Abstract
Immobilization material has slowly revolutionized since 3000 BCE from traditional plaster to modern day synthetic casting tape, including other sustainable immobilization material. This revolution is driven by the search for superior casting material that possesses excellent mechanical and load-bearing properties, non-toxicity, excellent healing rates, patient satisfaction and eco friendliness. Even though the new materials have been evolved, the traditional plaster still remains a material of choice owing to its excellent skin conformability, low cost and availability. This paper aims to present a comprehensive review on the technique of immobilization, existing orthopedic immobilization (casting and splinting) materials and complications associated with immobilization (mainly casting) which aimed to assist the medical practitioners and researchers in casting material improvements and selection.
Nine immobilization materials are comprehensively discussed for their desirable properties, drawbacks and the required improvements to the composition, along with the most common cast complications ranging from superficial pressure sores to compartment syndrome and Deep Vein Thrombosis. . This paper identifies that among the existing material, plaster casts are still highly used due to their cost benefit and the ability to fit patients into full body casts, while synthetic material is too rigid and has a higher probability of causing complications such as compartment syndrome and deep vein thrombosis. Patients show a higher preference in using synthetic casts for short term and body extremity casting as they are comparatively more comfortable. New materials such as Woodcast shows good promise but their mechanical characteristics and comfort are yet to be critically analyzed. However, there exists an imminent requirement to upgrade existing material as well as to introduce novel promising sustainable material for long term immobilization.
Keywords: Plaster of Paris, Immobilization, Synthetic, Cast complications, Fiberglass
Highlights
-
•
Plaster of paris is the benchmark material for orthopedic fracture immobilization.
-
•
SynThetic and novel ecofriendly orthopedic casting materials are gaining popularity due to their superior strength properties and growing interest in sustainability.
-
•
Cast complications arise from poor casting techniques, rigid casting and cast removal, among other patient factors.
-
•
Further modifications t are required for superior strength gain, material characteristics, and patient comfort.
1. Introduction
In modern day orthopedics fracture healing, post-operative treatment related to orthopedic trauma and support for structural deformities are conventionally treated by immobilization using casting or splinting. The process of immobilization, promotes fracture healing by limiting motion at the fracture site, preventing damage to the surrounding soft tissues and the neurovasculature [1]. Limiting movement at the fracture site under rigid stabilization enables direct osteonal remodeling with little to no external callus formation. This is known as direct bone repair under primary healing [2,3]. Direct bone repair also occurs when a small degree of movement takes place under less rigid interfragmentary stabilization. Here, endochondreal healing at the fracture site or intramembraneous ossification in the periosteum takes place as shown in Fig. 1 [4,5]. This condition is generally seen in orthopedic cast or splint immobilization and external fixtures [6]. With higher degrees of interfragmnetary movement hypertrophic nonunion can occur at the fracture site which can have deleterious effects on the fractured bone.
Fig. 1.
The main stages of the fracture healing process 1) Hematoma formation 2) Fibrocartilaginous callus formation 3) Bony callus formation 4) Bone remodeling [5].
Even though pin fixation through operative proceedures are recommended for treatment of unstable severe fractures, cast immobilization is still carried out as a follow up procedure [6,7]. Generally, the immobilization period for a fracture is 4–6 weeks but this varies with the bone where the fracture is located in and the nature of complications [3,6]. Table 1 refers to the minimum immobilization periods recommended for commonly fractured bones.
Table 1.
The most common immobilization periods and the average period for fracture union for commonly fractured bones for cases with no complications [8].
| Bone | Most common immobilization periods with no complications (weeks) |
Average period for fracture union with no complications (weeks) |
||
|---|---|---|---|---|
| Adult | Children <10 years | Adult | Children <10 years | |
| Metacarpal | 4–6 | 2–3 | 6 | 4–6 |
| Scaphoid | 8–12 | 8–10 | 15–20 | 12 |
| Carpal | 4–6 | 2–3 | 6 | 4–6 |
| Ulna | 4–6 | 3–4 | 6–8 | 4–6 |
| Radius | 4–6 | 3–4 | 6–8 | 4–6 |
| Humerus | 4–6 | 3–4 | 6–8 | 4–6 |
| Clavicle | 4 | 2–3 | 4 | 2–3 |
| Scapula | 4 | 2–3 | 4 | 2–3 |
| Ribs | 4–6 | 2–4 | 4 | 2–3 |
| Vertebral bones | 6–8 | 4–6 | 12 | 6–8 |
| Pelvic bones | 6–8 | 4–6 | 6–8 | 4–6 |
| Femur | 6–8 | 4–6 | 12 | 6–8 |
| Tibia | 6–8 | 4–6 | 12 | 6–8 |
| Talus | 6–8 | 4–6 | 12 | 6–8 |
| Calcaneuss | 6–8 | 4–6 | 12 | 6–8 |
| Phalanges | 4–6 | 2–3 | 6 | 4–6 |
Casting is the preferred technique for long term immobilization of definitive or complex fractures, whereas splinting is preferred for short term immobilization of stable or simple fractures and soft tissue strains and sprains [9,10]. Due to the rigid circumferential immobilization provided by casts, acute post-injury immobilization that accommodates swelling is carried out by splinting [11,12]. Casts and splints are also used in correction of congenital deformities associated with the musculoskeletal system [109] such as clubfoot, hip displacements and scoliosis [3] and in diabetic foot care [13].
1.1. Research gap
The first recorded use of orthopedic cast material dates back to the early 3000BCE and has evolved to the current benchmark materials-plaster impregnated bandages and synthetic fiber glass tape [14,15]. These materials have slowly evolved throughout the years registering higher strength, better patient comfort, non-toxicity and higher concern for the environment. It is crucial for medical practitioners and researchers to have a comprehensive understanding of the existing orthopedic cast material. The awareness of their advantages and drawbacks is mandatory for the selection of the best fit material for immobilization requirements. However, there is a significant gap between the most widely used orthopedic immobilization material and the newer material entering the market.
This gap is primarily due to plaster based material being widely used and covered under insurance schemes while newer material are not widely used nor covered under medical insurance in many countries. The plaster based material is however dormant in their material characteristic improvement over the decades, despite their extensive usage, nor is the medical practitioners aware of the materials available along with their pros and cons. Knowledge on the available material allows better utilization of the material for fracture immobilization, minimizing medical costs and patient discomfort.
1.2. Aims and objectives based on existing literature
This paper presents a comprehensive review of the technique of immobilization, existing orthopedic immobilization (casting and splinting) material and complications associated with immobilization (mainly casting), aimed to assist medical practitioners and researchers casting material selection and improvement. However, the main focus of this paper is immobilization through casting, although splinting is discussed where appropriate to provide a holistic overview on the revolution in immobilization materials.
In lieu with the research gap identified, this paper broadly reviews existing orthopedic cast material highlighting the advantages and drawbacks of each material. This aims to point out the mandatory specifications of an ideal cast material for extended immobilization, in order to promote the development of more suitable immobilization material in future. Further, the paper aims to bring together the current immobilization material and the complications of casting for the better utilization of the existing material to reduce patient discomfort, enabling medical practitioners to identify the best material for the immobilization requirement, based on the length of immobilization, nature of the fracture, affordability and patient factors such as skin sensitivity.
Hence, a systematic review investigating the evolution of the orthopedic casting materials and the techniques is important to identify challenges and future opportunities in this area.
2. Material evolution and technique of casting
2.1. Evolution of casting and splinting
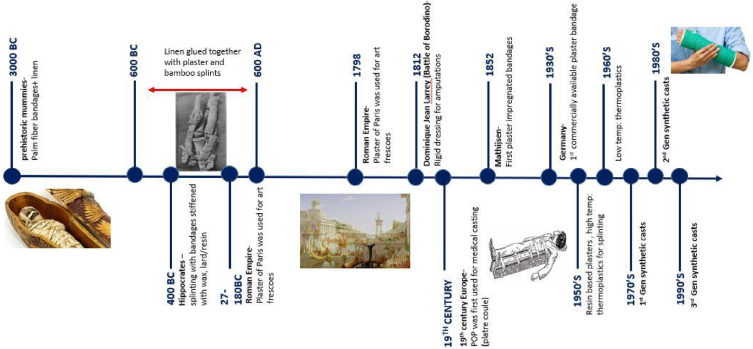
According to the Edwin Smith's surgical papyrus, the earliest use of fracture immobilization techniques dates back to 3000 BCE, where splints designed from sticks and palm fiber bandages and linen were found from prehistoric mummies [16]. Hippocrates had documented the importance of splinting and use of bandages stiffened with wax, lard or resin and multiple bandage layers for fracture care. It is manuscripted that linen glued together with plaster and splints made of bamboo were in use from 600 BCE to 600AD [9,15].
Although plaster came to existence with the development of art plaster frescoes during the Roman Empire, it was not used for medical care at the time [17]. “Plaster of Paris” (POP) was introduced as a replacement for lime used in bandage stiffening in 1798 [16].
During the battle of Borodino, Dominique Jean Larrey (1766–1842) applied a rigid dressing to a wounded infantryman following an amputation. Three months later, upon the removal of the dressing, the residual limb had healed. This concluded that the rigid dressing immobilization accelerated fracture healing [[14], [15], [16],18]. With the acceptance of limb immobilization for fracture healing, the first use of Plaster of Paris as a cast was carried out at the beginning of the 19th century in Europe. They poured Plaster of Paris around fractured limbs encased in a wooden construct, which was known as plâtre coulé. The technique of plâtre coulé has refined patients to one place during the entire healing process due to the weight of the cast [14,15]. The first Plaster of Paris bandage was invented in 1852 by A. Mathijsen, a medical officer of the Dutch army. Mathijsen manually rubbed Plaster of Paris on coarse cotton bandages in cast preparation, a method similar to the plaster impregnated bandages used today [19,20]. Since the Crimean war (1854–1856) Plaster of Paris (POP) bandages have been extensively used in fracture immobilization, through which the modern fracture immobilization technique emerged [2,18]. The early plaster bandages were made by soaking woven bandages in freshly prepared plaster by the hospital staff. The first commercially manufactured plaster bandages were available in Germany during the early 1930s, where plaster was mixed with minor quantities of volatile liquids on soft cloth bandages [2,15].
During the 1950s resin-based plasters were introduced along with high temperature thermoplastic material gaining popularity in splinting. Low-temperature thermoplastics were introduced during the 1960s replacing these high temperature thermoplastics and the 1970s mark the first generation of synthetic casts [19] with the 1980s demarking the second generation of synthetic casts. Generation 2 synthetic casts were commercialized with improved conformability and better handling ability with low tack Polyurethane (PU) resins. Since the 1990s, the third generation of synthetic casts has evolved with materials such as high strength polyester, registering enhanced conformability and elasticity [2] (Refer Fig. 2).
Fig. 2.
Timeline of evolution of orthopedic immobilization material developed by the authors.
2.2. Cast application and techniques
Prior to immobilization, it is important to have a clear idea of the neurovasculature and the surrounding anatomy. The skin must be observed for any inflammation, lesions or soft tissue injuries [21,22]. According to Smeltzer et al. assessing the five Ps: pain, pallor, pulselessness, paresthesia and paralysis is mandatory [22,23] to minimize cast complications. In high risk patient groups such as pediatric patients, multi-trauma patients, comatose patients, patients under anesthesia and patients with developmental disorders where effective communication is impassable, certain casting material and techniques are recommended to avoid morbidity. However, when the fracture site is prone to swelling and bleeding, temporary immobilization using a splint is recommended [1].
To ensure proper fracture alignment and minimal complications, cast application has to be carried out by practiced orthopedic technicians or medical practitioners. The proper application technique includes the placement of the limb in the desired position for comfort[108]. Casts are applied as a total contact cast, which is directly applied to the skin, or applied over a stockinette sleeve and layers of padding [22,24].
The application of plaster casts is technically simple compared to other cast materials [25]. Initially, a stockinette is applied to the skin followed by padding wrap for patient comfort. Practitioners can slit the padding along the entire length of the limb and apply a layer over the slit about ¾ the circumference, to accommodate any post traumatic swelling. The ends of the stockinette sleeve can be folded back over the padding to provide cushioned ends and better ventilation into the cast [24,26,27]. Next, the plaster/synthetic tape bandages are then dipped in tepid water. The ideal dip water temperature is maintained between 32 and 39 °C [28,29]. It is important to change the dip water after each application to remove plaster/synthetic resin deposits that can increase the dip water temperature. Practitioners are recommended to use gloves when using synthetic cast material to avoid exfoliative dermatitis and skin irritation [26].
Plaster/synthetic bandages are stretch-relaxed and laid up not pulled. As casts are generally applied over a layer of padding, the use of a very thick layer of padding can weaken the plaster. An ideal cast is required to be thin and strong. Generally, 7 to 8 layers of bandage are used in plaster casts and 3–4 layers of synthetic tape in a fiberglass and other synthetic casts [28]. Rubbing each layer before the next layup ensures bonding between layers for stronger and lighter casts [22,24,27]. According to Luck [18] rubbing under pressure can damage the underlying neurovasculature hence adequate force has to be applied during the process. Alternatives to the general application process of fiberglass tape was discussed by Smith et al. [26], where they applied fiberglass tape without water immersion and used a water-based gel on fiberglass tape to activate the resins.
As plaster and fiberglass bandages are dipped in tepid water, fast dipping and aggressive squeezing off of the water can result in making the casts hotter due to water inadequacy, resulting in cast burns. Luck [18] states that longer soaking times (>4 min) associated with higher dip water temperatures (>95 °F) results in a steady decline of the compressive strength of plaster casts. Hence, dip water temperature and soaking time must be properly managed by technicians for stronger and safer cast application.
For proper immobilization, the casts must completely dry. This depends on the cast thickness and the material used. The complete drying of a plaster cast takes 36–72 h and can become weight bearing only after about 48 h in the case of long leg casts and hip spica casts. However, the curing time for fiberglass and other synthetic cast material is 25–30 min and can immediately bear weight upon hardening.
As pointed out under aims and objectives, the selection of the cast material is based on the application, length of immobilization, material availability and cost concerns.
3. Methods
To fulfill the aim of this study, the authors applied a six-step method (refer Fig. 3).
Fig. 3.
Research steps followed.
Under step 1, the authors carried out a preliminary review to understand the evolution of the immobilization material, their history, techniques in cast application, to build up the basis for the review. Precise identification of terminology and techniques of casting and splinting was carried out. The detailed outcomes of the preliminary review are presented under Section 2. The authors have identified that the technique of fracture immobilization dates back to the early 3000 BCE, while understanding the underlying anatomy and accounting for the 4Ps is important before the application of the casts and splints.
Based on the results obtained in Step 1, the existing immobilization material were identified through secondary data sources. The secondary data sources used under Step 2 are, technical papers, journal articles, medical journals, MSDs, manufacturer's websites and reports and standard medical articles and books. The nature of the immobilization material along with their benefits and drawbacks are identified under Step 2.
Under Step 3, the complications associated with immobilization is broadly discussed. This provides a complete understanding of the most critical complications that immobilization is associated with. Similar to Step 2, the data was gathered through secondary data sources inclusive of patient feedback forms.
Step 4 provides a systematic review of the journal papers selected in Steps 2and 3. This systematic literature review was carried out via the “Preferred reporting Items for Systematic Reviews and meta-Analyses (PRISMA)” that provides a higher transparency into the reported systematic review [30].
The search for literature was done using abstract and citation databases such as PubMed, Medline, Science Direct, Scopus and IEEE Xplore digital Library for Peer-reviewed articles published from 1980 to 2022. This time frame was decided as studies on plaster modifications were carried out earlier on and a comprehensive understanding on gypsum plaster casts is crucial to this review. The literature search comprised of fields “article title”; ”abstract”; and “keywords”. The selection of the keywords was done through the preliminary examination of accessible literature and former reviews carried out on related titles and through the identification of recurring themes. The authors agreed upon a set of keywords to increase the probability of identifying of all relevant publications. The Boolean operators AND and OR were used in the syntax. The following search terms were used for the query: (“casting*” or “splinting*”) and (“immobilization*” or (“plaster” and “gypsum”) or (“synthetic” and “casting” and “material”)) and ((“fibreglass” or “hybrid” or “polyester” or “polyurethane” or “thermoplastics” or “silicone” or “rubber”) or (“casting” or “orthopedic” or “novel”) or (“new” or “composites” or “bioplastic” or “PLA” or “wood” or “sustainable” or “flammability” or “radiolucency” or “comfort” or biodegradable”)) or ((“thermal” and “burns” or “pressure” and “sores” or “thrombosis” or “swelling” or “hemotoma”) or (“cast” and“saw” and “burns”) or (“venous” and“congestion”) (“casting” and “complications”)).
The initial database searches generated 948 publications, and 31 additional studies were sought from reference lists of retrieved studies and review articles (Fig. 3). Following the removal of duplicates, records published before 1980, and irrelevant citations based on fast screening, 135 publications were further assessed for selection. Given the study scope and data acquisition requirements, original studies were eligible to be included in this review when: 1) the immobilization material was examined and analyzed; 2) the complications associated with immobilization was discussed. A total of 77 studies met all the selection criteria and were included in the final data synthesis of the review. The AI assisted tool, Research Rabbit [31] was used to search more refined papers with higher relevance to be added to the 77 papers already chosen through the selection criteria. Relevant documents were collected and organized in EndNote 20. Lastly, the findings were analyzed and discussed under Step 5.
PRISMA flowchart is given in Fig. 4.
Fig. 4.
PRISMA flowchart.
4. Findings
Based on the findings a detailed analysis of the existing orthopedic immobilization material and the complications associated with immobilization are comprehensively analyzed.
4.1. Existing orthopedic immobilization material
4.1.1. Plaster of paris
Plaster of Paris (POP) is the most popularly used casting and splinting material to this date. Casts and splints fabricated from Plaster is used not only for immobilization of fractures but also in the correction of bone related deformities such as clubfoot and as a support for sprained ligaments and inflamed or infected soft tissues [15].
To refine Plaster of Paris, gypsum is heated to about 120 °C, which removes most of the water from the crystals resulting in a powdery residue [32].
| (1) |
Plaster, promptly absorbs water and undergo crystallization in a rapid exothermic reaction. The complete reaction happens in two steps. Initially there is a slight expansion of volume during the setting stage and becomes a porous mass at the hardening stage [15].
When observed under a microscope, the rapid forming crystals during the setting stage, tightly interlock resulting in good mechanical strength gain and rigidity. For proper crystal interlocking, immobility of the plaster cast is essential. This is called “The Critical Point”, where immobility of the plaster is imperious for it to set properly and become rigid for ultimate strength gain. According to Luck [18], plaster casts that undergo bending after reaching the critical point, showed a 77% reduction of overall strength.
Post crystallization, the excess water evaporates from the surface giving plaster a grainy appearance. The setting time of POP casts depend on the additives [33] used and manufacturers generally provide the cast setting times of the product [16]. Commercially available plaster bandages comprise approximately 85% Calcium Sulphate by weight and binders which enable strong adhesion of the plaster to the bleached cotton fabric. According to Burrows and Milne [34], plaster detaches from the cotton bandage when wetted. To enable strong cohesion of the plaster to the bandage, additives such as hydroxypropylmethylcellulose (dry powder) is added. The composition of the additives is to be maintained at 0.1%–5% by weight to preserve the properties of plaster. To avoid dry plaster detaching from the bandage, a fine mist of propylene glycol is uniformly sprayed on to the plaster bandages [34]. According to Abbood et al. [35], additives such as Tree Glue Powder (TGP) and Polyvinyl Acetate (PVA) have been used to improve the setting time of plaster casts.
Plaster casts show steady strength gain during the first three to six days post application as more and more excess water comes to the surface to evaporate. It is highly recommended to dry plaster casts by natural methods, as artificially generated heat can cause thermal damage to the patient. Upon complete drying, plaster gains porosity, thus allowing the moisture to be absorbed from the underlying skin. The “wick effect” created due to micro cracking also adds to the vapor permeability of plaster. Perspiration, blood and puss are readily absorbed by plaster casts, thus preventing skin maceration [36].
Plaster encompasses many advantages such as availability and low cost, making it the standard casting material. Plaster has a superior ability to confirm to body contours than synthetic material [37,38]. Hence, it is highly recommended when casting extremities. The increased conformity of plaster provides a low tendency to create pressure areas than other materials and less shear, reducing skin irritation. Plaster retains body heat, creating a gentle and neutral warmth with a low probability of resulting in skin lesions during long term immobilization [16].
Practically, plaster is brittle and has poor mechanical properties compared to its synthetic alternatives. Hence, the addition of additives like Calcium Oxide, Ferric Oxide and Gum Arabic significantly improves abrasion resistance, tensile, compressive and transverse strength and reduces the possibility of material fracture upon hardening [32,[39], [40], [41]]. According to Mihalko et al. [42] plaster casts show bilinear characteristics as the plaster and the gauze bandage behaves as two separate units when subjected to tensile testing. Mechanical testing carried out by Agisparayan et al. [43] shows that plaster is susceptible to degradation under impact forces and can only absorb 1.222 J of impact energy when subjected to Charpy impact testing. Although additives have been effective in reducing plaster setting times, lengthy setting times is still a major drawback of plaster casts in comparison to synthetic material. The low fatigue strength of plaster results in increased cast breakage during weight bearing and movement [44] in long leg and hip spica casts. Another major drawback of plaster is its fast degradation when wetted, limiting the ability of patients to bathe and swim while fitted to the cast. Hence, waterproofing plaster casts is a major issue during long term immobilization.
The high mineral content and crystallization of plaster, attenuates and scatters x-rays [45], resulting in a thick radiopaque layer. This makes it difficult to carry out proper x-ray diagnosis of the underlying bone without cast removal [46].
Today gypsona impregnated leno-weave gauze bandages are popularly used than traditional plaster. Gypsona is plaster of paris with minimal additives with a much creamier consistency, but traditional plaster casts are more durable and water resistant than their gypsona alternatives [47]. According to Rowley et al. [49] the nature of the bandage weave has no significant impact on the durability and strength of the cast. Newer variations of plaster such as resin augmented plaster has considerable durability and higher water resistance [48].
4.1.2. Synthetic cast material
Overcoming the drawbacks of the traditional plaster casts, new synthetic cast materials have evolved. These mainly include polyester/cotton knit, fiberglass and thermoplastic [49] cast material. Studies have shown that many water activated synthetic material decrease in stiffness following water immersion (<53%) but regained up to 90% within 24 h [36]. Synthetic material that undergo exothermic reaction [2] upon water immersion record very high temperature levels between the initial 7–10 min, which can result in first degree burns. Hence, caution is necessary when applying synthetic casts on fresh fractures where swelling is highly likely.
Many synthetic casts are porous. Therefore it allows air circulation through the cast reducing the risk of skin maceration. Synthetic materials are better alternatives to traditional plaster in water resistance and radiolucency. Some studies [44,50] have shown that patients prefer synthetic casts to plaster due to better patient comfort, lightweight and superior mechanical properties[107].
4.1.2.1. Fiberglass casts
Today, fiberglass is one of the most commonly used materials for casting and splinting in developed countries. According to Lisa [19] the use of fiberglass casts dates back to the 1970s. The earliest was knitted Fiberglass bandages impregnated with a light-activated resin, which was recorded as the first synthetic cast material to be developed. The earliest fiberglass bandages hardened upon exposure to UV light forming a hard cast. In 1978, the first water activated synthetic fiberglass bandages were introduced by Cutter Biomedical. Dyncast XR (Smith and Nephew), Delta-Lite ‘S’ (Johnson and Johnson), Ortho tape, Scotch cast, Scotchflex (3 M), and Zim-flex are some of the widely used commercial fiberglass brands [46,48,51,52]. Apart from the Urethane pre-polymer, some commercial brands such as Delta- Lite ‘S’ (Johnson and Johnson) and Scotchcast Plus (3 M) include additives such as Silicone [50,52].
Modern fiberglass tape is impregnated with polyurethane or a polyurethane resin. Stretch-relaxed fiberglass tape is dipped in water at 20–25 °C to activate the resin and applied in the same manner as plaster casts. It is best to carry out moulding and conforming after 3–6 min of application before hardening. Some studies [28,53] have shown that temperatures above 49 °C beneath the cast over an extended period of time can cause superficial thermal injury. Hence, extra caution is required when applying extra fast setting and thicker casts with dip water temperatures above 32 °C.
The main advantage of fiberglass casts is their lightweight (i.e. 1/3 the weight of plaster casts) [3], water resistance, durability, quicker setting times and strength (2–3 times stronger than plaster). Load displacement curves for fiberglass tape shows linear characteristics [54] concluding that the resin and bandage act as one unit with yield strengths twice that of plaster [42]. Generally, fiberglass bandages are stronger and rigid across the bandage than along the length [36]. According to Rowley et al. [48] the fast curing nature of fiberglass allows weight bearing earlier on. They further state that in fiberglass and resin bandages, the type of the weave shows a significant impact on the lamination and flexibility of the end product. This is due to the nature of the weave aiding cross –bonding between molecules on either side of the laminate. According to Martin et al. [52] the differences in the mechanical strength and performance among the many varieties of fiberglass tapes mainly vary due to the anisotropy of the material caused by irregular resin distribution along the tapes.
Generally, fiberglass is a porous material hence it reduces the risk of skin related issues making it suitable for long term immobilization [3]. Past studies have proven that fiberglass casts can withstand water degradation [55,56] and can be wetted during bathing, but the drying process has to be carried out with caution adhering to provided instructions. Clinical trials conducted by Inglis et al. [44] recorded that patients treated with fiberglass casts reported no skin complications after swimming and bathing. According to Lisa [19] it takes approximately an hour to completely dry the cast. Commercially available fiberglass casting tape comes in different colors which provide the user with a cosmetic advantage apart from rigid fracture immobilization.
Flammability tests carried out by Wytch et al. [36,45] show that fiberglass casts have a low ignition temperature and a heat transfer rate of 29, although polyurethane used in them is highly flammable. Although other PU resin casts burn easily, the fiberglass in PU coated fiberglass bandages causes a heat sink effect which reduces the local temperature. Thus, fiberglass casts impose a low to moderate risk when operating near naked flames. According to Ref. [48], urethane pre-polymer impregnated fiberglass bandages have higher radiolucency than plaster. Radiographic assessments carried out by Wytch et al. [46], show that fiberglass, although radiolucent than plaster, records varying interference patterns based on the mesh pattern of the bandage. Also, the level of attenuation increased with the increasing number of layers.
Unlike plaster casts which can be handled or moulded without the requirement of gloves, technicians require gloves during application and moulding of fiberglass casts [47]. This is mainly due to the adherence of the water activated resin to the skin [26,49] which facilitates free isocyanate groups to come in contact with the applicator's skin [45], causing exfoliative dermatitis. The vapor permeability of fiberglass-polyurethane bandages is much less compared to the cotton and polyester alternatives available. This makes them unsuitable in the presence of wounds on the skin surface and can result in skin maceration [36].
There is growing concern regarding airborne fiberglass particles and dust during cast removal using cast saws. Past Studies have identified that isocyanates in synthetic cast material can cause occupational asthma, coughing and laryngospasm [36,[45], [57]]. Hence, modern cast saws are equipped with a vacuum to absorb the airborne fiberglass dust.
In order to tackle latex sensitivity of pediatric patients and patients with higher skin sensitivity, newer latex-free casts such as Delta-cast and Flashcast-Elite are available. These comprise of a polyester substrate with extensible yarn encompassing all the properties of the fiberglass casts but with an improved finish and conformability with no fiberglass dust generation during removal.
4.1.2.2. Plaster - fiberglass hybrid
Previous studies have shown that fiberglass and plaster pose qualities that are placed far apart in the spectrum of parameters analyzed for a good fracture casting material [43,55,59,60]. To achieve a feasible solution, Terry and Mark designed a hybrid cast combining traditional plaster and fiberglass, thus optimizing the advantages of both materials [60].
Plaster provides better conformity, economic feasibility and patient comfort while fiberglass provides durability, strength and significantly reduces the weight of the resulting cast [55].
Terry and Mark's hybrid cast consists of two layers of plaster bandage overlaid by two layers of Fiberglass tape. An improved version of the hybrid cast was designed by Agisparayan et al. [43] where 4 layers of fiberglass bandages were laid over 4 layers of plaster placed over a layer of cotton padding. According to Kelly [60] fiberglass tape was used as a reinforcement to enhance durability and water permeability of plaster casts, resulting in a plaster-fiberglass hybrid.
According to Terry and Mark the hybrid cast is more stable and registered fewer fatigue lines compared to traditional plaster when subjected to a Tukey test [55]. Charpy impact testing carried out by Agisparayan et al. [43] showed that plaster is brittle hence fractures with no significant deformation, while fiberglass underwent ductile failure due to fiber rupture. The hybrid cast was able to withstand moderate impact energy and failed under both brittle failure from the inner plaster layers and fiber rupture from the outer fiberglass layers [43]. This proves that the impact forces are predominantly borne by the outer fiberglass tapes before being transferred to the plaster layers beneath. This study showed that no delamination between the two materials were observed at failure. This ensures that the toughness and the impact resistance of the hybrid cast is superior to plaster. However, on removal with a cast saw, the fiberglass tapes completely separate from the underlying plaster suggesting that no significant chemical bond occurs between the two mediums [60].
The hybrid bandages could withstand 15 to 25 times more flexural loads than plaster, before undergoing failure. This was almost similar to the flexural strength of fiberglass with a flexural modulus 3.8–4 times higher than plaster, mainly due to a majority of the flexural loads being borne by the outermost fiberglass layers. Thus, making it suitable for fractures near joints and areas experiencing high stress. The indentation hardness of the hybrid bandages showed almost equal values to that of fiberglass. The main reason being the mesh-like surface of the knitted fiberglass tape of the hybrid cast [43].
According to Charles and Yen [59], the hybrid casts constructed of 2 inner plaster layers and a single fiberglass layer were two times stronger than plaster when subjected to 3-point bending and shear tests. The shear strength of the plaster-fiberglass interface was higher by a factor of 3 than the 3 point bending strength of the construct. The hybrid constructs weighed 14% less than plaster but 42% more than fiberglass. In terms of cost comparison hybrid constructs were 2.5 times the cost of plaster and half the cost of fiberglass.
Although the hybrid alternative is not tough as fiberglass tape, it aids to overcome the brittleness and low impact resistance of plaster and provides a cheaper and sustainable alternative to a fully fiberglass cast [43,59], with the added comfort of plaster. Studies have shown the hybrid cast to be suitable for extremity casting, especially lower extremity where plaster casts fail upon weight bearing and fatigue [59,60].
4.1.2.3. Polyester-polyurethane and other polyurethane impregnated casts
Apart from fiberglass, advances in the polymer industry have put forth fabric material impregnated with polyurethane resin such as polyurethane-polyester. These materials are gaining popularity due to their low cost, availability, high-strength-to-weight ratio, extensibility and low hygroscopicity [36,61].
Delta cast conformable is an excellent example for a polyester- PU cast [2]. Other commercially available polyester-polyurethane resin bandages go by the names of Dyncast, Deltacast plus, Deltacast®, Baycast etc. These follow similar activation by immersion in water as plaster and fiberglass bandages.
All polyurethane impregnated bandages use similar resins, which in the presence of a catalyst combines di-isocyanate with polyol to form a prepolymer urethane (PU) resin [61]. The Urethane prepolymer when combined with water liberates heat and carbon dioxide forming crude polyurethane [58].
| Prepolymer (with isocyanate end groups) +H2O = polyurethane polymer + CO2 | eq.(2) |
Unlike fiberglass casts, the prepolymer contains negligible quantities of free di-isocyanate due to the presence of excess polyol added during the manufacturing process. Chemicals such as stabilizers remove water molecules to avoid curing during storage and anti-foaming agents remove carbon dioxide liberated following water immersion [36]. Although Polyols and antifoaming agents added are non-toxic, the catalysts and stabilizers show a strong irritant and toxic nature even in very small quantities (<1–2%). Therefore it requires immersion in excess water.
The application process of PU resin bandages is similar to that of plaster but manufacturer's instructions are to be closely followed. Polyurethane resins are sticky and adhere to the skin. Hence, it requires the use of gloves and are difficult to handle. PU resins can cause skin irritation after prolonged exposure. New low-tack resins with added surfactants to reduce the surface tension are currently commercialized. This reduces very high adherence and promotes ease in handling and application.
Polyurethane-Polyester casts record higher elongation and diametral compression strength in comparison to polyurethane-fiberglass casts. The tack free time and setting time can be effectively reduced by increasing the NCO/OH molar ratio in the prepolymer. This simultaneously results in an increase in diametral compression strength, although increasing the molecular weight in polyol in PU resins resulted in contrasting results [61]. Clinical studies evaluating the mechanical properties of casting material show that PU resin bandages are weak across the width of the bandage making them weaker candidates for load bearing casts. In terms of conformity and moldability Polyester-PU resin casts were rated lower than plaster [62].
Due to the elastic and flexible nature of polyester and polypropylene casts, these record very low cast breakages when compared to traditional plaster. Petty and Wardman [63] concluded that patients fitted to polyesters casts benefit from the flexibility of the cast in attending to their day to day activities. In contrast hardened PU bandages can result in rough surfaces and sharp edges. These can get caught in patient's clothing and cause discomfort in day to day activities [2]. Although performance of cotton base PU resin bandages is not a satisfactory improvement on plaster bandages, the cotton base provides lower dead weight [64].
Radio attenuation assessments carried out by Wytch et al. [46] concludes that polyurethane resin on knitted polyester bandages shows very low x-ray attenuation, ensuring better radio diagnostics of the underlying bone. Hence, polyester-polyurethane casts show superior radiolucency to that of fiberglass tape and POP and requires no cast replacement [2,64].
Polyester-polyurethane and polypropylene-polyurethane bandages are extremely flammable (ignition time 6s and 45s respectively), thus making them a potential fire hazard [45,65]. Although the heat transfer rate is adequate enough to alert the wearer to remove from the proximity, it is cautious to avoid cast exposure to naked flames [65]. Especially polyester casts that have a heat transfer rate of 18. PU impregnated bandages liberate Carbon Monoxide (CO) and Hydrogen Cyanide (HCN) upon ignition [45]. However, the concentrations of these gases are low and unlikely to cause respiratory difficulties to the wearer.
Clinical studies show that PU resin casts have recorded skin sensitization in patients. Similar to fiberglass -PU resin bandages, these too use isocyanates and other irritant chemical additives making them toxic and hazardous. In comparison to fiberglass, these produce lower amounts of dust during cast removal. Thus, the risk of work-related asthma is lower in other PU resin bandages than fiberglass-PU tape [2,45]. Further, the non-biodegradable nature of PU resin casts is an environmental burden that results in landfills and toxin leakage to ground water.
4.1.2.4. Thermoplastics
Thermoplastic materials are formable when heated and acquire rigidity when cooled [66]. These are widely used in splinting than casting. Thermoplastic casts are made out of an open-weave fabric comprising of a linear thermoplastic polyester polymer. Some thermoplastic and polyester composite casts such as the commercially available product Hexcelite NS contains a thermoplastic linear polyester polymer with an inorganic filler on a cotton base [25,51].
Fillers, elastomers and resins like polycaprolactone are added to thermoplastics to increase memory, stiffness, durability and moldability, while inorganic fillers improve the rate of cooling and heating [67]. The cast bandages have to be preheated in water at 170 °C before application. This makes them soft and moldable for conformity. Low temperature softening thermoplastics are mainly used in casting and splinting to avoid thermal damage to the patient during the casting process [67]. These thermoplastics activate when immersed in hot water at 70–75 °C for 1–2 min. They have a low curing time of 3 min and can bear weight after 15 min [51]. The application procedure of thermoplastic casts is similar to that of plaster but they offer much cleaner working conditions compared to plaster casting [2]. Casts fabricated from thermoplastics can be reheated at relatively low temperatures [67] for remolding to alter the cast position without removal, enhancing their cost benefit.
In testing for moldability and durability of low temperature thermoplastics, Breger-Lee and Buford [67] concluded that thermoplastics show 0%–86% permanent deformation based on their ability to retain elasticity during load cycling. Thermoplastics with higher elastomeric properties showed the least deformation while those with higher plasticity undergo large viscous energy dissipation. Low temperature thermoplastics provide longer working times and can be stretched to the required length when heated but returns to the original dimensions upon cooling due to elastic memory [66]. Hence, enhancement of low temperature thermoplastics with elastomers enables flexibility in casts and splints. A majority of low temperature thermoplastics can be easily cut with a pair of scissors while the material is warm and be conferred into the desired shape. However, stiffer thermoplastics sometimes require a coping saw.
Clinical studies carried out by Jø et al. [68] show that bandage comfort was significantly higher in Hexcelite than plaster casts when used for below-knee walking casts. Some past studies have shown that the weight bearing ability of a majority of thermoplastic casts is lower than that of their fiberglass alternatives [51,52].
Although thermoplastic casts are lightweight and have good water tolerance, they bear many drawbacks. The temperature required to activate a thermoplastic cast material is high as 70–80 °C and can cause thermal damage to the technician as well as the patient. Shorter moulding durations are available as the casts harden while cooling. Hence, it demands special training for proper application [67,68]. These have low conformity compared to plaster casts and can result in sharp prominences and wrinkling of the material that can cause pressure sores [68,69]. The material cost is significantly higher than plaster but lower than or equal to that of fiberglass [45].
Many thermoplastic material are radiolucent but the underlying meshwork appears in the x-ray images making it difficult to clearly identify fracture lines [46,64]. The material expense and its drawbacks have made it less popular in fracture immobilization [25] in developing countries and for long term immobilization.
4.1.2.5. Silicone rubber
Silicone rubber- Room Temperature Vulcanizing (RTV) is widely used in splints and casting in sport related injuries where more flexibility with adequate immobilization is required [70,71]. These casts and splints are generally used for upper extremity injuries such as wrist, thumb and the carpal navicular.
Bassett III et al. [70] was the first to describe a cast made of medical gauze impregnated with RTV Silicone Rubber. A similar splint was tested by DeCarlo et al. [71] with added reinforcement such as aluminum, concluding that the hardness and flexibility of Silicone rubber can be controlled enabling a thin durable cast. According to Bassett III et al. [70] the degree of immobilization depends on the amount of silicone and the supporting materials used.
In preparation of Silicone Rubber, catalysts are mixed to provide curing times of 2–4 h. Further, Urethane foam padding is used as an underlying material in Silicone rubber splints [72]. Bergfled et al. [73] using a splint constructed of Silicone rubber (RTV 700), curing agent -Beta 2 red, medical gauze and Ensolite foam between the layers of silicone concluded that the required rigidity and shock absorbing qualities were present in Silicone rubber splints making them adequate candidates for sport related injuries.
According to Bassett III et al. [70] silicone rubber although not hard as fiberglass or plaster is rigid enough for short term stabilization for injured athletes. Silicone rubber results in soft casts but when combined with the padding under layer has superior shock absorbing qualities than their alternatives [71]. The non-porous nature of silicone rubber allows them to be used only for short term casts and splints.
4.1.3. Other cast material
Synthetic cast materials pose a wide array of environmental and health risks. Hence, ecofriendly sustainable alternatives are emerging [74]. These substitutes are developed to overcome the issues faced by traditional plaster while providing adequate immobilization at the fracture site.
4.1.3.1. Woodcast
Modern day synthetic cast material contains Methylene Diphenyl Diisocyanate (MDI) which has shown links to occupational asthma and toxicity [58]. Hence, Onbone Oy, Finland has developed a new wood-plastic composite material, Woodcast. Woodcast is fabricated from a biodegradable thermoplastic polymer (bio plastic) and woodchips [75]. It is a fabric like material made from fine-chipped woodchips acquired mainly from the Finnish aspen or spruce trees. The fine-chipped wood is certified for medical purposes [76]. This mass of fine-chipped wood is heated up to remove spores from cryptogams before combining it with the bio plastic. Woodcast which is a non-toxic alternative to commercially available casting and splinting material comes in two alternatives; the semi-rigid and the rigid model in thicknesses of 2 and 4 mm.
The material becomes pliable and self-adhesive when heated to 65 °C and hardens as it cools down, similar to a thermoplastic. Icepacks can be used for rapid cooling of the cast [77]. The heat insulating properties of woodchips in the cast prevents the skin from heat damage and the fabric like nature of the material provides the ability to cut with a pair of scissors without material tearing or wrinkling [77,78]. The material possesses 3D modality for better conformity.
Similar to plaster, this material can be handled without gloves and releases no aerosols during hardening [78,79]. According to Hirsimäki et al. [79], the material is self-adhesive and shows some adhesion to padding and other bandages as it possesses strong self-adhesive properties. Woodcast exhibit adhesion forces over 100 N [77] making it difficult to be debonded without the use of a cast saw. Casts made out of Woodcast become load-bearing after 5–15 min of application with a stress yield of 15 MPa and Young's modulus of 500 MPa. Further, the material can withstand water exposure, possibly because the polymer matrix shields the woodchips from water absorption [58].
The design of Woodcast splints can be formed in such a manner that it can be removed during showering and reattached using brackets. Woodcast sheets contain parallel rows (17 mm apart) of circular holes of diameter ≈ 9 mm. Thus, allowing skin breathability. A study conducted on the biocompatibility and patient comfort of Woodcast on long term wear (6 weeks) using 33 patients with distal radius fractures showed that there were no allergic reactions and minimal skin abrasion [77].
According to Pirhonen et al. [80] the material shows equal stiffness to that of fiberglass tape. Studies conducted by researchers [58,78] showed Woodcast performs better than polyester and fiberglass under compression (at a width of 4 mm when compared to 5–6 layers of Fiberglass tape), but performs two times lower than fiberglass in tension. Exposure to water, length of the curing and exposure to heat has no recorded negative effects on the material strength. Generally, cast material undergo more compressive loads than tension and the satisfactory strength characteristics of Woodcast makes it a safer alternative to synthetic casts.
Unlike plaster and fiberglass Woodcast has better radiolucency, enabling better x-ray diagnosis. The cast appears almost invisible in x-ray images generating clear images of the underlying trabecular bone [77]. The open design of the Woodcast enables the direct use of ultrasound imaging while in cast. Another major benefit of the Woodcast, is the ability to biodegrade after use either as bioenergy or compostable waste, causing no distress to the environment.
At present the design is limited to limb immobilization especially lower limbs and scaphoid casts. Existing studies have not directly proven the ability to treat wounds underneath the cast. Whereas, the ability to create open casts without full enclosure of the limb is possible. The ease in cast removal also provides some ability to treat those superficial wounds [58], [78,79].
4.1.3.2. Cellulose based casting material
A cellulose-compound cast was developed by Dr. PH Leavitt in 1933. The material immediately faced drawbacks of being extremely coarse and inflammable. Based on this development Thorndike and Garrey [81] constructed a cast material based on industry available cellulose product used to manufacture box toes for shoes.
Thorndike and Garrey [81] added Boric acid to cellulose to construct the light weight, hard and waterproof material. The cellulose compound was impregnated into fabric and applied as a cast after wetting it with a quick drying solvent. Although the developed cast is crude in nature and contains pyroxylin, boric acid and acetone, it can be applied similar to a plaster bandage. The cast is applied over a stockinet under adequate ventilation for drying.
The cellulose compound cast is far lighter than plaster, has extreme hardness, and is waterproof and translucent to x-rays. This cast can be worn in swimming and bathing activities due to its waterproof and lightweight. Compared to plaster only a small quantity of this material is required for adequate rigidity, but in comparison to synthetic material requires more drying time. It takes an average of 30–40 min to dispatch the patient but cannot be indented until fully dried. Upon drying, the material has a higher inflammability index similar to that of dry wood [81]. The cellulose cast provides good workability and ease of application. However, it requires gloves to protect the appliers’ hands from collodion-like films that flake off later on.
The cast has been tested for upper extremity fractures including Colles's fractures, fractured clavicles and double leg spica casts in children. Constructing jackets for arthritic spines and splints for deformity correction can be satisfactorily designed using this material.
During cast removal cutting and abrasion of the patient skin is a major risk. Hence, a felt strip (¼ inch) is included to provide protection during cast removal. Clinical trials have shown that the odour of acetone was unbearable when the cast was still wet but the odour subsided upon complete drying. No skin irritation was reported but flammability was recorded as a potential risk inherent to the material [81].
4.1.3.3. RECAST
According to Ref. [82] RECAST is a bio-based PolyLactic Acid (PLA) plastic for fracture immobilization. RECAST splint material is developed by the Fraunhofer Institute for Applied Polymer Research (IAP), Germany.
A bio-based PLA plastic enhanced with necessary fillers is used in fabricating the splints with the ability to be easily reshaped and confirm to body contours.
The low thermal softening point of PLA allows the splint to be malleable when heated to 55–65 °C. The material is reported to stay malleable from 30 s followed by 3 min post heating, before gradual hardening.
Similar, to most thermoplastic material this too can be reheated and reshaped. Further, the splint has fleece padding of PLA and Viscose for better patient comfort. As per manufacturer information, the splint can be applied only after swelling of the fracture site subsides which makes it inapplicable to fresh fracture sites and with swelling and inflammation.
The material is yet to be evaluated based on its mechanical properties, the load bearing capacity, patient comfort and biocompatibility. RECAST splints and liners are biodegradable using industrial composters [82]. Hence, this minimizes the environmental impact of hospital waste.
4.2. Cast padding and liners for skin shielding from direct cast contact
Simple cotton padding is the most commonly used cast liner. The affordability and higher degree of skin comfort have made cotton padding popular since the early 1852s [1]. Cotton padding readily absorbs water, perspiration and blood from the skin surface.
Cotton fibers upon absorption of blood, hardens constricting the immobilized limb [1]. Hence, incases of soft tissue swelling and edema, sterile cast padding that can stretch to accommodate swelling is recommended.
Today newer synthetic padding material is known to prevent skin irritation and are water resistant. Thus, allowing patients to bathe and swim. These are usually paired with synthetic casts such as fiberglass resulting in expensive casts that are low in affordability.
When used directly adjacent to the skin, closed cell foam padding is not recommended as it cannot effectively absorb moisture and urine from the skin. Hence, water permeable liners such as Gortex, are specially required in hip spica casts in pediatric and high risk patient groups. Studies conducted by Stevenson et al. [83] show that waterproof cast padding is preferred by patients over cotton padding, specially the commercial brand Wet and Dry (3 M) due to its odour and water resistance, while technicians prefer the commercial brand Delta Dry (BSN Medical) due to its ease in application and removal. It is still unclear whether waterproof padding or cotton padding is most suited in fracture alignment in unstable fractures although it shows no significance in stable fracture healing.
Studies have shown that thicker layers of padding act as an insulator entrapping body heat and increasing the temperature under the cast. Hence, the number of padding/webril layers and the cast thickness must be effectively managed to achieve higher patient comfort and reduce the risk of thermal injury [28]. Thicker layers of padding require plaster application under tension. This results in high friction between the cast and the skin, which can result in cast complications such as skin maceration. Hence, the proper application technique is desired for proper fracture healing and long term wearability of the cast.
Today, water proof cast liners are available in the market but some manufacturers still advise against the willful wetting of the cast.
4.3. Complications associated with immobilization
Immobilization and cast removal are not without complications [1]. The major complications associated with casting are mainly investigated here.
Patients usually experience increased hair growth [8,84] and decreased muscle bulk in the immobilized limb [85]. However, through proper techniques and attention to patient factors such as obesity, fracture location, age, skin condition and the intended activity level of the wearer, certain complications can be avoided. When applied over traumatic or surgical wounds extra attention should be provided to avoid the progression of complications. Proper assessment is mandatory in post-operative patients fitted to casts/splints [8]. Hence, all patient complaints regarding the cast must be examined by a medical practitioner and nurses have to closely monitor the patient condition after immobilization [22].
The major complications associated with cast immobilization discussed here, range from superficial nature to damaged underlying vasculature which requires surgical treatment.
4.3.1. Thermal burns
Lipid membranes of human tissues are highly sensitive to thermal energy [86]. Due to prolonged exposure to heat; proteins, enzymes, non-enzymatic metabolic pathways and physical alternations can occur in living tissues. Skin when exposed to elevated temperatures undergo transepidermal necrosis. Hence, prolonged exposure to heat has to be avoided during immobilization. Many commercially available cast materials undergo exothermic reactions when activated [28,53,86].
The risk of thermal injury is strongly associated with elevated temperatures underneath the cast. The contributing factors include high dip water temperature (>50 °C) [29,69], thicker casts (more than 24 layers) and lower ventilation during application. It is ideal to use no more than 12 layers of bandages and maintain dip water temperatures at 24 °C [28,85,87] to reduce the risk of thermal injury. To avoid thicker casts, applicators have to be mindful of bandage overlap in concavities of extremities such as the antecubital fossa and ankle dorsum [28].
Halanski et al. [86] showed that placing a curing cast on a pillow and folding the edges of a splint can significantly increase the risk of thermal injury. Thermal injury is common from any cast material that reaches high elevated temperatures during curing and has longer curing periods [53], more significantly in thicker plaster casts with long curing periods due to prolonged exposure to elevated temperature [87,88]. Risk of thermal injury is higher in faster setting plaster casts than in slow setting thinner plaster [87]. Fiberglass tape is relatively safer than plaster, as fewer layers are required to achieve the required strength characteristics. Application of fiberglass reinforcement in hybrid casts, before complete curing of the plaster bandages can elevate the risk of thermal injury due to the inability of effective heat dissipation [53,86].
In total body casts such as hip-spica casts adequate layers of padding and cast material has to be used to avoid thermal injury to patients. It is conventionally presumed that the increase of the layers of padding beneath the cast reduces the risk of thermal injury but studies have shown that thicker layers of padding entraps more heat. Using isopropyl alcohol to reduce the temperature of a curing cast is effective on the outer surface but inner cast temperatures are minimally affected by this, with no significant reduction in thermal injury [86].
Use of ice packs between the limb and the pillow, holding the limb or allowing it to hang free can lower the risk of thermal injury during cast curing [86,89].
4.3.2. Pressure sores
Areas of increased pressure leads to decreased perfusion which results in pressure sores. Children suffering from cerebral palsy related to surgery[[90], [106]] [], casting for knee flexion contracture and hip instability have recorded higher probability of pressure sore development [1]. The signs and symptoms of pressure sore progression include pain and burning sensation, localized heat, cast odour, staining through the cast and pyrexia (in children) [22].
It may be rationalized that the use of extra layers of padding can relieve pressure sores but this results in a loose fitting cast and risk of skin irritation due to high shear stress build up and fracture malalignment. Hence, application of adequate padding over bony prominences, pressure points and cast edges is recommended. Hence, the use of self-adhesive foam padding such as Reston self-adhering foam (3 M) over pressure sensitive areas is recommended.
A well-trained technician or physician should carry out cast application to prevent pressure sore development, as this is generally associated with poor casting techniques. The cast should be a well moulded fitting cast to minimize excessive pressure buildup. Wrinkling of the cast material and movement before complete curing creates edgy prominences which can result in pressure sores. This is commonly seen in synthetic casts with low curing times [22].
The detection and prevention of pressure sores can be achieved through properly placed cast windows in high risk patient groups and to examine open wounds [15]. Cast windows are prominently required to release pressure when Kirschner wires and external fixation devices are included in limb immobilization. Univalving and bivalving are also commonly used to release pressure entrapped underneath the cast [53].
4.3.3. Skin irritation and maceration
Patients often suffer from itching due to wetting of the casts or sweating, which results in foul odour and skin irritation [85]. This is critical in hip-spica casts where cast soiling is a major issue due to water and excretion coming into contact. Thus, it requires waterproofing.
Blisters, erythema, contact dermatitis are common among 13% of the cast wearers. Clinical studies record dermatitis associated with plaster casts is due to Benzalkonium Chloride used to improve plaster binding characteristics and melamine formaldehyde resin used in plaster resin casts [91].
Traditionally, casts are lined with a stockinet and cotton or synthetic padding. Cotton padding when exposed to blood and water retains moisture causing skin irritation and maceration [1]. Loosely fitted casts result in higher degrees of skin irritation and abrasion due to shear stress build up in the skin-padding interface. In case of cast wetting patients and their caretakers are instructed to dry the outer cast with a towel and use a blow dryer on a low temperature setting when drying the inside of the cast [92]. This extracts the moisture that otherwise can cause skin maceration and irritation. Patients are warned against using sharp objects to scratch beneath the cast as this can risk infection and skin abrasion.
Temporary treatments for cast related skin irritation include antihistamine medication, blowing cool air down the cast and applying calamine lotion [93] before cast application. New synthetic material and waterproof liners have shown improvements in preventing skin irritation, cast odour, patient satisfaction and allowing patients to swim and bathe. Studies show that covering the cast with a plastic bag while bathing is most effective against cast wetting [94]. Although improvements are underway, the optimal cast liner and material to reduce itching and skin maceration are yet to be determined.
4.3.4. Compartment syndrome
Inflammation/swelling of the fracture site and soft tissues may or may not be present during cast application. Increased tissue pressure within a limited space, compromising circulation and tissue function can lead to compartment syndrome. Compressed neurovasculature limits blood flow damaging muscles and nerves which can lead to extreme pain. Patients with nerve damage may not experience pain due to compartment syndrome, but may exhibit sensory and ischemia related symptoms. After the onset of nerve related ischemia full recovery is impossible.
Compartment syndrome can lead to major complications such as limb amputations and even mortality. Hence, special attention has to be paid when applying casts of low elastic modulus like fiberglass and tacky PU resin bandages, to avoid limb constriction and avoid areas of high-pressure buildup [95].
Compartment syndrome is an anticipated complication in hip spica casts. Hence, it is recommended to apply traction or stretch-relax [95] the cast material using hand and end the cast in the supramalleolar region with the hip and knee flexed at 45° [96]. Many studies show that stretch relaxing synthetic tape and relieving circumferential pressure via cast splitting is effective to reduce compartment syndrome [1,22,95].
Clinical studies have shown that plaster cast cutting and univalvling reduces pressure by 40–60%. A further 10–20% pressure reduction can be achieved by splitting the underlying layer of padding. Zaino et al. [97] show that casts made of fiberglass applied without stretch-relaxation is two times tighter than plaster casts. Hence, bivalving is required for a similar reduction in pressure entrapment. For stretch-relaxed fiberglass casts univalving is adequate to spread and keep the cast in open. As synthetic casts have the tendency to spring back to their original position after univalving, plastic cast wedges are required to hold them in an open position [64].
After pressure relieving, it is recommended to elevate the extremity no higher than the heart to maintain arterial perfusion. In extreme situations fasciotomy is required to relieve pressure to prevent the progression of tissue necrosis and nerve ischemia [3,22].
4.3.4.1. Deep vein thrombosis (DVT)
Deep Vein Thrombosis (DVT) is significantly seen in the adult population with prolonged lower extremity immobilization [1,15]. Studies have shown that the probability of DVT is 15–36% in adults, when placed in lower extremity casts for an average of 21 days.
Low molecular weight heparin use does not show any significant reduction in DVT even when administered daily [1]. Secondary complications such as pulmonary emboli or death have not been recorded due to cast induced DVT. It is recommended to use less rigid methods of immobilization such as splints and softer casting material such as Softcast (3 M) to reduce the risk of DVT.
4.3.5. Soft tissue swelling
Soft tissue swelling is common in the fracture site and generally subsides after 48 h. Long term prevalence of such post traumatic edema can result in excessive cast tightening and soft tissue injury, which can lead to secondary complications such as muscle atrophy, compartment syndrome, Volkmann contracture and transdermal necrosis. Fast setting casts such as PU resin and Fiberglass accommodates less soft tissue swelling than plaster casts that has a longer curing time.
Zaino et al. [97] with a stimulated edema-induced pressure setup for a short arm fiberglass cast concluded that single cut (bivalving and ace wrapping) method could reduce skin surface pressure caused by edema by 70.8%, while the most effective is the triple cut method (bivalving, spreading, webril cut and ace wrapping) which recorded a 99.9% reduction in edema induced pressure in fiberglass casts. The study suggested that women register higher skin surface pressure (104.4 mmHg) than men (81.1 mmHg). Hence, women are at a higher risk of succumbing to edema induced pressure complications when placed in casts [97].
Contrastingly, the gradual decrease in edema results in cast loosening which can cause significant displacement of the well aligned fracture which may require cast replacement or reapplication. This is a common complication in unstable fractures especially in lower extremities. Studies have shown that cotton padding best behaves in pressure release in splitting and accommodation of swelling than synthetic padding [98]. Cotton padding has its drawbacks when exposed to blood. The cotton fibers harden and can be constrictive of the encased limb resulting in edema [1].
Proper elevation to subside swelling and the use of adequate padding can reduce the risk of complications associated with cast loosening or development of high pressure areas. In cases of significant soft tissue swelling associated with unstable fractures percutaneous pin fixation is recommended primarily. However, percutaneous pin fixation has its own merits and drawbacks [99].
4.3.6. Venous congestion and hematoma
Impaired venous return due to tight casts can cause swelling or bluish discoloration of the immobilized limb. This venous congestion induced bluish skin discoloration has to be properly identified apart from bruising in patients to avoid poor perfusion [1,15]. Tight fitted rigid casts can lead to the development of venous congestion. Thus, in patients with a risk or a history of venous congestion, rigid immobilization has to be carried out by licensed practitioners. Orthopedic practitioners recommend the use of splints and cast univalving to lower the risk of venous congestion.
Hematoma or bleeding under the cast is seen especially in post-operative patients with trauma wounds. The brownish staining of the plaster casts can be observed in cases of hematoma. Studies have shown that significant brownish staining depicting blood underneath the plaster cast can only be seen after 24 h of immobilization and complete drying of plaster casts. The staining of the cast depends on the cast and padding thickness, post-operative patient positioning, the extent of trauma and the hemostasis technique. Mild hematoma can easily go unnoticed. Therefore, extra caution is required when fitting post-operative patients to casts. Existing literature shows a lack in the identification of hematoma in relation to synthetic casts [8].
4.3.6.1. Cast saw burns
Rigid casts specially fiberglass and PU resin casts require cast saws for removal. Studies report a 0.72%–1% frequency of cast saw burns during cast removal, which are thermal or abrasive in nature [1,85]. Technicians have to take precautions to avoid cast removal complications especially when using cast saws for removal of casts with waterproofing liners.
Keeping the patient steady and calm is required to prevent vibration related injuries especially in pediatric groups. Puddy et al. [100] have shown that bulkier casts require higher oscillation speeds resulting in high blade temperatures. Technicians have to be careful not to overheat the saw blades when cutting long casts and allow the blades to cool during the procedure to avoid burns and cuts. Proper inspection of the blades, frequent blade changes, use of sharp blades, avoiding sliding of the oscillating saw along the cast and using proper cutting techniques can greatly reduce the risk.
Studies [100,101] show that cast saw blades can reach dangerously high temperatures like 70 °C or 123–130 °C during operation that can even result in second- or third-degree burns. It takes a minimum of 2 min for the blades to cool down to safer operating temperatures. Application of 70% isopropyl alcohol, ultrasound gel and water to the heated blades can speed up the cooling process. According to Puddy et al. [100] the most effective cooling technique is the application of 70% isopropyl alcohol to the padding/dressing.
Cutting along safety strips which are less heat resistant than the cotton padding results in low heat generation during removal. Use of plaster shears, built in metal/plastic strips and easy wrap cast-made of thermosetting polymer that is mounted between the two edges of the cast material are other alternatives to cast saws [3,100] are recommended. Cutting underlying padding using trauma shears ensures no damage to the patient's skin [3], whereas in Thermoplastic cast removal can be done by heating and cutting with scissors. In clubfoot plaster casts, cast wetting is recommended for removal.
4.3.7. Cast dust and cast ingredient associated complications
Long term exposure to dust particles and ingredients such as isocyanates that arise from crude cast material can result in occupation related asthma and other breathing disorders. This is mainly seen in fiberglass and PU resin casts. There is a very low probability of asthmatic reactions related to Methylene Diphenyl Diisocyanate (MDI) in casting of plaster casts. Hence, it is precautionary to avoid exposure to even very low concentrations of MDI [[102], [103]][]. Similar consequences are reported due to exposure to cast dust that arise during cast removal. To minimize occupational asthma and ensure user safety, modern cast saws are equipped with a vacuum extraction accessory [2].
Studies have reported carcinogenic risks associated with the manufacturing of synthetic casts such as PU resin bandages and Fiberglass tape [1]. Cases of acetone poisoning have [104] been reported in synthetic casts. Acetone is used as a solvent evaporator in synthetic casts. There are no records of acetone absorption through the skin hence inspiration of acetone by the lungs can result in acute acetone poisoning. This can even result in comatose if inhaled in large quantities over a prolonged time. Thus, synthetic casts are eliminating the use of aerosol and breathable toxic agents.
5. Discussion
Based on the findings of the systematic literature review and the comprehensive analysis provided under Section 4, the following details can be extracted. A comprehensive summary is given in Table 2.
Table 2.
Comparison of modern-day immobilization material analyzed under the review.
| Material | Description | Activation | Cast layers required (10 cm wide) | Setting time | Curing time | Time for load bearing (s) | **General weight of cast (g) | x-ray permeability | Cost/package ($) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plaster of Paris bandages | Plaster of Paris impregnated leno weave fabric bandage with accelerators and binders. | Dip 10s in water at 20–25 °C | 5-12 layers | 4–8 min–30 min * 4–8 min for fast setting casts |
24–48 h | 48–60 h | >1000g | Radiopaque | $20 | Rigid but brittle. Undergo water degradation and messy application. Suitable for long term immobilization. |
| Fiberglass - PU resin bandages | Polyurethane or PU resins impregnated fiberglass bandage | Dip water at 15–27 °C for 2–10s | 3-6 layers | 3–5 min | 5–7 min | 15–30 min | <500g | Radiolucent | $70-$89. | Rigid waterproof bandage, contains isocyanates which can cause occupational asthma due to high dust generation during removal. Used as a secondary cast material. |
| Plaster of Paris, Fiberglass hybrid | Plaster of Paris casting tape overlaid with Fiberglass-PU bandage | Respective activation for each plaster of Paris and fiberglass bandages | 4 layers of plaster overlaid with 4 layers of fiberglass bandage | Respective set times for each material. Total cast takes ∼30 min | Respective set times for each material. Total cast takes ∼24 h | Respective set times for each material. Total cast takes ∼48 h |
>500g Depends on the number of layers |
Radiopaque plaster layers | 10% cost reduction compared to a total fiberglass cast. | Hybrid casts uses the benefits of both plaster and fiberglass but not radiolucent due to radiopaque plaster layers. |
| Polyester-PU resin and other PU resin bandages | Polyurethane polymer impregnated open weave polyester bandage (most common) *bandage type can vary according to commercial product. | Cold water immersion | 3-8 layers | 3–5 min | ∼30 min | 20–30 min | ∼650g | Radiolucent As they are weaker than fiberglass require more layers, hence attenuates more | $158 | Polyester offers more elongation and good flexibility to the cast. Contains isocyanates and tacky resins. Should handle using lubricated gloves. Used as a secondary cast material. |
| Thermo-plastic | Thermoplastic impregnated polyester tape with inorganic fillers. (available as thermoplastic impregnated bandages and thermoplastic sheets) | Immerse in hot water at 70–85 °C for 1–5 min | 3-5 layers (∼5 layers) | 2–3 min | 3–30 min | ∼15–30 min | ∼700g | Radiolucent but shadows and artefacts present | < $56 | Low temperature setting thermoplastics are available to reduce thermal damage. Low weight bearing ability than fiberglass bandages. |
| RTV Silicone rubber | RTV Silicone rubber + medical gauze with added curing agents and catalysts | Applied onto medical gauze until desired thickness is obtained and cut using bandage scissors to form the splint | 2-5 layers | 1 h | 2–4 h or 24 h. Depends on curing agent used |
∼5–24 h | 450–600g | Splints are removed during radiological assessments | $ 12 per can | Absorbs impact forces and offers high flexibility. Used in short term (<4 h) splinting of sport related injuries. Not suitable for longer term circumferential casting |
| Woodcast | Woodchips from Finnish Aspen or Spruce in Biodegradable thermoplastic polymer matrix | Heated to 62 °C | 1 layer of 4 mm material | 15–30 min when cooled at ambient temp (22 °C) | 15 min | 5–15 min | Lightweight material. Exact weight not provided. | Good radiological properties, cast almost invisible in x-ray images | $71-$ 180 | Sustainable Wood-plastic composite with good radiographical properties and rigidity. Can be reheated. No toxic materials available. |
| Cellulose based cast material | Pyroxylin + Boric acid impregnated fabric wetted by acetone | Cellulose impregnated bandage wetted using a quick drying solvent at room temperature | ∼3 layers | 30–40 min | Prolonged curing time | TBA | Lighter than plaster bandages | Radiolucent | TBA | Waterproof lightweight material. Prolonged drying times exceeding 30mins. Adequate ventilation required for cast drying. High flammability and shrinkage. |
| RECAST | Bio-based Poly Lactic Acid (PLA) plastic | Heated to 55–65 °C | 1 layers (thickness TBA) | After 3 min | TBA | TBA | TBA | Radiolucent, exact details not provided so far | TBA | Biodegradable sustainable material, to reduce the number of synthetic cast waste ending in landfills. |
TBA- To Be Added.
*The table provides generalized details of the cast material, values provided can slightly alter depending on the commercial product and additives.
**Only generalized cast weights are provided, weights can differ according to the number of layers used.
Table 3 shows each immobilization material against the risk of common complications they are likely to be associated with.
Table 3.
Existing immobilization material against the complications they are associated with.
| Material | Risk of complications |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thermal burns | Pressure Sores | Skin irritation and maceration | Compartment Syndrome | Deep Vein Thrombosis | Soft Tissue Swelling | Venous Congestion | Hematoma | Cast Saw burns | Dust associated issues | |
| Plaster of Paris bandages | Moderate (thicker and fast setting casts) | Low – Moderate probability (Improper technique) | Low – moderate (Hip Spica casts have a higher risk) | Moderate (High in hip Spica casts) | Low | Good accommodation to swelling | Moderate | Accommodates low hematoma | Low-Moderate | Moderate-High |
| Fiberglass - PU resin bandages | Low | Moderate -High (depends on application technique and curing time) | Low- Moderate (on short term casting) | High | High | Low accommodation | High | NDA* | High | High |
| Plaster of Paris, Fiberglass hybrid | Present in thicker and fast setting plaster | Low- Moderate | Moderate-High | Moderate | Moderate accommodation | High | NDA* | High | High | |
| Polyester-PU resin and other PU resin bandages | Higher than plaster | High – increased risk in prolonged exposure | High | Moderate-High | Low-Moderate accommodation (depends on cast flexibility) | High | NDA* | Low | Low | |
| Thermo-plastic | Moderate-High | Moderate -high | Moderate | High Not used in full body or high enclosure casts |
High | Low accommodation | High | NDA* | Very Low | Very Low |
| RTV Silicone rubber | NDA* | NDA* | Moderate-High | NDA* | NDA* | High accommodation (only used in short term splinting) | NDA* | NDA* | Nil | Nil |
| Woodcast | Low-Moderate | Low-Moderate | Low | NDA** | Low | Low accommodation | Low | NDA* | Nil | Nil |
| Cellulose based cast material | NDAno* | Moderate-High | High | Moderate- High | Moderate- High | Nil | High | NDA* | High | NDA* |
| RECAST | Low-Moderate | NDA* | NDA* | NDA* | Nil | NDA* | NDA* | Nil | Nil | |
NDA * No Data Available due to lack of literature findings.
Hence, it can be seen that plaster shows moderate probability of causing common complications while synthetic and other material are highly likely to cause serious complications. Further, plaster is still the material of choice in full body casting while newer material are more used for short term extremity casting and splinting.
6. Challenges and opportunities
Based on the findings and the discussion it is identified that the most common challenges in immobilization are;
-
1.
Ability to accommodate swelling and treating the wounds under the cast is a huge challenge which can lead to complications when fitted to casts. This is a requirement in diabetic foot care and accident trauma management.
-
2.
Proper disposal of synthetic casts and upcycling of synthetic cast material is challenging due to their toxic ingredients and nonbiodegradable nature.
-
3.
Waterproofing of casts and cast permeability to moisture on skin opposes each other. Both factors are sought after in terms of patient comfort.
-
4.
The low conformity of synthetic casts causes pressure entrapment in extremity casting.
-
5.
Majority of immobilization material have tacky ingredients that makes it messy in application and removal.
-
6.
Toxic ingredients in casts and dust cause asthma and other respiratory distress to users.
-
7.
Plaster has undergone minimal alterations to its performance despite its high use especially in full body casting.
-
8.
Monitoring the progress of fracture healing and wound healing is problematic without cast removal due to low radiolucency of casts.
Despite these challenges the following opportunities are identified by the authors:
-
1.
Development of material with low risk of complications, higher patient comfort and ecofriendliness with adequate mechanical performance.
-
2.
Improving the strength characteristics and waterproofing ability of plaster especially for full body casts
-
3.
Development of a cast lining or casting material that can accommodate swelling and hematoma under the cast for trauma patients and post-operative patients.
-
4.
Using sustainable fillers to improve strength and comfort of immobilization material.
-
5.
Upcycling and circular economy for synthetic and other immobilization material.
-
6.
Development of waterproof liners and highly breathable casts for full body and long body casts.
-
7.
Improving the radiolucency of casts and splints to avoid cast removal in patient monitoring.
7. Conclusions
This paper discusses the development of immobilization materials, their properties and patient comfort and satisfaction. The following conclusions can be made:
-
1.
The history of immobilization materials extend to 3000 BCE, which initiated with splints designed from sticks and palm fiber bandages, gradually transitioning to plaster in 1798. Developments in casting have taken place considering patient satisfaction, mobility and better mechanical properties.
-
2.
Even though there are new materials such as polyurethane impregnated fiberglass, polyurethane resin, thermoplastics and silicone rubber are available, still the most favorable and cost effective solution for immobilization in many countries is plaster.
-
3.
An immobilization material should possess required material properties such as adequate mechanical strength with tensile strength >20 MPa, flexural modulus >2 kN/mm2 and failure loads >10 kN with the ability to bear cyclic loads, faster setting times, waterproofing ability and quick drying time, faster and less messy application, composition of non-toxic, non-flammable and non-irritant constituents that can be activated using low heat or undergo mild to moderate exothermic reactions when immersed in water, good cast breathability and vapor permeability, ability to accommodate swelling and edema, ability to be used in long term immobilization (exceeding 4 weeks), excellent conformity with no pressure point generation, good radiographical properties and economic feasibility, for higher patient comfort and an efficient healing process. Out of the available materials the synthetic materials only satisfy the strength requirements.
-
4.
The patient comfort is a compulsory matter in the process of long term immobilization. In this regard breathability, thermal comfort, water resistance, lightweight and non –toxicity (non-irritant) are important. Only Woodcast material possess a majority of these characteristics.
-
5.
The most common complications in fracture immobilization are skin irritation and maceration, pressure sores, thermal burns, soft tissue swelling and cast saw burns. These complications mainly occur due to early stage heat generation, low porosity, and low conformity to body contours of immobilization materials. Hence, it is important to consider these properties as the top most priority, in the process of developing an immobilization material.
-
6.
Setting time is another key factor considered in the development of immobilization material. If a cast possesses a low setting time the patient can be discharged to his familiar environment which ensures the psychological well-being of the patient and is also beneficial to maximize routine activities. This can be achieved through the addition of accelerators in the development process of the cast material.
-
7.
In comparison of the available cast material under low cost, patient comfort, lower cast complications and setting time, the following order of choice can be achieved arranged in ascending order:
-
➢
Cost: plaster of paris < silicone rubber < fiberglass-plaster hybrid < thermoplastic < fiberglass < PU resin < Woodcast
-
➢
Patient comfort: Silicone rubber < thermoplastics < plaster of paris < PU resin < fiberglass < Woodcast
-
➢
Lower cast complications: synthetic casts < plaster of Paris
-
➢
Setting time: RECAST < thermoplastics < PU resin ≤ Fiberglass < Woodcast < plaster + fiberglass hybrid < plaster of paris < cellulose based material < Silicone rubber
(Properties can vary according to the brand and additives used.) (Refer Table 2)
-
8.
Further modifications to the existing orthopedic immobilization materials are required for superior strength gain, improved material characteristics, lightweight and superior patient comfort. Therefore, it is required to modify the abundantly used plaster cast material to gain the aforementioned properties. Graphene Oxide is considered in the work carried out in the University of Moratuwa to achieve this objective.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no competing interest.
Acknowledgments
The authors thank and appreciate the support of Ceylon Graphene Technologies (CGT) for their contribution to provide Graphene Oxide for futher studies carried out at the University of Moratuwa to improve the strength properties of plaster casts.
Acknowledgments
The authors thank and appreciate the support of Ceylon Graphene Technologies (CGT) for their contribution to provide Graphene Oxide for futher studies carried out at the University of Moratuwa to improve the strength properties of plaster casts.
Contributor Information
Chathushika Ekanayake, Email: ekanayakeemjc.21@uom.lk.
J.C.P.H. Gamage, Email: kgamage@uom.lk.
P. Mendis, Email: pamendis@unimelb.edu.au.
P. Weerasinghe, Email: pasinduw@uom.lk.
References
- 1.Halanski M., Noonan K. Cast and splint immobilization: complications. JAAOS-J. Am. Acad. Orthop. Surg. 2008;16(1):30–40. doi: 10.5435/00124635-200801000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Capper C. Synthetic casting tapes: benefits and uses of Delta-Cast Conformable. Br. J. Nurs. 1988;7(19):1162–1166. doi: 10.12968/bjon.1998.7.19.5574. [DOI] [PubMed] [Google Scholar]
- 3.Satryb S., Wilson T., Patterson M. Casting: all wrapped up. Orthop. Nurs. 2011;30(1):37–41. doi: 10.1097/NOR.0b013e31820574f9. [DOI] [PubMed] [Google Scholar]
- 4.Tofighi M. 2008. Do All Fractures Need Full Immobilisation? [DOI] [Google Scholar]
- 5.Sathyendra V., Darowish M. Basic science of bone healing. Hand Clin. 2013;29(4):473–481. doi: 10.1016/j.hcl.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Aiyer Amiethab. "Fracture Healing," 16 06 2021. https://www.orthobullets.com/basic-science/9009/fracture-healing [Online]. Available: Accessed.
- 7.Marsell R., Einhorn T. The biology of fracture healing. Injury. 2011;42(6):551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankin F., Gragg A., Kaufer H. Bleeding beneath postoperative plaster casts. Orthop. Nurs. 1983;2(1):27–32. doi: 10.1097/00006416-198301000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Raoufi M., Abedtash H., Mohagheghzadeh A. The historical background of plaster cast. Arch. Iran. Med. 2017;20(7):461–464. [PubMed] [Google Scholar]
- 10.Boyd A., Benjamin H., Asplund C. Splints and casts: indications and methods. Am. Fam. Physician. 2009;80(5):491–499. [PubMed] [Google Scholar]
- 11.Hohn R. Principles and application of plaster casts. Vet. Clin. N. Am. 1975;5(2):291–303. doi: 10.1016/s0091-0279(75)50037-7. https://doiorg/10.1016/s0091-0279(75)50037-7 [DOI] [PubMed] [Google Scholar]
- 12.Althoff A., Reeves R. 2020. Splinting. [PubMed] [Google Scholar]
- 13.Sinacore D. Healing times of diabetic ulcers in the presence of fixed deformities of the foot using total contact casting. Foot Ankle Int. 1998;19(9):613–618. doi: 10.1177/107110079801900908. [DOI] [PubMed] [Google Scholar]
- 14.DeMaio M., KathleenMcHale, Lenhart M., Garland J., McIlvaine C., Rhode M. Plaster: our orthopaedic heritage. J. Bone Joint Surg. 2012;94-A(20):1–8. doi: 10.2106/JBJS.L.00183. [DOI] [PubMed] [Google Scholar]
- 15.Szostakowski B., Smitham P., Khan W. Plaster of paris–short history of casting and injured limb immobilzation. Open Orthop. J. 2017;11:291–296. doi: 10.2174/1874325001711010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz J.C. Plaster of paris:the forgotten hand splinting material. J. Hand Ther. 2002;15:144–157. doi: 10.1053/hanthe.2002.v15.015014. [DOI] [PubMed] [Google Scholar]
- 17.Risdonne V., Hubbard C., López Borges V., Theodorakopoulos C. Materials and techniques for the coating of nineteenth-century plaster casts: a review of historical sources. Stud. Conserv. 2021:1–23. doi: 10.1080/00393630.2020.1864896. [DOI] [Google Scholar]
- 18.Luck J. Plaster of Paris casts: an experimental and clinical analysis. J. Am. Med. Ass. 1944;124(1):23–29. https://doi:10.1001/jama.1944.02850010025005 [Google Scholar]
- 19.Adkins L. Cast changes: synthetic versus plaster. Pediatr. Nurs. 1997;23(4):422–426. [PubMed] [Google Scholar]
- 20.Afshar A., Steensma D., Kyle R. Antonius mathijsen and plaster casts. Mayo Clin. Proc. 2017;92(No. 5):E83. doi: 10.1016/j.mayocp.2017.01.028. Elsevier Limited. [DOI] [PubMed] [Google Scholar]
- 21.Fisher T. Casts. Canad. Med. Assoc. J. 1969;101(11):79. [PMC free article] [PubMed] [Google Scholar]
- 22.Prior M., Miles S. Casting: part one. Emerg. Nurse. 1999;2(7) [Google Scholar]
- 23.Smeltzer S.C., Bare B.G., H. J. L. Cheever K.H. eleventh ed. Lippincott, Williams & Wilkins; Philadelphia: 2008. Textbook of Medical-Surgical Nursing. [Google Scholar]
- 24.Young C.F., Haddad F. Principles of plaster application. Br. J. Hosp. Med. 2005;68:M13–M14. doi: 10.12968/hmed.2007.68.Sup1.22666. 2007. Sup. 1. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen K., Lauritzen J. A new thermoplastic casting material: a comparison between plaster of paris and hexelite. Acta Orthop. Scand. 1981;52(1):27–29. doi: 10.3109/17453678108991753. [DOI] [PubMed] [Google Scholar]
- 26.Smith G., Hart R., Tsai T. Fiberglass cast application. Am. J. Emerg. Med. 2005;23(3):347–350. doi: 10.1016/j.ajem.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Warren G. Plaster casts. Lepr. Rev. 1971;42(1):55–59. doi: 10.5935/0305-7518.19710007. [DOI] [PubMed] [Google Scholar]
- 28.Hutchinson M., Hutchinson M. Factors contributing to the temperature beneath plaster or fiberglass cast material. J. Orthop. Surg. Res. 2008;3(1):1–8. doi: 10.1186/1749-799X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavalette R., Pope M., Dickstein H. Setting temperatures of plaster casts. The influence of technical variables. J. Bone Jt. Surg. Am. Vol. 1982;64(6):907–911. [PubMed] [Google Scholar]
- 30.Hutton B., Catala-Lopez F., Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: prisma-NMA. Med. Clin. 2016;147(6):262–266. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Research Rabbit, "Research Rabbit," 2022. [Online]. Available: https://www.researchrabbit.ai/. [Accessed 11 10 2022].
- 32.Selesnick H., Griffiths G. A waterproof cast liner earns high marks. Physician Sportsmed. 1997;25(9):67–74. doi: 10.3810/psm.1997.09.1507. [DOI] [PubMed] [Google Scholar]
- 33.Abd Kati F., Y. I. Razak W. Effect of adding some additives and drying method on compressive strength of gypsum products. Tikrit J. Dent. Sci. 2017;5(1):25–32. [Google Scholar]
- 34.Burrows A.J., Milne A.J. France Patent WO1990002538A1; 22 03 1990. Plaster of Paris Bandage Containing an Additive. [Google Scholar]
- 35.Abbood A., Atshan A., AL-Ridha A. Improvement of local gypsum plaster setting time by the combined usage of (TGP) and (PVA) additives. Mater. Sci. Eng. 2020;870(No. 1):12106. doi: 10.1088/1757-899X/870/1/012106. June. [DOI] [Google Scholar]
- 36.Wytch R., Ross N., Wardlaw D. Glass fibre versus non-glass fibre splinting bandages. Injury. 1992;23(2):101–106. doi: 10.1016/0020-1383(92)90042-Q. [DOI] [PubMed] [Google Scholar]
- 37.Schuind F., Moulart F., Liegeois J., Dejaie L., Strens C., Burny F. Orthopedic immobilization. Acta Orthop. Belg. 2002;68(5):439–461. [PubMed] [Google Scholar]
- 38.Vaughan L. The use of bandages and splints for the support of limbs in cats and dogs. J. Small Anim. Pract. 1964;5(3):235–243. doi: 10.1111/j.1748-5827.1964.tb04245.x. [DOI] [Google Scholar]
- 39.Hatim N.A.A., Al-Khayat I.K., Abdulla M.A. Modification of gypsum products (Part I): physical and mechanical properties of adding some additives on different types of gypsum products. Al-Rafidain Dent. J. 2007;7(2):206–212. doi: 10.33899/rden.2007.39747. [DOI] [Google Scholar]
- 40.Sanad M., Combe E., Grant A. The use of additives to improve the mechanical properties of gypsum products. J. Dent. Res. 1982;61(6):808–810. doi: 10.1177/00220345820610063201. [DOI] [PubMed] [Google Scholar]
- 41.Hatim N., Al-Khayat I., Abdulla M. Modification of gypsum products (Part II): the effect of drying methods on the compressive strength and surface hardness of modified gypsum products. Al-Rafidain Dent. J. 2009;9(2):162–167. doi: 10.33899/rden.2009.9107. [DOI] [Google Scholar]
- 42.Mihalko W., Beaudoin A., Krause W. Mechanical properties and material characteristics of orthopaedic casting material. J. Orthop. Trauma. 1989;3(1):57–63. doi: 10.1097/00005131-198903010-00011. [DOI] [PubMed] [Google Scholar]
- 43.Agisparayan C., Low K., Lim S., Wong K. Impact behaviour of hybrid bandage casts. Innov. Corros. Mater. Sci. (Formerly Recent Patents on Corrosion Science) 2019;9(1):66–73. [Google Scholar]
- 44.Inglis M., McClelland B., Sutherland L., Cundy P. Synthetic versus plaster of Paris casts in the treatment of fractures of the forearm in children: a randomised trial of clinical outcomes and patient satisfaction. Bone Joint J. 2013;95(9):1285–1289. doi: 10.1302/0301-620X.95B9.30666. [DOI] [PubMed] [Google Scholar]
- 45.Wytch R., Mitchell C., Ritchie I., Wardlaw D., Ledingham W. New splinting materials. Prosthet. Orthot. Int. 1987;11(1):42–45. doi: 10.3109/03093648709079380. [DOI] [PubMed] [Google Scholar]
- 46.Wytch R., Ashcroft G., McKenzie G., Wardlaw D., Ledingham W. Radiographic assessment of splinting bandages. Injury. 1991;22(1):41–44. doi: 10.1016/0020-1383(91)90160-G. [DOI] [PubMed] [Google Scholar]
- 47.Cresswell T., Flowers M., Barton L., Martindale V. Staff opinions on casting material brands: a prospective study. Injury. 2008;39(12):1467–1473. doi: 10.1016/j.injury.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Rowley D., Pratt D., Powell E., Norris S., Duckworth T. The comparative properties of plaster of Paris and plaster of Paris substitutes. Arch. Orthop. Trauma. Surg. 1985;103(6):402–407. doi: 10.1007/BF00435449. [DOI] [PubMed] [Google Scholar]
- 49.Lane P., Lee M. New synthetic casts: what nurses need to know. Orthop. Nurs. 1982;1(6):13–22. [PubMed] [Google Scholar]
- 50.Zhu M., Lokino E., Chan C., Gan A., Ong L., Lim K. Cast immobilisation for the treatment of paediatric distal radius fracture: fibreglass versus polyolefin. Singap. Med. J. 2019;60(4):183. doi: 10.11622/smedj.2018118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowker P., Powell E. A clinical evaluation of plaster-of-Paris and eight synthetic fracture splinting materials. Injury. 1992;23(1):13–20. doi: 10.1016/0020-1383(92)90118-C. [DOI] [PubMed] [Google Scholar]
- 52.Martin P., Weimann D., Orrxy J., Bahranixys A. A comparative evaluation of modern fracture casting materials. Eng. Med. 1988;17(2):63–70. doi: 10.1243/EMED_JOUR_1988_017_019_02. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed S., Carmichael K. Plaster and synthetic cast temperatures in a clinical setting: an in vivo study. Orthopedics. 2011;34(2) doi: 10.3928/01477447-20101221-11. [DOI] [PubMed] [Google Scholar]
- 54.Yalçınozan M., Sarı E. The impact of different application techniques on fiberglass casts: a mechanical experimental study. Indian J. Orthop. 2020;54(1):178–182. doi: 10.1007/s43465-020-00180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philbin T., Gittins M. Hybrid casts: a comparison of different casting materials. J. Osteopath. Med. 1999;99(6):311. doi: 10.7556/jaoa.1999.99.6.311. 311. [DOI] [PubMed] [Google Scholar]
- 56.B. AT and P. BG. A comparison of the mechanical properties of fiberglass cast materials and their clinical relevance. J. Orthoped. Tra. 1990;4(1):85–92. doi: 10.1097/00005131-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Stenton S. Review series: occupational and environmental lung disease: occupational asthma. Chron. Respir. Dis. 2010;7(1):35–46. doi: 10.1177/1479972309346757. [DOI] [PubMed] [Google Scholar]
- 58.Chatzistergosa P.E., Ganniari-Papageorgioub E., Chockalingam N. Comparative study of the strength characteristics of a novel wood-plastic composite and commonly used synthetic casting materials. Clin. BioMech. 2020;77 doi: 10.1016/j.clinbiomech.2020.105064. [DOI] [PubMed] [Google Scholar]
- 59.Charles M., Yen D. Properties of a hybrid plaster-fibreglass cast. Can. J. Surg. 2000;43(5):365. [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly D. The use of fiberglass as reinforcement plaster cast. Orthop. Nurs. 1983;2(6):33–36. [PubMed] [Google Scholar]
- 61.Gogoi R., Niyogi U., Alam M., Mehra D. Study of effect of NCO/OH molar ratio and molecular weight of polyol on the physico-mechanical properties of polyurethane plaster cast. World Appl. Sci. J. 2013;21(2):276–283. doi: 10.5829/idosi.wasj.2013.21.2.2549. [DOI] [Google Scholar]
- 62.Bullen M., Kinealy J., Blanchard R., Rodda C., Pivonka P. Comparison of the moulding ability of Plaster of Paris and polyester cast material in the healthy adult forearm. Injury. 2017;48(11):2586–2589. doi: 10.1016/j.injury.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Petty A., Wardman C. A randomized, controlled comparison of adjustable focused rigidity primary casting technique with standard plaster of Paris/synthetic casting technique in the management of fractures and other injuries. J. Orthop. Nurs. 1998;2(2):95–102. doi: 10.1016/S1361-3111(98)80076-9. [DOI] [Google Scholar]
- 64.Kunze K., Haberer K. Comparative studies of synthetic and nonsynthetic cast dressings. Unfallchirurg. 1994;97(6):325–331`. [PubMed] [Google Scholar]
- 65.Ritchie I., Wytch R., Wardlaw D. Flammability of modern synthetic bandages. Injury. 1988;19(1):31–32. doi: 10.1016/0020-1383(88)90171-4. [DOI] [PubMed] [Google Scholar]
- 66.Compton J., Edelstein J. New plastics for forming directly on the patient. Prosthet. Orthot. Int. 1978;2(1):43–47. doi: 10.3109/03093647809146297. [DOI] [PubMed] [Google Scholar]
- 67.Breger-Lee D., Buford W., Jr. Properties of thermoplastic splinting materials. J. Hand Ther. 1992;5(4):202–211. doi: 10.1016/S0894-1130(12)80274-2. [DOI] [Google Scholar]
- 68.Jø. gensen U., Nordkild P. Hexcelite (R) versus plaster of Paris: a controlled trial of the below-knee walking cast. Prosthet. Orthot. Int. 1986;10(3):114–116. doi: 10.3109/03093648609164513. [DOI] [PubMed] [Google Scholar]
- 69.Pope M., Callahan G., Lavalette R. Setting temperatures of synthetic casts. J. Bone Jt. Surg. Am. Vol. 1985;67(2):262–264. [PubMed] [Google Scholar]
- 70.Bassett F., III, Malone T., Gilchrist R. A protective splint of silicone rubber. Am. J. Sports Med. 1979;7(6):358–360. doi: 10.1177/036354657900700611. [DOI] [PubMed] [Google Scholar]
- 71.DeCarlo M., Malone K., Darmelio J., Rettig A. Casting in sport. J. Athl. Train. 1994;29(1):37. [PMC free article] [PubMed] [Google Scholar]
- 72.Canelon M. Silicone rubber splinting for athletic hand and wrist injuries. J. Hand Ther. 1995;8(4):252–257. doi: 10.1016/S0894-1130(12)80117-7. [DOI] [PubMed] [Google Scholar]
- 73.B. JA, W. GG, A. JT and H. R. Soft playing splint for protection of significant hand and wrist injuries in sports. Am. J. Sports Med. 1982;10:293–296. doi: 10.1177/036354658201000506. [DOI] [PubMed] [Google Scholar]
- 74.Müller K., Zollfrank C., Schmid M. Natural polymers from biomass resources as feedstocks for thermoplastic materials. Macromol. Mater. Eng. 2019;304(5) doi: 10.1002/mame.201800760. [DOI] [Google Scholar]
- 75.woodcast . Onbone Oy; 2021. The Safe Way to Do Casting.https://www.woodcast.com/ [Online]. Available: Accessed. [Google Scholar]
- 76.fi Forest. Splinting material made from wood and bioplastics. Onbone. 2016 https://forest.fi/products-services/splinting-material-made-from-wood-and-bioplastics/#373380b3 [Online]. Available: Accessed. [Google Scholar]
- 77.L. NC. and S. J A novel nontoxic wood-plastic composite cast. Open Med. Dev. J. 2012;4(1):1–5. doi: 10.2174/1875181401204010001. [DOI] [Google Scholar]
- 78.Lindfors N., Salo J. New ecological wood–plastic composite materials for scaphoid-type casting: material properties and clinical evaluation. Hand Ther. 2014;19(3):67–72. doi: 10.1177/1758998314538241. [DOI] [Google Scholar]
- 79.Hirsimäki J., Lindfors N., Salo J. Novel ankle cast designs with non-toxic material. Foot Ankle Online J. 2014;7:1–5. doi: 10.3827/faoj.2014.0704.0005. [DOI] [Google Scholar]
- 80.Pirhonen E., Pärssinen A., Pelto M. Comparative study on stiffness properties of WOODCAST and conventional casting materials. Prosthet. Orthot. Int. 2013;37(4):336–339. doi: 10.1177/0309364612465885. [DOI] [PubMed] [Google Scholar]
- 81.Thorndike A., Jr., Garrey W. A useful type of light, waterproof cast: preliminary report. N. Engl. J. Med. 1938;218(5):205–211. doi: 10.1056/NEJM193802032180501. [DOI] [Google Scholar]
- 82.Fraunhofer . fraunhofer; 2021. Thermoplastic, Compostable Splint for Bone Fractures.https://www.fraunhofer.de/en/research/current-research/bioeconomy/health/splint-for-bone-fractures.html [Online]. Available: Accessed. [Google Scholar]
- 83.Aw S., Ad G., A. G, P. B, S. LM. C. P Waterproof cast liners in paediatric forearm fractures: a randomized trial. J. Child Orthop. 2013;7:123–130. doi: 10.1007/s11832-012-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shugar J. Hair growth and plaster casts. Canad. Med. Assoc. J. 1983;128(11):1271. [PMC free article] [PubMed] [Google Scholar]
- 85.Shirley E., Maguire K., Mantica A., Kruse R. Alternatives to traditional cast immobilization in pediatric patients. JAAOS-J. Am. Acad. Orthop. Surg. 2020;28(1):e20–e27. doi: 10.5435/JAAOS-D-18-00152. [DOI] [PubMed] [Google Scholar]
- 86.Halanski M., Halanski A., Oza A., Vanderby R., Munoz A., Noonan K. Thermal injury with contemporary cast-application techniques and methods to circumvent morbidity. JBJS. 2007;89(11):2369–2377. doi: 10.2106/JBJS.F.01208. [DOI] [PubMed] [Google Scholar]
- 87.Goto M., Ogata K. Experimental study on thermal burns caused by plaster bandage. Nihon Seikeigeka Gakkai Zasshi. 1986;60(6):671–680. [PubMed] [Google Scholar]
- 88.Read J., Ferguson N., Ricketts D. Plaster cast burns: the reality. Emerg. Med. J. 2008;25(12):827–828. doi: 10.1136/emj.2007.053983. [DOI] [PubMed] [Google Scholar]
- 89.Metzman L., Gamble J., Rinsky L. Effectiveness of ice packs in reducing skin temperature under casts. Clin. Orthop. Relat. Res. 1996;330:217–221. doi: 10.1097/00003086-199609000-00029. [DOI] [PubMed] [Google Scholar]
- 90.Autti-Rämö I., Suoranta J., Anttila H., Malmivaara A., Mäkelä M. Effectiveness of upper and lower limb casting and orthoses in children with cerebral palsy: an overview of review articles. Am. J. Phys. Med. Rehabil. 2006;85(1):89–103. doi: 10.1097/01.phm.0000179442.59847.27. [DOI] [PubMed] [Google Scholar]
- 91.Lewis F., Cork M., Mcdonagh A., Gawkrodger D. Allergic contact dermatitis from resin-reinforced plaster. Contact Dermatitis. 1993;28(1):40–41. doi: 10.1111/j.1600-0536.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 92.Silfverskiold J. Fiberglass versus plaster casts: how to choose between them. PGM (Postgrad. Med.) 1989;86(5):71–74. doi: 10.1080/00325481.1989.11704430. [DOI] [PubMed] [Google Scholar]
- 93.M. MF, L. W and M. A. Calamine lotion to reduce skin irritation in children with cast immobilisation. J. Orthop. Surg. 2013;21:221–225. doi: 10.1177/230949901302100222. [DOI] [PubMed] [Google Scholar]
- 94.Dm N., R. LG. Dm R. Keeping plaster casts dry: what works? Injury. 2005;36:73–74. doi: 10.1016/j.injury.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Davids J., Frick S., Skewes E., Blackhurst D. Skin surface pressure beneath an above-the-knee cast: plaster casts compared with fiberglass casts. JBJS. 1997;79(4):565–569. doi: 10.2106/00004623-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 96.Alexandre V., Hodax J. The Orthopedic Consult Survival Guide. Springer; Cham.: 2017. Splinting and casting techniques; pp. 25–39. [DOI] [Google Scholar]
- 97.Zaino C., Patel M., Arief M., Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. JBJS. 2015;97(5):374–380. doi: 10.2106/JBJS.N.00579. [DOI] [PubMed] [Google Scholar]
- 98.Roberts A., Shaw K., Boomsma S., Cameron C. Effect of casting material on the cast pressure after sequential cast splitting. J. Pediatr. Orthop. 2017;37(1):74–77. doi: 10.1097/BPO.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 99.Miller B., Taylor B., Widmann R., Bae D., Snyder B., Waters P. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J. Pediatr. Orthop. 2005;25(4):490–494. doi: 10.1097/01.bpo.0000158780.52849.39. [DOI] [PubMed] [Google Scholar]
- 100.Puddy A., Sunkin J., Aden J., Walick K., Hsu J. Cast saw burns: evaluation of simple techniques for reducing the risk of thermal injury. J. Pediatr. Orthop. 2014;34(8):e63–e66. doi: 10.1097/BPO.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 101.Killian J., White S., Lenning L. Cast-saw burns: comparison of technique versus material versus saws. J. Pediatr. Orthop. 1999;19(5):683. [PubMed] [Google Scholar]
- 102.Rezaei R. Rochester Institute of Technology; 2017. The Easy Wrap Orthopedic Cast. [Google Scholar]
- 103.Suojalehto H., Linström I., Henriks‐Eckerman M., Jungewelter S., Suuronen K. Occupational asthma related to low levels of airborne methylene diphenyl diisocyanate (MDI) in orthopedic casting work. Am. J. Ind. Med. 2011;54(12):906–910. doi: 10.1002/ajim.21010. [DOI] [PubMed] [Google Scholar]
- 104.Chatterton C., Elliott R. Acute acetone poisoning from leg casts of a synthetic plaster substitute. J. Am. Med. Ass. 1946;130(17):1222–1223. doi: 10.1001/jama.1946.02870170028006b. [DOI] [PubMed] [Google Scholar]
- 106.Daines S., Aronsson D., Beynnon B., Sturnick D., Lisle J., Naud S. What is the best material for molding casts in children? J. Pediatr. Orthop. 2014;34(7):743–748. doi: 10.1097/BPO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 107.Marshall P., Dibble A., Walters T., Lewis D. When should a synthetic casting material be used in preference to plaster-of-Paris? A cost analysis and guidance for casting departments. Injury. 1992;23(8):542–544. doi: 10.1016/0020-1383(92)90156-M. [DOI] [PubMed] [Google Scholar]
- 108.Austin R. Treatment of broken legs before and after the introduction of gypsum. Injury. 1983;14(5):389–394. doi: 10.1016/0020-1383(83)90089-X. [DOI] [PubMed] [Google Scholar]
- 109.Hill C., Masters J., Perry D. A systematic review of alternative splinting versus complete plaster casts for the management of childhood buckle fractures of the wrist. J. Pediatr. Orthop. B. 2016;25(2):183–190. doi: 10.1097/BPB.0000000000000240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.