Abstract
Cytotoxic necrotizing factor 1 (CNF) is a toxin produced by some isolates of Escherichia coli that cause extraintestinal infections. CNF can initiate signaling pathways that are mediated by the Rho family of small GTPases through a covalent modification that results in constitutive activation. In addition to regulating the assembly of actin stress fibers and focal adhesion complexes, RhoA can also regulate gene expression at the level of transcription. Here we demonstrate for the first time, by using a luciferase-based reporter system, that the transcription of cyclooxygenase-2 (COX-2) is strongly upregulated in NIH 3T3 fibroblasts treated with CNF and that this effect is dependent upon the activation of RhoA by the toxin. Subsequent protein tyrosine phosphorylation events modulate the induction, but the transcription signal is not mediated by Rho-associated kinase (p160/ROCK) and so must rely upon another effector that is activated by RhoA. CNF therefore induces COX-2 expression via a RhoA-dependent signaling pathway that diverges from the pathway that regulates cytoskeletal rearrangements in response to RhoA activation.
Escherichia coli cytotoxic necrotizing factor 1 (CNF) covalently modifies members of the Rho subfamily of the Ras small GTPases and so brings about their constitutive activation (13, 15, 27, 28, 41, 49, 50). RhoA, Rac1, and Cdc42 are the Rho proteins that have been most extensively studied, and each of these proteins has pivotal roles in regulating signal transduction pathways. The active, GTP-bound forms of the Rho proteins interact with protein effectors that modulate many aspects of cellular behavior, particularly the organization of the actin cytoskeleton (16, 42, 43, 44, 57).
CNF action stimulates striking actin rearrangements, the induction of DNA synthesis in quiescent cells, and a block in cytokinesis (5, 9). The signaling mechanisms that link activation of the Rho proteins to these events are not entirely clear. Through its action on Rho, CNF is known to induce the tyrosine phosphorylation of focal adhesion complex proteins that result in actin stress fiber formation (25). We have recently demonstrated that CNF action promotes focal adhesion kinase (FAK) autophosphorylation and src-FAK association, which results in a complex in which potentially both kinases are active (54). It is likely that other signaling pathways are also perturbed by activation of the Rho proteins. Recently, it has been shown that activation of Rho via receptors that couple to the heterotrimeric G protein, G13, induces expression of the cyclooxygenase-2 (COX-2) gene (52).
The two isoforms of cyclooxygenase (COX-1 and COX-2) catalyze the rate-limiting step in the production of prostaglandins and other eicosinoids from membrane arachidonic acid (11, 29). Prostaglandins are lipid signaling molecules that participate in physiological processes as diverse as the maintenance of vascular integrity, pain transmission, inflammation, and bone remodeling (10, 22). COX-1 has a role in cellular homeostasis and is constitutively expressed in many tissues (40). COX-2 is inducibly expressed in response to inflammatory cytokines, lipopolysaccharide, mitogens, and reactive oxygen intermediates (58). The expression of COX-2 at abnormally high levels has been detected in cancers of the lung, breast, gallbladder, prostate, and stomach (17–19, 32, 46, 53). The association between COX-2 overexpression and tumor progression has, however, been established most effectively in colorectal cancers (48).
The induction of COX-2 gene expression in NIH 3T3 cells can be stimulated by several intracellular signaling pathways. Platelet-derived growth factor, serum, and v-src each activate COX-2 via Ras/Rac1/MEKK-1-mediated activation of the c-Jun NH2-terminal kinase (JunK) and also extracellular signal-related kinase (ERK) pathways (59, 60, 61). The JunK and ERK signals, together with cyclic AMP-mediated signals, converge on the ATF/CRE region within the COX-2 promoter (61). Some G protein-coupled receptors induce COX-2 expression through the activation of protein kinase C (PKC) (63). The induction of COX-2 expression by Gαa13-linked receptors involves the activation of the RhoA small GTPase but follows a pathway independent of actin stress fiber assembly and PKC activation (52).
In this study we investigated whether CNF was able to induce COX-2 gene expression. Here we demonstrate for the first time that CNF induces COX-2 expression through a RhoA-dependent pathway that was not dependent on the activation of Rho-associated kinase (p160/ROCK).
MATERIALS AND METHODS
Cell culture and transfection.
NIH 3T3 cells were obtained from the American Type Culture Collection (Rockville, Md.) and were maintained in Dulbecco modified Eagle medium (DMEM; Sigma-Aldrich, Poole, United Kingdom) supplemented with 10% fetal bovine serum in a humidified atmosphere containing 10% CO2 at 37°C. For experimental purposes, the cells were plated onto 35-mm six-well culture plates at a density of 105 cells per well. The next day, transfections were performed in triplicate by using the Lipofectamine (+) system (Life Technologies, Paisley, United Kingdom) according to the manufacturer's directions. Briefly, 1 μg of DNA was combined with 100 μl of Optimem medium (Life Technologies) and 3 μl of Plus reagent. Lipofectamine (6 μl) was combined with 100 μl of Optimem medium and added dropwise to the DNA-Plus reagent suspension. After 15 min, each transfection mix was diluted with 1.8 ml of Optimem medium and put onto the cells. After 6 h, the transfection medium was replaced with DMEM without serum, and the cells were incubated for 18 h. The cells were subjected to the treatments described and then harvested and assayed for luciferase activity.
Northern blotting.
NIH 3T3 cells were seeded in T75 tissue cultures at a density of 106 cells per flask. Once 60 to 80% confluency had been reached, the cells were made quiescent by serum depletion for 36 h. The cells were treated with lysates from recombinant E. coli that either expressed CNF or harbored pBluescript. The lysates were diluted in serum-free DMEM at 1 μg/ml and added to the cells for 8 h. The cells were trypsinized and harvested. Poly(A+) RNA extraction and Northern blot analyses were carried out as described previously (56). Each blot contained 5 μg per lane of poly(A+) RNA. Probes were labeled with [32P]dCTP (NEN, Boston, Mass.) to a high specific activity by using Ready-To-Go oligonucleotide-labeling beads and purified by using ProbeQuant G-50 microcolumns (Amersham Pharmacia, St. Albans, United Kingdom). COX-2 mRNA was detected by using a specific 2.2-kb cDNA probe (obtained from H. R. Herschman, University of California at Los Angeles School of Medicine). After the COX-2 probe was stripped from the blot, it was reprobed with a 1-kb glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific cDNA probe (obtained from A. E. Grigoriadis, Department of Craniofacial Development, King's College, London, United Kingdom) as a control to demonstrate equal loading of mRNA.
Luciferase assay.
Luciferase activity was measured using the luciferase assay system (Promega, Southampton, United Kingdom) according to the manufacturer's directions. Briefly, the cells in each well were washed twice in phosphate-buffered saline (PBS) and then lysed in 500 μl of luciferase lysis buffer. After lysis, insoluble material was removed by centrifugation, and 20 μl of each lysate was combined with 90 μl of luciferase assay reagent. The light produced by each assay reaction was measured by using a Turner TD20/20 luminometer. The values obtained were standardized for protein concentration by using the bicinchoninic acid-protein assay system (Pierce-Warriner, Chester, United Kingdom). The data presented here represent typical experiments in which each treatment was performed in triplicate and repeated at least twice. The histograms show the mean of a single experiment ± standard error where a fold induction above the background level of luciferase production for untreated cells has been calculated.
Preparation of bacterial toxins.
E. coli strain pISS392 harboring pGEM3 plasmid expressing CNF (12) and E. coli XL1-Blue harboring pBluescript SK(−) plasmid were grown overnight from a fresh colony (25). The bacteria were harvested by centrifugation and then lysed by ultrasonication in PBS containing lysozyme and protease inhibitors. The lysate was treated with DNase and RNase at 10 μg/ml, cleared by centrifugation, and passed through a 0.2-μm-pore-size nitrocellulose filter.
Actin stress fiber staining.
Quiescent NIH 3T3 cells in chamber slides were treated with bacterial lysates diluted in DMEM from E. coli expressing CNF or harboring pBluescript for 12 h at 37°C. For the inhibitor experiments, the cells were incubated in DMEM containing the ROCK inhibitors HA1077 or Y-27632 at a final concentration of 10 μM, cytochalasin D at a final concentration of 2 μM, or genistein at a final concentration of 1, 10, or 50 μM for 1 h prior to the addition of the lysates. The degree of polymerization of the actin cytoskeleton in the treated cells was visualized by using phalloidin conjugated to rhodamine 16 h after the addition of the toxin. The cells were washed in PBS, fixed in 3.7% paraformaldehyde in PBS, and permeabilized in 0.5% Triton X-100–4% polyethylene glycol–1 mM EGTA in 100 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 6.9]. The cells were blocked in 1% bovine serum albumin–100 mM glycine and stained with phalloidin-rhodamine (Sigma-Aldrich) at 0.5 μg/ml in PBS for 30 min. The cells were washed three times in PBS and mounted in Vectashield antifading reagent (Vector Laboratories, Burlingame, Calif.), and fluorescence was visualized by using an Olympus BH2 fluorescence microscope.
Expression vectors.
The induction of COX-2 gene expression was analyzed by using the reporter plasmid pTIS-10s, which carries the luciferase cDNA under the control of the murine COX-2 promoter (52). The plasmids pEF-lacZ and pEF-C3 were a gift from R. Treisman (Imperial Cancer Research Fund, London, United Kingdom).
Agonists and inhibitors.
Forskolin, cytochalasin D, and genistein were from Sigma-Aldrich. HA1077 was from Calbiochem-Novabiochem Corp., Nottingham, United Kingdom. Y27632 was a gift from Welfide Corporation, Osaka, Japan.
RESULTS
COX-2 induction by CNF.
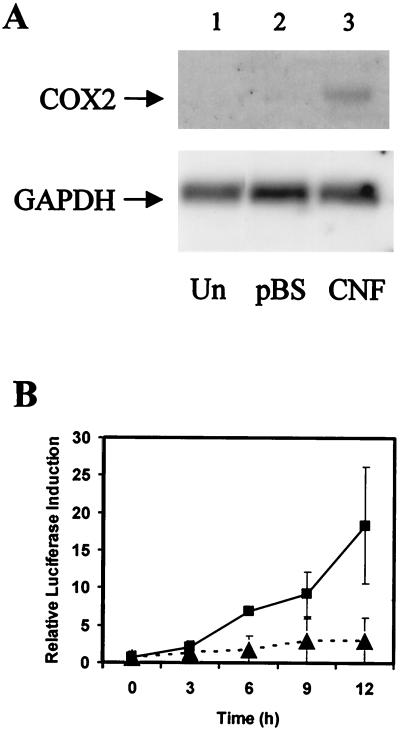
Previous studies have demonstrated that the induction of COX-2 gene transcription by agonists that activate G α13 is mediated by a RhoA-dependent signaling pathway (52). In addition, work from our laboratories has shown that CNF activates RhoA, resulting in cytoskeletal remodeling and focal adhesion formation (25). We tested whether the treatment of quiescent NIH 3T3 cells with CNF would lead to elevated levels of COX-2 expression. Northern blot analysis showed that transcription of the COX-2 gene was induced to a detectable level at 8 h after toxin treatment (Fig. 1A). We further analyzed the induction of COX-2 by CNF by using a luciferase-based reporter construct transfected into NIH 3T3 cells prior to toxin treatment (52). We found that a lysate from recombinant E. coli expressing CNF did induce the expression of COX-2, with the level of expression increasing sharply over 12 h after toxin treatment (Fig. 1B) and continuing to increase for at least 24 h (data not shown). The level of induction achieved was typically equivalent to 10 or 20 times that observed for cells not treated with toxin, and a 30-fold induction above background levels of transcription was also occasionally observed. A high concentration of a control E. coli lysate without CNF also slightly stimulated COX-2 expression, but this only reached one-tenth the level of induction observed for CNF at 12 h (Fig. 1B). The slight induction observed for the control was possibly due to nonspecific interactions between receptors in the fibroblast cell membranes and lipids present in the bacterial lysates.
FIG. 1.
CNF induces COX-2 gene transcription. (A) Northern blot of RNA extracted from NIH 3T3 cells either treated with lysates from E. coli expressing CNF (CNF) or harboring pBluescript (pBS) for 8 h or left untreated (Un). The blot was sequentially hybridized with specific [32P]dATP-labeled cDNA probes to demonstrate COX-2 and GAPDH gene expression. (B) Cells transfected with 1 μg of a COX-2 promoter-luciferase reporter construct were serum starved overnight and then treated with lysates from E. coli expressing CNF (▪) or harboring pBluescript (▴) at a final concentration of 1 μg/ml in serum-free DMEM. Samples were collected at 3-h intervals after the lysates were added and then assayed for luciferase production.
Transcriptional activation of COX-2 by CNF is RhoA dependent.
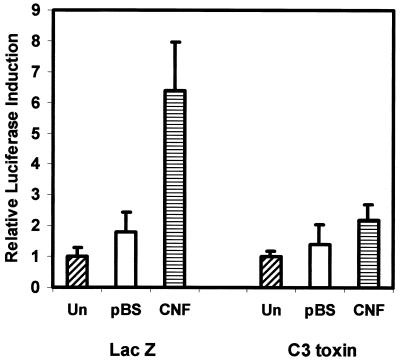
To determine which of the Rho family proteins activated by CNF is involved in the induction of COX-2 expression, we cotransfected cells with the COX-2 luciferase reporter construct and with a plasmid expressing Clostridium botulinum C3 toxin. C3 toxin ADP-ribosylates and inactivates RhoA, -B, and -C proteins, thus blocking the signaling pathways that are downstream of these proteins but not affecting the other small GTPases that can be activated by CNF. We found that C3 toxin effectively blocked the CNF induction of COX-2 (Fig. 2), and we conclude that the toxin induces COX-2 transcription through its action on Rho and not by its constitutive activation of Rac1 or cdc42.
FIG. 2.
The induction of COX-2 transcription by CNF is Rho dependent. NIH 3T3 cells were cotransfected with 1 μg of a COX-2 promoter-luciferase reporter construct and 0.1 μg of a pCDNA3 construct expressing either LacZ or C. botulinum C3 toxin. The cells were serum starved overnight and then treated with lysates from E. coli expressing CNF (CNF) or harboring pBluescript (pBS) at a final concentration of 1 μg/ml in serum-free DMEM or left untreated (Un). The cells were harvested 8 h after treatment and assayed for luciferase activity.
Inhibition of COX-2 induction by cytochalasin D.
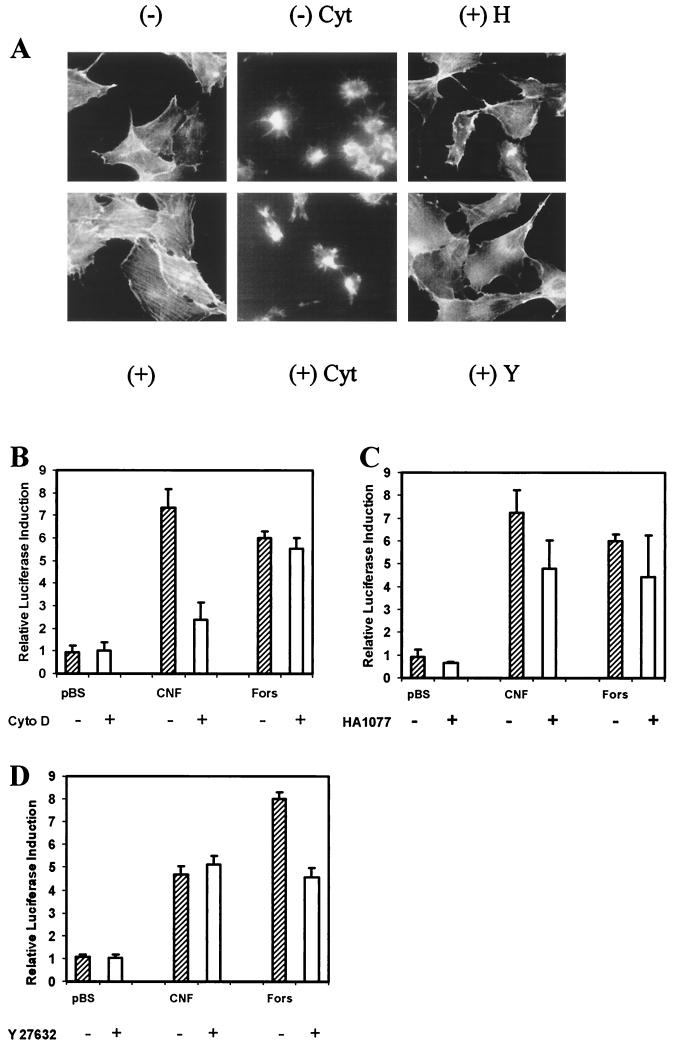
The formation of actin stress fibers and the assembly of the focal adhesion complex are the most obvious effects of Rho activation in quiescent fibroblasts. The induction of COX-2 expression by constitutively active Gα13 is not affected by cytochalasin D, an inhibitor that disrupts the actin cytoskeleton (52). Cytochalasin D is known to inhibit the actin stress fiber formation and tyrosine phosphorylation of FAK and paxillin induced by CNF treatment of Swiss 3T3 fibroblasts (25). To examine whether the polymerization of the actin cytoskeleton was a prerequisite for the induction of COX-2 transcription in NIH 3T3 fibroblasts by CNF, we treated the cells with cytochalasin D before adding the toxin. Under the experimental conditions used here there was considerable disruption of the actin cytoskeleton and, consequently, cell morphology by cytochalasin D in cells not treated with CNF. Furthermore, stress fibers did not develop in cells after toxin treatment (Fig. 3A). Even though cytochalasin D effectively disrupted the actin cytoskeleton, there was no effect on the levels of COX-2 transcription induced by forskolin. Forskolin is an agonist that induces COX-2 expression via a cyclic AMP-dependent pathway, which is independent of Rho and the pathway stimulated by CNF. The induction of COX-2 transcription by CNF was, however, attenuated by pretreatment with cytochalasin D (Fig. 3B). This may be due to the involvement of actin polymerization in the signal transduction process or to the need for cytoskeletal reorganization in order to permit toxin entry into the cells or trafficking after internalization. The entry of ricin toxin into differentiated HT-29 cells requires actin polymerization that can be blocked by cytochalasin D (8), while the entry of pertussis toxin into Chinese hamster ovary cells appears to rely upon cytochalasin D-independent receptor-mediated endocytosis (62).
FIG. 3.
The induction of COX-2 transcription by CNF is independent of p160/ROCK. (A) Rhodamine-phalloidin actin staining of NIH 3T3 cells pretreated with cytochalasin D (Cyt) at 2 μM or with the p160/ROCK inhibitors HA1077 (H) or Y27632 (Y) at 10 μM for 1 h prior to treatment with CNF (+) or left untreated (−). (B) NIH 3T3 cells were pretreated with 2 μM cytochalasin D (Cyto D) for 1 h (+) or left untreated (−). Lysates from E. coli expressing CNF or harboring pBluescript (pBS) at a final concentration of 1 μg/ml, or forskolin (Fors) at a final concentration of 10 μM in serum-free DMEM was then added. The cells were harvested 8 h after treatment and assayed for luciferase activity. This experiment was similarly performed for the p160/ROCK inhibitors HA1077 (C) and Y27632 (D), each at a final concentration of 10 μM.
The induction of COX-2 transcription by CNF is independent of p160/ROCK.
Activated RhoA in its GTP-bound form interacts with several protein effectors, including a number of protein kinases that are responsible for initiating different signaling pathways downstream of Rho. The activation of p160/ROCK by Rho leads to the formation of actin stress fibers through the phosphorylation of myosin light chain (3). Rho covalently modified by DNT, a toxin with a similar activity to CNF, interacts with p160/ROCK in a similar way to Rho that has been activated by conventional signaling processes (34). Inhibitors of p160/ROCK block the phosphorylation of myosin light chain phosphatase and LIM kinase, which are required for the RhoA-mediated formation of actin stress fibers (33, 39). The treatment of quiescent NIH 3T3 cells with two structurally unrelated inhibitors of p160/ROCK (HA1077 and Y27632) blocked the induction of stress fiber formation by CNF in those cells (Fig. 3A). However, when we examined the effects of these inhibitors on COX-2 expression, neither inhibitor blocked the induction of COX-2 transcription by CNF or forskolin (Fig. 3C and D). Thus, the Rho-dependent transcriptional activation of COX-2 by CNF does not depend upon activation of p160/ROCK and probably depends upon another protein kinase that can be activated by GTP bound or constitutively active Rho. Since p160/ROCK is responsible for stress fiber formation but is not needed for COX-2 induction by CNF, this would suggest that actin rearrangement blocked by cytochalasin D is not required in the actual signaling process. The inhibition of COX-2 induction by cytochalasin D, therefore, probably results from the inhibition of physical processes that depend upon the cytoskeleton. Endocytosis is required for toxin entry into the cell; proteolytic processing of the toxin may require endosome-lysosome fusion, or an actin-dependent translocation process may be required to bring the toxin into contact with its substrate at the cell membrane.
The induction of COX-2 transcription by CNF is affected by tyrosine phosphorylation.
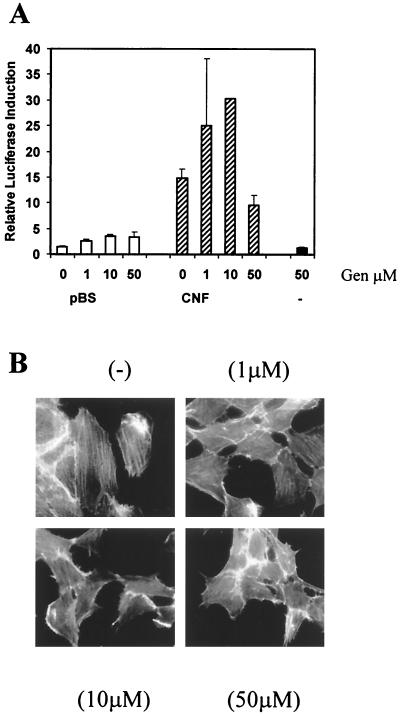
To investigate whether tyrosine kinase activity was needed for the induction of COX-2 expression by CNF, cells transfected with the COX-2 reporter construct were incubated with genistein for 1 h at different concentrations prior to toxin treatment. Genistein is a nonspecific inhibitor of tyrosine kinase activity. There was a slight reduction in COX-2 transcription at a genistein concentration of 50 μM (Fig. 4A), and at this concentration there was also inhibition of actin stress fiber formation (Fig. 4B). At lower concentrations that did not completely block stress fiber formation, there was an enhancement of the levels of COX-2 induction by CNF. Since genistein alone did not induce COX-2 expression, we conclude that a tyrosine phosphorylation event was involved in a negative feedback control pathway to downregulate the COX-2 expression stimulated by CNF.
FIG. 4.
The induction of COX-2 transcription by CNF is not blocked by inhibition of tyrosine phosphorylation. (A) NIH 3T3 cells were pretreated with genistein (Gen) at final concentrations of 1, 10, or 50 μM for 1 h prior to the addition of lysates from E. coli expressing CNF (CNF) or harboring pBluescript (pBS) at a final concentration of 1 μg/ml or in serum-free DMEM alone (−). The cells were harvested 8 h after treatment and assayed for luciferase activity. (B) NIH 3T3 cells were treated with a lysate from E. coli expressing CNF diluted in DMEM to a final concentration of 1 μg/ml for 16 h; the cellular actin was then stained with rhodamine-phalloidin (−). Cells were also treated with genistein at 1, 10, and 50 μM for 1 h prior to the addition of the CNF lysate.
DISCUSSION
The Rho proteins are targets for several bacterial toxins. Such toxins have proved to be extremely useful tools with which to analyze signaling pathways that are regulated by the small GTPases (1, 7, 21). It was previously shown that CNF treatment of Swiss 3T3 cells activated Rho family proteins, which induced the tyrosine phosphorylation of FAK and paxillin (25). As a result, focal adhesions are formed on the cell surface and cytoskeletal actin becomes reorganized into stress fibers. We have recently shown that the stimulation of actin stress fiber formation in Swiss 3T3 fibroblasts treated with CNF is via members of the p160/ROCK kinase family and that the autophosphorylation of FAK in such cells leads to stable FAK-src association (54). It is known that in its active, GTP-bound form or after covalent modification by CNF, Rho binds to and activates p160/ROCK (20, 34). This serine-threonine kinase is also involved in the regulation of serum response factor and NF-κB-dependent mRNA transcription, suggesting that CNF is likely to affect gene expression in toxin-sensitive cells. Here we demonstrate for the first time that CNF treatment of NIH 3T3 cells leads to activation of the COX-2 gene and that this activation is mediated through a Rho-dependent pathway.
We found that COX-2 induction by CNF was suppressed by C. botulinum C3 toxin mediated inactivation of RhoA, but not by either Y27632 or HA1077, which are chemical inhibitors of p160/ROCK. It has previously been shown that the induction of COX-2 by constitutively active Gα13 is also mediated by RhoA and is inhibited by C3 toxin but is independent of stress fiber formation (52). CNF also activates JunK in HeLa cells through its modification of the Rho proteins, cdc42 and Rac1 (28). The transcriptional activation of COX-2 by v-src can occur through a c-JunK-dependent pathway (59). However, such a pathway does not require Rho and so would not be sensitive to C3 toxin.
CNF-1 is most often expressed by uropathogenic E. coli (UPEC) strains that cause cystitis, pyelonephritis, and particularly prostatitis (4, 36). The murine model for UPEC infection has demonstrated that significant numbers of bacteria can be detected in the bladder 6 weeks after challenge and that the UPEC multiplies inside cells of the bladder epithelium (38). The role of CNF in the disease process is unclear, although mutation of the toxin gene resulted in less-extensive inflammation and greater susceptibility to neutrophil killing compared to the wild-type strain (45). CNF suppresses apoptosis in HEp-2 cells via a Rho- and actin-dependent pathway (14) but induces apoptosis in other epithelial cell lines that do not form stress fibers in response to the toxin (35). The ability of UPEC to regulate apoptosis may be important in allowing intracellular replication during chronic infection.
COX-2 expression induces a spectrum of different physiological effects that are cell type dependent and that might play a role in CNF-induced pathology. In cells of the immune system, COX-2 expression has been shown to upregulate interleukin-10 (IL-10) expression, while suppressing expression of IL-12 and so downregulating cell-mediated immunity (19). Thus, it is possible that CNF exerts a pathogenic effect by interfering with immune function. Many other bacterial toxins such as Staphylococcus aureus toxic shock syndrome toxin, Actinobacillus actinomycetemcomitans leukotoxin, and E. coli alpha-hemolysin also affect immune function (6, 18, 26). In addition, the suppression of apoptosis by CNF may be the result of COX-2 overexpression, and this in turn may allow the establishment of a chronic infection.
CNF induces DNA synthesis via a mitogen-activated protein kinase-independent pathway in cells grown in culture (25), although cytokinesis is subsequently blocked, and after a few days the cells become large and multinucleated. Whether CNF affects the regulation of cell cycle progression in vivo is currently unknown. Indeed, the effect of the toxin on different cell types may vary according to the pattern of expression of the Rho proteins and also the downstream effectors that are in turn activated by Rho, Rac, or cdc42, etc. The effect of CNF on the cell cycle may be due at least in part to COX-2, which is known to influence cell cycle progression. Induction of COX-2 by epidermal growth factor in polarized colon cancer cells stimulates prostaglandin release and mitogenesis, while overexpression in ECV-304 cells induces a prostaglandin-independent cell cycle arrest. In ECV-304, HEK 293, and COS7 cells, overexpression of COX-2 results in abnormal nuclear division, with more than 10% of the cells becoming multinucleated (55).
Transgenic mice overexpressing COX-2 under the control of the murine mammary tumor virus promoter displayed an enhanced tendency to develop mammary tumors (30). This tumorigenesis correlated with a suppression of apoptosis in the mammary glands of the transgenic animals. The expression of COX-2 in prostate cancer cells correlates with angiogenesis and tumor growth (31), and the treatment of such cells with inhibitors of COX-2 resulted in apoptosis (23). Persistent schistosomiasis and the resultant chronic inflammation are major predisposing factors to the development of squamous cell carcinoma of the bladder (37). It has also been shown that overexpression of COX-2 is associated with schistosomal-bilharzial bladder cancer (2). The localized overexpression of COX-2 at the site of a persistent infection has been suggested as the means by which Helicobacter pylori contributes to the development of gastric cancers. It has been demonstrated that H. pylori upregulates COX-2 transcription in murine, gastric mucosa cells, with a resultant increase in prostaglandin E2 synthesis (47). It has recently been shown that COX-2 is overexpressed in 38% of advanced bladder epithelial carcinomas (24) and that expression correlates with localized invasion by the tumor cells (51). The release of CNF by E. coli in the urogenital tract could potentially lead to accelerated development of urothelial carcinomas and deserves further investigation.
ACKNOWLEDGMENTS
This work was supported by Biotechnology and Biological Sciences Research Council grant 18/ICR07622 (to A.J.L.), by a travel award from Boehringer Ingelheim Fonds (to W.T.), and by National Institutes of Health grant DK 59630 (to E.R.).
REFERENCES
- 1.Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial toxins. Biol Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- 2.Alhasso A, Madaan S, Abel P D, El Baz M, Stamp G W H, Lalani E N. Over-expression of cyclooxygenase-2 in schistosomiasis-associated bladder cancer. J Pathol. 2000;192(Suppl.):8A. [Google Scholar]
- 3.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 4.Andreu A, Stapleton A E, Fennell C, Lockman H A, Xercavins M, Fernandez F, Stamm W E. Urovirulence determinants in Escherichia coli strains causing prostatitis. J Infect Dis. 1997;176:464–469. doi: 10.1086/514065. [DOI] [PubMed] [Google Scholar]
- 5.Caprioli A, Falbo V, Roda L G, Ruggeri F M, Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chance T D. Toxic shock syndrome: role of the environment, the host and the microorganism. Br J Biomed Sci. 1996;53:284–289. [PubMed] [Google Scholar]
- 7.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein RhoC is ADP ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chazaud B, Muriel M P, Bauvy C, Codogno P, Aubery M, Decastel M. Requirement for either the NH4Cl-sensitive or the cytochalasin D-sensitive pathway for ricin toxicity depends upon the endocytic state of differentiation of HT-29 cells. Eur J Cell Biol. 1994;64:15–28. [PubMed] [Google Scholar]
- 9.De Rycke J, Phan-Thanh L, Bernard S. Immunochemical identification and biological characterization of cytotoxic necrotizing factor from Escherichia coli. J Clin Microbiol. 1989;27:983–988. doi: 10.1128/jcm.27.5.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois R N, Abramson S B, Crofford L, Gupta R A, Simon L S, van De Putte L B A, Lipsky P E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 11.Eberhart C E, DuBois R N. Eicosinoids and the gastrointestinal tract. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 12.Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentini C, Matarrese P, Straface E, Falzano L, Donelli G, Boquet P, Malorni W. Rho-dependent cell spreading activated by E. coli cytotoxic necrotizing factor 1 hinders apoptosis in epithelial cells. Cell Death Differ. 1998;5:921–929. doi: 10.1038/sj.cdd.4400422. [DOI] [PubMed] [Google Scholar]
- 15.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 16.Fujisawa K, Madaule P, Ishizaki T, Wantanabe G, Bito H, Saito Y, Hall A, Narumiya S. Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J Biol Chem. 1998;273:18943–18949. doi: 10.1074/jbc.273.30.18943. [DOI] [PubMed] [Google Scholar]
- 17.Grossman E M, Longo W E, Panesar N, Mazuski J E, Kaminski D L. The role of cyclooxygenase enzymes in the growth of human gall bladder cancer cells. Carcinogenesis. 2000;21:1403–1409. [PubMed] [Google Scholar]
- 18.Hendricks A, Leibold W, Kaever V, Schuberth H J. Prostaglandin E2 is variably induced by bacterial super antigens in bovine mononuclear cells and has a regulatory role for the T cell proliferative response. Immunobiology. 2000;201:493–505. doi: 10.1016/S0171-2985(00)80069-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Stolina M, Sharma S, Mao J T, Zhu L, Miller P W, Wollman J, Herschman H, Dubinett S M. Nonsmall cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 20.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160-kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 21.Just I, Fritz G, Aktories K, Giry M, Popoff M R, Boquet P, Hegenbarth S, von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 22.Kage K, Fujita N, Ohhara T, Ogata E, Fujita T, Tsuruo T. Basic fibroblast growth factor induces cyclooxygenase-2 expression in endothelial cells derived from bone. Biochem Biophys Res Commun. 1999;254:259–263. doi: 10.1006/bbrc.1998.9875. [DOI] [PubMed] [Google Scholar]
- 23.Kamijo T, Sato T, Nagatomi Y, Kitamura T. Induction of apoptosis by cyclooxygenase-2 inhibitors in prostate cancer cell lines. Int J Urol. 2001;8:S35–S39. doi: 10.1046/j.1442-2042.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 24.Komhoff M, Guan Y, Shappell H W, Davis L, Jack G, Shyr Y, Koch M O, Shappell S B, Breyer M D. Enhanced expression of cyclooxygenase-2 in high-grade human transitional cell bladder carcinomas. Am J Pathol. 2000;157:29–35. doi: 10.1016/S0002-9440(10)64513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacerda H M, Pullinger G D, Lax A J, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21rho-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J Biol Chem. 1997;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- 26.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Ribinson M K, Billings P C. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 27.Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol Microbiol. 1997;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- 28.Lerm M, Selzer J, Hoffmeyer A, Rapp U R, Aktories K, Schmidt G. Deamidation of cdc42 and rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect Immun. 1999;67:496–503. doi: 10.1128/iai.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy G N. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs and colon cancer. FASEB J. 1997;11:234–247. [PubMed] [Google Scholar]
- 30.Liu C H, Chang S H, Narko K, Trifan O C, Wu M T, Smith E, Haudenschild C, Lane T F, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 31.Liu X H, Kirschenbaum A, Yao S, Lee R, Holland J F, Levine A C. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 32.Liu X H, Yao S, Krischenbaum A, Levine A C. NS398, a selective cyclooxygenase-2 inhibitor induces apoptosis and downregulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 33.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 34.Masuda M, Betancourt L, Matsuzawa T, Kashimoto T, Takao T, Shimonishi Y, Horiguchi Y. Activation of rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 2000;19:521–530. doi: 10.1093/emboj/19.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills M, Meysick K C, O'Brien A D. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect Immun. 2000;68:5869–5880. doi: 10.1128/iai.68.10.5869-5880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsumori K, Terai A, Yamamoto S, Ishitoya S, Yoshida O. Virulence characteristics of Escherichia coli in acute bacterial prostatitis. J Infect Dis. 1999;180:1378–1381. doi: 10.1086/314976. [DOI] [PubMed] [Google Scholar]
- 37.Mostafa M H, Sheweita S A, O'Connor P J. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulvey M A, Schilling J D, Hultgren S J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- 40.O'Neil G P, Ford-Hutchinson A W. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissue. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 41.Oswald E, Sugai M, Labigne A, Wu H C, Fiorentini C, Boquet P, O'Brien A D. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP binding proteins rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for rho bearing homology to a serine/threonine kinase PKN and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- 43.Ridley A J, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 44.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 45.Rippere-Lampe K E, O'Brien A D, Conran R, Lockman H A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect Immun. 2001;69:3954–3964. doi: 10.1128/IAI.69.6.3954-3964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 47.Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva A M, Del Vecchio-Blanco C, Bruni C B, Zarrilli R. Helicobacter pylori upregulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–28563. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- 48.Sano H, Kawahito Y, Wilder R, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 49.Schmidt G, Selzer J, Lerm M, Aktories K. The rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 51.Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:188–193. doi: 10.1002/1097-0142(20010701)92:1<188::aid-cncr1308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Slice L W, Walsh J H, Rozengurt E. Gα13 stimulates Rho-dependent activation of the cyclooxygenase-2 promoter. J Biol Chem. 1999;274:27562–27566. doi: 10.1074/jbc.274.39.27562. [DOI] [PubMed] [Google Scholar]
- 53.Subbaramaiah K, Telang N, Ramonetti J T, Araki R, DeVito B, Weksler B B, Dannenberg A J. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 54.Thomas W, Pullinger G D, Lax A J, Rozengurt E. Escherichia coli cytotoxic necrotizing factor and Pasteurella multocida toxin induce FAK autophosphorylation and src association. Infect Immun. 2001;69:5931–5935. doi: 10.1128/IAI.69.9.5931-5935.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trifan O C, Smith R M, Thompson B D, Hla T. Overexpression of cyclooxygenase-2 induces cell cycle arrest. J Biol Chem. 1999;274:34141–34147. doi: 10.1074/jbc.274.48.34141. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z Q, Grigoriadis A E, Mohle-Steinlein U, Wagner E F. A novel target cell for c-fos-induced oncogenesis: development of chondrogenic tumors in embryonic stem cell chimeras. EMBO J. 1991;10:2437–2450. doi: 10.1002/j.1460-2075.1991.tb07783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wantanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuki A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets for GTPase rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 58.Williams C S, DuBois R N. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270:G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 59.Xie W, Herschman H R. v-src induces prostaglandin synthase-2 gene expression by activation of the c-Jun N terminal kinase and c-Jun transcription factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- 60.Xie W, Herschman H R. Transcriptional regulation of prostaglandin synthase-2 expression by platelet derived growth factor and serum. J Biol Chem. 1996;271:31742–31748. doi: 10.1074/jbc.271.49.31742. [DOI] [PubMed] [Google Scholar]
- 61.Xie W, Fletcher B S, Andersen R D, Herschman H R. V-src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol. 1994;14:6531–6539. doi: 10.1128/mcb.14.10.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y R, Barbieri J T. Pertussis toxin-catalysed ADP-ribosylation of Gi-2 and Gi-3 in CHO cells is modulated by inhibitors of intracellular trafficking. Infect Immun. 1996;64:593–599. doi: 10.1128/iai.64.2.593-599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshinobu H, Rao C V, Simi B A, Cooma L, Rigas B, Reddy B S. Proceedings of the American Association for Cancer Research 91st Annual Meeting. American Association for Cancer Research; 2000. Induction of inducible isoforms of cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in rat epithelial cells by a PKC-dependent mechanism; pp. 60–61. [Google Scholar]