Abstract
Purpose
The aim of this study is to describe the demographics and clinical features of patients with young onset (YO) CRC.
METHODS
A retrospective review of patients with CRC diagnosed between ages 20 and 49 years evaluated at Memorial Sloan Kettering Cancer Center from 1/2004 to 6/2019. We excluded those with a hereditary CRC syndrome, inflammatory bowel disease, or prior CRC diagnosis.
Patient demographics, presenting symptoms, medical, surgical and smoking history, family history of cancer, tumor characteristics, and pathology were obtained from the electronic medical record.
RESULTS
We identified 3856 YO CRC patients (median age CRC diagnosis 43; 52.5% male). 59.1% were overweight or obese (32.2% and 26.9%, respectively). Most (90.1%) had no family history of CRC in a first degree relative. 56.3% of patients reported being never smokers. 5.2% had diabetes. Most common presenting symptoms were rectal bleeding (47.7%), abdominal pain/bloating (33.1%) and change in bowel habits (24.7%). The majority presented with left sided cancers (77.3%), at late stage disease (68.4% at stages 3 or 4).
CONCLUSION
Most YO CRC patients presented with rectal bleeding or abdominal pain, left sided cancers, later stage disease and had no family history of CRC in a first degree relative. Over half were overweight and obese, and were more likely to have never smoked. More data are needed to better understand YO CRC risk factors, and to help identify high-risk populations who may benefit from earlier screening.
Keywords: Young onset colorectal cancer, early onset colorectal cancer, colorectal cancer, colorectal cancer screening
INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer-related death in men and women in the United States (US).1 Since the mid-1980s, the incidence of CRC in the US has been decreasing. This has mainly been ascribed to CRC screening with identification and removal of adenomatous polyps.2 The incidence of CRC in young adults (<50 years old) who have traditionally not been routinely screened for CRC is increasing.3,4,5,6 The cause of this unexpected increase remains unclear, and the characteristics of these younger patients are not fully understood. Although young onset cancers were traditionally thought to have a high likelihood of underlying hereditary predisposition, it has instead been shown that hereditary CRC syndromes account for only a minority of these cases.7 Studies also suggest that CRC in this young age group present at later stages, are more likely to demonstrate signet-ring cell and poor histologic differentiation, are more commonly located in the left colon and rectum and often associated with metachronous and synchronous colorectal neoplastic lesions.5,8,9,10.
The well-established risk factors associated with CRC, including diets high in red meat and low in fiber, smoking tobacco, excess alcohol consumption and physical inactivity do not appear to explain the increasing incidence of young onset CRC (YO CRC).11 Although obesity has recently been implicated as a possible risk factor in young females12, a subsequent large analysis did not correlate obesity with increasing YO CRC trends.11
The aim of this study is to describe the demographics, clinical and pathologic features of patients with YO CRC. To better elucidate the clinical underpinnings of YO CRC, this study also assessed symptoms leading to a diagnosis of CRC and potential risk factors in a large population of YO CRC patients with comparison to a healthy nationally representative cohort.
METHODS
We performed a retrospective review of all patients between ages 20 and 49 with a pathologic diagnosis of CRC who were evaluated at Memorial Sloan Kettering Cancer Center (MSKCC) from January 2004 to June 2019. Patients were identified by International Classification of Diseases for Oncology (ICD-O) site and histology codes. To capture sporadic YO CRC patients, those with a known mutation for a hereditary CRC syndrome (defined as a known history of or genetic workup consistent with a hereditary CRC syndrome or immunohistochemistry (IHC) of tumor with mismatch repair deficiency (dMMR)) were excluded, as were those with a history of inflammatory bowel disease (IBD), or appendiceal, neuroendocrine, and carcinoid tumors.
Using the electronic medical record, we abstracted information on demographics (including age at diagnosis, gender, height, weight, race/ethnicity), family history of cancer, medical and surgical history, tumor characteristics, pathology, smoking history and symptoms leading to diagnosis. Data abstraction was done through chart review. Following data abstraction, we recoded, performed logic checks, and reviewed missing data across variables.
To explore whether the distributions of these variables in YO CRC varied from the general population of US young adults, we compared body mass index, hypertension (yes vs. no), diabetes (self-report of doctor-diagnosed), and smoking history (ever vs. never) in YO-CRC patients to a population of adults (ages 20–49) without a cancer history from the National Health and Nutrition Examination Survey (NHANES), a nationally representative, cross-sectional survey of civilian, non-institutionalized persons living in the US (see Supplemental Table 1 for NHANES characteristics). MSKCC data were standardized to the NHANES population based on age, race, and sex prior to comparison of clinical characteristics. NHANES data were used from the 2003–2016 survey cycles. Standardization was performed by deriving post-stratification weights for each combination of age group, sex, and race/ethnicity using NHANES as the reference population and then applying these weights to the MSKCC data. An additional sensitivity analysis was then conducted limiting MSKCC data to the years that overlapped with the available NHANES survey cycles (2004–2016), since NHANES data were not publicly available after 2016 at the time of analysis13. Due to the complex, four-stage sample design methodology of NHANES, formal statistical comparisons to MSKCC data could not be made. Analysis of NHANES data requires careful incorporation of survey sampling weights for appropriate variance estimation; given that MSKCC data would contain no such weights, formal comparisons of the two groups would not produce statistically valid results14.
The study was approved by the MSKCC Institutional Review Board/Privacy Board.
RESULTS
We identified a total of 4,180 patients, ages 20–49, who were diagnosed with CRC. Of these, 324 were excluded (190 for a known genetic syndrome associated with CRC, 123 for IBD and 11 for appendiceal, neuroendocrine and carcinoid tumors). A total of 3,856 patients were included in this analysis.
Baseline patient characteristics are shown in Table 1. The overall median age of CRC diagnosis was 43 years (range 20–49), with 52.4% male, 79.3% white and 88.6% non-Hispanic. 59.1% of patients were either overweight or obese (32.2% and 26.9%, respectively). Most (90.1%) had no family history of CRC in a first degree relative. 56.3% of patients reported being never smokers. 81.3% of patients had never undergone prior abdominal surgery for other indications.
Table 1-.
Demographic characteristics of patients (N = 3,856)
| Characteristic | N (%) |
|---|---|
|
| |
| Age(y) | |
| 20–29 | 203 (5.3) |
| 30–39 | 957 (24.8) |
| 40–49 | 2696 (69.9) |
| Sex | |
| Male | 2015 (52.4) |
| Female | 1832 (47.6) |
| Race | |
| White | 3058 (79.3) |
| Black | 294 (7.6) |
| Asian | 290 (7.5) |
| Other/Unknown | 214 (5.6) |
| Ethnicity | |
| Non-Hispanic | 3411 (88.6) |
| Hispanic | 216 (5.6) |
| Unknown | 225 (5.8) |
| Body Mass Index (kg/m2) | |
| Underweight (<18.5) | 95 (3.0) |
| Normal (18.5− <25) | 1191 (37.8) |
| Overweight (25− <30) | 1015 (32.2) |
| Obese (30+) | 847 (26.9) |
| Smoking | |
| Never | 2169 (56.3) |
| Ever | 1075 (27.9) |
| Unknown | 612 (15.9) |
| Family History | |
| No | 2693 (70.4) |
| Yes, 1st degree relative | 380 (9.9) |
| Yes, 2nd degree or unknown degree relative | 753 (19.7) |
Patients presented with a variety of symptoms including some with multiple symptoms (see Figure 1). Most commonly, patients reported rectal bleeding (47.7%), abdominal pain or bloating (33.1%), or change in bowel habits (24.7%). Most (77.3%) had left sided cancers, including 69% in the sigmoid and rectum, and most presented with later stage disease (including at least 68.4% with Stage III-IV) (see Table 2).
Figure 1.
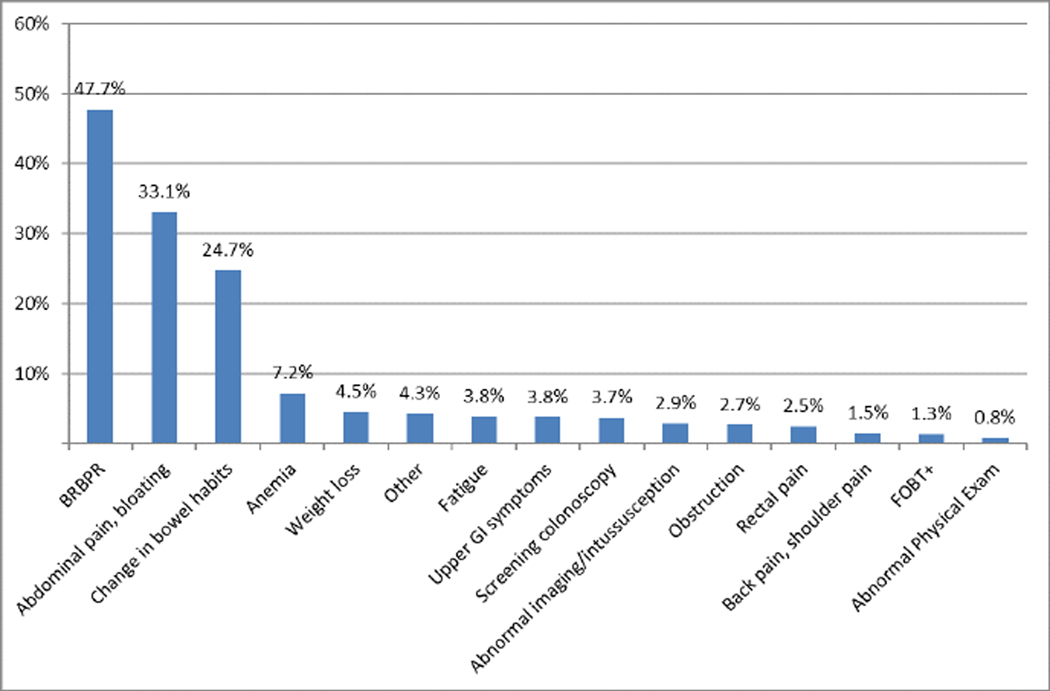
Percentage of patients who presented with a given symptom
Table 2-.
Cancer characteristics of MSK patients overall and by age decade (N= 3,856)
| Age Category | ||||
|---|---|---|---|---|
|
| ||||
| Cancer Characteristic | Overall | 20–29 | 30–39 | 40–49 |
|
| ||||
| Stage | ||||
| 0 | 46 (1.2) | 1 (0.5) | 9 (0.94) | 36 (1.34) |
| I | 181 (4.7) | 9 (4.4) | 28 (2.9) | 144 (5.3) |
| II | 386 (10.0) | 17 (8.4) | 80 (8.4) | 289 (10.7) |
| II or III | 448 (11.6) | 17 (8.4) | 106 (11.1) | 325 (12.1) |
| III | 1049 (27.2) | 64 (31.5) | 273 (28.5) | 712 (26.4) |
| IV | 1587 (41.2) | 87 (42.9) | 423 (44.2) | 1077 (40.0) |
| Unknown | 159 (4.1) | 8 (3.9) | 38 (4.0) | 113 (4.2) |
| Location | ||||
| Cecum | 334 (8.7) | 14 (6.9) | 89 (9.4) | 231 (8.6) |
| Ascending colon | 308 (8.0) | 20 (9.9) | 60 (6.3) | 228 (8.5) |
| Hepatic Flexure | 52 (1.4) | 0 (0.0) | 14 (1.4) | 38 (1.4) |
| Transverse colon | 153 (4.0) | 7 (3.5) | 42 (4.4) | 104 (3.9) |
| Splenic flexure of colon | 85 (2.2) | 4 (2.0) | 22 (2.3) | 59 (2.2) |
| Descending colon | 235 (6.1) | 18 (8.9) | 77 (8.1) | 140 (5.2) |
| Sigmoid/rectosigmoid | 1404 (36.5) | 71 (35.0) | 355 (37.3) | 978 (36.3) |
| Rectum | 1249 (32.5) | 69 (34.0) | 283 (29.8) | 897 (33.3) |
| Colon, NOS | 25 (0.7) | 0 (0.0) | 9 (1.0) | 16 (0.6) |
| Pathology | ||||
| Adenocarcinoma, NOS | 3359 (87.6) | 156 (76.9) | 817 (86.1) | 2386 (89.0) |
| Mixed | 39 (1.0) | 8 (3.9) | 18 (1.9) | 13 (0.5) |
| Mucinous | 233 (6.1) | 18 (8.9) | 63 (6.6) | 152 (5.7) |
| Mucinous >50% | 138 (3.6) | 9 (4.4) | 29 (3.1) | 100 (3.7) |
| Signet ring cell carcinoma | 65 (1.7) | 12 (5.9) | 22 (2.3) | 31 (1.2) |
Compared to NHANES, a nationally representative cohort of patients without cancer, (see supplemental figure 1), our YO CRC patients were less likely to have ever smoked tobacco cigarettes (41.5% vs. 37.7%, respectively). A higher proportion of our patients had diabetes (5.2% vs. 3.4%, respectively). Though the majority of our patients were overweight or obese, our group had lower proportions of overweight and obese patients than the NHANES group (29.2% and 23.7% vs. 31.8% and 33.7% respectively) even when we conducted a sensitivity analysis excluding patients who presented with weight loss.
DISCUSSION
To date, this is the largest single center study describing the clinical features of young onset CRC patients. We looked at 3,856 patients with YO CRC age 20 to 49 without a known hereditary predisposition to CRC and found that most patients had no family history of CRC in a first degree relative, presented with clinical symptoms of rectal bleeding, were found to have left sided cancers and at later stage disease (III-IV) which is consistent with prior studies.3,5,9 The underlying cause of these patterns remains unclear. Some studies suggest that this increase may be attributed to increased rates of obesity.4, 12 We therefore sought to look at traditional risk factors such as smoking and obesity and did not find an increased prevalence in our YO CRC study group compared to individuals without cancer in a nationally representative population of adults (NHANES) restricted to ages 20–49. History of prior abdominal surgery, namely cholecystectomy, has also been reported to be associated with a higher risk of sporadic CRC.15 The majority of our patients did not have a history of prior abdominal surgery.
A recent Swedish cohort study suggested that diabetes independently increased an individual’s risk of developing CRC at a younger age.16 Though our numbers are small, our group did have a higher prevalence of diabetes. Diabetes may therefore be a signal which should be investigated further as a potential risk factor for YO CRC.
Given that the incidence of YO CRC continues to increase, and to date the mechanism remains unclear, it is imperative that clinicians and patients be aware of YO CRC. We found that our patient population presented with a myriad of symptoms, with most reporting rectal bleeding, abdominal pain/bloating and change in bowel habits. In the past when young patients without risk factors for CRC were evaluated for these symptoms, many were thought to be due to benign causes that may not warrant further workup. Given that the majority of our patients presented with rectal bleeding, it is important to consider CRC in the differential diagnosis in symptomatic individuals under age of 50, to ensure appropriate further workup and follow-up as to not miss potential underlying CRC in this young population.
The major strength of this study is the large number of patients included. In addition, our comprehensive medical record afforded us the ability to accurately review the demographics, clinical and pathologic features of this study group. Several limitations should be noted. First are those inherent to retrospective studies and chart reviews as we are limited to what is included in the chart and essential information may be missing or mis-classified. There may be recall bias in patients reporting symptoms. In addition, although we excluded patients with dMMR found on IHC of tumor to reduce the chance of including potentially missed cases of Lynch syndrome, because a large portion of our study predated universal tumor testing by IHC for dMMR, we may have underestimated the number of patients with genetic syndromes. Second, this is a single institution tertiary cancer center and therefore may not be representative of the general population. Third, we compared our study group of YO CRC patients to a nationally representative cohort of patients without cancer (NHANES) to better understand the magnitude of risk factors in our patients. Although we standardized age, race, and sex, there may still be underlying differences in other important factors such as socioeconomic status and insurance status. We therefore used this comparison as a qualitative analysis and did not make any direct statistical comparisons.
In summary, we found that the majority of our YO CRC patients presented with rectal bleeding, abdominal pain, and change in bowel habits, had left sided cancers and presented with later stage disease (III-IV). Until we have a better understanding of the biology and risk factors of CRC in this population, CRC should be considered in young patients who present with these symptoms, and should be followed closely to determine whether further evaluation may be warranted. In asymptomatic individuals under age 50, important questions remain regarding who, when and how to screen for CRC. If confirmed with further studies, diabetes may be a potential risk factor in young individuals and could help define a high risk group that may be offered specialized CRC screening recommendations in the future. Prospective data are clearly needed to better define and understand the risk factors and causes for YO CRC to help guide future screening recommendations in this cohort in efforts to reduce the increasing incidence and significant mortality of this potentially preventable disease.
Supplementary Material
Supplemental Figure 1. Comparison of clinical variables for MSK patient population vs. NHANES cancer-free population <50 years old (Full MSK Cohort)
ACKNOWLEDGEMENTS:
This work was supported by grant P30 CA008748 from the National Cancer Institute of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST:
Leslie Park: none
Kelli O’Connell: none
Keri Herzog: none
Walid Chatila: none
Henry Walch: none
Randze Lerie D. Palmaira: none
Andrea Cercek:
Advisory Board: Bayer, GSK, Merck, Janssen, Seagen
Research Funding: GSK, RGenix, Seagen
Jinru Shia:
Consultant: Paige AI.
Moshe Shike: none
Arnold Markowitz: none
Julio Garcia Aguilar:
Honorarium as a speaker: Intuitive Surgical, Medtronic, and Johnson & Johnson
Mark Schattner:
Consultant: Boston Scientific
Advisory Board: Novo Nordisk
Elizabeth Kantor: none
Mengmeng Du: none
Robin B. Mendelsohn:
Advisory Board: Exact Sciences
Speaker: Medscape
STATEMENTS AND DECLARATIONS
Leslie Park: none
Kelli O’Connell: none
Keri Herzog: none
Walid Chatila: none
Henry Walch: none
Randze Lerie D. Palmaira: none
Andrea Cercek:
Advisory Board: Bayer, GSK, Merck, Janssen, Seagen
Research Funding: GSK, RGenix, Seagen
Jinru Shia:
Consultant: Paige AI.
Moshe Shike: none
Arnold Markowitz: none
Julio Garcia Aguilar:
Honorarium as a speaker: Intuitive Surgical, Medtronic, and Johnson & Johnson
Mark Schattner:
Consultant: Boston Scientific
Advisory Board: Novo Nordisk
Elizabeth Kantor: none
Mengmeng Du: none
Robin B. Mendelsohn:
Advisory Board: Exact Sciences
Speaker: Medscape
References
- 1.Siegel RL, Miller KD Fuchs HE et al.Cancer Statistics, 2021. CA Cancer J Clin 2021. Jan;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK., Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017; 109 (8): djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL., Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–8 [DOI] [PubMed] [Google Scholar]
- 5.Meyer JE., Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(18):4354–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You YN., Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(3):287–9 [DOI] [PubMed] [Google Scholar]
- 7.Silla IO, Rueda D, Rodríguez Y, García JL, de la Cruz Vigo F, Perea J. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol 2014; 20: 17288–17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg 2011; 213: 352–361 [DOI] [PubMed] [Google Scholar]
- 9.Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014; 89: 216–224 [DOI] [PubMed] [Google Scholar]
- 10.Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS, Wang SM. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg 2003; 90: 205–214 [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and tends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2186l [DOI] [PubMed] [Google Scholar]
- 12.Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019;5(1):37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. NHANES 2003–2004 Public Data General Release File Documentation. https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf. Accessed October 30, 2021.
- 14.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2013. [https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/99-10-analytic-guidelines.pdf]. [Google Scholar]
- 15.Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001. Sep;121(3):542–7. [DOI] [PubMed] [Google Scholar]
- 16.Ali Khan U, Fallah M, Sundquist K et al. Risk of colorectal cancer in patients with diabetes mellitus: A Swedish nationwide cohort study. PLoS Med. 2020;17(11):e10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of clinical variables for MSK patient population vs. NHANES cancer-free population <50 years old (Full MSK Cohort)


