Abstract
Infection with Neisseria meningitidis serogroup B is responsible for fatal septicemia and meningococcal meningitis. The severity of disease directly correlates with the production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8. However, the source of these cytokines has not been clearly defined yet. Since bacterial infection involves the activation of dendritic cells (DCs), we analyzed the interaction of N. meningitidis with monocyte-derived DCs. Using N. meningitidis serogroup B wild-type and unencapsulated bacteria, we found that capsule expression significantly impaired neisserial adherence to DCs. In addition, phagocytic killing of the bacteria in the phagosome is reduced by at least 10- to 100-fold. However, all strains induced strong secretion of proinflammatory cytokines TNF-α, IL-6, and IL-8 by DCs (at least 1,000-fold at 20 h postinfection [p.i.]), with significantly increased cytokine levels being measurable by as early as 6 h p.i. Levels of IL-1β, in contrast, were increased only 200- to 400-fold at 20 h p.i. with barely measurable induction at 6 h p.i. Moreover, comparable amounts of cytokines were induced by bacterium-free supernatants of Neisseria cultures containing neisserial lipooligosaccharide as the main factor. Our data suggest that activated DCs may be a significant source of high levels of proinflammatory cytokines in neisserial infection and thereby may contribute to the pathology of meningococcal disease.
The bacterial pathogen Neisseria meningitidis is the cause of septicemia and meningococcal meningitis. Worldwide, ca. 500,000 to 1 million cases of meningococcal disease occur every year. The incidence of meningococcal sepsis and meningitis varies from 1 to 5 per 100,000 in most industrialized countries to up to 50 per 100,000 in developing countries (8). Despite intensive efforts at prophylactic intervention and intensive care, mortality resulting from profound shock remains unacceptably high (26). Effective vaccines for N. meningitidis serogroups A, C, Y, and W135 have been developed, but there is no vaccine available for serogroup B, which is responsible for most meningococcal disease in the United States and Europe (1, 2, 16, 23, 39). To reduce the mortality and morbidity associated with meningococcal infection, novel therapeutic strategies and the development of effective vaccines against all pathogenic serogroups of N. meningitidis remain urgent needs.
Meningococci are mostly harmless colonizers of the respiratory tract, but under some not yet fully understood circumstances, they disseminate from locally infected tissues into the bloodstream and penetrate the blood-brain barrier to cause intense inflammation in the central nervous system (6). The severity of disease directly correlates with the production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8 (10, 37, 59, 58). A critical pathogenic role of cytokines and chemokines has been thoroughly established with different experimental models of bacterial meningitis. Thus, while the injection of TNF-α and IL-1 directly into the cerebrospinal fluid (CSF) results in an inflammatory response, antibodies neutralizing these cytokines are able to mitigate the extent of inflammation in experimental meningitis (34, 35, 42, 51). Interestingly, the cytokines are produced locally, with high concentrations in the CSF of meningitis patients and high concentrations in serum in cases of septicemia (4, 59, 60). TNF-α, IL-1, and IL-6 induce local inflammation (5), which may in turn allow an additional bacterial exit from the bloodstream by upregulating the expression of adhesion molecules (33). Potential sources of cytokines and chemokines have been identified during meningeal inflammation, both within the brain parenchyma and in meningeal inflammatory cells. Among these, endothelial cells, microglial cells, astrocytes, and particularly infiltrating monocytes are considered to be major origin sites of cytokines and chemokines (28). Indeed, monocytes have been shown to produce TNF-α, IL-1, IL-6, and IL-8 during meningococcal infection (38, 53, 59, 60).
The mononuclear phagocyte system is considered to be a continuum linking circulating pluripotent monocytes with differentiated effector cells such as tissue-based macrophages or specialized antigen-presenting cells (APCs). Dendritic cells (DCs) are the most potent APCs playing a crucial role in initiation and modulation of specific immune responses (3, 36). While the infection of macrophages by N. meningitidis has been characterized in great detail (32, 40, 43), their interaction with DCs and the influence of DC function had not been investigated thus far. Principally, DCs are located as a trace population in most tissues and, upon activation, DCs start to capture and process antigens. DC activation induces upregulation of costimulatory molecules and abundant surface expression of major histocompatibility complex (MHC) class II resulting in so-called “mature” DCs, which are potent stimulators of naive T cells (3, 7, 41, 54). During maturation, DCs migrate to lymphoid organs, the spleen, and the lymph nodes, where they liaise with and activate antigen-specific T cells. All of these DC activities can be induced by infectious agents and inflammatory products, so that DCs are mobile sentinels which bring antigens to T cells and express costimulators for the induction of immunity. Among APCs, DCs have also been shown to play a key role in determining the type of immune response (3, 41, 54). In most tissues, DCs are present in an immature state, lacking the signals for T-cell activation such as CD40, CD54, CD80, and CD86. Immature DCs can capture antigens by phagocytosis (21), macropinocytosis (48), and endocytosis (47, 48), making them masters of antigen surveillance. Once a DC has captured an antigen, however, its ability to do so rapidly declines, and the maturation process leads to efficient presentation of antigens in the context of MHC class I and class II complexes rather than antigen acquisition.
In this study, we investigated the interplay of DCs with N. meningitidis serogroup B by assessing the effect of bacterial infection on the activation of DCs and their functional maturation. We furthermore determined the role of the neisserial serogroup B polysaccharide capsule on the outcome of the interaction of DCs and N. meningitidis. Capsule synthesis in N. meningitidis has previously been shown to be a major mechanism of immune evasion by meningococci, in particular by making the bacteria resistant to human serum (reviewed in reference 57) and by inhibiting phagocytosis by macrophage cells (32, 43). The neisserial capsule may therefore represent the key determinant in neisserial defense against innate immune mechanisms.
MATERIALS AND METHODS
Bacteria.
The meningococcal strains used in this study were piliated N. meningitidis serogroup B strains MC58 and H44/76. Strain MC58 is a clinical isolate that was isolated in 1985 in the United Kingdom, and strain H44/76 was isolated in Norway in 1978 (20, 55). Both strains are B:15:P1.7,16 immunotype L3 and belong to the ET-5 complex. Capsule-deficient mutant strains of these serogroup B strains were constructed by insertional inactivation of the siaD gene encoding the α-2,8 polysialyltransferase which is necessary for capsule synthesis. To this end, the bacteria were transformed with vector pGH15, which contains the siaD gene carrying a chloramphenicol resistance cassette replacing an internal fragment of the siaD open reading frame. Chloramphenicol-resistant mutant bacteria were tested by Southern blotting and sequence analysis for correct insertional inactivation of siaD. In addition, siaD mutant strains were assessed for capsule expression by enzyme-linked immunosorbent assay (ELISA) and were found to have a capsule-negative phenotype. All strains were shown by Western blotting to express identical amounts of pili, Opa, and Opc. The pili expressed were detected with the monoclonal antibody SM1, demonstrating that they are class 1 pili.
Neisserial lipooligosaccharide (LOS) used for treatment of human DCs was prepared as described previously (18).
Generation of human DCs from PBMC.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized leukocyte-enriched buffy coats of healthy adult donors by Lymphoprep (1.077 g/ml; Nycomed, Oslo, Norway) density gradient centrifugation, applying 400 × g at room temperature. PBMC were plated on tissue culture dishes (3003; Falcon Labware, Oxnard, Calif.) at a density of 5 × 106 cells/ml in RPMI 1640 medium (Gibco/Life Technologies, Karlsruhe, Germany), supplemented with l-glutamine (2 mM), 1% autologous human plasma, and 100 U of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml for 45 min at 37°C. Nonadherent cells were washed free with phosphate-buffered saline (PBS), and adherent cells were cultured for 7 days without antibiotics in RPMI 1640 medium supplemented with 1% autologous human plasma, 2 mM l-glutamine, 1,000 U of recombinant human IL-4 (PBH, Hanover, Germany)/ml, and 800 U of recombinant human GM-CSF (Leukomax; Sandoz, Basel, Switzerland)/ml. Cytokines were replenished every other day.
Infection of DCs.
On day 7, nonadherent DCs were collected prior to infection by moderately vigorous aspiration and transferred to new 24-well plates at a density of 5 × 105 cells/ml (24). Bacteria were grown overnight in 5% CO2 on gonococcal complex (GC) agar with 1% supplement and used to inoculate supplemented Proteose Peptone medium (PPM+). They were grown to mid-log phase for infection. After two washes with PBS, the bacteria were diluted in RPMI 1640 medium and added at a multiplicity of infection (MOI) of ∼1 to each well. The cultures were incubated in RPMI 1640 medium with 1% autologous human plasma (unless otherwise indicated) at 37°C for different time spans before the numbers of adherent and invasive bacteria were assessed. The numbers of nonadherent bacteria were determined by plating serial dilutions of DC supernatant on GC agar plates, followed by incubation at 37°C and 5% CO2 for 24 h. For assessment of intracellular bacteria, cells were washed three times with PBS, followed by incubation of DC for 1 h in fresh RPMI 1640 medium containing 100 μg of gentamicin (Gibco/Life Technologies)/ml and 2% autologous human plasma. Cells were then washed three times with PBS, followed by the addition of 1% saponin in PBS to lyse DCs. The numbers of CFU were determined by plating appropriate dilutions of the lysates on GC agar. For assessment of cell-associated (adherent-plus-intracellular) bacteria per well, the assay was performed as described above except that the incubation step with gentamicin was omitted. All samples were tested in triplicate, and experiments were repeated at least twice.
Transmission electron microscopy.
DCs were infected with different bacteria as described above. At 6 h postinfection (p.i.), cells were washed, fixed in 2.5% glutaraldehyde, postfixed in 2% osmium tetroxide, stained with 0.5% uranyloacetate, dehydrated in graded alcohols, and finally embedded in Lowicryl K4M 812 overnight. Electron micrographs were taken with Zeiss EM900 and EM10 microscopes.
Flow cytometry.
Flow cytometry was used to monitor the expression of surface markers of uninfected and infected DCs. Indirect immunofluorescence was performed according to standard techniques, using murine MAbs revealed by phycoerythrin-conjugated anti-mouse immunoglobulin (Dianova, Hamburg, Germany). The primary Abs used were anti-HLA class II DR (L243) and anti-HLA class II DR/DQ (9.3F10) (ATCC, Manassas, Va.), CD25 (clone MA 251; Pharmigen, Hamburg, Germany), CD83 (HB15a; Immunotech, Hamburg, Germany), CD80 (Pharmingen), and CD86 (IT2.2; Pharmingen). The stained cells were analyzed on an EPICS XL-MCL (Coulter Immunotech Diagnotics, Krefeld, Germany).
Cytokine assessment by ELISA of DC supernatants.
To assess the amounts of cytokines and chemokines secreted by DCs after infection with N. meningitidis, DCs were infected with serogroup B strains, and supernatants were sampled at 6 and 20 h p.i. For supernatants sampled at 20 h p.i., 1% penicillin and streptomycin was added to all wells (including noninfected control cells) at 6 h p.i. for the killing of the bacteria. Without addition of antibiotics, neisserial cells exhibited unlimited growth resulting in lysis of DCs (data not shown). Supernatants were snap-frozen in liquid nitrogen and stored at −80°C. The concentrations of TNF-α, IL-1β, and IL-6 were determined twice in each supernatant by the following ELISA systems. For IL-6 and IL-1β, ELISA systems were established with the anti-human IL-6 and anti-human IL-1β matched antibody pairs (both from Endogen, Woburn, Mass.) and the streptavidin-horseradish peroxidase (HRP) conjugate (BD Pharmingen, Heidelberg, Germany) to a sensitivity of 5 pg of the cytokine in question/ml. For TNF-α, the OptEIA human TNF-α set (BD Pharmingen) was established according to the manufacturer's instructions to a sensitivity of 4 pg/ml. For all ELISA systems, the 3,3′,5,5′-tetramethyl(benzidine) (TMB) substrate reagent set (BD Pharmingen) was used to detect the HRP reaction.
RESULTS
Adherence of N. meningitidis serogroup B to DCs.
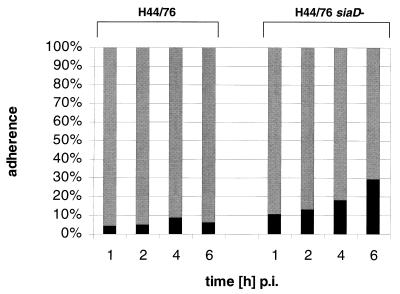
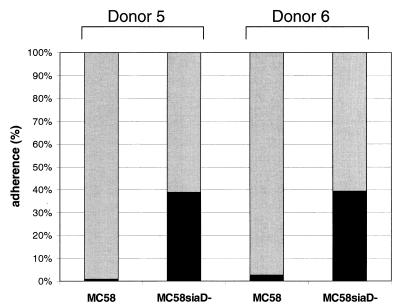
Immature DCs were produced from human PBMC by a standard protocol (46). Prior to infection, we checked the quality and purity of the DCs. According to the forward scatter-side scatter of the flow cytometry, 80 to 90% of the cells were CD1a+ and HLA class II+ DCs. These cells were infected with N. meningitidis serogroup B strain H44/76 wild-type bacteria, as well as the isogenic unencapsulated mutant strain H44/76 siaD, and cell association was determined. Proportions of cell-associated bacteria relate to all bacteria present in the well at the particular time point. Infections were performed in the presence of 1% autologous human plasma. At all time points bacteria were found to adhere to DCs (Fig. 1) with an increase in the proportion of DC-associated bacteria in a time-dependent manner for both strains. Unencapsulated bacteria, however, adhered more efficiently than the capsule expressing the wild-type strain (H44/76 wild-type, 4% at 1 h p.i. and 6% at 6 h p.i.; H44/76 siaD, 11% at 1 h p.i. and 30% at 6 h p.i.). Depending on the PBMC donor, the numbers of cell-associated bacteria showed some variation, with proportions of cell-associated bacteria at 6 h p.i. ranging from 2 to 10% for wild-type H44/76 bacteria and from 20 to 70% for the unencapsulated H44/76 siaD mutant strain (data not shown). For all donors tested, however, the proportion of DC-associated meningococci was significantly higher for the capsule-deficient strain in comparison to wild-type H44/76. This inhibition of DC adherence by expression of the serogroup B capsule polysaccharide was not restricted to strain H44/76, since infection of DCs with strain MC58 revealed a similar pattern of differential cell adherence for wild-type and siaD mutant bacteria (Fig. 2). For two different donors, the proportions of DC-associated bacteria at 6 h p.i. were 2.7% (donor 5) and 0.64% (donor 6) for the wild-type strain MC58 and 63.4% (donor 5) and 64.6% (donor 6) for MC58 siaD bacteria.
FIG. 1.
Adherence of N. meningitidis serogroup B to DCs. Adherence of capsulate (H44/76) and noncapsulate (H44/76 siaD) N. meningitidis to human DCs was determined. A total of 5 × 105 DCs per well were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Adherence was determined at 1, 2, 4, and 6 h p.i. The proportion of cell-adherent bacteria was calculated by dividing numbers of DC-adherent meningococci by the combined numbers of DC-adherent bacteria and meningococci in the supernatant. Shaded bar, supernatant; solid bar, adherence.
FIG. 2.
Adherence of N. meningitidis strain MC58 to DCs. The figure shows the association of wild-type strain MC58 and the capsule-deficient mutant strain MC58 siaD with DCs derived from two different donors (donors 5 and 6). A total of 5 × 105 DCs per well were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Adherence was determined at 6 h p.i. Shaded bar, supernatant; solid bar, adherence.
In all experiments, the meningococci showed some replication over the 6-h incubation period. However, the differential adherence of wild-type and capsule-deficient meningococci is not due to a stronger replication of the siaD bacteria since the wild-type H44/76 and MC58 strains showed a stronger replication than the siaD strains in the presence of human DCs. In RPMI medium without the presence of DCs instead, the replication of wild-type and capsule-negative strains is the same for strains H44/76 and MC58 (data not shown).
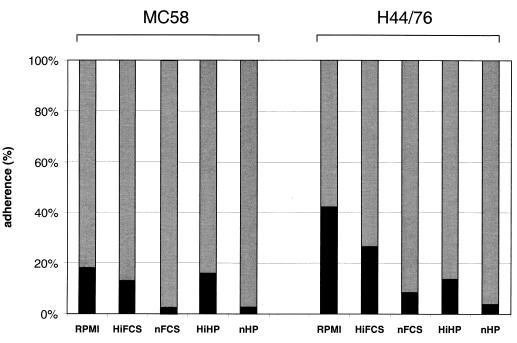
Does the presence of plasma or serum influence neisserial adherence to DC? Strains MC58 and H44/76 showed significantly decreased adherence to DC in the presence of 1% native or heat-inactivated autologous human plasma in comparison to infection in RPMI medium without plasma. The same was true for infection in the presence of 10% fetal calf serum (FCS) (Fig. 3). This also applied to the infection of DCs with unencapsulated siaD mutant strains MC58 and H44/76 in the presence of heat-inactivated human plasma or FCS in comparison to infections in medium alone. However, in the absence of plasma, the adherence of capsule-deficient meningococci was again higher than the adherence of the wild-type bacteria, showing that the capsule-meditated inhibition of cell association does not depend on the presence of plasma (data not shown).
FIG. 3.
Influence of presence of plasma or serum on adherence of N. meningitidis serogroup B to DCs. A total of 5 × 105 DCs were infected with wild-type strains MC58 and H44/76. DCs were infected with both strains at an MOI of 1 in the presence of RPMI or RPMI containing 1% native (nHP) or heat-inactivated (HiHP) autologous human plasma or 10% native (nFCS) or heat-inactivated (HiFCS) FCS. At 6 h p.i., neisserial adherence to DCs was determined. Shaded bar, supernatant; solid bar, adherence.
Phagocytosis of meningococci by DCs.
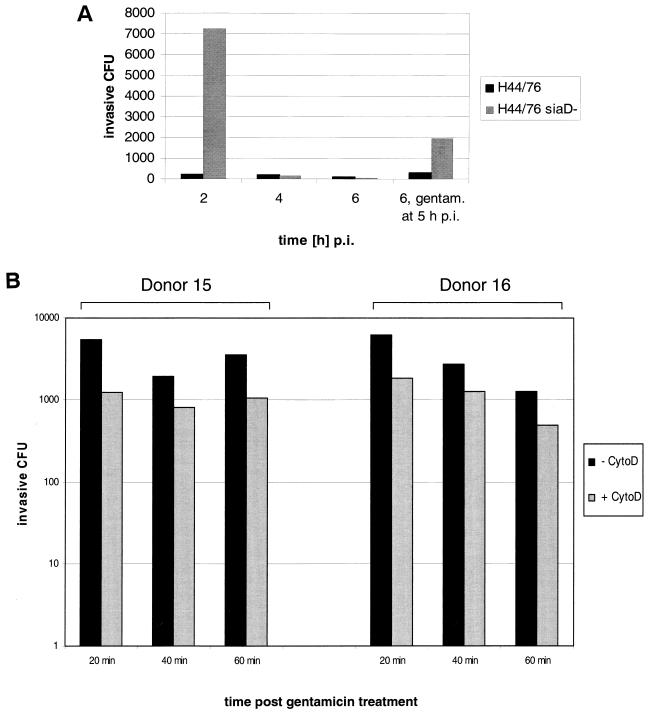
To determine phagocytosis of meningococci by DCs, we assessed the numbers of live intracellular bacteria by a gentamicin assay. In this assay, gentamicin was added to the infections at 1 h p.i. At 2 h p.i., we found ca. 7.5 × 103 colony-forming intracellular meningococci per 5 × 105 infected DCs for the capsule-deficient strain H44/76 siaD, which is equivalent to ca. 3.8% of the cell-associated bacteria at this time point (Fig. 4). For the capsulate H44/76 strain, we found only 265 intracellular bacteria on average per 5 × 105 DCs, equivalent to ca. 0.2% of the DC-associated bacteria. The intracellular bacteria are rapidly killed by DCs. At 4 h p.i., the gentamicin assay revealed only about 240 and 200 intracellular bacteria for the capsule-deficient and the capsulate strains, respectively. At 6 h p.i., these numbers decreased to only ca. 100 intracellular bacteria for both meningococcal strains. DCs retain their phagocytic capacity during the course of infection. When gentamicin was added to the infected DCs at 5 h p.i., 1.8 × 103 H44/76 siaD and 325 H44/76 wild-type meningococci were located inside DCs at 6 h p.i. (Fig. 4A). DCs thus retained their phagocytic capacity during several hours of infection.
FIG. 4.
Phagocytosis of N. meningitidis serogroup B by DCs. (A) DCs were infected with N. meningitidis serogroup B strain H44/76 wild-type bacteria, as well as the isogenic unencapsulated mutant strain H44/76 siaD. A total of 5 × 105 DCs per well were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Gentamicin was added at 1 h p.i., and the numbers of colony-forming intracellular bacteria were determined at 2, 4, and 6 h p.i. To some wells, gentamicin was added 5 h p.i. and the numbers of colony-forming intracellular bacteria were determined at 6 h p.i. (B) DCs were infected with N. meningitidis serogroup B strain H44/76 siaD. A total of 5 × 105 DCs per well were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Gentamicin was added at 5 h p.i., and the numbers of colony-forming intracellular bacteria were determined at 20, 40, and 60 min after the addition of gentamicin. To some wells, cytochalasin D was added at a concentration of 2 μg/ml at 4.5 h p.i. (30 min prior to gentamicin treatment), and the numbers of colony-forming intracellular bacteria were determined at 20, 40, and 60 min after the addition of gentamicin.
The intracellular killing of the phagocytosed meningococci is not due to endocytic uptake of gentamicin by the DCs. The addition of cytochalasin D, an inhibitor of endocytosis, 30 min prior to treatment of infected DCs with gentamicin, did not lead to higher numbers of intracellular CFU (Fig. 4B). In this assay, gentamicin was added at 5 h p.i. to the infected cells and cytochalasin D accordingly at 4.5 h p.i. Intracellular bacterial CFU were assayed at 20, 40, and 60 min after gentamicin treatment. Cytochalasin D even led to a reduced number of intracellular CFU, potentially due to the inhibition of bacterial uptake after addition of cytochalsin D. These results were found for DCs derived from two different donors.
Phagocytosis of capsulate and capsule-deficient meningococci was also assessed by transmission electron microscopy. Electron microscopy analysis of infected DCs showed, surprisingly, that the numbers of intracellular meningococci were markedly higher than those revealed by gentamicin killing assays, in particular for the H44/76 siaD strain (Fig. 5). For infection with this strain, we observed on average 20 to 30 intracellular bacteria per individual DC (Fig. 5B to D), giving a number of intracellular bacteria 104 times higher than that observed with the gentamicin killing assays. The wild-type strain was phagocytosed in lower numbers by DCs, with rarely any intracellular bacteria observed by electron microscopy. A maximum of 10% of DCs were found to contain phagocytosed encapsulated meningococci, and most of these DCs contained only one or two intracellular meningococci (Fig. 5A). Nevertheless, the numbers of phagocytosed encapsulated bacteria were ca. 103 times higher than the numbers calculated by gentamicin assays. Concerning the intracellular localization within DCs, both meningococcal strains were always found exclusively in the phagosomal compartment. Phagosomal meningococci are depicted by white arrows in Fig. 5A, C, and D. All microscopy data were confirmed by using alternate strain MC58. The differences observed by microscopy and in gentamicin killing assays demonstrate that most of the phagocytosed bacteria are efficiently lysed by DCs in a short period of time. Indeed, electron microscopy of the phagocytosed meningococci reveals a large number of meningococci at different stages of lytic degradation, which is deducible from the decrease of the optical density of single bacteria (indicated by a white asterisk in Fig. 5B). Despite 1,000-fold-higher numbers of intracellular capsule-deficient meningococci, comparable numbers of viable neisseriae could be obtained 6 h p.i. with both strains by gentamicin killing assays. Thus, to some extent, capsule expression seems to inhibit killing of the bacteria in the DC phagosome as well. These data demonstrate two phenomena: (i) the enormous capacity of human DCs to phagocytose meningococci and (ii) their ability to efficiently kill these bacteria.
FIG. 5.
Transmission electron microscopy of N. meningitidis-infected DCs. Electron microscopic images show the interaction of N. meningitidis H44/76 wild-type strain (A) and the capsule-deficient mutant H44/76 siaD (B to D) with DCs. (A) Electron microscopic image showing the weak adherence of H44/76 wild-type bacteria to DCs and the small number of intracellularly located meningococci within a vacuole (white arrow), with one phagocytosed bacterium per cell on average. (B) Electron microscopic image illustrating a large number of intracellularly located H44/76 siaD bacteria at different stages of lytic degradation, as revealed by a decrease of the optical density; single bacteria exhibit a high optical density and remain intact. A bacterium exhibiting a low optical density is indicated by a white asterisk. (C) Detail of a section of DCs incubated with the capsule-deficient mutant strain H44/76 siaD. The micrograph shows a number of intracellular bacteria present in membrane-bound vacuoles within DCs (white arrow). (D) The micrograph shows an H44/76 siaD meningococcus (white arrow) that is partially enclosed by the cytoplasmatic membrane of a DC during the process of phagocytosis.
Infection with N. meningitidis induces DC maturation.
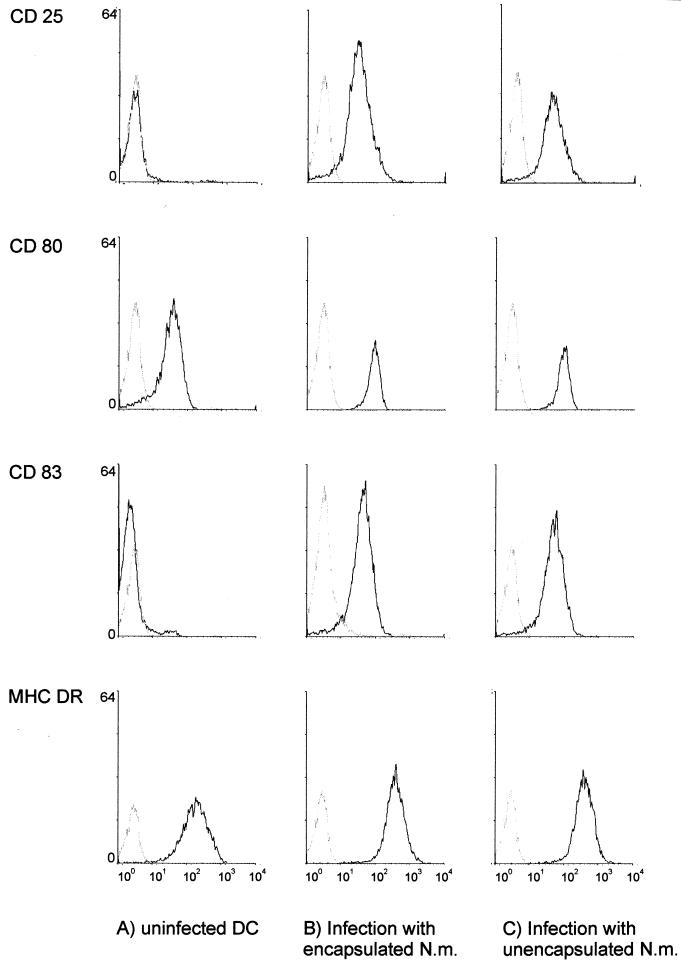
To investigate maturation effects of N. meningitidis-infected DCs, we assessed the surface marker expression of DCs. Infection with encapsulated and capsule-deficient MC58 bacteria had the same effect on surface marker profiles of DCs. CD83, a specific mature-phase marker of human DCs, was strongly upregulated 18 h after infection. About 95% of DCs expressed CD83, indicating maturation of N. meningitidis-infected DC. Infection also led to strong upregulation of MHC class II, the costimulatory molecules CD80 and CD86, as well as CD25 (IL-2-receptor α-chain) (Fig. 6). Infection of DCs with capsulated and capsule-deficient H44/76 bacteria caused DC maturation to a similar extent (data not shown). Parallel control infection of the DCs of the same donors with the four different meningococcal strains demonstrated that adherence and invasiveness of all strains exhibited the patterns observed in previous experiments (data not shown). These data demonstrate that both wild-type and capsule-deficient serogroup B meningococci cause the maturation of DCs. Moreover, they indicate that DC maturation is independent of the serogroup B meningococcal strain.
FIG. 6.
DC maturation after infection with N. meningitidis serogroup B. Flow cytometry profiles of surface marker expression of CD25, CD80, CD83, and MHC DR on human DCs of either uninfected DCs (A) or 24 h after infection with MC58 wild-type bacteria (B) or the unencapsulated mutant strain MC58 siaD (C) are shown. DCs were infected at an MOI of 1. The x axis of each histogram shows the log fluorescent intensity; the y axis shows the relative cell number. Gray histograms represent staining with matched-isotype antibodies.
Infection with N. meningitidis stimulates a burst of proinflammatory cytokines in DCs.
Since N. meningitidis is a potent inducer of DC maturation, we wondered about cytokine production of infected DCs. To this end, 5 × 105 DCs per well were infected with N. meningitidis strains MC58 and MC58 siaD at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. DC supernatants were sampled at 6 and 20 h p.i. and assessed for the cytokines TNF-α, IL-1β, IL-6, and IL-8 by ELISA. All infected DCs produced large amounts of TNF-α, IL-6, and IL-8, compared to uninfected DCs (Table 1). Depending on the donor, infection with strain MC58 causes an increase in TNF-α production of 150- to 500-fold at 6 h p.i. and 6,000- to 20,000-fold at 20 h p.i., while infection with strain MC58 siaD increased the production of TNF-α by 720- to 750-fold at 6 h p.i. and 10,000- to 22,000-fold by 20 h p.i. (Table 1). Similarly, amounts of IL-6 produced were increased 50- to 750-fold at 6 h p.i. and 16,000- to 110,000-fold at 20 h p.i. for strain MC58 and 200- to 3,400-fold (6 h p.i.) and 16,000- to 110,000-fold (20 h p.i.) for strain MC58 siaD. Finally, secretion of IL-8 had increased 15- to 20-fold (infection with strain MC58) and 35- to 60-fold (infection with MC58 siaD) at 6 h p.i. and more than 1,000-fold for both strains at 20 h p.i. Amounts of TNF-α, IL-6, and IL-8 produced after infection with strains H44/76 and H44/76 siaD were in a similar range to values after infection with strains MC58 and MC58 siaD (data not shown).
TABLE 1.
Cytokine production of DCs after infection with N. meningitidis serogroup B strains MC58 and MC58siaD
| Cytokine | Infecting strain | Mean cytokine concn (pg/ml) ± SD in:
|
|||
|---|---|---|---|---|---|
| Donor 11 at 6 h p.i. | Donor 12 at 6 h p.i. | Donor 11 at 20 h p.i. | Donor 12 at 20 h p.i. | ||
| TNF-α | MC58 | 680 ± 53 | 2,364 ± 164 | 27,420 ± 580 | 82,125 ± 460 |
| MC58siaD | 3,363 ± 124 | 3,405 ± 215 | 45,372 ± 620 | 93,409 ± 530 | |
| None | 4.5 ± 0.2 | 4.7 ± 0.1 | 4.4 ± 0.2 | 4.2 ± 0.1 | |
| IL-1β | MC58 | 18.1 ± 0.7 | <1 | 170 ± 24 | 293 ± 18 |
| MC58 siaD | 22.9 ± 0.9 | <1 | 226 ± 33 | 421 ± 21 | |
| None | <1 | <1 | <1 | <1 | |
| IL-6 | MC58 | 2,062 ± 210 | 1,502 ± 35 | 58,657 ± 2,720 | 113,709 ± 3,305 |
| MC58 siaD | 8,347 ± 202 | 6,837 ± 316 | 59,119 ± 2,158 | 111,368 ± 3,466 | |
| None | 38.4 ± 1.1 | 2.0 ± 0.1 | 3.6 ± 0.1 | <1 | |
| IL-8 | MC58 | 3,402 ± 377 | 2,277 ± 105 | >200,000 ± 8,500 | >200,000 ± 11,000 |
| MC58 siaD | 10,923 ± 802 | 5,043 ± 257 | >200,000 ± 12,000 | >200,000 ± 9,000 | |
| None | 175 ± 12 | 147 ± 19 | 165 ± 11 | 189 ± 14 | |
Interestingly, the amounts of IL-1β were only slightly increased after infection with N. meningitidis serogroup B bacteria at 6 h p.i. While the IL-1β levels of donor 11 had increased ca. 20-fold after infection with MC58 or MC58 siaD, neither infected nor uninfected DCs of donor 12 exhibited significant production of IL-1β. Overnight infection, however, led to IL-1β production in cells from both donors. Infection with strain MC58 had caused a 170- to 290-fold increase in IL-1β levels; infection with strain MC58 siaD led to a 230- to 420-fold increase. The cytokines TNF-α, IL-6, and IL-8 are produced rapidly after infection with N. meningitidis serogroup B (as early as 6 h p.i.); the production of IL-1β, however, is retarded and does not reach the levels of TNF-α, IL-6, and IL-8. Again, parallel infections of DCs of the same donors revealed the patterns of cell adherence and invasiveness for the four strains used that had been observed previously.
The increase in cytokine production after infection with capsule-deficient in comparison to encapsulated meningococci may suggest that cell adherence is critical for the induction of cytokine production. We therefore tested whether the cytokine production of DCs can also be induced by soluble factors present in the supernatant of N. meningitidis cultures. Capsulate (H44/76) and noncapsulate (H44/76 siaD) N. meningitidis were cultured in PPM+, and culture supernatants were sampled at an optical density at 600 nm of 1.5. The bacteria were removed by centrifugation and treatment with the antibiotics penicillin and streptomycin. Then, 10 μl of bacterium-free supernatants was added to 5 × 105 DCs per well cultured in 1 ml of RPMI 1640 containing 1% (vol/vol) autologous human plasma, and cytokine production was determined by ELISA 6 h after treatment (Table 2). Control DCs were treated with 10 μl of PPM+/ml or left untreated. Supernatants of both bacterial strains induced stronger production of TNF-α (H44/76, 60- to 290-fold; H44/76 siaD, 65- to 170-fold), IL-6 (H44/76, 50- to 1,900-fold; H44/76 siaD, 70- to 1,950-fold), and IL-8 (25- to 60-fold for strains H44/76 and H44/76 siaD). DCs treated with 1% (vol/vol) PPM+ produced 6- to 27-fold-increased levels of TNF-α and no increase in the levels of IL-6 or IL-8 (Table 2). In contrast, production of IL-1β was not significantly induced by neisserial supernatants, irrespective of the strain. Boiling of the bacterial supernatants before addition to DCs reduced the production of TNF-α, IL-6, and IL-8 only slightly (data not shown), suggesting that the cytokine-inducing factor is heat stable. For further characterization of the cytokine-inducing factor, we treated DCs with purified LOS of N. meningitidis serogroup B. A total of 5 × 105 DCs per well were treated with 100 ng of neisserial LOS or Salmonella LPS per 1 ml of RPMI 1640 medium containing 1% autologous human plasma. At 6 h, supernatants were sampled and the cytokine levels were determined by ELISA. LOS-treated cells were found to produce 300- to 800-fold increased amounts of TNF-α, 130- to 1,600-fold more IL-6, and 25- to 75-fold more IL-8, depending on the donor (Table 3). Cells treated in parallel with Salmonella LPS (100 ng/ml) were found to produce similarly increased amounts of these cytokines, whereas IL-1β was not induced by neisserial LOS or Salmonella LPS. These data clearly demonstrate that neisserial LOS may be a crucial factor for production of the cytokines TNF-α, IL-6, and IL-8 by DCs after neisserial infection, whereas it does not lead to a rapid increase in IL-1β levels.
TABLE 2.
Cytokine production after treatment with culture supernatants of strains H44/76 (H44/76-SN) and H44/76 siaD (H44/76siaD-SN)
| Cytokine | Treatment | Mean cytokine concn (pg/ml) ± SD in:
|
|
|---|---|---|---|
| Donor 11 | Donor 12 | ||
| TNF-α | H44/76-SN | 270 ± 11 | 1,340 ± 23 |
| H44/76siaD-SN | 780 ± 15 | 300 ± 12 | |
| PPM+ | 27 ± 0.4 | 60 ± 1.1 | |
| None | 4.5 ± 0.2 | 4.7 ± 0.1 | |
| IL-1β | H44/76-SN | <1 | 17.9 ± 0.6 |
| H44/76siaD-SN | <1 | 18.7 ± 0.8 | |
| PPM+ | <1 | 16.2 ± 0.3 | |
| None | <1 | <1 | |
| IL-6 | H44/76-SN | 1,831 ± 245 | 3,771 ± 157 |
| H44/76siaD-SN | 2,632 ± 94 | 3,893 ± 198 | |
| PPM+ | 13.5 ± 0.2 | <1 | |
| None | 38.4 ± 1.1 | 2.0 ± 0.1 | |
| IL-8 | H44/76-SN | 4,295 ± 248 | 7,607 ± 485 |
| H44/76siaD-SN | 4,740 ± 358 | 7,742 ± 525 | |
| PPM+ | 198 ± 14 | 143 ± 11 | |
| None | 175 ± 12 | 147 ± 19 | |
TABLE 3.
Cytokine production after treatment with neisserial LOS
| Cytokine | Treatment | Mean cytokine concn (pg/ml) ± SD in:
|
|
|---|---|---|---|
| Donor 11 | Donor 12 | ||
| TNF-α | Neisseria LOS | 1,380 ± 22 | 3,810 ± 47 |
| Salmonella LPS | 910 ± 68 | 2,210 ± 35 | |
| None | 4.5 ± 0.2 | 4.7 ± 0.1 | |
| IL-1β | Neisseria LOS | <1 | 17.3 ± 0.5 |
| Salmonella LPS | 15.9 ± 1.2 | 116 ± 12 | |
| None | <1 | <1 | |
| IL-6 | Neisseria LOS | 4,976 ± 280 | 7,153 ± 346 |
| Salmonella LPS | 3,203 ± 137 | 4,754 ± 221 | |
| None | 38.4 ± 1.1 | 2.0 ± 0.1 | |
| IL-8 | Neisseria LOS | 4,556 ± 210 | 11,165 ± 856 |
| Salmonella LPS | 6,971 ± 476 | 10,563 ± 631 | |
| None | 175 ± 12 | 147 ± 19 | |
DISCUSSION
The interaction of pathogenic neisseriae with PBMC and human macrophages and the cytokines produced by these cells in meningococcal disease have been well characterized. Macrophages and PBMC were shown to be induced to secrete proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8 by infection with N. meningitidis (22, 27, 30, 40, 43, 52). Here, we investigated the interaction of pathogenic N. meningitidis with human DCs and the induction of proinflammatory responses in these APCs upon exposure to meningococci.
Serogroup B meningococci were found to adhere to DCs with high efficiency, with up to 70% of the bacteria being cell associated at 6 h p.i. Major differences were seen, however, between capsulated and unencapsulated bacteria. While capsule-deficient meningococci were found to be highly DC adherent, encapsulated wild-type bacteria were found to adhere to DCs to a lesser extent. In line with these results, the N. meningitidis serogroup B capsule prevents phagocytosis of the bacteria by DCs. Whereas intracellular bacteria were found rarely in cells infected with wild-type meningococci, the unencapsulated bacteria were phagocytosed in high numbers. The phagocytosis of N. meningitidis by human DCs leads to efficient killing of the bacteria, since only a low proportion of the intracellular bacteria that were observed after examination of the infected DCs by electron microscopy were found to be alive in gentamicin assays determining the numbers of colony-forming intracellular neisseriae (ca. 0.1% for encapsulated and 0.01% for unencapsulated meningococci). These data show that human DCs are capable of efficiently eliminating N. meningitidis by phagocytosis and suggest that DCs may play an important role in controlling neisserial infections by their bactericidal activity. On the other hand, expression of the polysaccharide capsule prevents adherence of the bacteria to a great extent and prevents phagocytic killing of the meningococci. For the adherence of the bacteria, similar observations had previously been made for the infection of human macrophages by N. meningitidis serogroups A (32) and B (43). Capsulate bacteria of both serogroups failed to interact with human macrophages in significant numbers. For capsule-deficient bacteria, however, high levels of cell association were found (32, 43). Similar to our results, proportionately higher numbers of capsule-deficient serogroup A bacteria were internalized by human macrophages, while both capsulate and capsule-deficient bacteria exhibited a gradual decrease in viability (32). In the case of serogroup B meningococci, however, the expression of the capsule did not influence the numbers of bacteria phagocytosed by macrophages, and capsulate bacteria were killed even more efficiently by macrophages than unencapsulated neisseriae (43). While human macrophages were able to limit the growth of internalized bacteria only to some extent, human DCs seem to be more efficient in killing phagocytosed neisseriae and may play a more important role for controlling neisserial infections than was previously assumed.
In contrast to infection of human macrophages by serogroup B meningococci (43), our data provide evidence that the presence of plasma or serum plays only a limited role for bacterial adherence and phagocytosis, suggesting that nonopsonic phagocytosis of N. meningitidis by DCs may be an important innate immune response. Nonopsonic phagocytosis of serogroup A meningococci by human monocytes (32), of serogroup B meningococci by human macrophages (43), and of group C meningococci by human neutrophils (13) has previously been observed. Our data and those of Read et al. (43) demonstrate, however, that the serogroup B capsule provides N. meningitidis with a means to escape this type of host defense. Moreover, capsule expression has been shown to be responsible for resistance of N. meningitidis to human serum (reviewed in reference 57). The neisserial capsule is therefore the key mechanism of N. meningitidis to avoid innate immune mechanisms. Since the expression of Opa and, to a greater extent, Opc correlated with phagocytosis of capsule-deficient serogroup A meningococci by human monocytes (32), the role of the capsule may therefore be to mask these surface antigens to prevent phagocytosis by macrophages and DCs.
Infection with meningococci induced maturation of DCs and production of proinflammatory cytokines. Under steady-state conditions, DCs are present in an immature state, highly efficient in antigen capture and processing, but exhibiting only moderate to low efficiency in antigen presentation and immune modulation. Once the DC has captured antigen, however, it begins to mature, expressing large amounts of MHC molecules and costimulatory surface markers, as well as proinflammatory cytokines, thereby shaping the immune response (3, 48). All of these DC activities can be induced by infectious agents (54), while the cytokine-inducing ability depends on the type of stimuli or subsets of DCs (45). Bacterial LPS is especially well known to be a strong inducer of DC maturation (56). Our data clearly demonstrate that infection of DCs with N. meningitidis leads to maturation of DCs derived from human PBMC. Shortly after infection, DCs were expressing surface markers characteristic of mature DCs, such as CD83. Infection also resulted in strong upregulation of surface molecules involved in antigen presentation and immune regulation, including MHC class II, the costimulatory molecules CD80 and CD86, as well as CD25. Moreover, infection of DCs with N. meningitidis serogroup B causes strong production of the cytokines TNF-α, IL-6, IL-8 and, to a lesser extent, IL-1β. This suggests that DCs may indeed be associated with the pathology seen due to meningococcal infection.
Our data show that soluble factors relased into the supernatant of neisserial cultures rapidly induce the production of TNF-α, IL-6, and IL-8. We could furthermore demonstrate that neisserial LOS is a key player in the induction of these cytokines. IL-1β, instead, is induced only after overnight infection and does not seem to be induced strongly by neisserial LOS, not even after incubation for 20 h (data not shown). Similar results were reported very recently by Dixon et al. (12). In that study, incubation of human DCs with a LOS-negative mutant of strain H44/76 induced significantly weaker cytokine production than did incubation with the wild-type strain. The initial differences in cytokine induction observed herein after infection with encapsulated versus capsule-deficient meningococci may therefore be caused by the locally higher amount of LOS due to the stronger DC adherence of the capsule-deficient mutant strains. The outer membrane of N. meningitidis is well known to constantly release membrane blebs containing a full complement of outer membrane proteins and LPS in their natural conformation (11). It has been documented that LPS can lead to the direct upregulation of CD80/86 costimulatory molecules on DCs (10) and the production of proinflammatory cytokines (17, 44, 54). Neisserial LOS has been demonstrated to induce cytokine production in human macrophages and PBMC (22, 40, 52, 56, 61), as well as in DCs (12).
DCs are one of the most important immune modulators, and the outcome of an immune response is influenced by whether the DC has been activated by microbial products, such as LPS. In the case of meningococcal infections, however, activation of DCs may give rise to an exaggerated immune response being responsible for the fatal outcome. Severity of meningococcal disease directly correlates with the production of proinflammatory cytokines by mononuclear cells (37, 53, 59, 60): patients having TNF-α concentrations of >440 U/ml in their blood invariably die (58). It is intriguing that Neisseria-infected DCs produce the cytokines that are correlated with severe meningococcal disease: TNF-α, IL-6, and IL-8, as well as IL-1β to a lesser extent. Here, we studied the cytokine production by DCs which had been derived from human PBMC. However, DCs are also present at the blood-brain barrier and even within the brain (14, 31, 49). The production of cytokines resembling the one observed in our studies can be expected for brain DCs or blood-brain barrier DCs, and the entry of an enormous number of monocytes into the subarachnoid space is a hallmark of neisserial meningitidis (28). While the clinical symptoms of meningitis and septicemia are not mutually exclusive and often overlap, studies of the levels of cytokines, collected simultaneously from blood and CSF, suggest separate compartmentalized intravascular and intracranial inflammatory responses to infection (4, 19, 50, 59, 60).
Several approaches to treat meningococcal meningitis or to attenuate the severity of symptoms are based on the inhibition of the exacerbated inflammatory response (9, 15, 25, 29, 35, 38), but only some of these strategies have been shown to decrease complications of meningococcal disease (9, 29). To control the production of the inflammatory mediators to amounts that provide only beneficial immunostimulatory effects may be a valuable strategy. However, for targeted prevention of the catastrophic inflammatory responses associated with meningococcal disease, a precise characterization of the interplay of the cells producing these proinflammatory cytokines and the bacteria is absolutely necessary. Our future studies will therefore focus on the identification of meningococcal factors responsible for the activation of human DCs and the receptor(s) on the surface of DCs mediating the recognition of these molecules.
ACKNOWLEDGMENTS
A. Kolb-Mäurer and A. Unkmeir contributed equally to this study.
We thank M. Dietrich and S. Kurz for critical reading of the manuscript and W. Goebel and I. Gentschev for fruitful discussions. We are grateful to E. R. Moxon and D. A. Caugant for providing strains MC58 and H44/76, respectively; to M. Virji and J. E. Heckels for antobody SM1; to G. Krohne and C. Gehrig for electron microscopy; and to K. Ott for expert technical assistance. We also thank U. Vogel for his support and the generous gift of vector pGH15 and A. Leimbach for excellent assistance with phagocytosis assays.
This work was supported by a grant within Sonderforschungsbereich 479 (Erregervariabilität und Wirtsreaktion bei infektiösen Krankheitsprozessen), project B2, as well as by a fellowship from the Bundesministerium für Bildung and Forschung (A201K59603) to A.K.-M. and grant A16 to U.K. and E.K. within the scope of IZKF Würzburg.
REFERENCES
- 1.Al'Aldeen A A, Cartwright K A. Neisseria meningitidis: vaccines and vaccine candidates. J Infect. 1996;33:153–157. doi: 10.1016/s0163-4453(96)92081-2. [DOI] [PubMed] [Google Scholar]
- 2.Artenstein M S, Gold R, Zimmerly J G, Wyle F A, Schneider H, Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Ovsteboo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650–652. doi: 10.1093/infdis/166.3.650. [DOI] [PubMed] [Google Scholar]
- 5.Brouckaert P, Libert C, Everaerdt B, Takahashi N, Cauwels A, Fiers W. Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiology. 1993;187:317–329. doi: 10.1016/S0171-2985(11)80347-5. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright K A, Ala'Aldeen D A. Neisseria meningitidis: clinical aspects. J Infect. 1997;34:15–19. doi: 10.1016/s0163-4453(97)80004-7. [DOI] [PubMed] [Google Scholar]
- 7.Cassell D J, Schwartz R H. A quantitative analysis of APC function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994;180:1829–1834. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly M, Noah N. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993–1996. Epidemiol Infect. 1999;122:41–49. doi: 10.1017/s0950268898001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derkx B, Wittes J, McCloskey R. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin Infect Dis. 1999;28:770–777. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 10.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devoe I W, Gilchrist J E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973;138:1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon G L, Newton P J, Chain B M, Katz D, Andersen S R, Wong S, van der Ley P, Klein N, Callard R E. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin 12 production depends on lipopolysaccharide expression in intact bacteria. Infect Immun. 2001;69:4351–4357. doi: 10.1128/IAI.69.7.4351-4357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estabrook M M, Zhou D, Apicella M A. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect Immun. 1998;66:1028–1036. doi: 10.1128/iai.66.3.1028-1036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer H G, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 15.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N J, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 16.Gotschlich E C, Austrian R, Cvjetanovic B, Robbins J B. Prospects for the prevention of bacterial meningitis with polysaccharide vaccines. Bull W H O. 1978;56:509–518. [PMC free article] [PubMed] [Google Scholar]
- 17.Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 18.Hammerschmidt S, Birkholz C, Zahringer U, Robertson B D, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–996. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 19.Hazelzet J A, Risseeuw-Appel I M, Kornelisse R F, Hop W C, Dekker I, Joosten K F, de Groot R, Hack C E. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost. 1996;76:932–938. [PubMed] [Google Scholar]
- 20.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingalls R R, Lien E, Golenbock D T. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through Toll-like receptor 2. Infect Immun. 2001;69:2230–2236. doi: 10.1128/IAI.69.4.2230-2236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones D M. Meningococcal vaccines. J Med Microbiol. 1993;38:77–78. doi: 10.1099/00222615-38-2-77. [DOI] [PubMed] [Google Scholar]
- 24.Kolb-Mäurer A, Gentschev I, Fries H-W, Fiedler F, Bröcker E-B, Kämpgen E, Goebel W. Listeria monocytogenes-infected human dendritic cells: invasion and host cell response. Infect Immun. 2000;66:3680–3688. doi: 10.1128/iai.68.6.3680-3688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornelisse R F, de Groot R, Neijens H J. Bacterial meningitis: mechanisms of disease and therapy. Eur J Pediatr. 1995;154:85–96. doi: 10.1007/BF01991906. [DOI] [PubMed] [Google Scholar]
- 26.Kreger B E, Craven D E, Carling P C, McCabe W R. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980;68:332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- 27.Lapinet J A, Scapini P, Calzetti F, Pérez O, Cassatella M A. Gene expression and production of tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-8, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect Immun. 2000;68:6917–6923. doi: 10.1128/iai.68.12.6917-6923.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leib S L, Tauber M G. Pathogenesis of bacterial meningitis. Infect Dis Clin North Am. 1999;13:527–548. doi: 10.1016/s0891-5520(05)70093-3. [DOI] [PubMed] [Google Scholar]
- 29.Levin M, Quint P A, Goldstein B, Barton P, Bradley J S, Shemie S D, Yeh T, Kim S S, Cafaro D P, Scannon P J, Giroir B P. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356:961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen D R, Düx F, Wölk U, Tsipouchtsidis A, Haas G, Meyer T F. Immunoglobulin A1 protease, an exoenzyme of pathogenic neisseriae, is a potent inducer of proinflammatory cytokines. J Exp Med. 1999;190:1049–1058. doi: 10.1084/jem.190.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMenamin P G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 32.McNeil G, Virji M, Moxon E R. Interactions of Neisseria meningitidis with human monocytes. Microb Pathog. 1994;16:153–163. doi: 10.1006/mpat.1994.1016. [DOI] [PubMed] [Google Scholar]
- 33.Muenzner P, Dehio C, Fujiwara T, Achtman M, Meyer T F, Gray-Owen S D. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect Immun. 2000;68:3601–3607. doi: 10.1128/iai.68.6.3601-3607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustafa M M, Ramilo O, Olsen K D, Franklin P S, Hansen E J, Beutler B, McCracken G H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Investig. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nassif X, Mathison J C, Wolfson E, Koziol J A, Ulevitch R J, So M. Tumour necrosis factor α antibody protects against lethal meningococcaemia. Mol Microbiol. 1992;6:591–597. doi: 10.1111/j.1365-2958.1992.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 36.Nelson D J, Holt P G. Defective regional immunity in the respiratory tract of neonates is attributable to hyporesponsiveness of local dendritic cells to activation signals. J Immunol. 1995;155:3517–3524. [PubMed] [Google Scholar]
- 37.Ohga S, Aoki T, Okada K, Akeda H, Fujioka K, Ohshima A, Mori T, Minamishima I, Ueda K. Cerebrospinal fluid concentrations of interleukin-1β, tumour necrosis factor-α, and interferon gamma in bacterial meningitis. Arch Dis Child. 1994;70:123–125. doi: 10.1136/adc.70.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohga S, Okada K, Ueda K, Takada H, Ohta M, Aoki T, Kinukawa N, Miyazaki S, Hara T. Cerebrospinal fluid cytokine levels and dexamethasone therapy in bacterial meningitis. J Infect. 1999;39:55–60. doi: 10.1016/s0163-4453(99)90103-2. [DOI] [PubMed] [Google Scholar]
- 39.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 40.Pridmore A C, Wyllie D H, Abdillahi F, Steeghs L, van Der Ley P, Dower S K, Read R C. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Maraskovsky E, Bancherau J, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41–47. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 42.Ramilo O, Saez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor α/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Read R C, Zimmerli S, Broaddus C, Sanan D A, Stephens D S, Ernst J D. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reis e Sousa C, Germain R N. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J Immunol. 1999;162:6552–6561. [PubMed] [Google Scholar]
- 45.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 46.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serot J M, Bene M C, Foliguet B, Faure G C. Monocyte-derived IL-10-secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol. 2000;105:115–119. doi: 10.1016/s0165-5728(99)00240-4. [DOI] [PubMed] [Google Scholar]
- 50.Spanaus K S, Nadal D, Pfister H W, Seebach J, Widmer U, Frei K, Gloor S, Fontana A. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997;158:1956–1964. [PubMed] [Google Scholar]
- 51.Tuomanen E I, Saukkonen K, Sande S, Cioffe C, Wright S D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989;170:959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uronen H, Williams A J, Dixon G, Andersen S R, Van Der Ley P, Van Deuren M, Callard R E, Klein N. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide. Clin Exp Immunol. 2000;122:312–315. doi: 10.1046/j.1365-2249.2000.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Deuren M, van der Ven-Jongekrijg J, Bartelink A K, van Dalen R, Sauerwein R W, van der Meer J W. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 54.Viney J L. Immune fate decided by dendritic cell provocateurs. Trends Immunol. 2001;22:8–10. doi: 10.1016/s1471-4906(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 55.Virji M, Kayhty H, Ferguson D J P, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 56.Visintin A, Mazzoni A, Spitzer J H, Wyllie D H, Dower S K, Segal D M. Regulation of toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 57.Vogel U, Frosch M. Mechanisms of neisserial serum resistance. Mol Microbiol. 1999;32:1133–1139. doi: 10.1046/j.1365-2958.1999.01469.x. [DOI] [PubMed] [Google Scholar]
- 58.Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;i:355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 59.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin 1, and interleukin 6 in meningococcal meningitis: relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock: association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler E J, Fisher C J, Jr, Sprung C L, Straube R C, Sadoff J C, Foulke G E, Wortel C H, Fink M P, Dellinger R P, Teng N N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]