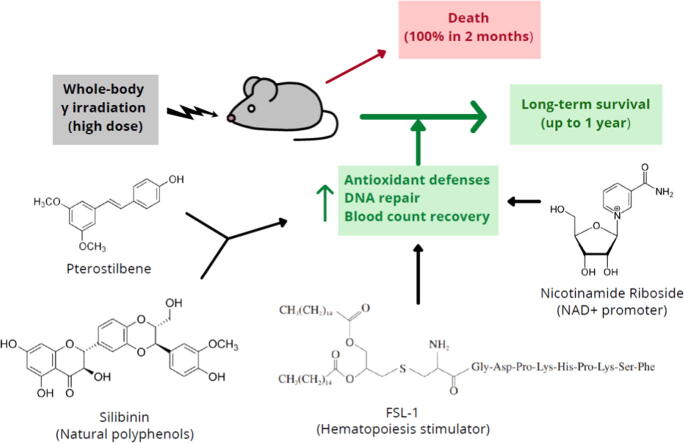
Graphical abstract
Keywords: Radioprotection, Ionizing radiations, Natural polyphenols, NAD+ precursors, Toll-like receptor 2/6 ligands
Abbreviations: BIRC5, baculoviral IAP repeat containing 5; BMNCs, bone marrow nucleated cells; BRCA1, breast cancer 1; CAT, catalase; CD2+L, CD2+ lymphocytes; CFU-GEMM: colony forming unit - granulocyte, erythrocyte, monocyte, megakaryocyte; CFU-GM, colony forming unit - granulocyte–macrophage, DMEM, Dulbecco′s modified Eagle′s medium; DTT, dithiothreitol; DSBs, double-strand DNA breaks; FBS, fetal bovine serum; FOXM1, forkhead box protein M1; FSL1, fibroblast-stimulating lipoprotein 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GCL, γ-glutamyl-cysteine ligase; GPX, glutathione peroxidase; GSH, glutathione; HEP, hepatocytes; HPLC, high performance liquid chromatography; IEC, intestinal epithelial cells; KHBM, Krebs-Henseleit bicarbonate medium; LC-MS/MS, liquid chromatography–mass spectrometry; LD50/30, lethal dose 50/30; MnNCE, micronucleated normochromatic cells; MnPCE, micronucleated polychromatic cells; NAMPT, nicotinamide phosphoribosyltransferase; NCE, normochromatic erythrocytes; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; NRF2, nuclear factor erythroid 2-related factor 2; 8OHdG, 8-hydroxy-2′-deoxyguanosine; PARP1, poly [ADP-ribose] polymerase 1; PCE, polychromatic erythrocytes; PGC-1α, peroxisome proliferator-activated receptor γ co-activator 1 α; PNA, Cy3-labeled peptide nucleic acid; PT, pterostilbene; RESV, resveratrol; ROS, reactive oxygen species; SIL, silibinin; SIRT, sirtuin; SOD, superoxide dismutase; STAT3, signal transducer and activator of transcription 3; TLR, toll–like receptor
Highlights
-
•
Effective, long-term protection of normal tissues from ionizing radiations remains an unmet need.
-
•
This work demonstrates that a combination of two radioprotectors, pterostilbene and silibinin, with two radiomitigators, nicotinamide riboside and fibroblast-stimulating lipoprotein 1, can confer long-term protection of normal tissues against a lethal dose of γ radiation in mice.
-
•
The mechanism involves induction of the Nrf2-dependent cellular antioxidant defense, reduction of NF-kB signaling, upregulation of the PGC-1α/sirtuins 1 and 3 axis and the PARP1-dependent DNA repair, and stimulation of hematopoietic cell recovery.
-
•
This strategy is potentially capable of protecting mammals against ionizing radiations.
Abstract
Introduction
Effective agents that could confer long-term protection against ionizing radiation in vivo would have applications in medicine, biotechnology, and in air and space travel. However, at present, drugs that can effectively protect against lethal ionizing radiations are still an unmet need.
Objective
To investigate if combinations of natural polyphenols, known for their antioxidant potential, could protect against ionizing radiations.
Methods
Plant-derived polyphenols were screened for their potential ability to confer radioprotection to mice given a lethal whole-body γ radiation (137Cs) dose expected to kill 50% of the animals in 30 days. Telomere and centromere staining, Q-FISH and comet assays were used to investigate chromosomal aberration, micronuclei formation and DNA breaks. Molecular oxidations were investigated by enzyme immunoassays and UPLC-MS/MS. RT-PCR, western blotting and siRNA-induced gene silencing were used to study signaling mechanisms and molecular interactions.
Results
The combination of pterostilbene (PT) and silibinin (SIL) was the most effective against γ-irradiation, resulting in 100% of the mice surviving at 30 days and 20% survival at one year. Treatment post γ-irradiation with two potential radiomitigators nicotinamide riboside (NR, a vitamin B3 derivative), and/or fibroblast-stimulating lipoprotein 1 (FSL1, a toll-like receptor 2/6 agonist), did not extend survival. However, the combination of PT, SIL, NR and FSL1 achieved a 90% survival one year post γ-irradiation. The mechanism involves induction of the Nrf2-dependent cellular antioxidant defense, reduction of NF-kB signaling, upregulation of the PGC-1α/sirtuins 1 and 3 axis, PARP1-dependent DNA repair, and stimulation of hematopoietic cell recovery. The pathway linking Nrf2, sirtuin 3 and SOD2 is key to radioprotection. Importantly, this combination did not interfere with X-ray mediated killing of different tumor cells in vivo.
Conclusion
The combination of the radioprotectors PT and SIL with the radiomitigators NR and FSL1 confer effective, long-term protection against γ radiation in vivo. This strategy is potentially capable of protecting mammals against ionizing radiations.
Introduction
Potential protective agents against harmful radiations have been investigated for decades. However, effective radioprotectors against lethal ionizing radiation remains an unmet need. Gamma radiation causes molecular damages in cells directly as well as indirectly through the generation of reactive oxygen species (ROS) [1], [2]. The response to radiation exposure depends on the cell type and dose of irradiation, inherent tissue sensitivity and repair capacity, as well as on regulatory factors specific to each cell type that include cell cycle status, O2 pressure, and the intracellular concentration of non-protein thiols and other antioxidants[3], [4]. A wide range of phytochemicals are antioxidants and, thus, potentially radioprotectors [5]. We previously reported that topical administration of pterostilbene (3,5-dimethoxy-4′-hydroxystilbene, PT, a natural stilbene) protects the skin against the burning and irritation effects of acute UVB doses as well as protecting against skin carcinogenesis caused by chronic UVB radiation [6]. The protection to UVB provided by PT is not due to physicochemical screen or direct antioxidant effects of the molecule itself, but rather to an elevtaion of the physiological antioxidant defenses [6]. Therefore, it is plausible that PT, if administered systemically, could also exert protection against other types of ionizing radiation.
NAD+-dependent enzymes such as poly(ADP-ribose) polymerases (PARP) and sirtuins (Sirt) are involved in chromatin repair and in epigenetics/transcriptional adaptions [7]. The effects of nicotinamide, an NAD+ precursor, treatment on DNA repair following γ and UV irradiation were originally assayed in several repair-proficient and repair-deficient cell lines [8]. Nicotinamide was shown to increase the repair activity in a concentration-dependent manner. Therefore, NAD+ precursors, if efficient under in vivo conditions, could be potential radiomitigators. Moreover, recovery of hematopoietic sites is key for survival after a deleterious irradiation. Interestingly, a recent report shows that the Toll–like receptor (TLR) 2/6 agonist, fibroblast-stimulating lipopeptide 1 (FSL1), therapeutically mitigates acute radiation syndrome (ARS) by promoting hematopoietic recovery [9]. Our initial strategy against γ radiation-induced systemic damage was to combine the administration of PT as a radioprotector, with nicotinamide riboside [NR, an NAD+ precursor] [10] and FSL1 as radiomitigators.
Material and Methods
Mice and treatment procedures
Swiss albino mice (male, 9–10 weeks old, 30–32 g) were obtained from Charles River Laboratories (Wilmington, MA). This strain is among the most commonly used in radiation studies [11]. Mice were fed a standard laboratory diet (SD, A03 from Panlab, Barcelona, Spain) before irradiation. The SD has 3,200 kcal/kg (23.5% crude protein, 4.3% crude fat, 3.7% crude fiber, and 51.1% carbohydrates, vitamins and minerals were as in the NIH-7 formula for rodents). This diet meets the requirements for adult mice recommended by the American Institute of Nutrition. Mice were fed ad libitum and kept in individual metabolic cages, thus allowing body weight and food ingestion be monitored daily. The animal room was routinely kept at 22 °C (12 h light/12 h dark). Care of irradiated mice followed the recommendation of the Robert Wood Johnson Medical School (Piscataway, NJ) for mouse total body irradiation (policy 15), which includes: a) use of antibiotics in the drinking water (134 mg ampicillin/kg/day, 40 mg enrofloxacin/kg/day, and 220/42 mg sulfamethoxazole/trimethoprim/kg/day), b) making drinking water readily available, c) Napa nectar Systems Engineering Lab Group Inc. (Napa, CA) provided on the bottom of the cage during the first 14 days and replaced daily, d) provision of softened food served in a small Petri dish on the cage floor, e) completely sterile environment (cage, food, and water) to avoid potential immunosuppression-associated infections. A free-standing wheel inside the cage was also included to facilitate the physical exercise of the animals. All experiments started at 10.00 h. Mice received γ-radiation (137Cs) (total body irradiation, TBI) in a BIOBEAM 8000 (Gamma-Service Medical GmbH, Leipzig, Germany). Total doses ranging from approx. 5.0 to 10.0 Gy, were administered at a rate of approx. 0.5–0.6 Gy/min (single fraction radiotherapy). The irradiation distance from the source was limited to 12 cm. Radiation dosimetry was controlled using alanine dosimeters from Bruker (Billerica, MA) with dosimetry variation of +/− 0.09 Gy. For oral administration,PT (Merck Chemicals GmbH, Darmstadt, Germany) and SIL (Abmole Bioscience Inc., Houston, TX) were solubilized in 2-hydroxypropyl-β-cyclodextrin, then suspended in 0.3% carboxymethylcellulose. For IP administration, a PT salt (pterostilbene phosphate disodium salt, SYNCOM, Groningen, The Netherlands) and SIL salt (silibinin-C-2′,3-dihydrogen succinate, disodium salt, Rottapharm/Madaus, Cologne, Germany) were dissolved in sterile vaccination-grade water. All other polyphenolic phytochemicals assayed (Merck Chemicals GmbH; and Cayman Chemical Co., Ann Arbor, MI) represent main flavonoids and non-flavonoids [12], i.e. gallic acid, caffeic acid, curcumin, epigallocatechin gallate, genistein, quercetin, luteolin, naringenin, delphindin, and phloridzin were solubilized as PT and SIL before oral administration [13]. NR (nicotinamide riboside, 3-aminocarbonyl-1-β-D-ribofuranosyl-pyridinium, Elysium Health, Inc., New York, NY) was dissolved in vaccination-grade water, and the pH of the solution neutralized (pH 7.0) before oral administration (185 mg/kg × day) [14]. FSL1 (InvivoGen, San Diego, CA) was resuspended in sterile vaccination-grade water, and administered IP (0.25 mg/kg × day, the volume administered was of 50–60 μL depending on the weight of the mouse, as previously reported [9]. All compounds used for in vivo treatments were greater than 99% pure by HPLC analysis based on the certificate of analysis provided by the suppliers.
Ethics statement
Procedures involving animals respected international laws and policies (EEC Directive 86/609, OJ L 358. 1, December 12, 1987; and NIH Guide for the Care and Use of Laboratory Animals, NIH Publ. No. 85–23, 1985). All procedures were approved by the Committee of Ethics in Animal Experimentation of the University of Valencia (approval number A1469427422649).
Pterostilbene levels
The analysis was performed by liquid chromatography and mass spectrometry (LC-MS/ MS) as previously described [15].
Silibinin levels
Measurement was based on the method of Song et al [16]. Briefly, samples (injection volume of 5 μL) were analyzed through HPLC (Ultimate 3000, ThermoFisher Scientific) and MS/MS (Waters TQ detector triple quadrupole) systems on a HypurityTM C18 column (50 × 4.6 mm). The mobile phases consisted of water (A) and acetonitrile (B). The gradient elution system was optimized as follows: 0–3.0 min 20% − 100% B, 3.0–5.0 min 100% − 100% B, 5.0–5.5 min 100% − 20% B, 5.5–7.5 min 20% B. The flow rate was 0.5 mL/min. MS/MS data were obtained under negative electrospray ionization mode. The multiple reaction monitoring mode was employed to monitor SIL with the precursor-to-product ion transition of m/z 481.3/125.0. The fragmentary voltage was 150 V, and the collision energy 15 eV. Tissue sample preparation was as described for determination of PT levels (see above).
Blood NAD+ levels
Measurement by HPLC following the method of Yoshino and Imai [17]. We used a Supelco LC-18-T column (15 cm × 4.6 cm; Sigma Aldrich, Saint Louis, MO) and a Hypercarb column (15 cm × 4.6 cm; ThermoFisher Scientific, Waltham, MA). Whole-blood samples (0.5 mL) were collected in sodium citrate tubes at 4 °C. Then, 0.1 mL were transferred to a cryovial containing 0.9 mL of 0.5 M ice-cold perchloric acid to lyse all cells and, after centrifugation (10,000 g × 10 min at 4 °C), supernatants were stored at −80 °C until analyzed.
Pathology
Mice were removed either dead, moribund, or scheduled for sacrifice. All mice were necropsied. Femurs were isolated 14 days after the irradiation. Bone marrow cells were flushed from isolated femurs using 26-gauge needle with pre-chilled PBS. The number of viable bone marrow nucleated cells (BMNCs) was assessed using a hemocytometer (Neubauer, Marienfeld, Germany).
To determine the effect of whole-body γ-irradiation in testes, right testes of individual mice were dissected out on day 1 and 7 after irradiation, and extra tissues were removed in pre-chilled PBS and fixed in 10 % formalin at room temperature. After embedding, 5 μM thick sections were cut, stained with hematoxylene and eosin and mounted. To determine the effect of γ-irradiation on quantitative changes in testes, five sections were scored per slide with ten seminiferous tubules in each section for a total of 5 × 10 seminiferous tubules per mouse. Under microscopic analysis spermatogonia appear round and pale, with prominent nucleoli.
To assess the effect of irradiation on motility of sperm cells, 20 μL single cell suspensions (approx. 106 cells/ml) were transferred to a hemocytometer and observed under inverted microscopy. All sperms were observed individually under the microscope (magnification of 400x) and considered as motile if they showed any movement. Results presented as sperm motility index (%) was determined by dividing the total number of motile sperm by the sum of motile plus non-motile sperm.
To assess the effect of irradiation on the intestine, the jejunum was collected and fixed in 4% paraformaldehyde for 48 h and then embedded in paraffin. Sections were cut in 4 μm of thickness and used for hematoxylin and eosin staining. These sections were visualized using a Leica DM1000 microscope (Leica Microsystems, Wetzlar, Germany).
Evaluation of therapy-induced in vivo toxicity
Blood cell count and chemistry were based on NIH standard methodology.
Chromosomal aberrations and double-strand DNA breaks
We followed the technique described by M’kacher et al [18], where telomere and centromere staining allows the detection of dicentrics, centric rings, and acentric chromosomes of premature chromosome condensation in lymphocytes. Blood was collected 8 h after irradiation [18], thus allowing time for DNA repair and the occurrence of chromosomal aberrations.
Peripheral blood lymphocytes were isolated as described here below. Premature chromosome condensation (PCC) was performed using CHO cells (ATCC) [19]. The condensed human chromosomes were differentiated from the CHO chromosomes according to their morphology [18]. This procedure required approximately 4 h.
Telomeres and centromeres of PCC fusions were stained using the Q-FISH technique [18]. Telomere and centromere-stained fusions were automatically recorded using the Autocapt software from Metasystems (Altlussheim, Germany) and a high-resolution CCD camera (C91002-23B, Hamamatsu Photonics, Herrsching, Germany).
In the same slide two different manual procedures were performed based on M’kacher et al. [18]. Briefly, first using the reverse 4′,6-diamidino-2-phenylindole, and scoring rings and excess acentric chromosomes. Second using the telomeres and centromeres staining to score dicentrics, centric rings, and acentric chromosomes. Resulting double-strand DNA breaks (DSBs) were calculated [18].
Micronuclei formation
For this purpose, the femoral bone marrow was collected from sacrificed mice, and smear was fixed utilizing methanol. After drying, a double staining with May Grunwald and Giemsa stains was prepared [20]. According to this method, polychromatic erythrocytes (PCE) stained reddish-blue and normochromatic erythrocytes (NCE) stained orange, while nuclear materials were dark purple. The micronucleated polychromatic (MnPCE) and normochromatic (MnNCE) cells were counted out of 2,000 cells under oil immersion.
Isolation and incubation of lymphocytes, hepatocytes, and intestinal epithelial cells
Isolation and incubation of hepatocytes followed previously reported methodology [21]. Blood mononuclear cells were obtained from heparinized blood by Accuspin-Histopaque (Sigma-Aldrich, St. Louis, MO) gradient centrifugation. Positive selection of lymphocyte subsets by monodispersed immunomagnetic Dynabeads (Dynal) was performed at 4 °C, as described elsewhere [22].
Cell volumes were measured as previously described [23].
Isolated lymphocytes were incubated (106 cells/flask) at 37 °C in Krebs-Ringer medium with 5% fat-free bovine serum albumin and in the presence of glucose (5 mM) and glutamine (2 mM). Cells were lysed using 0.2 mL of 25% perchloric acid. Protein was removed by centrifugation and the supernatant was neutralized with a 40% KOH solution and a Tris-(hydroxymethyl) aminomethane/KOH (0.5–2.0 M) before metabolite determination.
Primary intestinal epithelial cells (IECs) were isolated using colon tissue and the methodology of Graves et al [24]. The isolated intestinal crypts were suspended in the complete growth media (DMEM, 8.5 g/l sodium pyruvate, 2% v/v FBS, 0.25 U/mL insulin, 100 U penicillin, 100 μg/mL streptomycin, 25 μg/mL gentamycin, 5 μg/mL transferrin, and 10 ng/mL EGF containing 2% w/v D-sorbitol), plated at a density of approx. 2000 crypts/mL/well in type I collagen-coated culture dishes (ThermoFisher Scientific), and incubated at 37 °C.
Glucose and glutamine utilization were measured as previously described [25].
Cell culture
Isolated hepatocytes were cultured in William’s complete medium at a concentration of 106 cells/mL. Isolated C2D+ lymphocytes were cultured in PB-MAX medium (GIBCO, ThermoFisher Scientific) at a concentration of 2 × 106 cells/mL. IECs (106 cells/mL) were cultured in DMEM supplemented with 10% heat-inactivated FBS, and 300 mg/mL L-glutamine. All cells were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
DNA oxidation
Tissue samples were homogenized in 10 mM Tris (pH 7.0) + 1 mM EDTA + 150 mM NaCl, and processed as previously described [26]. Levels of 8-hydroxy-2′-deoxyguanosine (8OHdG) were analyzed by UPLC–MS/MS.
Lipid peroxidation
Tissue samples were homogenized in 0.1 M phosphate buffer (pH 7.4) + 1 mM EDTA + 0.005% butylated hydroxytoluene. Isoprostanes were measured using the 8-isoprostane EIA kit (Cayman Chemical Co.) and based on the manufacturer’s protocol.
Protein carbonylation
Carbonylation of proteins was measured using the Protein Carbonyl Fluorometric Assay Kit (Cayman Chemical Co.) and based on the manufacturer's protocol.
Enzyme activities and metabolite levels
Isolated cells were homogenized in 0.1 M phosphate buffer (pH 7.2) at 4 °C. γ-glutamylcysteine ligase (GCL), glutathione peroxidase (GPX), catalase (CAT), and superoxide dismutase 1 and 2 (SOD1 and SOD2) activities were measured as previously described [27]. Glutathione (γ-L-glutamyl-L-cystenyl-glycine, GSH) was determined by LC/MS as previously reported [28]. Cell processing followed published methodology [29]. NAD+ and NADH in isolated cells were quantitated using kits from MyBiosource (San Diego, CA).
RT-PCR and detection of mRNA
This procedure followed a previously reported methodology [27]. Real-time quantification of mRNA relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also performed as previously described [27].
Comet assay
Comet assay is a single cell gel electrophoresis assay for simple evaluation of cellular DNA damage. We used the assay kit from Abcam (ab238544) (Cambridge, UK) according to the manufacturer's instructions.
Nicotinamide phosphoribosyltransferase activity assay
Formation of 14C-nicotinamide mononucleotide (NMN) from 14C-nicotinamide (NAM¸ (GE Healthcare Life Sciences, Björkgatan, Uppsala, Sweden) can be measured by precipitation of 14C-NMN in acetone on filter paper and scintillation counting. Cell dispersion was performed by trypsinization (in Mg2+- and Ca2+-free PBS supplemented with 0.2% trypsin, 0.5 mM EDTA, and 5 mM glucose for 3 min at 37 °C). Cell pellets were obtained by centrifugation (800 g × 5 min at 4 °C), and then 100 μL of phosphate buffer [0.01 M Na2HPO4/NaH2PO4 buffer, pH 7.4, containing protease inhibitor (Sigma-Aldrich)] was added to each cell sample. Samples were further processed, and NAMPT activity was determined on 30 μg of protein as described previously [30], [31]. Radioactivity (disintegrations/min) was measured using a Tri-Carb® Liquid Scintillation Counter from Perkin-Elmer. Background disintegrations/min from scintillation fluid was subtracted from each measurement.
Colony forming cells in the bone marrow
Mice were sacrificed and femurs were isolated on day 7 post irradiation. Bone marrow cells were flushed from the isolated femurs (see above) with phosphate-buffered saline (4 °C) containing 5% fetal bovine serum. Single-cell suspension was passed through 100 μm nylon mesh strainer to remove debris and clumps. Cells were washed twice with phosphate-buffered saline (4 °C) and counted in a hemocytometer. Cells were plated at a concentration of 5 × 104 cells per 35 mm cell culture dish in Methocult GF M3434 (Stemcell Technologies, Vancouver, Canada) growth medium and incubated for 14 days at 37 °C. Colonies were visualized on day 14 of culture using light microscopy (AxioCam MRc5, Zeiss, Oberkochen, Germany) under a polarized light and scanned in a 60 mm gridded scoring Petri dish (Stemcell Technologies). Hematopoietic colony forming cells CFU-GM and CFU-GEMM were quantified.
Preparation of cell, nuclear, and mitochondrial extracts
Cell extracts were prepared using the Whole Cell Extraction Kit of Sigma Aldrich. The NE-PER™ Nuclear and Cytoplasmic Extraction kit (ThermoFisher Scientific) was used to prepare cytoplasmic and nuclear extracts. Mitochondrial extracts were prepared following the Cell Fractionation and Organelle Isolation procedures standardized by ThermoFisher Scientific.
Gene silencing
The PSilencer 3.1-H1 linear vector from Ambion Inc. (Austin, TX) was used to obtain long term gene silencing. To target mouse Nfkbie, a siRNA Oligo Duplex (Locus ID 18037) from Origen (Rockville, MD) was used. The siRNAs to target mouse Nfe2l2 (RefSeq NM_010902.3), Ppargc1 (RefSeq NM_008904.2), Sirt1 (RefSeq NM_019812.2), Sirt3 (RefSeq MN_022433.2), Parp1 (RefSeq NM_007415.2), SOD2 (RefSeq NM_013671.3), GPX1 (RefSeq NM_008160.6), and PRDX2 (peroxiredoxin 2) (RefSeq NM_009116.2) were from Invitrogen (San Diego, CA). In all cases silencing procedures followed the technical recommendations of the manufacturers. In the control experiments we used equivalent amounts of the corresponding sense oligonucleotides and scrambled oligonucleotides with the same base composition and a randomized sequence. Silencing was confirmed by immunoblotting. Invitrogen Lipofectamine RNAiMAX Transfection Reagent and manufactureŕs protocol were used for delivery of siRNA.
Cell death analysis
Apoptotic and necrotic cells were differentiated using Hoescht 33,342 (10 mM; stains all nuclei), propidium iodide (10 mM; stains nuclei of cells with a disrupted plasma membrane), and a fluorescence microscope (Diaphot 300, Nikon, Tokyo, Japan) with excitation at 360 nm. Approx. 103 cells were counted each time. Apoptotic cell death was also analyzed based on labeling of DNA strand breaks and using the in situ cell death detection kit from Promega (DeadEndTM Fluorometric TUNEL System) (Madison, WI).
Western blots
This procedure followed previously described methodology [27]. Protein bands were quantified using laser densitometry.
Tumor xenografts
Human A549 and MDA-MB-231 cells (ATCC, Manassas, VA) were grown in DMEM (Invitrogen), pH 7.4, supplemented with 10% heat-inactivated FCS (Biochrom KG, Berlin, Germany), 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were plated (20,000 cells/cm2) and cultured at 37 °C. Cells were allowed to attach for 12 h before any treatment addition. Cell number and viability were measured in a BioRad (Hercules, CA) TC20 Automated Cell Counter.
Nude (nu/nu) mice (female, 9–10 weeks old, Charles River Laboratories) were fed ad libitum on a standard diet (Letica, Rochester Hills, MI), and kept at 22 °C (12-h-light/12-h-dark). Tumor cells were harvested from culture flasks by adding 0.02% EDTA (5 min at 37 °C), washed twice and resuspended in DMEM, and injected SC on the back of the animal (5 × 106 cells/mouse). Local tumor growth was determined every 2 days using calipers and was expressed in mm3 (volume = 0.5a × b2, being a and b the long and short diameters).
Statistical analysis
The G*Power 3.1.9.2 software was used to determine the sample size (ANOVA test, effect size = 0.21, α = 0.05, β = 0.80 and the indicated groups). Survival data were analyzed with Kaplan-Meier curves and LogRank (Mantel-Cox) test. All other data are mean values ± SD for the number of different experiments. The Student’s t-test was used for statistical analyses.
Results
Pre-irradiation administration of polyphenols increases survival in mice subjected to whole-body γ-irradiation.
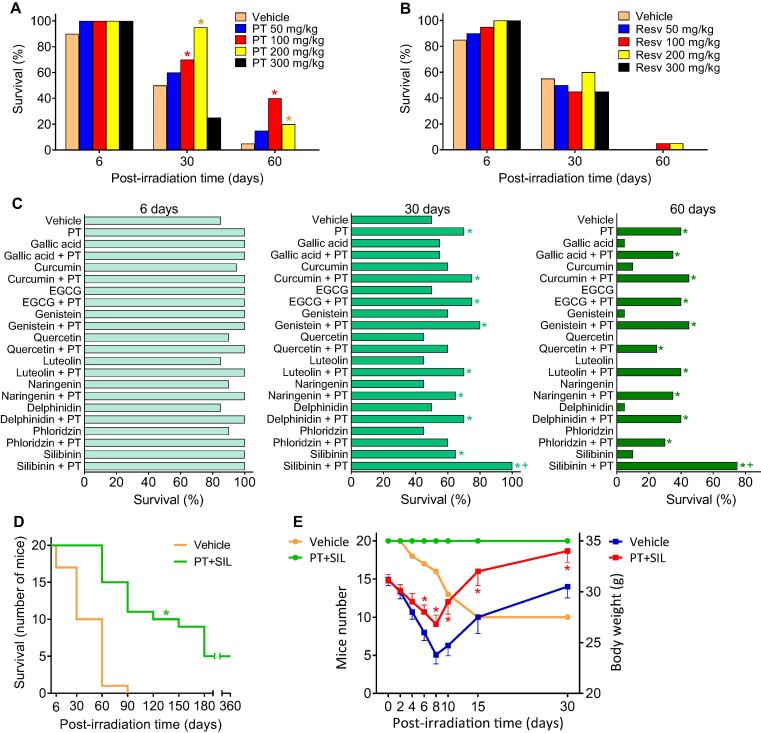
As shown in Supplementary Fig. S1, the whole-body γ radiation (137Cs) dose expected to kill 50% of the Swiss albino mice in 30 days (LD50/30) is approx. 7.2 Gy (administered as a single dose, 05–0.6 Gy/min). The potential protective effect of PT was assayed at different doses. In a 60-day experiment the PT dose showing the highest survival rate was 100 mg PT/kg (Fig. 1A). With respect to mouse survival, PT was found to be superior to resveratrol (Resv), its natural analog (Fig. 1B). PT has greater lipophilicity and a higher potential for cellular uptake than Resv [32].
Fig. 1.
Effect of pterostilbene and silibinin on the survival of γ-irradiated mice. (A) Thirty-days survival of mice treated with γ rays (LD50/30) and PT. Mice (n = 20/group) were treated with PT (dissolved in 2-hydroxypropyl-b-cyclodextrin, then suspended in 0.3% carboxymethylcellulose; administered orally, once per day, 5 days before and 2 days after irradiation). Mice received whole-body γ radiation (one single dose of 7.2 Gy (137Cs) at 0.5–0.6 Gy/min). Data were analyzed with LogRank (Mantel-Cox) test (*p < 0.05, comparing all groups vs. controls treated with vehicle). (B) Thirty-days survival of mice treated with γ rays (LD50/30) and resveratrol (Resv). Mice (n = 20/group) were treated with Resv (dissolved and administered exactly as PT). Mice received whole-body γ radiation as indicated for PT-treated mice. (C) Combination of PT and other natural polyphenols in the prevention of the lethality induced by γ radiation (LD50/30). Mice (n = 20/group) were treated with PT and/or other polyphenols. PT was solubilized and administered as above. All other polyphenolic phytochemicals, representing main flavonoids and non-flavonoids were solubilized and administered exactly as PT. Doses for each phytochemical were selected from the max non-toxic doses published. Data were analyzed with LogRank (Mantel-Cox) test (*p < 0.05, comparing all groups vs. controls treated with vehicle; +p < 0.05, comparing all groups vs. the group treated with PT alone). (D) Effect of the combination of PT and SIL on the long-term survival of mice subjected to whole-body γ irradiation (LD50/30). Mice (n = 20/group) were treated IP with PT (100 mg/kg) and SIL (70 mg/kg) × 7 days (5 days before and 2 days after γ irradiation). For IP administration, a PT salt (PT phosphate disodium salt) and SIL salt (SIL-C-2′,3-dihydrogen succinate, disodium salt) were dissolved in sterile vaccination-grade water. The IP administration was selected to facilitate the regular administration to a high number of mice. Data were analyzed with Kaplan-Meier curves and LogRank (Mantel-Cox) test (*< 0.05, comparing the PT + SIL-treated group vs. controls treated with vehicle). (E) Survival of γ-irradiated (LD50/30) mice treated with PT and SIL and the effect on body weight. Mice (n = 20/group) were treated IP with PT and SIL (as in D). Statistical analyses (body weight data) were performed using Student’s t-test (*p < 0.01 comparing PT + SIL vs. vehicle-treated controls).
Since the combination of different polyphenols may result in potential synergies (see e.g. [33]), we investigated this possibility by combining PT with different polyphenolic phytochemicals representing a wide array of polyphenols including gallic acid (a phenolic acid), caffeic acid (a hydroxycinnamic acid), curcumin (a curcuminoid), epigallocatechin gallate (a flavanol), genistein (an isoflavone), quercetin (a flavonol), luteolin (a flavone), naringenin (a flavanone), delphindin (an anthocyanidin), and phloridzin (a chalcone). The highest 60-day survival in mice subjected to γ radiation (LD50/30) was demonstrated to be the combination of PT and silibinin [SIL, (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one, a natural flavanone] (Fig. 1C). The combination of PT + SIL was also assayed for long-term survival (360-day experiment) and was found to significantly increase the survival compared to vehicle-treated controls (Fig. 1D). Our data up to day 30 post-irradiation are similar to those obtained by others (e.g. in McCart et al. using 7.5 Gy, [34]; our DL50/30 was approx. 7.2 Gy). Gastrointestinal and hematopoietic injuries are behind the deaths within this first period. The deaths between days 30 and 60 mainly reflect the animals' inability to overcome bone marrow damages. However, as evidenced by the study of post-mortem pathology, mice in the polyphenols-treated group died by a variety of causes: 25% (within the first 60 days post-irradiation) due to hematopoietic injuries; 50% (60–360 days post-irradiation) due to myeloid leukemia, lung cancer, infection/inflammation, or nephropathy (all reflecting long-term consequences of the radiation-induced damages). The survival attributed to the radioprotection elicited by PT + SIL is higher if both polyphenols are administered before the irradiation (Supplementary Fig. S2). Moreover, as shown in Fig. 1E, treatment with PT + SIL minimized the loss of body weight, which is a common side effect associated with radiotherapy that may compromise recovery and survival [35].
Post-irradiation administration of nicotinamide riboside and FSL1 lipopeptide further promotes survival in mice previously treated with polyphenols
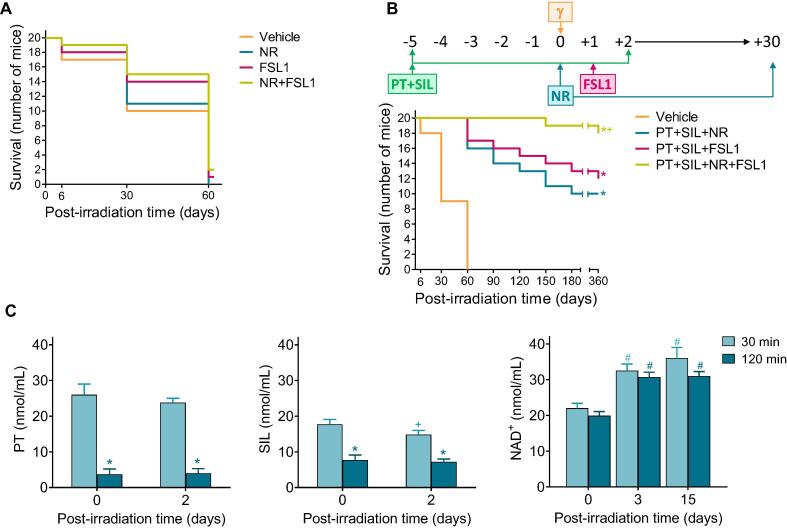
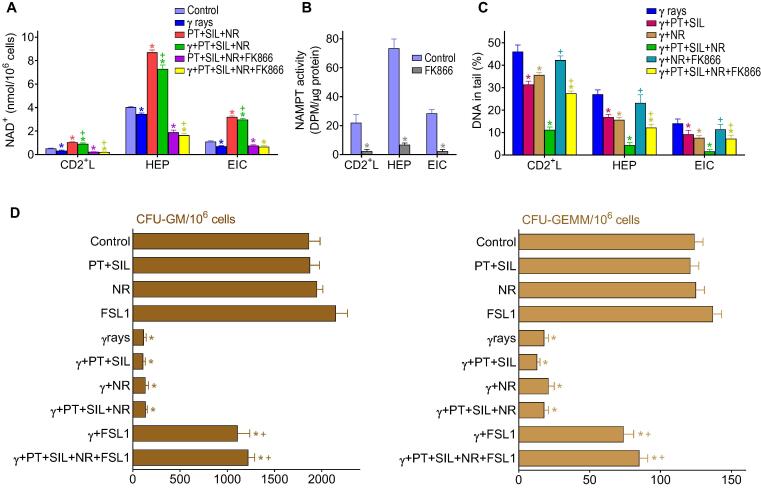
Post irradiation administration of NR and/or FSL1 (two potential radiomitigators) to mice subjected to γ irradiation (LD50/30), did not significantly reduce mortality (Fig. 2A). However, the combination of PT + SIL and NR + FSL1 dramatically increased long-term survival (360 days after γ-irradiation) up to 90% (Fig. 2B). The one year survival in PT + SIL + NR (60%) or PT + SIL + FSL1 (50%) treated groups was also higher than in vehicle-treated controls (0%) (Fig. 2B). As indicated in the scheme (Fig. 2B), both polyphenols were administered for 1 week (5 days before irradiation and up to 2 days after). Based on the bioavailability of these polyphenols at the doses used, that period of time (5 days before irradiation) ensures the maximum possible effect on the antioxidant defenses. After irradiation, we decided to maintain the administration for 2 extra days to ensure that the antioxidant defenses remain overexpressed. This is important to minimize non-irradiation derived ROS, which may arise as a consequence of the tissue damage and stress caused by the irradiation. We did not extend the administration of polyphenols beyond a week to avoid any possible side effect at the doses administered. Regarding FSL1, the TLR2/6 Ligand Lipopeptide, we followed the protocol recommended by Kurkjian et al. [9] and a single dose was administered one day after irradiation. Nicotinamide riboside (NR) was administered for a longer period of time (one month). This was based on three principles: a) the increase in blood NAD+ elicited by NR is limited (Fig. 2C) and, thus, beneficial effects require a much longer period of administration; b) maintenance of high cellular levels of NAD+ (compared to controls) favors DNA repair and also limits the appearance of mutations that can develop over time; and c) chronic NR supplementation is well-tolerated and elevates NAD+ [10]. Moreover, studies on the in vivo γ radiation-induced toxicity, via hematology, clinical chemistry and metabolism-related parameters in isolated CD2 + lymphocytes, reveal that the full treatment also improves all parameters affected by the ionizing radiation (Supplementary Table S1). These data support the safety of the protocol applied. Furthermore, in order to complement our findings, the effect of our radioprotective combination was also tested in two typically radiosensitive tissues. A shown, in Supplementary Fig. S3, the combined treatment with polyphenols, NR and FSL1, as compared to controls, improved the number of spermatogonia and the motility index in the seminiferous tubules (testes), as well as the depth and number of crypts in the jejunum in mice subjected to γ irradiation.
Fig. 2.
Combination of polyphenols with potential radiomitigators (nicotinamide riboside and fibroblast-stimulating lipopeptide 1) further increases the survival of γ-irradiated mice. (A) Effect of NR and FSL1 on the γ-irradiation (LD50/30)-induced mortality. Mice (n = 20/group) were treated with NR and/or FSL1. NR was dissolved in vaccination-grade water, and the pH of the solution neutralized (pH 7.0) before oral administration (185 mg/kg × day). FSL-1 was resuspended in sterile vaccination-grade water and administered IP (0.25 mg/kg × day). The volume administered in each case was of 50–60 mL depending on the weight of the mouse. NR was administered daily for 30 days, one single dose per day, and starting 1 h after the irradiation. FSL1 was administered only once, 24 h after the irradiation. Data were analyzed with LogRank (Mantel-Cox) test, but no significant differences were found comparing each treated-group vs. controls. (B) Effect of PT + SIL + NR + FSL1 on the survival of γ-irradiated mice (LD50/30). Mice (n = 20/group) were administered exactly as indicated in previous experiments [(A) and Fig. 1)]. Data were analyzed with Kaplan-Meier curves and LogRank (Mantel-Cox) test (*comparing all groups vs. vehicle-treated controls; +comparing the PT + SIL + NR + FSL1-treated group vs. the PT + SIL + NR- or the PT + SIL + FSL1-treated group). (C) PT, SIL and NAD+ levels in the circulating blood of treated and irradiated mice. Mice (n = 7/group) were treated with PT, SIL, or NR as scheduled previously and at the doses indicated in previous experiments (a). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing 120 min vs. 30 min; +comparing 2 vs. 0 days; #comparing 3 or 15 days vs. 0 days).
Fig. 2C shows levels of PT, SIL and NAD+ measured, in circulating blood of treated and irradiated mice, at two different time points (PT, SIL and NR were administered as in Fig. 2B). PT and SIL levels are higher at 30 min than at 120 min after their administration either the day of irradiation (day 0) or 2 days after irradiation (Fig. 2C). Whereas NAD+ levels were found similar 3 or 15 days after the irradiation, and at both time points after NR administration (Fig. 2C).
Pterostilbene and silibinin upregulate the antioxidant defense system and decrease the γ radiation-induced molecular damage in different cell types
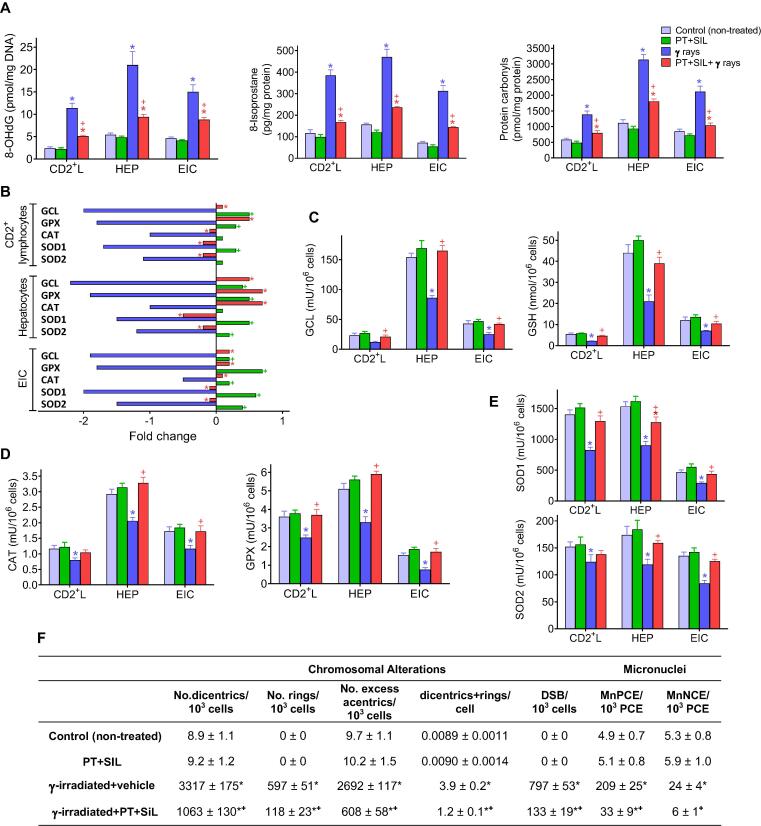
As shown in Fig. 3A, PT and SIL administration significantly diminishes the γ-radiation-induced increase in oxidative stress-related molecular markers in 3 different cell types isolated from control and treated mice. PT + SIL decreased γ ray-induced oxidative damage to DNA (8-hydroxy-2′-deoxyguanosine), proteins (protein carbonyls) and lipids (isoprostanes). PT + SIL also maintains high GSH levels (a prevalent non-protein thiol and antioxidant) (Fig. 3C). Additonally, PT + SIL also prevented the γ irradiation-associated decrease of several key oxidative stress-related enzyme activities: GCL, GPX, CAT, SOD1 and SOD2 (Fig. 3C-E). Moreover, antioxidant enzyme activities measured in untreated and treated mice correlated with their gene expression levels (Fig. 3B). Thus, suggesting a close relationship between the radioprotection elicited by the polyphenols and their capacity to modulate the biological antioxidant response. Therefore, the polyphenol-induced upregulation of our antioxidant defenses will counteract the effect of the radiation-induced oxidative stress, thus preventing oxidative molecular damages. Furthermore, as shown in Fig. 3F, PT + SIL also decreased the γ radiation-induced chromosomal aberrations and micronuclei formation in circulating CD2+ lymphocytes, both widely used cytogenetic bioindicators for monitoring exposure to ionizing radiation. All the parameters displayed in Fig. 3 were measured 2 days after irradiation (see the protocol shown in Fig. 2B). Nevertheless, the concomitant administration of PT + SIL + NR + FSL1 did not significantly change any of the results shown in Fig. 3 (data not shown).
Fig. 3.
Protective effect of pterostilbene and silibinin on the γ-radiation-induced oxidative and cytogenetic damage. (A) Effect of PT + SIL on the levels of different oxidative damage-related molecular markers in cells (CD2+ lymphocytes, hepatocytes and epithelial intestinal) isolated from control and γ-irradiated mice. PT, SIL and γ rays were administered exactly as previously indicated in Fig. 2B (n = 5 per group, cell type, and treatment). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing PT + SIL + γ rays-treated mice vs. mice treated with γ rays alone). (B) Expression (RT-PCR) of different antioxidant defense-related enzyme activities in isolated cells from PT + SIL- and/or γ-rays-treated mice. Fold change versus the expression rate of control (untreated) cells. PT, SIL and γ rays were administered as Fig. 2B (n = 5 per group, cell type, and treatment). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing PT + SIL + γ rays-treated mice vs. mice treated with γ rays alone; +comparing PT + SIL-treated mice vs. controls treated with vehicle). (C) Protective effect of PT + SIL on the γ radiation-induced damage in glutathione-related antioxidant defenses. PT, SIL and γ rays were administered as Fig. 2b (n = 5 per group, cell type, and treatment). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing PT + SIL + γ rays-treated mice vs. mice treated with γ rays alone). (D) and (E) Protective effect of PT + SIL on the γ radiation-induced damage in CAT and GPX enzyme activities (D), and SOD enzyme activities (E). PT, SIL and γ rays were administered as Fig. 2B (n = 5 per group, cell type, and treatment). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing PT + SIL + γ rays-treated mice vs. mice treated with γ rays alone). (F) Effect of PT + SIL on the γ radiation-induced chromosomal aberrations and micronuclei formation in circulating CD2+ lymphocytes. Lymphocytes were isolated from mice and procedures were as described under Experimental Section. DSBs, double-strand DNA breaks. Micronucleated polychromatic (MnPCE) and normochromatic (MnNCE) cells. Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing PT + SIL + γ rays-treated mice vs. mice treated with γ rays alone).
Pterostilbene, silibinin and nicotinamide riboside decrease cellular DNA damage, and FSL1 lipopeptide promotes recovery of the hematopoietic system
As shown in Fig. 4A, NR treatment increases the NAD+ content in different cell types and also prevents the decrease of NAD + induced by γ radiation. PT + SIL administered alone did not affect the NAD+ levels in any of the cells studied and did not affect the increase elicited by NR (results not shown). Administration of FK866, a specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase (NAMPT), decreased NAD+ levels in mice treated with PT + SIL + NR or PT + SIL + NR + γ rays (Fig. 4A). FK866-induced NAD + depletion associated with a drastic decrease in NAMPT activity (Fig. 4B) (PT, SIL, NR, FSL1 and/or γ radiation did not influence the rate of FK866-induced inhibition of the NAMPT activity, not shown). Importantly, as shown in Fig. 4C using the DNA damage COMET assay, PT + SIL or NR favored DNA recovery and their combination was the most effective (Fig. 4C). Presumably, the effect of the polyphenols is an antioxidant-related mechanism, whereas the NR-induced increase in NAD+ levels directly promote DNA repair activity. As shown in Fig. 4D, FSL-1 increases the hematopoietic colony forming cells CFU-GM (colony forming unit-granulocyte, macrophage) and CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte). These results confirm the post-irradiation hematopoietic recovery suggested by the data under Hematology in Supplementary Table S1.
Fig. 4.
Effect of radioprotectors and radiomitigators on cellular NAD+ content, DNA damage, and hematopoietic recovery in γ-irradiated mice. (A) Effect of PT, SIL, NR and the specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase (NAMPT) FK866 on the NAD+ content of cells isolated from γ-irradiated mice (n = 5 per group, cell type, and treatment). FK866 was administered in vivo (15 mg/kg, twice daily by IP injection, during 6 days and starting 3 days prior to irradiation, see Fig. 2B), and was also present in the cell cultures (100 nM) [36]. Cells were isolated 72 h after irradiation. Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing PT + SIL + NR + γ rays-treated mice or PT + SIL + NR + FK866 + γ rays-treated mice vs. mice treated with γ rays alone). (B) Effect of FK866 on the cellular NAMPT activity (p < 0.01; *comparing FK866-treated mice vs. controls, n = 5). Mice were treated with the inhibitor for 6 days as above (A). (C) Effect of PT, SIL, NR and FK866 on the DNA damage in cells isolated from γ-irradiated mice (n = 5 per group, cell type, and treatment). Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. γ-irradiated controls; +comparing γ-irradiated groups where FK866 was present vs. their respective controls). Mice were treated and cells isolated as above (A). (D) Effect of PT, SIL, NR and FSL1 on the hematopoietic recovery in γ-irradiated mice. Hematopoietic colony forming cells CFU-GM and CFU-GEMM were quantified in femurs isolated from control and treated mice (n = 5 per group and treatment). Mice were treated as in Fig. 2B and bone marrow cells isolated 7 days after irradiation. Statistical analyses were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing the different treatments + γ irradiation vs. γ irradiation alone).
Nrf2, NF-kB, PGC-1α and PARP1 interactions in the radioprotective mechanism
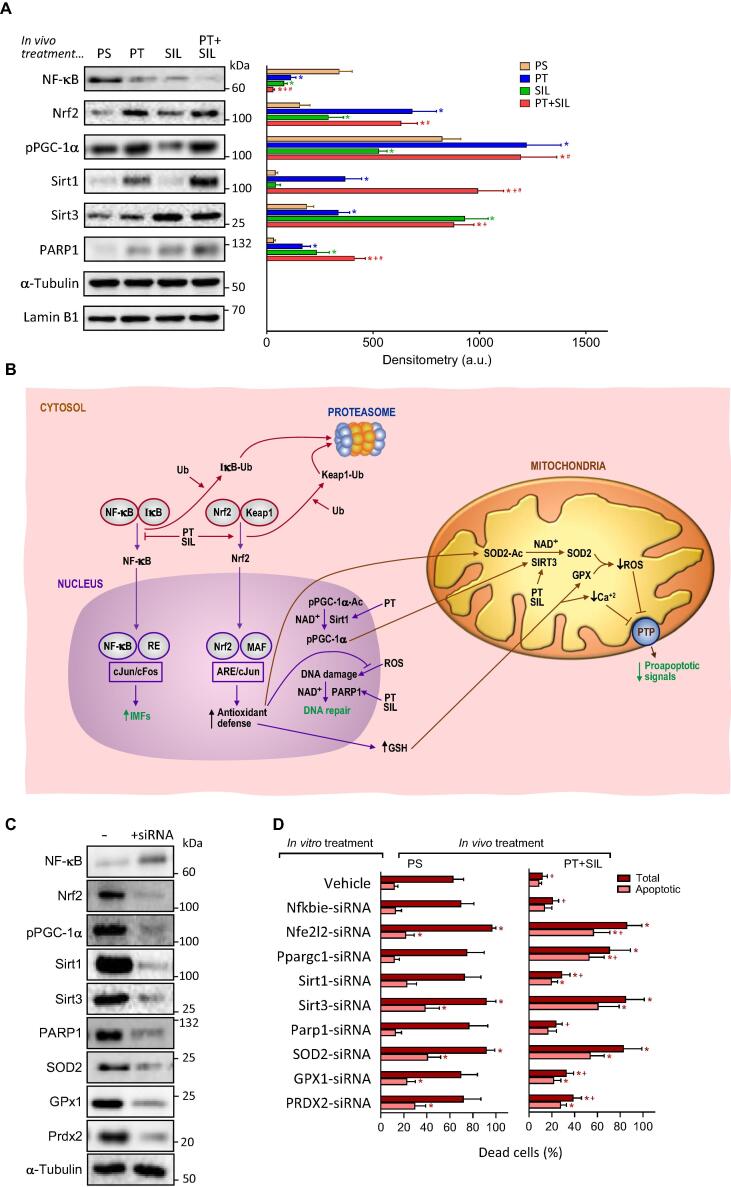
Isolated epithelial intestinal cells were used to investigate potential signaling pathways involved in the radioprotective effect elicited by the combination of PT + SIL. Based on the data reported in Fig. 3, PT + SIL exert protection against γ radiation-induced oxidative damage. Presumably, the observed protection is due to induction of Nrf2 (nuclear factor erythroid 2-related factor 2)-dependent antioxidant defenses (Fig. 3). Anti-inflammatory properties have been reported previously for both polyphenols (e.g. [37], [38]), thus suggesting the involvement of NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells)-dependent signaling. NR-induced increase of NAD+ will also favor PARP1 (Poly [ADP-ribose] polymerase 1)-dependent DNA repair [39], Sirt activity [40], and PGC-1α(peroxisome proliferator-activated receptor γ co-activator 1α)activation [41]. Moreover, PT may induce PGC-1α [42], Nrf2 activation [43], and Sirt1 expression [44]; whereas SIL could influence Sirt3 expression [45] and PARP1 activity [46].
As shown in Fig. 5A, as compared to controls, both PT and SIL increased nuclear Nrf2 while decreasing nuclear NF-kB levels. In the case of Nrf2, the effect of PT is more pronounced than that exerted by SIL. These effects, as a whole, suggest a cooperative effect in promoting the antioxidant defenses while downregulating the NF-kB-dependent proinflammatory response. pPGC-1α was also increased by PT (Fig. 5A). PT also increased Sirt1 levels. Interestingly, although SIL alone did not affect Sirt1 levels, the combination of the two polyphenols increased Sirt1 levels more than PT alone (Fig. 5A). Both PT and SIL increased Sirt3 levels, although the effect of SIL on Sirt3 was more robust (Fig. 5A). Both PT and SIL increased PARP1 (Fig. 5A). Importantly, addition to the in vivo treatment of NR and/or FSL1 (as in Fig. 2B) did not change significantly any of the data reported in Fig. 5A (not shown) indicating that neither of the two radiomitigators used interfere with the radioprotective effects elicited by the combination of PT and SIL. Nevertheless, it is key to remark that the combination of radioprotectors and radiomitigators is essential to achieve long-term post-irradiation survival (Fig. 2B). Fig. 5B outlines the possible molecular interrelationships between these signaling cascades (see also data in Fig. 3B), and the steps where PT and SIL may exert their effects. In order to further investigate which specific molecules may be involved in the radioprotective effect, we used a gene silencing-based approach on isolated IECs (from control or PT + SIL-treated mice, as in Fig. 2B). As shown in Fig. 5C, siRNAs targeting mouse Nfkbie (encodes the NFKB Inhibitor Epsilon, which inhibits the NF-κB-directed transactivation via cytoplasmic retention of REL proteins), Nfe2l2 (encodes the Nrf2), Ppargc1 (encodes the PGC-1α), Sirt1, Sirt3, Parp1, SOD2, GPX1 or PRDX2 gene expression facilitate the increase of free NF-kB (as a consequence of the downregulation of its inhibitor) and the downregulation of all the other molecular targets. PRDX2 was included in our analyses because this specific enzyme has been shown to exert a protective role in neutralization of oxidative stress induced by ionizing radiation [47]. Isolated IECs were cultured for 24 h in the absence or presence of PT (20 μM) + SIL (15 μM) which mimicked, the in vivo-induced radioprotective defense. As shown in Fig. 5D and S4, PT and SIL drastically decreased the γ irradiation-induced cell death observed in control (vehicle-treated) cells. Cell death analysis, based on the effect of specific RNAs in cells subjected to γ rays, shows that Nrf2, pPGC-1α, SOD2 and Sirt3 are mainly responsible (Fig. 5D and S4) for the radioprotective effect elicited by the treatment with polyphenols.
Fig. 5.
Effect of the treatment with pterostilbene and silibinin on Nrf2-, NF-kB-, PARP1- and PGC1α-dependent mechanisms. Epithelial intestinal cells were isolated, right before the irradiation, from mice subjected to the protocol displayed in Fig. 2B. (A) Western blots for the detection of p65NF-kB, Nrf2, PARP1 and Sirt1 in nuclear extracts, Sirt3 in mitochondrial extracts, and phosphorylated PGC-1α in total extracts. Densitometric analysis (western blots) represents the mean values ± SD for 5–6 different mice per molecule and experimental condition (p < 0.01; *comparing PT-, SIL- or PT + SIL-treated mice versus physiological saline, PS-treated mice, +comparing PT + SIL- versus PT-treated mice; #comparing PT + SIL- versus SIL-treated mice). In vivo treatment (as in Fig. 2B) with PT and/or SIL and NR, FSL1 or NR + FSL1 did not alter significantly any of the data reported in the western blot analysis here displayed (results not shown). (B) Potential molecular interactions and interrelationships between different signaling mechanisms. Ub, ubiquitin; IkB, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; Keap1, Kelch-like ECH-associated protein 1; RE, response element; ARE, antioxidant response element; MAF, musculoaponeurotic fibrosarcoma oncogene homolog; Ac, acetylated; IMFs, inflammation mediating factors. (C) Gene silencing of specific signaling-related molecules. The Fig. displays a representative experiment for n = 5–6 different experiments and experimental condition. PS, physiological saline. (D) Isolated IECs were cultured for 24 h in the presence or in the absence of specific siRNAs, and thereafter were irradiated with γ rays (same dose used for the in vivo experiments). Cell death was analyzed, as described under Methods, in isolated and cultured IEC cells 12 h after γ irradiation. PT (20 μM) and SIL (15 μM) were present in the cultures containing IECs isolated from PT + SIL-treated mice. Thereby, the effects induced by the in vivo treatment could be preserved under in vitro conditions. Data are mean values ± SD for 4–5 different experiments per molecule and experimental condition (p < 0.01; *comparing, under PS or PT + SIL, treatment with a specific siRNA versus controls -treatment with vehicle-; p < 0.01; +comparing data under PT + SIL versus their equivalents under PS).
The combination of radioprotectors and radiomitigators does not diminish the therapeutic efficacy of anti-cancer radiotherapy (X rays) in lung and mammary gland carcinomas
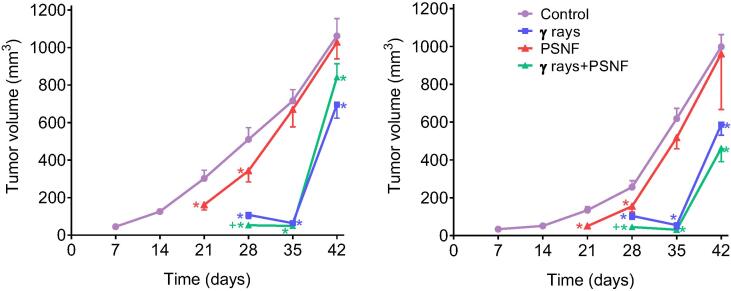
Finally, we investigated if our strategy could interfere with the efficacy of standard radiotherapy against in vivo growing tumors. Two different mouse xenograt models of human cancers (A549 lung adenocarcinoma and MDA-MB-231 triple negative mammary carcinoma) were treated with X rays and the full PSNF combination (PT + SIL + NR + FSL1). As shown in Fig. 6, the effect of radiotherapy on the growth of both tumors was similar in the absence or in the present of PSNF treatment. These results suggest that the efficacy of anti-cancer radiotherapy is not impaired by this radioprotective/radiomitigating formula.
Fig. 6.
Combined administration of the radioprotecting/radiomitigating combination (PT + SIL + NR + FSL-1; PSNF) and X rays to growing human A549 (lung adenocarcinoma) and MDA-MB-231 (triple negative mammary carcinoma) xenografts. Tumors were irradiated with X rays (10 Gy, single fraction, 5.0 Gy/min) using a 6-keV SL75 linear accelerator from Philips. The protocol for PSNF administration was identical to that followed in previous experiments (Fig. 2B), and the radiotherapy was administered on day 21. Statistical analyses (n = 5 mice per group) were performed using Student’s t-test (p < 0.01; *comparing all groups vs. controls; +comparing the X rays + PSNF-treated group vs. the group treated with X rays alone).
Discussion
The radioprotective effects of natural polyphenols and their possible applications under in vivo conditions were proposed more than 20 years ago (e.g. [48]). However, their implementation in practice has not been realized, probably due to problems related to their low in vivo bioavailability and short-half life [12]. The starting point for the strategy that led to the proposed formulation herein was the strong protection exerted by topically administered PT against UVB radiation-induced skin damage and carcinogenesis [6]. Here, we investigated the effect of systemically administered PT against γ radiation (Fig. 1A) and found a high percentage of survival by combining PT and SIL (Fig. 1C). Potential anticancer synergies between polyphenols are known (e.g. [15]), however possible synergies against the deleterious effects of ionizing radiation had not been previously explored. Moreover, the association of potential radioprotectors and radiomitigators as a strategy to decrease radiation-induced damage and enhance survival still is an underdeveloped field. The present work shows the potency of a four compound combination capable of achieving long-term survival in mice subjected to lethal (LD50/30) γ irradiation (Fig. 2B). This novel strategy, which demonstrates prolonged survival, may serve as a paradigm moving forward. Thus, considering the large number of molecules being tested as radioprotectors and/or radiomitigators [49], this paradigm may help to open up the field.
PT and SIL upregulate antioxidant defenses in different cell types (Fig. 3B-E) and decrease γ radiation-induced oxidative damage (Fig. 3A) as well as cytogenetic alterations (chromosomal aberrations and micronuclei formation) (Fig. 3F). These results are consistent with previous observations showing that protection exerted by PT against UVB radiation-induced skin damage also associates with upregulation of the skin antioxidant defenses [6]. As shown in Fig. 5, the protection elicited by PT and SIL may involve different signaling mechanisms which are interrelated. Oxidative stress and inflammation are direct pathophysiological mechanisms activated by exposure of cells to harmful ionizing radiations [50], [51]. PT and SIL increase nuclear Nrf2 and the antioxidant response in different cell types (Fig. 3 and Fig. 5), whereas they decrease NF-kB (Fig. 5) and, presumably, the radiation-associated inflammatory response. In addition, PT increases Sirt1 levels, which facilitates deacetylation of PGC-1α (Fig. 5). PGC-1α is an inducer of Sirt3 expression [52], and as shown in Fig. 5, SIL treatment also increases Sirt3 levels. Mitochondrial SOD2 (deacetylated by Sirt3) and GSH peroxidase (which depends on the import of GSH from the cytosol) would both work to decrease mitochondria-derived ROS and prevent apoptosis. Importantly, as shown in Fig. 5C and 5D, a gene silencing-based approach identified Nrf2, Sirt3 and SOD2 as key for the radioprotective effect elicited by the combined treatment with PT and SIL. In addition, NR generates NAD+ to support Sirt1 and Sirt3 activities [53] and PARP1-dependent DNA repair [54] (Fig. 5). PARP1 activity is also induced by both polyphenols (Fig. 5). NR is a precursor to NAD+ showing a superior pharmacokinetic profile as compared to other forms of B3 (nicotinic acid and nicotinamide) [55], therefore it represents an optimum NAD+ booster. The PT- and SIL-associated increase in Sirt1 and Sirt3 levels (Fig. 5) do not necessarily reflect a direct activation of the enzymes. In fact, PT did not activate Sirt1 in vitro in the presence of either a p53-derived peptide substrate or acetylated PGC-1α isolated from intestinal epithelial cells (results not shown). Nevertheless, it is still unclear which transcription factor(s) may mediate the increase in expression levels of these sirtuins. Furthermore, treatment with PT, SIL and NR needs to be complemented by the administration of FSL1 to stimulate hematopoiesis, which is critical to mitigate the bone marrow suppression linked to the acute radiation syndrome [9] (Fig. 4D).
In this study, IP administration was used when PT and SIL were given in combination with NR (orally administered) and FSL1 (IP single administration). This therapeutic regimen was selected to avoid forcing the animals to take large amounts of these molecules orally (due to low bioavailability), and for the ease of treating them IP on a daily basis. This regimen also has the advantage of avoiding the limitations imposed by the intestinal bacterial metabolism and the absorption through the gut barrier [12]. Nevertheless, different options regarding pharmaceutical formulations, structural modifications and delivery systems, can help to facilitate the oral administration of both polyphenols. Some examples include prodrugs (carboxyesters, sulfonates, sulfates, phosphates, acetals, or carbamates or carbonates), nanoemulsions, polyionic/polymeric shells encapsulating nanoparticles, liposomes, or exosomes [56]. NR can be given orally to humans with no problems that could limit its efficacy [55].
The results showing that combination of radioprotectors and radiomitigators does not diminish the therapeutic efficacy of anti-cancer radiotherapy (Fig. 6) must be further investigated in depth. However, it is possible that the differential response observed could be due to differences between normal and tumor cells such as the relative oxygen enhancement ratio. Since cancer cells proliferate rapidly, they usually exceed their vascular supply, thus resulting in necrotic and/or hypoxic regions, which are more resistant to radiation. This implies that higher doses of radiation must be applied to target the tumor. As the surrounding normal tissues remain well vascularized and oxygenated, they will need protection the more they are exposed to radiation. If radioprotectors may help normal tissues to tolerate higher doses of radiation, then (at least in theory) the radioresistance of hypoxic tumor cells would not be treatment limiting. In addition, it is also possible that the anticancer properties of the polyphenols may increase the efficacy of radiotherapy (see [56], [57]). For instance, PT was found to decrease mutant p53 protein expression while increasing the expression of the pro-apoptotic Bax protein in breast cancer cells [58]. In another example, the effect of SIL against different non-small cell lung cancers involves some prominent targets of STAT3 (BIRC5, FOXM1, BRCA1) [46].
Possible toxic side effects of the proposed combination must be also taken into account. As suggested by the toxicity-related parameters displayed in Supplementary Table S1 and the long-term survival (Fig. 2B), the doses and schedule used appeared safe for the irradiated mice. In mice fed with a PT-enriched diet at doses up to 3,000 mg/kg body weight/day (x 4 weeks) histopathology, hematology, clinical chemistry, and urinary balance studies found no alterations induced by PT as compared to controls [59]. SIL toxicity is also very low, the oral 50% lethal dose being 10,000 mg/kg in rats [60]. The dose of SIL recommended for humans, in case of Amanita Phalloides poisoning, is of 20 mg IV/kg × day [61]. Safety assessment studies (90-day tox study) of NR in rats showed that the no observed adverse effect level for NR was 500 mg/kg/day for males and 1,200 mg/kg/day in females [62]. In humans, no adverse effects of orally administered NR have been reported for a dose up to 2,000 mg/day [63]. Furthermore, FSL1, at the dose used in our study, had no adverse effect on mouse survival, clinical score, or body weight when compared to control mice [9]. Based on this background and our own data (Supplementary Table S1 and Fig. 2B), we may conclude that the combination of PT + SIL + NR + FSL1 has clear options to be adapted for human protection.
Ideal radioprotectors or radiomitigators should be stable, easy to administer, show no significant systemic toxicity, and protect normal tissues from radiation-induced damages. The proposed combination meets these criteria, while demonstrating high efficacy.
Conclusion
This work demonstrates that a combination of two radioprotectors, pterostilbene and silibinin, with two radiomitigators, nicotinamide riboside and fibroblast-stimulating lipoprotein 1, can confer long-term protection of normal tissues against a lethal dose of γ radiation in mice. The established safety profiles of these four molecules clearly warrants continued research to translate this combination for its application in humans.
Funding
This work was supported by grants from the MICINN (Ministerio de Ciencia e Innovación, Spain) (SAF2017-83458-R), and from Elysium Health Inc. (NY, USA) (OTR2017-17899INVES).
Availability of data materials
All the data generated during this study are recorded in electronic laboratory notebooks and are accessible upon request to the authors.
Compliance with Ethics Requirements
This study was performed in accordance with ethical standards, according to the Declaration of Helsinki, and according to national and international guidelines. The University of Valencia ethics committee in animal experimentation approved the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.05.005.
Contributor Information
Elena Obrador, Email: elena.obrador@uv.es.
José M. Estrela, Email: jose.m.estrela@uv.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Riley P.A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 2.Reisz J.A., Bansal N., Qian J., Zhao W., Furdui C.M. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21:260–292. doi: 10.1089/ars.2013.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavragani I.V., Laskaratou D.A., Frey B., Candéias S.M., Gaipl U.S., Lumniczky K., et al. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review Toxicol Res (Camb) 2016;5:12–33. doi: 10.1039/c5tx00222b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patyar R.R., Patyar S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur J Pharmacol. 2018;819:207–216. doi: 10.1016/j.ejphar.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Fischer N., Seo E.-J., Efferth T. Prevention from radiation damage by natural products. Phytomedicine. 2018;47:192–200. doi: 10.1016/j.phymed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Sirerol J.A., Feddi F., Mena S., Rodriguez M.L., Sirera P., Aupí M., et al. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic Biol Med. 2015;85:1–11. doi: 10.1016/j.freeradbiomed.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Cantó C., Sauve A.A., Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med. 2013;34:1168–1201. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riklis E., Kol R., Marko R. Trends and developments in radioprotection: the effect of nicotinamide on DNA repair. Int J Radiat Biol. 1990;57:699–708. doi: 10.1080/09553009014550871. [DOI] [PubMed] [Google Scholar]
- 9.Kurkjian C.J., Guo H., Montgomery N.D., Cheng N., Yuan H., Merrill J.R., et al. The Toll-Like Receptor 2/6 Agonist, FSL-1 Lipopeptide, Therapeutically Mitigates Acute Radiation Syndrome. Sci Rep. 2017;7:17355. doi: 10.1038/s41598-017-17729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams J.P., Brown S.L., Georges G.E., Hauer-Jensen M., Hill R.P., Huser A.K., et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estrela J.M., Mena S., Obrador E., Benlloch M., Castellano G., Salvador R., et al. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J Med Chem. 2017;60:9413–9436. doi: 10.1021/acs.jmedchem.6b01026. [DOI] [PubMed] [Google Scholar]
- 13.Wani T.A., Shah A.G., Wani S.M., Wani I.A., Masoodi F.A., Nissar N., et al. Suitability of Different Food Grade Materials for the Encapsulation of Some Functional Foods Well Reported for Their Advantages and Susceptibility. Crit Rev Food Sci Nutr. 2016;56:2431–2454. doi: 10.1080/10408398.2013.845814. [DOI] [PubMed] [Google Scholar]
- 14.Obrador E., Salvador R., Marchio P., López-Blanch R., Jihad-Jebbar A., Rivera P., et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1 G93A Mice. Mol Neurobiol. 2020:1–27. doi: 10.1007/s12035-020-02188-7. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer P., Asensi M., Segarra R., Ortega A., Benlloch M., Obrador E., et al. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y., Sun H., Xiao J., Wang F., Ding Y., Zhao J., et al. Development of a liquid chromatography-tandem mass spectrometric (LC-MS/MS) method for simultaneous determination of epigallocatechin-3-gallate, silibinin, and curcumin in plasma and different tissues after oral dosing of Protandim in rats and its application in pharmacokinetic and tissue distribution studies. J Pharm Biomed Anal. 2019;170:54–62. doi: 10.1016/j.jpba.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Yoshino J, Imai S. Accurate Measurement of Nicotinamide Adenine Dinucleotide (NAD+) with High-Performance Liquid Chromatography. In: Hirschey MD, editor. Sirtuins: Methods and Protocols, Totowa, NJ: Humana Press; 2013, p. 203–15. 10.1007/978-1-62703-637-5_14. [DOI] [PMC free article] [PubMed]
- 18.M’kacher R, El Maalouf E, Terzoudi G, Ricoul M, Heidingsfelder L, Karachristou I, et al. Detection and automated scoring of dicentric chromosomes in nonstimulated lymphocyte prematurely condensed chromosomes after telomere and centromere staining. Int J Radiat Oncol Biol Phys 2015;91:640–9. 10.1016/j.ijrobp.2014.10.048. [DOI] [PubMed]
- 19.Pantelias G.E., Maillie H.D. A simple method for premature chromosome condensation induction in primary human and rodent cells using polyethylene glycol. Somatic Cell Genet. 1983;9:533–547. doi: 10.1007/BF01574257. [DOI] [PubMed] [Google Scholar]
- 20.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 21.Berry M.N., Friend D.S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton AM. Fractionation of T and B cells using magnetic beads. Curr Protoc Immunol 2003;Chapter 3:Unit 3.5A. 10.1002/0471142735.im0305as57. [DOI] [PubMed]
- 23.Estrela J.M., Hernandez R., Terradez P., Asensi M., Puertes I.R., Viña J. Regulation of glutathione metabolism in Ehrlich ascites tumour cells. Biochem J. 1992;286(Pt 1):257–262. doi: 10.1042/bj2860257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graves C.L., Harden S.W., LaPato M., Nelson M., Amador B., Sorenson H., et al. A method for high purity intestinal epithelial cell culture from adult human and murine tissues for the investigation of innate immune function. J Immunol Methods. 2014;414:20–31. doi: 10.1016/j.jim.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newsholme P., Gordon S., Newsholme E.A. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J. 1987;242:631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crain P.F. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990;193:782–790. doi: 10.1016/0076-6879(90)93450-y. [DOI] [PubMed] [Google Scholar]
- 27.Benlloch M., Obrador E., Valles S.L., Rodriguez M.L., Sirerol J.A., Alcácer J., et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid Redox Signal. 2016;24:974–990. doi: 10.1089/ars.2015.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obrador E., Valles S.L., Benlloch M., Sirerol J.A., Pellicer J.A., Alcácer J., et al. Glucocorticoid receptor knockdown decreases the antioxidant protection of B16 melanoma cells: an endocrine system-related mechanism that compromises metastatic cell resistance to vascular endothelium-induced tumor cytotoxicity. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asensi M., Sastre J., Pallardó F.V., García de la Asunción J., Estrela J.M., Viña J. A high-performance liquid chromatography method for measurement of oxidized glutathione in biological samples. Anal Biochem. 1994;217:323–328. doi: 10.1006/abio.1994.1126. [DOI] [PubMed] [Google Scholar]
- 30.Elliott G.C., Ajioka J., Okada C.Y. A rapid procedure for assaying nicotinamide phosphoribosyltransferase. Anal Biochem. 1980;107:199–205. doi: 10.1016/0003-2697(80)90512-6. [DOI] [PubMed] [Google Scholar]
- 31.Schuster S., Penke M., Gorski T., Petzold-Quinque S., Damm G., Gebhardt R., et al. Resveratrol differentially regulates NAMPT and SIRT1 in Hepatocarcinoma cells and primary human hepatocytes. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Seo K.-H., Yokoyama W. Chemistry of Pterostilbene and Its Metabolic Effects. J Agric Food Chem. 2020;68:12836–12841. doi: 10.1021/acs.jafc.0c00070. [DOI] [PubMed] [Google Scholar]
- 33.Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 34.McCart E.A., Lee Y.H., Jha J., Mungunsukh O., Rittase W.B., Summers T.A., et al. Delayed Captopril Administration Mitigates Hematopoietic Injury in a Murine Model of Total Body Irradiation. Sci Rep. 2019;9:2198. doi: 10.1038/s41598-019-38651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paixão E.M.S., Gonzalez M.C., Nakano E.Y., Ito M.K., Pizato N. Weight loss, phase angle, and survival in cancer patients undergoing radiotherapy: a prospective study with 10-year follow-up. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-00799-w. [DOI] [PubMed] [Google Scholar]
- 36.Chini C.C.S., Guerrico A.M.G., Nin V., Camacho-Pereira J., Escande C., Barbosa M.T., et al. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res. 2014;20:120–130. doi: 10.1158/1078-0432.CCR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiou Y.-S., Tsai M.-L., Nagabhushanam K., Wang Y.-J., Wu C.-H., Ho C.-T., et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 38.Raina K., Agarwal C., Agarwal R. Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-κB signaling. Mol Carcinog. 2013;52:195–206. doi: 10.1002/mc.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bürkle A. Poly(APD-ribosyl)ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett. 2001;163:1–5. doi: 10.1016/s0304-3835(00)00694-7. [DOI] [PubMed] [Google Scholar]
- 40.Weidele K., Beneke S., Bürkle A. The NAD+ precursor nicotinic acid improves genomic integrity in human peripheral blood mononuclear cells after X-irradiation. DNA Repair (Amst) 2017;52:12–23. doi: 10.1016/j.dnarep.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Tang J., Lu L., Liu Y., Ma J., Yang L., Li L., et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J Cell Biochem. 2019;120:9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Ma Z, Xu L, Zhang X, Qiao S, Yuan J. PGC1α activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging (Albany NY) 2019;11:10061–73. 10.18632/aging.102418. [DOI] [PMC free article] [PubMed]
- 43.Wang G., Ren X., Yan H., Gui Y., Guo Z., Song J., et al. Neuroprotective Effects of Umbilical Cord-Derived Mesenchymal Stem Cells on Radiation-Induced Brain Injury in Mice. Ann Clin Lab Sci. 2020;50:57–64. [PubMed] [Google Scholar]
- 44.Cheng Y., Di S., Fan C., Cai L., Gao C., Jiang P., et al. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis. 2016;21:905–916. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Ye Z., Lai W., Rao J., Huang W., Zhang X., et al. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front Pharmacol. 2017;8:178. doi: 10.3389/fphar.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaipa J.M., Starkuviene V., Erfle H., Eils R., Gladilin E. Transcriptome profiling reveals Silibinin dose-dependent response network in non-small lung cancer cells. PeerJ. 2020;8 doi: 10.7717/peerj.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharapov M.G., Novoselov V.I., Penkov N.V., Fesenko E.E., Vedunova M.V., Bruskov V.I., et al. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic Biol Med. 2019;134:76–86. doi: 10.1016/j.freeradbiomed.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Castillo J., Benavente-García O., Lorente J., Alcaraz M., Redondo A., Ortuño A., et al. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (Procyanidins) from grape seeds (Vitis vinifera): comparative study versus other phenolic and organic compounds. J Agric Food Chem. 2000;48:1738–1745. doi: 10.1021/jf990665o. [DOI] [PubMed] [Google Scholar]
- 49.Obrador E., Salvador R., Villaescusa J.I., Soriano J.M., Estrela J.M., Montoro A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines. 2020;8 doi: 10.3390/biomedicines8110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrot L., Jones C., Perez P., Meunier J.-R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 51.Ha Y.M., Chung S.W., Kim J.M., Kim D.H., Kim J.Y., Lee E.K., et al. Molecular activation of NF-kappaB, pro-inflammatory mediators, and signal pathways in gamma-irradiated mice. Biotechnol Lett. 2010;32:373–378. doi: 10.1007/s10529-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 52.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cantó C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batra V., Kislay B. Mitigation of gamma-radiation induced abasic sites in genomic DNA by dietary nicotinamide supplementation: metabolic up-regulation of NAD(+) biosynthesis. Mutat Res. 2013;749:28–38. doi: 10.1016/j.mrfmmm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Trammell S.A.J., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obrador E., Salvador-Palmer R., Jihad-Jebbar A., López-Blanch R., Dellinger T.H., Dellinger R.W., et al. Pterostilbene in Cancer Therapy. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuli H.S., Mittal S., Aggarwal D., Parashar G., Parashar N.C., Upadhyay S.K., et al. Path of Silibinin from diet to medicine: A dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Elsherbini A.M., Sheweita S.A., Sultan A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr Cancer. 2020:1–9. doi: 10.1080/01635581.2020.1817513. [DOI] [PubMed] [Google Scholar]
- 59.Ruiz M.J., Fernández M., Picó Y., Mañes J., Asensi M., Carda C., et al. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem. 2009;57:3180–3186. doi: 10.1021/jf803579e. [DOI] [PubMed] [Google Scholar]
- 60.Fraschini F., Demartini G., Esposti D. Pharmacology of Silymarin. Clin Drug Investig. 2002;22:51–65. doi: 10.2165/00044011-200222010-00007. [DOI] [Google Scholar]
- 61.Ye Y., Liu Z. Management of Amanita phalloides poisoning: A literature review and update. J Crit Care. 2018;46:17–22. doi: 10.1016/j.jcrc.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Marinescu A.G., Chen J., Holmes H.E., Guarente L., Mendes O., Morris M., et al. Safety Assessment of High-Purity, Synthetic Nicotinamide Riboside (NR-E) in a 90-Day Repeated Dose Oral Toxicity Study, With a 28-Day Recovery Arm. Int J Toxicol. 2020;39:307–320. doi: 10.1177/1091581820927406. [DOI] [PubMed] [Google Scholar]
- 63.Dollerup O.L., Christensen B., Svart M., Schmidt M.S., Sulek K., Ringgaard S., et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108:343–353. doi: 10.1093/ajcn/nqy132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.