Abstract
The capacity of Shiga toxigenic Escherichia coli (STEC) to adhere to the intestinal mucosa undoubtedly contributes to pathogenesis of human disease. The majority of STEC strains isolated from severe cases produce attaching and effacing lesions on the intestinal mucosa, a property mediated by the locus of enterocyte effacement (LEE) pathogenicity island. This element is not essential for pathogenesis, as some cases of severe disease, including hemolytic uremic syndrome (HUS), are caused by LEE-negative STEC strains, but the mechanism whereby these adhere to the intestinal mucosa is not understood. We have isolated a gene from the megaplasmid of a LEE-negative O113:H21 STEC strain (98NK2) responsible for an outbreak of HUS, which encodes an auto-agglutinating adhesin designated Saa (STEC autoagglutinating adhesin). Introduction of saa cloned in pBC results in a 9.7-fold increase in adherence of E. coli JM109 to HEp-2 cells and a semilocalized adherence pattern. Mutagenesis of saa in 98NK2, or curing the wild-type strain of its megaplasmid, resulted in a significant reduction in adherence. Homologues of saa were found in several unrelated LEE-negative STEC serotypes, including O48:H21 (strain 94CR) and O91:H21 (strain B2F1), which were also isolated from patients with HUS. Saa exhibits a low degree of similarity (25% amino acid [aa] identity) with YadA of Yersinia enterocolitica and Eib, a recently described phage-encoded immunoglobulin binding protein from E. coli. Saa produced by 98NK2 is 516 aa long and includes four copies of a 37-aa direct repeat sequence. Interestingly, Saa produced by other STEC strains ranges in size from 460 to 534 aa as a consequence of variation in the number of repeats and/or other insertions or deletions immediately proximal to the repeat domain.
Shiga toxigenic Escherichia coli (STEC) is an important cause of gastrointestinal disease in humans, particularly since such infections may result in life-threatening sequelae, such as the hemolytic uremic syndrome (HUS) (13, 20, 28). It has been recognized for a number of years that STEC strains causing human disease may belong to a very broad range of O serogroups. However, a subset of these (notably O157 and O111) appear to be responsible for the majority of serious cases (those complicated by HUS) (13, 28). These STEC have the capacity to produce attaching and effacing (A/E) lesions on intestinal mucosa, a property mediated by the locus of enterocyte effacement (LEE) pathogenicity island. The presence of LEE is not essential for pathogenesis, as a minority of sporadic cases of HUS are caused by LEE-negative STEC strains. These cases are frequently reported to be at the severe extreme of the clinical spectrum. Nevertheless, until 1998, virtually all recorded outbreaks of STEC disease complicated by HUS had been caused by LEE-positive STEC belonging to serogroups O157 or O111 (28). The only exception to this was an outbreak of HUS in a German child care center caused by a Shiga toxigenic strain of Citrobacter freundii (39). In April 1998, there was a small outbreak involving three cases of HUS in South Australia, Australia. Culture, PCR, and serological analyses indicated that a LEE-negative O113:H21 STEC strain was responsible (26). This is one of the more common LEE-negative STEC serotypes associated with human disease (13, 28). Indeed, O113:H21 was among the first STEC serotypes to be causally linked to cases of HUS by Karmali et al. (14).
Although Stx is a sine qua non of virulence, adherence to the intestinal epithelium and colonization of the gut by the STEC is also an important component of the pathogenic process. The mechanism whereby LEE-positive strains adhere intimately and generate A/E lesions has been the subject of intensive study in recent years (for review, see reference 20). A/E lesions are characterized phenotypically by localized destruction of the apical microvilli of intestinal epithelial cells resulting from rearrangement of the cytoskeleton and intimate attachment of bacteria to the enterocyte surface. All of the genes required for this are located on LEE, which encodes a type III secretion system, several secreted effector proteins (Esp), the outer membrane protein intimin, and a receptor (Tir) which is translocated into the host cell membrane. Ebel et al. (8) have shown that during lesion formation, initial binding of STEC to host cells is mediated by filamentous structures, of which EspA is a major component. EspD is essential for the formation of these EspA filaments (17), which in turn are required for the translocation of EspB and Tir into epithelial cells and for the activation of epithelial signal transduction (15, 16, 36). It has been proposed that while EspA appendages mediate the initial interaction of STEC with host cells, this is later replaced by the intimate attachment mediated by intimin and Tir (8).
Two other recent studies have described additional putative adhesins from LEE-positive STEC. Tarr et al. (35) have described a chromosomally encoded 67-kDa homologue of Vibrio cholerae IrgA, termed Iha, which mediated adherence of O157:H7 STEC to HeLa cells. Also, Nicholls et al. (21) have described a very large (365-kDa) adhesin, termed Efa1, which mediated adherence to CHO (Chinese hamster ovary) cells. However, although adherence phenotypes have been examined for several LEE-negative STEC strains (5, 7, 31), virtually nothing is known of the actual mechanisms involved. In a previous study, we showed that the capacity of the O113:H21 STEC responsible for the South Australian HUS outbreak to adhere to Henle 407 and HEp-2 cells was similar to that of LEE-positive STEC reference strains (26). In the present study, we describe a novel autoagglutinating adhesin isolated from the outbreak strain, the first adhesin to be characterized from a LEE-negative STEC.
MATERIALS AND METHODS
Bacterial strains and cloning vectors.
The O113:H21 STEC strain 98NK2 was isolated from a patient with HUS at the Women's and Children's Hospital, South Australia, Australia, as previously described (26). All other STEC strains used in this study, as well as the O111 enteropathogenic E. coli (EPEC) strain 87A, were also isolated at the Women's and Children's Hospital and have been described previously (27), except for the O157:H7 strain EDL933 (provided by R. Robins-Browne), the O91:H21 strain B2F1 (provided by A. Melton-Celsa), and the O113 strains 3848 and 1183 (provided by J. Bennet). E. coli K-12 strains DH1 and JM109 have been described previously (10, 41). The cosmid vector pHC79 has also been described previously (11). The phagemids pBluescript SK (encoding ampicillin resistance) and pBC SK (encoding chloramphenicol resistance) were obtained from Stratagene, La Jolla, Calif. All cells of E. coli strains were routinely grown in Luria-Bertani (LB) medium (19) with or without 1.5% Bacto-Agar (Difco Laboratories, Detroit, Mich.). Where appropriate, ampicillin or chloramphenicol was added to growth media at concentrations of 50 and 40 μg/ml, respectively.
HEp-2 adherence assay.
HEp-2 adherence assays were carried out essentially as described previously for Henle 407 cells (27). Briefly, E. coli cells were grown overnight at 37°C in LB broth and diluted to a density of 104 CFU/ml (confirmed by viable count) in Dulbecco's modified Eagle's medium (DMEM), buffered with 20 mM HEPES and supplemented with 10% fetal calf serum and 2 mM l-glutamine. Washed HEp-2 monolayers in 24-well tissue culture plates were then infected with 1-ml aliquots of bacterial suspension. After incubation at 37°C for 3 h, the culture medium was removed and the monolayers were washed four times with phosphate-buffered saline (PBS) to remove nonadherent bacteria. The cell monolayers were then detached from the plate by treatment with 100 μl of 0.25% trypsin–0.02% EDTA. Cells were then lysed by addition of 400 μl of 0.025% Triton X-100, and 50-μl aliquots (and serial 10-fold dilutions thereof) were plated on LB agar to determine the total number of adherent bacteria per well. Assays were carried out in quadruplicate, and results were expressed as the mean (± standard error) CFU/well. The significance of differences in adherence was analyzed using Student's unpaired t test (two-tailed).
For Giemsa staining, HEp-2 cells were grown on 1.0-cm-diameter coverslips in 24-well tissue culture trays and were used when approximately 50% confluent. Wells were infected with approximately 106 CFU of bacteria suspended in 1 ml of tissue culture medium and incubated at 37°C for 3 h. Monolayers were then washed four times in PBS, fixed with 70% methanol, and stained with Giemsa stain. After washing, the coverslips were mounted on glass slides and examined by light microscopy.
DNA manipulations.
Routine DNA manipulations (restriction digestion, agarose gel electrophoresis, ligation, transformation, Southern hybridization analysis, etc.) were carried out essentially as described by Maniatis et al. (19).
Construction of cosmid gene bank.
Construction of the cosmid gene bank of 98NK2 DNA has been described previously (24). Briefly, high-molecular-weight chromosomal DNA was extracted as described previously (25) from STEC 98NK2 and was digested partially with Sau3A1 so as to optimize the yield of fragments in the size range of 35 to 40 kb. This DNA was ligated with a fivefold molar excess of pHC79 DNA, which had been digested with BamHI. Ligated DNA was packaged into lambda heads by using a Packagene kit (Promega Biotec, Madison, Wis.) and was transfected into E. coli DH1 cells which had been grown in LB broth–2% maltose. The cells were then plated onto LB agar supplemented with ampicillin, and after incubation, clones were stored in LB broth–ampicillin–15% glycerol in microtiter plates at −70°C.
DNA sequencing.
For DNA sequencing, plasmid DNA template was purified using a QIAPrep Spin miniprep kit (Qiagen, Hilden, Germany); alternatively, PCR products were purified using the Bresaspin PCR purification kit (Bresagen, Adelaide, Australia). The sequences of both strands were then determined using dye-terminator chemistry and either universal M13 sequencing primers or custom-made oligonucleotide primers on an Applied Biosystems model 377 automated DNA sequencer. The sequences were analysed using DNASIS and PROSIS Version 7.0 software (Hitachi Software Engineering, South San Francisco, Calif.).
Isolation of megaplasmid-cured derivative of 98NK2.
98NK2 was cured of its megaplasmid by direct electrotransformation with a temperature-sensitive suicide plasmid pCACTUS, which encodes chloramphenicol resistance and carries sacB (40). Transformants were selected by plating on minimal agar containing 25 μg of chloramphenicol/ml at 30°C and were subcultured three times in LB broth containing 25 μg of chloramphenicol/ml at 30°C. Serial dilutions were then plated on LB agar and incubated at 42°C, and single colonies were then replated on LB agar supplemented with 6% sucrose and incubated at 42°C. Loss of the megaplasmid from the 98NK2 derivative (designated 98NK2-Cu) was confirmed by the absence of a product after PCR using ehxA-specific primers (23) and the absence of high-molecular-weight plasmid DNA in ethidium bromide-stained agarose gels of genomic DNA extracts; loss of pCACTUS was also confirmed by the absence of a product after PCR using pCACTUS-specific primers.
PCR amplification of saa.
The complete saa genes from various STEC strains were amplified by PCR using an Expand High Fidelity PCR kit (Roche Molecular Diagnostics) and primers SaaF (5′-CCTCACATCTTCTGCAAATACC-3′) and SaaR (5′-GTTGTCCTGCAGATTTTACCATCCAATGGACATG-3′). These primers directed amplification of the region from nucleotides (nt) 1107 to 2795 of the insert of pJCP562, which incorporates the complete saa open reading frame as well as upstream sequences containing the putative promoter and ribosome binding site. When required, PCR products were purified and blunt cloned into the SmaI site of pBluescript SK.
Construction of saa-negative derivative of 98NK2.
To generate a saa-negative derivative of 98NK2, the 3,758-bp 98NK2 DNA insert was excised from pJCP562 with PstI, cloned into pCACTUS, and electroporated into E. coli DH5α cells. Transformants were selected by plating on LB agar containing 25 μg of chloramphenicol/ml at 30°C. Inverse PCR was then used to generate a deletion derivative of this construct lacking 1,402 bp from the saa open reading frame (equivalent to nt 1298 to 2699 of the insert of pJCP562). The primers used were 5′-CCGTAGATCTGCTGCATGAGTACTCGTTGCC-3′, which incorporates a BglII site (underlined), and 5′-TCCCGTAGATCTAACACCGTCGTCAGACTAAACACC-3′, which also contains a BglII site (underlined). PCR was performed using the Expand long template PCR system (Roche) and plasmid DNA template; the product was digested with BglII, recircularized with T4 ligase, and electroporated into E. coli DH5α cells. The plasmid containing the deleted saa operon was then extracted and linearized with BglII, end-filled with Klenow fragment, and blunt-ligated with a 1,252-bp HindII fragment from pUC4K (Roche), which contains a functional kanamycin resistance gene. Sequence analysis of the resultant construct (designated pΔsaa::kan) confirmed deletion of the major proportion of saa and insertion of the kanamycin cartridge. This construct was then electroporated into 98NK2 cells, and cells were plated on LB agar supplemented with 25 μg of chloramphenicol/ml and 50 μg of kanamycin/ml at 30°C. A randomly selected transformant was grown in LB broth-kanamycin-chloramphenicol at 30°C overnight, and serial dilutions in PBS were plated on LB agar-kanamycin and grown at 42°C (which is nonpermissive for pCACTUS derivatives) to select for cointegrants. Ten randomly selected colonies were then grown overnight at 37°C in LB broth-kanamycin, and serial dilutions were grown at 30°C on LB agar without NaCl, supplemented with 6% sucrose and kanamycin, in order to isolate recombinants lacking pCACTUS-derived sacB.
Purification of Saa and preparation of antiserum.
Purification of Saa was carried out using a QIAexpress kit (Qiagen). In order to construct an N-terminal His6-Saa fusion protein, the region of the insert of pJCP562 encoding amino acids 29 to 516 was amplified by high-fidelity PCR using primers SaaH6F (5′-CATAATGGATCCACTTATGAAAGCCTTGAAAAAGGG-3′) and SaaR. The two primers incorporate BamHI and PstI sites near their respective termini, enabling the resultant product to be cloned between the BamHI and PstI sites of pQE30 (Qiagen). Correct insertion of the saa fragment was confirmed by sequence analysis. The recombinant plasmid was then transformed into the expression host E. coli SG13009[pREP4]. For purification of His6-Saa, transformants were grown in 1 liter of LB broth supplemented with 50 μg of ampicillin/ml, and when the culture reached an A600 of 0.5, the culture was induced with 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and incubated for a further 3 h. Cells were harvested by centrifugation, resuspended in 24 ml of buffer A (6 M guanidine-HCl, 0.1 M NaH2PO4, 10 mM Tris, pH 8.0), and stirred at room temperature for 1 h. Cell debris was removed by centrifugation at 10,000 × g for 25 min at 4°C. The supernatant was then loaded (at a rate of 15 ml/h) onto a 2-ml column of Ni-nitrilotriacetic acid resin (ProBond; Invitrogen), which had been pre-equilibrated with 20 ml of buffer A supplemented with 0.5 M NaCl and 15 mM imidazole. The column was then washed with 40 ml of buffer A, followed by 20 ml of buffer B (8 M urea, 0.1 M NaH2PO4, 10 mM Tris, pH 8.0) and then 16 ml of buffer C (8 M urea, 0.1 M NaH2PO4, 10 mM Tris, pH 6.3), which was supplemented with 0.25 M NaCl and 5 mM imidazole. Saa was then eluted with a 30-ml gradient of 0 to 500 mM imidazole in buffer C; 3-ml fractions were collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Peak fractions were pooled, and the denatured Saa protein was refolded by dialysis against 100 ml of buffer B to which 1 liter of PBS was added dropwise at a rate of 60 ml/h. This was followed by dialysis against two changes of PBS. The total yield of purified Saa was approximately 5 mg, which was >95% pure, as judged by SDS-PAGE after staining with Coomassie brilliant blue R250.
Six BALB/c mice were then immunized by intraperitoneal injection of three 10-μg doses of purified Saa in 0.2 ml of PBS containing 5 μg of alum adjuvant (Imjectalum; Pierce, Rockford, Ill.) at 2-week intervals. Mice were exsanguinated by cardiac puncture 1 week after the third immunization, and pooled immune serum was stored in aliquots at −15°C.
Western blot analysis.
Crude lysates of STEC or other E. coli strains were separated by SDS-PAGE as described by Laemmli (18), and antigens were electrophoretically transferred onto nitrocellulose filters as described by Towbin et al. (38). Filters were probed with mouse anti-Saa antibody (used at a dilution of 1:5,000), followed by goat anti-mouse immunoglobulin G (IgG) conjugated to alkaline phosphatase (Bio-Rad Laboratories). Labeled bands were visualized using a chromogenic nitroblue tetrazolium/X-phosphate substrate system (Roche Molecular Diagnostics).
Immunogold electron microscopy.
E. coli cells suspended in PBS were applied to poly-l-lysine-coated grids for 5 min, washed twice in PBS-bovine serum albumin (BSA), and reacted with polyclonal anti-Saa antibody diluted 1:20 in PBS-BSA for 15 min. Grids were then washed twice with PBS-BSA and reacted with protein G–20-nm gold conjugate (ICN Biomedicals, Aurora, Ohio) diluted 1:40 in PBS-BSA for 15 min. The grids were then washed twice in PBS and examined without further staining using a Philips CM100 electron microscope at 80 kV.
Nucleotide sequence accession number.
The DNA sequence described in this paper has been deposited with GenBank under accession number AF325220.
RESULTS
Adherence phenotype of O113:H21 STEC strain 98NK2 and isolation of adherent clones.
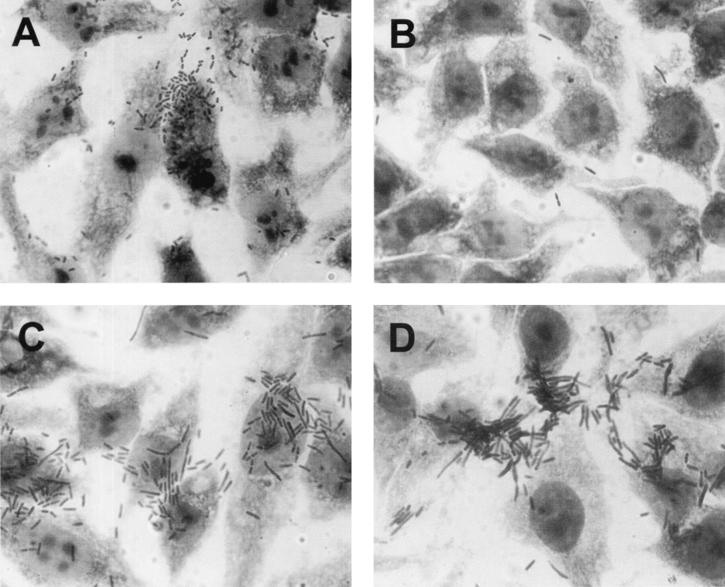
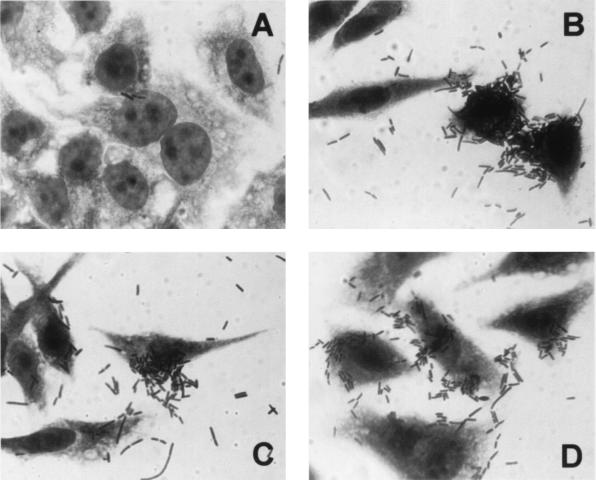
As a first step, the adherence properties of 98NK2 to HEp-2 cells were examined using a quantitative adherence assay and Giemsa staining as described in Materials and Methods. Total adherence after 3 h of incubation was (mean ± standard error) 1.16 (± 0.13) × 105 CFU per well, and Giemsa staining indicated large clusters of adherent bacteria on the epithelial cell surface (Fig. 1A). We describe this adherence pattern as “semilocalized” to distinguish it from the “localized” adherence phenotype associated with LEE-positive enteropathogenic E. coli (EPEC) strains, which is characterized by tightly bound bacterial microcolonies on the cell surface. Macroscopically, 98NK2 cells autoagglutinated when grown at 37°C for 16 h in DMEM, a property coincidentally also observed for the EPEC strain 87A.
FIG. 1.
Adherence of E. coli strains to HEp-2 cells (Giemsa stain). (A) STEC strain 98NK2; (B) E. coli DH1:pHC79; (C) E. coli DH1:pJCP561; (D) E. coli JM109:pJCP562.
To eliminate the possibility that the adherence mechanism of 98NK2 was related to that for EPEC strains, restricted genomic DNA from 98NK2 was subjected to low-stringency Southern hybridization analysis using a digoxigenin-labeled probe specific for bfpA, which encodes the EPEC bundle-forming pilus (32, 33). A strong hybridization signal was obtained for digests of DNA from EPEC strain 87A, but no probe-reactive fragments were detected for 98NK2 (result not shown). 98NK2 DNA did, however, react with probes specific for the irgA-related gene iha, and a library of 98NK2 DNA constructed in E. coli DH1 cells by using cosmid pHC79 (see Materials and Methods) was screened for clones reacting with this probe. One of these (designated pJCP561) was selected for further analysis.
The adherence of E. coli DH1:pJCP561 to HEp-2 cells in a standard 3-h assay was (mean ± standard error) 6.9 (± 0.89) × 104 CFU per well: this was significantly greater than that for DH1:pHC79, which was 1.15 (± 0.14) × 104 CFU per well (P < 0.001). Furthermore, examination of Giemsa-stained infected HEp-2 cells indicated that pJCP561 but not pHC79 conferred a semilocalized adherence phenotype upon DH1 similar to that seen for 98NK2 (Fig. 1B and C). A series of random subclones of pJCP561 were then constructed in pBC. One of these (designated pJCP562) contained a 3.8-kb PstI fragment and conferred semilocalized adherence upon E. coli JM109, as judged by Giemsa staining (Fig. 1D). In the quantitative HEp-2 adherence assay, JM109:pJCP562 exhibited a total adherence of 5.58 (± 0.26) × 104 CFU per well. This was significantly greater than that for JM109:pBC, which was 5.75 (± 0.63) × 103 CFU per well (P < 0.001). Both DH1:pJCP561 and JM109:pJCP562, but not JM109:pBC, autoagglutinated when grown in DMEM.
Sequence analysis of pJCP562.
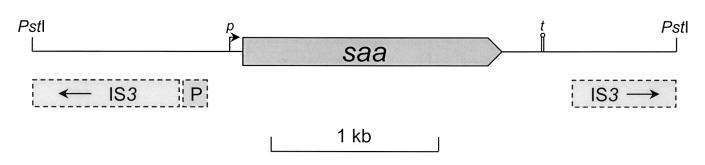
Sequence analysis indicated that the 98NK2 DNA insert in pJCP562 was 3,758 nt long and did not contain the irgA/iha-related gene. It contained only one intact open reading frame (nt 1230 to 2780) sufficient to encode a 516-aa protein. As shown in Fig. 2, the insert was flanked by two oppositely oriented incomplete copies of an IS3 element (nt 1 to 885 and 3183 to 3758); indeed, the last 576 nt of the insert is 99% identical to an inverted repeat of the first 576 nt. A 140-bp region from nt 890 to 1030 was 87% identical to nt 22485 to 22627 (a noncoding region) of pO157, the large virulence plasmid of O157:H7 STEC strain EDL933 (2), but the remainder of the pJCP562 insert had no homology with any pO157 sequences. The open reading frame which we have designated saa (STEC autoagglutinating adhesin) is preceded by a strong ribosome binding site (the sequence of nt 1216 to 1223 is AGGAAGAA). The NNPP program (29) (available at http://dot.imgen.bcm.tmc.edu) predicted that within the entire plus strand of the insert of pJCP562, the region most likely to be a promoter sequence was immediately upstream of the ribosome binding site for saa (nt 1156 to 1201); transcription was predicted to start at T1196. Immediately downstream of saa (nt 2980 to 3003) is a potential stem-loop element (ΔG = −15.2 kcal/mol) which may function as a transcription terminator.
FIG. 2.
Map of the 98NK2 DNA insert of pJCP562 showing the location of the saa open reading frame (thick arrow) and its putative promoter (p) and transcription termination site (t). The regions with DNA sequence homology with portions of IS3 elements and pO157 (P) are shown beneath the map.
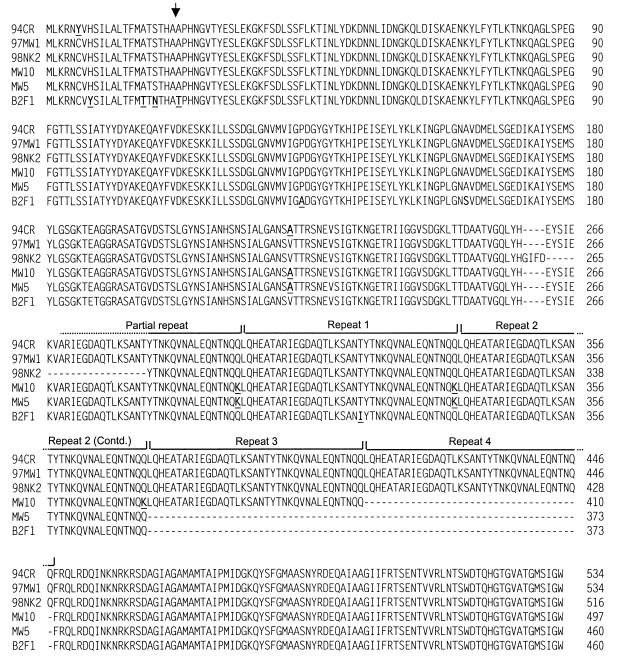
BLASTP analysis (1) indicated that the first 155 aa (30%) of the 516-aa Saa protein had no similarities with any known protein. However, a low but significant degree of sequence similarity was detected between the distal 70% of Saa and YadA, a plasmid-encoded outer membrane protein of Yersinia enterocolitica implicated in epithelial cell adherence and invasion (34), as well as a recently described family of phage-encoded immunoglobulin-binding proteins (Eib) of E. coli (30). Amino acids 155 to 516 of Saa exhibited 24% identity and 40% similarity with aa 53 to 455 of YadA, albeit with 13% gaps; aa 187 to 500 of Saa exhibited 27% identity and 42% similarity to EibD, with 22% gaps (slightly lesser similarities were seen between Saa and EibC or EibE). The N terminus of Saa comprises charged residues followed by a hydrophobic domain typical of signal peptides, and the program SignalP V1.1 (22) predicted a signal peptidase cleavage site between two alanine residues (A22 and A23). Interestingly, the C-terminal half of Saa comprises four complete copies and one partial copy of a 37-aa repeated element (LQHEATARIEGDAQTLKSANTYTNKQVNALEQNTNQQ) (covering the region from aa 266 to 429). There is absolute sequence identity between the various copies of the repeat unit at both the DNA and amino acid levels. This region of Saa is strongly helical and has a high probability of forming coiled-coil structures, as predicted by the program COILS (available at http://www.ch.embnet.org) with a 14-aa window setting. The latter half of each 37-aa repeat unit and a stretch of 15 residues (aa 430 to 444) immediately following the repeat domain had the highest coiled-coil potential. The extreme C-terminal portion of Saa is also hydrophobic and so may represent a membrane anchorage domain.
Presence of saa in other STEC strains.
Southern hybridization analysis was used to determine the presence of saa-related genes in other E. coli strains, including 32 STEC isolates which had previously been tested for presence of the LEE pathogenicity island and the plasmid-carried enterohemolysin gene ehxA (also referred to as EHEC-hlyA) (27) (Table 1). No hybridization signal was obtained at either high or low stringency with the E. coli K-12 strain C600 or C600 lysogenized with the Stx2-converting bacteriophage 933W. Neither was any signal obtained with the EPEC strain 87A or with any of the LEE-positive STEC strains tested, including representatives belonging to serotypes O157:H7, O157:H−, O111:H−, and O26. However, high-stringency saa homologues were present in 19 of the 26 LEE-negative STEC strains. All of these were also positive for ehxA. Homologues of saa were not present in any of the seven LEE-negative STEC strains which were negative for ehxA. Thus, it appears that saa is only present in LEE-negative STEC strains that also carry the plasmid-carried ehxA, implying that saa is located on the respective large virulence plasmid. This was confirmed by PCR analysis of DNA extracted from 98NK2 and a derivative (designated 98NK2-Cu) which had been cured of its megaplasmid (see Materials and Methods), using saa- and ehxA-specific primers. PCR was also used to test additional LEE-positive STEC isolates (three O157, five O111, and three O26 strains), all of which were positive for ehxA but negative for saa (results not shown).
TABLE 1.
Presence of saa homologues in various E. coli strains
| E. coli strain | Serotype | Sourcea | Presence of saab | Presence of LEEc | Presence of ehxAc |
|---|---|---|---|---|---|
| 98NK2 | O113:H21 | HUS | + | − | + |
| C600 | − | − | − | ||
| C600:933W | − | − | − | ||
| EPEC 87A | O111 | D | − | + | − |
| EDL933 | O157:H7 | BD | − | + | + |
| 96GR1 | O157:H− | HUS | − | + | + |
| 95ZG1 | O26 | BD | − | + | + |
| 96RO1 | O111:H− | HUS | − | + | + |
| 95NR1 | O111:H− | HUS | − | + | + |
| 99BG1 | O111:H− | BD | − | + | + |
| 97MW1 | O113:H21 | BD MHA/T | + | − | + |
| 3848 | O113:H21 | HUS | + | − | + |
| 1183 | O113:H21 | HUS | + | − | + |
| MW10 | O113:H21 | Food | + | − | + |
| 94CR | O48:H21 | HUS | + | − | + |
| 99AM1 | O23 | BD | + | − | + |
| MW5 | Ontd | Food | + | − | + |
| 00MC2 | O6 | BD | + | − | + |
| B2F1 | O91:H21 | HUS | + | − | + |
| MW13 | O98 | Food | + | − | + |
| MW2 | Ont | Food | + | − | + |
| MW9 | ORd | Food | + | − | + |
| MW14 | NDd | Food | + | − | + |
| MW8 | Ont | Food | + | − | + |
| MW6 | OR | Food | + | − | + |
| MW3 | O82:H8 | Food | + | − | + |
| MW7 | Ont:H11 | Food | + | − | + |
| 95HE4 | O91 | D | + | − | + |
| MW15 | O141 | Food | − | − | − |
| MW4 | Ont | Food | − | − | − |
| 95MV1 | OR:H9 | HUS | − | − | − |
| MW11 | ND | Food | − | − | − |
| 95AS1 | O128 | D | − | − | − |
| 031 | OX3:H21 | SIDS | − | − | − |
| MW12 | O159 | Food | − | − | − |
STEC strains were isolated either from foods or from the feces of patients with uncomplicated diarrhea (D), bloody diarrhea (BD), microangiopathic hemolytic anemia and thrombocytopenia (MHA/T), hemolytic uremic syndrome (HUS), or sudden infant death syndrome (SIDS).
Determined by Southern hybridization analysis using a digoxigenin-labeled PCR product corresponding to the complete saa open reading frame as probe.
Ont, O non-typable; OR, O rough; ND, serotype not determined.
As a further test of the contribution of saa to adherence of STEC, in vitro adherence of 98NK2 to HEp-2 cells was compared with that of 98NK2-Cu; total adherence after a 3-h incubation was (mean ± standard error) 9.87 (± 0.82) × 104 and 4.66 (± 0.47) × 104 CFU/well, respectively (P < 0.01). Geimsa staining also indicated that 98NK2-Cu displayed a more diffuse adherence pattern (results not shown).
Size heterogeneity of saa.
The complete saa genes were amplified by PCR from genomic DNA extracted from STEC strains 94CR, MW10, MW5, 97MW1, and B2F1, using primers SaaF and SaaR, as described in Materials and Methods. Interestingly, the various PCR products differed in length. Those obtained using 94CR and 97MW1 DNA templates were slightly larger (approximately 1,720 bp) than the 98NK2 PCR product (1,689 bp), whereas the PCR products from the MW10, MW5, and B2F1 templates were smaller (approximately 1,630, 1,500, and 1,500 bp, respectively) (results not shown). This was confirmed by direct sequence analysis of the various amplicons, which demonstrated that the products of the saa genes from 94CR and 97MW1 were 534 aa, compared with 516 aa for 98NK2. On the other hand, saa from MW10 encoded a 497-aa product, while saa from MW5 and B2F1 encoded 460-aa products. The alignment of these sequences (Fig. 3) indicates that the size differences result from variations in the number of 37-aa repeat units, as well as deletions and insertions immediately upstream of this domain. However, apart from these differences, the Saa proteins were highly conserved amongst the various strains.
FIG. 3.
Alignment of the deduced amino acid sequences of Saa proteins from various STEC strains was carried out using CLUSTAL W (37) with manual adjustment. The locations of the repeat sequences are indicated. -, absence of a residue. Residues which differ from the consensus sequence are shown in bold and are underlined. The numbers on the right indicate the position within the amino acid sequence for each protein. The arrow indicates the predicted signal peptidase cleavage site.
The in vitro adherence of these saa-positive STEC strains was then compared using the quantitative HEp-2 assay (Fig. 4). The three strains whose Saa proteins contained four copies of the repeat unit (98NK2, 94CR, and 97MW1) did not differ significantly in adherence. However, MW5 and B2F1, whose Saa proteins contain only two copies of the repeat unit, exhibited significantly reduced adherence (41 and 27%, respectively) relative to that for 98NK2 (P values of <0.01 and <0.001, respectively). Adherence of MW10, whose Saa protein contains three copies of the repeat unit, exhibited intermediate adherence (73% of that for 98NK2).
FIG. 4.
Adherence of saa-positive STEC strains to HEp-2 cells. The indicated STEC strains were subjected to the quantitative HEp-2 adherence assay as described in Materials and Methods. Data are the mean ± standard error of quadruplicate assays (∗, significantly different from 98NK2 [P < 0.01]; ∗∗, significantly different from 98NK2 [P < 0.001]).
Expression of saa.
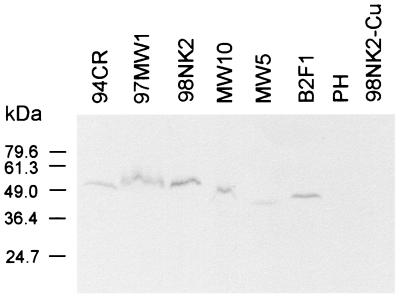
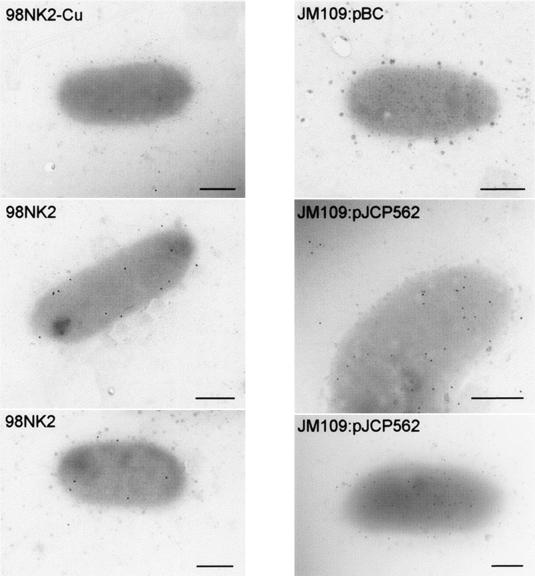
The weaker adherence of MW10, MW5, and B2F1 could be directly attributable to the shorter predicted Saa proteins or, alternatively, to poor expression of saa in these strains. To examine this, Saa from 98NK2 was purified as a His6-tagged fusion protein and was used to raise a polyclonal mouse antiserum as described in Materials and Methods. This antiserum was used for Western immunoblot analysis of lysates from various wild-type STEC strains grown in LB broth at 37°C (Fig. 5). Immunoreactive bands of the expected size (approximately 54 to 56 kDa) were seen in lysates of the fully adherent STEC strains 98NK2, 97MW1, and 94CR. Lysates of the STEC strains with shorter saa genes also contained immunoreactive species, the apparent sizes of which (approximately 50 kDa for MW10 and 45 to 47 kDa for MW5 and B2F1) were essentially consistent with their respective gene sequences. However, there was a slight difference between the apparent SDS-PAGE mobility of Saa from B2F1 and MW5, even though their deduced amino acid sequences are the same length. There are a total of nine predicted amino acid differences between the two proteins. Three of these are in the signal peptide and one is at position +1 of the mature protein, raising the possibility of defective cleavage by the signal peptidase. However, the SignalP program predicts that the signal peptide of Saa from B2F1 will still be cleaved at the same position, albeit with a lower probability score. Two of the five other amino acid differences are K/Q substitutions; thus, the resultant difference in net charge could affect the SDS-PAGE mobility of the respective proteins. The overall level of the expression of saa cannot account for the difference in adherence between the various STEC strains, as the intensity of the anti-Saa immunoreactive species in the lysate of B2F1 (the strain with the poorest adherence) was similar to that in the lysates from the highly adherent strains. As expected, no immunoreactive species were observed in lysates of the saa-negative, LEE-positive O111:H− STEC strain PH or in 98NK2-Cu (Fig. 5). Interestingly, no anti-Saa-reactive species were detected in lysates of any of the above saa-positive STEC strains when cells were grown in LB broth at 25°C (result not shown). A purified outer membrane protein fraction from 94CR (prepared as described previously [27]) was also subjected to Western blot analysis, and this confirmed that like YadA and the Eib proteins, Saa is an outer membrane protein (results not shown). Surface exposure of Saa was also confirmed using polyclonal anti-Saa antibody and immunogold electron microscopy (see Materials and Methods). Gold particles were clearly visible on the surfaces of 98NK2 and E. coli JM109:pJCP562 cells, but no labeling whatsoever was detected on the surface of either 98NK2-Cu or JM109:pBC (Fig. 6).
FIG. 5.
Western blot analysis of STEC strains. STEC strains were grown in LB broth at 37°C, and lysates were separated by SDS-PAGE, blotted, and probed with anti-Saa antibody as described in Materials and Methods. The mobilities of protein size markers (in kilodaltons) are also shown.
FIG. 6.
Electron micrographs of STEC strains after staining with anti-Saa and gold-labeled (20 nm) protein M conjugate. Bar, 0.5 μm.
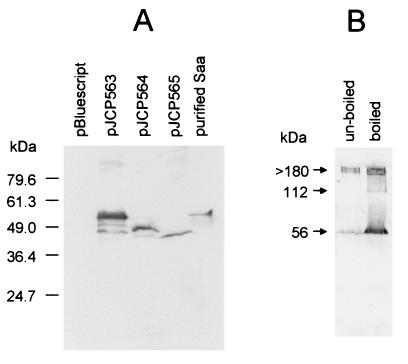
Expression of saa was also examined in E. coli JM109 derivatives carrying PCR-amplified saa genes from 98NK2, MW10, or MW5 cloned in pBluescript (designated pJCP563, pJCP564, and pJCP565, respectively) (Fig. 7A). In all three clones, the saa gene was oriented in the opposite direction of the vector lac promoter. The lysate of JM109:pJCP563 contained a strongly immunoreactive band which comigrated with Saa purified from 98NK2. JM109:pJCP564 and JM109:pJCP565 expressed slightly smaller immunoreactive proteins, as predicted from their respective DNA sequences. The JM109:pJCP563 and JM109:pJCP564 lysates both contained smaller immunoreactive species which may reflect proteolytic degradation of the overexpressed proteins. The JM109:pJCP563 lysate also contained a small amount of a much larger immunoreactive species. Separate Western blot analysis indicated that JM109:pJCP563 lysates which had not been boiled prior to SDS-PAGE contained much larger amounts of high-molecular-weight anti-Saa-reactive material, with an apparent size of >180 kDa (Fig 7B). Boiled lysates contained relatively more Saa monomer (56 kDa), as well as traces of an immunoreactive species with a size of 112 kDa, which might be a Saa dimer. Interestingly, all the saa-expressing clones exhibited a semilocalized adherence pattern on HEp-2 cells, as judged by Giemsa staining (Fig. 8), and (unlike JM109:pBluescript) autoagglutinated when grown in DMEM.
FIG. 7.
Western blot analysis of E. coli JM109 expressing cloned saa genes. (A) Lysates of JM109 carrying the indicated plasmids, or purified Saa, were separated by SDS-PAGE, blotted, and probed with anti-Saa antibody as described in Materials and Methods. The mobilities of protein size markers (in kilodaltons) are also shown. (B) A fresh lysate of E. coli JM109:pJCP563 was subjected to SDS-PAGE with or without boiling, blotted, and probed with anti-Saa antibody. The apparent size of immunoreactive species (arrows) was calculated with reference to the mobility of size markers.
FIG. 8.
Adherence of E. coli JM109 expressing cloned saa genes to HEp-2 cells (Giemsa stain). (A) E. coli JM109:pBluescript; (B) JM109:pJCP563; (C) JM109:pJCP564; (D) JM109:pJCP565.
Effect of Saa on serum resistance.
Given that the two proteins most closely related to Saa (YadA and Eib) both confer serum resistance, aliquots of 98NK2, 98NK2-Cu, JM109:pJCP562, and JM109:pBC were tested for susceptibility to the bactericidal effects of fresh human serum (or heat-inactivated serum) as described by El Tahir et al. (9). However, no differences in resistance were observed between the Saa-expressing strains and their respective Saa-negative controls (results not presented).
Construction and analysis of saa-negative 98NK2.
A derivative of 98NK2 in which a 1,402-bp internal portion of saa was deleted and replaced with a kanamycin resistance cartridge was constructed by allelic exchange using the suicide vector pCACTUS, as described in Materials and Methods. Mutagenesis of the saa operon in the mutant (designated 98NK2-S) was confirmed by PCR amplification using primers SaaF and SaaR. These primers direct amplification of a 1,689-bp fragment from 98NK2. However, a single 1,538-bp PCR product was obtained using 98NK2-S DNA as template. Sequence analysis confirmed that this fragment comprised the 1,252-bp kanamycin cartridge flanked by 190- and 96-bp fragments derived from the 5′ and 3′ termini of the saa operon. Absence of any 1,689-bp PCR product indicated that all copies of the megaplasmid in 98NK2-S contained the mutagenized saa operon. Retention of the megaplasmid was also confirmed by PCR using ehxA-specific primers and by agarose gel electrophoresis of extracted plasmid DNA (results not presented). Western blot analysis using polyclonal anti-Saa antibody was also used to confirm that 98NK2-S did not produce the Saa protein (Fig. 9A).
FIG. 9.
Analysis of saa-negative derivative of 98NK2. (A) Western blot analysis of 98NK2 and 98NK2-S lysates using polyclonal anti-Saa antibody. (B) Adherence of 98NK2 and 98NK2-S to HEp-2 cells (Giemsa stain).
The adherence phenotypes of 98NK2 and 98NK2-S were then compared. In the quantitative HEp-2 assay, total adherence after a 3-h incubation was (mean ± standard error) 3.07 (± 0.12) × 105 and 1.48 (± 0.05) × 105 CFU/well, respectively (P < 0.001). Geimsa staining also indicated that 98NK2-S displayed a diffuse, rather than the semilocalized, adherence pattern (Fig. 9B).
Effect of anti-Saa antibody or exogenous purified Saa on adherence to HEp-2 cells.
To examine the capacity of antibodies to Saa to inhibit adherence of STEC, HEp-2 monolayers were infected with STEC strain 94CR in the presence or absence of anti-Saa serum diluted 1:100 or 1:400. In the absence of antibody, total adherence after a 3-h incubation was (mean ± standard error) 1.89 (± 0.26) × 105 CFU/well. In the presence of anti-Saa antibody, adherence was reduced in a dose-dependent fashion (6.97 [± 0.98] × 104 CFU/well and 1.41 [± 0.17] × 105 CFU/well for 1:100 and 1:400 dilutions, respectively). For the 1:100 serum dilution, the reduction in adherence was statistically significant (P < 0.01).
The effect of exogenous purified Saa on STEC adherence was also investigated. HEp-2 monolayers were preincubated with or without Saa at concentrations of 5 or 25 μg/ml for 30 min and then infected with 98NK2. After a 3-h incubation, total adherence was (mean ± standard error) 1.97 (± 0.17) × 105 CFU/well in the absence of Saa, compared with 3.52 (± 0.21) × 105 and 4.38 (± 0.25) × 105 CFU/well in the presence of 5 and 25 μg/ml of Saa, respectively. At both concentrations, the stimulation of adherence by Saa was statistically significant (P values of <0.01 and <0.001, respectively).
DISCUSSION
Although a wide variety of STEC strains are capable of causing severe human gastrointestinal disease and life-threatening systemic complications, such as HUS, research into pathogenic mechanisms has been focused largely upon STEC belonging to serogroup O157 (particularly O157:H7). This has been prompted by the fact that strains belonging to this serogroup are the most frequent causes of STEC disease in humans in many parts of the world. However, this apparent predominance is due at least in part to the fact that O157 strains are much easier to detect than all other STEC strains (they are unable to ferment sorbitol). STEC belonging to serogroup O157 and a limited range of other groups, including O111 and O26, carry the LEE pathogenicity island, which encodes the capacity to adhere intimately to enterocytes. Although LEE is unquestionably an important accessory virulence factor for these strains, it is not essential for STEC virulence, and many strains lacking LEE are capable of causing life-threatening disease in humans. A substantial body of information has been accumulated on the complex molecular interactions between LEE-positive STEC and the intestinal epithelium. However, virtually nothing is known of adherence mechanisms of LEE-negative STEC, in spite of the fact that they are capable of highly efficient colonization of the human gut (28). Dytoc et al. (7) examined the in vitro and in vivo adherence phenotype of a different O113:H21 STEC strain, also isolated from a patient with HUS. This strain adhered diffusely to HEp-2 cells and caused microvillus effacement of rabbit caecum epithelial cells, but without generating the cytoskeletal rearrangements associated with LEE-positive strains. The presence of pili on the surface of this strain was also demonstrated by transmission electron microscopy, but the role of these in adherence was not investigated.
In the present study, we have described a gene we have designated saa, which encodes a novel autoagglutinating adhesin unique to LEE-negative STEC. The saa gene was isolated from cosmid pJCP561, which also contained a homologue of iha which has been reported by Tarr et al. (35) to facilitate adherence of E. coli O157:H7. E. coli DH1:pJCP561 had sixfold-higher adherence to HEp-2 cells relative to that of DH1:pHC79 and exhibited a semilocalized adherence phenotype. E. coli JM109 carrying a subclone of this cosmid in pBC (pJCP562) was 9.7 times more adherent than JM109:pBC. However, this subclone did not contain the iha-related gene, and the only intact open reading frame in the 3.8-kb 98NK2 DNA insert in pJCP562 was saa. JM109:pJCP562 also exhibited a semilocalized adherence phenotype indistinguishable from that of DH1:pJCP561. High-stringency homologues of saa were found in a number of unrelated STEC strains, all of which were LEE negative. All of these strains were positive for the STEC-associated enterohemolysin gene ehxA. All saa-negative, LEE-negative STEC cells were also negative for ehxA, suggesting that saa might also be plasmid carried, a fact confirmed by the absence of saa in a derivative of 98NK2 which had been cured of its large virulence plasmid. Interestingly, the vast majority of LEE-positive STEC strains also carry large ehxA-carrying plasmids, but the absence of saa from these strains indicates that there are important differences between the plasmids of these two STEC classes.
Saa appears to be located in the outer membrane and is exposed on the cell surface, as judged by immunogold electron microscopy. It exhibits a low degree of similarity (24 to 27% identity) with two other outer membrane proteins, notably YadA of Y. enterocolitica and EibD from E. coli. YadA is an important virulence factor in Y. enterocolitica, contributing to autoagglutination, serum resistance, complement inactivation, epithelial cell adherence, and resistance to phagocytosis and binding to a variety of extracellular matrix proteins (3, 9, 34). Like Saa, YadA is expressed at 37°C, but not at 25°C, and is encoded on a large, virulence-related plasmid (4). EibD is a member of a family of four related proteins encoded by a prophage in E. coli strain ECOR-9, responsible for nonimmune IgG binding and serum resistance (30). However, in the present study, we found no evidence that Saa contributes to serum resistance either in 98NK2 or when expressed by E. coli K-12 clones.
Saa exhibits interesting structural features, in particular, the presence of four copies of a 37-aa repeat unit in the C-terminal region, which contains a motif predicted to form coiled coils, as does a region immediately downstream of the repeat domain. Such coiled-coil motifs are frequently implicated in protein-protein interactions. SDS-PAGE analysis of unboiled extracts suggests that Saa, which lacks cysteines, is capable of forming multimers with masses of >180 kDa. Interestingly, YadA of Y. enterocolitica also lacks cysteines and contains a region with a high probability of forming coiled coils. Recent structural analysis of YadA (12) suggests that the protein is oligomeric (probably trimeric or tetrameric) with a tripartite “lollipop” organization comprising an N-terminal oval head (responsible for functions such as adherence and autoagglutination) followed by a coiled-coil stalk and a C-terminal membrane anchor. EspA, which is produced by LEE-positive STEC and EPEC strains, also has a coiled-coil domain, and this region is essential for assembly of the EspA filaments which mediate initial attachment of these bacteria to epithelial cells and deliver other secreted effector proteins into the host cell (6).
Interestingly, saa genes from diverse STEC strains exhibited marked variation in size, due largely to deletion of one or two of the direct repeat units, such that the encoded Saa proteins ranged from 460 to 534 aa in length. Wild-type STEC strains MW10, MW5, and B2F1, which carried the shorter saa genes, exhibited lower adherence to HEp-2 cells. Western blot analysis using a Saa-specific polyclonal antiserum confirmed that these strains produce smaller Saa proteins, as predicted. When the shorter saa genes from these strains were cloned into E. coli JM109 using pBluescript, Saa was produced and the recombinant bacteria exhibited a semilocalized adherence phenotype, indicating that these gene products are still functional, at least when overexpressed from a high-copy-number plasmid. Unfortunately, however, these clones grew poorly in vitro with respect to the parental strain, preventing direct quantitative examination of the effect of the number of repeat units on adherence in an otherwise isogenic background.
Three further pieces of evidence support the hypothesis that Saa functions as an adhesin in LEE-negative STEC. Firstly, both the plasmid-cured derivative 98NK2-Cu and the defined saa-negative mutant 98NK2-S exhibited significantly reduced adherence relative to 98NK2. Adherence was reduced to a similar extent in both derivatives (47 and 48% of the wild-type level, respectively). It should be emphasized, however, that in neither case was adherence abolished, which indicates that additional adherence mechanisms also operate.
Secondly, a polyclonal antiserum raised against purified Saa from 98NK2 significantly inhibited in vitro adherence of the saa-positive STEC 94CR in a dose-dependent fashion. This strain was chosen to confirm that anti-Saa antibody was capable of interfering with adherence of heterologous STEC serotypes, an important consideration if Saa is to be examined as a potential vaccine antigen for LEE-negative STEC. The reduction in adherence only reached statistical significance at the lowest serum dilution tested (1:100), which may imply poor antibody avidity. When overexpressed as a His6-tagged fusion protein, Saa was insoluble, presumably due to formation of inclusion bodies. It was purified after solubilization with 6 M guanidine-HCl, and after Ni-nitrilotriacetic acid chromatography, the purified protein was refolded by dialysis against decreasing concentrations of urea. This resulted in a soluble antigen, which elicited antibodies capable of reacting with (denatured) Saa on Western blots after separation by SDS-PAGE. However, there is no guarantee that the final conformation accurately reflects that of the native protein, and important conformational epitopes may have been disrupted.
Finally, exogenous purified Saa significantly enhanced in vitro adherence of 98NK2 to HEp-2 cells. Purified exogenous protein might have been expected to compete with the endogenous adhesin for available binding sites on the epithelial cell surface, thereby reducing adherence. However, given that Saa contains predicted coiled-coil domains, it is also plausible that exogenous protein might be capable of directly interacting with surface-localized Saa and enhance interactions either with the epithelial surface or with other bacterial cells.
An understanding of the adherence mechanisms of STEC may be crucial to the development of effective anti-STEC vaccines. Vaccines based on inactivated Shiga toxins are likely to prevent the systemic complications, but they will not prevent transmission of STEC from person to person. For this reason, vaccines targeting products of the LEE locus are being developed to block colonization of either humans or animal reservoirs by LEE-positive STEC strains. However, in spite of the long-recognized capacity of LEE-negative STEC strains to cause life-threatening human disease, there have been very few studies of adherence mechanisms. Saa is the first adhesin to be characterized for this important class of human pathogens, and it warrants further investigation as a candidate vaccine antigen.
ACKNOWLEDGMENT
This work was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burland V, Shao Y, Perna N T, Plunkett G, Siofia H J, Blattner F R. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 1998;26:4196–4204. doi: 10.1093/nar/26.18.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.China B, Sory M P, N'Guyen B T, De Bruyere M, Cornelis G R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Azavedo J, McWhirter E, Louie M, Brunton J. Eae-negative verotoxin-producing Escherichia coli associated with haemolytic uremic syndrome and hemorrhagic colitis. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 265–268. [Google Scholar]
- 6.Delahay R M, Knutton S, Shaw R K, Hartland E L, Pallen M J, Frankel G. The coiled-coil domain of EspA is essential for the assembly of the type III secretion translocon on the surface of enteropathogenic Escherichia coli. J Biol Chem. 1999;274:35969–35974. doi: 10.1074/jbc.274.50.35969. [DOI] [PubMed] [Google Scholar]
- 7.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebel F, Podzadel T, Rohde M, Kresse A U, Krämer S, Deibel C, Guzman C, Chakraborty T. Initial binding of shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol Microbiol. 1998;30:147–161. doi: 10.1046/j.1365-2958.1998.01046.x. [DOI] [PubMed] [Google Scholar]
- 9.El Tahir Y, Kuusela P, Skurnik M. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol Microbiol. 2000;37:192–206. doi: 10.1046/j.1365-2958.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 12.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The asociation between idiopathic hemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 15.Kenny B, Lai L-C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherchia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 16.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kresse A U, Rohde M, Guzman C A. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect Immun. 1999;67:4834–4842. doi: 10.1128/iai.67.9.4834-4842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 20.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls L, Grant T H, Robins-Browne R M. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol Microbiol. 2000;35:275–288. doi: 10.1046/j.1365-2958.2000.01690.x. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Paton A W, Paton J C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton A W, Paton J C. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O-antigen of Escherichia coli serotype O113. Infect Immun. 1999;67:5930–5937. doi: 10.1128/iai.67.11.5930-5937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton A W, Paton J C, Heuzenroeder M W, Goldwater P N, Manning P A. Cloning and nucleotide sequence of a variant Shiga-like toxin II gene from Escherichia coli OX3:H21 isolated from a case of Sudden Infant Death Syndrome. Microb Pathog. 1992;13:225–236. doi: 10.1016/0882-4010(92)90023-h. [DOI] [PubMed] [Google Scholar]
- 26.Paton A W, Woodrow M C, Doyle R M, Lanser J A, Paton J C. Molecular characterization of a Shiga-toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton A W, Voss E, Manning P A, Paton J C. Shiga toxin-producing Escherichia coli isolates from cases of human disease show enhanced adherence to intestinal epithelial (Henle 407) cells. Infect Immun. 1997;65:3799–3805. doi: 10.1128/iai.65.9.3799-3805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reese M G, Harris N L, Eeckman F H. Large scale sequencing specific neural networks for promoter and splice site recognition. In: Hunter L, Klein T E, editors. Biocomputing: Proceedings of the 1996 Pacific Symposium. Singapore, Singapore: World Scientific Publishing Co.; 1996. [Google Scholar]
- 30.Sandt C H, Hill C W. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect Immun. 2000;68:2205–2214. doi: 10.1128/iai.68.4.2205-2214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scotland S M, Knutton S, Said B, Rowe B. Adherence to Caco-2 cells of Vero cytotoxin-producing strains of Escherichia coli belonging to serogroups other than O157. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 257–260. [Google Scholar]
- 32.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 34.Tamm A, Tarkkanen A-M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 35.Tarr P I, Bilge S S, Vary J C, Jr, Jelacic S, Habeeb R L, Ward T R, Baylor M R, Besser T E. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun. 2000;68:1400–1407. doi: 10.1128/iai.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor K A, O'Connell C B, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschape H, Prager R, Streckel W, Fruth A, Tietze E, Bohme G. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: green butter as the infection source. Epidemiol Infect. 1995;114:441–450. doi: 10.1017/s0950268800052158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Den Bosch L, Manning P A, Morona R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- 41.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13 mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]