Abstract
We sought to determine the infectious dose of Helicobacter pylori during primary and secondary infection in the rhesus monkey and to determine whether preinoculation acid suppression is necessary to produce colonization. Mixed inoculation with three human-derived strains showed that H. pylori J166 is particularly adapted to colonization of rhesus monkeys, since it outcompeted two other strains. The minimum infectious dose of H. pylori J166 was 104 bacteria in specific-pathogen (H. pylori)-free monkeys. Rechallenge of these monkeys after antibiotic therapy was characterized by a 10- to 100-fold decrease in bacterial load compared to primary infection, but with little change in the infectious dose. Acid suppression prior to inoculation was not necessary for colonization to occur. These results provide a basis for future animal experiments using more ecologically relevant conditions of inoculation and suggest that reduction in bacterial load rather than complete protection may be a more realistic goal for H. pylori vaccination.
Helicobacter pylori causes chronic active gastritis in virtually all infected individuals. Approximately 15% of infected persons will go on to develop peptic ulcer disease or gastric adenocarcinoma. The variables that determine why some people develop disease while most do not are poorly understood, but they clearly involve both bacterial and host factors (12). Since infection can cause life-threatening disease and therapy is neither 100% effective nor universally available, animal models are critical to our understanding of this and other fundamental questions in H. pylori pathogenesis.
The rhesus monkey (Macaca mulatta) provides an attractive model of H. pylori that in many respects mimics human infection. Captive rhesus monkeys are naturally infected with H. pylori that is indistinguishable from that which infects humans (7, 9–11, 31). Infection is acquired at a young age and is nearly universal in adult animals. Once acquired, infection persists and is associated with chronic gastritis that resembles that seen in humans, although neutrophils are somewhat less common. Some animals may go on to develop atrophic gastritis, the histologic precursor to gastric adenocarcinoma. Similar to chronic infection in humans, H. pylori infection in the rhesus monkey induces a predominantly Th1-type immune response (25).
Since natural infection of rhesus monkeys with H. pylori is so common, it was surprising when initial attempts to produce experimental infection met with little success. Although persistent infection was produced with inoculation of rhesus monkey-derived H. pylori strains, inoculation with human-derived strains was often unsuccessful or resulted in only transient colonization (9). Recently, it was reported that H. pylori J166 preferentially colonized four rhesus monkeys when they were inoculated with a mixed culture (8). This work suggested that human-derived H. pylori J166 may be host adapted to rhesus monkeys relative to other human strains and might serve as a type strain for studies of H. pylori in the rhesus monkey model.
The purposes of this study were fourfold: (i) confirm the observation that H. pylori J166 preferentially colonizes rhesus monkeys; (ii) determine the infectious dose of H. pylori in rhesus monkeys, which, short of human inoculation, offer the best opportunity to estimate the infectious dose in humans; (iii) examine whether prior infection with H. pylori J166 alters the infectious dose and the bacterial load following subsequent challenge with the same strain; and (iv) assess whether acid suppression, which has been used almost universally in animal models of Helicobacter, is required for effective colonization after experimental H. pylori challenge.
MATERIALS AND METHODS
Animals.
Male and female rhesus macaques (n = 24) were located at the California Regional Primate Research Center, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Animals were housed individually and fed commercial primate chow (Purina) and fruit, with water available ad libitum. All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals (26a) and were approved by the Research Advisory Committee of the California Regional Primate Research Center.
All monkeys were specific pathogen (H. pylori) free (SPF). They were hand raised in the nursery from the day of birth using methods previously described (31). These monkeys were documented to be free of H. pylori by serology and by culture and histology of gastric biopsy specimens. Despite nursery rearing, some animals were infected with small numbers of bacteria resembling “H. heilmannii,” an uncultivated Helicobacter that commonly infects nonhuman primates and a wide range of other animals (32). Since monkeys infected with “H. heilmannii”-like bacteria have little or no inflammation and do not seroconvert to H. pylori (31), these animals were included in the study. In order to preclude the possibility that “H. heilmannii”-infected animals might react differently to H. pylori inoculation, all 24 SPF monkeys were treated with antibiotic therapy prior to challenge. Clearance of “H. heilmannii”-like bacteria was documented by a negative [14C]urea breath test performed 4 weeks after completion of antibiotic therapy using methods previously described (31). Equal numbers of previously “H. heilmannii”-infected monkeys were assigned to each group in a given experiment.
Antibiotic therapy.
Animals were treated twice daily for 10 days with omeprazole (0.3 mg/kg of body weight), clarithromycin (11 mg/kg), bismuth subsalicylate (20 mg/kg), and amoxicillin (14 mg/kg). Omeprazole was suspended in 8% Na2CO3 at 1 mg/ml. All drugs were delivered orogastrically without anesthesia.
Bacterial strain.
Three low-passage clinical isolates of human-derived H. pylori were used. H. pylori J166 (kindly provided by Guillermo Perez-Perez, New York University, New York, N.Y.) was previously shown to preferentially colonize rhesus monkeys (8). H. pylori D5127 (provided by Benjamin Gold, Emory University, Atlanta, Ga.) and 88-23 (provided by Guillermo Perez-Perez) have not been examined in the rhesus monkey. All strains contained the CagA pathogenicity island (5) and the S1 allele of the VacA cytotoxin, which was demonstrated by PCR with primers and conditions previously described (33). Strains were aliquoted and frozen at −70°C in brucella broth with 20% glycerol prior to use.
H. pylori inoculation.
Low-passage H. pylori aliquots were subcultured once on brucella agar with 5% newborn calf serum (Gibco-BRL, Gaithersburg, Md.) supplemented with TVPA (trimethoprim, 5 mg/liter; vancomycin, 10 mg/liter; polymyxin B, 2.5 IU/liter; amphotericin B, 4 mg/liter [all from Sigma]) and incubated at 37°C in 5% CO2. The subculture was then used to inoculate brucella broth (Difco Laboratories, Detroit, Mich.) with 5% newborn calf serum and TVPA. The liquid culture was incubated at 37°C in 5% CO2 until the optical density at 600 was approximately 0.2 to 0.4 (about 15 h) and then centrifuged and resuspended in brucella broth at the appropriate concentration per 2 ml of inoculum. Serial dilutions were prepared as needed. Prior to each inoculation, the culture was examined by Gram stain, wet mount, and rapid urease assay with urea-indole medium (21). Quantitation of the inoculum was confirmed by plating serial dilutions. Unless otherwise indicated, animals were pretreated with cimetidine (10 mg/kg) 1 h prior to challenge in order to neutralize gastric acid and then were orogastrically inoculated with a 2-ml bacterial inoculum three times on alternate days. The orogastric tube was flushed with 5 ml of phosphate-buffered saline after each inoculation.
Endoscopy and quantitative culture.
Endoscopy was performed after an overnight fast under ketamine anesthesia (10 mg/kg) administered intramuscularly. Three mucosal biopsy specimens each were obtained from the gastric antrum and corpus and were processed for culture. The three biopsy specimens from the antrum (or corpus) were placed together in preweighed vials containing brucella broth, weighed again, and transported immediately to the laboratory. The tissue was homogenized with a sterile ground-glass pestle, and 100 μl of undiluted or 1:10-, 1:100-, and 1:1,000-diluted tissue was inoculated onto brucella agar containing 5% bovine calf serum (Gibco-BRL) and TVPA. CFU per gram of gastric mucosa was calculated by enumerating colonies, adjusting for the dilution, and dividing by the tissue weight. All plates were incubated in an atmosphere of 5% CO2 for up to 10 days. H. pylori was identified in the conventional manner by colony morphology (pinhead-sized translucent colonies), microscopy (gram-negative curved organisms), and biochemistry (oxidase, catalase, and urease positive).
DNA fingerprinting.
Repetitive extragenic palindromic PCR (Rep-PCR) was used in order to identify each strain isolated from an inoculated monkey. Methods for Rep-PCR were as previously described (15).
RESULTS
Mixed inoculation in SPF monkeys.
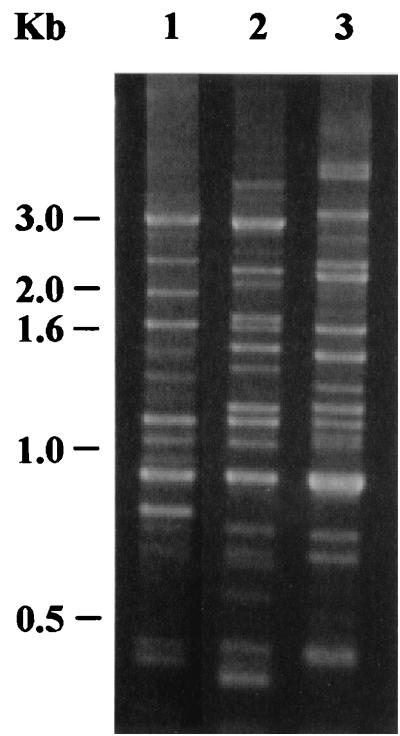
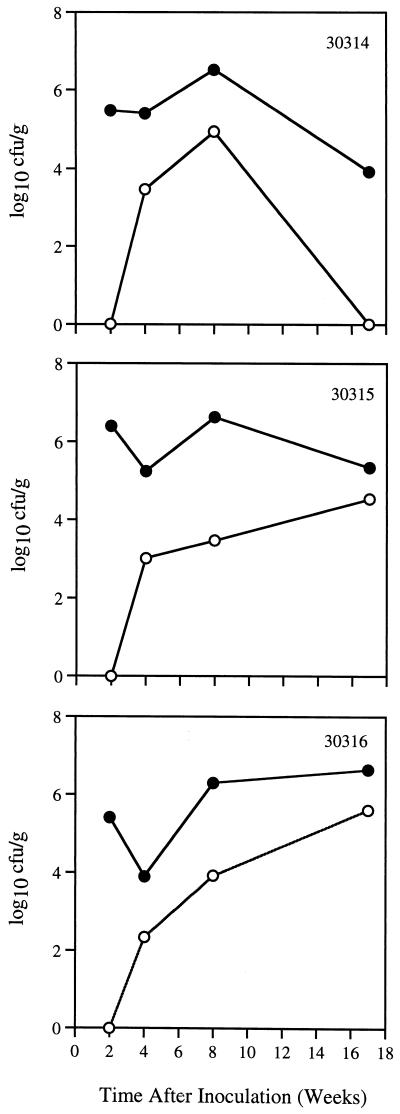
Since previous work suggested that human-derived H. pylori J166 may be adapted to rhesus monkeys, we inoculated this strain in combination with H. pylori strains D5127 and 88-23, which could each be distinguished from one another by Rep-PCR (Fig. 1). Three SPF rhesus monkeys were inoculated with 109 CFU containing an equal mixture of strains J166, D5127, and 88-23. Quantitative cultures of gastric biopsies were examined 2, 4, 8, and 17 weeks after inoculation. At 2 weeks postinoculation, H. pylori was recovered from the gastric antrum but not from the corpus in all animals (Fig. 2). Although the number of H. pylori CFU per gram of gastric tissue subsequently increased in the gastric corpus, it remained consistently lower than that in the antrum. Rep-PCR performed on four to eight colonies of H. pylori isolated from each monkey showed that all three animals were colonized with strain J166 at all time points. H. pylori strain D5127 was seen in monkey 30314 at 2 weeks (two of eight colonies) and at 8 weeks (three of eight colonies) postinoculation, while strain 88-23 was never recovered in any monkey. These results confirm previous work and suggest that H. pylori J166 is relatively adapted to colonization of the rhesus monkey.
FIG. 1.
Rep-PCR products electrophoresed in a 1.5% agarose gel stained with ethidium bromide. Template in lanes 1, 2, and 3 was chromosomal DNA from H. pylori strains 88-23, D5127, and J166, respectively. DNA kilobase ladder is shown at left.
FIG. 2.
Quantitative H. pylori cultures from gastric biopsies in three SPF monkeys 2, 4, 8, and 17 weeks after experimental inoculation with a mixture of three human-derived H. pylori strains (J166, 88-23, and D5127). Results for cultures from the gastric antrum (solid circles) and corpus (open circles) are shown. Strain J166 was isolated exclusively from animals 30315 and 30316 and was the predominant strain in animal 30314. H. pylori D5127 was also identified in a minority of colonies from animal 30314 at 2 weeks and at 8 weeks, but not at 4 or 17 weeks postinoculation. All animals were culture negative for H. pylori before inoculation (0 weeks). Animal number is shown in the upper right corner of each panel.
Single inoculation of H. pylori J166 in SPF monkeys.
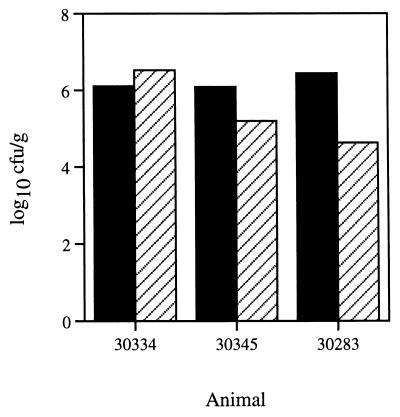
Since to this point we and others had inoculated monkeys with strain J166 only in mixed cultures, we next asked if this strain would colonize when inoculated singly. Furthermore, we reasoned that rhesus monkey-passaged H. pylori J166 might be more effective at colonization of monkeys than the initial human isolate. We therefore inoculated three SPF animals with 109 CFU containing an equal mixture of rhesus monkey-passaged stocks of J166 derived from six monkeys previously infected with this strain (three described above and three additional animals). Quantitative cultures were performed 4 weeks later. All animals were infected with, in most cases, 105 CFU/g or more (Fig. 3). Again, colony counts were generally greater in the gastric antrum than in the corpus.
FIG. 3.
Quantitation of H. pylori cultures from biopsy specimens of the gastric antrum (black bars) and corpus (hatched bars) obtained from three SPF monkeys 4 weeks after inoculation with human-derived H. pylori J166.
Infectious dose of H. pylori J166 in primary infection.
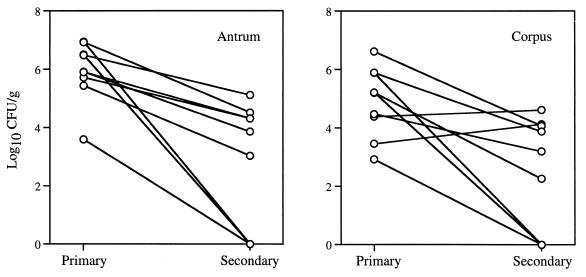
Three SPF monkeys per group with no prior H. pylori infection were each inoculated on a single occasion with 109, 107, 105, 104, or 103 CFU of H. pylori J166, which consisted of a mixture of the six rhesus-passaged derivatives. Quantitative cultures were obtained 2 weeks after inoculation. All animals inoculated with 105 or more bacteria were infected with H. pylori (Table 1). One of three animals inoculated with 104 was infected, while none of the animals that received 103 CFU was infected. Comparison of the proportion of animals infected that received above and below the median dose (105) showed that six of six and one of six were infected, respectively (Table 1). This difference is statistically significant by Fisher's exact test (P = 0.015) and suggests that infectivity was related to dose. Post hoc comparison of the proportion of animals infected by 105 or more (nine of nine) to the proportion infected by 104 or fewer (one of six) is also highly significant (Fisher's exact test, P = 0.005). Bacterial load varied but was usually between 104 and 106 CFU/g, with more bacteria typically seen in the gastric antrum than in the corpus (Fig. 4).
TABLE 1.
Inoculum size and colonization during primary and secondary infection
| Inoculum (CFU) | No. of monkeys infected (n = 3)
|
|
|---|---|---|
| Primary infection | Secondary infection | |
| 109 | 3 | NDa |
| 107 | 3 | ND |
| 106 | ND | 3 |
| 105 | 3 | 2 |
| 104 | 1 | 1 |
| 103 | 0 | ND |
ND, not done.
FIG. 4.
H. pylori J166 in the gastric antrum and corpus during primary and secondary infection.
Quantitative cultures were compared in J166-infected animals according to whether or not they had prior “H. heilmannii ” infection. In monkeys with (n = 4) or without (n = 6) prior “H. heilmannii,” the geometric means for CFU per gram in the antrum were 5.90 and 5.68 (t = 0.29; P = 0.39, one-tailed), respectively; in the corpus, geometric means for CFU per gram were 4.05 and 4.31 (t = −0.20; P = 0.42, one-tailed), respectively. These results suggest that prior infection with “H. heilmannii ” conferred no immunity to challenge with H. pylori. This is consistent with the absence of a cellular or humoral inflammatory response to “H. heilmannii ” infection (31).
Infectious dose of H. pylori J166 in secondary infection.
After 8 weeks of infection with H. pylori J166, all nine monkeys inoculated with 105 CFU or more of H. pylori were treated with antibiotics as described above. Cultures of endoscopic biopsy specimens from the gastric antrum and corpus obtained 4 weeks after completion of therapy were negative for H. pylori. After an additional 4 weeks, three monkeys in each group were inoculated on a single occasion with 106, 105, or 104 CFU of H. pylori J166. Endoscopy with quantitative cultures was performed 2 weeks later (approximately 20 weeks after the primary inoculation). All monkeys inoculated with 106 bacteria were infected, while inoculation with 105 and 104 bacteria infected two of three and one of three animals, respectively (Table 1).
Bacterial load in both the antrum and corpus during secondary infection was markedly lower than after primary infection (Fig. 4). The difference in bacterial load between primary and secondary infection was analyzed by a paired t test, using only the monkeys that were successfully infected twice (n = 6). The difference was highly significant in the antrum (mean, 6.1 versus 4.2 log10 CFU/g; P < 0.001, two-tailed) and approached significance in the corpus (mean, 5.0 versus 3.7 log10 CFU/g; P = 0.08, two-tailed). Inclusion of animals that remained uninfected during secondary infection showed a larger difference (data not shown). Analysis by paired t test also confirmed the impression from Fig. 4 that the antrum had a significantly higher bacterial load than the corpus (mean, 5.2 versus 4.4 log10 CFU/g; P = 0.009). There was no correlation between dose and bacterial load in either the antrum (r = 0.10; P = 0.7) or the corpus (r = 0.35; P = 0.2).
Infection without acid suppression.
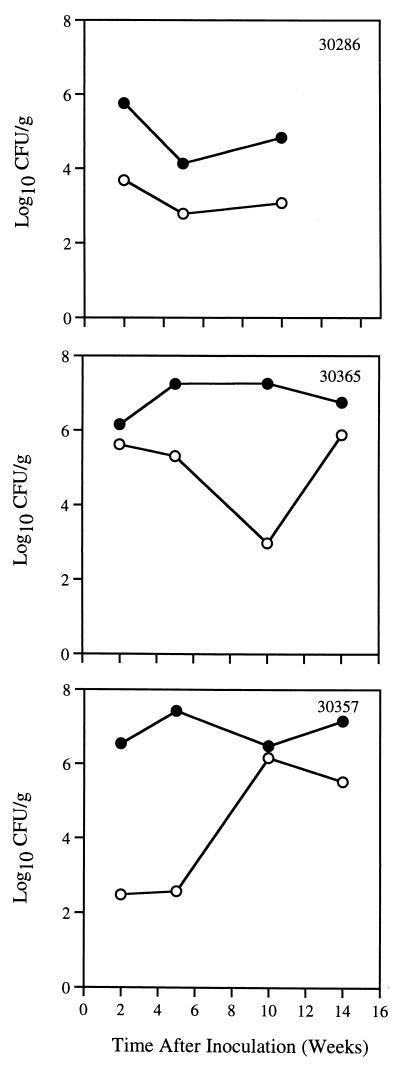
These results showed that 104 bacteria is the minimum dose of H. pylori J166 that will infect SPF rhesus monkeys. We next asked whether monkeys could be colonized with H. pylori J166 in the absence of preinoculation acid suppression. Three SPF monkeys were inoculated with 105 CFU of H. pylori J166 in the same manner as for studies of primary and secondary infection, but without administration of cimetidine. Serial quantitative cultures from the gastric antrum and corpus were performed up to 14 weeks after inoculation. All three monkeys were infected at each time point (Fig. 5). As seen previously, bacterial density was greater in the gastric antrum than in the corpus. No consistent trends in bacterial load over time were observed.
FIG. 5.
H. pylori J166 in the gastric antrum (solid circles) and corpus (open circles) over 14 weeks in three SPF monkeys that were given 105 CFU without preinoculation acid suppression.
DISCUSSION
We found that inoculation of H. pylori J166 resulted in colonization of all 15 rhesus monkeys inoculated with 105 or more CFU. This confirmation of the original report of Dubois et al. (8), performed at a different center with a large number of animals, strongly suggests that H. pylori J166 has a propensity for colonization of rhesus monkeys. This point is further emphasized by our recent finding that J166 recovered from the rhesus monkeys inoculated with a mixed culture had between 100- and 250-fold increased urease activity compared to the inoculated J166 (19). This was found to result from selection of urease-positive clones from a heterogenous inoculum, which was predominantly urease negative due to a 1-bp insertion in the ureA gene. Thus, J166 colonized preferentially despite being present in much smaller numbers than the other strains. We are currently using whole-genome DNA microarray to analyze the differences among the three strains used in this study and to compare preinoculation to postinoculation isolates of J166.
Use of strain J166 to examine the infectious dose of H. pylori showed that 104 bacteria is the minimum required to colonize rhesus monkeys that have not previously been infected with H. pylori. Few other data are available for comparison. The 50% infective dose for H. pylori SS1 in C57BL/6Ntac mice was recently reported to be less than 5 × 105 (28), while 102 CFU of Helicobacter felis was sufficient to colonize Swiss SPF mice (13). However, the ecological relevance of these observations is unclear, since neither H. felis nor H. pylori naturally colonizes mice. It is interesting to consider our results in light of recent findings that vomitus from H. pylori-infected persons frequently contains more than 103 and sometimes as high as 104 CFU/ml (27). H. pylori was not found in stools unless a cathartic was administered, in which case the quantity was markedly lower than that in vomitus. Our data on the minimum infectious dose together with studies of bacterial shedding and naturally acquired H. pylori in the rhesus model will further our understanding of the role of fecal-oral versus oral-oral transmission.
The results of rechallenge with a homologous strain showed that secondary H. pylori infection was typically characterized by a 10- to 100-fold decrease in bacterial load compared to primary infection, but with little change in the infectious dose (Fig. 4; Table 1). Although the inoculum was lower in secondary than in primary infection, this is an unlikely explanation for this effect since bacterial load was not associated with size of the inoculum. More likely, the lower bacterial load in secondary than in primary infection reflects development of a host immune response, which we have previously shown is characterized predominantly by Th1-type cytokines (25). These results are consistent with recent H. pylori vaccine studies with rhesus monkeys, which have shown that protective immunity is usually not achieved (22, 23, 30), though in some cases there may be 1- to 2-log reductions in bacterial load (22, 23). Since the density of H. pylori infection may be related to the extent of the inflammatory response and to the development of duodenal ulcer (2), quantitative reductions in bacterial load may be clinically significant. However, sterilizing immunity is probably not a realistic goal for H. pylori vaccine development.
The infectious dose of enteric bacteria is generally regarded as lower when gastric pH is raised, owing to the bactericidal effects of the gastric acid barrier. Some support for this has been obtained in experimental Campylobacter jejuni infection in humans (3). Both human experimental challenges with H. pylori also administered acid suppression therapy prior to inoculation (24, 26), as have virtually all studies with nonhuman primates (8, 9, 14, 25, 29). However, we found that preinoculation acid suppression is unnecessary to colonize rhesus monkeys with H. pylori J166. This probably reflects the fact that H. pylori expresses large amounts of a potent urease, which makes it uniquely suited to exploit the gastric niche. In vitro evidence in fact suggests that in physiologic concentrations of urea, H. pylori is intolerant of pH above 3.5 due to a rise in local pH from hydrolysis of urea (4). Although we did not systematically examine the relationship between acid suppression and infectious dose, the ecology of H. pylori infection and our finding that animals are readily infected with 105 CFU without acid suppression suggest to us that raised gastric pH does not increase colonization with H. pylori.
H. pylori density was greater in the gastric antrum than in the corpus. This has been observed previously in humans (1, 17, 18, 20) and in animals (6), including the rhesus monkey (10). The striking antral predominance that we sometimes found very early in acute infection (Fig. 2) has not been systematically described in humans or in animal models. However, similar findings have been reported in retrospective reports of presumed acute H. pylori infection in humans (16) and in one human inoculation (26). The hypothesis that local acid production plays an important role in the topography of H. pylori infection (6, 34) is a subject of ongoing studies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI42081 to J.V.S. from the National Institute of Allergy and Infectious Diseases.
We thank Anne Canfield for help with animal procedures.
REFERENCES
- 1.Atherton J C, Gonzalez-Valencia G, Dubois A, Blaser M. The vacA product from rhesus monkey Helicobacter pylori is cytolethal but causes little vacuolation. Gastroenterology. 1996;110(Suppl.):A882. [Google Scholar]
- 2.Atherton J C, Tham K T, Peek R M, Jr, Cover T L, Blaser M J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 3.Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 4.Clyne M, Labigne A, Drumm B. Helicobacter pylori requires an acidic environment to survive in the presence of urea. Infect Immun. 1995;63:1669–1673. doi: 10.1128/iai.63.5.1669-1673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covacci A, Falkow S, Berg D E, Rappuoli R. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 1997;5:205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- 6.Danon S J, O'Rourke J L, Moss N D, Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995;108:1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- 7.Drazek E S, Dubois A, Holmes R K. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol. 1994;32:1799–1804. doi: 10.1128/jcm.32.7.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Del Valle J, Yang M, Wirth H P, Perez-Perez G I, Blaser M J. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology. 1999;116:90–96. doi: 10.1016/s0016-5085(99)70232-5. [DOI] [PubMed] [Google Scholar]
- 9.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Perez-Perez G I, Blaser M J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G I, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 11.Dubois A, Fiala N, Weichbrod R H, Ward G S, Nix M, Mehlman P, Taub D M, Perez-Perez G I, Blaser M J. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J Clin Microbiol. 1995;33:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero R L, Thiberge J-M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiyama K, Fujioka T, Murakami K, Nasu M. Effects of Helicobacter pylori infection on gastric mucosal defense factors in Japanese monkeys. J Gastroenterol. 1995;30:441–446. doi: 10.1007/BF02347558. [DOI] [PubMed] [Google Scholar]
- 15.Go M F, Chan K Y, Versalovic J, Koeuth T, Graham D Y, Lupski J R. Cluster analysis of Helicobacter pylori genomic DNA fingerprints suggests gastroduodenal disease-specific associations. Scand J Gastroenterol. 1995;30:640–646. doi: 10.3109/00365529509096306. [DOI] [PubMed] [Google Scholar]
- 16.Graham D, Alpert L, Smith J, Yoshimura H H. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988;83:974–980. [PubMed] [Google Scholar]
- 17.Graham D Y, Genta R, Evans D G, Reddy R, Clarridge J E, Olson C A, Edmonds A L, Siepman N. Helicobacter pylori does not migrate from the antrum to the corpus in response to omeprazole. Am J Gastroenterol. 1996;91:2120–2124. [PubMed] [Google Scholar]
- 18.Hackelsberger A, Günther T, Schultze V, Labenz J, Roessner A, Malfertheiner P. Prevalence and pattern of Helicobacter pylori gastritis in the gastric cardia. Am J Gastroenterol. 1997;92:2220–2224. [PubMed] [Google Scholar]
- 19.Hansen L M, Solnick J V. Selection for urease activity during Helicobacter pylori infection of rhesus macaques (Macaca mulatta) Infect Immun. 2001;69:3519–3522. doi: 10.1128/IAI.69.5.3519-3522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuipers E J, Uyterlinde A M, Peña A S, Hazenberg H J, Bloemena E, Lindeman J, Klinkenberg-Knol E C, Meuwissen S G. Increase of Helicobacter pylori-associated corpus gastritis during acid suppressive therapy: implications for long-term safety. Am J Gastroenterol. 1995;90:1401–1406. [PubMed] [Google Scholar]
- 21.Labigne A, Courcoux P, Tompkins L. Cloning of Campylobacter jejuni genes required for leucine biosynthesis, and construction of leu-negative mutant of C. jejuni by shuttle transposon mutagenesis. Res Microbiol. 1992;143:15–26. doi: 10.1016/0923-2508(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 22.Lee C K, Soike K, Giannasca P, Hill J, Weltzin R, Kleanthous H, Blanchard J, Monath T P. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–3082. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee C K, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P, Ermak T, Weltzin R, Blanchard J, Monath T P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 24.Marshall B J, Armstrong J A, McGechie D B, Glancy R. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattapallil J J, Dandekar S, Canfield D R, Solnick J V. A predominant Th-1 type of immune response is induced early during acute Helicobacter pylori infection in the rhesus monkey. Gastroenterology. 2000;118:307–315. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- 26.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 26a.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 27.Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 28.Salama N, Otto G, Tompkins L S, Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun. 2001;69:730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuto R, Fujioka T, Kubota T, Nasu M. Experimental gastritis induced by Helicobacter pylori in Japanese monkeys. Infect Immun. 1993;61:933–939. doi: 10.1128/iai.61.3.933-939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solnick J V, Canfield D R, Hansen L M, Torabian S Z. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta) Infect Immun. 2000;68:2560–2565. doi: 10.1128/iai.68.5.2560-2565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solnick J V, Canfield D R, Yang S, Parsonnet J. The rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific pathogen free monkeys. Lab Anim Sci. 1999;49:197–201. [PubMed] [Google Scholar]
- 32.Solnick J V, Schauer D B. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001;14:59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telford J L, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Zanten S J, Dixon M F, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]