Abstract
Objective: To identify the independent risk factors of gastric cancer (GC) lymph node metastasis and to determine whether the preoperative neutrophil and lymphocyte ratio (NLR) and the platelet and lymphocyte ratio (PLR) can be used as the indicators of gastric cancer lymph node metastasis. Methods: The pathological data of 221 patients with gastric cancer were retrospectively analyzed, and the risk factors of lymph node metastasis were evaluated. The relationship between preoperative NLR and PLR and the clinical pathology of patients were analyzed, and the effect of these two indexes on lymph node metastasis was predicted through receiver operating characteristic (ROC) curve. Results: Lymph node metastasis correlated with tumor diameter, depth of invasion, Tumor-Node-Metastasis (TNM) stage, preoperative NLR and preoperative PLR (all P<0.05), but not with gender, age and tumor location (all P>0.05). According to the result of multivariate analysis, the degree of differentiation, depth of invasion, TNM staging and NLR were independent risk factors for GC lymph node metastasis. Conclusion: The sensitivity and specificity of PLR, tumor staging and tumor size are lower than NLR. Preoperative NLR can be used as an independent risk factor for the prediction of lymph node metastasis, and one of the effective indicators for predicting the prognosis of patients. Preoperative NLR may be an effective auxiliary tool to assess lymph nodes in GC patients.
Keywords: Lymph node metastasis, NLR, PLR, prognosis
Introduction
Gastric cancer (GC) is a highly malignant tumor of the digestive tract, and the third risk factor of tumor death in the world [1,2]. Limited by the current gastric cancer screening methods, the detection rate of early gastric cancer in China only accounts for about 20% of new onset gastric cancers, about 80% of gastric cancers are already in the advanced stage when they are diagnosed, and the overall survival rate is less than 50% [3]. The lymph node metastasis is the main and impressive factor for the prognosis of GC [4]. Accurate assessment of lymph node metastasis before surgery is of great value for the selection of surgical methods and the evaluation of the prognosis of gastric cancer [5].
Inflammation is one of the hallmark features of tumors, and the body’s inflammatory response reflects the nonspecific response of tumor tissue to hypoxia, tissue damage, and necrosis [6]. In the early stage of tumors, cytokines promote the massive accumulation of inflammatory cells around the tumor, providing a very favorable tumor microenvironment for tumor initiation and progression, thereby promoting tumor angiogenesis, cell proliferation, and metastasis [7]. At present, a large number of studies have shown that inflammation plays a key role in the occurrence and development of tumors, especially in tumor invasion and metastasis to other organs and lymph nodes, which is directly related to the prognosis of patients [8]. Therefore, identification of the inflammation markers, which are closely related to tumor occurrence and development, has become the focus of research.
White blood cells such as neutrophils are markers of inflammation [9]. Neutrophils can produce cytokines to stimulate tumor blood vessel growth, promote tumor progression, and enhance tumor metastasis and invasiveness [10]. On the other hand, neutrophils, as an inflammatory cell, can inhibit lymphocytes [11]. Activated T cells and natural killer cells can suppress the immune system and reduce the body’s immune defense capabilities. Compared with the traditional single inflammatory indicators such as white blood cells, neutrophils, lymphocytes, and monocytes, more attention has been paid to the composite indicators of inflammation such as NLR and PLR in tumor applications [12,13]. Studies have found that the increase in NLR and PLR are prognostic factors for many malignant tumors [14,15]. Therefore, the present study aimed to investigate the association between NLR or PLR and the prediction of the lymph node metastasis.
Materials and methods
General data
This is a retrospective study. Data of 221 GC patients admitted to our hospital from January 2018 to January 2021 were collected. All study subjects had provided informed consent form, and the research was also reviewed and approved by the Medical Ethics Committee of Affiliated Bozhou Hospital of Anhui Medical University (Bozhou Hospital Ethical Approval-2022-17). The influencing factors that were observed and recorded were the patient’s gender, age, tumor size, degree, depth of invasion, Tumor-Node-Metastasis (TNM) staging, preoperative NLR and preoperative PLR. Specifically: gender (male/female), age (<60/≥60), tumor diameter (<4/≥4), tumor location (cardia/gastric body), depth of invasion, TNM staging (I-II/III-IV), preoperative NLR (<2.26/≥2.26) and preoperative PLR (<142.37/≥142.37).
Inclusion and exclusion criteria
The inclusion criteria are as the following: diagnosed as gastric cancer by pathology or imaging; no active bleeding or coagulation dysfunction within four weeks before surgery, and no blood transfusion treatment; no other tumor-related medical history; no serious perioperative complications; Not accompanied by other serious organic diseases.
Exclusion criteria: Active infection; Chronic inflammatory; Long-term use of steroids or immunosuppressants; Hematological diseases, preoperative data is not adequate; Collect patients including gender, age, tumor location, and clinical data mainly include tumor size, depth of tumor invasion, lymph node metastasis and TNM staging. We collected the routine blood indicators before surgery, such as neutrophil and lymphocyte count, platelet count, and calculate NLR and PLR.
Research content
Univariate analysis of the relationship between lymph node metastasis in this kind of patients and gender, age, tumor diameter, tumor location, depth of invasion, TNM stage, NLR and PLR. Multivariate variable assignment of lymph node metastasis (LNM) in GC patients. Receiver operating characteristic (ROC) curve analysis NLR and PLR were used to predict the area under the curve (AUC) of lymph node metastasis (LNM), the sensitivity and specificity of it.
Statistical methods
Experiment data were analyzed using SPSS20.0 software. Chi-square test was used to analyze the relationship between GC lymph node metastasis and clinical and pathological characteristics, significant difference was judged based on P<0.05. The clinical variables with result of P<0.05 were included into the multivariate logistic regression analysis. GraphPad was used to draw ROC to judge the predictive function of NLR.
Results
Univariate analysis of lymph node metastasis in patients with gastric cancer
Lymph node metastasis in patients with GC was related to tumor size, tumor location, depth of invasion, TNM stage, and preoperative NLR and PLR (all P<0.05), but has no relation with gender and age, such as (Table 1) shows.
Table 1.
Univariate analysis of factors affecting lymph node metastasis
| Factor | Lymph node metastasis | χ2 | P | ||
|---|---|---|---|---|---|
|
| |||||
| LN (-, n = 56) | LN (+, n = 165) | ||||
| Gender | Male | 37 | 91 | 0.716 | 0.529 |
| Female | 28 | 65 | |||
| Age | <60 | 39 | 62 | 0.485 | 0.637 |
| ≥60 | 45 | 75 | |||
| Tumor diameter (mm) | <4 | 23 | 46 | 23.257 | <0.001 |
| ≥4 | 60 | 92 | |||
| Tumor location | Cardia | 17 | 30 | 4.864 | 0.027 |
| Gastric body | 69 | 105 | |||
| NLR | <2.26 | 29 | 59 | 32.693 | <0.001 |
| ≥2.26 | 53 | 70 | |||
| PLR | <142.37 | 32 | 49 | 7.974 | 0.039 |
| ≥142.37 | 51 | 89 | |||
| Infiltration depth | T1-T2 | 27 | 47 | 35.863 | <0.001 |
| T3-T4 | 53 | 94 | |||
| TNM staging | I-II | 38 | 43 | 36.286 | <0.01 |
| III-IV | 45 | 95 | |||
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging.
Multivariate analysis of lymph node metastasis
The variable assignments of multivariate analysis are shown in Table 2, according to the result, it can be inferred that tumor size, TNM stage, depth of invasion and preoperative NLR were risk factors for GC (Table 3).
Table 2.
Variable assignment table for multivariate analysis
| Factor | Variable | Assignment |
|---|---|---|
| Gender | X1 | Male = 1, Female = 2 |
| Age | X2 | <60 y = 1, ≥60 y = 2 |
| Tumor diameter | X3 | <4 mm = 1, ≥4 mm = 2 |
| Tumor location | X4 | Cardia = 1, Gastric body = 2 |
| NLR | X5 | <2.26 = 1, ≥2.26 = 2 |
| PLR | X6 | <142.37 = 1, ≥142.37 = 2 |
| TNM staging | X7 | I-II = 1, III-IV = 2 |
| Infiltration depth | X8 | T1-T2 = 1, T3-T4 = 2 |
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging.
Table 3.
Multivariate analysis of factors affecting lymph node metastasis in GC patients
| Factor | B | SE | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Tumor diameter | 1.512 | 0.725 | 5.846 | 0.019 | 2.896 | 1.354-7.231 |
| Tumor location | 0.472 | 0.479 | 0.832 | 0.673 | 1.298 | 0.325-2.896 |
| NLR | 1.326 | 0.489 | 5.635 | 0.019 | 2.853 | 1.563-7.025 |
| PLR | 0.284 | 0.376 | 0.618 | 0.594 | 1.627 | 0.386-2.637 |
| TNM staging | 1.864 | 0.896 | 7.986 | 0.015 | 3.428 | 1.386-6.297 |
| Infiltration depth | 1.196 | 0.378 | 5.365 | 0.023 | 2.426 | 1.065-5.276 |
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging; OR, Odds Ratio; CI, Confidence Interval.
ROC curve analysis result
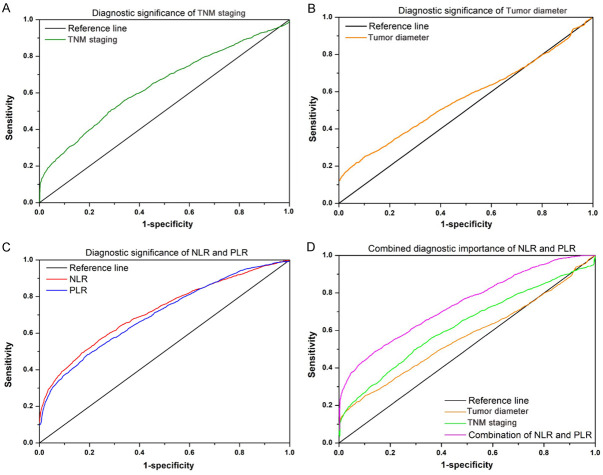
The AUC for predicting lymph node metastasis by preoperative NLR was 0.729 (0.576-0.881), the best cut-off value was 2.26, the sensitivity was 90.0%, and the specificity was 21.5%. The sensitivity and specificity of PLR, tumor staging and tumor size are lower than NLR (Table 4; Figure 1).
Table 4.
NLR and PLR predictive value of gastric cancer prognosis
| Index | Optimal threshold | AUC (95% CI) | Sensitivity (%) | Specificity (%) | Positive value (%) | Negative value (%) |
|---|---|---|---|---|---|---|
| NLR | 2.38 | 0.736 (0.529-0.916) | 81.7 | 32.6 | 81.4 | 92.5 |
| PLR | 136.25 | 0.533 (0.386-0.662) | 72.8 | 29.3 | 52.6 | 89.6 |
| TNM staging | 37.5 | 0.426 (0.312-0.587) | 75.3 | 28.4 | 54.1 | 75.6 |
| Tumor diameter | 49.3 | 0.398 (0.256-0.572) | 65.1 | 31.8 | 42.9 | 78.2 |
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging; AUC, Area Under the Curve; CI, Confidence Interval.
Figure 1.
The receiver operating characteristic curve of the (A) TNM staging, (B) tumor diameter, (C) NLR/PLR, and (D) the combining NLR and PLR. Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging.
Predictive of NLR in subsets of patients
We performed the analysis in the different subgroups of patients to explore the association between NLR and lymph node metastasis. The results are presented in the Figure 2. We found that high level of NLR was significantly associated with the status of the lymph node. Patients with a high level of NLR were at high risk of lymph node metastasis.
Figure 2.
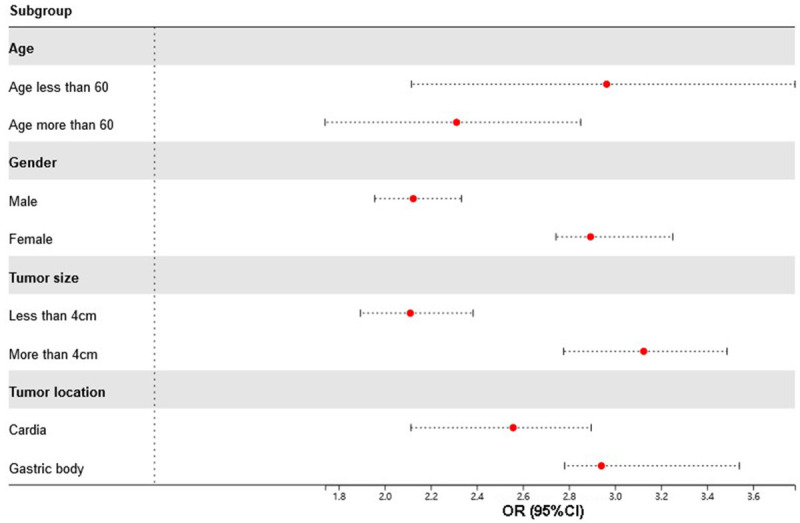
Subgroup analysis of the predictive value of NLR. Abbreviation: NLR, Neutrophil/Lymphocyte Ratio.
A nomogram to predict the lymph node metastasis
After having obtained the independent risk factors for the lymph node metastasis in multivariate analysis, we used the tumor diameters, NLR and tumor stage to perform the nomogram analysis. The nomogram for the prediction of the lymph node metastasis are presented in the Figure 3.
Figure 3.
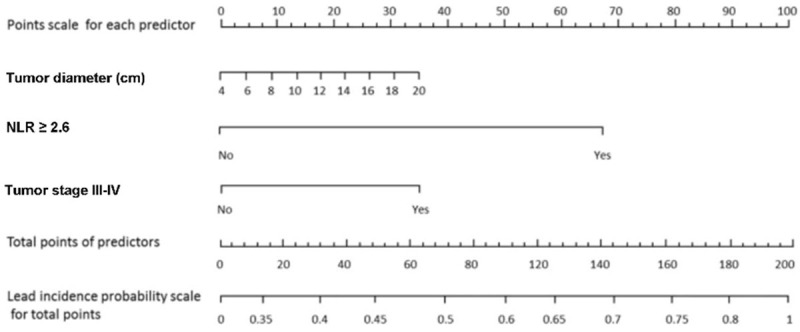
Nomogram for the prediction of lymph node metastasis. Abbreviation: NLR, Neutrophil/Lymphocyte Ratio.
Lymph node metastasis and survival of patients
All included patients were followed for their survival status. Then we performed the survival analysis to explore if the lymph status was associated with the survival of the patients. The results are presented in the Figure 4. As the Kaplan-Meier (KM) curve showed, significantly worse survival was observed in patients with the lymph nodes metastasis.
Figure 4.
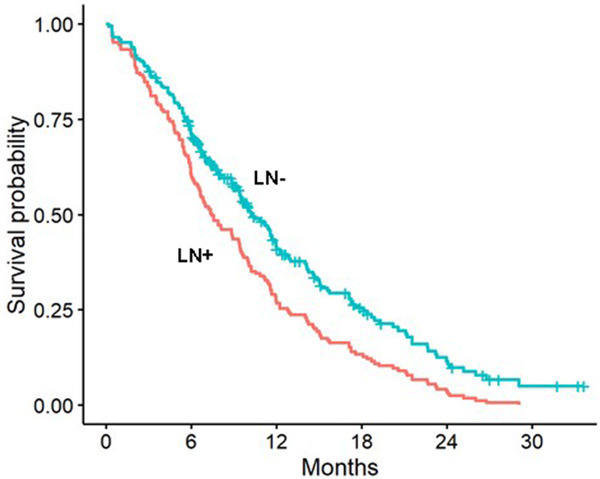
Kaplan-Meier curve of survival for the lymph node metastasis. Abbreviations: LN+, Lymph Node positive; LN-, Lymph Node negative.
NLR/PLR and survival of patients
We subsequently explored the clinical factors that were associated with the survival of the patients. Survival analysis with Cox regression analysis was performed. The univariate analysis showed that both NLR and PLR were associated with the overall survival (Table 5). The multivariate analysis showed that the tumor diameters, tumor location, NLR, tumor stage, and infiltration depth were independently associated with the survival of patients (Table 6).
Table 5.
Univariate analysis of factors affecting survival in patients with gastric cancer
| Factor | P | HR | 95% CI |
|---|---|---|---|
| Age | 0.084 | 1.231 | 0.887-1.557 |
| Gender male | 0.241 | 1.521 | 0.674-1.941 |
| Tumor diameter | 0.002 | 2.212 | 1.776-2.743 |
| Tumor location | 0.013 | 1.894 | 1.234-2.412 |
| NLR | 0.001 | 2.412 | 1.447-3.312 |
| PLR | 0.023 | 1.723 | 1.212-2.561 |
| TNM staging | 0.004 | 1.852 | 1.652-2.345 |
| Infiltration depth | 0.003 | 2.144 | 1.899-2.564 |
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging; HR, Hazard Ratio; CI, Confidence Interval.
Table 6.
Multivariate analysis of factors affecting survival in patients with gastric cancer
| Factor | P | HR | 95% CI |
|---|---|---|---|
| Tumor diameter | 0.003 | 2.344 | 1.987-2.784 |
| Tumor location | 0.002 | 2.564 | 1.452-3.122 |
| NLR | 0.003 | 2.512 | 1.347-3.411 |
| PLR | 0.052 | 1.621 | 0.798-2.322 |
| TNM staging | 0.004 | 1.859 | 1.655-2.447 |
| Infiltration depth | 0.002 | 2.213 | 1.778-2.664 |
Abbreviations: NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; TNM staging, Tumor-Node-Metastasis staging; HR, Hazard Ratio; CI, Confidence Interval.
Discussion
It was previously found that the proportion of neutrophils in peripheral blood increased in patients with advanced cancer, and regression analysis confirmed the correlation between NLR and the prognosis of GC patients. In recent years, the effect and mechanism of inflammatory factors on occurrence and development of cancer has attracted more and more attention [16-18]. Although the mechanism by which neutrophils affect gastric cancer is not fully understood, it is clinically believed that neutrophils can release cell growth factors, oxygen free radicals, proteases, chemokines and other components, and activate related nuclear factors to promote tumor cells growth [19,20]. At the same time, neutrophils can also inhibit the body’s immune response to tumor cells by secreting inflammatory factors, promote angiogenesis around the tumor and induce tumor cell metastasis [21-26]. Lymphocyte is a key link in human cellular immunity and humoral immunity, and it has a certain inhibitory effect on the proliferation of cancer cells, as well as hinders the metastasis of cancer cells. Therefore, the balance of inflammatory environment and anti-tumor immunity on tumor growth can be reflected by NLR [27-30].
As an indicator of inflammatory response, NLR can predict the gastric cancer risk effectively and a higher PLR also indicates a poor prognosis for GC [31-36]. It has been shown that inflammation caused by tumors may lead to changes in NLR. A low NLR indicates that the tumor is in non-active state, and thus a better prognosis of tumor patients. A higher NLR indicates the risk of tumor recurrence, and the preoperative and postoperative NLR values are equally important. Shimada et al. The preoperative average NLR of lymph nodes in GC was significantly higher than that in N0 patients, but they have not studied the predictive ability of NLR in lymph node metastasis in detail [37-39].
In this study, Tumor diameter, TNM stage and preoperative NLR were risk factors for gastric cancer lymph node metastasis. Although previous studies have shown the association between both NLR and PLR and the prediction of the lymph node metastasis [40]. However, in our study, the multivariate analysis result showed that there is no correlation between lymph node metastasis and PLR in gastric cancer. This is possibly due to the fact that small number of patients with gastric cancer were tested before surgery. The AUC for predicting lymph node metastasis by preoperative NLR was 0.729 (0.576-0.881), the sensitivity is 90.0%, while specificity is 21.5%. The limit values of NLR and PLR were determined based on ROC. The sensitivity of PLR, tumor staging and tumor size are lower than NLR. This is more objective for the assessment of lymph node metastasis in GC patient. Additionally, we found there was a significant higher predictive value when we combined NLR and PLR together for the prediction of lymph node metastasis than the TNM staging and also tumor diameter. To the best of our knowledge, this is the first study for the evaluation of the predictive value with the combination of the NLR and PLR in the clinical practice.
There exist some limitations for this study and the specific mechanism of the relationship between NLR, PLR and LNM has not been clarified. However, routine blood testing is simple, fast, low-cost, and does not increase the patient’s physical pain and economic burden. The preoperative blood routine testing obtains NLR and PLR, which can be applied to assess LNM in patients with GC and guide surgery. There was one more limitation that we could not prove that NLR is just a proxy or a true prognostic indicator of more chance of lymph node metastasis in gastric cancer, which needs further exploration.
Conclusion
In summary, the lymph node metastasis of GC is related to many factors. Preoperative NLR can predict that precisely, and the increase of NLR is related to the aggressiveness of the tumor. Therefore, besides other factors, NLR and related inflammatory indicators should be tested to guide the treatment plan. This study affirmed the value of preoperative NLR in evaluating the prognosis of GC patients, but meanwhile, other immunonutritional indicators (such as prognostic nutritional index) may also be useful predictors of lymph nodes. The proper NLR threshold needs to be verified in further studies with a larger sample size.
Acknowledgements
This work was supported by the Research Project of BZWJ2022a001, Project of Bozhou Municipal Health Commission, The Research Project of BZZC2022008, Project of Bozhou Science and Technology Bureau.
Disclosure of conflict of interest
None.
References
- 1.Urabe M, Yamashita H, Watanabe T, Seto Y. Comparison of prognostic abilities among preoperative laboratory data indices in patients with resectable gastric and esophagogastric junction adenocarcinoma. World J Surg. 2018;42:185–194. doi: 10.1007/s00268-017-4146-9. [DOI] [PubMed] [Google Scholar]
- 2.de Jong MC, Mihai R, Khan S. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as possible prognostic markers for patients undergoing resection of adrenocortical carcinoma. World J Surg. 2021;45:754–764. doi: 10.1007/s00268-020-05868-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Zheng R, Zhang S, Chen R, Wang S, Sun K, Li M, Lei S, Zhuang G, Wei W. Gastric and esophageal cancer in China 2000 to 2030: recent trends and short-term predictions of the future burden. Cancer Med. 2022;11:1902–1912. doi: 10.1002/cam4.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit. 2019;25:3537–3541. doi: 10.12659/MSM.916475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li GZ, Doherty GM, Wang J. Surgical management of gastric cancer: a review. JAMA Surg. 2022;157:446–454. doi: 10.1001/jamasurg.2022.0182. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galdiero MR, Marone G, Mantovani A. Cancer inflammation and cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028662. doi: 10.1101/cshperspect.a028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. 2019;99:1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 11.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, You Q, Li Z, Ma Y, Li C, Song H, Shi H, Zhang Y, Yu X, Gao H, Sun Y, Xie R, Xue Y. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. 2020;2020:9146042. doi: 10.1155/2020/9146042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, Johansson M. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36:841–848. doi: 10.1007/s10654-021-00752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xin Y, Yang Y, Liu N, Chen Y, Wang Y, Zhang X, Li X, Zhou X. Prognostic significance of systemic immune-inflammation index-based nomogram for early stage hepatocellular carcinoma after radiofrequency ablation. J Gastrointest Oncol. 2021;12:735–750. doi: 10.21037/jgo-20-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahng AW, Reicher S, Chung D, Varela D, Chhablani R, Dev A, Pham B, Nieto J, Venegas RJ, French SW, Stabile BE, Eysselein VE. Staining for p53 and Ki-67 increases the sensitivity of EUS-FNA to detect pancreatic malignancy. World J Gastrointest Endosc. 2010;2:362–368. doi: 10.4253/wjge.v2.i11.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Wang H, Ma J, Hao J, Zhang C, Ma Q, Wang B. Association between the platelet to lymphocyte ratio, neutrophil to lymphocyte ratio and axillary lymph node metastasis in cT1N0 breast cancer patients. Am J Transl Res. 2021;13:1854–1861. [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid HKS, Davis GN, Trejo-Avila M, Igwe PO, Garcia-Marín A. Prognostic and predictive value of neutrophil-to-lymphocyte ratio after curative rectal cancer resection: a systematic review and meta-analysis. Surg Oncol. 2021;37:101556. doi: 10.1016/j.suronc.2021.101556. [DOI] [PubMed] [Google Scholar]
- 18.Nemoto T, Endo S, Isohata N, Takayanagi D, Nemoto D, Aizawa M, Utano K, Togashi K. Change in the neutrophil-to-lymphocyte ratio during chemotherapy may predict prognosis in patients with advanced or metastatic colorectal cancer. Mol Clin Oncol. 2021;14:107. doi: 10.3892/mco.2021.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamoto S, Ikeda M, Kubo S, Yamamoto M, Yamashita T, Notsu A. Systemic immunity markers associated with lymphocytes predict the survival benefit from paclitaxel plus bevacizumab in HER2 negative advanced breast cancer. Sci Rep. 2021;11:6328. doi: 10.1038/s41598-021-85948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uludag SS, Sanli AN, Zengin AK, Ozcelik MF. Systemic inflammatory biomarkers as surrogate markers for stage in colon cancer. Am Surg. 2022;88:1256–1262. doi: 10.1177/0003134821995059. [DOI] [PubMed] [Google Scholar]
- 21.Stamatopoulos N, Espada Vaquero M, Leonardi M, Nadim B, Bailey A, Condous G. Pre-operative classification of molar pregnancy: how good is ultrasound? Aust N Z J Obstet Gynaecol. 2020;60:698–703. doi: 10.1111/ajo.13130. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi Y, Tsujimoto H, Hiraki S, Kouzu K, Tsuchiya S, Itazaki Y, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Kishi Y, Ueno H. Predictive value of immuno-inflammatory and nutritional measures modulated by neoadjuvant chemotherapy on the response of neoadjuvant chemotherapy and long-term outcomes in patients with esophageal cancer. Oncol Lett. 2020;19:487–497. doi: 10.3892/ol.2019.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, Kim JM, Chin YJ, Chong GO, Park SH, Lee YH, Hong DG, Lee YS. Prognostic value of hematological parameters in locally advanced cervical cancer patients treated with concurrent chemoradiotherapy. Anticancer Res. 2020;40:451–458. doi: 10.21873/anticanres.13973. [DOI] [PubMed] [Google Scholar]
- 24.Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in non-muscle invasive bladder cancer patients: initial intravesical bacillus calmette-guerin treatment after transurethral resection of bladder tumor setting. Front Oncol. 2018;8:642. doi: 10.3389/fonc.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Liu Y, Yang C, Li X, Pan C, Rao J, Li N, Liao W, Lin L. Combined neutrophil/platelet/lymphocyte/differentiation score predicts chemosensitivity in advanced gastric cancer. BMC Cancer. 2018;18:515. doi: 10.1186/s12885-018-4414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue D, Ozaka M, Matsuyama M, Yamada I, Takano K, Saiura A, Ishii H. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45:61–66. doi: 10.1093/jjco/hyu159. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2014;13:474–481. doi: 10.1016/s1499-3872(14)60284-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Li D, Ou X, Yi Q, Feng Y. Diagnostic accuracy of Ber-EP4 for metastatic adenocarcinoma in serous effusions: a meta-analysis. PLoS One. 2014;9:e107741. doi: 10.1371/journal.pone.0107741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cihan YB, Ozturk A, Mutlu H. Relationship between prognosis and neutrophil: lymphocyte and platelet: lymphocyte ratios in patients with malignant pleural mesotheliomas. Asian Pac J Cancer Prev. 2014;15:2061–2067. doi: 10.7314/apjcp.2014.15.5.2061. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed Z, Pinato DJ, Mauri FA, Chen KW, Chang PM, Sharma R. Inflammation as a validated prognostic determinant in carcinoma of unknown primary site. Br J Cancer. 2014;110:208–213. doi: 10.1038/bjc.2013.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang L, Zhao S, Liu W, Parchim NF, Huang J, Tang Y, Gan P, Zhong M. Diagnostic accuracy of circulating tumor cells detection in gastric cancer: systematic review and meta-analysis. BMC Cancer. 2013;13:314. doi: 10.1186/1471-2407-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 33.Chua TC, Chong CH, Liauw W, Zhao J, Morris DL. Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg. 2012;256:342–349. doi: 10.1097/SLA.0b013e3182602ad2. [DOI] [PubMed] [Google Scholar]
- 34.Tsiodras S, Georgoulakis J, Chranioti A, Voulgaris Z, Psyrri A, Tsivilika A, Panayiotides J, Karakitsos P. Hybrid capture vs. PCR screening of cervical human papilloma virus infections. Cytological and histological associations in 1270 women. BMC Cancer. 2010;10:53. doi: 10.1186/1471-2407-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Solanki SL, Kaur J, Gupta AM, Patkar S, Joshi R, Ambulkar RP, Patil A, Goel M. Cancer related nutritional and inflammatory markers as predictive parameters of immediate postoperative complications and long-term survival after hepatectomies. Surg Oncol. 2021;37:101526. doi: 10.1016/j.suronc.2021.101526. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa H, Nagasaka T, Cullings HM, Notohara K, Hoshijima N, Young J, Lynch HT, Tanaka N, Matsubara N. Efficient molecular screening of Lynch syndrome by specific 3’ promoter methylation of the MLH1 or BRAF mutation in colorectal cancer with high-frequency microsatellite instability. Oncol Rep. 2009;21:1577–1583. doi: 10.3892/or_00000390. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011;35:1861–1866. doi: 10.1007/s00268-011-1130-7. [DOI] [PubMed] [Google Scholar]
- 40.Gawiński C, Hołdakowska A, Wyrwicz L. Correlation between lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and extramural vascular invasion (EMVI) in locally advanced rectal cancer. Curr Oncol. 2022;30:545–558. doi: 10.3390/curroncol30010043. [DOI] [PMC free article] [PubMed] [Google Scholar]