Abstract
Background:
Hematopoietic stem cell transplantation using bone marrow as the graft source, is a common treatment for hematopoietic malignancies and disorders. For allogeneic transplants, the processing of bone marrow requires the depletion of ABO mismatched red blood cells to avoid transfusion reactions. Here, we tested the use of a closed-system, automated instrument for depleting red blood cells from the bone marrow and compared results to a semi-automated platform that is more commonly used in transplant centers today. We found that fully automated processing using the Sepax instrument resulted in comparable depletion of red blood cells and total mononuclear cell recovery to the COBE 2991 semi-automated process.
Methods:
We optimized the fully automated closed-system Sepax SmartRedux protocol. Three reduction folds (10x, 12x, and 15x) were tested on the Sepax. Each run was compared to the standard processing performed in our center on the COBE 2991. Given that bone marrow is difficult to acquire for these purposes, we opted to create a surrogate that is more easily obtainable, which consisted of cryopreserved peripheral blood stem cells that were thawed, and mixed with red blood cells, and supplemented with PlasmaLyte-A +4% Human Serum Albumin. This “bone marrow-like" product was split into two starting products of approximately 600mLs, and these were loaded onto the COBE and Sepax for direct comparison testing. Samples were taken from the final products for cell counts and flow cytometry. We also tested a 10x Sepax reduction using human bone marrow supplemented with human liquid plasma and red cells.
Results:
Red blood cell reduction increased as the Sepax reduction rate increased with an average of 86.06% in the 10x (70.85-96.39%), 98.80% in the 12x (99.5-98.1%), and 98.89% in the 15x (98.80-98.89). Reduction rate on the COBE ranged from an average of 69.0%-93.15%. However, white blood cell (WBC) recovery decreased as the Sepax reduction rate increased with an average of 47.65% (38.9-62.35%) in the 10x, 14.56% (14.34-14.78%) in the 12x, and 27.97% in the 15x (24.7-31.23%). The COBE WBC recovery ranges on an average of 53.17%-76.12%. Testing a supplemented human bone marrow sample using a 10x Sepax reduction resulted in an average of 84.22 (84.0-84.36) red cell reduction and WBC recovery of 43.37% (37.48-49.26). Flow cytometry analysis also showed that 10x Sepax reduction resulted in higher purity and better recovery of CD34+, CD3+ and CD19+ cells as compared to 12x and 15x reduction. Therefore, 10x reduction rate was selected for Sepax process.
Conclusions:
The fully automated closed-system SmartRedux program on the Sepax was shown to be effective in reducing red blood cells from “bone marrow-like" products and a supplemented bone marrow product using a 10x reduction rate.
Keywords: Bone Marrow, transplantation, Sepax, COBE, CD34 PBSC, Red Blood Cells
Introduction:
Hematopoietic stem cells from bone marrow or mobilized peripheral blood stem cells (PBSC’s) are commonly used in transplantation for hematological malignancies and immunodeficiencies. The goal of bone marrow transplant is to replace a patient’s diseased hematopoietic compartment using a healthy donor marrow graft [1]. Hematopoietic stem cell transplants can be challenging mainly due to graft rejection and graft-vs-host disease [2]. Although transplantation using mobilized PBSC has all but replaced BM as the source of healthy progenitor cells, bone marrow still accounts for approximately 25% of all allogeneic transplants in the U.S. [3]. Bone marrow is still preferred in some scenario’s due to a decreased risk of graft-versus-host disease (GvHD) when compared with allogeneic PBSC transplants, especially in children [4-6].
Major complications associated with the infusion of the bone marrow graft, such as acute hemolysis of donor erythrocytes, can be observed that are due to ABO blood group incompatibility among the marrow donor and recipient and the presence of pre-existing recipient antibodies [7-9]. In addition, incompatible ABO antibodies in the donor plasma present in the marrow graft can cause hemolysis of the recipient red blood cells. Therefore, it is important that red blood cells and plasma are removed from ABO incompatible grafts prior to infusion [10]. Currently, this process is performed at most centers using centrifugation or density sedimentation methods [5]. This manipulation of bone marrow is an important factor as it can directly affect the quality of the graft and the overall engraftment and patient survival. It is important during the red blood cell depletion process that the maximum amount of CD34+ hematopoietic progenitor cells are recovered [11-13]. There are no official limits on what constitutes a ‘safe criteria’ of what the maximum volume of RBCs may be in the graft, but most institutions have agreed to aim for 20 to 30mLs [7, 8, 14].
Since the quantity of CD34+ cells in bone marrow samples are generally very small, it is crucial to ensure processing events do not lead to an excessive loss and that CD34 cell recovery is high enough to reach the transplant dose. The COBE 2991 and the Sepax C Pro, herein referred to as COBE and Sepax, both use centrifugation of the starting product to produce a buffy coat with the desired cells, and remove the unwanted ABO mismatched red blood cells. The Sepax instrument utilizes a specific processing kit (CT49.1), which includes a tubing set and a 220mL separation chamber that will perform multiple centrifugation cycles for bone marrow products, which may be larger in volume than the chamber volume [7, 15, 16]. The Sepax is a fully-closed system and automated process while the COBE is semi-automated and requires significant user interaction to complete a process. The Sepax instrument uses SmartRedux software, which is listed as a class 1 medical device software with the FDA. The instrument and software applications are used for research and in clinical and commercial manufacturing environments where the process is validated by the end user. Since large volume human bone marrow products are extremely difficult to obtain for research purposes, we optimized the Sepax SmartRedux software with a “bone marrow-like" product to demonstrate reproducibility [7, 17]. This product consisted of red blood cells and cryopreserved/thawed PBSC’s from healthy donors, such that, the red blood cell content was fixed to 30% hematocrit to have similar characteristics to what is observed in clinical bone marrow products [17]. We then supplemented this “bone marrow-like” product with PlasmaLyte-A+ 4% Human Serum Albumin (HSA) to increase the volume.
The Sepax cell processing system, is a fully automated, closed-system that uses a controlled speed centrifugation to separate blood components. A pneumatic force and optical sensor is then employed to separate each fraction through movement of the piston in the chamber. The SmartRedux protocol is flexible, allowing the user to manipulate the starting volume, fixed volume, proportional volume, reduction rate, reprocessed buffy coat, and reprocessed volume.
We tested the Sepax to perform a red blood cell reduction of a “bone marrow-like" product and compared the performance of the red blood cell reduction to the standard COBE processing. The reduction rates on the Sepax were evaluated on the basis of RBC reduction, white blood cell (WBC), and CD34 cell recoveries, changes in cell populations during the process, and an optimal reduction rate was determined. A 10x reduction on the Sepax was tested using an actual human bone marrow product supplemented with human plasma and red blood cells to ensure the process was consistent and efficient. The Sepax SmartRedux software was confirmed to be an alternative process to the standard COBE processing in reducing the volume of red blood cells in bone marrow while not affecting the recovery of the CD34+ stem cells.
Materials and Methods:
“Bone Marrow-like” cell preparation
“Bone Marrow-like” products were prepared by using patient derived cryopreserved PBSC’s and unmatched healthy donor red blood cells from the NIH Clinical Center Blood Bank. Cryopreserved PBSC’s were thawed and transferred to a 3L transfer pack (Terumo). The PBSC bag was rinsed with a wash solution of plasmalyte-A (Baxter) and 4% HSA (Shire) then transferred to the 3L transfer pack. The healthy donor red blood cells (RBCs) were transferred to the 3L pack containing PBSCs, and each RBC bag was rinsed with the wash solution. The product was brought up to approximately 1200mLs and split into two 600mL starting products for each instrument. Before splitting the product, a sample was collected from the original bag to process a complete blood count (CBC) to ensure the hematocrit was approximately 30%.
Semi-Automated Cell processing on the COBE
“Bone Marrow-like” products were processed on the COBE 2991 using a blood processing set with a single bag. The COBE 2991 cell processor is a semi-automated instrument that concentrates/separates cells based on centrifugation. It consists mainly of a centrifuge and a flexible diaphragm. When the centrifuge is spinning, the flexible diaphragm located inside the centrifuge bowl is inflated with hydraulic fluid. This presses against the bottom of the cell processing bag to expel fluids and/or cells for removal or collection during centrifugation. “Bone Marrow-like” products were processed on the COBE 2991 for RBC reduction. A single donut bag is used for the process with centrifugation speed at 3000 rpm. Process duration per run varies depending on the starting bone marrow volume. In addition, there is a limit to packed RBC loading at 125 - 200 mL per run. If a marrow product contains >200 mL of packed RBC, it needs to be divided and processed on multiple COBE 2991 instruments. The starting product for our purposes contained at least 125mLs of RBC’s and at least a total nucleated cell (TNC) count of 5x109.
Fully Automated Cell processing on the Sepax
“Bone Marrow-like” products were processed using the SmartRedux software (Cytiva) on the Sepax C Pro. The Sepax uses a rotating syringe in the chamber of the closed CS-49.1 kit to separate the blood components. An optical sensor detects the plasma, buffy coat, and red blood cell layer and transfers each into separate bags connected to the kit. The SmartRedux protocol can process 30-3300mLs using one kit to get to either a user selected fixed final volume or a proportional final volume. The proportional volume is calculated as a percentage of the initial volume, while the fixed is selected by the technician [5]. A 10x, 12x, and 15x fixed reductions were tested in this study with using a reprocessed volume of 20mLs and reprocessed buffy coat of either 20 or 25mLs. Testing parameters were set per suggestions from the instrument manufacturer (Cytiva). A fully automated process of about 600mLs takes approximately 120 minutes or 4 cycles. The final buffy coat product was collected in a 150mL transfer pack (Terumo).
Testing a Supplemented Bone Marrow Product on the Sepax
A human bone marrow product (Stem Express) was processed on the Sepax C Pro using a 10x reduction. The initial bone marrow volume of 100mLs was transferred to a 2L transfer pack (Terumo) and the container was rinsed with human plasma from the NIH Clinical Center Blood Bank. Two bags of red blood cells and a bag of human plasma was added to the 2L transfer pack for a final volume of 1340.9mLs. A sample from this “large bag pre-sample” was collected before it was split into two bags. Samples were collected from each smaller pre-bag before they were run on the Sepax for a CBC and flow cytometry. Pre-bag 1 had a volume of 679.3mLs and pre-bag 2 had an initial volume of 663.8mLs. The final buffy coat product was collected in a 150mL transfer pack (Terumo) and post samples were collected from each bag for a cell counts and flow cytometry analysis.
Evaluation of samples
Values of pre and post samples were compared to determine the efficiency of the Sepax C Pro SmartRedux software. Samples were collected before the large bag was split into two (large bag pre), for the two smaller pre-samples (Sepax pre, COBE pre), and all final samples (Sepax buffy coat, Sepax RBC, Sepax plasma, COBE buffy coat, COBE packed red cells (PRC)). Complete blood counts were performed before and after processing using an automated blood cell analyzer (Advia 2120i Hematology systems, Siemens). The proportion of cell populations was determined using a 2-tube multicolor antibody staining strategy which consisted of the following: Tube 1 – CD4 (FITC), CD34 (PE), CD3 (PECy7), CD8 (APC), CD45 (APCCy7); and Tube 2 – CD14 (FITC), CD16 (PE), CD56 (PECy7), CD19 (APC), CD45 (APCCy7), CD3 (BV421), CD15 (BV510). Flow was performed on a 10-color BD FACS Canto X, BD Biosciences, San Jose, CA after labeling with fluorescent labeled antibodies (BD and Biolegend). 7-aminoactinomycin D (BD Pharmingen) was used to gate out dead cells. Measurements of CD34 cell counts was performed using a dual platform method, consisting of cell counts obtained from a hematology analyzer (Advia 2120i, Siemens) and a flow cytometer (BD FACS Canto™ X). Cells were gated on singlets, CD45+ cells, live cells (7AAD−), and then finally CD34+ cells. Calculations were made to determine red blood cell depletion, red blood cell volume in buffy coats, and recovery of WBC and each immune cell population.
Graphs and statistical analysis
GraphPad Prism 7 software was used to generate the graphs. Unpaired t tests were used in Figure 1 to compare groups.
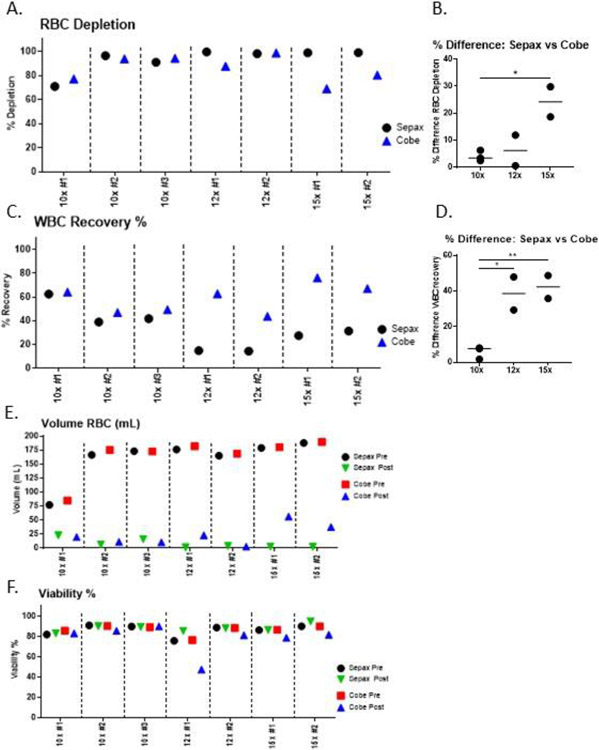
Figure 1. Comparison of RBC depletion and WBC recovery from “bone-marrow-like” products from Sepax versus COBE.
For each run, a single donor product was split into two pre-samples that were processed on the Sepax and COBE. The 10x, 12x and 15x Sepax setting were evaluated for optimization. A) Red blood cell depletion percentages in the buffy coat compared to the starting pre-product. B) To account for donor-donor variability among samples, the percent difference of red blood cell depletion between Sepax and the COBE devices were calculated. C) White blood cell recovery percentage in the buffy coat. D) To account for donor-donor variability among samples, the percent difference of WBC recovery between Sepax and the COBE devices were calculated. E) Total volume of red cells in the starting and buffy coat samples. F) WBC Viability of pre-processing and buffy coat samples from the Sepax and the COBE. For parts B and D, an unpaired t-test was used to determine significance between groups.
Results
“Bone marrow-like” Products:
Red Blood Cell Volume Reductions
Although the COBE is capable of depleting RBC’s from bone marrow products for the purposes of transplantation, it requires multiple technicians to process one donor. Since bone marrow starting products can be up to 1200mLs, two COBE machines are required and multiple people are needed to operate each instrument. We decided to test the SmartRedux protocol on the Sepax to provide a safer, faster, more consistent and efficient process, and to potentially reduce the number of technicians needed. Since the Sepax allows for the input of a fixed final volume of the buffy coat, we were able to test different reduction rates. We compared a 10x, 12x, and 15x reduction on the Sepax with the standard COBE process using split-paired “bone marrow-like” products. Total volumes on starting samples used in this study was 600mL, and the final processing volume on the Sepax was a fixed volume of 120mL, while the COBE final processing volume ranged between 150-200mL. Initial and final RBC volumes were measured for each product run on the different instruments, along with the percentage of the volume reduction for RBC’s.
While the 10x and 12x reductions on the Sepax were comparable to the COBE in depleting RBCs in the buffy coat, there was a larger difference between the two processes for the 15x (average Sepax 98.89% vs. COBE 74.65%) (Table 1). There was an increase in RBC depletion in the Sepax in the 12x and 15x reductions compared to the 10x reduction (Fig 1A). The Sepax had an average of 98.8% red blood cell reduction at 12x and 98.89% red blood cell reduction at 15x, while having a slightly lower average 86.06% red blood cell reduction at the 10x. Even though the 10x red cell blood reduction was not as efficient as the 12x or 15x, this was comparable to the standard COBE reduction process which was an average of 88.43% red blood cell reduction. It is important to point out that the COBE’s process was unchanged for these paired samples and only the Sepax process was modified. The variance in recovery of the COBE between runs was likely due to donor variability, and served as a good control as to what would be expected from our standard process. In effort to determine significance between the different methods, we normalized RBC depletion by calculating percent difference of RBC depletion. We show that the largest difference between the Sepax and the COBE in terms of % RBC depletion was when comparing the 15x Sepax to the COBE, and the smallest difference was when comparing the 10x Sepax to the COBE (Figure 1B).
Table 1. Sepax and COBE Red Blood Cell Volume Reductions.
Red blood cell (RBC) volumes before and after processing for both the Sepax and the COBE. Values from each run are shown along with the average of each individual column. Three runs were carried out for the 10x comparison, and two runs for the 12x and 15x comparison runs.
| Reduction | N | Sepax RBC pre- Volume (mL) |
Sepax RBC post Volume (mL) |
COBE RBC pre- Volume (mL) |
COBE RBC post Volume (mL) |
Sepax RBC Volume Reduction (%) |
COBE RBC Volume Reduction (%) |
|---|---|---|---|---|---|---|---|
| 10x comparison | 3 | 76.82 | 22.39 | 84.60 | 19.37 | 70.85 | 77.10 |
| 166.40 | 6.00 | 175.10 | 10.70 | 96.39 | 93.89 | ||
| 173.04 | 15.66 | 172.54 | 9.83 | 90.95 | 94.30 | ||
| Avg: 138.75 | Avg: 14.68 | Avg: 144.08 | Avg: 13.3 | Avg: 86.06 | Avg: 88.43 | ||
| 12x comparison | 2 | 176.00 | 0.83 | 182.00 | 22.56 | 99.53 | 87.60 |
| 164.85 | 3.20 | 168.44 | 2.194 | 98.06 | 98.70 | ||
| Avg: 170.42 | Avg: 2.02 | Avg: 175.22 | Avg: 12.38 | Avg: 98.80 | Avg: 93.15 | ||
| 15x comparison | 2 | 178.84 | 2.06 | 180.24 | 55.92 | 98.80 | 69.00 |
| 187.78 | 1.92 | 189.55 | 37.26 | 98.98 | 80.30 | ||
| Avg: 183.31 | Avg: 1.99 | Avg: 184.89 | Avg: 46.59 | Avg: 98.89 | Avg: 74.65 |
White Blood Cell (WBC) Recovery and Viability
The WBC recovery was highest on the Sepax at the 10x reduction (average: 47.65%). There was no significant difference between the 10x Sepax and the COBE for WBC recovery. The 12x and 15x reductions on the Sepax suffered from significantly decreased WBC recovery. The 12x and 15x had WBC recoveries of 14% and 27.97%, respectively (Figure 1C). There was a significant difference in WBC recovery for the 12x and 15x reduction between the Sepax and the COBE (p<0.05). To account for donor-to-donor variability between runs, we plotted the % difference between the Sepax vs the COBE. Upon doing this, the data shows that the 10x process was most similar to the COBE (had the least amount of difference), and 12x and 15x processes were the most different from the COBE and not significantly different from one another (Figure 1D). The absolute total nucleated cell (TNC) numbers of the Sepax and the COBE are shown in Table 2. The difference between the viable TNC pre and post processing from the Sepax and the COBE at the 10x reduction rate was minimal (3.53x109 (S) vs. 3.46x109 (C)). As the reduction rate was increased on the Sepax, however, these differences also increased due to a much higher loss in recovery (12x; 7.66x109 (S) vs. 4.10x109 (C) and 15x; 7.85x109 (S) vs. 3.07x109 (C)).
Table 2. TNC differences in each reduction conditions.
Viable total cell counts before and after processing for the Sepax and the COBE. Values from each run are shown along with the average of each individual column.
| Reduction | N | Sepax TNC pre X109 |
Sepax TNC buffy coat post X109 |
COBE TNC pre X109 |
COBE TNC buffy coat post X109 |
|---|---|---|---|---|---|
| 10x comparison | 3 | 2.52 | 1.58 | 4.24 | 2.71 |
| 10.01 | 3.89 | 10.91 | 5.12 | ||
| 6.05 | 2.52 | 6.10 | 3.01 | ||
| Avg: 6.19 | Avg: 2.66 | Avg: 7.08 | Avg: 3.61 | ||
| 12x comparison | 2 | 11.30 | 170 | 11.10 | 7.00 |
| 7.63 | 1.90 | 7.29 | 3.18 | ||
| Avg: 9.46 | Avg: 1.80 | Avg: 9.20 | Avg: 5.09 | ||
| 15x comparison | 2 | 9.66 | 2.70 | 9.51 | 7.28 |
| 12.65 | 3.95 | 11.90 | 7.98 | ||
| Avg: 11.16 | Avg: 3.32 | Avg: 10.71 | Avg: 7.63 |
The RBC volume in the Sepax buffy coat samples were comparable for all reduction rates and below 25mLs. The RBC volume in the COBE buffy coat samples were comparable in the 10x and 12x reductions. However, there was 55mLs and 37mLs in the 15x comparison samples (Figure 1E), indicating that the Sepax may be more consistent among donors than the COBE. Except for one run, the viability of the cells post-processing was consistently high for each Sepax reduction tested and comparable to the viability of the COBE samples. Samples were taken immediately after the Sepax and COBE was complete and processed within one hour. The decrease in viability on the 12x Sepax reduction for the COBE post sample may be explained by the fact that this particular sample was not analyzed within this time frame, but was instead analyzed several hours later (Figure 1F).
Frequency and recovery of different cell populations
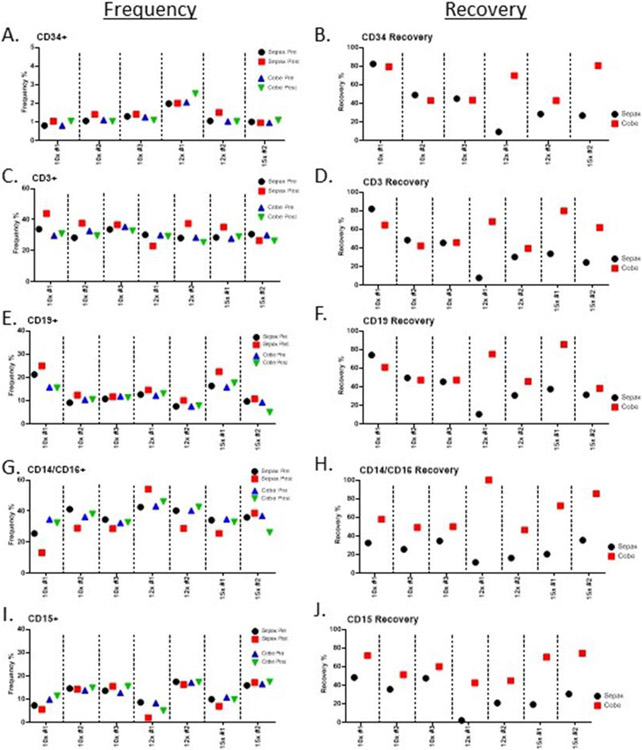
Flow cytometry was performed to examine the frequencies of various cell populations (CD34, CD3, CD19, CD14/CD16, CD15, CD56) between the two processing methods. There was little difference in the frequency of CD34 cells among the groups (Figure 2A), whereas the differences in CD34 recovery were more apparent (Figure 2B). The inadvertent exclusion of anti-CD34 in the 15x 01 sample resulted in a reduction of sample size, however, the other two samples analyzed (15x 02, 15x 03) showed a high level of consistency. The recovery of 10x Sepax groups were very similar among the donors with a slight increase as compared to the COBE. At the 12x and 15x reduction rates, however, we observed a dramatic loss in the recovery of CD34+ cells when compared to the COBE. Frequencies of CD3+ T-cells and CD19+ B-cells generally increased from pre-to-post processing on the Sepax, while the frequencies of these populations on the COBE-processed samples remained more similar (Figure 2C, E). Again, recovery of CD3+ T-cells and CD19+ B-cells was higher in the 10x Sepax-processed samples, but dropped significantly in the 12x-and 15x-processed samples (Figure 2D, F). Frequencies of monocytes (CD14/CD16+) were mostly decreased in post-processing Sepax samples, whereas they remained more similar in the COBE (Figure 2G). Frequencies of granulocytes (CD15+) remained more constant, with the exception of decrease in the 12x-1 Sepax post sample (Figure 2I). The recovery of monocytes and granulocytes was higher among COBE-processed samples in all patients at all comparisons (Figure 2H, J). Overall, these data show that the 10x processing on the Sepax resulted in higher purity and better recovery of CD34+, CD3+ and CD19+ cells than the 12x and 15x processing, and this process was used for further experiments. Due to the poor recovery of cells at the end of the process, the 12x and 15x processing on the Sepax was not continued.
Figure 2. Flow cytometry analysis of buffy coats from “bone-marrow-like” products using the Sepax and the COBE.
The buffy coats were evaluated by flow cytometry. A, C, E, G, I) Frequencies of CD34, CD32, CD19, CD14/CD16, CD15 cell populations in pre and post processing samples were plotted for each run. B, D, F, H, J) Cell recovery percentages were calculated for each run using the pre-processing and buffy coat samples for both the Sepax and COBE.
Human Bone Marrow Product on the Sepax
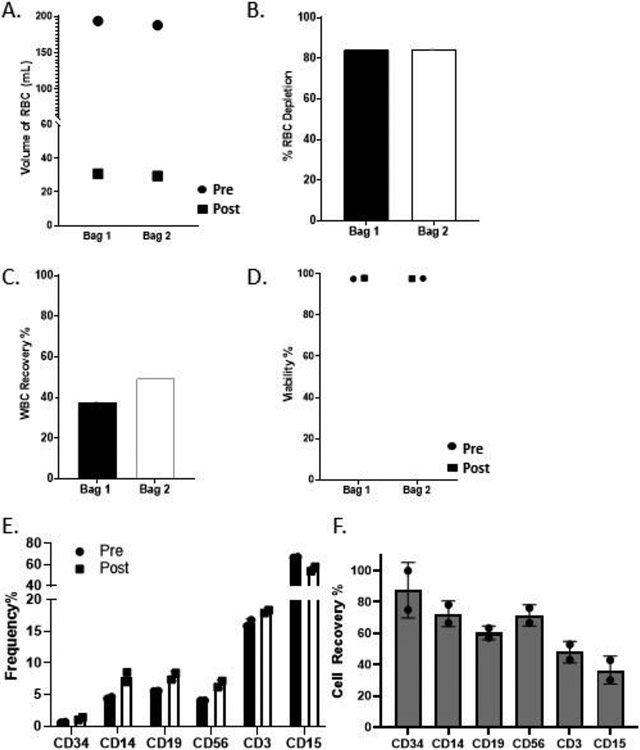
To confirm that the optimized 10x Sepax reduction is suitable for a bone marrow product, we tested a supplemented human bone marrow on the Sepax using the 10x reduction. After supplementing the 100mLs of donor bone marrow with human plasma and red blood cells the large bag was split into two bags for separate runs with a starting hematocrit of 28.3% and 28.5% for run 1 and run 2, respectively. The total volume of red blood cells in the buffy coat was significantly reduced from an average of 190mL down to an average of 30mL (Figure 3A). This red blood cell reduction in volume resulted in a red blood cell depletion percentage of 84.04% and 84.36% for bag 1 and bag 2, respectively (Figure 3B). The average WBC recovery from both bags was approximately 43.4%, with bag 2 being slightly higher (37.48% vs 49.26%; Figure 3C). The recovery observed here was comparable to the “bone marrow-like" samples in the 10x reduction Sepax runs. The viability of the two products was between 97% and 99% for post processing samples (Figure 3D).
Figure 3. Results of 10x Sepax reduction of human Bone Marrow are comparable to “bone marrow-like" products.
One bone marrow donor sample was split into two bags for separate Sepax runs. A) Total volume of red cells in the starting and buffy coat samples. B) Red blood cell depletion percentages in the Buffy Coat from the starting product for each bag. C) White blood cell recovery percentage in the buffy coat. D) Viability of pre-processing and buffy coat samples from each bag. E) Percentages of different cell populations from pre- and post samples were plotted for each run. F) Cell recovery percentages were calculated for both runs using the pre-processing and buffy coat samples for the Sepax.
Flow cytometry was performed to examine the frequency of various cell populations pre and post processing (CD34, CD14, CD19, CD56, CD3, CD15). The frequencies of these cell populations all had a slight increase, except CD15 granulocytes, where we observed a decrease in frequency (Figure 3E). Importantly, there was a 75% and 100% recovery of CD34+ cells for both bags tested, and the recovery of CD14, CD19, CD56, CD3, and CD15 cells ranged from 40-80% and was inline with what was observed for the “bone marrow-like” product (Figure 3F).
Discussion
ABO incompatible bone marrow transplant risks, such as intravascular immune hemolysis and delay of red cell engraftment, can be minimized by depletion of red blood cells in the product before the transplantation [18, 19]. The study described herein highlights the use of the automated closed system red cell depletion protocol for clinical scale reductions of bone marrow for stem cell transplantation. Current methods used at our center and elsewhere, utilize the COBE 2991 (Terumo BCT) to deplete red blood cells from bone marrow products prior to transplantation [18, 20, 21]. We tested and optimized a fully automated closed-system method to reduce the number of laboratory staff required for processing, and to enhance the consistency of producing a reduced volume of red blood cells from the bone marrow product.
We report here a detailed analysis between the Sepax and the COBE 2991 processing of split paired-products. Such a detailed analysis has been very difficult to perform due to the lack of volunteers willing to contribute a large volume of bone marrow required for this research [22]. One of the novelties of this body of work is that we devised a method whereby we were able to mimic a large volume bone marrow product by mixing red cells with mobilized PBSC. This large 1200mL product was split into two identical 600mL starting products to test the Sepax and COBE red blood cell volume reductions head-to-head. The clinical manufacturing process that was used for the COBE 2991 instrument was developed in our center and has been in use for these purposes since 2003. The Sepax is more customizable and has many different options. Per manufacturers suggestion, we tested 10x, 12x, and 15x volume reductions on the Sepax using the SmartRedux protocol. This software allows for a wide range of initial volumes to be used to initiate the reduction process and provides a final buffy coat volume based on the initial volume. Once each run was complete, samples were collected for CBC and flow cytometric analysis.
We report here that the 10x volume reduction on the Sepax is comparable to the reduction we observe using the COBE 2991. A 10x reduction had an average red blood cell volume reduction of 86.06% compared to 88.43% on the COBE. There was an increase in red blood cell volume reduction on the Sepax as the reduction rates increased to 12x and 15x. However, the increase in red blood cell reduction was at the cost of a lower white blood cell recovery. The 10x reduction rate on the Sepax has similar white blood cell recovery as the COBE. However, the Sepax had a drastic decrease in white blood cell recovery when performing a 12x or 15x reduction compared to the COBE. The viability remained consistent amongst all samples for each reduction comparison. Each Sepax reduction was able to reduce the red blood cell volume in the buffy coat to under 25mLs.
We then tested a healthy donor bone marrow on the Sepax to verify that our “bone marrow-like" product behaved similarly to an actual bone marrow product. The bone marrow product (100mLs) was supplemented with red cells and human liquid plasma and split in half to increase our N using the same donor. Both runs had a nearly identical red blood cell reduction, having 84.2±0.1% depletion and 29mL and 30mL of red cells in the buffy coat. Both runs also had a white blood cell recovery that was comparable to the “bone marrow-like" 10x reduction runs. Flow cytometry results showed that the pre and post buffy coat viability was between 97% and 99%. Cell recovery for CD34 cells very high at 87.5±12%.
Currently, there are only two publications on the topic of red blood cell reductions of bone marrow that utilize the Sepax SmartRedux software. Fantin et al. compared bone marrow red blood cell reduction using two different software programs on the Sepax: NeatCell, which is a ficoll-Paque-based process that we opted not to test versus SmartRedux utilized here [7]. Their results for the latter are similar to what we show here for human bone marrow product in terms of erythrocyte depletion (95.3±2% vs. ours at 84.2±0.1%) and CD34 recovery (86.4±14.6% vs. ours at 87.5±12%). Similarly, in the second manuscript by Mazzanti et al., the Sepax SmartRedux system was used in the manufacture of 8 separate products [5]. Here again we fall within the range reported by this group for erythrocyte depletion and CD34 cell recovery, which was 30-80% and 81-134%, respectively. These studies give us more confidence in our results given that we did not have a large number of samples to test.
In terms of impact on staff, we found that the Sepax instrument is much more conducive to smaller processing centers that may not operate with a large number of staff. Even though bone marrow processing on the COBE has been utilized for many years, there are limitations to the instrument. For example, at our center, due to the large volume usually associated with this type of product (2 liters), three instruments are required to be run simultaneously with multiple technicians operating each instrument. Specifically, for each instrument used, two technicians are tasked with performing the hands-on work, while one technician acts to verify and record data in the batch record. The amount of time spent on one product could be up to three hours, multiplied by 9 technicians (if using three instruments as in most circumstances). Other limitations include potential breaches in sterility of the product. The connection of the sample/buffer bag to the COBE kit by spiking is performed under non-aseptic conditions, which increases the chances of microbial contamination. In the same vein, on at least 6 separate occasions spanning approximately 15 years, we have observed leaks or breakages in the bags, some of which were reportedly due to contact with the internal mechanisms in the instrument that resulted in loss of the product. In comparison, due to its fully automated and closed-system nature, the Sepax instrument does not suffer from these attributes. The Sepax tubing set (CS49.1) associated with this process can be easily sterile connected to the product and buffer bag using a sterile tubing welder, eliminating non-aseptic spiking of bags. The SmartRedux Software is 21 CFR Part 11 compliant, and the electronic record keeping on the instrument suffices in place of handwritten batch records. Lastly, the manufacturer reports that the instrument is capable of processing a large volume product (up to 3300mLs) on a single instrument, although we were unable to verify this due to the lack of availability of such a large product. Cumulatively, this all results in the ability to reduce staff to one technician that is required per instrument for processing a large bone marrow product.
Conclusions
In conclusion, we demonstrate the utility of using a “bone marrow-like” product for testing equipment such as this in instances where bone marrow may not be available. Our data supports the idea that the Sepax is comparable to our standard process using the COBE 2991 for red blood cell reduction of bone marrow products. Due to its closed-system nature and lack of necessity to spike bags under non-sterile conditions, the product is less prone to contamination. Finally, since the Sepax is fully automated, it is better suited for clinical manufacturing when performing red blood cell reductions and there is a significant savings in the overall cost to process one product.
Acknowledgements
We thank the staff of the Center for Cellular Engineering, Department of Transfusion Medicine, National Institutes of Health (NIH) for help in preparing samples and operating the COBE. We also thank the staff of the Clinical Center Blood Bank for providing red cells and human liquid plasma. We also thank Dr. Eu Han Lee, Sonia Bulsara, and Zachary Nelson for their helpful discussion and providing insight on the Sepax Instrument.
Funding
Open Access funding provided by the National Institutes of Health (NIH). This project was funded by the Intramural Research Program of the NIH, Clinical Center. Human Bone Marrow was purchased by the Center for Cellular Engineering.
This study was supported by the National Institutes of Health with awards from the Intramural Research Program of the NIH Clinical Center [Z99CL999999] and the National Center for Advancing Translational Sciences [UL1TR000445].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest
The authors declare they have no disclosure of interests. The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
References
- 1.Georges GE, Doney K, and Storb R, Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv, 2018. 2(15): p. 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson E and Dazzi F, Bone Marrow Transplantation 1957-2019. Front Immunol, 2019. 10: p. 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korbling M and Freireich EJ, Twenty-five years of peripheral blood stem cell transplantation. Blood, 2011. 117(24): p. 6411–6. [DOI] [PubMed] [Google Scholar]
- 4.Dal Pozzo S, et al. , High recovery of mesenchymal progenitor cells with non-density gradient separation of human bone marrow. Cytotherapy, 2010. 12(5): p. 579–86. [DOI] [PubMed] [Google Scholar]
- 5.Mazzanti B, et al. , Fully automated, clinical-grade bone marrow processing: a single-centre experience. Blood Transfus, 2017. 15(6): p. 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimosato Y, et al. , Allogeneic Bone Marrow Transplantation versus Peripheral Blood Stem Cell Transplantation for Hematologic Malignancies in Children: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transplant, 2020. 26(1): p. 88–93. [DOI] [PubMed] [Google Scholar]
- 7.Fantin L, et al. , A comparison of two protocols for optimal red blood cell depletion using Sepax-2 device for ABO-major incompatible transplantation in adults. Curr Res Transl Med, 2019. 67(3): p. 107–111. [DOI] [PubMed] [Google Scholar]
- 8.Staley EM, Schwartz J, and Pham HP, An update on ABO incompatible hematopoietic progenitor cell transplantation. Transfus Apher Sci, 2016. 54(3): p. 337–44. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin PI, et al. , Transplantation of major ABO-incompatible bone marrow depleted of red cells by hydroxyethylstarch. Vox Sang, 1985. 48(2): p. 89–104. [DOI] [PubMed] [Google Scholar]
- 10.Soydan E, et al. , Impact of harvest product volume in erythrocyte depletion of allogeneic or autologous bone marrow using COBE spectra. Transfus Apher Sci, 2007. 36(3): p. 269–73. [DOI] [PubMed] [Google Scholar]
- 11.Bensinger WI, et al. , Peripheral blood stem cells (PBSCs) collected after recombinant granulocyte colony stimulating factor (rhG-CSF): an analysis of factors correlating with the tempo of engraftment after transplantation. Br J Haematol, 1994. 87(4): p. 825–31. [DOI] [PubMed] [Google Scholar]
- 12.Guttridge MG, et al. , Factors affecting volume reduction and red blood cell depletion of bone marrow on the COBE Spectra cell separator before haematopoietic stem cell transplantation. Bone Marrow Transplant, 2006. 38(3): p. 175–81. [DOI] [PubMed] [Google Scholar]
- 13.Korbling M, et al. , Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood, 1995. 86(7): p. 2842–8. [PubMed] [Google Scholar]
- 14.Rowley SD, Donato ML, and Bhattacharyya P, Red blood cell-incompatible allogeneic hematopoietic progenitor cell transplantation. Bone Marrow Transplant, 2011. 46(9): p. 1167–85. [DOI] [PubMed] [Google Scholar]
- 15.Aktas M, et al. , Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy, 2008. 10(2): p. 203–11. [DOI] [PubMed] [Google Scholar]
- 16.Gee AP, et al. , Multicenter cell processing for cardiovascular regenerative medicine applications: the Cardiovascular Cell Therapy Research Network (CCTRN) experience. Cytotherapy, 2010. 12(5): p. 684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorg N, et al. , Red blood cell depletion from bone marrow and peripheral blood buffy coat: a comparison of two new and three established technologies. Transfusion, 2015. 55(6): p. 1275–82. [DOI] [PubMed] [Google Scholar]
- 18.Blacklock HA, et al. , ABO-incompatible bone-marrow transplantation: removal of red blood cells from donor marrow avoiding recipient antibody depletion. Lancet, 1982. 2(8307): p. 1061–4. [DOI] [PubMed] [Google Scholar]
- 19.Del Fante C, et al. , Automated red blood cell depletion in ABO incompatible grafts in the pediatric setting. Transfus Apher Sci, 2017. 56(6): p. 895–899. [DOI] [PubMed] [Google Scholar]
- 20.Jin NR, et al. , Preparation of red-blood-cell-depleted marrow for ABO-incompatible marrow transplantation by density-gradient separation using the IBM 2991 blood cell processor. Exp Hematol, 1987. 15(1): p. 93–8. [PubMed] [Google Scholar]
- 21.Preti RA, et al. , Hemopoietic stem cell processing: comparison of progenitor cell recovery using the Cobe 2991 cell washer and the Haemonetics V50 apheresis system. Bone Marrow Transplant, 1992. 9(5): p. 377–81. [PubMed] [Google Scholar]
- 22.Kim-Wanner SZ, et al. , Erythrocyte depletion from bone marrow: performance evaluation after 50 clinical-scale depletions with Spectra Optia BMC. J Transl Med, 2017. 15(1): p. 174. [DOI] [PMC free article] [PubMed] [Google Scholar]