Abstract
Tumor necrosis factor alpha (TNF-α) and TNF-β are key mediators in bacterial inflammation. We therefore examined the role of TNF-α and its two receptors in murine pneumococcal central nervous system infection. TNF-α knockout mice and age- and sex-matched controls and TNF receptor (p55 and p75)-deficient mice and heterozygous littermates were infected intracerebrally with a Streptococcus pneumoniae type 3 strain. Mice were monitored until death or were killed 36 h after infection. Bacterial titers in blood, spleen, and brain homogenates were determined. Leukocyte infiltration and neuronal damage were assessed by histological scores. TNF-α-deficient mice died earlier than the controls after intracerebral infection although overall survival was similar. TNF-α deficiency did not inhibit leukocyte recruitment into the subarachnoid space and did not lead to an increased density of bacteria in brain homogenates. However, it caused a substantial rise of the concentration of S. pneumoniae cells in blood and spleen. Spleen bacterial titers were also increased in p55- and p75-deficient mice. TNF receptor-deficient mice showed decreased meningeal inflammation. Neuronal damage was not affected by either TNF-α or TNF receptor deficiency. In a murine model of pneumococcal peritonitis, 102 CFU of S. pneumoniae produced fatal peritonitis in TNF-α-deficient, but not wild-type, mice. Early leukocyte influx into the peritoneum was impaired in TNF-α-deficient mice. The lack of TNF-α or its receptors renders mice more susceptible to S. pneumoniae infections.
The high virulence of Streptococcus pneumoniae to mice has been known since the beginning of the 20th century (18). Intraperitoneal and intracerebral injections of small numbers of viable bacteria caused the death of animals within 1 to 2 days, whereas intravenous administration resulted in rapid clearance from the blood not leading to infection (6, 18, 43).
Tumor necrosis factor alpha (TNF-α) and TNF-β (lymphotoxin-α) are critically involved in inflammation and the cellular immune response (3). TNF-α and TNF-β are key mediators of the septic shock syndrome caused by lipopolysaccharides or staphylococcal superantigens (2).
TNF-α mediates its action via two distinct receptors, the p55 and p75 receptors. Induction of septic shock by lipopolysaccharides is prevented by p55 deficiency as well as p55 and p75 deficiency, whereas p75-deficient mice are not protected, suggesting a major role of the p55 TNF receptor in endotoxin-induced shock (30). Furthermore, p55-mediated TNF-α effects have been shown to be important for neutrophil recruitment into lungs challenged with Micropolyspora faeni cells, whereas p75 appeared to have a modulating effect (30). When only p75 was lacking in this model, the number of neutrophils was increased strongly, possibly due to the absence of downregulation of the inflammatory response by TNF-α-induced apoptosis. In human meningitis, TNF-α is increased in cerebral spinal fluid (CSF) and correlates with indices of meningeal inflammation as well as blood brain barrier disruption (36).
The present study addressed the question of whether deficiency for TNF-α or both its receptors alters the course of S. pneumoniae central nervous system (CNS) infection after intracerebral inoculation, the susceptibility to pneumococci after intraperitoneal inoculation, and the clearance of bacteria from the bloodstream.
(This work was presented, in part, at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Animals.
Female TNF-α knockout mice possessing an intact TNF-β gene on a C57BL6 background were bred in the Central Animal Care Facility of the Faculty of Medicine, University of Göttingen, and had originally been presented by George Kollias, Department of Molecular Genetics, Hellenic Pasteur Institute, Athens, Greece (27). Sex- and age-matched wild-type C57BL6 controls were obtained from the Central Animal Care Facility of the Faculty of Medicine, University of Göttingen. Male knockout mice for the p55 and p75 TNF receptors as well as heterozygous control littermates on a C57BL6 × 129 background were a kind gift from H. Bluethmann, Hoffmann-LaRoche, Basel, Switzerland. The method used for breeding the p55 and p75 knockout mice was described elsewhere (35). Water and food were available ad libitum. The experiments were approved by the Animal Care Committee of the Medical Faculty of the University of Göttingen and by the District Government of Braunschweig, Braunschweig, Lower Saxony, Germany.
Inoculum.
A S. pneumoniae type 3 strain originally isolated from an adult with meningitis (gift from M. G. Täuber, Division of Infectious Diseases, University of Bern, Bern, Switzerland) was used for inoculations. The inoculum was grown on sheep blood agar plates, stored at −70°C, and diluted in 0.9% NaCl.
Induction of CNS infection.
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg). Then, 25 μl of 0.9% NaCl containing 104 CFU (TNF-α knockout mice and controls, n = 6; TNF receptor knockout mice and control littermates, n = 8) of S. pneumoniae was injected into the right frontal lobe of the cerebral cortex with a 27-gauge disposable needle (24). With TNF-α knockout mice and controls, survival experiments were performed with a smaller inoculum of 100 CFU (n = 15). All animals resumed their normal behavior after awaking from anesthesia. TNF-α knockout mice and controls were followed up by determining the clinical score (0, appearing healthy; 1, slightly lethargic; 2, moderately lethargic, able to walk; 3, severely lethargic, unable to walk; 4, dead) 12, 20, and 36 h after infection. Mice with a clinical score of 3 were killed for ethical reasons. p55 and p75 knockout mice and their control littermates were assessed 12, 24, 32, and 36 h after infection. For survival analysis, animals were also followed up at 52, 60, 74, 84, and 98 h. For animals that were dead or were killed because of a clinical score of 3, blood bacterial titers were determined to ensure that infection was the cause of death.
Sample processing.
Mice which were killed 36 h after infection were anesthetized with ether. Blood was drawn by cardiac puncture, and mice were exsanguinated by perfusion with 0.9% saline. Then, brain and spleen were removed. The left frontal lobe of the cerebral cortex (TNF-α-deficient mice and their respective controls) or the cerebellum (TNF receptor-deficient mice and controls) and the ventral half of the spleen were homogenized in 0.9% saline (1/20 [wt/wt]). Bacterial titers in homogenates and blood were determined by plating serial 10-fold dilutions in 0.9% saline on sheep blood agar plates. The right cerebral hemisphere and the liver were placed in 4% paraformaldehyde and then embedded in paraffin.
For TNF receptor-deficient mice and controls, serum C-reactive protein was determined using a commercial turbidimetric test kit (Rolf Greiner BioChemica, Flacht, Germany).
Induction of sepsis and peritonitis.
TNF-α knockout mice and controls (n = 5 [each group]) received intraperitoneally 40 μl of 0.9% NaCl containing approximately 100 CFU of S. pneumoniae with a 24-gauge disposable needle. Mice were then observed 24 and 36 h after infection and then daily for 1 week by using the same clinical score system described above. For samples from mice that were alive 1 week after infection and that were behaving normally, blood and homogenates of the spleen were plated onto sheep blood agar. For another set of mice (n = 8 [each group]), leukocyte influx into the peritoneum was assessed 4 h after infection. Mice were killed by decapitation, and 5 ml of phosphate-buffered saline was instilled into the peritoneum and recollected after 10 s of massaging the abdomen. Leukocytes per milliliter of lavage fluid were counted using a Fuchs-Rosenthal chamber.
Histology.
Five-micrometer sections of the liver and coronary sections of the brain were stained with hematoxylin and eosin and examined by light microscopy. Brain sections were scored semiquantitatively for inflammation and neuronal damage. The scores were as follows.
Inflammation score.
Using a 40-fold magnification field, three meningeal, the two temporobasal, and the interhemispheral regions and the third ventricle were assessed for the number of leukocytes. A score was given in respect to the number of leukocytes present: 0, no leukocytes; 1, 1 to 10 leukocytes; 2, 11 to 50 leukocytes; 3, more than 50 leukocytes. All regional scores were added, thereby allowing a maximum score of 21 to be reached (14).
Neuronal damage score.
Neuronal damage was assessed in four regions: hippocampus, dentate gyrus, basal ganglia, and cortex. The number of damaged neurons assessed by morphological changes typical for necrosis or apoptosis was estimated as follows: 0, no damaged cells; 1, ≤10%; 2, 11 to 30%; 3, more than 30% of neuronal cells with necrotic or apoptotic morphology (14).
Statistics.
Data were described as means ± standard deviations if normally distributed, and groups were compared by the two-tailed t test for independent samples. In the absence of a normal distribution, the median and the 25th and 75th percentiles were used, and groups were compared by the two-tailed Mann-Whitney U test. For survival analysis, a log-rank test based on a Kaplan-Meier plot was used.
RESULTS
CNS infection in TNF-α-deficient mice.
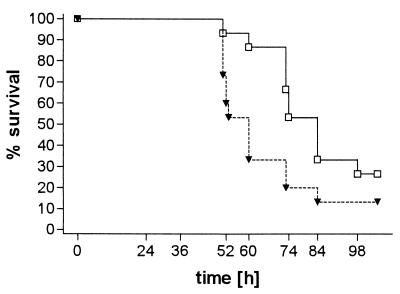
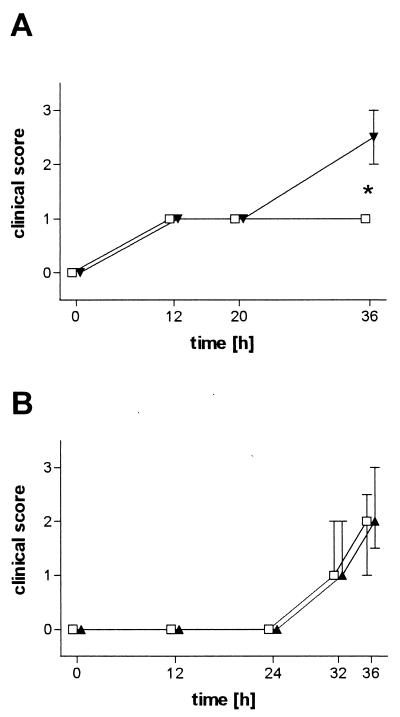
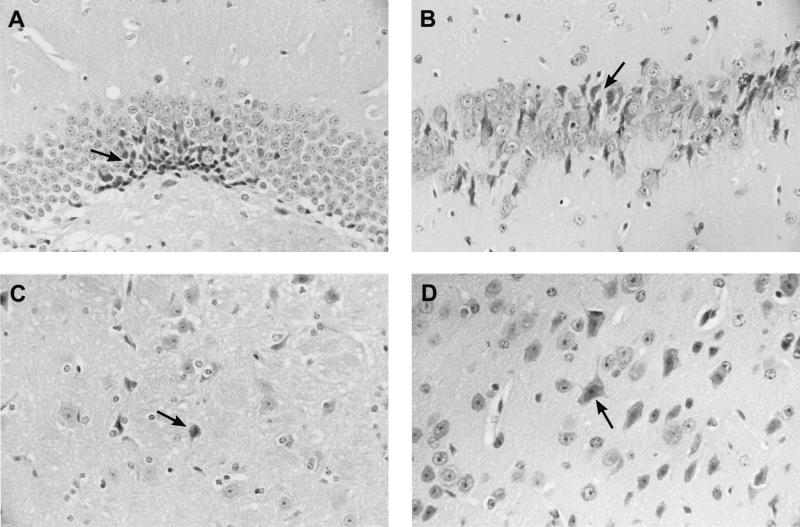
An inoculum of 104 CFU produced fatal infection in both wild-type and TNF-α-deficient mice. After intracerebral infection with 100 CFU, 13 of 15 TNF-α-deficient mice and 11 of 15 control mice died. TNF-α-deficient mice died earlier than their wild-type controls (P = 0.04, log-rank test; Fig. 1). Twenty hours after inoculation with 104 CFU, TNF-α-deficient and immunocompetent animals were slightly lethargic but able to walk and to feed. Thirty-six hours after infection, clinical scores were significantly worse in TNF-α-deficient mice when compared to those of controls (median [25th percentile, 75th percentile]) (2.5 [2, 3] versus 1 [1, 1], P = 0.004, Mann-Whitney U test; Fig. 2). The densities of viable bacteria in blood and spleen were significantly higher in TNF-α-deficient mice than in C57BL6 mice (mean ± standard deviation) (5.9 ± 0.6 and 6.4 ± 0.6 log CFU/ml versus 4.0 ± 0.7 and 4.9 ± 0.6 log CFU/ml, P = 0.0007 and 0.001, respectively, t test; Table 1). On the contrary, the bacterial densities in the brain homogenates of the two groups were almost identical (Table 1). Histological examination of the brains revealed leukocyte recruitment into the subarachnoid space in TNF-α-deficient mice which was not distinguishable from that in wild-type animals (median [25th percentile, 75th percentile]) (meningeal inflammation score, TNF-α-deficient versus wild-type mice, 12 [9, 14.8] versus 12.5 [11.5, 16.5], P = 0.48, Mann-Whitney U test; Table 1). In the liver, fatty degeneration of hepatocytes suggesting severe septic shock was more prominent in TNF-α-deficient animals than in control animals. Neuronal damage was seen predominantly in the neocortex and hippocampal formation. Rarely, damaged neurons could be morphologically characterized as apoptotic, and the vast majority of damaged neurons showed signs of necrosis (Fig. 3). There were no significant differences between groups (Table 1).
FIG. 1.
Survival (Kaplan-Meier plots) of TNF-α-deficient mice (▾; n = 15) and C57BL6 control mice (□; n = 15) after intracerebral inoculation of 100 CFU of S. pneumoniae. P = 0.04 (log-rank test).
FIG. 2.
Clinical disease scores of mice after intracerebral infection with 104 CFU of S. pneumoniae. (A) TNF-α-deficient mice (▾; n = 6) versus C57BL6 mice (□, n = 6). (B) TNF receptor (p55 and p75)-deficient mice (▴; n = 8) versus C57BL6 × 129 mice (□; n = 8). Error bars denote the 25th and 75th percentiles. Please note that the clinical symptoms were more severe in TNF-α-deficient mice, but not in TNF receptor-deficient mice, than in wild-type mice. ∗, P = 0.004
TABLE 1.
Density of viable bacteria, inflammation, and neuronal damage in TNF-α-deficient and C57BL6 mice 36 h after intracerebral injection of 104 CFU of S. pneumoniae
| Parameter | Values fora:
|
P | |
|---|---|---|---|
| TNF-α-deficient mice (n = 6) | C57BL6 mice (n = 6) | ||
| Bacterial titers (log CFU/ml) | |||
| Blood | 5.9 ± 0.6 | 4.0 ± 0.7 | 0.0007 |
| Spleen | 6.4 ± 0.6 | 4.9 ± 0.6 | 0.001 |
| Brain | 8.0 ± 0.9 | 8.0 ± 0.6 | 0.96 |
| Inflammation score | 12 [9, 14.8] | 12.5 [11.5, 16.5] | 0.48 |
| Neuronal damage score | |||
| Dentate gyrus | 0.5 [0, 1] | 1 [1, 1] | 0.59 |
| Hippocampus | 0 [0, 1.5] | 1 [1, 1] | 0.59 |
| Cortex | 1.5 [1, 2] | 1.5 [1, 2] | 1.00 |
| Basal ganglia | 1 [1, 1] | 1 [0.5, 1.5] | 1.00 |
For bacterial titers, data are shown as means ± standard deviations and were tested for significance by using the t test. For inflammation and neuronal damage scores, data are shown as medians [25th percentile, 75th percentile] and were tested for significance by using the Mann-Whitney U test.
FIG. 3.
Neuronal damage after intracerebral infection with 104 CFU of S. pneumoniae. Shown is necrotic neuronal damage (arrow) in the dentate gyrus (A), the CA1 region of the hippocampus (B), the striatum (C), and the neocortex (D) of TNF-α-deficient mice. Magnifications, ×100. Hematoxylin and eosin staining was used.
CNS infection in p55- and p75-deficient mice.
Mice deficient for both TNF receptors showed no difference compared to controls in regard to clinical score after inoculation of 104 CFU at 12, 24, 32, and 36 h (Fig. 2). Bacterial titers in the spleen were significantly higher in p55 and p75 knockout mice than in control littermates (mean ± standard deviation) (5.7 ± 0.9 versus 4.9 ± 0.4 log CFU/ml, P = 0.04, t test; Table 2). The differences of bacterial titers in blood and cerebellum did not reach statistical significance (p55 and p75 knockout mice versus controls: 7.1 ± 1.0 and 9.8 ± 1.1 versus 6.1 ± 1.0 and 8.9 ± 1.0 log CFU/ml, respectively, P = 0.07 and P = 0.08, respectively, t test; Table 2). Neuronal damage was not significantly different between the groups (Table 2). However, meningeal inflammatory scores were lower in p55- and p75-deficient animals (median [25th percentile, 75th percentile]) (12.5 [10.75, 14.5] versus 16 [14.5, 17.68]; P = 0.03; Mann-Whitney U test; Table 2). C-reactive protein was significantly lower in the serum of p55- and p75-deficient mice when compared to their control littermates (mean ± standard deviation) (6.84 ± 2.62 μg/ml versus 13.76 ± 2.95 μg/ml, P = 0.0002, t test; Table 2).
TABLE 2.
Density of viable bacteria, inflammation, C-reactive protein, and neuronal damage in p55- and p75-deficient and control mice 36 h after intracerebral injection of 104 CFU of S. pneumoniae
| Parameter | Values fora:
|
P | |
|---|---|---|---|
| p55- and p75- deficient mice (n = 8) | C57BL6 × 129 mice (n = 8) | ||
| Bacterial titers (log CFU/ml) | |||
| Blood | 7.1 ± 1.0 | 6.1 ± 1.0 | 0.07 |
| Spleen | 5.7 ± 0.9 | 4.9 ± 0.4 | 0.04 |
| Cerebellum | 9.8 ± 1.1 | 8.9 ± 1.0 | 0.08 |
| Inflammation score | 12.5 [10.8, 14.5] | 16 [14.5, 17.6] | 0.03 |
| Serum C-reactive protein (μg/ml) | 6.84 ± 2.62 | 13.76 ± 2.95 | 0.0002 |
| Neuronal damage score | |||
| Dentate gyrus | 1 [1, 1] | 1 [1, 1] | 1.0 |
| Hippocampus | 1 [1, 2] | 1 [1, 2] | 1.0 |
| Cortex | 2 [1.5, 2] | 1.5 [1, 2] | 0.44 |
| Basal ganglia | 1 [1, 1] | 1 [1, 1] | 1.0 |
For bacterial titers and protein concentration, data are shown as means ± standard deviations and were tested for significance by using the t test. For inflammation and neuronal damage scores, data are, shown as medians [25th percentile, 75th percentile] and were tested for significance by using the Mann-Whitney U test.
Peritonitis.
Twenty-four hours after intraperitoneal infection with 100 CFU, no behavioral changes were observed in wild-type mice, whereas three of five TNF-α-deficient mice were slightly lethargic. Between 24 and 36 h after infection, all TNF-α-deficient animals, but none of the wild-type controls, had died (P = 0.003; log-rank test). The C57BL6 mice remained healthy for the following week. Autopsy of TNF-α-deficient animals showed fatty degeneration of the liver and ischemic damage of pyramidal cells in the CA1 sector of the hippocampus typical for septic shock but no evidence of meningitis or encephalitis. Blood and spleen homogenates from C57BL6 mice killed 1 week after infection were sterile. Leukocyte influx into the peritoneum was higher 4 h after infection in controls when compared to TNF-α-deficient animals (mean ± standard deviation) (2,745 ± 952/μl versus 1,797 ± 435/μl; P = 0.02; t test).
DISCUSSION
Mice deficient for either TNF-α or the p55 TNF receptor are less sensitive to endotoxin but readily succumb to infections caused by Listeria monocytogenes, i.e., a bacterium able to survive and multiply intracellularly (27, 31, 32). The presence of an intact p55 TNF receptor has been shown to increase survival from S. pneumoniae infections in mice (25). A protective effect of TNF-α was demonstrated in murine salmonellosis (22) and peritonitis produced by the endogenous bowel flora (10). The susceptibility of TNF-α knockout mice possessing an intact TNF-β gene to S. pneumoniae, a pathogen with primarily extracellular localization, had not been studied previously. In the present study, all TNF-α-deficient animals died after intraperitoneal challenge with 100 CFU of S. pneumoniae within 36 h, whereas normal controls survived. Accordingly, TNF-α-deficient mice showed a significantly lower peritoneal influx of leukocytes 4 h after intraperitoneal infection, suggesting reduced peritoneal bacterial clearance. Thirty-six hours after intracerebral infection, TNF-α-deficient mice were sicker than wild-type mice. Their survival time was significantly shorter. After intracerebral infection, no difference in cerebral bacterial titers or leukocyte recruitment into the CSF was observed between TNF-α-deficient mice and wild-type controls. The clearance of S. pneumoniae from the bloodstream, however, was severely affected in TNF-α-deficient mice, resulting in bacterial titers in the blood and spleen, which were 2 logs higher than in wild-type animals.
Also, in animals that lacked both TNF receptors, bacterial titers were significantly higher in the spleen, whereas the differences in blood and cerebellum just failed to reach significance. Leukocyte influx into the subarachnoid space was, however, impaired in these animals. The clinical course of infection in animals lacking both TNF receptors was unchanged compared to that of controls.
TNF-α is a pleiotropic cytokine affecting the proliferation, differentiation, and function of virtually every cell type involved in the immune response. Many of the bioactivities of TNF-α are shared by other cytokines, particularly interleukin-1 (3, 31). TNF-α enhances the ability of granulocytes and macrophages to phagocytose and to kill pathogens (9, 21, 26, 44). TNF-α via its p55 receptor participates in the development of splenic follicular germinal centers (28). TNF-α- and p55-deficient mice lack splenic primary B-cell follicles and are unable to form germinal centers. Upon primary immunization, the production of immunoglobulin G (IgG) and IgE antibodies was reduced in comparison to wild-type controls, but upon secondary immunization, the magnitude of the IgE and IgG response was similar to that of wild-type controls with the exception of the IgG2a response, which remained severely compromised in TNF-α-deficient mice (27). In immunocompetent individuals, pneumococci are ingested and killed by macrophages mainly in the spleen and liver (6) and by neutrophilic granulocytes (5, 12). Opsonization by antibodies directed against capsular antigens is not essential but accelerates phagocytosis (6). The lower early influx of leukocytes into the peritoneum after intraperitoneal injection of pneumococci observed in TNF-α-deficient mice probably is one reason for their increased susceptibility to S. pneumoniae. However, TNF-α-deficient mice did not differ from their controls in respect to meningeal inflammation assessed by a histological score. Conversely, with p55- and p75-deficient mice, we found reduced meningeal inflammation. p55 TNF receptor-deficient and p55 and p75 TNF receptor-deficient mice also showed a decreased pulmonary inflammatory response when challenged intranasally with M. faeni cells. On the contrary, p75 deficiency led to an increased influx of leukocytes into the lungs, i.e., the inflammatory response was mainly dependent on the p55 receptor, whereas the p75 receptor probably limited leukocyte influx (30). Neutrophil influx into the peritoneum was, however, not different between p55-deficient mice and controls 24 h after infection with S. pneumoniae cells (25). In addition, decreased splenic clearance of pneumococci probably further increased susceptibility of TNF-α-deficient mice to pneumococcal infection. To what extent developmental abnormalities of the spleen due to TNF-α or TNF receptor deficiency contribute to decreased bacterial clearance remains unclear. TNF-α deficiency leads to increased bacterial load in all organs, including the spleen, resulting in the death of all animals in response to infection with a low dose of Mycobacterium bovis bacillus Calmette-Guérin (BCG) (1). This could, in part, be abolished when the same TNF-α-deficient mice were inoculated with a BCG strain enabled to produce TNF-α by a plasmid containing the cDNA for TNF-α. This shows for mycobacterial infection that developmental abnormalities in the spleen of TNF-α-deficient mice probably are no major handicap for the clearance of bacteria and survival of infection.
In the CNS, both wild-type and TNF-α-deficient mice were susceptible to low doses of S. pneumoniae. The lack of TNF-α did not lead to an increase of bacterial titers in the CNS. Similarly, in the CSF of rabbits rendered neutropenic by irradiation or nitrogen mustard, the growth of S. pneumoniae was unchanged in comparison to untreated rabbits (11, 41). These observations imply that (i) in the physiologically immunocompromised subarachnoid space, immunocompetent animals are not able to inhibit bacterial replication, and (ii) immunosuppression does not accelerate the multiplication of S. pneumoniae in the subarachnoid space. After an intracerebral injection of 100 CFU, TNF-α-deficient mice died earlier than wild-type controls, probably by their reduced ability to clear S. pneumoniae from systemic circulation.
For resistance against pneumococcal infection, the p55 TNF receptor plays a more important role than the p75 TNF receptor (25). The lack of the p55 TNF receptor (or both TNF receptors) abolishes all effects in response to activation of the p55 receptor (due to TNF-α as well as lymphotoxin-α [TNF-β]) completely, whereas in TNF-α deficiency, some of these effects could be partially compensated for by TNF-β (15). Mortality in M. bovis BCG-infected mice deficient for p55, therefore, was higher than in mice deficient for TNF-α (16).
The severity of clinical symptoms during the infection depends on the bacterial load and the host response. In mice deficient for both TNF receptors, the clinical course did not differ from the course in the respective controls because the decreased meningeal inflammatory reaction of the host outweighed the higher bacterial load. In TNF-α-deficient mice, the bacterial titers in blood and spleen after intracerebral infection were higher than in control animals, and these higher titers resulted in more severe disease symptoms, more pronounced sepsis-induced histological changes in the liver, and a higher mortality. In contrast, TNF-α- deficient and p55 and p75 receptor-deficient mice appeared to be less susceptible to lipopolysaccharides of gram-negative organisms and to infections by some gram-negative bacteria (19, 27, 30). The absence of TNF-α or the p55 receptor, however, does not prevent severe sepsis due to gram-positive bacteria (1). This points to a slightly different role of TNF-α in infections with gram-positive and gram-negative bacteria: the TNF-α response in human sepsis with gram-positive bacteria is lower than in sepsis with gram-negative bacteria, and infections with gram-positive bacteria respond less well to anti-TNF therapies than infections with gram-negative bacteria (8, 38). For this reason, the uninhibited bacterial growth caused by the lack of TNF-α or the p55 receptor in infections with gram-positive bacteria may be more detrimental than the strong immune response in wild-type controls. Control mice in the first series developed lethargy earlier in the course of meningitis than control mice in the second series. They, however, differed in their sex as well as their genetic background.
C-reactive protein is part of the acute-phase response in humans. In mice, it increases to a lesser extent in response to bacterial infection or stimulation with endotoxins (29, 37, 39). Human C-reactive protein has been shown to protect mice against lethal S. pneumoniae infection (20, 39). C-reactive protein binds to teichoic acid on pneumococcal cell walls (17). This complex can activate the classical complement pathway and is phagocytosed by granulocytes and monocytes (17, 40) or by the reticuloendothelial system of the spleen (23). The decreased C-reactive protein response in p55- and p75-deficient mice upon pneumococcal infection may therefore be an additional factor compromising an effective host response against S. pneumoniae in our model. This may not reflect a generally decreased acute-phase response: the increase in serum amyloid P—the main acute phase reactant in mice—after infection with S. pneumoniae was similar in p55-deficient mice when compared to controls (25). Similarly, p55- and p75-deficient mice were able to produce a normal serum amyloid P response after challange with lipopolysaccharides (30).
A monoclonal antibody against TNF-α reduced hippocampal injury in a neonatal rat model of Streptococcus group B meningitis (4). Mice lacking the p55 receptor or both TNF receptors showed increased neuronal damage after focal cerebral ischemia (7, 13). In the present study, neuronal damage in all regions investigated was not reduced in TNF-α- or TNF receptor-deficient mice. Since heat-inactivated pneumococci and their products lipoteichoic and teichoic acids, DNA, and peptidoglycans are able to elicit apoptotic and necrotic neuronal death in organotypic cultures of the hippocampal formation (34), one possible explanation for the similar neuronal damage in wild-type and knockout mice is the higher bacterial load in TNF-α- or TNF receptor-deficient mice. Furthermore, with a rabbit model of S. pneumoniae meningitis, we were unable to confirm a relation between TNF-α activity in CSF and neuronal damage (33, 42).
In conclusion, TNF-α deficiency did not influence bacterial growth and leukocyte invasion in the subarachnoid space after intracerebral S. pneumoniae infection. However, it increased the density of S. pneumoniae in blood and spleen and the severity of infection, decreased the interval between infection and death, and rendered mice more susceptible to intraperitoneal infection with S. pneumoniae. This was, in part, explained by a decreased early influx of leukocytes. Deficiency of both TNF receptors led to higher bacterial counts in the spleen after induction of meningitis and to reduced leukocyte invasion into the subarachnoid space. This did, however, not influence the clinical course of infection. Neuronal damage in animals deficient for TNF-α or for both TNF receptors was not reduced.
ACKNOWLEDGMENT
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Na 165/4-1).
REFERENCES
- 1.Bekker L-G, Moreira A L, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–6961. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan I, Leib S L, Bergeron M, Chow L, Täuber M G. Tumor necrosis factor-α contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 5.Braconier J H, Odeberg H. Granulocyte phagocytosis and killing virulent and avirulent serotypes of Streptococcus pneumoniae. J Lab Clin Med. 1982;100:279–287. [PubMed] [Google Scholar]
- 6.Brown E J, Hosea S W, Frank M M. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis. 1983;5(Suppl. 4):797–805. doi: 10.1093/clinids/5.supplement_4.s797. [DOI] [PubMed] [Google Scholar]
- 7.Bruce A J, Boling W, Kindy M S, Peschon J, Kraemer P J, Carpenter M K, Holtsberg F W, Mattson M P. Altered neuronal and microglial responses to exitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Abraham E. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:116–121. doi: 10.1086/314839. [DOI] [PubMed] [Google Scholar]
- 9.Collins H L, Bancroft G J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 10.Echtenacher B, Falk W, Mannel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 11.Ernst J D, Decazes J M, Sande M A. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect Immun. 1983;41:275–279. doi: 10.1128/iai.41.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frimodt-Möller N. The mouse peritonitis model: present and future use. J Antimicrob Chemother. 1993;31(Suppl. D):55–60. doi: 10.1093/jac/31.suppl_d.55. [DOI] [PubMed] [Google Scholar]
- 13.Gary D S, Bruce-Keller A J, Kindy M S, Mattson M P. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A, Brück W, Nau R. A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol. 2001;101:499–508. doi: 10.1007/s004010000326. [DOI] [PubMed] [Google Scholar]
- 15.Gruss H J. Molecular, structural, and biological characteristics of the tumor necrosis factor ligand superfamily. Int J Clin Lab Res. 1996;26:143–159. doi: 10.1007/BF02592977. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs M, Marino M W, Brown N, Abel B, Bekker L G, Quesniaux V J, Fick L, Ryffel B. Correction of defective host response to Mycobacterium bovis BCG infection in TNF-deficient mice by bone marrow transplantation. Lab Investig. 2000;80:901–914. doi: 10.1038/labinvest.3780094. [DOI] [PubMed] [Google Scholar]
- 17.Kim J O, Romero-Steiner S, Sorensen U B S, Blom J, Carvalho M, Barnard S, Carlone G, Weiser J N. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindborg A. Die Pneumokokken. Vergleichende Untersuchungen mit besonderer Berücksichtigung der Agglutination. Z Hyg. 1905;51:197–232. [Google Scholar]
- 19.Leon L R, White A A, Kluger M J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–R277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- 20.Mold C, Nakayama S, Holzer T J, Gewurz H, Du Clos T W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981;154:1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nacy C A, Meierovics A I, Belosevic M, Green S J. Tumor necrosis factor-alpha: central regulatory cytokine in the induction of macrophage antimicrobial activities. Pathobiology. 1991;59:182–184. doi: 10.1159/000163640. [DOI] [PubMed] [Google Scholar]
- 22.Nakano Y, Onozuka K, Terada Y, Shinomiya H, Nakano M. Protective effect of recombinant tumor necrosis factor-alpha in murine salmonellosis. J Immunol. 1990;144:1935–1941. [PubMed] [Google Scholar]
- 23.Nakayama S, Gewurz H, Holzer T, Du Clos T W, Mold C. The role of the spleen in the protective effect of C-reactive protein in Streptococcus pneumoniae infection. Clin Exp Immunol. 1983;54:319–326. [PMC free article] [PubMed] [Google Scholar]
- 24.Nau R, Wellmer A, Soto A, Koch K, Schneider O, Schmidt H, Gerber J, Michel U, Brück W. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J Infect Dis. 1999;179:1557–1560. doi: 10.1086/314760. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien D P, Briles D E, Szalai A J, Tu A H, Sanz I, Nahm M H. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect Immun. 1999;67:595–601. doi: 10.1128/iai.67.2.595-601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogle J D, Noel J G, Sramkoski R M, Ogle C K, Alexander J W. The effects of cytokines, platelet activating factor, and arachidonate metabolites on C3b receptor (CR1, CD35) expression and phagocytosis by neutrophils. Cytokine. 1990;2:447–455. doi: 10.1016/1043-4666(90)90054-w. [DOI] [PubMed] [Google Scholar]
- 27.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasparakis M, Kousteni S, Peschon J, Kollias G. Tumor necrosis factor and the p55TNF receptor are required for optimal development of the marginal sinus and for the migration of follicular dendritic cell precursors into splenic follicles. Cell Immunol. 2000;201:33–41. doi: 10.1006/cimm.2000.1636. [DOI] [PubMed] [Google Scholar]
- 29.Patterson L T, Higginbotham R D. Mouse C-reactive protein and endotoxin-induced resistance. J Bacteriol. 1965;90:1520–1524. doi: 10.1128/jb.90.6.1520-1524.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 31.Pfeffer K, Matsuyama T, Kündig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T W. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 32.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H, Zysk G, Reinert R R, Brück W, Stringaris A, Stuertz K, Bartels R, Schaper K, Weinig S, Nau R. Rifabutin for experimental pneumococcal meningitis. Chemotherapy. 1997;43:264–271. doi: 10.1159/000239577. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt H, Tlustochowska A, Stuertz K, Djukic M, Gerber J, Schütz E, Kuhnt U, Nau R. Organotypic hippocampal cultures—a model of brain tissue damage in Streptococcus pneumoniae meningitis. J Neuroimmunol. 2001;113:30–39. doi: 10.1016/s0165-5728(00)00402-1. [DOI] [PubMed] [Google Scholar]
- 35.Schumann J, Bluethmann H, Tiegs G. Synergism of Pseudomonas aeruginosa exotoxin A, superantigen, or TNF results in TNFR1- and TNFR2-dependent liver toxicity in mice. Immunol Lett. 2000;74:165–172. doi: 10.1016/s0165-2478(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 36.Sharief M K, Ciardi M, Thompson E J. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-alpha but not interleukin-1 beta. J Infect Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 37.Siboo R, Kulisek E. A fluorescent immunoassay for the quantification of C-reactive protein. J Immunol Methods. 1978;23:59–67. doi: 10.1016/0022-1759(78)90109-6. [DOI] [PubMed] [Google Scholar]
- 38.Sriskandan S, Cohen J. Gram-positive sepsis. Mechansims and differences from gram-negative sepsis. Infect Dis Clin N Am. 1999;13:397–412. doi: 10.1016/s0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- 39.Szalai A J, Briles D E, Volanakis J E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 40.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Täuber M G, Borschberg U, Sande M A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988;157:456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- 42.Trostdorf F, Reinert R R, Schmidt H, Nichterlein T, Stuertz K, Schmitz-Salue M, Sadowski I, Brück W, Nau R. Quinupristin/dalfopristin attenuates the inflammatory response and reduces the concentration of neuron-specific enolase in the cerebrospinal fluid of rabbits with experimental Streptococcus pneumoniae meningitis. J Antimicrob Chemother. 1999;43:87–94. doi: 10.1093/jac/43.1.87. [DOI] [PubMed] [Google Scholar]
- 43.Tsai Y H, Williams E B, Hirth R S, Price K E. Pneumococcal meningitis—therapeutic studies in mice. Chemotherapy. 1975;21:342–357. doi: 10.1159/000221879. [DOI] [PubMed] [Google Scholar]
- 44.Van Strijp J A, van der Tol M E, Miltenburg L A, van Kessel K P, Verhoef J. Tumour necrosis factor triggers granulocytes to internalize complement-coated virus particles. Immunology. 1991;73:77–82. [PMC free article] [PubMed] [Google Scholar]