Abstract
Purpose
Positional obstructive sleep apnoea (POSA) is of important clinical significance, as positional treatment can augment or obviate continuous positive airway pressure. This study aimed to determine the prevalence of POSA and its characteristics using different definitions.
Methods
We retrospectively examined a cohort of patients who underwent polysomnography (PSG) between 2013 and 2019 at two sleep centres. Demographic data and PSG data were collected from 624 patients with an apnoea–hypopnea index (AHI) ≥ 5. POSA was defined using different criteria as follows: (1) AHI of at least twice as high in the supine position as in the lateral position (Cartwright’ s definition). (2) A supine AHI ≥ 10 and a lateral AHI < 10 (Marklun’s definition). (3) AHI of at least twice as high in the supine position than in the lateral position, with the lateral AHI not exceeding 5 (Mador’s definition or Exclusive POSA; e-POSA). (4) AHI ≥ 15/h; a supine AHI ≥ twice that of the nonsupine AHI ≥ 20 min of sleep in the supine and nonsupine positions; and a nonsupine AHI < 15 (Bignold’s definition).
Results
The prevalence of POSA was 54% (Cartwright), 38.6% (Mador), 33.8% (Marklund) and 8.3% (Bignold). Multivariate regression analysis showed a body mass index (BMI) < 35 kg/m2 was the only significant predictor of POSA. Mador’s definition had the highest diagnostic yield (sensitivity 63%; specificity 100%; area under the receiver operating characteristic curve 90.2%).
Conclusion
POSA is common, but its prevalence depends on the definition used. Low BMI was identified as a significant predictor.
Keywords: OSA, POSA, Definition, Prevalence, Predictors
Introduction
Sleep-disordered breathing (SDB) is a spectrum of sleep disorders characterized by abnormal breathing during sleep; the most common type of SDB, obstructive sleep apnoea (OSA), affects more than 85% of SDB patients [1]. The prevalence of OSA among American adults is estimated to be 37% (170 million people), whereas in Saudi Arabia, OSA is estimated to affect 8.8% of adults [2, 3]. When left untreated, OSA can increase the risk of hypertension, diabetes and cardiovascular diseases [4]. OSA treatment is also important for the management of hypertension, atrial fibrillation, congestive heart failure and neurological conditions, including epilepsy and stroke [5].
OSA can be subdivided into positional obstructive sleep apnoea (POSA) and nonpositional sleep apnoea (non-POSA) based on an individual’s predominant sleeping position. POSA, also known as supine predominant sleep apnoea, is a condition in which patients exhibit an increased rate of respiratory events specifically during sleep in the supine position [6]. The apnoea–hypopnea index (AHI) measures the average number of apnoea and hypopnea events per hour during sleep and is used to diagnose OSA and classify its severity [7]. In 1984, Cartwright suggested a unique phenotype of OSA that was positional and associated with an AHI that was at least twice as high in the supine position than in the lateral position [8]. This was followed by three other definitions of POSA by Marklund, Mador and Bignold, resulting in a discrepancy in the reported prevalence of POSA in the literature [9]. Overall, POSA seems to be common and is predominantly seen in patients with mild OSA. A recent Swiss study of 1719 subjects in the general population estimated the prevalence of OSA and POSA to be 71% and 53%, respectively [10].
Continuous positive airway pressure (CPAP) is effective in treating OSA by preventing airway collapse, and if used daily for at least six hours, it can decrease sleepiness, improve daily functioning and restore memory to normal levels [11]. Unfortunately, 46–83% of patients may not be compliant with more than four hours of CPAP due to associated side effects, which include nasal symptoms and xerostomia [11]. On the other hand, positional therapy (PT) in those with POSA may augment and sometimes replace conventional CPAP and may even be curative in patients with mild OSA [12]. PT and lifestyle modification (LM) are effective tools for treating OSA; however, they are underutilized in clinical practice [13, 14]. The paucity of randomized controlled trials to support PT combined with the different definitions of POSA and the ongoing search for clinical predictors to guide patient selection might make it challenging for guidelines to strongly recommend PT [12]. There is an increasing body of evidence to support PT as an effective strategy in treating OSA, especially mild to moderate OSA [12, 15]. PT also effectively lowers AHI and reduces CPAP pressures, and it was found to be equivalent to CPAP in patients with POSA [16–18]. Furthermore, patients with POSA and e-POSA had a significantly lower likelihood of treatment adherence (PAP daily use ≥ 4 h) at 6 months and were at higher risk of PAP treatment withdrawal than those without POSA [19]. Heinzer et al. [10] reported in a large population-based study that POSA accounted for 75% of OSA subjects, while e-POSA was present in 36% of OSA subjects, recommending that a large proportion of OSA patients could be treated with PT and again underscoring the importance of establishing the diagnosis of POSA. Furthermore, Oksenberg et al. [15] reported that 35.3% of severe OSA patients had POSA. A total of 75.7% of these patients reported significant improvement with postural therapy by adopting the lateral posture. Moreover, nearly one-fifth of patients (18.2%) gained more benefit from postural therapy than from standard CPAP therapy. These data support the efficacy of postural therapy even in severe cases of POSA. This again emphasizes the importance of determining the phenotype status of OSA, particularly in those who cannot tolerate CPAP therapy.
Hence, identifying POSA as a phenotype may play an important role in the management of patients with OSA. Accordingly, the purpose of this study was to evaluate the prevalence and clinical predictors of POSA in a sample of the Saudi population using the common available definitions.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Sleep Medicine and Research Center (SMRC) at King Abdulaziz University Hospital (KAUH) and King Abdulaziz Medical City (KAMC), National Guard Health Affairs in Jeddah, Saudi Arabia. The study was approved by the Institutional Review Boards (IRBs) of KAUH and KAMC.
Study Population
All adult patients aged > 18 years referred to SMRC or KAMC who underwent complete polysomnography (PSG) between 2013 and 2019 were included in the study. In all studies, patients must have slept on supine and nonsupine positions. Patient with central sleep apnea or AHI of less than 5 were excluded. In addition, those diagnosed using a split night protocol were also excluded. The records of 379 patients from SMRC, KAUH and 245 patients from KAMC fulfilled the above criteria.
Data Collection Instruments
Patients’ medical records were reviewed for demographic data [age, sex, body mass index (BMI)]; Epworth Sleepiness Scale (ESS) score [20], which is a self-administered questionnaire routinely used to assess daytime sleepiness; and polysomnographic data. The scoring was standardized by following the American Academy of Sleep medicine (AASM) guidelines. In both centres, certified sleep technologists scored PSG records manually and certified sleep physicians reviewed them in accordance with AASM scoring rules [21]. All data were entered and configured using Microsoft Excel (2016).
In our study, the diagnosis of sleep apnoea was based on full polysomnography when the AHI was ≥ 5 events per hour of sleep [21]. POSA was defined in four different ways:
An AHI that was at least twice as high in the supine position than in the lateral position [8].
A supine AHI ≥ 10, together with a lateral AHI < 10 [22].
An AHI that was at least twice as high in the supine position than in the lateral position, but with a lateral AHI not exceeding 5 [6]; this is also called exclusive POSA (e-POSA).
An overall AHI ≥ 15; a supine AHI ≥ twice that of the nonsupine AHI ≥ 20 min of sleep in the supine and nonsupine positions; and a nonsupine AHI < 15 [23].
Furthermore, the following parameters were obtained from the polysomnographic data:
AHI: (number of apnoea events + number of hypopnoea events)/total sleep time (h)
Supine AHI: (number of apnoea events + number of hypopnoea events) while in the supine position/total sleep time (h) in the supine position
Nonsupine AHI: (Number of apnoea events + number of hypopnea events) while in the nonsupine position/total sleep time (h) in the nonsupine position
AHI in REM sleep: (number of apnoea events + number of hypopnoea events)/total sleep time (h) in REM sleep
AHI in non-REM sleep: (number of apnoea events + number of hypopnoea events)/total sleep time (h) in non-REM sleep
Time in bed (TIB): total time spent in bed from the lights off and lights on markers
Total sleep time (TST): period of sleep time between the lights off and lights on markers, excluding all wake stages.
Sleep efficiency (%): TST/TIB.
Mean O2 saturation: average value of the complete SpO2 curve.
Time spent with O2 saturation less than 90%: percentage of sleep time with oxygen saturation < 90%.
Statistical Analysis
Several characteristics of patients who developed POSA were compared to patients who did not develop POSA. For continuous variables, we started by assessing whether they fit a normal distribution using the visual approach (density plot and quantile‒quantile plot) and the Shapiro‒Wilk method. The mean and standard deviation were used for continuous variables with normal distribution. The median and interquartile range were used for continuous variables that were not normally distributed. To compare the continuous variables, we used either the Welch two-sample t test or the Wilcoxon rank sum test. For the categorical variables, frequencies and percentages were used. To evaluate the associations between the categorical variables, we applied the Chi-square or Fisher’s exact test. The Kruskal–Wallis test was applied for ordinal variables.
We initially performed an ANOVA to ascertain the overall differences between the groups. Then, variables with p values indicating significance in the ANOVA testing were further examined with the pairwise t test accounting for multiple testing with a Bonferroni correction of the p value. Because of the small number of comparisons (less than 5), we elected to use the Bonferroni correction; it is more conservative, which should reduce the false positive rate. Univariate and bivariate analyses were used to identify risk factors that influence the development of POSA based on each definition. Binary logistic regression was then used to evaluate the influence of several independent risk factors on the development of POSA based on each definition. This was reported using odds ratios (ORs) with 95% confidence intervals (CIs).
Sensitivity analysis was performed to compare the general performance of each definition of POSA compared to the standard definition (Definition 1) as the gold standard. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy were compared. To assess the predictive accuracy of each definition, the area under the receiver operating characteristic curve (AUROC) with standard error and 95% CIs was calculated. Because there is no gold standard definition that is accepted for POSA, we compared each definition with all the other definitions. All statistical tests were two-tailed, and p values < 0.05 were considered to indicate significance. All statistical analyses were performed using R software, version 4.0.2 [24].
Results
Patient Characteristics
The study cohort included 624 patients. The mean age was 50.2 years (SD 13.7), the mean BMI was 36.6 kg/m2 (SD 9.6), and 52.08% of the patients were male. The mean ESS score of the available data was 11.1 (SD = 5.6). Medical comorbidities were also obtained from patients’ records (Table 1).
Table 1.
General and sleep-related characteristics of the study population
| Variable | Full dataset (n = 624) |
|---|---|
| Age in years, mean (SD) | 50.2 (13.7) |
| Male sex, n (%) | 325 (52.08) |
| BMI in kg/m2, mean (SD) | 36.6 (9.6) |
| DM, n (%) | 205 (32.85) |
| COPD, n (%) | 230 (36.8) |
| Asthma, n (%) | 244 (39.1) |
| HTN, n (%) | 308 (49.4) |
| IHD, n (%) | 259 (41.5) |
| Epworth Sleepiness Scale score, mean (SD) | 11.1 (5.6) |
| TST (min), mean (SD) | 268.1 (66.2) |
| Sleep efficiency (%), mean (SD) | 69.7 (16.5) |
| Non-REM minutes, mean (SD) | 227 (54.6) |
| REM minutes, mean (SD) | 39.1 (23.3) |
| Time spent in supine position (min), mean (SD) | 10.7 (28.8) |
| AHI, mean (SD) | 22.2 (17) |
| AHI in REM sleep, mean (SD) | 36.5 (21.5) |
| AHI in non-REM sleep, mean (SD) | 19.4 (17.7) |
| AHI in the supine position, mean (SD) | 25.4 (20.8) |
| AHI in the nonsupine position, mean (SD) | 14 (16.3) |
| Mean O2 saturation, mean (SD) | 94.7 (2.3) |
| Minimum O2 saturation, mean (SD) | 50.2 (39.8) |
| Time spent < 90% O2 saturation (min), mean (SD) | 7 (14.3) |
BMI body mass index, ESS Epworth Sleepiness Scale, DM diabetes mellitus, COPD chronic obstructive pulmonary disease, HTN hypertension, IHD ischaemic heart disease, TST total sleep time, TIB time in bed, REM rapid eye movement, AHI apnoea–hypopnea index
Sleep-Related Characteristics of the Full Population
The polysomnographic data of all 624 patients were reviewed. The mean AHI was 22.2 (SD 17). The mean TIB and TST were 387.17 min (SD 45.9) and 268.1 min (SD 66.2), respectively (Table 1).
Characteristics of Patients Based on POSA Definition 1
Using the standard definition (Definition 1), the patients were divided into two groups: 46% of patients met the definition of non-POSA (non-POSA-Def 1), and 54% of patients met the definition of POSA (POSA-Def 1) Table 2.
Table 2.
General characteristics of patients classified according to POSA Definition 1
| Patient characteristics | All patients (n = 624) | Non-POSA* (n = 287, 46%) | POSA** (n = 337, 54%) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 50.2 (13.7) | 51.3 (13.9) | 49.3 (13.5) | 0.28 |
| Male sex, n (%) | 325 (52.08) | 137 (47.7) | 188 (55.8) | 0.045* |
| BMI (kg/m2), mean (SD) | 36.6 (9.6) | 38.1 (8.5) | 34.2 (7.7) | < 0.001* |
| DM, n (%) | 205 (32.85) | 83 (36.8) | 123 (43.6) | 0.12 |
| COPD, n (%) | 230 (36.8) | 105 (47.3) | 125 (44.2) | 0.484 |
| Asthma, n (%) | 244 (39.1) | 108 (47.6) | 136 (47.2) | 0.936 |
| HTN, n (%) | 308 (49.4) | 87 (36.2) | 123 (44.2) | 0.0646 |
| IHD, n (%) | 259 (41.5) | 124 (52.3) | 135 (48) | 0.332 |
| Epworth Sleepiness Scale score, mean (SD) | 11.1 (5.6) | 11.7 (5.8) | 10.8 (6.1) | 0.211 |
| AHI, mean (SD) | 22.2 (17) | 25.5 (19.2) | 19.5 (14.4) | < 0.001* |
| AHI in REM sleep, mean (SD) | 36.5 (21.5) | 40.7 (22) | 33 (20.5) | < 0.001* |
| AHI in the supine position, mean (SD) | 25.4 (20.8) | 22.5 (22.8) | 27.7 (18.9) | < 0.001* |
| Mean O2 saturation, mean (SD) | 94.7 (2.3) | 94.4 (2.4) | 95 (2.2) | 0.061 |
| Time spent < 90% O2 saturation, mean (SD) | 7 (14.3) | 8.7 (16) | 5.6 (12.5) | 0.009* |
*p value of <0.05 is considered to be significant
Males were more likely than females to suffer from POSA-Def 1 (55.8%, p value < 0.001). Patients with POSA-Def 1 had lower BMIs than patients with non-POSA-Def 1 (32.9 kg/m2 and 34.2 kg/m2 vs. 38.1 kg/m2, p value < 0.001). The time spent with an oxygen saturation of less than 90% during sleep was significantly shorter in patients with POSA-Def 1 than in patients with non-POSA-Def 1 (5.6 vs. 8.7 min, p value = 0.009). There was no significant difference between POSA-Def 1 patients and non-POSA-Def 1 patients in terms of comorbidities (Table 2). Based on the univariate logistic regression assessment of significant predictors of POSA, four variables were predictors: BMI ≤ 35 kg/m2 with an OR 1.76 (95% CI 1.27–2.44, p value < 0.001), male sex with an OR 1.38 (95% CI 1.01–0.1.89, p value = 0.0451), AHI > 10 with an OR 0.63 (95% CI 0.44–0.9, p value = 0.0123), and an AHI in REM > 20 with an OR 0.64 (95% CI 0.44–0.92, p value = 0.1717) (see Table 3 for all definitions in the univariate logistic regression analysis).
Table 3.
Univariate analysis of significant variables using logistic regression
| Variable | Units | Def 1 | Def 2 | Def 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI 95% | p value | OR | CI 95% | p value | OR | CI 95% | p value | ||
| Age in years | ≤ 50 | Ref | Ref | Ref | ||||||
| > 50 | 0.87 | [0.63;1.21] | 0. 4134 | 1.23 | [0.88;1.71] | 0.2209 | 0.75 | [0.53;1.05] | 0.0922 | |
| Gender | Female | Ref | Ref | Ref | ||||||
| Male | 1.38 | [1.01;1.89] | 0.04505 | 1.57 | [1.13;2.17] | 0.0068 | 1 | [0.72;1.40] | 0.9859 | |
| BMI in kg/m2 | > 35 | Ref | Ref | Ref | ||||||
| ≤ 35 | 1.76 | [1.27;2.44] | < 0.001 | 0.63 | [0.45;0.88] | 0.0061 | 1.52 | [1.08;2.15] | 0.0165 | |
| DM | No | Ref | Ref | Ref | ||||||
| Yes | 1.33 | [0.93;1.91] | 0.1202 | 0.73 | [0.50;1.06] | 0.0949 | 1.8 | [1.24;2.60] | 0.0019 | |
| COPD | No | Ref | Ref | Ref | ||||||
| Yes | 0.88 | [0.62;1.25] | 0.4837 | 1.12 | [0.78;1.61] | 0.5426 | 1 | [0.69;1.44] | 0.9971 | |
| Asthma | No | Ref | Ref | Ref | ||||||
| Yes | 0.99 | [0.70;1.40] | 0.9362 | 0.95 | [0.66;1.36] | 0.785 | 0.99 | [0.69;1.43] | 0.974 | |
| HTN | Yes | Ref | Ref | Ref | ||||||
| No | 0.72 | [0.50;1.02] | 0.06495 | 1.03 | [0.72;1.48] | 0.8733 | 0.60 | [0.42;0.87] | 0.0069 | |
| IHD | Yes | Ref | Ref | Ref | ||||||
| No | 1.19 | [0.84;1.68] | 0.3321 | 0.88 | [0.61;1.25] | 0.4687 | 1.1 | [0.70;1.44] | 0.999 | |
| AHI | < 10 | Ref | Ref | Ref | ||||||
| > 10 | 0.63 | [0.44;0.90] | 0.0123 | 92.56 | [22.67;377.89] | < 0.001 | 3.41 | [2.36;4.93] | < 0.001 | |
| AHI in REM | ≤ 20 | Ref | Ref | Ref | ||||||
| > 20 | 0.64 | [0.44;0.92] | 0.01717 | 5.13 | [3.19;8.26] | < 0.001 | 3.01 | [2.12;4.27] | < 0.001 | |
| Mean SaO2 in % | ≤ 95 | Ref | Ref | Ref | ||||||
| > 95 | 1.42 | [0.93;2.18] | 0.1061 | 0.55 | [0.35;0.86] | 0.0083 | 1.08 | [0.69;1.68] | 0.7482 | |
| Time SaO2 Less 90% | ≤ 2 | Ref | Ref | Ref | ||||||
| > 2 | 0.65 | [0.46;0.92] | 0.01386 | 1.97 | [1.39;2.79] | < 0.001 | 0.65 | [0.45;0.94] | 0.0223 | |
BMI body mass index, DM diabetes mellites, COPD chronic obstructive pulmonary disease, HTN hypertension, IHD ischaemic heart disease, AHI apnoea–hypopnea index, SaO2 oxygen saturation, REM rapid eye movement
Characteristics of Patients Based on POSA Definition 2
Using Definition 2, the patients were divided into two groups: 61.4% met the definition of non-POSA (non-POSA-Def 2), and 38.6% met the definition of POSA (POSA-Def 2) Table 4.
Table 4.
General characteristics of patients classified according to POSA Definition 2
| Patient characteristics | All patients (n = 624) | Non-POSA (n = 383, 61.4%) | POSA (n = 241, 38.6%) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 50.2 (13.7) | 49.8 (14) | 51 (13.1) | 0.934 |
| Male sex, n (%) | 325 (52.08) | 183 (47.8) | 142 (58.9) | 0.007* |
| BMI (kg/m2), mean (SD) | 36.6 (9.6) | 34.9 (8.3) | 37.7 (7.95) | < 0.001* |
| DM, n (%) | 205 (32.85) | 138 (43.4) | 67 (35.8) | 0.945 |
| COPD, n (%) | 230 (36.8) | 142 (44.5) | 88 (47.3) | 0.543 |
| Asthma, n (%) | 244 (39.1) | 155 (47.8) | 89 (46.6) | 0.785 |
| HTN, n (%) | 308 (49.4) | 131 (40.8) | 79 (40.1) | 0.873 |
| IHD, n (%) | 259 (41.5) | 157 (48.8) | 102 (52) | 0.496 |
| Epworth Sleepiness Scale score, mean (SD) | 11.1 (5.6) | 10.5 (5.7) | 11.9 (5.3) | 0.026* |
| AHI, mean (SD) | 22.2 (17) | 15.9 (13.2) | 32.6 (17.5) | < 0.001* |
| AHI in REM sleep, mean (SD) | 36.5 (21.5) | 29.5 (19.6) | 48.1 (19.5) | < 0.001* |
| AHI in the supine position, mean (SD) | 25.4 (20.8) | 16.5 (15) | 40.3 (20.6) | < 0.001* |
| Mean O2 saturation, mean (SD) | 94.7 (2.3) | 94.9 (2.29) | 94.5 (2.22) | 0.168 |
| Time spent < 90% O2 saturation, mean (SD) | 7 (14.3) | 5.8 (13.3) | 8.9 (15.6) | 0.012* |
*p value of <0.05 is considered to be significant
Males were still more likely to suffer from POSA-Def 2 (58.9%, p value < 0.001). Patients with POSA-Def 2 had a lower BMI than patients with non-POSA-Def 2 (32.9 kg/m2 and 34.9 kg/m2 vs. 37.7 kg/m2, p value < 0.001). There was no significant difference between POSA-Def two patients and non-POSA-Def 2 patients in terms of comorbidities. The time spent with a oxygen saturation of less than 90% during sleep was significantly longer in patients with POSA-Def 2 than in patients with non-POSA-Def 2 (8.7 vs. 5.6 min, p value = 0.012) (Table 4). Based on the univariate logistic regression assessment of significant predictors of POSA based on definition 2, six variables were associated with POSA: male sex with an OR 1.57 (95% CI 1.13–2.17, p value = 0.0068), BMI ≤ 35 kg/m2 with an OR 0.63 (95% CI 0.45–0.88, p value = 0.0061), AHI > 10 with an OR 92.6 (95% CI 22.7–377.9, p value < 0.001), AHI in REM > 20 with an OR 5.13 (95% CI 3.19–8.26, p value < 0.001), mean oxygen saturation > 95% with an OR 0.55 (95% CI = 0.35–0.86, p value = 0.008), and sleep time with an SaO2 less than 90% with an OR 1.97 (95% CI 1.39–2.79, p value < 0.001) (see Table 3 for all definitions in the univariate logistic regression analysis).
Characteristics of Patients Based on POSA Definition 3
Using Definition 3, the patients were divided into two groups: 66.2% met the definition of non-POSA (non-POSA-Def 3), and 33.8% met the definition of POSA (POSA-Def 3) Table 5.
Table 5.
General characteristics of patients classified according to POSA Definition 3
| Patient characteristics | All patients (n = 624) | Non-POSA (n = 413, 66.2%) | POSA (n = 211, 33.8%) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 50.2 (13.7) | 50.9 (13.5) | 49 (13.9) | 0.029* |
| Male sex, n (%) | 325 (52.08) | 215 (52.1) | 110 (52.1) | 0.986 |
| BMI (kg/m2), mean (SD) | 36.6 (9.6) | 37 (8.3) | 34.1 (7.9) | < 0.001* |
| DM, n (%) | 205 (32.85) | 115 (35.5) | 90 (49.7) | 0.002* |
| COPD, n (%) | 230 (36.8) | 148 (45.5) | 82 (45.6) | 0.997 |
| Asthma, n (%) | 244 (39.1) | 157 (47.4) | 87 (47.3) | 0.974 |
| HTN, n (%) | 308 (49.4) | 123 (36.3) | 87 (48.6) | 0.007* |
| IHD, n (%) | 259 (41.5) | 170 (50) | 89 (50) | 1 |
| Epworth Sleepiness Scale score, mean (SD) | 11.1 (5.6) | 11.3 (5.38) | 10.6 (5.93) | 0.698 |
| AHI score, mean (SD) | 22.2 (17) | 27.6 (21.4) | 18.9 (19.9) | < 0.001* |
| AHI score in REM sleep, mean (SD) | 36.5 (21.5) | 39.7 (21.8) | 30.3 (19.6) | < 0.001* |
| AHI in the supine position, mean (SD) | 25.4 (20.8) | 27.9 (23) | 20.6 (14.6) | < 0.001* |
| Mean O2 saturation, mean (SD) | 94.7 (2.3) | 94.7 (2.2) | 94.8 (2.4) | 1 |
| Time spent < 90% O2 saturation, mean (SD) | 7 (14.3) | 7.36 (14.6) | 6.2 (13.6) | 0.829 |
*p value of <0.05 is considered to be significant
Patients with POSA-Def 3 were younger than patients with non-POSA-Def 3 (49 years vs. 50.9 years, p value = 0.029). There was no male predominance as in the first two definitions. Patients with POSA-Def 3 had lower BMIs than patients with non-POSA-Def 3 (34.1 kg/m2 vs. 37 kg/m2, p value < 0.001). Based on Definition 3, diabetes mellitus (DM) and hypertension became statistically significant predictors (p value = 0.002 and 0.007, respectively), with a lower distribution in the non-POSA-Def 3 group (35.5% compared to 49.7% for DM, and 36.3% compared to 48.6% for hypertension in the POSA-Def 3 group). The time spent with an oxygen saturation of less than 90% during sleep was not significantly different in patients with POSA-Def 3 compared to patients with non-POSA-Def 3 (7.4 vs. 6.2 min, p value = 0.829). Based on the univariate logistic regression assessment of significant predictors of POSA, six variables were associated with POSA: BMI less than or equal to 35 kg/m2 with an OR = 1.52 (95% CI 1.08–2.15, p value = 0.017), history of DM with an OR 1.8 (95% CI 1.24–2.6, p value = 0.0019), history of hypertension with an OR 0.6 (95% CI 0.42–0.87, p value = 0.007), AHI > 10 with an OR 3.42 (95% CI 2.36–4.93, p value < 0.001), AHI in REM > 20 with an OR 3.01 (95% CI 2.12–4.27, p value < 0.001), and a time spent with an oxygen saturation less than 90% during sleep of more than 2 min with an OR 0.65 (95% CI 0.45–0.94, p value = 0.223) (see Table 3 for all definitions in the univariate logistic regression analysis).
Characteristics of Patients Based on POSA Definition 4
Using Definition 4, the patients were further divided into two groups: 91.7% of patients met the definition of non-POSA (non-POSA-Def 4), and 8.3% of patients met the definition of POSA (POSA-Def 4) Table 6.
Table 6.
General characteristics of patients classified according to POSA Definition 4
| Patient characteristics | All patients (n = 624) | Non-POSA (n = 572, 91.7%) | POSA (n = 52, 8.3%) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 50.2 (13.7) | 50.1 (13.8) | 51.8 (12.9) | 1 |
| Male sex, n (%) | 325 (52.08) | 292 (51) | 33 (63.5) | 0.086 |
| BMI (kg/m2), mean (SD) | 36.6 (9.6) | 36.2 (8.4) | 34 (6.6) | 0.214 |
| DM, n (%) | 205 (32.85) | 187 (40.5) | 18 (41.9) | 0.86 |
| COPD, n (%) | 230 (36.8) | 213 (46.1) | 17 (39.5) | 0.408 |
| Asthma, n (%) | 244 (39.1) | 230 (48.8) | 14 (31.8) | 0.031* |
| HTN, n (%) | 308 (49.4) | 189 (39.6) | 21 (51.2) | 0.147 |
| IHD, n (%) | 259 (41.5) | 241 (50.7) | 18 (41.9) | 0.265 |
| Epworth Sleepiness Scale score, mean (SD) | 11.1 (5.6) | 11.1 (5.61) | 10.3 (5.32) | 1 |
| AHI, mean (SD) | 22.2 (17) | 21.6 (17.4) | 25 (10.8) | 0.424 |
| AHI in REM sleep, mean (SD) | 36.5 (21.5) | 36.3 (21.6) | 38.3 (20.3) | 1 |
| AHI in the supine position, mean (SD) | 25.4 (20.8) | 24.2 (20.8) | 38 (15.5) | < 0.001* |
| Mean O2 saturation, mean (SD) | 94.7 (2.3) | 94.7 (2.3) | 95.4 (1.88) | 0.149 |
| Time spent < 90% O2 saturation, mean (SD) | 7 (14.3) | 7.18 (14.6) | 4.57 (9.6) | 0.488 |
*p value of <0.05 is considered to be significant
Interestingly, in the pairwise comparison, there was no significant difference between POSA-Def 4 patients and non-POSA-Def 4 patients in terms of demographic parameters, oxygenation parameters and comorbidities except for asthma (48.8% in non-POSA vs. 31.8% in POSA patients with a p value = 0.031) (Table 6). Because of the significant imbalance between positive and negative cases based on Definition 4, a simple univariate logistic regression was noninformative and was not used, as most of the variables had very wide confidence intervals mainly due to the small representation of positive cases in the dataset, which made finding a stable statistical solution impossible for the algorithm.
Differences Between the Four Sets of Criteria
There was no significant difference between the four sets of criteria with regard to age, sex, or comorbidities except for DM, which was significant in Definition 3, and ESS score, which was significant in Definition 2.
Sensitivity Analysis of the Four Definitions of POSA
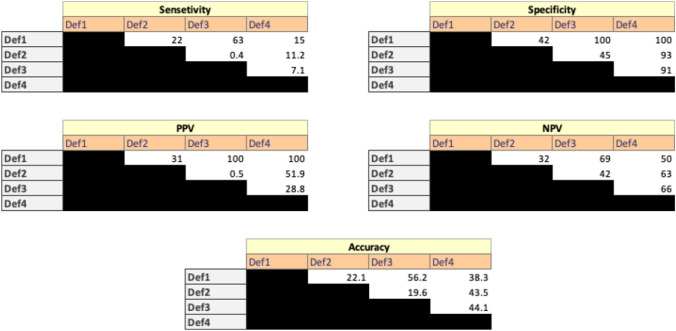
Since there is no standard definition for POSA and to determine the performance of each definition, a sensitivity analysis was conducted. Definition 1 was assumed to be the basic standard definition against which the other three definitions were compared. The following parameters were compared: sensitivity, specificity, PPV, NPV and accuracy. Figure 1 depicts the matrix of these comparisons. When assessing different combinations of definitions, starting with one definition as a screening tool, followed by another definition as a confirmatory tool, the combination of Definition 1 as a screening tool and Definition 3 as a confirmatory tool generated the best overall results (sensitivity 63%, specificity 100%, PPV 100%, NPV 69% and overall accuracy 56.2%). Other combinations were significantly worse. Since Definition 1 is part of Definition 3, and based on the sensitivity analysis, Definition 3 has the highest diagnostic yield for patients with POSA. Using the AUROC analysis, Fig. 2 redemonstrates that the combination of Definitions 1 and 3 resulted in the best diagnostic value of patients with POSA (AUC 90.2%).
Fig. 1.
Analysis matrix of all definitions
Fig. 2.
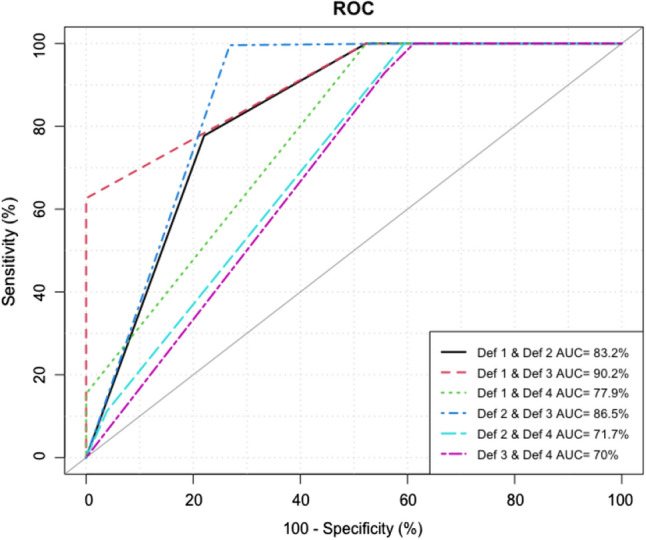
ROC-AUC for all possible definition combinations
Multivariate Logistic Regression
Based on the multivariate logistic regression, all definitions agreed that a BMI less than or equal to 35 kg/m2 was the only predictive variable for POSA. All other variables in Definitions 1 and 3 became nonsignificant. For Definition 2, male sex remained a significant predictor, and a mean oxygen saturation > 95% remained a significant negative predictor, but AHI and AHI in REM became nonsignificant. All multivariate models had a good predictive value (AUROC between 77.7 and 88.8%) (see Table 7 for all definitions in the multivariate logistic regression analysis).
Table 7.
Multivariate analysis using logistic regression
| Variable | Units | Def 1 | Def 2 | Def 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI 95% | p value | OR | CI 95% | p value | OR | CI 95% | p value | ||
| Age in years | ≤ 50 | Ref | Ref | Ref | ||||||
| > 50 | 0.74 | [0.31;1.80] | 0.5076 | 0.98 | [0.34;2.83] | 0.9679 | 0.74 | [0.31;1.80] | 0.5076 | |
| Gender | Female | Ref | Ref | Ref | ||||||
| Male | 1.21 | [0.50;2.90] | 0.6696 | 3.17 | [1.05;9.53] | 0.04 | 1.21 | [0.50;2.90] | 0.6696 | |
| BMI in kg/m2 | > 35 | Ref | Ref | Ref | ||||||
| ≤ 35 | 3.89 | [1.60;9.45] | 0.0027 | 0.21 | [0.07;0.70] | 0.0102 | 3.89 | [1.60;9.45] | 0.0027 | |
| DM | No | Ref | Ref | Ref | ||||||
| Yes | 2.53 | [0.90;7.11] | 0.0784 | 0.71 | [0.21;2.39] | 0.5769 | 2.53 | [0.90;7.11] | 0.0784 | |
| COPD | No | Ref | Ref | Ref | ||||||
| Yes | 1.16 | [0.08;17.23] | 0.9160 | 4,395,779.65 | [0.00;Inf] | 0.9942 | 1.16 | [0.08;17.23] | 0.9160 | |
| Asthma | No | Ref | Ref | Ref | ||||||
| Yes | 0.45 | [0.16;1.22] | 0.1154 | 1.64 | [0.47;5.71] | 0.4376 | 0.45 | [0.16;1.22] | 0.1154 | |
| HTN | Yes | Ref | Ref | Ref | ||||||
| No | 0.50 | [0.18;1.39] | 0.1817 | 1.98 | [0.61;6.42] | 0.2540 | 0.50 | [0.18;1.39] | 0.1817 | |
| IHD | Yes | Ref | Ref | Ref | ||||||
| No | 3.82 | [0.80;18.31] | 0.0932 | 0.31 | [0.05;1.91] | 0.2078 | 3.82 | [0.80;18.31] | 0.0932 | |
| AHI | < 10 | Ref | Ref | Ref | ||||||
| > 10 | 1.08 | [0.41;2.79] | 0.8812 | 0.00 | [0.00;Inf] | 0.9915 | 1.08 | [0.41;2.79] | 0.8812 | |
| AHI in REM | ≤ 20 | Ref | Ref | Ref | ||||||
| > 20 | 0.46 | [0.17;1.24] | 0.1245 | 3.63 | [1.00;13.27] | 0.0508 | 0.46 | [0.17;1.24] | 0.1245 | |
| Mean SaO2 | ≤ 95 | Ref | Ref | Ref | ||||||
| > 95 | 1.31 | [0.44;3.86] | 0.6283 | 0.20 | [0.05;0.84] | 0.0276 | 1.31 | [0.44;3.86] | 0.6283 | |
| Time SaO2 less 90% | ≤ 2 | Ref | Ref | Ref | ||||||
| > 2 | 0.38 | [0.13;1.17] | 0.0912 | 0.77 | [0.19;3.15] | 0.7158 | 0.38 | [0.13;1.17] | 0.0912 | |
| ROC-AUC | 77.7 | [69.5;85.8] | 88.8 | [83.3;94.4] | 80.6 | [72.2;88.9] | ||||
Discussion
Different studies have shown that more than 50% of patients with OSA are likely to have POSA. It was also found that in approximately 80% of OSA patients, the AHI was higher in the supine position than in the nonsupine position. Unfortunately, despite the high prevalence of POSA, clinicians focus mainly on CPAP, and PT is usually ignored. Therefore, in this study, we tried to determine the prevalence of POSA using the four commonly applied definitions and address its positive predictors. The prevalence of POSA in our study was 54% (Definition 1; Cartwright), 38.6% (Definition 2; Marklund), 33.8% (Definition 3; Mador), and 8.3% (Definition 4; Bignold). Based on the sensitivity analysis, the Mador definition had the highest diagnostic yield for POSA, with a sensitivity and specificity of 63% and 100%, respectively. Furthermore, with multivariate regression analysis, a BMI < 35 kg/m2 was the only significant predictor of PSA across all applied definitions.
The prevalence of POSA in the literature, as shown in our study, depends primarily on the chosen definition. In our study, we found that with the Cartwright definition, the prevalence of POSA was 54%, which was similar to that reported in several studies. Studies from the United Arab Emirates (UAE), Australia, France, Switzerland and Denmark reported the following prevalence rates of POSA: 53%, 61%, 53.5%, 53% and 62.3%, respectively [10, 19, 25–27]. However, using the Mador definition, we reported a prevalence of 33.8%, which was again close to that reported in the literature. Studies from France, Switzerland and Denmark reported prevalence rates of 20.1%, 26%, and 29.1%, respectively [10, 19, 27].
We went further and tried to identify the predictors of POSA. Our study revealed that male sex, younger age, a lower BMI, time spent with an oxygen saturation less than 90% during sleep, DM, hypertension and a history of asthma were significant factors associated with POSA. However, when a multivariate regression analysis was used, a low BMI of less than 35 kg/m2 and male sex remained positive predictors for POSA. It is not clear why low BMI is a predictor of POSA. It is expected that high BMI rather than low BMI will be associated with POSA. High BMI may make the patient symptomatic regardless of the position, and hence it may be difficult to differentiate between symptoms of the patient, regardless of the position during sleep. It is possible that low BMI may be associated with mild OSA, which is only obvious during POSA. Indeed, this link between low BMI, mild OSA and POSA was already reported in the literature and again shown in the current study according to Definition 1 of POSA. Nevertheless, more studies are required to clarify this association. Indeed, our study has shown that an AHI > 10 and an AHI during REM > 20 were associated with a low OR (0.63 and 0.64, respectively), which indicates that the milder the disease, the more likely POSA will develop (Definition 1, Cartwright). The same findings were seen with Definition 2 (Marklund), although the parameters measuring the severity of sleep apnoea showed conflicting results. Moreover, with Definition 3 (Mador), low BMI, DM and severe OSA according to a AHI > 10 and REM AHI > 20 were identified predictors for POSA, while hypertension was identified as a negative predictor for POSA. Similarly, when multivariate regression analysis was applied taking into consideration all other variables, only a low BMI of less than 35 kg/m2 remained a predictive variable across all definitions. Compared with other definitions, Marklund (Definition 2) also revealed that male sex and better oxygenation according to a mean oxygen saturation of > 95% remained significant predictors for POSA. Nevertheless, our findings seem to be in agreement with the findings in the literature. Studies have shown that male sex, younger age, lower AHI, lower BMI and time in the supine position are associated with POSA [10, 19, 25]. Moreover, the Mallampati score and heavy alcohol consumption were found to be associated factors in previous studies [10, 25]. Zinchuk et al. [28] also reported that patients with POSA tend to be younger, have a lower BMI, and have lower AHIs than their nonpositional counterparts. Uzer et al. [29] also emphasized that POSA patients have a lower BMI than REM-related OSA patients. In the UAE study, age, BMI, diastolic blood pressure, Mallampati score, and Berlin score were found to be the best predictive factors for POSA, with an AUC of 0.71 (95% CI [0.63, 0.78]) [26]. Oksenberg et al. [15] found that POSA patients were less obese and had less severe OSA (p < 0.001) than non-POSA patients among the severe OSA patients they studied. Hence, our study agrees with the literature in that patients with POSA are less obese and have milder disease. Furthermore, our study failed to show a link between POSA and comorbidities regardless of which definition was used. This finding supports the notion that POSA is more likely to be present in patients with relatively less severe OSA. In pure POSA, non-CPAP PT may obviate the need for the use of CPAP. Moreover, in severe OSA with associated elements of POSA, using PT tends to help reduce pressure steering in CPAP.
However, do patients with POSA convert to non-POSA upon follow-up? Oksenberg et al. [30] reported that approximately two-thirds of POSA patients remained in the supine position predominantly upon follow-up for a mean of 6.6 years; however, the remaining patients converted to non-POSA. This information highlights the importance of close follow-up of these patients and that most POSA patients would benefit from postural therapy if they remained compliant with therapy.
This study to our knowledge is the first investigation of the prevalence of POSA using four different commonly applied definitions. The results align with the observed trend that a lower BMI is a positive predictor of POSA. The limitations of our study include its cross-sectional, the retrospective nature, and PT interventions were not studied. Also, it is not multi-centre study and based on hospital based data. Further randomized controlled trials are needed to investigate the positive effects of PT on OSA and to confirm the patient characteristics that are predictive of POSA.
Conclusion
POSA is common, and its prevalence depends on the definition used. It seems to be associated with male sex, milder disease and a relatively low BMI. It seems that Mador’s definition of e-POSA yields the highest sensitivity, specificity and a stable AUROC. Regardless of the definition used, a lower BMI is a strong predictor of POSA. This finding emphasizes the importance of non-CPAP PT, which is currently relatively underutilized in clinical practice. Nevertheless, POSA remains a common condition, with variable prevalence depending on the definition used.
Acknowledgements
The authors are grateful to Mrs. Walaa Abuzahra, Research Coordinator, Sleep Medicine and Research Center, for coordinating all study procedures.
Abbreviations
- AHI
Apnoea–hypopnea index
- AUROC
Area under the receiver operating characteristic curve
- BMI
Body mass index
- CIs
Confidence intervals
- CPAP
Continuous positive airway pressure
- DM
Diabetes mellitus
- e-POSA
Exclusive POSA
- ESS
Epworth Sleepiness Scale
- IRBs
Institutional review boards
- KAMC
King Abdulaziz Medical City
- KAUH
King Abdulaziz University Hospital
- LM
Lifestyle modification
- non-POSA
Nonpositional sleep apnoea
- NPV
Negative predictive value
- ORs
Odds ratios
- OSA
Obstructive sleep apnoea
- POSA
Positional obstructive sleep apnoea
- POSA
Positional obstructive sleep apnoea
- PPV
Positive predictive value
- PSG
Polysomnography
- PT
Positional therapy
- SDB
Sleep-disordered breathing
- SMRC
Sleep Medicine and Research Center
- TIB
Time in bed
- TST
Total sleep time
- UAE
United Arab Emirates
Author Contributions
SW, SQ, FA and RS designed the study and wrote the manuscript. MA, MN and RB collected the data. HM performed the statistical analyses. All the authors reviewed the manuscript.
Funding
None.
Data Availability
Data is available upon request.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval and consent to participate
Ethical approval was obtained from the Unit of Biomedical Ethics at King Abdulaziz University Hospital, Jeddah, Saudi Arabia, with reference number 365-20.
Consent for Publication
This is a retrospective study, no consent needed.
References
- 1.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 2.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/s2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wali SO, Abalkhail B, Krayem A. Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Ann Thorac Med. 2017;12:88–94. doi: 10.4103/1817-1737.203746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22:6. doi: 10.1007/s11886-020-1257-y. [DOI] [PubMed] [Google Scholar]
- 5.Ho ML, Brass SD. Obstructive sleep apnea. Neurol Int. 2011;3:e15. Doi:10.4081/ni.2011.e15 [DOI] [PMC free article] [PubMed]
- 6.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–2137. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 7.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 9.Schiza SE, Mermigkis C, Bouloukaki I. The influence of a clinical classification system on the management of patients with positional dependent obstructive sleep apnoea. Sleep Breath. 2015;19:741–743. doi: 10.1007/s11325-014-1037-2. [DOI] [PubMed] [Google Scholar]
- 10.Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med. 2018;48:157–162. doi: 10.1016/j.sleep.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao YN, Wu YC, Lin SY, Chang JZ, Tu YK. Short-term efficacy of minimally invasive treatments for adult obstructive sleep apnea: a systematic review and network meta-analysis of randomized controlled trials. J Formos Med Assoc. 2019;118:750–765. doi: 10.1016/j.jfma.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Ravesloot MJ, Van Maanen JP, Dun L, De Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17:39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkas A, Duce B, Töyräs J, Seppä J, Leppänen T. Comparison of the effect of weight change, simulated computational continuous positive airway pressure treatment and positional therapy on severity of sleep apnea. J Sleep Res. 2021;30:e13070. 10.1111/jsr.13070 [DOI] [PubMed]
- 15.Oksenberg A, Gadoth N, Töyräs J, Leppänen T. Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA. Sleep Breath. 2020;24:551–559. doi: 10.1007/s11325-019-01897-1. [DOI] [PubMed] [Google Scholar]
- 16.Oksenberg A, Gadoth N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res. 2014;23:204–210. doi: 10.1111/jsr.12097. [DOI] [PubMed] [Google Scholar]
- 17.Permut I, Diaz-Abad M, Chatila W, Crocetti J, Gaughan JP, D'Alonzo GE, Krachman SL. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238–243. doi: 10.5664/jcsm.27820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry RB, Uhles ML, Abaluck BK, Winslow DH, Schweitzer PK, Gaskins RA, Doekel RC, Emsellem HA. Night Balance sleep position treatment device versus auto-adjusting positive airway pressure for treatment of positional obstructive sleep apnea. J Clin Sleep Med. 2019;15:947–956. doi: 10.5664/jcsm.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabil A, Blanchard M, Trzepizur W, Goupil F, Meslier N, Paris A, Pigeanne T, Priou P, Le Vaillant M, Gagnadoux F. Positional obstructive sleep apnea within a large multicenter French cohort: prevalence, characteristics, and treatment outcomes. J Clin Sleep Med. 2020;16:2037–2046. doi: 10.5664/jcsm.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Ver. 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012.
- 22.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–1635. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 23.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–383. doi: 10.5664/jcsm.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
- 25.Garg H, Er XY, Howarth T, Heraganahally SS. Positional sleep apnea among regional and remote Australian population and simulated positional treatment effects. Nat Sci Sleep. 2020;12:1123–1135. doi: 10.2147/nss.s286403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oulhaj A, Al Dhaheri S, Su BB, Al-Houqani M. Discriminating between positional and non-positional obstructive sleep apnea using some clinical characteristics. Sleep Breath. 2017;21:877–884. doi: 10.1007/s11325-017-1499-0. [DOI] [PubMed] [Google Scholar]
- 27.Laub RR, Mikkelsen KL, Ønnesen P. Prevalence of positional obstructive sleep apnea and patients characteristics using various definitions. Eur Respir J. 2015;46:2372. 10.1183/13993003.congress-2015.PA2372
- 28.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev. 2017;35:113–123. doi: 10.1016/j.smrv.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzer F, Toptas AB, Okur U, Bozkurt S, Dogrul E, Turhan M, Cilli A. Comparison of positional and rapid eye movement-dependent sleep apnea syndromes. Ann Thorac Med. 2018;13:42–47. doi: 10.4103/atm.ATM_184_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oksenberg A, Goizman V, Eitan E, Nasser K, Gadoth N, Leppänen T. Obstructive sleep apnea: do positional patients become nonpositional patients with time? Laryngoscope. 2020;130:2263–2268. doi: 10.1002/lary.28387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.