Abstract
Objective: To assess whether the composite dietary antioxidant index (CDAI) is associated with osteoporosis (OP) in middle-aged and older US populations. Methods: We conducted a cross-sectional survey and identified individuals aged 40-85 years (n=11,664) from secondary datasets from the 2007-2010, 2013-2014, and 2017-2018 National Health and Nutrition Examination Survey (NHANES). Dual-energy X-ray absorptiometry was used to measure bone mineral density (BMD), and OP was defined as a BMD T-score ≤-2.5 at the femoral neck or lumbar spine. The CDAI score was calculated based on dietary data from the first NHANES 24-hour dietary recall. Multivariate logistic regression models were used to evaluate the association between CDAI and OP. Results: Among the 11,664 participants, the average age was 60.3 (11.8), 5,898 (50.6%) were female, and 925 (7.9%) had OP. The median CDAI was -2.0 (interquartile range, -6.9 to 4.2). After adjusting for age, sex, race, family income, body mass index, physical activity, calorie intake, estimated glomerular filtration rate, smoking and drinking status, hypertension, and diabetes, the CDAI was associated with OP (odds ratio (OR), 0.98; 95% CI: 0.96-0.99). Participants in the highest CDAI quantile were at low risk of osteoporosis (OR, 0.61; 95% CI: 0.44-0.85) versus those in the lowest quantile. Moreover, this association was stable in the subgroup and sensitivity analyses. Conclusion: Dietary antioxidant ability assessed by using the CDAI was inversely associated with OP among US adults aged 40-85 years.
Keywords: Osteoporosis, composite dietary antioxidant index, dietary recall, NHANES, cross-sectional study
Introduction
Osteoporosis (OP) is one of the most prevalent public health issues, especially in older adults, that manifests as decreased bone mass and destruction of bone microstructures. It affects an estimated 200 million people worldwide and contributes to a disease burden greater than that of hypertension [1,2]. A proper lifestyle and proper nutrition are needed to prevent OP. Diet is crucial since it is one of the few healthy changes people can make. The risk of OP has been linked to the intake of multiple nutrients, including calcium, zinc [3,4], vitamin D [5], vitamin E [6], and other nutrients [7]. Several researchers have also discovered that adherence to certain dietary patterns, such as the Mediterranean diet [8,9], promotes bone health and reduces OP risk. In addition, diet quality plays an essential role in determining health outcomes because all dietary intakes often work in concert [10-14]. To investigate the influence of overall diet quality on the bone health of middle-aged and older US populations, we used the Composite Dietary Antioxidant Index, which is considered a factor in preventing OP [15].
Diet is an important source of exogenous antioxidants, which provides an effective auxiliary function for the body’s antioxidant system. Previous studies have found that a higher intake of dietary antioxidants, such as carotenoids and vitamin E, is associated with decreased OP risk [16,17]. However, these studies focused on specific dietary antioxidants, and the effect of the whole dietary antioxidant capacity on OP remains unclear. The composite dietary antioxidant index (CDAI) is a scoring system developed by Wright et al. to quantify the potential dietary antioxidant capacity of a daily diet [18]. To better understand the relationship between dietary antioxidant capacity and OP, we aimed to explore the association between CDAI and OP in the US population aged 40 and older using data from the National Health and Nutrition Examination Survey (NHANES) in this cross-sectional study.
Materials and methods
Study population
Research ethics review board approval was obtained from the National Centers for Health Statistics Research Ethics Review Board for the NHANES study protocol.
The NHANES is an ongoing nationwide survey to evaluate the health and nutritional status of the non-institutionalized US civilian population. Bone mass density (BMD) data were available only in the NHANES 2007-2010, 2013-2014, and 2017-2018. Thus, we combined the 8-year data for our analysis.
A total of 40,115 participants completed the survey.
Participants aged <40 (n=24,258) were excluded. The following participants were also excluded: those with unreliable or incomplete dietary recall data (n=576), implausible total energy intake (<500 or >5,000 kcal/day; n=218) [19], missing BMD data (n=3,399). Finally, 11,664 participants who completed a 1-day 24 h dietary recall were included in the final analysis. Prior to participating in the study, participants provided written consent to the National Center for Health Statistics Research Ethics Review Board. Observational studies were reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology-nutritional Epidemiology Guidelines.
Assessment of OP and CDAI
In the NHANES, BMD was examined using dual-energy X-ray absorptiometry (DXA), and details are provided in the NHANES protocol (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). This study defined OP as a BMD T-score ≤-2.5 at the femoral neck or lumbar spine. Dietary intake information was acquired through the first NHANES 24-hour dietary recall. The CDAI was developed by Wright et al. [18]. It is the sum of dietary intakes of six antioxidants (selenium, magnesium, zinc, vitamin A, vitamin C, and vitamin E) computed by subtracting the global mean and dividing the result by the global standard deviation to estimate CDAI. The CDAI was calculated by summing the standardized intake of these vitamins and minerals and equal weight, as described below [20-22] (Equation 1):
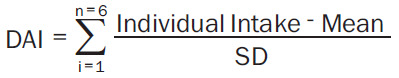 |
The dietary antioxidant quality score (DAQS) was used to calculate the antioxidant nutrient intake [23]. The score refers to the intake of certain vitamins and minerals that have been proven to act as dietary antioxidants: selenium, zinc, vitamin A, vitamin C, and vitamin E. Daily nutrient intake was compared with the recommended daily intake (RDI) for the study population. The intake of each of the five antioxidant nutrients was evaluated separately by assessing potential covariates. Each nutrient was assigned values of 0 or 1. When the intake was below 2/3 of the RDI, it was assigned a value of 0, whereas when it was higher than 2/3, it was assigned a value of 1. The quality of the dietary antioxidants ranged from 0 (very poor) to 5 (high).
Assessment of potential covariates
Based on the literature, the following covariates were included: age, sex, race, family income, caloric intake, body mass index (BMI), estimated glomerular filtration rate (eGFR), self-reported hypertension and diabetes, and smoking and drinking status [24,25]. Family income was categorized into three levels based on the family poverty income ratio (PIR): low income (≤1.3), medium income (1.3-3.5), and high income (>3.5). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [26]. Smoking status was based on self-reports and divided into three levels (never smoked, ex-smoker, and current smoker). Drinking status was determined through self-reporting. Individuals who drank ≥12 standard drinks in any year were considered drinkers, and others were considered non-drinkers [27]. Moreover, those without drinking data were imputed as non-responders. Based on a previous study, physical activity was categorized into three groups: no physical activity, low-intensity physical activity, and high-intensity physical activity [14]. The details of all the variables can be obtained at http://www.cdc.gov/nchs/nhanes/.
Statistical analysis
All statistical analyses were performed with R, version 4.0.5 (R Project for Statistical Computing) using the survey package, version 4.1-1, and with Free Software Foundation statistics software, version 1.3. In all tests, P<0.05 (two-sided) was considered statistically significant. Normally distributed and skewed continuous variables were reported as mean (SD) and median (interquartile range (IQR)), respectively. Categorical variables were reported as counts and proportions. Comparisons among CDAI quartiles were performed using a one-way analysis of variance for continuous variables and the chi-square test for categorical variables. Four models were constructed using logistic regression analysis to investigate the association between CDAI and OP. Multicollinearity was tested using the variance inflation factor (VIF) method. A VIF ≥10 indicated the presence of multicollinearity in the analysis. Model 1 was the crude model, not adjusted for covariates. Model 2 was adjusted for age, sex, race/ethnicity, and family income. Model 3 (the main model) was based on Model 2 and the eGFR, BMI, calorie intake, physical activity, and drinking status. A fully adjusted model was used for Model 4, which was adjusted for all covariates in Model 3 and diabetes, hypertension, and smoking status. To further explore the potential associations, the CDAI score was also classified by quantile for multivariable logistic regression analyses, and a trend test was performed. Stratified and interaction analyses were performed according to age, sex, race, and ethnicity. Finally, three sensitivity analyses were conducted to assess the robustness. First, considering the potential association between medication and BMD, we excluded participants taking medicines that potentially affect BMD, including thiazide diuretics, glucocorticoids, bisphosphonates, allopurinol, sex hormone therapy, thyroid replacement therapy, bone resorption inhibitors, and participants with chronic kidney disease, diabetes, and rheumatoid arthritis. Second, considering that dietary recalls over 24 h may not be adequate to estimate average intakes, the CDAI score was calculated based on the averages of two NHANES dietary recalls. Third, the dietary antioxidant capacity was accessed by the dietary antioxidant quality (DAQ) score, a scoring system developed by Rivas et al. [22].
Results
Characteristics of the study population: CDAI was significantly different between the highest and lowest quantiles
Of the 40,115 participants aged 0-85, 28,451 were excluded due to age (<40) and unavailable or implausible data. A total of 11,664 participants were included in the analysis (Figure 1). The baseline characteristics are shown in Table 1. The mean age of the included participants was 60.3 years old, 5,898 (50.6%) were female, and 925 (7.9%) had OP. The median CDAI was -2.0 (IQR, -6.9 to 4.2). Compared to those in the lowest quantiles, participants in the highest CDAI quantile were more likely to be women, younger, non-Hispanic black, high-income, with higher eGFR, high-intensity physical activity, higher BMI and daily calorie intake, and without hypertension, alcohol drinking, or diabetes.
Figure 1.
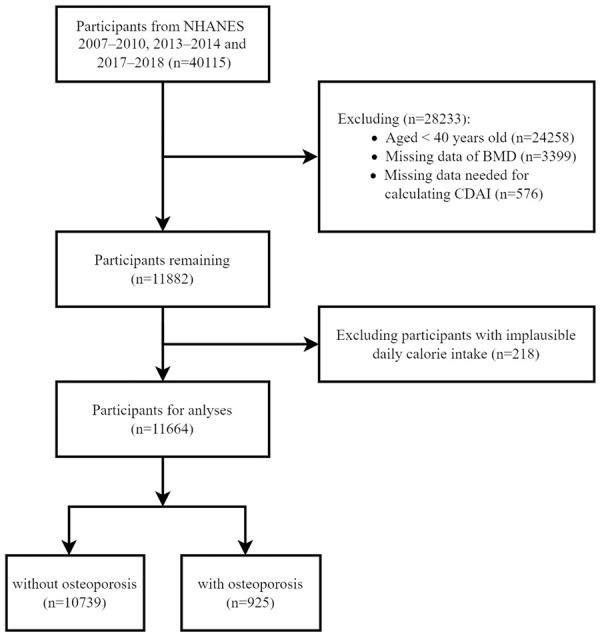
Flow diagram of the screening and enrollment of study participants. NHANE: National Health and Nutrition Examination Survey; BMD: Bone Mass Density; CDAI: Composite Dietary Antioxidant Index.
Table 1.
Distribution of characteristics of participants
| Quartiles of composite dietary antioxidant index (CDAI) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total (n=11664) | Q1 (n=2916) | Q2 (n=2916) | Q3 (n=2916) | Q4 (n=2916) | P value | |
| CDAI score | -2.0 (-6.9, 4.2) | -9.8 (-11.7, -8.2 | -4.4 (-5.6, -3.2) | 0.7 (-0.6, 2.3) | 9.6 (6.5, 14.9) | <0.001 |
| Age (years) | 60.3±11.8 | 61.7±11.8 | 60.9±11.9 | 60.0±11.8 | 58.6±11.5 | <0.001 |
| Sex (%) | <0.001 | |||||
| Female | 5898 (50.6) | 1846 (63.3) | 1664 (57.1) | 1394 (47.8) | 994 (34.1) | |
| Male | 5766 (49.4) | 1070 (36.7) | 1252 (42.9) | 1522 (52.2) | 1922 (65.9) | |
| Race (%) | <0.001 | |||||
| Asian | 577 (4.9) | 115 (3.9) | 147 (5.0) | 136 (4.7) | 179 (6.1) | |
| Mexican American | 1724 (14.8) | 446 (15.3) | 432 (14.8) | 443 (15.2) | 403 (13.8) | |
| Non-hispanic Black | 2283 (19.6) | 726 (24.9) | 545 (18.7) | 511 (17.5) | 501 (17.2) | |
| Non-hispanic White | 5492 (47.1) | 1222 (41.9) | 1361 (46.7) | 1434 (49.2) | 1475 (50.6) | |
| Other hispanic | 1191 (10.2) | 315 (10.8) | 337 (11.6) | 277 (9.5) | 262 (9.0) | |
| Others | 397 (3.4) | 92 (3.2) | 94 (3.2) | 115 (3.9) | 96 (3.3) | |
| Family income (%) | <0.001 | |||||
| BMI (kg/m2) | 29.1±6.0 | 29.1±6.1 | 29.2±5.9 | 29.1±6.0 | 28.9±5.9 | 0.23 |
| Calorie intake (kcal/day) | 1963.0±821.6 | 1217.9±401.0 | 1704.8±466.9 | 2126.4±571.2 | 2802.8±801.2 | <0.001 |
| eGFR (ml/(min*1.73 m2)) | 83.8±20.5 | 81.5±22.3 | 83.5±20.4 | 84.4±20.2 | 85.7±18.7 | <0.001 |
| Osteoporosis (%) | <0.001 | |||||
| No | 10739 (92.1) | 2603 (89.3) | 2668 (91.5) | 2718 (93.2) | 2750 (94.3) | |
| Yes | 925 (7.9) | 313 (10.7) | 248 (8.5) | 198 (6.8) | 166 (5.7) | |
| BMI (kg/m2) | 29.1±6.0 | 29.1±6.1 | 29.2±5.9 | 29.1±6.0 | 28.9±5.9 | 0.23 |
| Low | 2950 (27.9) | 970 (37.0) | 768 (28.9) | 647 (24.6) | 565 (21.2) | |
| Medium | 4065 (38.4) | 1027 (39.2) | 1079 (40.5) | 1004 (38.1) | 955 (35.9) | |
| High | 3565 (33.7) | 624 (23.8) | 814 (30.6) | 984 (37.3) | 1143 (42.9) | |
| Drinking status (%) | <0.001 | |||||
| Non-drinker | 3950 (33.9) | 1111 (38.1) | 1018 (34.9) | 956 (32.8) | 865 (29.7) | |
| Drinker | 6457 (55.4) | 1441 (49.4) | 1579 (54.1) | 1679 (57.6) | 1758 (60.3) | |
| Missing | 1257 (10.8) | 364 (12.5) | 319 (10.9) | 281 (9.6) | 293 (10) | |
| Smoking status (%) | <0.001 | |||||
| Never smoked | 6013 (51.6) | 1479 (50.7) | 1551 (53.2) | 1513 (51.9) | 1470 (50.4) | |
| Ex-smoker | 3569 (30.6) | 800 (27.4) | 861 (29.5) | 946 (32.5) | 962 (33.0) | |
| Current smoker | 2080 (17.8) | 637 (21.8) | 503 (17.3) | 456 (15.6) | 484 (16.6) | |
| Diabetes (%) | <0.001 | |||||
| No | 9600 (82.3) | 2314 (79.4) | 2378 (81.6) | 2422 (83.1) | 2486 (85.3) | |
| Yes | 2058 (17.7) | 601 (20.6) | 536 (18.4) | 491 (16.9) | 430 (14.7) | |
| Hypertension (%) | <0.001 | |||||
| No | 7028 (60.3) | 1655 (56.9) | 1748 (60.1) | 1784 (61.3) | 1841 (63.2) | |
| Yes | 4618 (39.7) | 1255 (43.1) | 1161 (39.9) | 1128 (38.7) | 1074 (36.8) | |
| Physical activity (%) | <0.001 | |||||
| No | 3626 (31.1) | 1184 (40.6) | 971 (33.3) | 774 (26.5) | 697 (23.9) | |
| Low | 4929 (42.3) | 1139 (39.1) | 1233 (42.3) | 1318 (45.2) | 1239 (42.5) | |
| High | 3109 (26.7) | 593 (20.3) | 712 (24.4) | 824 (28.3) | 980 (33.6) | |
Note: BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; CDAI: Composite Dietary Antioxidant Index.
Multivariable regression analyses: high-quartile CDAI had a lower risk of OP
Table 2 summarizes the results of the logistic regression analyses. In all four models, CDAI was associated with OP. In the crude model (Model 1), the odds ratio (OR) of CDAI for OP was 0.97 (95% CI, 0.97-0.98). Participants in the highest CDAI quantile were at a relatively lower risk of OP than those in the lowest CDAI quantile (OR=0.5 (95% CI, 0.41-0.61)). In the main model (Model 3) adjusted for age, sex, race, ethnicity, family income, BMI, eGFR, calorie intake, physical activity, and drinking status, the OR of CDAI for OP was 0.98 (95% CI, 0.96-0.99). For CDAI quantiles 2, 3, and 4, the ORs were 0.77 (95% CI, 0.62-0.96), 0.64 (95% CI, 0.50-0.83), and 0.59 (95% CI, 0.42-0.81), respectively (p for trend <0.001). This relationship remained in the fully adjusted model.
Table 2.
Association between CDAI and osteoporosis
| Osteoporosis/n | Prevalence/% | Model 1a | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|---|---|
| CDAI (continuous) | 925/11664 | 7.9 | 0.97 (0.97-0.98) | 0.99 (0.98-1.00) | 0.97 (0.96-0.99) | 0.98 (0.96-0.99) |
| CDAI categories | ||||||
| Quantile 1 | 313/2916 | 10.7 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Quantile 2 | 248/2916 | 8.5 | 0.77 (0.65-0.92) | 0.83 (0.68-1.01) | 0.77 (0.62-0.96) | 0.79 (0.63-0.98) |
| Quantile 3 | 198/2916 | 6.8 | 0.61 (0.50-0.73) | 0.75 (0.61-0.93) | 0.64 (0.50-0.83) | 0.66 (0.51-0.85) |
| Quantile 4 | 166/2916 | 5.7 | 0.50 (0.41-0.61) | 0.82 (0.65-1.03) | 0.59 (0.42-0.81) | 0.61 (0.44-0.85) |
| P for trend | <0.001 | 0.03 | <0.001 | 0.001 |
Model 1: crude model;
Model 2: adjusted for age, sex, race/ethnicity and family income;
Model 3: model 2 + BMI, eGFR, calorie intake, physical activity and drinking status;
Model 4 (the fully adjusted model): model 3 + diabetes, hypertension and smoking status.
BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; CDAI: Composite Dietary Antioxidant Index.
Subgroup analyses: in different populations, CDAI did not interact with OP
Figure 2 shows the results of the subgroup analysis. There was an inverse relationship between OP and CDAI among participants aged 60 or older (OR, 0.97; 95% CI, 0.95-0.99), among females (OR, 0.97; 95% CI, 0.95-0.99), and among non-Hispanic whites (OR, 0.97; 95% CI, 0.95-0.99). No significant interactions were observed.
Figure 2.
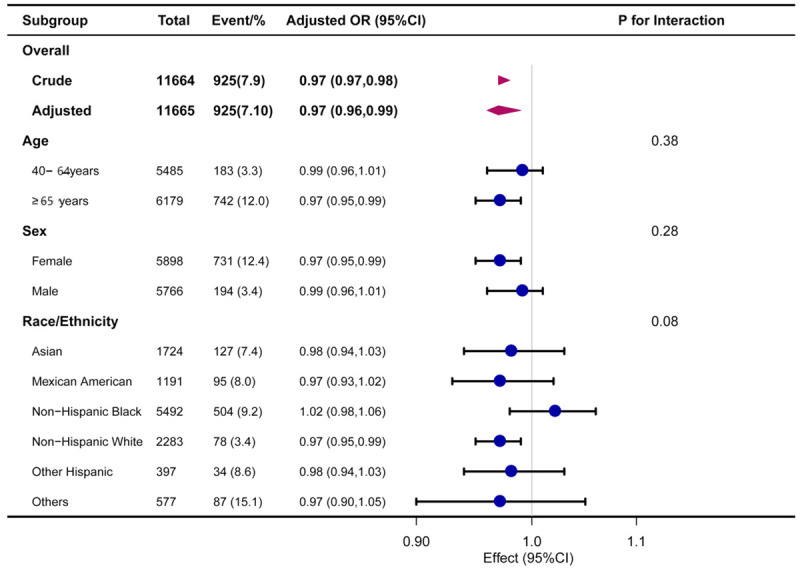
Association between CDAI and osteoporosis. Each stratification was adjusted for age, sex, race/ethnicity and family income, BMI, eGFR, caloric intake, physical activity, and drinking status, except the stratification factor itself. BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; CDAI: Composite Dietary Antioxidant Index; CI: Confidence Interval; OR: Odd Ratio.
Sensitivity analyses: the results of CDAI and OP were still robust by re-analysis of 3 models
The results of the sensitivity analysis are presented in Table 3. After excluding participants who received bone resorption inhibitors, glucocorticoids, bisphosphonates, thiazide diuretics, allopurinol, sex hormone therapy, and thyroid replacement therapy and excluding those with chronic kidney disease, diabetes, and rheumatoid arthritis, CDAI score was associated with OP (OR, 0.97; 95% CI, 0.96-0.99). The adjusted OR suggested an inverse association between the highest CDAI quartile and OP compared to the lowest quartile (OR, 0.57; 95% CI, 0.35-0.92). After redefining the CDAI score based on the averages of two NHANES dietary recalls (n=10,554), the association remained (OR, 0.96; 95% CI, 0.95-0.98). For CDAI quantile 4, the OR was 0.66 (95% CI, 0.47-0.94). In addition, when dietary antioxidant capacity was assessed using the DAQ score (n=18,935), it was still associated with OP (OR, 0.90; 95% CI, 0.84-0.95).
Table 3.
Sensitivity analyses
| Osteoporosis/n | Prevalence/% | Adjusted OR (95% CI)a | |
|---|---|---|---|
| Sensitivity analyses 1b | |||
| CDAI (continuous) | 415/6716 | 6.7 | 0.97 (0.96-0.99) |
| CDAI categories | |||
| Quantile 1 | 132/1544 | 8.5 | 1.00 (Ref.) |
| Quantile 2 | 113/1544 | 7.3 | 0.90 (0.65-1.25) |
| Quantile 3 | 96/1544 | 6.2 | 0.71 (0.49-1.04) |
| Quantile 4 | 74/1544 | 4.8 | 0.57 (0.35-0.92) |
| Sensitivity analyses 2c | |||
| CDAI (continuous) | 830/10554 | 7.9 | 0.96 (0.95-0.98) |
| CDAI categories | |||
| Quantile 1 | 281/2636 | 10.7 | 1.00 (Ref.) |
| Quantile 2 | 225/2636 | 8.5 | 0.84 (0.66-1.06) |
| Quantile 3 | 181/2636 | 6.9 | 0.80 (0.61-1.05) |
| Quantile 4 | 143/2636 | 5.4 | 0.66 (0.47-0.94) |
| Sensitivity analyses 3d | |||
| DAQ (continuous) | |||
| DAQ categories | 915/10989 | 8.3 | 0.90 (0.84-0.95) |
| <5 | 355/4241 | 8.4 | 1.00 (Ref.) |
| ≥5 | 560/6748 | 8.3 | 0.79 (0.65-0.97) |
All models are adjusted for age, sex, race/ethnicity, family income, BMI, eGFR, calorie intake, physical activity and drinking status;
Excluding participants taking medicines that may affect BMD which include thiazide diuretics, glucocorticoids, bisphosphonates, allopurinol, sex hormone therapy, thyroid replacement therapy, bone resorption inhibitors and excluding participants with chronic kidney disease, diabetes, and rheumatoid arthritis;
Calculating CDAI based on the averages of two NHANES dietary recall;
Accessing dietary antioxidant capacity by using DAQ score.
CDAI: Composite Dietary Antioxidant Index; DAQ: Dietary Antioxidant Quality; BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; BMD: Bone Mass Density; NHANE: National Health and Nutrition Examination Survey.
Discussion
This study found an inverse association between composite dietary antioxidant index (CDAI) and osteoporosis (OP) risk in US adults aged 40 and older. To our knowledge, this is the first large population-based study to explore the association between dietary antioxidant capacity and OP in the US.
Related research has shown that oxidative stress is an independent risk factor for OP [28]. Oxidative stress refers to a state in which the generation and elimination of reactive oxygen species (ROS) are unbalanced, and the level of ROS increases. Elevated ROS levels can affect various enzymes, proteins, and cytokines to disrupt the coupling of osteoblasts and osteoclasts, thus suppressing the osteogenic lineage. In addition, ROS affects osteoblasts, osteoclasts, and the bone matrix and promotes the development of OP. Antioxidants can inhibit oxidative stress. When the antioxidant balance in the body is disrupted, exogenous antioxidants can prevent or delay OP development.
Previous studies usually focus on the effects of antioxidant supplementation alone on bone health [29-38]. A meta-analysis indicated that greater dietary vitamin C intake was associated with a lower risk of OP and higher BMD [30]. Several studies have also indicated a protective effect of vitamin E supplementation against OP [29-31]. However, a clinical review [39] showed mixed observational findings on the association between vitamin A intake or serum concentration and bone mass density (BMD) or fracture. In addition, zinc, magnesium, selenium, and other trace elements, which are essential components of antioxidants, play a role in bone growth, development, and maintenance. There are also conflicting reports regarding the association between serum magnesium (Mg) levels and postmenopausal OP. A meta-analysis [40] showed that low serum Mg levels appeared to be a risk factor for OP in postmenopausal women. However, the subgroup analysis found contradictions in terms of ethnicity and geography, such as in China and Turkey. The uncertain results of previous studies may be due to the different study populations and lifestyles. It is important to note that although individual dietary antioxidants may contribute to the development and progression of OP, it would be more meaningful to assess their combined effects on OP risk. Currently, there are limited epidemiologic studies on the relationship between dietary antioxidant capacity and bone health. A recent cross-sectional study of 280 Spanish women reported a positive association between DAQ and BMD [5]. Among premenopausal women, dietary total antioxidant capacity positively correlated with lumbar spine and total femur BMC. In middle-aged and older populations in the US, HEI-2015 total and component food scores were associated with a reduced risk of OP [41]. Regu et al., in a study of 8,022 Korean adults, showed that postmenopausal women in the highest quintile of daily beta-carotene intake had a lower risk of osteopenia in the lumbar spine [34]. These results are consistent with our findings. The CDAI, designed to measure the total amount of antioxidants in a diet, captures a dietary antioxidant profile more precisely, can reduce exposure to misclassification, and shows a protective effect. By calculating the CDAI in the diet, the present study (n=11,664) investigated the association between OP and dietary antioxidant capacity as a whole and can complement previous studies.
The present study has some limitations. First, as with other cross-sectional studies, this study cannot establish a causal inference regarding the association between dietary antioxidant capacity and OP. Additional cohort studies are needed in the future. Second, an analysis of dietary intake using a 1-day 24-hour recall at baseline might not be representative of the participants’ cumulative usual diet. Nonetheless, 24-hour dietary recalls can provide an adequate estimate of a population’s average intake if the sample size is sufficiently large. Furthermore, we conducted a sensitivity analysis based on the average of the two dietary recalls to reduce bias. Third, since this study did not describe dietary supplements in detail, we did not consider the CDAI. Finally, although we considered the effects of medicine and diseases that can affect BMD, unidentified confounders still exist. In summary, this cross-sectional analysis found an inverse association between dietary antioxidant capacity and OP among US adults aged 40-85. This relationship may provide evidence for future dietary guidelines for patients with OP.
Acknowledgements
We would like to thank Dr. Ruan Zhijie (Department of Dermatology, First Affiliated Hospital of Shantou University Medical College, Shantou 515000, China), Dr. Cai Shaoyan (Department of Anesthesiology, Shantou Central Hospital, Shantou 515000, China), Prof. Jun Lyu (Department of Clinical Research, The First Affiliated Hospital of Jinan University, Guangzhou 510630, China) and the clinical team of scientists for their consultation in data analysis and article writing. This research was funded by the Dongguan Bureau of Science and Technology (project No. 202050715035199).
Disclosure of conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Quattrini S, Pampaloni B, Gronchi G, Giusti F, Brandi ML. The mediterranean diet in osteoporosis prevention: an insight in a peri- and post-menopausal population. Nutrients. 2021;13:531. doi: 10.3390/nu13020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher CM, Kovach JS, Meliker JR. Urinary cadmium and osteoporosis in U.S. Women >or= 50 years of age: NHANES 1988-1994 and 1999-2004. Environ Health Perspect. 2008;116:1338–1343. doi: 10.1289/ehp.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormick G, Belizán JM. Calcium intake and health. Nutrients. 2019;11:1606. doi: 10.3390/nu11071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Is zinc an important trace element on bone-related diseases and complications? A meta-analysis and systematic review from serum level, dietary intake, and supplementation aspects - PubMed. no date. doi: 10.1007/s12011-020-02193-w. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Kuang X, Li K, Guo X, Deng Q, Li D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020;11:10817–10827. doi: 10.1039/d0fo00787k. [DOI] [PubMed] [Google Scholar]
- 6.Fusaro M, Cianciolo G, Brandi ML, Ferrari S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La Manna G, Gallieni M, Vettor R, Aghi A, Gasperoni L, Giannini S, Sella S, M Cheung A. Vitamin K and osteoporosis. Nutrients. 2020;12:3625. doi: 10.3390/nu12123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erem S, Atfi A, Razzaque MS. Anabolic effects of vitamin D and magnesium in aging bone. J Steroid Biochem Mol Biol. 2019;193:105400. doi: 10.1016/j.jsbmb.2019.105400. [DOI] [PubMed] [Google Scholar]
- 8.Zupo R, Lampignano L, Lattanzio A, Mariano F, Osella AR, Bonfiglio C, Giannelli G, Pergola G. Association between adherence to the Mediterranean Diet and circulating Vitamin D levels. Int J Food Sci Nutr. 2020;71:884–890. doi: 10.1080/09637486.2020.1744533. [DOI] [PubMed] [Google Scholar]
- 9.Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, Feriche B, Lorenzo ML, Olea F. Mediterranean diet and bone mineral density in two age groups of women. Int J Food Sci Nutr. 2013;64:155–161. doi: 10.3109/09637486.2012.718743. [DOI] [PubMed] [Google Scholar]
- 10.Byberg L, Bellavia A, Larsson SC, Orsini N, Wolk A, Michaëlsson K. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098–2105. doi: 10.1002/jbmr.2896. [DOI] [PubMed] [Google Scholar]
- 11.Shin S, Sung J, Joung H. A fruit, milk and whole grain dietary pattern is positively associated with bone mineral density in Korean healthy adults. Eur J Clin Nutr. 2015;69:442–448. doi: 10.1038/ejcn.2014.231. [DOI] [PubMed] [Google Scholar]
- 12.Zeng FF, Xue WQ, Cao WT, Wu BH, Xie HL, Fan F, Zhu HL, Chen YM. Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: a case-control study. Osteoporos Int. 2014;25:2131–2141. doi: 10.1007/s00198-014-2741-2. [DOI] [PubMed] [Google Scholar]
- 13.Benetou V, Orfanos P, Pettersson-Kymmer U, Bergström U, Svensson O, Johansson I, Berrino F, Tumino R, Borch KB, Lund E, Peeters PH, Grote V, Li K, Altzibar JM, Key T, Boeing H, von Ruesten A, Norat T, Wark PA, Riboli E, Trichopoulou A. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587–1598. doi: 10.1007/s00198-012-2187-3. [DOI] [PubMed] [Google Scholar]
- 14.Hardcastle AC, Aucott L, Fraser WD, Reid DM, Macdonald HM. Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur J Clin Nutr. 2011;65:378–385. doi: 10.1038/ejcn.2010.264. [DOI] [PubMed] [Google Scholar]
- 15.Odai T, Terauchi M, Hirose A, Kato K, Miyasaka N. Bone mineral density in premenopausal women is associated with the dietary intake of α-tocopherol: a cross-sectional study. Nutrients. 2019;11:2474. doi: 10.3390/nu11102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 17.Tarantino U, Capone A, Planta M, D’Arienzo M, Letizia Mauro G, Impagliazzo A, Formica A, Pallotta F, Patella V, Spinarelli A, Pazzaglia U, Zarattini G, Roselli M, Montanari G, Sessa G, Privitera M, Verdoia C, Corradini C, Feola M, Padolino A, Saturnino L, Scialdoni A, Rao C, Iolascon G, Brandi ML, Piscitelli P. The incidence of hip, forearm, humeral, ankle, and vertebral fragility fractures in Italy: results from a 3-year multicenter study. Arthritis Res Ther. 2010;12:R226. doi: 10.1186/ar3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160:68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 19.Ha K, Kim K, Sakaki JR, Chun OK. Relative validity of dietary total antioxidant capacity for predicting all-cause mortality in comparison to diet quality indexes in US adults. Nutrients. 2020;12:1210. doi: 10.3390/nu12051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luu HN, Wen W, Li H, Dai Q, Yang G, Cai Q, Xiang YB, Gao YT, Zheng W, Shu XO. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signal. 2015;22:951–959. doi: 10.1089/ars.2014.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolarzyk E, Pietrzycka A, Zając J, Morawiecka-Baranek J. Relationship between dietary antioxidant index (DAI) and antioxidants level in plasma of Kraków inhabitants. Adv Clin Exp Med. 2017;26:393–399. doi: 10.17219/acem/61834. [DOI] [PubMed] [Google Scholar]
- 22.Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, López G, Lorenzo ML, Ocaña-Peinado FM, Olea-Serrano F. Association between dietary antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr Hosp. 2012;27:1886–1893. doi: 10.3305/nh.2012.27.6.6039. [DOI] [PubMed] [Google Scholar]
- 23.Tur JA, Romaguera D, Pons A. Does the diet of the Balearic population, a Mediterranean-type diet, ensure compliance with nutritional objectives for the Spanish population? Public Health Nutr. 2005;8:275–283. doi: 10.1079/phn2004693. [DOI] [PubMed] [Google Scholar]
- 24.Brzezińska O, Łukasik Z, Makowska J, Walczak K. Role of vitamin C in osteoporosis development and treatment-a literature review. Nutrients. 2020;12:2394. doi: 10.3390/nu12082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilesanmi-Oyelere BL, Kruger MC. Nutrient and dietary patterns in relation to the pathogenesis of postmenopausal osteoporosis-a literature review. Life (Basel) 2020;10:220. doi: 10.3390/life10100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, Xie X. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. 2022;158:745–753. doi: 10.1001/jamadermatol.2022.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Han A, Park Y. Association of dietary total antioxidant capacity with bone mass and osteoporosis risk in Korean women: analysis of the Korea national health and nutrition examination survey 2008-2011. Nutrients. 2021;13:1149. doi: 10.3390/nu13041149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malmir H, Shab-Bidar S, Djafarian K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br J Nutr. 2018;119:847–858. doi: 10.1017/S0007114518000430. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ramos M, Vargas LA, Fortoul Van der Goes TI, Cervantes-Sandoval A, Mendoza-Nunez VM. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J Nutr Health Aging. 2010;14:467–472. doi: 10.1007/s12603-010-0099-5. [DOI] [PubMed] [Google Scholar]
- 32.Shuid AN, Mohamad S, Muhammad N, Fadzilah FM, Mokhtar SA, Mohamed N, Soelaiman IN. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res. 2011;29:1732–1738. doi: 10.1002/jor.21452. [DOI] [PubMed] [Google Scholar]
- 33.Ostman B, Michaëlsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med. 2009;47:668–673. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Regu GM, Kim H, Kim YJ, Paek JE, Lee G, Chang N, Kwon O. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30-75 years using data from the fourth and fifth Korean national health and nutrition examination surveys (2008-2011) Nutrients. 2017;9:1025. doi: 10.3390/nu9091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Xie D, Li J, Long H, Wu J, Wu Z, He H, Wang H, Yang T, Wang Y. Association between dietary selenium intake and the prevalence of osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. 2019;20:585. doi: 10.1186/s12891-019-2958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Lazarenko OP, Kang J, Blackburn ML, Ronis MJ, Badger TM, Chen JR. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS One. 2013;8:e70438. doi: 10.1371/journal.pone.0070438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano M, Nakamura Y, Miyazaki A, Takahashi J. Zinc pharmacotherapy for elderly osteoporotic patients with zinc deficiency in a clinical setting. Nutrients. 2021;13:1814. doi: 10.3390/nu13061814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Liu J, Cao Y, Zhu Y, Guan K, Chen Y. Association of dietary and serum vitamin E with bone mineral density in middle-aged and elderly Chinese adults: a cross-sectional study. Br J Nutr. 2016;115:113–120. doi: 10.1017/S0007114515004134. [DOI] [PubMed] [Google Scholar]
- 39.Zhou P, Shao R, Wang H, Miao J, Wang X. Dietary vitamin A, C, and E intake and subsequent fracture risk at various sites: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2020;99:e20841. doi: 10.1097/MD.0000000000020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groenendijk I, van Delft M, Versloot P, van Loon LJC, de Groot LCPGM. Impact of magnesium on bone health in older adults: a systematic review and meta-analysis. Bone. 2022;154:116233. doi: 10.1016/j.bone.2021.116233. [DOI] [PubMed] [Google Scholar]
- 41.Fan Y, Ni S, Zhang H. Association between healthy eating index-2015 total and component food scores with osteoporosis in middle-aged and older Americans: a cross-sectional study with U.S. national health and nutrition examination survey. Osteoporos Int. 2022;33:921–929. doi: 10.1007/s00198-021-06247-0. [DOI] [PubMed] [Google Scholar]


