Abstract
Aim
To explore the knowledge, attitude and practices of cervical cancer screening among HIV‐infected women in public health facilities in Lusaka, Zambia.
Design
Cross‐sectional study.
Methods
The study was conducted from 1st January 2020 to 28th February 2020. We used a structured questionnaire for data collection. The Structural Equation Modelling (SEM) was used to analyse relationships among latent variables (knowledge, attitude and practice).
Results
The overall knowledge, attitude, and practice scores of cervical cancer screening among women living with HIV were 6.86/11 (62.4%), 6.41/7 (91.6%) and 2.92/8 (36.5%), respectively. Overall, knowledge was positively and significantly associated with attitude (r = .53, p < .001) and practice (r = .38, p < 0.001). Additionally, attitude and practice were significantly associated (r = 0.29, p < .001). Our findings support the reinforcement of current public health interventional programmes to improve the knowledge about cervical cancer and screening uptake.
Keywords: attitude, cervical cancer, HIV infection, knowledge, screening
1. INTRODUCTION
Cervical cancer is one of the most typical cancers worldwide, with an estimated 604,000 new cases and 342,000 deaths in 2020 (Sung et al., 2021). It disproportionately affects Human Immunodeficiency Virus (HIV) infected women compared to uninfected ones (Sung et al., 2021). Improved knowledge, attitude and practice towards cervical cancer screening have been shown to improve diagnosis rates and outcomes (Taneja et al., 2021).
2. BACKGROUND
Invasive cervical cancer (ICC) is a public health problem responsible for increased morbidity and mortality worldwide (Burchell et al., 2018). An estimated 570,000 women worldwide were diagnosed with cervical cancer, and about 311,000 of them died from the disease in 2018 (Piñeros et al., 2018). Nearly 5% of all cervical cancer cases are attributable to HIV infection (Stelzle, Tanaka, Lee, Khalil, et al., 2021). Sub‐Saharan Africa (SSA) has the highestburden, where over 71% of women living with HIV develop cervical cancer (Shrestha et al., 2018). Globally, Zambia has the third‐highest incidence rate of cervical cancer, with 66.4 new cases per 100,000 women in 2018 (WHO, 2020). Moreover, cervical cancer is the commonest female cancer in Zambia (Arbyn et al., 2020), with 1,839 associated deaths in 2018 (Matambo et al., 2018).
Cervical cancer arises following a human papillomavirus (HPV) persistent infection with oncogenic or high‐risk types (Schiffman et al., 1993). HPV types 16 and 18 cause nearly 75% of cervical cancer cases, while HPV types 31 and 45 are responsible for 10% of cervical cancers worldwide (Qureshi et al., 2015). HPV spreads from skin to skin or through sexual contact between mucous membranes of people with the infection (Nindl & Stockfleth, 2020). In persistent “high‐risk” HPV infections, the virus can damage the deoxyribonucleic acid (DNA) and cause cells to divide and continue growing out of control leading to cancer (Arbyn et al., 2018).
The development of cervical cancer is linked to various risk factors, including multiple sexual partners, unprotected sex, and coitus with uncircumcised sexual partners (Small et al., 2017). Other factors include smoking, prolonged use of combined oral contraceptives, and engaging in early sexual practices (Torre et al., 2016). Women with HIV infection tend to have a higher risk of persistent oncogenic HPV infection due to lowered immunity (Jacob, 2015). Women living with HIV infection are six times more likely to get cervical cancer than those without HIV infection (Stelzle, Tanaka, Lee, Ibrahim Khalil et al., 2021). The reported high incidence of cervical cancer in Zambia is linked to the heavy burden of HIV infection (Nyambe & Lubeya, 2021; Palefsky et al., 2018). HPV infection may present without symptoms or might be detected due to abnormal cervical cytology (Bennett & Edmonds, 2012). If cervical cancer symptoms are present, they might include post‐coital bleeding, offensive blood‐stained vaginal discharge, postmenopausal bleeding and dyspareunia (i.e. painful sexual intercourse) (Mwaka et al., 2015).
Cervical cancer prognosis and clinical outcomes are significantly improved by screening and testing, making it possible to diagnose and treat cervical cancer in the early stages (Deverakonda & Gupta, 2016). However, even when diagnosed early, in many low resource settings like Zambia, there is a long turnaround time to treatment (Mumba et al., 2021). Regular screening at different ages is now recommended in Zambia and other SSA countries as a secondary prevention strategy for cervical cancer (Brisson et al., 2020; WHO, 2020). From 21 to 29 years, it is recommended that women have a Pap smear every 3 years (WHO, 2020). Every 5 years for those from 30 to 65 years combined with HPV testing (WHO, 2020). However, after 65 years, it is recommended that women who have had regular screening can stop screening (Campos et al., 2017). Nevertheless, in the 2019–2023 Zambia National Strategic Plan on preventing and controlling cervical cancer, all eligible women will be screened every 3 years regardless of HIV serostatus (WHO, 2020). Another management criteria used is vaccination, one of the most commonly used public health strategies to reduce the risk of infection and minimize the prevalence of the disease‐causing agent (HPV) in the environment (Landy et al., 2018).
Although cervical cancer early screening and treatment can decrease morbidity and mortality, most women in Zambia report to the hospital late (Chanda et al., 2020; WHO, 2020). We speculate that this could partly be explained by a lack of awareness, knowledge, and poor women's attitude towards cervical cancer screening. Between 2019 and 2023, Zambia targets to provide 2,275,621 women with cervical cancer screening services and attain a national coverage rate of 65% (WHO, 2020). This ambitious rollout will require massive sensitisation of the population, particularly the women living with HIV infection, due to their high risk of developing cervical cancer.
We, therefore, sought to explore the knowledge, attitude and practices towards cervical cancer screening, including its prevention, treatment and symptoms among women living with HIV infection.
3. MATERIALS AND METHODS
3.1. Study design, site and population
A cross‐sectional study design was used among women living with HIV attending antiretroviral therapy (ART) clinics in the public health facilities (Chipata, Kanyama, Chawama, Matero and Chilenje) in Lusaka, Zambia. Lusaka is the capital, and largest city in Zambia, with an extensive network of primary health clinics (Mukosha et al., 2021), where HIV‐related services are mostly provided free from user fees. Primary health clinics are staffed by doctors, clinical officers and nurses, who provide HIV services to the surrounding populations. The five public health facilities were selected because of their comparable cervical cancer screening services and population density (Lusaka City Council, 2008).
A systematic random sampling technique was conducted based on the average clinic attendance of women living with HIV per day for 2 months. We determined that we needed to enrol every fifth woman at each public health facility who met the study's inclusion criteria. To be eligible, a woman had to be 18 years and above, living with HIV, should have been attending an ART clinic at one of the five public health facilities and should have signed informed consent. The enrolment was done after assigning the sample size to each health facility using probability proportional to size. The study was conducted between 1st January 2020 to 28th February 2020.
3.2. Sample size
We used Stata version 16.1 to calculate the sample size, based on previously reported knowledge (cervical cancer causes, symptoms and prevention) of 20% (Lyimo & Beran, 2012) in a similar setting to Zambia. We needed to enrol at least 248 participants to determine the true proportion with precision 0.05, power 80%, 10% non‐response and 95% confidence level. This study was a preliminary, and therefore, we did not consider the clustering effect for the different health facilities and the expected heterogeneity of the target population in terms of education level and wealth.
3.3. Data collection tool
We used a structured self‐administered questionnaire for data collection. The questionnaire was adapted from a study done in Ethiopia on the knowledge about cervical cancer and barriers towards screening among women living with HIV infection (Shiferaw et al., 2018) and a local study done in the general population (Nyambe et al., 2019). Before adaptation, we pre‐tested the questionnaire among 12 HIV‐positive women in local public health sector clinics that were not included in the study. The questionnaire was prepared in English and contained closed‐ended questions only. To address language barriers, we translated the questionnaire into Nyanja and Bemba, the two local languages that are commonly spoken in Lusaka. We did not back translate the questionnaires because the questions were all quantitative. A qualified local languages translator translated the questionnaire, which comprised four sections. Section A focused on socio‐demographic information to elicit intrapersonal and interpersonal attributes. Questions in this section included: the participants' age (date from last birthday), an education level (tertiary, secondary, primary), religion (Catholic, Protestant, Muslim or other), employment status (employed or unemployed), marital status (married, single), household income per month in Zambian kwacha (rate to dollar at the time of data collection was 19 ZMW to $1), HIV serostatus, how long they had been living with HIV. Section B focused on knowledge about cervical cancer, including its symptoms, treatment and prevention (risk factors, i.e. multiple partners, vaginal douching, HPV infection, low immunity due to HIV, early sexual debut, symptoms, i.e. vaginal bleeding, pain during sex; prevention, i.e. how often and when to screen if HIV positive, attending a regular screening, HPV vaccine and treatment options, i.e. chemotherapy, radiotherapy or surgery). At the end of section B, all respondents were given basic information on cervical cancer causes, risks and prevention to bridge the knowledge gap.
Section C focused on the attitude towards cervical cancer screening (ever been screened before, when last time screened, willingness to be screened again, the importance of screening, willingness to travel a long distance to access screening services, willingness to pay for screening services and willingness to recommend someone to go for screening) and section D focused on practices questions: Embarrassing to go for screening, no health facility in the catchment area, lack of awareness about screening, fear of positive results, the procedure painful, lack of support from the partner, no time for screening, health care providers have a negative attitude towards women who seek screening services.
Each question item for knowledge was assigned a score of one for a correct response and 0 for a wrong response. For attitude and practices, we assigned a score of one for a positive/favourable response to the questions and a score of zero for a negative response. The score of each item was used for the final analysis.
3.4. Study measures
In traditional education, it is assumed that an individual's knowledge can influence attitude, which affects behaviour. We made several assumptions; First, based on traditional education theory, we proposed a model as shown in Figure 1. We assumed that the expected covariance matrix in the theory mentioned above does not differ from our sample covariance matrix. We made several hypotheses. First, knowledge regarding cervical cancer directly and positively influences both the attitude towards cervical cancer (H1 > 0) and practice to screen for cervical cancer among women living with HIV(H3>0), referred to in short as attitude and practice. Second, an attitude directly and positively affects practice to screen for cervical cancer (H2 > 0); Third, knowledge indirectly affects the practice to screen for cervical cancer significantly and positively through attitude.
FIGURE 1.
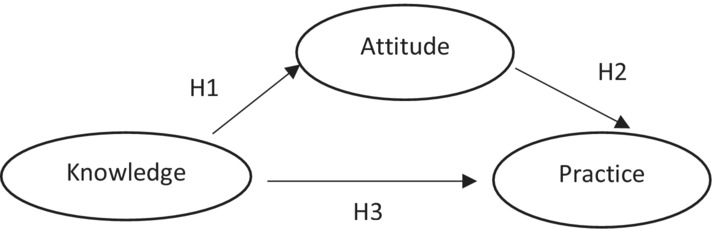
Hypothetical model of knowledge, attitude and practice towards cervical cancer screening among HIV positive women, Lusaka, Zambia.
3.5. Statistical analysis
The descriptive data were presented using frequencies with percentages and mean scores with standard deviation (SD). The Shapiro–Wilk test was used to confirm the normality of continuous data. The ANOVA test assessed the mean differences in the overall scores for knowledge and attitude towards cervical cancer screening. ANOVA was followed by the Bonferroni posthoc test where appropriate to assess pairwise comparison. Finally, we compared proportions of correct responses for knowledge, practice and attitude questions between the health facilities using the Pearson chi‐square test or Fisher's exact test as appropriate.
Structural Equation Modelling (SEM) was used as knowledge, attitude, and practices (KAP) cover several facets, and SEM can model several explanatory variables and multiple outcome variables. As previously demonstrated (Mufwambi et al., 2021), we fitted direct and indirect pathways to KAP. We used direct variables (questions on cervical cancer screening knowledge, practices and attitude) to estimate the latent variables (knowledge, attitude and practices).
A p‐value of < .05 was considered statistically significant at a 95% confidence level for all statistical analysis. We analysed the data using Stata/IC version 16.1 (Stata Corp., College Station, Texas, USA).
3.6. Ethical statement
We obtained ethical clearance from University of Zambia School of Health Sciences Research Ethics committee. Additional permission to conduct the study was sought from selected public health facilities where the study was conducted and National Health Research Authority (NHRA). The study's purpose, risks, and nature were explained to the study participants. Those who declined to participate were reassured that no privileges would be taken away from them. Participants who agreed were requested to sign a consent form. Participants in the study did not receive any remuneration for participating. There was a possibility of stigma by community members or family members if a woman's HIV status is intentionally or unintentionally disclosed. Therefore, care was taken to ensure enrolment and discussions were done in a separate private room by the research staff. Additionally, talking about sensitive issues such as cervical cancer screening and HIV status made psychological distress possible. We encouraged participants not to answer questions they were not comfortable with or did not wish to answer.
The confidentiality of all study records was safeguarded to the extent legally possible. All listings or data forms that linked participant ID numbers to other identifying information was stored in a separate, locked, fireproof safe cabinet in a locked local office. All data analysis was performed using datasets which have only study ID numbers as unique identifiers.
4. RESULTS
Table 1 shows the baseline characteristics of participants concerning their knowledge and attitude towards cervical cancer screening. Of the 248 study participants, the majority were from the Kanyama health facility, 71(28.6%) and were in the age group 18–30 years, 107 (43.2%). More than a third of them were living with HIV infection for more than 5 years, 96 (38.7%) and earned between 150 and 900 Zambian kwacha ($8 and $48) at the time of data collection, 77 (31.1%). In addition, at least more than half attained a secondary level of education, 124 (50.0%) were single, 128 (51.8%) and were unemployed, 143 (57.7%).
TABLE 1.
Baseline characteristics
| Factor | Total population N = 248 (%) | Mean knowledge score (SD) | p‐Value a | Mean attitude score (SD) | p‐Value a |
|---|---|---|---|---|---|
| Health facility | |||||
| Chawama | 64 (25.8) | 6.40 (2.71) | .160 | 6.71 (1.18) | .047 |
| Chilenje | 35 (14.1) | 7.17 (2.68) | 6.66 (1.30) | ||
| Chipata | 25 (10.1) | 6.28 (2.47) | 6.04 (1.34) | ||
| Kanyama | 71 (28.6) | 7.72 (1.74) | 6.16 (1.34) | ||
| Matero | 53 (21.4) | 6.93 (3.26) | 6.42 (1.24) | ||
| Age (years) | |||||
| 18–30 | 107 (43.2) | 6.34 (2.98) | .029 | 6.30 (1.30) | .079 |
| 31–40 | 85 (34.3) | 7.36 (2.37) | 6.67 (1.20) | ||
| 41–50 | 56 (22.6) | 7.09 (2.76) | 6.25 (1.38) | ||
| HIV duration (years) | |||||
| <1 | 37 (14.9) | 6.35 (2.99) | .147 | 6.14 (1.40) | .073 |
| 2–3 | 65 (26.2) | 6.12 (3.21) | 6.24 (1.20) | ||
| 4–5 | 50 (20.2) | 6.80 (2.59) | 6.64 (1.40) | ||
| >5 | 96 (38.7) | 7.58 (2.25) | 6.52 (1.24) | ||
| Income (ZMW) b | |||||
| 150–900 | 77 (31.1) | 6.23 (2.54) | .001 | 6.40 (1.29) | .769 |
| 1,000–1,900 | 70 (28.2) | 6.47 (3.06) | 6.49 (1.29) | ||
| 2,000–2,900 | 33 (13.3) | 6.91 (2.80) | 6.55 (1.54) | ||
| ≥3,000 | 68 (27.4) | 7.94 (2.37) | 6.29 (1.18) | ||
| Education | |||||
| Primary | 76 (30.8) | 5.93 (2.74) | <.001 | 6.39 (1.34) | .663 |
| Secondary | 124 (50.2) | 6.87 (2.62) | 6.36 (1.39) | ||
| Tertiary | 47 (19.0) | 8.28 (2.60) | 6.62 (0.92) | ||
| Marital status | |||||
| Married | 119 (48.2) | 6.79 (2.81) | .693 | 6.52 (1.23) | .1535 |
| Single | 128 (51.8) | 6.93 (2.74) | 6.30 (1.34) | ||
| Employment status | |||||
| Employed | 105 (42.3) | 7.37 (2.72) | .012 | 6.61 (1.10) | .043 |
| Unemployed | 143 (57.7) | 6.48 (2.74) | 6.27 (1.41) | ||
Abbreviations: SD, standard deviation; ZMW, Zambian Kwacha.
p‐values from student T test or One‐way analysis of variance as appropriate.
Rate to dollar at the time of data collection was 19 ZMW to $1.
There was evidence of a difference in mean scores for knowledge of cervical cancer risks, causes and prevention by age (p = .029), income (p = .001), an education level (p < .001) and employment status (p = .012). Conversely, average attitude scores were significantly different by employment status (p = .043) and health facility (p = .047).
4.1. Knowledge of cervical cancer
The overall knowledge score of cervical cancer among women living with HIV was 6.86(2.76) mean (SD), translating to 6.86/11 (62.4%). They were highest, 7.72(1.74) for Chipata and lowest, 6.28(2.47) for Chawama. There was no evidence of a difference in overall cervical cancer knowledge score between the health facilities (p = .161) (Table 2). The majority of the respondents, 182 (73.4%), correctly responded to the causative agent for cervical cancer. However, the least correctly responded to 110 (45.5%) was having multiple sexual partners as a risk of cervical cancer. When different questions about cervical cancer knowledge were compared among the health facilities, a significant association was found regarding vaginal discharge as a symptom of cervical cancer (p = .004).
TABLE 2.
Knowledge of cervical cancer questions
| Knowledge questions |
Total n = 248 (%) |
Chawama n = 64 (%) |
Chilenje n = 35 (%) |
Chipata n = 25 (%) |
Kanyama n = 71 (%) |
Matero n = 53 (%) |
p‐Value a |
|---|---|---|---|---|---|---|---|
| The possible causative agent for cervical cancer | 111 (44.8) | 12 (34.3) | 29 (45.3) | 16 (30.2) | 7 (28.00) | 47 (66.2) | <.001 |
| Having multiple sexual partners is a risk factor for cervical cancer | 110 (45.5) | 17 (53.1) | 30 (46.9) | 17 (33.3) | 10 (40.0) | 36 (51.4) | .273 |
| Infection by HPV is a risk of cervical cancer | 182 (73.4) | 24 (68.6) | 53 (82.8) | 39 (73.6) | 19 (76.0) | 47 (66.2) | .258 |
| Vaginal discharge is a symptom for cervical cancer | 103 (41.7) | 11 (31.4) | 31 (48.4) | 12 (23.1) | 10 (40.0) | 39 (54.9) | .004 |
| Vaginal bleeding is a symptom for cervical cancer | 132 (53.2) | 17 (48.6) | 36 (56.3) | 23 (43.4) | 13 (52.0) | 43 (60.6) | .386 |
| Cervical cancer is preventable | 143 (57.7) | 20 (57.1) | 37 (57.8) | 26 (49.1) | 17 (68.0) | 43 (60.6) | .566 |
| Vaccination with HPV vaccine prevents cervical cancer | 151 (60.9) | 21 (60.0) | 39 (60.9) | 28 (52.8) | 18 (72.0) | 45 (63.4) | .568 |
| Screening can detect cervical cancer early | 160 (64.5) | 21 (60.0) | 44 (68.6) | 30 (56.6) | 21 (84.0) | 44 (61.9) | .158 |
| Cervical cancer screening for HIV‐positive women is at any age | 171 (68.9) | 24 (68.6) | 44 (68.8) | 35 (66.0) | 19 (76.0) | 49 (69.0) | .939 |
| Overall score, mean (SD) | 6.86 (2.76) | 6.40 (2.76) | 7.17 (2.68) | 6.28 (2.47) | 7.72 (1.74) | 6.92 (3.26) | .161 b |
Abbreviation: SD, standard deviation.
p‐values from Pearson chi‐square test or Fisher's exact test as appropriate.
One‐way analysis of variance (ANOVA).
4.2. The attitude towards cervical cancer screening
Overall, the respondents showed a positive attitude towards cervical cancer screening. The overall mean score was 6.41 (1.29), mean (SD), translating to 6.41/7 (91.6%). Being highest among respondents from the Chawama health facility 6.71 (1.18), and lowest among respondents from Chipata health facility 6.04 (1.34). The majority of the respondents, 233 (93.9%), 227 (91.5%) and 232 (93.6%) agreed that it was important to detect cervical cancer at an early stage, would undergo screening if made available and would practice screening in any catchment health centre, respectively. We noted a significant association between the health facility and willingness to be screened again after the first screening visit (p = .018), willingness to practice screening even if it required payment (p < .001) and willingness to travel long distances to areas where there are cervical cancer screening services (p = .017) (Table 3).
TABLE 3.
Attitude towards cervical cancer screening
| Attitude questions |
Total n = 248 (%) |
Chawama n = 64 (%) |
Chilenje n = 35 (%) |
Chipata n = 25 (%) |
Kanyama n = 71 (%) |
Matero n = 53 (%) |
p‐Value a |
|---|---|---|---|---|---|---|---|
| I have been screened before | 168 (67.7) | 21 (60.0) | 47 (73.44) | 30 (56.6) | 18 (72.0) | 52 (73.2) | .191 |
| Willing to be screened again | 161 (64.9) | 19 (54.3) | 45 (70.3) | 26 (49.1) | 18 (72.0) | 53 (74.7) | .018 |
| It important to detect cervical cancer at an early stage | 233 (93.9) | 35 (100) | 61 (95.3) | 48 (90.6) | 24 (96.0) | 65 (91.6) | .350 |
| Willing to undergo for cervical cancer screening | 227 (91.5) | 35 (100) | 57 (89.1) | 49 (92.5) | 22 (88.0) | 64 (90.1) | .357 |
| Willing to practice screening in any catchment health centre | 232 (93.6) | 35 (100) | 61 (95.3) | 49 (92.5) | 21 (84.0) | 66 (92.9) | .155 |
| Willing to practice screening with payment | 50 (20.2) | 3 (8.6) | 11 (17.2) | 5 (9.4) | 4 (16.0) | 27 (38.0) | <.001 |
| Willing to travel long distances to areas where there are cervical cancer screening services | 90 (36.3) | 16 (45.7) | 13 (20.3) | 24 (45.3) | 7 (28.0) | 30 (42.3) | .017 |
| Overall score, mean (SD) | 6.41 (1.29) | 6.71 (1.18) | 6.66 (1.30) | 6.04 (1.34) | 6.16 (1.34) | 6.42 (1.24) | .047 b |
p‐values from Pearson chi‐square test or Fisher's exact test as appropriate.
One‐way analysis of variance (ANOVA).
4.3. Practices towards HPV vaccine
The practices of respondents towards cervical cancer screening were below average. Overall, the average score of cervical cancer screening practices was 2.92 (1.24) mean (SD), translating to 2.92/8 (36.5%). The highest mean score 3.39 (1.36), were recorded for Matero and the lowest 2.29 (0.84) for Chawama hospital. The majority of the respondents said they were not embarrassed to screen for cervical cancer 239/248 (96.4%), with 28/248 (11.3%) fearing a positive result. We noted significant differences between health facilities on all practice questions except whether or not it was embarrassing to go for cervical cancer screening.
4.4. Associations among knowledge, attitudes, and practice
In this study, parameter estimation was conducted using the maximum likelihood method. The sample size required for this type of analysis should not be less than 100 (Boomsma & Hoogland, 2001), and the sample size in this study was 248, which satisfied this requirement. SEM confirmed our hypothesised theoretical framework (Figure 1). The overall model had a good fitness of data: The Chi‐square was not significant (p = .109), Root Mean Standard Error of Approximation (RMSEA) = 0.048 (95% CI: 0.042–0.054), Tucker–Lewis Index (TLI) = 0.959 and Comparative Fit Index (CFI) = 0.962 all suggesting model fit.
Overall, knowledge was positively and significantly associated with attitude (r = .53, p < .001) and practice (r = .38, p < .001). Attitude and practice were significantly associated (r = .29, p < .001). All nine knowledge questions (Table 2) were positively correlated with overall knowledge (p < .001) except question eight (p = .021). With regard to attitude questions (Table 3), the first four questions were positively and significantly correlated with attitude (p < .001) but question four (p = .008). Questions five to seven were negatively and significantly correlated with overall attitude score (p < .001). For practice (Table 4), there was positive and significant correlation with all questions (p < .001) except questions two to eight (p = .034), as shown (Figure 2).
TABLE 4.
Practices of cervical cancer screening
| Practice questions |
Total n = 248(%) |
Chawama n = 64(%) |
Chilenje n = 35(%) |
Chipata n = 25(%) |
Kanyama n = 71(%) |
Matero n = 53(%) |
p‐Value |
|---|---|---|---|---|---|---|---|
| It is embarrassing to go for screening | 239 (96.4) | 32 (91.4) | 60 (93.6) | 51 (96.2) | 25 (100) | 71 (100) | .072 a |
| No health facility in the catchment area | 129 (52.0) | 10 (28.6) | 26 (40.6) | 27 (50.9) | 16 (64.0) | 50 (70.4) | <.001b |
| Lack of awareness about screening | 206 (83.1) | 32 (91.4) | 54 (84.4) | 41 (77.4) | 16 (64.0) | 63 (88.7) | .034 a |
| Fear of positive results | 28 (11.3) | 2 (5.7) | 2 (3.1) | 3 (5.7) | 4 (16.0) | 17 (23.9) | .001 a |
| Is the procedure painful | 19 (7.7) | – | (1.6) | 2 (3.8) | 6 (24.0) | 10 (14.1) | <.001 a |
| Lack of support from the partner | 33 (13.3) | – | 4 (6.3) | 12 (22.6) | 2 (8.0) | 15 (21.1) | .002 a |
| I do not have time am always busy | 34 (13.7) | 1 (2.9) | 8 (12.5) | 13 (24.5) | 6 (24.0) | 6 (8.4) | .012 a |
| Health care workers negative attitude towards women who seek screening services | 36 (14.5) | 3 (8.6) | 6 (9.4) | 10 (18.9) | 8 (32.0) | 9 (12.7) | .069 |
| Overall score mean (SD) | 2.92 (1.24) | 2.29 (0.84) | 2.51 (0.98) | 3.00 (1.29) | 3.32 (1.25) | 3.39 (1.36) | <.001 |
p‐values from Pearson chi‐square test or Fisher's exact test as appropriate.
FIGURE 2.
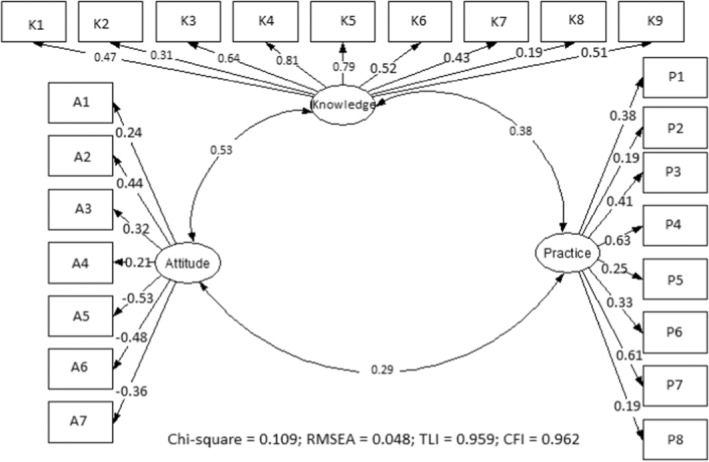
Structure Equation Model (SEM) on cervical cancer knowledge, attitude and practice among HIV positive women in Lusaka, Zambia. K1–K9 are questions on knowledge, A1–A7 are questions on attitude and P1–P8 are questions on practice. CFI, comparative fit index; RMSEA, root mean squared error of approximation; TLI, Tucker – Lewis index.
5. DISCUSSION
Most developing countries lack data on factors influencing cervical cancer screening uptake. We explored knowledge of cervical cancer, attitude and practices towards screening among women living with HIV in public health facilities in Lusaka, Zambia. Our study confirmed the initial hypothesised theoretical framework based on the KAP model, suggesting that the knowledge on cervical cancer screening shapes attitude and practice. SEM results show that knowledge plays an essential role in shaping attitude and practice. Also, knowledge via attitude may enhance practice. Better knowledge may increase attitude, and this may eventually result in increased uptake of cervical cancer screening. This suggests that one potential pathway to increase cervical cancer screening uptake and favourable attitude among HIV‐positive women in Lusaka is to increase knowledge.
The overall knowledge score of cervical cancer risk factors, causes and prevention was above average (62.4%). The overall attitude and practice scores towards screening among women living with HIV were 91.6% and 36.5%, respectively. The knowledge scores were highest for Kanyama and lowest for Chawama. In contrast, the highest scores for attitude were recorded in Chawama and the lowest in Chipata. Furthermore, the highest scores practices of cervical cancer screening were recorded for Matero and lowest for Chawama hospital. Knowledge was positively and significantly associated with attitude and practice of cervical cancer screening. Additionally, women who reported a good attitude were more likely to have good practices towards screening.
The average knowledge score in the index study is slightly higher than most regional estimates (Mutyaba et al., 2006; Okunowo et al., 2018). A study in Ethiopia, Uganda and Sudan reported a suboptimal knowledge score of 51%, 52% and 47%, respectively, about cervical cancer regarding its risk factors, signs and symptoms, prevention and treatment (Mitiku & Tefera, 2016; Mutyaba et al., 2006). Very low scores have been published for Tanzania 19% (Lyimo & Beran, 2012), Nigeria 18% (Amu et al., 2019) and Zimbabwe 34% (Makurirofa et al., 2019). These findings indicate that the knowledge levels about cervical cancer are above average and could significantly impact cervical cancer screening uptake in Zambia.
Our findings show an increase of 50% from reported estimates in Lusaka 5 years ago (Nyambe et al., 2019). This points towards the successful implementation of interventional programs to reduce the incidence of cervical cancer. Zambia has run one of the most robust screening programs in SSA (WHO, 2020). The recently launched 2019 to 2023 national health strategic plan targets a robust community health sensitisation for cervical cancer screening as one of the main strategies to reduce cervical cancer apart from the rollout of HPV vaccinations in all 10 provinces. Other reasons for the differences in the reported knowledge scores in the region could be the differences in the measurement of “knowledge” of cervical cancer and the questions that make up the matrix for knowledge score. Moreover, the populations of interest are not always similar. In the index study, we enrolled the high‐risk group for cervical cancer, which is more likely to recall information about cervical cancer than the general population. Few studies have explored the interaction of knowledge, attitude and practices of cervical cancer screening in the context of high HIV prevalence in SSA.
Women living with HIV infection reported high scores of attitude in the present study. Few studies have explored this aspect in settings with high HIV prevalence. In Nigeria, a much lower percentage was reported of, 65% in the general population of pregnant women (Okunowo et al., 2018). Using the traditional education theory, we assumed that an individual's knowledge could influence attitude, affecting behaviour in the index study. Our findings were consistent with this theory. Women with high knowledge scores were more likely to have high scores of attitude and good practices towards cervical cancer screening.
Furthermore, women who reported a good attitude were more likely to have good practices towards screening. This theory is critical when designing interventions to improve the uptake of public health services. Knowledge is key to the education theory, and based on the levels we found in the index study, there is a need for reinforcements of current programmes on cervical cancer screening, mainly focusing on the most at‐risk group of women living with HIV infection. Having complete information and a good attitude about the risks of cervical cancer and its prevention has previously been reported to give women the capacity to make an informed decision to undergo screening (Rosenstock, 2005). Therefore, our findings indicate that women in this setting will be willing to undergo cervical cancer screening with the observed good attitude.
This study also found that women who were attending Kanyama and Chipata health facilities had the highest knowledge scores of cervical cancer and the highest attitude scores towards cervical cancer screening. The differences could be explained in terms of the socio‐economic status of the women who access the various health facilities in the Lusaka region. For instance, Chilenje hospital serves mainly the middle‐class population from the surrounding central parts of Lusaka city compared to Chawama hospital. The Chawama hospital, on the other hand, serves the majority of the population falling under the lower economic class and are from a highly densely populated region of Lusaka city. The difference in socio‐economic status between residents from the different parts of Lusaka indicates that their monthly income and education level are different and could partly explain the observed results. This assumption is in line with what we found on education level and corroborated by other similar studies that reported monthly income and education level to significantly determine uptake of cervical cancer screening (Arrossi, 2007; Fru et al., 2020; Morema et al., 2014; Murfin et al., 2020).
5.1. Policy implications
The campaign for increasing cervical cancer screening uptake as one method to reduce the cancer burden should take a holistic approach. This should address factors associated with both knowledge and attitudes of HIV positive women. Interventional packages should focus on increasing knowledge, which can ultimately improve attitude and practice. Healthcare professionals attending to HIV positive women should constantly be imparting knowledge and encourage them to screen for cervical cancer. Government and other stakeholders should play a significant role and develop models on how best to strategise in the campaign against cervical cancer and possibly include screening in the HIV care continuum. Improved cervical cancer literacy and engagement of all HIV positive women can help foster a better health‐seeking behaviour environment, resulting in greater uptake of the screening services.
5.2. Study limitations
The paucity of research in this area among women living with the HIV population in SSA indicates a need for further extensive research. We used SEM to assess direct and indirect relationships between knowledge attitude and cervical cancer screening practices, which is more robust for latent variables. This study is hypothesis‐generating for future researchers to replicate the study with a larger sample size, which may add to our understanding of screening practices and barriers to screening in this high‐risk population.
The above‐average knowledge of cervical cancer risks, causes and prevention and the high scores of attitude found in this study may not be generalized to the general population. Further, the results may not be used to inform policy changes as this was a preliminary study.
6. CONCLUSION
Women living with HIV in this study showed above‐average knowledge and attitude towards cervical cancer screening. Conversely, cervical cancer screening practices among women living with HIV was below average. The knowledge, attitude and practices were highly correlated among the HIV‐infected women. Our findings support reinforcing current public health interventional programmes to improve cervical cancer awareness and screening uptake knowledge. They also indicate the need for targeted intervention for women's high‐risk groups to optimize programme outcomes. Finally, expanding coverage of simple and effective interventions like extended screening services to over 114 facilities is critical to reducing the risk of morbidity and mortality for women living with HIV infection in Zambia and other low‐resource settings.
AUTHOR CONTRIBUTIONS
Moses Mukosha, contributed to the study design, data collection, data analysis, data interpretation, and manuscript writing. Patrick Kaonga, data analysis, data interpretation, and manuscript writing. Mwansa Ketty Lubeya, Andrew Kumwenda, Steward Mudenda and Luwi Mwangu contributed to the data curation and writing of the manuscript. All authors read and approved the final draft of the manuscript.
FUNDING INFORMATION
This was a self‐funded study and did not receive any form of funding from anywhere.
CONFLICT OF INTEREST
All the authors declare no competing interests.
ACKNOWLEDGEMENT
The authors would like to thank the women from the public health facilities who participated in this study and members of staff from the facilities who facilitated the recruitment process. Moses Mukosha and Mwansa Ketty Lubeya would like to acknowledge that some of their time is supported by the UNC‐UNZA‐Wits Partnership for HIV and Women's Reproductive Health, (D43 TW010558).
Mukosha, M. , Muyunda, D. , Mudenda, S. , Lubeya, M. K. , Kumwenda, A. , Mwangu, L. M. , & Kaonga, P. (2023). Knowledge, attitude and practice towards cervical cancer screening among women living with human immunodeficiency virus: Implication for prevention strategy uptake. Nursing Open, 10, 2132–2141. 10.1002/nop2.1460
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
REFERENCES
- Amu, E. , Ndugba, S. , & Olatona, F. (2019). Knowledge of cervical cancer and attitude to cervical cancer screening among women in Somolu Local Government Area, Lagos, Nigeria. Journal of Community Medicine and Primary Health Care, 31(1), 76–85. [Google Scholar]
- Arbyn, M. , Weiderpass, E. , Bruni, L. , de Sanjosé, S. , Saraiya, M. , Ferlay, J. , & Bray, F. (2020). Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. The Lancet Global Health, 8(2), 191–203. 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn, M. , Xu, L. , Simoens, C. , & Martin‐Hirsch, P. P. (2018). Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database of Systematic Reviews, 5(5), CD009069. 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrossi, S. (2007). Determinants of women's participation in cervical cancer screening trial, Maharashtra, India. Bulletin of the World Health Organization, 85, 264–272. 10.2471/blt.06.031195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, P. , & Edmonds, D. (2012). Preterm labour, Dewhurst's textbook of obstetrics and gynaecology (pp. 338–355). Wiley‐Blackwell. [Google Scholar]
- Boomsma, A. , & Hoogland, J. J. (2001). The robustness of LISREL modeling revisited. Structural Equation Models: Present and Future. A Festschrift in Honor of Karl Jöreskog, 2(3), 139–168. [Google Scholar]
- Brisson, M. , Kim, J. J. , Canfell, K. , Drolet, M. , Gingras, G. , Burger, E. A. , Martin, D. , Simms, K. T. , Bénard, É. , Boily, M. C. , Sy, S. , Regan, C. , Keane, A. , Caruana, M. , Nguyen, D. T. N. , Smith, M. A. , Laprise, J. F. , Jit, M. , Alary, M. , … Hutubessy, R. (2020). Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low‐income and lower‐middle‐income countries. The Lancet, 395(10224), 575–590. 10.1016/S0140-6736(20)30068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell, A. N. , Kendall, C. E. , Cheng, S. Y. , Lofters, A. , Cotterchio, M. , Bayoumi, A. M. , Glazier, R. H. , Antoniou, T. , Raboud, J. , Yudin, M. H. , & Loutfy, M. (2018). Cervical cancer screening uptake among HIV‐positive women in Ontario, Canada: A population‐based retrospective cohort study. Preventive Medicine, 107, 14–20. 10.1016/j.ypmed.2017.11.023 [DOI] [PubMed] [Google Scholar]
- Campos, N. G. , Tsu, V. , Jeronimo, J. , Mvundura, M. , Lee, K. , & Kim, J. J. (2017). To expand coverage, or increase frequency: Quantifying the tradeoffs between equity and efficiency facing cervical cancer screening programs in low‐resource settings. International Journal of Cancer, 140(6), 1293–1305. 10.1002/ijc.30551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda, S. L. , Liusha, N. , & Chansa, A. (2020). Medical ward in‐patient mortality patterns at a tertiary hospital in Urban Zambia: A one year review June 2018‐June 2019. Medical Journal of Zambia, 47(2), 132–142. [Google Scholar]
- Deverakonda, A. , & Gupta, N. (2016). Diagnosis and treatment of cervical cancer: A review. Journal of Nursing and Health Science, 2(3), 1–11. [Google Scholar]
- Fru, C. N. , Tassang, A. , Cho, F. N. , Tassang, T. , & Fru, P. N. (2020). Socio‐economic determinants influencing cervical cancer screening in Buea: A cross‐sectional study. International Journal of Tropical Disease & Health, 41(11), 14–22. 10.9734/IJTDH/2020/v41i1130331 [DOI] [Google Scholar]
- Jacob, T. (2015). Cervical cancer awareness and attitudes towards screening among HIV positive women at United Bulawayo Hospitals. Bindura University of Science Education. [Google Scholar]
- Landy, R. , Windridge, P. , Gillman, M. S. , & Sasieni, P. D. (2018). What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. International Journal of Cancer, 142(4), 709–718. 10.1002/ijc.31094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusaka City Council . (2008). Lusaka City State of Environment (SoE) outlook report .
- Lyimo, F. S. , & Beran, T. N. (2012). Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: Three public policy implications. BMC Public Health, 12(1), 22. 10.1186/1471-2458-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makurirofa, L. , Mangwiro, P. , James, V. , Milanzi, A. , Mavu, J. , Nyamuranga, M. , & Kamtauni, S. (2019). Women's knowledge, attitudes and practices (KAP) relating to breast and cervical cancers in rural Zimbabwe: A cross sectional study in Mudzi District, Mashonaland East Province. Bmc Public Health, 19(1), 109. 10.1186/s12889-018-6333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matambo, J. , Manasyan, A. , & Kapambwe, S. (2018). A decade of cervical cancer screening: Trends of incidence in Zambia (2007‐2017). American Society of Clinical Oncology. doi: 10.1200/jgo.18.63400 [DOI] [Google Scholar]
- Mitiku, I. , & Tefera, F. (2016). Knowledge about cervical cancer and associated factors among 15‐49 year old women in Dessie town, Northeast Ethiopia. PloS One, 11(9), e0163136. 10.1371/journal.pone.0163136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morema, E. N. , Atieli, H. E. , Onyango, R. O. , Omondi, J. H. , & Ouma, C. (2014). Determinants of cervical screening services uptake among 18–49 year old women seeking services at the Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, Kenya. BMC Health Services Research, 14(1), 335. 10.1186/1472-6963-14-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufwambi, W. , Stingl, J. , Masimirembwa, C. , Manasa, J. , Nhachi, C. , Stadler, N. , Mwila, C. , Kalungia, A. C. , Mukosha, M. , Mutiti, C. S. , Kamoto, A. , Kaonga, P. , Godman, B. , & Munkombwe, D. (2021). Healthcare professionals' knowledge of pharmacogenetics and attitudes towards antimicrobial utilization in zambia: Implications for a precision medicine approach to reducing antimicrobial resistance. Frontiers in Pharmacology, 11, 2183. 10.3389/fphar.2020.551522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukosha, M. , Jacobs, C. , Musonda, P. , Zulu, J. M. , Masaku, S. , Nkwemu, C. , Vwalika, B. , Kapembwa, K. M. , & Kaonga, P. (2021). Determinants of preterm births at a national hospital in zambia: Application of partial proportional odds model. Obstetrics and Gynecology Research, 4, 117–130. 10.26502/ogr061 [DOI] [Google Scholar]
- Mumba, J. M. , Kasonka, L. , Owiti, O. B. , Andrew, J. , Lubeya, M. K. , Lukama, L. , Kasempa, C. , Msadabwe, S. C. , & Kalinda, C. (2021). Cervical cancer diagnosis and treatment delays in the developing world: Evidence from a hospital‐based study in Zambia. Gynecologic Oncology Reports, 37, 100784. 10.1016/j.gore.2021.100784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfin, J. , Irvine, F. , Meechan‐Rogers, R. , & Swift, A. (2020). Education, income and occupation and their influence on the uptake of cervical cancer prevention strategies: A systematic review. Journal of Clinical Nursing, 29(3–4), 393–415. 10.1111/jocn.15094 [DOI] [PubMed] [Google Scholar]
- Mutyaba, T. , Mmiro, F. A. , & Weiderpass, E. (2006). Knowledge, attitudes and practices on cervical cancer screening among the medical workers of Mulago Hospital, Uganda. BMC Medical Education, 6(1), 13. 10.1186/1472-6920-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaka, A. D. , Okello, E. S. , Wabinga, H. , & Walter, F. M. (2015). Symptomatic presentation with cervical cancer in Uganda: A qualitative study assessing the pathways to diagnosis in a low‐income country. BMC Women's Health, 15(1), 15. 10.1186/s12905-015-0167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nindl, I. , & Stockfleth, E. (2020). Human papilloma virus infections. Braun‐Falco´ s . Dermatology, 16, 1–12. 10.1007/s00147-002-0525-7 [DOI] [Google Scholar]
- Nyambe, A. , Kampen, J. K. , Baboo, S. K. , & Van Hal, G. (2019). Knowledge, attitudes and practices of cervical cancer prevention among Zambian women and men. BMC Public Health, 19(1), 1–15. 10.1186/s12889-019-6874-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambe, A. , & Lubeya, M. K. (2021). Cervical cancer and HIV in Zambian women. The Lancet Global Health, 9(6), 734–735. 10.1016/s2214-109x(21)00230-8 [DOI] [PubMed] [Google Scholar]
- Okunowo, A. A. , Daramola, E. S. , Soibi‐Harry, A. P. , Ezenwankwo, F. C. , Kuku, J. O. , Okunade, K. S. , & Anorlu, R. I. (2018). Women's knowledge of cervical cancer and uptake of Pap smear testing and the factors influencing it in a Nigerian tertiary hospital. Journal of cancer Research and Practice, 5(3), 105–111. 10.1016/j.jcrpr.2018.02.001 [DOI] [Google Scholar]
- Palefsky, J. M. , Hirsch, M. , & Bloom, A. (2018). Human papillomavirus infections: Epidemiology and disease associations. UpToDate. [Google Scholar]
- Piñeros, M. , Mery, L. , Soerjomataram, I. , Bray, F. , Steliarova‐Foucher, E. , Piñeros, M. , & Bray, F. (2018). Global cancer observatory: Cancer today (Vol. 113, pp. 9–15). International Agency for Research on Cancer. 10.1093/jnci/djaa069 [DOI] [Google Scholar]
- Qureshi, R. , Arora, H. , & Rizvi, M. (2015). EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Letters, 356(2), 321–331. 10.1016/j.canlet.2014.09.021 [DOI] [PubMed] [Google Scholar]
- Rosenstock, I. M. (2005). Why people use health services. The Milbank Quarterly, 83(4), 94–127. 10.1111/j.1468-0009.2005.00425.x [DOI] [Google Scholar]
- Schiffman, M. H. , Bauer, H. M. , Hoover, R. N. , Glass, A. G. , Cadell, D. M. , Rush, B. B. , Scott, D. R. , Sherman, M. E. , Kurman, R. J. , & Wacholder, S. (1993). Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. JNCI: Journal of the National Cancer Institute, 85(12), 958–964. 10.1093/jnci/85.12.958 [DOI] [PubMed] [Google Scholar]
- Shiferaw, S. , Addissie, A. , Gizaw, M. , Hirpa, S. , Ayele, W. , Getachew, S. , Kantelhardt, E. J. , Assefa, M. , & Jemal, A. (2018). Knowledge about cervical cancer and barriers toward cervical cancer screening among HIV‐positive women attending public health centers in Addis Ababa city. Ethiopia. Cancer Medicine, 7(3), 903–912. 10.1002/cam4.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha, A. D. , Neupane, D. , Vedsted, P. , & Kallestrup, P. (2018). Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pacific Journal of Cancer Prevention: APJCP, 19(2), 319–324. 10.22034/APJCP.2018.19.2.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, W., Jr. , Bacon, M. A. , Bajaj, A. , Chuang, L. T. , Fisher, B. J. , Harkenrider, M. M. , Jhingran, A. , Kitchener, H. C. , Mileshkin, L. R. , Viswanathan, A. N. , & Gaffney, D. K. (2017). Cervical cancer: A global health crisis. Cancer, 123(13), 2404–2412. 10.1002/cncr.30667 [DOI] [PubMed] [Google Scholar]
- Stelzle, D. , Tanaka, L. F. , Lee, K. K. , Ibrahim Khalil, A. , Baussano, I. , Shah, A. S. , McAllister, D. , Gottlieb, S. L. , Klug, S. J. , Winkler, A. S. , Bray, F. , Baggaley, R. , Clifford, G. M. , Broutet, N. , & Dalal, S. (2021). Estimates of the global burden of cervical cancer associated with HIV. The Lancet Global Health, 9(2), 161–169. 10.1016/S2214-109X(20)30459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzle, D. , Tanaka, L. F. , Lee, K. K. , Khalil, A. I. , Baussano, I. , Shah, A. S. , McAllister, D. A. , Gottlieb, S. L. , Klug, S. J. , & Winkler, A. S. (2021). Estimates of the global burden of cervical cancer associated with HIV. The Lancet Global Health, 9(2), 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. [DOI] [PubMed] [Google Scholar]
- Taneja, N. , Chawla, B. , Awasthi, A. A. , Shrivastav, K. D. , Jaggi, V. K. , & Janardhanan, R. (2021). Knowledge, attitude, and practice on cervical cancer and screening among women in India: a review. Cancer Control, 28, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre, L. A. , Sauer, A. M. G. , Chen, M. S., Jr. , Kagawa‐Singer, M. , Jemal, A. , & Siegel, R. L. (2016). Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA: A Cancer Journal for Clinicians, 66(3), 182–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2020). Global strategy to accelerate the elimination of cervical cancer as a public health problem .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.


