Abstract
The selC tRNA gene is a common site for the insertion of pathogenicity islands in a variety of bacterial enteric pathogens. We demonstrate here that Escherichia coli that produces Shiga toxin 2d and does not harbor the locus of enterocyte effacement (LEE) contains, instead, a novel genomic island. In one representative strain (E. coli O91:H− strain 4797/97), this island is 33,014 bp long and, like LEE in E. coli O157:H7, is integrated 15 bp downstream of selC. This E. coli O91:H− island contains genes encoding a novel serine protease, termed EspI; an adherence-associated locus, similar to iha of E. coli O157:H7; an E. coli vitamin B12 receptor (BtuB); an AraC-type regulatory module; and four homologues of E. coli phosphotransferase proteins. The remaining sequence consists largely of complete and incomplete insertion sequences, prophage sequences, and an intact phage integrase gene that is located directly downstream of the chromosomal selC. Recombinant EspI demonstrates serine protease activity using pepsin A and human apolipoprotein A-I as substrates. We also detected Iha-reactive protein in outer membranes of a recombinant clone and 10 LEE-negative, Shiga toxin-producing E. coli (STEC) strains by immunoblot analysis. Using PCR analysis of various STEC, enteropathogenic E. coli, enterotoxigenic E. coli, enteroaggregative E. coli, uropathogenic E. coli, and enteroinvasive E. coli strains, we detected the iha homologue in 59 (62%) of 95 strains tested. In contrast, espI and btuB were present in only two (2%) and none of these strains, respectively. We conclude that the newly described island occurs exclusively in a subgroup of STEC strains that are eae negative and contain the variant stx2d gene.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains contain genes encoding proteins of the major Stx groups, Stx1 and Stx2. Stx2 is a family of structurally related proteins, including Stx2 and its variants, designated Stx2c (52), Stx2d (33, 40), Stx2e (22), and Stx2f (51). Pathogenic STEC strains usually contain genes encoding Stx1, Stx2, or both, whereas E. coli strains containing genes encoding Stx2 variants are more frequently isolated from asymptomatic carriers (Stx2d) (55) or from animals such as pigs (Stx2e) (22) or pigeons (Stx2f) (16, 51). Stx2c is also produced by human isolates but mostly in strains that also contain genes encoding Stx1, Stx2, or both.
STEC strains possess several different pathogenicity islands (26). The locus of enterocyte effacement (LEE) (32, 39) is usually integrated adjacent to either the selC or the pheU tRNA locus and consists of three parts (32, 39, 43, 53). Proteins of a type III secretion system export effector molecules and are encoded at one end of the island. The secreted proteins EspA, -B, and -D, which apparently function as part of the type III secretion apparatus, are encoded at LEE's opposite end (39). The central portion of LEE encodes intimin, which mediates intimate attachment to the host cell, and Tir, the best-characterized effector protein of LEE. A P4 phage integrase family gene is adjacent to the LEE-chromosome junction (39, 56). E. coli O157:H7 and other STEC strains also possess a genomic island termed the tellurite resistance and adherence-conferring island (TAI). The adherence-mediating iha gene is detected in eae-positive and eae-negative STEC strains (57). A third genomic island found in STEC O26 strains is similar in size and sequence to the high-pathogenicity island of yersiniae (29).
Pathogenicity islands are found to be integrated at the selC gene in additional enteric pathogens. For example, the SHI-2 island of Shigella flexneri (35, 62), the SPI-3 pathogenicity island of Salmonella enterica (8), and PAI-I of uropathogenic E. coli (UPEC) (46) are all integrated at this locus.
We investigated the selC region of a variety of eae-negative, Stx2d-producing E. coli strains to determine if this frequently used insertion site was occupied in these organisms, in lieu of LEE. Here we describe a novel genomic island adjacent to the selC tRNA gene in LEE-negative STEC strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Thirty-five STEC strains of different serotypes were recovered from stools of patients with nonbloody diarrhea (n = 16) and asymptomatic carriers (n = 19) and investigated in this study. The strains were isolated between 1996 and 2000 at the Institute for Hygiene and Microbiology of the University of Würzburg, Würzburg, Germany. They were all eae negative and contained stx2d but were negative by PCR with primers GK3-GK4 (stxB2, stxB2c), FK1-FK2 (stx2e), and 128-1–128-2 (stx2f). However, PCR with 20 of 35 Stx2d-positive isolates revealed a PCR product with primers LP30-LP31 (stxA1). PCRs were performed as described previously (40, 51). Enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), and UPEC strains (10 strains each) and enteroinvasive E. coli (EIEC) strains (5 strains) were chosen from our collection. E. coli O157:H7 strain EDL933 and E. coli laboratory strain DH5α (Gibco-BRL, Eggenstein, Germany) were used as controls for cytotoxicity tests and PCR analyses. E. coli ORN172 was used as a host for recombinant iha plasmids (57, 66) in outer membrane protein (OMP) analyses and adherence assays. EspP was prepared from E. coli DH5α/pB9 as described previously (14).
Plasmids pK18 (42), pACYC184 (15), pBluescript II KS(+) (Stratagene Europe, Amsterdam, The Netherlands), and the cosmid SuperCos I (Stratagene) were used as cloning vectors. pIha contains the cloned iha gene (iha86-24) from E. coli O157:H7 strain 86-24 inserted into pSK+ (57). E. coli strains were grown in Luria broth (LB) (47) and glycerol-Dulbecco's modified Eagle's medium (Gly-DMEM). Gly-DMEM contains in 1 liter DMEM powder (8.3 g; Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany), glycerol (0.45%), HEPES (25 mM), sodium pyruvate (10 mM), NaOH (4.5 mM), l-glutamine (4 mM), and sodium bicarbonate (44 mM); the pH of this solution was 7.4 at room temperature. To maintain recombinant plasmids, media were supplemented with 100 or 200 μg of ampicillin (Roth, Karlsruhe, Germany) ml−1, 50 μg of kanamycin (Sigma-Aldrich) ml−1, or 34 μg of chloramphenicol (Sigma-Aldrich) ml−1.
DNA techniques.
Chromosomal DNA was prepared either by a standard method (47) or with Nucleobond AXG100 columns (Macherey & Nagel, Düren, Germany) for the cosmid cloning procedure. Plasmids and cosmids were purified using Qiagen plasmid Midi kits (Qiagen GmbH, Hilden, Germany). SuperCos I was digested with XbaI, dephosphorylated with shrimp alkaline phosphatase (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany), phenol extracted, and digested with BamHI (Gibco-BRL), according to the manufacturer's recommendations.
To prepare a cosmid library, E. coli 4797/97 DNA was partially digested with Sau3AI (Gibco-BRL) and the fragments were shrimp alkaline phosphatase dephosphorylated, ligated into the cosmid arms, and then packaged in phage heads with the Gigapack III XL-4 system (Stratagene), according to the manufacturer's instructions. E. coli DH5α transductants were selected on LB agar containing 100 μg of ampicillin ml−1.
PCR.
PCR was performed in the GeneAmp PCR System 9600 (Perkin-Elmer Applied Biosystems, Weiterstadt, Germany) in 50 μl containing 5 μl of bacterial suspension (104 bacteria), 200 μM deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP), 30 pmol of each primer, 5 μl of 10-fold-concentrated polymerase synthesis buffer, 1.5 mM MgCl2, and 2.0 U of AmpliTaq DNA polymerase (Perkin-Elmer Applied Biosystems). All PCR primers used in this study are described in Table 1.
TABLE 1.
PCR primers and conditions used in this study
| Primer designation | Primer sequence (5′—3′) | Target | PCR condition
|
Length of PCR product (bp) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Denaturing
|
Annealing
|
Extension
|
||||||||
| Temp (°C) | Time (s) | Temp (°C) | Time (s) | Temp (°C) | Time (s) | |||||
| K260 | GAG CGA ATA TTC CGA TAT CTG GTT | selC | 94 | 90 | 60 | 90 | 72 | 120 | 2,173 | 65 |
| K295 | CGC CGA TTT TTC TTA GCC CA | |||||||||
| K255 | GGT TGA GTC GAT TGA TCT CTG G | selC-LEE left junction | 94 | 90 | 60 | 90 | 72 | 120 | 405 | 65 |
| K260 | GAG CGA ATA TTC CGA TAT CTG GTT | |||||||||
| K295 | CGC CGA TTT TTC TTA GCC CA | selC-LEE right junction | 94 | 90 | 60 | 90 | 72 | 120 | 418 | 65 |
| K296 | CAT TCT GAA ACA AAC TGC TC | |||||||||
| SK1 | CCC GAA TTC GGC ACA AGC ATA AGC | eae | 94 | 30 | 52 | 60 | 72 | 60 | 863 | 49 |
| SK2 | CCC GGA TCC GTC TCG CCA GTA TTC G | |||||||||
| ANK-49 | ATG TTA TCC TCA TAT AAA ATA AAC | pas | 94 | 20 | 50 | 60 | 72 | 120 | 1,221 | 21 |
| ANK-50 | TTA ATA CGA CAG TGG AAT ATG | |||||||||
| escC-U | AGA CAA CAC CAG GGA CGA C | escC | 94 | 60 | 56 | 60 | 72 | 90 | 575 | This study |
| escC-L | ACA AGC TGC CCC GTC CTC T | |||||||||
| escF-U | ATG GCG GAT TGA GAC ACC T | escF | 94 | 60 | 52 | 60 | 72 | 90 | 518 | This study |
| escF-L | TCA ACA TTC CAC TTT CTA CG | |||||||||
| espI-I | ATG GAC AGA GTG GAG ACA G | espI | 94 | 30 | 52 | 60 | 72 | 60 | 560 | This study |
| espI-II | GCC ACC TTT ATT CTC ACC A | |||||||||
| iha-I | CAG TTC AGT TTC GCA TTC ACC | iha | 94 | 30 | 56 | 60 | 72 | 90 | 1,305 | This study |
| iha-II | GTA TGG CTC TGA TGC GAT G | |||||||||
| btuB-I | GCC CCT TCC CAC TGT TTA CT | btuB | 94 | 30 | 55 | 60 | 72 | 90 | 1,032 | This study |
| btuB-II | GGT ATT GAT TGA TGG AGT GCG | |||||||||
| espI-B | GAG AAA GAC TGG AAA AAT CAA G | espI-iha | 94 | 30 | 54 | 60 | 72 | 240 | 3,916 | This study |
| iha-A | GGC AAG GAC AAC CCC ATC T | |||||||||
| iha-B | CGT GAT GGT GAT AAC AAA GG | iha-btuB | 94 | 30 | 54 | 60 | 72 | 90 | 1,325 | This study |
| btuB-A | CGG AAA AGA GTA AAC AGT GG | |||||||||
Nucleotide sequencing and analysis.
Taq cycle sequencing was performed in an automatic sequencer (model 377A; Perkin-Elmer Applied Biosystems).
Sequencing was initially performed with universal and reverse primers for M13/pUC vectors from cosmid pWZK6-6 and corresponding subclones. Gaps and overlaps were sequenced using internal primers, designed with Oligo 4.0 software (National BioSciences Inc., Plymouth, United Kingdom). Nucleotide sequence analysis was performed with the HUSAR program package (Heidelberg Unix Sequence Analysis Resources; German Cancer Research Center, Heidelberg, Germany), the DNASIS program (Hitachi Software, San Bruno, Calif.), and Lasergene (DNAStar Inc., Madison, Wis.). Homology searches in the EMBL-GenBank databases were performed with the BLAST algorithm (3) at the National Center for Biotechnology Information, Bethesda, Md. (http: //www.ncbi.nlm.nih.gov).
Secreted proteins.
Extracellular proteins were prepared from culture supernatants for gel electrophoresis and activity tests as described previously (13, 14).
OMP analysis.
OMPs were prepared according to the method of Achtman et al. (1) Briefly, bacteria were grown to mid-exponential phase in LB (for EspI analysis), or for 20 h in 80 ml of LB or Gly-DMEM (for Iha analysis), without agitation. Bacteria were harvested by centrifugation (3,800 × g, 15 min, 4°C) and suspended in 10 mM Tris-HCl, pH 8.0. Cells were disrupted by sonication, unbroken cells were removed by centrifugation (3,800 × g, 15 min, 4°C), and the supernatant was again centrifuged (48,000 × g, 4°C, 60 min). The pellet was suspended in distilled water and extracted with an eightfold volume of detergent solution (1.67% N-laurylsarcosine, 11.1 mM Tris-HCl, pH 7.6). The insoluble outer membranes were sedimented by centrifugation (46,000 × g, 90 min, 20°C) and suspended in 50 μl of phosphate-buffered saline (PBS).
Iha detection.
Iha antigen was sought in OMPs from 10 stx2d+ STEC strains and in E. coli ORN172 transformed with pIha86-24 (57) and with pIha4797. pIha4797 is pACYC184 with a 3.7-kb EcoRV fragment of cosmid pWZK6-6 which contains open reading frame (ORF) L13, but no other ORFs, cloned into the unique EcoRV site. Negative controls consisted of E. coli ORN172 transformed with pACYC184 and pSK+. OMP concentrations were determined using the protein assay kit from Bio-Rad (Hercules, Calif.). Two or four micrograms of protein was loaded in each lane of a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel for immunoblotting or Coomassie blue analysis, respectively. The separated OMPs were either transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore, Bedford, Mass.) or stained with Coomassie blue.
For immunoblot analysis, membranes were blocked (4 h) at 4°C in antibody buffer (PBS with 0.05% [vol/vol] Tween 20) containing 5% nonfat dried milk and 0.02% sodium azide. The membranes were then washed once with antibody buffer and incubated overnight with anti-Iha antibodies diluted 1:2,000 in antibody buffer. This antibody was raised against the peptide sequence (C)YTWTRSEQRDGDNKG-COOH of Iha86-24 of E. coli O157:H7 (57). A similar sequence (YTWTQSEQRDGDNKG) is found in Iha of E. coli O91:H−.
Following incubation with the antibody, the blots were washed three times with antibody buffer, incubated (30 min) with affinity-purified goat anti-rabbit immunoglobulin G (IgG; heavy plus light chains) peroxidase conjugate (Boehringer Mannheim, Indianapolis, Ind.) diluted 1:2,000 in antibody buffer, and washed three times in antibody buffer. All washes and incubations were performed at room temperature after the blocking step. Bound anti-Iha antibodies were detected using the SuperSignal chemiluminescent substrate for Western blotting (Pierce, Rockford, Ill.), according to the manufacturer's instructions.
Amino acid sequencing.
N-terminal sequencing of proteins was performed by automated Edman degradation using an Applied Biosystems 476A sequencer (Perkin-Elmer Applied Biosystems) (14).
Protease assays.
Pepsin A from porcine stomach mucosa, IgA1, hemoglobin, α2-macroglobulin, haptoglobin, apolipoprotein A-I, lactoferrin, collagen type III, trypsin, high-density lipoprotein, low-density lipoprotein, and very low density lipoprotein were purchased from Sigma-Aldrich Chemie GmbH. Bovine serum albumin and transferrin were purchased from Serva Electrophoresis GmbH (Heidelberg, Germany), and thrombin was purchased from Roche Diagnostics (Mannheim, Germany).
To investigate the putative protease activity, ca. 5 μg of EspI-containing supernatant or an equal amount of precipitated culture supernatant from the vector control strain DH5α/pK18 was mixed with 30 μg of the above substrates or with 1 μl of diluted human plasma. PBS (150 mM) was then added to a final volume of 10 μl, and the tubes were incubated overnight. The samples were then analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich) and EDTA (Applichem, Darmstadt, Germany) were used as protease inhibitors in final concentrations of 10 μg ml−1 and 50 mM, respectively. One microliter of EspI and 10 μl of PBS were mixed with either PMSF or EDTA and incubated for 1 h at 37°C. After this preincubation, 2 μl of pepsin A (5 mg/ml) and 10 μl of PBS were added to the samples and incubated overnight at 37°C.
Gelatinase and caseinase zymogram analyses were performed by electrophoresing EspI from recombinant strain DH5α/pZH4 and culture supernatants from vector control strain DH5α/pK18 in precast zymogram gels (Invitrogen, Groningen, The Netherlands). Gels were renatured, developed, and stained, and proteolysis of gelatin and casein was identified by the appearance of clear bands against a blue background of nonhydrolyzed protein. Ten nanograms of trypsin (Serva Electrophoresis GmbH) and 10 ng of collagenase from Clostridium histolyticum (Sigma-Aldrich) were used as positive controls for casein and gelatin hydrolysis, respectively.
Cytotoxicity assay.
Vero cell toxicities of bacterial culture supernatants were determined as described previously (23, 48). E. coli O157:H7 strain EDL933 and E. coli K-12 strain DH5α were used as positive and negative controls, respectively.
Nucleotide sequence accession number.
The complete nucleotide sequence of the genomic island of E. coli O91:H− strain 4797/97 has been entered into the EMBL database library (accession no. AJ278144).
RESULTS
selC insertion site is occupied in a subset of LEE-negative STEC strains.
Thirty-five stx2d-positive E. coli strains were negative when subjected to PCR with primers GK3 and GK4 (stxB2 and stxB2c) FK1 and FK2 (stx2e), and 128-1 and 128-2 (stx2f). All 35 isolates were also PCR negative for eae (50) and other LEE genes (i.e., escF, escC, and pas [Table 1]) that flank eae, suggesting that LEE is largely or completely absent from these isolates. Twenty of these 35 stx2d-positive strains were also positive by PCR with LP30-LP31 (stxA1).
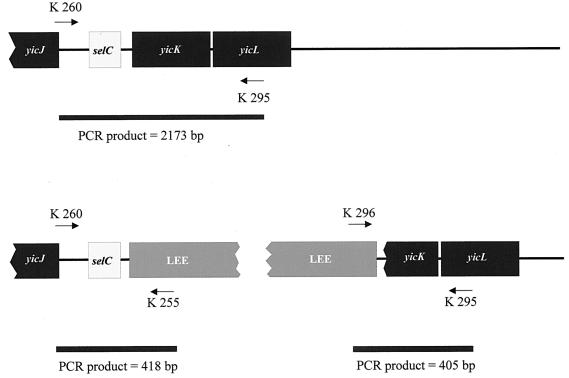
To investigate the selC region, the common integration site of LEE, of these LEE-negative STEC strains, we subjected their genomic DNA to selC tRNA amplification analyses (Fig. 1). Twelve of 35 strains investigated yielded a PCR product of about 2,100 bp with primers K260 and K295, implying an intact (i.e., K-12-like) selC locus. Ten of the remaining 23 strains yielded PCR products with primers K260 and K255, suggesting the presence of left-border LEE sequences (39) in the vicinity of the selC gene; 13 strains yielded no product. Primers K296 and K295 failed to elicit PCR products in any strain tested. Taken together, these data suggest that, in selected eae-negative STEC strains, foreign DNA is present distal to the 3′ end of selC that contains left, but not right, border elements of LEE (compared with data in reference 39). To characterize the DNA inserted adjacent to selC in these LEE-negative STEC strains, we constructed a cosmid library from E. coli O91:H− strain 4797/97 for analysis. Of ca. 900 screened cosmid clones, two yielded a PCR product with primers K260 and K255. One of these two cosmid clones, pWZK6-6, was subcloned and sequenced. The subclones were generated by digesting pWZK6-6 with EcoRI, ligating these fragments in pBluescript II KS(+), and transforming these clones in E. coli DH5α. DNA sequences which were not covered by these subclones were sequenced bidirectionally by using internal primers with pWZK6-6 as template.
FIG. 1.
Amplification scheme to detect LEE-specific DNA integrated adjacent to selC. The selC tRNA locus is located between yicJ and yicK in the E. coli K-12 chromosome. Primer K260 is specific to chromosomal sequences directly upstream of selC, and primer K295 is complementary to yicL. Amplification of this region in its original configuration in E. coli K-12 yields a PCR product of 2,173 bp. Lack of a PCR product suggests that foreign DNA occupies that region. Primers K255 and K296 are specific for the left and right borders of the LEE, respectively. Primer combinations K260-K255 and K296-K295 can detect LEE-specific DNA integrated into the selC locus by amplifying 418- or 405-bp PCR products, respectively.
Sequence analysis of the inserted DNA in E. coli O91:H−. (i) Size and site of inserted DNA.
Cosmid pWZK6-6 has 37,710 bp of inserted DNA (deposited in GenBank under accession no. AJ278144). This insert contains at one end the selC tRNA gene and at the other end yicK, yicL, and nlpA (Fig. 2). Homology to the K-12 chromosome ceases 15 bp downstream of the 3′ end of selC. The region comprising the 3′ end of selC, and 35 bp of adjacent DNA from E. coli O91:H− strain 4797/97 including the putative insertion point (position 3000), is homologous to corresponding regions of S. flexneri strain SA100 (35), E. coli O157:H7 strain EDL933 (39), E. coli O6:K15:H31 strain 536 (10), and E. coli K-12 (9). It is most closely related to a similar region of the pathogenicity island SHI-2 of S. flexneri strain SA100. These analyses show that the selC insertion site of E. coli O91:H− is occupied by 33,014 nucleotide pairs that are absent from this site in E. coli K-12. The G+C content of the inserted sequence is 47.4%.
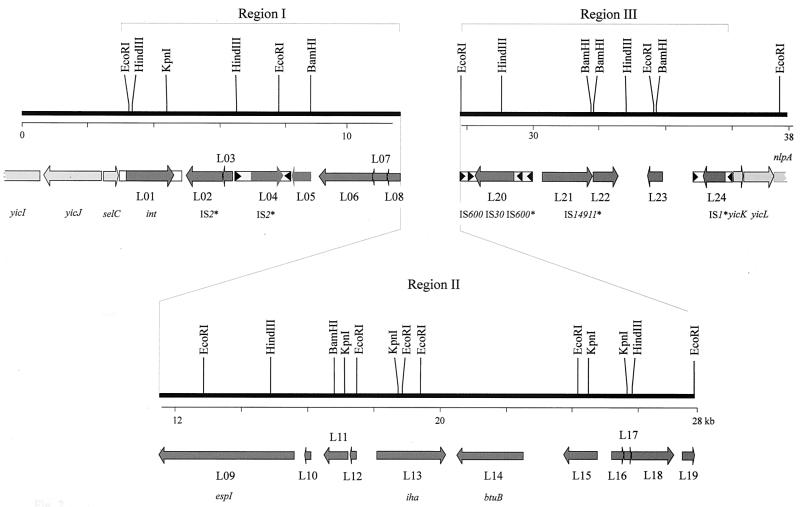
FIG. 2.
Genetic structure of the genomic island of E. coli 4797/97. Broken bars indicate partially sequenced chromosomal genes at the cosmid pWZK6-6 insert border. Chromosomal genes are indicated by light grey shading, and island genes are indicated by dark grey shading. Arrows indicate size, location, and transcriptional orientation of ORFs present on the island. Designations below the scheme represent either chromosomal genes (yicI, yicJ, selC, yicK, and yicL), genes which are functionally characterized (espI and iha4797), genes with extensive homologies to other bacterial alleles (int and btuB), or ISs (IS2-like, IS600, IS30, and IS1). Asterisks indicate that designations were selected according to the most homologous IS element. ORFs present in the island are labeled consecutively from L01 to L24. Boxes with triangles represent insertion elements with terminal repeats. Region I and region III contain primarily mobilizable elements, and region II contains mainly genes that encode proteins that putatively interact with host eukaryotic cells.
(ii) Mosaic structure of inserted DNA.
Within the DNA that is inserted adjacent to selC in E. coli strain 4797/97, there are four segments longer than 100 bp that possess extensive (>90%) identity to DNA in other enteric pathogens. Between positions 3000 and 4764, there is a region that has 99.5% identity to the SHI-2 island of S. flexneri (62). This region is also homologous to the left end of LEE of E. coli O157:H7 (39), but the LEE homology ceases at position 4468. The DNA between positions 8580 and 11021 is 99.8% identical to a region in LEE of E. coli O157:H7, which is part of the prophage 933L (39). A third region of extensive homology is observed between positions 17314 and 19979 and sequences within TAI of E. coli O157:H7 strain 86-24 (57). Finally, the DNA between positions 20059 and 20199 downstream of the TAI homologue is 92% identical to DNA of UPEC strain 366-11 (69).
ORF analysis of the DNA inserted adjacent to selC in E. coli O91:H−. (i) ORF categories.
The DNA inserted downstream of selC contains 24 ORFs encoding proteins with ≥50 deduced amino acids (Fig. 2; Table 2). These ORFs are numbered sequentially from L01 to L24. The ORFs in this island can be categorized as those that presumably cause DNA to mobilize (i.e., ORFs encoding proteins with homology to known or postulated integrases, prophages, or insertion sequences [ISs]), those that play a role in bacterium-host interactions (i.e., ORFs encoding proteins that can affect eukaryotic cells or molecules or that can mediate bacterial adherence to eukaryotic cells), and ORFs encoding proteins with unknown function.
TABLE 2.
Characteristics of ORFs and deduced amino acid sequences present in the sequenced DNA fragmenta
| Label | ORF characteristic
|
Amino acid
characteristic
|
|||||
|---|---|---|---|---|---|---|---|
| Position | Direction | Size (bp) | Sequence to which homologous (% identity) | Accession no. of homologue | No. of amino acid residues | Sequence to which homologous (% identity; no. of identical residues/total no. of residues) | |
| 1–1205 | C | 1,203 | yicI (99.8) | AE000443 | 401b | YicI (98) | |
| 1215–2654 | C | 1,440 | yicJ (100) | AE000443 | 479 | YicJ (100; 479/479) | |
| 2889–2983 | D | 95 | selC (100) | Y00299 | |||
| L01 | 3284–4468 | D | 1,185 | int (99) | AF071034 | 394 | CP4 integrase (98; 390/394) |
| L02 | 4802–5683 | C | 882 | EAEC sequence (88)c | Z32523/P51026 | 293 | 48.2-kDa protein of IS2 (61; 175/283) |
| L03 | 5641–6036 | C | 396 | P51026 | 131 | 48.2-kDa protein of IS2 (76; 87/113) | |
| L04 | 6567–7358 | D | 792 | ORF of IS2 (97.9) | AE000386/P19777 | 263 | 34.4-kDa protein of IS2 (97; 255/263) |
| L05 | 7941–8315 | C | 375 | NSH | 124 | NSH | |
| L06 | 8616–10154 | C | 1,539 | L0015 (99) | AAC31494/AF071034 | 512 | L0015 of LEE0157 (99; 511/512) |
| L07 | 10204–10551 | C | 348 | L0014 (100) | AF071034/CA A11510.1| | 115 | Hypothetical protein of E. coli EB1 (100; 115/115) |
| L08 | 10548–10928 | C | 381 | L0013 (99) | AAC31492.1| | 126 | L0013 of LEEO157 (89; 113/126) |
| L09 | 11248–15339 | C | 4,092 | espP (94) | AF074613/S57664 | 1,363 | SepA of S. flexneri (51; 710/1,389) |
| L10 | 15774–15589 | C | 186 | NSH | 61 | NSH | |
| L11 | 16252–16989 | C | 738 | NSH | AAC74572 | 245 | AraC-type regulator (38; 89/229) |
| L12 | 17164–17316 | C | 153 | TAI | AF215931 | 50 | NSH |
| L13 | 17821–19908 | D | 2,088 | iha (93.5) | AF126104 | 695 | Iha of E. coli O157:H7 (91; 637/696) |
| L14 | 20254–22098 | C | 1,845 | btuB (77–86)d | AE000471/P06129 | 614 | BtuB of E. coli (79; 486/614) |
| L15 | 23467–24498 | C | 1,032 | NSH | AE000420 | 343 | GntR of E. coli (32; 97/294) |
| L16 | 24769–25212 | D | 444 | NSH | P32058 | 147 | Mannitol-specific phosphotransferase enzyme II, A component (42; 58/136) |
| L17 | 25228–25515 | D | 288 | P39302 | 95 | Unknown phosphotransferase system enzyme II, B component (26; 25/29) | |
| L18 | 25528–26784 | D | 1,257 | NSH | TR7066/P39301 | 418 | SgaT of E. coli/PIMP of S. coelicolor (41; 181/434) |
| L19 | 27457–27771 | D | 315 | S. flexneri invasion plasmid (95) | SFU28354/JC5053 | 104 | 11.5-kDa protein of S. flexneri (87; 50/57) |
| L20 | 28273–29424 | C | 1,152 | R0046 (100) | AF250878 | 383 | IS30 transposase (100; 383/383) |
| L21 | 30274–31815 | D | 1,542 | NSH | AAB48471 | 513 | orf1, transposase subunit of P. alcaligenes (50.5; 257/508) |
| L22 | 31827–32576 | D | 750 | NSH | AAC45210 | 249 | orf2, transposase subunit of P. alcaligenes (42; 101/239) |
| L23 | 33472–34089 | C | 618 | SauE4.E10 (96) | AF222142 | 205 | |
| L24 | 35261–35764 | C | 504 | insB (99.8) | U18997/P03830 | 167 | InsB (99; 167/167) |
| 36014–36390 | D | Truncated (377) | yicK (100) | AE000443, P31436 | 125 | YicK (100; 87/87) | |
| 36390–37313 | D | 924 | yicL (99) | AE000443 | 307 | YicL (99.4; 307/307) | |
| 37318–37711 | D | 394 | nlpA (99) | AE000443 | 131 | NlpA (100; 131/131) | |
ORFs located in the island of E. coli O91:H− strain 4797/97 are labeled L01 to L24. Abbreviations: C, complementary strand; D, direct strand; NSH, no significant homology; PIMP, putative integral membrane protein.
Protein deduced from truncated ORF.
Eighty-eight percent nucleotide sequence identity to a 303-bp segment of Z32523, which contains aggR of EAEC. The region of identity was not in the aggR gene but in a sequence which was not described.
No full-length homology.
Seven of the eight leftmost ORFs, and four of the five rightmost ORFs, of the insert (Fig. 2) appear to encode molecules that can mobilize DNA. We have designated these segments of the island as regions I and III, respectively. Region II contains 11 ORFs encoding deduced proteins with homology to proteins in the database with potential pathogenic functions (i.e., EspP of the large plasmid of E. coli O157:H7 and Iha of TAI of E. coli O157:H7 [Fig. 2; Table 2]), as well as proteins with less well characterized roles.
(ii) Analysis of region I and III ORFs.
Regions I and III contain ORFs with putative mobility-associated functions. In addition to int (L01), region I ORFs L02 and L03 appear to be fragments of transposases, because their corresponding proteins are homologous to a hypothetical 48.2-kDa protein of IS2 of E. coli (accession no. P51026) and to TnpA, a putative transposase of IS1087 of Ralstonia sp. (59), respectively. ORF L04 might encode an IS2 element-homologous protein, because its corresponding protein is similar to an IS2-like element of Burkholderia glumae (accession no. BAA24290).
In region III, there are two intact IS elements with inverted repeats, which are nearly identical to IS1 and IS30 of E. coli, the latter of which divided an IS600 sequence in region III into two parts (Fig. 2) The deduced amino acid sequences of L21 and L22 were 50 and 42% identical to ORFs 1 and 2, respectively, of the Pseudomonas alcaligenes transposon IS14911 (68). In addition, the deduced amino acid sequence of L24 is 99% identical to insB present in IS1 of E. coli (61).
Region II has ORFs that encode proteins with potential virulence roles and ORFs without known functions. (i) L09 (EspI).
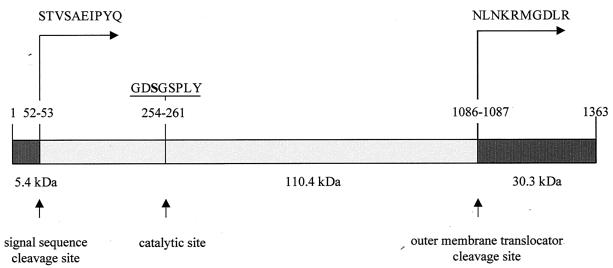
ORF L09 contains 4,092 bp and is located between positions 11248 and 15339. An 842-bp segment at the 3′ end of L09 is 94% identical to the 3′ end of the serine protease gene espP of E. coli O157:H7 (14). The most homologous holoprotein in the database is S. flexneri SepA (5), with 51% identity. Proteins with lesser homology include the S. flexneri mucinase (accession no. AAB58244) (50%), E. coli O157:H7 EspP and the identical E. coli O26:H− PssA (47%) (17), avian E. coli temperature-sensitive hemagglutinin (54), E. coli secreted hemoglobinase (32%) (37), S. flexneri SigA (42%) (2), and Sat (42%), an autotransporter protein of UPEC (24). These homologies suggest that L09 encodes a serine protease. Furthermore, the octapeptide GDSGSPLY between positions 254 and 261, inclusive, within the deduced amino acid sequence resembles closely the catalytic site of serine proteases (4, 12), where S-256 is the active-site serine (Fig. 3). A potential signal sequence cleavage (63) site is found at amino acid position 52 of L09 (Fig. 3). The presumptive secreted molecule of 1,310 amino acids has a calculated molecular mass of 140.6 kDa. Moreover, L09 has an outer membrane translocator cleavage site between N-1086 and N-1087 (Fig. 3), as do the homologues SepA and EspP, and the carboxy-terminal cleavage motif of the helper domain KRMGDLR is also present between positions 1090 and 1096 in L09. After cleavage of the signal peptide and the outer membrane translocator, the remaining protein would have a molecular mass of 110.4 kDa. Because of the similarity between EspP and the protein encoded by L09, and its determination in a genomic island, we propose the term EspI (for E. coli secreted protease, island encoded) for this latter molecule.
FIG. 3.
Structure of EspI. The signal sequence cleavage site, the catalytic site, the serine protease motif, and the outer membrane translocator site are shown. Amino acid sequences shown represent the first amino acids obtained by N-terminal amino acid sequencing.
(ii) L11 (AraC homologue).
ORF L11 encodes a putative protein of 245 amino acids with similarity to members of the AraC family of transcriptional regulators (Table 2). From positions 190 to 231 of the AraC homologue, there is a motif similar to the helix-turn-helix motif of the AraC family (20) with the sequence RMHNARQLISSKLSVNQIAIRCGYASTPYFISVFREYFGITP.
(iii) L13 (Iha4797).
We designated the protein specified by L13 as Iha4797, because of its homology of 91% to Iha of E. coli O157:H7 (Iha86-24). Iha4797 and Iha86-24 each have putative TonB boxes with the sequence DVMIVSA at their N terminus. The peptides recognized by antibodies to Iha86-24 are located at positions 546 to 560 in Iha68-24 and positions 547 to 561 in Iha4797 and are largely conserved between the two strains.
(iv) L14 (BtuB homologue).
L14 is minimally homologous (86% over a length of 168 nucleotides) to the E. coli btuB gene, but its deduced amino acid sequence has considerably more relatedness to the vitamin B12 receptor of E. coli (27) and S. enterica serovar Typhimurium (64). A 300-bp segment upstream of L14 (positions 22089 to 22389) is highly homologous to the E. coli btuB promoter region (accession no. X17416).
(v) L15 (GntR homologue).
ORF L15 encodes a deduced protein of 343 amino acid residues that is 32% identical to the GntR protein regulator of the gluconate (gnt) operon of E. coli (60) (P46860). GntR is in the GalR-LacI family of proteins, which regulate transcription of inducible genes and contain a helix-turn-helix motif in the N terminus (18, 36). We detected the corresponding helix-turn-helix motif LQEVANFAGVGTMTVSRAL in L15 between positions 20 and 38, inclusive, within the deduced amino acid sequence, suggesting that the L15 protein also belongs to this family.
(vi) L16 to L18 (phosphotransferase homologues).
ORFs L16 and L17 encode putative proteins with 42 and 26% identity to a mannitol-specific phosphotransferase system enzyme, component 2A (P32058/S36122), and a phosphotransferase system enzyme, component 2B, of E. coli, respectively (45). The deduced amino acid sequence encoded by ORF L18 has 41% identity to a putative transmembrane phosphotransferase of Streptomyces coelicolor (accession no. T37066).
Expression analysis of proteins encoded by two region II ORFs. (i) EspI.
To investigate the serine protease activity of EspI, we cloned an 8,073-bp BamHI fragment from cosmid pWZK6-6 in plasmid vector pK18, yielding pZH4. This fragment contains the entire ORF L09, termed espI, and the smaller ORFs L06, L07, L08, and L10. We detected a protein with an apparent molecular mass of ca. 110 kDa in the culture supernatant of E. coli DH5α/pZH4 (Fig. 4). This protein is not present in the supernatants of E. coli control strains DH5α and DH5α/pK18 (Fig. 4).
FIG. 4.
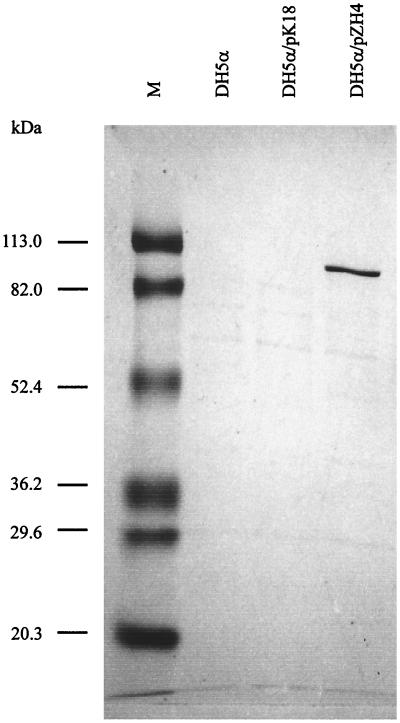
Coomassie blue-stained SDS-PAGE of culture supernatants of E. coli strains DH5α, DH5α/pK18, and DH5α/pZH4. M, molecular mass markers.
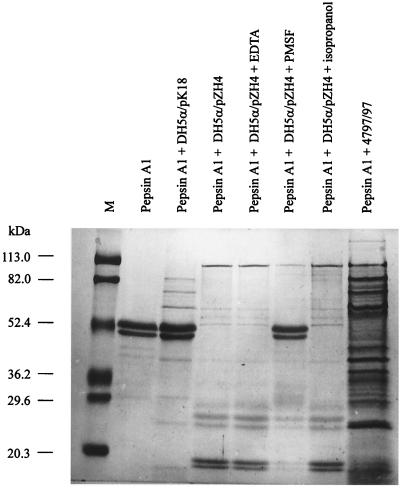
This molecular mass correlates well with the molecular mass of 110.4 kDa of the deduced structure of the mature EspI protein, from which the signal sequence and the outer membrane translocator sequence have been cleaved (Fig. 3). The secreted protein has an N-terminal sequence of STVSAEIPYQ, as predicted from the nucleotide sequence adjacent to the postulated signal cleavage site between positions 52 and 53. Also, outer membranes of recombinant laboratory strains expressing cloned L09 contain a ca. 30.5-kDa protein that has an N-terminal sequence of NLNKRMGDL (data not shown), as predicted from the espI nucleotide sequence starting at the putative outer membrane translocator cleavage site at position 1087. This cleavage pattern is consistent with the presence of a residual outer membrane locator as identified in EspP of E. coli O157:H7 (14). We also characterized the proteolytic activity of EspI from E. coli DH5α/pZH4. Substrate degradation could not be detected using IgA1, hemoglobin, α2-macroglobulin, haptoglobin, thrombin, lactoferrin, transferrin, bovine serum albumin, collagen type 3, trypsin, high-density lipoprotein, low-density lipoprotein, or very low density lipoprotein. Zymogram analyses for gelatinase or caseinase activity were also negative, and EspI is not toxic to Vero cells. However, the double band of swine pepsin A1 (Fig. 5) was degraded by the supernatant of E. coli DH5α/pZH4 but not by the supernatant of DH5α containing the vector control (Fig. 5). The degradation fragments produced by EspI were the same size as those produced by EspP of E. coli O157:H7 (14; data not shown). Preincubation for 1 h at 37°C with 50 mM PMSF abolishes the proteolytic activity (Fig. 5), whereas preincubation with EDTA has no influence (Fig. 5). Thus, EspI and EspP are functionally related. Culture supernatant from the wild-type E. coli strain 4797/97 has the same proteolytic activity on pepsin A (Fig. 5) and was similarly inhibited by PMSF but not by EDTA (data not shown). The PMSF used for the experiment was dissolved in isopropanol; therefore, isopropanol was used as a control (Fig. 5) and could be shown not to inhibit protease activity.
FIG. 5.
Coomassie blue-stained SDS-PAGE. The gel shows cleavage of pepsin A1 by EspI and inhibition by protease inhibitors. M, molecular mass markers.
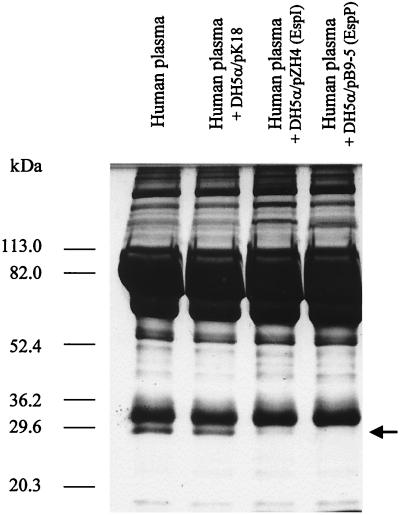
Like EspP, EspI degrades a 28.5-kDa band in human plasma (Fig. 6). We excised the 28.5-kDa band from a gel and determined its amino-terminal sequence. The sequence obtained, DEPPQSPWD, was identical to the first nine amino acids of the mature chain of apolipoprotein A-I, corresponding to position 25 of the published sequence (accession no. P02647). The supernatant of DH5α/pZH4, but not the supernatant of DH5α containing the vector control, cleaved purified apolipoprotein A-I into small fragments (data not shown).
FIG. 6.
Coomassie blue-stained SDS-PAGE analysis of human plasma incubated with EspI and EspP. The arrow indicates the 28.5-kDa band cleaved by EspI and EspP.
(ii) Expression of Iha4797.
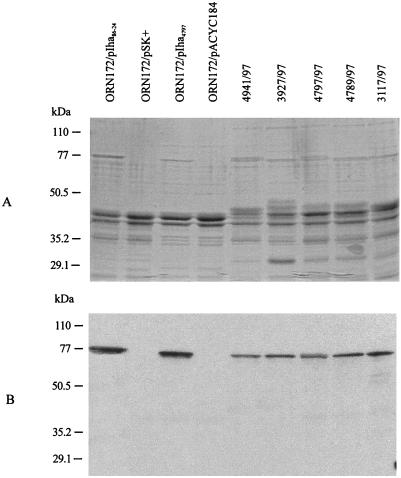
ORF L13 was cloned on a 3.7-kb EcoRV fragment of pWZK6-6, yielding pIha4797. Outer membranes of E. coli ORN172 transformed with pIha86-24 contain a ca. 77-kDa protein that is visible with Coomassie blue staining and is also detected by anti-Iha antibody in protein immunoblots (Fig. 7). This protein is also identified in the OMPs of E. coli ORN172 transformed with pIha4797 (Fig. 7) and in all of the 10 wild-type stx2d-positive, LEE-negative STEC strains tested, which were also positive by PCR with primers K250 and K260 (five representative strains are shown in Fig. 7). This protein is demonstrated better by wild-type E. coli grown in Gly-DMEM than by that grown in LB (data not shown). Vector control strains ORN172/pSK+ and ORN172/pACYC184 grew only poorly in Gly-DMEM, and so OMPs prepared from LB are shown in Fig. 7.
FIG. 7.
SDS-PAGE analysis of recombinant and wild-type Iha. Coomassie blue staining (A) and protein immunoblotting (B) analyses of OMPs prepared from E. coli ORN172/pIha86-24, ORN172/pSK+, ORN172/pIha4797, ORN172/pACYC184, ONT:H− strain 4941/97, O62:H− strain 3927/97, O91:H− strain 4797/97, O96:H− strain 4789/97, and O128:H− strain 3117/97.
Presence and linkage of espI, iha, and btuB in enteric E. coli.
We used PCR analysis to determine the presence or absence of selected loci of the genomic island in other E. coli strains. We chose ORFs L09 (espI), L13 (iha), and L14 (btuB) as targets. To determine the linkage, if any, among these genes, we also used primer pairs espI-B and iha-A and iha-B and btuB-A (Table 1).
Nine additional, K260- and K255-positive strains from our strain collection of 35 LEE-negative, stx2d-positive strains possessed espI, iha, and btuB, and all PCRs demonstrated that these loci were linked. These nine strains belong to serotypes O128:H2 (three strains), O128:H− (two strains), O128:HNT (one strain), O62:H− (one strain), O96:H− (one strain), and ONT:H− (one strain).
In none of 10 STEC strains of serotypes O26:H−/H11, O103:H2, O145:H−, O111:H−, and O157:H7 and no EAEC, EIEC, ETEC, EPEC, and UPEC strains did we detect btuB (Table 3). The espI gene was detected in only 2 of 10 EAEC strains. However, all STEC O26:H−/H11, O111:H11/H, O145:H−, and O157:H7 strains and 42% of ETEC, EAEC, EIEC, and UPEC strains tested possessed iha (Table 3). However, this allele was absent from STEC O103:H2.
TABLE 3.
Detection of espI, iha, and btuB in different E. coli pathogroups by PCR
| Pathogroup and serotype | Total no. of strains | No. of strains PCR positive for:
|
||
|---|---|---|---|---|
| espI | iha | btuB | ||
| STEC | ||||
| O26:H11/H− | 10 | 0 | 10 | 0 |
| O111:H− | 10 | 0 | 10 | 0 |
| O145:H− | 10 | 0 | 10 | 0 |
| O157:H7/H− | 10 | 0 | 10 | 0 |
| O103:H2/H− | 10 | 0 | 0 | 0 |
| EPEC | 10 | 0 | 0 | 0 |
| ETEC | 10 | 0 | 2 | 0 |
| EAEC | 10 | 2 | 6 | 0 |
| EIEC | 5 | 0 | 3 | 0 |
| UPEC | 10 | 0 | 8 | 0 |
DISCUSSION
Bacterial genomes are mosaics, with regions encoding specialized functions scattered as islands between regions encoding common housekeeping genes. If such specialized DNA regions are present in the genomes of pathogens, they are termed pathogenicity islands (25, 26, 34). Pathogenicity islands generally are distinct genetic units, include virulence loci, are present in pathogens but absent from nonpathogens, usually have a different G+C content from that of the core genome, and occupy large DNA regions (>30 kb). These islands are often flanked by direct repeats, frequently associate with tRNA genes and/or insertion elements, possess cryptic mobility genes, and may be unstable (25, 26). Recently, the definition of pathogenicity islands has been expanded to include selected metabolic regions and O-antigen-encoding regions (26, 58).
The genomic island described here fulfills most of the criteria proposed for pathogenicity islands (26). Most notably, this island is integrated adjacent to selC, a site that is preferentially occupied in STEC and EPEC by LEE, in UPEC by PAI-I (26, 46), in ETEC by TIA (19), in S. flexneri by the SHI-2 island (35, 62), and in S. enterica serovar Typhimurium by SPI-3 (8). The E. coli O91:H− island is remarkable because it is present in STEC strains that do not contain LEE. The overall G+C content is lower than that found in the rest of the E. coli chromosome, and its size is >30 kb. Direct repeats were not identified, but insertion elements are present throughout the island, and even at the border, distal to selC, there is an IS element, which probably has led to the deletion of a putative repeat. This raises the possibility that this island has had a complex evolutionary history. However, an intact integrase is present, suggesting that the island might have recently been mobilized into the strain studied.
Two ORFs in region II are plausibly related to the virulence of the STEC strains in which they are found. EspI is homologous to SepA of S. flexneri, which is thought to be involved in invasion and destruction of the host intestinal epithelium (6). Because of the function of EspI as a protease, we propose “locus of proteolysis activity” (LPA) as the name for this novel genomic island of E. coli O91:H− strain 4797/97.
It is probable that a family of Iha homologues confers adherence. The Iha described here (Iha4797) differs slightly in its sequence from Iha expressed by E. coli O157:H7. It has yet to be demonstrated that Iha4797 also mediates adherence.
The particular genetic background in which the putative effector ORFs are found is worthy of note. Iha in E. coli O157:H7 is encoded in an island other than LEE, termed TAI, but the iha homologue described in this study lacks neighboring tellurite resistance genes. However, DNA surrounding iha4797 is similar to the corresponding DNA of TAI adjacent to iha of E. coli O157:H7. This suggests that horizontal gene transfer has played a role in the acquisition of iha4797. In addition, espI, which is homologous to sepA and espP, is encoded on the chromosome and not on a plasmid. This could mean that lateral gene transfer occurred not only between different bacteria but also between different genetic elements.
There are interesting contrasts between LPA of E. coli 4797/97 and pathogenicity islands from related members of the family Enterobacteriaceae. In LPA, genes that may alter metabolic functions or express virulence are located in the central portion of the island and are surrounded by mobility-associated genes (Fig. 2). In contrast, mobile genetic elements are interspersed throughout SHI-2, and prophage sequences flank LEE, but LEE is devoid of IS-related sequences.
These observations may have consequences for the mode of pathogenicity island acquisition. At present, it is not known if pathogenicity islands are acquired intact, probably by phage transduction, or by stepwise insertion of genes into the island. It is also possible that pathogenicity islands use diverse mechanisms of horizontal transfer. For example, the Vibrio cholerae pathogenicity island and the Staphylococcus aureus toxic shock syndrome toxin island were probable mobilized by transduction, because both islands are associated with phage sequences (28, 31), whereas the high-pathogenicity island of STEC O26 might utilize direct repeats located at both junctions with the chromosome for mobilization (29). Because prophage sequences were also present in the island of 4797/97 and in LEE, the phage origin of these islands is plausible. We have found many complete and incomplete ISs, suggesting dynamic recombinational activity in this locus. Thorough analyses of other related islands are needed to determine if this structure is stable in other STEC strains. Because we investigated only three marker genes, the genetic structure of most of the islands remains to be determined. Detailed sequence analysis of these islands will shed light on the extent of genetic variability of this element.
This work extends to LEE-negative STEC the observation that the selC locus is a common integration site for pathogenicity islands (7, 26, 35). In E. coli and Shigella strains, pathogenicity islands carry adjacent to selC an integrase gene which is homologous to that found in bacteriophage P4. This phage, which has been described as a satellite bacteriophage, utilizes an attB site close to selC (41). Therefore, it is expected that islands that are associated with such integrase genes can recombine into this site. These investigations support the hypothesis that phages or phage-derived sequences may be involved in the mobility of pathogenicity islands.
The virulence of stx2d-positive, eae-negative E. coli strains may be less than that of other STEC strains. We and others (55) isolated such strains from patients with watery diarrhea and from asymptomatic carriers. The presence of LPA might prevent the insertion of the LEE in selC and thus prevent the development of a more pathogenic strain. However, it is important to note that eae-negative strains can nonetheless cause severe illness (38, 67) and that eae-negative E. coli O91:H−, the same serotype as that of the prototype strain in this study, has also been isolated from patients with hemolytic-uremic syndrome (11).
In a recent study, Ramachandran et al. (44) have characterized the stx2 subtype of 146 STEC strains from ovine sources in Australia. The stx2 subtype found most often was stx2d, and it was predominantly associated with the serotypes O75:H−/H8/H40, O91:H−, O123:H−, O128:H2, and OR:H2. In most of these strains, eae was absent and stx2d occurred frequently together with stx1. The authors reinforced with their results the hypothesis of association with certain serotypes and of stx types with their animal host. However, we do not have enough data to speculate whether the Stx2d-producing E. coli strains described in our study originated from ovine reservoirs.
It is tempting to speculate on the functions of other region II genes, which were not subjected to expression analysis. The btuB homologue raises the question why these strains need two such genes. One hypothesis might be that the island-encoded BtuB represents the phage receptor for the Stx2d-encoding phage or that the chromosomal btuB gene is defective, probably because of prophage insertion (30).
Pathogenicity islands might provide the genetic versatility needed for the survival of E. coli in diverse environments. A rapid mode of gene acquisition and, potentially, loss or exchange may also confer a selective advantage as E. coli occupies these different niches. The genomic island described here is another example of a genetic element that alters its bacterial host and might also widen the bacterial ability to colonize and to grow in diverse environments. Future studies are necessary to understand the origin of such islands, their transmission, and their exact role in pathogenicity.
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 479) and the National Institutes of Health (NIH R01 AI47499).
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coliK1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hasani K, Henderson I R, Sakellaris H, Rajakumar K, Grant T, Nataro J P, Robins B R, Adler B. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect Immun. 2000;68:2457–2463. doi: 10.1128/iai.68.5.2457-2463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzaeby peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. [PubMed] [Google Scholar]
- 5.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 6.Benjelloun-Touimi Z, Tahar M S, Montecucco C, Sansonetti P J, Parsot C. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology. 1998;144:1815–1822. doi: 10.1099/00221287-144-7-1815. [DOI] [PubMed] [Google Scholar]
- 7.Blanc P A, Groisman E A. The Salmonella selClocus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc P A, Solomon F, Kayser J, Groisman E A. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado V J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coliK-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 10.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coliwild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnet R, Souweine B, Gauthier G, Rich C, Livrelli V, Sirot J, Joly B, Forestier C. Non-O157:H7 Stx2-producing Escherichia colistrains associated with sporadic cases of hemolytic-uremic syndrome in adults. J Clin Microbiol. 1998;36:1777–1780. doi: 10.1128/jcm.36.6.1777-1780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988;334:528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- 13.Brunder W, Schmidt H, Karch H. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coliO157:H7. Microbiology. 1996;142:3305–3315. doi: 10.1099/13500872-142-11-3305. [DOI] [PubMed] [Google Scholar]
- 14.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coliO157:H7, cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dell'Omo G, Morabito S, Quondam R, Agrimi U, Ciuchini F, Macri A, Caprioli A. Feral pigeons as a source of verocytotoxin-producing Escherichia coli. Vet Rec. 1998;142:309–310. doi: 10.1136/vr.142.12.309. [DOI] [PubMed] [Google Scholar]
- 17.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 18.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coliof serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckenstein J M, Lindler L E, Elsinghorst E A, Dale J B. Identification of a gene within a pathogenicity island of enterotoxigenic Escherichia coli H10407required for maximal secretion of the heat-labile enterotoxin. Infect Immun. 2000;68:2766–2774. doi: 10.1128/iai.68.5.2766-2774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallegos M T, Michan C, Ramos J L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993;21:807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallien P, Much C, Perlberg K W, Protz D. Detection of pas gene in shiga toxin producing Escherichia coli(STEC) Berl Münch Tierärztl Wochenschr. 1999;112:127–130. [PubMed] [Google Scholar]
- 22.Gannon V P, Gyles C L. Characteristics of the Shiga-like toxin produced by Escherichia coliassociated with porcine edema disease. Vet Microbiol. 1990;24:89–100. doi: 10.1016/0378-1135(90)90054-y. [DOI] [PubMed] [Google Scholar]
- 23.Gentry M K, Dalrymple J M. Quantitative microtiter cytotoxicity assay for Shigellatoxin. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyer D M, Henderson I R, Nataro J P, Mobley H L. Identification of Sat, an autotransporter protein toxin produced by uropathogenic Escherichia coli. Mol Microbiol. 2000;38:53–66. doi: 10.1046/j.1365-2958.2000.02110.x. [DOI] [PubMed] [Google Scholar]
- 25.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 26.Hacker J, Kaper J B. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol. 2000;54:641–679. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- 27.Heller K, Kadner R J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985;161:904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaolis D K, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 29.Karch H, Schubert S, Zhang D, Zhang W, Schmidt H, Ölschlager T, Hacker J. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coliclonal lineages. Infect Immun. 1999;67:5994–6001. doi: 10.1128/iai.67.11.5994-6001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kholodii G Y, Mindlin S Z. Integration of bacteriophages lambda and phi 80 in wild-type Escherichia coliat secondary attachment sites. I. Formation of secondary lysogens. Mol Gen Genet. 1984;197:104–108. doi: 10.1007/BF00327929. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay J A, Ruzin A, Ross H F, Kurepina N, Novick R P. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 32.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melton C A, Darnell S C, O'Brien A D. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coliO91:H21 isolates in orally infected, streptomycin-treated mice. Infect Immun. 1996;64:1569–1576. doi: 10.1128/iai.64.5.1569-1576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morschhäuser J, Vetter V, Emody L, Hacker J. Adhesin regulatory genes within large, unstable DNA regions of pathogenic Escherichia coli: cross-talk between different adhesin gene clusters. Mol Microbiol. 1994;11:555–566. doi: 10.1111/j.1365-2958.1994.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 35.Moss J E, Cardozo T J, Zychlinsky A, Groisman E A. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol Microbiol. 1999;33:74–83. doi: 10.1046/j.1365-2958.1999.01449.x. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen C C, Saier M H. Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett. 1995;377:98–102. doi: 10.1016/0014-5793(95)01344-x. [DOI] [PubMed] [Google Scholar]
- 37.Otto B R, van Dooren S J, Nuijens J H, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia colistrain EB1. J Exp Med. 1998;188:1091–1103. doi: 10.1084/jem.188.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton A W, Woodrow M C, Doyle R M, Lanser J A, Paton J C. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eaeresponsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perna N T, Mayhew G F, Posfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coliO157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierard D, Muyldermans G, Moriau L, Stevens D, Lauwers S. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coliisolates. J Clin Microbiol. 1998;36:3317–3322. doi: 10.1128/jcm.36.11.3317-3322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierson L S, Kahn M L. Integration of satellite bacteriophage P4 in Escherichia coli. DNA sequences of the phage and host regions involved in site-specific recombination. J Mol Biol. 1987;196:487–496. doi: 10.1016/0022-2836(87)90026-x. [DOI] [PubMed] [Google Scholar]
- 42.Pridmore R D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 43.Rahn K, Renwick S A, Johnson R P, Wilson J B, Clarke R C, Alves D, McEwen S, Lior H, Spika J. Persistence of Escherichia coliO157:H7 in dairy cattle and the dairy farm environment. Epidemiol Infect. 1997;119:251–259. doi: 10.1017/s0950268897007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran V, Hornitzky M A, Bettelheim K A, Walker M J, Djordjevic S P. The common ovine Shiga toxin 2-containing Escherichia coliserotypes and human isolates of the same serotypes possess a Stx2d toxin type. J Clin Microbiol. 2001;39:1932–1937. doi: 10.1128/JCM.39.5.1932-1937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reizer J, Charbit A, Reizer A, Saier M H., Jr Novel phosphotransferase system genes revealed by bacterial genome analysis: operons encoding homologues of sugar-specific permease domains of the phosphotransferase system and pentose catabolic enzymes. Genome Sci Technol. 1996;1:53–75. [Google Scholar]
- 46.Ritter A, Blum G, Emody L, Kerenyi M, Bock A, Neuhierl B, Rabsch W, Scheutz F, Hacker J. tRNA genes and pathogenicity islands: influence on virulence and metabolic properties of uropathogenic Escherichia coli. Mol Microbiol. 1995;17:109–121. doi: 10.1111/j.1365-2958.1995.mmi_17010109.x. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H, Plaschke B, Franke S, Rüssmann H, Schwarzkopf A, Heesemann J, Karch H. Differentiation in virulence patterns of Escherichia colipossessing eae genes. Med Microbiol Immunol. 1994;183:23–31. doi: 10.1007/BF00193628. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt H, Rüssmann H, Karch H. Virulence determinants in nontoxinogenic Escherichia coliO157 strains that cause infantile diarrhea. Infect Immun. 1993;61:4894–4898. doi: 10.1128/iai.61.11.4894-4898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler L, Karch H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coliisolated from pigeons. Appl Environ Microbiol. 2000;66:1205–1208. doi: 10.1128/aem.66.3.1205-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt C K, McKee M L, O'Brien A D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperandio V, Kaper J B, Bortolini M R, Neves B C, Keller R, Trabulsi L R. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli(STEC) serotypes. FEMS Microbiol Lett. 1998;164:133–139. doi: 10.1111/j.1574-6968.1998.tb13078.x. [DOI] [PubMed] [Google Scholar]
- 54.Stathopoulos C, Provence D L, Curtiss R. Characterization of the avian pathogenic Escherichia colihemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect Immun. 1999;67:772–781. doi: 10.1128/iai.67.2.772-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephan R, Hoelzle L E. Characterization of Shiga toxin type 2 variant B-subunit in Escherichia colistrains from asymptomatic human carriers by PCR-RFLP. Lett Appl Microbiol. 2000;31:139–142. doi: 10.1046/j.1365-2672.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Inouye M, Inouye S. Association of a retroelement with a P4-like cryptic prophage (retronphage φR73) integrated into the selenocystyl tRNA gene of Escherichia coli. J Bacteriol. 1991;173:4171–4181. doi: 10.1128/jb.173.13.4171-4181.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarr P I, Bilge S S, Vary J C, Jr, Jelacic S, Habeeb R L, Ward T R, Baylor M R, Besser T E. Iha: a novel Escherichia coliO157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun. 2000;68:1400–1407. doi: 10.1128/iai.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarr P I, Schoening L M, Yea Y-L, Ward T R, Jelacic S, Whittam T S. Acquisition of the rfb-gnd cluster in evolution of Escherichia coliO55 and O157. J Bacteriol. 2000;182:6183–6191. doi: 10.1128/jb.182.21.6183-6191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tibazarwa C, Wuertz S, Mergeay M, Wyns L, van der Lelie D. Regulation of the cnr cobalt and nickel resistance determinant of Ralstonia eutropha (Alcaligenes eutrophus) CH34. J Bacteriol. 2000;182:1399–1409. doi: 10.1128/jb.182.5.1399-1409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong S, Porco A, Isturiz T, Conway T. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntUgenes of GntI, the main system for gluconate metabolism. J Bacteriol. 1996;178:3260–3269. doi: 10.1128/jb.178.11.3260-3269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umeda M, Ohtsubo E. Four types of IS1 with differences in nucleotide sequence reside in the Escherichia coliK-12 chromosome. Gene. 1991;98:1–5. doi: 10.1016/0378-1119(91)90096-t. [DOI] [PubMed] [Google Scholar]
- 62.Vokes S A, Reeves S A, Torres A G, Payne S M. The aerobactin iron transport system genes in Shigella flexneriare present within a pathogenicity island. Mol Microbiol. 1999;33:63–73. doi: 10.1046/j.1365-2958.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 63.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei B Y, Bradbeer C, Kadner R J. Conserved structural and regulatory regions in the Salmonella typhimurium btuB gene for the outer membrane vitamin B12 transport protein. Res Microbiol. 1992;143:459–466. doi: 10.1016/0923-2508(92)90091-2. [DOI] [PubMed] [Google Scholar]
- 65.Wieler L H, McDaniel T K, Whittam T S, Kaper J B. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia colidiffers in relation to the clonal phylogeny of the strains. FEMS Microbiol Lett. 1997;156:49–53. doi: 10.1111/j.1574-6968.1997.tb12704.x. [DOI] [PubMed] [Google Scholar]
- 66.Woodall L D, Russell P W, Harris S L, Orndorff P E. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5promoter. J Bacteriol. 1993;175:2770–2778. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodward M J, Gavier W D, McLaren I M, Wray C, Sozmen M, Pearson G R. Infection of gnotobiotic calves with Escherichia coliO157:H7 strain A84. Vet Rec. 1999;144:466–470. doi: 10.1136/vr.144.17.466. [DOI] [PubMed] [Google Scholar]
- 68.Yeo C C, Wong D T, Poh C L. IS1491 from Pseudomonas alcaligenes NCIB 9867: characterization and distribution among Pseudomonasspecies. Plasmid. 1998;39:187–195. doi: 10.1006/plas.1997.1331. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Foxman B, Manning S D, Tallman P, Marrs C F. Molecular epidemiologic approaches to urinary tract infection gene discovery in uropathogenic Escherichia coli. Infect Immun. 2000;68:2009–2015. doi: 10.1128/iai.68.4.2009-2015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]