Abstract
When broilers cannot adapt to a high-temperature environment through self-regulation, it will cause heat stress, resulting in a large number of deaths and substantial economic losses. Studies have shown that thermal manipulation (TM) during the embryonic stage can improve broilers' ability to resist heat stress later. However, different TM strategies produce different results on broilers' growth. In this study, yellow-feathered broiler eggs were selected and randomly divided into 2 groups between E10 and E18, which the control group was incubated at 37.8°C with 56% humidity, and the TM group was subjected to 39°C with 65% humidity. After hatching, all broilers were reared normally until slaughtered at 12 d of age (D12). During D1 to D12, body weight, feed intake, and body temperature were recorded. The results showed that TM significantly decreased (P < 0.05) the final body weight, weight gain, and average daily feed intake of broilers. Meanwhile, the serum levels of Triiodothyronine (T3) and free T3 were significantly decreased in the TM group (P < 0.05). The expressions of hepatic growth regulation-associated genes, growth hormone receptor (GHR), insulin-like growth factor1, and 2 (IGF1 and IGF2) were significantly down-regulated in the TM group (P < 0.05). In addition, TM altered hepatic DNA methylation, resulting in a significant increase (P < 0.05) in the methylation of the IGF1 and GHR promoter regions. The above results indicated that TM during the embryonic stage decreased the serum thyroid hormone level and increased the methylation level of the IGF1 and GHR promoter regions to down-regulate the expression of growth-related genes, resulting in early growth inhibition of broilers.
Key words: embryonic stage, thermal manipulation, growth inhibition, DNA methylation
INTRODUCTION
Metabolic programming, initially proposed by Barker in 1990 (Barker, 1990), refers to animals subjected to various nutritional and environmental factors during the sensitive life stages of development that may significantly influence later health, including poor growth, metabolic dysfunction, reproductive dysfunction, and low immunity (Reynolds et al., 2010, 2019). Research has shown that epigenetics, defined as altering the phenotype of an organism without changes to genetic DNA sequence, is involved in the process of metabolic programming. DNA methylation, the most well-studied epigenetic modification, plays a vital role in gene expression regulation, genome stability, genomic imprinting, and X chromosome inactivation (Mittelstaedt et al., 2021). In mammals, DNA methylation refers to adding a methyl group to the C-5 position of the cytosine ring of DNA, which is catalyzed by DNA methyltransferase (DNMT) and using S-adenosyl methionine as a methyl donor (Moore et al., 2013). DNA methylation includes 2 types. One is de novo methylation, namely Dnmt3a and Dnmt3b, which can establish new methylation on unmethylated DNA (Okano et al., 1999). Another type of reserved replication methylation is that DNA methylation already exists on the DNA strand, but during DNA replication, Dnmt1 can copy DNA methylation from the parent DNA strand to the newly synthesized daughter strand (Hermann et al., 2004). Previous research showed that DNA methylation is involved in the epigenetic regulation of oxidative phosphorylation and mitochondrial β-oxidation genes in chickens under chronic stress (Hu et al., 2017).
Heat stress is one of the most common types of environmental stress. With global warming, the temperature of the external environment continues to rise. In addition, broilers do not have sweat glands, which can easily lead to heat stress. Heat stress could lead to reduced feed intake, slow growth, reduced meat quality in broilers (Zaboli et al., 2019), and even to the large-scale death of chickens, resulting in tremendous economic losses (Liu et al., 2020). Studies have recently reported that thermal manipulation (TM) during the embryonic period could improve the heat resistance capability without negatively affecting the broiler's growth (Piestun et al., 2013; Piestun et al., 2015; Al-Zghoul et al., 2019). For instance, research on TM in broiler embryos has found that TM could elevate the final body weight of broilers (Al-Zghoul et al., 2013; Saleh et al., 2020). Moreover, this approach involved epigenetic regulation, including DNA methylation (Cramer et al., 2019). However, other studies have found that TM could significantly reduce broiler body weight during growth (Vitorino Carvalho et al., 2020; El-Shater et al., 2021). Thus, whether TM during the embryonic period is effective is still debatable. In addition, we did not find that this method has been tested on yellow-feathered broilers unique to China. It is also unknown whether it can affect the growth performance of yellow-feathered broilers and improve their ability to resist heat stress and whether the treatment conditions need to be changed to cope with yellow-feathered broilers.
Growth is a complex process involving multiple factors such as genes, hormones, and the environment (Singhal, 2017). For livestock and poultry, growth mainly depends on regulating the hypothalamic-pituitary-thyroid axis (HPT) and the somatotropic axis. The HPT axis can affect the development and growth of the body through the regulation of thyroid hormones. Thyrotropin-releasing hormone, secreted by the hypothalamus, stimulates the anterior pituitary to secrete thyroid-stimulating hormone (TSH), which acts on the thyroid to stimulate the synthesis and secretion of thyroid hormone (T4) and triiodothyronine (T3) (Flach et al., 2021). Studies show that T4 and T3 can regulate metabolism and affect muscle growth (Ambrosio et al., 2017) and bone development (Williams, 2013). The poultry thyroid gland mainly secretes T4, so T3 in peripheral blood and tissues is mainly formed by removing single iodine by outer ring deiodination (ORD) or inner ring deiodination (IRD) of T4. T3 is deiodized again and becomes inactive T2. Poultry has 3 types of deiodiases: Deiodinase1∼3 (DIO1, DIO2, and DIO3). DIO1 is mainly present in the liver, kidney, and small intestine, which can catalyze ORD and IRD reactions simultaneously and participate in the generation and degradation of peripheral T3. DIO2 is mainly expressed in the brain and only catalyzes ORD reaction, promoting the transformation of T4 to T3 in the brain. DIO3 can be detected in all tissues and only participates in IRD response, protecting cells from T3 overenrichment under special conditions such as early bird development, disease, and hunger (Darras et al., 2006). Thyroid hormones circulating in vertebrates are transported by binding proteins; however, birds lack thyroxin-binding globulin, which is present in the blood of large mammals (Bartalena and Robbins, 1993). The main thyroid hormone-binding protein in birds is transthyretin (TTR), which has a higher binding affinity with T3 than T4(Chang et al., 1999). Monocarboxylate transporter 8 (MCT8) and L-type amino acid transporter 1 (LAT1) is required for thyroid hormone uptake (Bourgeois et al., 2016). Thyroid hormone effects in birds are mediated by thyroid hormone receptors (TRs), commonly referred to as T3 receptors, because they have a high affinity for T3. Thyroid hormone receptors in vertebrates are encoded by 2 genes, the thyroid hormone receptor α (TRα) and the thyroid hormone receptor β (TRβ). When T3 does not bind TRs, synergistic regulatory proteins inhibit the transcription of thyroid regulatory genes. Thus, TRs binds to T3 can stimulate its transcription (Decuypere et al., 2005; Cheng et al., 2010). For the somatotropic axis, the hypothalamus produces growth hormone-releasing hormone (GHRH) or somatostatin (SST) to stimulate or inhibit the secretion of growth hormone (GH) in the pituitary. GH binds to the growth hormone receptor (GHR) on the surface of the cell membrane of the target organ, thereby initiating the intracellular signal transduction mechanism and promoting the expression of insulin-like growth factor 1 (IGF1), which enters various tissues of the body through the blood circulation to play a role (Kuhn et al., 2005). Both thyroid hormones and IGF can act on the pituitary or hypothalamus through negative feedback regulation to maintain the metabolic homeostasis of the entire body (Berelowitz et al., 1981). The study on the connection between the 2 axes found that thyroid hormone could affect the production of IGF1 through liver GHR, and the thyrotropic and somatotropic axes could interact with each other to influence the body's growth (Tollet et al., 1990; Tsukada et al., 1998; Wang et al., 2007). However, it was unclear whether embryonic TM affects the HPT axis and somatotropic axis and its detailed mechanism.
Until now, TM-associated research has been mainly conducted in white-feathered broilers, but no reports were found in Chinese domestic, yellow-feathered broilers. In China, yellow-feathered broilers account for more than 40% of broilers and are reared under high humidity and temperature. Thus, whether TM affects yellow-feathered broiler growth is unknown. Thus, this study aimed to explore TM's effects during the embryonic period on growth performance, serum T3 and T4 levels, regulation of the HPT axis and somatotropic axis, and hepatic DNA methylations on specific genes in yellow-feathered broilers.
MATERIALS AND METHODS
Animals and Experimental Design
All the experiments were approved by the Animal Ethics Committee of Nanjing Agricultural University. The sampling procedures followed the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
Two hundred yellow-feathered broiler eggs were obtained from Jiangsu Lihua Husbandry Company, LTD, China. All eggs were randomly divided into control (CON) or TM groups on E10. The eggs in CON were maintained at 37.8°C and 56% relative humidity (RH) throughout the incubation period. In contrast, the eggs in the TM were subjected to 39°C for 18h a day and 65% RH from E10 to E18. Eggs were illuminated at E18 to remove unfertilized and dead embryos. The remaining eggs from both groups were incubated at 37°C and 65% humidity until hatching. After hatching, the 1-day-old broilers' weight and wing temperature were recorded. The broilers were reared at 35°C during the first week, then dropped by 3°C every week. The RH was maintained at 40 to 60% during the whole experiment. The first stage feed of New Hope Liuhe was fed (ash ≤8%, crude fiber ≤5%, crude protein ≥21%), and the feeding process complies with animal welfare standards. The body temperature and body weight were recorded every 3 d. On the 12th d of age, 7 broilers were selected from each group to be weighed and sacrificed by rapid decapitation. Blood samples were collected and centrifugated to separate the serum stored at -20°C for further analysis. The liver (without a gallbladder), hypothalamus, and pituitary were rapidly frozen in liquid nitrogen and then transferred to -80°C for storage until use.
Determination of Serum Concentration of Thyroid Hormones
Serum T4, T3, FT4, and FT3 were measured using radioimmunoassay kits purchased from Beijing North Institute of Biological Technology Co., Ltd., China, following the manufacturer's instructions. The sensitivity of T3 was 0.2 ng/mL, that of T4 was 5 ng/mL, that of FT3 was 0.5 fmol/mL, and that of FT4 was 1 fmol/mL. The in-batch coefficient of variation and inter-batch coefficient of variation of all kits were 10% and 15%, respectively.
Total RNA Isolation and Real-Time PCR
Total RNA was isolated from samples using TRIzol Reagent (Tsingke, China), treated with RNase-free DNase, and reverse-transcribed to cDNA using random hexamer primers (TransGen Biotech, China). Two microliters of diluted cDNA (1:20, vol/vol) were used for real-time PCR with an Mx3000 P Real-Time PCR System (Stratagene). All primers (Table S1) were synthesized by Tsingke (Nanjing, China). Several reference genes were tested, and PPIA (Peptidylprolyl Isomerase A) was chosen as the final reference gene. Data were analyzed using the method of 2−ΔΔCT.
Methylated DNA Immunoprecipitation Analysis
The methylated DNA immunoprecipitation (MeDIP) analysis was conducted as previously published (Hu et al., 2017). Briefly, genomic DNA was extracted from liver tissue, which was sonicated to generate small fragments of 300 to 500 bp, which was heat-denatured for immunoprecipitation with a 5mC antibody (ab10805, Abcam, UK) overnight (4°C). Antibody/DNA complexes were captured with pretreated protein A/G agarose beads (sc-2003, Santa Cruz, CA). The agarose beads bound to the immune complexes were washed to remove the nonspecific binding and resuspended in a working solution containing proteinase K. The MeDIP and negative control DNA were amplified by fluorescent quantification to amplify the proximal promoter sequences of broilers IGF1, IGF2, and GHR genes, and the primers were listed in Table S2. The prediction of GHR and IGF2 promoter region transcription factors was made by hTFtarget (http://bioinfo.life.hust.edu.cn/hTFtarget/#!/prediction) to find whether THR binding sites in their promoter regions. Data were normalized and presented as the fold change relative to the mean of the CON group.
Western Blotting
Total protein extraction and western blotting were performed as previously described (Zhao et al., 2021). Following the instructions, the protein concentration was measured using the BCA Protein Assay kit (Rockford, IL). Thirty micrograms of total liver protein were subjected to electrophoresis on the 10% SDS-PAGE gel and transferred to the nitrocellulose membrane for subsequent immunoblotting. Western blotting analysis for DNMT1 (Abclonal, A19679) and tubulin-α (Bioworld, BS1699) were carried out according to the manufacturer's protocols. The immunoblot images were captured using VersaDoc 4000MP system (Bio-Rad). The band density was analyzed by Quantity One software (Bio-Rad).
Statistical Analysis
All data are presented as means ± SEM, and the statistical differences between groups were analyzed using Student t test with SPSS 22.0 software (SPSS Inc., Chicago, IL). The differences were considered statistically significant when P < 0.05 and P < 0.01 indicates very significant differences.
RESULTS
Body Weight, Body Temperature, and Daily Feed Intake
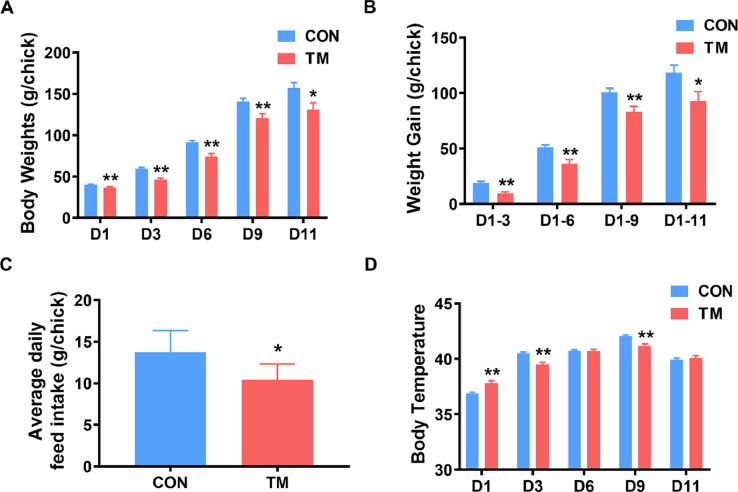
Compared with the CON group, the body weight (Figure 1A), weight gain (Figure 1B), and average daily feed intake (Figure 1C) of the broilers in the TM group were significantly decreased (P < 0.05). TM significantly increased the body temperature of broilers after hatching. In contrast, body temperature on D3 and D9 was significantly decreased (P < 0.05), and there was no significant difference in body temperature on D6 and D11 (Figure 1D).
Figure 1.
Effect of embryonic thermal manipulation on the growth performance of broilers. (A) Body weight change; (B) weight gain change; (C) average daily feed intake; (D) body temperature change. Values are mean ± SEM, CON: n = 30, TM: n = 24. *P < 0.05, **P < 0.01, determined by an unpaired t test. Abbreviations: CON, control group; TM, thermal manipulation during embryonic development.
Serum Thyroid Hormone Levels and Expression of Genes Related to Thyroid Hormone Metabolism in the Liver
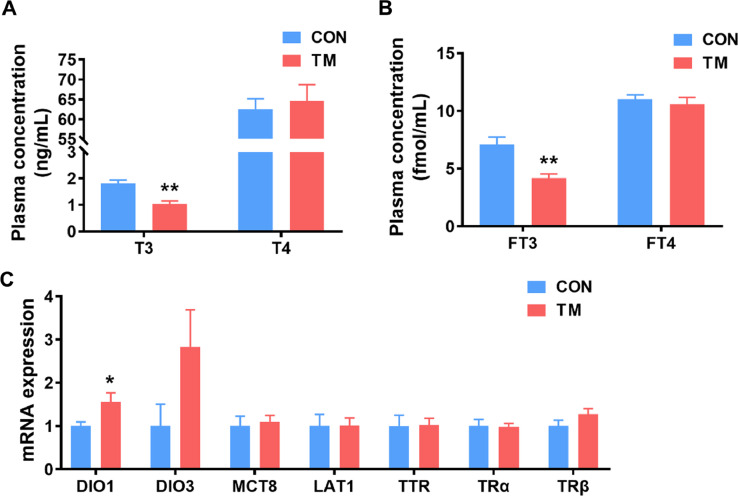
Serum levels of T3 and FT3 in broiler broilers in TM were significantly lower (P < 0.01) than those in the CON group (Figures 2A and 2B), whereas there was no difference in the levels of T4 and FT4 between the 2 groups. TM could significantly up-regulate the mRNA expression of DIO1 (P < 0.01). However, no significant differences were found in the mRNA expression of genes related to thyroid hormone metabolism: DIO3, monocarboxylate transporter 8, L-type amino acid transporter 1, transthyretin, TRα, and TRβ (Figure 2C).
Figure 2.
Effect of embryonic thermal manipulation on serum thyroid hormones levels and expression levels of thyroid hormone-related gene in the liver in the broiler. (A) Serum T3 and T4 levels; (B) serum FT3 and FT4 levels; (C) mRNA expression level of thyroid hormone-related gene in the liver. Values are mean ± SEM, n = 7. *P < 0.05, **P < 0.01, determined by an unpaired t test. PPIA served as the final reference gene in (C). Abbreviations: CON, control group; DIO1and DIO3, deiodinase1/3; LAT1, L-type amino acid transporters 1; MCT8, monocarboxylate transporters 8; PPIA, Peptidylprolyl Isomerase A; TM, thermal manipulation during embryonic development; TRα, thyroid hormone receptor Α; TRβ, thyroid hormone receptor Β; TTR, transhyretin.
Expression of Genes Related to the Somatotropic Axis in Liver
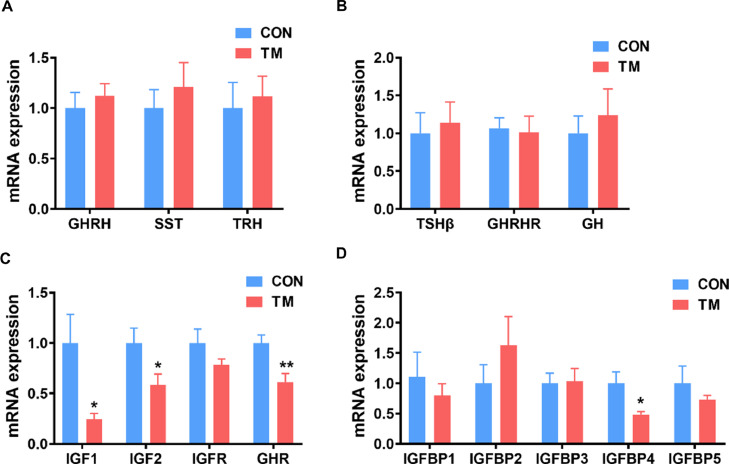
Embryonic TM had no significant effect on the mRNA expression of GHRH, SST, and Thyrotropin-releasing hormone in the hypothalamus (Figure 3A) or TSHβ, GH, and GHRHR in the pituitary (Figure 3B). However, the mRNA expressions of IGF1, IGF2, GHR, and IGFBP4 in the liver were significantly down-regulated in the TM group (P < 0.05) (Figure 3C and 3D). In comparison, no significant differences between the CON and TM groups were found in the mRNA expressions of IGFBP1, IGFBP2, IGFBP3, and IGFBP5 (Figure 3D).
Figure 3.
Effect of embryonic thermal manipulation on the expression of growth-related genes in the broilers. (A) the mRNA expression level of growth-related genes in the hypothalamus; (B) the mRNA expression level of growth-related genes in the pituitary; (C) the mRNA expression level of growth-related genes in the liver; (D) the mRNA expression level of IGF-binding proteins in the liver. Values are mean ± SEM, n = 7. *P < 0.05, **P < 0.01, determined by an unpaired t test. PPIA served as the final reference gene. Abbreviations: CON, control group; GH, growth hormone; GHR, growth hormone receptor; GHRH, growth hormone-releasing hormone; GHRHR, growth hormone-releasing hormone receptor; IGF1/2, insulin-like growth factor1/2; IGFBP1∼5, insulin-like growth factor binding protein 1∼5; IGFR, insulin-like growth factor receptor; PPIA, Peptidylprolyl Isomerase A; SST, somatostatin; TM, thermal manipulation during embryonic development; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone; TSHβ, thyroid-stimulating hormone beta.
Expression of DNA Methyltransferase-Related Genes in the Liver and DNA Methylation Levels in Promoter Regions of IGF1, IGF2, and GHR
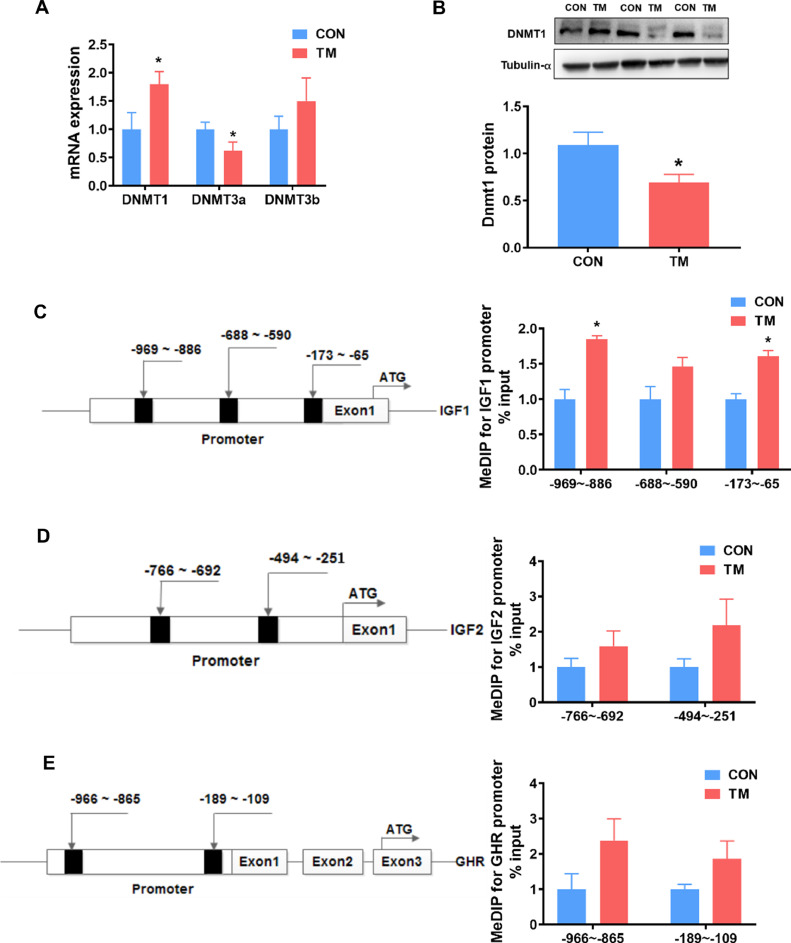
Embryonic TM significantly decreased (P < 0.05) the mRNA level of DNMT3a (Figure 4A) and the protein level of DNMT1 (Figure 4B). However, that's all we tested for because of the lack of antibody availability in the liver. MeDIP analysis found that TM significantly increased (P < 0.05) methylation levels in the promoter region of IGF1 (Figure 4C). In contrast, no significant changes were found in the promoter region of IGF2 and GHR (Figures 4D–4E).
Figure 4.
Effect of embryonic thermal manipulation on DNA methylation levels on the promoter regions of differently expressed growth-related genes in broiler liver. (A) mRNA expression level of DNA methylation-related enzymes in liver, n = 7; PPIA served as a the final reference gene. (B) Protein expression level of DNMT1 in liver, n = 7; Tubulin-α served as a loading control. (C) DNA methylation level on promoter region of IGF1 gene, n = 4; (D) DNA methylation level on promoter region of IGF2 gene, n = 4; (E) DNA methylation level on promoter region of GHR gene, n = 4. Values are mean ± SEM. *P < 0.05, determined by an unpaired t test. Abbreviations: CON, control group; DNMT1, DNA methyltransferase1; DNMT3a/b, DNA methyltransferase3a/b; GHR, growth hormone receptor; IGF1/2, insulin-like growth factor1/2; PPIA, Peptidylprolyl Isomerase A; TM, thermal manipulation during embryonic development.
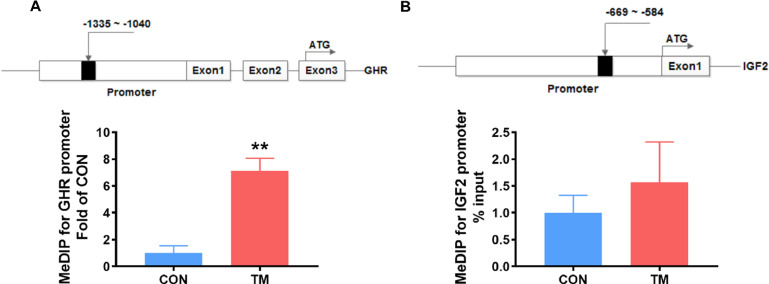
In addition, we predicted the transcription factors of GHR and IGF2 promoter regions and found THR binding sites in their promoter regions. By detecting the methylation in the region of the THR binding site, we found that TM significantly up-regulated (P < 0.01) the methylation in the GHR promoter region (Figure 5A), but the methylation changes in the IGF2 promoter region were not detected (Figure 5B).
Figure 5.
Effect of embryonic thermal manipulation on DNA methylation levels of TRE on the promoter regions of GHR and IGF2 in broiler liver. (A) DNA methylation level on the promoter region of the GHR gene, n = 4; (B) DNA methylation level on the promoter region of the IGF2 gene, n = 4. Values are mean ± SEM. **P < 0.01, determined by an unpaired t test. Abbreviations: CON, control group; GHR, growth hormone receptor; IGF2, insulin-like growth factor2; TM, thermal manipulation during embryonic development; TRE, thyroid hormone response element.
DISCUSSION
Many studies have found that TM during the incubation period of broilers embryos can improve the heat stress resistance of broilers without reducing production performance, an essential requirement of the poultry industry. To analyze the effect of TM on hatchability, different researchers had different settings for embryo age, temperature, and duration of embryonic treatment. Thus, the hatchability varies among different research settings. In white-feathered broilers, Al-Zghoul et al. conducted a gradient study on the temperature (Al-Zghoul et al., 2015a) and duration of embryonic treatment (Al-Zghoul et al., 2015b). They found that treatment at 39°C for 18 h every day during E10∼18 was the most effective because it did not reduce hatchability and hatch weight. Moreover, body weights on D14 and the carcass, breast muscle, heart, and liver weights on D35 were increased significantly after TM during E10∼18 (Al-Zghoul and El-Bahr, 2019). However, in the current study, we treated the embryos of yellow-feather broilers with the same parameters and obtained entirely different results. The weight after hatching was significantly decreased, and the weight loss persisted until the end of the experiment (D12). This type of embryonic and neonatal growth inhibition, known in mammals as intrauterine growth retardation, is likely to result from stress during fetal development, resulting in low birth weight (Heinrich, 1992). Our experiment found that the broilers' body weight decreased significantly after the treatment, indicating that the treatment probably caused harm to broilers during the embryonic period. However, the way used in the current experiment is the treatment method that has been repeatedly verified in the literature, which does not affect growth. The difference between the 2 broiler breeds may cause different outcoming. Compared with white-feathered broilers, which are slaughtered at D42, the yellow-feathered broilers will not be slaughtered until D65. Perhaps the early growth inhibition after hatching may compensate for the later growth stage in yellow-feathered broilers.
Thyroid hormones, essential for embryonic and early postnatal development, can promote muscle and bone growth by regulating cell proliferation (Lademann et al., 2020) and energy metabolism (Mullur et al., 2014). In this experiment, embryonic TM had no significant effect on serum T4 and FT4 but significantly reduced serum T3 and FT3 levels, indicating that TM may not directly affect thyroid function but the activity of deiodinase DIO1 and DIO3. A DIO1 conversion of T4 to T3 or rT3 and a DIO3 conversion of T4 to rT3 should be expressed in most tissues (Sabatino et al., 2021). In this study, TM during the embryonic stage only significantly increased the expression of DIO1 and had no significant effect on DIO3 and other thyroid hormone-related transporters and binding proteins. However, we only detected the mRNA expression of related genes, and the changes in the activities of proteins and associated enzymes were unclear, especially with DIO1 and DIO3 changes in the blood. Thyroid hormone receptors present a high affinity for T3 (Brtko, 2021). When the level of T3 in the blood circulation is low, cell proliferation and energy metabolism will be inhibited, thus affecting the growth and development of the entire body. This study found that TM did not affect the expression of TR, which indicated that TM during the embryonic period might not affect the binding of thyroid hormone to receptors.
In addition to thyroid hormones, the somatotropic axis (GH-IGF) is an essential hormonal axis that regulates body metabolism, growth, and development. GH and IGF1 can directly act on tissues in paracrine and autocrine ways; meanwhile, GH can also combine with GHR to initiate intracellular responses and control the secretion of IGF1 (Dixit et al., 2021). The current study found that embryonic TM significantly suppressed the expression of hepatic GHR, IGF1, and IGF2, consistent with the early-stage slow-growth phenotype. Compared with common broiler breeds, the dwarf chickens presented mutation in GHR, which disrupts its interaction with GH and further down-regulates the secretion of IGF (Lin et al., 2018). In mammals, intrauterine growth retardation is also mainly due to significantly reduced IGF levels (Setia and Sridhar, 2009). In this experiment, there was no significant change in the expression of pituitary GH, whereas the expression of GHR and IGF1/2 in the liver was down-regulated. We suspect that the low GHR likely combined with GH abnormally, resulting in the inability of IGF to be secreted normally, thereby may inhibiting growth. As 2 major players in regulating growth and development, there is a complex relationship between the thyrotropic and somatotropic axes. For instance, when T3 secretion is inhibited, it also affects the expression of GHR and IGF in the liver (Setia and Sridhar, 2009). In this experiment, the transcription factors that may be bound to the GHR promoter region were predicted, and the existence of the binding region of TR was found, indicating that thyroid hormone can directly regulate GHR. In addition, the methylated binding region of GHR after TM was extremely high, which may prevent the transcription factors binding, resulting in the down-regulation of GHR expression.
Embryonic TM means regulation during the critical window of growth and development, which involves epigenetic modifications, especially DNA methylation. DNMT1, DNMT3a, and DNMT3b are the major DNMT, and their increased expression significantly increases DNA methylation levels (Hermann et al., 2004; Okano et al., 1999). High methylation in the promoter region of a functional gene will inhibit or silence its expression. In this experiment, embryo TM significantly down-regulated the expression of DNMT1 and DNMT3a in the liver, indicating that TM affects the methylation level of the broiler. However, this change does not represent the methylation of the promoter regions of GHR and IGF1. In addition, MeDIP analysis showed that the promoter region of IGF1 was highly methylated, which contributed to the downregulation of IGF1 mRNA in the liver. The hepatic IGF2 and GHR mRNA were significantly down-regulated in the embryonic TM group; however, DNA methylation levels were not changed in their promoter region. In addition, studies reported that early TM alters total H3K27me2 (Kisliouk et al., 2020) or H3K27me2 (Rosenberg et al., 2021) levels in the hypothalamus. Thus, the down-regulation of the above genes may be caused by other epigenetic modifications, such as histone modifications, instead of DNA methylation.
In this experiment, in order to improve their heat resistance without reducing the production performance, the embryos of yellow-feather broilers were treated at 39°C for 18 h/d during E10∼18. It was found that the TM in the embryonic stage significantly reduced the body weight of the broilers, whereas the blood T3 and FT3 levels were decreased, and the liver GHR, IGF1, and IGF2 mRNA expressions were down-regulated. Downregulation of hepatic IGF1 and GHR mRNA was associated with hypermethylation in the promoter region. The above results indicated that TM during the embryonic period had a negative impact on the early growth of yellow-feather broilers. However, whether this early growth inhibition is related to the timing of TM and whether it can be eliminated at the later stage of broiler growth by compensating growth requires further research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of the Key Laboratory of Animal Physiology & Biochemistry (College of Veterinary Medicine, Nanjing Agricultural University) and funds from the National Natural Science Foundation of China (no. 31972638) and the Natural Science Foundation of Jiangsu Province (no. BK20221016).
DISCLOSURES
Ruqian Zhao reports financial support was provided by National Natural Science Foundation of China. Lei Wu reports financial support was provided by Natural Science Foundation of Jiangsu Province.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102562.
Appendix. Supplementary materials
REFERENCES
- Al-Zghoul M.B., Alliftawi A.R.S., Saleh K.M.M., Jaradat Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019;98:4113–4122. doi: 10.3382/ps/pez249. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Dalab A.E., Ababneh M.M., Jawasreh K.I., Al Busadah K.A., Ismail Z.B. Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance. Res. Vet. Sci. 2013;95:502–507. doi: 10.1016/j.rvsc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., El-Bahr S.M. Thermal manipulation of the broilers embryos: expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC Vet. Res. 2019;15:166. doi: 10.1186/s12917-019-1917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., El-Bahr S.M., Al-Rukibat R.K., Dalab A.E., Althnaian T.A., Al-Ramadan S.Y. Biochemical and molecular investigation of thermal manipulation protocols during broiler embryogenesis and subsequent thermal challenge. BMC Vet. Res. 2015;11:292. doi: 10.1186/s12917-015-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Ismail Z.B., Dalab A.E., Al-Ramadan A., Althnaian T.A., Al-Ramadan S.Y., Ali A.M., Albokhadaim I.F., Al Busadah K.A., Eljarah A., Jawasreh K.I., Hannon K.M. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Res. Vet. Sci. 2015;99:105–111. doi: 10.1016/j.rvsc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Ambrosio R., De Stefano M.A., Di Girolamo D., Salvatore D. Thyroid hormone signaling and deiodinase actions in muscle stem/progenitor cells. Mol. Cell Endocrinol. 2017;459:79–83. doi: 10.1016/j.mce.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Barker D.J. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalena L., Robbins J. Thyroid hormone transport proteins. Clin. Lab. Med. 1993;13:583–598. [PubMed] [Google Scholar]
- Berelowitz M., Szabo M., Frohman L.A., Firestone S., Chu L., Hintz R.L. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279–1281. doi: 10.1126/science.6262917. [DOI] [PubMed] [Google Scholar]
- Bourgeois N.M., Van Herck S.L., Vancamp P., Delbaere J., Zevenbergen C., Kersseboom S., Darras V.M., Visser T.J. Characterization of chicken thyroid hormone transporters. Endocrinology. 2016;157:2560–2574. doi: 10.1210/en.2015-2025. [DOI] [PubMed] [Google Scholar]
- Brtko J. Thyroid hormone and thyroid hormone nuclear receptors: history and present state of art. Endocr. Regul. 2021;55:103–119. doi: 10.2478/enr-2021-0012. [DOI] [PubMed] [Google Scholar]
- Chang L., Munro S.L., Richardson S.J., Schreiber G. Evolution of thyroid hormone binding by transthyretins in birds and mammals. Eur. J Biochem. 1999;259:534–542. doi: 10.1046/j.1432-1327.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- Cheng S.Y., Leonard J.L., Davis P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T., Rosenberg T., Kisliouk T., Meiri N. PARP inhibitor affects long-term heat-stress response via changes in DNA methylation. Neuroscience. 2019;399:65–76. doi: 10.1016/j.neuroscience.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Darras V.M., Verhoelst C.H., Reyns G.E., Kühn E.R., Van der Geyten S. Thyroid hormone deiodination in birds. Thyroid. 2006;16:25–35. doi: 10.1089/thy.2006.16.25. [DOI] [PubMed] [Google Scholar]
- Decuypere E., Van As P., Van der Geyten S., Darras V.M. Thyroid hormone availability and activity in avian species: a review. Domest. Anim. Endocrinol. 2005;29:63–77. doi: 10.1016/j.domaniend.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Dixit M., Poudel S.B., Yakar S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell Endocrinol. 2021;519 doi: 10.1016/j.mce.2020.111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shater S.N., Rizk H., Abdelrahman H.A., Awad M.A., Khalifa E.F., Khalil K.M. Embryonic thermal manipulation of Japanese quail: effects on embryonic development, hatchability, and post-hatch performance. Trop. Anim. Health Prod. 2021;53:263. doi: 10.1007/s11250-021-02726-y. [DOI] [PubMed] [Google Scholar]
- Flach E., Koenig J., van der Venne P., Parzer P., Resch F., Kaess M. Hypothalamic-pituitary-thyroid axis function in female adolescent nonsuicidal self-injury and its association with comorbid borderline personality disorder and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;111 doi: 10.1016/j.pnpbp.2021.110345. [DOI] [PubMed] [Google Scholar]
- Heinrich U.E. Intrauterine growth retardation and familial short stature. Baillieres Clin. Endocrinol. Metab. 1992;6:589–601. doi: 10.1016/s0950-351x(05)80114-4. [DOI] [PubMed] [Google Scholar]
- Hermann A., Goyal R., Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Liu J., Jia Y., Cai D., Idriss A.A., Omer N.A., Zhao R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Reports. 2017;7:40251. doi: 10.1038/srep40251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisliouk T., Rosenberg T., Ben-Nun O., Ruzal M., Meiri N. Early-life m(6)A RNA demethylation by fat mass and obesity-associated protein (FTO) influences resilience or vulnerability to heat stress later in life. eNeuro. 2020;7 doi: 10.1523/ENEURO.0549-19.2020. ENEURO.0549-0519.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E.R., Geelissen S.M., Van der Geyten S., Darras V.M. The release of growth hormone (GH): relation to the thyrotropic- and corticotropic axis in the chicken. Domest. Anim. Endocrinol. 2005;29:43–51. doi: 10.1016/j.domaniend.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Lademann F., Tsourdi E., Hofbauer L.C., Rauner M. Thyroid hormone actions and bone remodeling—the role of the wnt signaling pathway. Exp. Clin. Endocrinol. Diabetes. 2020;128:450–454. doi: 10.1055/a-1088-1215. [DOI] [PubMed] [Google Scholar]
- Lin S., Li C., Li C., Zhang X. Growth hormone receptor mutations related to individual dwarfism. Int. J. Mol. Sci. 2018;19:1433. doi: 10.3390/ijms19051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Ren M., Ren K., Jin Y., Yan M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult. Sci. 2020;99:6205–6211. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt N.N., Becker A.L., de Freitas D.N., Zanin R.F., Stein R.T., Duarte de Souza A.P. DNA methylation and immune memory response. Cells. 2021;10:2943. doi: 10.3390/cells10112943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullur R., Liu Y.Y., Brent G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Druyan S., Brake J., Yahav S. Thermal manipulations during broiler incubation alter performance of broilers to 70 days of age. Poult. Sci. 2013;92:1155–1163. doi: 10.3382/ps.2012-02609. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Yahav S., Halevy O. Thermal manipulation during embryogenesis affects myoblast proliferation and skeletal muscle growth in meat-type chickens. Poult. Sci. 2015;94:2528–2536. doi: 10.3382/ps/pev245. [DOI] [PubMed] [Google Scholar]
- Reynolds L.P., Borowicz P.P., Caton J.S., Crouse M.S., Dahlen C.R., Ward A.K. Developmental programming of fetal growth and development. Vet. Clin. North Am. Food Anim. Pract. 2019;35:229–247. doi: 10.1016/j.cvfa.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Reynolds L.P., Borowicz P.P., Caton J.S., Vonnahme K.A., Luther J.S., Hammer C.J., Maddock Carlin K.R., Grazul-Bilska A.T., Redmer D.A. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 2010;88:E61–E72. doi: 10.2527/jas.2009-2359. [DOI] [PubMed] [Google Scholar]
- Rosenberg T., Kisliouk T., Ben-Nun O., Cramer T., Meiri N. Cross-tolerance: embryonic heat conditioning induces inflammatory resilience by affecting different layers of epigenetic mechanisms regulating IL6 expression later in life. Epigenetics. 2021;16:228–241. doi: 10.1080/15592294.2020.1795596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino L., Vassalle C., Del Seppia C., Iervasi G. Deiodinases and the three types of thyroid hormone deiodination reactions. Endocrinol. Metab. (Seoul) 2021;36:952–964. doi: 10.3803/EnM.2021.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh K.M.M., Tarkhan A.H., Al-Zghoul M.B. Embryonic thermal manipulation affects the antioxidant response to post-hatch thermal exposure in broiler chickens. Animals (Basel) 2020;10:126. doi: 10.3390/ani10010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia S., Sridhar M.G. Changes in GH/IGF-1 axis in intrauterine growth retardation: consequences of fetal programming? Horm. Metab. Res. 2009;41:791–798. doi: 10.1055/s-0029-1231026. [DOI] [PubMed] [Google Scholar]
- Singhal A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann. Nutr. Metab. 2017;70:236–240. doi: 10.1159/000464302. [DOI] [PubMed] [Google Scholar]
- Tollet P., Enberg B., Mode A. Growth hormone (GH) regulation of cytochrome P-450IIC12, insulin-like growth factor-I (IGF-I), and GH receptor messenger RNA expression in primary rat hepatocytes: a hormonal interplay with insulin, IGF-I, and thyroid hormone. Mol. Endocrinol. (Baltimore, Md.) 1990;4:1934–1942. doi: 10.1210/mend-4-12-1934. [DOI] [PubMed] [Google Scholar]
- Tsukada A., Ohkubo T., Sakaguchi K., Tanaka M., Nakashima K., Hayashida Y., Wakita M., Hoshino S. Thyroid hormones are involved in insulin-like growth factor-I (IGF-I) production by stimulating hepatic growth hormone receptor (GHR) gene expression in the chicken. Growth Horm. IGF Res. 1998;8:235–242. doi: 10.1016/s1096-6374(98)80116-0. [DOI] [PubMed] [Google Scholar]
- Vitorino Carvalho A., Hennequet-Antier C., Crochet S., Bordeau T., Courousse N., Cailleau-Audouin E., Chartrin P., Darras V.M., Zerjal T., Collin A., Coustham V. Embryonic thermal manipulation has short and long-term effects on the development and the physiology of the Japanese quail. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Carré W., Saxton A.M., Cogburn L.A. Manipulation of thyroid status and/or GH injection alters hepatic gene expression in the juvenile chicken. Cytogenet. Genome Res. 2007;117:174–188. doi: 10.1159/000103178. [DOI] [PubMed] [Google Scholar]
- Williams G.R. Thyroid hormone actions in cartilage and bone. Eur. Thyroid. J. 2013;2:3–13. doi: 10.1159/000345548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality?—a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhuang Y., Shi Y., Xu Z., Zhou C., Guo L., Liu P., Wu C., Hu R., Hu G., Guo X., Xu L. Effects of N-acetyl-l-cysteine on heat stress-induced oxidative stress and inflammation in the hypothalamus of hens. J. Thermal Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.