Abstract
Objective: To compare the clinical effect and safety of transcatheter arterial chemoembolization (TACE) combined with lenvatinib versus TACE combined with sorafenib in the treatment of intermediate-advanced hepatocellular carcinoma. Methods: In this retrospective study, 84 patients with intermediate-advanced hepatocellular carcinoma admitted to the First Affiliated Hospital of Anhui Medical University and the First Affiliated Hospital of USTC from June 2019 to June 2021 were enrolled. The control group was given TACE combined with sorafenib, and the experimental group was given TACE combined with lenvatinib. The clinical efficacy, tumor markers, liver function indexes, and occurrence of toxic and side effects were compared between the two groups. Results: The disease control rate (DCR) and the objective remission rate (ORR) of the experimental group was higher than that of the control group, and the difference was statistically significant (P<0.05). Before treatment, there were no significant differences in the levels of alpha fetoprotein (AFP) and des-gamma carboxyprothrombin (DCP) between the two groups (both P>0.05); after the treatment, the levels of AFP and DCP in both groups decreased, and those in the experimental group were lower than the control group (all P<0.05). Before treatment, there were no significant differences in the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) or lactate dehydrogenase (LDH), bilirubin (BIL) between the two groups (all P>0.05); after treatment, the levels of ALT, AST and LDH, BIL in both groups decreased, with the experimental group lower than the control group (all P<0.05). The overall survival (OS) and progression-free survival (PFS) in the experimental group were significantly higher than in the control group (both P<0.05). The incidences of symptoms of diarrhea, hand-foot syndrome, hypertension and rash in the experimental group were higher than those in the control group (all P<0.05). Fatigue, digestive tract reaction, bone marrow suppression and abnormal liver function of the two groups were similar (all P>0.05). Conclusion: Compared with TACE plus sorafenib, TACE plus lenvatinib can better control disease progression, reduce the levels of tumor markers, and stabilize the liver function of patients with intermediate-advanced hepatocellular carcinoma.
Keywords: Transcatheter arterial chemoembolization, lenvatinib, sorafenib, clinical effect, safety, comparative analysis
Introduction
Hepatocellular carcinoma (HCC) is a malignancy with poor prognosis. The incidence rate ranks fourth among malignant tumors in China [1]. Recent studies have found that the risk factors for liver cancer include diabetes, obesity, smoking and drug-induced liver damage [2,3]. At present, surgical resection is the first choice for early liver cancer. For advanced hepatocellular carcinoma or patients who decline or cannot tolerate surgery, local ablation or precise radiotherapy are alternatives [4].
For advanced hepatocellular carcinoma patients, comprehensive treatment is advocated, such as local ablation (radio frequency, microwave, Pei, cryoablation), transcatheter hepatic artery chemoembolization, liver transplantation, targeted drugs, biotherapy, traditional Chinese medicine or other treatments [5]. Transcatheter arterial embolization (TAE), transcatheter arterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) are mainly divided into three categories [6-8]. Hepatic artery chemoembolization (TACE) is an important treatment for patients with advanced HCC [9,10]. Avritscher et al. [11] proposed that repeated TACE treatment will lead to tumor resistance to chemotherapy drugs, which significantly increases the risk of tumor recurrence and metastasis. The proliferation and invasion of tumor cells will be enhanced, which will lead to tumor recurrence and distant metastasis [12].
In recent years, molecular targeted drugs and immunotherapy have made great breakthroughs in the field of liver cancer [13]. Aiming at carcinogenic sites, corresponding targeted drugs have been developed, which can lead to specific death of tumor cells [14]. Compared to traditional treatment methods, targeted drugs have the advantages of selective and efficient killing of tumor cells and less damage to normal tissues. Molecular targeted drugs have become an important method for the treatment of advanced HCC in recent years [15]. The European SHARP (sorafenib hepatecellular carcinoma assessment randomized protocol) trial confirmed for the first time that sorafenib, a multi-target small molecule tyrosine kinase inhibitor, can improve the median survival of patients with unresectable HCC [16]. Sorafenib has become the first systemic molecular targeted drug approved for advanced HCC [17]. However, two randomized controlled trials reported a complete remission rate of 0% [18,19]. Lenvatinib mesylate is a new first-line drug for the treatment of liver cancer. As a targeted therapeutic drug, its role in liver cancer is mainly to inhibit vascular endothelial growth factor receptor [20]. However, the evidence of the efficacy and safety of lenvatinib in clinical application is still limited. Whether to choose lenvatinib or sorafenib has become a problem faced by clinicians.
Therefore, the aim of this study was to compare the clinical effect and safety of TACE combined with lenvatinib versus TACE combined with sorafenib in treatment of intermediate-advanced HCC.
Data and methods
Study population
Totally 84 patients with intermediate-advanced HCC in the First Affiliated Hospital of Anhui Medical University and the First Affiliated Hospital of USTC from June 2019 to June 2021 were enrolled in this retrospective analysis. Among them, patients who received TACE combined with lenvatinib (n=43) were assigned to the experimental group, and those who received TACE combined with sorafenib (n=41) were the control group. This study was approved and recognized by the ethics committee of the First Affiliated Hospital of Anhui Medical University.
Inclusion and exclusion criteria
Inclusion criteria: ① Patients with an age ≥18; ② Patients with primary liver cancer indicated by imaging or pathological diagnosis; ③ Patients with at least one recist1.1 measurable lesion; ④ Patients with BCLC stage B (suitable for TACE treatment) or stage C; ⑤ Patients with Eastern Cooperative Oncology Group (ECOG) PS score of 0 to 2; ⑥ Patients with liver function child Pugh of A or B (≤9 points); ⑦ Patients with expected survival time of more than 2 months; ⑧ Patients with no previous treatment before admission; ⑨ Patients with complete clinical data.
Exclusion criteria: ① Patients with a history of solid organ transplantation or bone marrow suppression; ② Patients with autoimmune disease or autoimmune deficiency, for whom steroids or other treatments leading to immunosuppression are required; ③ Patients with serious dysfunction of heart, brain, lung and other important organs; ④ Patients with uncontrollable hypertension, gastrointestinal bleeding or coagulation dysfunction; ⑤ Patients with incomplete clinical data.
Transcatheter arterial chemoembolization (TACE) treatment
The appropriate puncture catheter was chosen. The right femoral artery was punctured by Seldinger method to establish the femoral artery channel, and the catheter was sent to the celiac artery and mesentery to reach the tumor blood supply artery. The tumor size and blood supply vessels were determined by digital subtraction angiography (DSA), and interventional therapy was performed after evaluation. Chemotherapy drugs were infused through the catheter. Finally, according to the angiographic results, embosphere was used for microsphere vascular embolization to observe the stagnation of blood flow before the end of the operation. After the operation, anti-inflammatory, liver protection, and symptomatic support treatment were given according to the doctor’s advice. The end point of treatment was intolerable adverse reaction or tumor progression.
Method
The experimental group was treated with TACE with lenvatinib. Lenvatinib mesylate capsule (manufacturer: Eisaico., Ltd.; Registration Certificate No.: h20180052) was orally taken on an empty stomach or with food at a fixed time every day from the fourth day after TACE treatment, with 30 days as a course of treatment. For those weighing <60 kg, 8 mg/time, once a day; For those weighing ≥60 kg, 12 mg/time, once a day; the dose was adjusted according to the individual tolerance. Before repeating TACE treatment, lenvatinib should be suspended for 3 days. Figure 1A-C showed the patients received treatment before and after TACE. The end point of treatment was intolerance to adverse reactions or tumor progression.
Figure 1.
Patients with liver cancer before and after TACE treatment. A: The patients in the experimental group before TACE treatment; B: The patients in the experimental group during TACE treatment; C: The patients in the experimental group after treatment; D: The patients in the control group before TACE treatment; E: The patients in the control group during TACE treatment; F: The patients in the control group after treatment.
The control group was treated with TACE combined with sorafenib. After TACE treatment, sorafenib was orally administered, 400 mg/time, twice a day. If the patient had strong and intolerable side effects, the dose of sorafenib could be halved or stopped for 2 weeks. After the relief of symptoms, it would be applied again. The drug was used for at least 3 months or until the progression of disease. Figure 1D-F shows the patients who received treatment before and after TACE.
Follow-up
Progression-free survival (PFS) and overall survival (OS) were determined based on a review of the electronic medical records. For the patients who were seen last but had missing dates of death in the clinical records, we conducted a telephone follow-up, and those who were not contacted were recorded as lost. PFS was defined as the time from treatment initiation to progression. OS was defined as the time from treatment initiation to death or the last follow-up.
Evaluation index
Efficacy evaluation criteria [21]: Complete remission (CR), all tumor lesions completely disappeared and this was maintained for more than 4 weeks; Partial remission (PR), the total diameter of all tumor lesions decreased by more than 30% and this was maintained for more than 4 weeks; Stable (SD), tumor focus shrinkage did not meet the PR standard or enlargement did not meet the PD standard; Progression (PD), the total diameter of all tumor lesions increased by 20% or new lesions appeared. Total effective rate = (CR + PR)/total number of cases.
The classification of adverse reactions refers to the NCI-CTC (version 4.0) classification standard for adverse reactions of anticancer drugs [22]: Grade 0, none; Grade I, mild; Grade II, moderate; Grade III, severe; Grade IV, very serious.
Tumor markers: Before and after treatment, the elbow vein blood of the two groups of patients was taken, and the supernatant was taken after centrifugation. The levels of alpha fetoprotein (AFP), des-gamma carboxyprothrombin (DCP), were detected. The serum AFP and DCP levels were measured by electro-chemiluminescence immunoassay using the Roche Cobas E602 system (Roche Diagnostics GmbH, Mannheim, Germany) and the ARCHITECT i2000 immunoassay analyzer (Abbott Laboratories, North Chicago, IL) per the manufacturer’s instructions, respectively. The cut-off value of AFP for HCC was set at 25 ng/mL according to a previous study [23]. The cut-off value of DCP was determined to be 40 mAU/mL for the differentiation of HCC and nonmalignant liver disease based on previous research [24]. Approximately 5 ml of fasting elbow venous blood was extracted from all subjects in the early morning and centrifuged at 3,000×g for 10 min to separate serum.
Quality of life: The quality of life was assessed by SF-36 questionnaire, which was developed by the American Medical Outcomes Research Group in 1992. The scale includes eight dimensions: physiological function, psychological function, physical pain, emotional function, social function, and mental health. According to the different weights of each item in the scale, the sum of the scores of each item in the subscale was calculated and converted into the standard score of 0-100. The higher the score, the higher the quality of life.
Liver function: Blood samples were collected after a 12 h fasting period. Venous blood samples were drawn between 6:00 and 8:00 and were immediately analyzed. Beckman Coulter AU5800 devices were used to determine the levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH), bilirubin (BIL) according to manufacturer’s guidelines for clinical laboratory investigations.
Statistical analysis
All data were analyzed by SPSS 25.0. The statistical results were expressed as mean ± standard deviation (x̅ ± SD), and the data comparison was conducted by t-test and the correlation analysis was conducted by Pearson linear phase. P<0.05 was considered to be statistical significance. Analyses were performed using Graph Pad Prism 7 Software (Graph Pad Prism, San Diego, CA).
Results
Clinical data
As shown in Table 1, the mean age was (55±12.26) in the experimental group and (52.5±11.47) in the control group (P=0.55). The BMI was (19.4±1.66) kg/m2 in the experimental group and (19.1±1.76) kg/m2 in the control group it was (P=0.12). Furthermore, there was no significant difference between two group in terms of HBV infection, smoking, cerebral infarction, hypertension, diabetes, coronary heart disease, ECOG PS score, BCLC stages, Child-Pugh classification, invasion of blood vessels, distant metastasis or AFP level between both groups (all P>0.05).
Table 1.
Comparison of clinical data between the two groups
| Experimental group (n=43) | Control group (n=41) | t/X2 | P | |
|---|---|---|---|---|
| Age (years) | 55±12.26 | 52.5±11.47 | 2.15 | 0.55 |
| Sex | 1.156 | 0.64 | ||
| Male (n %) | 25 (58.1%) | 24 (58.5%) | ||
| Female (n %) | 18 (41.9%) | 17 (41.5%) | ||
| BMI | 19.4±1.66 | 19.1±1.76 | 5.74 | 0.21 |
| Prior HBV infection | 6.85 | 0.34 | ||
| Yes | 38 (88.4%) | 36 (87.8%) | ||
| No | 5 (11.6%) | 5 (12.2%) | ||
| ECOG PS score | 8.76 | 0.11 | ||
| 0-1 | 20 (46.5%) | 23 (56.1%) | ||
| 2 | 23 (53.4%) | 18 (43.9%) | ||
| BCLC stages | 5.72 | 0.31 | ||
| B | 11 (25.6%) | 12 (29.3%) | ||
| C | 32 (74.4%) | 29 (70.7%) | ||
| Child-Pugh classification | 3.21 | 0.54 | ||
| Child A | 32 (74.4%) | 28 (68.3%) | ||
| Child B | 11 (25.6%) | 13 (31.7%) | ||
| Invading blood vessels | 4.23 | 0.61 | ||
| No | 17 (39.5%) | 15 (36.5%) | ||
| Yes | 26 (60.4%) | 26 (43.4%) | ||
| Distant metastasis | 5.21 | 0.56 | ||
| No | 11 (25.6%) | 12 (29.3%) | ||
| Yes | 32 (74.4%) | 29 (70.7%) | ||
| AFP | 2.43 | 0.62 | ||
| <400 ng/ml | 23 (53.4%) | 26 (63.4%) | ||
| ≥400 ng/ml | 20 (46.5%) | 15 (36.5%) | ||
| Smoking | 16 (37.2%) | 16 (39.0%) | 2.71 | 0.35 |
| Cerebral infarction | 7 (16.3%) | 7 (17.1%) | 2.96 | 0.33 |
| Hypertension | 17 (39.5%) | 17 (41.5%) | 1.79 | 0.26 |
| Diabetes | 10 (23.3%) | 11 (26.8%) | 1.29 | 0.19 |
| Coronary heart disease | 4 (9.3%) | 6 (14.6%) | 2.48 | 0.32 |
Note: Significant difference if P<0.05. ECOG: Eastern Cooperative Oncology Group.
The level of tumor markers
Before treatment, there was no significant difference in the levels of AFP or DCP between the two groups (all P>0.05). After the treatment, the levels of AFP and DCP in the two groups were decreased, and the experimental group showed lower levels than those of the control group (all P<0.05) (Table 2).
Table 2.
Comparison of tumor vascular factors and tumor markers indexes between the two groups
| Index | Time | Experimental group (n=43) | Control group (n=41) | t | P |
|---|---|---|---|---|---|
| AFP (ng/mL) | Before treatment | 489.8±13.5 | 486.7±13.4 | 0.873 | 0.315 |
| After treatment | 55.9±13.9 | 72.4±14.1 | 7.943 | 0.022 | |
| t | 14.128 | 8.416 | - | - | |
| P | 0.011 | 0.052 | - | - | |
| DCP (mAU/L) | Before treatment | 834.7±24.5 | 840.1±26.4 | 0.785 | 0.432 |
| After treatment | 9.8±15.3 | 23.6±14.3 | 8.194 | 0.021 | |
| t | 18.628 | 15.116 | - | - | |
| P | 0.006 | 0.013 | - | - |
Note: Significant difference as P<0.05. DCP: Des-gamma Carboxyprothrombin; AFP: Alpha Fetoprotein.
Clinical effectiveness
As shown in Table 3, the DCR and ORR in the experimental group were statistically higher than that of the control group (both P<0.05). This indicated that TACE combined with lenvatinib had a better clinical effect than TACE combined with sorafenib in the treatment of intermediate-advanced HCC.
Table 3.
Comparison of clinical effect between the two groups (%)
| Group | Number of cases | CR | PR | SD | PD | DCR | ORR |
|---|---|---|---|---|---|---|---|
| Experimental group | 43 | 7 (16.3%) | 20 (46.5%) | 10 (23.3%) | 6 (13.9%) | 37 (86%) | 27 (62.8%) |
| Control group | 41 | 5 (12.2%) | 14 (34.1%) | 10 (24.4%) | 12 (29.3%) | 29 (70.7%) | 19 (46.3%) |
| t | - | 6.32 | 2.92 | 3.42 | 5.43 | 3.29 | 5.47 |
| P | - | 0.054 | 0.68 | 0.06 | 0.056 | 0.03 | 0.027 |
Note: Significant difference if P<0.05. CR: Complete Remission; PR: Partial Remission; SD: Disease Stability; PD: Progressive Disease; DCR: Disease Control Rate; ORR: Objective Remission Rate.
The side effects of intervention therapy
The incidences of symptoms of diarrhea, hand-foot syndrome, hypertension and rash in the experimental group were higher than those in the control group (all P<0.05). Fatigue, digestive tract reaction, bone marrow suppression and abnormal liver function of the two groups were similar (all P>0.05) (Table 4).
Table 4.
Compared side effects between the two groups [cases (%)]
| Experimental group (n=43) | Control group (n=41) | χ2 | P | |
|---|---|---|---|---|
| Fatigue | 21 (50.0) | 13 (33.3) | 1.097 | 0.295 |
| Diarrhea | 15 (36.7) | 4 (10.0) | 4.565 | 0.033 |
| Hand foot syndrome | 15 (36.7) | 1 (3.3) | 8.438 | 0.004 |
| hypertension | 8 (20.0) | 1 (3.3) | 4.191 | 0.045 |
| Alopecia | 24 (56.7) | 21 (53.3) | 0.000 | 0.99 |
| Rash | 12 (30.0) | 2 (6.7) | 4.007 | 0.045 |
| Myelosuppression | 17 (40.0) | 13 (33.3) | 0.072 | 0.789 |
| Digestive tract reaction | 22 (53.3) | 19 (46.7) | 0.067 | 0.796 |
| Abnormal liver function | 12 (30.0) | 10 (33.3) | 0.000 | 0.99 |
Note: Significant difference as P<0.05.
Liver function indexes
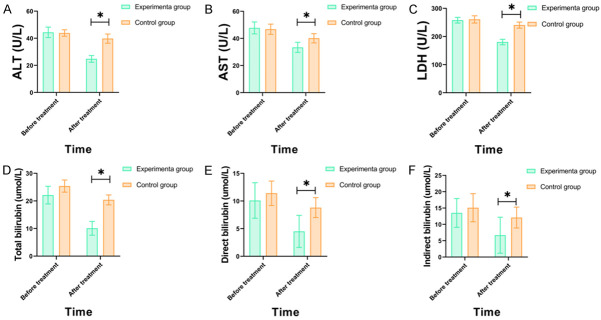
There was no significant difference in the levels of ALT, AST, LDH, total bilirubin, direct bilirubin or indirect bilirubin between the two groups before the treatment (all P>0.05). After the treatment, the levels of ALT, AST, LDH, total bilirubin, direct bilirubin, and indirect bilirubin in the two groups were decreased, and the levels in experimental group were lower than those of the control group (all P<0.05) (Figure 2).
Figure 2.
Comparison of liver function indexes between the two groups before and after treatment. Note: Compared to the control group, *P<0.05. A: ALT (alanine aminotransferase); B: AST (aspartate aminotransferase); C: LDH (lactate dehydrogenase); D: Total bilirubin; E: Direct bilirubin; F: Indirect bilirubin.
Quality of life (SF-36 questionnaire)
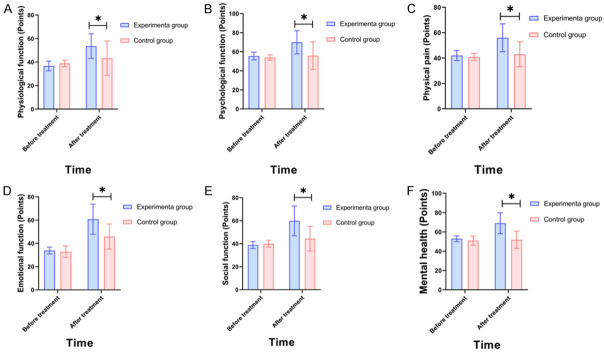
The SF-36 questionnaire of patients (physiological function, psychological function, physical pain, emotional function, social function, and mental health) in the experimental group improved more significantly compared to the control group (all P<0.05) (Figure 3).
Figure 3.
Comparison of quality of life between the two groups (SF-36 questionnaire). A: Physiological function; B: Psychological function; C: Physical pain; D: Emotional function; E: Social function; F: Mental health.
Overall survival
Median OS was 13 months in the experimental group and 8 months in the control group. The cumulative OS rates of 1 year and 2 years in the experimental group were significantly higher than that in the control group (P < 0.01) (Figure 4A).
Figure 4.
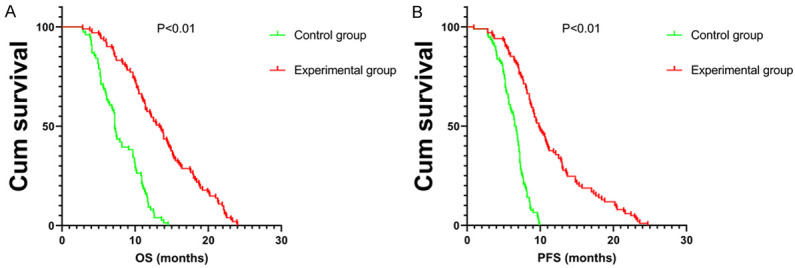
Kaplan-Meier curves of cumulative survival (A) and progression-free survival (PFS) (B) of the two groups with liver cancer.
Progression-free survival
Median PFS was 10 months in the experimental group and 6.5 months in the control group. The cumulative PFS rates at 1 year and 2 years in the experimental group were higher than that in the control group (P<0.01, Figure 4B).
Discussion
Primary liver cancer is a solid tumor with high malignancy, and surgical resection is effective. However, it is difficult to implement surgical treatment for HCC patients who have liver cirrhosis [25]. Extrahepatic metastasis of hepatocellular carcinoma mostly occurs in advanced patients. TACE is an effective treatment for this disease. The main mechanism of action of TACE is to block tumor blood supply by embolizing tumor blood vessels, causing local ischemia and hypoxia of tumor and inhibiting tumor growth [26-28]. Tai et al. [29] pointed out that TACE alone cannot achieve the effect of radical cure for patients with advanced liver cancer. The main reasons: on the one hand, most patients have distant metastasis when receiving treatment, while TACE is only for local treatment of liver tumors; On the other hand, there are two kinds of blood supply for hepatocellular tumors. Even if the hepatic artery is completely embolized, the tumor still has a source of blood supply to grow [30].
Sorafenib is an inhibitor of many receptor tyrosine kinases, including Raf kinase, vascular endothelial growth factor receptor, and other kinases. Lenvatinib [31] targets vascular endothelial growth factor receptor 1-3, fibroblast growth factor receptor 1-4, and platelet-derived growth factor α Receptors. Compared to sorafenib, the strong activity of lenvatinib on fibroblast growth factor receptor is a remarkable feature [32,33]. Recent studies [34-36] have shown that lenvatinib has immunomodulatory activity. In immunodeficient mice, there was no difference in the antitumor activity between lenvatinib and sorafenib, but the former showed a stronger antitumor activity in mice with normal immune function. Therefore, the combination of lenvatinib and immunotherapy may bring new hope to patients with advanced HCC [37]. Compared to sorafenib, cost utility analysis found that lenvatinib can obtain better effects at a lower cost [38]. The emergence of lenvatinib had a positive impact on the conversion treatment of unresectable HCC patients [39]. In some case reports [40], patients with advanced HCC and recurrent HCC with vascular invasion successfully underwent hepatectomy after using lenvatinib.
The results of this study showed that the DCR and ORR of the experimental group was higher than that of the control group. Before treatment, there was no significant difference in the levels of AFP or DCP between the two groups; After treatment, the levels of AFP and DCP in the two groups decreased with a more significant decrease in the experimental group, indicating that TACE combined with lenvatinib can improve the anti-tumor effect and effectively reduce the levels of tumor vascular factors and tumor markers. Liver cancer is a typical vascular tumor, and its development and metastasis are closely related to tumor neovascularization. The formation of tumor neovascularization mainly depends on the VEGF/VEGFR signal pathway. VEGFR-2 is a common vascular growth factor in the VEGF/VEGFR signaling pathway, which can stimulate proliferation of vascular endothelial cells, significantly promoting neovascularization, and accelerating tumor development [41]. AFP and DCP are markers of liver cancer that are closely related to the development of liver cancer [42]. In this study, after the treatment with TACE combined with lenvatinib, the disease progression was effectively controlled, and the levels of tumor vascular factors and tumor markers were significantly inhibited. The reason may be that TACE can directly inject chemotherapy drugs into the target artery supplying blood to the tumor in the liver, so that the drugs can directly act on the tumor and play an effective anti-tumor effect, prevent the growth of tumor cells, and block the blood supply at the tumor through embolization [43]. Lenvatinib combined with TACE is a targeted anti-angiogenesis drug that can inhibit the activity of VEGFR-2, block the related signal transduction pathways of VEGFR-3 and FGFRL-4, and effectively improve disease control [44].
The results of this study also showed that there was no significant difference in the levels of ALT, AST, LDH, total bilirubin, direct bilirubin or indirect bilirubin between the two groups before treatment; and after the treatment, the levels of ALT, AST, LDH, total bilirubin, direct bilirubin, and indirect bilirubin in the two groups decreased, with a more significant decrease in the experimental group, indicating that the combined treatment can effectively stabilize the liver function of patients. The development of tumor will damage the liver function of patients, resulting in the increase of ALT, AST, LDH, total bilirubin, direct bilirubin, and indirect bilirubin [45]. TACE has the dual effects of blocking tumor blood supply and directly killing tumor cells. Combined with the targeted treatment of lenvatinib, it can significantly control tumor growth and reduce the damage to liver function. In addition, lenvatinib can make up for the defects of TACE, avoid hypoxia stimulation caused by embolization, effectively prevent tumor cell proliferation and tumor angiogenesis, and stabilize liver function [46]. Therefore, the levels of ALT, AST, LDH, total bilirubin, direct bilirubin, and indirect bilirubin were lower than those of the control group. Previous studies have shown that some patients have more toxic and side effects treated with lenvatinib [47,48]. In this study, the toxic and side effects of patients include hypertension, renal insufficiency, gastrointestinal reactions, liver toxicity, hand foot syndrome, and fatigue, which were all relieved after timely symptomatic treatment. Therefore, most patients can tolerate it in the process of medication, and the safety is worthy of affirmation.
Our research has limitations. First of all, this study is a retrospective analysis, and the treatment choices of patients were determined according to the preferences of the competent doctors or patients, which may lead to the selection bias of our study population. Secondly, the sample size is relatively small. Thirdly, the study is a single-center study.
In conclusion, compared with TACE plus sorafenib, TACE plus lenvatinib for advanced hepatocellular carcinoma can control the disease progression, reduce the levels of tumor markers, stabilize the liver function of patients, and the toxic and side effects are controllable, suggesting clinical application and promotion.
Disclosure of conflict of interest
None.
References
- 1.An M, Wang W, Zhang J, Till BG, Zhao L, Huang H, Yang Y, Li T, Han L, Zhang X, Qin P, Wang Y, Zhang M, Zhang M, Gao Q, Wang Z. Association of hepatitis B virus DNA levels with overall survival for advanced hepatitis B virus-related hepatocellular carcinoma under immune checkpoint inhibitor therapy. Cancer Immunol Immunother. 2022;72:385–395. doi: 10.1007/s00262-022-03254-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefanini B, Bucci L, Santi V, Reggidori N, Rampoldi D, Lani L, Granito A, Sangiovanni A, Cabibbo G, Farinati F, Campani C, Foschi FG, Svegliati-Baroni G, Raimondo G, Gasbarrini A, Mega A, Biasini E, Sacco R, Morisco F, Caturelli E, Vidili G, Azzaroli F, Giannini EG, Rapaccini GL, Brunetto MR, Masotto A, Nardone G, Di Marco M, Magalotti D, Trevisani F Italian Liver Cancer (ITA.LI.CA) Group. Potential feasibility of atezolizumab-bevacizumab therapy in patients with hepatocellular carcinoma treated with tyrosine-kinase inhibitors. Dig Liver Dis. 2022;54:1563–1572. doi: 10.1016/j.dld.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Zhu T, Zhang Y, Bai J, Xue Y, Xu G, Lu L, Peng Q. Pan-cancer analysis of the prognostic and immunological role of BRCA1-associated protein 1 gene (BAP1): friend or foe? Gene. 2022;840:146765. doi: 10.1016/j.gene.2022.146765. [DOI] [PubMed] [Google Scholar]
- 4.Nehlsen AD, Sindhu KK, Wolken T, Khan F, Kyriakakos CK, Ward SC, Moshier E, Taouli B, Buckstein M. Characterization and prediction of signal intensity changes in normal liver parenchyma on gadoxetic acid-enhanced MRI scans after liver-directed radiation therapy. Radiol Imaging Cancer. 2022;4:e210100. doi: 10.1148/rycan.210100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H, Zhong T, Jiang S. H2AFX might be a prognostic biomarker for hepatocellular carcinoma. Cancer Rep (Hoboken) 2023;6:e1684. doi: 10.1002/cnr2.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wang J. Serum T cell immunoglobulin mucin 3 predicts worse prognosis in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Med Sci Monit. 2022;28:e935326. doi: 10.12659/MSM.935326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao Z, Liu X, Peng C, Wang L, Xu D. Combination of transcatheter arterial chemoembolization and portal vein embolization for patients with hepatocellular carcinoma: a review. World J Surg Oncol. 2021;19:293. doi: 10.1186/s12957-021-02401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suoh M, Hagihara A, Kageyama K, Yamamoto A, Enomoto M, Tamori A, Kawada N. Successful transcatheter arterial embolization for hemothorax from a spontaneous rupture of hepatocellular carcinoma metastasis to the chest wall in an elderly patient. Intern Med. 2021;60:2223–2228. doi: 10.2169/internalmedicine.6003-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terasawa M, Allard MA, Golse N, Sa Cunha A, Cherqui D, Adam R, Saiura A, Vibert E. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization alone before major hepatectomy for patients with large hepatocellular carcinoma: an intent-to-treat analysis. Surgery. 2020;167:425–431. doi: 10.1016/j.surg.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Tsai WL, Sun WC, Chen WC, Chiang CL, Lin HS, Liang HL, Cheng JS. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Medicine (Baltimore) 2020;99:e21489. doi: 10.1097/MD.0000000000021489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avritscher R, Jo N, Polak U, Cortes AC, Nishiofuku H, Odisio BC, Takaki H, Tam AL, Melancon MP, Yevich S, Qayyum A, Kaseb A, Kichikawa K, Gupta S, Goldberg SN, Chang SH. Hepatic arterial bland embolization increases Th17 cell infiltration in a syngeneic rat model of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2020;43:311–321. doi: 10.1007/s00270-019-02343-1. [DOI] [PubMed] [Google Scholar]
- 12.Noh SY, Gwon DI, Park S, Yang WJ, Chu HH, Kim JW. Diaphragmatic weakness after transcatheter arterial chemoembolization of the right inferior phrenic artery for treatment of hepatocellular carcinoma: a comparison of outcomes after N-butyl cyanoacrylate versus gelatin sponge embolization. Acta Radiol. 2022;63:48–58. doi: 10.1177/0284185120981771. [DOI] [PubMed] [Google Scholar]
- 13.Fan WZ, Zhang YQ, Yao W, Wang Y, Tan GS, Huang YH, Yang JY, Li JP. Is emergency transcatheter hepatic arterial embolization suitable for spontaneously ruptured hepatocellular carcinoma in child-pugh C cirrhosis? J Vasc Interv Radiol. 2018;29:404–412. e3. doi: 10.1016/j.jvir.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Imamura A, Taguchi H, Takano H, Funatsu H, Nakamura K, Arimitsu H, Chiba S. Whole-liver transcatheter arterial chemoinfusion and bland embolization with fine-powder cisplatin and trisacryl gelatin microspheres for treating unresectable multiple hepatocellular carcinoma. Jpn J Radiol. 2021;39:494–502. doi: 10.1007/s11604-020-01078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura A, Taguchi H, Takano H, Funatsu H, Nakamura K, Arimitsu H, Chiba S. Whole-liver transcatheter arterial chemoinfusion and bland embolization with fine-powder cisplatin and trisacryl gelatin microspheres for treating unresectable multiple hepatocellular carcinoma. Jpn J Radiol. 2021;39:494–502. doi: 10.1007/s11604-020-01078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang N, Shi C, Liu Q, Song J, Ye X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J Cancer Res Ther. 2021;17:733–739. doi: 10.4103/jcrt.JCRT_1848_20. [DOI] [PubMed] [Google Scholar]
- 17.Zhang A, Xiao Z, Liu Q, Li P, Xu F, Liu J, Tao H, Feng L, Song S, Liu Z, Huang G. CaCO3-encapuslated microspheres for enhanced transhepatic arterial embolization treatment of hepatocellular carcinoma. Adv Healthc Mater. 2021;10:e2100748. doi: 10.1002/adhm.202100748. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Bai H, Xia W, Wang D, Zhou B, Zhao X, Yang G, Xu L, Zhang W, Liu P, Xu J, Meng S, Liu R, Gao X. Predicting the outcome of transcatheter arterial embolization therapy for unresectable hepatocellular carcinoma based on radiomics of preoperative multiparameter MRI. J Magn Reson Imaging. 2020;52:1083–1090. doi: 10.1002/jmri.27143. [DOI] [PubMed] [Google Scholar]
- 19.He C, Ge N, Wang X, Li H, Chen S, Yang Y. Conversion therapy of large unresectable hepatocellular carcinoma with ipsilateral portal vein tumor thrombus using portal vein embolization plus transcatheter arterial chemoembolization. Front Oncol. 2022;12:923566. doi: 10.3389/fonc.2022.923566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YS, Lin CY, Chuang MT, Tsai YS, Wang CK, Ou MC. Nitroglycerine use in transcatheter arterial (chemo) embolization in patients with hepatocellular carcinoma: five-year retrospective study. Clin Res Hepatol Gastroenterol. 2018;42:542–552. doi: 10.1016/j.clinre.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Yoshiya S, Iwaki K, Sakai A, Fujita S, Kawasaki T, Yoshizumi F, Hiroshige S, Okamoto M, Fukuzawa K, Motohiro A, Maehara Y. Laparoscopic left hepatectomy for ruptured hepatocellular carcinoma controlled after transcatheter arterial embolization: case report and review of the literature. In Vivo. 2018;32:659–662. doi: 10.21873/invivo.112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi F, Zhang L, Li S, Lin CJ, Shen LJ, Li CF, Jie M, Li ZW, Wu PH. Chemolipiodolization with or without embolization in transcatheter arterial chemoembolization combined with radiofrequency ablation for hepatocellular carcinoma-propensity score matching analysis. Oncotarget. 2016;7:31311–21. doi: 10.18632/oncotarget.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, Lau DT. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 2022;28:216–229. doi: 10.3748/wjg.v28.i2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ao J, Chiba T, Kanzaki H, Kanayama K, Shibata S, Kurosugi A, Iwanaga T, Kan M, Sakuma T, Qiang N, Ma Y, Kojima R, Kusakabe Y, Nakamura M, Kobayashi K, Kiyono S, Kanogawa N, Saito T, Nakagawa R, Kondo T, Ogasawara S, Suzuki E, Nakamoto S, Muroyama R, Tawada A, Kato J, Kanda T, Maruyama H, Kato N. Serum angiopoietin 2 acts as a diagnostic and prognostic biomarker in hepatocellular carcinoma. J Cancer. 2021;12:2694–2701. doi: 10.7150/jca.56436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan X, Xie P, Liu J, Wu H, Xie Y. Therapeutic value of transcatheter arterial chemoembolization combined with portal vein embolization for primary hepatocellular carcinoma with portal vein tumor thrombus: a pilot study. Asia Pac J Clin Oncol. 2015;11:e6–e12. doi: 10.1111/ajco.12272. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Zhang Y, Chen H, Li W, Tsochatzis E. The combination of transcatheter arterial chemoembolisation (TACE) and thermal ablation versus TACE alone for hepatocellular carcinoma. Cochrane Database Syst Rev. 2022;1:CD013345. doi: 10.1002/14651858.CD013345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe Y, Tokue H, Taketomi-Takahashi A, Tsushima Y. Imaging findings and complications of transcatheter interventional treatments via the inferior phrenic arteries in patients with hepatocellular carcinoma. Eur J Radiol Open. 2018;5:171–176. doi: 10.1016/j.ejro.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa C, Minami Y, Morita M, Noda T, Arasawa S, Izuta M, Kubo A, Matsunaka T, Tamaki H, Shibatoge M, Kudo M. Prediction of embolization area after conventional transcatheter arterial chemoembolization for hepatocellular carcinoma using synapse vincent. Dig Dis. 2016;34:696–701. doi: 10.1159/000448859. [DOI] [PubMed] [Google Scholar]
- 29.Tai CJ, Huang MT, Wu CH, Tai CJ, Shi YC, Chang CC, Chang YJ, Kuo LJ, Wei PL, Chen RJ, Chiou HY. Contrast-enhanced ultrasound and computed tomography assessment of hepatocellular carcinoma after transcatheter arterial chemo-embolization: a systematic review. J Gastrointestin Liver Dis. 2016;25:499–507. doi: 10.15403/jgld.2014.1121.254.tai. [DOI] [PubMed] [Google Scholar]
- 30.Li JX, Deng WX, Huang ST, Lin XF, Long MY, Zhang J, Su TS, Li LQ, Pang YD, Liang CF, Zhou HM, Lu HY, Liang SX, Xiang BD. Efficacy and safety of radiotherapy plus anti-PD1 versus transcatheter arterial chemoembolization plus sorafenib for advanced hepatocellular carcinoma: a real-world study. Radiat Oncol. 2022;17:106. doi: 10.1186/s13014-022-02075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Tian H, Xiang H. Is transcatheter arterial chemoembolization plus sorafenib better than chemoembolization plus placebo in the treatment of hepatocellular carcinoma? Tumori. 2021;107:292–303. doi: 10.1177/0300891620945029. [DOI] [PubMed] [Google Scholar]
- 32.Lee WC, Hung HC, Lee JC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM. Treatment strategy of adding transcatheter arterial chemoembolization to sorafenib for advanced stage hepatocellular carcinoma. Cancer Rep (Hoboken) 2021;4:e1294. doi: 10.1002/cnr2.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muto H, Kuzuya T, Ito T, Ishizu Y, Honda T, Ishikawa T, Ishigami M, Fujishiro M. Complete response of advanced hepatocellular carcinoma achieved by sorafenib dose re-escalation after failure of long-term low-dose-sorafenib treatment combined with transcatheter arterial chemoembolization: a case report. Clin J Gastroenterol. 2020;13:397–402. doi: 10.1007/s12328-019-01066-7. [DOI] [PubMed] [Google Scholar]
- 34.Kuang J, Wan D, Wan P, Wu D. Efficacy of sorafenib combined with transcatheter hepatic arterial chemoembolization in treating intermediate-advanced hepatocellular carcinoma. J BUON. 2021;26:868–874. [PubMed] [Google Scholar]
- 35.Kodama K, Kawaoka T, Aikata H, Uchikawa S, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Masaki K, Honda Y, Mori N, Takaki S, Tsuji K, Kohno H, Kohno H, Moriya T, Nonaka M, Hyogo H, Aisaka Y, Chayama K. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J Gastroenterol Hepatol. 2018;33:1780–1786. doi: 10.1111/jgh.14152. [DOI] [PubMed] [Google Scholar]
- 36.Su D. The transcatheter arterial chemoembolization combined with targeted nanoparticle delivering sorafenib system for the treatment of microvascular invasion of hepatocellular carcinoma. Bioengineered. 2021;12:11124–11135. doi: 10.1080/21655979.2021.2001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Shi M, Shen L, Qi H, Wan W, Cao F, Xie L, Wu Y, Chen G, Mo J, Zhu G, Ye D, Zhang Y, Feng Z, Xu L, Fan W. Microwave ablation versus sorafenib for intermediate-stage hepatocellular carcinoma with transcatheter arterial chemoembolization refractoriness: a propensity score matching analysis. Int J Hyperthermia. 2020;37:384–391. doi: 10.1080/02656736.2020.1752400. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Meng Q. Drug effect analysis of sorafenib combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma. Pak J Pharm Sci. 2018;31:1751–1755. [PubMed] [Google Scholar]
- 39.Hatooka M, Kawaoka T, Aikata H, Morio K, Kobayashi T, Hiramatsu A, Imamura M, Kawakami Y, Murakami E, Waki K, Honda Y, Mori N, Takaki S, Tsuji K, Kohno H, Kohno H, Moriya T, Nonaka M, Hyogo H, Aisaka Y, Chayama K. Comparison of outcome of hepatic arterial infusion chemotherapy and sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Anticancer Res. 2016;36:3523–9. [PubMed] [Google Scholar]
- 40.Zhao RC, Zhou J, Wei YG, Liu F, Chen KF, Li Q, Li B. Cost-effectiveness analysis of transcatheter arterial chemoembolization with or without sorafenib for the treatment of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2017;16:493–498. doi: 10.1016/S1499-3872(17)60009-2. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Li L, Li B, Wang W. Safety and efficacy of endovascular implantation of a portal vein stent combined with iodine-125 seed-strips followed by transcatheter arterial chemoembolization with sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Br J Radiol. 2020;93:20190279. doi: 10.1259/bjr.20190279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu S, Chen S, Liang C, Liu Z, Zhu Y, Li Y, Lu L. Texture analysis of intermediate-advanced hepatocellular carcinoma: prognosis and patients’ selection of transcatheter arterial chemoembolization and sorafenib. Oncotarget. 2017;8:37855–37865. doi: 10.18632/oncotarget.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park W, Cho S, Ji J, Lewandowski RJ, Larson AC, Kim DH. Development and validation of sorafenib-eluting microspheres to enhance therapeutic efficacy of transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol Imaging Cancer. 2021;3:e200006. doi: 10.1148/rycan.2021200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai R, Song R, Pang P, Yan Y, Liao Y, Zhou C, Wang S, Zhou X, Wang H, Zhang H, Sun H, Ma H. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2017;17:714. doi: 10.1186/s12885-017-3707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takano M, Kokudo T, Miyazaki Y, Kageyama Y, Takahashi A, Amikura K, Sakamoto H. Complete response with sorafenib and transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma. World J Gastroenterol. 2016;22:9445–9450. doi: 10.3748/wjg.v22.i42.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448–58. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Li J, Ai DL, Fu JL, Peng XM, Zhang LZ, Wang JY, Zhao Y, Yang B, Yu Q, Liu CZ, Wang HM. Enhanced therapeutic efficacy of combined use of sorafenib and transcatheter arterial chemoembolization for treatment of advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2014;44:711–7. doi: 10.1093/jjco/hyu068. [DOI] [PubMed] [Google Scholar]
- 48.Lee TY, Lin CC, Chen CY, Wang TE, Lo GH, Chang CS, Chao Y. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: a post hoc analysis of the START trial. Medicine (Baltimore) 2017;96:e7655. doi: 10.1097/MD.0000000000007655. [DOI] [PMC free article] [PubMed] [Google Scholar]