filtering according to GATK4 best practice workflows of germline short variant discovery [36].
For the dataset by Ducie et al. (GSE 102094) [37], processed gene expression profiles derived from RNA-seq and SNP array raw data were obtained from GEO website. Starting from the CEL files, HRD score was calculated using Penn-CNV [28], ASCAT [17] and scarHRD [20].
Validation datasets on the correlation between the HRDness signature and overall survival
To evaluate the association between the HRDness signature and survival outcomes in HGSC, we selected GEO datasets that met the following criteria: (i) high-grade serous carcinoma on histopathological diagnosis, (ii) overall survival data is available, (iii) raw data without or before quantile normalisation is available, and (iv) specimen is derived from frozen tissue, and (v) there are at least 100 samples. As a result, GSE9891, GSE26712, GSE32062, GSE53963 were selected. GSE32063, which was derived from the same study as GSE32062 but with a different batch, was integrated into GSE32062 using SVA::ComBat [33]. Quantile normalisation, conventionally used in microarray analysis, tends to smooth out extremely high or low values. To detect samples with low BRCA1 expression, we obtained raw data and re-normalised gene expression levels for each dataset. For GSE9891 and GSE26712, using the Affymetrix Human Genome Array, the MAS5 method was applied for normalisation with the ‘affy’ R package [38]. For GSE32062, GSE32063, and GSE53963 using the Agilent-014850 Whole Human Genome Microarray, the locally weighted scatterplot smoothing method was applied with limma [29].
Analysis workflow including all the datasets is provided in Supplementary Fig. S3.
Statistical analysis
Unless otherwise noted, all statistical analyses in this study were performed using Python (3.8.8). Spearman’s rank correlation test, ROC curve, and the Brunner–Munzel test were performed using SciPy (1.7.2). Machine-learning analyses were performed using Scikit-learn (1.0.1). Survival analyses including the Kaplan–Meier curve, the multivariate log-rank test, and the Cox proportional hazard regression analysis were performed using Lifelines (0.26.3). The multivariate log-rank test of this package is a generalisation of the log-rank test, where the null hypothesis is that the hazard ratios are the same in all groups, while the alternative hypothesis is that there is at least one group that differs from the other groups. A P value <0.05 was considered statistically significant. Visualisation of the results was performed using Matplotlib (3.4.3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Identification of gene expression signatures assessing HRD in TCGA ovarian cancer
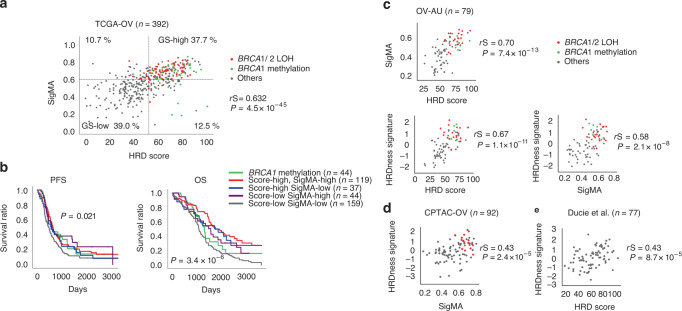
In TCGA ovarian cancer (TCGA-OV) cohort, the HRD score derived from SNP arrays and the mutational signature 3 score [26] (SigMA, see “Materials and methods”) derived from whole-exome sequencing-based somatic mutation profiles were significantly positively correlated (Spearman correlation rS = 0.632, P = 4.5 × 10−45, Fig. 1a). Using ROC curves, we calculated the cut-off value in each of the two scores that optimally distinguished tumours with loss-of-function gene mutations with loss of the wild-type allele (LOH) in BRCA1/2. In the HRD score, the AUC, optimal cut-off, sensitivity, and specificity at the cut-off were 0.770, 52.0, 0.929 and 0.408, respectively, while in the SigMA, 0.790, 0.616, 0.917 and 0.380, respectively (Supplementary Fig. S1A). Notably, there were no tumours with BRCA1/2 mutations with LOH or BRCA1 methylation in tumours below both cutoffs (Fig. 1a).
Fig. 1. Analysis of TCGA-OV dataset for genomic scar, survival and HRDness gene expression signature.
a Association between HRD score, SigMA ratio and BRCA1/2 mutations with LOH, and BRCA1 methylation in TCGA-OV. The two dotted lines indicate the optimal cutoffs for each score (see also Supplementary Fig. S1A). rS Spearman correlation coefficient. b The prognostic impact of HRD status. First, the cases with BRCA1 methylation were divided, then the remaining cases were divided by HRD score and SigMA ratio following the above cut-off lines. As a result, a total of five groups were compared. Multivariate log-rank test results showed significant differences between groups in both analyses (P = 0.021, 3.4 × 10−6, respectively). c–e Validation of the HRDness signature score in OV-AU, CPTAC-OV, and Ducie et al. [37].
We then performed a comparative survival analysis betweesn HRD and non-HRD tumours based on genomic scar. We previously reported that tumours with BRCA1 methylation had a poor prognosis among HRD tumours [39]. Therefore, we first separated tumours with BRCA1 methylation, and then classified the remaining tumours into four groups according to the above two cut-off values: HRD score-high/SigMA-high, HRD score-high/SigMA-low, HRD score-low/SigMA-high, and HRD score-low/SigMA-low, for a total of five groups for comparative survival analysis. Progression-free survival (PFS) and overall survival (OS) were significantly different among the groups, but the P value was smaller in the latter analysis (multivariate log-rank test, P = 0.021, 3.4 × 10−6, respectively, Fig. 1b). In the OS analysis, the group with either of the two scores higher had a better prognosis than the group with both lower scores. Notably, the survival curve of the BRCA1 methylation group declined sharply, eventually having the second worst survival after the group with the lower scores for both.
Next, we examined the gene expression profiles associated with HRD. Differential gene expression analysis was performed between tumours with high vs. low genomic scar scores, both for SigMA and HRD scores (see “Materials and methods” and Supplementary Fig. S1B). We defined the HRDness signature for the 173 high- and 76 low-expressed genes in tumours with high HRD genomic scar scores that are commonly found in the RNA-seq and gene expression microarray data (Supplementary Table S2). BRCA1 was one of the low-expressed genes. The HRDness signature enrichment score was calculated using ssGSEA [32] and its association with genomic scar score was tested on external datasets, which showed significant positive correlations between these scores (Fig. 1c–e).
Analysis of the HRD status in the JGOG3025-TR2 dataset
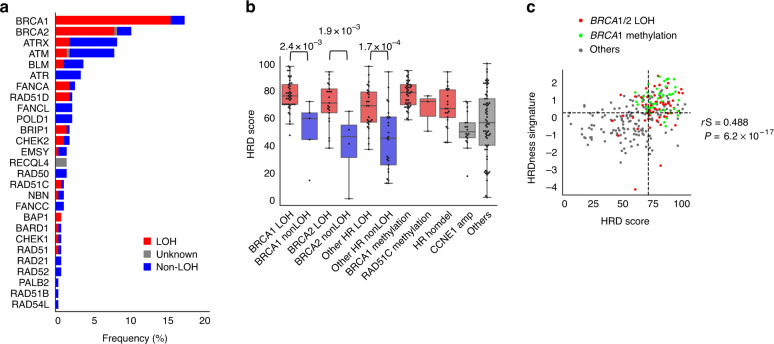
Clinical information on the 274 HGSCs collected in the JGOG3025-TR2 study is shown in Supplementary Table S3. Gene mutations in 29 HRR-related genes (Supplementary Table S1) were examined for their wild-type allele loss (allele-specific LOH) (see “Materials and methods”). The LOH involvement rate was 89.1% (41/46) in BRCA1 mutations and 80.8% (21/26) in BRCA2 mutations (Fig. 2a), consistent with our previous report [18]. The HRD score calculated from the OncoScan SNP array has been reported to correlate strongly with the HRD score calculated from the FDA-approved HRD test, but to be higher [40]. The median HRD score calculated from the OncoScan analysis in this study was also high, at 67.5. In BRCA1, BRCA2 and HR-related genes other than BRCA1/2, tumours having mutations with the allele-specific LOH had higher HRD scores than those without (Fig. 2b and Supplementary Fig. S4). HRD scores were relatively higher in tumours with BRCA1 methylation and homozygous deletions in HR-related genes and relatively lower in those with CCNE1 amplification (Fig. 3b). The gene mutations with LOH in HRR-related genes and BRCA1 methylation tended to be mutually exclusive (Supplementary Fig. S5). The HRDness signature enrichment score calculated for the JGOG3025-TR2 cohort was positively correlated with the HRD score (Spearman r = 0.488, P = 6.2e-17, Fig. 2c). Tumour purity required for proper analysis is reported to be at least 15% for chromosomal structural aberrations by SNP array, 10% for gene mutation detection, and 35% for DNA methylation and mRNA expression [10]. In the JGOG3025-TR2 dataset, tumour purity estimated by ASCAT was at least 35% in all cases. No obvious bias in tumour purity, HRD score or HRDness signature score was observed by sampling site (Supplementary Fig. S6).
Fig. 2. Generation of the JGOG-TR2 dataset and validation of the HRDness signature.
a Mutation frequency and involvement of locus-specific LOH in HRR-related genes. b Association between HR-related gene alteration and mean HRD score. In BRCA1, BRCA2 and HR-related genes other than BRCA1/2, tumours with locus-specific LOH had significantly higher HRD scores than those without P values; Brunner–Munzel test. c Positive correlation between HRD score and HRDness signature. rS Spearman correlation coefficient.
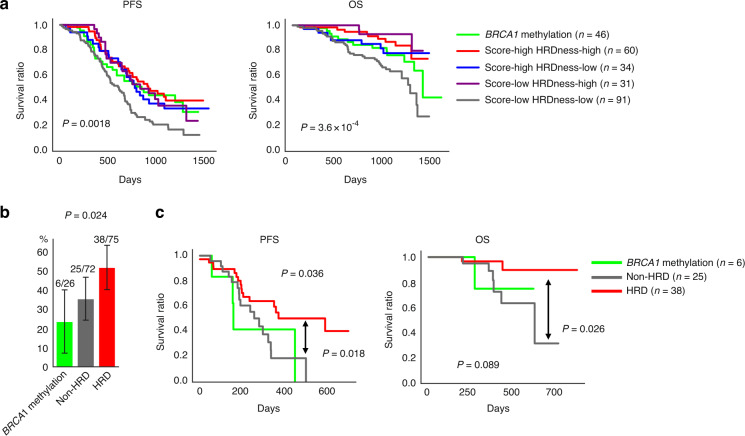
Fig. 3. Prognostic analysis of the JGOG3025-TR2 dataset.
a Survival time from the start of initial treatment. First, the cases with BRCA1 methylation were divided, then the remaining cases were divided by the median HRD score and HRDness signature score (Fig. 2c, dotted lines). Score; HRD score, HRDness; HRDness signature score. b Percentage of patients who used olaparib after relapse. non-HRD (dark grey); both scores low. HRD (red): no BRCA1 methylation and either score high. c Survival time after olaparib administration stratified by BRCA1 methylation and HRD status.
Survival analysis of the JGOG3025-TR2 cohort divided by median scores
JGOG3025-TR2 cases were divided by the presence or absence of BRCA1 methylation, and then by the medians of the HRD score and the HRDness signature score, for a total of five groups for survival analysis (Fig. 2c, dotted lines). Tumours with both low scores had no BRCA1 methylation (Fig. 2c). PFS analysis showed that the group with both low scores had a worse prognosis, and no clear differences were seen between the other groups (multivariate log-rank test, P = 0.0018, Fig. 3a, left). In the OS analysis, the dual low-scoring group showed the worst, and the group with BRCA1 methylation showed the second worst survival (multivariate log-rank test, P = 3.6 × 10−4, Fig. 3a, right). In HGSC, gene expression-based molecular subtypes were reported to correlate with survival outcome [41]. Cox proportional hazard multivariate analysis using the HRD score, HRDness signature score, BRCA1 methylation, HGSC molecular subtypes [24] (consensusOV subtype, see “Materials and methods”), and other clinical information as covariates showed that the HRDness-high status was an independent favourable prognostic factor for OS (Table 1, hazard ratio [95% CI] = 0.40 [0.21–0.76], P = 0.0055) and PFS (Supplementary Table S4, hazard ratio [95% CI] = 0.64 [0.43–0.86], P = 0.0049 for PFS). BRCA1 methylation was not a significant prognostic factor in the univariate analysis for OS (hazard ratio [95% CI] = 1.02 [0.56–1.86], P = 0.96) but was an independent poor prognostic factor in the multivariate analysis after adjusting for HRD status (hazard ratio [95% CI] = 2.93 [1.37–6.27], P = 0.0055). This represents that BRCA1 methylation was a negative confounding factor, that is, most of the tumours with BRCA1 methylation were in the HRD group (Fig. 2c) but had as poor a prognosis as non-HRD tumours (Fig. 3a).
Table 1.
Cox proportional hazard analysis for overall survival in the JGOG-TR2 cohort.
| Univariate | Multivariate (n = 253) | Multivariate (n = 156) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | N = 274 | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | <62 | 137 | |||||||||
| >=62 | 137 | 1.52 | (0.95–2.43) | 0.084 | 1.28 | (0.72–2.25) | 0.4 | 0.95 | (0.48–1.87) | 0.89 | |
| Performance status | 0 | 202 | |||||||||
| >= 1 | 64 | 2.05 | (1.23–3.43) | 0.0063 | 1.68 | (0.94–3.00) | 0.083 | 1.07 | (0.51–2.25) | 0.85 | |
| Surgery | PDS | 125 | |||||||||
| IDS | 120 | 2.34 | (1.32–4.14) | 0.0036 | 3.05 | (1.51–6.17) | 0.0019 | 4.2 | (1.67–10.59) | 0.0023 | |
| NDS | 29 | 7.52 | (3.78–14.98) | 9.3 × 10−9 | 3.17 | (1.28–7.89) | 0.013 | 8.69 | (2.82–26.74) | 1.6 × 10−4 | |
| Residual tumour | R0 | 162 | |||||||||
| R1 | 44 | 1.73 | (0.91–3.30) | 0.093 | 1.47 | (0.76–2.86) | 0.25 | 1.53 | (0.73–3.20) | 0.26 | |
| R2 | 68 | 3.26 | (1.94–5.50) | 9.0 × 10−6 | 3.36 | (1.58–7.10) | 0.0016 | 4.26 | (1.64–11.03) | 0.0028 | |
| BRCA1 methylation | No | 219 | |||||||||
| Yes | 46 | 1.02 | (0.56–1.86) | 0.96 | 2.93 | (1.37–6.27) | 0.0055 | 2.86 | (1.08–7.60) | 0.035 | |
| HRD score (median) | Low | 132 | |||||||||
| High | 133 | 0.58 | (0.36–0.94) | 0.028 | 0.64 | (0.36–1.13) | 0.12 | ||||
| HRD score (ROC curve) | Low | 120 | |||||||||
| High | 145 | 0.61 | (0.38–0.98) | 0.039 | 1.54 | (0.63–3.75) | 0.34 | ||||
| HRDness signature | Low | 135 | |||||||||
| High | 136 | 0.48 | (0.30–0.79) | 0.0039 | 0.4 | (0.21–0.76) | 0.0055 | ||||
| HRDness prediction | Negative | 76 | |||||||||
| Positive | 93 | 0.4 | (0.23–0.70) | 0.0012 | 0.16 | (0.06–0.42) | 2.2 × 10−4 | ||||
| ConsensusOV | IMR | 71 | |||||||||
| MES | 77 | 1.55 | (0.83–2.90) | 0.17 | 1.57 | (0.78–3.14) | 0.2 | 1.85 | (0.73–4.65) | 0.19 | |
| DIF | 80 | 0.79 | (0.39–1.62) | 0.53 | 1.57 | (0.69–3.56) | 0.28 | 1.59 | (0.59–4.25) | 0.36 | |
| PRO | 43 | 1.52 | (0.73–3.16) | 0.26 | 0.96 | (0.41–2.24) | 0.93 | 0.91 | (0.32–2.58) | 0.86 | |
HR hazard ratio, 95% CI 95% confidence interval, PDS primary debulking surgery, IDS interval debulking surgery, NDS non-debulking surgery, R0 no residual tumour, R1 residual tumour under 1 cm in diameter, R2 residual tumour more than 1 cm in diameter, IMR immunoreactive, MES mesenchymal, DIF differentiated, PRO proliferative.
We also analysed the prognosis after relapse. Because of the limited number of cases, we divided the patients into three groups: BRCA1 methylation, HRD (no BRCA1 methylation and at least one of the two scores is high) and non-HRD (tumours with both low scores). After the first recurrence, the proportion of patients treated with olaparib as maintenance therapy was lower in the BRCA1 methylation group and higher in the HRD group (Chi-square test, P = 0.024, Fig. 3b). PFS from the start of olaparib treatment was better in the HRD group than in the other two groups, and OS showed a similar trend (multivariate log-rank test, P = 0.036, 0.089, respectively, Fig. 3c).
Analysis using HRDness prediction and optimised HRD score cut-off
To explore the clinical applicability of the HRDness gene signature, we developed a machine-learning method for binary classification of the HRDness status based on the tumour gene expression (see “Materials and methods” for details). The leave-one-out cross-validation in the TCGA-OV training dataset showed that the binary classification was successful in 74.9% (137/183), 72.3% (215/295) and 79.0% (228/289) of the cases, and the accuracy of the classification in those cases was 93.4% (128/137), 89.3% (192/215), 92.5% (211/228), in the RNA-seq, Affymetrix microarray and Agilent microarray, respectively (Supplementary Fig. S7AB). Within the cases with successful binary classification, the cross-platform concordance of that classification was greater than 99% (Supplementary Fig. S7C).
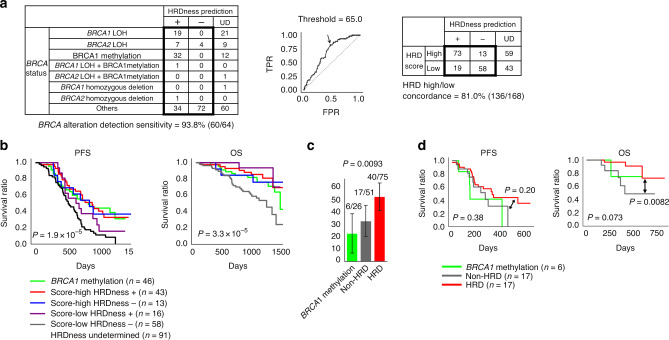
In the JGOG3025-TR2 cohort, 62.0% (170/274) cases were successfully classified into the HRDness positive or negative, where 93.8% (60/64) cases with BRCA1/2 alterations were assigned to HRDness positive (Fig. 4a, left). Considering that the determination of HRD status by the current HRD testing (MyChoice CDx) was established from the HRD score of tumours with BRCA alterations [11], we used the ROC curve to determine the HRD score cut-off to optimise cases with BRCA1/2 mutations with LOH in this analysis. The concordant rate of the classifications between HRDness and HRD score was 81.0% (136/168) (Fig. 4a, right). In other external datasets, including OV-AU and CPTAC-OV cohorts, the results were comparable to those in the JGOG-TR2 cohort (Supplementary Fig. S8).
Fig. 4. Survival analysis using the HRDness prediction and the optimised HRD score cut-off in the JGOG-TR2 cohort.
a Relationship between HRDness prediction results and BRCA status and the optimised HRD score cut-off. UD; undetermined b Stratified survival analysis using BRCA1 methylation, HRDness predictions, and the optimised HRD score cut-off. c Percentage of patients who used olaparib after relapse. d Survival time after olaparib administration stratified by BRCA1 methylation and HRD status.
Similar to Fig. 3a, stratified survival analysis using the HRDness prediction and the optimised HRD score cut-off showed significant differences between groups in PFS (multivariate log-rank test, P = 1.9 × 10−5, Fig. 4b, left) and OS (multivariate log-rank test, P = 3.3 × 10−5, Fig. 4b, right). Cox proportional hazard multivariate analysis showed that the predicted HRDness status was an independent favourable prognostic factor for OS (Table 1, hazard ratio [95% CI] = 0.16 [0.06–0.42], P = 2.2 × 10−4) and PFS (Supplementary Table S4. hazard ratio [95% CI] = 0.51 [0.32–0.81], P = 0.004). Furthermore, the same analysis as in Fig. 3b showed a significant difference in the rate of olaparib maintenance therapy after relapse between the groups divided by HRD status (Chi-square test, P = 0.0093, Fig. 4c). Probably due to the reduced sample size through HRDness prediction, there was no significant difference in PFS or OS after olaparib initiation, while there was a difference in OS between HRD tumours excluding BRCA1 methylation and non-HRD tumours (log-rank test, P = 0.0082, Fig. 4d).
Association between HRDness signature and survival outcome in GEO datasets
To further validate the association between HRDness signature and survival outcomes, we obtained four datasets from the NCBI Gene Expression Omnibus (GEO) with survival information, genome-wide gene expression profiles, and the sufficient number of HGSC cases (n > 100).
Samples with very low BRCA1 gene expression were frequently associated with BRCA1 methylation, and most tumours entering below −1.5 SD were associated with BRCA1 methylation (Supplementary Fig. S9A). Therefore, we divided each dataset into two groups based on the median HRDness signature score (HRDness-high or low), and defined BRCA1 low tumours as those with BRCA1 gene expression less than −1.5 SD among HRDness-high tumours (Supplementary Fig. S9B). In an integrated analysis of the four datasets, HRDness-high tumours had a better survival than HRDness-low tumours, and BRCA1 low tumours tended to have an unfavourable outcome (Fig. 5a). Cox proportional hazard analysis using the common clinical information of surgical completion and HRDness signature status, BRCA1 low, and consensusOV subtype as covariates showed that HRDness signature score-high was an independent favourable prognostic factor (Fig. 5b, hazard ratio [95% CI] = 0.63 [0.52–0.77], P = 7.5 × 10−6). The multivariate analysis using the clinical information available for each dataset showed similar results, where HRDness-high status was a significant favourable prognostic factor (Supplementary Table S5). Although not statistically significant, the median hazard ratio for BRCA1 low status exceeded 1 in all datasets, supporting the possibility that it is a poor prognostic factor (Supplementary Table S5).
Fig. 5. Survival analysis of the GEO datasets.
a Overall survival analysis in the integrated GEO dataset stratified by median HRDness signature and low BRCA1 expression (n = 867). In each dataset, cases were first divided into two groups by the median of HRDness signature score, and then BRCA1 expression low (<−1.5 SD) cases were separated among HRDness signature score-high cases, resulting in three groups. b Cox proportional hazard analysis for overall survival in the integrated GEO datasets. c Overall survival analysis in the integrated GEO dataset stratified by HRDness prediction and low BRCA1 expression (n = 616). HR hazard ratio, 95% CI 95% confidence interval.
We additionally performed HRDness prediction in the GEO datasets. Among the successfully classified cases, the frequency of HRDness-positive tumours ranged from 45.2 to 56.4% (Supplementary Table S6). In an integrated analysis of the four datasets, HRDness-positive tumours had a better survival than HRDness-negative tumours (Fig. 5c). Cox proportional hazard analysis showed that HRDness prediction was an independent favourable prognostic factor (Fig. 5b, hazard ratio [95% CI] = 0.59 [0.46–0.75], P = 1.6 × 10−5). Multivariate analysis of each dataset still yielded similar results (Supplementary Table S5).
Discussion
Previously, a total of 1058 cases, consisting of 295 breast cancer cases and 434 HGSC cases from TCGA, plus 127 ovarian cancer cases and 202 breast cancer cases from custom SNP arrays, were collectively investigated and a HRD score ≥42, which can select 95% of tumours with BRCA mutations or BRCA1 methylation, was set as the HRD tumour cut-off [11]. Based on the results of this research, the MyChoice diagnostic system was developed and has recently been widely used as a companion diagnostic to PARP inhibitors in ovarian cancer. In another clinical trial of ovarian cancer, a HRD score > = 33, which can select 99% of cases with BRCA mutations, was used for the cut-off [42]. We previously found that the cut-off for HRD scores to enrich BRCA1/2 mutations is ≥63 when the analysis is limited to TCGA-OV [39]. Among BRCA1/2 mutations in HGSCs, monoallelic mutations are present in about 10%, and such tumours are HR proficient [43]. This study differs from our previous one in that it limited BRCA1/2 mutations to only those with LOH and used mutational signature 3 in combination with HRD score. Surprisingly, none of the tumours with both scores below the cutoffs had BRCA1/2 mutations with LOH or BRCA1 methylation (Fig. 1a). This result indicates that the combination of both SNP array and exome sequencing can more accurately identify tumours with BRCA functional deficiency.
We identified a novel HRDness gene expression signature using the TCGA-OV data as a training set, and demonstrated that it correlated with the genomic scars in the JGOG3025-TR2 dataset and three other datasets (Figs. 1c–e and 2c). This is a much more robust analysis compared to the previous report for the BRCAness signature [15]. And a high HRDness score correlated with a better patient outcome in the JGOG3025-TR2 dataset and in other four datasets (Figs. 3a, 4b, 5, Table 1, Supplementary Tables S4 and S5). The score also correlated with survival after relapse in patients who received olaparib in the JGOG3025-TR2 cohort (Fig. 3c). Gene expression profiles can also be used to identify molecular subtypes that can reflect tumour sensitivity to drugs including bevacizumab [44] and taxane [45]. Gene expression-based HRD analysis may have different significance than current DNA-based genomic scar scaling in the assessment of HRD properties in HGSC and may be useful for patient treatment stratification.
We previously reported in TCGA data that overall survival in BRCA1 methylation cases was as poor as in HR proficient cases despite being classified as HRD by genomic scar [39], and this was replicated in the present analysis (Fig. 1b). The relatively poor prognosis of BRCA1 methylation cases has also been shown in a large meta-analysis [7]. BRCA1 promotor methylation silencing can cause HRD and sensitivity to platinum and PARP inhibitors [46], but it is often lost after chemotherapy [33, 46–48] which causes chemo-resistant relapse. In this study, the group with BRCA1 methylation had the similar PFS but worse OS than the other HRD groups (Figs. 1b and 3a). This suggests that demethylation of BRCA1 resulted in drug resistance after relapse. In the JGOG3025-TR2 cohort, the poor prognosis in the BRCA1 methylation group could also be attributed to the relatively infrequent use of olaparib (Fig. 3b), probably due to poor response to chemotherapy after relapse. Furthermore, cases with BRCA1 expression low had as unfavourable outcomes as HRDness-low tumours (Fig. 5). Among recurrent/metastatic triple-negative breast cancers, tumours with BRCA1 expression low as well as BRCA1 methylation were reported to be resistant to carboplatin [49]. HRD analysis based on gene expression profile may have the advantage of predicting BRCA1 methylation, which is difficult to assay in routine practice.
Currently, the use of PARP inhibitors is the standard of care in the first-line treatment of ovarian cancer [50]. One of limitations of this study is that the cohort of ovarian cancer patients analysed in this study did not receive PARP inhibitors as first-line therapy, and the relationship between the efficacy of PARP inhibitors in such a setting could not be analysed. In addition, the currently approved HRD test (MyChoice CDx) was not approved at the time this study cohort was enrolled and its data were not included in the analysis. In the future, the utility of adding mutational signature 3, HRDness signature, and BRCA1 methylation/expression information to the current HRD test in terms of predicting PARP inhibitor sensitivity at the time of first-line treatment should be examined. Whether these HRD assessments are truly predictive of the response to PARP inhibitors in post-relapse therapy also needs to be validated in the datasets of randomised controlled trials [51–53] conducted to date.
In conclusion, in HGSCs, we (i) generated the JGOG3025-TR2 multi-omics dataset, (ii) established a HRDness gene expression signature that correlates with survival outcomes and (iii) demonstrated that among HRD tumours, those with BRCA1 methylation or BRCA1 low have a worse prognosis. This study will advance HRD research in HGSC and promote personalised treatment of HGSC.
Supplementary information
Acknowledgements
We would like to thank all the JGOG members who participated in the JGOG3025-TR2 study; Drs. Makio Shozu, Hiroyuki Shigeta, Kazuhiro Takehara, Akira Kikuchi, Toyomi Sato, Akinori Oki, Shinya Yoshioka, Shinya Sato, Ryuji Kawaguchi, Hisafumi Okura, Takeshi Iwasa, Shoji Kamiura, Masato Kamitomo, Yoichi Aoki, Nao Suzuki, Yoshio Yoshida, Tadashi Kimura, Daisuke Aoki, Kazuyoshi Kato, Hiroaki Kobayashi, Hidemichi Watari, Etsuko Miyagi, Tsuyoshi Saito, Yoshihito Yokoyama, Tsunekazu Kita, Takashi Matsumoto, Satoshi Nagase, Toshiya Yamamoto, Yukio Hirano, Tomoaki Ikeda, Shiro Suzuki, Keiya Fujimori, Nagamasa Maeda, Naohiko Umesaki, Masatoshi Sugita, and Akira Kouyama.
Author contributions
ST: data analysis and writing the manuscript; KY: sample collection and data analysis; TB: sample collection and review of the manuscript; MS: sample collection and review of the manuscript; HY: sample collection and review of the manuscript; AO: sample collection and review of the manuscript; HK: sample collection and review of the manuscript; KO: sample collection and data analysis; MM: data analysis and review of the manuscript; TE: design of this study; NM: data analysis and writing the manuscript.
Funding
This work was supported by AstraZeneca K.K. and Merck Sharp & Dohme Corp as the programme of Externally Sponsored Research (ESR-19-14550).
Data availability
The data from SNP array, RNA-seq, DNA methylation array in this study are available from the corresponding authors upon reasonable request. Data sources other than JGOG3025 were summarised in Supplementary Table S7. Controlled access data were obtained through dbGaP access permission (phs000178, phs000892). Data and codes to reproduce the results are available from the corresponding author upon reasonable request.
Competing interests
NM received a research grant from AstraZeneca. NM received lecture fees from AstraZeneca and Takeda Pharmaceutical. NM is also an outside director of Takara Bio. TB received lecture fees from AstraZeneca. KY received lecture fees and a research grant from AstraZeneca. There are no other competing interests related to this paper.
Ethics approval and consent to participate
For the JGOG3025 study, i.e., clinical data analysis, frozen tumour tissue collection, target sequencing, and future analyses of tumour tissue, written informed consent was obtained from all patients with approval from the Institutional Review Board at each JGOG participating site prior to the start of the study [16]. The JGOG3025-TR2 study was then conducted with the approval of the Ethics Committee of JGOG and the Institutional Ethics Committee of Kindai University (approval number; 29-167), with opt-out patient consent. This study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shiro Takamatsu, Kosuke Yoshihara.
Contributor Information
Takayuki Enomoto, Email: enomoto@med.niigata-u.ac.jp.
Noriomi Matsumura, Email: noriomi@med.kindai.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02122-9.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous Recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murai J, Huang SN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min A, Im SA. PARP inhibitors as therapeutics: beyond modulation of PARylation. Cancers. 2020;12:394. doi: 10.3390/cancers12020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinopoulos PA, Matulonis UA. Targeting DNA damage response and repair as a therapeutic strategy for ovarian cancer. Hematol Oncol Clin North Am. 2018;32:997–1010. doi: 10.1016/j.hoc.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Kalachand RD, Stordal B, Madden S, Chandler B, Cunningham J, Goode EL, et al. BRCA1 promoter methylation and clinical outcomes in ovarian cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2020;112:1190–203. doi: 10.1093/jnci/djaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–75. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–62. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- 10.Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–73. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 13.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 14.Polak P, Kim J, Braunstein LZ, Karlic R, Haradhavala NJ, Tiao G, et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476–86. doi: 10.1038/ng.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–61. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosuke Y, Tsukasa B, Muneaki S, Koji N, Masayuki S, Shiro T, et al. Homologous recombination inquiry through ovarian malignancy investigations: The Japanese Gynecologic Oncology Group study JGOG3025. medRxiv 2022.07.06.22277241. [Preprint]. Available from: 10.1101/2022.07.06.22277241
- 17.Van Loo P, Nordgard SH, Lingjærde OC, Russnes HG, Rye IH, Sun W, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci USA. 2010;107:16910–5. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takamatsu S, Brown JB, Yamaguchi K, Hamanishi J, Yamanoi K, Takaya H, et al. Utility of homologous recombination deficiency biomarkers across cancer types. JCO Precis Oncol. 2022;6:e2200085. doi: 10.1200/PO.22.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12:R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sztupinszki Z, Diossy M, Krzystanek M, Reiniger L, Csabai I, Favero F, et al. Migrating the SNP array-based homologous recombination deficiency measures to next generation sequencing data of breast cancer. npj Breast Cancer. 2018;4:16. doi: 10.1038/s41523-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquard AM, Eklund AC, Joshi T, Krzystanek M, Favero F, Wang ZC, et al. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark Res. 2015;3:9. doi: 10.1186/s40364-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babraham Bioinformatics. Taking appropriate QC measures for RRBS-type or other -Seq applications with Trim Galore! https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/.
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen GM, Kannan L, Geistlinger L, Kofia V, Safikhani Z, Gendoo DMA, et al. Consensus on molecular subtypes of high-grade serous ovarian carcinoma. Clin Cancer Res. 2018;24:5037–47. doi: 10.1158/1078-0432.CCR-18-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellrott K, Bailey MH, Saksena G, Covington KR, Kandoth C, Stewart C, et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 2018;6:271–81. doi: 10.1016/j.cels.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulhan DC, Lee JJK, Melloni GEM, Cortés-Ciriano I, Park PJ. Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat Genet. 2019;51:912–9. doi: 10.1038/s41588-019-0390-2. [DOI] [PubMed] [Google Scholar]
- 27.Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang J, Oh C, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173:355–70. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, et al. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17:1665–74. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson O, Wahlestedt C, Timmons JA. Considerations when using the significance analysis of microarrays (SAM) algorithm. BMC Bioinforma. 2005;6:129. doi: 10.1186/1471-2105-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 35.McDermott JE, Arshad OA, Petyuk VA, Fu Y, Gritsenko MA, Clauss TR, et al. Proteogenomic Characterization of Ovarian HGSC Implicates Mitotic Kinases, Replication Stress in Observed Chromosomal Instability. Cell Rep Med. 2020;1:100004. [DOI] [PMC free article] [PubMed]
- 36.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ducie J, Dao F, Considine M, Olvera N, Shaw PA, Kurman RJ, et al. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat Commun. 2017;8:990. doi: 10.1038/s41467-017-01217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–15. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 39.Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep. 2020;10:2757. doi: 10.1038/s41598-020-59671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cristescu R, Liu XQ, Arreaza G, Chen C, Albright A, Qiu P, et al. Genomic instability metric concordance between OncoScanTM, CytoSNP and an FDA-approved HRD test. Int J Gynecol Cancer. 2020;30:A130–1. [Google Scholar]
- 41.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123:517–25. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–15. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxwell KN, Wubbenhorst B, Wenz BM, De Sloover D, Pluta J, Emery L, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8:319. doi: 10.1038/s41467-017-00388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kommoss S, Winterhoff B, Oberg AL, Konecny GE, Wang C, Riska SM, et al. Bevacizumab may differentially improve ovarian cancer outcome in patients with proliferative and mesenchymal molecular subtypes. Clin Cancer Res. 2017;23:3794–801. doi: 10.1158/1078-0432.CCR-16-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami R, Matsumura N, Mandai M, Yoshihara K, Tanabe H, Nakai H, et al. Establishment of a novel histopathological classification of high-grade serous ovarian carcinoma correlated with prognostically distinct gene expression subtypes. Am J Pathol. 2016;186:1103–13. doi: 10.1016/j.ajpath.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Kondrashova O, Topp M, Nesic K, Lieschke E, Ho GY, Harrell MI, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9:3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prieske K, Prieske S, Joosse SA, Trillsch F, Grimm D, Burandt E, et al. Loss of BRCA1 promotor hypermethylation in recurrent high-grade ovarian cancer. Oncotarget. 2017;8:83063–74. doi: 10.18632/oncotarget.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elazezy M, Prieske K, Kluwe L, Oliveira-Ferrer L, Peine S, Müller V, et al. BRCA1 promoter hypermethylation on circulating tumor DNA correlates with improved survival of patients with ovarian cancer. Mol Oncol. 2021;15:3615–25. doi: 10.1002/1878-0261.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakai H, Matsumura N. Individualization in the first-line treatment of advanced ovarian cancer based on the mechanism of action of molecularly targeted drugs. Int J Clin Oncol. 2022;27:1001–12. doi: 10.1007/s10147-022-02163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 52.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 53.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from SNP array, RNA-seq, DNA methylation array in this study are available from the corresponding authors upon reasonable request. Data sources other than JGOG3025 were summarised in Supplementary Table S7. Controlled access data were obtained through dbGaP access permission (phs000178, phs000892). Data and codes to reproduce the results are available from the corresponding author upon reasonable request.