Abstract
Objective: To explore the relationship between serum circular RNA ERBB2 (CircERBB2) levels and clinicopathological features and prognosis of patients with non-small cell lung cancer (NSCLC). Methods: In this retrospective study, 87 patients with NSCLC (NSCLC group) and 85 patients with benign lung disease (benign lung disease group) in the First Affiliated Hospital of Yangtze University from March 2016 to February 2019 were enrolled; in addition, another 87 healthy subjects were selected as the control group. Serum levels of CircERBB2, cytokeratin 19 fragment (CYFRA21-1) and carcinoembryonic antigen (CEA) in each group were detected, and their relationship with the prognosis of NSCLC patients as well as their diagnostic value was analyzed. The serum CircERBB2 levels in NSCLC patients with different clinicopathological characteristics were compared, and the correlation of serum level of CircERBB2 with CircERBB2 as well as CYFRA21-1 was analyzed. Results: The serum CircERBB2, CYFRA21-1 and CEA levels in the control group, benign lung disease group and NSCLC group were gradually increased (P<0.05). In the NSCLC group, serum CircERBB2 level was correlated with lymph node metastasis, tumor diameter and TNM stage (P<0.05). The areas under the curve (AUCs) of serum CircERBB2, CYFRA21-1, and CEA in diagnosing NSCLC were 0.871, 0.693, and 0.861, with cut-off values of 2.27, 4.45 ng/mL, and 18.49 ng/mL, respectively. The sensitivity was 80.5%, 65.5%, 78.2%, the specificity was 87.1%, 74.1%, 88.2%, respectively. The AUC and sensitivity of CircERBB2 combined with CEA in diagnosing NSCLC was 0.938 and 94.3%, respectively. The serum CircERBB2 level was positively correlated with CYFRA21-1 and CEA in the NSCLC group (P<0.05); and the levels of the above three serum indexes were significantly higher in the death group than those in the survival group (all P<0.05). The 36-month cumulative survival rate of patients in the CircERBB2 low expression group was longer than that in the CircERBB2 high expression group (P<0.05). Conclusion: Serum CircERBB2 is highly expressed in NSCLC patients, and closely related to disease progression, so it has good prognostic value.
Keywords: Circular RNA ERBB2, non-small cell lung cancer, clinicopathological features, diagnosis, prognosis
Introduction
Due to the lack of effective clinical screening methods, most non-small cell lung cancer (NSCLC) patients are likely to miss the optimal timing for surgery, thus the prognosis is poor [1-3]. Pathological biopsy is the gold standard for diagnosing NSCLC, but it cannot be used for early diagnosis due to its invasive nature. Traditional tumor markers, such as cytokeratin-19-fragment (CYFRA21-1) and carcinoembryonic antigen (CEA), are useful in judging disease progression; however, their diagnostic value is limited [4,5]. Therefore, it is of great significance to find serum markers that can predict disease progression and prognosis as well as assist clinical diagnosis. Some circular RNAs (circRNA) are dysregulated in NSCLC that have potential value in diagnosing NSCLC and assessing the prognosis of NSCLC [6,7]. Circular RNA ERBB2 (CircERBB2), a member of the circRNA family, is upregulated in lung cancer and may be a valuable therapeutic target [8]. Based on this, we aimed to investigate the diagnostic value of CircERBB2 for NSCLC by measuring the serum CircERBB2 level in NSCLC patients, and to analyze its relationship with the clinicopathological features of NSCLC.
Materials and methods
General information
In this retrospective study, 87 patients with NSCLC admitted to the First Affiliated Hospital of Yangtze University from March 2016 to February 2019 were selected as the NSCLC group. In addition, 85 patients with benign lung diseases during the same period were included as the benign disease group, including 67 cases of pneumonia and 18 cases of pulmonary fibroma. Besides, 87 healthy subjects were included as the healthy controls.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Yangtze University (20160235).
Inclusion criteria
(1) NSCLC patients with pathological confirmation; (2) Patients with normal function of major organs; (3) Patients who underwent radical resection; (4) Patients without preoperative anti-tumor therapy, such as immunotherapy, chemotherapy, or molecular targeted therapy.
Exclusion criteria
(1) Patients with hematological diseases or other malignant tumors; (2) Patients with contraindications to surgery; (3) Patients with poor compliance and incomplete examination data; (4) Patients with psychiatric diseases or immune system diseases; (5) Patients with pneumonia, tuberculosis, sepsis or other infectious diseases; or (6) Patients who were lost to follow-up.
Methods
Sample collection
The fasting peripheral blood (4-6 mL) was drawn from each participant and centrifuged for 5 min at 5500 r/min. The obtained serum was stored in aliquots (-70°C freezer).
qRT-PCR
The serum was thawed on ice, and total RNA was extracted with Trizol LS Reagent (Invitrogen, 10296010). cDNA was prepared using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, K1621). Subsequently, cDNA was amplified by qRT-PCR instrument (ABI, 7300 Plus), and the cycle threshold (CT) was determined. GAPDH was used as the internal reference, and it was calculated by the 2-∆∆CT method. The primer sequences are shown in Table 2.
Table 2.
Primers used for qPCR
| Primer | Sequence |
|---|---|
| CircERBB2 | F: 5’-ACGTTTGAGTCCATGCCCAA-3’ |
| R: 5’-TTGTGAGCGATGAGCACGTA-3’ | |
| GAPDH | F: 5’-CTCTCTGCTCCTCCTGTTCGACAG-3’ |
| R: 5’-AGGGGTCTTACTCCTTGGAGGCCA-3’ |
Abbreviation: CircERBB2, circular RNA ERBB2; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Electrochemiluminescence
The serum CYFRA21-1 and CEA levels were detected by electrochemiluminescence after thawing samples at a suitable temperature [electrochemiluminescence instrument (Roche, Cobas e602)].
Follow up
The 87 NSCLS patient were followed up for 7-36 months from the day after the operation and follow up ended on the day of death or February 2022. The follow-up was carried out by telephone or home surveys. According to the survival data, the NSCLC patients were divided into a survival group (47 cases) and a death group (40 cases).
Statistical analysis
SPSS 25.0 was used for data analysis. Count data were expressed by number (%) and analyzed using chi-square test. The measurement data were expressed as mean ± standard deviation and compared among three groups using ANOVA, while the pairwise comparison was conducted using t-test. Pearson method was used to for correlation analysis. The diagnostic value was evaluated by receiver operating characteristic (ROC) curve and compared by Z test. The relationship between CircERBB2 level and the prognosis of NSCLC patients was analyzed by Kaplan-Meier (K-M) and log-rank test. P<0.05 was considered statistically significant.
Results
Comparison of baseline data among the three groups of patients
There was no statistical difference among the three groups in terms of age, gender, smoking history, underlying diseases such as hypertension, hyperlipidemia and diabetes etc. (P>0.05, Table 1).
Table 1.
Comparison of general data among the three groups
| Group | n | Male/female | Age (<60/≥60 years) | Smoking (Yes/No) | Hyperlipidemia | Diabetes | Hypertension | Age (years) |
|---|---|---|---|---|---|---|---|---|
| Control group | 87 | 50/37 | 41/46 | 45/42 | - | - | - | 58.93±6.07 |
| Benign lung disease group | 85 | 49/36 | 40/45 | 47/38 | 5 | 8 | 6 | 59.78±6.21 |
| NSCLC group | 87 | 53/34 | 39/48 | 51/36 | 6 | 10 | 9 | 60.56±6.80 |
| χ2/F | - | 0.270 | 0.119 | 0.837 | 0.074 | 0.199 | 0.583 | 1.425 |
| P | - | 0.874 | 0.942 | 0.658 | 0.786 | 0.656 | 0.445 | 0.242 |
NSCLC, non-small cell lung cancer.
Comparison of serum CircERBB2, CYFRA21-1 and CEA levels among the three groups
Compared to the control group, the serum CircERBB2, CYFRA21-1 and CEA levels in the benign lung disease group and the NSCLC group were increased (all P<0.05) with significantly higher levels in the NSCLC group (P<0.05) (Table 3).
Table 3.
Comparison of serum CircERBB2, CYFRA21-1 and CEA levels among three groups (mean ± SD)
| Group | n | CircERBB2 | CYFRA21-1 (ng/mL) | CEA (ng/mL) |
|---|---|---|---|---|
| Control group | 87 | 1.01±0.34 | 0.78±0.26 | 3.36±1.12 |
| Benign lung disease group | 85 | 1.63±0.54a | 2.45±0.82a | 10.69±3.56a |
| NSCLC group | 87 | 2.92±1.05a,b | 6.31±1.58a,b | 27.23±6.22a,b |
| F | - | 163.503 | 646.382 | 739.504 |
| P | - | <0.001 | <0.001 | <0.001 |
Compared with the control group, P<0.05;
compared with the benign lung disease group, P<0.05.
Abbreviation: CircERBB2, circular RNA ERBB2; CYFRA21-1, cytokeratin-19-fragment; CEA, carcinoembryonic antigen.
Comparison of serum CircERBB2 expression in NSCLC patients with different clinicopathological features
As shown in Table 4, the serum CircERBB2 level was significantly higher in NSCLC patients with tumor diameter >4 cm, lymph node metastasis, and stage III disease (all P<0.05), but there was no statistical difference in patients with different age, gender, smoking history, differentiation degree or pathological type.
Table 4.
Comparison of serum CircERBB2 expression levels in NSCLC patients with different clinicopathological features (mean ± SD)
| Clinical information | Case (n=87) | CircERBB2 | t | P |
|---|---|---|---|---|
| Gender | 1.657 | 0.101 | ||
| Male | 53 | 3.07±1.12 | ||
| Female | 34 | 2.69±0.91 | ||
| Age | 1.604 | 0.113 | ||
| <60 years old | 39 | 2.72±0.92 | ||
| ≥60 years old | 48 | 3.08±1.13 | ||
| Smoking | 1.817 | 0.073 | ||
| Yes | 51 | 2.75±0.99 | ||
| No | 36 | 3.16±1.10 | ||
| Differentiation | 0.856 | 0.395 | ||
| Low differentiated | 32 | 3.05±1.12 | ||
| Medium and high differentiated | 55 | 2.85±1.01 | ||
| Tumor diameter | 3.749 | <0.001 | ||
| ≤4 cm | 44 | 2.53±0.84 | ||
| >4 cm | 43 | 3.32±1.11 | ||
| TNM staging | 2.939 | 0.004 | ||
| Phase I~II | 57 | 2.69±0.89 | ||
| Phase III | 30 | 3.36±1.21 | ||
| Pathological type | 0.778 | 0.439 | ||
| Adenocarcinoma | 60 | 2.86±1.08 | ||
| Squamous cell carcinoma | 27 | 3.05±0.99 | ||
| Lymph node metastasis | 2.787 | 0.007 | ||
| Yes | 38 | 3.26±1.10 | ||
| No | 49 | 2.65±0.94 |
NSCLC, non-small cell lung cancer.
The diagnostic value of serum CircERBB2, CYFRA21-1 and CEA for NSCLC
In the diagnosis of NSCLC, the AUC of serum CircERBB2 and CEA was 0.871 and 0.861 respectively, which were higher than the 0.693 of CYFRA21-1 (ZCircERBB2-CYFRA21-1, ZCEA-CYFRA21-1=3.646, 3.360, P<0.001, P=0.001, respectively), and the AUC of combined detection of CircERBB2 and CEA was 0.938, which was higher than each of the two alone (Zcombined-CircERBB2, Zcombined-CEA=2.013, 2.201, P=0.044, 0.028, respectively). The sensitivity and specificity of the combined detection was 94.3% and 85.9%, respectively (Table 5; Figure 1).
Table 5.
Diagnostic efficacy of each index for NSCLC
| Variables | AUC | 95% CI | Cutoff value | Sensitivity (%) | Specificity (%) | Youden Index |
|---|---|---|---|---|---|---|
| CircERBB2 | 0.871 | 0.815-0.926 | 2.27 | 80.5 | 87.1 | 0.676 |
| CYFRA21-1 | 0.693 | 0.614-0.772 | 4.45 ng/mL | 65.5 | 74.1 | 0.396 |
| CEA | 0.861 | 0.803-0.919 | 18.49 ng/mL | 78.2 | 88.2 | 0.664 |
| Joint detection of CircERBB2 and CEA | 0.938 | 0.902-0.974 | - | 94.3 | 85.9 | 0.802 |
NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen.
Figure 1.
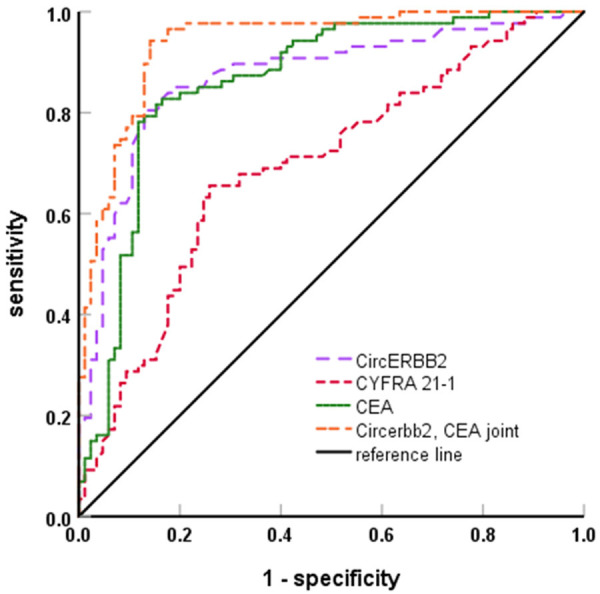
ROC curve of serum CircERBB2, CYFRA21-1 and CEA in the diagnosis of NSCLC. NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen.
Correlation of serum CircERBB2 level with CYFRA21-1 and CEA
Pearson correlation analysis showed that there was a positive correlation between the expression of CircERBB2 and CYFRA21-1 as well as CEA in NSCLC patients (r=0.555, 0.576, P<0.05) (Figure 2).
Figure 2.
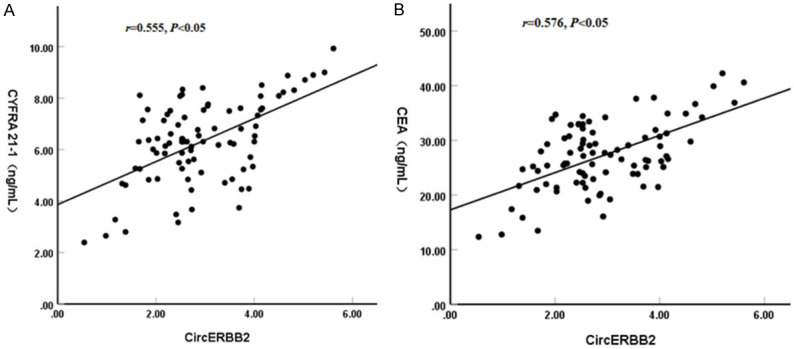
Correlation between serum CircERBB2 expression and CYFRA21-1, CEA in NSCLC patients. A. Relation between CircERBB2 and CYFRA21-1. B. Relation between CircERBB2 and CEA. NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen.
Comparison of serum CircERBB2, CYFRA21-1 and CEA levels with different prognosis
Compared with the survival group, the serum levels of CircERBB2, CYFRA21-1 and CEA were all increased in the death group (all P<0.05) (Table 6).
Table 6.
Comparison of serum CircERBB2, CYFRA21-1 and CEA levels in NSCLC patients with different prognosis outcome
| Group | n | CircERBB2 | CYFRA21-1 (ng/mL) | CEA (ng/mL) |
|---|---|---|---|---|
| Survival group | 47 | 2.56±0.86 | 5.65±1.63 | 24.63±5.95 |
| Death group | 40 | 3.29±1.14 | 7.08±1.12 | 30.27±5.08 |
| t | - | 3.399 | 4.685 | 4.709 |
| P | - | 0.001 | 0.049 | 0.010 |
NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen.
The relationship between serum CircERBB2 expression and prognosis of NSCLC patients
Based on the median value of serum CircERBB2 (2.71) in the NSCLC group, the NSCLC patients were divided into a CircERBB2 low expression group (n=44) and CircERBB2 high expression group (n=43). The mean survival time in the CircERBB2 low expression group was 34.21 months (95% CI: 32.76-35.65), which was significantly longer than the 24.67 months in the CircERBB2 high expression group (95% CI: 21.97-27.38). Compared with high-expression group, the cumulative survival rate of the CircERBB2 low-expression group was significantly higher (84.10% vs 23.3%) (Figure 3).
Figure 3.
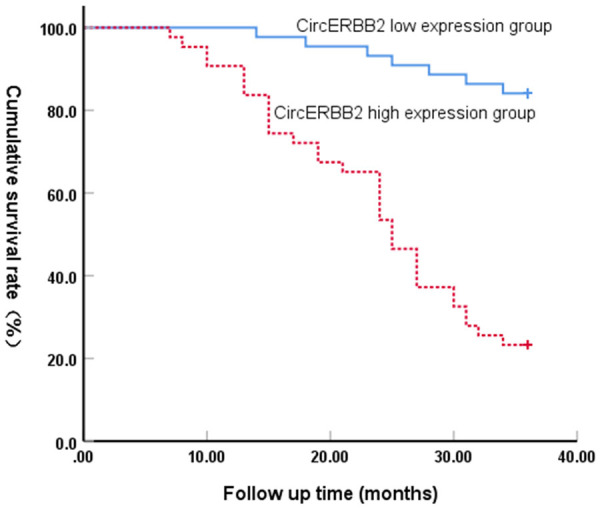
Relationship between serum CircERBB2 expression and prognosis of NSCLC. NSCLC, non-small cell lung cancer.
Discussion
NSCLC is a high morbidity type of tumor with a high degree of malignancy. Currently, surgery is still an important method for the treatment of NSCLC [9-11]. Therefore, it is of great significance to find markers for the early screening of NSCLC as well as the prediction of the prognosis of NSCLC patients.
Circular RNAs (cirRNA) can participate in protein translation and regulate the expression of miRNAs. It has been reported that cirRNAs are associated with the progression of asthma, pneumonia, NSCLC and other diseases [12-14]. Recent reports have shown that circRNA_001846 is up-regulated in NSCLC and presumably beneficial for clinical diagnosis [15]. In addition, circ_SEC31A was highly expressed in NSCLC patients, and is also a risk factor for poor prognosis of patients [16]. As a member of the circRNA family, CircERBB2 is overexpressed in gastric cancer, and its expression level is higher in the plasma of relapsed patients, making it a potential prognostic predictor [17]. CircERBB2 is elevated in the plasma of NSCLC patients, and its expression profile is similar to that in gastric cancer [17] and asthma [18], suggesting that the serum CircERBB2 expression level might be related to the pathological development of NSCLC.
In this study, by comparing serum CircERBB2 level in NSCLC patients with different clinicopathological characteristics, it was found that the serum CircERBB2 levels was correlated with tumor diameter, stage and lymph node metastasis of NSCLC patients, suggesting that serum CircERBB2 expression could be used for clinical assessment of disease severity and the development of individualized treatment plans for NSCLC patients. In this study, compared with the death group, serum CircERBB2 level was lower in the survival group, and the cumulative survival rate of low-CircERBB2 expression group was higher than that in the high-CircERBB2 expression group, suggesting that high serum CircERBB2 expression level is associated with poor prognosis of NSCLC patients. Gao et al. [8] explored the expression level and role of CircERBB2 in lung cancer from the perspective of cytology and histology, in which the relationship between CircERBB2 level and disease progression was analyzed. By comparing the diagnostic value of CircERBB2 and traditional tumor markers, it was found that CircERBB2 might be an auxiliary marker for diagnosing NSCLC and evaluating the prognosis of NSCLC patients.
CYFRA21-1 is an acidic protein widely present in breast, lung and other tissues. Its level in normal human peripheral blood is low and can maintain tissue stability. When tumor cells are necrotic and lysed, CYFRA21-1 can be released into the blood, which can be used as a marker for the diagnosis of NSCLC and other tumors [19-21]. CEA is an acidic glycoprotein located in the urinary tract and respiratory tract, and is a common indicator for clinical diagnosis of breast cancer, NSCLC and other tumors [22,23]. This study showed that the serum CYFRA21-1 and CEA levels in the NSCLC group were higher than the other two groups, which was in line with the findings of Li et al. [4] and Sun et al. [24]. These two indicators in the death group were also higher, suggesting that CYFRA21-1 and CEA might be related to disease progression. Moreover, serum CircERBB2 level was positively correlated with CYFRA21-1 and CEA by correlation analysis, further suggesting that CircERBB2 was significantly associated with the development of NSCLC, and CircERBB2 might be a therapeutic target for NSCLC. In addition, the diagnostic value of serum CircERBB2, CYFRA21-1 and CEA was analyzed by ROC curve. The results showed that the AUC of serum CircERBB2 and CEA in diagnosing NSCLC was 0.871 and 0.861, respectively, which were higher than the AUC of CYFRA21-1 in the diagnosis of NSCLC. Subsequently, the diagnostic value of combined detection of CircERBB2 and CEA for NSCLC was investigated, and the results showed an AUC of 0.938, a sensitivity of 94.3%, and a specificity of 85.9%, suggesting that the combination of CircERBB2 and CEA can well diagnose NSCLC, which provided a more effective method for early clinical diagnosis of NSCLC.
In conclusion, CircERBB2 is highly expressed in the serum of NSCLC patients and it is positively associated with the expression of CYFRA21-1 and CEA. The combined detection of CircERBB2 and CEA has high performance in diagnosing NSCLC and provides a new strategy for early clinical diagnosis. However, there are still some drawbacks in this study. First, this is a retrospective study with limited sample size (less than 100 case in each group), which may cause some selection bias. Second, the mechanism of CircERBB2 in NSCLC was not explored. We will expand the sample size and explore this underlying mechanism through in vivo animal experiments and in vitro cellular experiments in our future studies.
Disclosure of conflict of interest
None.
References
- 1.Xu C, Liu W, Li L, Wang Y, Yuan Q. Serum tumour M2-pyruvate kinase as a biomarker for diagnosis and prognosis of early-stage non-small cell lung cancer. J Cell Mol Med. 2021;25:7335–7341. doi: 10.1111/jcmm.16762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baran K, Brzezianska-Lasota E. Proteomic biomarkers of non-small cell lung cancer patients. Adv Respir Med. 2021;89:419–426. doi: 10.5603/ARM.a2021.0089. [DOI] [PubMed] [Google Scholar]
- 3.Dong X, Chang M, Song X, Ding S, Xie L, Song X. Plasma miR-1247-5p, miR-301b-3p and miR-105-5p as potential biomarkers for early diagnosis of non-small cell lung cancer. Thorac Cancer. 2021;12:539–548. doi: 10.1111/1759-7714.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Chen Y, Wang X, Wang C, Xiao M. The value of combined detection of CEA, CYFRA21-1, SCC-Ag, and pro-GRP in the differential diagnosis of lung cancer. Transl Cancer Res. 2021;10:1900–1906. doi: 10.21037/tcr-21-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieta O, Varela-Santoyo E, Cardona AF, Sanchez-Reyes R, Lara-Mejia L, Bassarmal SS, Valle-Bautista D, Corrales-Rodriguez L, Motola-Kuba D, Cabrera-Miranda L, Martin C. Association of carcinoembryonic antigen reduction with progression-free and overall survival improvement in advanced non-small-cell lung cancer. Clin Lung Cancer. 2021;22:510–522. doi: 10.1016/j.cllc.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Zhao D, Liu H, Liu H, Zhang X, Zhang M, Kolluri VK, Feng X, He Z, Wang M, Zhu T, Yan X, Zhou Y. Downregulated expression of hsa_circ_0037515 and hsa_circ_0037516 as novel biomarkers for non-small cell lung cancer. Am J Transl Res. 2020;12:162–170. [PMC free article] [PubMed] [Google Scholar]
- 7.Bao Q, Li F, Zheng H, Chen S, Song X. Prognostic role of dysregulated circRNAs in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2020;12:823–829. doi: 10.21037/jtd.2019.12.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Ding C, Zhou J, Wu G, Han Z, Li J, Hei F. Propofol suppresses lung cancer tumorigenesis by modulating the circ-ERBB2/miR-7-5p/FOXM1 axis. Thorac Cancer. 2021;12:824–834. doi: 10.1111/1759-7714.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y, Feng Y, Qiao T, Han Y. Identifying prognostic biomarkers of non-small cell lung cancer by transcriptome analysis. Cancer Biomark. 2020;27:243–250. doi: 10.3233/CBM-190222. [DOI] [PubMed] [Google Scholar]
- 10.Kajikawa S, Ohashi W, Kato Y, Fukami M, Yonezawa T, Sato M, Kosaka K, Kato T, Tanaka H, Ito S, Yamaguchi E, Kubo A. Prognostic impact of serum procalcitonin in non-small cell lung cancer. Tumori. 2021;107:385–391. doi: 10.1177/0300891620966647. [DOI] [PubMed] [Google Scholar]
- 11.Yong J, Huang L, Chen G, Luo X, Chen H, Wang L. High expression of Stabilin-2 predicts poor prognosis in non-small-cell lung cancer. Bioengineered. 2021;12:3426–3433. doi: 10.1080/21655979.2021.1943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Fu B, Qi X, Xu Y, Mou Y, Zhou M, Cao Y, Wu G, Xie J, Zhao J, Wang Y, Xiong W. Diagnostic and therapeutic value of Hsa_circ_0002594 for T helper 2-mediated allergic asthma. Int Arch Allergy Immunol. 2021;182:388–398. doi: 10.1159/000511612. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Yang T, Ding Z, Cao Y. Circ_0026579 alleviates LPS-induced WI-38 cells inflammation injury in infantile pneumonia. Innate Immun. 2022;28:37–48. doi: 10.1177/17534259211069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Sun D, Li X, Yang B, Zhang W. Identification of key circRNAs in non-small cell lung cancer. Am J Med Sci. 2021;361:98–105. doi: 10.1016/j.amjms.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Ma C, Qiu J, Feng X, Yang K. Identification of circRNA_001846 as putative non-small cell lung cancer biomarker. Bioengineered. 2021;12:8690–8697. doi: 10.1080/21655979.2021.1991161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin M, Shi C, Hua Q, Li T, Yang C, Wu Y, Zhao L, Yang H, Zhang J, Hu C, Huang G. High circ-SEC31A expression predicts unfavorable prognoses in non-small cell lung cancer by regulating the miR-520a-5p_GOT-2 axis. Aging (Albany NY) 2020;12:10381–10397. doi: 10.18632/aging.103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanishi K, Konishi H, Shoda K, Arita T, Kosuga T, Komatsu S, Shiozaki A, Kubota T, Fujiwara H, Okamoto K, Ichikawa D, Otsuji E. Circulating circERBB2 as a potential prognostic biomarker for gastric cancer: an investigative study. Cancer Sci. 2020;111:4177–4186. doi: 10.1111/cas.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang JQ, Wang F, Wang LT, Li YM, Lu JL, Chen JY. Circular RNA ERBB2 contributes to proliferation and migration of airway smooth muscle cells via miR-98-5p/IGF1R signaling in asthma. J Asthma Allergy. 2021;14:1197–1207. doi: 10.2147/JAA.S326058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao J, Liu W, Zhao C, Xia T. The diagnostic significance of 64-slice spiral CT combined with serological CA19-9, Bcl-2, CYFRA21-1 detection in thoracic esophageal carcinoma. Transl Cancer Res. 2021;10:5383–5389. doi: 10.21037/tcr-21-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Liu X, Shang X, Qi K, Zhang S. The diagnostic value of the combination of carcinoembryonic antigen, squamous cell carcinoma-related antigen, CYFRA21-1, neuron-specific enolase, tissue polypeptide antigen, and progastrin-releasing peptide in small cell lung cancer discrimination. Int J Biol Markers. 2021;36:36–44. doi: 10.1177/17246008211049446. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Yuan Q, Zou YT, Su T, Lin Q, Zhang YQ, Shi WQ, Liang RB, Ge QM, Li QY, Shao Y. CA-125, CA-153, and CYFRA21-1 as clinical indicators in male lung cancer with ocular metastasis. J Cancer. 2020;11:2730–2736. doi: 10.7150/jca.36238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farahani H, Amri J, Alaee M, Mohaghegh F, Rafiee M. Serum and saliva levels of cancer antigen 15-3, carcinoembryonic antigen, estradiol, vaspin, and obestatin as biomarkers for the diagnosis of breast cancer in postmenopausal women. Lab Med. 2020;51:620–627. doi: 10.1093/labmed/lmaa013. [DOI] [PubMed] [Google Scholar]
- 23.Cheng C, Yang Y, Yang W, Wang D, Yao C. The diagnostic value of CEA for lung cancer-related malignant pleural effusion in China: a meta-analysis. Expert Rev Respir Med. 2022;16:99–108. doi: 10.1080/17476348.2021.1941885. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Chen X, Wang Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol Lett. 2020;20:3046–3052. doi: 10.3892/ol.2020.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]


