Graphical abstract

Keywords: Mesenchymal stromal/stem cells (MSCs), Metabolism, Immunomodulatory properties, Therapy
Highlights
-
•
Metabolism plays critical roles in the MSCs immunomodulatory properties.
-
•
Manipulation the metabolism in MSCs can enhance their therapeutic effects.
-
•
MSCs can affect the immune cells metabolism and determine their behaviors.
-
•
The more direct evidence of MSCs immunometabolism is needed to be uncovered.
-
•
The mechanism of metabolism influencing immunomodulatory properties in MSCs is unclear.
Abstract
Background
Mesenchymal stromal/stem cells (MSCs) are the most promising stem cells for the treatment of multiple inflammatory and immune diseases due to their easy acquisition and potent immuno-regulatory capacities. These immune functions mainly depend on the MSC secretion of soluble factors. Recent studies have shown that the metabolism of MSCs plays critical roles in immunomodulation, which not only provides energy and building blocks for macromolecule synthesis but is also involved in the signaling pathway regulation.
Aim of Review
A thorough understanding of metabolic regulation in MSC immunomodulatory properties can provide new sights to the enhancement of MSC-based therapy.
Key scientific Concepts of Review
MSC immune regulation can be affected by cellular metabolism (glucose, adenosine triphosphate, lipid and amino acid metabolism), which further mediates MSC therapy efficiency in inflammatory and immune diseases. The enhancement of glycolysis of MSCs, such as signaling molecule activation, inflammatory cytokines priming, or environmental control can promote MSC immune functions and therapeutic potential. Besides glucose metabolism, inflammatory stimuli also alter the lipid molecular profile of MSCs, but the direct link with immunomodulatory properties remains to be further explored. Arginine metabolism, glutamine-glutamate metabolism and tryptophan-kynurenine via indoleamine 2,3-dioxygenase (IDO) metabolism all contribute to the immune regulation of MSCs. In addition to the metabolism dictating the MSC immune functions, MSCs also influence the metabolism of immune cells and thus determine their behaviors. However, more direct evidence of the metabolism in MSC immune abilities as well as the underlying mechanism requires to be uncovered.
Introduction
Stem cells including pluripotent embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and tissue-resident adult stem cells (ASCs), are characterized by the capacities of self-renewal and multipotent differentiation. The application of ESCs and iPSCs is greatly restricted due to the ethical issues and the risk of tumor formation. In this context, ASCs are widely used in stem cell-based therapy for tissue regeneration and repair in the clinic. Mesenchymal stromal/stem cells (MSCs) including the bone marrow, adipose tissue and umbilical cord-derived MSCs are most commonly used in stem cell-based therapy. In addition to differentiating into resident cells to replace the injured tissues, MSCs possess potent immunomodulatory properties to regulate the adjacent cells and empower the tissue repair, which have been well demonstrated in various disease treatments, such as lung injury, arthritis, colitis, acute graft versus host disease (GVHD) et al [1], [2], [3]. In fact, the immunomodulation of MSCs is mainly achieved by autocrine and paracrine secretion including releasing variant immuno-regulating cytokines or extracellular vesicles, which has been reviewed elsewhere [1], [4].
Recent evidence has proven that the immune regulation of MSCs could also be affected by metabolism, which not only provides energy and building blocks for macromolecule synthesis but is also involved in the signaling pathway regulation. Environmental stimuli induce metabolic alteration and subsequently influence the MSC immune capacities and repair abilities in inflammatory and immune diseases. Manipulations of the MSC metabolism, such as gene or enzyme regulation, cytokines priming, or environmental control, serve as promising and feasible targets to enhance MSC immunomodulation and therapeutic effects. Therefore, a thorough understanding of MSC immunometabolism and subsequent explorations of the possible mechanism are prerequisites.
In this review, we comprehensively focus on the glucose, adenosine triphosphate (ATP), and amino acid metabolism in MSC immunomodulatory functions. At the same time, we exhibit how the mentioned metabolisms mediate the MSC therapy efficiency in various inflammatory and immune diseases. Then, we also describe how MSCs regulate the metabolism of the immune cells to dictate their functions in disease therapy. Finally, we discuss the clinical application of MSCs in the regenerative field.
Glucose metabolism
The main energy metabolism is glucose catabolism, of which glycolysis is the extraordinarily critical part, occurring in the cytosol. Following a series of redox reactions, the main terminal products of glycolysis are pyruvate and ATP molecules. In most cell types, the pyruvate can be further broken down into lactate via lactate dehydrogenase (LDH) or into acetyl-CoA via pyruvate dehydrogenase (PDH). The manipulation of these two dehydrogenases to shunt pyruvate into distinct flux determines the stem cell fate [5]. Furthermore, the intermediates in glycolysis can also be shunted into amino acid, lipid and nucleotide synthesis to link the energy metabolism. Recent studies have demonstrated that the enhancement of glycolysis of MSCs elevated their therapeutic effects in diseases including cardiovascular diseases, bone defect disease and ischemic disease by promoting survival after transplantation [6], [7], upregulating the proliferation and differentiation capacities [8] or increasing the secretion of pro-growth factors [9] for tissue regeneration.
Beyond regulating MSC proliferation and differentiation, glycolysis is reported to improve and maintain MSC immune function. The immunomodulatory activation of MSCs is not constitutive but licensed by exogenous stimulation, such as pro-inflammatory cytokines (IFN-γ, TNF-α, LPS) and hypoxia. This enhanced immunomodulatory capacity of MSCs requires a metabolic switch towards glycolysis (reducing the TCA cycle). As demonstrated by R. Contreras-Lopez, MSCs pretreated with oligomycin (a known inhibitor of ATPase and oxidative phosphorylation (OXPHOS) metabolism) exhibited an increment of extracellular acidification rate (ECAR) (an indicator of aerobic glycolysis) and a down-regulated oxygen consumption rate (OCR), which was associated with HIF-1α expression, consequently resulting in inhibition of pro-inflammatory Th1 and Th17 proliferation and increase of the Treg cell population, explaining the therapeutic potentials of MSCs in the Delayed-Type Hypersensitivity (DTH) murine model [10]. Likely, co-culture with peripheral blood mononuclear cells (PBMCs) promoted the glycolytic activity of MSCs, which was accompanied by enhanced suppression of T cell proliferation [11]. Glycolysis augmentation provides an emerging avenue to improve the immunomodulatory properties of MSCs. Strategies that can promote glycolytic metabolism such as signaling molecular regulation, inflammatory factors stimulation and external environmental manipulation facilitate MSC immune properties and thus enhance therapeutic efficiency (Fig. 1).
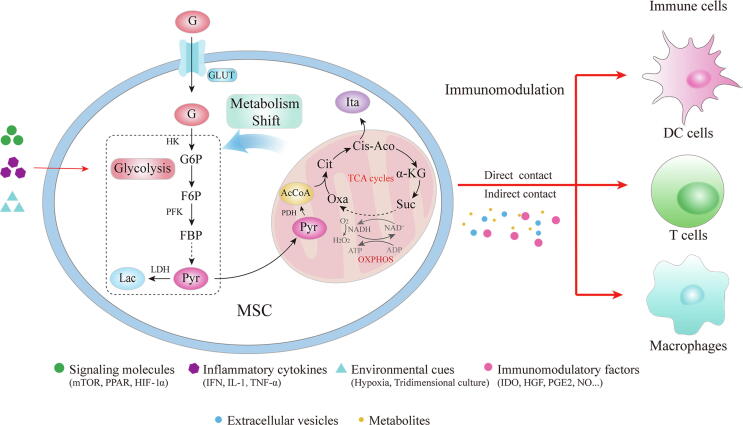
Fig. 1.
Glucose metabolism in MSC regulating immunomodulation. Signaling molecules, inflammatory cytokines and environmental cues can enhance the metabolic shift from OXPHOS to glycolysis in MSCs and thus promote the immunoregulatory capacities. The effect of MSCs on immune cells can be achieved by direct cell–cell contact or releasing metabolites and secretomes including immunomodulatory factors, extracellular vesicles. G, glucose; GLUT, glucose transporters; HK, hexokinase; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; PFK, phosphofructokinase; FBP, fructose bisphosphate; Pyr, pyruvate; Lac, lactate; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; AcCo, acetyl-CoA; Cit, citrate; Cis-Aco, cis-Aconitate; Suc, succinate; Oxa, oxaloacetate; Ita, Itaconate; TCA, tricarboxylic acid.
Signaling molecules regulate glycolysis-associated immunomodulation
Mammalian target of rapamycin (mTOR)
mTOR, an evolutionary conserved serine-threonine kinase serves as a sensor of numerous stimulations such as O2, nutrients, and growth factors. Recently, its role in regulating metabolism to promote MSC immune functions has provoked great interest. Accumulating evidence has revealed that activation of the mTOR signaling pathway facilitates a switch in glucose metabolism from OXPHOS to glycolysis, which enhances MSC immunosuppressive capacities. As clarified by Y. Liu et al, the PI3-kinase/AKT signaling cascade and its downstream effector mTORC1 mediated the metabolic shift to glycolysis in IFN-γ treated MSCs, increasing the production of immune-regulatory factors (indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2)). However, the mTOR inhibitor, rapamycin, which was an immune-suppressive drug applied in the clinic reversed this effect [11]. Additionally, the AMP-activated protein kinase (AMPK), a cellular energy sensor, which supported the ATP supply, served as the upstream of mTOR and was also involved in the immunometabolism regulation of MSCs. Recent research conducted by R. Contreras-Lopez et al demonstrated that AMPK signaling pathway mediating glycolytic reprogramming of MSCs, enhanced the immunosuppressive functions and increased therapeutic benefits in the DTH and the GVHD murine models [12]. Mechanically, mTOR induced glycolysis flux by targeting two critical transcription factors: HIF-1α and myelocytomatosis oncogene (c-Myc). mTORC1 enhanced the abundance of HIF-1α protein under hypoxia and increased the transcriptional activity of HIF-1α by S6K1/S6 and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4EBP1)/eIF4E pathways or by promoting the interaction of HIF-1α with the transcriptional co-activator p300 [13], [14], [15]. HIF-1α promoted glycolysis through activation of glycolytic enzymes (such as glucose transporters (GLUT1) and glucose-6-phosphate transporter (G6PT)) [16] and through inhibition of mitochondrial respiration by targeting pyruvate dehydrogenase kinase 2 (PDK2) and PDK4 to suppress PDH and shunt pyruvate flux to lactate [17]. Using a novel chemically synthesized sphingosine metabolite, o-cyclic phytosphingosine-1-phosphate (cP1P), activated mTOR-HIF-1α signaling pathway, contributing to glycolysis enhancement and therapeutic improvement of MSCs [15]. On the other hand, c-Myc, a potent oncogene, well-known for its ability to boost glycolytic metabolism and glutaminolysis [18], regulated the critical glycolytic genes (GLUT1, LDH, pyruvate kinase type M (PKM) and hexokinase (HK)2) and glutamine transporters proteins, which were required for T cell proliferation and activation. Deletion of Myc inhibited the glycolysis and thus suppressed T cell proliferation [19]. mTOR-Myc axis played critical roles in metabolic associated immunomodulation [20] in immune cells, however, little was known regarding its roles in MSC metabolism regulation and immunomodulation.
Peroxisome proliferator-activated receptor (PPAR)
PPAR family members are nuclear-receptors consisting of PPARα, PPARβ/δ and PPARγ, ubiquitously expressed in MSCs, serving as transcription factors upon ligand activation and initiating cell transcriptional programming. PPAR plays important roles in lipid metabolism. In general, PPARα and PPARβ/δ are responsible for fatty acid oxidation (FAO) through participating in substrate delivery and OXPHOS while PPARγ focuses on lipogenesis [21]. Evidence indicated that PPARγ was identified to block the osteogenic differentiation and promote the adipogenic differentiation of MSCs while PPARβ/δ was found to induce osteoblast differentiation by the Wnt signaling pathway [22]. Not surprisingly, inhibiting the PPARγ pathway in periodontal ligament stem cells (PDLSCs) using the cannabinoid receptor I (CB1) promoted MSC osteogenesis and enhanced their regeneration in periodontitis [23]. Interestingly, B.E. Heck et al exhibited that PPARδ agonist alleviated the osteoarthritis (OA) by enhancing the MSC chondrogenesis and blunting the inflammation. The mix of PPARδ agonist, hyaluronic acid (HA) gel and bone marrow mesenchymal stromal/stem cells (BMSCs) significantly rescued the formation of type II collagen cartilage in the human OA synovial fluid [24].
Additionally, PPAR also regulated glucose metabolism. The emerging research has found that the PPAR affected the glycolysis in MSCs and thus regulated their immunosuppressive properties and therapeutic efficiency [25]. For example, Contreras‑Lopez et al unveiled that PPARβ/δ invalidation switched MSC metabolic state towards glycolysis, which promoted the inhibition of Th1 and Th17 proliferation [26]. Compared with PPARβ/δ+/+ MSCs, the glycolytic-associated factors including the ratio of ECAR to OCR, the glucose consumption, lactate production as well as the GLUT1 expression level significantly elevated in PPARβ/δ-/- MSCs, contributing to immune function enhancement. In line with this, Luz-Crawford et al reported that PPARβ/δ deficiency dictated immunosuppressive and therapeutic properties of MSCs in arthritis through increasing vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1 and nitric oxide (NO) production [3].
Although the critical roles of PPAR in the immune system have been well-reviewed elsewhere [21], the direct evidence of PPAR-mediated MSC immunometabolism (either glucose or lipid metabolism or both) is deficient and further research is needed.
Hypoxia-inducible factor (HIF) −1α
HIF-1α serves as a well-known glycolytic regulator, which is mainly regulated by hypoxia. Upon stimulation, HIF-1α translocates into the nucleus to form a heterodimer, binding to hypoxia-response element (HRE) sequence and targeting glycolytic enzymes as mentioned above [16].
Evidence has proven that HIF-1α played predominant roles in MSC immunomodulation and metabolism. In addition to hypoxia stimulation, R. Contreras-Lopez et al discovered that TNF-α and IFN-γ primed MSCs exhibited a significant increase of HIF-1α expression and nuclear translocation and enhanced therapeutic effects in DTH. They further confirmed that after silencing HIF-1α of MSCs, the inhibitory capacities on Th1 and Th17 cells and the secretion of immunomodulatory factors were significantly diminished, which was also related to the metabolic alteration from glycolysis to OXPHOS [10]. Overexpression of HIF-1α of human dental mesenchymal stromal/stem cells inhibited dendritic cell differentiation and promoted monocytes toward immunosuppressive types with reduced TNF-α and increased IL-10 production [27]. In consistent with this, HIF-1α overexpression also promoted MSC extracellular vesicles (EVs) secretion and immunosuppressive factors (IDO, COX2 and PD-L1) expression. Furthermore, EVs derived from HIF-1α overexpressed MSCs presented better therapeutic effects on DTH murine models, with M1 macrophages decreasing and M2 macrophages increasing [28]. Mechanically, the increased secretion of exosomes in MSCs induced by HIF-1α was attributed to the activation of notch ligand Jagged-1, which was also involved in the regulation of immune cells [29], [30]. Interestingly, a dramatical increase of HIF-1α expression induced by tridimensional (3D) compaction of MSCs [31], which will be discussed below, could contribute to enhanced immunoregulation of MSCs [32]. Taken together, HIF-1α overexpression or induced by hypoxia, inflammation or 3D culture promotes a metabolic shift towards glycolysis and enhances immunomodulatory properties in MSCs.
Inflammatory cytokines enhance immune functions through glycolysis
Interferons (IFNs)
IFNs are polypeptides produced by infected cells, regulating the host antimicrobial defense and orchestrating the immune responses [33]. As well investigated, priming MSCs with IFN notably increased immune functions by inducing IDO, which is an initial rate-limiting enzyme of tryptophan catabolism and a critical immune-regulatory factor in MSCs [34]. Naive MSCs expressed undetectable IDO unless they were induced by IFN-γ and the catabolic activity of this enzyme licensed MSC immunoregulatory effects [1]. The tryptophan depletion and kynurenine (the product of tryptophan by IDO) increase contributed to the immunomodulation of MSCs, especially on T cell suppression [35].
Recently, IFN-enhanced immunomodulatory abilities of MSCs have been reported to be associated with glycolytic metabolism. As shown in a proteomic and metabolomic experiment, MSCs primed with IFN-γ exhibited elevated expression of the glycolytic enzyme (GLUT5 and HK2) and enhanced immune regulatory factors such as IDO, TGF-β, antigen-presenting proteins, and chemokines [36]. The IFN-γ-primed MSCs notably attenuated the symptoms of GVHD and improved survival in the mouse model. To further investigate the signaling pathway, Y. Liu et al discovered that IFN-γ exposure led to ECAR increase but mitochondrial electron transport activity decrease, indicating that the metabolic state of MSCs shifted from OXPHOS to glycolysis [11]. Mechanically, they claimed that IFN-γ activated AKT/mTOR signaling pathway, which was crucial in metabolic reconfiguration from OXPHOS to glycolysis, boosted the IDO and PGE2 expression in MSCs and consequently suppressed T cell proliferation [11]. Besides, another study described that IFN-β induced rapid signal transducer and activator of transcription 1 (STAT1) and STAT3 activation in MSCs and upregulated its downstream hepatocyte growth factor (HGF) expression, mediating T-cell proliferation inhibition. This regulatory process was associated with IFN-β stimulated-mTOR signaling, facilitating glycolytic capacity and concomitantly promoting immune function of MSCs, which might be the mechanism of the experimental autoimmune encephalomyelitis (EAE) treatment [37].
Interleukin-1 (IL-1)
IL-1 is mainly produced by immune cells such as macrophages, neutrophils, dendritic cells and NK cells, which is involved in pro-inflammatory and immunomodulatory actions. As an inflammatory stimulus, unequivocal evidence has shown that pretreatment with IL-1 endowed MSCs with increased immunomodulatory and anti-inflammatory capacities, improving the treatment outcomes. For example, the precondition of IL-1 alone or combined with other inflammatory stimuli increased the production of immunomodulatory factors (IDO-1 and PGE2) or exosomes secretion, enhancing MSC immunomodulatory and anti-inflammatory capacities in osteoarthritis and septic [38], [39], [40], [41].
Besides immune-regulation, IL-1 has been reported to target the glycolytic process by stimulating glucose uptake or activating glycolytic enzyme, which was reviewed elsewhere [42]. For example, both IL-1α and IL-1β were identified to enhance the glucose uptake in different cells, including rheumatoid synovial cells [43], human gingival fibroblasts [44], astrocytic brain cells and human chondrocytes [45]. A recent study discovered that IL-1β enhanced glycolysis in lung adenocarcinoma cells [46]. In contrast, blockage of IL-1 signaling pathway inhibited glycolysis in Th17 cells, alleviating GVHD severity [47]. Mechanically, evidence showed that IL-1β-dependent signal was associated with PI3K/AKT/mTOR signaling pathway [48], which was a regulatory pathway in glycolysis (discussed above). As for stem cells, research has discovered that priming with β-glucan, a prototypical trained-immunity-inducing agonist, induced immune effects of hematopoietic stem cells (HSCs) through enhancing glycolysis, which was mediated by IL-1β [49]. Collectively, given that IL-1 could regulate glycolysis in various cells and IL-1 could enhance MSC immunomodulatory properties, it is possible that IL-1 regulates MSC immunometabolism through glycolysis.
Tumor necrosis factor-α (TNF-α)
Another inflammatory cytokine TNF-α has been widely discussed in the activation of MSC immunomodulation [50] since it was believed to supply the initial stimulus for MSC priming [51]. TNF-α precondition strengthened a plethora of immunomodulatory factors expression of MSCs, including IDO, PGE2, IL-6 [52], [53], NO [54] and exosomes secretion [55], enhancing MSC therapeutic efficiency in colitis and rheumatoid arthritis [52], [56].
Interestingly, these TNF-α-triggering immunomodulation of MSCs could be affected by glycolysis, since recently, studies have reported that TNF-α participated in the regulation of glycolysis. For instance, TNF-α could induce metabolism toward glycolysis, with the increment of HIF-1α and GLUT1 expression through TNF/TAK1/HIF-1α/glycolysis signaling axis [57]. Furthermore, A.H. Remels et al demonstrated that TNF-α activated the NF-κB pathway, promoted HIF-1α activation and thus elevated glycolytic metabolism [58]. Similarly, as discovered by Q. Xu et al, exogenous TNF-α or endogenous TNF-α produced by LPS-stimulated macrophages promoted glycolysis and lactate secretion in human lung fibroblast cells [59]. Besides glycolytic regulation, TNF-α could also participate in tryptophan metabolism since it induced IDO in MSCs, however, more evidence should be added.
Environmental factors promote glycolytic metabolism
Hypoxia
MSCs reside in a hypoxic niche in vivo, preferring to use glycolysis to sustain a quiescent state with low ROS [60]. However, when delivered into a non-physical culture environment in vitro or injured sites in vivo, MSCs experience “culture shock” which requires metabolic reconfiguration in response to the environmental cues to dictate their immunomodulatory functions. Exposed to hypoxia MSCs exhibited higher immunomodulatory and anti-inflammatory capacities. For example, hypoxia-pretreatment MSCs produced more immune-regulating factors of IDO, PGE2 and PD-L1, exerting enhanced inhibiting effects on CD4+/CD8+ T lymphocyte proliferation and increasing more regulatory T cells (T-reg) [61], [62], [63]. In the humanized mouse model of GVHD, MSCs primed with mild hypoxia (5%) produced more immunomodulatory factors to inhibit T cell proliferation and improved the survival of GVHD compared with naive MSC-injected mice [64]. The underlying mechanism associated with the metabolic pathway was that hypoxia stimulated glycolysis in the MSCs, which promoted immunomodulatory capacities. Hypoxia stabilized HIF-1α, which translocated to the nucleus and enhanced glycolysis in MSCs by activating glycolytic genes or PDKs as mentioned above. Collectively, hypoxia pretreatment promisingly becomes viable strategies of promoting MSC immune-regulatory potentials through the metabolic pathway.
3D culture
3D culture mimics the natural environment where MSCs reside and preserves or even promotes some cellular characteristics, including enhancing the immunomodulatory properties. Several lines of evidence have uncovered that aggregation of MSCs into 3D spheroids promoted the secretion of anti-inflammatory or immunoregulatory factors, strengthened regulation on immune cells and thus elevated MSC therapeutic potentials [65], [66]. For example, 3D aggregation of MSCs exhibited enhancing anti-inflammatory regulation on activated macrophages [67]. Similarly, intraluminally injected MSC spheroids downregulated inflammatory cytokines and numbers of macrophages, ameliorating dextran sulphate sodium (DSS)-induced colitis [68]. In another study shown by J.A. Zimmermann et al, spheroidal MSC aggregates combined with microparticle delivery of IFN-γ increased and sustained suppression on T-cell activation and proliferation, which maximized the effects of IFN-γ on MSC immunomodulation [69].
Interestingly, these benefits of 3D aggregation could be attributed to metabolic reconfiguration, which altered mitochondrial morphology, reduced OXPHOS and switched metabolic flux toward glycolysis. As demonstrated by Y. Liu et al, 3D aggregation upregulated glycolytic associated gene expression (HK2, PKM2, and lactate dehydrogenase A (LDHA)) and reduced mitochondrial membrane potential [32]. Mechanically, previous views held that the oxygen restriction in the aggregates resulting in the hypoxic milieu caused the alteration of cellular properties [70], [71]. However, studies have shown only a slight reduction of oxygen tension in the 3D aggregates. Instead, metabolic reconfiguration (metabolic shift to glycolysis) of MSCs mainly resulted from size-dependent mechanical compaction, which activated the PI3K pathway [72]. Taken together, the 3D culture alters the morphology, cell to cell contact and external environment of MSCs, which leads to a metabolic shift toward glycolysis and elevates immunomodulatory capacity.
ATP metabolism
ATP supplies energy for cellular biological behaviors, determining cellular functions. The majority of ATP is produced by mitochondrial through respiration and OXPHOS (Fig. 1). Metabolism of ATP including ATP production and hydrolysis regulates MSC immunomodulatory properties [73]. As reported by M. Pasztorek et al, mitochondrial dynamics (including ATP supply) could influence MSC immunomodulatory abilities [74]. Similarly, the serum-free medium significantly induced the ATP release of MSCs, which prevented apoptosis [75]. Interestingly, studies found that the hydrolysis of ATP into adenosine monophosphate (AMP) and adenosine also mediated the immune functions of MSCs. For example, S.S. Jeske et al discovered that MSCs hydrolyzed ATP into adenosine dependent of ectonucleotidases CD39 and CD73 expression, mediating their immunosuppressive properties [76]. Similarly, when MSCs were cocultured with activated T cells, which expressed a high level of CD39, there was a significant increase of adenosine production from ATP compared with T cells or MSCs alone, resulting in the suppression of T cells [77]. In addition to ATP metabolism in MSCs themselves, MSCs could also influence the mitochondrial dynamic and ATP metabolism in immune cells, regulating their functions. As several studies demonstrated that, MSCs transferred mitochondrial to immune cells to modulate their functions by restoring the mitochondrial membrane potential, mitochondrial function and ATP production [73], [78], which will be discussed below. Collectively, changes in the cell culture environment or exposure to external stimuli (such as coculturing with activated immune cells) result in ATP metabolic alteration (synthesis and hydrolysis), which further influences the immunomodulation of MSCs.
Lipid metabolism
Lipid metabolism, which is comprised of fatty acid (FA) anabolism and catabolism (especially fatty acid oxidation (FAO)) plays a pivotal role in cell biological behaviors. Fatty acid synthesis (FAS), mediated by fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC) participates in the membrane glycerophospholipids (GPL) formation, which is required for cell division and expansion [79]. On the other hand, FAs serve as energy storage in the form of triglycerides (TAG) in lipid droplets (LDs) and are ready to oxidize in the mitochondria to fuel the Krebs cycles to release ATP. In addition to determining cell proliferation or differentiation, both FAS and FAO significantly affect the immune cell functions, which has been reviewed elsewhere [80], [81], suggesting that lipid metabolism can influence the immune capacities.
Studies on MSC-based therapy on metabolic diseases such as type II diabetic diseases and non-alcoholic fatty liver disease discovered that MSCs ameliorated the disease progression through regulation of lipid metabolism [82], [83]. With the technique improvement, a lipidomic analysis of MSCs conducted by Campos et al emerged and reported that after pro-inflammatory stimulation, the lipid molecular profile of MSCs significantly changed, with the phosphatidylcholine (PC) species with shorter fatty acids (FAs) decreasing and lysoPC (LPC 18:0) levels increasing. Simultaneously, the levels of phosphatidylethanolamine (PE), phosphatidylserine (PS) and sphingomyelin (SM) in MSCs also dramatically altered compared with the untreated counterpart [84]. These suggested that the immune and anti-inflammatory capacities of MSCs might be associated with lipid metabolism (Fig. 2).
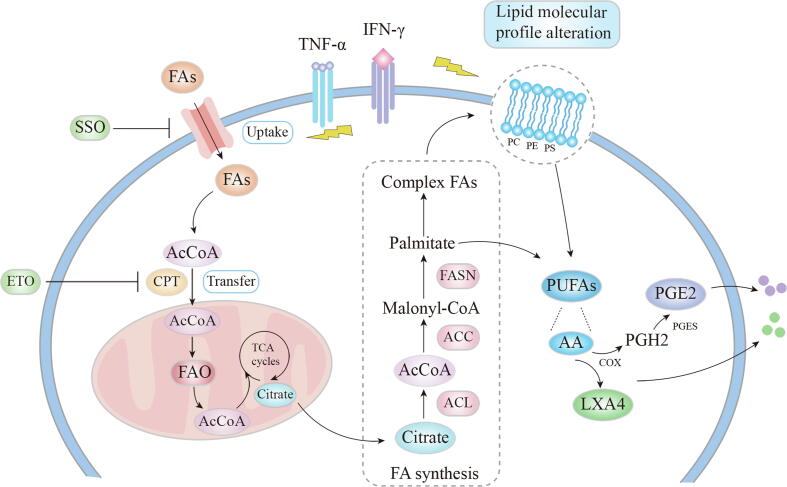
Fig. 2.
The lipid metabolism associated immune-regulation in MSCs. Inflammatory stimuli (TNF-α and IFN-γ) can influence the lipid molecular profile and the fatty acid uptake and thus FAO in MSCs. The phospholipids on cell membranes can produce a polyunsaturated fatty acid, AA, which can be catalyzed to PGE2, or LXA4 through COX, both mediating the immunomodulation of MSCs. FAs, Fatty acids; AcCo, acetyl-CoA; CPT, palmitoyltransferase; FAO, fatty acid oxidation; SSO, sulfosuccinimidyl oleate; ETO, etomoxir; PUFAs, polyunsaturated fatty acids; AA, arachidonic acid; COX, cyclooxygenase; LXA4, lipoxin A4; PGH2, Prostaglandin H2; PGES, prostaglandin E synthases; PGE2, prostaglandin E2; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine.
The widely investigated lipid-associated immune-regulation of MSCs was the PGE2, which was a major metabolite produced by a long-chain polyunsaturated fatty acid (arachidonic acid) through cyclooxygenase (COX) catalyzation [85]. Indeed, PGE2 was recognized as a key factor secreted by MSCs when exposed to various populations of immune cells including T cells, NK cells, DCs and macrophages and it mediated MSC immunosuppressive or anti-inflammatory functions through binding to the prostaglandin receptors [86], which had been discussed at large elsewhere [87], [88], [89], [90], [91]. Another polyunsaturated fatty acid, lipoxin (LX), also derived from arachidonic acid [92], was demonstrated to be involved in the MSC-based therapy on acute lung injuries. The secretion of lipoxin A4 (LXA4) by MSCs decreased the TNF-α in the lung injury mice models and improved the mice survival, whereas, the therapeutic effects were reversed by the LXA4 receptor antagonist [93]. Moreover, as mentioned above, manipulation of PPARβ/δ in MSCs exerted anti-inflammatory effects in different tissues [94], dictating their immune abilities and therapeutic potential of various diseases. Since PPARβ/δ was a master regulator of FAO, the link between lipid metabolism and MSC immunomodulation seemed to be predictable.
However, there existed a contradictory result. A study performed by R. Jitschin displayed that the lipid metabolism seemed not to affect the MSC immune capacities. They found that primed MSCs with inflammatory cytokines exhibited enhanced FA uptake and increased FAO [95]. Whereas, this increased dependency on FAO after inflammatory stimulation did not result in elevated MSC immunomodulation. The block of the carnitine palmitoyltransferase (CPT), transferring fatty Acyl-CoA to mitochondria for FAO, with etomoxir (ETO) and inhibition of exogenous FA uptake with sulfosuccinimidyl oleate (SSO) did not affect T cell suppression. Moreover, ETO and SSO did not downregulate the expression of immunomodulatory factors (IDO1 and COX2) of MSCs [95].
Taken together, although the lipid metabolism (FAS and FAO) as well as the biologic lipid such as the polyunsaturated fatty acids play a critical role in the immune regulation, the direct evidence that the lipid metabolism mediates MSC immune functions is scant and controversial. More thorough work is required to uncover this interacting link and the underlying mechanism.
Amino acid metabolism
Amino acids are fundamental units, the metabolism of which is essential for MSC proliferation and biologic functions. The emerging evidence reflects that amino acid metabolism plays important roles in the immune regulations of MSCs (Fig. 3).
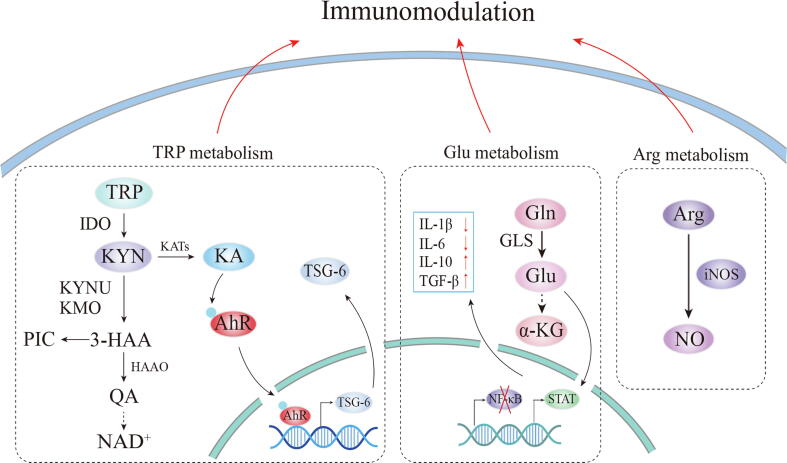
Fig. 3.
Amino acid metabolism in MSC immune functions. TRP can be converted into KYN by IDO, which is then further catalyzed into KA through KAT. The TRP depletion and KYN production contribute to immunomodulation. KA is the ligand of the AhR, promoting AhR to enter into the nucleus and directly bind to the TSG-6 promoter. KYN can be also metabolized to 3-HAA and finally produces NAD+. The intermediates along the pathway have immunomodulatory effects, which have not been demonstrated in MSCs. Moreover, Glu can decrease the IL-1β, IL-6 production and increase IL-10 and TGF-β secretion through activation of STAT and inhibition of NK-κB. Arg can be converted into NO by iNOS, regulating immune cells. TRP, tryptophan; KYN, kynurenine; IDO, indoleamine 2,3-dioxygenase; KA, kynurenic acid; KATs, kynurenine aminotransferases; KYNU, kynureninanse; KMO, kynurenine 3-monooxygenase; 3-HAA, 3-hydroxyanthranilic acid; PIC, picolinic acid; HAAO, 3-hydroxyanthranilate 3,4-dioxygenase; QA, quinolinic acid; Gln, glutamine; Glu, glutamate; GLS, glutaminase; Arg, arginine.
Arginine metabolism
The metabolic pathways of arginine are divided into two ways: decomposed into urea and ornithine by arginase, which is critical for cell growth and proliferation; Oxidized into biologically active monoxide nitrogen (NO) through nitric oxide synthase (NOS), which participates in various biological regulation. A recent study illustrated that the electrical stimulation promoted the chondrogenesis of ADSCs, partially by upregulation of arginine catabolism, contributing to the enhanced therapy for the cartilage defects [96]. Additionally, arginine affected immunomodulatory functions. For example, T cells required it for activation and one of the mechanisms that MSCs inhibiting T cell proliferation was creating an arginine exhaustion environment through upregulating iNOS [97], [98]. Laila Rashed et al demonstrated that the administration of MSCs combined with arginine, which induced NO production, downregulated the apoptosis and inflammation in rats with injured gastric mucosa [99]. In fact, the iNOS-mediated MSC immunomodulation was demonstrated in several research. iNOS-mediated immunosuppression enhanced the secretion of NO in MSCs, inhibiting T lymphocyte proliferation by blocking STAT5 phosphorylation [34], [100]. MSCs lack of iNOS exhibited impaired therapeutic effects in acute liver injury and fibrosis [1], [101]. Although there was no direct evidence demonstrating that MSCs depended on arginine metabolism to regulate immune functions, this link was possible and promising to construct considering that NOS is involved in arginine catabolism and serves as the immune-regulator of the MSCs.
Glutamine metabolism
Glutamine, albeit an unnecessary amino acid, can be utilized in almost every cell as a substrate to provide the demanding energy and precursors for the synthesis of crucial macromolecules for growth. Besides, glutamine is regarded as an immunonutrient since in infection or high catabolic conditions, the consumption rate of the glutamine in immune cells exceeds the glucose [102], suggesting its critical roles in the immune system. For example, glutamine metabolism is required for lymphocytes proliferation and cytokine production through activation of ERK and JNK kinases, which finally upregulated the transcription of cell proliferation-related genes [102]. In neutrophils, the consumption of glutamine was the highest compared with other immune cells [103], most of which was converted into glutamate. The glutamine metabolism in neutrophils enhanced superoxide production and promoted bacterial killing. As for macrophages, their activation was largely affected by glutamine metabolism. The synthesis and release of pro-inflammatory cytokines in macrophages relied on glutamine availability while the glutamine metabolite α-KG was reported to promote M2 macrophage differentiation [104].
Previous researches reported that BMSCs required glutamine metabolic product α-KG for proliferation, specification, and differentiation. Stimulation of the enzyme glutaminase (Gls), which broke down glutamine to form glutamate and α-KG, enhanced BMSC osteogenesis whereas diminished adipogenesis [105]. The role of glutamine metabolism in regulating MSC immune functions was discovered by G.G. Dos Santos, demonstrating that treatment with glutamate downregulated IL-1β and IL-6 production while upregulated immune factors (TGF-β, IL-10) in BMSCs through NF-κB inhibition and STAT-3 activation [106]. Moreover, the addition of glutamine in the BMSC conditional medium decreased the pro-inflammatory factors and increased the anti-inflammatory cytokine of lymphocytes and macrophages. Collectively, the glutamine metabolism of MSCs can become a novel target to dictate MSC immune capacities and the further mechanism is worthy of studying.
Tryptophan-kynurenine metabolism
The essential amino acid tryptophan is mainly catabolized by the kynurenine pathway (KP), of which kynurenine (KYN) is the first stable metabolite mediated by IDO or tryptophan 2,3-dioxygenase (TDO). Subsequently, during the metabolic process, several other intermediates are generated, including 3-hydroxyanthranilic acid (3-HAA), quinolinic acid (QUIN), kynurenic acid (KA) and picolinic acid (PIC).
The metabolic activity of IDO, which participated in the activation of the kynurenine pathway, has been widely investigated as a potential mechanism of the MSC immunosuppressive effect on the immune cells. Meisel et al demonstrated that IFN-γ induced IDO expression in MSCs in a dose-dependent manner. The primed MSCs showed increased IDO activity and enhanced immunosuppressive capacities, especially inhibition of T cell proliferation [107], [108], [109], which was widely investigated in immune and inflammatory diseases such as GVHD [110], EAE [111] and CCl4-induced liver injury [112]. Moreover, the application of competitive inhibitors of IDO, such as 1-methyl-tryptophan (1-MT) rescued the inhibition effects of MSCs on immune cells, including T cells [108]. In vivo, IDO also mediated MSC immune function and therapy effects. As validated in the murine model of EAE, MSC-treated mice presented the greater expression of IDO in the spleens and showed greater recovery, which was not observed in the 1-MT treatment mice [113]. Besides adaptive immune cells, S. Lee et al claimed that the upregulated IDO of MSCs converted innate immune cells, macrophages, from pro-inflammatory M1 to anti-inflammatory M2 subsets and limited inflammatory cytokines secretion [114].
In addition to tryptophan depletion, following the induction of IDO expression, tryptophan was further broken down into variant and immune-active metabolites. The initial stable product was KYN, which was elaborately investigated for its immunomodulatory roles in T cells, natural killer cells (NKT) [115], dendritic cells (DC) [116], monocytes [117], and macrophages. For example, KYN suppressed CD4+ and CD8+ T cell proliferation in a dose manner through binding to the Aryl hydrocarbon receptor (AhR) [118]. Furthermore, L.F. Campesato et al uncovered that KYN promoted Tregs generation, resulting in M2-like macrophage expansion. The IDO-KYN-AhR-mediated immunosuppression was modulated by crosstalk between Tregs and tumor-associated macrophages [119]. Likewise, also acted as the AhR agonist, KA, which was transformed from KYN by kynurenine aminotransferases (KATs), had a similar immune regulation. G. Wang et al discovered the immunosuppressive effect on T cells of MSCs was attributed to IDO mediated TSG-6 upregulation and more specifically, to its metabolite KA [2]. As illustrated in the chromatin immunoprecipitation, KA activated AhR and enhanced its direct bind to TSG-6 promoter, which exerted immune function in the acute liver injury (ALI) mice model. This promotion of TSG-6 was reversed through knocking down KATs, preventing the KA production [2]. Moreover, KA was described to have anti-inflammatory capacities in several mental diseases [120], [121], inflammatory bowel disease [122] and atherosclerotic disease [123]. Through the PPARδ-HO-1 pathway, KA ameliorated LPS-induced inflammatory response in macrophages, reducing the TNF-α and IL-6 secretion [124]. Mechanically, KA bound to GPR35, which was widely expressed in the immune cells and mediated its immune regulation.
On the other way, KYN was catalyzed by kynurenine 3-monooxygenase (KMO) and kynureninanse (KYNU) into 3-HAA, which was also reported to exert immune regulatory effects. For example, 3-HAA modulated the immune system by inhibiting T cell growth and reducing macrophage-mediated inflammation [125]. In atherosclerosis, 3-HAA could disturb inflammasome activation and IL-1β production in macrophages. In this context, targeted inhibition of HAAO, which prevented 3-HAA from breaking down and endogenously increased the level of 3-HAA, attenuated hypercholesterolemia and atherosclerosis [126].
The final product NAD+ was essential in the immune cell energy metabolism. A recent study revealed that the KYN-derived NAD+ influenced energy metabolism by maintaining mitochondrial respiration in macrophages, which mediated their anti-inflammatory and phagocytic capability.
However, the evidence of these metabolites in the kynurenine pathway mediating the MSC immune functions is little, which needs to be further demonstrated. If so, the utilization of agonist or antagonist or combinations of the different enzymes in the tryptophan-kynurenine metabolism of MSCs turns out to be a promising avenue to regulate immune functions and enhance therapeutic potentials.
Other amino acids
Studies showed that amino acids could tune the functions of immune cells by diverse mechanisms [127]. For example, serine, leucine and valine influenced the energy metabolism (glycolysis and OXPHOS); Cysteine, glutamine, and glycine maintained the intracellular redox balance and thus regulated the ROS production; The intermediates of methionine, arginine, glutamine, the leucine, valine and isoleucine affected the post-translational modifications (PTMs) of proteins. However, whether these amino acids are involved in MSC immune function is as yet unknown and more amino acids remained to be uncovered.
MSCs regulate immune cell metabolism
The emerging evidence has shown that metabolism in immune cells predicts and dictates their fate and function. Their functional alteration depends on the metabolic switch from quiescent to an activated state, shunting nutrients into distinct directions to instruct the appropriate immune polarization. For instance, a metabolic shift towards aerobic glycolysis was required for T cell activation, which provided sufficient substrates for the production of lipids, proteins, nucleic acids, and other carbohydrates. After stimulation, DCs underwent a metabolic switch to aerobic glycolysis [128]. As for macrophages, the distinct arginine metabolism defined the two types of the cell, with the pro-inflammatory M1 subsets utilizing it through iNOS, while M2 macrophages through arginase1 [129]. Moreover, M2 macrophage differentiation heavily relied on OXPHOS, notably FAO, to fuel their long-term function. Consistently, induction of PPARβ/δ, the upstream of FAO, promoted alternatively activated macrophages through STAT6 and suppressed inflammation [130], [131].
MSCs regulate immune cell metabolism
Recent studies show that MSCs exert their immune-regulation and therapeutic effects through triggering the metabolic alteration in immune cells. For example, L. Pan et al displayed that the therapeutic effects of human umbilical cord Wharton’s jelly-derived MSCs (hWJ-MSCs) on fulminant hepatitis relied on the metabolic regulation of T cells. Specifically, hWJ-MSCs inhibited T cell activation and proliferation by blunting the glycolytic pathway [132]. Consistently, M. Bottcher et al reported that MSCs mimicked the effects of mTOR inhibitor, blunting the glycolysis in T cells and therefore suppressing T cell activation, which could be mediated by IDO metabolic activity [133]. Furthermore, EVs derived from MSCs were incorporated by lymphocytes, suppressing T cell proliferation and Th1 differentiation concomitant with promoting Treg cell differentiation. M. Bottcher further demonstrated that the glycolytic enzyme, PKM2 expression and mitochondria membrane potential decreased in MSC-EV-priming T cells, suggesting metabolic regulation in T cells [134]. Another study focused on the macrophage metabolism indicated that the conditioned medium from the murine ADSCs promoted AKT/mTOR pathway activation and upregulated PPARγ expression and lipid droplet biogenesis in M2 macrophages, which mediated their therapy in experimental colitis and sepsis [135]. Likewise, when cocultured with macrophages, MSCs skewed metabolism of macrophages to M2-like bioenergetic state, with decreasing glycolysis while increasing CPT1α expression, a key enzyme for FAO (mentioned above). Meanwhile, the metabolic signaling factor, p-mTOR was also significantly reduced by MSCs both in M1 and M2 macrophages [136]. In the ALI mice model, MSCs administration profoundly transformed the metabolomic profile of the liver-resident immune cells and remarkably ameliorated liver damage. In their high-performance chemical isotope labeling liquid chromatography-mass spectrometry (CIL LC-MS) experiment, three major metabolic pathways (arginine, glutamate and aspartate metabolism) exhibited the most affected, suggesting that MSC therapeutic potential might be attributed to disturbing these metabolic pathways in immune cells in ALI [137]. MSCs administration reversed the level of citrulline and ornithine in untreated ALI, priming the immune cells to shunting arginine into arginase (Arg) 1 hydrolysis and mitigating ALI severity. Similarly, MSCs reduced the pro-inflammatory factors (IL-6, TNF-α) by altering the metabolites in the cecal ligation and puncture (CLP) induced lung and liver injury in septic rat models and thus improved the survival rates [138].
Metabolic regulation through mitochondria transfer
Interestingly, one possible mechanism of the metabolic regulation might be mitochondria transferred from MSCs to immune cells either by direct cell–cell contacts or paracrine secretion within EVs [25]. Of note, mitochondria served as a primary site of OXPHOS and played key roles in energy balance and cell metabolism, the function of which determined the immune cell functions. For example, in the model of acute respiratory distress syndrome (ARDS) and sepsis, macrophages received the mitochondria from MSCs through tunneling nanotubes (TNT)-like structures, increasing OCR and promoting phagocytic capabilities [139]. Since M2 macrophage differentiation heavily relied on OXPHOS, Y. Yuan et al uncovered that MSCs delivered mitochondria to macrophages, decreasing the glycolysis while increasing the basal respiration and thus promoting anti-inflammatory M2 macrophages polarization in diabetic nephropathy mice, which was regulated by PGC-1α/TFEB mediated autophagy [140]. Furthermore, mitochondria trafficking to mouse splenocytes via F-actin nanotubes significantly promoted Treg cells and inhibited effector T cells with increasing OCR and ATP production [141]. In addition to direct-contact delivery, mitochondria could be normally encapsulated in EVs to the immune cells. Following engulfing the EVs derived from BMSCs, which contained mitochondria, alveolar macrophages in an ARDS environment showed enhanced anti-inflammatory functions and phagocytosis. These mitochondria-enriched EVs abrogated inflammation and lung injury in vivo [142]. As reported by A.C. Court et al, MSCs delivered mitochondria through EVs mainly to CD4+ cells rather than CD8+ T cells or CD19+ B cells. The artificial transfer of mitochondria promoted CD4+ T cells activation and Treg cell differentiation by upregulating FOXP3, IL2RA, CTLA4, and TGFb1 expression in the GVHD murine model [143]. Mechanically, a recent study discovered that the mitochondria delivered from ADSCs depended on the HLA expression and were associated with HLA-C and HLA-DRB1 eplet mismatch load between ADSC and Treg donors [144]. Furthermore, CD39/CD73 signaling drove the MSC mitochondria trafficking and subsequently stabilize BACH2 and SENP3 to induce Treg cell expression of FOXP3 [145]. Additionally, the mitochondria status of MSCs determined the mitochondrial transfer. The delivery and function of mitochondrial of human synovial MSCs from rheumatoid arthritis patients to Th17 cells reduced compared with healthy BMSCs [146].
In summary, MSCs regulate immune cell functions through metabolic reconfiguration by mitochondria transferring, which improves the mitochondrial respiratory function in immune cells, resulting in immunosuppressive effects.
MSCs in regenerative clinical application
MSC-based cellular therapies have been applied and achieved great results in various clinical settings. ADSCs which are easy to obtain and possess high immunomodulation functions, have been widely applied in regenerative conditions including wound healing and scar repairs [147], [148], [149], hair growth [150], [151], face rejuvenation [152], [153], soft tissue regeneration [154], [155] and breast reconstruction [153], [156].
Regenerative plastic surgery
In the systematic review and clinical research, the safety and efficacy of ADSCs or stromal vascular fraction (SVF) and fat grafting which contained ADSCs have been confirmed in wound and scar healing and face rejuvenation treatments [148], [152]. Additionally, adding platelet-rich plasma (PRP), a high-concentration platelet-oriented plasma, containing abundant nutritional and growth factors, could significantly enhance the fat grafting survival [157] and regenerative effects of ADSCs [158], which promoted proliferation and multiple differentiation of ADSCs [159]. Similar regenerative results could be seen in soft tissue reconstruction, using fat graft with ADSCs or PRP [154], [155], [160]. Mechanically, in addition to secreting various growth factors or pro-angiogenic factors, the regenerative abilities of ADSCs could also be attributed to immunomodulatory and anti-inflammatory effects. The potent immunomodulatory properties of ADSCs was promoting macrophage phenotype from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype, which was crucial for wound and scar repairs [161], [162].
Hair regrowth
Autologous micrografts enriched with human hair follicle mesenchymal stromal/stem cells (HF-MSCs) or ADSCs exerted favorable outcomes with an increase of hair count and hair density [150], [151]. Similarly, PRP could exert nearly equal effects as HF-MSCs or clinical drugs in hair regrowth [163], [164]. Since androgenic alopecia (AGA) could trigger an inflammatory and immune response, the anti-inflammatory and immunomodulatory properties of PRP or HF-MSCs or ADSCs could be one of the important mechanisms for AGA treatment and hair regrowth [150], [165].
A new application of ADSCs in coronavirus disease 2019 (COVID-19)
Recently, MSC-based therapy on COVID-19 pneumonia achieved satisfying results through immunomodulation [166]. The applied MSCs included dental pulp stromal/stem cells, umbilical cord stromal/stem cells, mesenchymal stromal/stem cells and ADSCs [167]. Compared with drugs and vaccines, ADSC treatment on patients with COVID-19 showed satisfying results attributed to potent immune-regulatory and antiviral effects of ADSCs [168].
Taken together, these regenerative effects of MSCs in the clinical application might be associated with metabolism, which needs more evidence to explore.
Conclusion and future perspectives
The metabolism has emerged as a central hub influencing MSC biological functions and therapeutic potentials. In this review, we discuss the metabolic-mediated MSC modulation on the immune cells and their therapeutic effects. Therefore, metabolism manipulation provides a promising target to enhance the MSC therapeutic efficiency (Table 1). Although, the evidence is clear that the enhancement of glycolysis of MSCs contributes to their immunomodulatory improvement and thus promotes the therapeutic effects, the exact mechanism of how this metabolism affects the immune functions of MSCs is still under exploration. More specific signal pathways which are associated with glycolysis in MSCs and subsequently result in their immune regulations require to be further explored. On the other hand, given the profound effect of the metabolic intermediates on the immune cells, further studies are required to explore whether these metabolites derived from MSCs exert similar effects on the immune system and how they mediate the immune regulation of MSCs. Notably, the metabolites regulating the functions of immune cells through epigenetic modifications seem to provide a new sight in the MSC therapy, which however, requires more evidence.
Table 1.
Direct evidence of MSC-based therapy for disease models associated with immunometabolism.
| Animal models of diseases or injury | Associated metabolism | Findings | Year | MSC source | Reference |
|---|---|---|---|---|---|
| Delayed-type hypersensitivity (DTH) murine model | Shift from glycolysis to OXPHOX | HIF knocking down exhibiting reduced inhibition on Th1 and Th17 and limited potential of Treg production; reducing intercellular adhesion molecule (ICAM), IL-6, and nitric oxide (NO); imparing therapeutic effects on mice syndrome and decreased Treg cells production | 2020 | Murine MSC isolated from BW | [10] |
| DTH in mice | Glycolysis | Enhanced immunosuppresion on Th1 and Th17 proliferation and elevated PD-L1, PGE2 Production; alleviating mice syndrome and reducing number of Th1 and Th17 by AMPK pathway | 2021 | Murine MSC and human MSC | [12] |
| Cecal ligation and puncture (CLP) sepsis in mice | PGE2 | Improving the survial of mice; decreasing TNF-α and IL-6 concentrations; increasing IL-10 from macrophages | 2009 | Murine BM-MSCs | [90] |
| Graft-versus-host disease (GvHD) in mice | PGE2 | Improving the survival of mice with GVHD; decreaseing IFNγ and TNFα and inhibiting T-cell expansion; | 2015 | Human bone marrow-derived (BM) MSCs | [87] |
| Ischemia-reperfusion acute kidney injury (IRI-AKI) in mice | PGE2 | IL-17A pretreated MSCs improved the symptom of mice with IRI-AKI through PGE2 secretion; decreased serum IL-6, TNF-α, and IFN-γ levels, a higher serum IL-10 level, and higher spleen and kidney Treg percentages | 2018 | Murine BM- MSCs | [88] |
| DSS-induced colitis in mice | PGE2 | Inhibiting T cell proliferation and activation; reducing IL-6, TNF-α, IL-17a; increasing the number of regulatory T cells and IL10, TGF-β; skewing macrophages into the anti-inflammatory M2 phenotype; | 2019 | Human ADSCs | [89] |
| Inflammatory bowel disease (IBD) in mice | PGE2 | Reducing inflammatory responses, promoting the functional and structural recovery of colitis; inducing M2 macrophage polarization | 2020; | Human placenta-derived MSCs (hP-MSCs) | [91] |
| LPS-induced acute lung injury (ALI) in mice | Lipoxin A4 | Improving the survial of ALI mice partly by secrtion of LXA4; increasing the LXA4 level in BAL fluid of ALI mice and decresing the TNF-α and MIP-2. | 2015 | Human MSCs | [93] |
| Experimental autoimmune encephalomyelitis (EAE) in mice | Tryptophan-kynurenines | A significant reduction in Th1 and Th17 lymphocytes; increasing IL-27 and cyp1a1 expression and presence of IL-10-secreting T CD4 + cells. | 2021 | Murine endometrial-derived MSCs | [111] |
| ALI mice models | Kynurenic acid, the metabolite of tryptophan catabolism | Acvation of TSG-6; reducing neutrophil infiltration in ALI | 2017 | Human umbilical cord-derived MSC (hUC-MSC) | [2] |
| Indomethacin-induced gastric ulcer (GU) in rats | L-arginine | MSC combined with arginine decreasing caspase-3, BAX genes expression and PGE2 and TNF-α serum levels in the gastric ulcer rats; increasing Bcl-2 and eNOS gene expression | 2016 | Rat BM-MSCs | [99] |
Previous investigations focus on glucose metabolism. The recent emerging studies have revealed that the lipid and amino acid metabolism in MSCs are of equal importance to dictate the immune functions. However, the direct evidence is scant and must be further uncovered. Since the metabolism is interactional and not independent, the full understanding of the complex metabolism-mediated immune functions in MSCs is challenging and needs more effort. Besides the metabolic regulation in MSCs itself, MSCs also influence the metabolism in immune cells to exert their therapeutic functions. Interestingly, the mitochondria delivered from MSCs to immune cells influence the cell energy metabolism and thus regulate immune functions, turning out to be a striking mechanism of immunometabolism in MSCs. Collectively, metabolism influences the immune functions of MSCs. The metabolic regulation provides a new avenue for MSC therapy, although more work needs to be done.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [Grant No. 82071072].
Biographies

Hanyue Li was born in Sichuan province, China in 1993. She obtained her bachelor’s degree in Xiangya Stomatological Hospital, Central South University, Changsha, China in 2017. Subsequently, she completed her Master’s degree in orthodontics from the Hospital of Stomatology, Wuhan University, Wuhan, China, in 2020. During that period, she was working on the orthodontic tooth movement and relapse and orthodontic tooth resorption. She received the national scholarship in 2019. She has participated in numerous academic seminars and obtained many awards. She is currently pursuing a Doctor’s degree in the College of Stomatology, Chongqing Medical University, Chongqing, China and doing research in the Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China. She is actively working on the mesenchymal stem cell (MSC)-based therapy study and the periodontal regeneration research. She focuses on the metabolism of MSCs and the exploration of the molecular mechanism of MSC-based therapy in periodontitis. Up to now, along with laboratory work, she has published 6 scientific articles under the guidance of supervisors. 5 articles have cited from her published articles and the H-index was 1 from the Scopus.

Hongwei Dai is a professor of orthodontics, PhD. tutor. He has supervised a large number of master and doctoral students. He is the vice president of the Stomatological Hospital of Chongqing Medical University, Chongqing, China. Professor Dai was graduated from the West China Hospital of Stomatology, Sichuan University, China. He was selected as a member of the Orthodontics Professional Committee of the Chinese Stomatological Association and a member of the Stomatological Education Professional Committee of the Chinese Stomatological Association. He is the editorial board of the Journal of Oral Science Research and Chinese Journal of Orthodontics. He is a fellow of the International College of Dentists. He also serves as a vice president and secretary general of Chongqing Stomatological Association. The topic of his research has focused mainly on mesenchymal stem cell research in the periodontal regeneration as well as the drug-targeted delivery of biomimetic biomaterials. He had published more than 20 original research papers and review articles, including orthodontic tooth movement and relapse, orthodontic tooth resorption, alveolar bone reconstruction and biomimetic biomaterials. 114 articles have been cited from his published articles and the H-index was 6 from the Scopus.

Jie Li received his PhD. degree in Biochemistry and Molecular Biology from Sichuan University, Chengdu, China, in 2016. He was also a visiting PhD. student in Center for Stem Cell Research and Regenerative Medicine, Tulane University sponsored by China Scholarship Council from 2014-2015. He is currently a research Associate at the Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, College of Stomatology, Chongqing Medical University. He is a member of the International Association for Dental Research, member of the Chinese Stomatological Association and former F1000Prime Associate Faculty Member. His main research interests are stem cell biology, tissue engineering, bone and tooth regeneration. To date, he has published more than 30 peer-reviewed journal articles, including 17 co-first/co-communication authors, and 3 book chapters in 2 Springer books. His H-index was 13 from the Scopus. He is also a reviewer for many journals such as Advanced Healthcare Materials, Frontiers in Cell and Developmental Biology, Experimental Cell Research, and Biochimi. He holds several grants to support his research including the National Natural Science Foundation of China, Chongqing Research Program of Basic Research and Frontier Technology, and Scientific and Technological Research Program of Chongqing Municipal Education Commission.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 2.Wang G., Cao K., Liu K., Xue Y., Roberts A.I., Li F., et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25(7):1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luz-Crawford P., Ipseiz N., Espinosa-Carrasco G., Caicedo A., Tejedor G., Toupet K., et al. PPARbeta/delta directs the therapeutic potential of mesenchymal stem cells in arthritis. Ann Rheum Dis. 2016;75:2166–2174. doi: 10.1136/annrheumdis-2015-208696. [DOI] [PubMed] [Google Scholar]
- 4.Song N., Scholtemeijer M., Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–664. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyh-Chang N.g., Ng H.-H. The metabolic programming of stem cells. Genes Dev. 2017;31(4):336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang F., Li B., Yang Y., Huang M., Liu X., Zhang Y., et al. Leptin enhances glycolysis via OPA1-mediated mitochondrial fusion to promote mesenchymal stem cell survival. Int J Mol Med. 2019 doi: 10.3892/ijmm.2019.4189. [DOI] [PubMed] [Google Scholar]
- 7.Moya A., Larochette N., Paquet J., Deschepper M., Bensidhoum M., Izzo V., et al. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells. 2017;35(1):181–196. doi: 10.1002/stem.2493. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Dong Z., Li D., Li B., Liu Y., Zheng X., et al. Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway. Cell Prolif. 2021;54(7) doi: 10.1111/cpr.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L.-C., Wang H.-W., Huang C.-C. Modulation of inherent niches in 3D multicellular MSC spheroids reconfigures metabolism and enhances therapeutic potential. Cells. 2021;10(10):2747. doi: 10.3390/cells10102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras-Lopez R., Elizondo-Vega R., Paredes M.J., Luque-Campos N., Torres M.J., Tejedor G., et al. HIF1alpha-dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. FASEB J. 2020;34:8250–8264. doi: 10.1096/fj.201902232R. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Yuan X., Munoz N., Logan T.M., Ma T. Commitment to aerobic glycolysis sustains immunosuppression of human mesenchymal stem cells. Stem Cells Transl Med. 2019;8:93–106. doi: 10.1002/sctm.18-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras-Lopez R., Elizondo-Vega R., Luque-Campos N., Torres M.J., Pradenas C., Tejedor G., et al. The ATP synthase inhibition induces an AMPK-dependent glycolytic switch of mesenchymal stem cells that enhances their immunotherapeutic potential. Theranostics. 2021;11(1):445–460. doi: 10.7150/thno.51631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K., Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd K.M., Yang J., Shen M.H., Sampson J.R., Tee A.R. mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34:2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.J., Jung Y.H., Choi G.E., Kim J.S., Chae C.W., Lim J.R., et al. O-cyclic phytosphingosine-1-phosphate stimulates HIF1alpha-dependent glycolytic reprogramming to enhance the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2019;10:590. doi: 10.1038/s41419-019-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lord-Dufour S., Copland I.B., Levros L.C., Jr., Post M., Das A., Khosla C., et al. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells. 2009;27:489–497. doi: 10.1634/stemcells.2008-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takubo K., Nagamatsu G.o., Kobayashi C., Nakamura-Ishizu A., Kobayashi H., Ikeda E., et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12(1):49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masui K., Tanaka K., Akhavan D., Babic I., Gini B., Matsutani T., et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18(5):726–739. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnanaprakasam J.N.R., Sherman J.W., Wang R. MYC and HIF in shaping immune response and immune metabolism. Cytokine Growth Factor Rev. 2017;35:63–70. doi: 10.1016/j.cytogfr.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Xu D., Xie R., Xu Z., Zhao Z., Ding M., Chen W., et al. mTOR-Myc axis drives acinar-to-dendritic cell transition and the CD4(+) T cell immune response in acute pancreatitis. Cell Death Dis. 2020;11(6) doi: 10.1038/s41419-020-2517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christofides A., Konstantinidou E., Jani C., Boussiotis V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021;114:154338. doi: 10.1016/j.metabol.2020.154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magadum A., Engel F.B. PPARbeta/delta: linking metabolism to regeneration. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan W., Cao Y., Yang H., Han N., Zhu X., Fan Z., et al. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Prolif. 2019;52(6) doi: 10.1111/cpr.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heck B.E., Park J.J., Makani V., Kim E.C., Kim D.H. PPAR-delta agonist with mesenchymal stem cells induces type II collagen-producing chondrocytes in human arthritic synovial fluid. Cell Transplant. 2017;26:1405–1417. doi: 10.1177/0963689717720278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen C., Khoury M. Musculoskeletal progenitor/stromal cell-derived mitochondria modulate cell differentiation and therapeutical function. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.606781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras-Lopez R.A., Elizondo-Vega R., Torres M.J., Vega-Letter A.M., Luque-Campos N., Paredes-Martinez M.J., et al. PPARbeta/delta-dependent MSC metabolism determines their immunoregulatory properties. Sci Rep. 2020;10:11423. doi: 10.1038/s41598-020-68347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez V.G., Ontoria-Oviedo I., Ricardo C.P., Harding S.E., Sacedon R., Varas A., et al. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1) doi: 10.1186/s13287-017-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Ferrer M., Villanueva-Badenas E., Sanchez-Sanchez R., Sanchez-Lopez C.M., Baquero M.C., Sepulveda P., et al. HIF-1alpha and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22073416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-King H., Garcia N.A., Ontoria-Oviedo I., Ciria M., Montero J.A., Sepulveda P. hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 30.Cahill E.F., Tobin L.M., Carty F., Mahon B.P., English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19. doi: 10.1186/s13287-015-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domnina A., Ivanova J., Alekseenko L., Kozhukharova I., Borodkina A., Pugovkina N., et al. Three-dimensional compaction switches stress response programs and enhances therapeutic efficacy of endometrial mesenchymal stem/stromal cells. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Munoz N., Tsai A.C., Logan T.M., Ma T. Metabolic reconfiguration supports reacquisition of primitive phenotype in human mesenchymal stem cell aggregates. Stem Cells. 2017;35:398–410. doi: 10.1002/stem.2510. [DOI] [PubMed] [Google Scholar]
- 33.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Castro L.L., Lopes-Pacheco M., Weiss D.J., Cruz F.F., Rocco P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J Mol Med (Berl) 2019;97(5):605–618. doi: 10.1007/s00109-019-01776-y. [DOI] [PubMed] [Google Scholar]
- 35.Meisel R., Zibert A., Laryea M., Gobel U., Daubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 36.Wobma H.M., Tamargo M.A., Goeta S., Brown L.M., Duran-Struuck R., Vunjak-Novakovic G. The influence of hypoxia and IFN-gamma on the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials. 2018;167:226–234. doi: 10.1016/j.biomaterials.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigo T., La Rocca C., Faicchia D., Procaccini C., Ruggieri M., Salvetti M., et al. IFNbeta enhances mesenchymal stromal (Stem) cells immunomodulatory function through STAT1-3 activation and mTOR-associated promotion of glucose metabolism. Cell Death Dis. 2019;10:85. doi: 10.1038/s41419-019-1336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M., Shin D.I., Choi B.H., Min B.H. Exosomes from IL-1beta-primed mesenchymal stem cells inhibited IL-1beta- and TNF-alpha-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18:525–536. doi: 10.1007/s13770-020-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D., et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 40.Yao M., Cui B., Zhang W., Ma W., Zhao G., Xing L. Exosomal miR-21 secreted by IL-1beta-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y., Yoo S.M., Park H.H., Baek S.Y., Kim Y.-J., Lee S., et al. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13(10):1792–1804. doi: 10.1002/term.2930. [DOI] [PubMed] [Google Scholar]
- 42.Tan Q., Huang Q., Ma Y.L., Mao K., Yang G., Luo P., et al. Potential roles of IL-1 subfamily members in glycolysis in disease. Cytokine Growth Factor Rev. 2018;44:18–27. doi: 10.1016/j.cytogfr.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Taylor D.J., Feldmann M., Evanson J.M., Woolley D.E. Comparative and combined effects of transforming growth factors alpha and beta, interleukin-1 and interferon-gamma on rheumatoid synovial cell proliferation, glycolysis and prostaglandin E production. Rheumatol Int. 1989;9:65–70. doi: 10.1007/BF00270247. [DOI] [PubMed] [Google Scholar]
- 44.Bird T.A., Davies A., Baldwin S.A., Saklatvala J. Interleukin 1 stimulates hexose transport in fibroblasts by increasing the expression of glucose transporters. J Biol Chem. 1990;265(23):13578–13583. [PubMed] [Google Scholar]
- 45.Lee G.M., Tioran M.E., Jansen M., Graff R.D., Kelley S.S., Lin P. Development of selective tolerance to interleukin-1beta by human chondrocytes in vitro. J Cell Physiol. 2002;192:113–124. doi: 10.1002/jcp.10122. [DOI] [PubMed] [Google Scholar]
- 46.Tan Q., Duan L., Huang Q., Chen W., Yang Z., Chen J., et al. Interleukin -1beta promotes lung adenocarcinoma growth and invasion through promoting glycolysis via p38 pathway. J Inflamm Res. 2021;14:6491–6509. doi: 10.2147/JIR.S319433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park M.J., Lee S.H., Lee S.H., Lee E.J., Kim E.K., Choi J.Y., et al. IL-1 receptor blockade alleviates graft-versus-host disease through downregulation of an interleukin-1beta-dependent glycolytic pathway in Th17 cells. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/631384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C., Zeng L., Zhang T., Liu J., Wang W. Tenuigenin prevents IL-1beta-induced inflammation in human osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-kappaB signaling pathway. Inflammation. 2016;39:807–812. doi: 10.1007/s10753-016-0309-3. [DOI] [PubMed] [Google Scholar]
- 49.Mitroulis I., Ruppova K., Wang B., Chen L.-S., Grzybek M., Grinenko T., et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172(1-2):147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Garcia L., Castro-Manrreza M.E. TNF-alpha and IFN-gamma participate in improving the immunoregulatory capacity of mesenchymal stem/stromal cells: importance of cell-cell contact and extracellular vesicles. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22179531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorronsoro A., Ferrin I., Salcedo J.M., Jakobsson E., Fernandez-Rueda J., Lang V., et al. Human mesenchymal stromal cells modulate T-cell responses through TNF-alpha-mediated activation of NF-kappaB. Eur J Immunol. 2014;44:480–488. doi: 10.1002/eji.201343668. [DOI] [PubMed] [Google Scholar]
- 52.Sayegh S., El Atat O., Diallo K., Rauwel B., Degboe Y., Cavaignac E., et al. Rheumatoid synovial fluids regulate the immunomodulatory potential of adipose-derived mesenchymal stem cells through a TNF/NF-kappaB-dependent mechanism. Front Immunol. 2019;10:1482. doi: 10.3389/fimmu.2019.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann J.A., Mcdevitt T.C. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16(3):331–345. doi: 10.1016/j.jcyt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Nogueira-Pedro A., Makiyama E.N., Segreto H.R.C., Fock R.A. The role of low-dose radiation in association with TNF-alpha on immunomodulatory properties of mesenchymal stem cells. Stem Cell Rev Rep. 2021;17:968–980. doi: 10.1007/s12015-020-10084-9. [DOI] [PubMed] [Google Scholar]
- 55.Nakao Y., Fukuda T., Zhang Q., Sanui T., Shinjo T., Kou X., et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song W.-J., Li Q., Ryu M.-O., Nam A., An J.-H., Jung Y.C., et al. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res Vet Sci. 2019;125:176–184. doi: 10.1016/j.rvsc.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koedderitzsch K., Zezina E., Li L., Herrmann M., Biesemann N. TNF induces glycolytic shift in fibroblast like synoviocytes via GLUT1 and HIF1A. Sci Rep. 2021;11:19385. doi: 10.1038/s41598-021-98651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Remels A.H., Gosker H.R., Verhees K.J., Langen R.C., Schols A.M. TNF-alpha-induced NF-kappaB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1alpha. Endocrinology. 2015;156:1770–1781. doi: 10.1210/en.2014-1591. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q., Mei S., Nie F., Zhang Z., Feng J., Zhang J., et al. The role of macrophage-fibroblast interaction in lipopolysaccharide-induced pulmonary fibrosis: an acceleration in lung fibroblast aerobic glycolysis. Lab Invest. 2022;102(4):432–439. doi: 10.1038/s41374-021-00701-7. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y., Ma T. Metabolic regulation of mesenchymal stem cell in expansion and therapeutic application. Biotechnol Prog. 2015;31(2):468–481. doi: 10.1002/btpr.2034. [DOI] [PubMed] [Google Scholar]
- 61.Jiang C.M., Liu J., Zhao J.Y., Xiao L., An S., Gou Y.C., et al. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. 2015;94(1):69–77. doi: 10.1177/0022034514557671. [DOI] [PubMed] [Google Scholar]
- 62.Kadle R.L., Abdou S.A., Villarreal-Ponce A.P., Soares M.A., Sultan D.L., David J.A., et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE. 2018;13(3):e0193178. doi: 10.1371/journal.pone.0193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bobyleva P.I., Andreeva E.R., Gornostaeva A.N., Buravkova L.B. Tissue-related hypoxia attenuates proinflammatory effects of allogeneic PBMCs on adipose-derived stromal cells in vitro. Stem Cells Int. 2016;2016:1–13. doi: 10.1155/2016/4726267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y., Jin H.J., Heo J., Ju H., Lee H.-Y., Kim S., et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018;32(12):2672–2684. doi: 10.1038/s41375-018-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartosh T.J., Ylöstalo J.H., Mohammadipoor A., Bazhanov N., Coble K., Claypool K., et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrenko Y., Sykova E., Kubinova S. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8:94. doi: 10.1186/s13287-017-0558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartosh T.J., Ylostalo J.H., Bazhanov N., Kuhlman J., Prockop D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1) Stem Cells. 2013;31:2443–2456. doi: 10.1002/stem.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molendijk I., Barnhoorn M.C., de Jonge-Muller E.S.M., Mieremet-Ooms M.A.C., van der Reijden J.J., van der Helm D., et al. Intraluminal injection of mesenchymal stromal cells in spheroids attenuates experimental colitis. J Crohns Colitis. 2016;10(8):953–964. doi: 10.1093/ecco-jcc/jjw047. [DOI] [PubMed] [Google Scholar]
- 69.Zimmermann J.A., Hettiaratchi M.H., McDevitt T.C. Enhanced immunosuppression of T cells by sustained presentation of bioactive interferon-gamma within three-dimensional mesenchymal stem cell constructs. Stem Cells Transl Med. 2017;6:223–237. doi: 10.5966/sctm.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shearier E., Xing Q.i., Qian Z., Zhao F. Physiologically low oxygen enhances biomolecule production and stemness of mesenchymal stem cell spheroids. Tissue Eng Part C Methods. 2016;22(4):360–369. doi: 10.1089/ten.tec.2015.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai A.-C., Liu Y., Yuan X., Ma T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng Part A. 2015;21(9-10):1705–1719. doi: 10.1089/ten.tea.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bijonowski B.M., Daraiseh S.I., Yuan X., Ma T. Size-dependent cortical compaction induces metabolic adaptation in mesenchymal stem cell aggregates. Tissue Eng Part A. 2019;25(7-8):575–587. doi: 10.1089/ten.tea.2018.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan W., Diao S., Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther. 2021;12:140. doi: 10.1186/s13287-021-02194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasztorek M., Rossmanith E., Mayr C., Hauser F., Jacak J., Ebner A., et al. Influence of platelet lysate on 2D and 3D amniotic mesenchymal stem cell cultures. Front Bioeng Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berlier J.L., Rigutto S., Dalla Valle A., Lechanteur J., Soyfoo M.S., Gangji V., et al. Adenosine triphosphate prevents serum deprivation-induced apoptosis in human mesenchymal stem cells via activation of the MAPK signaling pathways. Stem Cells. 2015;33(1):211–218. doi: 10.1002/stem.1831. [DOI] [PubMed] [Google Scholar]
- 76.Jeske S.S., Theodoraki M.N., Boelke E., Laban S., Brunner C., Rotter N., et al. Adenosine production in mesenchymal stromal cells in relation to their developmental statusAdenosinproduktion in mesenchymalen Stromazellen in Bezug auf deren Entwicklungsstatus. HNO. 2020;68(2):87–93. doi: 10.1007/s00106-019-00805-z. [DOI] [PubMed] [Google Scholar]
- 77.Kerkelä E., Laitinen A., Räbinä J., Valkonen S., Takatalo M., Larjo A., et al. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells. 2016;34(3):781–790. doi: 10.1002/stem.2280. [DOI] [PubMed] [Google Scholar]
- 78.Mohammadalipour A., Dumbali S.P., Wenzel P.L. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.603292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clemot M., Senos Demarco R., Jones D.L. Lipid mediated regulation of adult stem cell behavior. Front Cell Dev Biol. 2020;8:115. doi: 10.3389/fcell.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lochner M., Berod L., Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36(2):81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Yan J., Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020;30(12):979–989. doi: 10.1016/j.tcb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Ma Y., Wang L., Yang S., Liu D., Zeng Y.i., Lin L., et al. The tissue origin of human mesenchymal stem cells dictates their therapeutic efficacy on glucose and lipid metabolic disorders in type II diabetic mice. Stem Cell Res Ther. 2021;12(1) doi: 10.1186/s13287-021-02463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng L., Yu P., Li F., Jiang X., Jiao X., Shen Y., et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34(6):1697–1708. doi: 10.1007/s13577-021-00593-1. [DOI] [PubMed] [Google Scholar]
- 84.Campos A.M., Maciel E., Moreira A.S.P., Sousa B., Melo T., Domingues P., et al. Lipidomics of mesenchymal stromal cells: understanding the adaptation of phospholipid profile in response to pro-inflammatory cytokines. J Cell Physiol. 2016;231(5):1024–1032. doi: 10.1002/jcp.25191. [DOI] [PubMed] [Google Scholar]
- 85.Casati S., Giannasi C., Niada S., Bergamaschi R.F., Orioli M., Brini A.T. Bioactive lipids in MSCs biology: state of the art and role in inflammation. Int J Mol Sci. 2021;22(3):1481. doi: 10.3390/ijms22031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fontaine M.J., Shih H., Schäfer R., Pittenger M.F. Unraveling the mesenchymal stromal cells' paracrine immunomodulatory effects. Transfus Med Rev. 2016;30(1):37–43. doi: 10.1016/j.tmrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 87.Auletta J.J., Eid S.K., Wuttisarnwattana P., Silva I., Metheny L., Keller M.D., et al. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells. 2015;33(2):601–614. doi: 10.1002/stem.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai M., Zhang L., Fu B., Bai J., Zhang Y., Cai G., et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93:814–825. doi: 10.1016/j.kint.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 89.Kawata Y., Tsuchiya A., Seino S., Watanabe Y., Kojima Y., Ikarashi S., et al. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019;376(2):257–271. doi: 10.1007/s00441-018-02981-w. [DOI] [PubMed] [Google Scholar]
- 90.Németh K., Leelahavanichkul A., Yuen P.S.T., Mayer B., Parmelee A., Doi K., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao X., Duan L., Hou H., Liu Y., Chen S., Zhang S., et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics. 2020;10(17):7697–7709. doi: 10.7150/thno.45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das U.N. Ageing: Is there a role for arachidonic acid and other bioactive lipids? A review. J Adv Res. 2018;11:67–79. doi: 10.1016/j.jare.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fang X., Abbott J., Cheng L., Colby J.K., Lee J.W., Levy B.D., et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol. 2015;195(3):875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 94.Neels J.G., Grimaldi P.A. Physiological functions of peroxisome proliferator-activated receptor beta. Physiol Rev. 2014;94:795–858. doi: 10.1152/physrev.00027.2013. [DOI] [PubMed] [Google Scholar]
- 95.Jitschin R., Böttcher M., Saul D., Lukassen S., Bruns H., Loschinski R., et al. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia. 2019;33(7):1783–1796. doi: 10.1038/s41375-018-0376-6. [DOI] [PubMed] [Google Scholar]
- 96.Chang C.Y., Park J.H., Ouh I.-O., Gu N.-Y., Jeong S.Y., Lee S.-A., et al. Novel method to repair articular cartilage by direct reprograming of prechondrogenic mesenchymal stem cells. Eur J Pharmacol. 2021;911:174416. doi: 10.1016/j.ejphar.2021.174416. [DOI] [PubMed] [Google Scholar]
- 97.Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 98.Chiu M., Taurino G., Bianchi M.G., Bussolati O. The role of amino acids in the crosstalk between mesenchymal stromal cells and neoplastic cells in the hematopoietic niche. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.714755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rashed L., Gharib D.M., Hussein R.E., Tork O., Abusree A. Combined effect of bone marrow derived mesenchymal stem cells and nitric oxide inducer on injured gastric mucosa in a rat model. Tissue Cell. 2016;48(6):644–652. doi: 10.1016/j.tice.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 100.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 101.Chen X., Gan Y., Li W., Su J., Zhang Y., Huang Y., et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014;5(1):e1009. doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10 doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pithon-Curi T.C., De Melo M.P., Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: a comparative study. Cell Biochem Funct. 2004;22(5):321–326. doi: 10.1002/cbf.1109. [DOI] [PubMed] [Google Scholar]
- 104.Liu P.S., Wang H., Li X., Chao T., Teav T., Christen S., et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 105.Yu Y., Newman H., Shen L., Sharma D., Hu G., Mirando A.J., et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29(4):966–978.e4. doi: 10.1016/j.cmet.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.dos Santos G.G., Hastreiter A.A., Sartori T., Borelli P., Fock R.A. L-glutamine in vitro modulates some immunomodulatory properties of bone marrow mesenchymal stem cells. Stem Cell Rev Rep. 2017;13(4):482–490. doi: 10.1007/s12015-017-9746-0. [DOI] [PubMed] [Google Scholar]
- 107.He H., Takahashi A., Mukai T., Hori A., Narita M., Tojo A., et al. The immunomodulatory effect of triptolide on mesenchymal stromal cells. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.686356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee H.-J., Jung H., Kim D.-K. IDO and CD40 may be key molecules for immunomodulatory capacity of the primed tonsil-derived mesenchymal stem cells. Int J Mol Sci. 2021;22(11):5772. doi: 10.3390/ijms22115772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haghighitalab A., Matin M.M., Amin A., Minaee S., Bidkhori H.R., Doeppner T.R., et al. Investigating the effects of IDO1, PTGS2, and TGF-beta1 overexpression on immunomodulatory properties of hTERT-MSCs and their extracellular vesicles. Sci Rep. 2021;11:7825. doi: 10.1038/s41598-021-87153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim D.S., Jang I.K., Lee M.W., Ko Y.J., Lee D.H., Lee J.W., et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-gamma. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manganeli Polonio C., Longo de Freitas C., Garcia de Oliveira M., Rossato C., Nogueira Brandão W., Ghabdan Zanluqui N., et al. Murine endometrial-derived mesenchymal stem cells suppress experimental autoimmune encephalomyelitis depending on indoleamine-2,3-dioxygenase expression. Clin Sci (Lond) 2021;135(9):1065–1082. doi: 10.1042/CS20201544. [DOI] [PubMed] [Google Scholar]
- 112.de Witte S.F.H., Merino A.M., Franquesa M., Strini T., van Zoggel J.A.A., Korevaar S.S., et al. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res Ther. 2017;8(1) doi: 10.1186/s13287-017-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matysiak M., Stasiołek M., Orłowski W., Jurewicz A., Janczar S., Raine C.S., et al. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J Neuroimmunol. 2008;193(1-2):12–23. doi: 10.1016/j.jneuroim.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S., Zhang Q.Z., Karabucak B., Le A.D. DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/IDO axis. J Dent Res. 2016;95:1274–1281. doi: 10.1177/0022034516657817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trikha P., Moseman J.E., Thakkar A., Campbell A.R., Elmas E., Foltz J.A., et al. Defining the AHR-regulated transcriptome in NK cells reveals gene expression programs relevant to development and function. Blood Adv. 2021;5(22):4605–4618. doi: 10.1182/bloodadvances.2021004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Manni G., Mondanelli G., Scalisi G., Pallotta M.T., Nardi D., Padiglioni E., et al. Pharmacologic induction of endotoxin tolerance in dendritic cells by L-kynurenine. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zang X., Zheng X., Hou Y., Hu M., Wang H., Bao X., et al. Regulation of proinflammatory monocyte activation by the kynurenine-AhR axis underlies immunometabolic control of depressive behavior in mice. FASEB J. 2018;32(4):1944–1956. doi: 10.1096/fj.201700853R. [DOI] [PubMed] [Google Scholar]
- 118.Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S., et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 119.Campesato L.F., Budhu S., Tchaicha J., Weng C.-H., Gigoux M., Cohen I.J., et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plitman E., Iwata Y., Caravaggio F., Nakajima S., Chung J.K., Gerretsen P., et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(4):764–777. doi: 10.1093/schbul/sbw221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lovelace M.D., Varney B., Sundaram G., Lennon M.J., Lim C.K., Jacobs K., et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373–388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 122.Zhang S., Fang J., Liu Z., Hou P., Cao L., Zhang Y., et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res Ther. 2021;12(1) doi: 10.1186/s13287-020-02118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baumgartner R., Berg M., Matic L., Polyzos K.P., Forteza M.J., Hjorth S.A., et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J Intern Med. 2021;289(1):53–68. doi: 10.1111/joim.13142. [DOI] [PubMed] [Google Scholar]
- 124.Lee T., Park H.S., Jeong J.H., Jung T.W. Kynurenic acid attenuates pro-inflammatory reactions in lipopolysaccharide-stimulated endothelial cells through the PPARdelta/HO-1-dependent pathway. Mol Cell Endocrinol. 2019;495 doi: 10.1016/j.mce.2019.110510. [DOI] [PubMed] [Google Scholar]
- 125.Lee K., Kwak J.H., Pyo S. Inhibition of LPS-induced inflammatory mediators by 3-hydroxyanthranilic acid in macrophages through suppression of PI3K/NF-kappaB signaling pathways. Food Funct. 2016;7:3073–3082. doi: 10.1039/c6fo00187d. [DOI] [PubMed] [Google Scholar]
- 126.Berg M., Polyzos K.A., Agardh H., Baumgartner R., Forteza M.J., Kareinen I., et al. 3-Hydroxyanthralinic acid metabolism controls the hepatic SREBP/lipoprotein axis, inhibits inflammasome activation in macrophages, and decreases atherosclerosis in Ldlr-/- mice. Cardiovasc Res. 2020;116(12):1948–1957. doi: 10.1093/cvr/cvz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kelly B., Pearce E.L. Amino assets: how amino acids support immunity. Cell Metab. 2020;32(2):154–175. doi: 10.1016/j.cmet.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 128.Kelly B., O'Neill L.AJ. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27(1):451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 130.Kang K., Reilly S.M., Karabacak V., Gangl M.R., Fitzgerald K., Hatano B., et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galvan-Pena S., O'Neill L.A. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pan L., Liu C., Liu Q., Li Y., Du C., Kang X., et al. Human Wharton's jelly-derived mesenchymal stem cells alleviate concanavalin A-induced fulminant hepatitis by repressing NF-kappaB signaling and glycolysis. Stem Cell Res Ther. 2021;12:496. doi: 10.1186/s13287-021-02560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Böttcher M., Hofmann A.D., Bruns H., Haibach M., Loschinski R., Saul D., et al. Mesenchymal stromal cells disrupt mTOR-signaling and aerobic glycolysis during T-cell activation. Stem Cells. 2016;34(2):516–521. doi: 10.1002/stem.2234. [DOI] [PubMed] [Google Scholar]
- 134.Franco da Cunha F., Andrade-Oliveira V., Candido de Almeida D., Borges da Silva T., Naffah de Souza Breda C., Costa Cruz M., et al. Extracellular vesicles isolated from mesenchymal stromal cells modulate CD4(+) T lymphocytes toward a regulatory profile. Cells. 2020;9(4):1059. doi: 10.3390/cells9041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Souza-Moreira L., Soares V.C., Dias S., Bozza P.T. Adipose-derived mesenchymal stromal cells modulate lipid metabolism and lipid droplet biogenesis via AKT/mTOR -PPARgamma signalling in macrophages. Sci Rep. 2019;9:20304. doi: 10.1038/s41598-019-56835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vasandan A.B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi X., Liu J., Chen D., Zhu M., Yu J., Xie H., et al. MSC-triggered metabolomic alterations in liver-resident immune cells isolated from CCl4-induced mouse ALI model. Exp Cell Res. 2019;383(2):111511. doi: 10.1016/j.yexcr.2019.111511. [DOI] [PubMed] [Google Scholar]
- 138.Cui Y., Liu S., Zhang X., Ding X., Duan X., Zhu Z., et al. Metabolomic analysis of the effects of adipose-derived mesenchymal stem cell treatment on rats with sepsis-induced acute lung injury. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jackson M.V., Morrison T.J., Doherty D.F., McAuley D.F., Matthay M.A., Kissenpfennig A., et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan Y., Yuan L., Li L., Liu F., Liu J., Chen Y., et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1alpha activation. Stem Cells. 2021;39:913–928. doi: 10.1002/stem.3375. [DOI] [PubMed] [Google Scholar]
- 141.Agrawal M., Rasiah P.K., Bajwa A., Rajasingh J., Gangaraju R. Mesenchymal stem cell induced Foxp3(+) tregs suppress effector T cells and protect against retinal ischemic injury. Cells. 2021;10(11):3006. doi: 10.3390/cells10113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Court A.C., Le‐Gatt A., Luz‐Crawford P., Parra E., Aliaga‐Tobar V., Bátiz L.F., et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2) doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Piekarska K., Urban-Wójciuk Z., Kurkowiak M., Pelikant-Małecka I., Schumacher A., Sakowska J., et al. Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-28338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Do J.-s., Zwick D., Kenyon J.D., Zhong F., Askew D., Huang A.Y., et al. Mesenchymal stromal cell mitochondrial transfer to human induced T-regulatory cells mediates FOXP3 stability. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luz-Crawford P., Hernandez J., Djouad F., Luque-Campos N., Caicedo A., Carrère-Kremer S., et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res Ther. 2019;10(1) doi: 10.1186/s13287-019-1307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gentile P., Garcovich S. Systematic review: adipose-derived mesenchymal stem cells, platelet-rich plasma and biomaterials as new regenerative strategies in chronic skin wounds and soft tissue defects. Int J Mol Sci. 2021;22(4):1538. doi: 10.3390/ijms22041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gentile P., Sterodimas A., Calabrese C., Garcovich S. Systematic review: advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res Ther. 2021;12:318. doi: 10.1186/s13287-021-02397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gentile P., Sterodimas A., Pizzicannella J., Dionisi L., De Fazio D., Calabrese C., et al. Systematic review: allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int J Mol Sci. 2020;21(14):4982. doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gentile P., Scioli M.G., Cervelli V., Orlandi A., Garcovich S. Autologous micrografts from scalp tissue: trichoscopic and long-term clinical evaluation in male and female androgenetic alopecia. Biomed Res Int. 2020;2020:1–10. doi: 10.1155/2020/7397162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gentile P. Autologous cellular method using micrografts of human adipose tissue derived follicle stem cells in androgenic alopecia. Int J Mol Sci. 2019;20(14):3446. doi: 10.3390/ijms20143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gentile P., Sterodimas A., Calabrese C., De Angelis B., Trivisonno A., Pizzicannella J., et al. Regenerative application of stromal vascular fraction cells enhanced fat graft maintenance: clinical assessment in face rejuvenation. Expert Opin Biol Ther. 2020;20(12):1503–1513. doi: 10.1080/14712598.2020.1815703. [DOI] [PubMed] [Google Scholar]
- 153.Gentile P., Garcovich S. Adipose-derived mesenchymal stem cells (AD-MSCs) against ultraviolet (UV) radiation effects and the skin photoaging. Biomedicines. 2021;9(5):532. doi: 10.3390/biomedicines9050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grimaldi M., Gentile P., Labardi L., Silvi E., Trimarco A., Cervelli V. Lipostructure technique in Romberg syndrome. J Craniofac Surg. 2008;19:1089–1091. doi: 10.1097/SCS.0b013e318176354a. [DOI] [PubMed] [Google Scholar]
- 155.Cervelli V., Gentile P. Use of cell fat mixed with platelet gel in progressive hemifacial atrophy. Aesthetic Plast Surg. 2009;33(1):22–27. doi: 10.1007/s00266-008-9223-x. [DOI] [PubMed] [Google Scholar]
- 156.Gentile P. Breast silicone gel implants versus autologous fat grafting: biomaterials and bioactive materials in comparison. J Clin Med. 2021;10(15):3310. doi: 10.3390/jcm10153310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gentile P., Scioli M.G., Bielli A., Orlandi A., Cervelli V. Concise review: the use of adipose-derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells. 2017;35:117–134. doi: 10.1002/stem.2498. [DOI] [PubMed] [Google Scholar]
- 158.Cervelli V., Bocchini I., Di Pasquali C., De Angelis B., Cervelli G., Curcio C.B., et al. P.R.L. platelet rich lipotransfert: our experience and current state of art in the combined use of fat and PRP. Biomed Res Int. 2013;2013:1–9. doi: 10.1155/2013/434191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scioli M.G., Bielli A., Gentile P., Cervelli V., Orlandi A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med. 2017;11(8):2398–2410. doi: 10.1002/term.2139. [DOI] [PubMed] [Google Scholar]
- 160.Gentile P., Casella D., Palma E., Calabrese C. Engineered fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: clinical, histological and instrumental evaluation in breast reconstruction. J Clin Med. 2019;8(4):504. doi: 10.3390/jcm8040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gentile P., Garcovich S. Concise review: adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and proMote wound repair. J Clin Med. 2019;8(6):855. doi: 10.3390/jcm8060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gentile P. New strategies in plastic surgery: autologous adipose-derived mesenchymal stem cells contained in fat grafting improves symptomatic scars. Front Biosci (Landmark Ed) 2021;26:255–257. doi: 10.52586/4940. [DOI] [PubMed] [Google Scholar]
- 163.Gentile P., Scioli M.G., Bielli A., De Angelis B., De Sio C., De Fazio D., et al. Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7(2):27. doi: 10.3390/biomedicines7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gentile P., Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil((R)), finasteride((R)), and adult stem cell-based therapy. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trink A., Sorbellini E., Bezzola P., Rodella L., Rezzani R., Ramot Y., et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169(3):690–694. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- 166.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gentile P., Sterodimas A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin Biol Ther. 2020;20(7):711–716. doi: 10.1080/14712598.2020.1761322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gentile P., Sterodimas A., Pizzicannella J., Calabrese C., Garcovich S. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVID-19 disease. Aging Dis. 2020;11:1191–1201. doi: 10.14336/AD.2020.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]