Abstract
Differential hypoxaemia (DH) is common in patients supported by femoral veno-arterial extracorporeal membrane oxygenation (V-A ECMO) and can cause cerebral hypoxaemia. To date, no models have studied the direct impact of flow on cerebral damage. We investigated the impact of V-A ECMO flow on brain injury in an ovine model of DH. After inducing severe cardiorespiratory failure and providing ECMO support, we randomised six sheep into two groups: low flow (LF) in which ECMO was set at 2.5 L min−1 ensuring that the brain was entirely perfused by the native heart and lungs, and high flow (HF) in which ECMO was set at 4.5 L min−1 ensuring that the brain was at least partially perfused by ECMO. We used invasive (oxygenation tension—PbTO2, and cerebral microdialysis) and non-invasive (near infrared spectroscopy—NIRS) neuromonitoring, and euthanised animals after five hours for histological analysis. Cerebral oxygenation was significantly improved in the HF group as shown by higher PbTO2 levels (+ 215% vs − 58%, p = 0.043) and NIRS (67 ± 5% vs 49 ± 4%, p = 0.003). The HF group showed significantly less severe brain injury than the LF group in terms of neuronal shrinkage, congestion and perivascular oedema (p < 0.0001). Cerebral microdialysis values in the LF group all reached the pathological thresholds, even though no statistical difference was found between the two groups. Differential hypoxaemia can lead to cerebral damage after only a few hours and mandates a thorough neuromonitoring of patients. An increase in ECMO flow was an effective strategy to reduce such damages.
Subject terms: Circulation, Physiology, Cardiology
Introduction
Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) is an increasingly used rescue therapy for patients with refractory cardiogenic shock (CS)1,2. It provides mechanical circulatory support for both heart and lungs by generating blood flow, oxygenating, and removing carbon dioxide. Although a life-saving therapy, V-A ECMO exposes patients to several complications, in particular bleeding, infections, and cerebrovascular accidents3. Peripheral femoral cannulation (the most used configuration) also brings a physiological paradigm with a retrograde flow along the abdominal and thoracic aorta. This potentially causes two specific complications: increase in left ventricle (LV) afterload4—which can cause LV dilation, secondary cardiac damage and pulmonary oedema—and differential hypoxaemia (DH).
DH occurs when the upper body, perfused with deoxygenated blood by the native heart and lungs, has a lower oxygen saturation than the lower body perfused with oxygenated blood from the ECMO circuit5. This happens in the case of concomitant pulmonary failure, which commonly ensues in the context of pulmonary oedema and/or pneumoniae. DH causes coronary and cerebral hypoxaemia and is a complication frequently encountered by ECMO clinicians. The effects of such DH on cerebral structures and integrity have been, however, poorly investigated.
We hypothesised that increasing V-A ECMO flow would lead to an optimized cerebral oxygen delivery and reduce brain injury risks assessed by biological, physiological and histological markers.
Thus, in a novel experimental model of severe cardiopulmonary failure supported by V-A ECMO, we aimed to study the impact of the ECMO flow on brain injury.
Methods
Our methods are reported according to the ARRIVE guidelines6. Six Merino crossbred non-pregnant, female sheep, between the age of 1 and 3 years, weighing between 45 and 60 kg, were used in this study. Animals were sourced from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and housed at the Queensland University of Technology (QUT) Medical Engineering Research Facility, with ad libitum access to shade, food and water. The animals were fasted overnight prior to the experiment. The study was approved by the QUT Office of Research Ethics and Integrity (QUT: 1,800,000,337). All experiments were performed in accordance with NHMRC Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and the Animal Care 8th Edition 2013 and Protection Act 2001 (QLD).
Instrumentation, cardio-pulmonary failure and ECMO canulation
The experimental set-up used in this study has been published previously7 and details are available in Supplementary Materials. In summary, animals underwent general anaesthesia using propofol for induction and were placed on mechanical ventilation with the following settings: tidal volume (VT) of 8 mL kg−1, positive end-expiratory pressure (PEEP) of 5 cmH2O, respiratory rate (RR) and inspiratory fraction of oxygen (FiO2) adjusted to maintain an arterial partial pressure of oxygen (PaO2) between 60 and 100 mmHg, and an arterial partial pressure of carbon dioxide (PaCO2) between 35 and 45 mmHg. Anaesthesia was maintained using and midazolam and sufentanil. Cardiogenic shock was induced with multiple injections of 96% ethanol into the subepicardial layer of the left ventricle in order to obtain the following parameters (corresponding to cardiogenic shock definition according to the latest guidelines1): LV ejection fraction (EF) < 30%, systolic arterial pressure (SAP) < 90 mmHg for at least 10 min, arterial blood lactate > 4 mmol L−1 and/or urine output < 0.5 mL kg−1 h−1. Acute respiratory failure (ARF) was induced by reducing VT to 4 mL kg−1, PEEP to 0 cmH2O, RR to 5 per min and FiO2 to 21% to achieve a PaO2 below 60 mmHg and/or an arterial saturation of oxygen (SaO2) below 60%.
A detailed description of the ECMO equipment and set-up has been previously reported7 and is detailed in Supplementary Materials. Animals were cannulated using a 19-Fr right jugular venous access and a 15-Fr femoral arterial return cannula, the positions of which were assessed using fluoroscopy to obtain the tip of the access cannula in the inferior vena cava and the return cannula in the abdominal aorta below the renal arteries. Thereafter and until randomization, ECMO was started and set as follows: pump speed adjusted to achieve a flow rate of 1 L min−1 (i.e. the “ECMO flow” which corresponds to the blood flow at the end of the arterial tip), and fresh gas flow set at 2 L min−1 (i.e. the amount of gas necessary to adjust PaCO2). Blender was set to deliver 100% oxygen throughout the experiment.
Randomized interventions
After instrumentation, sheep were randomly allocated to one of the following groups (n = 3 per group):
Low flow (LF): ECMO was set at 2.5 L min−1 to ensure that the brain was entirely perfused by the native heart and lungs, while V-A ECMO flow only reached the abdominal aorta close to the diaphragm8;
High flow (HF): ECMO was set up at 4.5 L min−1 to ensure that the brain was partially perfused by the ECMO.
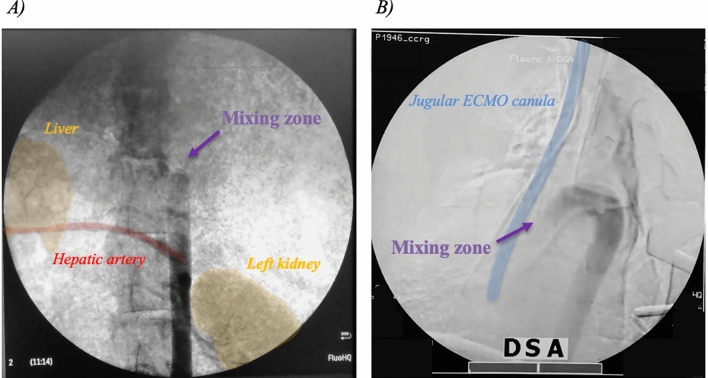
The position of the mixing zone along the aorta, where LV and ECMO flows blend, was confirmed with fluoroscopy by infusing 40–60 mL of contrast medium (Ultravist® 370, iopromide, Bayer, USA) in the systemic circulation, via the lateral port of the arterial return cannula (Fig. 1, supplementary videos online). All other parameters were kept constant during the experiment.
Figure 1.
X-ray images of the mixing zone. Legend: The mixing zone corresponds to the zone where the LV and the ECMO meet. (A) Low flow: the mixing zone is in the abdominal aorta above the hepatic artery. (B) High flow: the mixing zone is in the ascending aorta (the whole aortic arch is opacified). ECMO = extracorporeal membrane oxygenation; LV = left ventricle.
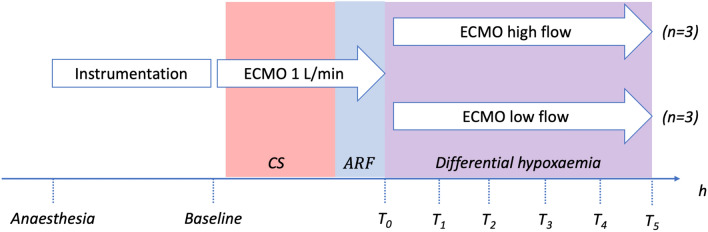
Baseline was defined as the first assessment after one hour from completion of sheep instrumentation, and T0 the time upon randomisation when differential hypoxaemia (i.e., CS + ARF) criteria were met. After randomisation, data was collected hourly until completion of the experiment after five hours. (Fig. 2).
Figure 2.
Study design and protocol. Legend: Instrumentation corresponds to anesthesia, establishing invasive monitoring and ECMO cannulation which took approximately 3 to 4 h. CS corresponds to the induction of cardiogenic shock by repeated injection of 96% ethanol in the LV. ARF corresponds to the induction of hypoxemia. Therefore, T0 is the time where differential hypoxemia (CS + ARF) criteria were met. T1 to T5 correspond to the study period, during which each group received either a low flow (2.5 L min−1) where brain is perfused entirely by the native heart and lungs, or a high flow (4.5 L min−1) where brain is partially perfused by the ECMO. ARF = acute respiratory failure, CS = cardiogenic shock, ECMO = extracorporeal membrane oxygenation, LV = left ventricle.
Cerebral assessments
We appraised effects of intervention on the brain using four distinct methods: haemodynamics, oxygenation, metabolism, and histology. For this purpose, during the instrumentation phase, after exposing the skull we drilled two burr holes on the right side of the skull, 5 mm lateral to the sagittal suture, from either side of the lambdoid suture.
Brain haemodynamics. We continuously monitored cerebral perfusion pressure (CPP) (defined as mean arterial pressure (MAP) minus intracranial pressure (ICP)), and regional flow using laser-doppler velocity (OxyFlo, Oxford Optronics, Oxford, UK).
Brain oxygenation. We measured non-invasive regional saturation (rSO2) obtained via near-infrared spectroscopy (NIRS) sensors placed on the frontal lobe (ForeSight Elite, Edwards Lifesciences, Nyon, Switzerland). Invasive oxygen saturation was monitored using oxygen tension (PbTO2) (OxyLite, Oxford Optronics). A change of 25% in the baseline values of rSO2 was considered abnormal, and these values were compared with rSO2 measured in the lower limb (opposite from the ECMO canulation), which served as reference.
Brain metabolism. We performed microdialysis (CMA Microdialysis AB, Stockholm, Sweden) using a 20,000 Dalton cut-off perfused with CNS perfusion fluid (Microdialysis AB) at a rate of 0.3 μ L min−1. Micro-dialysates samples were collected hourly in capped microvials. Lactate, pyruvate, and glucose levels were monitored hourly using a CMA-600 microdialysis analyser (CMA Microdialysis AB). Based on the 2014 Consensus statement from International Microdialysis Forum9, we considered as abnormal the following values: glucose < 0.8 mmol L−1, lactate > 4 mmol L−1, pyruvate > 200 mmol L−1, lactate/pyruvate ratio (LPR) > 25. We kept systemic glucose and CO2 constant to ensure that variation in microdialysis parameters be related to cerebral flow and oxygenation. We considered the following metabolic patterns: normal (LPR ≤ 25 and lactate ≤ 4), ischemia (LPR > 25, lactate > 4, pyruvate ≤ 200), mitochondrial dysfunction (LPR > 25, lactate ≤ 4, pyruvate > 200).
Brain histology. Animals were euthanized at the end of the experiment using 150 mg/kg of phenobarbitone (Lethabarb®), and their brains were immediately harvested to confirm appropriate position of intra-cerebral catheters and histological analysis. Brain tissue was fixed in 10% buffered formalin for at least 48 h, processed and embedded in paraffin. Sections (5 µm) were stained with haematoxylin and eosin and examined by light microscopy by a specialist veterinary pathologist (CP) blinded to the group allocation. Each sample consisted of eight sections (four from the frontal lobe and four from the parietal lobe) and ten fields per section were analysed. Seven parameters were then evaluated to score brain injury: neuronal shrinkage and hyperacidophilia, spongy state, congestion, perivascular oedema, perivascular haemorrhages, neutrophilic inflammation degree, and fibrin thrombi. A semi-quantitative analysis was performed with values being 0 (no lesion observed), 1 (< 5 lesions per field) or 2 (≥ 5 lesions per field) for each parameter (Supplementary Table 1).
Kidney assessments
We inserted a microdialysis probe, invasive oxygen tension (PkO2) and flow probes (OxyLite/OxyFlo, Oxford Optronics) into the renal cortex through left mini-laparotomy. Kidney was monitored hourly and post-mortem histology was carried out. Nine parameters were then evaluated to score kidney injury: two in the glomeruli (fibrin and dilation of the Bowman’s space), four in the tubules (dilation, swelling, necrosis and hyaline casts presence within the lumen), and three in the interstitium (inflammation, congestion, and haemorrhage). A semi-quantitative analysis was performed with values being 0 (no lesion observed) to 2 (maximum lesions observed) for each parameter. (Supplementary Table 2).
Outcomes
-
Primary outcome
Primary outcome was cerebral damage evaluated through the global cerebral injury score.
Secondary outcomes
Secondary outcomes were:
Brain metabolism parameters (glucose, pyruvate, lactate and LPR);
Kidney histological assessment;
Kidney metabolism parameters (glucose, pyruvate, lactate and LPR).
Statistical analysis
For all tests, normality was tested with Kolmogorov–Smirnov test, and data was expressed as mean ± standard deviation. Data was expressed as median ± interquartile range when not distributed normally. Data was analysed using linear mixed effects model with a Geisser-Greenhouse correction, with time and group as fixed effects and sheep as random effect. Analysis was followed by multiple comparisons test with Tukey correction, both for differences between groups (high flow vs low flow) and along time (all time points vs T0). Histological analyses were considered as categorical variables and compared with a chi-squared test. All statistical analyses were two-sided, with a significance level set at 0.05, and were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA).
Results
All sheep survived procedures with no complications and completed follow-up. Systemic glucose, and carbon dioxide levels were kept constant throughout the experiment.
Cardiopulmonary failure characteristics
All sheep achieved pre-defined CS criteria with no statistical differences between groups or timepoints (Table 1). Hypoxaemia was successfully achieved in all sheep with a mean PaO2 at randomization of 48 ± 15 versus 42 ± 11 mmHg in the low flow and high flow group, respectively (p > 0.99).
Table 1.
Hemodynamic variables at different timepoints for each study group.
| Low flow (N = 3) | High flow (N = 3) | |
|---|---|---|
| Heart rate (beats min−1) | ||
| Baseline | 91 ± 7 | 110 ± 18 |
| T0 | 86 ± 17 | 85 ± 56 |
| T1 | 91 ± 21 | 68 ± 23 |
| T2 | 96 ± 22 | 80 ± 11 |
| T3 | 89 ± 17 | 78 ± 10 |
| T4 | 89 ± 1 | 96 ± 27 |
| T5 | 92 ± 8 | 92 ± 6 |
| SAP (mmHg) | ||
| Baseline | 65 ± 13 | 82 ± 7 |
| T0 | 69 ± 8 | 81 ± 39 |
| T1 | 69 ± 4 | 86 ± 20 |
| T2 | 72 ± 7 | 90 ± 13 |
| T3 | 70 ± 7 | 89 ± 11 |
| T4 | 73 ± 4 | 93 ± 15 |
| T5 | 77 ± 6 | 94 ± 17 |
| MAP (mmHg) | ||
| Baseline | 58 ± 1 | 69 ± 7 |
| T0 | 62 ± 3 | 70 ± 45 |
| T1 | 66 ± 6 | 81 ± 14 |
| T2 | 66 ± 6 | 85 ± 11 |
| T3 | 63 ± 2 | 81 ± 11 |
| T4 | 70 ± 8 | 81 ± 6 |
| T5 | 72 ± 2 | 84 ± 11 |
| Pulse pressure (mmHg) | ||
| Baseline | 17 ± 6 | 19 ± 1 |
| T0 | 14 ± 9 | 16 ± 9 |
| T1 | 7 ± 4 | 8 ± 8 |
| T2 | 12 ± 7 | 8 ± 6 |
| T3 | 11 ± 8 | 11 ± 1 |
| T4 | 12 ± 6 | 18 ± 17 |
| T5 | 11 ± 10 | 15 ± 12 |
| Lactate (mmol L−1) | ||
| Baseline | 1.5 ± 0.7 | 1.6 ± 0.4 |
| T0 | 6.0 ± 0.8 | 5.7 ± 1.6 |
| T1 | 7.3 ± 1.0 | 8.0 ± 3.5 |
| T2 | 7.3 ± 1.1 | 9.0 ± 4.8 |
| T3 | 7.3 + 1.8 | 9.1 ± 5.9 |
| T4 | 7.7 ± 3.3 | 8.6 ± 7.3 |
| T5 | 8.0 ± 4.3 | 8.4 ± 8.3 |
| LVEF (%) | ||
| Baseline | 46 ± 18 | 47 ± 5 |
| T0 | 28 ± 7 | 16 ± 6 |
LVEF left ventricular ejection fraction; MAP mean arterial pressure; SAP systolic arterial pressure.
Low flow versus high flow characteristics
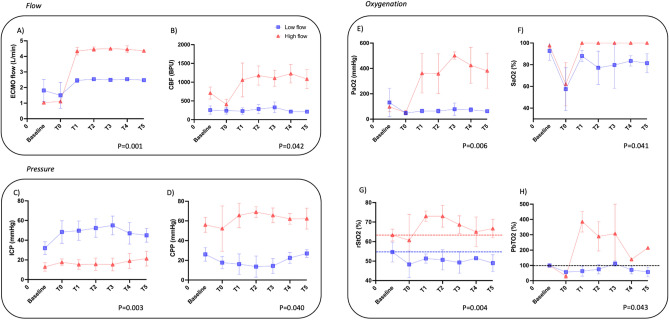
Figure 3 shows data obtained in terms of flow (V-A ECMO flow and cerebral brain flow), pressure (ICP and CPP) and oxygenation (PaO2, SaO2, rSO2 and PbTO2) in both groups during experimentation. V-A ECMO flows achieved predefined values (mean flow of 2.50 ± 0.06 and 4.42 ± 0.15 L min−1, respectively) which translated into significantly different cerebral blood flows of 205 ± 76 and 970 ± 400 BPU respectively (p = 0.042). Cerebral oxygenation parameters were also significantly higher in the high flow when compared to low flow group in terms of PaO2 (p = 0.006), SaO2 (p = 0.041), rSO2 (p = 0.004) and PbTO2 (p = 0.043). At baseline, animals had similar PbTO2 values in both the low (16.72 ± 19.7 mmHg) and the high flow (11.94 ± 5.7 mmHg) group (p = 0.96). SaO2 was 82.6 ± 12.6% in the low flow group throughout the experiment.
Figure 3.
Flow, pressure, and oxygenation parameters at different timepoints for each study group. Legend: (A) ECMO flow in L min−1; (B) local cerebral blood flow in blood per units (BPU) obtained by OXYFLOW; (C) ICP in mmHg; (D) CPP in mmHg; (E) PaO2 in mmHg; (F) SaO2 in %; (G) rStO2 in % obtained by NIRS; (H) PbTO2 variation from baseline (in %) obtained by OXYLITE. Arterial blood gases were drawn from the right radial artery. Red lines represent results from the high flow group and blue lines from the low flow group. Dotted lines for rStO2 and PbTO2 represent the initial (“baseline”) levels. Data are represented as mean ± SD. BPU = blood per units; CPP = cerebral perfusion pressure; ECMO = extracorporeal membrane oxygenation; ICP = intracranial pressure; NIRS = near-infrared spectroscopy; PaO2 = partial arterial pressure of oxygen; PbTO2 = partial brain oxygen tension; SaO2 = arterial saturation of oxygen; rStO2 = tissue oxygen saturation.
Primary outcome
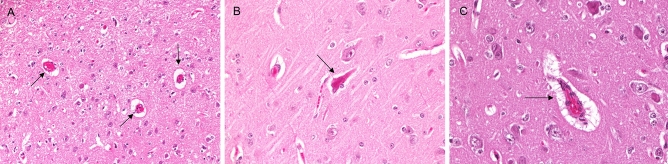
Cerebral global histological injury score was significantly higher in the low flow group compared to the high flow group (p = 0.0003). Histology showed some degrees of brain damage in both groups (Fig. 4.), except for inflammation and thrombosis which were absent. Neuronal shrinkage, congestion, and perivascular oedema were significantly higher in the low flow group (p < 0.0001) (Fig. 5).
Figure 4.
Hematoxylin/eosin—stained sections of the brain. Legend: Examples of scores brain pathologies: (A) vascular congestion (arrow); (B) neuronal shrinkage (arrow); (C) congested blood vessel with perivascular edema (arrow).
Figure 5.
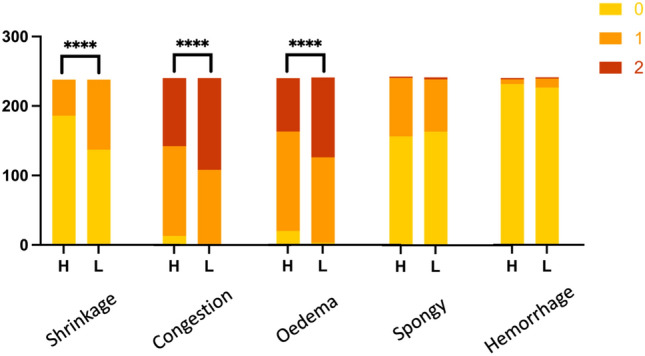
Histological scoring differences of the brain between low flow (L) and high flow (H) groups. Legend: Histograms represent the proportion of each score for all 240 samples (y-axis) analyzed. A minimum score of 0 (in yellow) corresponds to the absence of damage, whereas a maximum score of 2 (in red) corresponds to a high level of damage (> 5 lesions per field). Inflammation and thrombosis were not observed and are therefore not represented in this figure. **** p < 0.0001.
Secondary outcomes
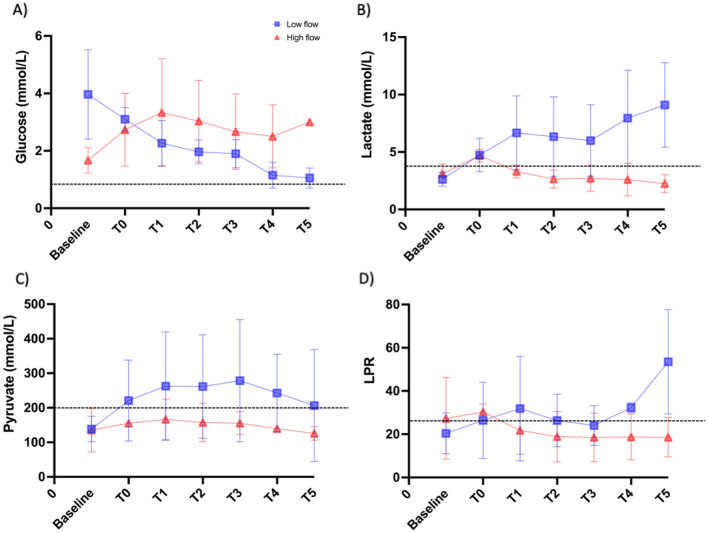
Brain metabolism parameters obtained from microdialysis are summarized in Fig. 6. Analysis found no significant difference between the two groups but values in the low flow group all reached predefined pathological threshold.
Figure 6.
cerebral microdialysis values at different timepoints for each study group. Legend: Concentrations of (A) glucose (mmol L−1), (B) lactate (mmol L−1), and (C) pyruvate (mmol L−1), and (D) lactate-pyruvate ratio (LPR). Red lines represent results from the “high flow” group and blue lines from the “low flow” group. Dotted lines represent pathological thresholds as defined by the 2014 Consensus statement from International Microdialysis Forum8.
Kidney histopathological examination reported some degree of injury in both groups, especially vascular congestion (74%) and dilation of the Bowman’s space (50%). Cloudy swelling of the tubular epithelium was significantly higher in the high flow group (p < 0.0001) and accumulation of granular material/hyaline casts within the tubular lumen were significantly higher in the low flow group (p < 0.001). Kidney microdialysis showed no statistical difference between groups for glucose (p = 0.61) nor LPR (p = 0.95). (Supplementary Fig. 1 and Fig. 2).
Discussion
Differential hypoxemia leads to significant complications in patients supported by VA-ECMO, with a decrease in cerebral oxygenation and therefore, damage to cerebral cells being one of the major challenges. Using a previously published cardio-pulmonary failure supported by VA-ECMO model, two clinically relevant ECMO flows were tested which translated into different degrees of differential hypoxaemia as shown by both invasive (PbTO2) and non-invasive (NIRS) cerebral oxygenation. Although limited by its sample size, this study demonstrated significantly less pronounced histological brain damage in the group receiving high flow support compared to the low flow group. This could be explained by relative conservation of the cerebral aerobic state as shown by the lower lactate levels and LPR in the cerebral microdialysis analysis.
Cerebral complications occurring while on V-A ECMO are classically described as acute vascular accidents (ischemic and/or haemorrhagic) or microbleeds10, reported between 13 and 20% in recent literature11,12. This is mostly due to the relative short time spent by patients on ECMO (few days to weeks). Nevertheless, a more chronic malperfusion and/or hypoxaemia could also participate in the cognitive decline seen in some ECMO survivors13. These complications are probably underestimated because of the lack of standardized reporting criteria and the broad variety of severity ranging from seizures to brain death14. In addition, it is not always possible to determine if those injuries were favoured by factors present before ECMO implantation, such as hypoxia. Nevertheless, these neurological complications are acknowledged to be responsible for a high morbidity and mortality.
Differential hypoxaemia corresponds to relative hypoxaemia in the upper body (perfused by the LV) compared to the lower body (perfused by the ECMO). Data regarding DH are lacking, which can be explained by several factors: (1) DH is a highly variable and dynamic condition, (2) multiple factors can contribute to these variations, such as the proportion between native LV and ECMO flows15,16, arterial cannula tip position5, and venous drainage position17, and (3) the carotids of most large animals (bovine, pigs, primates) depart from a common bi-carotid trunk (also known as bovine arch) making cerebral studies during VA ECMO less translatable to humans. As a result, it is unclear if DH creates additional complications by causing cerebral hypoxia18 or if a relative level of hypoxia is acceptable, as suggested by some authors19.
We investigated cerebral lesions using histology as our primary outcome. Of other possibilities to screen for cerebral damage, this choice was made for several reasons: magnetic resonance imaging is contra-indicated with ECMO, computerized tomography scan shows anomalies with a few hours delay, electroencephalography was not available in sheep. Thus, histology seemed the best choice to investigate early brain lesions. We used a multimodal cerebral monitoring comprising of oxygenation, pressure, and metabolism to explore potential mechanisms explaining these lesions.
Indeed, studying cerebral metabolism was essential as one could have argued that the increase in ECMO flow—shifting from a “low pulsatile hypoxemic flow” to a “high pulseless hyperoxemic flow”—could have induced additional damage similar to an ischemia–reperfusion-like syndrome with hyperoxia, as observed in resuscitated cardiac arrest, post-carotid stenosis revascularisation, traumatic brain injury or stroke20,21. In the latter situations, the negative effects of hyperoxaemia are thought to be generated via reactive oxygen species exacerbating the initial reperfusion injury via the recruitment of neutrophils, causing a disproportionate inflammatory response22. Effects of hyperoxaemia alone (without an initial brain injury) are currently debated as results are not equivocal23–25. A recent study found that hyperoxemia was independently associated with an increase in mortality in patients undergoing V-A ECMO for cardiogenic shock26.
We observed cerebral lesions after only a few hours spent in DH and a reduction of those when ECMO flow was increased. These results could be due to an increase in cerebral flow, oxygenation, or a combination of both. All our results are compatible with typical hypoxic-ischemic lesions: histological findings27,28, regional variation of NIRS, and microdialysis results9,29.
One last parameter was modified between low and high flow: pulsatility. Indeed, an increase in flow will increase LV afterload and mechanically decrease pulsatility, although we did not observe a significant change in pulse pressure (common definition of pulsatility). As no study has yet reported an impact of pulsatility alone on brain or circulating cells damage except for von Willebrand factor30,31, and given the fact that the arterial tip of the ECMO is almost facing the renal arteries, pulsatility alone is unlikely to explain changes in brain or kidney histology we observed.
We decided to use kidney as control for our experiment as they experienced ischemia–reperfusion injuries like the brain (from the induction to cardiogenic shock to the ECMO support) but without any hypoxaemia. Histology showed some degree of injury which are non-specific and could be related to several factors known to induced acute kidney injury such as ischemia–reperfusion, systemic inflammation or non-pulsatile flow32,33. Microdialysis found no difference between the two groups and in particular, we did not observe an increase in kidney damage with a higher flow. Overall, these results tend to confirm that brain lesions were triggered by differential hypoxaemia and not solely by ischemia–reperfusion.
This is the first experiment assessing the effect of the V-A ECMO flow consequences on the brain in a context of DH. We used a cardiogenic shock model developed specifically for this study7, allowing us to keep native LV output constant—where other CS models (especially acute myocardial infarction models) suffer from a decrease in LV cardiac output over time34. We confirmed arterial cannula tip position and mixing zone location for each animal using fluoroscopy which allowed our results to be consistent and robust. Our decision to use an ovine model was made to avoid bovine arch thus enhancing clinical translation of our results. Animals were randomised to avoid selection bias. We demonstrated that some degree of cerebral damage appeared already after 5 h of DH for upper body PaO2 levels that can be considered not “profound”, and that an increase in ECMO flow—a very common strategy used in case of DH—was effective in reducing these damages. Interestingly, even though systemic arterial lactate was relatively constant in both groups, brain lactate kept diminishing in the high flow group, suggesting a cerebral adaptation to DH in the high flow group. Nevertheless, DH complications could not be entirely reversed.
Apart from its small sample size, our study suffers several limitations. First, the follow-up period was relatively short (5 h). Nevertheless, we considered this to be clinically relevant as most patients would benefit from an intervention to reverse DH in such delays. Also, we were able to obtain significant results despite a short period of time, and temporal trends are in favour of the effect of treatment. Second, the effects of the position of the cannulas (i.e. superior vena cava or inferior vena cava for the drainage and a higher position for the return canula) were not investigated. Indeed, Hou et al.17 demonstrated that the position of the venous canula improved upper body oxygenation, yet this was not an objective in our study and venous cannulas were systematically in the inferior vena cava. Third, to better characterise brain damage, neurobiomarkers such as neuron specific enolase (NSE), glial fibrillary acidic protein (GFAP) or S-100B would have been beneficial. However, antibodies assays are not all available in sheep, have not been fully investigated, and their use/threshold have been poorly investigated in patients undergoing ECMO35–37. Fourth, we have not been able to study cerebral autoregulation, an important parameter to fully understand brain hemodynamic, as transcranial doppler is not feasible in sheep. Finally, our model investigated previously healthy, young, female animals which may be a limitation to generalisation of results.
Our conclusion—“protect the brain”—may be simple, but advocates for continuous neuromonitoring during V-A ECMO, which is not currently performed in clinical practices38. In the absence of better tools, we suggest using frontal NIRS to help clinicians guide their behaviour in case of suspected differential hypoxaemia. In case of differential hypoxaemia, increasing ECMO flow could be considered an option and hyperoxia should be avoided. Nevertheless, increasing the ECMO flow comes with a price of increasing LV afterload and should only be considered as a safety procedure while waiting for a sustainable intervention. Since we did not assess mortality or long-term outcome, it is not guaranteed that the benefits of improving cerebral oxygenation overcome the LV damage caused by afterload increase. Indeed, a recent meta-analysis found that LV unloading significantly decreased mortality39.
Conclusion
Among animals undergoing peripheral V-A ECMO support, differential hypoxaemia lead to cerebral damage after only a few hours, which could be partially corrected by increasing ECMO flow. Neuromonitoring of these patients is therefore of great importance. This strategy could help clinicians experiencing differential hypoxaemia although left ventricle afterload should be carefully monitored.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the staff at QUT-MERF for assistance with animal care. The authors would like to thank Ms Marie Lyager for her English proofreading.
Abbreviations
- ARF
Acute respiratory failure
- CPP
Cerebral perfusion pressure
- CS
Cardiogenic shock
- DH
Differential hypoxaemia
- ECMO
Extracorporeal membrane oxygenation
- EF
Ejection fraction
- FiO2
Inspired fraction of oxygen
- HF
High flow
- ICP
Intracranial pressure
- LF
Low flow
- LPR
Lactate-pyruvate ratio
- LV
Left ventricle
- MAP
Mean arterial pressure
- NIRS
Near-infrared spectroscopy
- PaCO2
Arterial partial pressure of carbon dioxide
- PaO2
Arterial partial pressure of oxygen
- PbTO2
Partial brain oxygen tension
- PEEP
Positive end-expiratory pressure
- RR
Respiratory rate
- rStO2
Regional saturation of oxygen
- SAP
Systolic arterial pressure
- V-A
Veno-arterial
- VT
Tidal volume
Author contributions
S.R., J.Y.S., G.L.B. and J.F.F. designed the study. S.R., S.H., K.W., J.S.J., S.M.C., K.S., C.A., X.W., G.A., K.H., N.S., S.A.L. and B.V. conducted the study and the sample collection. S.R., W.B.D., L.H., N.B., M.B., M.P., J.D.R., D.M. and E.S.W. performed analysis, reporting and interpretation of the data. C.P. conducted the histological analysis. S.R. drafted the manuscript. I.R., R.L., M.S,. J.Y.S., G.L.B. and J.F.F. revised it critically for important intellectual content before submission. All authors read and approved the final manuscript.
Funding
This study was funded by the National Health and Medical Research Council (Australia) Centre for Research Excellence in Advanced Cardio-respiratory Therapies Improving OrgaN Support (CRE ACTIONS) and the 2019 The Prince Charles Hospital Foundation (Australia) New Investigator Grant (Grant no NI2019-12). SH and SL received a PhD scholarship from The Prince Charles Hospital Foundation (Australia) for their PhD. JR, KS, and KW received a PhD scholarship from the University of Queensland (Australia) for their PhD. NB received a postgraduate fellowship from the Prince Charles Hospital Foundation (Australia). JYS received a research fellowship from the Advance Queensland Industry Research Fellowships program (Australia). Australian governments fund Australian Red Cross Lifeblood for the provision of blood, blood products and services to the Australian community.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sacha Rozencwajg, Email: sacha.rozencwajg@aphp.fr.
Gianluigi Li Bassi, Email: g.libassi@uq.edu.au.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30226-6.
References
- 1.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Price S, Slutsky AS, Brodie D. Temporary circulatory support for cardiogenic shock. Lancet. 2020;396:199–212. doi: 10.1016/S0140-6736(20)31047-3. [DOI] [PubMed] [Google Scholar]
- 3.Karagiannidis C, et al. Extracorporeal membrane oxygenation: Evolving epidemiology and mortality. Intensive Care Med. 2016 doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 4.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Falk L, et al. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion. 2019;34:22–29. doi: 10.1177/0267659119830513. [DOI] [PubMed] [Google Scholar]
- 6.Rive A. The ARRIVE guidelines 20: Updated guidelines for reporting animal research. PLOS Biol. 2020;40:1769–1777. doi: 10.1177/0271678X20943823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinsar S, et al. An innovative ovine model of severe cardiopulmonary failure supported by veno-arterial extracorporeal membrane oxygenation. Sci. Rep. 2021;11:20458. doi: 10.1038/s41598-021-00087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens MC, Callaghan FM, Forrest P, Bannon PG, Grieve SM. Flow mixing during peripheral veno-arterial extra corporeal membrane oxygenation—A simulation study. J. Biomech. 2017;0:64–70. doi: 10.1016/j.jbiomech.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson PJ, et al. Consensus statement from the 2014 International microdialysis forum. Intensive Care Med. 2015 doi: 10.1007/s00134-015-3930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Guennec L, et al. Diffuse cerebral microbleeds after extracorporeal membrane oxygenation support. Am. J. Respir. Crit. Care Med. 2015;191:594–596. doi: 10.1164/rccm.201411-2118LE. [DOI] [PubMed] [Google Scholar]
- 11.Lorusso R, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation. Crit. Care Med. 2016;44:e964–e972. doi: 10.1097/CCM.0000000000001865. [DOI] [PubMed] [Google Scholar]
- 12.Xie A, Lo P, Yan TD, Forrest P. Neurologic complications of extracorporeal membrane oxygenation: A review. J. Cardiothorac. Vasc. Anesth. 2017;31:1836–1846. doi: 10.1053/j.jvca.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Khan IR, et al. Cognitive, psychiatric, and quality of life outcomes in adult survivors of extracorporeal membrane oxygenation therapy: A scoping review of the literature. Crit. Care Med. 2020;48:e959–e970. doi: 10.1097/CCM.0000000000004488. [DOI] [PubMed] [Google Scholar]
- 14.Chiarini G, Cho S-M, Whitman G, Rasulo F, Lorusso R. Brain injury in extracorporeal membrane oxygenation: A multidisciplinary approach. Semin. Neurol. 2021;41:422–436. doi: 10.1055/s-0041-1726284. [DOI] [PubMed] [Google Scholar]
- 15.Stevens MC, Callaghan FM, Forrest P, Bannon PG, Grieve SM. A computational framework for adjusting flow during peripheral extracorporeal membrane oxygenation to reduce differential hypoxia. J. Biomech. 2018;79:39–44. doi: 10.1016/j.jbiomech.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Rozencwajg S, et al. A mock circulation loop to evaluate differential hypoxemia during peripheral venoarterial extracorporeal membrane oxygenation. Perfusion. 2022 doi: 10.1177/02676591211056567. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, et al. Superior vena cava drainage improves upper body oxygenation during veno-arterial extracorporeal membrane oxygenation in sheep. Crit. Care Lond. Engl. 2015;19:68. doi: 10.1186/s13054-015-0791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada H, et al. Cerebral tissue oxygen saturation during percutaneous cardiopulmonary support in a canine model of respiratory failure. Artif. Organs. 2000;24:640–643. doi: 10.1046/j.1525-1594.2000.06601.x. [DOI] [PubMed] [Google Scholar]
- 19.Holzgraefe B, et al. Does permissive hypoxaemia during extracorporeal membrane oxygenation cause long-term neurological impairment?: A study in patients with H1N1-induced severe respiratory failure. Eur. J. Anaesthesiol. 2017;34:98–103. doi: 10.1097/EJA.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 20.Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: Pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47:1393–1414. doi: 10.1007/s00134-021-06548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: From pathophysiology to therapeutic strategies. Int. J. Mol. Sci. 2021;23:14. doi: 10.3390/ijms23010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer M, et al. Dangers of hyperoxia. Crit. Care. 2021;25:440. doi: 10.1186/s13054-021-03815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X, et al. Should hyperoxia be avoided during sepsis? An experimental study in ovine peritonitis*. Crit. Care Med. 2017;45:e1060–e1067. doi: 10.1097/CCM.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 24.Terraneo L, et al. Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol. 2017;11:12–20. doi: 10.1016/j.redox.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J. Cereb. Blood Flow Amp. Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moussa MD, et al. Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: A bicenter retrospective propensity score-weighted analysis. Crit. Care. 2022;26:257. doi: 10.1186/s13054-022-04133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oechmichen M, Meissner C. Cerebral hypoxia and ischemia: The forensic point of view: A review. J. Forensic Sci. 2006;51:880–887. doi: 10.1111/j.1556-4029.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 28.Rahaman P, Del Bigio MR. Histology of brain trauma and hypoxia-ischemia. Acad. Forensic Pathol. 2018;8:539–554. doi: 10.1177/1925362118797728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carteron L, Bouzat P, Oddo M. Cerebral microdialysis monitoring to improve individualized neurointensive care therapy: An update of recent clinical data. Front. Neurol. 2017;8:103–110. doi: 10.3389/fneur.2017.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heilmann C, et al. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS) Intensive Care Med. 2012;38:62–68. doi: 10.1007/s00134-011-2370-6. [DOI] [PubMed] [Google Scholar]
- 31.Vincent F, Rauch A, Loobuyck V, Robin E, Nix C, Vincentelli A, Susen S. Arterial pulsatility and circulating von Willebrand factor in patients on mechanical circulatory support. J. Am. College Cardiol. 2018;71(19):2106–2118. doi: 10.1016/j.jacc.2018.02.075. [DOI] [PubMed] [Google Scholar]
- 32.Villa G, Katz N, Ronco C. Extracorporeal membrane oxygenation and the kidney. Cardiorenal. Med. 2016;6:50–60. doi: 10.1159/000439444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostermann M, Lumlertgul N. Acute kidney injury in ECMO patients. Crit. Care. 2021;25:313. doi: 10.1186/s13054-021-03676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinsar S, et al. Heart failure supported by veno-arterial extracorporeal membrane oxygenation (ECMO): A systematic review of pre-clinical models. Intensive Care Med. Exp. 2020;8:16. doi: 10.1186/s40635-020-00303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen DN, et al. Serum S100B protein could help to detect cerebral complications associated with extracorporeal membrane oxygenation (ECMO) Neurocrit. Care. 2013;20:367–374. doi: 10.1007/s12028-013-9874-6. [DOI] [PubMed] [Google Scholar]
- 36.Bembea MM, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation*. Crit. Care Med. 2015;43:2202–2211. doi: 10.1097/CCM.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 37.Schrage B, et al. Neuron-specific-enolase as a predictor of the neurologic outcome after cardiopulmonary resuscitation in patients on ECMO. Resuscitation. 2019;136:14–20. doi: 10.1016/j.resuscitation.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Cvetkovic M, et al. International survey of neuromonitoring and neurodevelopmental outcome in children and adults supported on extracorporeal membrane oxygenation in Europe. Perfusion. 2021 doi: 10.1177/02676591211042563. [DOI] [PubMed] [Google Scholar]
- 39.Schrage B, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: Results from an international, Multicenter Cohort Study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.