Abstract
Background:
Hopefulness, whether inherently present (“dispositional hope”) or augmented (by enhancement techniques) may impact outcomes. We set out to determine the association of dispositional hope with survival among patients diagnosed with advanced cancer.
Methods:
Data from ENABLE, a palliative care intervention, were re-analyzed to determine the association of higher dispositional hope and patient survival. This is a secondary analysis of data combined from the ENABLE II and ENABLE III randomized controlled trials (RCTs) relative to dispositional hope and survival. A dispositional hope index was created from three “hope” items from two validated baseline questionnaires. Dispositional hope and survival data were collected during the two RCTs. In ENABLE II, participants were randomly assigned to the ENABLE intervention or to usual care. In ENABLE III, participants were randomly assigned to receive the intervention immediately or 12 weeks after enrollment.
Results:
529 persons were included in Cox proportional-hazards regression analyses to model the effects of dispositional hope on survival. An initial analysis without covariates yielded a significant effect of hope, (Wald = 8.649, HR = 0.941, CI: 0.904–0.980, p = 0.003), such that higher dipositional hope was associated with longer survival. In a subsequent analysis that included all covariates, the effect of dispotional hope approached statistical significance (Wald = 2.96, HR = 0.933, CI: 0.863–1.010, p = 0.085).
Conclusion:
Higher levels of dispositional hope was associated with longer survival in patients with advanced cancer. Prospective trials are needed to determine the effects of dispositional and augmented hope on outcomes of patients with advanced cancer.
Keywords: Hope, palliation, survival, ENABLE, advanced cancer
Precis:
Within the ENABLE database, composed of information pooled from randomized trials, dispositional hope was associated with longer survival among patients with advanced cancer. Accordingly, hope may be an important stratification parameter in the design of clinical trials and workshops to augment hopefulness might be considered for such individuals.
INTRODUCTION
In the 2020 update of cancer statistic (1), the American Cancer Society reported a decline in death rates of leading cancers including malignancies of breast, lung prostate and colon. Although the trend has been evident since 1991, Siegel et al emphasized that the drivers of this progress are difficult to decipher. While it is likely that these improvements are related to preventative measures (e.g., reductions in smoking) and novel therapies (e.g., combination treatments, checkpoint blockade immunotherapy and targeted therapies), the authors noted that additional factors may contribute to these results.
Recently, Corn, Feldman, and Wexler (2) have discussed dispositional hope (i.e., inherent hopefulness) and the ability to enhance hope (3) as one potential factor. Although the term hopefulness was previously associated with amorphous ideas and imprecise meanings, the concept has recently acquired a rigorous definition (4). Based on the work of Snyder et al (5) hopefulness is considered a measurable cognitive, goal-directed construct defined as “having goals” (hoped-for ends or objectives), “agency” (the energy or motivation to reach goals) and “pathways” (perceived routes and plans to approach goals) (6). Work by Feldman & Dreher (3) demonstrated that a single-session hope-enhancement intervention can increase dispositional hopefulness and raise healthy adults’ likelihood of goal accomplishment. In oncology, goals are not restricted to cure; but might also include a desire for symptom control or reaching a personal life goal.
Several investigators have suggested that interventions designed to enhance quality of life, by the very nature of the positive outlook created, may allow one to directly “will” a better outcome (7). Corn, Feldman, and Wexler (2) discuss possible biological mediators of a “direct” path, which are funneled through a psycho-neuro-immunological axis. Conversely, indirect mediators through the effects of hopefulness on behavior such as health-promoting behaviors (quitting smoking, adherence to exercise routines and dietary control), and pursuit of health-related knowledge (8–11) may have survival ramifications. Such salutary practices have demonstrated improved survival among patients with colorectal cancer who engaged in physical activity (12) and individuals diagnosed with cancers of the breast or bladder who experienced lower rates of mortality when smoking ceased (13, 14). Moreover, there could be an indirect benefit of hopefulness among patients who are demotivated to adhere to prescribed cancer therapeutics when such medications diminish quality of life (15, 16). In the latter scenario a hopeful person, or one who can be made more hopeful, might be able to persevere despite iatrogenic hardship and thereby adhere to toxic yet beneficial interventions. To date, the relationship between dispositional hope and survival among patients with cancer has not been extensively studied. The current analysis tested whether there is an association between dispositional hope and survival in studies of early palliative care designed to enhance quality of life.
Project ENABLE (Educate, Nurture, Advise, Before Life Ends), a nurse-led telehealth early palliative care intervention for patients with advanced cancer (17–19) demonstrated improved quality of life, mood, and survival benefits in two randomized controlled trials. Patient reported outcomes included items measuring hope. Therefore, we combined data from these trials to examine the relationship between baseline dispositional hope (as measured by a “hope index” created from single hope items), intervention status (if and when patients received the ENABLE intervention), and survival among patients with advanced cancer. Based on the theoretical linkage of hope with improved survival as mediated by a combination of biological and behavioral pathways (2), we hypothesized that baseline dispositional hope would be associated with longer survival.
METHODS
This study is a secondary analysis using a correlational design to test novel hypotheses about the association between dispositional hope and survival. The data were derived from two previously published randomized controlled trials. In ENABLE II (hereafter referred to as Study 1 in which enrollment took place between November 2003 and May 2007) (18) participants were randomly assigned to either the ENABLE intervention or (b) usual cancer care. In ENABLE III (hereafter referred to as Study 2, in which enrollment took place between October 2009 and March 2013) (19) using a wait-list control design, all participants received the ENABLE intervention but were randomly assigned to receive the intervention either early (upon enrollment) or after a delay of 12 weeks. Target sample sizes of 400 (Study 1) and 360 (Study 2) were chosen to provide 80% power to identify key differences in outcomes of interest in the original studies (quality of life, symptom intensity, mood) (18). Due to slower accrual than anticipated, the final enrollment totals were 322 (Study 1) and 207 (Study 2). The protocols, data, and safety monitoring board (DSMB) plans for both studies were approved by the Dartmouth College and the Veterans Affairs Medical Center (VAMC), White River Junction, Vermont institutional review boards (IRB). The trials were registered in clinicaltrials.gov (Identifier Study 1: NCT00253383; Identifier Study 2: NCT01245621).
In both studies, the primary dependent variables were patient-reported quality of life, mood, symptom intensity, and resource use. In Study 1, participant survival was examined in a post-hoc analysis. In Study 2, survival was examined as an a priori primary outcome. In the current analyses, we focus on the intervention and hope effects on survival. Specifically, we examine the association between participants’ baseline hope index and subsequent survival time as well as the moderating effect of receiving the ENABLE intervention on this association.
Participants
For both studies, participants were recruited from the Norris Cotton Cancer Center at Dartmouth-Hitchcock Medical Center or the WRJ VAMC within 60 days of a new diagnosis of advanced cancer. Eligibility criteria included diagnosis of a new advanced solid tumor or hematological malignancy (only ENABLE III), recurrence, or new disease progression following stable disease, and an oncologist-predicted prognosis of approximately 6–24 months. In addition, all participants had to be English-speaking and over age 18. Individuals were excluded if they had impaired cognition, or a severe psychiatric disease (e.g., schizophrenia, bipolar disorder) or active substance abuse disorder. As noted, pursuant to enrollment, patients were randomly assigned to receive the intervention vs. usual care (Study 1) or early vs. delayed intervention (Study 2). Full details of the respective studies are described elsewhere (18, 19).
The combined sample of this analysis included 529 patients, of whom 161 received usual care (Study 1 only), 265 received the early intervention (161 from Study 1; 104 from Study 2), and 103 received the delayed intervention (Study 2 only).
Intervention
The ENABLE intervention has been described in detail elsewhere (18, 19) and consists of a psychoeducational approach to encourage patient activation and enhanced quality of life. In brief, after an initial in-person palliative care consult, trained nurse coaches specializing in palliative care facilitated 4 (Study 1) or 6 (Study 2) semi-structured psychoeducational telephone coaching sessions with patients using an author-developed informational guidebook called Charting Your Course©, followed by monthly check-in calls until the patient died or the study ended. Topics discussed during sessions included problem-solving, coping, self-care, symptom management, building a support system, communication skills, decision-making, advanced care planning, and life review. Importantly, affecting the level of hope was not included as a specific intervention goal.
Data Collection and Instruments
The primary variables of interest in these analyses were self-reported dispositional hope and observed survival. A 3-item hope index was created by summing one item from the Center for Epidemiological Studies-Depression (CES-D) measure (during the past week “I felt hopeful about the future;” 0 = Rarely, 1 = Some or a little, 2 = Occasionally or Moderately, 3 = Most of the Time) and two items from the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-PAL), one from the Additional Concerns Subscale (“I feel hopeful;” 0 = Not at All, 1 = A little bit, 2 = Somewhat, 3 = Quite a bit, 4 = Very much) and one from the Emotional Well Being subscale (“I am losing hope in the fight against my illness” reverse scored such that 0 = Very Much, 1 = Quite a bit, 2 = Somewhat, 3 = A little bit, 4 = Not at All). This 3-item index showed acceptable reliability, Cronbach’s alpha = .778. In a principal component’s analysis, all three items loaded on a single factor above .80.
Survival time was calculated from the date of enrollment to the date of death; patients who were still alive at study closure (Study 1: May 1, 2008; Study 2: September 5, 2013) were censored on that date.
Given the correlational nature of the primary independent variables (individual differences in dispostional hope and an intervention that spanned two RCT), results were assessed in the context of a variety of statistical covariates. Specifically, self-reported demographics (e.g., age, gender, ethnicity/race, marital status, employment status, level of education and rural/urban residence) were collected at baseline. Cancer site was identified from the electronic health record. Furthemore, we collected variables representing illness severity characteristics (resource use 3 months prior to study enrollment (days spent in the hospital and intensive care unit [ICU] and emergency department [ED] visits), chart review variables pertaining to the use of chemotherapy or radiation, the presence of advance directives (AD) as well as do not resuscitate (DNR) orders. All analyses were conducted using SPSS software, Version 24 (SPSS Inc., Chicago, IL).
Analysis
The two study samples were compared on dispositional hope and all statistical covariates (described below) using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. For all continuous variables (hope index, days in hospital, days in ICU, ED visits, and age), the mean and standard deviation were calculated. The remaining variables were categorical: AD (yes/no), DNR (yes/no), undergoing chemotherapy at enrollment (yes/no), undergoing radiotherapy at enrollment (yes/no), gender (male/female), residence (rural vs. urban), education (college graduate vs. not), married (yes/no), employment (employed full or part time vs. not currently employed), and race (white vs. other). Cancer site was dummy-coded and each diagnosis site (lung, gastrointestinal, genitourinary, breast, hematological, and other) treated as a separate variable. For all dichotomous variables a count and percentage of the total were calculated. Pearson’s bivariate correlations between the baseline hope index and all other continuous covariates were calculated; point-biserial correlations were used for dichotomous covariates. Cox proportional-hazards regression analyses (20) were used to model the effects of dispositional hope on survival, with and without adjustment for baseline covariates.
RESULTS
Comparisons of Baseline Characteristics Between Study 1 and Study 2
Table 1 lists baseline characteristics for the total combined sample and for Study 1 and Study 2 separately for the hope index, demographics, cancer site, and illness severity-related variables. Participants had a mean age of 65, were 56% male, 68% married, 98% white race, 58% rural residence, and often had lung (39%) or gastrointestinal (35%) cancers. Of the 21 baseline variables examined, 8 differed between studies: Study 1 compared to Study 2 had a lower proportion of college graduates, a greater number of ICU days and ED visits. Further, Study 1 was characterized by more patients receiving chemotherapy, a higher proportion of patients with gastrointestinal cancer, and more patients receiving radiation treatments. Of note, Study 1 had no patients with hematological malignancies or cancers classified as “other” because only patients with four solid tumor types were eligible.
Table 1.
Baseline characteristics of combined sample & comparisons between Study 1 and Study 2
| Combined Sample | Comparison between study groups | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Study 1 (N = 322) | Study 2 (N = 207) | ||||||
|
|
|
||||||
| N | Mean (SD) or No. (%) | N | Mean (SD) or No. (%) | N | Mean (SD) or No. (%) | p * | |
|
| |||||||
| Demographic variables | |||||||
| Age | 529 | 64.7 (10.7) | 322 | 65.0 (11.2) | 207 | 64.3 (9.9) | .453 |
| Gender (male) | 529 | 296 (56%) | 322 | 187 (58.1%) | 207 | 109 (52.7%) | .221 |
| Rural residence | 529 | 306 (57.8%) | 322 | 181 (56.2%) | 207 | 125 (60.4%) | .343 |
| College graduate | 482 | 166 (34.4%) | 275 | 81 (29.5%) | 207 | 85 (41.1%) | .008 |
| Married | 526 | 356 (67.7%) | 319 | 221 (69.3%) | 207 | 135 (65.2%) | .331 |
| Employed | 525 | 112 (21.3%) | 318 | 63 (19.8%) | 207 | 49 (23.7%) | .291 |
| White | 485 | 475 (97.9%) | 279 | 275 (98.6%) | 206 | 200 (97.1%) | .257 |
|
| |||||||
| Cancer site | |||||||
| Lung (NSCLC) | 529 | 205 (38.8%) | 322 | 117 (36.3%) | 207 | 88 (42.5%) | .155 |
| Gastrointestinal | 529 | 183 (34.6%) | 322 | 133 (41.3%) | 207 | 50 (24.2%) | .000 |
| Genitourinary | 529 | 55 (10.4%) | 322 | 39 (12.1%) | 207 | 16 (7.7%) | .107 |
| Breast | 529 | 56 (10.6%) | 322 | 33 (10.2%) | 207 | 23 (11.1%) | .753 |
| Hematological | 529 | 10 (1.9%) | 322 | 0 (0%) | 207 | 10 (4.8%) | .000 |
| Other cancer | 529 | 20 (3.8%) | 322 | 0 (0%) | 207 | 20 (9.7%) | .000 |
|
| |||||||
| x Days in hospital | 528 | 2.79 (5.2) | 322 | 2.95 (5.2) | 206 | 2.54 (5.2) | .381 |
| Days in ICU | 404 | .18 (.92) | 322 | .03 (.25) | 82 | .74 (1.9) | .001 |
| ED visits | 529 | .43 (.84) | 322 | .34 (.78) | 207 | .57 (.90) | .003 |
| Advanced directives | 527 | 238 (45.2%) | 322 | 149 (46.3%) | 205 | 89 (43.4%) | .520 |
| Do not resuscitate | 515 | 43 (8.3%) | 322 | 23 (7.1%) | 193 | 20 (10.4%) | .201 |
| Chemotherapy | 529 | 427 (80.7%) | 322 | 271 (84.2%) | 207 | 156 (75.4%) | .012 |
| Radiation | 529 | 81 (15.3%) | 322 | 41 (12.7%) | 207 | 40 (19.3%) | .040 |
|
| |||||||
| Hope | |||||||
| Baseline HOPE | 465 | 8.1 (2.7) | 264 | 8.1 (2.6) | 201 | 8.1 (2.8) | .958 |
Abbreviations: SD = Standard Deviation; % = Percent of total patients; ICU = Intensive Care Unit; ED = Emergency Medicine Department.
Independent samples t-tests were used for continuous variables (Age, Days in hospital, Days in ICU, ER visits, and Baseline HOPE); Chi-Squared analyses were used for categorical variables.
Association Between Baseline Hope Index and Covariates
Being employed (p=.004) and having a breast cancer diagnosis (p=.044) was associated with a higher hope index. Being diagnosed with lung cancer (p=.016), having completed an AD (p=.012), and having a DNR order on file (p=.009) was associated with lower hope index. No other associations achieved statistical significance (Table 2).
Table 2.
Correlation of baseline Hope with control covariates
| N | r | p | |
|---|---|---|---|
|
| |||
| Demographic variables | |||
| Age | 465 | −.004 | .938 |
| Gender (male) | 465 | .001 | .986 |
| Rural residence | 465 | −.024 | .610 |
| College graduate | 461 | −.028 | .545 |
| Married | 462 | .057 | .220 |
| Employed | 461 | .135 | .004 |
| White | 464 | .077 | .097 |
|
| |||
| Cancer site | |||
| Lung | 465 | −.111 | .016 |
| Gastrointestinal | 465 | −.022 | .633 |
| Genitourinary | 465 | .028 | .548 |
| Breast | 465 | .093 | .044 |
| Hematological | 465 | .088 | .058 |
| Other cancer | 465 | .072 | .120 |
|
| |||
| Illness-related variables | |||
| Days in hospital | 464 | .008 | .869 |
| Days in ICU | 344 | .029 | .590 |
| ED visits | 465 | −.008 | .864 |
| Advanced directives | 463 | −.117 | .012 |
| Do not resuscitate | 452 | −.122 | .009 |
| Chemotherapy | 465 | .020 | .672 |
| Radiation | 465 | .034 | .471 |
ICU = Intensive Care Unit; ER = Emergency Medicine Department. Analyses used pairwise deletion and significance values are not corrected for multiple comparisons.
Association Between Baseline Hope Index and Survival
An initial Cox regression survival analysis with no covariates yielded a significant effect of dispositional hope such that a higher baseline hope index was associated with longer survival (Wald = 8.649, HR = 0.941, CI: 0.904–0.980, p = 0.003).
A subsequent analysis was used to model the effects of intervention status, hope index, and their interaction adjusting for all 19 baseline covariates. The design involved survival over time among a control group that never received the intervention (usual care), an experimental group that received the intervention upon enrollment (early intervention), and an experimental group that received the intervention 12 weeks after enrollment (delayed intervention). Because the intervention was the same for the latter two groups and simply involved implementation at different points in time, the data were handled using a Cox regression survival analysis with intervention as a time-varying covariate (Cox, 1972). Patients with missing covariate data were excluded as needed from each Cox model. As can be seen in Table 3, in this analysis the effect of dispositional hope did not achieve conventional levels of significance (p = .085). As is also evident in Table 3, neither the effect of the intervention (p = 0.246) nor the interaction between dispositional hope and the intervention (p = 0.778) achieved statistical significance. Five baseline covariates achieved significance: number of hospital days 3 months prior to enrollment (p < 0.001), DNR order on file (p < 0.001), lung cancer diagnosis (p = 0.045), gastrointestinal cancer diagnosis (p = 0.041), hematological cancer diagnosis (p = 0.025) and gender (p = 0.033).
Table 3.
Results of Cox Proportional Hazards Regression Analysis including all statistical covariates
| 95% CI for HR | |||||
|---|---|---|---|---|---|
|
|
|||||
| Variables in the Equation | Wald | HR | p | Lower | Upper |
|
| |||||
| Intervention | 1.24 | 0.601 | .265 | 0.246 | 1.471 |
| HOPE (at baseline) | 2.96 | 0.933 | .085 | 0.863 | 1.010 |
| Intervention x HOPE | 0.08 | 1.015 | .778 | 0.915 | 1.127 |
|
| |||||
| Demographic variables | |||||
| Age | 0.11 | 0.998 | .737 | 0.984 | 1.012 |
| Gender (male) | 4.53 | 0.701 | .033 | 0.506 | 0.972 |
| Rural | 0.04 | 0.973 | .849 | 0.732 | 1.294 |
| College graduate | 2.60 | 0.778 | .107 | 0.574 | 1.056 |
| Married | 3.68 | 0.727 | .055 | 0.524 | 1.007 |
| Employed | 0.00 | 1.001 | .996 | 0.690 | 1.453 |
| White | 1.36 | 0.427 | .244 | 0.102 | 1.786 |
|
| |||||
| Cancer site | |||||
| Lung | 4.05 | 0.297 | .044 | 0.091 | 0.969 |
| Gastrointestinal | 4.19 | 0.289 | .041 | 0.088 | 0.948 |
| Genitourinary | 1.53 | 0.458 | .216 | 0.133 | 1.578 |
| Breast | 1.40 | 0.455 | .236 | 0.124 | 1.675 |
| Hematological | 5.03 | 0.198 | .025 | 0.048 | 0.816 |
|
| |||||
| Illness-related variables | |||||
| Days in hospital | 17.98 | 1.060 | < .001 | 1.032 | 1.088 |
| Days in ICU | 1.02 | 0.922 | .313 | 0.789 | 1.079 |
| ED visits | 0.10 | 0.973 | .753 | 0.822 | 1.153 |
| Advanced directives | 0.17 | 1.065 | .677 | 0.793 | 1.430 |
| Do not resuscitate | 22.49 | 0.293 | < .001 | 0.177 | 0.487 |
| Chemotherapy | 1.71 | 0.771 | .190 | 0.522 | 1.138 |
| Radiation | 0.85 | 0.820 | .358 | 0.538 | 1.251 |
CI = Confidence Interval; HR = Hazard Ratio (risk of death); Wald = Wald statistic; Intervention = having the palliative care intervention (vs. not having it) entered as a time-varying covariate; ICU = Intensive Care Unit; ER = Emergency Medicine Department. All variables were entered into a Cox proportional hazards regression model simultaneously.
The Cox analysis was repeated including only covariates that correlated significantly with the hope index (ADs, DNR order on file, lung cancer diagnosis, breast cancer diagnosis, and being employed) (see Table 2) as a test of whether these variables rather than the hope index, could be responsible for the relationships of interest. In this analysis, dispostional hope was once again associated with longer survival (Wald = 5.435, HR = 0.952, CI: 0.913–0.992, p = 0.020).
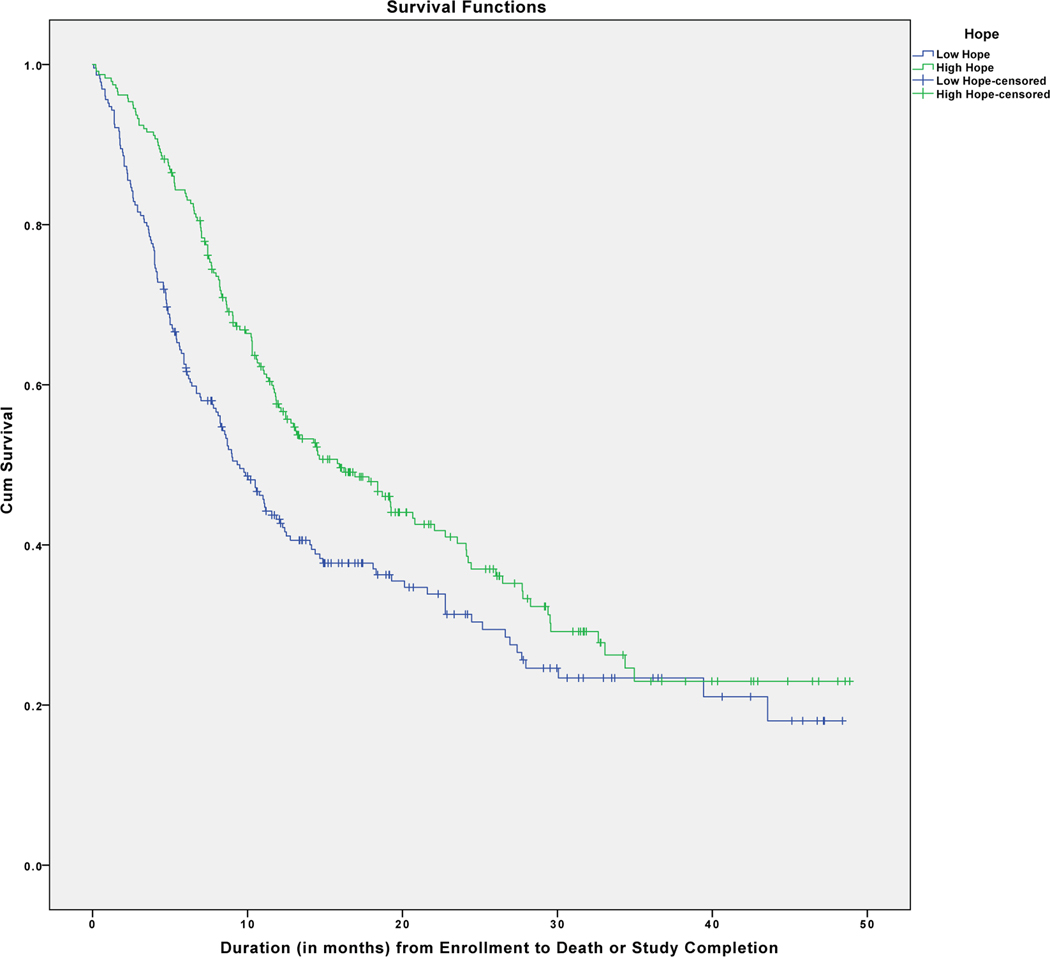
In order to provide a better understanding of these results, patients were divided into groups as a function of their categorization as having relatively high (Hope Index > 8.0) or low (Hope Index < 8.0) dispositional hope as determined by a median split. As can be seen in Figure 1, those high in hope survived longer than those low in hope. A Mantel-Cox Log Rank test applied to these data was significant, c2(1) = 9.258, p = .002, with median survival time duration (in months) greater in the high hope (Mdn = 15.933, CI: 11.858–20.009) than in the low hope group (Mdn = 9.500, CI: 7.70–11.30). That said, hope is treated as a continuous variable using applicable covariate adjustments in our primary statistical hypothesis tests, and patients were categorized into these high and low hope groups primarily for illustrative purposes.
Figure 1.
Kaplan-Meier survival curves for patients with relatively high versus low hope (determined by a median split on the entire patient sample). Cross-hatch lines indicate censored data. Not corrected for covariates
DISCUSSION
This is one of the first studies examining and finding a positive association between dispositional hope and overall survival among patients diagnosed with advanced cancer. However, other investigators have explored linkages between hopefulness and survival in chronic disease. Moskowitz et al (21), analyzed data from the National Health Epidemiologic Follow-up Study (NHEFS) and noted that among older patients (>65) with diabetes, hopefulness was associated with a lower mortality risk. Those authors speculated that contextual factors, including the likelihood of increased medication adherence among hopeful patients (22), may have an influence on the relationship of positive affect and survival.
The present analysis of the combined samples of the ENABLE II and III RCTs, although exploratory, was conducted among 529 patients who were diagnosed with advanced stages of relatively common cancers (e.g., lung, breast, colon, etc.). Although there are multiple limitations of a retrospective secondary analysis, the results reported herein are statistically significant, may be clinically meaningful, and are hypothesis-generating for future clinical trials.
We are not advocating that positive psychology be invoked as an oncologic strategy (e.g., that hopefulness should in any way substitute for proven clinical treatments.) Rather we consider that there are several direct and indirect theory-based explanations for why dispositional hope may favorably impact survival. First, there may be a direct effect of a “hope phenotype” in which a positive outlook, whether by sheer will or as an epigenetic phenomenon, could result in better survival (7). Indeed, in the context of patients with cervical cancer, Nelson et al suggested a mechanism that activates the psychoneuroimmunologic axis. Since cancer management is complex and heterogeneous, it may be prudent to measure and examine the impact of baseline dispositional hope within prospective trials assessing new therapeutic interventions for patients with advanced malignancies.
It is also arguable, however, that patients who can access hope might have indirect benefits via health-promoting behaviors such as exercising, consuming nutritionally-sound diets, or enhancing medication adherence. The influence of hope in these contexts is easily assessed by a prospective trial design in which patients are randomized to a therapy with or without hope-enhancement training. For example, endocrine therapy is known to be associated with a survival advantage among patients with breast cancer, yet non-adherence has been consistently described in approximately 45% of women for whom Level I evidence dictates prescription of such medications (23). Recently, the Southwest Oncology Group (SWOG) conducted a phase III trial to determine if text messaging increases adherence to such aromatase inhibitors. Although the idea had merit, twice-weekly reminders via smartphone did not improve adherence. The SWOG is now poised to study whether hope enhancement techniques based on Feldman and Dreher’s (3) work could increase adherence to endocrine therapy. The premise of such an approach is that hope techniques may enable patients to see beyond the toxicity of aromatase inhibitors while pursuing the dual objectives of improving survival and minimizing recurrence. The latter can only be achieved if the admittedly toxic drugs are taken (Mark O’Rourke, personal communication, August 2020).
Another possible mechanism for the impact of hope on survival pertains to the role of pain. Several authors have speculated that there is an inverse relationship between pain levels and survival outcomes in patients diagnosed with pancreatic cancer (24, 25). Furthermore, in vivo models imply that animals with implanted tumors have accelerated tumor growth and increased mortality rates when subjected to pain (26, 27). Conversely, hope appears to be related to heightened pain tolerance and pain coping. In a laboratory study, Snyder and colleagues (28) found that healthy adults with high hope were able to tolerate the pain associated with having their hands submerged in freezing water better than low-hope participants. This connection between hope and pain perception appears to be mediated by “pain catastrophization,” with research demonstrating that higher-hope individuals spend less time ruminating on their pain than lower-hope individuals (29).
Our results should be viewed in the context of other recent advances in the management of cancer. During the past two decades, oncologists have witnessed an unprecedented number of new drugs or pharmacologic combinations that have been recognized as standard therapy by regulatory bodies (30–34). Remarkably, this progress has not been restricted to rare malignancies but rather has changed practice for the most frequently encountered entities including tumors of the lung, breast, and gastrointestinal tract. In reviewing the divergence of the survival curves documented in these phase III studies, we noted that the benefits were, in several instances, of similar orders of magnitude to those associated with hopefulness in our report. While the data presented herein are derived from post-hoc analyses, this finding seems important to examine formally in prospective clinical trials. Moreover, the financial commitment to achieve the advances with the aforementioned medications has been enormous (35, 36). In contrast to the costs of contemporary cancer care, the dissemination of hope techniques requires a minimal investment of money and time. What’s more, the benefits derived from hope enhancement techniques are realized immediately (3) and are likely to be tumor agnostic.
This study has several notable limitations. First the ENABLE investigators did not define dispositional hope as an a priori entity to be assessed vis-à-vis survival. This limitation is not simply an admission of the retrospective nature of our analysis but also an acknowledgment of the fact that established scales specifically designed for measuring hopefulness were not employed (5). Rather, within the instruments administered by ENABLE investigators (i.e., CES-D and FACIT-PAL) elements existed which allowed patients to declare whether they deem themselves to be hopeful. Future research must use validated tools of hopefulness to verify the purported association with survival. Notwithstanding, the simple metric of dispositional hope which we employed seems intuitive with internally-reassuring correlations (e.g., associations between hopefulness and employment; linkage between hopelessness and DNR orders). Second, the ENABLE sample was majority white race and these results should not be considered valid in a more racially diverse sample (19). As such, future research will be mandatory to test whether the present results can be generalized for more diverse populations.
Third, we focused on the levels of hopefulness that were determind at baseline. The presence of hope is not a static entity. Rather, hope is dynamic as manifest by the changing degrees of hope that human beings experience on a daily basis. Unfortunatgely, missing data with regards to hopefulness at follow-up visits precludes a more sophisticated analysis which looks at hope as it varies with time.
Another limitation pertains to our dichotimization of the sampe in terms of “high” and “low” hope. In the ENABLE database, hope was measured as a continuous item using Likert scales. For ease of analysis we divided patients into low and high hope gropus by using a median split. The median split was based on the scores of our particular sample. Therefore, other samples would be expected to have a different median and a slightly different categorization of high and low hopefulness. This shortcoming highlights the value of replicating and extending these findings in other samples of patients with different stages of disease and illness trajectories.
Our report is observational and, by definition, cannot establish a direct causal relationship between increased hopefulness and improvements in overall survival. As such, we cannot conclude from the present study whether hopeful people are more likely to live longer, or whether individuals with a better prognosis are likely to be more hopeful. There are four reasons to believe the former rather than the latter argument. First, research shows that patients with cancer often do not know or correctly understand their prognosis (37–39) hence it is unlikely that progrostic understanding played a significant role in this sample. Second, in the present study hope was measured at baseline, and its relationship with mortality approached statistical significance (p = .085) even adjusting for all 19 covariates, including indicators of illness severity. When the analysis was repeated, adjusting only for the covariates that manifested statistically significant correlations with hope, the relationship between dispositional hope and survival retained statistical significance (p = .02). Third, it should be mentioned that past research on hope outside of the cancer domain has shown reciprocal relationships between hope and life outcomes (40, 41). As such, future research may be necessary to explore reciprocal relationships in the context of advanced cancers. For instance, hopefulness (or lack thereof) may predict later cancer progression which, in turn, predicts subsequent levels of hopefulness (whether increased or decreased). Finally, past research indicates that psychosocial interventiosn can potentially have causal impacts on surivival (42–46).
To determine a causal relationship between hopefulness and survival, future investigations should include randomized controlled trials of hope enhancement techniques to determine if hope might be susceptible to favorable intervention (3). At this juncture, such trials are being designed to gauge whether hope enhancement techniques can be adopted by physicians and patients as another component of the clinical armamentarium against cancer. We recognize that this view constitutes a departure from conventional trial design. However, hope enhancement offers a very low-cost intervention that might influence the duration and the quality of life of patients with cancer. The clinical impact of such measures may be considerable.
Acknowledgments
Funding: Support was provided by the National Institute of Nursing Research (R01NR011871–01), the National Cancer Institute (grant R01 CA101704), and the Pamm Gross Kahane Research Institute of Life’s Door.
Footnotes
Conflicts of Interest: None.
Trial Registration: The trials were registered in clinicaltrials.gov (Identifier Study 1: NCT00253383; Identifier Study 2: NCT01245621).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Corn BW, Feldman DB, Wexler ID: The science of hope. Lancet Oncol 2020; 21: e452–e459. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DB, Dreher DE: Can hope be changed in 90 minutes? Testing the efficacy of a single-session goal-pursuit intervention for college students. J Happiness Stud 2012; 13: 745–759. [Google Scholar]
- 4.Snyder CR. Hope theory: Rainbows in the mind. Psychol Inq 2002; 13: 249–275. [Google Scholar]
- 5.Snyder CR, Harris C, Anderson JR, et al. The will and the ways: Development and validation of an individual differences measure of hope. J Pers Soc Psychol 1991; 60: 570–585. [DOI] [PubMed] [Google Scholar]
- 6.Feldman DB, Rand KL, Kahle-Wrobleski K. Hope and goal attainment: Testing a brief basic prediction of hope theory. J Soc Clin Psychol 2009; 28: 479–497. [Google Scholar]
- 7.Nelson EL, Wenzel LB, Osann K, et al. Stress immunity and cervical cancer: Biobehavioral outcomes of a randomized clinical trial. Clin Cancer Res 2008; 14: 2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg AT, Shauer GG, Rodgers K, Narula SK. College student smokers: Former versus current and non-smokers. Am J Prev Med 2012; 43: S229–236. [DOI] [PubMed] [Google Scholar]
- 9.Nothwehr F, Clark DO, Perkins A. Hope and the use of behavioral strategies related to diet and physical activity. J Hum Nutr Diet 2013; 26: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irving LM, Snyder CR, Crowson JJ Jr. Hope and coping with cancer by college women. J Pers 1998; 66: 195–214. [DOI] [PubMed] [Google Scholar]
- 11.Feldman DB, Sills JR. Hope and cardiovascular health-promoting behavior: Education alone is not enough. Psychology & Health 2013; 28: 727–45. [DOI] [PubMed] [Google Scholar]
- 12.Guerico BJ, Zhang S, Ou F, et al. Associations of physical activity with survival and progression in metastatic colorectal cancer: Results from CALGB (Alliance)/SWOG 80405. J Clin Oncol 2019; 37: 2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: Mortality from breast cancer and smoking related diseases. J Clin Oncol 2016; 34: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cacciamani GE, Ghodoussipour S, Mari A, et al. Association between smoking exposure, neoadjuvant chemotherapy response and survival outcomes following radical cystectomy: Systematic review and meta-analysis. J Urol 2020; 204: 649–660. [DOI] [PubMed] [Google Scholar]
- 15.Hershman DL, Unger JM, Hillyer GC, et al. Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol 2020; 38: 2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia S, Hageman L, Chen Y et al. Effect of a daily messaging and directly supervised therapy intervention on oral mercaptopurine adherence in children with Acute Lymphoblastic Leukemia: A randomized clinical trial. JAMA Network Open 2020;3(8): e2014205. doi: 1001/jamanetworkopen.2020.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakitas M, Stevens M, Ahles T, et al. Project ENABLE: A palliative care demonstration project for advanced cancer patients in three settings. J Palliat Med 2004; 7: 363–372. [DOI] [PubMed] [Google Scholar]
- 18.Bakitas M, Lyons K, Hegel M, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009; 302: 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakitas M, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015; 33: 1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society: Series B, Methodological, 1972; 34: 187–220. [Google Scholar]
- 21.Moskowitz JT, Epel E, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychology 2008; 27: S73–81. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez JS, Penedo FJ, Antoni M, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology 2004; 23: 413–18. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010; 28: 4120–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchicectomy in patients with unresectable pancreatic cancer: A prospective randomized trial. Ann Surg 1993; 217: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelsen DP, Portenoy R, Thaler H, et al. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery 1997; 122: 53–59. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JW, Shavit Y, Terman GW, Gale RP, Liebskind JC. Stress and morphine affect survival of rats challenged with a mammary ascites tumor (MAT 13762B). Nat Immun Cell Growth Regul 1983; 3: 43–50. [PubMed] [Google Scholar]
- 27.Page GG, Ben-Eliyahu S, Yirmiya R, Liebskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain 1993; 54: 21–28. [DOI] [PubMed] [Google Scholar]
- 28.Snyder CR, Berg C, Woodward JT, et al. Hope against the cold: individual differences in trait hope and acute pain tolerance on the cold pressor task. J Pers 2005; 73: 287–312. [DOI] [PubMed] [Google Scholar]
- 29.Hood A, Pulvers K, Carrillo J, Merchant G, Thomas M. Positive traits linked to less pain through lower pain catastrophizing. Personality and Individual Differences 2012; 52: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slamon D, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against Her-2 for metastatic breast cancer that overexpresses Her-2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 31.Socinski MA, Cappuzzo JF, Orlandi F, et al. Atezolizumab for first-line treatment of metastatic non-squamous NSCLC. N Engl J Med 2018; 378: 2288–301. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Byoung CC, Masanobu T, et al. Nivolumab vs chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multi-centre, randomized, open-label phase 3 trial. Lancet Oncology 2019; 20: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 33.Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 2019; 381: 1632–43. [DOI] [PubMed] [Google Scholar]
- 34.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX vs Gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 35.Mariotto AB, Yabroff KR, Shao Y, Fever EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011; 103: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 2020; 323: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? Supportive Care in Cancer. 1994. Jul 1;2(4):242–4. [DOI] [PubMed] [Google Scholar]
- 38.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012; 367: 1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein AS, Prigerson HG, O’Reilly EM, Maciejewski PK. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol 2016; 34: 2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman DB, Rand KL, Kahle-Wrobleski K. Hope and goal attainment: Testing a basic prediction of hope theory. Journal of Social and Clinical Psychology. 2009; 28: 479–97. [Google Scholar]
- 41.Van Ryzin MJ. Protective factors at school: Reciprocal effects among adolescents’ perceptions of the school environment, engagement in learning, and hope. Journal of youth and adolescence. 2011; 40: 1568–80. [DOI] [PubMed] [Google Scholar]
- 42.Faller H, Schuler M, Richard M, Heckl U, Weis J, Kuffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013; 31: 782–793. [DOI] [PubMed] [Google Scholar]
- 43.Spiegel D, Kraemer HC, Bloom JR, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989; 2 (8668): 888–891. [DOI] [PubMed] [Google Scholar]
- 44.Goodwin P, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastic breast cancer. N Engl J Med. 2001; 345: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 45.Kuchler T, Bestmann B, Rappat S, Henne-Bruns D, Wood-Dauphinee S. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-yer survival results of a randomized trial. J Clin Oncol. 2007; 25: 2702–2708. [DOI] [PubMed] [Google Scholar]
- 46.Andersen B, Yang H, Farrar W, et al. Psychologic interventions improve survival for berast cancer patients: a ransomized clincal trial. Cancer. 2008; 113: 3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]