Abstract
Background
Few studies have simultaneously examined the effect of long-term exposure to air pollution and ambient temperature on the rate of hospital admissions with cardiovascular and respiratory disease using causal inference methods.
Methods
We used a variation of a difference-in-difference (DID) approach to assess the effects of long-term exposure to warm-season temperature, cold-season temperature, NO2, O3, and PM2.5 on the rate of hospital admissions for cardiovascular disease (CVD), myocardial infarction (MI), ischemic stroke, and respiratory diseases from 2001 to 2016 among Medicare beneficiaries who use fee-for-service programs. We computed the rate of admissions by zip code and year. Covariates included demographic and socioeconomic variables which were obtained from the decennial Census, the American Community Survey, the Behavioral Risk Factor Surveillance System, and the Dartmouth Health Atlas. As a secondary analysis, we restricted the analysis to zip code-years that had exposure to low concentrations of our pollutants.
Results
PM2.5 was associated with a significant increase in the absolute rate of annual admissions with cardiovascular disease by 47.71 admissions (95% CI: 41.25–56.05) per 100,000 person-years, myocardial infarction by 7.44 admissions (95% CI: 5.53–9.63) per 100,000 person-years, and 18.58 respiratory admissions (95% CI: 12.42–23.72) for each one μg/m3 increase in two-year average levels. O3 significantly increased the rates of all the studied outcomes. NO2 was associated with a decreased rate of admissions with MI by 0.83 admissions (95% CI: 0.10–1.55) per 100,000 person-years but increased rate of admissions for respiratory disease by 3.16 admissions (95% CI: 1.34–5.24) per 100,000 person-years. Warmer cold-season temperature was associated with a decreased admissions rate for all outcomes.
Conclusion
Air pollutants, particularly PM2.5 and O3, increased the rate of hospital admissions with cardiovascular and respiratory disease among the elderly, while higher cold-season temperatures decreased the rate of admissions with these conditions.
Keywords: Air pollution, Temperature, Cardiovascular Disease, Respiratory Disease, Epidemiology
Graphical Abstract
1. Introduction
There have been two major recent trends in air pollution epidemiology research: the first is the use of multi-pollutant models to look at the effect of long-term exposure to a pollutant while simultaneously adjusting for confounding by others(Beelen et al., 2014; Crouse et al., 2015; Klompmaker et al., 2021) and the second is the use of causal methodology to look at exposure-outcome relationships (Abu Awad et al., 2019; Schwartz et al., 2021; Wang et al., 2017, 2016; Wei et al., 2021; Wu et al., 2020; Yitshak-Sade et al., 2019). These developments address concerns common to all epidemiological studies, namely, potential unmeasured confounding. The use of multi-pollutants models and the use of causal methodology ameliorates these concerns and lends weight to the results obtained from large longitudinal cohort studies. Such advances are necessary in order to convince policymakers of the importance of air pollution and climate research and the need for urgent action to address these exposures.
While some progress has been made, there have been fewer studies looking at the effects of long-term exposure to air pollution and hospitalizations with cardiovascular and respiratory outcomes using causal methods and controlling for multiple pollutants (Danesh Yazdi et al., 2021, 2019; Klompmaker et al., 2021). This leaves an incomplete picture of the full effects of air pollution on human health.
On the other hand, the relationship between temperature and cardiovascular disease is also of great interest given global climate change. It is important, however, to examine this relationship in the context of air pollution as these variables are related to one another. Previous studies have found that the effect of air pollution on cardiovascular disease may be modified by temperature(Klompmaker et al., 2021). However, the effect of air pollution on health might also be confounded by temperature. Some of the negative impacts of temperature on health may be mediated through air pollution and it is important to account for this.
We conducted a study examining the relationship between long-term exposure to air pollution and temperature and hospital admission rates with cardiovascular and respiratory disease. We used a causal difference-in-difference multi-pollutant analysis which would account for both measured and unmeasured between-zip code confounding in the method itself. This strategy reduces the risk of unmeasured confounding causing bias in our effect estimates. We also used an additive model to ease the interpretation of our results.
2. Materials and Methods
2.1. Study Population
We used the Medicare denominator file and hospital admissions data from the Medicare Provider Analysis and Review (MEDPAR) files to construct a dataset of rates of hospital admissions for fee-for-service Medicare beneficiaries aged 65 years and over by zip code and year from 2000 to 2016 in the contiguous United States. MEDPAR data were available on a ZIP code spatial resolution.
2.2. Exposure Assessment
Air pollution levels for PM2.5, NO2, and O3, were estimated for each zip code-year using predictions from previously published models. Each pollutant was predicted from an ensemble spatio-temporal model incorporating three machine learning algorithms(Di et al., 2019a, 2019b; Requia et al., 2020). Input variables included land use terms, meteorological data, satellite data, and chemical transport models. These variables were used to generate predicted pollutant levels using a random forest (RF), a gradient boosting machine (GBM), and a neural network (NN). These predictions were in turn used as independent variables in a geographically-weighted generalized additive model (GAM) against monitored values obtained from the Environmental Protection Agency (US EPA) and other monitors (IMPROVE, SEARCH, CASTNET, etc.) to estimate the final pollutant levels. These estimations were on a 1 km2 spatial scale and a daily temporal scale. All models demonstrated strong performance, with ten-fold cross-validation R2 values of 0.89 for PM2.5, 0.84 for NO2, and 0.86 for O3 for annual averages(Di et al., 2019a, 2019b; Requia et al., 2020). We then aggregated these annual levels to a zip code spatial scale and a two-year calendar average temporal scale for our analysis.
For ozone, we used the average warm-season O3 levels (i.e., levels averaged from April 1st through September 30th) as our exposure of interest, because O3 is more readily formed in the warm season, and most monitoring occurs then. In this analysis, any future reference to “ozone” or “O3” refers to the two-year warm-season average.
Our temperature data was derived from the gridMET dataset, which estimated levels of various meteorological parameters on a 4 km by 4 km scale(Abatzoglou, 2013). We aggregated these levels to zip code and calculated warm-season averages (April through the end of September) and cold-season averages (January through the end of March and October through the end of December). We averaged these values over two calendar years to create the exposures in our study.
2.3. Outcome Definition
We looked at four outcomes in this study: annual rates of hospital admissions with cardiovascular disease (CVD), myocardial infarction (MI), ischemic stroke, and respiratory disease. We used ICD-9 and ICD-10 codes in the primary discharge code positions to identify our outcomes. CVD was defined as ICD-9 codes 390–459 and ICD-10 codes beginning with “I”. Myocardial infarction was defined as ICD-9 codes 410.X0 and 410.X1 and ICD 10-code: I21. Ischemic stroke was defined as ICD-9 codes: 433.X1, 434.X1, 436, and ICD-10 code: I63. Finally, respiratory disease was defined as ICD-9 codes 460–519 and ICD-10 codes beginning with “J”. To calculate annual rates, we divided the number of cases of admissions with these four outcomes by the number of Medicare beneficiaries enrolled in fee-for-service programs that year in that ZIP code.
2.4. Covariate Assessment
We included demographic information for each zip code-year based on data in the Medicare denominator file. We estimated the proportion of the study population who were female, identified as black, identified as race other than black or white, were Medicaid-eligible, and by age group (65–74,75–84).
We obtained zip code-level socioeconomic variables from survey data generated by the US Decennial Census and the American Community Survey (ACS) in 2000, 2010, 2011, 2012, 2013, 2014, 2015, and 2016. The variables we included in our models for each zip code and year were: population density, percent of the population over 65 living below the poverty line, percentage of the housing occupied by the owners, median value of housing occupied by owners, median household income, and percentage of the elderly population who did not graduate from high school. We further derived information on the percentage of the population who have ever smoked and the mean body mass index (BMI) at a county level from the respondents to the Behavioral Risk Factor Surveillance System (BRFSS). We used the Dartmouth Health Atlas to obtain information on: proportion of Medicare beneficiaries with at least one Hgb A1c test in a year, the proportion of diabetic beneficiaries over 65 years of age who had a lipid panel test in a year, the proportion of beneficiaries who had an eye examination in a year, proportion beneficiaries with at least one ambulatory doctor’s visit in a year, proportion of female beneficiaries who had a mammogram over a two-year period. We took these variables as indicative of access to care. We filled in the values in years not measured using linear interpolation and extrapolation. Any remaining missingness was assumed to be at random, and those observations were excluded from further study. We further calculated the distance to the nearest hospital using the distance from the centroid of the postal code to the nearest facility based on data from ESRI in 2010 (ESRI Data and Maps; United States Geological Survey, 2010).
2.5. Statistical Analysis
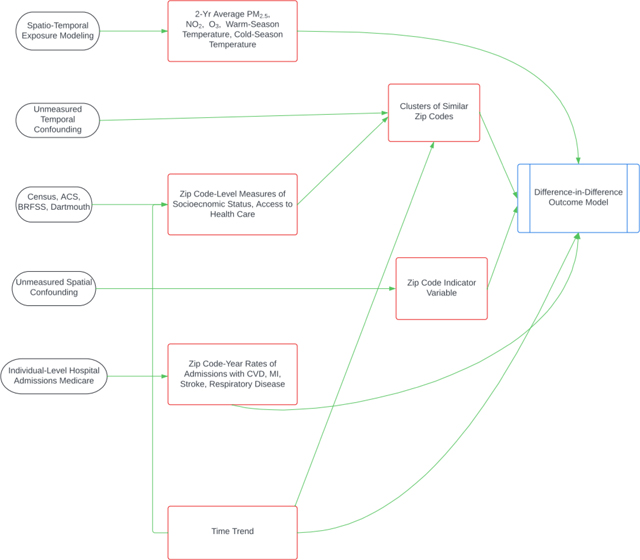
We used a variation of a difference-in-difference analysis, adapted from Schwartz, et al. 2021. In this model, we looked at year-to-year within-zip code changes in the rate of hospital admissions with each of our outcomes, given our exposures of interest and the covariates. By conditioning on zip code, we removed any confounding by between-zip code variables including slowly changing individual characteristics, measured or unmeasured. Time trends in outcomes due to time-varying common factors (improvement in medical care, etc.) are captured using a natural spline for time. The main limitation in using this modeling approach is the parallel trends assumption, which states that each zip code would have had the same change in the outcome rate over time, except those differences due to changes in exposure (i.e. pollution level). This cannot be verified directly. To address this concern, first, we added time-varying socioeconomic and demographic variables in the model to account for any differences in time trends in outcome that may differ among zip codes due to different trends in those variables. Second, to further relax the parallel trends assumption, we classified each zip code into one of five clusters whose long-term trends might differ, using Ward’s Hierarchical Cluster Analysis and average values for the following socioeconomic and demographic variables: percent of the population who identify as black, percent of the population who identify as Hispanic, median household income, median house value, the proportion with at least one ambulatory doctor’s visit in a year, percent of the population over 65 living below the poverty line, percentage of the elderly population who did not graduate from high school, smoking rate, population density, and distance of zip code to the nearest hospital. Five clusters were chosen based on an optimization of Euclidean distance and thirty indices in the “NbClust” package(Charrad et al., 2014). The characteristics of the clusters can be seen in Supplemental Table 1. Cluster 1 seems to represent fairly white, rural, middle-income zip codes with a relatively low poverty rate and good access to health care. Cluster 2 seems to represent slightly more diverse, probably suburban, high-income zip codes with a low poverty rate. Clusters 3, 4, and 5 represent zip codes with larger minority populations. Clusters 3 and 4 are lower income and have higher poverty rates. Cluster 5 seems to indicate densely populated cities with wide disparities (i.e. high median house values but also high poverty and lower rates of high school graduation).
We included an interaction term between the assigned cluster and a smooth year term to allow for differences in outcome rate over time by cluster that previously may not have been controlled. If the assumption of parallel trends in admissions rates now holds for the zip codes within each cluster, this would be considered a causal model. The equation for the final model was:
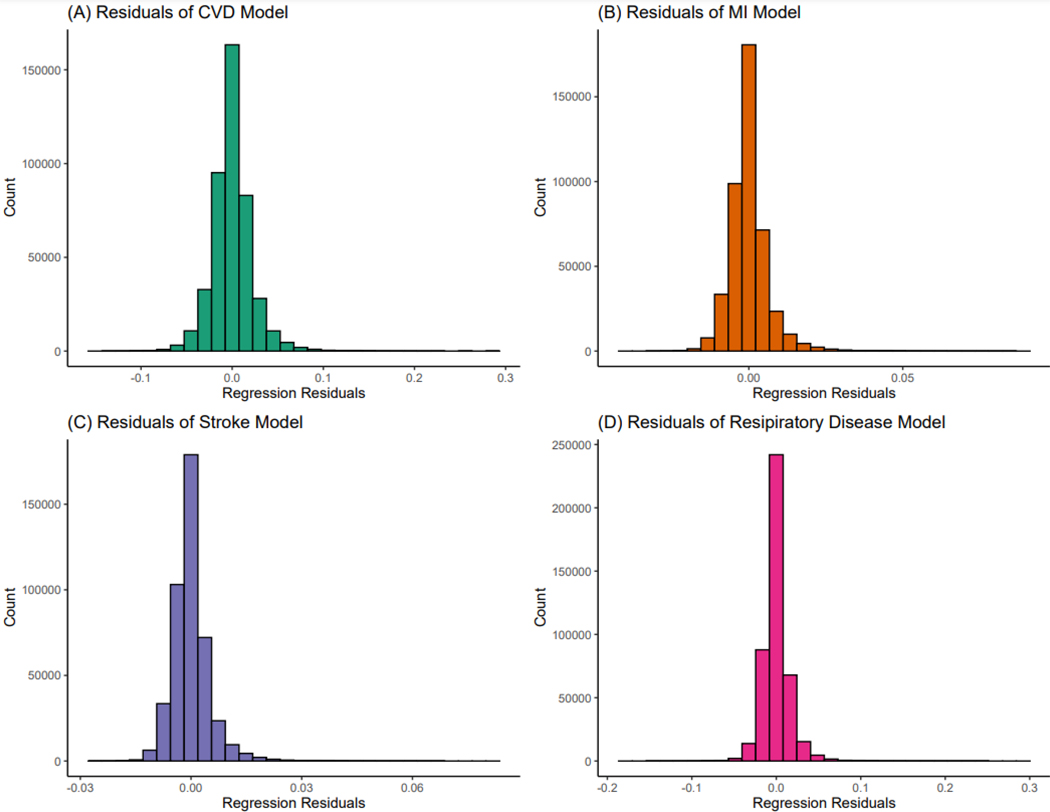
where ARij is the admission rate for zip code i in year j, ns is the natural spline of the year term (with three degrees of freedom) and δi is an indicator term for each zip code and εij is the error term. Linear rate models give unbiased estimates, but because rates do not have constant variance, may have biased confidence intervals. Consequently, to calculate empirical confidence intervals, we used 1000 bootstraps. We chose a linear model to assess our outcome as the residuals from the model were fairly normally distributed (Figure 1). Furthermore, a linear model is an additive model which eases the interpretation of the results. Our effect estimates would represent the absolute change in the average rate of hospital admissions with our outcomes given a one unit increase in the exposure within a zip code, after adjusting for the covariates.
Figure 1.
Distribution of residuals for each outcome in main model
Our primary model was a multi-pollutant model, which included all five exposures of interest to adjust for confounding between exposures. We restricted the data to zip code-years that had at least 100 individuals enrolled in Medicare to have enough population to give rise to cases. We further conducted subgroup analyses looking at zip code-years where the two-year levels of PM2.5 were ≤ 10 μg/m3, the levels of NO2 were ≤ 53 ppb, and levels of O3 were ≤ 50 ppb. Due to the high correlation between warm-season temperature and cold-season temperature (Figure S1), we also conducted a sensitivity analysis where we looked at multi-pollutant models with only warm-season temperature but not cold-season temperature and vice versa. We also ran single pollutant analyses and looked at effect measure modification by region and cluster using interaction terms for our temperature exposures and these variables. Since our models used a two-year average of exposures and our data began in 2000, our statistical analysis was based on data from 2001 through 2016. All data cleaning and statistical analyses were conducted in R Statistical Software Version 3.5.1. In particular the “NBClust” package and “gnm” package were used(Charrad et al., 2014; Turner and Firth, 2020).
3. Results
We analyzed a dataset of 436,684 zip code-years which included 28,992 unique zip codes. The demographic characteristics of the population can be seen in Table 1. The proportion of women in each zip code-year was on average 55.2%, most observations were for individuals between 65–74 years of age (55.7%), and on average, 13.1% were also eligible for Medicaid. Blacks and other minorities constituted about 12% of the individuals in each zip code-year (Table 1). The lower exposure analyses had largely similar demographic characteristics and can be seen in Supplemental Table S2. The zip code-years with lower values of PM2.5 tended to have a lower proportion of individuals who identified as black which indicates that a larger proportion of those who identified as black lived in zip code-years which have higher PM2.5 values.
Table 1.
Demographic Characteristics of Data (2001–2016)
| Total Observations (N) | 436,684 |
| Zip Codes | 28,992 |
| Demographic Characteristic | Average Percentage Across Zip Code-Years |
| Sex: Female | 55.2% |
| Race: Black | 7.5% |
| Race: Other/Unknown | 4.4% |
| Medicaid-Eligible | 13.1% |
| Age Group: 65 ≤ Age < 75 | 55.7% |
| Age Group: 7 5 ≤ Age < 85 | 32.0% |
The average temperature was 20.26 °C for the warm-season and 6.28 °C for the cold-season. Average levels of air pollution were 16.22 ppb for NO2, 45.29 ppb for O3, and 9.77 μg/m3 for PM2.5 (Table 2) over a two-year period. These air pollutants had relatively low concentrations across the contiguous US.
Table 2.
Distribution of Exposures Over a Two-year Period (2001–2016)
| Exposure | Minimum | 10th Percentile | 25th Percentile | Mean | Median | 75th Percentile | 90th Percentile | Maximum |
|---|---|---|---|---|---|---|---|---|
| Warm-Season Temperature (°C) | 6.49 | 15.67 | 17.49 | 20.26 | 19.92 | 22.98 | 25.61 | 31.92 |
| Cold-Season Temperature (°C) | −9.73 | −0.58 | 1.94 | 6.28 | 5.38 | 10.55 | 14.69 | 24.01 |
| NO2 (ppb) | 0.03 | 7.15 | 9.63 | 16.22 | 13.86 | 20.63 | 29.14 | 98.59 |
| O3 (ppb) | 19.75 | 38.76 | 42.29 | 45.29 | 45.19 | 48.56 | 51.66 | 80.44 |
| PM2.5 (μg/m3) | 0.79 | 5.78 | 7.82 | 9.77 | 9.73 | 11.76 | 13.71 | 28.85 |
The main results of our study can be seen in Table 3. An increase in two-year warm-season temperature of 1°C was associated with an increase in the overall absolute rate of respiratory disease (rate difference: 62.13 admissions (95% CI: 51.68–73.02) per 100,000 person-years) but was not associated with a significant change in the rate of CVD, MI, or stroke. An increase in two-year cold-season temperature was associated with a decreased rate of all studied outcomes. An increase of two-year average NO2 by one ppb was associated with a decreased rate of MI and an increased rate of respiratory disease. O3 increased the rate of all our outcomes, with an increase of 9.08 admissions (95% CI: 4.78–13.19) per 100,000 person-years in the rate for CVD, 2.23 admissions (95%CI: 1.12–3.37) per 100,000 person-years in the rate for MI, 1.18 admissions (95% CI: 0.24–2.13) per 100,000 person-years in the rate for stroke, 9.51 admissions (95% CI: 6.59–12.13) per 100,000 person-years in the rate for respiratory disease for each ppb increase in pollution levels over two calendar years. PM2.5 increased the rate of hospitalizations with cardiovascular diseases in general (rate difference: 47.71 admissions (95% CI: 41.25–56.05) per 100,000 person-years) and MIs in particular (rate difference: 7.44 admissions (95% CI: 5.53–9.63) per 100,000 person-years) as well as respiratory disease (rate difference: 18.58 admissions (95% CI: 12.42–23.72) per 100,000 person-years). The comparison of single-pollutant and multi-pollutant models showed a slightly lower effect estimate in the multi-pollutant model (Table S3). This is expected as in the multi-pollutant model the air pollutants adjust for one another.
Table 3.
Main Results-Difference-in-Difference Analysis of Air Pollution and Temperature and Hospital Admissions
| Cardiovascular Disease1,2 | Myocardial Infarction1,2 | Stroke1,2 | Respiratory Disease1,2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate Difference | Lower 95% CI | Upper 95% CI | Rate Difference | Lower 95% CI | Upper 95% CI | Rate Difference | Lower 95% CI | Upper 95% CI | Rate Difference | Lower 95% CI | Upper 95% CI | |
| Warm Temp | 3.79 | −12.18 | 16.77 | −3.76 | −7.92 | 0.31 | 0.74 | −2.86 | 4.09 | 62.13 | 51.68 | 73.02 |
| Cold Temp | −50.48 | −60.18 | −40.20 | −5.01 | −7.67 | −2.18 | −9.66 | −11.95 | −7.45 | −66.42 | −73.73 | −59.93 |
| NO2 | −0.84 | −3.79 | 1.61 | −0.83 | −1.55 | −0.10 | 0.49 | −0.12 | 1.11 | 3.16 | 1.34 | 5.24 |
| O3 | 9.08 | 4.78 | 13.19 | 2.23 | 1.12 | 3.37 | 1.18 | 0.24 | 2.13 | 9.51 | 6.59 | 12.13 |
| PM2.5 | 47.71 | 41.25 | 56.05 | 7.44 | 5.53 | 9.63 | 1.75 | −0.10 | 3.60 | 18.58 | 12.42 | 23.72 |
All results are presented per 100,000 person-years
Results reflect the effect for each one unit increase in annual exposure levels. For warm and cold-season temperature the units are degrees Celsius. For NO2 and O3, the units are parts per billion (ppb) and for PM2.5 the units are μg/m3
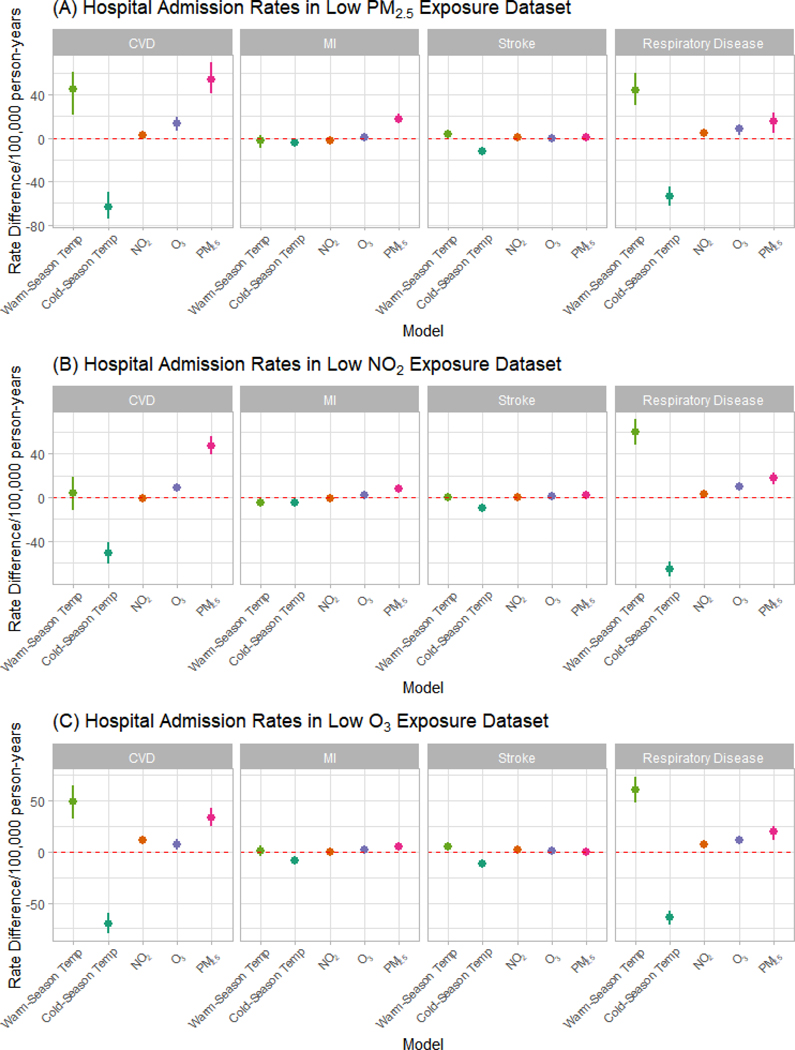
In the subgroup analysis, we restricted our dataset to lower exposure values. For zip code-years with two-year PM2.5 levels ≤ 10 μg/m3, we found that PM2.5 had a larger effect on hospital admission rates for CVD (53.89 vs. 47.71 admissions per 100,000 person-years) and MI (17.66 vs. 7.44 admissions per 100,000 person-years), but not respiratory disease (15.21 vs. 18.58 admissions per 1000,000 person-years), as compared to the full analysis. In the low NO2 dataset, NO2 was slightly less harmful for respiratory disease (2.97 vs. 3.16 admissions per 100,000 person-years). In the low ozone dataset, ozone was less harmful for CVD (7.35 vs. 9.08 admissions per 100,000 person-years), stroke (0.49 vs. 1.18 admissions per person-years), and MI (1.97 vs. 2.23 admissions per 100,000 person-years), but more harmful for respiratory disease (11.18 vs. 9.51 admissions per 100,000 person-years) though the effects for stroke were no longer significant in the low exposure dataset (Figure 2 and Table S4a–4c).
Figure 2.
Hospital Admission Rates in Low Exposure Analyses
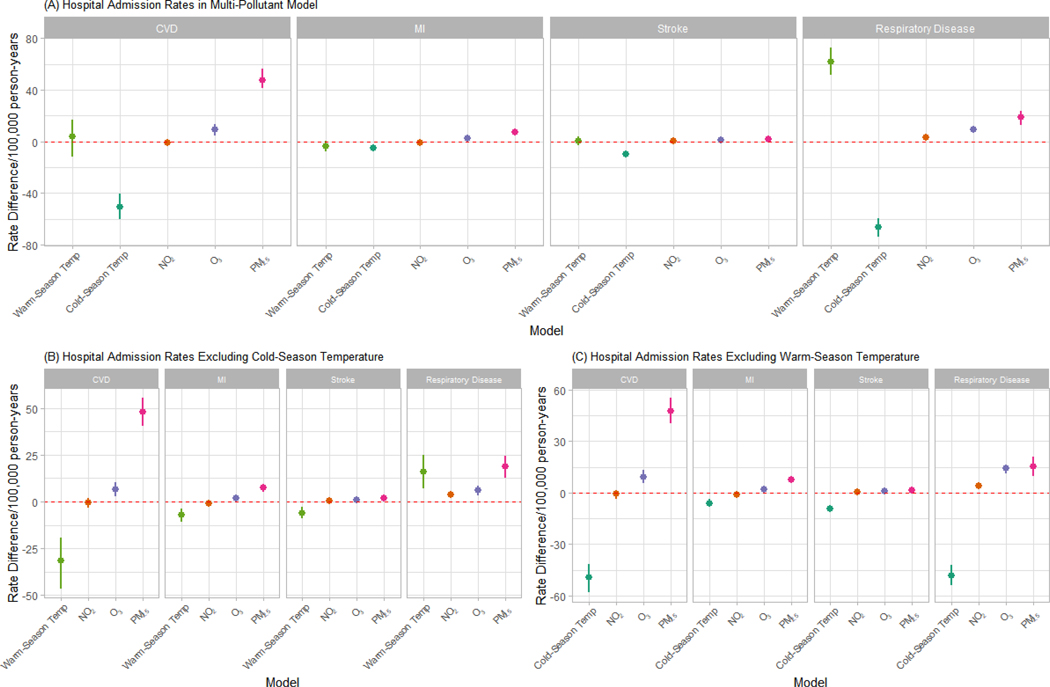
Since there was a high correlation between warm-season temperature and cold-season temperature (ρ=0.85), we ran multi-pollutant models with only one of these variables as a sensitivity analysis. We found that increased two-year warm-season temperature was associated with a lower rate of admissions with CVD, MI, and stroke but a higher rate of admissions with respiratory disease. In the main analyses, the relationship between warm-season temperature and CVD, MI, and stroke was non-significant. An increase in two-year cold-season temperature was still significantly associated with a decrease in the rate of hospital admissions with all outcomes (Figure 3 and Table S5a–5b). In the analyses with interaction terms between seasonal temperature and region, we find that though the main effect of higher cold-season temperatures is generally protective, it is less protective in the Western region, where temperatures remain mild during the cold season. For clusters, it seems that warmer cold-season temperatures are most protective for Cluster 4 as compared to the other clusters, which tended to have lower socioeconomic characteristics, particularly in terms of admissions for respiratory diseases (Figure S3 & Figure S4).
Figure 3.
Hospital Admission Rates and Exposure to Air Pollution and Temperature. A) All exposures included in multipollutant model. B) Cold-season temperature excluded from multipollutant model. C) Warm-season temperature excluded from multipollutant model.
4. Discussion
This study looked at the relationship between long-term exposures to PM2.5, NO2, O3, warm-season temperature, and cold-season temperature and annual hospital admission rates with cardiovascular disease, myocardial infarction, ischemic stroke, and respiratory disease using a variation of the difference-in-difference approach. We found that among the air pollutants, PM2.5 increased the rate of CVD, MI, and respiratory disease and O3 was harmful for all our outcomes. NO2 was protective for MI but harmful for respiratory disease. However, when we restricted to lower levels of NO2, the protective effect on MI became non-significant and harmful (Supplemental Figure S2). An increase in warm-season temperature was found to be harmful for overall admission rates with respiratory disease, and a decrease in cold-season temperature was found to increase admission rates for all our outcomes. For some of our significantly harmful air pollution effects, we found more substantial effects at lower concentration exposures, consistent with previous studies(Danesh Yazdi et al., 2021, 2019; Di et al., 2017). Our study design adds additive effect estimates and considerable assurance by controlling for all unmeasured confounders that differ spatially, that differ temporally due to temporal changes in socio-demographic variables and captures unmeasured temporal confounders whose trends differ by clusters based on socioeconomic and racial factors. It provides a causal interpretation if the assumptions of the model are met.
Our results reinforce conclusions from other literature that air pollution, even at low concentrations, below national standards, results in adverse health outcomes. This is particularly true of PM2.5 and O3 which consistently showed harmful effects across outcomes. The US Environmental Protection Agency (EPA) does not currently have any guidelines for long-term exposure to O3 and is re-assessing PM2.5 standards. Our study points to the need for stricter air pollution regulations.
It is difficult to compare our results to other studies as most other literature assesses these relationships on a multiplicative scale or with adjustment for different sets of co-exposures if any at all. A previous study looking at long-term air pollution and cardiovascular and respiratory outcomes using a doubly robust additive model in the same population found PM2.5 to be harmful for MI, stroke, and pneumonia. In this study, the PM2.5 effects on stroke while not significant, were positive. The lack of significance may reflect the lower power in this study where all difference in pollution between zip codes are controlled away by the indicator variables for every zip code. In the prior study, ozone was harmful for pneumonia admissions and protective in MI and stroke. NO2 had a protective effect for pneumonia and MI and was harmful for stroke. However, those models did not adjust for temperature and also looked at ozone as annual average ozone and not warm-season average ozone(Danesh Yazdi et al., 2021). In another study, also conducted among Medicare participants across the US from 2000–2016, the researchers found that PM2.5 and NO2 increased the risk of cardiovascular admissions (PM2.5: HR 1.041 (95% CI: 1.038–1.045) and NO2: HR 1.033 (95% CI: 1.028–1.037) per IQR increase) and cerebrovascular admissions (PM2.5: HR 1.405 (1.380– 1.430) and NO2: HR 1.237 (1.215–1.259) per IQR increase) but ozone had a protective effect. All the pollutants were harmful at lower concentrations(Klompmaker et al., 2021). We also found PM2.5 and ozone to be harmful for cardiovascular disease, though in our study we found non-significant effects for NO2. It is important to note that in that study, a non-causal multiplicative approach was used as well as other covariates such as greenness and oxidant capacity. Another study in the Medicare population in the southeast region using a marginal structural model approach found significantly harmful effects for both PM2.5 and ozone on myocardial infarction and stroke, though those models did not adjust for NO2 or temperature(Danesh Yazdi et al., 2019). A meta-analysis looking at the effect of long-term exposure to PM2.5 on cardiovascular events and mortality found a relative risk of 1.09 (95% CI: 0.99–1.20) for each 10 μg/m3 increase in pollution levels for incident myocardial infarction and a relative risk of 1.13 (95% CI: 1.11–1.15) for incident stroke, which is consistent with the direction of effects in our results, though the effect for MI was significant and the effect for stroke was non-significant in our study(Alexeeff et al., 2021). Another meta-analysis looking at long-term exposure to PM2.5 and MI found a pooled relative risk of 1.18 (95% CI: 1.11–1.26) for each 10 μg/m3 increase in pollution levels(Zou et al., 2021). A systemic review of literature on ambient temperature and cardiopulmonary outcomes found higher winter temperatures to be associated with lower mortality from cardiovascular and respiratory disease which is consistent with what we found for our outcomes as well(Zafeiratou et al., 2021). Methodologically speaking, other difference-in-difference papers have looked at mortality as the outcome of interest as opposed to hospital admission. The most directly comparable study which looked at long-term exposure to PM2.5 and mortality found a harmful effect, an increase in the mortality rate of 3.85 × 10−4 for each one μg/m3 increase in PM2.5 levels. This is similar our results which found a 4.77 × 10−4 increase in rate of CVD admissions for each unit increase in two-year PM2.5 levels(Schwartz et al., 2021).
Air pollution is generally believed to cause harm to the cardiovascular and respiratory systems by penetrating into lung tissue and the systemic circulation and causing increased levels of inflammation and oxidative stress(Hajat et al., 2015; Viehmann et al., 2015). There is also evidence that air pollution can accelerate biological aging(Ward-Caviness et al., 2016). It is further believed that exposure to particulate matter can lead to vasoconstriction, hypertension, and plaque destabilization, all of which may increase the risk of cardiovascular disease(Fiordelisi et al., 2017). Ozone has also been linked to increased vasoconstriction, peripheral blood pressure, as well as autonomic dysregulation(Srebot et al., 2009). Lower temperatures have also been associated with an increase in inflammatory biomarkers such C-reactive protein (CRP) and interleukin-6 (IL-6) which is consistent with the protective effects we saw with higher cold-season temperatures(Halonen et al., 2010; Schneider et al., 2008). Lower temperatures have also been associated with higher blood pressure which is also a risk factor for cardiovascular disease(Halonen et al., 2011).
Our study had several strengths. Firstly, we used a very large national cohort to look at the exposure-outcome relationship. Secondly, we looked at multiple exposures simultaneously, which better reflects real-life exposure to air pollutants. Thirdly, our model was on an additive scale, which means that our effect estimates can be directly interpreted as the change in rate. Moreover, we used a causal modeling approach with an indicator term for zip code which would have eliminated confounding by any variables that may vary between zip codes, both measured and unmeasured, leaving only potential confounding within a zip code over time. Finally, we adjusted for several measures of socioeconomic status on a time-varying basis, which would be the main confounders of concern in the air pollution and hospital admissions relationship. This likely accounted for a large portion of the within-zip code confounding. We also included an interaction term between a smooth term for year and zip code cluster to account for time trends by unmeasured confounders whose trends were similar within clusters defined by socioeconomic characteristics. This should also account for confounding by these characteristics and relax the parallel trends assumption of the model.
Our approach also had some limitations. We assigned exposure based on spatio-temporal models derived from machine learning estimations. While these exposures had strong validation measures, there is still some residual measurement error that may influence the results. Furthermore, we used an administrative dataset and billing codes to identify our outcomes which is subject to potential misclassification, though we would expect this to be non-differential with regards to pollution exposure. Further, in a linear model, the misclassification error tends to be absorbed into the residual error and produce no bias, which is another advantage of the linear rate model. Finally, our causal approach relies on the parallel trends assumption, which we cannot verify. However, we did attempt to address it by including numerous time-varying socioeconomic variables in the model and by including an interaction term between time and zip code socioeconomic cluster.
5. Conclusion
Among the elderly population in the United States, air pollution generally increased the rate of hospital admissions with CVD, MI, stroke, and respiratory disease. PM2.5 and ozone, in particular, were found to increase the risk of admissions across several health comes. Increasing cold-season temperature reduced the rate of admissions with CVD, MI, stroke, and respiratory disease. These results strengthen the need for stricter air pollution guidelines in order to protect human health.
Supplementary Material
Highlights.
PM2.5 and O3 increased the rate of hospital admissions.
Warmer cold-season temperature reduces the rates of hospital admissions.
Effects were seen on an additive scale.
Harmful effects were seen at low air pollution concentrations as well.
Funding:
This publication was made possible by the United States Environmental Protection Agency (US EPA) grant RD-8358720. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This publication was also made possible by National Institutes of Health (NIH) grants R01ES032418-01 and ES-000002.
Footnotes
Declaration of Interests: Dr. Joel D. Schwartz has appeared as an expert witness on behalf of the US Department of Justice in cases involving violations of the Clean Air Act
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abatzoglou JT, 2013. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 33, 121–131. 10.1002/joc.3413 [DOI] [Google Scholar]
- Abu Awad Y, Di Q, Wang Y, Choirat C, Coull BA, Zanobetti A, Schwartz J, 2019. Change in PM(2.5) exposure and mortality among Medicare recipients: Combining a semi-randomized approach and inverse probability weights in a low exposure population. Environ. Epidemiol. (Philadelphia, Pa.) 3, e054–e054. 10.1097/EE9.0000000000000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S, 2021. Long-Term PM(2.5) Exposure and Risks of Ischemic Heart Disease and Stroke Events: Review and Meta-Analysis. J. Am. Heart Assoc. 10, e016890–e016890. 10.1161/JAHA.120.016890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Östenson C-G, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst-Hensch N, Künzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel-Chapelon F, Declercq C, Grioni S, Krogh V, Tsai M-Y, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G, 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383, 785–795. 10.1016/S0140-6736(13)62158-3 [DOI] [PubMed] [Google Scholar]
- Charrad M, Ghazzali N, Boiteau V, Niknafs A, 2014. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Software; Vol 1, Issue 6. 10.18637/jss.v061.i06 [DOI] [Google Scholar]
- Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS, Arden Pope C, Brauer M, Brook RD, Robichaud A, Menard R, Burnett RT, 2015. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the canadian census health and environment cohort (CanCHEC). Environ. Health Perspect. 10.1289/ehp.1409276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Wei Y, Requia WJ, Shi L, Sabath MB, Dominici F, Coull BA, Evans JS, Koutrakis P, Schwartz JD, 2021. Long-Term Association of Air Pollution and Hospital Admissions Among Medicare Participants Using a Doubly Robust Additive Model. Circulation 143, 1584–1596. 10.1161/CIRCULATIONAHA.120.050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J, 2019. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ. Int. 130, 104879. 10.1016/j.envint.2019.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J, 2019a. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 130, 104909. 10.1016/j.envint.2019.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern RF, Kelly JT, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J, 2019b. Assessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ. Sci. Technol. 10.1021/acs.est.9b03358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Yan, Zanobetti A, Wang Yun, Koutrakis P, Choirat C, Dominici F, Schwartz JD, 2017. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 376, 2513–2522. 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI Data and Maps; United States Geological Survey, 2010. USA Hospitals [WWW Document]. ArcGIS. URL https://www.arcgis.com/home/item.html?id=f114757725a24d8d9ce203f61eaf8f75 (accessed 9.1.19).
- Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D, 2017. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 22, 337–347. 10.1007/s10741-017-9606-7 [DOI] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD, 2015. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation a repeat-measures analysis in the multi-ethnic study of atherosclerosis (MESA). Epidemiology 26, 310–320. 10.1097/eDe.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J, 2011. Relationship between outdoor temperature and blood pressure. Occup. Environ. Med. 68, 296 LP – 301. 10.1136/oem.2010.056507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J, 2010. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ. Health 9, 42. 10.1186/1476-069X-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker JO, Hart JE, James P, Sabath MB, Wu X, Zanobetti A, Dominici F, Laden F, 2021. Air pollution and cardiovascular disease hospitalization – Are associations modified by greenness, temperature and humidity? Environ. Int. 156, 106715. 10.1016/j.envint.2021.106715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requia W, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, Sulprizio M, Amini H, Shi L, Schwartz J, 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ. Sci. Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Panagiotakos D, Picciotto S, Katsouyanni K, Löwel H, Jacquemin B, Lanki T, Stafoggia M, Bellander T, Koenig W, Peters A, Group, for the A.S., 2008. Air Temperature and Inflammatory Responses in Myocardial Infarction Survivors. Epidemiology 19. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Wei Y, Yitshak-Sade M, Di Q, Dominici F, Zanobetti A, 2021. A national difference in differences analysis of the effect of PM2.5 on annual death rates. Environ. Res. 194, 110649. 10.1016/j.envres.2020.110649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebot V, Gianicolo EAL, Rainaldi G, Trivella MG, Sicari R, 2009. Ozone and cardiovascular injury. Cardiovasc. Ultrasound 7, 30. 10.1186/1476-7120-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H, Firth D, 2020. Generalized nonlinear models in R: An overview of the gnm package. Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jöckel K-H, Hoffmann B, 2015. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup. Environ. Med. 72, 656 LP – 663. 10.1136/oemed-2014-102800 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kloog I, Coull BA, Kosheleva A, Zanobetti A, Schwartz JD, 2016. Estimating Causal Effects of Long-Term PM2.5 Exposure on Mortality in New Jersey. Environ. Health Perspect. 124, 1182–1188. 10.1289/ehp.1409671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lee M, Liu P, Shi L, Yu Z, Awad YA, Zanobetti A, Schwartz JD, 2017. Doubly Robust Additive Hazards Models to Estimate Effects of a Continuous Exposure on Survival. Epidemiology. 10.1097/EDE.0000000000000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, Wahl S, Colicino E, Trevisi L, Kloog I, Just AC, Vokonas P, Cyrys J, Gieger C, Schwartz J, Baccarelli AA, Schneider A, Peters A, 2016. Long-term exposure to air pollution is associated with biological aging. Oncotarget 7, 74510–74525. 10.18632/oncotarget.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yazdi MD, Di Q, Requia WJ, Dominici F, Zanobetti A, Schwartz J, 2021. Emulating causal dose-response relations between air pollutants and mortality in the Medicare population. Environ. Heal. 20, 53. 10.1186/s12940-021-00742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Braun D, Schwartz JD, Kioumourtzoglou M-A, Dominici F, 2020. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Sci. Adv. 5692, 1–13. 10.1126/sciadv.aba5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, Kloog I, Zanobetti A, Schwartz JD, 2019. Estimating the causal effect of annual PM(2.5) exposure on mortality rates in the Northeastern and mid-Atlantic states. Environ. Epidemiol. (Philadelphia, Pa.) 3, e052–e052. 10.1097/EE9.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafeiratou S, Samoli E, Dimakopoulou K, Rodopoulou S, Analitis A, Gasparrini A, Stafoggia M, De’ Donato F, Rao S, Monteiro A, Rai M, Zhang S, Breitner S, Aunan K, Schneider A, Katsouyanni K, 2021. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Sci. Total Environ. 772, 145383. 10.1016/j.scitotenv.2021.145383 [DOI] [PubMed] [Google Scholar]
- Zou L, Zong Q, Fu W, Zhang Z, Xu H, Yan S, Mao J, Zhang Y, Cao S, Lv C, 2021. Long-Term Exposure to Ambient Air Pollution and Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Med. 8, 616355. 10.3389/fmed.2021.616355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.