Graphical abstract
Keywords: COVID-19, Low molecular weight heparin, Outcomes, Prophylactic, Therapeutic
Abbreviations: COVID-19, COronaVIrus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome CoronaVirus 2
Thrombotic and thromboembolic phenomena are one of the leading complications and causes of poor outcomes in COVID-19 patients admitted to hospital [1]. In the pathophysiology of pulmonary and endothelial damage due to SARS-CoV-2 infection, an inflammatory and prothrombotic state with microvascular thrombosis has been described [2]. Therefore, early initiation of therapeutic low molecular weight heparin (LMWH) doses could be more effective than prophylactic doses not only in preventing thrombotic/thromboembolic complications (due to its anticoagulant effect), but also in improving overall outcomes (due to its anti-inflammatory properties) in patients admitted to hospital with non-severe COVID-19 pneumonia.
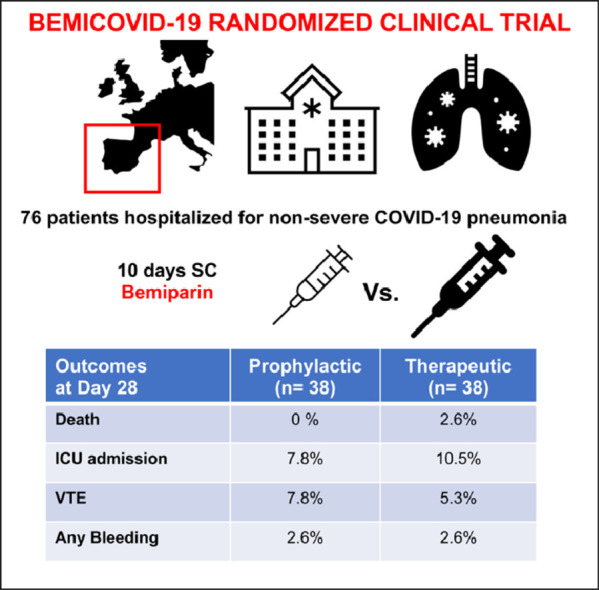
We conducted the BEMICOVID-19 study, a single-blind, randomized (1:1 ratio), controlled trial (NCT04420299) evaluating the efficacy and safety of therapeutic (115 IU/Kg once daily) vs. prophylactic (3.500 IU once daily) subcutaneous bemiparin for a 10-day period (after this 10-day phase, thromboprophylaxis management was left at investigators’ choice) in patients requiring hospital admission due to non-severe COVID-19 pneumonia (defined as CURB65≤2 points without criteria for intensive care unit [ICU] admission nor mechanical ventilation requirement) with baseline d-dimer above the upper limit of normal reference range (> 500 ng/mL), at two University Hospitals (HM-Monteprincipe/HM-Puerta-Sur) in Madrid Metropolitan Area, from August-2020 to June-2021 (the trial was prematurely stopped based on slow recruitment rate due to vaccination campaign). The primary efficacy outcome was a composite of death, ICU admission, need of mechanical ventilation support, and venous (VTE)/arterial (ATE) thromboembolism within 28±2 days of enrollment. Safety outcomes were major bleeding and non-major clinically relevant bleeding. The study involved three mandatory visits (baseline/start of treatment, 10-day/end of treatment, and 28-day follow-up), including computed tomography (CT) pulmonary angiography at inclusion and at day 10±1. A full list of participating centers, investigators, statistical-analysis, and eligibility-criteria are provided in Supplementary material.
Seventy-six patients (mean [SD] age: 60.6 [11.5] years; men: 60 [76.9%]) were randomized to standard thromboprophylaxis (n = 38) and therapeutic dose bemiparin (n = 38). Baseline characteristics and COVID-19 therapy are shown in Table 1a . All patients completed the follow-up period. Table 1b shows the outcomes. The primary efficacy outcome (death, ICU admission, mechanical ventilation support and VTE/ATE) was registered in 7 (18.4%) patients treated with therapeutic-dose of bemiparin and in 6 (15.8%) patients receiving prophylactic-dose. The relative risk (RR) was 1.17 (95%CI:0.43–3.15), the odds ratio (OR) was 1.20 (95%CI:0.38–3.83), and the absolute risk difference was 2.6% (95%CI:−14.3–19.6;p = 0.761). Neither major bleeding event nor serious adverse event (unrelated with COVID-19 evolution) were reported in any arm of treatment. Although reductions of d-dimer levels between randomization and end of treatment were higher in therapeutic-dose group compared to prophylactic-dose (median[IQR] reduction: 332 [70;521] Vs. 325 [126;430] ng/ml;p = 0.721), no association between d-dimer levels at randomization and VTE were observed (Supplementary material).
Table 1a.
Characteristics at baseline and COVID-19 therapy of randomized patients.
| Prophylactic Bemiparin | Therapeutic Bemiparin | |
|---|---|---|
| N = 38 | N = 38 | |
| Demographics | ||
| Age (years); mean±SD | 60.7 (±12.0) | 60.5 (±11.1) |
| Sex n (%) | ||
| Female | 6 (15.8%) | 10 (26.3%) |
| Male | 32 (84.2%) | 28 (73.7%) |
| Comorbidities | n (%) | n (%) |
| Diabetes Mellitus | 2 (5.3%) | 2 (5.3%) |
| Hypertension | 5 (13.2%) | 5 (13.2%) |
| Chronic pulmonary disease/asthma | 2 (5.3%) | 2 (5.3%) |
| Cardiopathy | 1 (2.6%) | 0 (0.0%) |
| Current smoker | 1 (2.6%) | 3 (7.9%) |
| Previous venous thrombosis | 0 (0.0%) | 1 (2.6%) |
| Vital signs | mean (SD) | mean (SD) |
| Systolic blood pressure (mmHg) | 121.6 (27.7) | 127.3 (22.2) |
| Diastolic blood pressure (mmHg) | 73.6 (17.5) | 75.1 (13.6) |
| Heart rate (bpm) | 80.0 (11.3) | 78.0 (13.5) |
| Temperature ( °C) | 36.3 (0.8) | 36.5 (0.8) |
| Respiratory assessment | mean (SD) | mean (SD) |
| Respiratory Rate (bpm) | 16.7 (4.4) | 17.3 (3.7) |
| Saturation O2 (%) | 94.6 (2.5) | 93.8 (3.6) |
| Severity Score | n (%) | n (%) |
| CURB65 Score | ||
| 0 | 18 (47.4%) | 21 (60.5%) |
| 1 | 19 (50.0%) | 15 (39.5%) |
| 2 | 1 (2.6%) | 0 (0.0%) |
| Laboratory analyses | median (IQR) | median (IQR) |
| IL6 levels | 5.8 (1.8–69.4) | 9.5 (2.2–62.6) |
| D Dimer levels | 852.0 (662.0–1139.0) | 730.0 (600.0–949.0) |
| Ferritin levels | 673.8 (546.4–1058.0) | 1218.9 (635.0–1711.5) |
| COVID-19 therapy | n (%) | n (%) |
| Steroids | 38 (100%) | 38 (100%) |
| Tocilizumab | 9 (23.7%) | 16 (42.1%) |
| Remdesivir | 6 (15.8%) | 5 (13.1%) |
SD: standard deviation; IL-6: interleukin-6; IQR: interquartile range.
Table 1b.
Clinical (efficacy and safety) outcomes during the 28-day post-randomization period.
| Prophylactic Bemiparin | Therapeutic Bemiparin | RR (95% CI) | OR (95% CI) | ARR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Outcome | N = 38 | N = 38 | ||||
| Primary efficacy outcome | ||||||
| Death, ICU admission, mechanical ventilation support, and VTE/ATE at day 28±2 days | 6 (15.8%) | 7 (18.4%) | 1.17 (0.43; 3.15) | 1.20 (0.38; 3.83) | 2.6 (−14.3–19.6) | 0.761 |
| Secondary efficacy outcomes | ||||||
| Death | 0 (0%) | 1 (2.6%) | – | – | 2.6 (−2.4; 7.7) | 0.314 |
| Intensive Care Unit (ICU) admission & Mechanical ventilation support | 3 (7.8%) | 4 (10.5%) | 1.33 (0.32; 5.56) | 1.37 (0.32; 5.91) | 5.3 (−6.8; 17.3) | 0.692 |
| VTE/ATE | 3 (7.8%) | 2 (5.3%) | 0.67 (0.12; 3.77) | 0.65 (0.12; 3.48) | −2.6 (−13.8; 8.5) | 0.644 |
| Discharge at day 10 | 18 (47.4%) | 17 (44.7%) | 0.94 (0.58; 1.54) | 0.90 (0.37; 2.20) | −2.6 (−25.1; 19.8) | 0.818 |
| Safety outcome | ||||||
| Bleeding | 1 (2.6%) | 1 (2.6%) | 1.0 (0.1; 15.4) | 1.0 (0.1; 16.6) | 0.0 (−0.1; 0.1) | 1.000 |
| Major bleeding | 0 | 0 | ||||
| Clinically relevant bleeding non-major bleeding | 1 | 0 | ||||
| Any bleeding | 0 | 1 | ||||
RR: Risk Ratio; OR: Odds ratio; ARR: Absolute Risk Reduction; CI 95%: 95% Confidence Interval.
ICU, Intensive Care Unit; VTE, Venous thromboembolism; ATE, Arterial thromboembolism.
Several randomized clinical trials (RCT) have been designed and performed addressing on the optimal intensity of anticoagulation for inpatients with COVID-19 and different results have been reported, so some questions are pending to be answered [3,4]. The current trial showed no benefit for full anticoagulation with LWMH Vs prophylactic dose in COVID-19 patients admitted to hospital with non-severe pneumonia, and our results are according to some of these RCT. Probably, baseline characteristics of the evaluated population with different degrees of severity and used therapies could explain, at least in part, the differences observed in clinical outcomes between trials. Additionally, in our trial higher basal inflammatory markers (interleukin-6 and ferritin) and more tocilizumab use was observed in therapeutic bemiparin dose group compared to prophylactic group. Probably, it reflects a slightly higher severity in full anticoagulation arm and can justify, in part, the slightly poorer outcomes.
Focusing on noncritically ill hospitalized COVID-19 patients with increased d-dimer levels, therapeutic anticoagulation (with heparin or direct oral anticoagulants) has showed controversial results compared with prophylactic anticoagulation. Some trials did not demonstrated improvement on primary clinical composite outcome of death, need of mechanical ventilation and/or ICU admission [5], [6], [7]. Other trials have showed any benefit with initial strategy of therapeutic-dose LMWH compared with standard heparin thromboprophylaxis among COVID-19 inpatients. Multiplatform randomized clinical trial REMAP-CAP/ACTIV-4a/ATTAC reported increased probability of survival to hospital discharge with reduced use of cardiovascular or respiratory organ support [8], HEP-COVID observed reduction in major thromboembolism and death in high-risk inpatients with very elevated d-dimer levels [9], while in RAPID trial the odds of death at 28 days was decreased, with low risk of major bleeding [6].
One of the reasons why anticoagulation at therapeutic doses has not been effective in the forementioned studies may be in the selected d-dimer cut-off. d-dimer level above the upper limit of normal reference range (> 500 ng/ml in our study) probably is not a truly effective marker to identify COVID-19 patients at increased risk of thrombotic phenomena and poor outcomes. Possibly, as suggested in ATTACC/ACTIV-4a/REMAP-CAP and HEP-COVID trials [8,9], higher d-dimer cut-off point could more reliably identify this type of patient. But in this case, the higher the cut-off point for d-dimer, the higher the percentage of patients with severe or critical clinical status at admission. In this setting, in critically ill COVID-19 patients, main RCTs have also not shown improvement with an initial strategy of full anticoagulation with heparin compared to usual-care pharmacologic thromboprophylaxis. Neither a greater probability of survival to hospital discharge nor a greater number of days free of cardiovascular or respiratory organ support was obtained in multiplatform REMAP-CAP/ACTIV-4a/ATTAC studies [10].
A unique specific characteristic in the design of our study is the inclusion of a CT pulmonary angiography at inclusion and at the end of treatment (10 days later), to accurately assess the incidence of pulmonary embolism in both treatment groups. Thus, two interesting data have been observed. In one hand, although not the objective of this trial, we have been able to observe a relatively high frequency of pulmonary embolism (5 cases in 78 subjects; 6,4%) in non-critically ill COVID-19 inpatients in a 10-day period from the admittance; all the cases were non-massive pulmonary embolism and with segmentary or sub-segmentary affectation. In the other hand, we observed similar incidence of pulmonary embolism in both arms, suggesting that therapeutic dose is not more effected in preventing pulmonary embolism in this setting compared to usual care thromboprophylaxis.
Regarding the risk of bleeding associated to therapeutic anticoagulation dose, the results are also controversial. Increased risk of bleeding with higher-intensity anticoagulation have been reported in some studies [5,8,10]. But other studies not observed significantly higher risk for major bleeding with therapeutical dose compared with usual thromboprophylaxis [6,7], according with our results. In our trial (with a short duration of full anticoagulation period), no major bleeding was registered and only 2 cases of non-major bleeding were observed, one in each arm.
Among the limitations of our study, we must point the relatively low number of patients and the short duration of study treatment (10 days), limiting the long-term impact assessment. Among our strengths we remark the homogeneity of the two participating sites, with the same protocol for management of COVID-19 patients, and the CT pulmonary angiography performance to diagnose pulmonary embolism during the treatment period.
In conclusion, in noncritically ill inpatients COVID-19 with increased d-dimer levels, a 10-day therapeutic-dose bemiparin course do not improve clinical outcomes compared to usual thromboprophylaxis. Thus, identifying subgroups of COVID-19 patients who could obtain benefit for full anticoagulation is necessary with further investigations.
Ethics approval
The study protocol was approved by the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) and the Drug Research Ethics Committee of HM Group (GHM) (Comité Ético de Investigación con Medicamentos de HM Hospitales) (ref. no. 20.04.1623-GHM). All patients provided a written informed consent prior to participation.
The applicable regulations and guidelines governing clinical study conduct were followed and the study was performed in compliance with the Declaration of Helsinki and Good Clinical Practice principles outlined in the International Conference on Harmonization.
CRediT authorship contribution statement
Jose F Varona: Visualization, Writing – original draft, Writing – review & editing, Formal analysis. Elena Núñez: Writing – review & editing. Borja M. Fernández Félix: Formal analysis. Jose María Castellano Vázquez: Visualization, Writing – original draft, Writing – review & editing. Antonio Cubillo: Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare they have no conflict of interest.
Acknowledgments
The study has been funded by Laboratorios Farmacéuticos Rovi (Madrid, Spain)
We are grateful to all site staff who contributed to the development of the study and all health-care professionals that enrolled participants or cared of them during the study
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.01.031.
Appendix. Supplementary materials
References
- 1.Jiménez D., García-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannis D., Douketis J.D., Spyropoulos A.C. Anticoagulant therapy for COVID-19: what we have learned and what are the unanswered questions? Eur J Intern Med. 2021;S0953-6205(21) doi: 10.1016/j.ejim.2021.11.003. [published online ahead of print, 2021 Nov 11] 00378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes R.D., de Barros E., Silva P.G.M., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated d-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sholzberg M., Tang G.H., Rahhal H., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. Published 2021 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcos M., Carmona-Torre F., Vidal Laso R., et al. Therapeutic vs. prophylactic bemiparin in hospitalized patients with non-severe COVID-19 (BEMICOP): an open-label, multicenter, randomized trial. Thromb Haemost. 2021 doi: 10.1055/a-1667-7534. [published online ahead of print, 2021 Oct 12] 10.1055/a-1667-7534. [DOI] [PubMed] [Google Scholar]
- 8.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators Therapeutic anticoagulation with heparin in noncritically Ill patients with COVID-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyropoulos A.C., Goldin M., Giannis D., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.6203. [published online ahead of print, 2021 Oct 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators Therapeutic anticoagulation with heparin in critically Ill patients with COVID-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


