Graphical abstract

Keywords: Rice, Cadmium, OsABCG43, Leaf bacteria, Phytotoxicity
Highlights
-
•
Rice OsABCG43 is functionally identified as a Cd importer controlling Cd accumulation.
-
•
Transgenic rice overexpressing OsABCG43 causes Cd accumulation resulting in phytotoxicity.
-
•
OsABCG43-mediated enrichment of Cd is harmful for leaf bacteria.
-
•
Accumulated Cd impairs the formation of virulence factors of leaf bacteria.
-
•
RNA-seq analysis reveals potential key genes involving in Cd and reactive oxygen species homeostasis.
Abstract
Introduction
Cadmium (Cd), one of the major toxic heavy metals, causes severe deleterious effects on all living organisms from prokaryotes to eukaryotes. Cadmium deposition affects bacterial diversity and bacterial population in soil. Cadmium accumulation in plants is mainly controlled by transporters and the resulting Cd enrichment gives rise to phytotoxicity.
Objective
This study aimed to mine transporters that control Cd import or accumulation in rice and uncover the underlying mechanisms that how accumulated Cd poses risks to host plant and leaf bacteria.
Methods
RNA-seq analysis, histochemical assays, and elemental quantification were carried out to reveal the biological roles of OsABCG43 for Cd import. Pathogen inoculation, IC50 value, and bacterial virulence assays were conducted to disclose the effects of Cd on leaf bacteria.
Results
OsABCG43 is characterized as a Cd importer controlling Cd accumulation in rice. OsABCG43 was induced under Cd stress and specifically expressed in the vasculature of leaves and roots. Overexpression of OsABCG43 caused Cd accumulation which inhibits photosynthesis and development and alters the antioxidant system, resulting in phytotoxicity. Moreover, overexpression of OsABCG43 resulted in retarded plant growth and enhanced rice sensitivity to Cd stress. Numerous differentially expressed genes were identified via RNA-seq analysis between the OsABCG43-overexpressing plants and wild type, which functioned in Cd or reactive oxygen species (ROS) homeostasis. In addition, OsABCG43 transcripts were induced by leaf bacteria Xanthomonas oryzae pv. oryzicola (Xoc) and X. oryzae pv. oryzae (Xoo). The enriched Cd directly impaired the formation of virulence factors for the leaf bacteria, preventing colonization or proliferation of Xoc or Xoo in rice leaves.
Conclusion
This work reveals that OsABCG43 is expressed specifically in the vascular and plasma membrane-localized OsABCG43 functions as a Cd importer. OsABCG43-mediated import of Cd is harmful for both rice and the corresponding leaf bacteria.
Introduction
Cadmium (Cd), a nonessential heavy metal that is widespread in nature, is documented as highly toxic to all living organisms from prokaryotes to eukaryotes [1]. Development in industries and agriculture accelerates Cd contamination in agricultural soils. The environmental survey reported that about 7% of the nationwide soil were heavily polluted by Cd in China [2], resulting in about 150 million kilograms of farm products were contaminated by Cd each year, of which one-third of them were rice [3], [4]; accordingly, the deposition of Cd in crops poses perpetual damage to the health of animals and humans. Specially, when Cd being concentrated in human body through the food chain, it can indefinitely pose health implications including anemia, cancer, cardiac failure, emphysema, hypertension, osteoporosis, proteinuria, renal dysfunction [5]. In addition, the toxicity of Cd irreversibly destroys the soil ecosystem by impacting microbial population or microbial community structure [6], [7].
Increasing evidences report that plants absorb Cd from the soil by roots and transport Cd from roots to different organs by many specific or non-specific transporters of essential nutrients [8], [9], [10], [11]. Several transporter families have been verified to be responsible for Cd transportation in plants, including ATP-binding cassette G subfamily protein (ABCG), natural resistance-associated macrophage protein (NRAMP), zinc/iron-regulated transporter-like protein (ZIP), and heavy metal ATPase (HMA) [8], [11], [12], [13], [14]. These transporters exhibiting tissue-specific expression patterns and different subcellular localization can uptake Cd from the soil, translocate Cd among different plant tissues, transport Cd among different cells, or pump Cd out of plants [8], [11], [12], [13], [14]. Within plants, the deposition of Cd can inhibit photosynthesis, plant growth or development, decrease nutrient uptake, alter the antioxidant system, resulting in phytotoxicity [11], [15]. The threshold of Cd phytotoxicity to rice varies with rice cultivars, the growth of Cd-sensitive rice is inhibited by 5.0 μM Cd, while the growth for Cd-tolerant rice is normal even over 25.0 μM Cd [11], [16].
All organisms from bacteria to flora and fauna contain ATP-binding cassette (ABC) protein genes in their genome. Eukaryotic ABC proteins can be divided into eight subfamilies (ABCA to ABCH) by phylogenetic analysis [1], [8]. Because some ABCG proteins are featured with depicted pleiotropic drug resistance (PDR) phenomena, they alternatively are named PDR proteins. According to new nomenclature, rice ABC superfamily contains over 130 members, of which, the largest subfamily ABCG has 50 members [17]. Mounting evidences show that ABCG/PDR proteins play critical roles in the transportation of toxic heavy metals, secondary metabolites, or phytohormones [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Several plant ABCG proteins function as toxic heavy-metal transporters [19], [20], [21]. Such as Arabidopsis (Arabidopsis thaliana) AtABCG32/AtPDR8 acting as a Cd transporter pumps Cd or Cd conjugates out of the plasma membrane [19]. AtABCG40/AtPDR12 is a Pb transporter pumping Pb complexes or Pb out of the cytoplasm [20]. Rice (Oryza sativa) OsABCG36/OsPDR9 exports Cd conjugates or Cd out of the root cells [21]. Additionally, these ABCG proteins play roles in plant growth or plant development or in response to biotic stress or abiotic stress [21], [22], [23], [24], [25], [26], [27], [28]. OsABCG36/OsPDR9 negatively regulates rice tolerance to Cd at high Cd concentrations [21]. OsABCG9 can transport epicuticular wax of rice leaves. The osabcg9 mutants showed retarded growth and were sensitive to low humidity [22]. However, the relationship between the substrates of plant ABCG proteins and their biological roles is largely unknown. A few plant ABCG proteins have been implicated in immunity that they possibly facilitate the influx or efflux of defense components to plasma membrane against pathogens invasion [23], [24]. AtABCG34 and AtABCG40/AtPDR12 secret camalexin to leaf surface preventing pathogens infection [24], [25]. Wheat Lr34, encoding an ABCG transporter, possibly regulates ABA redistribution to provide broad-spectrum and durable resistance against multiple fungal pathogens in major cereals [26]. Several ABCG genes can regulate phytohormone signaling pathways such as salicylic acid- or jasmonic acid-dependent defense pathways to boost plant immunity [27], [28]. However, whether rice OsABCG genes play roles against bacterial pathogens infection and how their potential substrates contribute to preventing the proliferation of bacteria are scarcely uncovered.
Here, we revealed that OsABCG43 was activated under Cd treatment and plasma membrane-localized OsABCG43 was a Cd importer. Overexpression of OsABCG43 caused Cd accumulation, resulting in retarded plant growth and enhanced rice sensitivity to Cd stress. Notably, OsABCG43 expression was induced upon challenge with leaf bacteria Xanthomonas oryzae pv. oryzicola (Xoc) and X. oryzae pv. oryzae (Xoo). Enrichment of Cd in leaves impaired the formation of virulence factors of the leaf bacteria, resulting in attenuated proliferation of leaf bacteria. Together, OsABCG43-mediated Cd import causes Cd accumulation which is harmful for both rice and the corresponding leaf bacteria.
Materials and methods
Plant materials, growth conditions and Cd treatments
Wild type rice (Oryza sativa ssp. Geng) cultivar Zhonghua 11 (ZH11) and the corresponding transgenic rice in ZH11 background were used as experimental materials. The transgenic rice OsABCG43-overexpressing (OsABCG43-OE) plants and osabcg43 knockout mutants were generated in ZH11 background. The transgenic rice and ZH11 seeds were sown on seedbeds, then the 30-day-old seedlings were cultivated in the experimental paddy fields of Huazhong Agricultural University from May to October in 2018 to 2021 under natural cultivation conditions.
For hydroponic experiment, the 10-day-old seedlings were planted on floating plates on complete nutrient solution in a greenhouse under a 14 h light/10 h dark photoperiod [29]. The solution for hydroponic culture was renewed every second day. For Cd treatment, rice seedlings were treated with 30 μM CdCl2 at the tillering stage for 20 days. After Cd treatment, different rice tissues (roots, leaves) were sampled for further analysis including gene expression quantification, shoot or root biomass, and Cd content.
Constructs and transgenic rice plants generation
OsABCG43 coding sequence was amplified by PCR using the specific primers from cDNA of ZH11 leaves (Table S1). The amplified PCR products were inserted into the pU1301 vector between the KpnI and BamHI restriction sites via restricting-ligation method to generate the OsABCG43-OE construct [30]. Two 20-bp sgRNA each matching the first exon of OsABCG43 were inserted into the sgRNA expression cassettes via overlapping PCR method, then the two cassettes were assembled into the pYLCRISPR/Cas9 vector via Gibson Assembly method to generate OsABCG43 knockout construct [31]. OsABCG43 promoter (2090 bp upstream from its translation initiation site) was amplified by PCR using the specific primers from DNA of ZH11 leaves (Table S1). The corresponding PCR products were inserted upstream of the reporter β-glucuronidase (GUS) gene into the pCAMBIA1381 vector via overlapping PCR method to generate OsABCG43pro:GUS construct [32]. After sequencing all the constructs using the vector-specific primers, they were transferred to Agrobacterium tumefaciens, which were subsequently transformed into rice calli prepared from ZH11 mature embryos, to generate transgenic rice plants [30]. PCR analysis was used for confirmation of the positive lines of OsABCG43-OE and OsABCG43pro:GUS plants using an OsABCG43-specific primer and a vector-specific primer. The OsABCG43 genome DNA was sequenced for null mutant confirmation of the osabcg43 mutants.
Leaf bacteria inoculation
Xoo or Xoc colonies were cultivated on nutrient agar medium (3 g/L beef extract, 1 g/L yeast extract, 10 g/L sucrose, 5 g/L polypeptone, 20 g/L agar [pH 7.0]) for 3 days at 28 °C. Then, bacterial cells were collected via centrifugation and suspended in sterilized water, adjusting to an OD600nm of 0.5 for inoculation. Xoo included Chinese strains (KS 1–21, Zhe173), Philippine strains (PXO99, PXO341, PXO61, PXO71, PXO347, PXO112), and Korean strain (KACC10331). Xoc included Chinese strains (RS105, HNB8-47, GX01, RH3). Five to ten leaves of each rice plant at the booting stage were inoculated with different Xoo strains by the leaf-clipping method or at the tillering stage inoculated with different Xoc strains by the penetration method [33]. Bacterial growth was assessed by measuring lesion length of rice leaves 14 d post Xoo or Xoc inoculation or counting colony-forming units every 3 d after Xoo or Xoc inoculation as described previously [34].
Gene expression analysis
The TRIzol solution (Invitrogen, USA) was used in the extraction of rice RNAs from different tissues including roots, stems, nodes, sheaths, leaves, and panicles of wild type, or leaves of transgenic plants. HiScript III Reverse Transcriptase (Vazyme, China) was used for cDNA synthesis. ChamQ SYBR qPCR Master Mix (Vazyme, China) was used for quantitative reverse transcription PCR (RT-qPCR) analysis. The RT-qPCR assays were conducted using 20 μL mixtures containing 10 μL of 2 × ChamQ Universal SYBR qPCR Master Mix, 5 μL of cDNA, 0.4 μL of each gene-specific primers (Table S2), and 4.2 μL of ddH2O in the 7500 Real-Time PCR System (Applied Biosystems, USA). Amplification steps were 95 °C for 30 s, 45 cycles of 95 °C for 10 s, and 60 °C for 20 s, followed by 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. The relative transcript levels of leaf bacteria gyrB gene and rice actin gene were used for gene transcription normalization [35].
Transcriptome analysis
Leaves of the OsABCG43-OE and wild type were sampled at the tillering stage. The assays including RNA extraction, RNA quantification, library construction, and Illumina HiSeq 2500 platform-based sequencing were carried out by Novogene (Beijing, China). Read quality was checked using FastQC v1.11.4, both the adapter sequences and the low-quality reads were trimmed using Cutadapt v3.4. All the clean reads were mapped to Nipponbare genome (MSU Release 7) using TopHat v2.1.1 and the transcript abundance of each transcript was evaluated using Cufflinks v2.2.1. Differentially expressed genes (DEGs) were assessed using DESeq v2.10 that were characterized with false discovery rate (FDR) < 0.05 and relative expression |log2(fold change)| ≥ 1. GOATOOLS was used for Gene Ontology (GO) analysis and KOBAS was used for Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The raw transcriptome data were deposited to Sequence Read Archive of NCBI with BioProject ID: PRJNA798458.
IC50 assay
To assess effects of Cd stress on leaf bacteria, Xoo strain PXO99 and Xoc strain RS105 were grown in m210 liquid medium (6.55 g/L K2HPO4·3H2O, 4 g/L yeast extract, 0.3 g/L MgSO4·7H2O, 8 g/L casein enzymatic hydrolysate, 5 g/L sucrose [pH 7.0]) entering the early logarithmic phase stage. After that, bacteria were transferred into m210 liquid medium with different concentrations of CdCl2 (0.0 μM, 0.5 μM, 1.0 μM, 1.5 μM, 2.0 μM). Bacterial density was measured spectrometrically at 600 nm using a Spark™ Multimode Microplate Reader (Tecan, Switzerland). The half-maximal inhibitory concentration (IC50) values were calculated in GraphPad Prism 8.02 as reported previously [35].
Twitching motility assays and swarming motility assays
Twitching motility assays were conducted according to a previously reported protocol [35]. In brief, Xoo strain PXO99 and Xoc strain RS105 were adjusted to an OD600 nm of 0.6, and 3 μL of the suspension were dotted onto PS plates (1 g/L L-glutamic acid, 10 g/L sucrose, 10 g/L peptone, 3 g/L agar [pH 7.0]) containing different concentrations of CdCl2 (0.0 μM, 0.5 μM, 1.0 μM, 2.0 μM). After incubation at 28 °C for 7 days, marginal sides for bacterial cell were photographed using a Leica stereo microscope (Wetzlar, Germany).
Swarming motility assays were carried out according to a published protocol [35]. In brief, Xoo strain PXO99 and Xoc strain RS105 were adjusted to an OD600 nm of 1.0, and 3 μL of bacterial cultures were dotted onto the center of NB plates (3 g/L beef extract, 5 g/L peptone, 3 g/L agar [pH 7.0]) premixed with different concentrations of CdCl2 (0.0 μM, 0.5 μM, 1.0 μM, 2.0 μM). After incubation at 28 °C for 7 days, the diameters of each swimming zone were photographed and calculated.
EPS production measurement and biofilm formation analysis
Bacteria Xoo strain PXO99 and Xoc strain RS105 were cultivated in m210 liquid medium premixed with different concentrations of CdCl2 (0.0 μM, 0.5 μM, 1.0 μM, 2.0 μM) for 24 h. After bacterial cells were centrifugated at 4 °C, the supernatants were transferred into new tubes, suspended in one volume of 1.0% potassium chloride and two volumes of 100% ethanol, then stored at −20 °C for 12 h. The suspensions were centrifugated at 4 °C and the precipitates were fully dried at 55 °C for 12 h, then the dry precipitated extracellular polysaccharides (EPS) were measured [35].
Biofilm formation assays for bacteria were conducted according to a previously reported PVC microplate method [35]. Xoo strain PXO99 and Xoc strain RS105 were cultivated in XOM2 medium (40 μM MnSO4, 14.7 mM KH2PO4, 240 μM Fe (III)-EDTA, 5 mM MgCl2, 10 mM sodium L-(+)-glutamate, 670 μM L-methionine, 0.18% xylose [pH 6.5]) premixed with different concentrations of CdCl2 (0.0 μM, 0.5 μM, 1.0 μM, 2.0 μM). Each bacterial suspension was subsequently diluted in 180 μL XOM2 and cultivated in a 96-well PVC microplate. Bacterial pellicles were suspended in 0.1% crystal violet for 2 h and then redissolved with 100% ethanol to remove the adherent dye. The absorbance of the dye was measured using a Spark™ Multimode Microplate Reader (Tecan, Switzerland) at 590 nm.
Subcellular localization assays
OsABCG43 coding sequence without the stop codon was amplified by PCR using the specific primers (Table S1). The corresponding PCR products were inserted into the transient expression vector pM999 between the KpnI and EcoRI restriction sites, resulting in that OsABCG43 is in-frame and upstream of the yellow fluorescent protein (YFP) gene where they were regulated by the cauliflower mosaic virus 35S promoter. After sequencing, the OsABCG43-GFP construct was transformed into rice protoplasts via polyethylene glycol (PEG)-mediated transient protoplast transformation and the protoplasts were incubated at 26 °C for 12 h [35]. The protoplasts were visualized for fluorescent signals by a Leica Microsystem (LAS AF, Germany).
Histochemical GUS staining assays
Histochemical GUS staining analysis were carried out as previously described [32]. In brief, rice tissues including roots, stems, leaves, panicles, and sheaths were submerged in GUS staining solution (1 mg/mL of X‐Gluc, 0.1% Triton X‐100, 10 mM EDTA, 50 mM sodium phosphate buffer [pH 7.0], 1 mM potassium ferrocyanide, 100 μg/mL of chloramphenicol, 1 mM potassium ferricyanide, 20% methanol) under vacuum infiltration for 30 min, then incubated at 37 °C for 24 h. After that, the stained samples were fixed in a fixation solution (5% acetic acid, 3.7% formaldehyde, 50% ethanol) at 25 °C for 24 h. Rice tissues were photographed using a Leica stereo microscope (Wetzlar, Germany).
DAB staining
Rice leaves at the booting stage were collected for 3,3′-diaminobenzidine (DAB) staining [11]. In brief, DAB was dissolved in a buffer containing 10 mM 2-(N-morpholinol) ethanesulfonic acid and 0.2% Tween 20, reaching a final concentration of 1 mg/mL. Leaves were submerged in DAB staining solution under a vacuum for 12 h in the dark, then immersed in ethanol to remove chlorophyll in a water bath at 60℃ for 12 h.
Elemental measurement
Rice leaves were dried at 80℃ for 6 days achieving a constant weight and ground to powder. About 0.2 g powder of each sample was treated with HNO3/HClO4 solution (85:15, v:v) in a Microwave Digestion System (MARS6, CEM, USA) under a temperature gradient for 60 min from 120℃ to 180℃ until completely digested. Contents of metal ions including Cd, arsenic (As), magnesium (Mg), iron (Fe), copper (Cu), molybdenum (Mo), manganese (Mn), lead (Pb), nickel (Ni), and zinc (Zn) were measured by an Agilent inductively coupled plasma mass spectrometry (ICP-MS) (Santa Clara, USA) according to a protocol established previously [11].
Histochemical experiments
Leaves of rice plants at the booting stage were collected and immediately frozen in liquid nitrogen. The activities of superoxide dismutase (SOD), hydrogen peroxide (H2O2), peroxidase (POD), glutathione (GSH), catalase (CAT), and malondialdehyde (MDA) were measured using assay kits with Cat. No. E-BC-K020-M, E-BC-K102-M, E-BC-K227-S, E-EL-0026, E-BC-K031-S, and E-EL-0060, respectively (Elabscience, USA), according to manufacturer protocols using a Spark Multimode Microplate Reader (Tecan).
All the measurements were biologically repeated with three times.
Determination of chlorophyll concentration and photosynthetic rate
Leaves of rice plants at the booting stage were collected for chlorophyll measurement. Samples were incubated in extraction buffer (absolute ethanol:acetone:water, 4.5:4.5:1, v:v:v) for 12 h, and the extraction was measured spectrophotometrically at 645 nm and 663 nm using a Spark Multimode Microplate Reader (Tecan). Chlorophyll content was calculated as previously described [11]. Leaf photosynthetic rate was quantified from 10:00 am to 11:00 am using the LI-6400XT portable photosynthesis system (LI-COR, USA). Quantum efficiency of photosystem II (Fv/Fm) was assessed using the MultispeQ V2.0 (PhotosynQ, USA).
Statistical data analysis
The statistical differences between transgenic plants and wild type were analyzed using the Student’s t-test at the P < 0.05 or P < 0.01 level in the Microsoft Office Excel program. The statistical differences of OsABCG43 transcription in roots or leaves of seedlings treated with Cd were analyzed using the Tukey’s multiple comparison test at the P < 0.05 level in the IBM SPSS program.
Results
OsABCG43 is specifically expressed in the vasculature
The subfamily G of rice ABC superfamily has 50 members [17], some of them showed increased expression in response to Cd treatment, such as OsABCG36, OsABCG37, OsABCG43, and OsABCG44 [36], [37]. To identify whether and even which of these OsABCG genes contribute to Cd accumulation, transcript levels for them were analyzed. Based on genome-wide high-throughput data [37] and RT-qPCR assays, OsABCG43, not only has the highest transcript in leaves when compared with other OsABCG genes (Fig. S1), and also is markedly induced by Cd treatment (Fig. S2). Thus, we were particularly interested in OsABCG43, and carried out series of assays to explore its potential role in Cd accumulation. To determine the spatial expression profile of OsABCG43, we examined its expression pattern in different tissues including roots, stems, nodes, sheaths, leaves, and panicles by RT-qPCR. The results showed that OsABCG43 was ubiquitously expressed, with the relatively highest transcript in leaves and the lowest expression in nodes (Fig. 1A). We also designed a construct OsABCG43pro:GUS where OsABCG43 promoter driving the reporter GUS gene expression and generated transgenic plants. GUS signals were detected in stems, roots, panicles, leaves, and sheaths, with the most abundant in leaves (Fig. 1B). The GUS staining results were almost consistent with results of RT-qPCR. Subsequently, cell-type-specific expression of OsABCG43 was carried out in roots and leaves. Transverse section analysis showed that GUS signals were located exclusively to the vasculature of roots and leaves, including xylem, phloem, vessel, and bundle sheath cell (Fig. 1C-F).
Fig. 1.
Expression patterns of OsABCG43 and subcellular localization of OsABCG43. (A) OsABCG43 expression in different tissues; (B) Histochemical staining of different tissues of the OsABCG43pro:GUS transgenic lines. Scale bars, 1 mm; (C) Transverse section of root. Scale bar, 50 μm; (D) GUS activity in the vascular bundle of root. Scale bar, 10 μm; (E) Transverse section of leaf. Scale bar, 50 μm, (F) GUS activity in the vascular bundle of leaf. Scale bar, 50 μm; (G) Subcellular localization of OsABCG43-YFP in rice protoplasts. SCAMP1-RFP is used as a plasma membrane-localized marker protein. Scale bars, 10 μm. Data represents mean (three replicates) ± SD.
In addition, we assayed subcellular localization of OsABCG43 by transient transfection of OsABCG43:YFP construct in rice protoplasts. OsABCG43:YFP exclusively colocalized with SECRETORY CARRIER MEMBRANE PROTEIN 1 (SCAMP1) which was fused to the red fluorescent protein (RFP) as a plasma membrane-localized marker protein (Fig. 1G), confirming OsABCG43 is a plasma membrane protein. Collectively, these results suggest that OsABCG43 encoding a plasma membrane protein is specifically expressed in the vasculature.
OsABCG43 is a Cd inducible gene
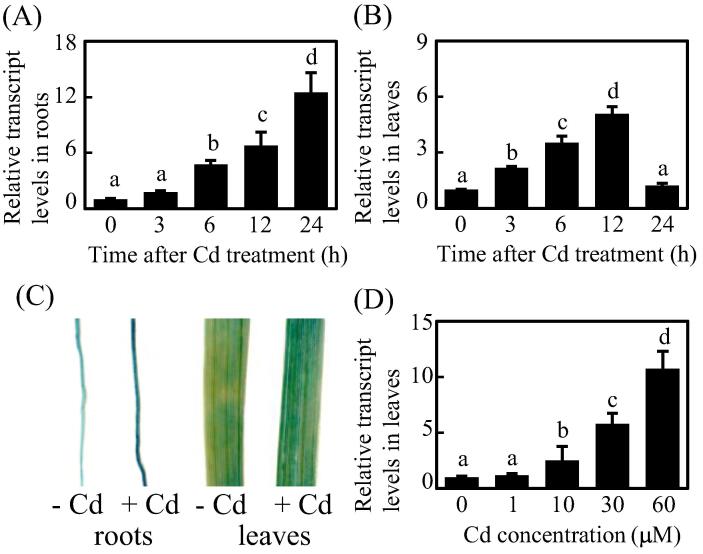
To explore the potential role of OsABCG43 in Cd accumulation, we quantified its expression pattern in response to Cd treatment. A time-course analysis revealed that OsABCG43 transcripts were upregulated in Cd-treated roots (Fig. 2A). A comparable expression pattern of OsABCG43 was also surveyed in Cd-treated leaves (Fig. 2B). There were about 6.8-fold and 5.1-fold increased OsABCG43 transcripts in roots and leaves after Cd treatment for 12 h. GUS staining further verified the upregulated expression patterns of OsABCG43 in roots and leaves in response to Cd stress (Fig. 2C). Moreover, transcript levels of OsABCG43 were quantified in the presence of different Cd concentrations, with that OsABCG43 transcript was in a concentration-dependent pattern in response to Cd treatment (Fig. 2D). Thus, OsABCG43 is a Cd inducible gene.
Fig. 2.
OsABCG43 is a Cd inducible gene. (A) OsABCG43 transcription in roots of 10-d-old seedlings treated with 30 μM Cd for the indicated time; (B) OsABCG43 transcription in leaves of 10-d-old seedlings treated with 30 μM Cd for the indicated time; (C) GUS staining of roots and leaves of the OsABCG43pro:GUS transgenic lines without (-) or with (+) Cd treatment; (D) OsABCG43 transcription in leaves of 10-d-old seedlings treated with different concentrations of Cd for 12 h. Data represents mean (three replicates) ± SD. The letters above each bar present statistically significant differences which were determined by one-way ANOVA analysis followed by Tukey’s multiple test (P < 0.05).
OsABCG43 antagonizes plant growth and development
OsABCG43 has been experimented with conferring Cd tolerance on yeast [38], whereas, its role in rice is unclear. To investigate its physiological role for Cd accumulation, we generated OsABCG43-overexpressing (OsABCG43-OE) plants and deletion of OsABCG43 (osabcg43) transgenic plants in ZH11 genetic background. Nine independent OsABCG43-OE T0 plants were obtained where OsABCG43 coding sequence was driven by the maize Ubiquitin promoter. Transcript levels of OsABCG43 were dramatically increased in these OsABCG43-OE plants (Fig. S3). Simultaneously, five heterozygous osabcg43 T0 mutations were obtained via clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 (CRISPR/Cas9)-mediated genome editing strategy after PCR amplification and sequencing the targeting regions of the total 18 transgenic plants (Fig. S4A). The homozygous osabcg43 mutations (designated osabcg43-1 and osabcg43-2) were obtained in T2 generation and had large genomic deletions of 207-bp or 173-bp, respectively (Fig. S4B, C). These two osabcg43 mutants had premature stop codons, resulting in both osabcg43-1 and osabcg43-2 are loss-of-function alleles.
When planting in the paddy field, plant heights of the OsABCG43-OE plants were lower than that of wild type ranging from 16-cm to 18-cm at the seedling stage and from 13-cm to 17-cm at the tillering stage (Fig. S5). However, plant heights of the osabcg43 mutants were similar to that of wild type (Fig. 3A, B).
Fig. 3.
Overexpression of OsABCG43 causes accelerated senescence and attenuated photosynthesis. (A) The whole plant of OsABCG43-OE plants and osabcg43 mutants at the tillering stage grown in the paddy field; (B) Plant height of the OsABCG43-OE plants and osabcg43 mutants; (C) Leaf showing senescence at the booting stage; (D) Chlorophyll levels in the OsABCG43-OE plants and osabcg43 mutants leaves; (E) Relative transcript levels of OsRbcL and OsCab1 in the OsABCG43-OE plants and osabcg43 mutants leaves; (F) Photosynthetic rates of the OsABCG43-OE plants and osabcg43 mutants leaves; (G) Fv/Fm values of the OsABCG43-OE plants and osabcg43 mutants leaves. Data represents mean (three replicates) ± SD. Asterisks present significant difference which were determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
The OsABCG43-OE plants exhibited spots or reddish-brown lesions on their leaves at the booting stage, and later, their leaves accelerated senescence, whereas, the osabcg43 mutants showed similar leaves as wild type without spots or lesions (Fig. 3C). In agreement, the OsABCG43-OE plants had lower contents of chlorophyll a (1.7-fold decreased), chlorophyll b (1.4-fold decreased), and total chlorophyll (1.6-fold decreased) than those of wild type or the osabcg43 mutants (Fig. 3D). Moreover, we analyzed transcript levels of OsRbcL and OsCab1, which two are representative chlorophyll-related genes involved in chlorophyll biosynthesis and chlorophyll binding, respectively [39], in leaves of these plants. Both OsRbcL and OsCab1 had significantly decreased transcript levels in the OsABCG43-OE plant leaves relative to those in wild type or the osabcg43 mutant leaves (Fig. 3E). In addition, the photosynthetic rate and Fv/Fm were 1.7- and 1.6-fold lower in the OsABCG43-OE plants than that of wild type or the osabcg43 mutants, respectively (Fig. 3F, G). These findings indicate that overexpression of OsABCG43 inhibits photosynthesis and accelerates senescence.
ROS abnormality of OsABCG43-OE plants
Because the OsABCG43-OE plants developed automatically spots or lesions on their leaves, we speculated that was the result of reactive oxygen species (ROS) accumulation. To confirm our hypothesis, we stained the OsABCG43-OE plants, osabcg43 mutants, and wild type leaves with DAB. The DAB staining showed that the obvious red-brown precipitates were exclusively observed in the OsABCG43-OE plant leaves, but no such precipitates were surveyed in the osabcg43 mutants and wild type leaves (Fig. 4A). As DAB is proverbially used to characterize H2O2 accumulation [11]. Therefore, we measured H2O2 content in these rice leaves. There were 2.4- to 2.6-fold higher of H2O2 contents in the leaves of OsABCG43-OE plants than osabcg43 mutants or wild type (Fig. 4B), indicating that accumulated H2O2 may directly cause lesions or spots on the OsABCG43-OE plant leaves.
Fig. 4.
Impaired ROS homeostasis in the OsABCG43-OE plants. (A) DAB staining for the transgenic rice plants (OsABCG43-OE plants and osabcg43 mutants) and wild type (WT) leaves; (B) H2O2 levels in the transgenic rice and WT leaves; (C) SOD activity in the transgenic rice and WT leaves; (D) CAT activity in the transgenic rice and WT leaves; (E) POD activity in the transgenic rice and WT leaves; (F) GSH content in the transgenic rice and WT leaves; (G) GRX activity in the transgenic rice and WT leaves; (H) MDA content in the transgenic rice and WT leaves; (I) Relative transcript levels of senescence responsive genes in the transgenic rice and WT leaves. The OsABCG43-OE plants, osabcg43 mutants and WT were planted in the paddy field and sampled for analysis at the booting stage. Data represents mean (three replicates) ± SD. Asterisks present significant differences between WT and OsABCG43-OE plants or osabcg43 mutants, as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
H2O2 accumulation is usually related to disruption of ROS scavenging system in plants. To confirm the possibility, we surveyed the activities of three primary anti-oxidative enzymes: catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), which separately catalyzes the decomposition of hydrogen peroxide (H2O2) to water, the dismutation of superoxide anion to H2O2, and oxidation–reduction reaction [40]. There were about 1.6-fold increased SOD, 1.3-fold increased POD, and 1.5-fold decreased CAT activities in the OsABCG43-OE plants than in wild type, while unchanged activities for these enzymes in the osabcg43 mutants compared with those in wild type (Fig. 4C-E).
Apart from anti-oxidative enzymes, plants also employ other oxidoreductases such as glutaredoxin (GRXs) or glutathione (GSH) to defend themselves from ROS burst-caused damage [41]. We simultaneously measured contents of GRX and GSH in the leaves of transgenic plants and wild type. There were 1.7-fold higher GRX and 1.5-fold lower GSH contents in the leaves of OsABCG43-OE plants than those of osabcg43 mutants or wild type (Fig. 4F, G).
The metabolic disorder of ROS can promote cell death leading to accelerated leaf senescence. Malondialdehyde (MDA) content indirectly reflects the changes of cell damage. Notably, the OsABCG43-OE plants had 1.5-fold increased MDA contents than wild type or the osabcg43 mutants (Fig. 4H). In addition, we examined transcript levels of four senescence-associated genes. RT-qPCR analysis revealed that the transcript level of OsDOS was lower, the expression levels of Osh69, SAG13, and sgr were higher in the OsABCG43-OE plant leaves than in wild type or the osabcg43 mutant leaves (Fig. 4I). Overall, these results indicate that impaired anti-oxidative enzymes system leads to disordered ROS homeostasis, which ultimately causes the appearance of lesions or spots and premature senescence of the OsABCG43-OE plants.
OsABCG43 functions in Cd import
To explore the effect of overexpression or deletion of OsABCG43 on Cd accumulation, we measured Cd levels in the leaves of OsABCG43-OE plants, osabcg43 mutants, and wild type when they were grown in the paddy field. The OsABCG43-OE plants had 1.9-fold higher Cd contents than wild type, whereas the osabcg43 mutants had unchangeable Cd levels compared with wild type (Fig. 5A). Meanwhile, contents of other metal ions including toxic metals Ni, Pb, and As, and nutrient metals Cu, Fe, Mg, Mo, Mn, and Zn were also measured. Neither the OsABCG43-OE plants nor the osabcg43 mutants had changeable contents of these metal ions in their leaves relative to wild type (Fig. S6), indicating that overexpression of OsABCG43 specifically enhances the accumulation of Cd, but not other metal ions.
Fig. 5.
Overexpression of OsABCG43 increased Cd accumulation in rice. (A) Cd contents in the OsABCG43-OE plants, osabcg43 mutants, and wild type (WT) leaves when they were planted in the paddy field; (B) Contents of Cd in shoots and roots, and translocation rate of Cd from root to shoot in OsABCG43-OE plants, osabcg43 mutants, and WT grown hydroponically with different concentrations of Cd for 10 d; (C) Cd levels in xylem sap of OsABCG43-OE plants, osabcg43 mutants, and WT grown hydroponically with 5 μM Cd for 10 d. Data represents mean (three replicates) ± SD. Asterisks present significant differences between WT and OsABCG43-OE plants or osabcg43 mutants, as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
Then, we assessed Cd contents in these plants grown hydroponically with different concentrations of CdCl2 (1.0 μM, 2.0 μM, 5.0 μM), Cd levels in both shoots and roots of the OsABCG43-OE plants were slightly higher than that of wild type. Moreover, at high Cd (2.0 or 5.0 μM) conditions, the OsABCG43-OE plants had significantly greater Cd contents in both shoots (1.7- to 2.0-fold increased) and roots (1.4- to 1.6-fold increased) relative than those in wild type (Fig. 5B). However, the osabcg43 mutants had similar Cd levels in shoots or roots as that in wild type when exposed to different concentrations of Cd. Comparing Cd contents in shoots and roots, the translocation rate of Cd from root to shoot was comparable in the OsABCG43-OE plants to those in wild type or the osabcg43 mutants under Cd stress, suggesting OsABCG43 may not function as a transporter controlling long-distance transportation of Cd from root to shoot (Fig. 5B). In addition, Cd levels in xylem sap were measured in these rice plants when treated with 5.0 μM Cd for 10 d. There was a noticeable accumulation (3.0-fold increased) of Cd in xylem sap from the OsABCG43-OE plants, but not from wild type or the osabcg43 mutants (Fig. 5C). Together, these data indicate that OsABCG43 possibly acts as a Cd importer.
Overexpression of OsABCG43 enhances rice sensitivity to Cd stress
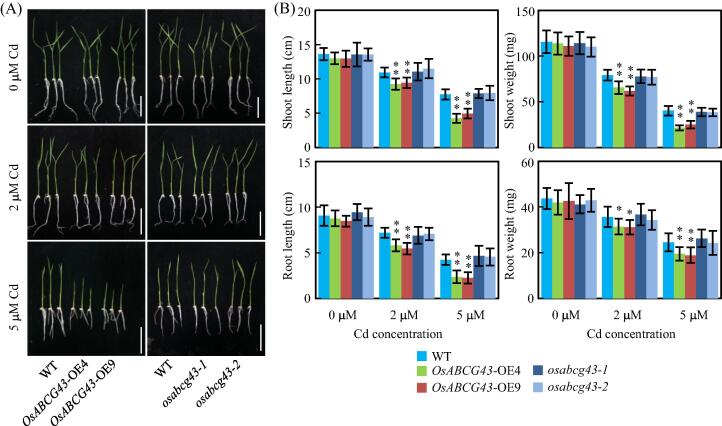
To investigate whether OsABCG43 has a role in Cd tolerance in rice, we evaluated rice plants grown in hydroponic solutions containing different concentrations of Cd (0.0 μM, 2.0 μM, 5.0 μM). In the absence of Cd, the weight and length of shoots and roots for the OsABCG43-OE plants and osabcg43 mutants were comparable to those of wild type. However, the growth of roots and shoots for these plants was observably inhibited along with Cd increased from 2.0 to 5.0 μM. Among these plants, the OsABCG43-OE plants showed significantly attenuated lengths and weights for roots or shoots compared with those of wild type after 2.0 and 5.0 μM Cd added. Moreover, no differences on growth of roots and shoots were surveyed between wild type and the osabcg43 mutants when exposed to excess Cd (Fig. 6A, B), suggesting that overexpression of OsABCG43 enhances rice sensitivity to Cd stress.
Fig. 6.
Overexpression of OsABCG43 enhanced rice sensitivity to Cd stress. (A) Phenotypes of seedlings of wild type (WT), OsABCG43-OE plants, and osabcg43 mutants when they were treated with different concentrations of Cd for 10 d. Scale bars, 5 cm; (B) Plants growth including shoot and root length, shoot and root weight of WT, OsABCG43-OE plants, and osabcg43 mutants seedlings when they were treated with different concentrations of Cd for 10 d. Data represents mean (three replicates) ± SD. Asterisks present significant differences between the OsABCG43-OE plants or osabcg43 mutants and WT, as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
Overexpression of OsABCG43 causes dramatic changes of transcriptome
For further elucidating roles of OsABCG43, we compared global gene expression in the leaves between OsABCG43-OE plant and wild type by RNA-seq analysis. A total of 10,603 differentially expressed genes (DEGs) which had significant changes in transcript levels (fold changes ≥ 2 and P ≤ 0.05) were identified in the OsABCG43-OE plant relative to wild type. Of which, 4180 DEGs were upregulated and 6423 DEGs were downregulated. We then performed GO and KEGG enrichment analysis to gain deeper insight into the DEGs in the OsABCG43-OE plants. GO enrichment analysis showed that enriched GO terms for molecular functions and biological processes were different for Up-DEGs and Down-DEGs. The primary enriched GO terms for Up-DEGs were oxidoreductase activity and transmembrane transport, while for Down-DEGs were carbohydrate metabolic process and kinase activity (Fig. S7A, B). Accordingly, the top enriched KEGG pathways for Up-DEGs were relevant to carbon fixation in photosynthetic tissues and biosynthesis of secondary metabolites, while for Down-DEGs were concerned with phenylpropanoid biosynthesis and glutathione metabolism (Fig. S7C, D).
A number of Cd transportation genes and Cd-responsive genes had different expression levels (Fig. 7A). Such as, OsMTP1 and OsZIP9, participating in heavy-metal transportation and upregulated by Cd treatment [42], [43], had significantly increased transcript levels in the OsABCG43-OE plants relative to them in wild type (Fig. 7B). Moreover, a great variety of ROS homeostasis-related genes showed distinct expressions. Nine glutaredoxin (GRX) family members which have been reported regulating and maintaining redox-dependent signaling pathways and cellular redox state [44], had different transcripts: six of them had increased transcript levels and three of them had decreased transcript levels in the OsABCG43-OE plants (Fig. 7A). However, twelve peroxidase (PRX) family genes which encode peroxidases catalyzing oxidation–reduction reaction all had decreased transcript levels in the OsABCG43-OE plants compared with in wild type (Fig. 7A). Among them, OsGRX6 and OsPRX38 which have been reported showing increased and decreased expressions, respectively, in shoots under Cd stress [45], had enhanced and suppressed expressions in the OsABCG43-OE plants in comparison to wild type (Fig. 7B). In addition, nine OsABCG genes had increased and eleven OsABCG genes had decreased expressions in the OsABCG43-OE plants than those in wild type (Fig. 7A). Such as, OsABCG48, showing induced expression under Cd stress [46], exhibited greater expression levels in the OsABCG43-OE plants than in wild type (Fig. 7B). Some of these DEGs that have altered expressions under Cd stress were randomly selected to verify their transcript levels by RT-qPCR assay. A good correlation of these representative DEGs was observed between RNA-seq and RT-qPCR (Fig. 7B), further supporting Cd accumulation in the OsABCG43-OE plants.
Fig. 7.
Validation of differentially expressed genes in the OsABCG43-OE plants, osabcg43 mutants, and wild type (WT) leaves. (A) Heat map of DEGs involving in Cd transportation, ROS homeostasis and rice ABCG family members; (B) Relative transcript levels of representative DEGs in the leaves of OsABCG43-OE plants, osabcg43 mutants, and WT. Data represents mean (three replicates) ± SD. Asterisks present significant differences between the OsABCG43-OE plants or osabcg43 mutants and WT, as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05). DEGs, differentially expressed genes.
OsABCG43 is induced by leaf bacteria
OsABCG43 transcript is induced where rice leaves were infected with leaf bacteria Xoo from our previous transcriptome data [47]. Here, we performed RT-qPCR assays to assess its dynamic expression profile, with the result that OsABCG43 transcripts were activated in the leaves after Xoo infection, reaching the highest level 12 h post infection, whereas its transcripts were not altered using water as a mock-inoculated control (Fig. 8A). In parallel, we investigated its dynamic expression profile in response to another leaf bacteria Xoc by RT-qPCR. In line with Xoo-activated expression pattern, OsABCG43 transcripts were also induced after Xoc infection (Fig. 8B), suggesting that OsABCG43 transcripts are induced upon different leaf bacteria challenges.
Fig. 8.
Expression patterns of OsABCG43 after leaf bacteria infection. (A) Relative transcript levels of OsABCG43 after Xoo PXO99 infection; (B) Relative transcript levels of OsABCG43 after Xoc RS105 infection. hpi, hours post infection. Data represents mean (three replicates) ± SD. Asterisks present significant differences between H2O inoculation and leaf bacteria, as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
Overexpression of OsABCG43 prevents Xoo or Xoc proliferation
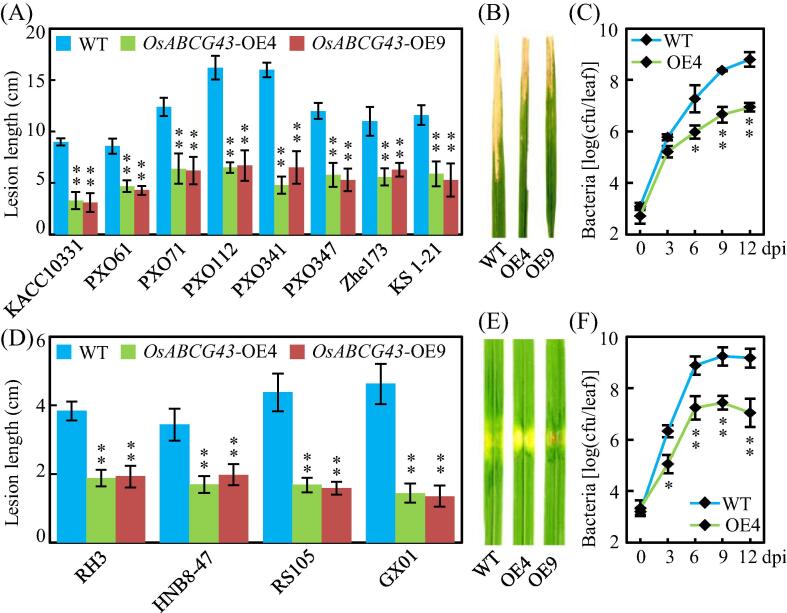
We evaluated responses of the OsABCG43-OE plants to Xoo at the booting stage by inoculating them with Xoo PXO99. The OsABCG43-OE T0 plants had significantly shorter lesion lengths ranging from 4.8 ± 0.8 cm to 6.9 ± 0.6 cm than wild type with 10.2 ± 0.6 cm 14 d after PXO99 inoculation (Fig. S8). T3 families from two independent OsABCG43-OE T2 plants were infected with a set of Xoo strains which were from Philippines (PXO71, PXO112, PXO341, PXO61, PXO347), China (KS 1–21, Zhe173), and Korea (KACC10331). The OsABCG43-OE plants exhibited markedly increased resistance to these different Xoo strains than those of wild type (Fig. 9A, B). In agreement, Xoo population were decreased in the leaves of OsABCG43-OE plants than in wild type, with about 73-fold lower bacterial population 12 d after Xoo infection (Fig. 9C).
Fig. 9.
Overexpression of OsABCG43 prevents leaf bacteria proliferation. (A) Response of the OsABCG43-OE plants to a set of Xoo strains; (B) Phenotype of the OsABCG43-OE plants against Xoo PXO99 infection; (C) Growth of Xoo PXO99 in the OsABCG43-OE4 plant leaves; (D) Response of the OsABCG43-OE plants to a set of Xoc strains; (E) Phenotype of the OsABCG43-OE plants against Xoc RS105 infection; (F) Growth of Xoc RS105 in the OsABCG43-OE4 plant leaves. Rice plants were inoculated with Xoc or Xoo at the tillering stage. dpi, days post infection. Data represents mean (three replicates) ± SD. Asterisks present significant differences between the OsABCG43-OE plants and wild type (WT), as determined by two-tailed Student’s t-test (**P < 0.01 or *P < 0.05).
In addition, we evaluated responses of the osabcg43 mutants to Xoo by inoculating them with different Xoo strains. The osabcg43 mutants showed comparable lesion lengths as wild type after a set of Xoo strains inoculation (Fig. S9A).
In parallel, we evaluated responses of the OsABCG43-OE plants and osabcg43 mutants to different Xoc strains. The OsABCG43-OE plants exhibited significantly increased resistance to all the tested Xoc strains with approximately ranging from 41.2% to 69.6% decreased lesion lengths and 61-fold lower Xoc growth rate relative to those in wild type (Fig. 9D-F). However, the osabcg43 mutants had unchangeable lesion lengths compared with wild type 14 d after different Xoc inoculation (Fig. S9B). Thus, these results together indicate that overexpression of OsABCG43 prevents the colonization and proliferation of Xoo and Xoc, implying that accumulated Cd in the OsABCG43-OE plant leaves may be harmful for these leaf bacteria.
Accumulated Cd prevents leaf bacteria proliferation
Since there were excessive Cd but restrained leaf bacteria growth in the OsABCG43-OE plants, therefore, we conjectured that Cd stress probably prevents the colonization or proliferation of leaf bacteria. To confirm our hypothesis, we investigated responses of the OsABCG43-OE plants, osabcg43 mutants, and wild type to Xoo after different concentrations Cd treatment. These plants were grown hydroponically and treated with 10 and 50 μM Cd for 20 days. After Cd treatment, rice plants were inoculated with leaf bacteria Xoo and Xoc, then lesion length and bacterial growth were calculated 14 d after inoculation. We found that exogenous application of Cd obviously prevents Xoo proliferation in all the rice plants as shown with shortened lesion lengths and decreased bacterial population. However, the OsABCG43-OE plants always exhibited shorter lesion lengths and lesser bacterial growth than those of wild type under different concentrations of Cd treatment, while there were no differences on lesion lengths and bacterial growth between wild type and the osabcg43 mutants (Fig. S10A, B).
Attenuated Xoc proliferation were also observed in all the rice plants when grown hydroponically with excess Cd. The OsABCG43-OE plants but not the osabcg43 mutants repeatedly had shorter lesion lengths and lesser bacterial growth when comparing with wild type after treatment with excess Cd (Fig. S10C, D). Collectively, higher concentration of Cd could prevent the proliferation of leaf bacteria in rice leaves.
Accumulated Cd impairs the virulence factors of leaf bacteria
Successful colonization and proliferation for bacteria in host plant tissues largely depends on their secreted virulence factors. Key virulence factors for leaf bacteria such as Xoo and Xoc include biofilm formation, EPS production, and secretion systems [48]. To acquire the detailed effect of accumulated Cd on secreted virulence factors of Xoo and Xoc, we analyzed key virulence factors of them in cultures that contained different concentrations of Cd. We found undiversified shapes of edges for Xoo colonies when grown in semi-solid medium that contained different concentrations of Cd, indicating unchanged twitching motility for Xoo (Fig. 10A). However, the diameters of swimming zone for Xoo were clearly decreased along with Cd was added in the medium, indicating that accumulated Cd weakens swarming motility of Xoo (Fig. 10B). We then measured biofilm formation and EPS production in Xoo. The results showed that Xoo had a significantly decreased accumulation of EPS (Fig. 10C), and production of biofilm (Fig. 10D), when grown in the culture medium with increasing concentrations of Cd. Furthermore, we analyzed the expression levels of key genes that function in type III secretion system for Xoo. The transcript levels of hpa1, hrcU, and hrpF were significantly decreased when Xoo were cultured in medium with Cd compared to without Cd (Fig. 10E).
Fig. 10.
Extracellularly accumulated Cd suppresses the secretion of virulence factors in Xoo PXO99. (A) Twitching motility of Xoo PXO99 on PS plates containing different concentrations of Cd. Scale bars, 200 μm; (B) Swarming motility of Xoo PXO99 on NB plates containing different concentrations of Cd; (C) Production of EPS of Xoo PXO99 in m210 medium containing different concentrations of Cd; (D) Biofilm formation of Xoo PXO99 in XOM2 medium containing different concentrations of Cd; (E) Relative transcript levels of key genes functioned in type III secretion system of Xoo PXO99 in XOM3 medium after different concentrations of Cd treatment; (F) Inhibition of Xoo PXO99 in liquid medium containing different concentrations of Cd. Data represents mean (three replicates) ± SD. The letters above each bar present statistically significant differences which were determined by one-way ANOVA analysis followed by Tukey’s multiple test (P < 0.05).
In parallel, we evaluated the effect of accumulated Cd on the virulence of Xoc by measuring these secreted virulence factors. There were similar attenuated virulence factors for Xoc as that for Xoo when grown in the culture medium with increasing concentrations of Cd, including decreased swarming motility, lessened biofilm formation and EPS production, and downregulated transcript levels of type III secretion system genes (Fig. S11).
Apart from impaired virulence factors, whether accumulated Cd could directly affect bacterial growth. To answer this conjecture, we examined the IC50 value for Cd of Xoc and Xoo, with the results that the IC50 values for Cd of Xoc was 0.51 μM, of Xoo was 0.56 μM, which were lower than Cd contents in the leaves or xylem sap from the OsABCG43-OE plants (Fig. 10F, S11F). Together, these data suggest that accumulated Cd not only impairs secreted virulence factors of Xoo and Xoc but also affects their growth, resulting in attenuated virulence or colonization of the leaf bacteria on host rice.
Discussion
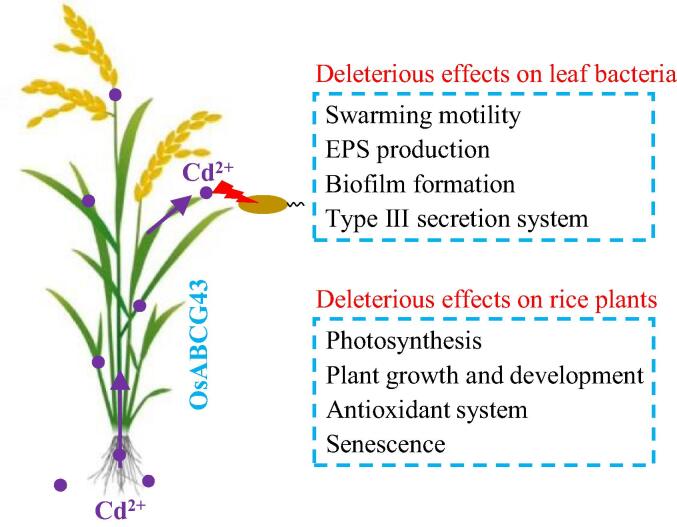
Cadmium enters plant roots and moves to different cells or tissues through transporters for chemically similar nutrients, whereas it has no biological function but great toxicity in plants. Here, we found that rice OsABCG43 functions as a Cd importer contributing to the accumulation of Cd in rice plants. Accumulated Cd in rice plants not only inhibited plant growth or development but also prevented colonization or proliferation for leaf bacteria that invade rice leaves (Fig. 11).
Fig. 11.
A putative model of OsABCG43-regulated Cd accumulation which is harmful for host rice and leaf bacteria. OsABCG43 acts as a Cd importer controlling Cd accumulation in rice. Accumulated Cd inhibits photosynthesis, plant growth and development, alters antioxidant system, and accelerates senescence, resulting in phytotoxicity. In addition, Cd excess impairs the formation of virulence factors for the leaf bacteria, preventing their colonization or proliferation in rice leaves.
OsABCG43 has the same expression pattern under Cd treatment as AtABCG32/AtPDR8 or OsABCG36/OsPDR9, that is Cd-induced (Fig. 2), and even they all encode plasma membrane localized proteins (Fig. 1G). However, OsABCG43 possibly acts as a Cd importer that is different from AtABCG32/AtPDR8 and OsABCG36/OsPDR9 which function as Cd exporters [19], [21]. Overexpression of OsABCG43 caused Cd accumulation in both leaves and roots (Fig. 5), resulting in enhanced rice sensitivity to Cd stress and phytotoxicity (Fig. 6), which is opposite to the roles for AtABCG32/AtPDR8 or OsABCG36/OsPDR9 as Cd exporter. Moreover, there were only increased contents of Cd but not other metals in the OsABCG43-OE plants (Fig. S6), suggesting that OsABCG43 specifically transport Cd in plants. In addition, some Cd-responsive genes had altered transcript levels in the OsABCG43-OE plants which further supports accumulated Cd in transgenic rice plants (Fig. 7A). These results together confirm that OsABCG43 is a Cd importer, implying the different roles of OsABCGs on Cd transportation.
The OsABCG43-OE plants accumulated excess Cd, while the osabcg43 mutants had unchanged levels of Cd in leaves or roots relative to those of wild type, which probably due to apparent functional redundancy of this ABCG family in rice. Based on RNA-seq analysis, at least 20 OsABCG genes had enhanced or suppressed transcript levels in the leaves of OsABCG43-OE plants. Therefore, we can not exclude the possibility that some of these OsABCG genes acting as Cd transporters complement the loss-of-function of OsABCG43 in the osabcg43 mutants. Theoretically, we could generate Cd-safe transgenic rice by deletion of OsABCG43 and the related OsABCG genes through gene editing.
The OsABCG43-OE plants had decreased transcript levels of chlorophyll-related genes, attenuated chlorophyll contents, lessened photosynthetic rate and Fv/Fm value (Fig. 3). These phenotypes were probably caused by Cd accumulation as Cd stress can destroy photosynthetic structure and affect carbon metabolism enzyme activity of photosynthetic apparatus of plants [49], [50]. The accumulated Cd in the OsABCG43-OE plants inhibited photosynthesis resulting in senescence. Moreover, Cd excess in plants can inhibit plant growth or development [15], [51]. The OsABCG43-OE plants had compromised plant height and lesions or spots on their leaves, exhibiting growth retardation. Furthermore, the OsABCG43-OE plants were sensitive to Cd stress, showing attenuated roots and shoots growth (Fig. 6). All phenotypes were the typical symptoms for Cd toxicity.
Notably, the common manifestation of Cd excess to plants is that to alter the antioxidant system and induce the oxidative stress response, such as ROS production [52]. Cd-induced overproduction of ROS was obviously observed in the OsABCG43-OE plants, characterizing by increased SOD, POD, and GRX activities, while decreased CAT and GSH activities (Fig. 4). When the level of ROS is too high, it can suppress photosynthetic electron transport chains, causing plant to die [15]. We conjectured that lesions or spots on the OsABCG43-OE plant leaves were the result of accumulated Cd-induced overproduction of ROS. Therefore, Cd accumulation leads to phytotoxicity in the OsABCG43-OE plants. Similarly, AtABCG32/AtPDR8 and OsABCG36/OsPDR9 knockout mutants have accumulated Cd exhibiting phytotoxicity [19], [21]. These results support that OsABCG43-mediated import of Cd is harmful for rice plants.
Apart from deleterious effect on plants, studies using culture dependent or independent approaches uncovered that high concentration levels of Cd impact the activity of soil bacteria [53]. Previous studies reported that Cd is an essential factor affecting bacterial diversity and bacterial population abundance in soil [54], [55]. Similarly, we here found that Cd overabundance in rice plants is harmful for leaf bacteria Xoo and Xoc. Evidence supporting the conclusion are 1) the growth rates for Xoo and Xoc were significantly suppressed in the OsABCG43-OE plants (Fig. 9); 2) the bacterial population decreased in leaves after rice growing hydroponically under Cd treatment (Fig. S10); and 3) the virulence factors for bacteria were markedly attenuated in the OsABCG43-OE plants (Fig. 10). Since EPS production and biofilm formation are essential for the soil bacterium’s resistance against heavy metals [56], [57], we here found Cd excess could dramatically suppress biofilm formation and EPS production for leaf bacteria Xoc and Xoo. It seems that Cd cause deleterious effects on soil bacteria and leaf bacteria by the similar mechanism.
Conclusion
We characterized that OsABCG43 expressing specifically in the vascular encodes a Cd importer. OsABCG43-mediated import of Cd causes deleterious effects on rice that transgenic plants showed accelerated senescence, retarded growth, exacerbated lesions or spots on leaves, and be sensitive to Cd stress, exhibiting phytotoxicity. In addition, OsABCG43-mediated import of Cd is harmful for leaf bacteria that accumulated Cd impaired the secreted virulence factors of Xoc and Xoo, preventing their colonization or proliferation in rice leaves.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Jingjing Tian: Investigation, Methodology, Data curation, Writing – original draft. Li Wang: Methodology, Data curation. Shugang Hui: Methodology, Data curation. Dan Yang: Investigation, Methodology. Yuqing He: Data curation. Meng Yuan: Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (31821005, 32172421), the National Science Foundation of Hubei Province (2020CFA058), and the earmarked fund for the China Agriculture Research System (CARS‐01‐01).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.05.010.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Shahid M., Dumat C., Khalid S., Niazi N.K., Antunes P.M.C. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol. 2017;241:73–137. doi: 10.1007/398_2016_8. [DOI] [PubMed] [Google Scholar]
- 2.The Ministry of Environmental Protection. The ministry of Land and Resources Report on the national soil contamination survey. 2014; http://www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm.
- 3.Hu Y., Cheng H., Tao S. The challenges and solutions for cadmium-contaminated rice in China: a critical review. Environ Int. 2016;92-93:515–532. doi: 10.1016/j.envint.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Yu H., Wang J., Fang W., Yuan J., Yang Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ. 2006;370(2-3):302–309. doi: 10.1016/j.scitotenv.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol. 2006;1(1) doi: 10.1186/1745-6673-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y.P., Liu Q., Liu Y.J., Jia F.A., He X.H. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci Rep. 2014;4:4287. doi: 10.1038/srep04287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S., El-Latif Hesham A., Qiao M., Rehman S., He J.-Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res Int. 2010;17(2):288–296. doi: 10.1007/s11356-009-0134-4. [DOI] [PubMed] [Google Scholar]
- 8.Huang X., Duan S., Wu Q.i., Yu M., Shabala S. Reducing cadmium accumulation in plants: structure-function relations and tissue-specific operation of transporters in the spotlight. Plants. 2020;9(2):223. doi: 10.3390/plants9020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y.u., Jin L., Wang X. Cadmium absorption and transportation pathways in plants. Int J Phytoremediation. 2017;19(2):133–141. doi: 10.1080/15226514.2016.1207598. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Zhu Y, Yu L, Yang M, Zou X, Yin C, et al. Research advances in cadmium uptake, transport and resistance in rice (Oryza sativa L.). Cells 2022;11:569. [DOI] [PMC free article] [PubMed]
- 11.Chu C, Huang R, Liu L, Tang G, Xiao J, Yoo H, et al. The rice heavy metal transporter OsNRAMP1 regulates disease resistance by modulating ROS homeostasis. Plant Cell Enrivon 2022;45:1109–26. [DOI] [PubMed]
- 12.Pottier M., Oomen R., Picco C., Giraudat J., Scholz-Starke J., Richaud P., et al. Identification of mutations allowing Natural Resistance Associated Macrophage Proteins (NRAMP) to discriminate against cadmium. Plant J. 2015;83(4):625–637. doi: 10.1111/tpj.12914. [DOI] [PubMed] [Google Scholar]
- 13.Liu X.S., Feng S.J., Zhang B.Q., Wang M.Q., Cao H.W., Rono J.K., et al. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019;19(1) doi: 10.1186/s12870-019-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi R., Bashir K., Ishimaru Y., Nishizawa N.K., Nakanishi H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal Behav. 2012;7(12):1605–1607. doi: 10.4161/psb.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haider F.U., Liqun C., Coulter J.A., Cheema S.A., Wu J., Zhang R., et al. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. doi: 10.1016/j.ecoenv.2020.111887. [DOI] [PubMed] [Google Scholar]
- 16.He J.-y., Ren Y.-F., Zhu C., Jiang D.-a. Effects of cadmium stress on seed germination, seedling growth and seed amylase activities in rice (Oryza sativa) Rice Sci. 2008;15(4):319–325. [Google Scholar]
- 17.Verrier P.J., Bird D., Burla B.o., Dassa E., Forestier C., Geisler M., et al. Plant ABC proteins-a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13(4):151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Nuruzzaman M., Zhang R.u., Cao H.-Z., Luo Z.-Y. Plant pleiotropic drug resistance transporters: transport mechanism, gene expression, and function. J Integr Plant Biol. 2014;56(8):729–740. doi: 10.1111/jipb.12196. [DOI] [PubMed] [Google Scholar]
- 19.Kim D.Y., Bovet L., Maeshima M., Martinoia E., Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Lee K, Lee J, Noh EW, Lee Y. AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol 2005;138:827–36. [DOI] [PMC free article] [PubMed]
- 21.Fu S, Lu Y, Zhang X, Yang G, Chao D, Wang Z, et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J Exp Bot 2019;70:5909–18. [DOI] [PMC free article] [PubMed]
- 22.Nguyen VNT, Lee SB, Suh MC, An G, Jung KH. OsABCG9 is an important ABC transporter of cuticular wax deposition in rice. Front Plant Sci 2018;9:960. [DOI] [PMC free article] [PubMed]
- 23.Campe R., Langenbach C., Leissing F., Popescu G.V., Popescu S.C., Goellner K., et al. ABC transporter PEN3/PDR8/ABCG36 interacts with calmodulin that, like PEN3, is required for Arabidopsis nonhost resistance. New Phytol. 2016;209:294–306. doi: 10.1111/nph.13582. [DOI] [PubMed] [Google Scholar]
- 24.He Y., Xu J., Wang X., He X., Wang Y., Zhou J., et al. The Arabidopsis pleiotropic drug resistance transporters PEN3 and PDR12 mediate camalexin secretion for resistance to Botrytis cinerea. Plant Cell. 2019;31:2206–2222. doi: 10.1105/tpc.19.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khare D., Choi H., Huh S.U., Bassin B., Kim J., Martinoia E., et al. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proc Natl Acad Sci USA. 2017;114:E5712–E5720. doi: 10.1073/pnas.1702259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krattinger S.G., Kang J., Bräunlich S., Boni R., Chauhan H., Selter L.L., et al. Abscisic acid is a substrate of the ABC transporter encoded by the durable wheat disease resistance gene Lr34. New Phytol. 2019;223:853–866. doi: 10.1111/nph.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell EJ, Schenk PM, Kaza, K, Penninckx IA, Anderson JP, Maclean DJ, et al. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 2003;133:1272–84. [DOI] [PMC free article] [PubMed]
- 28.Zhang H, Jing W, Zheng J, Jin Y, Wu D, Cao C, et al. The ATP-binding cassette transporter OsPDR1 regulates plant growth and pathogen resistance by affecting jasmonates biosynthesis in rice. Plant Sci 2020;298:110582. [DOI] [PubMed]
- 29.Yoshida S., Ohnishi Y., Kitagishi K. Role of silicon in rice nutrition. Soil Plant Food. 1959;5:127–133. [Google Scholar]
- 30.Yuan M, Chu Z, Li X, Xu C, Wang S. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 2010;22:3164–76. [DOI] [PMC free article] [PubMed]
- 31.Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 2015;8:1274–84. [DOI] [PubMed]
- 32.Yuan M, Chu Z, Li X, Xu C, Wang S. Pathogen-induced expressional loss of function is the key factor in race-specific bacterial resistance conferred by a recessive R gene xa13 in rice. Plant Cell Physiol 2009;50:947–55. [DOI] [PubMed]
- 33.Hui S., Shi Y., Tian J., Wang L.i., Li Y., Wang S., et al. TALE-carrying bacterial pathogens trap host nuclear import receptors for facilitation of infection of rice. Mol Plant Pathol. 2019;20(4):519–532. doi: 10.1111/mpp.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan M., Ke Y., Huang R., Ma L., Yang Z., Chu Z., et al. A host basal transcription factor is a key component for infection of rice by TALE-carrying bacteria. Elife. 2016;5 doi: 10.7554/eLife.19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z., Hui S., Lv Y., Zhang M., Chen D., Tian J., et al. miR395-regulated sulfate metabolism exploits pathogen sensitivity to sulfate to boost immunity in rice. Mol Plant. 2022;15(4):671–688. doi: 10.1016/j.molp.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Gupta B.B., Selter L.L., Baranwal V.K., Arora D., Mishra S.K., Sirohi P., et al. Updated inventory, evolutionary and expression analyses of G (PDR) type ABC transporter genes of rice. Plant Physiol Biochem. 2019;142:429–439. doi: 10.1016/j.plaphy.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Oono Y., Yazawa T., Kawahara Y., Kanamori H., Kobayashi F., Sasaki H., et al. Genome-wide transcriptome analysis reveals that cadmium stress signaling controls the expression of genes in drought stress signal pathways in rice. PLoS ONE. 2014;9(5):e96946. doi: 10.1371/journal.pone.0096946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oda K., Otani M., Uraguchi S., Akihiro T., Fujiwara T. Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci Biotechnol Biochem. 2011;75(6):1211–1213. doi: 10.1271/bbb.110193. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Makino A. Translational downregulation of RBCL is operative in the coordinated expression of rubisco genes in senescent leaves in rice. J Exp Bot 2013;64:1145–52. [DOI] [PMC free article] [PubMed]
- 40.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 2010;33:453–67. [DOI] [PubMed]
- 41.Rouhier N., Lemaire S.D., Jacquot J.-P. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol. 2008;59(1):143–166. doi: 10.1146/annurev.arplant.59.032607.092811. [DOI] [PubMed] [Google Scholar]
- 42.Yuan L., Yang S., Liu B., Zhang M., Wu K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 2012;31(1):67–79. doi: 10.1007/s00299-011-1140-9. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X., Chen L., Li X. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Bot Stud. 2018;59:22. doi: 10.1186/s40529-018-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg R., Jhanwar S., Tyagi A.K., Jain M. Genome-wide survey and expression analysis suggest diverse roles of glutaredoxin gene family members during development and response to various stimuli in rice. DNA Res. 2010;17(6):353–367. doi: 10.1093/dnares/dsq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L., Wang J., Song K.e., Sun Y., Qin Q., Xue Y. Transcriptome analysis of rice (Oryza sativa L.) shoots responsive to cadmium stress. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46684-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai X, Wang M, Jiang Y, Wang C, Ow DW. Overexpression of OsABCG48 lowers cadmium in rice (Oryza sativa L.). Agronomy 2021;11:918.
- 47.Hong H., Liu Y., Zhang H., Xiao J., Li X., Wang S., et al. Small RNAs and gene network in a durable disease resistance gene-mediated defense responses in rice. PLoS ONE. 2015;10(9):e0137360. doi: 10.1371/journal.pone.0137360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Büttner D., Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev. 2010;34(2):107–133. doi: 10.1111/j.1574-6976.2009.00192.x. [DOI] [PubMed] [Google Scholar]
- 49.Hasan S.A., Fariduddin Q., Ali B., Hayat S., Ahmad A. Cadmium: toxicity and tolerance in plants. J Environ Biol. 2009;30:165–174. [PubMed] [Google Scholar]
- 50.Wang Y., Jiang X., Li K., Wu M., Zhang R., Zhang L.u., et al. Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: physiological, biochemical and ultrastructural analyses. Biometals. 2014;27(2):389–401. doi: 10.1007/s10534-014-9720-0. [DOI] [PubMed] [Google Scholar]
- 51.Qadir S., Jamshieed S., Rasool S., Ashraf M., Akram N.A., Ahmad P. Modulation of plant growth and metabolism in cadmium-enriched environments. Rev Environ Contam Toxicol. 2014;229:51–88. doi: 10.1007/978-3-319-03777-6_4. [DOI] [PubMed] [Google Scholar]
- 52.Nagajyoti P.C., Lee K.D., Sreekanth T.V.M. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8(3):199–216. [Google Scholar]
- 53.Xie Y., Fan J., Zhu W., Amombo E., Lou Y., Chen L., et al. Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front Plant Sci. 2016;7:755. doi: 10.3389/fpls.2016.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H., Wang C., Xie Y., Luo Y., Sheng M., Xu F., et al. Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J Hazard Mater. 2020;392:122478. doi: 10.1016/j.jhazmat.2020.122478. [DOI] [PubMed] [Google Scholar]
- 55.Yu X., Zhao J., Liu X., Sun L., Tian J., Wu N. Cadmium pollution impact on the bacterial community structure of arable soil and the isolation of the cadmium resistant bacteria. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fones H., Preston G.M. The impact of transition metals on bacterial plant disease. FEMS Microbiol Rev. 2013;37(4):495–519. doi: 10.1111/1574-6976.12004. [DOI] [PubMed] [Google Scholar]
- 57.Pal A., Paul A.K. Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J Microbiol. 2008;48(1):49–64. doi: 10.1007/s12088-008-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.