Summary
Neuroimaging studies showing the adverse effects of air pollution on neurodevelopment have largely focused on smaller samples from limited geographical locations and have implemented univariant approaches to assess exposure and brain macrostructure. Herein, we implement restriction spectrum imaging and a multivariate approach to examine how one year of annual exposure to daily fine particulate matter (PM2.5), daily nitrogen dioxide (NO2), and 8-h maximum ozone (O3) at ages 9-10 years relates to subcortical gray matter microarchitecture in a geographically diverse subsample of children from the Adolescent Brain Cognitive Development (ABCD) Study℠. Adjusting for confounders, we identified a latent variable representing 66% of the variance between one year of air pollution and subcortical gray matter microarchitecture. PM2.5 was related to greater isotropic intracellular diffusion in the thalamus, brainstem, and accumbens, which related to cognition and internalizing symptoms. These findings may be indicative of previously identified air pollution-related risk for neuroinflammation and early neurodegenerative pathologies.
Subject areas: Health sciences, Public health, Environmental science, Environmental health, Neuroscience
Graphical abstract
Highlights
-
•
Air pollution exposure at ages 9-10 years relates to subcortical microarchitecture
-
•
PM2.5 is linked with intracellular isotropic diffusion in basal ganglia and brainstem
-
•
NO2 and O3 did not reliably associate with subcortical microarchitecture outcomes
-
•
Subcortical intracellular isotropic diffusion related to cognition and emotion
Health sciences; Public health; Environmental science; Environmental health; Neuroscience
Introduction
Outdoor air pollution is a serious environmental health concern that contributes to respiratory and cardiovascular morbidity and mortality.1 However, an emerging literature also suggests that outdoor air pollution can have harmful effects on the brain, including neurodevelopmental consequences as well as pathologies characteristic of neurodegenerative diseases (e.g., Alzheimer’s and Parkinson diseases).2,3,4 Children are thought to be especially vulnerable to these effects given their brains are continuing to grow and develop.5,6,7,8,9 Moreover, despite reductions in air pollution levels in the U.S. over the past few decades, adverse health effects are detected at low levels of exposure that fall well-below the U.S. Environmental Protection Agency’s (EPA) annual standards.10,11,12,13
Particulate matter (PM) consists of chemical mixtures of solids and liquid droplets that are suspended in the air, and is measured by the diameter of air particles. Outdoor ambient PM2.5 (i.e. particles that are smaller than 2.5 μm) largely comes from the combustion of gasoline, oil, diesel fuel, coal or wood; while ambient NO2 and O3 are gasses.14 The majority of NO2 comes from internal engine combustion from vehicles and the burning of coal and natural gas, whereas ground-level O3 results from a chemical reaction of nitrogen oxides and volatile organic compounds that occurs in the presence of heat and sunlight.14 Recent human neuroimaging and behavioral studies suggest childhood outdoor air pollution exposure, including PM2.5 and its constituents, NO2, and O3 relate to poor neurodevelopmental outcomes.7,8,15 Specifically, greater levels of outdoor air pollution during childhood have been associated with deficits in intelligence,2,16,17,18 memory,19 verbal functioning,20,21 and various executive functions (i.e. attention, working memory, inhibition)19,22,23,24,25,26 as well as subclinical mental health problems, including symptoms of anxiety, depression, delinquent behaviors, and attention deficit hyperactivity disorders (ADHD)27,28,29,30,31; albeit it is important to note others have failed to find these associations.32,33,34 Utilizing Magnetic Resonance Imaging (MRI), childhood ambient PM2.5 and NO2 exposure levels have also been linked to macrostructural brain differences in gray matter surface area, volume, and cortical thickness in both cortical and subcortical regions,11,35,36,37,38 as well as microstructural properties of white matter pathways.10,38,39 One notable limitation of the studies above is that since they rely on linear models that can only handle one exposure and its relationship with whole-brain measures, or many exposures and their relationship with one region, we are unable to capture multivariate relationships between air pollutants and brain measures. Multivariate neuroimaging approaches have the potential to clarify some of these associations between common outdoor pollutants and multiple brain outcomes, ultimately providing a more complete in vivo assessment of the underlying neurobiology.
Beyond these initial MRI studies in children, it remains to be determined how exposure to outdoor ambient air pollution in the U.S. may relate to potential changes to neural microarchitecture during childhood in subcortical gray matter brain regions important for cognitive and emotional functioning. Animal exposure studies strongly suggest this avenue of research is crucial as early life exposure to airborne particles, including PM2.5 and smaller nano-sized particles, results in decreases in dendritic spine density and branching in the hippocampus,40,41 impaired neurogenesis,42 as well as impaired spatial cognition and more depressive-like symptoms.40,42,43,44 Recent advancements in diffusion magnetic resonance imaging (dMRI) acquisition and biophysical modeling techniques bring forth the opportunity to probe these emerging associations between ambient air pollution and gray matter microstructure using restriction spectrum imaging (RSI). RSI applies a mathematical model to determine the proportion of the diffusion-weighted MRI signal stemming from either restricted or hindered water diffusion, which reflects water within intracellular or extracellular spaces, respectively45,46 (Figure 1A). The quantification of relative restricted diffusion (i.e., restricted normalized diffusion) can then be used to make inferences as to the biological properties of brain tissue, such as cellular and neurite density within gray matter regions. Specifically, the fraction of restricted components is believed to be directly proportional to tissue cellularity; an interpretation which has recently been histologically confirmed.46 Parsing the restricted component into isotropic and anisotropic (i.e., directional) diffusion can provide some insight into the organization of neurites in a region. Regions with unidirectionally organized neurites (e.g., of packed bi-polar or pyramidal cells with long directional processes) have high directional restricted diffusion (RND) and regions with larger or more cell bodies and/or tangled neurites (e.g., more or larger glia, tangled interneurons, Purkinje cells, or multipolar neurons) have high isotropic restricted diffusion (RNI). Given RSI’s potential for elucidating microstructure development,47,48 exposure effects,10 as well as neuroinflammation and neurodegenerative pathologies49,50,51,52 that have been previously noted in postmortem air pollution studies in children,2,3,4 its utility in assessing potential neurotoxic effects of PM2.5 and other pollutants in vivo has remained to be seen.
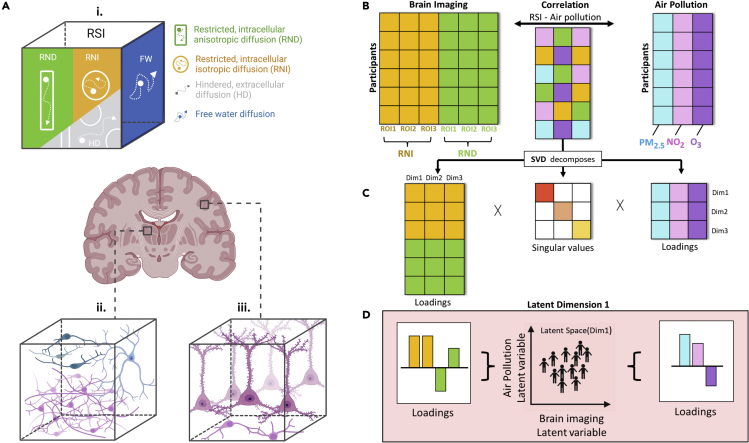
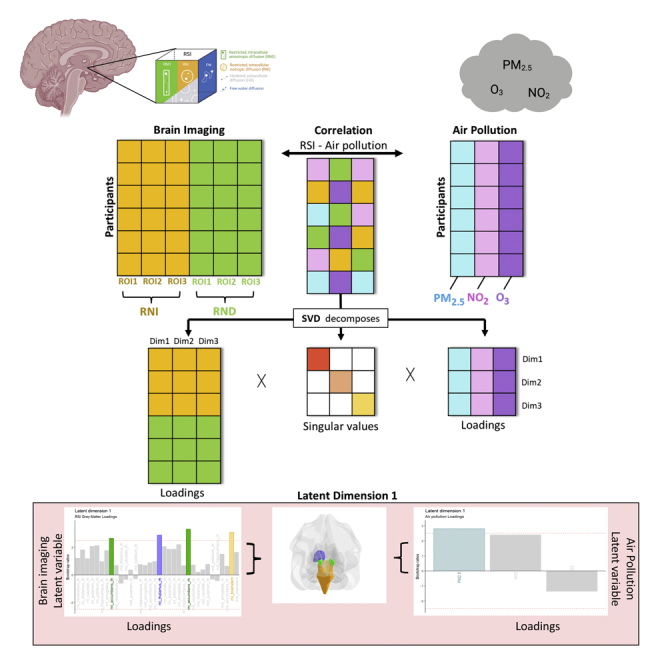
Figure 1.
Air pollution and gray matter microarchitecture relationship explored in the current study using Restriction Spectrum Imaging (RSI) and a Partial Least Squares Correlation (PLSC) framework
(1A) RSI identifies types of microstructural diffusion (1A.i), including restricted water molecules bounded by cell membranes representing intracellular diffusion that is either directional (RND) or isotropic (RNI), hindered water molecules primarily within the extracellular space (HD), and free water molecules that are neither hindered nor restricted in their movement. Around neurites, RNI and RND capture different aspects of gray matter microarchitecture in regions with intertwined, multidirectional neurites (i.e., high RNI, Figure 1A.ii), as compared to regions with elongated, organized neurites (i.e., high RND, Figure 1A.iii). (1B) PLSC relates shared information between two data blocks i.e. subcortical gray matter RSI measures (RNI; RND) and ambient air pollution (PM2.5; NO2; O3) by using Singular Value Decomposition (SVD) to decompose the correlation matrix of the two blocks into three matrices (1C). These three matrices include a square matrix of all the singular values and two latent variable matrices consisting of variable loadings for RSI brain measures and variable loadings for air pollution measures. Columns of the latent variable matrices correspond to a latent dimension of the PLSC solution and are ordered by decreasing the amount of cross-block covariance explained. (1D) The latent dimension and its associated two latent variables (one per data table). The latent variables are derived from linear combinations of the original variables (i.e. air pollutants and subcortical gray matter RSI values). The contribution of these original variables toward the latent variables is described by loadings.
The goal of the current study was to examine how simultaneous exposure to current low levels of outdoor ambient air pollutants, including one-year average of daily fine particulate matter (PM2.5), daily nitrogen dioxide (NO2), and 8-h maximum ground-level ozone (O3) concentrations, relate to subcortical gray matter microarchitecture as measured by RSI in 9-10-year-old children from the Adolescent Brain Cognitive Development (ABCD) Study® (Table 1) To account for simultaneous exposure to PM2.5, NO2, and O3, as well as our focus on gray matter microarchitecture of multiple subcortical regions of interest, as measured by directional (RND) and isotropic (RNI) intracellular diffusion, we applied a partial least squares correlation (PLSC) approach (Figure 1B–1D). This technique accommodates both multivariate exposures and outcomes to identify latent information and features in local brain structures.53 Moreover, PLSC provides an advantage over univariate methods as it takes into account the multicollinearity between environmental exposures, allowing us to model both independent and joint effects of each of the three ambient air pollutants.54 With this data-driven, multivariate analysis technique, this study aims to address whether subcortical gray matter microarchitecture is related to outdoor ambient air pollutant exposure in children ages 9-10 years old. We also then performed post hoc exploratory regression analyses using cognitive function from the NIH Toolbox and emotional problems reported by the caregiver on the Child Behavioral Checklist to assess the potential functional relevance of gray matter microstructure patterns in the identified regions from PLSC analyses.
Table 1.
Summary of participant characteristics and ambient air pollution exposure levels for the current study
| Na | %a | |
|---|---|---|
| Total | 8796 | |
| Mean Age, months (SD) | 119 | (7.52) |
| Sex | ||
| Male | 4539 | (51.6%) |
| Female | 4257 | (48.4%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 4781 | (54.4%) |
| Non-Hispanic Black | 1148 | (13.1%) |
| Hispanic | 1786 | (20.3%) |
| Non-Hispanic Asian | 172 | (2.0%) |
| Otherb | 909 | (10.3%) |
| Handedness | ||
| Right | 7,069 | (80.4%) |
| Left | 605 | (6.9%) |
| Mixed | 1122 | (12.8%) |
| Household Income | ||
| <$50K | 2234 | (25.4%) |
| ≥$50K < $100K | 2315 | (26.3%) |
| ≥$100K | 3,551 | (40.4%) |
| Don’t Know or Refuse | 696 | (7.9%) |
| Parental Education | ||
| < HS Diploma | 385 | (4.4%) |
| HS Diploma/GED | 753 | (8.6%) |
| Bachelor | 2,298 | (26.1%) |
| Some College | 2,239 | (25.5%) |
| Post Graduate Degree | 3,121 | (35.5%) |
| Parental Employment | ||
| Full-time/Part-time | 6203 | (70.5%) |
| Unemployed | 446 | (5.1%) |
| Other | 2147 | (24.4%) |
| Sibling Relationship | ||
| Single | 5964 | (67.2%) |
| Sibling | 1365 | (15.4%) |
| Twin | 1524 | (17.2%) |
| Triplet | 22 | (0.2%) |
| MRI Manufacturer | ||
| Siemens | 5,852 | (66.5%) |
| GE | 1,978 | (22.5%) |
| Philips | 966 | (11.0%) |
| Air Pollutants, Concentration (SD) | ||
| Annual PM2.5 (μg/m3) | 7.62 | (1.56) |
| Annual NO2 (ppb) | 18.70 | (5.82) |
| Annual O3 (8-h max, ppb) | 41.60 | (4.44) |
Data are expressed as the number (percentage) of participants unless otherwise indicated.
Abbreviations: GED, General Educational Development; HS, high school; MRI, magnetic resonance imaging.
Columns are N and percent of sample, unless otherwise specified in the preceding rows.
“Other” race/ethnicity category includes participants who were parent-identified as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, Other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian not listed, or Other Race not listed.
Results
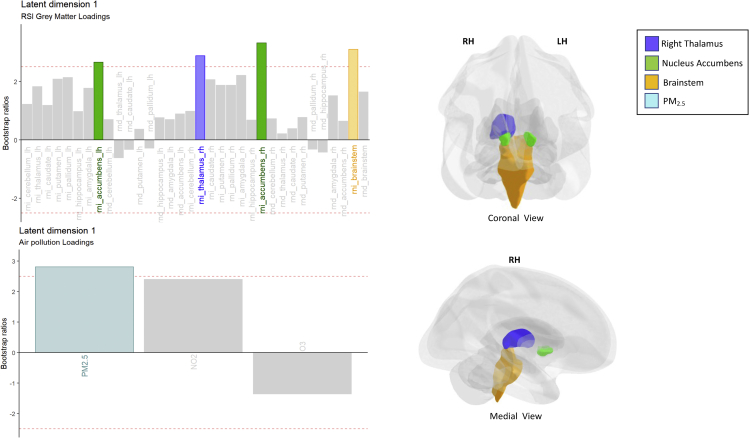
Descriptive statistics of gray matter microstructure across brain regions as well as outdoor air pollution exposure are shown in Figure S1. Across the cohort, one-year annual daily PM2.5 was 7.62 (1.56) μg/m3, daily NO2 was 18.7 (5.82) ppb, and 8-h maximum O3 was 41.6 (4.44) ppb. These values are significantly lower than the EPA standards of 12 μg/m3 for PM2.5 (t(8795) = 263.33, p < 0.0001), 53 ppb for NO2 (t(8795) = 552.73, p < 0.0001), and 70 ppb 8-h maximum of O3 (t(8795) = 599.90, p < 0.0001). In examining how PM2.5, NO2, and O3 were associated with the microarchitecture of the 9 subcortical gray matter regions of interest, one significant latent dimension was identified upon performing a permutation test, which explained 66% of the variance that is common between the latent variables of ambient air pollution and subcortical gray matter microarchitecture (Figures S2 and S3). Within this latent dimension we observed that, based on variable loadings that met a bootstrap ratio threshold of 2.5, exposure to PM2.5 significantly and reliably shared a positive association with RNI of both left and right hemisphere of the nucleus accumbens, the right thalamus, and the brainstem (Figure 2, Table S1). Observed associations with NO2 or O3 and any of the RND brain measures of interest did not reach our a priori statistical significance threshold of p < 0.01 for this latent dimension (Figure 2, Table S1). Robustness of the applied PLSC model was confirmed with the 10-fold CV process, in which the variance explained ranged from 64 to 74% (Table S2).
Figure 2.
Bootstrap ratio bar plots of the variable loadings for the identified latent dimension
PM2.5 was positively associated with specific restricted diffusion RSI imaging variables of subcortical gray matter, including RNI of the left and right accumbens, right thalamus, and the brainstem. Abbreviations: RNI, restricted isotropic diffusion; RND, restricted directional diffusion; lh, left hemisphere; rh, right hemisphere. See also Table S1.
In follow-up analyses, we found that restricted diffusion in these PM2.5-related brain regions was significantly related to both cognition and behavior (Tables 2 and S3–S7). Specifically, RNI in the right thalamus was positively associated with all three metrics of cognition (i.e., general cognitive ability, executive functioning, and learning and memory performance). Similarly, RNI within the brainstem was also found to significantly relate to a child’s general cognitive ability. In terms of behavioral problems, RNI in the left nucleus accumbens and the brainstem was positively related to internalizing problems, while RNI in the right nucleus accumbens was negatively related to externalizing behavior. Interestingly, similar to the left nucleus accumbens, RNI of the right nucleus accumbens positively related to internalizing problems, but negatively related to externalizing problems; albeit these associations did not reach significance.
Table 2.
Associations between restricted isotropic diffusion (RNI) in PLSC-identified brain regions and cognitive and emotional outcomes
| Restricted Isotropic Diffusion - RNI |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accumbens lh |
Accumbens rh |
Thalamus rh |
Brainstem |
|||||||||
| beta | Std error | CI | beta | Std error | CI | beta | Std error | CI | beta | Std error | CI | |
| Cognitive ability | ||||||||||||
| General Cognitive Ability | 0.36 | 0.36 | −0.34 – 1.07 | 0.12 | 0.36 | −0.58 – 0.83 | 1.41∗∗∗ | 0.39 | 0.64–2.18 | 1.39∗∗ | 0.43 | 0.54–2.24 |
| Executive Function | 0.57 | 0.42 | −0.25 – 1.39 | 0.49 | 0.42 | −0.32 – 1.31 | 1.46∗∗ | 0.45 | 0.57–2.34 | 0.51 | 0.50 | −0.47 – 1.50 |
| Learning & Memory | 0.40 | 0.37 | −0.32 – 1.13 | 0.31 | 0.37 | −0.41 – 1.03 | 1.42∗∗∗ | 0.40 | 0.64–2.21 | 0.72 | 0.45 | −0.16 – 1.60 |
| Emotional problemsa | ||||||||||||
| Internalizing | 9.14∗ | 3.85 | 1.58–16.69 | 5.38 | 4.07 | −2.60 – 13.36 | 2.11 | 5.00 | −7.69 – 11.92 | 18.40∗∗∗ | 4.42 | 9.74–27.06 |
| Externalizing | −8.15 | 4.49 | −16.95–0.65 | −11.72∗∗ | 3.94 | −19.45–−3.99 | −2.73 | 4.15 | −10.85–5.40 | 4.42 | 4.87 | −5.12 – 13.96 |
Estimates (beta), standard errors (Std. error), and 95% Confidence Intervals (CI) reported for each RSI variable of interest included in the model.
Abbreviations: lh, left hemisphere; rh, right hemisphere. See also Tables S3–S7.
∗ p < 0.05 ∗∗p < 0.01 ∗∗∗p < 0.001.
Utilized gamma distribution as link function in regression models to account for non-normal outcome distributions related to emotional problems.
Discussion
Here we apply a robust, multivariate data-driven approach and leverage a large, geographically diverse cohort of 9-10-year-old children across the United States, to uncover that subcortical microarchitecture is related to one-year annual ambient exposure levels (7.62 (1.56) μg/m3 for PM2.5, 18.7 (5.82) ppb for NO2, and 41.6 (4.44) ppb for 8-h maximum O3) that are significantly lower than the current U.S. EPA standards. Specifically, we found that ambient exposure was related to subcortical gray matter microstructure, with positive associations seen between PM2.5 and restricted isotropic diffusion (RNI) in regions of the accumbens, thalamus, and brainstem. Moreover, higher RNI in these identified brain areas were found to be linked to better performance on cognitive tests as well as greater internalizing, but less externalizing, problems. Below we discuss how these findings suggest that current low-level exposures to ambient air pollution in the U.S. may influence gray matter microarchitecture in specific regions of the basal ganglia and the brainstem, with potential implications for cognition and emotional functioning in children.
Previous child MRI studies have found that various brain features may be vulnerable to PM2.5 and its components with various regional and hemispheric differences.2,10,11,16,35,36,37,38,39,55,56,57,58,59,60 Until recently, the majority of these studies were limited by including participants from limited geographic locations and with relatively small sample sizes. However, by applying novel hybrid ambient air pollution models and leveraging the ABCD Study, our team has begun to decipher how current low-level ambient air pollution exposures are linked to brain structure in today’s youth. In doing so, we have previously found that one year of annual PM2.5 exposure largely below EPA standards is linked to hemisphere-specific differences in gray matter macrostructure, including thickness, surface area, and volume,11 as well as widespread altered patterns of microstructure, including RNI as measured by RSI, in key white matter tracts.10 The current research expands upon these initial studies by applying a multivariate analytic framework with three criteria co-pollutants to reveal that one year of annual PM2.5 exposure is also most strongly associated with gray matter RNI in the thalamus, accumbens, and brainstem. Interestingly, the current findings of differences in microarchitecture within these specific regions are in line with other studies showing the potential neurotoxic vulnerability of the basal ganglia and portions of the brainstem. Our previous studies in the ABCD cohort have found that PM2.5 exposure at ages 9-10 years is related to larger volumes in the right thalamus and left nucleus accumbens.11 Whereas PM2.5 components, such as polycyclic aromatic hydrocarbons (PAH) and copper have been linked to smaller volumes in the basal ganglia, including the caudate and nucleus accumbens, as well as higher gray matter tissue concentrations within the caudate in children37,55,59 and differences in thalamic volumes in older adults.61,62 Ecological studies have also shown differences in brainstem auditory evoked potentials between children from the more highly polluted Mexico City as compared to less polluted cities in Mexico.16 Thus, our current findings expand upon this literature to further suggest that in addition to differences in volumes and/or function, cellular microarchitecture in gray matter may also be impacted by PM2.5 exposure in childhood in these brain regions.
As previously mentioned, RSI can be used to make inferences about potential differences in cellular microstructure within brain tissue regarding the size, shape, and orientation of intracellular diffusion compartments.63 As such, increases in restricted normalized isotropic diffusion (RNI) using RSI may indicate differences in tissue morphology and composition, such as more or larger spherical structures, such as cell bodies or supporting glial cells.47 Thus, it is possible the associations between RNI and air pollution exposure identified here may be emblematic of neuroinflammatory response and/or early markers of risk for neurodegenerative diseases that have been detected in postmortem brains from children from highly polluted urban cities.2,3,4 Postmortem studies of air pollution exposure in Mexico City have identified neuropathologies in children’s white matter and cortex that are characteristic of neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease, including neuroinflammation, hyperphosphorylated tau, accumulation of amyloid plaques and α-synuclein, and imbalances in gene expression, and less prio-related proteins.2,3,4,64 Moreover, while the majority of these postmortem studies investigate ultramicroscopic aspects of exposure-related pathology that are far beyond the current spatial resolution of the in vivo imaging sequences used here, increased RNI of the thalamus and brainstem has recently been seen in adults affected by Parkinson disease as compared with healthy controls.49 Thus, these findings further support our interpretation that increased RNI in thalamus and brainstem in children exposed to higher concentrations to PM2.5 may relate to risk for such neuropathologies. The potential link between previous histological findings with air pollution and the current increases of RNI in the nucleus accumbens in the current study, however, are less straightforward. Exposure to concentrated ambient ultrafine particles has been associated with increased neuronal cell death in the nucleus accumbens,65 which one might expect would lead to less, rather than greater, RNI. Yet, other differences in cell morphology or changes in cell functioning might also occur and ultimately contribute to the association seen between PM2.5 and RNI in the nucleus accumbens. For instance, early life exposure to diesel exhaust particles, a major component of ambient particulate matter, is known to increase levels of dopamine, but decrease levels of serotonin, in the nucleus accumbens.66 Importantly, beyond the nucleus accumbens, animal studies also suggest air pollution exposure may alter the larger dopaminergic and serotonergic neurotransmitter systems, whose primary centers are located in the midbrain and brainstem (which both were included in the segmentation of the brainstem in the current study). Lastly, neuroinflammation is also characteristic of air pollution exposure67,68 with notable differences in reactive astrocytes and microglial activation seen in brain autopsies from children living in Mexico City.2,16 Interestingly, while RNI cannot differentiate between specific cell types (i.e. enlarged glia versus increased or larger neuronal cell bodies), a recent gene-wide association study found that RNI is strongly associated with genes linked to astrocyte-mediated neuroinflammation, characterized by the enlargement of activated glial cells69 and seen in children with suspected obesity-related neuroinflammation.48 Taken together, the associated increases in RNI with greater exposure seen here may also indicate exposure-related neuroinflammation; albeit further study is required to be certain. Future work is needed that integrates microstructural imaging and histological approaches to clarify the mechanisms underlying air pollution effects in these regions, and to what extent associations between air pollution and in vivo diffusion imaging capture potential risk for the development of neuroinflammation and neurodegeneration pathologies in children as identified by ex vivo microscopy.
Given that little is known about associations between RSI, cognition, and behavior, we also examined whether microarchitecture in identified regions of interest is related to a child’s cognitive performance and emotional problems. Subcortical gray matter microarchitecture of the right thalamus was related to improved cognitive functioning ability, whereas the nucleus accumbens microarchitecture was associated with greater internalizing, but less externalizing, problems. Gray matter microarchitecture in the brainstem was also associated with general cognitive performance, as well as internalizing behavior. While there is little research on how restricted, intracellular isotropic diffusion in the thalamus, nucleus accumbens, and brainstem may relate to cognitive and emotional outcomes, an existing body of brain-behavior literature suggests these relationships are congruent with the expected functions of these brain regions. For example, while historically referenced as playing a passive role as the brain’s primary relay station, the thalamus is now thought to play a more active role in regulating information through its bidirectional connections (i.e., thalamocortical circuits) and informational exchange with the cortex.70,71 The term “cognitive thalamus” references the central roles of various thalamic nuclei (e.g., anterior, mediodorsal) in functions such as ,the direction of attentional resources to tasks at hand, important for various executive functions including behavioral flexibility and task switching, as well as learning and memory.71 Alternatively, the nucleus accumbens regulates emotional and reward-based stimuli responses by integrating information from other cortical and limbic structures, such as the orbitofrontal cortex and amygdala.72,73,74 As such, MRI-based macrostructural and activational differences in the nucleus accumbens have been noted in individuals affected with a number of psychiatric disorders, including depression, anxiety, and attention deficit disorders.75,76 The identified link between brainstem microarchitecture and behavioral outcomes is also interesting given that the brainstem holds the aforementioned dopaminergic and serotonin neurotransmitter circuits that project to cortical and subcortical regions to modulate various cognitive and mood-related processes.77 Of course, these exploratory regression analyses also showed a few unexpected findings. Bilateral RNI of the nucleus accumbens was related positively to internalizing, but negatively to externalizing, problems; with stronger effects of internalizing in the right hemisphere and of externalizing in the left hemisphere. The lack of prior findings regarding hemispheric RNI differences and RNI-behavior associations makes these relationships difficult to interpret, but does suggest a need for further investigation. Moreover, brainstem RNI was associated with both greater cognitive performance and more internalizing problems, suggesting the complex relationships between intelligence and internalizing behavior78,79 may exist even at a neurobiological level. Finally, subnuclei of these structures themselves have complex associations both in terms of structure and function.80,81 Thus, it is beyond the scope of the current study to disentangle the various pathways by which these differential associations may emerge in relation to RNI patterns in both the accumbens and brainstem. Rather, together, prior research and our findings suggest that PM2.5-related patterns of gray matter microarchitecture (indexed by RNI) in these regions may be important to consider in future work focused on understanding potential biological links between air pollution exposure and various cognitive and emotional behaviors.
Conclusion
This study is the first large, multisite U.S. study to examine associations between one-year annual PM2.5, NO2, and O3 exposures, which were all well within the current EPA standards, and intracellular subcortical gray matter microarchitecture in children. Associations were largely driven by PM2.5 and effects were most prominent for microarchitecture in the thalamus, accumbens, and brainstem, with microarchitecture in these regions found to be associated with a child’s cognitive and emotional functioning. The current findings contribute to an emerging literature suggesting adverse health and developmental effects of outdoor ambient air pollution may exist at lower levels of exposure seen across the U.S. Moreover, the current findings may also provide additional support for the association between air pollution and emergence of risk for neuroinflammation and neurodegenerative pathologies in children.
Limitations of the study
While the ABCD Study sought to recruit a sample that mirrored the US population by design, the ABCD cohort is not a fully representative cohort of the U.S.82,83 As a whole, the ABCD cohort has under-representation of Asian, American Indian/Alaskan Native, and Native Hawaiian/Pacific Islander, as well as over-representation of families with higher total household incomes (∼64% with ≥$50,000) and those with highly educated caregivers (∼59% with bachelor degree or higher). Moreover, our final subsample of high-quality exposure and neuroimaging data included a higher proportion of non-Hispanic whites and higher socioeconomic status as compared to the larger ABCD Study cohort. Thus, it is important to note that while the current study captures how one-year exposure estimates for PM2.5, NO2, and O3 relate to gray matter microstructure in a large and diverse sample, the results may not be generalizable to all children in the U.S. For instance, the burden of health effects related to outdoor air pollution exposure is not ubiquitous. Individuals who are poorer, have completed fewer years of education, and are from historically minoritized communities are exposed to higher levels of pollution within the U.S.,84,85 and these groups may be more susceptible to air pollution-related health threats because of compounded effects of disadvantage (i.e. psychosocial stress, access to resources, proximity to industrial or roadway emission sources, and so forth).86,87 Additional studies are needed to assess the current findings within these populations underrepresented in the ABCD cohort. Another limitation is that the ABCD study currently lacks air pollution exposure beyond the one-year annual estimates that correspond with the 9-10-year age period for participants. While the current findings build upon previous studies showing one year of exposure is associated with brain outcomes in late childhood in both the ABCD study10,11 and BREATHE cohorts,59,60,88 key questions remain as to how exposures during earlier periods have unique and/or additive effects over time and to what degree these may contribute to the current findings. The current cross-sectional study design also limits the possibility of drawing causal inferences. Previously noted cytoarchitectonic differences both within and between subcortical regions might confound estimates of restricted isotropic and directional diffusion. While histological methods support the assignment of RNI to larger/more cell bodies and of RND to greater neurite organization/density, the resolution of diffusion-weighted MRI scans and the spatial scale of cell bodies, axons, and dendrites lends some uncertainty about the contribution of neurite organization and disorganization to RND and RNI, respectively. This matter is compounded by averaging across all voxels of large and cytoarchitectonically diverse regions, as is necessary for human MRI research. Future work may consider RNI and RND on a finer-grained, voxel-level to interrogate the consistency and sources of patterns identified here and integrate histological and imaging methods in animal models to complement this type of research. Furthermore, while PLSC analysis is useful for identifying patterns between multiple types of variables, further work is needed to investigate possible interactive effects between these pollutant exposures and how they impact neural structure and function.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| The Adolescent Brain Cognitive Development℠ Study (ABCD Study®) data, annual release 4.0 | NIMH Data Archive | https://doi.org/10.15154/1523041 |
| Software and algorithms | ||
| R | https://www.r-project.org/ | version 4.1.2 |
| TExposition | Beaton et al.89 | version 2.6.10.1 |
| TInPosition | Beaton et al.89 | version 0.13.6.1 |
| data4PCCAR | Abdi and Beaton90 | |
| Caret | https://cran.r-project.org/web/packages/caret/index.html | version 6.0-93 |
| PLSC Analysis Script | This paper | https://doi.org/10.6084/m9.figshare.21926088 |
| Regression Analysis | This Paper |
https://doi.org/10.6084/m9.figshare.21926157 https://doi.org/10.6084/m9.figshare.21861894 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Megan Herting (herting@usc.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study population
Data for the analysis were acquired from the larger ongoing ABCD Study® (NDA 4.0 data release 2021), which enrolled over 11,877 9- and 10-year-old participants across the USA from 2016-2018.91,92,93 The ABCD Study® comprises of 21 diverse study sites and implements an identical protocol for recruitment and neuroimaging of all participants.91,94,95,96,97,98,99 Study sites obtained approval from their local Institutional Review Board (IRB). Centralized (IRB) approval was obtained from the University of California, San Diego. Written informed consent was provided by each parent or caregiver; each child provided verbal assent. All ethical regulations were complied with during data collection and analysis. Primary inclusion criteria for participants were age (9.0 to 10.99 years) and fluency in English. Exclusionary criteria were MRI contraindications; history of significant traumatic brain injury or major neurological disorder (i.e. seizure disorder, cerebral palsy, or other conditions requiring neurological or medical care); non-correctable sensory and/or motor impairments that preclude the youth’s ability to participate in study procedures; current or persistent major Axis I psychiatric disorder (i.e. psychosis, bi-polar); current medication of antipsychotics or mood stabilizers; current or history of and persistent severe learning disorder, mental retardation, or pervasive developmental disorder or substance use disorder diagnosis and/or substance use disorder; parent report of intellectual disability; prematurity, very low birth weight, or perinatal complications; and knowledge at baseline of impending move to an area not within reasonable traveling distance to an ABCD Study site. For the current analysis, we utilized baseline data and had the additional inclusion criteria of having a valid primary residential address at baseline from the 21 study sites, high quality diffusion weighted imaging (i.e. exclusion of >2mm of head motion, images with artifacts, or abnormal clinical findings as determined by radiologist review (see Hagler et al.97 for details), and complete cases of data, including covariates, as required by PLSC (see Figure S4). This resulted in a final sample of 8796 participants. Children included in the final sample for analysis included a slightly higher proportion of non-Hispanic whites and those from higher socioeconomic status background as compared to the overall ABCD® study cohort (Table S8).
Method details
Estimation of ambient air pollution exposure
A one-year annual ambient air pollution concentration for PM2.5, NO2, and O3 were assigned to primary residential addresses of each child as described elsewhere.100 Briefly, daily estimates of PM2.5 (μg/m3), daily estimates of NO2 (ppb), and daily 8-hour maximums of ground-level O3 (ppb) were derived at a 1-km2 resolution across the USA using hybrid ensemble spatiotemporal modeling, which utilize satellite-based aerosol optical depth and other satellite measurements, land-use variables (i.e. land use coverage, road density, elevation, normalized difference vegetation, etc.), weather data, and chemical transport models,101,102,103 and averaged over the 2016 calendar year, corresponding with ABCD baseline data collection. The cross-validation of these models with EPA monitored levels across the U.S. were found to perform well, with R2 Root Mean Square Error (RMSE) of 0.89 for PM2.5 annual averages,101 0.84 for NO2 annual averages102 and 0.90 for daily 8-hour maximum O3.103 The Pearson correlation coefficients between the annual pollutant concentrations across all sites were: PM2.5 - NO2 = 0.20; PM2.5 - O3 = -0.16 ; NO2- O3 = 0.03.
Restriction spectrum imaging (RSI) acquisition and preprocessing
MRI Imaging methods and image acquisition performed in the ABCD Study® are harmonized across all 21 sites using 3 Tesla scanners (Siemens Prisma, General Electric 750, Philips).95 The imaging algorithm used by RSI to capture different water diffusion lengths is an extension of the linear spherical deconvolution model104,105,106,107 covering a range of diffusion length scales. Measures of interest derived from the RSI model for the current study included directional (RND) and isotropic (RNI) intra-cellular diffusion (i.e., restricted water bounded by membrane of cells).97 Each of these measures is normalized and defined as the Euclidean norm (square root of the sum of squares) for the corresponding model coefficients divided by the norm of all model coefficients. As such, these measures are unitless and range from 0 to 1 and the square of each measure is equivalent to the signal fraction for their respective model components. RNI is derived from the 0th order spherical harmonic coefficients of the restricted fraction and is the contribution of intracellular space to isotropic diffusion in a given voxel. RND is calculated from the 2nd order spherical harmonic coefficients of the restricted fraction and reflects oriented diffusion.97 These RSI metrics were then extracted for subcortical gray matter regions, which included left and right hemisphere of hippocampus, thalamus, amygdala, caudate, putamen, pallidum, nucleus accumbens, cerebellum as well as brainstem as defined by Freesurfer v5.3 atlas-based subcortical segmentation (ASEG). All images underwent quality control (QC) and rigorous inspection by trained technicians at the central ABCD Data Analysis, Informatics, and Resource Center (DAIRC).97 Only high-quality imaging data were used for the current study (see Figure S4 for details).
NIH toolbox
Children completed the cognitive battery from the NIH Toolbox98 at baseline which was comprised of ten cognitive measures, including: Picture Vocabulary Test, Oral Reading Recognition Test, Flanker Task, List Sorting working Memory Test, Dimensional Change Card Sort, Pattern Comparison Processing Speed Test, and the Picture Sequence Memory Test, The Little Man Task, Matrix Reasoning Test, Rey Auditory Verbal Learning Test, and the Cash Choice Task.98 All tests were administered using an iPad with one-on-one monitoring by a research assistant. As previously published, Bayesian Probabilistic Principal Components Analysis along with varimax rotation was implemented to obtain the principal components of the ABCD Baseline Cognitive Battery. The three derived PCA factor scores, known as general cognitive ability, executive function, and learning/memory, for each participant were included in the analysis.108
Child behavior checklist (CBCL)
Caregivers reported on children’s problem behaviors using a 3-point scale with the code of 0 if the item was not true, 1 if the item was sometimes true, and 2 if the item was often true. Internalizing and Externalizing subscales were utilized in this analysis. Internalizing and Externalizing CBCL outcomes were analyzed using t-scores corrected for age and sex.109
Covariates/confounders
Potential confounders were decided based on prior knowledge.110 The set of covariates which may predict subcortical gray matter development and exposure to ambient air pollutants were identified using a Directed Acyclic Graph (DAG) approach111 (see details in Figure S5). Specifically, disparity in air pollution exposures is observed across multiple domains including an individual’s socioeconomic status, race and ethnicity, and alongside neighborhood differences in crime rates.84,112,113,114 As previously reported,11 the distribution of one-year annual PM2.5 exposures are associated with both demographic and social covariates in the ABCD sample. Specifically, our analyses adjusted for child’s age and sex at birth, race and ethnicity, highest household education, total combined family income, parental employment status, perceived neighborhood safety, child’s handedness, MRI imaging manufacture, motion during the diffusion MRI sequence, and ABCD Study site.
Quantification and statistical analysis
All statistical analyses were conducted in R (version 4.1.2). Partial Least Squares Correlation (PLSC) was used to relate information and identify reliable patterns between air pollution exposures and microarchitectural features of subcortical gray matter regions. PLSC is a multivariate analysis technique that allows for the comparison of two multidimensional data blocks with possible cross-correlated features (formal expression of PLSC can be found in the supplemental methods). Reliable patterns between these data blocks, termed latent dimensions, are revealed by using partial least squares to compute the best fit correlation between the two data blocks and by also constraining solutions to the covariance structure that is attributable to the relationship between the two blocks of variables.53,115 That is, PLSC establishes whether there is a relationship between two datasets by maximizing the covariance between the datasets and identifies the sets of measures that best model this relationship. PLSC requires complete case data in order to perform the underlying statistical procedures. Thus, 80 participants with missing data were removed using listwise deletion in order to obtain complete case data for PLSC analysis. PLSC analysis was performed using the ‘TExposition’ and ‘TInPosition’ packages89 followed by the validation procedure of bootstrap executed with the Boot4PLSC() function in package ‘data4PCCAR’.116 Cross validation was performed using R package ‘Caret’.117
Prior to running PLSC, primary exposure and outcome variables were residualized, then mean-centered and normalized (to standard deviation of 1), to remove the influence of sociodemographic, scanner-related and confounding influences (see section: covariates/confounders for details). For each data block (brain variables and exposure variables), the residualized values were arranged into a matrix with each participant as a row, and each exposure or brain outcome as a column. Using Singular Value Decomposition (SVD), the PLSC analysis decomposed the correlation between the two data blocks into three matrices: a matrix describing variable loadings for RSI brain measures, a matrix describing variable loadings for air pollution measures and a square matrix of all the singular values. Each latent dimension consists of a latent variable pair (one column per data block), which are derived from linear combinations of the original variables (i.e., the variables measuring air pollution exposure and microstructural features of subcortical gray matter regions). Furthermore, variable loadings, also referred to as saliences, describe the contribution of the original variables towards the latent variables while the singular values explain the correlation expressed by the latent variable pairs. The percentage of variance explained by the latent dimension relative to other latent dimensions is obtained by the ratio of squared singular values of that latent dimension divided by the sum of singular values of all latent dimensions multiplied by 100.
Because the SVD procedure of our PLSC analysis gives a fixed number of solutions, we used permutation and bootstrapping computations to derive statistical tests for our PLSC.53,115,118 To assess statistical significance of the global model and each latent dimension, we performed a permutation test119 using 10,000 permutation samples without replacement in which the order of conditions was reassigned for each observation. Exact probabilities were then computed as the number of times the permuted singular values exceed the observed singular values. Based on permutations tests and the percentage of variance explained by the latent dimensions, we determined one latent dimensions was important for interpreting our results (Figures S2 and S5). To determine the significance of the specific variable loadings, we computed standard error estimates using a bootstrap procedure with 10,000 samples. We then derived a confidence bootstrap ratio by dividing the mean of the bootstrapped distribution of a variable by its standard deviation.120 Bootstrap ratios are approximately equivalent to a z-score, and thus we considered loadings with bootstrap ratios that were greater than 2.5 (corresponding to p < 0.01) as reliable and significant. As an additional analysis of the robustness of our PLSC model, we performed a 10-fold cross-validation (CV) procedure, which is described in full details in supplemental methods.
Lastly, since RSI is a novel neuroimaging biomarker with little known about potential functional implications, we explored whether gray matter microarchitecture in identified regions may relate to cognition and behavior. Specifically, linear mixed effect models were implemented to determine whether RSI diffusion variables of the identified subcortical gray matter regions of interest associate with neurocognitive function or behavioral problems, while adjusting for all identified confounding and precision covariates, including random effects to account for the structure of the sample including study site and family relationship.
Acknowledgments
Research described in this article was supported by the National Institutes of Health [NIEHS R01ES032295, R01ES031074, P30ES007048-23S1, 3P30ES000002-55S1] and EPA grants [RD 83587201, RD 83544101]. The Rose Hills Foundation also supported the efforts of MH and CCI. CCI would like to acknowledge scholars involved in NSP (R25 NS089462), BRAINS (R25 NS094094), and Diversifying CNS (R25 NS117356), as well as R25MH125545 and R25MH120869 for creating a supportive network of ABCD Study users.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health Grants [U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147]. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1523041.
Author contributions
Kirthana Sukamaran: project administration, formal analysis, writing - original draft and editing, visualization. Carlos Cardenas-Iniguez: conceptualization, statistical supervision, project administration, visualization, writing - original draft. Elisabeth Burnor: methodology, data curation, writing – review & editing. Katherine L. Bottenhorn – visualization, writing – original draft. Daniel Hackman: methodology, writing – review & editing. Jiu-Chiuan Chen: conceptualization, methodology, writing - review & editing. Rob McConnell: methodology, writing - review & editing. Kiros Berhane: methodology, writing - review & editing. Joel Schwartz: methodology, data curation, resources, writing - review & editing. Megan M. Herting: funding acquisition, conceptualization, methodology, supervision, project administration, writing - original draft.
Declaration of interests
Joel Schwartz has served as an expert witness for the US Department of Justice in cases involving Clean Air Act violations.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: January 31, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106087.
Supplemental information
Data and code availability
-
•
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD Study data used in this report came from https://doi.org/10.15154/1523041.
-
•
All original code is deposited at figshare.com and is now publicly available. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Brook R.D., Rajagopalan S., Pope C.A., Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C., Torres-Jardón R., Nuse B., Herritt L., Villarreal-Calderón R., Osnaya N., Stone I., García R., et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 3.Calderón-Garcidueñas L., Franco-Lira M., Henríquez-Roldán C., Osnaya N., González-Maciel A., Reynoso-Robles R., Villarreal-Calderon R., Herritt L., Brooks D., Keefe S., et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. 2010;62:91–102. doi: 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderón-Garcidueñas L., Reynoso-Robles R., Vargas-Martínez J., Gómez-Maqueo-Chew A., Pérez-Guillé B., Mukherjee P.S., Torres-Jardón R., Perry G., Gónzalez-Maciel A. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ. Res. 2016;146:404–417. doi: 10.1016/j.envres.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Brockmeyer S., D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl. Neurosci. 2016;7:24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderón-Garcidueñas L., Azzarelli B., Acuna H., Garcia R., Gambling T.M., Osnaya N., Monroy S., DEL Tizapantzi M.R., Carson J.L., Villarreal-Calderon A., Rewcastle B. Air pollution and brain damage. Toxicol. Pathol. 2002;30:373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 7.de Prado Bert P., Mercader E.M.H., Pujol J., Sunyer J., Mortamais M. The effects of air pollution on the brain: a review of studies interfacing environmental epidemiology and neuroimaging. Curr. Environ. Health Rep. 2018;5:351–364. doi: 10.1007/s40572-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herting M.M., Younan D., Campbell C.E., Chen J.-C. Outdoor air pollution and brain structure and function from across childhood to young adulthood: a methodological review of brain MRI studies. Front. Public Health. 2019;7:332. doi: 10.3389/fpubh.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters A., Veronesi B., Calderón-Garcidueñas L., Gehr P., Chen L.C., Geiser M., Reed W., Rothen-Rutishauser B., Schürch S., Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnor E., Cserbik D., Cotter D.L., Palmer C.E., Ahmadi H., Eckel S.P., Berhane K., McConnell R., Chen J.-C., Schwartz J., et al. Association of outdoor ambient fine particulate matter with intracellular white matter microstructural properties among children. JAMA Netw. Open. 2021;4:e2138300. doi: 10.1001/jamanetworkopen.2021.38300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cserbik D., Chen J.-C., McConnell R., Berhane K., Sowell E.R., Schwartz J., Hackman D.A., Kan E., Fan C.C., Herting M.M. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ. Int. 2020;143:105933. doi: 10.1016/j.envint.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuinn L.A., Wiggins L.D., Volk H.E., Di Q., Moody E.J., Kasten E., Schwartz J., Wright R.O., Schieve L.A., Windham G.C., Daniels J.L. Pre- and postnatal fine particulate matter exposure and childhood cognitive and adaptive function. Int. J. Environ. Res. Publ. Health. 2022;19:3748. doi: 10.3390/ijerph19073748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadogeorgou G., Kioumourtzoglou M.-A., Braun D., Zanobetti A. Low levels of air pollution and health: effect estimates, methodological challenges, and future directions. Curr. Environ. Health Rep. 2019;6:105–115. doi: 10.1007/s40572-019-00235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2021. Ambient (outdoor) air pollution.https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health [Google Scholar]
- 15.Lopuszanska U., Samardakiewicz M. The relationship between air pollution and cognitive functions in children and adolescents: a systematic review. Cognit. Behav. Neurol. 2020;33:157–178. doi: 10.1097/WNN.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 16.Calderón-Garcidueñas L., D’Angiulli A., Kulesza R.J., Torres-Jardón R., Osnaya N., Romero L., Keefe S., Herritt L., Brooks D.M., Avila-Ramirez J., et al. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 2011;29:365–375. doi: 10.1016/j.ijdevneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera F.P., Li Z., Whyatt R., Hoepner L., Wang S., Camann D., Rauh V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–e202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P., Tuvblad C., Younan D., Franklin M., Lurmann F., Wu J., Baker L.A., Chen J.-C. Socioeconomic disparities and sexual dimorphism in neurotoxic effects of ambient fine particles on youth IQ: a longitudinal analysis. PLoS One. 2017;12:e0188731. doi: 10.1371/journal.pone.0188731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu Y.-H.M., Hsu H.-H.L., Coull B.A., Bellinger D.C., Kloog I., Schwartz J., Wright R.O., Wright R.J. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ. Int. 2016;87:56–65. doi: 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderón-Garcidueñas L., Mora-Tiscareño A., Styner M., Gómez-Garza G., Zhu H., Torres-Jardón R., Carlos E., Solorio-López E., Medina-Cortina H., Kavanaugh M., D'Angiulli A. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J. Alzheimers Dis. 2012;31:183–191. doi: 10.3233/JAD-2012-120610. [DOI] [PubMed] [Google Scholar]
- 21.Calderón-Garcidueñas L., Mora-Tiscareño A., Franco-Lira M., Zhu H., Lu Z., Solorio E., Torres-Jardón R., D’Angiulli A. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. J. Alzheimers Dis. 2015;45:757–770. doi: 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- 22.Chiu Y.-H.M., Bellinger D.C., Coull B.A., Anderson S., Barber R., Wright R.O., Wright R.J. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ. Health Perspect. 2013;121:859–864. doi: 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris M.H., Gold D.R., Rifas-Shiman S.L., Melly S.J., Zanobetti A., Coull B.A., Schwartz J.D., Gryparis A., Kloog I., Koutrakis P., et al. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol. Teratol. 2016;57:60–70. doi: 10.1016/j.ntt.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kicinski M., Vermeir G., Van Larebeke N., Den Hond E., Schoeters G., Bruckers L., Sioen I., Bijnens E., Roels H.A., Baeyens W., et al. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ. Int. 2015;75:136–143. doi: 10.1016/j.envint.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Rivas I., Basagaña X., Cirach M., López-Vicente M., Suades-González E., Garcia-Esteban R., Álvarez-Pedrerol M., Dadvand P., Sunyer J. Association between early life exposure to air pollution and working memory and attention. Environ. Health Perspect. 2019;127:057002. doi: 10.1289/EHP3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S., Zhang J., Zeng X., Zeng Y., Wang S., Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ. Health Perspect. 2009;117:1612–1618. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman N.C., Ryan P., LeMasters G., Levin L., Bernstein D., Hershey G.K.K., Lockey J.E., Villareal M., Reponen T., Grinshpun S., et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 Years of age. Environ. Health Perspect. 2013;121:731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perera F.P., Tang D., Wang S., Vishnevetsky J., Zhang B., Diaz D., Camann D., Rauh V. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ. Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perera F.P., Chang H.w., Tang D., Roen E.L., Herbstman J., Margolis A., Huang T.-J., Miller R.L., Wang S., Rauh V. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One. 2014;9:e111670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddique S., Banerjee M., Ray M.R., Lahiri T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatr. 2011;170:923–929. doi: 10.1007/s00431-010-1379-0. [DOI] [PubMed] [Google Scholar]
- 31.Younan D., Tuvblad C., Franklin M., Lurmann F., Li L., Wu J., Berhane K., Baker L.A., Chen J.-C. Longitudinal analysis of particulate air pollutants and adolescent delinquent behavior in southern California. J. Abnorm. Child Psychol. 2018;46:1283–1293. doi: 10.1007/s10802-017-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorcano A., Lubczyńska M.J., Pierotti L., Altug H., Ballester F., Cesaroni G., El Marroun H., Fernández-Somoano A., Freire C., Hanke W., et al. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ. Int. 2019;131:104927. doi: 10.1016/j.envint.2019.104927. [DOI] [PubMed] [Google Scholar]
- 33.Kusters M.S.W., Essers E., Muetzel R., Ambrós A., Tiemeier H., Guxens M. Air pollution exposure during pregnancy and childhood, cognitive function, and emotional and behavioral problems in adolescents. Environ. Res. 2022;214:113891. doi: 10.1016/j.envres.2022.113891. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M., Wang C., Zhang X., Song H., Li Y. Association between exposure to air pollutants and attention-deficit hyperactivity disorder (ADHD) in children: a systematic review and meta-analysis. Int. J. Environ. Health Res. 2022;32:207–219. doi: 10.1080/09603123.2020.1745764. [DOI] [PubMed] [Google Scholar]
- 35.Beckwith T., Cecil K., Altaye M., Severs R., Wolfe C., Percy Z., Maloney T., Yolton K., LeMasters G., Brunst K., Ryan P. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS One. 2020;15:e0228092. doi: 10.1371/journal.pone.0228092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guxens M., Lubczyńska M.J., Muetzel R.L., Dalmau-Bueno A., Jaddoe V.W.V., Hoek G., van der Lugt A., Verhulst F.C., White T., Brunekreef B., et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatr. 2018;84:295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Lubczyńska M.J., Muetzel R.L., El Marroun H., Hoek G., Kooter I.M., Thomson E.M., Hillegers M., Vernooij M.W., White T., Tiemeier H., Guxens M. Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ. Res. 2021;198:110446. doi: 10.1016/j.envres.2020.110446. [DOI] [PubMed] [Google Scholar]
- 38.Peterson B.S., Bansal R., Sawardekar S., Nati C., Elgabalawy E.R., Hoepner L.A., Garcia W., Hao X., Margolis A., Perera F., Rauh V. Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. J. Child Psychol. Psychiatry. 2022;63:1316–1331. doi: 10.1111/jcpp.13578. [DOI] [PubMed] [Google Scholar]
- 39.Lubczyńska M.J., Muetzel R.L., El Marroun H., Basagaña X., Strak M., Denault W., Jaddoe V.W.V., Hillegers M., Vernooij M.W., Hoek G., et al. Exposure to air pollution during pregnancy and childhood, and white matter microstructure in preadolescents. Environ. Health Perspect. 2020;128:27005. doi: 10.1289/EHP4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fonken L.K., Xu X., Weil Z.M., Chen G., Sun Q., Rajagopalan S., Nelson R.J. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatr. 2011;16:987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivas-Arancibia S., Guevara-Guzmán R., López-Vidal Y., Rodríguez-Martínez E., Zanardo-Gomes M., Angoa-Pérez M., Raisman-Vozari R. Oxidative stress caused by ozone exposure induces loss of brain repair in the Hippocampus of adult rats. Toxicol. Sci. 2010;113:187–197. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- 42.Woodward N.C., Haghani A., Johnson R.G., Hsu T.M., Saffari A., Sioutas C., Kanoski S.E., Finch C.E., Morgan T.E. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl. Psychiatry. 2018;8:261–310. doi: 10.1038/s41398-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehsanifar M., Tameh A.A., Farzadkia M., Kalantari R.R., Zavareh M.S., Nikzaad H., Jafari A.J. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 44.Mokoena M.L., Harvey B.H., Viljoen F., Ellis S.M., Brink C.B. Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology. 2015;232:2921–2938. doi: 10.1007/s00213-015-3928-8. [DOI] [PubMed] [Google Scholar]
- 45.White N.S., McDonald C.R., Farid N., Kuperman J.M., Kesari S., Dale A.M. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR. Am. J. Neuroradiol. 2013;34:958–964. doi: 10.3174/ajnr.A3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White N.S., Leergaard T.B., D’Arceuil H., Bjaalie J.G., Dale A.M. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum. Brain Mapp. 2013;34:327–346. doi: 10.1002/hbm.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer C.E., Pecheva D., Iversen J.R., Hagler D.J., Sugrue L., Nedelec P., Fan C.C., Thompson W.K., Jernigan T.L., Dale A.M. Microstructural development from 9 to 14 years: evidence from the ABCD study. Dev. Cogn. Neurosci. 2022;53:101044. doi: 10.1016/j.dcn.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rapuano K.M., Laurent J.S., Hagler D.J., Hatton S.N., Thompson W.K., Jernigan T.L., Dale A.M., Casey B.J., Watts R. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc. Natl. Acad. Sci. USA. 2020;117:26977–26984. doi: 10.1073/pnas.2007918117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hope T.R., Selnes P., Rektorová I., Anderkova L., Nemcova-Elfmarkova N., Balážová Z., Dale A., Bjørnerud A., Fladby T. Diffusion tensor and restriction spectrum imaging reflect different aspects of neurodegeneration in Parkinson’s disease. PLoS One. 2019;14:e0217922. doi: 10.1371/journal.pone.0217922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reas E.T., Hagler D.J., White N.S., Kuperman J.M., Bartsch H., Cross K., Loi R.Q., Balachandra A.R., Meloy M.J., Wierenga C.E., et al. Sensitivity of restriction spectrum imaging to memory and neuropathology in Alzheimer’s disease. Alzheimer's Res. Ther. 2017;9:55. doi: 10.1186/s13195-017-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reas E.T., Hagler D.J., Kuperman J.M., Wierenga C.E., Galasko D., White N.S., Dale A.M., Banks S.J., McEvoy L.K., Brewer J.B. Associations between microstructure, amyloid, and cognition in amnestic mild cognitive impairment and dementia. J. Alzheimers Dis. 2020;73:347–357. doi: 10.3233/JAD-190871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reas E.T., Smirnov D.S., Banks S.J., Galasko D.R., Brewer J.B. Brain microstructure mediates effects of tau on cognitive decline in early Alzheimer’s disease. Alzheimer's Dementia. 2021;17:e055578. doi: 10.1002/alz.055578. [DOI] [Google Scholar]
- 53.Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Hamra G.B., Buckley J.P. Environmental exposure mixtures: questions and methods to address them. Curr. Epidemiol. Rep. 2018;5:160–165. doi: 10.1007/s40471-018-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mortamais M., Pujol J., van Drooge B.L., Macià D., Martínez-Vilavella G., Reynes C., Sabatier R., Rivas I., Grimalt J., Forns J., et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ. Int. 2017;105:12–19. doi: 10.1016/j.envint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 56.Mortamais M., Pujol J., Martínez-Vilavella G., Fenoll R., Reynes C., Sabatier R., Rivas I., Forns J., Vilor-Tejedor N., Alemany S., et al. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ. Res. 2019;178:108734. doi: 10.1016/j.envres.2019.108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Crespo L., Kusters M.S.W., López-Vicente M., Lubczyńska M.J., Foraster M., White T., Hoek G., Tiemeier H., Muetzel R.L., Guxens M. Exposure to traffic-related air pollution and noise during pregnancy and childhood, and functional brain connectivity in preadolescents. Environ. Int. 2022;164:107275. doi: 10.1016/j.envint.2022.107275. [DOI] [PubMed] [Google Scholar]
- 58.Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G., Walsh K., Miller R.L., Arias F., Semanek D., Perera F. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatr. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pujol J., Fenoll R., Macià D., Martínez-Vilavella G., Alvarez-Pedrerol M., Rivas I., Forns J., Deus J., Blanco-Hinojo L., Querol X., Sunyer J. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016;6:e00467. doi: 10.1002/brb3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I., Forns J., Blanco-Hinojo L., Capellades J., Querol X., et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 61.Casanova R., Wang X., Reyes J., Akita Y., Serre M.L., Vizuete W., Chui H.C., Driscoll I., Resnick S.M., Espeland M.A., Chen J.C. A voxel-based morphometry study reveals local brain structural alterations associated with ambient fine particles in older women. Front. Hum. Neurosci. 2016;10:495. doi: 10.3389/fnhum.2016.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Power M.C., Lamichhane A.P., Liao D., Xu X., Jack C.R., Gottesman R.F., Mosley T., Stewart J.D., Yanosky J.D., Whitsel E.A. The association of long-term exposure to particulate matter air pollution with brain MRI findings: the ARIC study. Environ. Health Perspect. 2018;126:027009. doi: 10.1289/EHP2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White N.S., McDonald C., Farid N., Kuperman J., Karow D., Schenker-Ahmed N.M., Bartsch H., Rakow-Penner R., Holland D., Shabaik A., et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014;74:4638–4652. doi: 10.1158/0008-5472.CAN-13-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calderón-Garcidueñas L., Kavanaugh M., Block M., D’Angiulli A., Delgado-Chávez R., Torres-Jardón R., González-Maciel A., Reynoso-Robles R., Osnaya N., Villarreal-Calderon R., et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J. Alzheimers Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 65.Cory-Slechta D.A., Sobolewski M., Marvin E., Conrad K., Merrill A., Anderson T., Jackson B.P., Oberdorster G. The impact of inhaled ambient ultrafine particulate matter on developing brain: potential importance of elemental contaminants. Toxicol. Pathol. 2019;47:976–992. doi: 10.1177/0192623319878400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yokota S., Oshio S., Moriya N., Takeda K. Social isolation-induced territorial aggression in male offspring is enhanced by exposure to diesel exhaust during pregnancy. PLoS One. 2016;11:e0149737. doi: 10.1371/journal.pone.0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang Y.J., Tan H.-Y., Lee C.Y., Cho H. An air particulate pollutant induces neuroinflammation and neurodegeneration in human brain models. Adv. Sci. 2021;8:2101251. doi: 10.1002/advs.202101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Genc S., Zadeoglulari Z., Fuss S.H., Genc K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012;2012:e782462. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan C.C., Loughnan R., Makowski C., Pecheva D., Chen C.-H., Hagler D.J., Thompson W.K., Parker N., van der Meer D., Frei O., et al. Multivariate genome-wide association study on tissue-sensitive diffusion metrics highlights pathways that shape the human brain. Nat. Commun. 2022;13:2423. doi: 10.1038/s41467-022-30110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fama R., Sullivan E.V. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci. Biobehav. Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff M., Vann S.D. The cognitive thalamus as a gateway to mental representations. J. Neurosci. 2019;39:3–14. doi: 10.1523/JNEUROSCI.0479-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. The contribution of the amygdala, nucleus accumbens, and prefrontal cortex to emotion and motivated behaviour. Int. Congr. Ser. 2003;1250:347–370. doi: 10.1016/S0531-5131(03)01013-6. [DOI] [Google Scholar]
- 73.Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Blair J., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 74.Goto Y., Grace A.A. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monk C.S., Klein R.G., Telzer E.H., Schroth E.A., Mannuzza S., Moulton J.L., Guardino M., Masten C.L., McClure-Tone E.B., Fromm S., et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatr. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 76.Sturm V., Lenartz D., Koulousakis A., Treuer H., Herholz K., Klein J.C., Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J. Chem. Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Hurley R.A., Flashman L.A., Chow T.W., Taber K.H. The brainstem: anatomy, assessment, and clinical syndromes. J. Neuropsychiatry Clin. Neurosci. 2010;22:iv. doi: 10.1176/jnp.2010.22.1.iv. 1-7. [DOI] [PubMed] [Google Scholar]
- 78.Karpinski R.I., Kinase Kolb A.M., Tetreault N.A., Borowski T.B. High intelligence: a risk factor for psychological and physiological overexcitabilities. Intelligence. 2018;66:8–23. doi: 10.1016/j.intell.2017.09.001. [DOI] [Google Scholar]
- 79.Koenen K.C., Moffitt T.E., Roberts A.L., Martin L.T., Kubzansky L., Harrington H., Poulton R., Caspi A. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am. J. Psychiatr. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venkatraman A., Edlow B.L., Immordino-Yang M.H. The brainstem in emotion: a review. Front. Neuroanat. 2017;11:15. doi: 10.3389/fnana.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Floresco S.B. The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 82.Compton W.M., Dowling G.J., Garavan H. Ensuring the best use of data. JAMA Pediatr. 2019;173:809–810. doi: 10.1001/jamapediatrics.2019.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heeringa S.G., Berglund P.A. A guide for population-based analysis of the adolescent brain cognitive development (ABCD) study baseline data. bioRxiv. 2020 doi: 10.1101/2020.02.10.942011. Preprint at. [DOI] [Google Scholar]
- 84.Hajat A., Hsia C., O’Neill M.S. Socioeconomic disparities and air pollution exposure: a global review. Curr. Environ. Health Rep. 2015;2:440–450. doi: 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tessum C.W., Paolella D.A., Chambliss S.E., Apte J.S., Hill J.D., Marshall J.D. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci. Adv. 2021;7:eabf4491. doi: 10.1126/sciadv.abf4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortes-Ramirez J., Wilches-Vega J.D., Paris-Pineda O.M., Rod J.E., Ayurzana L., Sly P.D. Environmental risk factors associated with respiratory diseases in children with socioeconomic disadvantage. Heliyon. 2021;7:e06820. doi: 10.1016/j.heliyon.2021.e06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munoz-Pizza D.M., Villada-Canela M., Reyna M.A., Texcalac-Sangrador J.L., Osornio-Vargas Á.R. Air pollution and children’s respiratory health: a scoping review of socioeconomic status as an effect modifier. Int. J. Publ. Health. 2020;65:649–660. doi: 10.1007/s00038-020-01378-3. [DOI] [PubMed] [Google Scholar]
- 88.Alemany S., Vilor-Tejedor N., García-Esteban R., Bustamante M., Dadvand P., Esnaola M., Mortamais M., Forns J., van Drooge B.L., Álvarez-Pedrerol M., et al. Traffic-related air pollution, APOE ε4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, Spain) Environ. Health Perspect. 2018;126:087001. doi: 10.1289/EHP2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beaton D., Chin Fatt C.R., Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Comput. Stat. Data Anal. 2014;72:176–189. doi: 10.1016/j.csda.2013.11.006. [DOI] [Google Scholar]
- 90.Abdi H., Beaton D. 2023. Principal Component and Correspondence Analyses Using R. Springer Cham.https://link.springer.com/book/9783319092577 [Google Scholar]
- 91.Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Jernigan T., Potter A., Thompson W., Zahs D. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jernigan T.L., Brown S.A., Dowling G.J. The adolescent brain cognitive development study. J. Res. Adolesc. 2018;28:154–156. doi: 10.1111/jora.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Pérez-Stable E.J., Riley W.T., Bloch M.H., Conway K., et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bagot K.S., Matthews S.A., Mason M., Squeglia L.M., Fowler J., Gray K., Herting M., May A., Colrain I., Godino J., et al. Current, future and potential use of mobile and wearable technologies and social media data in the ABCD study to increase understanding of contributors to child health. Dev. Cogn. Neurosci. 2018;32:121–129. doi: 10.1016/j.dcn.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feldstein Ewing S.W., Bjork J.M., Luciana M. Implications of the ABCD study for developmental neuroscience. Dev. Cogn. Neurosci. 2018;32:161–164. doi: 10.1016/j.dcn.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hagler D.J., Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Sutherland M.T., Casey B.J., Barch D.M., Harms M.P., et al. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luciana M., Bjork J.M., Nagel B.J., Barch D.M., Gonzalez R., Nixon S.J., Banich M.T. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 2018;32:67–79. doi: 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uban K.A., Horton M.K., Jacobus J., Heyser C., Thompson W.K., Tapert S.F., Madden P.A.F., Sowell E.R., Adolescent Brain Cognitive Development Study Biospecimens and the ABCD study: rationale, methods of collection, measurement and early data. Dev. Cogn. Neurosci. 2018;32:97–106. doi: 10.1016/j.dcn.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan C.C., Marshall A., Smolker H., Gonzalez M.R., Tapert S.F., Barch D.M., Sowell E., Dowling G.J., Cardenas-Iniguez C., Ross J., et al. Adolescent brain cognitive development (ABCD) study linked external data (LED): protocol and practices for geocoding and assignment of environmental data. Dev. Cogn. Neurosci. 2021;52:101030. doi: 10.1016/j.dcn.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Q., Amini H., Shi L., Kloog I., Silvern R., Kelly J., Sabath M.B., Choirat C., Koutrakis P., Lyapustin A., et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Q., Amini H., Shi L., Kloog I., Silvern R., Kelly J., Sabath M.B., Choirat C., Koutrakis P., Lyapustin A., et al. Assessing NO2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ. Sci. Technol. 2020;54:1372–1384. doi: 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Requia W.J., Di Q., Silvern R., Kelly J.T., Koutrakis P., Mickley L.J., Sulprizio M.P., Amini H., Shi L., Schwartz J. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ. Sci. Technol. 2020;54:11037–11047. doi: 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dell’Acqua F., Rizzo G., Scifo P., Clarke R.A., Scotti G., Fazio F. A model-based deconvolution approach to solve fiber crossing in diffusion-weighted MR imaging. IEEE Trans. Biomed. Eng. 2007;54:462–472. doi: 10.1109/TBME.2006.888830. [DOI] [PubMed] [Google Scholar]
- 105.Jian B., Vemuri B.C. A unified computational framework for deconvolution to reconstruct multiple fibers from diffusion weighted MRI. IEEE Trans. Med. Imag. 2007;26:1464–1471. doi: 10.1109/TMI.2007.907552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaden E., Knösche T.R., Anwander A. Parametric spherical deconvolution: inferring anatomical connectivity using diffusion MR imaging. Neuroimage. 2007;37:474–488. doi: 10.1016/j.neuroimage.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 107.Tournier J.-D., Calamante F., Gadian D.G., Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 108.Thompson W.K., Barch D.M., Bjork J.M., Gonzalez R., Nagel B.J., Nixon S.J., Luciana M. The structure of cognition in 9 and 10 year-old children and associations with problem behaviors: findings from the ABCD study’s baseline neurocognitive battery. Dev. Cogn. Neurosci. 2019;36:100606. doi: 10.1016/j.dcn.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Achenbach T.M., Dumenci L., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; 2001. Ratings of Relations between DSM-IV Diagnostic Categories and Items. [Google Scholar]
- 110.Weng H.-Y., Hsueh Y.-H., Messam L.L.M., Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am. J. Epidemiol. 2009;169:1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 111.Greenland S., Brumback B. An overview of relations among causal modelling methods. Int. J. Epidemiol. 2002;31:1030–1037. doi: 10.1093/ije/31.5.1030. [DOI] [PubMed] [Google Scholar]
- 112.Bell M.L., Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 2012;120:1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bondy M., Roth S., Sager L. Crime is in the air: the contemporaneous relationship between air pollution and crime. J. Assoc. Environ. Resour. Econ. 2018;7:555–585. [Google Scholar]
- 114.Miranda M.L., Edwards S.E., Keating M.H., Paul C.J. Making the environmental Justice grade: the relative burden of air pollution exposure in the United States. Int. J. Environ. Res. Publ. Health. 2011;8:1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 116.Hachay . 2021. data4PCCAR. [Google Scholar]
- 117.Kuhn M. 2022. Classification and Regression Training [R package caret version 6.0-92]https://CRAN.R-project.org/package=caret [Google Scholar]
- 118.Abdi H., Williams L.J. Partial least squares methods: partial least squares correlation and partial least square regression. Methods Mol. Biol. 2013;930:549–579. doi: 10.1007/978-1-62703-059-5_23. [DOI] [PubMed] [Google Scholar]
- 119.Berry K.J., Johnston J.E., Mielke P.W., Jr. Permutation methods. WIREs. Comp. Stat. 2011;3:527–542. doi: 10.1002/wics.177. [DOI] [Google Scholar]
- 120.Efron B., Tibshirani R. Bootstrap methods for standard errors, confidence Intervals, and other measures of statistical accuracy. Stat. Sci. 1986;1:54–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD Study data used in this report came from https://doi.org/10.15154/1523041.
-
•
All original code is deposited at figshare.com and is now publicly available. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.