Abstract
Objective: To observe the predictive values of plasma prothrombin time (PT), fibrinogen (FIB), activated partial thromboplastin time (APTT) and D-dimer (DD) levels for pregnancy outcome in parturients with hypertensive disorder complicating pregnancy (HDCP). Methods: A retrospective analysis was conducted on 107 parturients with gestational hypertension admitted to Xi’an International Medical Center Hospital from April 2018 to April 2021 (research group) and on 50 healthy parturients who underwent physical examination in the same period (control group). PT, FIB, APTT, and DD values of all parturients included in the study were examined at admission, and pregnancy outcomes were recorded. The working curve (ROC) of the relationship between coagulation function test indicators and pregnancy outcomes of parturients in the research group was analyzed. Results: Compared to the control group, PT and APTT values of parturients in the research group were lower, while FIB and DD levels were markedly higher (P < 0.05). Correlation analysis showed APTT and PT were negatively correlated with the severity of disease (both P < 0.001), while the expression of FIB and DD were positively correlated with it (both P < 0.001). Parturients were divided into an adverse outcome group and a normal outcome group. Logistic regression analysis showed that pre-pregnancy body mass index, PT, APTT, FIB, DD and other indicators were all risk factors for adverse outcome in HDCP parturients. ROC curve analysis showed that the area under the curve of these combined risk factors for predicting adverse outcome was 0.971. Conclusion: Levels of PT, FIB, APTT, and DD are abnormal in parturients with different degrees of HDCP. Regular coagulation function tests can effectively detect HDCP, enabling improvement of pregnancy outcome.
Keywords: Plasma prothrombin time, fibrinogen, activated partial thromboplastin time, D-dimer, hypertensive disorder complicating pregnancy, pregnancy outcome
Introduction
Pregnancy is a special physiologic period for women, and various physiologic measures change in order to facilitate the growth of the fetus and the delivery at a later stage [1]. Hypertensive disorder complicating pregnancy (HDCP) is a group of obstetric-specific diseases that seriously threaten maternal and fetal health [2]. Epidemiologic statistics show that the global incidence is around 7%-13%, and it accounts for 6%-10% of pregnant women in Europe and the United States and 5%-12% in China. HDCP has a very high mortality rate (0.042‰), and is one of the main causes of maternal and perinatal morbidity and mortality [3]. Statistically, the number of maternal deaths caused by HDCP accounts for 10%-16% of the total worldwide, while 50,000-60,000 maternal deaths are caused by preeclampsia of HDCP each year [4]. It has been revealed [5] that HDCP may lead to serious adverse pregnancy outcome. Therefore, in the course of hypertensive disorder complicating pregnancy, timely prediction, close attention to preeclampsia, and early intervention are critical to reduce harm and mortality.
D-dimer (DD) is a specific degradation product of cross-linked fibrin produced by the fibrinolytic system during coagulation and can be used as a molecular marker of a hypercoagulable state and secondary hyperfibrinolysis in vivo [6]. At present, DD level is considered an essential indicator for screening for deep venous thrombosis or pulmonary thrombosis, and for monitoring thrombolytic therapy [7]. Studies have found that [8] the expression of DD has in predicting pregnancy outcome. Increased sex hormone levels affect maternal coagulation function, causing a hypercoagulable state in the third trimester of pregnancy [9]. This state functions to stop bleeding promptly during parturition and to promote the formation of arterial and venous intravascular thrombi during hemostasis, which is very beneficial for the regeneration of the endometrium at a later stage [10,11]. However, if the body’s function is unbalanced, the blood coagulation level will be excessively high, causing adverse consequences such as pregnancy-induced hypertension, disseminated intravascular coagulation, and pulmonary embolism [12]. As routine coagulation tests, plasma prothrombin time (PT), fibrinogen (FIB), and activated partial thromboplastin time (APTT) are associated with HDCP [13]. However, it is not clear whether PT, FIB, and APTT have significance in pregnancy outcome.
At present, the risk factors for HDCP can be determined as age, Body Mass Index (BMI), and others, while there is controversy about the significance of coagulation values in HDCP. The aim of this study was to analyze the predictive value of PT, FIB, APTT, and DD for maternal pregnancy outcome in HDCP and provide a reference for disease observation and prognosis.
Methods and materials
Clinical information
A retrospective analysis was conducted on 107 parturients with gestational hypertension admitted to Xi’an International Medical Center Hospital from April 2018 to April 2021 (research group) and on 50 healthy parturients who underwent physical examination in the same period (control group). This study was approved by the Medical Ethics Committee of Xi’an International Medical Center Hospital (Ethical batch No.: 20190441).
Inclusion and exclusion criteria
Inclusion criteria: Parturients who were diagnosed with hypertensive disorder complicating pregnancy in Xi’an International Medical Center Hospital after the 20th week of pregnancy, which was in line with the relevant diagnostic guidelines of the German Society of Obstetrics and Gynecology (DGGG) in 2013 [14]; parturients who underwent prenatal examination and delivery in Xi’an International Medical Center Hospital; parturients with complete clinical data.
Exclusion criteria: Number of fetuses > 2; parturients with an age < 18 years; parturients with cardiovascular disease and abnormal liver and kidney function; parturients with poor compliance; or parturients with communication disorders.
Test method
Fasting peripheral venous blood (3 ml) was taken andplaced in a sodium citrate anticoagulant tube, and the blood was fully mixed with 0.109 mol/L sodium citrate at a ratio of 9:1. After being centrifuged at 3,000 r/min for 10 min, the upper serum was taken, and PT, FIB, APTT, and DD were detected within 2 h. DD was detected by immunoturbidimetry with the kit provided by Sysmex Medical Electronics (Shanghai) Co., Ltd. The procedures were in strict accordance with the instructions of the kit. PT, FIB, and APTT were detected by Sysmex CA-8000 automatic coagulation analyzer. All maternal pregnancy outcomes were recorded, including abnormal intrapartum hemorrhage, postpartum hemorrhage, premature delivery, placental abruption, and neonatal asphyxia.
Outcome measures
Main outcome measures
The expression of PT, FIB, APTT, and DD in the research group and the control group was observed. According to maternal pregnancy outcome, parturients were divided into an adverse pregnancy outcome group and a normal pregnancy outcome group. Logistic regression was used to analyze the risk factors of adverse maternal outcomes, and the prediction curves were plotted.
Secondary outcome measures
The clinical data of the two groups were compared. According to HDCP grade, the parturients were divided into a mild group, a moderate group and a severe group. The expression of each index according to different HDCP degrees was compared. The correlation between each index and the degree of HDCP was analyzed by Spearman test.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp, Armonk, NY, USA). The enumerated data were expressed as [n (%)], and were compared by chi-square test. The measured data were expressed as mean ± standard deviation (means ± sd), and were compared by Student t-test. Logistic regression was used to analyze the risk factors for pregnancy outcomes. Receiver operating curve (ROC) was used to analyze the clinical value of various indicators in predicting HDCP. The correlation between HDCP and PT, FIB, APTT, and DD was analyzed by Spearman analysis and expressed as r. Comparisons between multiple groups were performed using one-way analysis of variance and post hoc tests using LSD-t tests. Sample size was calculated using the formula:
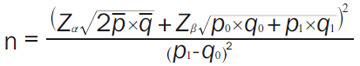 |
RR value was 0.5, and α = 0.05 and β = 0.1 were set. We first found that the incidence of adverse pregnancy outcomes in pregnant women with pregnancy-induced hypertension ranged from 35% to 47% through a literature search, and we took 41% as the average incidence for this study. Subsequently, we substituted the formula to calculate a total of 104 people. Considering that there may be an incomplete increase in sample data by 10%, a need for a total of 114 people was estimated, and a total of 57 people was estimated for the control group according to the ratio of 1:0.5. We included a total of 107 patients and 50 healthy people as samples in this study in combination with the actual clinical situation. A difference considered significant when P < 0.05.
Results
Clinical data
Comparison of the clinical data between the two groups suggested no statistical difference in terms of age, BMI, parity, mode of delivery, smoking history, or alcoholism history (P > 0.05), but there was a statistical difference in gestational age at delivery (P < 0.001, Table 1).
Table 1.
Baseline data comparison
| Variable | Control Group (n = 50) | Research Group (n = 107) | χ2 value | P value |
|---|---|---|---|---|
| Age | ||||
| ≥ 30 years | 17 | 35 | 0.025 | 0.872 |
| < 30 years | 33 | 72 | ||
| Prenatal BMI | ||||
| ≥ 23 kg/m2 | 31 | 57 | 1.054 | 0.305 |
| < 23 kg/m2 | 19 | 50 | ||
| Gestational age | ||||
| ≥ 36 weeks | 42 | 59 | 12.369 | < 0.001 |
| < 36 weeks | 8 | 48 | ||
| Parity | ||||
| Primipara | 22 | 50 | 0.102 | 0.749 |
| Multiparous | 28 | 57 | ||
| Mode of delivery | ||||
| Natural delivery | 23 | 57 | 0.720 | 0.395 |
| Cesarean section | 27 | 50 | ||
| Smoking history | ||||
| Yes | 8 | 12 | 0.709 | 0.402 |
| No | 42 | 95 | ||
| History of alcoholism | ||||
| Yes | 3 | 5 | 0.143 | 0.705 |
| No | 47 | 104 |
BMI: Body Mass Index.
Comparison of expression of PT, FIB, APTT, and DD between the research group and control group
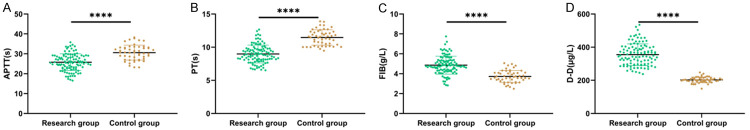
The levels of PT and APTT in the research group were lower than those of the control group, while its FIB and DD levels were higher than those of the control group (P < 0.05, Figure 1).
Figure 1.
Comparison of serum levels of APTT, PT, FIB, and DD between the two groups. A. Comparison of APTT between control group and research group. B. Comparison of PT between the control group and research group. C. Comparison of FIB level between control group and research group. D. Comparison of DD level between control group and research group. Note: **** means P < 0.0001. PT: Plasma Prothrombin Time; FIB: Fibrinogen; APTT: Activated Partial Thromboplastin Time; DD: D-Dimer; HDCP: Hypertensive Disorders of Pregnancy.
Expression of PT, FIB, APTT, and DD in parturients with different degrees of HDCP
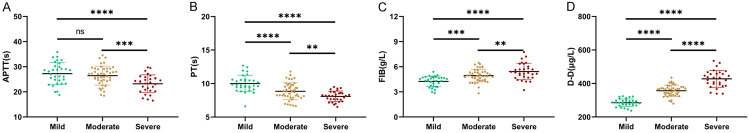
We further divided the parturients of the research group as mild (n = 32), moderate (n = 45), or severe (n = 30) according to their degree of HDCP. The levels of PT, FIB, APTT, and DD were further compared between the sub-groups and results indicated no difference in APTT between mild group and moderate group (P > 0.05). The APTT of the mild group and moderate group was higher than that of the severe group (P < 0.001). PT gradually decreased with increasing severity (P < 0.01), while FIB and DD rose with increasing severity (P < 0.01, Figure 2).
Figure 2.
Expression of APTT, PT, FIB, and DD in parturients with different degrees of HDCP. A. APTT in parturients of different severity of HDCP. B. PT in parturients of different severity of HDCP. C. FIB in parturients of different severity of HDCP. D. DD in parturients of different severity of HDCP. Note: ** means P < 0.01, *** means P < 0.001, **** means P < 0.0001. PT: Plasma Prothrombin Time; FIB: Fibrinogen; APTT: Activated Partial Thromboplastin Time; DD: D-Dimer; HDCP: Hypertensive Disorder of Pregnancy.
Correlation of PT, FIB, APTT, and DD with different degrees of HDCP
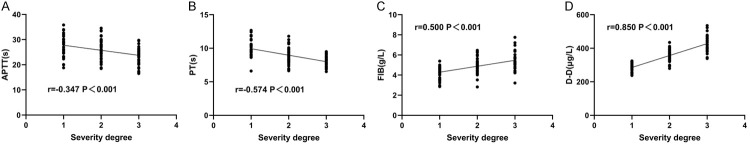
Spearman test was used to analyze the correlation between PT, FIB, APTT, and DD and different degrees of HDCP. The results showed that APTT and PT were negatively correlated with the severity of disease (both P < 0.001, Figure 3A, 3B), while the expression of FIB and DD were positively correlated with it (both P < 0.001, Figure 3C, 3D).
Figure 3.
Correlation between PT, FIB, APTT, and DD and severity of illness. A. APTT was associated with the degree of HDCP. B. PT was associated with the degree of HDCP. C. FIB correlated with the degree of HDCP. D. DD was associated with the degree of HDCP. Note: Plasma prothrombin time (PT), fibrinogen (FIB), activated partial thromboplastin time (APTT), D-dimer (DD), hypertensive disorder complicating pregnancy (HDCP), X-axis 1 indicates mild, 2 indicates moderate, 3 indicates severe, and 0 and 4 are not significant.
Analysis of risk factors of pregnancy outcome
According to pregnancy outcome, there were 16 cases of fetal growth restriction, 13 cases of fetal distress and 18 cases of premature infants in HDCP patients. Subsequently, patients were grouped into adverse pregnancy outcome group (n = 47) and normal pregnancy outcome group (n = 60). Univariate analysis revealed that age, prenatal BMI, gestational age at delivery, disease severity, PT, FIB, APTT and DD were all risk factors affecting adverse pregnancy outcomes in patients (Table 2, P < 0.05). Then, by multivariate logistic regression analysis, we identified that age, prenatal BMI, gestational age at delivery, PT, APTT, and DD were independent risk factors affecting adverse pregnancy outcomes (Table 3, P < 0.05). Furthermore, ROC curve was plotted for significant independent risk factors, and it was revealed that only the area under the curve of DD was > 0.8 among independent predictors, while the area under the combined curve was 0.971, which is an ideal predictor (Figure 4).
Table 2.
Univariate analysis of adverse pregnancy outcome
| Variable | Adverse pregnancy outcome group (n = 47) | Normal pregnancy outcome group (n = 60) | χ2 value | P value |
|---|---|---|---|---|
| Age | ||||
| ≥ 30 years old (n = 35) | 25 | 10 | 15.974 | < 0.001 |
| < 30 years old (n = 72) | 22 | 50 | ||
| Prenatal BMI | ||||
| ≥ 23 kg/m2 (n = 57) | 37 | 20 | 21.813 | < 0.001 |
| < 23 kg/m2 (n = 50) | 10 | 40 | ||
| Gestational age | ||||
| ≥ 36 weeks (n = 59) | 17 | 42 | 12.194 | 0.006 |
| < 36 weeks (n = 48) | 30 | 18 | ||
| Parity | ||||
| Primipara (n = 50) | 20 | 30 | 0.587 | 0.444 |
| Multiparous (n = 57) | 27 | 30 | ||
| Mode of delivery | ||||
| Natural delivery (n = 57) | 23 | 34 | 0.632 | 0.426 |
| Cesarean section (n = 50) | 24 | 26 | ||
| Smoking history | ||||
| Yes (n = 12) | 5 | 7 | 0.027 | 0.867 |
| No (n = 95) | 42 | 53 | ||
| History of alcoholism | ||||
| Yes (n = 5) | 4 | 1 | 2.771 | 0.096 |
| No (n = 104) | 43 | 59 | ||
| Degree of illness | ||||
| Mild (n = 32) | 10 | 22 | 14.670 | 0.001 |
| Moderate (n = 45) | 15 | 30 | ||
| Severe (n = 30) | 22 | 8 | ||
| PT (s) | 8.37±0.92 | 9.46±1.43 | 4.503 | < 0.001 |
| FIB (g/L) | 5.11±0.86 | 4.67±0.89 | 2.606 | 0.011 |
| APTT (s) | 23.81±3.29 | 27.36±4.22 | 4.860 | < 0.001 |
| DD (μg/L) | 401.83±47.82 | 318.71±52.71 | 8.429 | < 0.001 |
Note: BMI: Body Mass Index; PT: Plasma Prothrombin Time; FIB: Fibrinogen; APTT: Activated Partial Thromboplastin Time; DD: D-dimer.
Table 3.
Logistic multivariate risk analysis of adverse pregnancy outcome
| Variable | β value | Standard error | χ2 | P value | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| Age | 2.546 | 0.988 | 6.638 | 0.010 | 12.751 | 1.839 | 88.427 |
| Prenatal BMI | 2.566 | 1.006 | 6.509 | 0.011 | 13.009 | 1.812 | 93.372 |
| Gestational age | 2.631 | 1.030 | 6.532 | 0.011 | 13.893 | 1.847 | 104.516 |
| Degree of illness | 0.316 | 0.523 | 0.365 | 0.546 | 1.372 | 0.492 | 3.821 |
| PT | -1.351 | 0.441 | 9.368 | 0.002 | 0.259 | 0.109 | 0.615 |
| FIB | 0.275 | 0.461 | 0.355 | 0.551 | 1.317 | 0.533 | 3.253 |
| APTT | -0.271 | 0.112 | 5.858 | 0.016 | 0.762 | 0.612 | 0.950 |
| DD | 0.030 | 0.008 | 13.531 | < 0.001 | 1.030 | 1.014 | 1.047 |
Note: PT: Plasma Prothrombin Time; FIB: Fibrinogen; APTT: Activated Partial Thromboplastin Time; DD: D-dimer.
Figure 4.
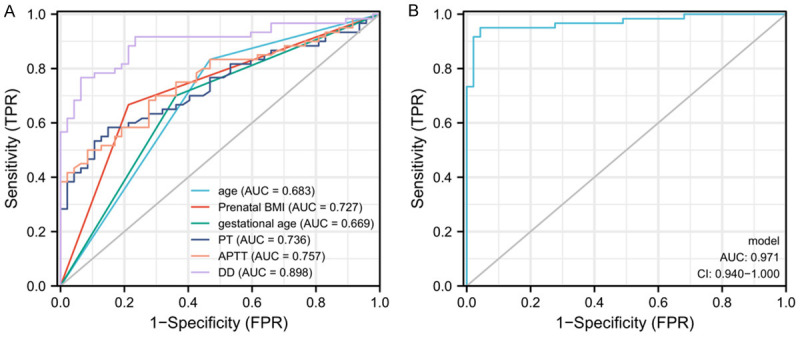
ROC curve analysis of independent risk factors in predicting adverse maternal pregnancy outcome. A. ROC curve analysis of each independent risk factor in predicting adverse maternal pregnancy outcome. B. ROC curve analysis of combined detection in predicting adverse maternal pregnancy outcome. Note: PT: Plasma Prothrombin Time; FIB: Fibrinogen; APTT: Activated Partial Thromboplastin Time; DD: D-Dimer; HDCP: Hypertensive Disorder Complicating Pregnancy; AUC: Area Under The Curve.
Discussion
Pregnancy is a physiologic process from conception to delivery, that takes a long time and can be affected by many interfering factors, and pregnant women are prone to complications from adverse factors [15]. Hypertension in pregnancy is a multiple obstetric disease that occurs mostly after 20 weeks of gestation and is specifically characterized by elevated maternal blood pressure, which can lead to spiral arterial spasm in the uterus if the hypertensive state is maintained for a long time and adversely affects the placental blood oxygen supply, resulting in intrauterine asphyxia or dysplasia of the fetus [16,17]. Therefore, it is of great significance to identify indicators to observe the condition of HDCP and predict the occurrence of adverse events.
Women in their childbearing age experience corresponding physiologic changes in their bodies with the occurrence of pregnancy, and among these changes, the most obvious ones are in coagulation function and blood circulation [18,19]. Studies have found that the blood system coagulation and anticoagulation in the body remain in a relatively dynamic balance under normal circumstances. When coagulation imbalance occurs due to in vivo or in vitro factors, patients are at high risk of hypercoagulation, If not treated timely this can cause thrombosis in the body and even death [20]. Many studies have found that the blood of pregnant women will be in a pathologic hypercoagulable state, while the dilatation of the veins and the increase of blood volume will compress the inferior vena cava, thus blocking the patient’s blood return, slowing blood flow, and even leading to the deterioration of varicose veins [21,22]. When the hypercoagulable state exceeds the normal range, it is prone to develop into HDCP, resulting in thrombosis, intrauterine growth restriction, placental abruption, and even intrauterine fetal death [23]. This suggests a possible link between coagulation values and HDCP.
APTT reflects the endogenous coagulation system and is a relatively sensitive screening index for clinical practice [24]. PT is an indicator to examine exogenous coagulation factors in clinical practice and is a common indicator for the detection of oral anticoagulants [25]. FIB, a frequently used indicator in clinical practice, is believed to be related to coagulation activity, which mainly reflects the content of fibrinogen [26]. DD, a unique marker of secondary fibrinolysis, is one of the end products of cross-linked fibrin produced by fibrin in response to coagulation factor XIIIa after degradation by plasma plasmin [27]. In this study, the levels of PT, APTT, FIB, and DD were analyzed in parturients with HDCP. It was found that the levels of PT and FIB in HDCP parturients were lower than those in healthy ones, while the levels of APTT and DD in HDCP parturients were markedly higher than those in control patients. In the study by Shi et al. [28], PT and APTT levels were found to be notably lower and FIB levels were significantly higher in HDCP patients compared to normal parturients. However, in the study of Chen et al. [29], DD level was observed to be significantly higher in HDCP parturients than in normal parturients, which was consistent with our findings. Altogether, these results suggest that PT, APTT, FIB, and DD are associated with the occurrence and development of HDCP parturients. In order to further understand the relationship between PT, APTT, FIB, DD, and HDCP condition, we categorized them according to the degree of HDCP. In this manner, it was found that the expression of APTT and PT gradually decreased with the severity of the disease, and the expression of FIB and DD gradually increased with the severity of the disease. This shows that PT, APTT, FIB, and DD participated in the development of HDCP.
HDCP often occurs after 20 weeks of gestation and can result in adverse pregnancy outcomes such as preterm delivery, neonatal asphyxia, and fetal distress, which seriously threaten the health of mothers and infants [30]. Therefore, early prediction of adverse pregnancy outcome in patients with HDCP and timely intervention play substantial roles in improving adverse pregnancy outcomes. To further identify factors influencing adverse maternal outcomes in HDCP, age, prenatal BMI, gestational age at delivery, PT, APTT, and DD were all found to be independent risk factors for adverse pregnancy outcomes. The relationship between age and adverse pregnancy outcome has been demonstrated in a large number of previous studies, and elderly pregnant women were found to be more inclined to decreased physical function and pregnancy complications such as HDCP and electrocardiographic changes during pregnancy, as well as a higher risk of adverse pregnancy outcomes such as stillbirth and preterm delivery [31,32]. Sugiyama et al. [33] found that both pre-pregnancy BMI and weight gain during pregnancy were risk factors for adverse neonatal outcome [34]; that is, the greater the pre-pregnancy BMI, the more weight gain during pregnancy, and the greater the risk of adverse neonatal pregnancy outcome. This may be related to the increased risk of cardiovascular disease in pregnant women due to prepregnancy obesity. Studies have found [35] that insufficient gestational age at delivery can lead to FGR, intrauterine distress, or death of the fetus. This is due to defects in the development of newborns with insufficient delivery cycles, who are prone to growth restriction and fetal distress. When the body is in a hypercoagulable state, this will cause a significant increase in DD in the blood, while PT and APTT time are shortened. However, increased DD content in the body can deposit on the surface of endothelial cells to form fibrin deposits, resulting in impaired nutrient and oxygen transport or exchange in the capillary bed. In severe cases, this leads to placental hypoperfusion, spiral artery embolism, and even placental thrombosis, which cause premature delivery [36]. At the end of this study, we analyzed the significant risk factors of adverse pregnancy by ROC curve in order to predict adverse pregnancy outcome and found that only the area under the curve of DD was > 0.8. However, by plotting the combined curve, the area under the curve rose to 0.971, which is an ideal predictor.
In this study, we found that PT, FIB, APTT, and DD were differently expressed in HDCP, and the levels of various indicators were correlated with the severity of HDCP. However, this study has some limitations. First, patients could not be randomly categorized as this study was a retrospective one. Second, whether combined prediction can be applied in clinical practice requires more samples for validation. Finally, we hope that our experiment will be further verified and improved with subsequent studies by increasing the sample size.
In summary, PT, FIB, APTT, and DD levels were abnormal in parturients with different degrees of HDCP, and regular coagulation tests could effectively detect HDCP, allowing relevant treatment measures to improve pregnancy outcome.
Disclosure of conflict of interest
None.
References
- 1.Sedgh G, Finer LB, Bankole A, Eilers MA, Singh S. Adolescent pregnancy, birth, and abortion rates across countries: levels and recent trends. J Adolesc Health. 2015;56:223–230. doi: 10.1016/j.jadohealth.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton ALM, Harper LM, Tita ATN. Hypertensive disorders in pregnancy. Obstet Gynecol Clin North Am. 2018;45:333–347. doi: 10.1016/j.ogc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Gupta A. Hypertensive disorders of pregnancy. Cardiol Clin. 2019;37:345–354. doi: 10.1016/j.ccl.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am. 2019;37:301–316. doi: 10.1016/j.emc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Wang T, Chen L, Zhang S, Chen L, Qin J. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: a meta-analysis of cohort studies. Pregnancy Hypertens. 2021;24:107–117. doi: 10.1016/j.preghy.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Little M. Is D-dimer the new test for venom-induced consumption coagulopathy after snakebite? Med J Aust. 2022;217:191–192. doi: 10.5694/mja2.51663. [DOI] [PubMed] [Google Scholar]
- 7.Sah RG, d’Esterre CD, Hill MD, Hafeez M, Tariq S, Forkert ND, Demchuk AM, Goyal M, Barber PA. Diffusion-weighted MRI stroke volume following recanalization treatment is threshold-dependent. Clin Neuroradiol. 2019;29:135–141. doi: 10.1007/s00062-017-0634-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Ahn HJ, Park JY, Kim BJ, Hwang KR, Lee TS, Jeon HW, Kim SM. The clinical significance of D-dimer concentrations in patients with gestational hypertensive disorders according to the severity. Obstet Gynecol Sci. 2017;60:542–548. doi: 10.5468/ogs.2017.60.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radwanska E. The role of reproductive hormones in vascular disease and hypertension. Steroids. 1993;58:605–610. doi: 10.1016/0039-128x(93)90102-s. [DOI] [PubMed] [Google Scholar]
- 10.Halici-Ozturk F, Ozturk M, Yakistiran B, Caglar AT, Engin-Ustun Y, Ozgu-Erdinc AS. Severe thrombocytopenia in pregnancy: a retrospective study. Blood Coagul Fibrinolysis. 2020;31:517–521. doi: 10.1097/MBC.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T, Ishikawa S, Kataoka S, Uda T, Iinuma Y, Hattori R, Yamada T, Morikawa M, Kaneuchi M, Minakami H. Coagulation/fibrinolysis and laboratory characteristics of pregnant women with severely depressed antithrombin activity. Hypertens Pregnancy. 2013;32:235–244. doi: 10.3109/10641955.2013.792346. [DOI] [PubMed] [Google Scholar]
- 12.Khalafallah AA, Ibraheem AR, Teo QY, Albarzan AM, Parameswaran R, Hooper E, Pavlov T, Dennis AE, Hannan T. Review of management and outcomes in women with thrombophilia risk during pregnancy at a single institution. ISRN Obstet Gynecol. 2014;2014:381826. doi: 10.1155/2014/381826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Wang J, Wang K, Peng T, Liu H, Zheng H, Hu Q. Correlation of component blood transfusion of hypertension patients with pregnancy and postpartum hemorrhage. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200849. [DOI] [PubMed] [Google Scholar]
- 14.Stepan H, Kuse-Fohl S, Klockenbusch W, Rath W, Schauf B, Walther T, Schlembach D. Diagnosis and treatment of hypertensive pregnancy disorders. Guideline of DGGG (S1-Level, AWMF Registry No. 015/018, December 2013) Geburtshilfe Frauenheilkd. 2015;75:900–914. doi: 10.1055/s-0035-1557924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy TF, Parks JA. Gestation as mothering. Bioethics. 2020;34:960–968. doi: 10.1111/bioe.12808. [DOI] [PubMed] [Google Scholar]
- 16.Turner K, Hameed AB. Hypertensive disorders in pregnancy current practice review. Curr Hypertens Rev. 2017;13:80–88. doi: 10.2174/1573402113666170529110024. [DOI] [PubMed] [Google Scholar]
- 17.Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20:229–239. doi: 10.1053/j.ackd.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JY, Wang PH, Vitale SG, Chen SN, Marranzano M, Cianci A, Lin LT, Tsui KH. Pregnancy-induced hypertension is an independent risk factor for meconium aspiration syndrome: a retrospective population based cohort study. Taiwan J Obstet Gynecol. 2019;58:396–400. doi: 10.1016/j.tjog.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S International Society for the Study of Hypertension in Pregnancy (ISSHP) The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Erez O, Mastrolia SA, Thachil J. Disseminated intravascular coagulation in pregnancy: insights in pathophysiology, diagnosis and management. Am J Obstet Gynecol. 2015;213:452–463. doi: 10.1016/j.ajog.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 21.Zununi Vahed S, Rahbar Saadat Y, Ardalan M. Thrombotic microangiopathy during pregnancy. Microvasc Res. 2021;138:104226. doi: 10.1016/j.mvr.2021.104226. [DOI] [PubMed] [Google Scholar]
- 22.de Maat MP, de Groot CJ. Thrombophilia and pre-eclampsia. Semin Thromb Hemost. 2011;37:106–110. doi: 10.1055/s-0030-1270335. [DOI] [PubMed] [Google Scholar]
- 23.Leaton MB, Martin PS. Dealing with coagulopathies of pregnancy-induced hypertension. Dimens Crit Care Nurs. 2001;20:14–17. doi: 10.1097/00003465-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Q, Yu L, Wang F. Efficacy of using thromboelastography to detect coagulation function and platelet function in patients with acute cerebral infarction. Acta Neurol Belg. 2021;121:1661–1667. doi: 10.1007/s13760-020-01456-6. [DOI] [PubMed] [Google Scholar]
- 25.Chee YL. Coagulation. J R Coll Physicians Edinb. 2014;44:42–45. doi: 10.4997/JRCPE.2014.110. [DOI] [PubMed] [Google Scholar]
- 26.Lowe A, Kitchen S, Jennings I, Kitchen DP, Woods TAL, Walker ID. Effects of Emicizumab on APTT, FVIII assays and FVIII inhibitor assays using different reagents: results of a UK NEQAS proficiency testing exercise. Haemophilia. 2020;26:1087–1091. doi: 10.1111/hae.14177. [DOI] [PubMed] [Google Scholar]
- 27.Halaby R, Popma CJ, Cohen A, Chi G, Zacarkim MR, Romero G, Goldhaber SZ, Hull R, Hernandez A, Mentz R, Harrington R, Lip G, Peacock F, Welker J, Martin-Loeches I, Daaboul Y, Korjian S, Gibson CM. D-dimer elevation and adverse outcomes. J Thromb Thrombolysis. 2015;39:55–59. doi: 10.1007/s11239-014-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi F, Yu A, Yuan L. Clinical significance of detection of coagulation indexes, immune factors and inflammatory factors in patients with pregnancy-induced hypertension syndrome in China. Iran J Public Health. 2019;48:681–687. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Chen Y, Wang X, Chu X, Ning W, Gu L, Li L, Xie Z, Wen C. Second trimester maternal serum D-dimer combined with alpha-fetoprotein and free beta-subunit of human chorionic gonadotropin predict hypertensive disorders of pregnancy: a systematic review and retrospective case-control study. J Transl Med. 2021;19:94. doi: 10.1186/s12967-021-02718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ukah UV, De Silva DA, Payne B, Magee LA, Hutcheon JA, Brown H, Ansermino JM, Lee T, von Dadelszen P. Prediction of adverse maternal outcomes from pre-eclampsia and other hypertensive disorders of pregnancy: a systematic review. Pregnancy Hypertens. 2018;11:115–123. doi: 10.1016/j.preghy.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;70:92–100. doi: 10.1016/j.bpobgyn.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2021;70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama T, Metoki H, Hamada H, Nishigori H, Saito M, Yaegashi N, Kusaka H, Kawano R, Ichihara K, Yasuhi I, Hiramatsu Y, Sagawa N Japan Gestational Diabetes Study Group. A retrospective multi-institutional study of treatment for mild gestational diabetes in Japan. Diabetes Res Clin Pract. 2014;103:412–418. doi: 10.1016/j.diabres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, Liu J, Wu S, Feng Y, Chen Y, Cai S, Shi Y, Ma L, Jiang Y. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth. 2020;20:390. doi: 10.1186/s12884-020-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yong HY, Mohd Shariff Z, Appannah G, Rejali Z, Mohd Yusof BN, Bindels J, Tee YYS, van der Beek EM. Rate of gestational weight gain trajectory is associated with adverse pregnancy outcomes. Public Health Nutr. 2020;23:3304–3314. doi: 10.1017/S1368980020002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng J, Li Y, Dong Y, Chen Y, Liu Y, Wang S, Zhu H, Liu J, Lu Y, Zhai Y, Cao Z. Predictive values of D-dimer for adverse pregnancy outcomes: a retrospective study. Clin Chem Lab Med. 2021;59:e99–e101. doi: 10.1515/cclm-2020-0392. [DOI] [PubMed] [Google Scholar]