Abstract
Systemic sporotrichosis is an emerging infection potentially fatal for immunocompromised patients. Adhesion to extracellular matrix proteins is thought to play a crucial role in invasive fungal diseases. Here we report studies of the adhesion of Sporothrix schenckii to the extracellular protein fibronectin (Fn). Both yeast cells and conidia of S. schenckii were able to adhere to Fn as detected by enzyme-linked immunosorbent binding assays. Adhesion of yeast cells to Fn is dose dependent and saturable. S. schenckii adheres equally well to 40-kDa and 120-kDa Fn proteolytic fragments. While adhesion to Fn was increased by Ca2+, inhibition assays demonstrated that it was not RGD dependent. A carbohydrate-containing cell wall neutral fraction blocked up to 30% of the observed adherence for the yeast cells. The biochemical nature of this fraction suggests the participation of cell surface glycoconjugates in binding by their carbohydrate or peptide moieties. These results provide new data concerning S. schenckii adhesion mechanisms, which could be important in host-fungus interactions and the establishment of sporotrichosis.
Infections caused by the dimorphic mycopathogen Sporothrix schenckii have increased in recent years mainly in immunocompromised patients (7, 17). S. schenckii is found as mycelium in its saprophytic form and as yeast cells in human lesions. S. schenckii can cause either limited cutaneous lesions or invasive, disseminated infections (17). Systemic sporotrichosis may be due to conidia inhalation (8) or bloodstream dissemination from a cutaneous lesion (4). Risk factors such as alcoholism, diabetes, and extensive use of immunosuppressive drugs may predispose to severe infections, including pulmonary and osteoarticular sporotrichosis (17).
Adherence of pathogenic microorganisms to host tissues is regarded as a prerequisite for dissemination. Microbial adherence has been studied extensively in pathogenic bacteria (15) and fungi such as Candida albicans (37), Aspergillus fumigatus (3), and Blastomyces dermatitidis (19), but little is known about the adherence mechanisms in S. schenckii.
Extracellular matrices (ECM) are covered by epithelial and endothelial cells. However, cell injury and exposure of subendothelial ECM may occur during infections (20). Fibronectin (Fn), a large (440 kDa) dimeric glycoprotein, is a multifunctional ECM protein with a central role in cell adhesion and spreading (29). Fn has been implicated in adherence of several pathogens such as Aspergillus fumigatus (31, 39), Candida albicans (21), Candida tropicalis (1, 5), and Pneumocystis carinii (33). Adherence to Fn is a virulence factor for Staphylococcus aureus, since an Fn binding protein is important for internalization of this bacterium by nonprofessional phagocytes (36). Different mechanisms seem to be involved in these adhesion processes. Either a protein-protein or a lectin-like interaction was described for several fungal pathogens (14, 35, 39). It was also reported that adherence of A. fumigatus to Fn varied in accordance with its morphology (12).
Our previous studies have shown that both conidia and yeast cells of S. schenckii are able to bind to the immobilized ECM proteins laminin, collagen type II, and Fn in vitro (24), suggesting the presence of cell surface adhesins recognizing these ECM proteins. In the present paper, we describe the adhesion mechanism of S. schenckii to human Fn and its proteolytic fragments. Inhibition experiments with purified cell wall fractions demonstrated for the first time the involvement of S. schenckii cell surface components in Fn binding. The effect of RGD peptides, divalent ions, and monosaccharides was also investigated. The results presented may help in understanding how this pathogen interacts with host cells.
MATERIALS AND METHODS
Reagents.
An antiserum against yeast cells of S. schenckii was raised in rabbits as described (23), according to the NIH Guide for Care and Use of Laboratory Animals. A purified immunoglobulin fraction of a rabbit anti-human Fn serum was from Dako S/A (Copenhagen, Denmark). A horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (whole molecule) was from Sigma (Saint Louis, Mo.). The α-chymotryptic 40-kDa and 120-kDa fragments of Fn and RGD peptides (RGDS and GRGESP) were from Gibco-BRL (Gaithersburg, Md.). Bacteriological peptone, yeast extract, and bacteriological agar were from Oxoid Ltd. (Hampshire, England).
Microorganism and growth conditions.
S. schenckii strain 1099-18 was used throughout this study. This strain was originally obtained from the Mycology Section, Department of Dermatology, Columbia University, New York, N.Y. The organism was routinely maintained on modified Sabouraud's dextrose agar (glucose 2%, peptone 1%, yeast extract 0.5%, agar 2%) slants at 4°C. The yeast form of S. schenckii was grown in brain heart infusion broth (BHI; Difco) at 37°C in a rotary shaker at 150 rpm. After 7 days in culture the cells were harvested and washed with sterile phosphate-buffered saline (PBS; 50 mM, pH 7.2). The mycelial phase of S. schenckii was grown in Sabouraud broth at 25°C for 7 days. Conidia were separated by gauze filtration and washed with sterile PBS. The yeast cells and conidia were quantified by Neubauer chamber counts. The cell suspension was adjusted to 108 cells/ml.
Cell wall fractions.
S. schenckii cell wall peptido-rhamnomannan (CWPR) was isolated from the yeast cell mass as previously described (25). Briefly, cell mass suspended in 0.02 M citrate buffer (pH 7.0) was autoclaved for 90 min at 120°C. After centrifugation at 15,000 × g for 10 min, the extract was isolated and dialyzed with two changes of distilled water, followed by ethanol precipitation. The precipitate was taken up in distilled water and then lyophilized. This fraction is described as the crude extract. The same procedure was used to isolate a crude extract (crude mannoprotein fraction [MP]) of Saccharomyces cerevisiae.
For the isolation of CWPR, the crude extract was further fractionated with Cetavlon (N,N-cetylhexadecyltrimethylammonium bromide). S. schenckii crude extract was also subjected to further fractionation by anion exchange chromatography on a MonoQ HR 5/5 (Pharmacia) column. The unbound fraction, eluted with the equilibration buffer (Tris-HCl, 50 mM, pH 7.2) was called the neutral fraction, and the bound one, eluted with an NaCl gradient, was called the acidic fraction.
The monosaccharide composition of each fraction was determined after acid hydrolysis (4 M trifluoroacetic acid, 6 h at 100°C) by gas-liquid chromatography as we described previously (23). Glucuronic acid was ascertained by high-pH anion exchange chromatography using a Dionex DX 500 system equipped with a CarboPac PA10 column eluted with 0.15 M sodium acetate in 0.1 M NaOH (16).
Isolation of plasma Fn.
Fn was freshly prepared in our laboratory according to Vuento and Vaheri (38). Briefly, human plasma (collected with informed consent from healthy donors of our department) was subjected to gelatin-Sepharose chromatography, and bound proteins were eluted by 0.05 M Tris buffer (pH 7.4) with increasing arginine concentrations. The fraction eluted with 1 M arginine was dialyzed against PBS and concentrated, and purity was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the method of Laemmli (22).
Binding assays.
The adherence of S. schenckii cells to Fn was evaluated by enzyme-linked immunosorbent assay (ELISA). Wells of polystyrene microtiter plates (Maxisorp; Nunc) were coated with human plasma Fn (1 to 75 μg/ml in 0.2 M bicarbonate buffer [pH 9.4]) by passive adsorption overnight at 4°C. Alternatively, microplates were also sensitized with proteolytic fragments of 40 kDa (frag 40) and 120 kDa (frag 120) in different concentrations. The plates were then washed with PBS containing 0.05% Tween 20 (PBS-Tween). Nonspecific binding was blocked by incubating each well for 2 h at 37°C with 0.5% bovine serum albumin (BSA) in PBS (pH 7.4). After a further washing with PBS-Tween, yeast cells or conidia (107 cells/well) were added, followed by incubation for 1 h at 37°C. The plates were then washed to remove unbound cells, and a rabbit anti-S. schenckii serum, diluted 1:500 in PBS-Tween/BSA 0.1%, was added. The plates were incubated for 1 h at 37°C, washed, and incubated with an HRP-conjugated goat anti-rabbit IgG. The plates were washed with PBS-Tween, and the reaction was developed with the substrate 0-phenylenediamine (OPD) (0.5 mg ml−1 and 0.005% H2O2 in 0.01 M sodium citrate buffer, pH 5.6). The reaction was stopped after 10 min with 0.2 M H2SO4 and measured at 490 nm using an automated reader (Bio-Rad ELISA reader). In addition, for each experiment, wells saturated with BSA were also overlaid with yeast cells or conidia. Adhesion to these wells was considered background and subtracted from Fn adhesion values. Each experiment was done in triplicate, and the results correspond to a typical experiment from at least three independent repeats.
To assess the effect of divalent cations on binding, this assay was performed with a PBS-Tween buffer containing different concentrations of Mg2+ or Ca2+ at the fungal incubation step.
Preparation of biotinylated fungal cells.
Biotinylated S. schenckii cells were prepared by using a commercial kit (Amersham). Briefly, S. schenckii cells were washed twice with cold PBS and incubated under gentle agitation with 40 mM sodium bicarbonate buffer containing 150 mM NaCl and 2 μl of biotin reagent for 30 min at 4°C, according to the supplier's instructions. After washing with PBS (50 mM, pH 7.2), the integrity of the cells was confirmed by optic microscopy. After centrifugation (6,000 × g for 5 min at 4°C), the cells were collected and used in binding assays.
Inhibition assays.
S. schenckii cells were pretreated with different concentrations (10 to 100 μg/ml) of the arginine-glycine-aspartic acid (RGD) peptides RGDS and GRGESP for 1 h at 37°C. After this incubation step, cells were allowed to adhere to Fn-coated wells, and the adhesion assays were performed as described above.
For carbohydrate inhibition experiments, Fn-coated wells were pretreated with 200 mM galactose (Gal), mannose (Man), glucuronic acid (GlcA), or rhamnose (Rha) for 1 h at 37°C or with different S. schenckii cell wall fractions diluted in PBS (pH 7.4) at a final concentration of 100 μg of carbohydrate per well. Afterward, 107 yeast cells were added to each well in the presence of sugars or cell wall fractions. After incubation, the unbound cells were removed by washing with PBS-Tween, and the assay was completed as already described.
Alternatively, biotinylated fungal cells were added to 96-well plates sensitized with Fn and previously treated with rabbit anti-human Fn antibodies (5 to 100 μg/ml) for 1 h at 37°C. After washing, plates were incubated with streptavidin-peroxidase conjugate (diluted 1:1,500 in PBS-Tween/BSA 0.1%). The plates were washed with PBS-Tween, and the reaction was developed with OPD as described.
Statistical analysis.
Values are reported as mean ± standard deviation (SD). The analysis of variance and Tukey-Kramer tests were used to compare differences between the binding capacity to ECM proteins and the binding to BSA, as well as to determine differences associated with the effect of potential inhibitors or between different fungus morphological phases. P < 0.05 was considered significant.
RESULTS
S. schenckii cells adhere to human Fn in vitro.
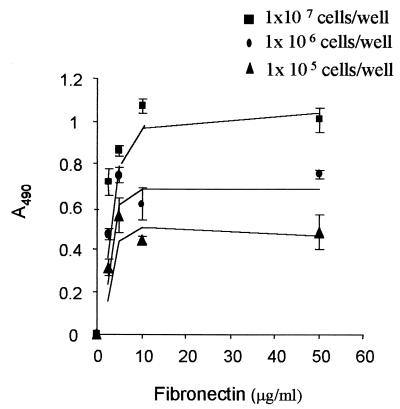
Adhesion to host tissue is an important step for dissemination. We evaluated the adherence of S. schenckii cells to Fn fixed in microtiter plates. Yeast cells of S. schenckii adhere to immobilized Fn in a dose-dependent manner (Fig. 1). Thus, the coating of wells with increasing concentrations of Fn resulted in saturation at 10 μg/ml of Fn.
FIG. 1.
Adhesion of S. schenckii to immobilized Fn. Different yeast cell concentrations were tested. The number of cells adhering was determined by measuring the optical density at 490 nm (A490), as described in the text. Results are representative of three independent experiments and the values are the means ± SD of triplicate wells.
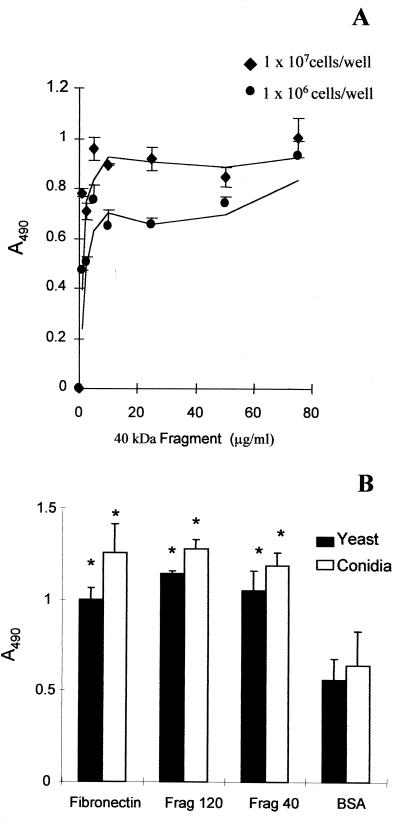
To further investigate the binding site of S. schenckii to Fn, we tested the ability of yeast cells and conidia to bind to its 120- and 40-kDa proteolytic fragments. The 120-kDa fragment contains the RGD cell-binding domain (32), and the 40-kDa one contains the heparin-binding domain (10). Yeast cells of S. schenckii adhere to the 40-kDa proteolytic fragment of Fn in a dose-dependent manner (Fig. 2A). As shown in Fig 2B, both morphological phases of S. schenckii adhere significantly to both fragments compared to BSA. There was no difference in fungus adhesion rates between fragments and the intact Fn. These results suggest that S. schenckii has more than one binding site in human Fn.
FIG. 2.
Adhesion of S. schenckii to Fn fragments. (A) Adhesion to the 40-kDa Fn fragment. Yeast cells were assayed at 107 and 106 cells/well. (B) Microtiter plates were coated with either Fn or Fn fragments: frag120 (containing the cell-binding domain) or frag40 (containing the GAG-binding domain). Control wells contained immobilized BSA only. Both conidia (open bars) and yeast cells (black bars) of S. schenckii were tested at a concentration of 107 cells per well. As described in the text, the number of bound cells was determined by measuring the optical density at 490 nm. The values are the means ± SD of triplicate wells, and this figure is representative of three independent experiments. ∗, P < 0.05.
S. schenckii conidia and yeast cells bind specifically to Fn.
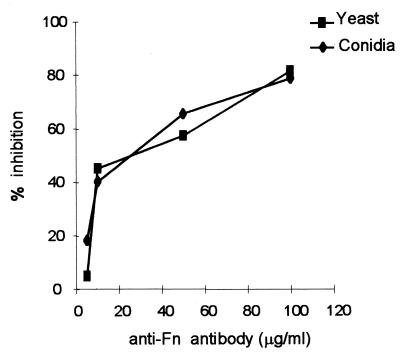
The specificity of S. schenckii adhesion was tested with an anti-Fn immunoglobulin fraction. This polyclonal antibody against human plasma Fn inhibited the adhesion of S. schenckii yeast cells and conidia (Fig. 3). Inhibition of yeast cell adherence was dose dependent, increasing from 4.8 to 81% as antibody concentration rose from 5 to 100 μg/ml. Similarly, assays with conidia demonstrated inhibition of 18% with the lowest antibody concentration and 78% with 100 μg/ml of anti-Fn antibody. Together these results indicate that Fn binding sites were recognized by the cell surface molecules of both morphological phases of S. schenckii.
FIG. 3.
S. schenckii yeast cells and conidia specifically bind to Fn. The adhesion of yeast cells and conidia to Fn at 75 μg/ml was tested in the presence of different concentrations of a purified immunoglobulin fraction of an anti-Fn rabbit serum. Binding in the absence of antibody was considered 100% adhesion. Negative controls were done with antibodies from normal rabbit serum. The values are the means ± SD of triplicate wells. The figure is representative of three independent experiments.
Effect of divalent ions in S. schenckii adhesion.
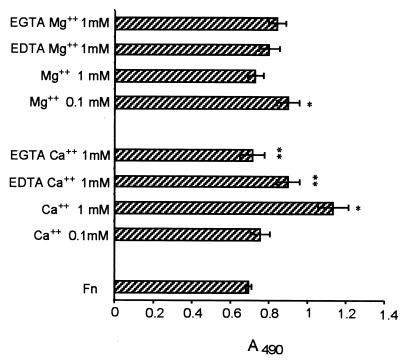
Interactions of Fn with mammalian adhesion receptors such as integrins and lectin-like receptors (selectins) are divalent-cation dependent. Since microorganisms may exhibit adhesins similar to eukaryotic cell adhesion molecules (18), we tested the effect of calcium and magnesium in S. schenckii adherence to Fn. Binding of S. schenckii yeast cells to Fn was enhanced in the presence of higher concentration of Ca2+ (1 mM), whereas 0.1 mM Ca2+ had no significant effect (Fig. 4). On the other hand, 0.1 mM Mg2+ only slightly increased adhesion, whereas 1 mM Mg2+ showed no effect. The addition of EDTA or EGTA (5 mM) significantly reversed the Ca2+ effect (P < 0.01).
FIG. 4.
Effect of calcium and magnesium in S. schenckii binding to Fn. S. schenckii yeast cells were added to Fn-coated microtiter wells (50 μg/ml) in the presence of Ca2+ or Mg2+ (0.1 or 1 mM) coincubated or not with EDTA or EGTA (5 mM). Unbound cells were removed by washing the plates with PBS-Tween, and the number of bound cells was determined by ELISA at 490 nm. The values are the means ± SD of triplicate wells, and the figure is representative of three independent experiments. ∗, P < 0.05 versus control (adhesion without ions); ∗∗, P < 0.01 versus adhesion values in presence of Ca2+ at 1 mM.
Effect of RGD-containing peptides and monosaccharides in S. schenckii adhesion to Fn.
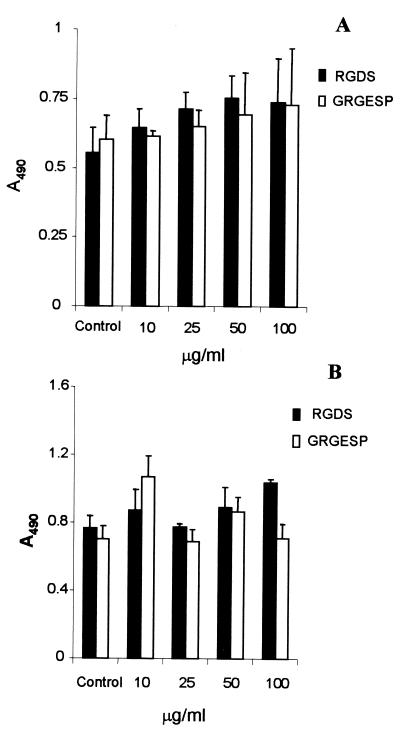
Studies on eukaryotic cells have shown that integrins recognize the tripeptide arginine-glycine-aspartic acid (RGD) as a binding site in the central region of the Fn molecule (32, 34). Recent data suggested that yeast species and filamentous fungi not only employ RGD-mediated interactions to adhere to host tissues but may also express a subset of adhesins that themselves contain an RGD sequence (13, 14). As S. schenckii cells bind to the 120-kDa fragment, which contains the RGD sequence, we performed inhibition assays with RGDS and GRGESP peptides (Fig. 5). Pretreatment of both conidia and yeast cells with RGDS or GRGESP peptide at 10 to 100 μg/ml failed to reduce the adherence to immobilized Fn compared to control wells. In binding assays, coincubation with RGDS was not able to significantly inhibit the adhesion of yeast cells to the 120-kDa Fn fragment (data not shown). This observation clearly indicates that the interactions between S. schenckii and Fn do not involve the RGD motif of the Fn molecule.
FIG. 5.
Effect of RGD peptides on the adhesion of S. schenckii to Fn. Conidia (A) or yeast cells (B) were allowed to adhere to Fn (50 μg/ml) in the absence of peptides (control) or after treatment with different concentrations of RGDS (black bars) or GRGESP peptides (open bars) for 1 h at 37°C. The number of bound cells was determined by ELISA at 490 nm. The values are the means ± SD of triplicate wells, and the figure is representative of three independent experiments.
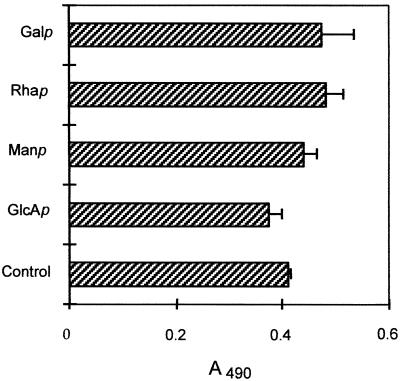
S. schenckii expresses on its cell wall a glycopeptide component known as peptido-rhamnomannan, composed mainly of Rha, Man, and also some GlcA, as well as a galactose-containing polysaccharide (25, 26, 28). Table 1 shows a qualitative analysis of monosaccharides present in the cell wall fractions that we have isolated in the present work. Considering that S. schenckii cell wall fractions were almost 80% carbohydrates (data not shown), we tested the role of the monosaccharides in cell adherence. Monosaccharides had no significant effect on yeast cell binding (Fig. 6). Furthermore, the negatively charged glycosaminoglycans (GAGs) heparin and heparan sulfate and sialic acid (200 mM) also failed to inhibit the attachment of S. schenckii to Fn (data not shown).
TABLE 1.
Carbohydrate composition of different fractions isolated from the cell wall of yeast cells of S. schenckii and Saccharomyces cerevisiaea
| Sugar |
S. schenckii
|
S. cerevisiae mannoprotein | ||
|---|---|---|---|---|
| CWPR | MonoQ neutral | MonoQ acidic | ||
| Rhamnose | ++ | ++ | ++ | − |
| Mannose | ++ | ++ | ++ | ++ |
| Glucose | ± | + | ± | − |
| Galactose | ± | ++ | − | − |
| Glucuronic acidb | ND | ± | ++ | ND |
Composition indicated: −, not detected; ± trace amounts; +, detected; ++, significant amount; ND, not done.
Glucuronic acid was identified by HPLC (Dionex) with a Carbopac PA10 column.
FIG. 6.
Effect of monosaccharides on the adhesion of S. schenckii to Fn. S. schenckii yeast cells were added to Fn-coated wells (50 μg/ml) together with 200 mM galactose (Galp), rhamnose (Rhap), mannose (Manp), or glucuronic acid (GlcAp). Unbound cells were removed by washing the plates with PBS-Tween, and the number of bound cells was determined by measuring the optical density at 490 nm. The figure is representative of three independent experiments, and the values are the means ± SD of triplicate wells.
Cell wall extracts of S. schenckii inhibit adhesion to Fn.
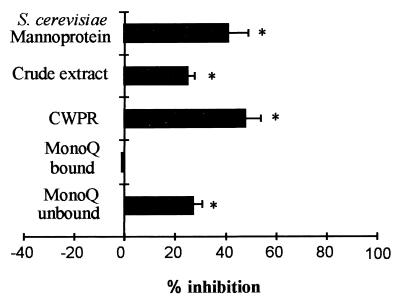
To investigate whether adhesins are present on the S. schenckii cell surface, several cell wall fractions were preincubated with immobilized Fn, followed by incubation with yeast cells, as described in Materials and Methods. The cell wall crude extract and the peptido-rhamnomannan purified fraction (CWPR) inhibited adhesion by 22 and 48%, respectively (Fig. 7). This inhibition did not seem to be related to negatively charged cell wall glycoconjugates, because only the neutral fraction derived from the crude cell wall extract was able to inhibit binding. The acidic fraction did not inhibit adhesion. A crude mannoprotein fraction (MP) from S. cerevisiae showed an inhibitory effect, suggesting the presence of similar adhesion molecules among these two fungi.
FIG. 7.
Inhibition of the adhesion of S. schenckii to Fn by cell wall fractions. S. schenckii yeast cells were added to Fn-coated microtiter wells (50 μg/ml) incubated with different cell wall fractions at 100 μg of carbohydrate per well. Unbound cells were removed by washing the plates with PBS-Tween, and the number of bound cells was determined by measuring the optical density at 490 nm. Binding in the absence of inhibitor was set as the control. Values shown are means ± SD of percent inhibition of binding in the presence of inhibitor in triplicate samples, and this figure is representative of three independent experiments. ∗, P < 0.01.
DISCUSSION
Adhesion of microorganisms to host cells and tissues represents a critical step in the process of infection (15). Likewise, binding events described in this paper could play an important role in S. schenckii invasion of host tissue. We demonstrated that S. schenckii adheres to human Fn in a dose-dependent and saturable manner. This binding was blocked by polyclonal anti-Fn antibodies, demonstrating the specificity of adherence of S. schenckii to Fn. Furthermore, this fungus binds at different sites on the Fn molecule. Support for this conclusion comes from the finding that conidia and yeast cells bind both to the 40-kDa fragment, which bears the GAG-binding domain, and to the 120-kDa fragment, bearing the cell binding domain and the RGD sequence.
Despite the increasing number of disseminated cases of sporotrichosis, mainly in immunocompromised patients (17), very little is known about virulence factors in this species. Our results reinforce previous data from our group suggesting that both morphological phases of S. schenckii adhere to ECM proteins (24). Other investigators have described Fn as binding to different fungal pathogens such as C. albicans (11, 21), C. tropicalis (1), and A. fumigatus (31). Binding to Fn could potentially confer advantages on invading cells, since Fn is found at high concentrations in body fluids and is abundantly expressed on vascular endothelium. In fact, previous work by our group has demonstrated that S. schenckii yeast cells adhere in vitro to human endothelial cells (unpublished data). Studies with Aspergillus species have already correlated adhesion capacity and virulence (39). In these experiments, pathogenic A. fumigatus conidia bound significantly better to Fn than do the nonpathogenic Aspergillus strains A. ornatus and A. wentii, whereas strain A. wentii ATCC 1023, a rare pathogen, showed intermediate levels of binding (39).
In recent years, several molecules with receptor-like characteristics have been described in pathogenic fungi such as C. albicans (9) and A. fumigatus (12). Most of these microbial molecules are glycoproteins present in the cell wall and are known as adhesins, displaying properties similar to integrins or lectins. They generally recognize RGD-containing peptides or fucosyl glycosides in different tissues (30). Many adhesion receptors bind divalent ions such as Ca2+ and Mg2+. Binding of S. schenckii yeast cells to Fn was increased in the presence of calcium, and this effect was reversed when the chelating agents EDTA and EGTA were added to the assay. These results suggest the presence of an integrin or a lectin-like adhesin on S. schenckii, as described for other fungi (2, 13).
The tripeptide RGD, first identified in Fn, is recognized by several integrins (α5β1, αIIbβ3, and all or most αv-containing integrins) but not by many others (6). Inhibition assays with the RGDS and GRGESP peptides showed that binding was unaffected by these peptides, suggesting that other sequences in the 120-kDa fragment are recognized by S. schenckii cells. Similar to our results, López-Ribot et al. (27) demonstrated that interactions between C. albicans and tenascin C did not occur through RGD-bearing peptides. However, our experimental approach neither discharged the presence of RDG sequences on cell wall-adhesive molecules of this fungus nor tested the possibility that such a class of adhesins could mediate interactions of this fungus with the surface of host cells. The extensive repertoire of microbial adhesins already described may reflect the range of sites that pathogens can invade during infection. Our work demonstrated that S. schenckii has adhesins that recognize human Fn and that different sites in the same molecule can be identified by this fungus as binding sites.
To understand the characteristics of interaction between S. schenckii and Fn, we performed inhibition assays with monosaccharides and tested the effect of different cell wall fractions in our assays. The S. schenckii cell wall is composed of β-glucans and a glycopeptide component, peptido-rhamnomannan. The chemical analysis of this glycopeptide has shown it to be 14.2% protein and 84.6% carbohydrate, and rhamnose and mannose were identified as the main sugars present in this molecule (25). We demonstrated that the glycopeptide peptido-rhamnomannan and its neutral MonoQ subfraction were both able to inhibit the binding to immobilized Fn. Mannose, rhamnose, and glucuronic acid residues tested individually had no effect. These data might be explained by the requirement for a correct three-dimensional structure for efficient S. schenckii-Fn interaction. Some adhesins on bacteria or fungi appear to act as lectins and recognize carbohydrate structures in the host tissue. A. fumigatus is known to adhere to laminin through sialic acids present in the laminin oligosaccharide moieties (2). Recent studies suggested that attachment of conidia to Fn and basal lamina proteins is mediated by negatively charged carbohydrates on the conidial surface (39). S. schenckii cells adhere to the 40-kDa proteolytic fragment of Fn, which contains the GAG-binding domain. However, neither heparan sulfate nor heparin was able to inhibit the interactions of this pathogen with this ECM protein (data not shown).
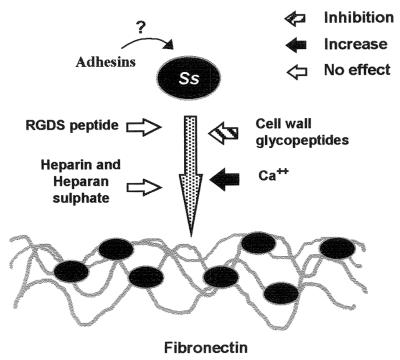
In summary, we postulate that interaction of S. schenckii with human Fn is mediated by adhesins present at the surface of fungal cells (Fig. 8) and report for the first time the involvement of cell wall components of this fungus in Fn recognition. Further studies are necessary to clarify the participation of carbohydrates in the observed binding events as well as to achieve the purification and chemical characterization of molecules responsible for S. schenckii adherence to the ECM matrix. These data may lead to a better comprehension of S. schenckii interactions with host tissues and sporotrichosis pathogenesis.
FIG. 8.
Proposed mechanism of adhesion of S. schenckii (Ss) adherence to Fn, based on the data presented here. The interaction of this fungus with Fn (represented by dark gray wavy lines) is increased by the addition of Ca2+ (black arrow) and inhibited by cell wall glycopeptides (hatched arrow). RDG peptides, heparan sulfate, and heparin had no effect on the binding (open arrows). Cell wall adhesins present on the S. schenckii surface are still uncharacterized.
ACKNOWLEDGMENTS
This work was supported by grants from FAPERJ, CNPq, MCT/CNPq/Pronex, and the CAPES Foundation (CAPES/COFECUB, proc 341/01). L.M.P. is an International Scholar from Howard Hughes Medical Institute. O.C.L. is supported by a fellowship from Fiocruz. C.C.F. is supported by a fellowship from CAPES Foundation.
We gratefully acknowledge Helena Nader (Escola Paulista de Medicina/UNIFESP, São Paulo) for the gift of heparan sulfate and heparin. We also acknowledge the skillful technical assistance of Maria Celia Machado, Gilson Fernando A. Gomes, and Orlando A Agrellos Filho.
REFERENCES
- 1.Bendel C M, Hostetter M K. Distinct mechanisms of epithelial adhesion for Candida albicans and Candida tropicalis. J Clin Investig. 1993;92:1840–1849. doi: 10.1172/JCI116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchara J P, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky J C, Tronchin G, Chabasse D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun. 1997;65:2717–2724. doi: 10.1128/iai.65.7.2717-2724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchara J P, Sanchez M, Esnault K, Tronchin G. Interactions between Aspergillus fumigatus and host matrix proteins. Contrib Microbiol. 1999;2:167–181. doi: 10.1159/000060293. [DOI] [PubMed] [Google Scholar]
- 4.Castrejón O V, Robles M, Arroyo O E Z. Fatal fungemia due to Sporothrix schenckii. Mycoses. 1995;38:373–376. doi: 10.1111/j.1439-0507.1995.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 5.DeMuri G P, Hostetter M K. Evidence for a β1 integrin fibronectin receptor in Candida tropicalis. J Infect Dis. 1996;174:127–132. doi: 10.1093/infdis/174.1.127. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza S E, Ginsberg M H, Burke T A, Plow E F. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990;265:3440–3446. [PubMed] [Google Scholar]
- 7.Durden F M, Elewski B. Fungal infections in HIV-infected patients. Semin Cutan Med Surg. 1997;16:200–212. doi: 10.1016/s1085-5629(97)80043-0. [DOI] [PubMed] [Google Scholar]
- 8.Farley M L, Fargan M F, Mabry L C. Presentation of Sporothrix schenckii in pulmonary cytology specimens. Acta Cytol. 1991;35:389–395. [PubMed] [Google Scholar]
- 9.Gale C A, Bendel C M, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Pardo A, Rostagno A, Frangione B. Primary structure of human plasma fibronectin. Characterization of 38 kDa domain containing the C-terminal heparin-binding site (HEP III site) and a region of molecular heterogeneity. Biochem J. 1987;241:923–928. doi: 10.1042/bj2410923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaur N K, Klotz S A, Henderson R L. Overexpression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect Immun. 1999;67:6040–6047. doi: 10.1128/iai.67.11.6040-6047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil M L, Peñalver M C, López-Ribot J L, O'Connor J E, Martinez J P. Binding of extracellular matrix proteins to Aspergillus fumigatus conidia. Infect Immun. 1996;64:5239–5247. doi: 10.1128/iai.64.12.5239-5247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hostetter M K. Integrin-like proteins in Candida spp. and other microorganisms. Fungal Genet Biol. 1999;28:135–145. doi: 10.1006/fgbi.1999.1165. [DOI] [PubMed] [Google Scholar]
- 14.Hostetter M K. RGD-mediated adhesion in fungal pathogens of humans, plants and insects. Curr Opin Cell Microbiol. 2000;3:344–348. doi: 10.1016/s1369-5274(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 15.Joh D, Wann E R, Kreikemeyer B, Speziale P, Höök M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 16.Jonef C, Wait R, Previato J O, Mendonça-Previato L. The structure of a complex glycosylphosphatidyl inositol-anchorage glucoxylan from the kinetoplastid protozoan Leptomonas samueli. Eur J Biochem. 2000;267:5387–5396. doi: 10.1046/j.1432-1327.2000.01581.x. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman C A. Sporotrichosis. Clin Infect Dis. 1999;29:231–237. doi: 10.1086/520190. [DOI] [PubMed] [Google Scholar]
- 18.Kerr J R. Cell adhesion molecules in the pathogenesis of and host defense against microbial infection. J Clin Pathol. 1999;52:220–230. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein B S. Molecular basis of pathogenicity in Blastomyces dermatitidis: the importance of adhesion. Curr Opin Microbiol. 2000;3:339–343. doi: 10.1016/s1369-5274(00)00100-4. [DOI] [PubMed] [Google Scholar]
- 20.Klotz S A, Maca R D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988;56:2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klotz S A, Smith R L. A Fn receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991;163:604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lima O C, Lopes Bezerra L M. A new concanavalin A binding antigen of the cell surface of Sporothrix schenckii. J Med Vet Mycol. 1997;35:167–172. doi: 10.1080/02681219780001101. [DOI] [PubMed] [Google Scholar]
- 24.Lima O C, Figueiredo C C, Pereira B A S, Coelho M G P, Morandi V, Lopes-Bezerra L M. Adhesion of the human pathogen Sporothrix schenckii to several extracellular matrix proteins. Braz J Med Biol Res. 1999;32:651–657. doi: 10.1590/s0100-879x1999000500020. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd K O, Bitoon M A. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii: structural and immunochemical studies. J Immunol. 1971;107:663–671. [PubMed] [Google Scholar]
- 26.Lopes L M, Mendonça-Previato L, Fournet B, Degrand P, Previato J. O-glycosidically linked oligosaccharides from peptidorhamnomannans of Sporothrix schenckii. Glycoconj J. 1992;9:75–81. doi: 10.1007/BF00731702. [DOI] [PubMed] [Google Scholar]
- 27.López-Ribot J L, Bikandi J, Millán R S, Chaffin W L. Interactions between Candida albicans and the human extracellular matrix component tenascin-C. Mol Cell Biol Res Commun. 1999;2:58–63. doi: 10.1006/mcbr.1999.0152. [DOI] [PubMed] [Google Scholar]
- 28.Mendonça-Previato L, Gorin P A J, Travassos L R. Galactose-containing polysaccharides from the human pathogens Sporothrix schenckii and Ceratocystis stenoceras. Infect Immun. 1980;29:934–939. doi: 10.1128/iai.29.3.934-939.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohri H. Fibronectin and integrin interactions. J Investig Med. 1996;44:429–441. [PubMed] [Google Scholar]
- 30.Patti J M, Höök M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6:752–758. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 31.Peñalver M, O'Connor J E, Martinez J P, Gil M L. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64:1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierschbacher M D, Hayman E G, Ruoslathi E. Location of cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981;26:259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- 33.Pottraz S T, Paulsrud J R, Smith J S, Martin W J., 2nd Evidence for Pneumocystis carinii binding to a cell-free substrate: role of the adhesive protein Fn. J Lab Clin Med. 1994;123:273–281. [PubMed] [Google Scholar]
- 34.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;2:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 35.Santoni G, Gismondi A, Liu J H, Punturieri A, Santoni A, Frati L, Piccoli M, Djeu J Y. Candida albicans expresses a fibronectin receptor antigenically related to α5β1 integrin. Microbiology. 1994;140:2971–2979. doi: 10.1099/13500872-140-11-2971. [DOI] [PubMed] [Google Scholar]
- 36.Sinha B, Francois P, Que Y A, Hussain M, Heilmann C, Moreillon P, Lew D, Krause K H, Peters G, Herrmann M. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect Immun. 2000;68:6871–6878. doi: 10.1128/iai.68.12.6871-6878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundström P. Adhesins in Candida albicans. Curr Opin Microbiol. 1999;2:353–357. doi: 10.1016/S1369-5274(99)80062-9. [DOI] [PubMed] [Google Scholar]
- 38.Vuento M, Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979;183:331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasylnka J A, Moore M M. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect Immun. 2000;68:3377–3384. doi: 10.1128/iai.68.6.3377-3384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]