Abstract
Background: The occurrence of extramedullary plasmacytoma (EMP) in the ovaries is rare. Here, we describe the clinical, pathological and radiological presentations of ovarian plasmacytomas to improve the differential diagnosis of this disease. Also, the reasons for misdiagnosis, clinical manifestations and radiological features were discussed through a literature review. Case presentation: A 54-year-old woman was suspected to have EMP upon routine ultrasound examination and was subsequently diagnosed using pathological examination of the left ovarian mass. The radiological features of this case included (1) a solitary soft-tissue mass in the left ovary with clear boundaries; (2) a homogeneous mass with medium-density without necrosis, which was homogeneously enhanced after contrast medium injection; and (3) magnetic resonance imaging showing a homogeneous lesion with isointense signals on T1- and T2-weighted imaging, restricted diffusion on diffusion-weighted imaging, and a low apparent diffusion coefficient value of approximately 0.72×10-3 mm2/s, which was significantly and homogeneously enhanced after contrast medium injection with a rapid rise-slow decay type, and with thickened vascular shadows around the lesion. Conclusions: EMP in the ovary is rare and only a few cases have been reported. We reviewed EMP-related literature and the summarized the clinical manifestations, radiological features and treatment strategies of this disease to help the diagnosis and management. Application of second-line drugs might be a viable strategy to improve the survival rate of patients and to prevent the progression to a certain extent.
Keywords: Extramedullary plasmacytoma, ovary, radiological presentations, differential diagnosis
Introduction
Plasma cell neoplasms (plasmacytomas) are characterized by neoplastic proliferation of plasma cells, synthesizing monoclonal immunoglobulins. Depending on the site, they can be categorized as single (solitary plasmacytoma) or multiple lesions (multiple myeloma, MM) [1]. Solitary plasmacytomas mostly occur in the bone (plasmacytoma of the bone) but can also occur in soft tissues outside the bone (extramedullary plasmacytoma, EMP). EMP may present as single or multiple lesions [1,2]. The clinical course, treatment and prognosis of solitary plasmacytoma of the bone, EMP, multiple or solitary plasmacytoma and MM are different, and they should be regarded as independent diseases rather than as a spectrum of the same disease [2,3].
EMPs most frequently develop in the upper aerodigestive tract, approximately 80% in the head and neck but rarely in the ovary [4,5]. Few cases of solitary ovarian EMP and a literature review have been reported [6-11]. In addition, EMP is a special form of plasmacytoma with unique biological behavior, clinical manifestations and treatment strategies [12]. Therefore, preoperative diagnosis is very important. Due to its rarity, there are few reports on the radiological features of ovarian plasmacytomas. We discussed the clinical manifestations, radiological features, diagnosis and differential diagnosis of EMPs in the ovary with a literature review, to improve the radiological understanding of this tumor and the diagnostic accuracy.
Case presentation
A 54-year-old woman with no obvious discomfort underwent routine ultrasound examination, which revealed a well-defined hypoechoic nodule, approximately 0.9×0.7 cm in size, on the left uterine wall and a well-defined cystic mass full of fine echoes about 4.9×4.2 cm in size in the left adnexa. A diagnosis of a uterine hypoechoic nodule (myoma) and cystic mass (chocolate cyst) in the left adnexa was considered.
Gynecological examination after admission revealed a mass of approximately 4 cm in diameter without tenderness in the left adnexa. Complete blood count revealed 77% neutrophils, human chorionic gonadotropin yielded negative for pregnancy, and reproductive hormone levels and biochemical tests were within normal ranges. Furthermore, bone marrow examination results were normal. Immunofixation electrophoresis, immunoglobulin and urinary protein were negative. Moreover, positron emission tomography-computed tomography (PET-CT) showed no other organ involvement.
Contrast-enhanced computed tomography (CT) of the whole abdomen revealed an oval, well-defined homogeneous and isodense mass measuring approximately 4.5×4.0 cm in the left pelvic cavity, with homogenous enhancement. A diagnosis of a left pelvic mass, which could have been a benign tumor developing from the left adnexa with the possibility of an ovarian fibrothecoma or a broad ligament fibroid, was considered. Contrast-enhanced magnetic resonance imaging (MRI) of the pelvis revealed a well-defined, roundish, homogeneous mass measuring 4.6×4.1×3.7 cm with isointense signals on T1-weighted imaging (T1WI) and T2-wei-ghted imaging (T2WI), hyperintense signals on diffusion-weighted imaging (DWI), and a low apparent diffusion coefficient (ADC) value of 0.72×10-3 mm2/s in the left adnexa. In addition, there was a significant homogeneous enhancement after contrast medium injection. A possibility of fibrothecoma was considered and broad ligament fibroid needed to be excluded (Figure 1).
Figure 1.
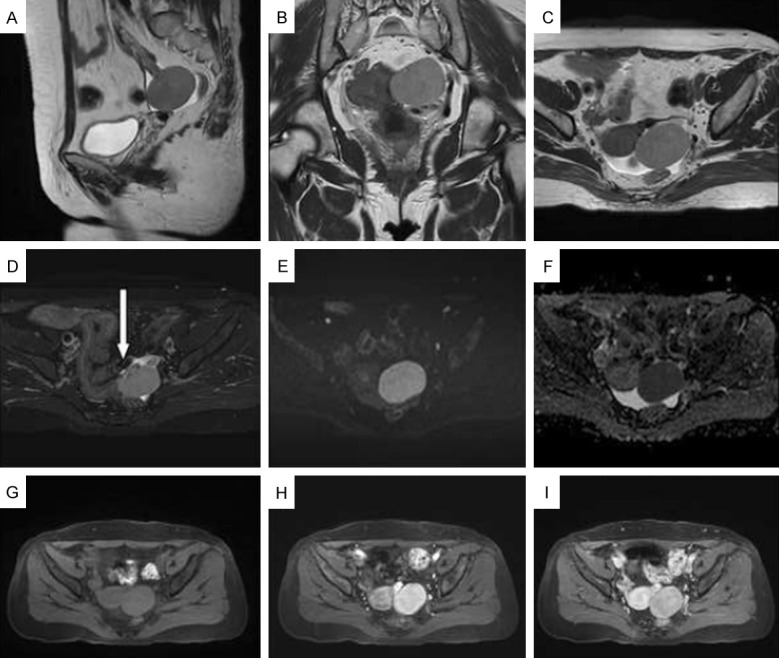
Magnetic resonance imaging. A-D: Sagittal, coronal, and transverse T2-weighted imaging (T2WI) images show an isolated soft-tissue mass in the left ovary, measuring 4.5×4.0 cm, with a clear boundary. On T2WI, the mass appears slightly higher in position and uniform, surrounded by thick, hollow vascular shadows (white arrow); E, F: Limited diffusion on diffusion-weighted imaging (DWI) and low signal on apparent diffusion coefficient (ADC). The ADC value was 0.72×10-3 mm2/s; G-I: T1-weighted imaging (T1WI) showing a uniform isointense signal on a plain scan. Enhancement was uniform in the early stage and then withdrew in the late stage, presenting with a rapid outflow pattern.
Intraoperatively, a 5×5 cm solid mass was found in the left ovary with a smooth outer surface and a relatively intact capsule, with gray-white cut surface, and the mass tissue was brittle and fragile for clamping. Hematoxylin-eosin staining of the tumor at high and medium magnifications revealed that the tumor was diffusely and loosely arranged. The cells were ovoid, slightly larger plasma cells with eccentric nuclei, coarse chromatin and basophilic cytoplasm arranged in a spoke-wheel pattern, along with binuclear cells (Figure 2). Intraoperative rapid frozen sections suggested a round cell tumor of the left ovary that was morphologically consistent with a plasma cell-derived tumor. However, sex cord-stromal tumors, such as the granulosa cell tumor, had to be excluded. The immunohistochemistry indicated CD138(+), CD163(-), L(+), R(-), CK(-), MuM1(+), CD79a(+), CD19(-), CD20(-), CD38(+), CD56(-), CD117(-), α-inhibin(-), SF1(-), Ki-67(+) 50%, Kappa(-), and Lambda(+) (Figure 2). The results indicated an ovarian plasmacytoma.
Figure 2.
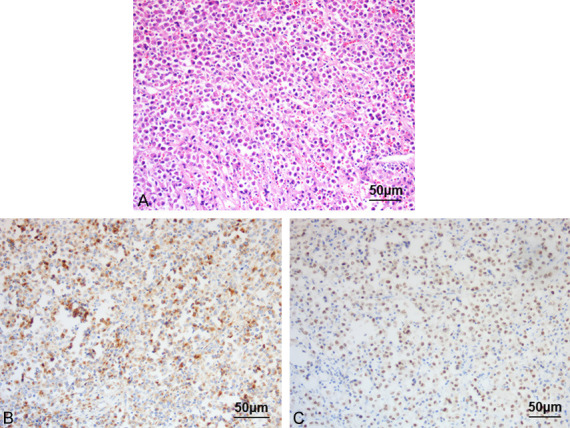
Hematoxylin and eosin staining at a medium high (×20) magnification. A: Hematoxylin and eosin staining showing the tumor tissue diffusely arranged and the ovoid, slightly larger than normal plasma cells with eccentric nuclei, coarse chromatin, and basophilic cytoplasm arranged in a spoke-wheel pattern, along with binuclear cells. B, C: Immunohistochemical staining showing MUM1(+) and CD138(+).
Repeated bone marrow flow cytometry revealed no abnormal clonal plasma cell population (<0.01%) and 1.1% of normal plasma cell phenotype (CD45+, CD38+, CD138+, CD19+, CD56-, CD28-, CD20-, and CD117-). Bone marrow cell examination indicated a plasmacytoma with occasionally discernible mature plasma cells (0.5%). Histological examination revealed predominant granulocytic lineage with good hematopoiesis, and plasma cells were not increased (plasma cells accounted for approximately 1% with occasionally discernible proplasmacytes). Plasmacytoma fluorescence in situ hybridization results showed that Iq21 amplification was positive, while RB1, IGH, P53 and D13S319 were negative. Karyotype analysis was 46, XX [13]. Blood immunofixation electrophoresis, immunoglobulin, troponin and urine protein were negative. Repeat whole-body PET-CT imaging revealed postoperative changes in the left ovary with increased fluorodeoxyglucose metabolism in the surrounding soft tissues, possibly due to inflammation.
Subsequently, the patient underwent five sessions of pomalidomide/cyclophosphamide/dexamethasone regimen (bortezomib 1.9 mg, cyclophosphamide 0.3 g, and dexamethasone 40 mg, all administered once per week), and two sessions of ixazomib/dexamethasone regimen (ixazomib capsules 4 mg once per week for 3 weeks combined with dexamethasone 20 mg once per week). The patient has been followed up regularly for 2 years and is currently in a stable condition. The manifestations of ovarian plasmacytoma in the current case are illustrated in Table 1.
Table 1.
Manifestations of the ovarian plasmacytoma reported in the present study
| Parameter | Value/Characteristic | |
|---|---|---|
| Info | Age/sex | 54, female |
| Site | L, ovary | |
| Symptoms | Mass, 4 cm, no pain | |
| US | L, uterine wall | Well-defined, hypoechoic nodule, 0.9×0.7 cm |
| L, adnexa | Well-defined, cystic mass with fine echoes, 4.9×4.2 cm | |
| Neutrophils (%) | 77 | |
| PET-CT | N/I | |
| CT | L, pel | oval, well-defined, homo, iso mass, 4.5×4.0 cm |
| MRI | pel | Roundish, well-defined, homo mass, 4.6×4.1×3.7 cm, hemo enhancement after CM |
| T1W1: isointense signals | ||
| T2W1: isointense signals | ||
| DWI: hyperintense signals | ||
| L, adnexa | ADC (mm2/s): 0.72×10-3 | |
| Visualization | L. ovary | 5×5 cm, mass, smooth, intact capsule |
| Histology | Tissue | Diffuse and loose |
| Cell | Eccentric nuclei, basophilic cytoplasm, spoke-wheel pattern | |
| IHC | + | CD138, L, MuM1, CD79a, CD38, Ki-67, Lambda |
| - | CD163, R, CK, CD19, CD20, CD56, CD117, α-inhibin, SF1, Kappa | |
| Plasma cell (%) | normal | 1.1% |
| abnormal | <0.01% | |
| FISH | + | Iq21 |
| - | RB1, IGH, P53, D13S319 | |
| Treatment | PCD-ID | |
| Follow-up | Stable within 2 yr | |
Note: Info: information; US: ultrasound; L: left; N/I: no other organ involvement; CT: Contrast-enhanced computed tomography; abd: abdomen; pel: pelvic cavity; homo: homologous; iso: isodense; CM: contrast medium; T1WI: T1-weighted imaging; T2WI: T2-weighted imaging; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; IHC: Immunohistochemistry; yr: years; FISH: fluorescence in situ hybridization; PCD: pomalidomide/cyclophosphamide/dexamethasone regimen; ID: ixazomib/dexamethasone.
Literature review and discussion
EMP refers to plasma cell tumors that originate outside the bone marrow hematopoietic tissues [14-16]. With the continuous advent of new drugs, myeloma patients’ survival time has significantly increased, but the incidence extramedullary lesion is increasing [17,18]. Using sensitive imaging technologies, such as PET-CT and MRI, the EMP diagnosis rate has significantly improved [19]. The medical community has provided an in-depth and comprehensive understanding on the classification, pathogenesis, clinical features and treatment of the disease [13,20-24].
Primary plasmacytoma, both inside and outside the bone, differs from MM due to the absence of hypercalcemia, renal insufficiency, anemia, and marrow plasma cell proliferation, with normal skeletal examination and serum or urine protein levels <2 g/dL [25]. In this case, multiple bone marrow examinations detected no clonal B lymphocytes or abnormal plasma cell populations, and immunofixation electrophoresis was negative. Both immunoglobulin and urine protein were negative, and PET-CT did not indicate involvement of other sites. Therefore, myeloma was excluded. The pathological diagnosis of the left ovarian mass was plasmacytoma, thus, EMP was considered.
EMPs most frequently develop in the upper aerodigestive tract, with approximately 80% in the head and neck, particularly in the nasal cavity/sinuses (30%-60%), followed by the nasopharynx/oropharynx (20%) [4,26]. In addition, approximately 10%-20% of patients have cervical lymph node metastasis at presentation time [14,27,28]. Other common sites include tonsils, lacrimal glands, lymph nodes, thyroid gland, gastrointestinal tract, genitourinary tract, testes, and breast [29-33], but the occurrence in the ovaries is rare. In the present study, we retrieved relevant literature using the terms ‘extramedullary plasmacytoma’ OR ‘EMP’ AND ‘case report’ OR ‘features’ OR ‘diagnosis’ OR ‘imaging findings’. In the end, 14 articles were included, in which a total of 406 cases of EMP were reported and analyzed (Table 2). Based on these reports we summarized the following characteristics.
Table 2.
Main clinical features and outcomes of extramedullary plasmacytomas in literature reviews
| Author | Cases | M/F | Range of ages | Site | Main symptoms | Treatment | Progression |
|---|---|---|---|---|---|---|---|
| Susnerwala [14], 1997 | 25 | 23/2 | 27~84 | Head & neck | Nasal-related diseases | RT/CT/RT-CT | 14 |
| Reed [26], 2011 | 25 | NA | NA | Head & neck, abd | NA | RT | 0 |
| Yang [34], 2006 | 46 | 32/14 | 4~85 | Head & neck | Pain, mass, swell | RT/RT-CT | NA |
| Tang [35], 2005 | 18 | 14/4 | 39~77 | Thorax | Pain, mass, cough | NA | NA |
| Ooi [37], 2006 | 12 | 8/4 | NA | Head & neck, thorax, abd | mass | NA | 5 |
| Wang [39], 2018 | 6 | 3/3 | 29~76 | Head & neck, thorax | Pain, mass, cough | NA | NA |
| Liu [40], 2017 | 8 | 6/2 | 43~86 | Head & neck | Pain, mass, vomit, hemorrhage | NA | NA |
| Tong [41], 2016 | 6 | 4/2 | 42~65 | Head & neck, thorax, abd, testis | Pain, mass | Surgery/Surgery-RT-CT | 1 |
| Nina [49], 1990 | 13 | 12/1 | 6~76 | Head & neck, lymph node | Surgery-RT | 5 | |
| Liebross [50], 1999 | 22 | 19/3 | 31~80 | Head & neck, colon, pleura | mass | Surgery/surgery-RT | 12 |
| Rangeard [51], 2006 | 17 | 14/3 | 39~80 | Head & neck | NA | Surgery/surgery-RT | 4 |
| Richardson [53], 2003 | 193 | 116/77 | 34~84 | NA | NA | Drug/SCT-drug | NA |
| Rolins [54], 1995 | 1 | 1/0 | 43 | Nose | Pain, mass | Surgery-RT | 1 |
| Holland [55], 1992 | 14 | 9/5 | 20~85 | Head & neck, thorax, abd, pelv | NA | RT | 5 |
Note: M: male; F: female; NA: not available; abd: abdomen; pelv: pelvic; RT: Radiation therapy; CT: chemotherapy; SCT: stem cell transplant.
Clinical manifestations
Clinical manifestations of EMP are more common in men than women, with a male-to-female ratio of 3:1. The onset age is mostly between 50 and 70 years. EMP is considered to lack characteristic clinical manifestations, and it usually manifests with pain or the corresponding symptoms caused by compression of adjacent tissues, with soft-tissue masses outside the bone marrow as the first symptom, leading to an easy misdiagnosis of masses with other features [34,35]. EMPs usually occur in patients with high tumor burdens and can exist independently or with MM [36]. EMP is classified into three clinical stages: stage I, limited to the original site; stage II, severe damage to surrounding tissues or involvement of nearby draining lymph nodes; stage III, distant metastasis. This classification is of great significance for guiding treatment and analyzing prognosis [14].
A definite EMP diagnosis needs to exclude MM since over 60% of EMP patients can be cured with only local therapies, while the 5-year survival rate for patients with MM is approximately 35% [37].
Pathology and immunohistochemistry
EMP is composed of neoplastic monoclonal plasma cells, but there are obvious differences in the degree of differentiation. Bartl et al. classified EMP into three grades according to the degree of cell differentiation: grade I (low grade), grade II (intermediate grade) and grade III (high grade) [38]. Immunohistochemistry usually shows positive expressions of CD79a, CD38, CD138 and Lambda/Kappa, with a Ki-67 proliferation index of about 40%, and negative expressions of CD3, CD5 and CD20. Immunohistochemistry of this case revealed CD38(+), CD138(+), CD79a(+), Ki-67(+) 50%, and Lambda(+), which supported the diagnosis of EMP.
Radiological features
EMP is easy to misdiagnose because of its low incidence and lack of specific radiological features. Based on the literature review, we summarized the radiological features of EMP as follows. (1) The mass is isolated, round or oval, smooth, well-circumscribed and homogeneous. (2) Plain CT-scan shows well-circumscribed soft-tissue masses with homogeneous density, presenting as large lesions with small necrosis. (3) Contrast-enhanced CT scan shows masses with moderate to significant homogeneous enhancement, with tortuous and thick vascular shadows within and around the tumors. (4) MRI scan shows masses with isointense or slightly hypointense signals on T1WI, isointense or slightly hyperintense homogeneous signals on T2WI, hyperintense signals on DWI, and an ADC value <1.2×10-3 mm2/s, suggesting malignancy [39]. Contrast-enhanced MRI scan shows moderate to prominent homogeneous enhancement with tortuous and thickened flow-empty vascular shadows within and around the tumor, and necrosis is rare [40]. Tong et al. reported that prominent enhancing septa of various shapes and different numbers could be seen inside the EMP after enhancement, histologically corresponding to the loose interstitial structure with abundant blood vessels [41]. However, this case showed homogeneous enhancement without the abovementioned phenomenon, which might be related to its rarity. Lastly, (5) destruction of the adjacent bone can be observed, while bone sclerosis is rare. Draining lymph nodes near the lesion can be enlarged with insignificant internal necrosis [39]. In this case, no destruction or sclerosis of the adjacent bone was observed, which might be related to the lesions originating in the pelvic cavity and far away from the pelvic bone, and there was no obvious enlargement of the lymph nodes adjacent to the drainage area.
The radiological features of this case were as follows. (1) A solitary soft-tissue mass was found in the left ovary with clear boundaries. (2) A homogeneous mass with medium-density without necrosis was found and homogeneously enhanced after contrast medium injection. (3) An MRI scan showed a homogeneous lesion with isointense signals on T1WI and T2WI, restricted diffusion on DWI, and a low ADC value of approximately 0.72×10-3 mm2/s, which was significantly and homogeneously enhanced after contrast medium injection with a rapid rise-slow decay type, and with thickened vascular shadows around the lesion. These features are consistent with the literature reports.
Analysis of the causes of misdiagnosis
We misdiagnosed this case as fibrothecoma and broad ligament fibroid, and here are the reasons. (1) The lesion presented as homogeneous hypointense signals on both T1WI and T2WI, leading to a consideration of fibrous components of the lesion, which was similar to fibrothecoma. (2) A solitary mass in the adnexa with homogeneous hypointense signals, which was significantly and homogeneously enhanced after contrast medium injection, was similar to a broad ligament fibroid.
Differential diagnosis
Fibrothecoma is mainly seen in perimenopausal and postmenopausal women. Some tumors can secrete estrogen to stimulate the endometrium, leading to endometrial hyperplasia [42]. The radiological features are as follows. (1) The tumors mostly appear as unilateral solid or cystic-solid masses [43]. (2) The masses are round or roundish and occasionally lobulated [41]. (3) The tumors usually have clear boundaries with intact capsules [44,45]. (4) The tumors appeared as masses with homogeneous density on CT and were slightly enhanced after contrast medium injection with a delayed accumulation of the contrast medium. On MRI scans, the tumors are mainly isointense or hypointense on T1WI and have complex signals on T2WI. The density on CT and signal intensity on MRI are related to tumor components [46,47]. The patient had no history of abnormal menstruation or irregular vaginal bleeding. The tumor appeared as a left ovarian mass with homogeneous isodensity on CT and homogeneous isointense signals on T1WI and T2WI. After enhancement, the tumor was significantly and homogeneously enhanced, with a rapid rise-slow decay type. Thus, this case was inconsistent with fibrothecoma.
The onset age of broad ligament fibroids is relatively early, and the tumor is closely related to the adnexa of the uterus. The tumors show similar density/intensity on CT/MRI to those of the myometrium, and the enhancement degree and pattern were also similar to those of the myometrium. The patient was an elderly woman. The enhancement degree of the mass was higher than that of the myometrium in the early phase and lower than the myometrium in the late phase, showing a rapid rise-slow decay type, which was not consistent with the broad ligament fibroid.
Lymphoma is usually characterized by large lesions with small necrosis, mild to moderate enhancement, simultaneous involvement of multiple sites, a high tendency toward fusion, easy formation of “sandwich sign”, and obviously restricted diffusion on DWI [34,48]. This case presented as a single solitary lesion with no enlarged and fused lymph nodes or masses in other body parts, which was different from lymphoma.
Stromal tumors are usually solid and closely associated with the intestine. They are significantly and continuously enhanced after contrast medium injection. However, in this case, the tumor was located in the left ovary with no close relationship with the intestinal tract, and a dynamic contrast-enhanced scan showed a rapid rise-slow decay type, which was inconsistent with the stromal tumors.
Ovarian cystadenocarcinoma is a common malignant tumor of the ovary. It can be irregular in shape, and the solid component presents as mural nodules. The solid component and the cyst wall were significantly enhanced after contrast medium injection. The tumor can invade surrounding tissues or metastasize into the omentum and lymph nodes, with significantly increased CA-125 levels but no obvious changes in hormones. However, the tumor markers in this case were normal, and the tumor was morphologically regular with homogeneous enhancement. No obvious cystic component was observed, and there was no obvious invasion or enlarged lymph nodes, with no signs of ascites, omental thickening, or lymph node metastasis.
Treatment and prognosis
There is no unified treatment regimen for EMP. However, a treatment regimen considering the specific site, size, differentiation and invasive degree of the lesion is needed. Radiotherapy is the first choice for head and neck lesions. For patients undergoing radiotherapy alone, a moderate radiation dose (40-60 Gy) can achieve a local control rate of 80-100% (overall local control rate 88%), with no obvious dose-dependent effect [26,49-51]. Moreover, for lesions in the gastrointestinal tract and testes, surgical resection has roughly equivalent effects as radiotherapy, and for patients with high malignancy and relapse after treatment, adjuvant chemotherapy may be considered. It has been reported that treatment with the targeted drug bortezomib has a good effect on EMPs [52]. Therefore, second-line drugs combined with the new drug bortezomib can be used as the first-line treatment in patients with MM and EMP [53].
EMP prognosis depends on many factors, including the patient’s general condition, tumor size, degree of differentiation and invasion, and whether the treatment is standardized and timely. The 5-year survival rate of EMP patients is approximately 50-70%, and the median survival time after treatment is approximately 6-8 years [54,55]. Some EMP patients progress to MM after a certain time [14,52]. This patient has been followed up regularly for two years and is currently stable with no signs of progression to MM.
EMP in the ovary is rare and lacks specific clinical and radiological features. The diagnostic criteria for EMP include: (1) Histologically confirmed solitary plasma cell lesion; (2) A bone marrow biopsy showing <5% of plasma cells; (3) Absence of damage to multiple organs, such as MM; (4) Skeletal survey excluding intramedullary diseases; (5) Except for possible monoclonal gammopathies, all laboratory tests were normal, including the beta-2 microglobulin test, complete blood count, electrolytes, serum free light chains and serum protein electrophoresis [6,56]. Ovarian EMP can be diagnosed when the clinical history is consistent and the following imaging features are present: a well-circumscribed solitary soft-tissue mass in unilateral or bilateral ovaries, CT showing homogeneous medium-density masses with significant and homogeneous enhancement after contrast medium injection, and MRI showing homogeneous lesions with slightly hypointense signals on T1WI, isointense signals on T2WI, restricted diffusion on DWI, and a low ADC value <1.2×10-3 mm2/s, while the contrast-enhanced scan displays significant and homogeneous enhancement with a rapid rise-slow decay type.
This article analyzed the clinical manifestations, pathology, radiological features and causes of misdiagnosis of ovarian EMPs via a literature review, and summarizes experiences in improving diagnostic accuracy and reducing missed diagnoses and misdiagnoses, so as to help clinicians in diagnosis and treatment.
Acknowledgements
This study was supported by the Jinhua Science Foundation Project (2020-4-021 and 2021-3-134). The study was approved by Ethics Committee of Jinhua Maternal and Child Health Care Hospital, Ethics: 2021 Ethics Review QT No. (012). The patient provided written informed consent for the publication of this case report and accompanying images.
Disclosure of conflict of interest
None.
Abbreviations
- EMP
Extramedullary plasmacytoma
- MM
Multiple myeloma
- PET-CT
Positron emission tomography-computed tomography
- CT
Contrast-enhanced computed tomography
- MRI
Magnetic resonance imaging
- DWI
Diffusion-weighted imaging
- ADC
Apparent diffusion coefficient
- T1WI
T1-weighted image
- T2WI
T2-weighted image
- FISH
Fluorescence in situ hybridization
References
- 1.Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-Cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. AJR Am J Roentgenol. 2000;174:1141–1145. doi: 10.2214/ajr.174.4.1741141. [DOI] [PubMed] [Google Scholar]
- 2.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the international myeloma working group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 3.Soesan M, Paccagnella A, Chiarion-Sileni V, Salvagno L, Fornasiero A, Sotti G, Zorat PL, Favaretto A, Fiorentino M. Extramedullary plasmacytoma: clinical behaviour and response to treatment. Ann Oncol. 1992;3:51–57. doi: 10.1093/oxfordjournals.annonc.a058070. [DOI] [PubMed] [Google Scholar]
- 4.Gerry D, Lentsch EJ. Epidemiologic evidence of superior outcomes for extramedullary plasmacytoma of the head and neck. Otolaryngol Head Neck Surg. 2013;148:974–981. doi: 10.1177/0194599813481334. [DOI] [PubMed] [Google Scholar]
- 5.Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, Arnold W. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- 6.Voegt H. Extramedulläre plasmocytome. virchows arch. Path Anat. 1928;302:497–508. [Google Scholar]
- 7.Bambirra EA, Miranda D, Magalhães GM. Plasma cell myeloma simulating Krukenberg’s tumor. South Med J. 1982;75:511–512. doi: 10.1097/00007611-198204000-00046. [DOI] [PubMed] [Google Scholar]
- 8.Hautzer NW. Primary plasmacytoma of ovary. Gynecol Oncol. 1984;18:115–118. doi: 10.1016/0090-8258(84)90014-3. [DOI] [PubMed] [Google Scholar]
- 9.Cook HT, Boylston AW. Plasmacytoma of the ovary. Gynecol Oncol. 1988;29:378–381. doi: 10.1016/0090-8258(88)90239-9. [DOI] [PubMed] [Google Scholar]
- 10.Emery JD, Kennedy AW, Tubbs RR, Castellani WJ, Hussein MA. Plasmacytoma of the ovary: a case report and literature review. Gynecol Oncol. 1999;73:151–154. doi: 10.1006/gyno.1998.5246. [DOI] [PubMed] [Google Scholar]
- 11.Shakuntala P, Praveen S, Shankaranand B, Rajshekar K, Umadevi K, Bafna U. A rare case of plasmacytoma of the ovary: a case report and literature review. Ecancermedicalscience. 2013;7:288. doi: 10.3332/ecancer.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham A, Mahindra A. Solitary plasmacytoma: a review of diagnosis and management. Curr Hematol Malig Rep. 2019;14:63–69. doi: 10.1007/s11899-019-00499-8. [DOI] [PubMed] [Google Scholar]
- 13.Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, Gertz M, Kumar S, Mikhael J, Roy V, Kyle RA, Lacy MQ. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susnerwala SS, Shanks JH, Banerjee SS, Scarffe JH, Farrington WT, Slevin NJ. Extramedullary plasmacytoma of the head and neck region: clinicopathological correlation in 25 cases. Br J Cancer. 1997;75:921–927. doi: 10.1038/bjc.1997.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagan R, Morris CG, Kirwan J, Mendenhall WM. Solitary plasmacytoma. Am J Clin Oncol. 2009;32:612–617. doi: 10.1097/COC.0b013e31819cca18. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong YP, Zhang JJ, Huang XN. Multiple myeloma with rupture of ovarian plasmacytoma. Chin Med J (Engl) 2012;125:2948–2950. [PubMed] [Google Scholar]
- 18.Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, Masszi T, Caillot D, Lenhoff S, Udvardy M, Crawley C, Arcese W, Mariette C, Hunter A, Leleu X, Schipperus M, Delforge M, Pioltelli P, Snowden JA, Itälä-Remes M, Musso M, van Biezen A, Garderet L, Kröger N. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the chronic malignancies working party of the EBMT. Haematologica. 2018;103:890–897. doi: 10.3324/haematol.2017.178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papamichail D, Hog R, Goldschmidt H, Dimitrakopoulou-Strauss A. Imaging features of multiple myeloma extramedullary lesions in the liver with 18F-FDG PET/CT, contrast-enhanced CT and MRI. Diagnostics (Basel) 2019;9:179. doi: 10.3390/diagnostics9040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, Alsayed Y, Waheed S, Haider S, Restrepo A, Van Rhee F, Crowley J, Barlogie B. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–1767. doi: 10.3324/haematol.2012.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bladé J, Fernández de Larrea C, Rosiñol L. Extramedullary disease in multiple myeloma in the era of novel agents. Br J Haematol. 2015;169:763–765. doi: 10.1111/bjh.13384. [DOI] [PubMed] [Google Scholar]
- 22.Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. 2019;36:32–39. doi: 10.1016/j.blre.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Avivi I, Cohen YC, Suska A, Shragai T, Mikala G, Garderet L, Seny GM, Glickman S, Jayabalan DS, Niesvizky R, Gozzetti A, Wiśniewska-Piąty K, Waszczuk-Gajda A, Usnarska-Zubkiewicz L, Hus I, Guzicka R, Radocha J, Milunovic V, Davila J, Gentile M, Castillo JJ, Jurczyszyn A. Hematogenous extramedullary relapse in multiple myeloma - a multicenter retrospective study in 127 patients. Am J Hematol. 2019;94:1132–1140. doi: 10.1002/ajh.25579. [DOI] [PubMed] [Google Scholar]
- 24.Beksac M, Seval GC, Kanellias N, Coriu D, Rosiñol L, Ozet G, Goranova-Marinova V, Unal A, Bila J, Ozsan H, Ivanaj A, Balić LI, Kastritis E, Bladé J, Dimopoulos MA. A real world multicenter retrospective study on extramedullary disease from balkan myeloma study group and barcelona university: analysis of parameters that improve outcome. Haematologica. 2020;105:201–208. doi: 10.3324/haematol.2019.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran CC, Anderson CC, Caldemeyer KS, Smith RR. Meningeal myelomatosis: CT and MR appearances. AJNR Am J Neuroradiol. 1995;16:1501–1503. [PMC free article] [PubMed] [Google Scholar]
- 26.Reed V, Shah J, Medeiros LJ, Ha CS, Mazloom A, Weber DM, Arzu IY, Orlowski RZ, Thomas SK, Shihadeh F, Alexanian R, Dabaja BS. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer. 2011;117:4468–4474. doi: 10.1002/cncr.26031. [DOI] [PubMed] [Google Scholar]
- 27.Strojan P, Soba E, Lamovec J, Munda A. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol Biol Phys. 2002;53:692–701. doi: 10.1016/s0360-3016(02)02780-3. [DOI] [PubMed] [Google Scholar]
- 28.Miller FR, Lavertu P, Wanamaker JR, Bonafede J, Wood BG. Plasmacytomas of the head and neck. Otolaryngol Head Neck Surg. 1998;119:614–618. doi: 10.1016/S0194-5998(98)70021-X. [DOI] [PubMed] [Google Scholar]
- 29.Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore) 1976;55:217–238. doi: 10.1097/00005792-197605000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Gao B, Wang X. An extramedullary plasmacytoma originating from the thoracic spinal cord: magnetic resonance imaging findings. Case report. J Neurosurg Spine. 2007;6:57–59. doi: 10.3171/spi.2007.6.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Wang DM, Li YH, Wang HF. Extramedullary plasmacytoma: report of two cases. Tianjin Med J. 2013;41:510–511. [Google Scholar]
- 32.Marom T, Goldfarb A, Vaknine H, Kravtsov V, Roth Y. Clinical Photograph. Sinonasal extramedullary plasmacytoma. Otolaryngol Head Neck Surg. 2009;141:533–534. doi: 10.1016/j.otohns.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Junquera L, Gallego L, Torre A, Hernando J, Fresno MF. Synchronous oral squamous cell carcinoma and extramedullary plasmacytoma of the tonsil. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:413–416. doi: 10.1016/j.tripleo.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Yang BT, Wang ZC, Jiang ZC, Xian JF, Liu ZL, Lan BS. CT and MRI diagnosis of lymphoma in sinonasal cavity. J Clin Radiol. 2006;25:518–523. [Google Scholar]
- 35.Tang Y, Li XJ, Liu JC, Chen GX. Image features of plasmacytic tumors in CT and MR. J Pract Med Tec. 2005;6:703–705. [Google Scholar]
- 36.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971–976. doi: 10.1182/blood-2015-07-635383. [DOI] [PubMed] [Google Scholar]
- 37.Ooi GC, Chim JC, Au WY, Khong PL. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am J Roentgenol. 2006;186:821–827. doi: 10.2214/AJR.04.1787. [DOI] [PubMed] [Google Scholar]
- 38.Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–355. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- 39.Wang TT, Dong JN, Lin TT, Wang PP. Analysis of the imaging features in patients with extramedullary plasmacytoma in head and neck region and thorax. Chin J CT MRI. 2018;16:30–32. [Google Scholar]
- 40.Liu YF, Zhan AL. Imaging findings of extramedullary plasmacytoma. Chin Imaging J Integr Trad West Med. 2017;15:724–726. [Google Scholar]
- 41.Tong YX, Zhang W, Du RB, Ma MP, Bao Q. CT and MRI Features of extramedullary plasmacytoma. Chin J Med Imaging. 2016;24:570–572. [Google Scholar]
- 42.Zhang XH, Shi XQ, Zeng C, Wang JJ, Du SL, Li YM. Analysis of imaging features of 32 cases of ovarian sex cord-stromal tumors. Chongqing Med. 2021;50:586–590. [Google Scholar]
- 43.Long YH, Song Y, Hao L. Atypical CT findings and differential diagnosis of ovarian thecoma. Guide Chin Med. 2020;18:134–135. [Google Scholar]
- 44.Tian Y, Zhu LL, Qiu LQ. Imaging features of extramedullary plasmacytoma. Chin Imaging J Integr Trad West Med. 2019;17:85–87. [Google Scholar]
- 45.Tanaka YO, Saida TS, Minami R, Yagi T, Tsunoda H, Yoshikawa H, Minami M. MR findings of ovarian tumors with hormonal activity, with emphasis on tumors other than sex cord-stromal Tumors. Eur J Radiol. 2007;62:317–327. doi: 10.1016/j.ejrad.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Ge FQ, Liu LL. CT differential diagnosis of the group of ovarian thecoma-fibroma and suberous uterine leiomyoma. Chin J CT MRI. 2018;16:106–108. [Google Scholar]
- 47.Huang B, Liu YF, Chen YL, Chen WJ, Song T. MRI findings of ovarian sex cord-stromal tumor and analysis of clinical pathological features. Chin Med Herald. 2018;15:135–138. [Google Scholar]
- 48.Ozgen B, Oguz KK, Cila A. Diffusion MR imaging features of skull base osteomyelitis compared with skull base malignancy. AJNR Am J Neuroradiol. 2011;32:179–184. doi: 10.3174/ajnr.A2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayr NA, Wen BC, Hussey DH, Burns CP, Staples JJ, Doornbos JF, Vigliotti AP. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol. 1990;17:293–303. doi: 10.1016/0167-8140(90)90003-f. [DOI] [PubMed] [Google Scholar]
- 50.Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol. 1999;52:245–249. doi: 10.1016/s0167-8140(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 51.Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, Mege A, Dolivet G, Toussaint B, Charra-Brunaud C, Hoffstetter S, Marchal C, Peiffert D. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: a dose greater than 45 gy to the target volume improves the local control. Int J Radiat Oncol Biol Phys. 2006;64:1013–1017. doi: 10.1016/j.ijrobp.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Gao W, Chen WM, Chen SL. A report of 9 multiple myeloma patients with extramedullary and extraosseous plasmocytoma and literature review. J Leuk Lymphoma. 2008;17:346–350. [Google Scholar]
- 53.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 54.Rolins H, Levin M, Goldberg S, Mody K, Forte FJ. Solitary extramedullary plasmacytoma of the epiglottis: a case report and review of the literature. Otolaryngol Head Neck Surg. 1995;112:754–757. doi: 10.1016/S0194-59989570189-3. [DOI] [PubMed] [Google Scholar]
- 55.Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992;69:1513–1517. doi: 10.1002/1097-0142(19920315)69:6<1513::aid-cncr2820690633>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 56.Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999;13:1249–1257. doi: 10.1016/s0889-8588(05)70124-6. [DOI] [PubMed] [Google Scholar]


