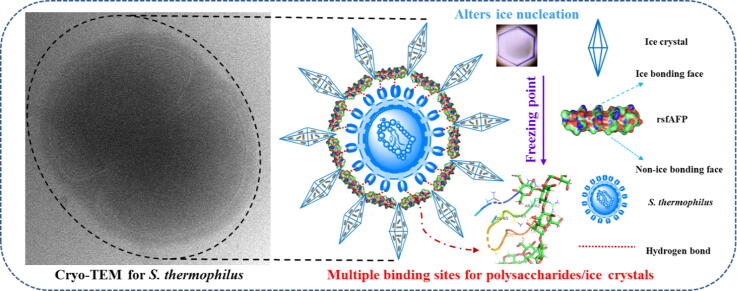
Graphical abstract
Keywords: Antifreeze peptide, Streptococcus thermophilus, Physiological function, Membrane structure, Cryoprotective mechanism
Highlights
-
•
This study provides new insights into the mechanism by which antifreeze peptides regulate cell physiological functions and apoptosis under freezing stress.
-
•
A novel interaction model between frozen cells and ice crystals with antifreeze peptide intervention was revealed.
-
•
This study provides a scientific basis for the use of an antifreeze peptide as an effective cryopreservation agent for LAB and other microbes.
Abstract
Introduction
Antifreeze peptides regulate the physiological functions of frozen cells and even their apoptosis; however, the mechanisms by which antifreeze peptides regulate these processes remain unclear, although the interactions between cell membranes and ice are well known to be important in this process.
Objectives
Our study aims to investigate how antifreeze peptides regulate cell physiological functions during the freezing process.
Methods
We investigated the cryoprotective effect of rsfAFP on the physiological functions of S. thermophilus under freezing stress by measuring cellular metabolism activity, intracellular enzyme activity, cell membrane characterization, and cell apoptosis. The mechanism by which rsfAFP impacts S. thermophilus physiological functions under freezing stress was investigated using multispectral techniques and cryo-TEM.
Results
We show that a recombinant antifreeze peptide (rsfAFP) interacts with the extracellular capsular polysaccharides and peptidoglycan of Streptococcus thermophilus and ice to cover the outer layer of the membrane, forming a dense protective layer that regulates the molecular structure of extracellular ice crystals, which results in reduced extracellular membrane damage, depressed apoptosis and increased intracellular metabolic activity. This interaction mechanism was indicated by the fact that S. thermophilus better maintained its permeability barrier, membrane fluidity, membrane structural integrity, and cytoplasmic membrane potential during freezing stress with rsfAFP treatment.
Conclusion
These results provide new insights into the mechanism by which rsfAFP regulates frozen cell physiological functions and apoptosis under freezing stress.
Introduction
The increasing variety of viruses in the human environment, such as influenza and coronavirus (COVID-19), requires the development of more antiviral vaccines. Currently, some studies have shown that an oral vaccine against coxsackievirus A16 (CVA16) has been developed by using safe food bacteria such as Lactococcus lactis [1]. However, cryopreservation agents will be inevitably used for the development of lyophilized oral vaccines when the cells need to be freeze-dried. In fact, cryopreservation of living cells and tissues is fundamental in modern biology, food, agriculture and medicinal research [2]. Lactic acid bacteria (LAB) are important microorganisms in the modern biological and food industries. Due to the fermentation characteristics and functions of probiotics [3], LAB play an increasingly important role in food fermentation and processing in industry. Moreover, LAB have been developed for vaccinal, medical and technological use as mucosal delivery vehicles that allow the expression of heterologous proteins and serve as various delivery systems and oral vaccine carriers [4]. In industry, lactobacilli are usually prepared into freeze-dried powder, which is convenient for preserving the functional stability of the LAB strain [5]. However, as ice crystals form on Lactobacillus cells at low temperature or during the freeze-drying process, antifreeze agents generally must be added to increase the activity of the bacteria in the freeze-dried powder [6]. At present, the antifreeze agents that can be used for cell cryopreservation mainly include glycerol, trehalose, skim milk, and sucrose. However, these antifreeze agents cannot regulate the formation of ice crystals during recrystallization or thawing, which is important because the mechanical stress damage caused by these processes is even greater than that caused by the freezing process [7] and decreases the recovery of all cells [8]. Moreover, organic solvents, such as glycerol and DMSO, are potentially cytotoxic and might irreversibly damage cells [9]. Therefore, innovative cryopreservation methods and cryoprotective agents need to be investigated.
Extremophilic organisms have evolved many strategies that enable them to survive in subzero environments, which may inspire new biomimetic strategies to improve cryopreservation [10], [11]. For example, arctic fish species produce a variety of antifreeze proteins (AFPs) that help arctic fish adapt to cold growing environments [12]. AFPs are specialized proteins that can inhibit the growth and recrystallization of ice, a phenomenon called thermal hysteresis (TH), and influence ice crystal morphology [13]. Moreover, AFPs can decrease the freezing points of solutions in a noncollagative manner due to the surface coverage of AFPs, which allows them to be used at a much lower concentration than other cryoprotectants [14]. Liu et al. (2016) reported that the ice nucleation temperature decreased significantly when the AFPs coverage was approximately 80% and increased when the coverage was less than 60% and higher than 90% [15]. Because of these desirable properties, AFPs can be used as cryoprotectants for living cells, tissues and organs as well as frozen foods [16]. However, the mechanism by which AFPs regulate cell physiological function in the frozen state is still unclear. To determine this mechanism, it is necessary to clarify how AFPs interact with ice, cells and both ice and cells and to explore the cryoprotection of cells by AFPs at the molecular level.
The snow flea AFP isolated from Canadian Hypogastrura harveyi was shown to be a hyperactive insect AFP, as its TH activity is one or two orders of magnitude greater than that of most AFPs found in fishes and plants [14]. The snow flea AFP has the characteristic of the Janus effect in structure; that is, it has both an ice-binding surface and a non-ice-binding surface. Due to the specificity of its structure, the snow flea AFP exhibits excellent recrystallization inhibitory activity [17], [18]. AFPs, especially the snow flea AFP, are widely used in cell cryopreservation, which protects the cellular integrity and physiological functions of LAB [19]. Nevertheless, to our knowledge, it is still unclear how the snow flea AFP binds to cells and binding sites. Considering the above, the aim of this study was to provide new insights into the mechanism by which the snow flea AFP regulates cell physiological functions under freezing stress. To this end, we first overexpressed and then purified recombinant snow flea antifreeze peptide (rsfAFP) in recombinant E. coli BL21 (DE3). We then investigated (1) the cryoprotective effect of this peptide on the physiological functions of S. thermophilus during frozen stress; (2) the mechanisms by which rsfAFP interacts with these cells and ice; and (3) the mechanism by which rsfAFP impacts S. thermophilus apoptosis under cold stress. This study offers insights into the mechanism by which rsfAFP regulates the physiological functions of living cells under freezing stress by binding extracellular capsular polysaccharides and peptidoglycan and thus coating the outsides of cells and provides a scientific basis for the use of this AFP as an effective cryopreservation agent for LAB or the lyophilized oral vaccines based on LAB for the benefit of humankind.
Material and methods
Materials
The E. coli BL21 (DE3) expression strain was purchased from MoBiTec (Göettingen, Germany). The pET28a plasmid cloning vector was purchased from Genscript Biotechnology Co., Ltd. (Nanjing, China). The restriction endonucleases XbaI and XhoI and DNA purification kits were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The S. thermophilus that was used throughout the study was obtained from Fuzhou University (Fuzhou, China). Other analytical reagents used in this work were purchased from Sigma (USA).
Thermal hysteresis activity (THA)
The THA of rsfAFP was quantified using an 8500 DSC (PerkinElmer, America), and the detailed procedures were as described previously by Wu et al. [20]. The enthalpy of melting (ΔHm) and exothermic enthalpy of refreezing (ΔHr) were calculated from the crystallization peak. The THA was calculated using Eq. (1).
| (1) |
where T0 is the initial freezing temperature calculated from the cold crystallization peak and Th is the holding temperature.
The fraction (φ) in the sample was estimated using Eq. (2).
| (2) |
Ice recrystallization inhibition (IRI) activity
The IRI activity of rsfAFP was determined using a polarizing optical microscope with a cold stage (Linkam Scientific Instruments Ltd. Tadworth, UK) attached to an Olympus BX41 microscope equipped with a CCD camera (Roper Scientific, USA) as described previously by Wang et al. [21]. Briefly, a small drop (3 μL) of pH 7.4 TBS buffer (5 mM Tris-HCl, 150 mM NaCl) with or without rsfAFP was placed between two circular glass cover slips. The cover slips were placed in the chamber of the cold stage and then rapidly cooled from room temperature to −50 °C at a rate of 10 °C/min and held for 5 min. The microscopic image of the sample at −50 °C was recorded, and then the temperature was gradually increased from −50 °C to −14 °C at a rate of 5 °C/min. The temperature was then held at −14 °C for 1 min and increased to −12 °C at a rate of 1 °C/min. Afterward, the samples were cycled 2 times between −14 °C and −12 °C at a rate of 1 °C/min. Ice crystal growth was monitored and photographed at the end of the 2 cycles using a CCD camera.
Nanoliter osmometer experiments
The morphologies of single ice crystals were analyzed with a nanoliter osmometer (Otago Osmometers Ltd., Dunedin, New Zealand) with a temperature precision of 0.01 °C. The temperature controller chamber was dehydrated with dry purified nitrogen (99.99%, 25 °C) throughout the manipulating procedure. The detailed procedures were as described previously by Geng et al. [22]. Briefly, a drop (1 nL) of sample was injected into an immersion oil B-filled sample well using a microscope, quickly cooled to −20 °C, and then slowly warmed to the melting temperature. Once a single ice crystal appeared, it was maintained for approximately 20 s, and the temperature was recorded as the melting temperature (Tm). Then, the temperature slowly decreased to the specific value (Tf) at which ice started to grow. This procedure was recorded by a high-speed camera (Zeiss, Stemi 2000).
Low-field nuclear magnetic resonance imaging
To determine the effects of rsfAFP on the melting of a frozen solution, 0.5 mg/mL solutions of rsfAFP were prepared in distilled water and 5 mM TBS or 20 mM TBS. Distilled water was used as a negative control, and glycerol (10%, w/w) and arginine (1.0 mg/mL, Arg) were used as positive controls. Glass tubes (inner diameter = 15 mm) holding 6.0 mL of each solution were frozen in a −20 °C freezer overnight for NMR microimaging experiments. The melting rates of the frozen solutions were determined, and the detailed procedures were as described previously by Li et al. [23].
S. thermophilus cryopreservation
The cryopreservation of S. thermophilus was performed according to the method described by Li et al. [23]. The concentration of rsfAFP used for cryoprotection was 0.5 mg/mL (Fig. S1). Commercial cryoprotectants (1 mg/mL sucrose, 1 mg/mL skim milk, and 10% glycerol (v/v)) were used for comparisons with rsfAFP, and 20 mM PBS was used as a negative control because it has no cryoprotective effect. The bacterial suspensions with or without cryoprotectant were frozen at −20 °C for 24 h and thawed once every 2 h during the period (2 times in total).
S. thermophilus cell viability
The cryoprotective activity of rsfAFP was analyzed using the method described by Chen et al. [7]. Briefly, 50 µL of each cell suspension (106 CFU) before and after freezing was incubated in 4 mL of M17 broth at 200 rpm and 37 °C for 7 h, after which the bacterial concentration was determined by measuring the OD600 using a UV–Vis spectrophotometer (UV-2600, UNICO Instrument Co. Ltd., Shanghai, China). The relative survival rate is expressed as ODA/ODB × 100, where ODA is the number of viable cells after freezing stress, and ODB is the number of viable cells before freezing.
Cell metabolic activity
After the freezing stress treatment with or without cryoprotectants described in the section of this paper entitled ‘S. thermophilus cryopreservation’, the acidification activity of S. thermophilus was investigated according the method of Meneghel et al. [24]. After thawing, the cells were washed twice and resuspended in 50 mM potassium phosphate buffer (pH 7.4). A 4 mM 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT) solution was added to the cell suspensions for a final concentration of 2 mM. Then, the bacterial suspensions were incubated at 37 °C for 2 h. The reduction of the colorless compound INT to red formazan was detected by measuring the absorbance at 584 nm.
Intracellular enzyme activity
After cryopreservation, the S. thermophilus samples were thawed at 37 °C for 10 min, and the cells were harvested by centrifugation (6,000 rpm, 10 min) after being washed twice with PBS. Cell-free extracts (CFEs) were prepared from the resuspended cells according to the method previously published by Wang et al. [25]. The activities of β-galactosidase, lactic dehydrogenase (LDH), pyruvate kinase (PK) and hexokinase (HK) were measured by using assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) as described by the manufacturer.
Cell membrane characterization
The cell membrane potential was determined using the cell-permeant lipophilic cationic dye RH123 [26], the cell membrane fluidity was determined using the fluorescent dye DPH according to the method described by Chen et al. [7], and the intracellular level of calcium ions with or without freezing was determined using Fluo-3/AM according to the method described by Leri et al. [27]. The membrane integrity of S. thermophilus cells was investigated with PI/CFDA fluorescent dyes (Sigma, China). Briefly, S. thermophilus samples were thawed at 37 °C for 10 min and harvested by centrifugation (6,000 rpm, 10 min). The harvested cells were washed twice with PBS (20 mM, pH 7.4) and resuspended in an equal volume of PBS to an approximate concentration of 107 CFU/mL. PI (1.496 mM) and CFDA (0.460 mM) were added, and the suspensions were incubated at 37 °C for 15 min in the dark. Fluorescence emission spectra (excitation, 470 nm; emission, 490 ∼ 670 nm; bandwidth, 10 nm) were recorded using an F-4500 fluorescence spectrofluorometer (Hitachi, Japan) at room temperature. The membrane integrity was calculated as the ratio of CFDA fluorescence (integrated intensity between 510 and 540 nm) to PI fluorescence (integrated intensity between 580 and 620 nm). These ratios were normalized to the value for the unfrozen cells, which were assigned a membrane integrity of 100%.
Flow cytometric analysis of S. thermophilus cell apoptosis
After the freezing treatment described in the section of this paper entitled ‘S. thermophilus cryopreservation’, S. thermophilus samples were thawed at 37 °C for 10 min and harvested by centrifugation (6,000 rpm, 10 min). The harvested cells were washed twice with PBS (20 mM, pH 7.4) and resuspended in an equal volume of PBS to an approximate concentration of 107 CFU/mL. Ten microliters of annexin V-FITC was first added to a cell suspension and incubated at 40 °C for 20 min in the dark. Subsequently, 10 μL of PI (50 μg/mL dissolved with 20 mM PBS) was added to the mixture and incubated at 40 °C for 10 min in the dark prior to the fluorescence measurement using a FACSort flow cytometer (BD Biosciences, Mountain View, CA, USA). Fluorescence emission spectra (excitation 470 nm, emission 490 ∼ 670 nm, BW 5 nm) were recorded, and the cell apoptosis was analyzed using Cell Quest 3.0 software (Becton Dickinson, Franklin Lakes, NJ, USA).
DNA fragmentation resulting from apoptotic signaling cascades after cold stress was detected with a TUNEL staining kit purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Furthermore, caspase activation was determined by flow cytometry after the cells had been stained with activated caspases using the CaspACE assay system (FITC-VAD-FMK, Sangon Biotech Co., Ltd, Shanghai, China).
Cryogenic transmission electron microscopy (cryo-TEM)
S. thermophilus cells were prepared for cryo-TEM according to the method described previously by Li et al. [23]. Cell solutions (2.5 μL) were dripped into Quantifoil holey carbon TEM grids (orthogonal, 300 mesh, copper). The excess liquid was then blotted, and the grids were vitrified in liquid ethane using a Vitrobot (FEI). These samples were then imaged on a Talos F200C G2 TEM instrument (FEI). The images were captured under low-dose conditions using a bottom-mount CCD camera (Ceta 4 K*4K). The concentration of rsfAFP used in the cryoprotective measurements was 0.5 mg/mL, and 20 mM PBS was used as a negative control.
Raman spectral analysis
An aqueous cell suspension (4 μL) was used to obtain the Raman spectra of ice and rsfAFP before and after crystallization. Each solution was sealed with a coverslip, rapidly cooled to −80 °C by an optical closed-cycle helium cryostat at a rate of 10 °C/min and then held for 20 min. Raman spectra of the samples at −50, −20, 0 and 25 °C were obtained using a confocal Raman microscope (TESCAN-MAIA3, Czech Republic). Laser light at 532 nm was transmitted to the microscope through a single fiber for excitation. The laser power at the objective was 8 mW, and a 100× objective lens (NA = 0.75) was used. The focal length was 4.6 mm, and the confocal pinhole diameter was 120 µm (equivalent to 1.2 µm in diameter). The longitudinal resolution (FWHM) was less than 12 µm, and the lateral resolution (PSF) was less than 2 µm. The spectral scanning range was 500–4000 cm−1.
Molecular docking simulations
The 3D structures of the snow flea AFP (2PNE) and a capsular polysaccharide from the lactobacillus cellular capsule (1CAP) were obtained from the Protein Data Bank. The structure of peptidoglycan in the lactobacillus cellular wall was drawn in GaussView 5.0. The interactions between rsfAFP and the polysaccharide were studied using SYBYL-X 2.1.1 software (Tripos Inc., St. Louis, USA). Their force field and charges were set as Tripos and Gasteiger-Hückel, the threshold and bloat were set as 0.5 and 0 A, respectively, and other parameters were set at their default values.
Data analysis
All experiments were conducted using at least three different technical replicates. Data analysis was performed using SPSS 17.0 (SPSS, Chicago, IL, USA), and data are reported as the mean ± standard deviation from at least three to four independent experiments. Statistical significance was determined by Duncan’s multiple range tests (P < 0.05).
Results
Expression, purification, and confirmation of rsfAFP
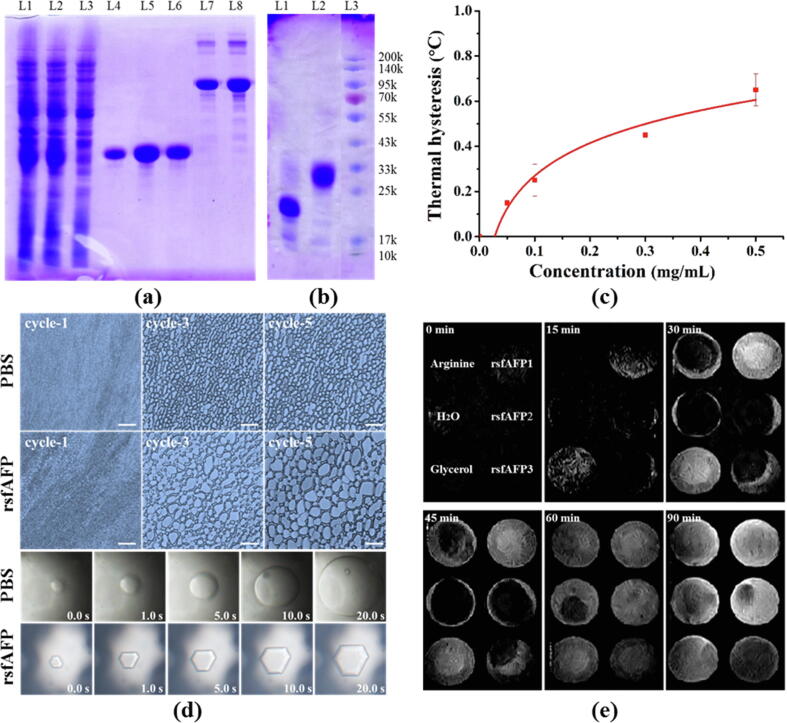
The DNA coding sequence of the snow flea AFP was synthesized and cloned into the pET28a-SUMO vector (Fig. S2). The protein rsfAFP was successfully expressed (1.5 g/L) with an N-terminal 6 × His-SUMO tag from the pET28a-SUMO vector in E. coli BL21 (DE23) upon induction with IPTG (Fig. 1a). As shown in Fig. 1b, SDS–PAGE analysis clearly showed a 27-kDa fusion product of the target protein (6 × His-SUMO-rsfAFP) and SUMO tag from the transgenic strain (the molecular weight of the 6 × His-SUMO tag is 20 kDa). The band in the SDS–PAGE gel containing the fusion protein was excised and in-gel digested with trypsin. Nano LC–MS/MS analysis identified the unique rsfAFP sequence CKGADGAHGVNGCPGTAGAAG-SVGGPGC- DGGHGGNGGNGNPGCAGGVGGAGGASGGTGVGGRGG********GADGAPGAP (Fig. S3), indicating that the target protein had been successfully expressed. The target protein (rsfAFP) was obtained from the fusion protein after digestion by SUMO protease and purification by RP-HPLC (Fig. S4).
Fig. 1.
Expression and identification of recombinant snow flea antifreeze peptide (rsfAFP) in E. coli BL21 (DE3). (a) Target protein purification profile. Lanes 1–2: loaded sample; lane 3: flow through; lanes 4–6: fractions eluted with 20 mM TBS buffer with 20, 50, and 250 mM imidazole, respectively; lanes 7–8: 2 μg and 4 μg BSA (66.4 kDa), respectively; (b) Enzymatic hydrolysis of fusion proteins with SUMO protease. L1: SUMO; L2: SUMO-rsfAFP; L3: Marker; (c) Thermal hysteresis activity of rsfAFP at different concentrations; (d) Optical images show the completely different growth behaviors and shapes of ice crystals with or without the addition of 0.5 mg/mL rsfAFP in PBS (20 mM, pH 7.4). The scale is 100 μm; (e) Time-dependent NMR microimaging of frozen aqueous solutions during melting as labeled in the top left frame. Solutions containing rsfAFP at concentration of 0.5 mg/mL dissolved in 20 mM TBS (labeled rsfAFP1 in the figure), 5 mM TBS (labeled rsfAFP2) and pure water (labeled rsfAFP3) are shown. Pure water was used as the negative control, and 1.0 mg/mL arginine and 10% glycerol were used as positive controls. In the proton density images, black represents solid ice, and white represents areas with high densities of mobile water.
Freezing point depression and the inhibition of ice growth and recrystallization are the main functions of snow flea AFPs [14]. TH activity, ice recrystallization inhibitory (IRI) activity, and single ice crystal growth and morphology were investigated to verify the existence of functioning rsfAFPs after recombinant expression. As shown in Fig. 1c, TH activity was positively associated with concentration. The presence of rsfAFP reduced the ice crystal size (Fig. 1d). Moreover, the ice crystals in PBS had a flat, disk-like shape, and the ice grew so rapidly that the observation window was occluded by ice within 20 s. Interestingly, ice crystal growth in a rsfAFP aqueous dispersion was different from that in pure water in two ways (Fig. 1d). First, the ice crystals were hexagonal; second, the ice crystals in the rsfAFP aqueous dispersion grew much slower than those in pure water, suggesting that rsfAFP inhibited ice growth. We obtained proton LF-NMR images from transverse sections of the frozen aqueous solutions containing rsfAFP (dissolved in different buffers) after they had melted for various durations. The ice melted significantly faster with the addition of rsfAFP than in the commercial cryoprotectant groups and negative group (Fig. 1e). Collectively, these results clearly show that rsfAFP is a typical hyperactive AFP.
Cytoprotective effects of rsfAFP on the physiological functions of S. thermophilus under freezing stress
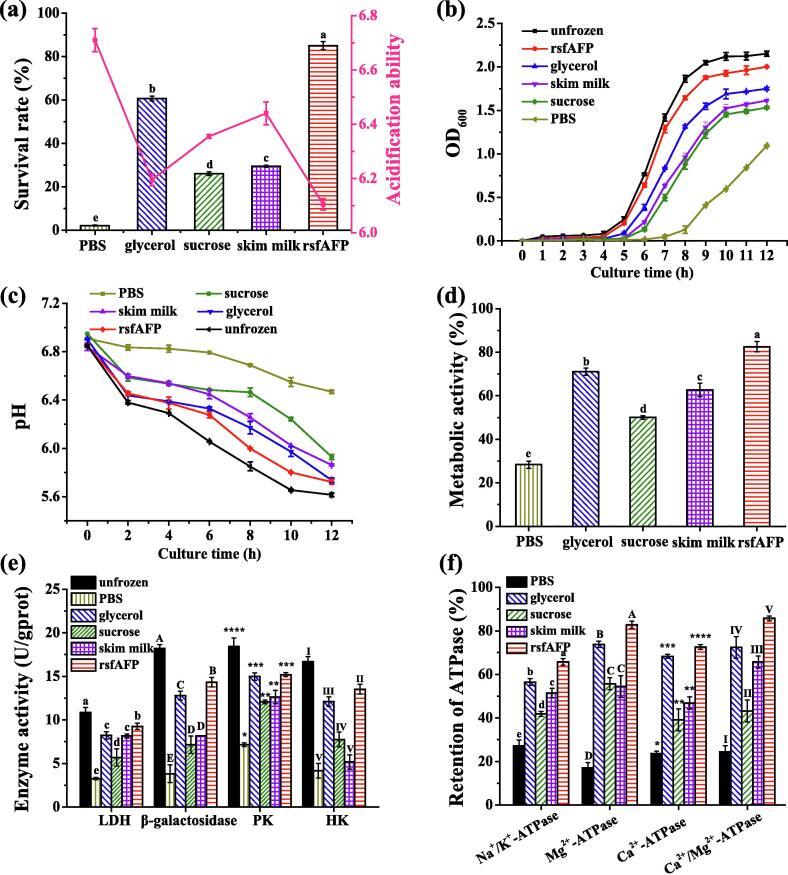
Cryopreservation is one of the most commonly used techniques for the preservation of LAB, but mechanical damage caused by ice crystals during the preservation process damages LAB, reducing their vitality and function. To determine the cryoprotective capacity of various cryoprotectants, the effects of different cryoprotectants on the viability and acidification ability of S. thermophilus cells exposed to freeze stress were tested (Fig. 2a). The survival rate and culture pH were negatively associated and significantly greater under the cryoprotection of rsfAFP than under commercial cryoprotectants (Fig. 2a). After freezing treatment, the PBS (negative control) samples showed an obvious growth lag (Fig. 2b). In addition, the acidification ability of S. thermophilus was significantly reduced compared to that without freezing (Fig. 2c). This shows that freezing stress treatment caused damage to the cells that they do not naturally repair, decreasing cell physiological function in terms of all tested metrics. However, the addition of cryoprotectants reduced the damage caused by freezing stress to varying degrees and maintained the physiological functions of S. thermophilus cells after resuscitation. Compared with the control treatment, rsfAFP most reliably maintained the physiological functions of S. thermophilus cells during the thawing process. In the paragraphs below, we detail the cryoprotective effects on various indicators of physiological function.
Fig. 2.
Viability of S. thermophilus cells after freezing at −20 °C for 24 h and 2 freeze–thaw cycles with various cryoprotectants. (a) Survival rate and culture pH of S. thermophilus after incubation for 7 h; (b) growth curve of S. thermophilus; (c) acidification ability of S. thermophilus; (d) S. thermophilus metabolic activity with various cryoprotectants; (e) effects of various cryoprotectants on the activity of lactic dehydrogenase (LDH), β-galactosidase, pyruvate kinase (PK) and hexokinase (HK); (f) effects of cryoprotectants on ATPase in S. thermophilus under cold stress. Different letters on columns indicate significant different values (P < 0.05).
The metabolic activity of S. thermophilus cells is related to the activities of key intracellular enzymes, including lactate dehydrogenase (LDH), β-galactosidase, pyruvate kinase (PK) and hexokinase (HK). The reduction of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT), an artificial electron receptor, by dehydrogenases and reductases after cryopreservation of S. thermophilus was one method we used to evaluate the metabolic activity, and the results were consistent with the experiment to deduce the acidification function of S. thermophilus (Fig. 2d). The metabolic activity of the cells in the PBS group after thawing was only 28.33 ± 1.67% that of the unfrozen cells, while the metabolic activity of S. thermophilus in the rsfAFP group was 82.54 ± 2.33%, which was significantly (P < 0.05) higher than those observed with the commercial cryoprotectants sucrose (50.04% ± 0.79%), skim milk (62.65% ± 3.08%) and glycerin (71.06% ± 1.60%). Therefore, the addition of rsfAFP to the solution during the freezing process increased the metabolic activity of S. thermophilus that was retained after thawing compared to that with other cryoprotectants.
The intracellular activities of LDH, β-galactosidase, PK and HK in S. thermophilus cells after thawing were significantly decreased compared with those of unfrozen cells (Fig. 2e). However, the activities of these enzymes were improved to varying degrees by rsfAFP and commercial cryoprotectants. Among the groups, the enzyme activities of the rsfAFP group were closest to those of the unfrozen cell group. Thus, these findings suggest that rsfAFP maintains cell metabolism by protecting the intracellular activities of specific enzymes in S. thermophilus cells under freezing.
In addition to the metabolic enzymes described above, membrane proteins in S. thermophilus play important roles in maintaining cell metabolism. Specifically, ATPase is an important physiological component that helps maintain the ion gradient and membrane potential; Ca2+-ATPase and Mg2+-ATPase regulate intracellular Ca2+ and Mg2+ concentrations, respectively; and Na+-K+-ATP maintains high extracellular Na+ and high intracellular K+ concentrations and catalyzes the hydrolysis of ATP to provide energy [28]. Here, to investigate the relationship between these membrane proteins and the cell metabolism of S. thermophilus, we measured the activities of these membrane proteins. The activities of Na+-K+-ATPase, Ca2+-ATPase and Mg2+-ATPase in S. thermophilus cells treated with different cryoprotectants were higher than those in S. thermophilus cells in the PBS group (Fig. 2f). Importantly, the ATPase activity of the rsfAFP group was also considerably higher than that of the commercial cryoprotectant groups. These results indicate that rsfAFP may maintain the steady-state balance of intracellular and extracellular ion concentrations under freezing stress by preserving ATPase activity to improve the physiological functions of S. thermophilus.
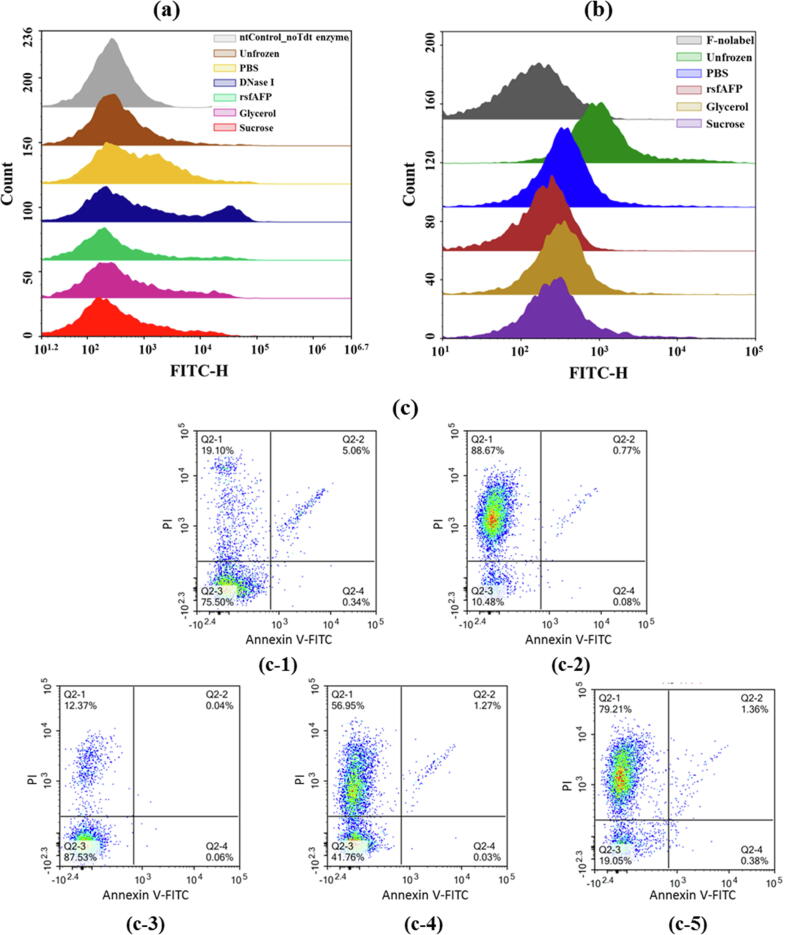
To determine whether apoptosis was induced by the cell membrane injury that occurred under freezing stress, S. thermophilus cell apoptosis was analyzed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and FITC-VAD-FMK analyses. The TUNEL (Fig. 3a) and FITC-VAD-FMK (Fig. 3b) analyses suggested that some S. thermophilus cell apoptosis occurred under freezing stress but it had no significant influence on physiological functions. Moreover, under the same freezing treatment conditions, rsfAFP inhibited the apoptosis of S. thermophilus cells more strongly than commercial cryoprotectants. However, the cells resuspended in PBS and stored at 4 °C for 7 days exhibited significant apoptosis (Table S1).
Fig. 3.
Effects of cryoprotectants on S. thermophilus apoptosis after freezing stress. (a) TUNEL staining; (b) FITC-VAD-FMK staining; (c) Annexin V-FITC/PI staining. (c-1) No cold-stress treatment and treatment with (c-2) 20 mM PBS (negative control), (c-3) 0.5 mg/mL rsfAFP, and (c-4) 10% (v/v) glycerol and (c-5) 1.0 mg/mL sucrose (positive controls). Q2-1: necrotic cells; Q2-2: late apoptotic cells; Q2-3: viable cells; Q2-4: early apoptotic cells.
The mechanical stress caused by ice crystals in the frozen state is the main cause of the decline in the physiological function of S. thermophilus after resuscitation. The cryoprotectants had significantly different effects on the viability of S. thermophilus cells (Fig. 3c). The recovery rates of the cells treated with commercial cryoprotectants after freezing stress were less than 45%. Compared with the PBS (negative control) group, the rsfAFP group showed an increase in the percentage of living cells from (12.18 ± 2.08)% to (87.12 ± 0.66)%. A significant 7.2-fold increase in the percentage of viable cells was observed in the rsfAFP group compared with the negative control group, and a 2.0-fold increase was observed in the rsfAFP group compared with the glycerol group (Table S2). Collectively, these results indicate that S. thermophilus showed no obvious apoptosis under freezing stress and instead mainly underwent necrosis caused by mechanical stress.
Influence of rsfAFP on the membrane structure and properties of S. thermophilus during freezing
Cell death often occurs due to the mechanical damage caused by ice crystals during freezing [29]. Therefore, we investigated the rsfAFP cryoprotective mechanism by determining the influence of rsfAFP on the membrane structure of S. thermophilus under freezing conditions.
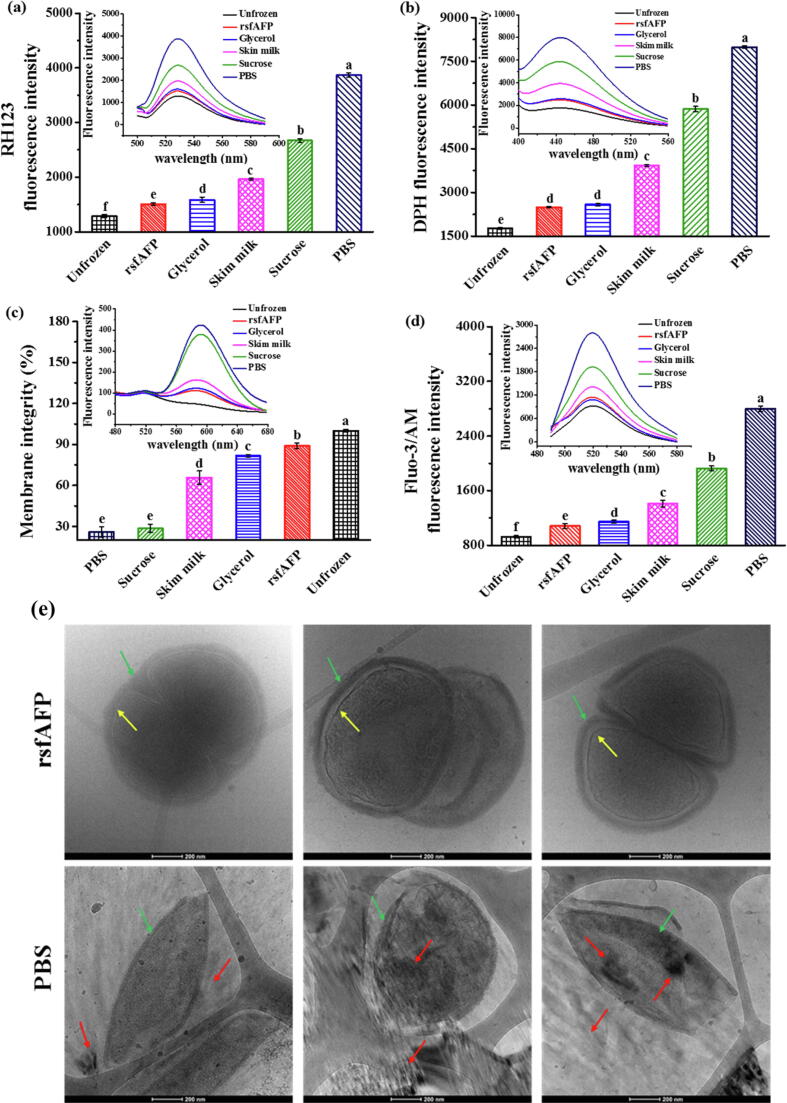
First, the cell membrane potential of S. thermophilus after freezing stress in the presence of different cryoprotectants was analyzed by the cell membrane lipid-soluble cationic fluorescent dye RH123, which has a fluorescence intensity that is positively correlated with cell membrane potential [26]. Freezing stress leads to an increase in the cell membrane potential, thereby opening potassium channels. The outflow of potassium ions leads to further membrane hyperpolarization and enhancement of the probe fluorescence. The fluorescence intensities of all groups after freezing stress treatment were increased compared to those of the unfrozen cells, and the fluorescence intensity of the PBS (negative control) group was the highest (Fig. 4a). This result indicated that the cell membrane potential increased after freezing treatment, but the membrane potential of the rsfAFP group was the closest to that of the unfrozen cells. Thus, rsfAFP helps maintain the normal permeability of cell membranes and ion concentration homeostasis to maintain the metabolic activity of S. thermophilus cells during cryopreservation. Moreover, the fluorescence intensity of DPH decreased obviously after freezing treatment, indicating decreased cell membrane fluidity, especially that of the negative control group (Fig. 4b). Furthermore, the cell membrane fluidity of the rsfAFP group was significantly higher than those of the groups supplemented with other cryoprotectants and the negative control group.
Fig. 4.
Cellular membrane properties of S. thermophilus after freezing at −20 °C for 24 h and 2 freeze–thaw cycles with different cryoprotectants. (a) RH123 probe fluorescence intensity indicating the cell membrane potential of S. thermophilus; (b) DPH fluorescence intensity indicating the cell membrane fluidity of S. thermophilus; (c) PI/CFDA fluorescence intensity indicating the cell membrane integrity of S. thermophilus; (d) Fluo-3/AM fluorescence intensity representing the intracellular calcium ion concentration; (e) Cryo-TEM of S. thermophilus cells treated with or without 0.5 mg/mL rsfAFP. The green, yellow and red arrows indicate cytoderm, cytomembrane and ice crystals, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Various physicochemical events occur when cells are exposed to freezing stress. The cellular membrane is sensitive to low temperatures and is the primary target of damage. Therefore, the membrane integrity of S. thermophilus cells was investigated by PI/CFDA fluorescent dyes. The membrane integrity of S. thermophilus cells treated with different cryoprotectants was higher than that of cells in the PBS group (Fig. 4c). Importantly, the membrane integrity of cells in the rsfAFP group was also considerably higher than those of the commercial cryoprotectant groups and closest to the unfrozen group. The mechanical damage caused by ice crystals is often cell membrane damage, which disrupts the intracellular Ca2+ balance. Therefore, we also analyzed the intracellular calcium ion concentration, which is positively correlated with the fluorescence intensity of the Fluo-3/AM fluorescent probe. The fluorescence intensity changed the least upon the addition of rsfAFP compared to the other cryoprotectants and negative control and was similar to the fluorescence level of the cells without freezing treatment (Fig. 4d). These results indicate that rsfAFP had a significant protective effect on S. thermophilus and that the physical parameters of the cell membrane were maintained under freezing stress.
Damage to the cell membrane caused by mechanical stress is irreversible. To better understand the changes in cell membranes caused by freezing stress, we conducted scanning electron microscopy (SEM) studies. The SEM results showed that the cells were disrupted and shriveled after cold stress and surrounded by intracellular components that had leaked from the cell (Fig. S5). However, the rsfAFP group showed the opposite result: the S. thermophilus cells were plump, and the cell surface was smooth and intact. This may have occurred because the mechanical stress caused by ice crystals caused the cell membrane to rupture, leading the cell contents to leak and deposit on the cell surface to form a continuous substrate [30], and the mechanical stress of S. thermophilus cells was reduced in the presence of rsfAFP.
To further investigate the mechanism by which rsfAFP interacts with the membrane of S. thermophilus cells, we first observed isolated S. thermophilus incubated with or without rsfAFP in situ via cryo-TEM. As shown in Fig. 4e, cells without rsfAFP exhibited serious rupturing and cellular deformation. An easily recognized cytoskeleton and typical cell structure were clearly observed for the samples treated with 0.5 mg/mL rsfAFP. This is the first report that clearly shows the whole-cell structure of S. thermophilus cells under freezing conditions with AFPs and ice crystals. Furthermore, compared to the control cells, the ice crystal content around the cells treated with rsfAFP was obviously lower, the ice crystals were smaller, and the cytoderm was significantly thicker. A layer of material seemed to be wrapped around the cytoderm, from which we inferred that rsfAFP bound the cell wall by interacting with molecules on the membrane, thus covering the cell surface and forming a protective layer to prevent the formation of large ice crystals. Since S. thermophilus can secrete extracellular capsular polysaccharides [31], we hypothesized that the interaction between rsfAFP and capsular polysaccharides leads to the formation of a tight membrane structure that protects the bacteria from damage caused by freezing. This result corroborates the results of previous studies on the physiological functions of cell membranes, which showed that rsfAFP effectively maintained the permeability and structural integrity of cell membranes, preventing cellular leakage caused by ice crystal-induced mechanical damage.
Investigation of the interaction of rsfAFP with S. thermophilus under freezing stress
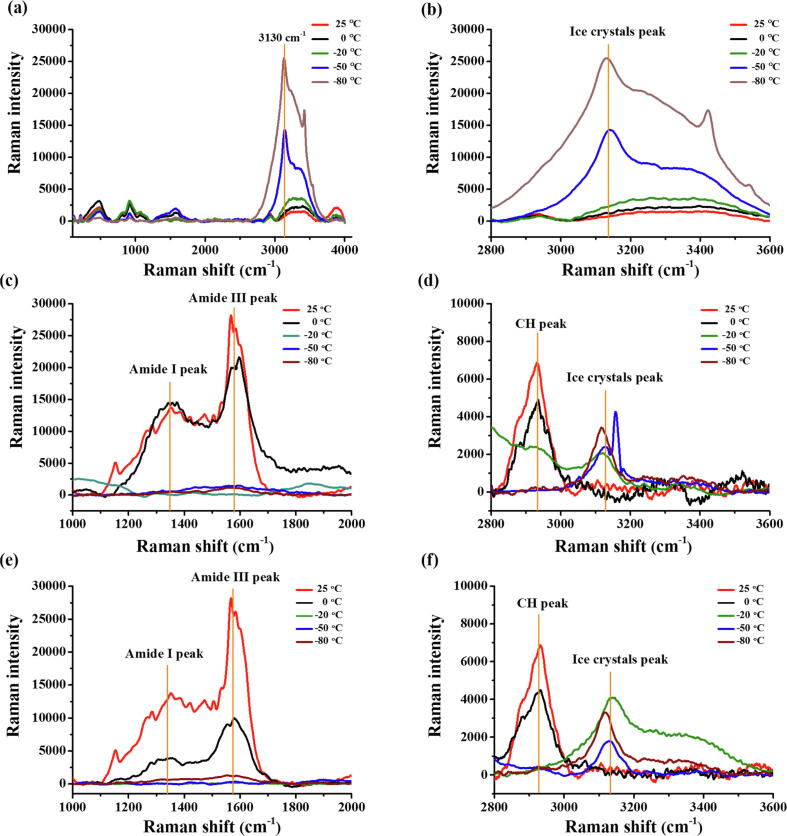
To assess whether rsfAFP reduces ice crystal-induced mechanical damage, we used Raman spectroscopy. Raman spectroscopy is a fast in situ sampling method for determining the structural variation of proteins and hydrates, so in situ Raman spectroscopy was employed to study the interactions between rsfAFPs and the extracellular ice of S. thermophilus. An ice Raman peak (3130 cm−1) appeared after the bacterial PBS suspension was frozen, and a crystallization peak (3430 cm−1) appeared when the temperature was below −20 °C (Fig. 5a, b), which can be used as an indicator of intracellular ice [32]. Furthermore, the stretching vibration peaks of the amide band (1200–1800 cm−1) and CH band (2840–3000 cm−1) typical of organic matter [33] became weaker or disappeared with decreasing temperature (Fig. 5c-f). During freezing, AFPs bind ice crystals by their hydrophobic groups, and hydrophobic groups repel liquid water molecules [15]. When the protein solution froze, the amide band disappeared, indicating that the molecular vibrations were limited because the protein had adsorbed on the ice crystals (Fig. 5c, d). In addition, the extracellular ice Raman signal from the sample containing S. thermophilus and rsfAFP (Fig. 5f) was significantly weaker than that of the control group (Fig. 5b), in which no intracellular ice Raman signal was observed.
Fig. 5.
In situ Raman spectroscopy analysis for the interaction between rsfAFP and extracellular ice at different temperatures. (a) and (b) Raman spectra of S. thermophilus suspended in PBS; (c) and (d) Raman spectra of 0.5 mg/mL rsfAFP; (e) and (f) S. thermophilus suspended in 0.5 mg/mL rsfAFP.
Based on cryo-TEM and Raman spectroscopy analyses, we speculated that rsfAFP may bind the extracellular capsular polysaccharides of S. thermophilus or peptidoglycan in the cell wall in some way. Therefore, the interaction between rsfAFP and cellular polysaccharides was further analyzed by using Fourier transform infrared spectroscopy (FTIR). By analyzing the FTIR spectra of rsfAFP and either peptidoglycan or cellular polysaccharides at different ratios, it was found that the presence of peptidoglycan (Fig. S6a) or capsular polysaccharides (Fig. S6b) changed the amide band spectrum of the protein. A new peak at 859 cm−1 appeared after rsfAFP interacted with peptidoglycan, and the signal intensity increased as the concentration of peptidoglycan increased (Fig. S6a). Otano et al. [34] reported that N-H and N in nucleic acids can form hydrogen bonds to produce characteristic infrared peaks at 859 cm−1. Therefore, we speculated that the new peaks (859.88 cm−1 and 859.03 cm−1) are likely to indicate hydrogen bonding between the N-H in rsfAFP and the N+ in peptidoglycan (Fig. S6a). The peak from 1038 cm−1 to 1058 cm−1 is characteristic of C-O in bacterial polysaccharides [35]. The peak undergoes a blueshift when the ratio of rsfAFP to polysaccharide increases from 0:1 to 1:1 but undergoes a redshift when this ratio decreases. Moreover, as the ratio of rsfAFP to capsular polysaccharide increased, the C-O peak intensity increased, indicating an interaction between capsular polysaccharide and rsfAFP that reduces the vibration frequency of C-O. This result confirmed the previous speculation that rsfAFP binds to the extracellular capsular polysaccharides of S. thermophilus or peptidoglycan in the cell wall.
Isothermal titration calorimetry (ITC) can measure the thermodynamic characteristics of biomolecules during the binding process and is used to analyze the interaction between biomolecules [36]. Therefore, we further analyzed the binding thermodynamic properties of rsfAFP to S. thermophilus cells by ITC. The results (Fig. S7) showed that the rsfAFP and S. thermophilus solution was exothermic during the titration process (ΔH < 0), indicating that rsfAFP and S. thermophilus had binding behavior, and the binding process generated heat. With the progress of the titration, the generated heat gradually decreased, the titration inflection point appeared at the sixth drop, and the heat release tended to 0 μJ/s after the seventh drop, indicating that the rsfAFP binding sites were saturated. Fig. S7b was obtained by fitting the binding process curve with Launch NanoAnalyze software. Although the binding constant of rsfAFP and S. thermophilus could not be obtained because the molar concentration of the S. thermophilus cell suspension could not be calculated, the fitted curve showed that the titration process of rsfAFP and S. thermophilus conformed to the SSIS model. In conclusion, the results of the ITC experiments proved that there is an interaction between rsfAFP and S. thermophilus cells and that rsfAFP can bind to S. thermophilus cells.
Molecular docking to simulate the interactions between rsfAFP and cellular polysaccharides
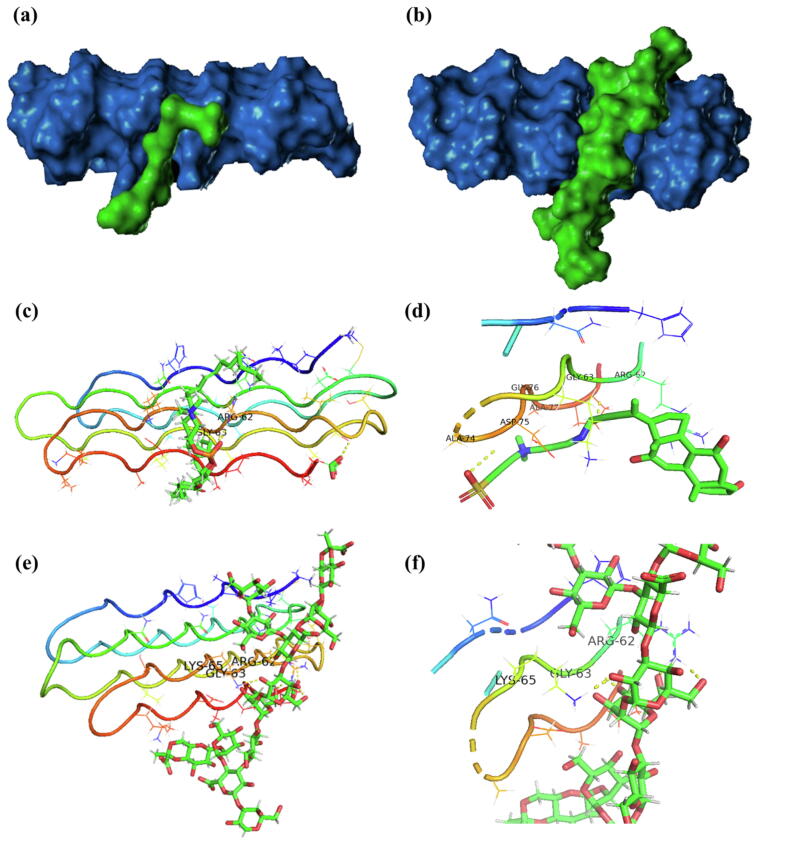
The positions of potential binding sites for different polysaccharides on rsfAFP are depicted in Fig. 6. The two polysaccharides bound a similar site on rsfAFP that is characterized by a wide and large groove. In the models, the bound polysaccharides differ in both orientation and conformation, as they selectively interact with specific binding site residues. Fig. 6 depicts the potential binding modes. Two hydrogen bonds were formed between peptidoglycan and rsfAFP (GLY63 and ASP75). Several hydrophobic interactions of peptidoglycan with the residues ARG62, ALA74, GLY76 and ALA77 in rsfAFP were also observed. In contrast, three hydrogen bonds were formed between the lactobacillus capsular polysaccharide and residues ARG62, GLY63 and LYS65 of rsfAFP. Thus, there are multiple hydrogen binding sites for polysaccharides on the surface of the hydrophilic helix, especially the binding site provided by GLY63 and LYS65. This observation suggested that rsfAFP predominantly binds cellular polysaccharides via hydrogen bonds and hydrophobic interactions.
Fig. 6.
Molecular docking simulations showing interactions of peptidoglycan and capsular polysaccharide (PDB: 1CAP) with rsfAFP (PDB: 2PNE). Visualization of the structure of rsfAFP and potential binding sites for (a) peptidoglycan and (b) capsular polysaccharide in rsfAFP. (c) General overview (left) and (d) magnified view (right) of the docking between rsfAFP and peptidoglycan; (e) general overview (left) and (f) magnified view (right) of the docking between rsfAFP and a capsular polysaccharide.
Discussion
Cryopreservation is one of the most commonly used techniques for the preservation of LAB, but mechanical damage caused by ice crystals during the preservation process damages LAB, reducing their vitality and function [7]. Given this, there is a continuing need for new cryoprotectants to investigate innovative cryopreservation methods. AFPs are proteins/peptides that regulate ice structure and promote the cryopreservation of live cells, which can reduce freezing damage and increase survival rates for cells under freezing conditions by regulating the ice crystal structure around cells during the freezing process [37]. However, the effects of AFPs on cell membrane structure, physiology and metabolism by regulating ice crystal formation during the freezing process has remained unclear.
Thus, to address this gap in knowledge, we aimed to investigate the mechanism by which rsfAFP regulates cell physiological functions and apoptosis under freezing stress. In this study, rsfAFP, an AFP with a defined structure and function, was recombinantly expressed, purified, and used to cryoprotect S. thermophilus. We found that rsfAFP inhibited the growth of ice crystals and caused them to adopt a hexagonal shape in frozen solution. Other studies have pointed out that AFPs bind a single ice crystal, forming a hexagonal unit by binding a specific ice crystal plane, which leads to the creation of a curved ice front on that plane between bound AFPs through the Gibbs-Thomson effect. Further adsorption of water on such an ice front is energetically unfavorable, which thus inhibits crystal growth [38]. Furthermore, we found that rsfAFP had significant THA, i.e., it lowered the freezing point and accelerated the melting of ice crystals into liquid water under low-temperature freezing conditions, thus shortening the freeze–thaw time of cells, which could decrease freezing-induced damage in frozen cells. The THA of AFPs is closely related their effect on the structure of ice crystals; it lowers the nonequilibrium freezing temperature (Tf) and slightly elevates the equilibrium melting temperature (Tm) of AFP solutions, resulting in a difference between Tf and Tm, which causes TH. Furthermore, the measured glass transition temperatures within cell suspensions showed that rsfAFP increased the vitrification transition temperature of S. thermophilus suspensions from −30.1 °C to −22.9 °C (Fig. S8), and, as a previous study showed, the vitrification transition temperature plays an important role in protecting cells at low temperatures [30]; this finding implies that rsfAFP could cryoprotect S. thermophilus by adjusting the vitrification transition temperature of S. thermophilus cell suspensions. Vitrification in cell cryoprotection is advantageous; for example, vitrification at a high subzero temperature can make cells osmotically unresponsive at all temperatures down to the Tg of the extracellular solution, which can decrease intracellular damage [39]. These results imply that rsfAFP has strong antifreeze activity and can be applied for the cryoprotection of microbes and similar applications in industry.
Furthermore, the structure-based cell death mechanism of the cryoprotective effect of rsfAFP on S. thermophilus was evaluated through analyses of the physiological function, membrane structure, and apoptotic signaling pathways of S. thermophilus. First, the results showed that rsfAFP maintained the growth of S. thermophilus through freezing treatment, decreased its acidification ability, and maintained the metabolic activities of LDH, β-galactosidase, PK and HK, which are key cell enzymes closely related to the physiological functions of cells, to a significantly greater extent than other cryoprotectants. rsfAFP also significantly reduced the activity of key enzymes in the membrane involved in ion transport: Na+-K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase. Moreover, through staining with fluorescence probes (RH123, DPH, and Fluo-3/AM) and SEM, we found that rsfAFP maintained the integrity of the cell membrane structure, reduced cell membrane potential and fluidity changes, and maintained normal membrane permeability and ion concentration gradients during freezing; thus, the metabolic activity of the cells was well maintained due to the cryoprotective effect of rsfAFP under freezing stress.
We further investigated the effects of cell death-triggering stress in S. thermophilus with rsfAFP intervention under freezing stress. Previous research has shown that the cell death of prokaryotes, such as Escherichia coli, is almost the same as eukaryotic apoptosis, which exhibits DNA fragmentation, phosphatidylserine exposure and chromosome condensation, which are characteristic features of apoptosis [40]. Thus, to determine the antiapoptotic effect of rsfAFP on S. thermophilus under freezing stress, TUNEL staining was performed, and cells treated with rsfAFP or glycerol showed weak TUNEL staining, as shown in Table S1. The results showed that the percentage of TUNEL-positive cells was only (5.02 ± 2.42)% in the rsfAFP treatment group, which was significantly lower than that in the other treatment groups. This result implies that the fragmentation of S. thermophilus DNA was inhibited upon treatment with rsfAFP. Moreover, the effect of extracellular phosphatidylserine exposure on S. thermophilus undergoing cell death was also analyzed, and the results indicated that the percentage of annexin V-positive cells, i.e., those with exposed phosphatidylserine, in the rsfAFP treatment was only 0.04%, which was significantly lower than that in the other treatment groups. These findings are the first to suggest that the typical features of eukaryotic apoptosis also occurred in S. thermophilus under freezing stress. In summary, the above experimental results confirmed that rsfAFP can effectively preserve the physiological and biochemical functions of S. thermophilus by reducing damage to cell membranes during freezing.
While this conclusion was made based on the effects of rsfAFP, we also wanted to determine the mechanism responsible for these effects. Thus, we investigated how rsfAFP directly interacts with the cell membrane of S. thermophilus. We evaluated the structure of S. thermophilus cells and their interactions with rsfAFP under freezing treatment by cryo-TEM. The cryo-TEM images clearly show that rsfAFP covered the outer layer of S. thermophilus cells to form a dense protective layer that inhibited the propagation of ice through the cell wall, which slowed ice crystal growth outside the cells and inhibited ice recrystallization and nucleation in the extracellular and intracellular regions of S. thermophilus cells. This phenomenon of rsfAFP covering the outer layer of cells has not been described in previous studies and could provide a clear, structure-based explanation for how rsfAFP maintains the structural integrity and physiological function of cell membranes under freezing stress. Furthermore, we elucidated the ability of rsfAFP to cover the outer layer of S. thermophilus cells. In situ Raman spectroscopy showed that the stretching vibration peaks of the amide band decreased or disappeared during freezing and that the signals for extracellular ice (3130 cm−1) and intracellular ice (3430 cm−1) decreased in intensity. The change in amide vibrations combined with the cryo-TEM data indicated that rsfAFP may react with extracellular components by hydrogen bonding or other molecular interactions. Moreover, ITC analysis further confirmed the interaction between rsfAFP and S. thermophilus cells.
Considering that LAB such as S. thermophilus have a capsular structure and contain capsular polysaccharides, peptidoglycans and other components [31], we speculated that rsfAFP may interact with these components. Therefore, the interaction between an AFP and these components was further investigated by using molecular dynamics simulations. The overall structure of the peptide is helical, and the peptide is divided into a hydrophilic side and a hydrophobic side. The inner side of the helix on the hydrophilic side of the peptide mainly has GLY as the skeleton, and hydrophilic amino acid residues such as LYS and HIS are evenly distributed on the outer side. The helical spacing coincides with the ice cell spacing [17]. Multiple binding sites for polysaccharides/ice crystals are present on the surface of rsfAFP. Among them, GLY63 and LYS65 provide the strongest binding sites on the helix. On the hydrophobic-side surface, VAL and ALA are evenly distributed, forming the hydrophobic surface. When the protein binds a polysaccharide, the polysaccharide site is occupied and can likely no longer bind an ice crystal; as a result, the hydrophobic surface can prevent ice crystals from growing from the polysaccharide.
Conclusion
In summary, this study clearly reveals that rsfAFP exhibits antifreeze activity and can interact with the extracellular capsular polysaccharides and peptidoglycan of S. thermophilus as well as ice crystals, regulate the molecular structure of ice crystals, cover the outer layers of S. thermophilus cell membranes to form protective dense layers that preserve membrane structural integrity, and maintain the normal physiological functions of the cells. In this manner, rsfAFP improved the survival rate of S. thermophilus cells under freezing stress. This study elucidated the mechanism of the cryoprotective activity of rsfAFP under freezing stress and provides a scientific basis for the use of this AFP as an effective cryopreservation agent for LAB and other microbes.
Author contributions
Xu Chen and Jinhong Wu conducted the literature research, conceptualized and synthetized information, and wrote the manuscript. Ruibin Wang and Fujia Yang conducted the Raman spectrum analysis. Yuzhi Rong, Mi Zhou and Jianhua Liu conducted the molecular simulation. Jianlian Huang and Shaoyun Wang provided financial support. Shaoyun Wang was responsible for the supervision of the entire manuscript.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support: This work was supported by the National Natural Science Foundation of China (No. 31972017, No. U1905202), Fujian Major Project of Provincial Science & Technology Hall (No. 2020NZ010008) and Xiamen Ocean and Fishery Development Special Fund Project (21CZP006HJ04). We thank Mina Liu from Nanjingjinsirui Science & Technology Biology Corp for her help with gene synthesis. We also thank Tingting Ji from Shanghai Jiao Tong University for her help with the cryo-TEM tests.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.05.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Jiang Z., Tian X., Lu X., Zhong B. An oral vaccine against CVA16 (Coxsackievirus A16) was developed by constructing a recombinant Lactococcus lactis. Trop J Pharm Res. 2020;19(5):927–932. [Google Scholar]
- 2.Woods E.J., Benson J.D., Agca Y., Critser J.K. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48(2):146–156. doi: 10.1016/j.cryobiol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Feng T., Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12(1):1801944. doi: 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pontes D.S., de Azevedo M.S.P., Chatel J.-M., Langella P., Azevedo V., Miyoshi A. Lactococcus lactis as a live vector: Heterologous protein production and DNA delivery systems. Protein Expr Purif. 2011;79(2):165–175. doi: 10.1016/j.pep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Reddy K.B.P.K., Awasthi S.P., Madhu A.N., Prapulla S.G. Role of cryoprotectants on the viability and functional properties of probiotic lactic acid bacteria during freeze drying. Food Biotechnol. 2009;23(3):243–265. [Google Scholar]
- 6.Zachariassen K.E., Kristiansen E. Ice nucleation and antinucleation in nature. Cryobiology. 2000;41(4):257–279. doi: 10.1006/cryo.2000.2289. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Wu J., Li L., Wang S. Cryoprotective activity and action mechanism of antifreeze peptides obtained from Tilapia scales on Streptococcus thermophilus during cold stress. J Agric Food Chem. 2019;67(7):1918–1926. doi: 10.1021/acs.jafc.8b06514. [DOI] [PubMed] [Google Scholar]
- 8.Cao L., Huang Q., Wu Z., Cao D.-D., Ma Z., Xu Q., et al. Neofunctionalization of zona pellucida proteins enhances freeze-prevention in the eggs of Antarctic notothenioids. Nat Commun. 2016;7(1) doi: 10.1038/ncomms12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capicciotti C.J., Kurach J.D.R., Turner T.R., Mancini R.S., Acker J.P., Ben R.N. Small molecule ice recrystallization inhibitors enable freezing of human red blood cells with reduced glycerol concentrations. Sci Rep. 2015;5:9692. doi: 10.1038/srep09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S., Stevens C.A., Vance T.D.R., Olijve L.L.C., Graham L.A., Campbell R.L., et al. Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Sci Adv. 2017;3(8) doi: 10.1126/sciadv.1701440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey R., Usui K., Livingstone R.A., Fischer S.A., Pfaendtner J., Backus E.H.G., et al. Ice-nucleating bacteria control the order and dynamics of interfacial water. Sci Adv. 2016;2(4) doi: 10.1126/sciadv.1501630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVries A.L., Wohlschlag D.E. Freezing resistance in some antarctic fishes. Science. 1969;163(3871):1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- 13.Bar Dolev M., Braslavsky I., Davies P.L. Ice-binding proteins and their function. Annu Rev Biochem. 2016;85(1):515–542. doi: 10.1146/annurev-biochem-060815-014546. [DOI] [PubMed] [Google Scholar]
- 14.Graham L.A., Davies P.L. Glycine-rich antifreeze proteins from snow fleas. Science. 2005;310(5747):461. doi: 10.1126/science.1115145. [DOI] [PubMed] [Google Scholar]
- 15.Liu K., Wang C., Ma J., Shi G., Yao X., Fang H., et al. Janus effect of antifreeze proteins on ice nucleation. Proc Natl Acad Sci USA. 2016;113(51):14739–14744. doi: 10.1073/pnas.1614379114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Wu J., Cai X., Wang S. Production, structure–function relationships, mechanisms, and applications of antifreeze peptides. Compr Rev Food Sci Food Saf. 2021;20(1):542–562. doi: 10.1111/1541-4337.12655. [DOI] [PubMed] [Google Scholar]
- 17.Treviño M.Á., Pantoja-Uceda D., Menéndez M., Gomez M.V., Mompeán M., Laurents D.V. The singular NMR fingerprint of a polyproline II helical bundle. J Am Chem Soc. 2018;140(49):16988–17000. doi: 10.1021/jacs.8b05261. [DOI] [PubMed] [Google Scholar]
- 18.Gates Z.P., Baxa M.C., Yu W., Riback J.A., Li H., Roux B., et al. Perplexing cooperative folding and stability of a low-sequence complexity, polyproline 2 protein lacking a hydrophobic core. Proc Natl Acad Sci USA. 2017;114(9):2241–2246. doi: 10.1073/pnas.1609579114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Wu J., Yang F., Huang D., Huang J., Wang S., et al. Snow flea antifreeze peptide for cryopreservation of lactic acid bacteria. npj Sci Food. 2022;6(1) doi: 10.1038/s41538-022-00128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J., Rong Y., Wang Z., Zhou Y., Wang S., Zhao B.o. Isolation and characterisation of sericin antifreeze peptides and molecular dynamics modelling of their ice-binding interaction. Food Chem. 2015;174:621–629. doi: 10.1016/j.foodchem.2014.11.100. [DOI] [PubMed] [Google Scholar]
- 21.Wang ShaoYun, Damodaran S. Ice-structuring peptides derived from bovine collagen. J Agric Food Chem. 2009;57(12):5501–5509. doi: 10.1021/jf900524y. [DOI] [PubMed] [Google Scholar]
- 22.Geng H., Liu X., Shi G., Bai G., Ma J., Chen J., et al. Graphene oxide restricts frowth and recrystallization of ice crystals. Angew Chem Int Ed. 2017;56(4):997–1001. doi: 10.1002/anie.201609230. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Wu J., Zhang L.i., Chen X., Wu Y., Liu J.-H., et al. Investigation of the physiochemical properties, cryoprotective activity and possible action mechanisms of sericin peptides derived from membrane separation. LWT - Food Sci Technol. 2017;77:532–541. [Google Scholar]
- 24.Meneghel J., Passot S., Dupont S., Fonseca F. Biophysical characterization of the Lactobacillus delbrueckii subsp. bulgaricus membrane during cold and osmotic stress and its relevance for cryopreservation. Applied Microbiology. Biotechnology. 2017;101(4):1427–1441. doi: 10.1007/s00253-016-7935-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Chen M., Wu J., Wang S. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. LWT - Food Sci Technol. 2015;63(2):878–885. [Google Scholar]
- 26.Xi D., Wang X., Teng D., Mao R., Zhang Y., Wang X., et al. Mechanism of action of the tri-hybrid antimicrobial peptide LHP7 from lactoferricin, HP and plectasin on Staphylococcus aureus. Biometals. 2014;27(5):957–968. doi: 10.1007/s10534-014-9768-x. [DOI] [PubMed] [Google Scholar]
- 27.Leri M., Natalello A., Bruzzone E., Stefani M., Bucciantini M. Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ1-42 aggregation. Food Chem Toxicol. 2019;129:1–12. doi: 10.1016/j.fct.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Belli W.A., Marquis R.E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57(4):1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan M., Fayter A.E.R., Gibson M.I. Ice recrystallization inhibiting polymers enable glycerol-Free gryopreservation of microorganisms. Biomacromolecules. 2018;19(8):3371–3376. doi: 10.1021/acs.biomac.8b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Wang S. Cryoprotective effect of antifreeze glycopeptide analogues obtained by nonenzymatic glycation on Streptococcus thermophilus and its possible action mechanism. Food Chem. 2019;288:239–247. doi: 10.1016/j.foodchem.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabhan A., Shah N.P. Structural characterization of exopolysaccharide from Streptococcus thermophilus ASCC 1275. J Dairy Sci. 2020;103(8):6830–6842. doi: 10.3168/jds.2019-17439. [DOI] [PubMed] [Google Scholar]
- 32.Dong J., Malsam J., Bischof J.C., Hubel A., Aksan A. Spatial distribution of the state of water in frozen mammalian cells. Biophys J. 2010;99(8):2453–2459. doi: 10.1016/j.bpj.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okotrub K.A., Surovtsev N.V. Raman scattering evidence of hydrohalite formation on frozen yeast cells. Cryobiology. 2013;66(1):47–51. doi: 10.1016/j.cryobiol.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Otano J., Bui M.-P., Seo S.S. Determination of DNA hybridization on gold nanoparticle conjugated polystyrene particle thin film using attenuated total reflectance fourier transform infrared spectroscopy. Anal Lett. 2014;47(1):167–177. [Google Scholar]
- 35.Lyu W., Shi Y., Zheng Y., Liu X.e. XPS and FTIR studies of fungus-stained Daemonorops margaritae. J For Res. 2019;30(2):739–743. [Google Scholar]
- 36.Zhang S., Xu Y., Guan H., Cui T., Liao Y., Wei W., et al. Biochemical and structural characterization of the BioZ enzyme engaged in bacterial biotin synthesis pathway. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-22360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Shi X., Cai X., Yang F., Li L., Wu J., et al. Ice-binding proteins: a remarkable ice crystal regulator for frozen foods. Crit Rev Food Sci Nutr. 2021;61(20):3436–3449. doi: 10.1080/10408398.2020.1798354. [DOI] [PubMed] [Google Scholar]
- 38.Rahman A.T., Arai T., Yamauchi A., Miura A.i., Kondo H., Ohyama Y., et al. Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci Rep. 2019;9(1) doi: 10.1038/s41598-018-36546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca F., Passot S., Gautier J., Cenard S., Morris J. 135 Measured glass transition temperatures within cells following slow cooling: Implications for cryopreservation and freeze drying. Cryobiology. 2013;67(3):436. doi: 10.1016/j.cryobiol.2013.09.141. [DOI] [Google Scholar]
- 40.Dwyer D., Camacho D., Kohanski M., Callura J., Collins J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012;46(5):561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.