Abstract
Background & Objective
High prevalence of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus results in deleterious complications and morbidities related to both diseases. Thus, we aimed to investigate dietary and anthropometric risk factors for progression of Non-alcoholic Fatty Liver Disease (NAFLD) in diabetic people.
Methods
Anthropometric, and dietary intakes, and hepatic steatosis and fibrosis were assessed in two hundred participants with type two diabetes (T2DM). Subjects with CAP score of more than 270 dB/m were considered to have NAFLD. Multivariable-adjusted ORs and 95% CIs were used to investigate the association between NAFLD and dietary inflammatory index (DII) score and anthropometric indices.
Results
Participants in the highest tertile of DII had 2.41 (95% CI:1.16-4.97), 2,53 (95% CI: 1.04-6.16), 2.78 (95% CI: 1.09-7.13) times higher odds of developing NAFLD in comparison to the lowest tertile in crude, adjusted model 1 and 2, respectively. Among those with the highest relative to the lowest tertile of trunk-to-leg fat ratio (TLR), ORs and 95% CI were OR = 1.88, 95% CI = 0.9-3.91, and OR = 7.99, 95% CI = 2.43-26.26 in crude and full-adjusted models. Odds of NAFLD in the third tertile of metabolic score for visceral fat (METS-VF) was higher than the first tertile in crude (OR = 9.5, 95% CI = 4.01-22.46) and full-adjusted models (OR = 4.55, 95% CI = 1.46-14.2).
Conclusions
In conclusion, this study highlighted an association between greater DII (pro-inflammatory diet) and higher NAFLD risk. Moreover, TLR and METS-VF are known as novel estimators of central obesity as a risk factor for NAFLD in diabetes.
Keywords: Type-2 diabetes, Diet, Inflammation, Obesity, TLR, METS-VF
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is recognized by excessive hepatic accumulation of fat especially triglycerides that is defined in the absence of secondary causes of hepatic steatosis including alcohol consumption, chronic viral hepatitis, and medications, among other things [1]. NAFLD is commonly accompanied with the other metabolic risk factors including insulin resistance, obesity and dyslipidemia [2]. There is an upward trend in the incidence of NAFLD worldwide, its prevalence is reported to be roughly 25% in western countries, and up to 40% in Asian populations [3]. Diabetes mellitus type 2 (T2DM) is known as a risk factor that increases in the prevalence and severity of fatty liver disease. Globally, 56% and 37% diabetic people are affected by NAFLD and NASH, respectively [4]. The coexistence of NAFLD and T2DM is associated with more severe liver tissue damage like NASH, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [5]. Moreover, it can lead to a worse metabolic profile, atherogenic dyslipidemia such as hypertriglyceridemia, higher LDL levels, decreased levels of HDL and ultimately an increased risk of cardiovascular diseases [6,7]. NAFLD can also increase the severity of T2DM and results in exacerbation of risks related to diabetes like microvascular complications [8]. Obesity is known as one of the main risk factors for NAFLD [9]. It is suggested that central obesity is a stronger factor for prediction of metabolic disorders and NAFLD may be developed in people with no general obesity [10,11]. Previous studies have revealed that people with NAFLD had higher visceral fat content and waist circumference compared with the control group after adjusting for body mass index (BMI) [[11], [12], [13]]. Metabolic dysfunction is influenced by distribution of body fat that cannot be detected using BMI alone [14]. A higher ratio of trunk-to-lower body fat as reported in obese subjects with steatosis is compared with those with no steatosis [15]. In addition, Cioffi et al. found that adolescents with higher trunk-to-leg fat ratio (TLR) had higher triglycerides, total cholesterol, homeostasis model assessment of insulin resistance (HOMA‐IR), c‐reactive protein, and alanine aminotransferase (ALT), and lower high‐density lipoprotein (HDL) cholesterol [16]. In Kim et al. study, subjects with metabolic syndrome showed a greater ratio of trunk to leg fat [17]. Mariani et al. found that higher TLR is positively associated with steatosis in individuals with severe hepatic fat accumulation [18]. Furthermore, metabolic score for visceral fat (METS-VF) is introduced as an efficient estimator of visceral fat and has been a good indicator to predict diabetes and hypertension [19,20]. It is also demonstrated that subjects who had higher METS-VF progressed from metabolically healthy to unhealthy phenotype and an increased visceral fat tissue (VAT) is associated with development of unhealthy phenotype even if weight loss occurs [21]. Inflammation is hypothesized as one of the underlying factors in pathogenesis of NAFLD that can cause stress response in hepatocytes and result in progression of NAFLD [22]. Dietary modifications, physical activity and weight loss are considered main tools to manage NAFLD [23]. Specific dietary patterns may play protective or destructive roles in development of NAFLD [24]. An unhealthy diet which is characterized by higher content of fat, refined carbohydrate and protein can increase inflammatory markers. In contrast, a healthy diet which is known as anti-inflammatory diet includes greater proportion of fruits, vegetables, fish, omega-3 and fiber that is associated with less inflammatory potential [25,26]. It is suggested that the key point of an anti-inflammatory diet is the stabilization of insulin and decreased omega-6 fatty acids consumption [27]. Dietary inflammatory index (DII) has been described as a tool to assess the contribution of dietary exposure to inflammation and by using 45 anti-inflammatory and pro-inflammatory dietary components represents how inflammatory potential of diet can influence chronic diseases [28]. Higher dietary inflammatory index (DII) was suggested in subjects with higher liver damage, and a direct association was observed between DII and fatty liver index values (>50th percentile FLI) [24]. Ramírez-Vélez et al. found that lower DII score (anti-inflammatory diet) can reduce AST:ALT ratio and FLI. Odds ratio (OR) for NAFLD decreased in the lowest DII tertile (anti-inflammatory) compared to the highest DII tertile (pro-inflammatory) [29]. The same findings showing the inverse link between lower DII and FLI were reported in a cross-sectional study [30]. Results of a case-control study revealed that participants in the third tertile of DII had 2.02 times risk for developing of NAFLD [31].
According to the high prevalence of NAFLD among diabetic people and negative complications following this coexistence, the focal purpose of this research was pinpointing the nutritional and anthropometrical risk factors leading to development of NAFLD in type-2 diabetes which is less addressed compared to each one individually. In fact, previous findings revealed that NAFLD can predict the development of T2DM and vice versa [32]. Therefore, we hypothesized to identify underlying risk factors that may pertain to progression of NAFLD in diabetic patients. This study was designed to evaluate the link between inflammatory burden of diet and severity of NAFLD in people with diabetes on account of influences that inflammatory pathways exert on NAFLD and its development. In addition, since obesity plays an outsize role in incidence of NAFLD, we aimed to assess the relation between central obesity using various examination tools and exacerbation of NAFLD in type-2 diabetes. Thus, current study is the first study that investigates the association of DII and central obesity with NAFLD in diabetic participants using TLR and METS-VF.
2. Methods
2.1. Study design
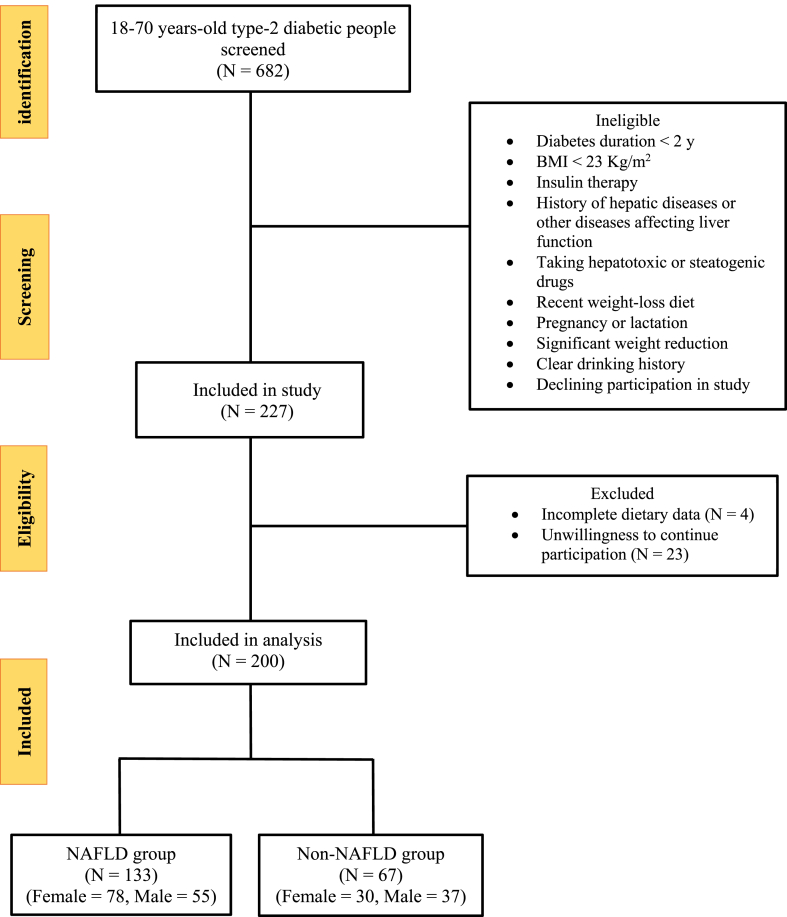
This cross-sectional study was conducted on two-hundred people with T2DM, aged 18–70 years who referred to the Institute of Diabetes and Metabolism, Iran University of Medical Sciences Fig. 1. Out Of these, 133 participants were diagnosed to have NAFLD, based on a controlled attenuation parameter (CAP) score more than 270 dB/m (NAFLD group) and 67 participants had a CAP ≤270 dB/m (control group). Subjects were excluded if they met the following criteria: (1) body mass index less than 23 kg/m2 (2) diabetes duration less than 2 years (3) alcohol consumption more than 21 units/week for men and 14 units/week for women (4) individuals with acute or chronic liver disorders (hepatitis), gallbladder diseases, cancer, autoimmune and hereditary diseases that affect liver status, chronic inflammatory diseases, renal diseases, and heart failure (5) pregnancy and lactation (6) taking insulin (7) use of certain drugs including anti-inflammatory and drugs affecting liver, hormones, corticosteroids (8) being on a weight loss diet and weight loss of more than 10% in the past six months. This study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (NO: IR. SBMU.NNFTRI.REC.1400.010) on May 12, 2021.
Fig. 1.
Flowchart of the study participants Clinical, paraclinical and physical activity assessment.
Hepatic steatosis and fibrosis were assessed by transient elastography (Fibroscan®) (Echosens, Paris, France) [33]. Blood pressure was measured using an automatic sphygmomanometer according to standard protocols (OMRON, Germany). Moreover, Metabolic equivalent of task (MET) questionnaire was employed to assess the physical activity of participants [34]. Serum fasting blood sugar (FBS), serum triglyceride (TG), serum high density lipoprotein (HDL), and serum cholesterol were measured by enzymatic assays method using standard biochemical kits (Pars Azmoon Co., Iran), and Roche Diagnostics kits (Roche Cobas 6000 analyzer) were used for serum insulin measurement via ECLIA method. Homeostatic model assessment for insulin resistance (HOMA) was calculated using the formula: fasting insulin (μU/L) × fasting glucose (nmol/L)/22.5.
2.2. Anthropometric assessment
Weight measurement was carried out using a digital scale (Seca 808, Germany) with an accuracy of 0.1 kg and height was measured by the use of a wallmounted stadiometer with a sensitivity of 0.1 cm (Seca, Germany). Afterwards, Body mass index (BMI = weight (kg)/height (m2)) was calculated. To measure waist circumference, the point between the lowest rib and the iliac crest was considered. Hip circumference was measured by a non-elastic tape considering the maximum level. Then, waist to hip ratio (WHR) and waist to height ratio (WHtR) were calculated.
Evaluation of body composition was implemented by dual-energy X-ray absorptiometry (DEXA). The fat mass in different regions of body was obtained. Then, trunk to leg fat mass ratio was calculated (Trunk to leg fat ratio = Trunk fat (kg)/Right and Left leg fat (kg)) [35].
Metabolic score for visceral fat (METS-VF) was designed to estimate VAT that includes a non-insulin-based metabolic score for insulin resistance (METS-IR). In comparison to imaging method, it indicated a better performance to predict incidence of type 2 diabetes and hypertension [36]. To calculate METS-IR, the formula below is used:
| METS-IR = [Ln ((2 × FG) + TG)] × BMI/ [Ln (HDL cholesterol)] | (1) |
By considering waist to height ratio (WHtR) and sex that is expressed by male (1) and female (0) and age which is given is years, the METS-VF can be calculated:
| METS-VF = 4.466 + 0.011[(Ln (METS-IR))3] + 3.239[(Ln (WHtR))3 + 0.319(Sex) + 0.594(Ln (Age)) | (2) |
The following transformation is required to estimate VAT:
| VAT (g) = e4.466 + 0.011[(Ln (METS-IR))3] + 3.239[(Ln (WHtR))3 + 0.319(Sex) + 0.594(Ln (Age)) | (3) |
2.3. Food intake assessment and calculation of the DII
A validated semi-quantitative food frequency questionnaire (FFQ) with 147 food items was used for dietary data collection [37]. All FFQ questionnaire was completed by a trained expert. Analysis of food items was conducted by Nutritionist 4 software (First Databank Inc., Hearst Corp, San Bruno, CA, USA) modified for Iranian foods. Dietary inflammatory index (DII) that explains a link between diet and inflammation was first introduced by Shivappa et al. In fact, it is suggested that specific nutrients and food items are associated with pro-inflammatory [(IL-1β), (IL-6), (TNF-α) or CRP] or anti-inflammatory [(IL-4) and (IL-10)] biomarkers [38]. A total of 31 food parameters were included to calculate DII (energy, carbohydrate, fat, protein, fiber, cholesterol, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), saturated fats (SFAs), trans fatty acids, omega-3, omega-6, thiamin, riboflavin, niacin, pyridoxine, folic acid, cobalamin, vitamin A, C, D, E, β-carotene, zinc, selenium, magnesium, iron, caffeine, garlic, onion, and black tea). First, the energy-adjusted amount of nutrients was calculated using residual method [39]. Second, the ratio of the standard global mean from the quantity of food parameters consumed by each individual to the global standard deviation was calculated in order to obtain the Z score for all the 31 parameters using Shivappa et al. data [38]. Then it was converted to the percentile score. The calculated value was multiplied by the effect score for all food items derived from Shivappa et al. [38]. In the end, sum of each DII score from all the components generated the overall DII score for every participant. DII score is either negative or positive. The more positive score is related with more pro-inflammatory effects of diet and more anti-inflammatory effects is indicated when the score is more negative [30].
2.4. Statistical analysis
To examine normality of the data, The Kolmogorov–Smirnov test was performed. Chi-square test was used for comparison of qualitative variables between the study groups as well as different tertiles of DII. The quantitative variables with normal and non-normal distribution were compared using independent t-test and Mann–Whitney test, respectively. Comparisons across tertiles of DII were performed using one-way ANOVA and Kruskal-Wallis H tests for normal and non-normal distributions, respectively. Binary logistic regression was used to evaluate the association of DII and different anthropometric indices with NAFLD as a dependent variable. We synthesized two regression models for DII and three models for anthropometric indices. The crude and adjusted odds ratios (95% CI) for NAFLD in tertiles 2 and 3 of each index were compared to the first tertile as the reference. SPSS Version 16.0; (SPSS Inc., Chicago, IL) was used to carry out statistical analysis and P < 0.05 was considered statistically significant.
3. Results
Two-hundred people with T2DM including 133 participants (78 women and 55 men) with (NAFLD group) and 67 (30 women and 37 men) without NAFLD (control group) were studied. The mean age of participants in the NAFLD and control groups were 52.19 ± 9.06 years and 52.24 ± 9.75 years, respectively. General characteristics, anthropometric indices and energy-adjusted dietary inflammatory index (E-DII) of the study participants are shown in Table 1.
Table 1.
comparison of participants' characteristics, anthropometric indices and DII between NAFLD and non-NAFLD group.a.
| NAFLD group (n = 133) | Non-NAFLD group (n = 67) | p-value∗ | |
|---|---|---|---|
| Age (years) | 52.19 (9.06) | 52.24 (9.75) | 0.846 |
| Female (%) | 58.64 | 44.77 | 0.072 |
| Diabetes duration (year) | 8.47 (5.26) | 9.74 (6.77) | 0.278 |
| CAP score (dB/m) | 320.75 (33) | 247.67 (21.66) | 0.000 |
| Physical activity (MET-min/week) | 950.83 (1757.85) | 738.06 (683.27) | 0.374 |
| Current smoker (%) | 18.04 | 16.41 | 0.846 |
| SBP (mmHg) | 123.14 (14.55) | 124.72 (16.03) | 0.581 |
| DBP (mmHg) | 77.86 (10.42) | 75.37 (9.02) | 0.116 |
| Weight (kg) | 81.4 (15.08) | 72.7 (10.91) | 0.000 |
| BMI (kg/m2) | 30.07 (4.06) | 26.17 (3.42) | 0.000 |
| WC (cm) | 103.56 (10.88) | 94.44 (7.67) | 0.000 |
| HC (cm) | 108 (8.06) | 101.73 (7.18) | 0.000 |
| WHR | 0.95 (0.06) | 0.92 (0.06) | 0.000 |
| WHtR | 0.63 (0.06) | 0.56 (0.05) | 0.000 |
| Trunk fat mass (kg) | 17.75 (4.29) | 13.15 (3.01) | 0.000 |
| Total leg fat mass (kg) | 9.81 (3.03) | 7.97 (3.04) | 0.000 |
| TLR | 1.89 (0.43) | 1.76 (0.4) | 0.045 |
| METS-VF | 7.2 (0.26) | 6.9 (0.34) | 0.000 |
| VAT (g) | 1387.88 (360.31) | 1054.89 (344.82) | 0.000 |
| E-DII | −2.72 (0.67) | −2.93 (0.75) | 0.041 |
CAP, controlled attenuation parameter; MET, metabolic equivalents; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; HC, hip circumference, WHR, waist circumference to hip circumference ratio, WHtR, waist to height ratio; TLR, trunk to leg fat ratio, METS-VF, metabolic score for visceral fat; VAT, visceral adiposity tissue; E-DII, energy-adjusted dietary inflammatory index.
Data were presented as mean (SD) except for gender and smoking status which (%) were given.
P values were obtained from Mann Whitney U test or chi-square test. Independent sample test was applied for TLR, METS-VF, VAT and E-DII.
Compared to the control group, participants in the NAFLD group had greater weight, BMI, waist circumference, hip circumference, waist to hip ratio (WHR), waist to height ratio (WHtR), trunk fat mass, and total leg fat mass. Moreover, trunk-to-leg fat ratio (TLR), metabolic score for visceral fat (METS-VF), and DII were significantly higher in participants with NAFLD compared to those without NAFLD. In addition, participants in NAFLD group were more likely to have higher visceral adiposity tissue (VAT) estimated from METS-VF. However, no significant difference was observed between the NAFLD and control groups in terms of age, sex, blood pressure, physical activity and smoking status. Also, participants' characteristics and anthropometric indices did not significantly differ in the two study groups across various tertiles of DII. Nevertheless, trunk fat mass was significantly higher in the third tertile of DII compared to the first tertile as presented in Table 2.
Table 2.
Participant characteristics and anthropometric indices across tertiles of E-DII.a
| Energy-adjusted dietary inflammatory index tertilesb |
||||
|---|---|---|---|---|
| T1 (n = 66) < −3.11 |
T2 (n = 67) (-3.11) - (−2.49) |
T3 (n = 67) −2.49 < |
P-value∗ | |
| Age (years) | 52.3 (9.39) | 51.37 (9.07) | 52.94 (9.43) | 0.616 |
| Female (%) | 57.57 | 53.73 | 50.74 | 0.731 |
| Diabetes duration (year) | 9.23 (5.4) | 8.5 (5.94) | 8.8 (6.16) | 0.576 |
| CAP score (dB/m) | 281.3 (43.24) | 306.56 (43.65) | 300.71 (46.41) | 0.004 |
| Physical activity (MET-min/week) | 14.98 (18.75) | 17.72 (36.32) | 11.27 (12.95) | 0.837 |
| Current smoker (%) | 15.15 | 29.85 | 29.85 | 0.198 |
| SBP (mmHg) | 123.94 (16.51) | 125.27 (14.34) | 121.79 (14.2) | 0.485 |
| DBP (mmHg) | 77.12 (11.39) | 77.49 (9.28) | 76.46 (9.4) | 0.548 |
| Weight (kg) | 75.04 (11.64) | 81.4 (14.88) | 78.98 (15.79) | 0.06 |
| BMI (kg/m2) | 27.93 (3.98) | 29.65 (4.2) | 28.7 (4.49) | 0.054 |
| WC (cm) | 98 (8.75) | 102.16 (11.94) | 101.31 (11.14) | 0.147 |
| HC (cm) | 104.52 (7.21) | 107.34 (8.32) | 105.82 (9.13) | 0.154 |
| WHR | 0.93 (0.06) | 0.95 (0.06) | 0.95 (0.07) | 0.218 |
| WHtR | 0.59 (0.05) | 0.61 (0.07) | 0.61 (0.06) | 0.196 |
| Trunk fat mass (kg) | 14.81 (3.65) | 16.82 (4.52) | 16.97 (4.86) | 0.011 |
| Total leg fat mass (kg) | 8.86 (3.16) | 9.39 (2.86) | 9.34 (3.43) | 0.466 |
| TLR | 1.77 (0.44) | 1.85 (0.39) | 1.9 (0.43) | 0.216 |
| METS-VF | 7.04 (0.33) (n = 65) |
7.13 (0.28) (n = 66) |
7.11 (0.36) (n = 66) |
0.192 |
| VAT (g) | 1202.37 (385.61) (n = 65) |
1309.79 (361.36) (n = 66) |
1310.64 (411.12) (n = 66) |
0.186 |
CAP, controlled attenuation parameter; MET, metabolic equivalents; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; HC, hip circumference, WHR, waist circumference to hip circumference ratio, WHtR, waist to height ratio; TLR, trunk to leg fat ratio, METS-VF, metabolic score for visceral fat; VAT, visceral adiposity tissue; E-DII, energy-adjusted dietary inflammatory index.
Data were presented as mean (SD) except for gender and smoking status which (%) were given.
DII score of individuals in first tertile was less than (−3.11), second tertile was between (−3.11) and (−2.49), third tertile was more than (−2.49).
P values were obtained from Kruskal-Wallis test, chi-square test and one-way ANOVA.
Table 3 demonstrates dietary intake of participants in different tertiles of DII. Consumption of energy, total fats, saturated fat, cholesterol, MUFA was significantly higher in the third tertile of DII than those in the first tertile. Furthermore, dietary intake of vitamin K, biotin, beta-carotene, alpha-carotene, and lutein significantly decreased from the first to the third tertile of DII. Third tertile of DII score was associated with lower intakes of whole-grains, vegetables, nuts, white meat, and olive oil as well as greater intakes of refined-grains, high-fat dairy, fast foods, and salt.
Table 3.
Dietary intake of study participants across tertiles of E-DII.a
| Energy-adjusted dietary inflammatory index tertilesb |
||||
|---|---|---|---|---|
| T1 (n = 66) < −3.11 |
T2 (n = 67) (-3.11) - (−2.49) |
T3 (n = 67) −2.49 < |
P-value∗ | |
| Energy (kcal/d) | 2331.61 (821.56) | 2378.21 (802.90) | 2787.87 (1086.38) | 0.007 |
| Nutrients | ||||
| Proteins (g/d) | 90.01 (32.41) | 117.38 (256.19) | 125.79 (261.20) | 0.599 |
| Carbohydrates (g/d) | 355.91 (135.17) | 352.66 (176.71) | 374.69 (163.98) | 0.690 |
| Total Fats (g/d) | 71.5 (27.88) | 82.11 (41.03) | 111.42 (58.77) | 0.000 |
| SFA (g/d) | 20.29 (7.01) | 26.82 (23.47) | 37.65 (21.93) | 0.000 |
| Cholesterol (mg/d) | 266.42 (251.85) | 236.9 (114.93) | 347.48 (275.75) | 0.001 |
| MUFA (g/d) | 24.77 (9.4) | 26.43 (8.91) | 36.57 (20.16) | 0.000 |
| PUFA (g/d) | 16.69 (7.34) | 17.05 (6.57) | 22.37 (16.18) | 0.149 |
| Omega 3 (g/d) | 1.06 (0.69) | 1.29 (1.79) | 1.75 (2.45) | 0.013 |
| Omega 6 (g/d) | 1.75 (2.61) | 2.17 (3.23) | 2.63 (4.79) | 0.297 |
| Vitamin A (RAE/d) | 713.48 (373.33) | 723.22 (667.4) | 755.28 (598.96) | 0.386 |
| Vitamin C (mg/d) | 189 (102.83) | 244.91 (545.18) | 213.72 (305.73) | 0.323 |
| Vitamin D (mcg/d) | 1.86 (1.63) | 1.74 (2.39) | 2.17 (3.09) | 0.468 |
| Vitamin E (mg/d) | 14.25 (5.66) | 13.86 (5.43) | 16.1 (10.67) | 0.861 |
| Vitamin K (mcg/d) | 318.14 (307.56) | 218.5 (141.31) | 178.33 (129.09) | 0.000 |
| Vitamin B12 (mcg/d) | 3.71 (2.17) | 18.26 (116.12) | 11.84 (52.58) | 0.016 |
| Biotin (mg/d) | 43.23 (18.53) | 33.89 (13.89) | 38.83 (23.02) | 0.006 |
| Zn (mg/d) | 13.69 (5.37) | 18.82 (49.7) | 19.86 (50.88) | 0.699 |
| Cu (mg/d) | 2.03 (0.75) | 2.06 (1.52) | 1.97 (1.25) | 0.224 |
| Mn (mg/d) | 10.37 (4.67) | 8.98 (4.44) | 7.49 (4.04) | 0.000 |
| Beta-carotene (mcg/d) | 5156.56 (2971.1) | 3798.28 (2415.14) | 3083.05 (1800.06) | 0.000 |
| Alpha-carotene (mcg/d) | 1312.59 (990.38) | 888.24 (849.13) | 713.33 (576.98) | 0.000 |
| Lutein (mcg/d) | 3391.32 (2417.81) | 2309.55 (1374.85) | 1936.78 (1199.59) | 0.000 |
| Food groups | ||||
| Whole grains (g/d) | 201.21 (159.47) | 146.27 (132.56) | 106.9 (132.14) | 0.000 |
| Refined grains (g/d) | 224.48 (138.09) | 268.1 (128.28) | 336.06 (168.76) | 0.000 |
| Vegetables (g/d) | 493.7 (282.99) | 375.81 (204.59) | 375.21 (240.96) | 0.006 |
| Fruits (g/d) | 505.3 (304.93) | 568.92 (552.71) | 565.96 (373.29) | 0.574 |
| High-fat dairy (g/d) | 47.87 (84.68) | 73.42 (113.29) | 92.16 (128.56) | 0.012 |
| Legumes (g/d) | 33.89 (46.23) | 29.49 (27.6) | 22.57 (22.77) | 0.079 |
| Nuts (g/d) | 10.44 (11.61) | 6.13 (6.62) | 8.05 (10.49) | 0.018 |
| White meat (g/d) | 45.23 (30.04) | 40.86 (36.81) | 42.53 (64.54) | 0.016 |
| Fast foods (g/d) | 3.23 (6.74) | 3.87 (5.71) | 7.34 (15.85) | 0.004 |
| Olive oil (g/d) | 1.56 (2.69) | 0.93 (1.6) | 0.79 (1.96) | 0.032 |
| Salt (g/d) | 4.29 (4.91) | 4.89 (5.07) | 8.43 (7.73) | 0.001 |
Data were presented as mean (SD).
DII score of individuals in first tertile was less than (−3.11), second tertile was between (−3.11) and (−2.49), third tertile was more than (−2.49). MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
P values were obtained from Kruskal-Wallis test and one-way ANOVA.
Multivariable-adjusted ORs and 95% CIs were used to investigate the association between NAFLD and DII score in different categories as displayed in Table 4. Overall, participants in highest tertile of DII had 2.41 (95% CI:1.16-4.97), 2,53 (95% CI: 1.04-6.16), 2.78 (95% CI: 1.09-7.13) times higher odds of developing NAFLD in comparison to lowest tertile in crude, model 1 (adjusted for age, sex, diabetes duration, smoking status, physical activity, energy intake, BMI and WHtR), model 2 (further adjustment for HOMA, serum fasting blood sugar, serum triglyceride and serum cholesterol), respectively. In addition, men in third tertile of DII showed nearly a three-fold (OR = 3.09, 95% CI = 1.08–8.81), five-fold (OR = 5.26, 95% CI = 1.22–22.62), and seven-fold (OR = 6.84, 95% CI = 1.32-35.48) increase in likelihood of NAFLD compared with the first tertile, respectively. The similar relation was not significant in women.
Table 4.
Odds ratios (ORs) and 95% confidence intervals (95% CIs) for NAFLD in diabetic people according to tertiles of energy-adjusted dietary inflammatory index.
| Energy-adjusted dietary inflammatory index quartilesa |
P trendb | |||
|---|---|---|---|---|
| T1 (n = 66) < −3.11 |
T2 (n = 67) (-3.11) - (−2.49) |
T3 (n = 67) −2.49 < |
||
| OR (95% CI) | OR (95% CI) P-value |
OR (95% CI) P-value |
||
| Crude | ||||
| Overall | 1 (ref.) | 2.41 (1.16-4.97) P = 0.017 |
2.41 (1.16-4.97) P = 0.017 |
0.015 |
| Women | 1 (ref.) | 1.75 (0.64-4.76) P = 0.274 |
2.25 (0.77-6.5) P = 0.134 |
0.124 |
| Men | 1 (ref.) | 3.77 (1.27-11.17) P = 0.016 |
3.09 (1.08-8.81) P = 0.035 |
0.039 |
| Model 1 | ||||
| Overall | 1 (ref.) | 2.05 (0.87-4.81) P = 0.096 |
2.53 (1.04-6.16) P = 0.04 |
0.035 |
| Women | 1 (ref.) | 1.46 (0.43-4.92) P = 0.538 |
2.2 (0.55-8.67) P = 0.26 |
0.255 |
| Men | 1 (ref.) | 3.51 (0.89-13.87) P = 0.073 |
5.26 (1.22-22.62) P = 0.025 |
0.025 |
| Model 2 | ||||
| Overall | 1 (ref.) | 2.52 (1.02-6.22) P = 0.04 |
2.78 (1.09-7.13) P = 0.032 |
0.025 |
| Women | 1 (ref.) | 2.12 (0.5-9.05) P = 0.3 |
1.56 (0.33-7.38) P = 0.574 |
0.5 |
| Men | 1 (ref.) | 5.45 (1.13-26.22) P = 0.034 |
6.84 (1.32-35.48) P = 0.022 |
0.022 |
Model 1: Adjusted for age (continuous), sex (except for sex-stratified models) (female/male), diabetes duration (continuous), smoking (smoker/non-smoker), physical activity (continuous), energy intake (kcal/d), BMI (continuous) and WHtR (continuous).
Model 2: further adjustments for serum fasting blood sugar (continuous), serum triglyceride (continuous), serum cholesterol (continuous) and HOMA (continuous).
DII score of individuals in first tertile was less than (−3.11), second tertile was between (−3.11) and (−2.49), third tertile was more than (−2.49).
Odds ratios (ORs) and 95% CIs were obtained using binary logistic regression models. The overall trend of ORs was examined by the use of tertiles of the DII as an ordinal variable in the model.
Table 5 shows multivariable-adjusted ORs and 95% CI for progression of NAFLD in various categories of anthropometric indices. Overall, different indices for assessment of central obesity were positively associated with development of NAFLD. The third tertile of WC was associated with approximately 9 (OR = 9.03, 95% CI = 3.82-21.34), 12 (OR = 12.41, 95% CI = 4.77-32.23), 14.5 (OR = 14.56, 95% CI = 5.28-40.16), 4 (OR = 3.71, 95% CI = 1.03-13.31) times higher risk of NAFLD compare with lowest tertile in crude, model 1 (age, sex, diabetes duration, smoking status, physical activity, energy intake), model 2 (additional adjustment for HOMA, serum fasting blood sugar, serum triglyceride, and serum cholesterol), model 3 (further adjustment for BMI), respectively. Risk of NAFLD increased in third tertile of WHR by factor of roughly 3 (OR = 2.69, 95% CI = 1.21-5.99), 4.5 (OR = 4.42, 95% CI = 1.74-11.21), 5 (OR = 4.64, 95% CI = 1.77-12.15), 3 (OR = 2.94, 95% CI = 1.05-8.25) in crude, model 1, model 2, model 3 respectively. Participants in highest tertile of WHtR had nearly 23 (OR = 23.18, 95% CI = 8.17–65.74), 29 (OR = 29.15, 95% CI = 9.45–89.86), 33 (OR = 33.06, 95% CI = 10.25–106.59), and 13 (OR = 13.1, 95% CI = 2.68-63.99) times greater odds of NAFLD in crude, model 1, model 2 and model 3, respectively. Among those with the highest relative to the lowest tertile of TLR, ORs and 95% CI were OR = 1.88, 95% CI = 0.9-3.91, OR = 4.66, 95% CI = 1.77–12.27, OR = 5.27, 95% CI = 1.93–14.39, and OR = 7.99, 95% CI = 2.43–26.26 in crude, model 1, model 2, model 3, respectively. Odds of NAFLD in third tertile of METS-VF were higher than first tertile in crude (OR = 9.5, 95% CI = 4.01–22.46), model 1 (OR = 10.87, 95% CI = 4.45–26.53), model 2 (OR = 13.85, 95% CI = 5.38–35.63) and model 3 (OR = 4.55, 95% CI = 1.46–14.2).
Table 5.
Odds ratios (ORs) and 95% confidence intervals (95% CIs) for NAFLD in diabetic individuals according to tertiles of anthropometric indices.
| Crude OR (95% CI) P-value |
Model 1 OR (95% CI) P-value |
Model 2 OR (95% CI) P-value |
Model 3 OR (95% CI) P-value |
|
|---|---|---|---|---|
| WC | ||||
| T1 (n = 63) < 95 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| T2 (n = 67) 95-104 | 2.72 (1.33-5.56) P = 0.006 |
2.97 (1.39-6.34) P = 0.005 |
3.53 (1.6-7.82) P = 0.002 |
1.88 (0.77-4.54) P = 0.161 |
| T3 (n = 70) > 104 | 9.03 (3.82-21.34) P = 0.000 |
12.41 (4.77-32.23) P = 0.000 |
14.56 (5.28-40.16) P = 0.000 |
3.71 (1.03-13.31) P = 0.044 |
| P trend* | 0.000 | 0.000 | 0.000 | 0.04 |
| WHR | ||||
| T1 (n = 66) < 0.91 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| T2 (n = 69) 0.91-0.97 | 0.79 (0.39-1.57) P = 0.508 |
0.97 (0.45-2.08) P = 0.946 |
0.94 (0.43-2.07) P = 0.889 |
0.83 (0.34-1.98) P = 0.678 |
| T3 (n = 65) > 0.97 | 2.69 (1.21-5.99) P = 0.015 |
4.42 (1.74-11.21) P = 0.002 |
4.64 (1.77-12.15) P = 0.002 |
2.94 (1.05-8.25) P = 0.04 |
| P trend* | 0.02 | 0.002 | 0.002 | 0.049 |
| WHtR | ||||
| T1 (n = 66) < 0.57 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| T2 (n = 67) 0.57-0.62 | 4.72 (2.26-9.83) P = 0.000 |
6.16 (2.73-13.91) P = 0.000 |
6.93 (2.92-16.47) P = 0.000 |
4.7 (1.76-12.49) P = 0.002 |
| T3 (n = 67) > 0.62 | 23.18 (8.17-65.74) P = 0.000 |
29.15 (9.45-89.86) P = 0.000 |
33.06 (10.25-106.59) P = 0.000 |
13.1 (2.68-63.99) P = 0.001 |
| P trend* | 0.000 | 0.000 | 0.000 | 0.001 |
| TLR | ||||
| T1 (n = 66) < 1.65 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| T2 (n = 67) 1.65-2.02 | 1.41 (0.69-2.87) P = 0.335 |
2.45 (1.05-5.68) P = 0.036 |
3.07 (1.26-7.51) P = 0.014 |
4.92 (1.65-14.64) P = 0.004 |
| T3 (n = 67) > 2.02 | 1.88 (0.9-3.91) P = 0.089 |
4.66 (1.77-12.27) P = 0.002 |
5.27 (1.93-14.39) P = 0.001 |
7.99 (2.43-26.26) P = 0.001 |
| P trend* | 0.088 | 0.002 | 0.001 | 0.001 |
| METS-VF | ||||
| T1 (n = 65) < 6.99 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| T2 (n = 66) 6.99-7.26 | 3.71 (1.79-7.68) P = 0.000 |
4.06 (1.91-8.61) P = 0.000 |
4.34 (1.97-9.53) P = 0.000 |
2.47 (1.04-5.84) P = 0.039 |
| T3 (n = 66) >7.26 |
9.5 (4.01-22.46) P = 0.000 |
10.87 (4.45-26.53) P = 0.000 |
13.85 (5.38-35.63) P = 0.000 |
4.55 (1.46-14.2) P = 0.009 |
| P trend* | 0.000 | 0.000 | 0.000 | 0.007 |
Model 1: age (except for METS-VF) (continuous), sex (except for METS-VF) (female/male), diabetes duration (continuous), smoking (smoker/non-smoker), physical activity (continuous), energy intake (kcal/d). Model 2: further adjustment for serum fasting blood sugar (except for METS-VF) (continuous), serum triglyceride (except for METS-VF) (continuous), serum cholesterol (continuous) and HOMA (continuous). Model 3: additionally adjusted for BMI (continuous). WC, waist circumference; WHR, waist circumference to hip circumference ratio; WHtR, waist circumference to height ratio; TLR, trunk to leg fat ratio; METS-VF, metabolic score for visceral fat. * Odds ratios (ORs) and 95% CIs were obtained using binary logistic regression models. The overall trend of ORs was examined by the use of tertiles of the anthropometric indices as an ordinal variable in models.
4. Discussion
The findings of this study support this hypothesis that central obesity and following a pro-inflammatory diet may be associated with higher odds of NAFLD in patients with diabetes. The associations between DII, central obesity and NAFLD in general population were reported in several studies previously [11,24,29]. A cohort study of 8520 adults revealed that DII score was associated with the non-invasive liver markers (ALT, AST, and GGT) [30]. Consistently, in examining the relationship between the Mediterranean dietary pattern as an anti-inflammatory diet and NAFLD in obese individuals, it was found that there is a positive association between DII and fatty liver index [24]. The study of Mazidi et al. [40] also disclosed a significant impact of adiposity on the relation between NAFLD and DII score. The association of DII with the rise in BMI has also been reported [30].
Pro-inflammatory diets have been proposed as potential threat for NAFLD because they might disturb hepatic β‐oxidation, increase endogenous lipid production, and up-regulate the expression of pro‐inflammatory molecules and oxygen reactive species [31]. Given that obesity is an inflammatory condition and is directly related to fatty liver, it seems that inflammatory dietary patterns and higher DII scores are positively associated to adiposity and NAFLD, which confirms the findings of the present study.
Furthermore, the present study revealed that higher DII score was associated with higher energy intake, total fat, saturated fat, cholesterol and MUFA. Also, the increase in DII score was significantly allied with lower intake of legumes, nuts, white meat, olive oil and higher intake of fast food and salt. Although inconstant, previous studies have reported similar findings. An inverse association of healthy foods with inflammation and inflammatory diseases has been reported frequently [24,41]. Furthermore, it has been reported previously that anti-inflammatory nutrients favorably affect NAFLD [42].
Another finding of this study was that mean TLR, VAT, METS-VF, weight, waist circumference and WHR were significantly higher in diabetic subjects with NAFLD compared to diabetic participants without NAFLD. A positive association has been also found between TLR, METS-VF, waist circumference, WHtR and WHR with odds of NAFLD. Previously, it has been reported that the association of trunk-to-peripheral fat ratio with cardiometabolic risk factors is independent of whole-body fat mass [43]. Visceral obesity appears to be a predictor of NAFLD, even mild disease, in non-obese individuals [44]. Visceral obesity is an important risk factor for NAFLD, not only in obese and diabetic patients but also in relatively healthy individuals [44]. It has been shown that the risk of NAFLD is higher in lean people with visceral obesity than in lean people with normal waist circumferences [45].
Although BMI is the most common parameter associated with obesity, it does not reflect body fat distribution. While, WHR, TLR, WHtR and METS-VF can reflect visceral fat distribution, independently of weight. These indicators are good predictors of cardiovascular risk factors and related diseases such as NAFLD [20,46]. Waist circumference and WHR have been introduced as two reliable indicators for assessing visceral adiposity and fatty liver disease [47]. In agreement with the findings of the present study, Atri et al. [48] in a study of 106 morbid obese women showed a link between waist circumference and WHtR with steatosis.
These findings support the idea that diet-induced inflammation and visceral adiposity may increase the risk of metabolic abnormalities and NAFLD. Similar to these results, recent examination of the relationship between DII and body fat percent (BF%) in boys showed that higher BF% is associated with higher DII [49]. Andrade et al. [50] found in 6-month follow-up of obese women who underwent bariatric surgery that women with higher DII experienced less weight loss and less fat loss. In a study conducted by Aslani et al. [51], the association of pro-inflammatory diets with higher obesity indices including waist circumference, hip circumference and abdominal obesity in children and adolescents was reported. Contradictorily, Correa-Rodriguez et al. [52] failed to show a correlation between DII and BMI and fat mass in adults.
The association between body fat and inflammation appears to be bidirectional. Pro-inflammatory diets increase adipose tissue, and increased fat mass in turn increases inflammation and related diseases such as NAFLD [53,54]. Underlying mechanism of association between DII, obesity and NAFLD is not yet fully understood. Previous studies, however, have indicated that pro-inflammatory diets stimulate the production of cytokines such as TNF-α, IL-1, and IL-6 which increase appetite and calorie intake [55].
Considering the role of inflammation in the pathogenesis of NAFLD and the high prevalence of NAFLD in people with T2DM, based on the findings of this study, it seems that anti-inflammatory diets can play a significant role in alleviating development of NAFLD in diabetic patients and central obesity using the assessed indices especially METS-VF and TLR is identified as a risk factor that can be conducive to progression of NAFLD in T2DM.
Our study had several strengths. The main strength of this study was its novelty and originality as this is the first study investigating the association between nutritional factors like DII and anthropometric factors related to central obesity and development of NAFLD in people with T2DM. Moreover, all confounding variables were identified and added to models. Also, a validated and reliable FFQ was applied to assess dietary intakes of participants via a face-to-face interview done by an expert nutritionist. Furthermore, Inclusion criteria were designed in a way to limit the possible effects of underlying factors to some extent. Lastly, Evaluation of body composition was done using DEXA as a gold standard. Our study faced several limitations as follows: it was not possible to infer the causality relationship because the study was designed to be cross-sectional. Using FFQ is inevitably associated with recall bias. Also in this study, inflammatory markers were not measured, which is suggested to be considered in future studies. For the first time, this study investigated the relationship between diet-induced inflammation and central obesity with severity of NAFLD in people with T2DM, so confirming the results requires further studies. It is suggested that in future studies, a larger sample size and prospective design should be considered.
5. Conclusion
In conclusion, this study highlighted an association between greater DII (pro-inflammatory diet) and higher risk of progression of NAFLD in diabetic patients. Also, it was shown that central obesity estimated specifically by TLR and METS-VF were positively associated with an increased risk of developing NAFLD in diabetic subjects.
Author contribution statement
Samira Soltanieh: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Marieh Salavatizadeh, Asieh Mansour, Hossein Poustchi, and Mojtaba Malek: Performed the experiments.
Zahra Yari: Analyzed and interpreted the data; Wrote the paper.
Mohammad E. Khamseh, and Fariba Alaei-Shahmiri: Conceived and designed the experiments.
Azita Hekmatdoost: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant Number: 26839).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
Contributor Information
Fariba Alaei-Shahmiri, Email: alaeishahmiri.f@iums.ac.ir.
Azita Hekmatdoost, Email: a_hekmat2000@yahoo.com.
References
- 1.Marjot T., et al. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr. Rev. 2020;41(1):66–117. doi: 10.1210/endrev/bnz009. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Muthiah M.D., Sanyal A.J. Burden of disease due to nonalcoholic fatty liver disease. Gastroenterol. Clin. 2020;49(1):1–23. doi: 10.1016/j.gtc.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Khneizer G., Rizvi S., Gawrieh S. Springer; 2020. Non-alcoholic Fatty Liver Disease and Diabetes Mellitus; pp. 417–440. (Diabetes: from Research to Clinical Practice). [DOI] [PubMed] [Google Scholar]
- 5.Bril F., Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 6.Bril F., et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J. Clin. Endocrinol. Metab. 2016;101(2):644–652. doi: 10.1210/jc.2015-3111. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui M.S., et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin. Gastroenterol. Hepatol. 2015;13(5):1000–1008. e3. doi: 10.1016/j.cgh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira Barbosa J., Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol. Commun. 2021;5(2):158–167. doi: 10.1002/hep4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi Z., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15(1):11. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 10.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Pang Q., et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J. Gastroenterol.: WJG. 2015;21(5):1650. doi: 10.3748/wjg.v21.i5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G., et al. Associations between liver histology and cortisol secretion in subjects with nonalcoholic fatty liver disease. Clin. Endocrinol. 2006;64(3):337–341. doi: 10.1111/j.1365-2265.2006.02466.x. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N., et al. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am. J. Gastroenterol. 2003;98(12):2771–2776. doi: 10.1111/j.1572-0241.2003.08767.x. [DOI] [PubMed] [Google Scholar]
- 14.Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 15.Vega G.L., et al. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology. 2007;46(3):716–722. doi: 10.1002/hep.21727. [DOI] [PubMed] [Google Scholar]
- 16.Cioffi C.E., et al. Truncal‐to‐leg fat ratio and cardiometabolic disease risk factors in US adolescents: NHANES 2003‐2006. Pediatric Obesity. 2019;14(7) doi: 10.1111/ijpo.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y.M., et al. Clinical and body compositional factors associated with metabolic syndrome in obese koreans: a cross-sectional study. Metab. Syndr. Relat. Disord. 2018;16(6):290–298. doi: 10.1089/met.2017.0174. [DOI] [PubMed] [Google Scholar]
- 18.Mariani S., et al. Plasma levels of SIRT1 associate with non-alcoholic fatty liver disease in obese patients. Endocrine. 2015;49(3):711–716. doi: 10.1007/s12020-014-0465-x. [DOI] [PubMed] [Google Scholar]
- 19.Antonio‐Villa N., et al. The combination of insulin resistance and visceral adipose tissue estimation improves the performance of metabolic syndrome as a predictor of type 2 diabetes. Diabet. Med. 2020;37(7):1192–1201. doi: 10.1111/dme.14274. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y., et al. Metabolic Score for Visceral Fat: a reliable indicator of visceral obesity for predicting risk for hypertension. Nutrition. 2022;93 doi: 10.1016/j.nut.2021.111443. [DOI] [PubMed] [Google Scholar]
- 21.Elías-López D., et al. 2020. Natural Course of Metabolically Healthy Phenotype and Risk of Developing Cardiometabolic Diseases: a Three Years Follow-Up Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi N., et al. Liver histology during Mipomersen therapy for severe hypercholesterolemia. J. Clin. Lipidol. 2014;8(6):606–611. doi: 10.1016/j.jacl.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantero I., et al. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin. Nutr. 2018;37(5):1736–1743. doi: 10.1016/j.clnu.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia N., et al. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39(2):99–110. doi: 10.1016/j.diabet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano D., Ceriello A., Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006;48(4):677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Sears B. Anti-inflammatory diets. J. Am. Coll. Nutr. 2015;34(sup1):14–21. doi: 10.1080/07315724.2015.1080105. [DOI] [PubMed] [Google Scholar]
- 28.Farhadnejad H., et al. The association between dietary inflammation scores and non-alcoholic fatty liver diseases in Iranian adults. BMC Gastroenterol. 2022;22(1):1–9. doi: 10.1186/s12876-022-02353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramírez‐Vélez R., et al. The Dietary Inflammatory Index and hepatic health in the US adult population. J. Hum. Nutr. Diet. 2021;35(5):968–979. doi: 10.1111/jhn.12962. [DOI] [PubMed] [Google Scholar]
- 30.Darbandi M., et al. Anti-inflammatory diet consumption reduced fatty liver indices. Sci. Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-98685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vahid F., et al. Association of pro-inflammatory dietary intake and non-alcoholic fatty liver disease: findings from Iranian case-control study. Int. J. Vitam. Nutr. Res. 2019;88(3–4):144–150. doi: 10.1024/0300-9831/a000571. [DOI] [PubMed] [Google Scholar]
- 32.Kosmalski M., et al. The coexistence of nonalcoholic fatty liver disease and type 2 diabetes mellitus. J. Clin. Med. 2022;11(5):1375. doi: 10.3390/jcm11051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malekzadeh R., Poustchi H. Fibroscan for assessing liver fibrosis: an acceptable alternative for liver biopsy: fibroscan: an acceptable alternative for liver biopsy. Hepat. Mon. 2011;11(3):157. [PMC free article] [PubMed] [Google Scholar]
- 34.Ainsworth B.E., et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32(9; SUPP/1):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 35.Wilson J.P., et al. Ratio of trunk to leg volume as a new body shape metric for diabetes and mortality. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bello-Chavolla O.Y., et al. Metabolic Score for Visceral Fat (METS-VF), a novel estimator of intra-abdominal fat content and cardio-metabolic health. Clin. Nutr. 2020;39(5):1613–1621. doi: 10.1016/j.clnu.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Esfahani F.H., et al. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J. Epidemiol. 2010;20(2):150–158. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivappa N., et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Publ. Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 40.Mazidi M., et al. Diet with greater inflammatory potential is associated with higher prevalence of fatty liver among US adults. Eur. J. Clin. Nutr. 2019;73(12):1653–1656. doi: 10.1038/s41430-018-0364-y. [DOI] [PubMed] [Google Scholar]
- 41.Thoma C., Day C.P., Trenell M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J. Hepatol. 2012;56(1):255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Harrison S.A., et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003;98(11):2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 43.Kouda K., et al. Associations between trunk-to-peripheral fat ratio and cardiometabolic risk factors in elderly Japanese men: baseline data from the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Environ. Health Prev. Med. 2021;26(1):1–12. doi: 10.1186/s12199-021-00959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ha Y., et al. Intimate association of visceral obesity with non‐alcoholic fatty liver disease in healthy A sians: a case‐control study. J. Gastroenterol. Hepatol. 2015;30(11):1666–1672. doi: 10.1111/jgh.12996. [DOI] [PubMed] [Google Scholar]
- 45.Fracanzani A.L., et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin. Gastroenterol. Hepatol. 2017;15(10):1604–1611. e1. doi: 10.1016/j.cgh.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 46.Huxley R., et al. Body mass index, waist circumference and waist: hip ratio as predictors of cardiovascular risk—a review of the literature. Eur. J. Clin. Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 47.Reis S.S., et al. Correlation between anthropometric measurements and non-alcoholic fatty liver disease in individuals with obesity undergoing bariatric surgery: cross-sectional study. Obes. Surg. 2021;31(8):3675–3685. doi: 10.1007/s11695-021-05470-2. [DOI] [PubMed] [Google Scholar]
- 48.Atri A., et al. The prevalence and predictors of non-alcoholic fatty liver disease in morbidly obese women–A cross-sectional study from Southern India. Eur. Endocrinol. 2020;16(2):152. doi: 10.17925/EE.2020.16.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gholamalizadeh M., et al. Associations between the dietary inflammatory index with obesity and body fat in male adolescents. BMC Endocr. Disord. 2022;22(1):1–8. doi: 10.1186/s12902-022-01001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade P.A., et al. Baseline pro-inflammatory diet is inversely associated with change in weight and body fat 6 months following-up to bariatric surgery. Obes. Surg. 2019;29(2):457–463. doi: 10.1007/s11695-018-3530-3. [DOI] [PubMed] [Google Scholar]
- 51.Aslani Z., et al. Association of dietary inflammatory index with anthropometric indices in children and adolescents: the weight disorder survey of the childhood and adolescence surveillance and prevention of adult non-communicable disease (CASPIAN)-IV study. Br. J. Nutr. 2019;121(3):340–350. doi: 10.1017/S0007114518003240. [DOI] [PubMed] [Google Scholar]
- 52.Correa-Rodríguez M., et al. Dietary inflammatory index, bone health and body composition in a population of young adults: a cross-sectional study. Int. J. Food Sci. Nutr. 2018;69(8):1013–1019. doi: 10.1080/09637486.2018.1446915. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz-Canela M., et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br. J. Nutr. 2015;113(6):984–995. doi: 10.1017/S0007114514004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehrdad M., et al. High dietary inflammatory index (DII) scores increase odds of overweight in adults with rs9939609 polymorphism of FTO gene. Clin. Nutr. ESPEN. 2021;42:221–226. doi: 10.1016/j.clnesp.2021.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Inui A., Meguid M.M. Cachexia and obesity: two sides of one coin? Curr. Opin. Clin. Nutr. Metab. Care. 2003;6(4):395–399. doi: 10.1097/01.mco.0000078989.18774.74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.